Abstract
Background:
Beyond sleep duration, other facets of sleep, such as variability and timing, may be associated with obesity risk in youth. Yet, data are limited.
Objective:
Using a longitudinal design, we tested whether multiple facets of sleep were associated with fat mass gain over 1 year.
Methods:
A convenience sample of non-treatment seeking youth (8-17y) wore actigraphy monitors for 14 days. Average weekly sleep duration, within-subject sleep duration variability, weekend catch-up sleep, bed and waketime shift, social jet lag, bedtime, waketime, and sleep midpoint were calculated. The association of each facet of baseline sleep with 1-year fat mass (1yFM), adjusting for baseline fat mass (BLFM) and height, was examined.
Results:
One-hundred-thirty-seven youths (54.0% female; 12.5±2.6y; 28.4% Non-Hispanic Black or African American; BLFM: 15.3±8.9kg; 1yFM: 17.0±10.0kg; 28.5% with baseline overweight/obesity) were studied. Waketime (p=.03) and sleep midpoint (p=.02) were inversely associated with 1yFM, such that earlier waketime and midpoint were associated with higher 1yFM. No other facet of sleep was significantly associated with 1yFM (ps>.09).
Conclusion:
Using objective measures, youth with earlier waketimes and sleep midpoints had greater gains in fat mass. Additional research is needed to determine if sleep timing may be a modifiable target to prevent pediatric obesity.
Keywords: youth, sleep, adiposity, obesity, actigraphy
Introduction
Research has demonstrated a consistent association between short nightly sleep duration and increased risk for higher body mass index (BMI), overweight, and obesity in youth (1, 2). A recent systematic review examining the associations between multiple dimensions of sleep and obesity in children found the majority of studies reported an inverse association between sleep duration (hours per night) and weight (3). This relationship has been observed both cross-sectionally, as well as prospectively (2, 3). For example, among children 6-12 years old across the weight spectrum, those who slept 7.5 hours or fewer per night had a three times greater risk of having obesity five years later than those who slept 9 hours or more each night (1). These data suggest that age-appropriate sufficient sleep may be particularly important for promoting healthy weight in youth.
However, the mechanisms linking sleep and obesity risk among youth are poorly understood. It is possible that the association between shorter sleep duration and high weight is at least partially due to increased energy intake and/or poor diet quality (4). Indeed, experimental studies have found that restricting nightly age-specific sleep recommendations is associated with increased energy intake in youth (5). Observational studies, using both objective (6) and self-report (7) measures of sleep, have also found an association between insufficient sleep duration and poorer diet quality, such as greater intake of added sugar, fats, and carbohydrates (6, 7). Yet, the data are not entirely consistent regarding the associations of sleep duration and food intake in youth [e.g., (8)]. Mixed results suggest that it may be important to use objective measures to examine other facets of sleep, in addition to sleep duration, in order to fully understand the associations between sleep and weight.
Sleep variability may be particularly salient for obesity risk in youth (7, 9, 10). There are several facets of sleep variability (Table 1), including variability of sleep duration [i.e., measures based on hours per night of sleep, such as weekend catch-up sleep, calculated as the difference between weekend sleep duration and weekday sleep duration (11)] and behavioral estimations of circadian rhythm shifts [i.e., metrics based on sleep timing such as such as social jet lag, calculated as the difference between weekend sleep midpoint and weekday sleep midpoint (12)]. Data generally support the role of sleep variability in energy intake, BMI, and adiposity beyond what is attributable to sleep duration alone (3, 4). Additionally, preliminary data in youth suggest that objectively measured (12) as well as parent-reported (13) social jet lag is associated with greater energy intake, higher BMIz, waist circumference, and fat mass. However, there is a relative paucity of data examining objectively measured sleep variability in children and adolescents.
Table 1.
Sleep Constructs
| Construct | Definition/ Calculation |
|---|---|
| Average Weekly Sleep Duration | Average hours per night of sleep over one week. |
| Bedtime | The time one falls asleep; when using actigraphy data, defined as the first minute of a five-minute period of no actigraphy-detected period of activity (6). |
| Bedtime Shift | The absolute difference in weekend and weekday bedtime, calculated as average weekend bedtime minus average weekday bedtime (7). |
| Sleep Midpoint | Halfway between sleep onset and wake time (12). |
| Social Jet Lag | Calculated as the absolute difference between the midpoint of nightly sleep on the weekends and midpoint of sleep on weekdays (12). |
| Waketime | The time one awakens; when using actigraphy data, defined as the first minute of a five-minute period of actigraphy-detected after a period of inactivity (6). |
| Waketime Shift | The absolute difference in weekend and weekday waketime calculated as average weekend waketime minus average weekday waketime (7). |
| Weekday Sleep | Average hours per night of sleep over Sunday night through Thursday night sleep (11). |
| Weekend Catch-Up Sleep | The difference between average hours per night of weekend sleep and weekday sleep; calculated as average hours per night of weekend (Friday and Saturday nights) sleep minus average hours per night of weekday (Sunday through Thursday nights) sleep (11). |
| Weekend Sleep | Average hours per night of sleep over Friday and Saturday nights’ sleep (6). |
| Within-Subject Sleep Duration Variability | Night-to-night variation in hours of sleep from the average; calculated in hours as the intra-subject standard deviation of average nightly sleep duration across a multi-day period (9). |
Sleep timing is another facet of sleep that may play a role in obesity risk in youth. For instance, bedtime (i.e. the time one falls asleep) has been associated with weight in youth (3), with data generally supporting a relationship between later self- and parent-reported bedtime and higher weight (14, 15). While waketime may also be important, few studies have examined the relationship between waketime and obesity risk in youth, with only one study finding a cross-sectional relationship between early waketime and greater odds of having obesity (16). It is also possible that the combination of bed and waketimes, or overall sleep timing pattern, may be particularly relevant for obesity risk in youth. A combination of self-reported later bed and wake times have been associated with higher weight in youth (17). Although the mechanisms remain unclear, preliminary data indicate that, similar to sleep variability, sleep timing may be associated with circadian rhythm misalignment (i.e., sleep patterns that are incongruent with physiologically-driven circadian preference), which is known to be associated with obesogenic behaviors and high weight in children and adolescents (18). However, studies utilizing objectively measured sleep timing are limited in youth (3, 4).
The majority of studies examining sleep characteristics are cross-sectional, thereby precluding an understanding of the temporal relationships between facets of sleep and obesity risk. Additionally, it is unknown which sleep facets may be most relevant to the development of high weight. Further, adiposity is known to be a better predictor of cardiometabolic health than BMI (19), and more data are needed examining the association between facets of sleep and body composition. Thus, longitudinal data examining different sleep facets are needed to fully understand the association between sleep, and adiposity gain in youth over time.
Therefore, we examined relationships between multiple facets of actigraphy-measured sleep (including average weekly sleep duration, within-subject sleep duration variability, weekend catch-up sleep, bed and waketime shift, social jet lag, bedtime, waketime, and sleep midpoint) and change in fat mass at 1-year follow-up among youth (8-17 years old). We hypothesized that shorter sleep duration, greater sleep variability, and later sleep timing would be associated with a greater increase in fat mass at 1-year follow-up.
Methods
Participants
Boys and girls (8-17 years old) in good general health across the weight spectrum were recruited to participate in the Children’s Growth and Behavior Study [Clinical Trials Identifier: NCT02390765 (6, 20)], an ongoing prospective study designed to understand the relationships between eating behaviors and body weight in children. Youth were recruited through mailings to parents in the greater Washington, D.C. area and flyers posted at local public facilities (e.g., libraries) in communities near Bethesda, M.D. Additionally, flyers were distributed through local primary and secondary schools and parent listservs.
Youth were eligible if they were 8-17 years old, cognitively capable of completing study procedures, and had a BMI ≥ 5th percentile for age and sex according to the Centers for Disease Control and Prevention 2000 U.S. standards (21). Individuals were excluded if they had a history of brain injury, major medical or psychiatric illness, current pregnancy or history of pregnancy, current and regular use of illicit drugs, greater than 5% body weight reduction during the three months prior to screening, or regular use of medications known to impact eating behavior, weight, autonomic functioning, or endocrine functioning. The study procedure was approved by the National Institutes of Health (NIH) Institutional Review Board.
Procedures
Following a telephone screen, potentially eligible families were seen at the NIH Hatfield Clinical Research Center for all visits. Participants completed measures at baseline and a 1-year follow-up. The study’s purposes, testing procedures, and possible study risks were reviewed in detail with families. Interested parents and youth provided written consent and assent, respectively. All data were collected prior to the COVID-19 pandemic. Participants underwent the following procedures and measures at each visit.
Baseline Visit
Anthropometric Measures. Fasting body weight was measured to the nearest 0.1 kg using a Scale-Tronix (Welch Allyn, St. Joseph, MI) calibrated digital scale which is widely used in pediatric populations (6, 20, 22) and appropriate for any individual who is able to stand (product expert, Welch Allyn, personal communication, 2021). Height (to the nearest 0.1 cm) was measured in triplicate by stadiometer and the average was used to calculate BMIz, adjusting for age and sex according to CDC growth standards (21).
Body Composition. Kilograms of lean mass and total fat mass were measured via dual-energy x-ray absorptiometry (iDEXA system, GE Healthcare, Madison, WI). Percent fat mass was calculated from the values obtained.
Physical Examination. Medical history (including parent-report of child snoring), vital signs, and history of past or current medical and psychiatric illness, and physical characteristics to determine pubertal stage were assessed by a pediatric endocrinologist or a nurse practitioner. For females, pubertal development was determined by breast development through observation and palpitation (23). For males, pubertal development was determined by palpation to measure testicular volume (pre-puberty: ≤ 3 mL, early- to mid-puberty: 4–12 mL, late-puberty: >12 mL) by orchidometer beads (24).
Children’s Depression Inventory. Depressive symptoms were assessed using the Children’s Depression Inventory - Second Edition (25). Respondents indicate to what degree they experienced depressive symptoms during the prior weeks. Each item response ranges from 0-2 and total scores range between 0 and 52, with higher total scores indicating more depressive symptoms. This measure is a widely used and demonstrated adequate internal consistency for the present sample (Cronbach’s α = .79).
Socioeconomic Status. Socioeconomic status was determined by parents’ reported educational and occupational status via the Hollingshead Two Factor Index of Socioeconomic Status. Possible scores range from 8-66 with higher scores indicating lower family socioeconomic status (26).
Sleep. Wrist-worn actigraphy was used to assess sleep behavior in youth for approximately 14 days following the baseline visit Participants were given an ActiGraph GT3X+ activity monitor (ActiGraph, Pensacola, FL), which is worn as a wristwatch and delivers 24-hour physical activity and sleep/wake measurements. In young adults, the GT3X+ is a valid and reliable device for detecting sleep/wake diurnal patterns and has good concordance with polysomnography (27). The GT3X+ wrist actigraphy has also been used in youth ages 8-17 years (12, 28). To be included in analyses, participants were required to have valid data from ≥3 weekday nights of sleep and ≥1 weekend night of sleep. On average, data were available for 13.7 (± 2.9) days of weekly sleep, 9.8 (± 2.2) days of weekday sleep, and 3.9 (± 1.0) days of weekend sleep. Additionally, on average, participants had 0.95 (± 1.88) days of missing sleep data. Data were included if participants provided more than 14 nights of sleep. Sleep data were downloaded from devices using Actilife software version 6.13.3 (Actigraph, Pensacola, FL). The Sadeh sleep detection algorithm, which has been previously validated for youth [e.g., (29)], was applied to data in order to detect periods of sleep. Data were visually inspected, and sleep periods were confirmed by cross-referencing self-reported sleep logs.
Average weekly sleep duration (hour/night) of all nights (Sunday-Saturday) was calculated. Additionally, weekend catch-up sleep was calculated by subtracting average hours per night of weekday (Sunday through Thursday nights) from average hours per night of weekend (Friday and Saturday nights) sleep (11). Further, within-subject sleep duration variability was calculated as the intra-subject standard deviation of average sleep duration across the data collection period (9, 10). Bedtime shift was calculated by taking the absolute value of average weekday sleep onset time minus average weekend sleep onset time. Similarly, waketime shift was calculated as the absolute value of average weekend waketime minus average weekday waketime (7). Based on sleep onset and waketimes, sleep midpoint was calculated as the time halfway between the start and end of the primary nightly sleep period (12). Social jet lag was then calculated as the absolute difference between mean sleep midpoint on weekend days minus weekdays (12).
1-Year Follow Up Visit
Approximately one year after completing the screening visits, participants returned to the NIH and repeated the following procedures: anthropometric measures, (fasting weight and height), body composition (via dual-energy x-ray absorptiometry), and physical examination.
Data Analytic Plan
All analyses were performed with IBM SPSS Statistics version 27 (SPSS, INC., Chicago, IL). Data were screened for normality and arcsine square root transformation was conducted for percent fat mass to enhance normality (6). Within-subject sleep duration variability, baseline fat mass, fat mass at one year follow-up, and depressive symptoms were not normally distributed; therefore, scores were log base 10 transformed to achieve normality. Additionally, weekend catch-up sleep and social jet lag were not normally distributed, and log base 10 transformation did not achieve normality. Therefore, outliers were recoded to within three standard deviations from the mean which resulted in normal distribution (30).
Nine multiple linear regressions were conducted to examine the association of facets of sleep with fat mass at one year follow-up (1-year fat mass). Several covariates were considered for these models. Given the differences in academic and social demands throughout the year, time of year when sleep data were obtained (dummy coded as four categorical seasons) was considered as a covariate for all models (12). Additionally, due to established links of depressive symptoms with sleep and weight status in children and adolescents (31), the total score on the Children’s Depression Inventory- Second Edition was considered as a covariate for all models. Studies have also demonstrated links between socioeconomic status and poor sleep and weight status in youth (32); therefore, socioeconomic status was considered as a covariate for all models. Given evidence suggesting that age and pubertal status differentially affect sleep in youth (33), both variables were considered as covariates. Adiposity also develops differentially across childhood and adolescence by sex (34); therefore, sex (0= male, 1= female) was also considered. Additionally, given the links between high weight and obstructive sleep apnea (35), snoring (0 = no snoring, 1 = snoring reported) was considered as a covariate in all models. The final covariate considered was race (0 = Other; 1 = Non-Hispanic Black or African American). In an effort to conserve power, models were all run twice: once with only those covariates significantly associated with 1-year fat mass at p< .05 (partially adjusted) and once with all aforementioned covariates (fully adjusted; data available upon request). No considered covariate, other than baseline fat mass, was significantly associated with 1-year fat mass (ps > .06; see Table 4 for zero-order correlations between all study variables and covariates), therefore partially adjusted models included baseline fat mass and height. All tests were two-tailed, and differences were considered significant when p < .05.
Table 4.
Zero-Order Correlations for All Study Variables and Covariates
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age | - | |||||||||||||||||||
| 2. Pubertal Status | .79** | - | ||||||||||||||||||
| 3. Sex | .12 | .16. | - | |||||||||||||||||
| 4. Race | .11 | .08. | .11 | - | ||||||||||||||||
| 5. Height | .80** | .77** | −.04 | .03 | - | |||||||||||||||
| 6. Depressive Symptomsa | −.02 | −.06. | .05 | .08 | −.05 | - | ||||||||||||||
| 7. Socioeconomic Status | −.10 | −.05. | −.07 | .32** | −.14 | .12 | - | |||||||||||||
| 8. Snoring | −.03 | .01. | −.11 | .14 | .10 | −.06 | .09 | - | ||||||||||||
| 9. Baseline Fat Mass (kg)a | .42** | .37** | .34** | .18* | .40** | −.02 | .02 | .11 | - | |||||||||||
| 10. 1-Year Fat Mass (kg)a | .37** | .35** | .34** | .16 | .34** | .01 | .02 | .13 | .95** | - | ||||||||||
| 11. BMIz Score | .04 | .12. | .10 | .31** | .10 | −.05 | .20* | .19* | .79** | .77** | - | |||||||||
| 12. Season | −.13 | −.17. | −.02 | .14 | −.11 | .08 | .13 | .10 | .01 | −.02 | .09 | - | ||||||||
| 13. Average Weekly Sleep Duration (hr) | −.39** | −.32** | −.02 | −.11 | −.30** | −.14 | −.12 | −.08 | −.18* | −.18* | −.11 | .21* | - | |||||||
| 14. Weekend Catch-Up Sleep (hr) | .27** | .21* | .16 | .02 | .25** | −.01 | −.09 | −.02 | .15 | .12 | .05 | −.03 | −.20* | - | ||||||
| 15. Within-Subject Sleep Duration Variabilitya | .41** | .40** | .12 | .27** | .31** | .00 | .16 | −.02 | .22* | .21* | .15 | −.12 | −.37** | .39** | - | |||||
| 16. Bedtime Shift | .07 | .11. | −.04 | .13 | .05 | −.01 | .25** | −.02 | .06 | .03 | .10 | −.09 | −.24** | −.13 | .10 | - | ||||
| 17. Waketime Shift | .26** | .23** | .10 | .08 | .24** | −.03 | .11 | .01 | .13 | .11 | .05 | −.17 | −.42** | .73** | .40** | .41** | - | |||
| 18. Social Jet Lag (hr) | .12 | .19* | .02 | .14 | .15 | .05 | .26** | .03 | .14 | .09 | .15 | −.18* | −.34** | .18* | .23** | .61** | .60** | - | ||
| 19. Bedtimeb | .42** | .39** | .00 | .33** | .45** | .07 | .07 | .13 | .25** | .18* | .15 | −.01 | −.49** | .23** | .58** | .07 | .26** | .13 | - | |
| 20. Waketimec | .23** | .18* | −.08 | .23** | .29** | .03 | −.02 | .10 | .10 | .03 | .03 | .20* | .11 | .11 | .41** | −.13 | −.03 | −.18* | .74** | - |
| 21. Sleep Midpointc | .35** | .33** | −.05 | .29** | .42** | .10 | .07 | .14 | .20* | .12 | .12 | .10 | −.24** | .19* | .57** | −.04 | .13 | .10 | .91** | .89** |
Note: Three participants had missing data for socioeconomic status and 3 different participants had missing data for pubertal status. Sex: 0= male, 1= female; Puberty: 1= no puberty development, 2= early-to-mid puberty development, 3= late puberty development; Race: 0 = Other; 1 = Non-Hispanic Black or African American; Snoring: 0 = no snoring, 1 = snoring present; Season: 1= winter, 2= spring, 3= summer, 4= fall.
Log-transformed values presented.
Times presented as fraction from noon.
Times presented as fraction from midnight.
p<.5
p<.01
Results
Participants
Of the 184 participants who completed a 1-year follow up visit, 30 were excluded from analyses due to non-compliance with actigraphy-wearing protocols (n= 13), technological issues downloading the data (n=11), or had sleep data from fewer than 3 weekdays and 2 weekends (n=6). An additional 17 participants did not provide baseline sleep data. Those with missing data did not significantly differ from the studied sample with regard to sex, race, age, or BMz (ps > .28). Therefore, a total of 137 youths (54.0% female; 12.5 ± 2.6y; 28.4% Non-Hispanic Black or African American; baseline fat mass: 15.3± 8.9 kg; 1-year fat mass: 17.0 ± 10.0 kg; 28.5% with overweight/obesity at baseline) were studied (Table 2). Characteristics of facets of sleep are described in Table 2.
Table 2.
Sample Characteristics
| Characteristica | Total Sample (N = 137) | Total Sample Range |
|---|---|---|
| Age (years) | 12.5 ± 2.6 | 8.0-17.0 |
| Sex (% female) | 54.0 | |
| Race/Ethnicity (%) | ||
| Hispanic or Latino | 6 | |
| Asian | 12.7 | |
| Black or African American | 28.4 | |
| Multiple Races | 7.5 | |
| White | 47 | |
| Unknown | 0.7 | |
| Children’s Depressive Inventory-2 Total Score | 6.6 ± 5.0 | 0.0 - 27.0 |
| Mild Depressive Symptoms (%) | 3.6 | |
| Moderate Depressive Symptoms (%) | 2.2 | |
| Severe Depressive Symptoms (%) | 0.7 | |
| Socioeconomic Status (SES) Quartiles (%) | ||
| First (lowest SES) | 9.8 | |
| Second | 30.3 | |
| Third | 36.4 | |
| Fourth (highest SES) | 23.5 | |
| Baseline Fat Mass (kg) | 15.3 ± 8.9 | 3.8 - 47.0 |
| 1-Year Fat Mass (kg) | 17.0 ± 10.0 | |
| BMIz Score | 0.5 ± 1.0 | −4.4 |
| Healthy weight (%) | 71.5 | |
| Overweight (%) | 12.4 | |
| Obesity (%) | 16.1 | |
| Time of Year Completing the Protocol (%) | ||
| Winter (December, January, February) | 17.5 | |
| Spring (March, April, May) | 32.1 | |
| Summer (June, July, August) | 25.5 | |
| Fall (September, October, November) | 24.8 | |
| Average Weekly Sleep Duration (hr/night) | 7.2 ± 0.9 | 4.6 - 8.8 |
| Weekend Catch-Up Sleep (hr/night) | 0.3 ± 1.0 | −1.5 - 3.7 |
| Within-Subject Sleep Duration Variability | 1.2 ± 0.5 | 0.4 - 3.7 |
| Bedtime Shift (hr) | 0.9 ± 0.7 | 0.0 - 3.2 |
| Waketime Shift (hr) | 1.3 ± 1.1 | 0.0 - 5.3 |
| Social Jet Lag (hr) | 1.1 ± 1.0 | 0.0 - 7.3 |
| Bedtime (hh:mm ± hr) | 11:31PM ± 1.3 | 8:52PM - 3:21AM |
| Waketime (hh:mm ± hr) | 7:53AM ± 1.1 | 5:02AM - 11:31AM |
| Sleep Midpoint (hh:mm ± hr) | 3:46AM ± 1.1 | 1:40AM - 7:26AM |
Values presented are mean ± standard deviation, unless otherwise noted as percentage. Four participants were missing data for socioeconomic status.
Facets of Sleep and 1-Year Fat Mass
Results of partially adjusted analyses [adjusting for baseline fat mass (kg) and baseline height (cm) only] are presented in Table 3. Adjusting for baseline fat mass and height, average weekly sleep duration was not significantly associated with 1-year fat mass [F(2,137) = 420.53, β = −0.01, p = .44, R2 = .91). Additionally, no facet of sleep variability [weekend catch-up sleep F(2,137) = 419.41, β = −0.003, p = .60, R2 = .91; within-subject sleep duration variability F(2,137) = 418.95, β = 0.02, p = .69, R2 = .91; bedtime shift F(2,137) = 421.05, β = −0.01, p = .38, R2 = .91; waketime shift F(2,137) = 418.65, β = −0.002, p = .79, R2 = .91; social jet lag F(2,137) = 424.48, β = −0.01, p = .19, R2 = .91] nor bedtime [F(2,137) = 428.71, β = −0.23, p = .09, R2 = .91] was significantly associated with 1-year fat mass. However, waketime [F(2,137) = 435.12, β = −0.32, p = .03, R2 = .91] was negatively associated with 1-year fat mass such that waking up earlier was associated with greater fat mass at 1-year follow-up (Figure 1a). Likewise, sleep midpoint was inversely associated with 1-year fat mass [F(2,137) = 438.64 β = −0.35, p = .02, R2 = .91], such that having an earlier sleep midpoint was associated with greater 1-year fat mass (Figure 1b).
Table 3.
Associations of Facets of Sleep and Fat Mass at 1-Year Follow-Up
| F | β | p | R2 | |
|---|---|---|---|---|
| Partially Adjusted Models | ||||
| Average Weekly Sleep Duration | 420.53 | −0.01 | .44 | .91 |
| Weekend Catch-Up Sleep | 419.41 | −0.003 | .60 | .91 |
| Within-Subject Sleep Duration Variability | 418.95 | 0.02 | .69 | .91 |
| Bedtime Shift | 421.05 | −0.01 | .38 | .91 |
| Waketime Shift | 418.65 | −0.002 | .79 | .91 |
| Social Jet Lag | 424.48 | −0.01 | .19 | .91 |
| Bedtime | 428.71 | −0.23 | .09 | .91 |
| Waketime | 435.12 | −0.32 | .03 | .91 |
| Sleep Midpoint | 438.64 | −0.35 | .02 | .91 |
Note. All models adjusted for baseline fat mass (kg) and baseline height (cm).
Figure 1.
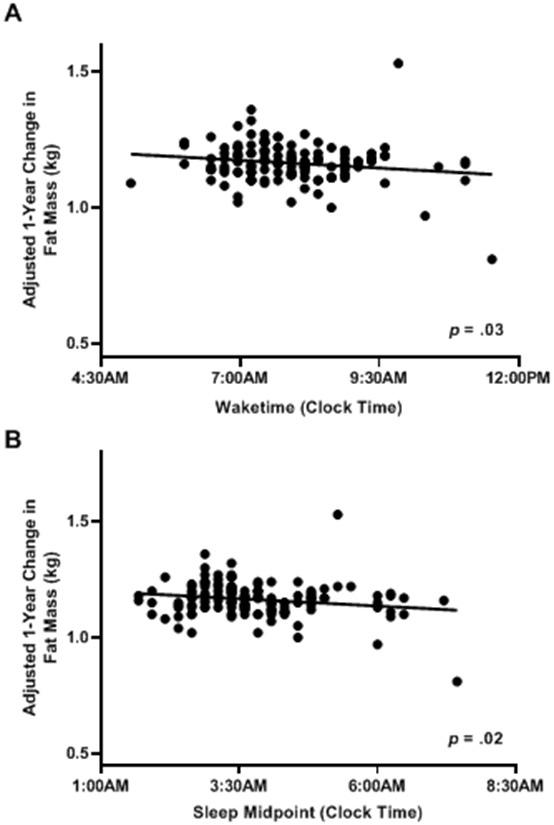
The association of waketime and sleep midpoint and 1-year change in fat mass. Waketime (A; p = .03) and sleep midpoint (B; p = .02) were significantly inversely associated with fat mass at 1-year follow-up. Analyses adjusted for baseline fat mass (kg) and height (cm). For ease of visualization, raw wake and sleep midpoint times were used rather than adjusted wake and sleep midpoint times.
Discussion
The current study utilized objective measures of sleep and fat mass to examine the associations between facets of sleep and fat mass at 1-year follow-up among youth of a broad age and weight range. Average weekly sleep duration, sleep variability (weekend catch-up sleep, within-subject sleep duration variability, bed and waketime shift, and social jet lag), and bedtime were not significantly associated with fat mass at 1-year follow-up. However, earlier waketime and earlier sleep midpoint were associated with greater fat mass at 1-year follow-up, after adjusting for baseline fat mass and height. These results suggest that wake timing may be associated with greater gains in fat mass over time.
Null results between average weekly sleep duration, sleep variability and bedtime and 1-year fat mass are somewhat inconsistent with prior literature among youth [e.g., (1, 9, 14)]. his It is possible that methodological differences, such as a somewhat smaller sample size of a wide age range could account for null findings. Alternatively, few studies among youth have utilized actigraphy to examine several nuanced facets of sleep over a 2-week period in relation to objectively measured adiposity (2, 3). It is possible that prior significant associations detected with the use of self-report measures [for review see (2, 3)], which may be biased or unreliable, are attenuated when utilizing objective measures of multiple facets of sleep. We used objective measures, which may have provided a more accurate representation of nuanced facets of sleep. Indeed, the majority of prior studies rely on child- or parent-report of sleep, with many using single item measures, such as “on an average night, how many hours of sleep do you get on weekdays and weekend days” (4). Asking participants to estimate a weekly average may not adequately capture nightly sleep differences. Additionally, relationships may have been attenuated due to the fact that our sample had relatively short average weekly sleep duration with little variability (7.2 hours ± 1.1) as well as low variability in other metrics of sleep. Finally, there are some data to support that the prospective relationship between short sleep duration and risk for high weight is most robustly observed in early childhood (2, 36, 37), and other facets of sleep may be more salient in middle childhood and adolescence. For example, Bell & Zimmerman (2010) found short sleep duration to be prospectively associated with increased risk for having overweight or obesity at 5-year follow up among children ages 0-4. Interestingly, this relationship was not present among older children [ages 5-13; (37)] which could potentially be due to the shifts observed in sleep habits during middle childhood [10-13 years; (38)]. Together, data from prior studies and as well as the observed null results in the present sample may suggest it is important for researchers and clinicians to consider more nuanced facets of sleep rather than duration alone when assessing obesity risk in youth. However, it remains unclear how assessment of multiple facets of sleep may impact clinical intervention. Future research should directly assess the utility of detailed clinical interviewing for treatment recommendations and subsequent sleep and weight outcomes. Additional objectively-measured longitudinal data among larger samples of youth across a wide age spectrum are needed to more fully understand these relationships.
Notably, both early waketime and sleep midpoint were associated with greater gains in fat mass. While contrary to our original hypotheses, these findings are consistent with one study in children that found earlier wake times to be associated with greater odds of having obesity (16). Additionally, Garipy et al. (2018) found that delayed school start time, and ostensibly later waketime, was associated with lower BMIz among youth 10-18 years old (39). Our results complement these previous data by highlighting that earlier waketime and sleep midpoint may be understudied components of broader sleep-timing, which may play a role in the promotion of high weight among youth. Emerging data suggest that sleep patterns that are incongruent with physiologically-driven circadian preference, which often shifts later beginning in middle childhood (40), are associated with higher BMIz, waist-to-height ratio, waist circumference, and fat mass (12). While preliminary data suggest that social jet lag may represent circadian rhythm misalignment (12), we did not observe a significant association between social jet lag and fat mass at 1-year follow-up. Given that in our sample, mean social jet lag was relatively low (1.1±1.0 hr), it is possible that participants were not able to sleep according to their body’s circadian preference on the weekends (which would increase social jet lag) due to social demands (e.g., waking early to attend sporting events). Therefore, we may not have accurately captured circadian rhythm misalignment through this construct and it may instead be better represented by early average waketime and sleep midpoint time. Supporting this notion, a recent study found the positive association between reported social jet lag and age-adjusted BMI percentiles to be highest among individuals with two or more hours of social jet lag (41). Data specifically examining waketime and sleep midpoint among youth are scarce. The current findings suggest these facets may serve as additional indicators of circadian rhythm misalignment and may therefore be important to examine in future research. Additional longitudinal data considering multiple facets of sleep timing throughout the seasons of the year as well as a measure of circadian preference are needed to better elucidate these associations. Finally, waketime and sleep midpoint are related yet distinct constructs. Indeed, they were highly correlated in our sample. Thus, additional data are needed to better understand how they may differentially affect obesity risk in youth.
Study strengths include use of actigraphy to examine several facets of sleep over two weeks, which provided an objective measure that more accurately quantifies sleep as compared to self-report assessments (42). Additionally, the current study included fat mass as measured by dual-energy x-ray absorptiometry, thereby minimizing the known limitations of using BMI (19). Finally, examination of youth in generally good health enabled us to study factors that may promote high weight before the development of overweight or obesity, thereby elucidating potential risk factors for the onset of pediatric obesity.
Study limitations include a relatively small sample size of a broad age strata which precluded us from further assessing varying sleep needs across the developmental trajectory (43). Similarly, our sample did not include enough youth with overweight or obesity to assess the influence of weight status on outcomes. Given that emerging data have found links between sleep timing and weight status (44), future studies should recruit samples enriched for children with overweight and obesity in order to better elucidate potential differences among groups. It is also possible that the 1-year timeframe was not sufficient to see meaningful changes in fat mass in our sample, especially given that adiposity develops differentially by sex across puberty (34), suggesting future studies should include a longer follow-up period. Further, participants in the current sample provided actigraphy data throughout the year, and while season of assessment was not a significant covariate in analyses, the sample size was likely not large enough to consider differences in results based on time of year. It is possible that participants completing the study protocol during summer months may have been more likely to sleep according to their biological preference, thereby reducing circadian misalignment and increasing sleep duration (45). Therefore, future research should aim to recruit samples large enough to compare results based on time of year, e.g., during the academic year versus summer break. Moreover, future studies could also consider use of repeated measures to investigate how social changes associated with season affects within-person sleep patterns and obesity risk. Finally, given that assessing the association between several facets of sleep and 1-year fat mass was not a primary aim of the original study, no a priori power estimates were made for the current study aims. This study also utilized nine regression analyses which could have inflated the chance of a type 1 error; thus, this study should be considered hypothesis-generating.
Conclusion
Sleep timing, specifically waketime and sleep midpoint, may be relevant for the development of excess fat gain in youth. No significant associations were detected between other facets of sleep, including average weekly sleep duration or sleep variability, and adiposity. Thus, sleep timing specifically may be a potentially modifiable factor in the development of excess weight gain in youth. More data among larger samples of youth across a wide age and weight spectrum collected throughout the entire year are needed to provide clarification on how circadian preference impacts the association between sleep timing and onset of obesity.
What is already known about this subject?
Facets of sleep beyond total sleep duration (e.g., sleep variability and sleep timing) have been linked to obesity risk in youth. However, there is a paucity of objectively measured longitudinal sleep data.
What are the new findings of this manuscript?
Using objective measures of sleep, we found that youth with earlier waketimes and sleep midpoints had greater gains in fat mass over one year. Average weekly sleep duration, sleep variability and bedtime were not associated with adiposity gain.
How might results change the direction of research or the focus of clinical practice?
These data support the possibility that modifying sleep timing behaviors might be a viable strategy for prevention of obesity among youth.
FUNDING:
This work was supported by Intramural Research Program (NICHD grant number Z1A-HD00641; Yanovski); Supplemental funding (OBSSR, NIH; Yanovski); National Research Service Award (grant number 1F32HD082982; NICHD, NIH; Kelly)
Footnotes
DISCLOSURE: The authors have no conflict to declare. The opinions and assertions expressed herein are those of the authors and are not to be construed as reflecting the views of the Uniformed Services University of the Health Sciences (USU), or the U.S. Department of Defense.
CLINICAL TRIAL REGISTRATION: NCT02390765
DATA AVAILABILITY STATEMENT:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Silva GE, Goodwin JL, Parthasarathy S, Sherrill DL, Vana KD, Drescher AA, et al. Longitudinal association between short sleep, body weight, and emotional and learning problems in Hispanic and Caucasian children. Sleep. 2011;34(9):1197–205. Epub 2011/09/03. doi: 10.5665/SLEEP.1238. PubMed PMID: 21886357; PubMed Central PMCID: PMCPMC3157661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller MA, Kruisbrink M, Wallace J, Ji C, Cappuccio FP. Sleep duration and incidence of obesity in infants, children, and adolescents: a systematic review and meta-analysis of prospective studies. Sleep. 2018;41(4). Epub 2018/02/06. doi: 10.1093/sleep/zsy018. PubMed PMID: 29401314. [DOI] [PubMed] [Google Scholar]
- 3.Morrissey B, Taveras E, Allender S, Strugnell C. Sleep and obesity among children: a systematic review of multiple sleep dimensions. Pediatric Obesity. 2020;15(4):e12619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kreitsch KN, Chardon ML, Beebe DW, Janicke DM. Sleep and weight-related factors in youth: a systematic review of recent studies. Sleep medicine reviews. 2019. [DOI] [PubMed] [Google Scholar]
- 5.Simon SL, Field J, Miller LE, DiFrancesco M, Beebe DW. Sweet/dessert foods are more appealing to adolescents after sleep restriction. PloS one. 2015;10(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mi SJ, Kelly NR, Brychta RJ, Grammer AC, Jaramillo M, Chen KY, et al. Associations of sleep patterns with metabolic syndrome indices, body composition, and energy intake in children and adolescents. Pediatric Obesity. 2019:e12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ievers-Landis CE, Kneifel A, Giesel J, Rahman F, Narasimhan S, Uli N, et al. Dietary intake and eating-related cognitions related to sleep among adolescents who are overweight or obese. J Pediatr Psychol. 2016;41(6):670–9. Epub 2016/03/21. doi: 10.1093/jpepsy/jsw017. PubMed PMID: 26994854. [DOI] [PubMed] [Google Scholar]
- 8.Appelhans BM, Fitzpatrick SL, Li H, Cail V, Waring ME, Schneider KL, et al. The home environment and childhood obesity in low-income households: indirect effects via sleep duration and screen time. BMC Public Health. 2014;14(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He F, Bixler EO, Liao J, Berg A, Imamura Kawasawa Y, Fernandez-Mendoza J, et al. Habitual sleep variability, mediated by nutrition intake, is associated with abdominal obesity in adolescents. Sleep Med. 2015;16(12):1489–94. Epub 2015/11/28. doi: 10.1016/j.sleep.2015.07.028. PubMed PMID: 26611945; PubMed Central PMCID: PMCPMC4662770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He F, Bixler EO, Berg A, Kawasawa YI, Vgontzas AN, Fernandez-Mendoza J, et al. Habitual sleep variability, not sleep duration, is associated with caloric intake in adolescents. Sleep Medicine. 2015;16(7):856–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee BH, Kang S-G, Choi J-W, Lee YJ. The association between self-reported sleep duration and body mass index among Korean adolescents. Journal of Korean Medical Science. 2016;31(12):1996–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cespedes EF, Rifas-Shiman S, Quante M, Redline S, Oken E, Taveras E. Chronotype, social jet lag, and cardiometabolic risk factors in early adolescence. JAMA Pediatrics. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petrov ME, Vander Wyst KB, Whisner CM, Jeong M, Denniston M, Moramarco MW, et al. Relationship of sleep duration and regularity with dietary intake among preschool-aged children with obesity from low-income families. Journal of Developmental and Behavioral Pediatrics: JDBP. 2017;38(2):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thivel D, Isacco L, Aucouturier J, Pereira B, Lazaar N, Ratel S, et al. Bedtime and sleep timing but not sleep duration are associated with eating habits in primary school children. J Dev Behav Pediatr. 2015;36(3):158–65. Epub 2015/01/22. doi: 10.1097/DBP.0000000000000131. PubMed PMID: 25607639. [DOI] [PubMed] [Google Scholar]
- 15.Amigo I, Peña E, Errasti JM, Busto R. Sedentary versus active leisure activities and their relationship with sleeping habits and body mass index in children of 9 and 10 years of age. Journal of Health Psychology. 2016;21(7):1472–80. [DOI] [PubMed] [Google Scholar]
- 16.Scharf RJ, DeBoer MD. Sleep timing and longitudinal weight gain in 4-and 5-year-old children. Pediatric Obesity. 2015;10(2):141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Venkatapoorna CM, Ayine P, Selvaraju V, Parra EP, Koenigs T, Babu JR, et al. The relationship between obesity and sleep timing behavior, television exposure, and dinnertime among elementary school-age children. Journal of Clinical Sleep Medicine. 2020;16(1):129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Broussard JL, Van Cauter E. Disturbances of sleep and circadian rhythms: novel risk factors for obesity. Curr Opin Endocrinol Diabetes Obes. 2016;23(5):353–9. Epub 2016/09/02. doi: 10.1097/MED.0000000000000276. PubMed PMID: 27584008; PubMed Central PMCID: PMCPMC5070789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vanderwall C, Clark RR, Eickhoff J, Carrel AL. BMI is a poor predictor of adiposity in young overweight and obese children. BMC Pediatrics. 2017;17(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LeMay-Russell S, Tanofsky-Kraff M, Schvey NA, Kelly NR, Shank LM, Mi SJ, et al. Associations of weekday and weekend sleep with children’s reported eating in the absence of hunger. Nutrients. 2019;11(7):1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000. CDC Growth Charts for the United States: methods and development. Vital Health Stat 11. 2002(246):1–190. PubMed PMID: 12043359. [PubMed] [Google Scholar]
- 22.Ranzenhofer LM, Hannallah L, Field SE, Shomaker LB, Stephens M, Sbrocco T, et al. Pre-meal affective state and laboratory test meal intake in adolescent girls with loss of control eating. Appetite. 2013;68:30–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Archives Of Disease In Childhood. 1969;44(235):291–303. PubMed PMID: 5785179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanner JM. Growth and maturation during adolescence. Nutrition Reviews. 1981;39(2):43–55. PubMed PMID: 7010232. [DOI] [PubMed] [Google Scholar]
- 25.Kovacs M The Children's Depression, Inventory (CDI). Psychopharmacol Bull. 1985;21(4):995–8. PubMed PMID: 4089116. [PubMed] [Google Scholar]
- 26.Hollingshead AB. Hollingshead two factor index of social position In: Miller DC, editor. Handbook of research design and social measurement. 5th ed. Newbury Park, CA: Sage Publications,1991; 1957. p. 351–9. [Google Scholar]
- 27.Kushida CA, Chang A, Gadkary C, Guilleminault C, Carrillo O, Dement WC. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med. 2001;2(5):389–96. Epub 2003/11/01. PubMed PMID: 14592388. [DOI] [PubMed] [Google Scholar]
- 28.Hjorth MF, Quist JS, Andersen R, Michaelsen KF, Tetens I, Astrup A, et al. Change in sleep duration and proposed dietary risk factors for obesity in Danish school children. Pediatric Obesity. 2014;9(6):e156–e9. Epub 2014/09/25. doi: 10.1111/ijpo.264. PubMed PMID: 25251317. [DOI] [PubMed] [Google Scholar]
- 29.Sadeh A, Sharkey M, Carskadon MA. Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep. 1994;17(3):201–7. [DOI] [PubMed] [Google Scholar]
- 30.Osborne JW, Overbay A. The power of outliers (and why researchers should always check for them). Practical Assessment, Research, and Evaluation. 2004;9(1):6. [Google Scholar]
- 31.Kohut T, Robbins J, Panganiban J. Update on childhood/adolescent obesity and its sequela. Current Opinion in Pediatrics. 2019;31(5):645–53. [DOI] [PubMed] [Google Scholar]
- 32.Breitenstein RS, Doane LD, Lemery-Chalfant K. Early life socioeconomic status moderates associations between objective sleep and weight-related indicators in middle childhood. Sleep Health. 2019;5(5):470–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lucien JN, Ortega MT, Shaw ND. Sleep and Puberty. Current Opinion in Endocrine and Metabolic Research. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glass NA, Torner JC, Letuchy EM, Burns TL, Janz KF, Eichenberger Gilmore JM, et al. The relationship between greater prepubertal adiposity, subsequent age of maturation, and bone strength during adolescence. Journal of Bone and Mineral Research. 2016;31(7):1455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verhulst SL, Schrauwen N, Haentjens D, Suys B, Rooman RP, Van Gaal L, et al. Sleep-disordered breathing in overweight and obese children and adolescents: prevalence, characteristics and the role of fat distribution. Archives of Disease in Childhood. 2007;92(3):205–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Storfer-Isser A, Patel SR, Babineau DC, Redline S. Relation between sleep duration and BMI varies by age and sex in youth age 8–19. Pediatric Obesity. 2012;7(1):53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bell JF, Zimmerman FJ. Shortened nighttime sleep duration in early life and subsequent childhood obesity. Archives of Pediatrics & Adolescent Medicine. 2010;164(9):840–5. [DOI] [PubMed] [Google Scholar]
- 38.Laberge L, Petit D, Simard C, Vitaro F, Tremblay R, Montplaisir J. Development of sleep patterns in early adolescence. Journal of Sleep Research. 2001;10(1):59–67. [DOI] [PubMed] [Google Scholar]
- 39.Gariépy G, Janssen I, Sentenac M, Elgar FJ. School start time and the healthy weight of adolescents. Journal of Adolescent Health. 2018;63(1):69–73. [DOI] [PubMed] [Google Scholar]
- 40.Kuula L, Pesonen A-K, Merikanto I, Gradisar M, Lahti J, Heinonen K, et al. Development of late circadian preference: sleep timing from childhood to late adolescence. The Journal of Pediatrics. 2018;194:182–9. e1. [DOI] [PubMed] [Google Scholar]
- 41.Cetiner O, Yildirim G, Kalyoncu ZB. Social jetlag is associated with the frequency of consumption of sugar-sweetened beverages and a high BMI percentile in adolescents: results of the cross-sectional family life, activity, sun, health, and eating (FLASHE) study. Journal of the Academy of Nutrition and Dietetics. 2021. [DOI] [PubMed] [Google Scholar]
- 42.Brychta RJ, Rögnvaldsdóttir V, Guðmundsdóttir SL, Stefánsdóttir R, Hrafnkelsdóttir SM, Gestsdóttir S, et al. Longitudinal change in adolescent bedtimes measured by self-report and actigraphy. Journal for the Measurement of Physical Behaviour. 2019;2(4):282–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maslowsky J, Ozer EJ. Developmental trends in sleep duration in adolescence and young adulthood: evidence from a national United States sample. J Adolesc Health. 2014;54(6):691–7. Epub 2013/12/24. doi: 10.1016/j.jadohealth.2013.10.201. PubMed PMID: 24361237; PubMed Central PMCID: PMCPMC4401462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hayes JF, Balantekin KN, Altman M, Wilfley DE, Taylor CB, Williams J. Sleep patterns and quality are associated with severity of obesity and weight-related behaviors in adolescents with overweight and obesity. Child Obes. 2018;14(1):11–7. Epub 2017/08/30. doi: 10.1089/chi.2017.0148. PubMed PMID: 28850274; PubMed Central PMCID: PMCPMC5743029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wing YK, Li SX, Li AM, Zhang J, Kong AP. The effect of weekend and holiday sleep compensation on childhood overweight and obesity. Pediatrics. 2009;124(5):e994–e1000. Epub 2009/10/28. doi: 10.1542/peds.2008-3602. PubMed PMID: 19858153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


