Abstract
Purpose of Review
Continuous glucose monitors (CGM) are transforming diabetes management, yet adolescents and young adults (AYA) with type 1 diabetes (T1D) do not experience the same benefits seen with CGM use in adults. The purpose of this review is to explore advances, challenges, and the financial impact of CGM use in AYA with T1D.
Recent Findings
CGM studies in young adults highlight challenges and suggest unique barriers to CGM use in this population. Recent studies also demonstrate differences in CGM use related to race and ethnicity, raising questions about potential bias and emphasizing the importance of patient-provider communication. Cost of these devices remains a significant barrier, especially in countries without nationalized reimbursement of CGM.
Summary
More research is needed to understand and address the differences in CGM utilization and to increase the accessibility of CGM therapy given the significant potential benefits of CGM in this high-risk group.
Keywords: Type 1 diabetes, Adolescents, Continuous glucose monitors, Barriers, Technology, Cost
Introduction
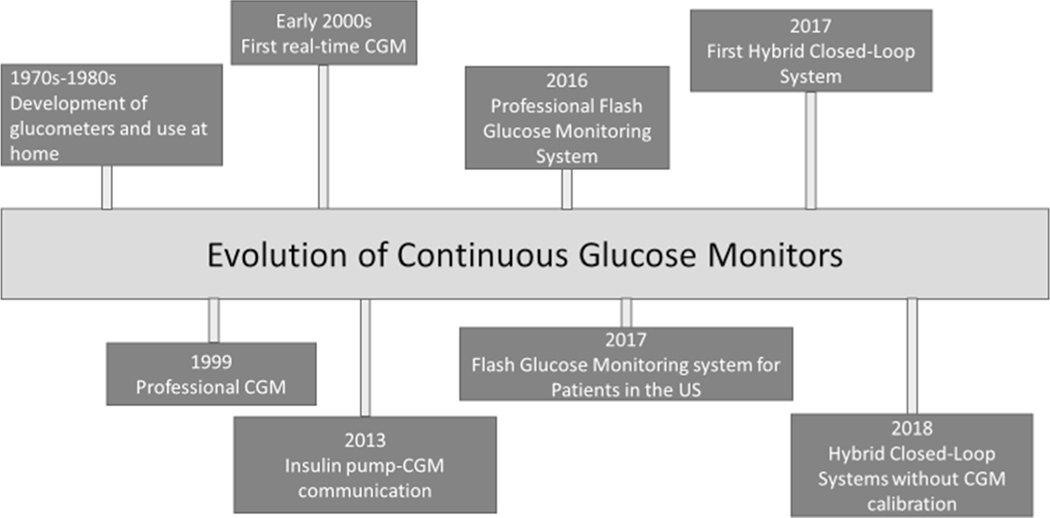
In 2015, the Type 1 Diabetes Exchange Clinical Registry reported that only 17% of adolescents and 14% of young adults (YA) with type 1 diabetes (T1D) met glycemic goals [1]. Updated data in 2019 demonstrated worsening glycemic control in these age groups [2••]. While technologies such as continuous glucose monitors (CGMs) have been shown to improve glycemic control, adolescents and young adults (AYAs) are the least likely age groups to use this technology [2••]. Over the last 20 years, CGM technology has significantly evolved, progressing from physician-use monitoring systems to personal devices that allow for real-time monitoring of interstitial glucose (Fig. 1). While AYAs are increasing their use of these devices, the 2016–2018 T1D Exchange analysis found only 22–24% of AYAs reported using CGM compared to 51% of children (<age 6) and 37% of 26–50-year-olds [2••]. Studies in adults using CGM have demonstrated improved glycemic control, decreased hypoglycemia, and improved patient-reported outcomes such as quality of life; however, early CGM studies in AYAs failed to show similar benefits despite using the same technology [3, 4]. Given the risk for poor glycemic control in this population, understanding barriers to using these devices is key to improving adherence and ultimately glycemic control. The purpose of this review is to explore benefits, barriers, and the financial impact of CGM use in AYAs with T1D.
Fig. 1.
Evolution of continuous glucose monitors and hybrid closed-loop technology
The Evolution of Continuous Glucose Monitoring Systems
Continuous glucose monitoring (CGM) systems utilize a minimally invasive glucose sensor to measure interstitial glucose levels [5]. CGMs consist of a sensor to detect glucose levels, a transmitter to send a signal from the sensor to the receiver, and a receiver which converts the signal into a glucose level which is then displayed on the device. Glucose levels are measured every 1–5 min and are continuously sent to devices such as physical receivers, smartphones, and insulin pumps [6]. In 1999, the FDA approved the first physician-use continuous glucose monitor [7, 8]. This device passively collected glucose values over a series of days, and results could be viewed by patients at their doctor’s office. Since then, CGM devices have changed dramatically (Fig. 1) with several FDA-approved devices now available for use by patients. Personal CGM systems currently approved for use in pediatrics in the USA are manufactured by Dexcom (the most recent Dexcom G6 is approved down to age 2), FreeStyle Libre 2 system (approved for age 4 and above), and the Medtronic Guardian Sensor 3 (approved for age 14 and above when used alone and down to age 2 when used with the Medtronic 770G system) [9–11].
Among these CGM devices, there are several differences; the Dexcom and Medtronic devices are real-time CGM (rtCGM) devices, while the FreeStyle Libre devices are intermittently scanned CGM (isCGM). RtCGMs automatically send glucose data measured continuously to patients and provide alarms for predicted high and low blood glucose levels based on the device algorithm [12]. In contrast, isCGMs must be scanned by a receiver to provide readings of glucose data and cannot predict high and low glucose levels, although the FreeStyle Libre 2 system does now offer certain alarm features [12, 13]. Other notable differences include the need for calibration; the Medtronic Guardian Sensor 3 requires calibration with finger stick blood glucose every 12 h, while the FreeStyle Libre and Dexcom G6 are factory calibrated systems. All three devices have the ability for realtime data sharing to clinicians, caregivers, family, and friends [14]. The latest advance in CGM technology is the development of hybrid closed-loop control (CLC) systems which allow for automated insulin delivery based on interstitial glucose readings provided by the CGM [15]. The first CLC system was introduced in 2013 and suspended insulin delivery with predicted low blood glucose; since then, the technology has progressed quickly with current devices automating basal insulin delivery and correction insulin boluses [16–18].
Over the last 20 years, CGM technology has changed significantly, progressing from devices primarily for professional use to personal devices and most recently to devices integrated with insulin pumps allowing for more automated insulin delivery. The hope is that with these advanced technologies, patients will be able to better manage their diabetes with improved glycemic control and quality of life, and decreased hypoglycemia. Although the technology has dramatically changed and there have been many well-documented benefits of these devices [3], barriers to successful and consistent use of these devices remain a continued challenge in the AYA population.
Benefits of Continuous Glucose Monitor Use
Advances in continuous glucose monitoring (CGM) systems have been exponential in the last few decades (Fig. 1) and have significantly changed the management of T1D. Research on benefits of CGM use in adults with T1D is widespread [3, 4, 19, 20]. For example, the landmark Juvenile Diabetes Research Foundation (JDRF) trial in 2008 demonstrated significant reduction in hemoglobin A1c (HbA1c) in adults age 25 or older using CGM (mean difference in HbA1c in CGM group −.53%, p<0.001 compared to controls) [3]. A 2017 study examined the effect of CGM use in adults using insulin injections and again found significantly improved glycemic control in the group wearing CGM at the end of the trial (HbA1c −0.6%, p<0.001) [21]. In 2014, Belgium piloted a prospective observational study of CGM reimbursement in patients with T1D using insulin pump therapy at select diabetes centers. The diabetes centers selected participants to participate in this pilot (participants were required to meet some minimum criteria including diagnosis >1 year, insulin pump therapy >6 months, poor glycemic control, and interest in CGM use). This prospective observational study in adults with T1D demonstrated improved HbA1c from 7.7±0.9% to 7.4±0.8% at 12 months after reimbursement of CGM (p<0.0001), with an increased proportion of patients achieving glycemic targets (HbA1c <7%), and significant improvement in those patients who started CGM due to poor glycemic control (8.2 to 7.6% after 12 months, p<0.0001) [4]. This study also found that fear of hypoglycemia decreased in the total study population, with the most prominent decrease in those adults who started on CGM because of problems related to hypoglycemia [4]. Further, CGM has been associated with decreased time in hypoglycemia and less fear of hypoglycemia [4, 19, 20]. Finally, in a randomized trial of patients age 18–75 years with impaired hypoglycemia awareness, CGM increased time spent in normoglycemia (65.0% versus 55.4%, p<0.0001), decreased both hypoglycemia and hyperglycemia, and decreased severe hypoglycemia [19] as compared to self-monitoring of blood glucose. Importantly, psychosocial factors such as quality of life and treatment satisfaction improved in studies examining effects of CGM use in adults as well [4].
Research in youth specifically focused on the potential benefits of CGM use is less prevalent than in adults, and the findings are less certain [22, 23]. The 2008 JDRF CGM study described above found no difference in HbA1c in young adults wearing CGM compared to those who did not [3]. A more recent study evaluating the effect of CGM on HbA1c in AYA found a small but significant decrease in those wearing CGM (−0.37%, p=0.01) [24••]. Similarly, in a large cross-sectional study of 1992 patients younger than 26 years old, CGM use was associated with lower HbA1c (HbA1c 9.1% compared to 8.7%, p<.008) [25]. CGM use has also been associated with less time spent in hypoglycemia, fewer episodes of severe hypoglycemia, and less overnight glucometer testing in AYA [24••, 26, 27].
Qualitative studies of CGM use in youth have demonstrated improved psychosocial outcomes, such as reduced adolescent, parental and familial distress, lower anxiety and fear of hypoglycemia, improved parental sleep quality, and improved quality of life [27–29]. Children and parents also reported feeling more confident with regard to blood glucose monitoring, allowing for more child independence and delegation of care to other caregivers [26]. In a recent study, Messer and colleagues found that adolescents with a higher perceived benefit of CGM use exhibited less diabetes distress, higher self-efficacy (i.e., perceived capability to carry out a certain behavior), and more positive attitudes toward technology, while those perceiving a higher burden of CGM displayed the opposite: more diabetes distress, less self-efficacy, and less positive technology attitudes [30].
From a broader perspective, several national prospective studies following full governmental subsidy of CGM for people with T1D showed significant benefits on multiple levels. For example, in a prospective cohort study in a tertiary hospital in Western Australia involving youth (age 18 and below) with T1D, there was a significant decrease in parental fear of hypoglycemia and improved hypoglycemia awareness in youth, as well as increased parental and child diabetes treatment satisfaction, better parental sleep quality, and less overnight glucometer testing [27]. Additionally, HbA1c in this sample significantly decreased from 8.4 to 8.1% (68 to 65 mmol/mol) [27]. Similarly, in a large, prospective multicenter study in adults in Belgium, improved glycemic control and psychological outcomes, such as quality of life, were observed after the implementation of a national reimbursement program [4, 20, 31].
Although research investigating benefits of CGM use in AYA is rapidly escalating, more studies with diverse populations are needed to understand why the benefits seen in adults with T1D who use CGM do not translate to this high-risk population. The difference in outcomes in these two groups suggests the presence of unique barriers to CGM use in the AYA population.
Challenges Associated with Continuous Glucose Monitor Use
Despite the many demonstrated benefits of CGM use, including improved glycemic control and reduced hypoglycemia, the 2019 Type 1 Diabetes Exchange Clinical Registry data show that AYA utilize this technology less than any other age group [2••, 24••, 32•], indicating that they face unique barriers to use. In 2008, the JDRF Continuous Glucose Monitoring Study Group conducted a randomized control trial examining the effect of CGM on HbA1c in children and adults [3]. They found significantly improved HbA1c in those over age 25 but not in younger participants, leading the authors to conclude that additional investigation to barriers of CGM use in the youth population was needed. In the 8to 14-year-old group, they observed benefits related to glycemic control (specifically more patients achieved glycemic targets of HbA1c of <7% and more patients had a 10% or more decrease in the HbA1c from baseline); however, this benefit was not observed in the 15–24-year age group. Only 30% of participants in this adolescent and young adult age group wore their sensor 6 days per week, compared to 50% in younger children and 83% in adults. Of note, the CGM devices commonly used at this time required frequent calibration, and CGM devices have changed significantly since this study was conducted (see Fig. 1). A subsequent study demonstrated that wearing a CGM 75% of the time or more results in improved glycemic control, suggesting the reason adolescents did not see the same reduction in HbA1c as adults in this study was primarily due to inconsistent use of the devices [33].
Twelve years later (2020), a study conducted in this same age group (14–24-year-olds) examining the effect of CGM on HbA1c found a small reduction in HbA1c (8.9% in non-CGM users compared to 8.4% in CGM users at the end of the study; p=0.01) [24••]. Again, CGM use declined substantially over time, with 82% of participants wearing a CGM 5 days per week or more at week 6 of the trial, and only 68% wearing it with this frequency at the end of the trial, with 14% completely stopping CGM use by the end of the trial [24••]. Of note, the CGM devices used in this study required calibration twice daily, which was likely a barrier to use. When applying these findings to a real-world setting, it is important to note that participants in these studies were provided with frequent research personnel contact and encouragement to adhere to the research intervention (CGM use), so it is likely that adherence to a CGM in a real-world setting would be even lower than what was observed in a rigorous study among people who agreed to use a CGM. Given these concerns, the barriers to CGM use must be examined and mitigated as much as possible for adolescents and young adults to realize the maximum benefits of these technologies.
Understanding unique barriers to CGM use in adolescents and young adults is critical to increasing CGM use in this population. While several studies have examined barriers to CGM use in adults [34, 35], few have studied barriers in AYA with diabetes. A recent cross-sectional online study of adolescents age 12–19 years within the T1D Exchange Clinical registry sought to explore barriers to diabetes device use in this population [36•]. Cost was identified as the biggest barrier to device (not specific to CGM) use reported in this group [36•]. The concern about cost (cost of device, insurance coverage, cost of supplies) is similar to what is reported in adults with T1D and is explored further in the next section of this review [37]. However, adolescents also reported concerns about their self-image, and how others may view the diabetes devices on their bodies, at a much higher rate than adults. This is likely related to the age and developmental stage of the adolescent or young adult [36•, 38, 39], and exploring ways to address this barrier (discussing different skin placement of these devices, adjusting alarm settings, etc.) may be key to improving CGM use in adolescents and young adults. Recent developments in CGM devices have addressed some of these concerns, with improved reliability, eliminating the need for calibration, and sleeker designs, so it will be important to determine whether use among AYAs remains low as the devices evolve.
While the studies described above focused on barriers to diabetes technology use, emerging evidence supports concerns about racial-ethnic differences in CGM use. A recent study examining factors that influenced glycemic control other than SES found that after accounting for the effect of SES on glycemic control, the Hispanic and White populations were similar, while the Black young adult group had the poorest glycemic control [40••]. The authors concluded that diabetes technology use was one of the key variables contributing to the differences in glycemic control in this population. Only 28% of Black participants had ever used CGM compared to 37% of Hispanic participants and 71% of white participants (p<0.001) [40••]. A related study examining diabetes technology use in young adults (age 18–28) found that after adjusting for SES, significant differences remained in CGM and insulin pump use among non-Hispanic Black young adults compared to non-Hispanic white and Hispanic young adults [41••]. Their model (adjusted for age, sex, study site, insurance, education, poverty level, health literacy, and more) predicted that, after adjusting for the above factors, 53% of non-Hispanic white patients would use a CGM device compared to 58% of Hispanic youth and 31% of non-Hispanic Black youth. The underlying reasons for these disparities are not clear but cannot be solely attributed to SES; contributing factors may include provider bias, patient preferences, and patient-provider relationships (that may change during the transition from pediatric to adult care) [41••].
As noted above, numerous studies in adults and youth show significant benefit of consistent CGM use with improved time in range, decreased hypoglycemia, and improved psychosocial factors [3, 4, 24••], but several factors continue to limit CGM use in this high-risk AYA population. While cost remains a significant barrier to use in the US medical system (see next section), recent studies suggest that provider bias may at least in part be driving disparities in CGM use in minority AYAs, specifically non-Hispanic Black youth. This finding is extremely concerning and requires more investigation into provider bias and ways to overcome both intentional and unintentional bias. Among those that are prescribed a CGM, several barriers to use remain, which may be largely due to the developmental stage of adolescents and young adults and their desire to not appear different from their peers. Given the poor glycemic control typically seen in this population and the potential improvement with continued and consistent CGM use [32•], further research is needed to investigate solutions to increase the use of this important technology in this high-risk population.
Cost-Related Challenges to Continuous Glucose Monitor Use
Compared to traditional finger stick blood glucose monitoring, CGMs have proven to offer significant improvement in diabetes management both for patients with diabetes and their diabetes providers [2••, 6, 42–44]. CGMs are easier for patients to use and provide dramatically more blood glucose data for patients and diabetes providers [27]. Given these significant benefits, it is not surprising that CGM technology has been met with enthusiasm by both groups. However, as with any new technology in health care, cost and insurance coverage are significant potential barriers to widespread adoption [45, 46]. Indeed, the introduction of home monitoring of blood glucose levels in the 1970s was met with similar patient enthusiasm, tempered by skepticism about the cost of the new technology [47, 48]. In our current complex health care landscape, determining how to balance costs and benefits of this technology is a much more complicated undertaking.
As CGM accuracy has improved, wear-times lengthened and calibration frequency decreased (and no longer required for some CGM systems) patient and provider enthusiasm has only grown for these devices [2••, 49]. With the results of the DIAMOND trial demonstrating significant reductions in hypoglycemia along with reductions in HbA1c in adults with T1D, multiple groups across the globe have sought to understand the potential economic impact of these devices [50••, 51–55]. In general, these studies have shown that if the cost impact over a lifetime is considered, these devices are likely to represent a reasonable investment for the medical system as a whole. Using the data from the DIAMOND trial, a US-based group estimated that CGM use would be conservatively estimated to cost $98,000 per additional Quality Adjusted Lifeyear with a cost as low as $33,500 if sensor wear duration was lengthened as it has been in the current generation of this device [50••]. In addition, this analysis included cost for twice daily finger stick calibrations which are no longer needed with the next-generation version of this device [56], suggesting that costs are even lower. Similar analyses in the UK, Italy, and France have also found that the improvement in health outcomes and quality of life are sufficiently improved with these devices to justify the cost [51, 53, 55]. Furthermore, at least one of these studies likely underestimated the benefit of CGM, as it only included patients with T1D and impaired awareness of hypoglycemia and did not include patients using CGM for other reasons [54]. Recent data from the T1D Exchange network suggest that CGM may also help patients avoid DKA with early alerts for sustained, elevated blood glucose levels [2••]. Given the cost associated with these admissions, future research examining the extent to which CGM may prevent these types of admissions would further bolster the economic case for CGM use.
Unfortunately, despite these analyses, obtaining insurance coverage for CGM technology in the USA remains disjointed and often difficult to navigate [2••, 36•, 37, 46]. A recent review of state Medicaid coverage plans found that 13 states required that patients show four times daily glucose testing prior to approval for CGM even among those with T1D [46]. Reserving CGM technology only for those patients who already test frequently not only mitigates the economic benefit of these devices but also prevents patients who may have other barriers to frequent testing—such as school or occupational restrictions—from benefitting from these devices [57]. Further, a patchwork of rules differing between various insurance providers and pharmacy benefit managers variously consider these devices as falling under pharmaceutical and durable medical equipment (DME) benefits. This means that while some patients are able to obtain needed supplies from a retail pharmacy, a significant portion are forced to navigate paperwork from a third-party DME supplier, further complicating the process of obtaining the device. Given the high cost of the devices, this creates a significant lag time—often months—between when CGM is prescribed and when it is ready for use. Indeed, in a survey of pediatric and adult clinicians, cost was identified as by far the single most significant barrier to increased device use among patients with nearly 50% of providers surveyed identifying this as a problem for patients [42].
In light of increasing integration between CGM and insulin pumps, as well as the clear benefit they provide to individual patients, it is increasingly clear that the future of diabetes care likely includes a majority, if not nearly all, of patients with T1D using CGM as their primary mode of glycemic monitoring. Data already show that these devices help patients avoid significant acute complications associated with intensive glycemic management of T1D such as severe hypoglycemia or ketoacidosis requiring hospitalization. Ongoing work is needed to further bolster the economic case for these devices so that all patients with T1D can have the option to use this lifechanging technology.
Conclusions
The development of continuous glucose monitoring technology over the last 20 years has transformed the management of type 1 diabetes, yet AYAs traditionally have not used these devices at the same rate nor seen the same degree of benefits reported in adults with T1D [3, 24••]. Multiple studies demonstrate that AYAs that use continuous glucose monitors have improved glycemic control and decreased hypoglycemia [24••, 32•]; however, consistent use of these devices continues to be a challenge in this population [3, 24••]. Certain barriers, such as worry about body image, exist to a higher degree in AYAs and need to be assessed and addressed by providers when discussing diabetes technologies [36•]. Recent studies also clearly demonstrate differences in CGM use related to race and ethnicity, specifically among Black young adults, and additional research is needed to identify the causes of this disparity [40••, 41••] so that targeted interventions can be developed. These studies suggest that provider bias is a key factor driving this inequality and interventions addressing this bias (whether conscious or unconscious) are critical. Cost remains a significant barrier to use, and until insurance uniformly and routinely covers CGM therapy, this will continue to remain a barrier [36•, 42]. International studies have found CGMs to be cost effective [50••] and to improve HbA1c and quality of life when fully reimbursed through a national healthcare system [4, 27]. CGMs are undoubtedly the future of care in T1D and have the potential to significantly improve glycemic control in adolescents and young adults, and therefore finding ways to mitigate barriers to CGM use in AYAs is critical to improving glycemic control in this high-risk population.
Footnotes
Declarations
Conflict of Interest Daniel R Tilden, Angelee M Parmar, and Eveline R Goethals declare that they have no conflict of interest. Karishma A Datye and Sarah S Jaser receive research funding through the NIDDK.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Miller KM, Foster NC, Beck RW, Bergenstal RM, DuBose SN, DiMeglio LA, et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care. 2015;38(6):971–8. [DOI] [PubMed] [Google Scholar]
- 2. Foster NC, Beck RW, Miller KM, Clements MA, Rickels MR, DiMeglio LA, et al. State of type 1 diabetes management and outcomes from the T1D Exchange in 2016–2018. Diabetes Technol Ther. 2019;21(2):66–72. •• Key study describing diabetes related outcomes in the large T1D Exchange Clinical Registry.
- 3.Tamborlane WV, Beck RW, Bode BW, Buckingham B, Chase HP, Clemons R, et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359(14):1464–76. [DOI] [PubMed] [Google Scholar]
- 4.Charleer S, Mathieu C, Nobels F, De Block C, Radermecker RP, Hermans MP, et al. Effect of continuous glucose monitoring on glycemic control, acute admissions, and quality of life: a realworld study. J Clin Endocrinol Metab. 2018;103(3):1224–32. [DOI] [PubMed] [Google Scholar]
- 5.Marks BE, Wolfsdorf JI. Monitoring of pediatric type 1 diabetes. Front Endocrinol (Lausanne). 2020;11:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dorando E, Haak T, Pieper D. Continuous glucose monitoring for glycemic control in children and adolescents diagnosed with diabetes type 1: a systematic review and meta-analysis. Exp Clin Endocrinol Diabetes. 2020. [DOI] [PubMed] [Google Scholar]
- 7.Garg SK, Akturk HK. A new era in continuous glucose monitoring: food and drug administration creates a new category of factory-calibrated nonadjunctive, interoperable class II medical devices. Diabetes Technol Ther. 2018;20(6):391–4. [DOI] [PubMed] [Google Scholar]
- 8.Medtronic. Innovation Milestones. 2021. Available from: https://www.medtronicdiabetes.com/about-medtronic-innovation/milestone-timeline. Accessed 12 March 2021.
- 9.FDA. FDA authorizes first fully Interoperable continuous glucose monitoring System, Streamlines REVIEW pathway for similar devices. 2018. Available from: https://www.fda.gov/news-events/press-announcements/fda-authorizes-first-fully-interoperable-continuous-glucose-monitoring-system-streamlines-review. Accessed 12 March 2021.
- 10.Abbott. FreeStyle Libre 2: Now Available in U.S. 2020. Available from: https://www.abbott.com/corpnewsroom/diabetes-care/freestyle-libre-2-now-available-in-us.html. Accessed 12 March 2021. [Google Scholar]
- 11.FDA. MiniMed 770G System P160017/S076. 2020. Available from: https://www.fda.gov/medical-devices/recently-approved-devices/minimed-770g-system-p160017s076. Accessed 12 March 2021.
- 12.Edelman SV, Argento NB, Pettus J, Hirsch IB. Clinical implications of real-time and intermittently scanned continuous glucose monitoring. Diabetes Care. 2018;41(11):2265–74. [DOI] [PubMed] [Google Scholar]
- 13.Abbott. Get Started Your Guide to the FreeStyle Libre 2 System. 2020. Available from: https://www.freestyle.abbott/content/dam/adc/fds/us-en/documents/get-started-guide.pdf. Accessed 12 March 2021. [Google Scholar]
- 14.Welsh JB, Derdzinski M, Parker AS, Puhr S, Jimenez A, Walker T. Real-time sharing and following of continuous glucose monitoring data in youth. Diabetes Ther. 2019;10(2):751–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galindo RJ, Aleppo G. Continuous glucose monitoring: the achievement of 100 years of innovation in diabetes technology. Diabetes Res Clin Pract. 2020;170:108502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berget C, Messer LH, Vigers T, Frohnert BI, Pyle L, Wadwa RP, et al. Six months of hybrid closed loop in the real-world: an evaluation of children and young adults using the 670G system. Pediatr Diabetes. 2020;21(2):310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lal RA, Basina M, Maahs DM, Hood K, Buckingham B, Wilson DM. One year clinical experience of the first commercial hybrid closed-loop system. Diabetes Care. 2019;42(12):2190–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinsker JE, Müller L, Constantin A, Leas S, Manning M, McElwee Malloy M, et al. Real-world patient-reported outcomes and glycemic results with initiation of control-IQ technology. Diabetes Technol Ther. 2021;23(2):120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Beers CA, DeVries JH, Kleijer SJ, Smits MM, GeelhoedDuijvestijn PH, Kramer MH, et al. Continuous glucose monitoring for patients with type 1 diabetes and impaired awareness of hypoglycaemia (IN CONTROL): a randomised, open-label, crossover trial. Lancet Diabetes Endocrinol. 2016;4(11):893–902. [DOI] [PubMed] [Google Scholar]
- 20.Charleer S, De Block C, Van Huffel L, Broos B, Fieuws S, Nobels F, et al. Quality of life and glucose control after 1 year of nationwide reimbursement of intermittently scanned continuous glucose monitoring in adults living with type 1 diabetes (FUTURE): a prospective observational real-world cohort study. Diabetes Care. 2020;43(2):389–97. [DOI] [PubMed] [Google Scholar]
- 21.Beck RW, Riddlesworth T, Ruedy K, Ahmann A, Bergenstal R, Haller S, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. Jama. 2017;317(4):371–8. [DOI] [PubMed] [Google Scholar]
- 22.Charleer S, Gillard P, Vandoorne E, Cammaerts K, Mathieu C, Casteels K. Intermittently scanned continuous glucose monitoring is associated with high satisfaction but increased HbA1c and weight in well-controlled youth with type 1 diabetes. Pediatr Diabetes. 2020;21(8):1465–74. [DOI] [PubMed] [Google Scholar]
- 23.Sherr JL, Tauschmann M, Battelino T, de Bock M, Forlenza G, Roman R, et al. ISPAD clinical practice consensus guidelines 2018: diabetes technologies. Pediatr Diabetes. 2018;19(Suppl 27): 302–25. [DOI] [PubMed] [Google Scholar]
- 24. Laffel LM, Kanapka LG, Beck RW, Bergamo K, Clements MA, Criego A, et al. Effect of continuous glucose monitoring on glycemic control in adolescents and young adults with type 1 diabetes: a randomized clinical trial. JAMA. 2020;323(23):2388–96. •• Key study examining continuous glucose monitor use in AYA.
- 25.Sheikh K, Bartz SK, Lyons SK, DeSalvo DJ. Diabetes device use and glycemic control among youth with type 1 diabetes: a single-center, cross-sectional study. J Diabetes Res. 2018;2018:5162162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pickup JC, Ford Holloway M, Samsi K. Real-time continuous glucose monitoring in type 1 diabetes: a qualitative framework analysis of patient narratives. Diabetes Care. 2015;38(4):544–50. [DOI] [PubMed] [Google Scholar]
- 27.Burckhardt MA, Abraham MB, Mountain J, Coenen D, Paniora J, Clapin H, et al. Improvement in psychosocial outcomes in children with type 1 diabetes and their parents following subsidy for continuous glucose monitoring. Diabetes Technol Ther. 2019;21(10): 575–80. [DOI] [PubMed] [Google Scholar]
- 28.Burckhardt MA, Roberts A, Smith GJ, Abraham MB, Davis EA, Jones TW. The use of continuous glucose monitoring with remote monitoring improves psychosocial measures in parents of children with type 1 diabetes: a randomized crossover trial. Diabetes Care. 2018;41(12):2641–3. [DOI] [PubMed] [Google Scholar]
- 29.Vesco AT, Jedraszko AM, Garza KP, Weissberg-Benchell J. Continuous glucose monitoring associated with less diabetesspecific emotional distress and lower A1c among adolescents with type 1 diabetes. J Diabetes Sci Technol. 2018;12(4):792–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Messer LH, Cook PF, Tanenbaum ML, Hanes S, Driscoll KA, Hood KK. CGM benefits and burdens: two brief measures of continuous glucose monitoring. J Diabetes Sci Technol. 2019;13(6): 1135–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Charleer S, De Block C, Nobels F, Radermecker RP, Lowyck I, Mullens A, et al. Sustained impact of real-time continuous glucose monitoring in adults with type 1 diabetes on insulin pump therapy: results after the 24-month RESCUE study. Diabetes Care. 2020;43(12):3016–23. [DOI] [PubMed] [Google Scholar]
- 32. Addala A, Maahs DM, Scheinker D, Chertow S, Leverenz B, Prahalad P. Uninterrupted continuous glucose monitoring access is associated with a decrease in HbA1c in youth with type 1 diabetes and public insurance. Pediatr Diabetes. 2020. • This study highlights the importance of uninterrupted CGM use.
- 33.Battelino T, Liabat S, Veeze HJ, Castañeda J, Arrieta A, Cohen O. Routine use of continuous glucose monitoring in 10 501 people with diabetes mellitus. Diabet Med. 2015;32(12):1568–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naranjo D, Tanenbaum ML, Iturralde E, Hood KK. Diabetes technology: uptake, outcomes, barriers, and the intersection with distress. J Diabetes Sci Technol. 2016;10(4):852–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lanning MS, Tanenbaum ML, Wong JJ, Hood KK. Barriers to continuous glucose monitoring in people with type 1 diabetes: clinician perspectives. Diabetes Spectr. 2020;33(4):324–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Messer LH, Tanenbaum ML, Cook PF, Wong JJ, Hanes SJ, Driscoll KA, et al. Cost, Hassle, and On-body experience: barriers to diabetes device use in adolescents and potential intervention targets. Diabetes Technol Ther. 2020;22(10):760–7. • Examines barriers to diabetes device use in adolescents.
- 37.Tanenbaum ML, Hanes SJ, Miller KM, Naranjo D, Bensen R, Hood KK. Diabetes device use in adults with type 1 diabetes: barriers to uptake and potential intervention targets. Diabetes Care. 2017;40(2):181–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borus JS, Laffel L. Adherence challenges in the management of type 1 diabetes in adolescents: prevention and intervention. Curr Opin Pediatr. 2010;22(4):405–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Comeaux SJ, Jaser SS. Autonomy and insulin in adolescents with type 1 diabetes. Pediatr Diabetes. 2010;11(7):498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Agarwal S, Kanapka LG, Raymond JK, Walker A, Gerard-Gonzalez A, Kruger D, et al. Racial-ethnic inequity in young adults with type 1 diabetes. J Clin Endocrinol Metab. 2020;105(8): e2960–9. •• Key study describing racial inequity in T1D.
- 41. Agarwal S, Schechter C, Gonzalez J, Long JA. Racial-ethnic disparities in diabetes technology use among young adults with type 1 diabetes. Diabetes Technol Ther. 2020. •• This study describes concering racial-ethnic disparities in diabetes technology use.
- 42.Tanenbaum ML, Adams RN, Hanes SJ, Barley RC, Miller KM, Mulvaney SA, et al. Optimal use of diabetes devices: clinician perspectives on barriers and adherence to device use. J Diabetes Sci Technol. 2017;11(3):484–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cappon G, Vettoretti M, Sparacino G, Facchinetti A. Continuous glucose monitoring sensors for diabetes management: a review of technologies and applications. Diabetes Metab J. 2019;43(4):383– 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DeSalvo DJ, Miller KM, Hermann JM, Maahs DM, Hofer SE, Clements MA, et al. Continuous glucose monitoring and glycemic control among youth with type 1 diabetes: International comparison from the T1D Exchange and DPV Initiative. Pediatr Diabetes. 2018;19(7):1271–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sumnik Z, Szypowska A, Iotova V, Bratina N, Cherubini V, Forsander G, et al. Persistent heterogeneity in diabetes technology reimbursement for children with type 1 diabetes: The SWEET perspective. Pediatr Diabetes. 2019;20(4):434–43. [DOI] [PubMed] [Google Scholar]
- 46.Anderson JE, Gavin JR, Kruger DF. Current eligibility requirements for CGM coverage are harmful, costly, and unjustified. Diabetes Technol Ther. 2020;22(3):169–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anhalt H. Limitations of continuous glucose monitor usage. Diabetes Technol Ther. 2016;18(3):115–7. [DOI] [PubMed] [Google Scholar]
- 48.Sonksen PH, Judd SL, Lowy C. Home monitoring of blood-glucose. Method for improving diabetic control. Lancet. 1978;1(8067): 729–32. [DOI] [PubMed] [Google Scholar]
- 49.van den Boom L, Karges B, Auzanneau M, Rami-Merhar B, Lilienthal E, von Sengbusch S, et al. Temporal trends and contemporary use of insulin pump therapy and glucose monitoring among children, adolescents, and adults with type 1 diabetes between 1995 and 2017. Diabetes Care. 2019;42(11):2050–6. [DOI] [PubMed] [Google Scholar]
- 50. Wan W, Skandari MR, Minc A, Nathan AG, Winn A, Zarei P, et al. Cost-effectiveness of continuous glucose monitoring for adults with type 1 diabetes compared with self-monitoring of blood glucose: the DIAMOND randomized trial. Diabetes Care. 2018;41(6):1227–34. •• Demonstrates the cost-effectiveness of CGM devices.
- 51.Roze S, Isitt JJ, Smith-Palmer J, Lynch P, Klinkenbijl B, Zammit G, et al. Long-term cost-effectiveness the Dexcom G6 real-time continuous glucose monitoring system compared with self-monitoring of blood glucose in people with type 1 diabetes in France. Diabetes Ther. 2021;12(1):235–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garcia-Lorenzo B, Rivero-Santana A, Vallejo-Torres L, CastillaRodriguez I, Garcia-Perez S, Garcia-Perez L, et al. Costeffectiveness analysis of real-time continuous monitoring glucose compared to self-monitoring of blood glucose for diabetes mellitus in Spain. J Eval Clin Pract. 2018;24(4):772–81. [DOI] [PubMed] [Google Scholar]
- 53.Chaugule S, Oliver N, Klinkenbijl B, Graham C. An economic evaluation of continuous glucose monitoring for people with type 1 diabetes and impaired awareness of hypoglycaemia within North West London Clinical Commissioning Groups in England. Eur Endocrinol. 2017;13(2):81–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chaugule S, Graham C. Cost-effectiveness of G5 Mobile continuous glucose monitoring device compared to self-monitoring of blood glucose alone for people with type 1 diabetes from the Canadian societal perspective. J Med Econ. 2017;20(11):1128–35. [DOI] [PubMed] [Google Scholar]
- 55.Nicolucci A, Rossi MC, D’Ostilio D, Delbaere A, de Portu S, Roze S. Cost-effectiveness of sensor-augmented pump therapy in two different patient populations with type 1 diabetes in Italy. Nutr Metab Cardiovasc Dis. 2018;28(7):707–15. [DOI] [PubMed] [Google Scholar]
- 56.Roze S, Isitt J, Smith-Palmer J, Javanbakht M, Lynch P. Long-term cost-effectiveness of Dexcom g6 real-time continuous glucose monitoring versus self-monitoring of blood glucose in patients with type 1 diabetes in the U.K. Diabetes Care. 2020;43(10):2411–7. [DOI] [PubMed] [Google Scholar]
- 57.Kraaijeveld SR. Continuous glucose monitoring as a matter of justice. HEC Forum. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]