Supplemental Digital Content is available in the text.
Keywords: SARS-CoV-2, symptoms, persistent, outcome, severity, neurologic, mental health, chronic fatigue, headache
Abstract
In children, the risk of coronavirus disease (COVID) being severe is low. However, the risk of persistent symptoms following infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is uncertain in this age group, and the features of “long COVID” are poorly characterized. We reviewed the 14 studies to date that have reported persistent symptoms following COVID in children and adolescents. Almost all the studies have major limitations, including the lack of a clear case definition, variable follow-up times, inclusion of children without confirmation of SARS-CoV-2 infection, reliance on self- or parent-reported symptoms without clinical assessment, nonresponse and other biases, and the absence of a control group. Of the 5 studies which included children and adolescents without SARS-CoV-2 infection as controls, 2 did not find persistent symptoms to be more prevalent in children and adolescents with evidence of SARS-CoV-2 infection. This highlights that long-term SARS-CoV-2 infection–associated symptoms are difficult to distinguish from pandemic-associated symptoms.
Children infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are usually asymptomatic or have mild coronavirus disease (COVID) with low rates of hospitalization (<2%) or death (<0.03%).1–9 Reported hospitalization rates might overestimate severity as many studies do not specify whether children are hospitalized with COVID or because of COVID.10 The disease burden is higher in adolescents, who are more frequently infected and hospitalized than younger children.9
Despite the low-risk that acute COVID poses in children in the short term, 2 longer term consequences of SARS-CoV-2 infection are of more concern. The first is “pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS)” or “multisystem inflammatory syndrome in children (MIS-C),” an immune-mediated disease that occurs in a small proportion (<0.1%) of children 2 to 6 weeks after being infected with SARS-CoV-2.11–20
The second is “long COVID,” also called “post-COVID syndrome” or “post-acute sequelae of SARS-CoV-2 (PASC).” These terms describe the persisting symptoms following COVID, described mainly in adults, affecting the sensory, neurologic, and cardiorespiratory systems, as well as mental health.21–23 To date, there is no clear definition for this syndrome and no agreement on the duration of symptoms that justify the diagnosis, which ranges from 4 to 12 weeks after the acute infection. Over 200 symptoms have been attributed to long COVID, many of them nonspecific and highly prevalent in the general population, such as fatigue, sleep disturbance, concentration difficulties, loss of appetite, and muscle or joint pain.24–26 In adults, reported risk factors for long COVID include female sex, middle age, white ethnicity and comorbidities, especially asthma.27–29 There is much less data on long COVID in children and adolescents.
The low-risk posed by the acute disease means that 1 of the key benefits of COVID vaccination of children and adolescents might be to protect them from long COVID. An accurate determination of the risk of long COVID is therefore crucial in the debate about the risks and benefits of vaccination in this age group. Here, we review and summarize studies that have reported long COVID symptoms in children and adolescents.
STUDIES OF LONG COVID IN CHILDREN AND ADOLESCENTS
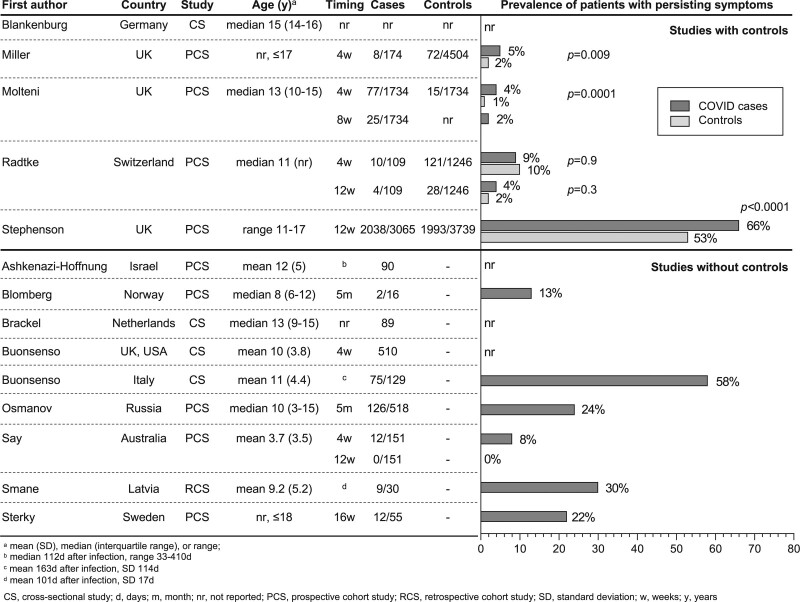
We identified 14 studies (4 cross-sectional studies,26,30–32 9 prospective cohort studies,33–41 1 retrospective cohort study42) investigating long COVID symptoms in a total of 19,426 children and adolescents (Table 1 and Fig. 1; Table, Supplementary Digital Content 1, http://links.lww.com/INF/E531). The number of children and adolescents in each study varied from 16 to 6804 (median 330, interquartile range 89–1533). All of the studies were done in high-income countries. Case reports, studies which followed children after a SARS-CoV-2 infection but did not evaluate symptoms of long COVID or studies which did not address predominantly children and adolescents were not included.43–50
TABLE 1.
Strengths and Limitations of Studies Which Investigated Persistent Symptoms After SARS-CoV-2 Infection in Children and Adolescents
| Limitations | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Control Group Without SARS-CoV-2 Infection | Includes Children With Moderate and Severe Disease | All Cases Laboratory Confirmed | Face-to-face Follow-up or Clinical Assessment | Further Strengths | Preprint (Not Peer-reviewed) | No Control Group | Small Cohort or Small Number Seropositive | No Data on Preexisting Medical Conditions | Large Number With Preexisting Medical Condition | Includes Self-reported SARS-CoV-2 Infection | Not All Children Laboratory Confirmed Infection | Includes Only/mostly Asymptomatic or Mild Infection | Timing of SARS-CoV-2 Infection Not Specified | Duration of Symptoms Not Specified | Self-/Parent-reported Symptoms, No Clinical Assessment | Few Clinical Outcomes Assessed | No Mental Health Outcomes | Selection Bias | Misclassification Bias | Recall Bias | Nonresponse Bias | Further Limitations |
| Studies with controls | |||||||||||||||||||||||
| Blankenburg et al30 | ✓ | ✓ | ✓† | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Includes participants up to 38y of age | |||||||||
| Miller et al36 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Molteni et al35 | ✓ | ✓ | Control group matched for age, sex, week of testing | ✓* | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Participants who did not answer every week were excluded (73%) | |||||||||||
| Radtke et al34 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Stephenson et al37 | ✓ | ✓ | ✓ | Largest cohort; control group matched for age, sex, month of testing, geographical area | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | More female and adolescent participants | ||||||||||||
| Studies without controls | |||||||||||||||||||||||
| Ashkenazi-Hoffnung et al39 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓‡ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Blomberg et al33 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Fatigue, concentration difficulties, memory problems assessed only in adults | |||||||||||
| Brackel et al32 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Buonsenso et al31 | Variety of questions asked | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||||
| Buonsenso et al26 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||||
| Osmanov et al40 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Only includes hospitalized children | ||||||||||||||||
| Say et al41 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Low median age | |||||||||||||
| Smane et al42 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||||
| Sterky et al38 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Only includes hospitalized children | |||||||||||
*Except asthma.
†Only for blood drawing.
‡Except overweight.
FIGURE 1.
Proportion of children and adolescents with persistent symptoms after SARS-CoV-2 infection.
There is marked heterogeneity between studies, including differences in design, inclusion criteria, outcomes, and follow-up times (Table 2). Children were evaluated for persistent symptoms for varying durations: more than 4 weeks (2 studies),31,36 more than 4 and 8 weeks (1 study),35 more than 4 and 12 weeks (2 studies),34,41 more than 12 weeks (1 study),37 more than 5 months (2 studies),33,40 and at arbitrary timepoints (6 studies).26,30,32,38,39,42 In 7 studies, evaluation of symptoms was done only through online questionnaires or phone interviews,26,31,32,34–36,40 while 5 studies included study visits.30,33,39,41,42
TABLE 2.
Heterogeneity and Methodological Limitations Found in Studies Investigating Children and Adolescents With Persistent Symptoms After SARS-CoV-2 Infection
| Heterogeneity between studies |
| Studies vary considerably in: |
| • Age range of participants |
| • Proportion of participants with preexisting medical conditions |
| • Inclusion criteria |
| ∘ Laboratory confirmed COVID |
| ∘ Severity of disease |
| • Time points of assessment |
| • Outcome measurement |
| ∘ Number and range of symptoms assessed |
| ∘ Duration of follow-up |
| • Data collection method |
| Methodologic limitations |
| • No control group |
| • Small cohort or small number seropositive |
| • No data on preexisting medical conditions |
| • Inclusion criteria |
| ∘ Includes self-reported SARS-CoV-2 infection |
| ∘ Not all cases laboratory confirmed infection |
| ∘ Includes only/mostly asymptomatic or mild infection |
| • Outcome |
| ∘ Timing of SARS-CoV-2 infection not specified |
| ∘ Duration of symptoms not specified |
| ∘ Self-/parent-reported symptoms, no clinical assessment |
| ∘ Few clinical outcomes assessed |
| ∘ No mental health outcomes |
| • Bias |
| ∘ Selection bias |
| ∘ Misclassification bias |
| ∘ Recall bias |
| ∘ Nonresponse bias |
RESULTS OF STUDIES OF LONG COVID IN CHILDREN AND ADOLESCENTS
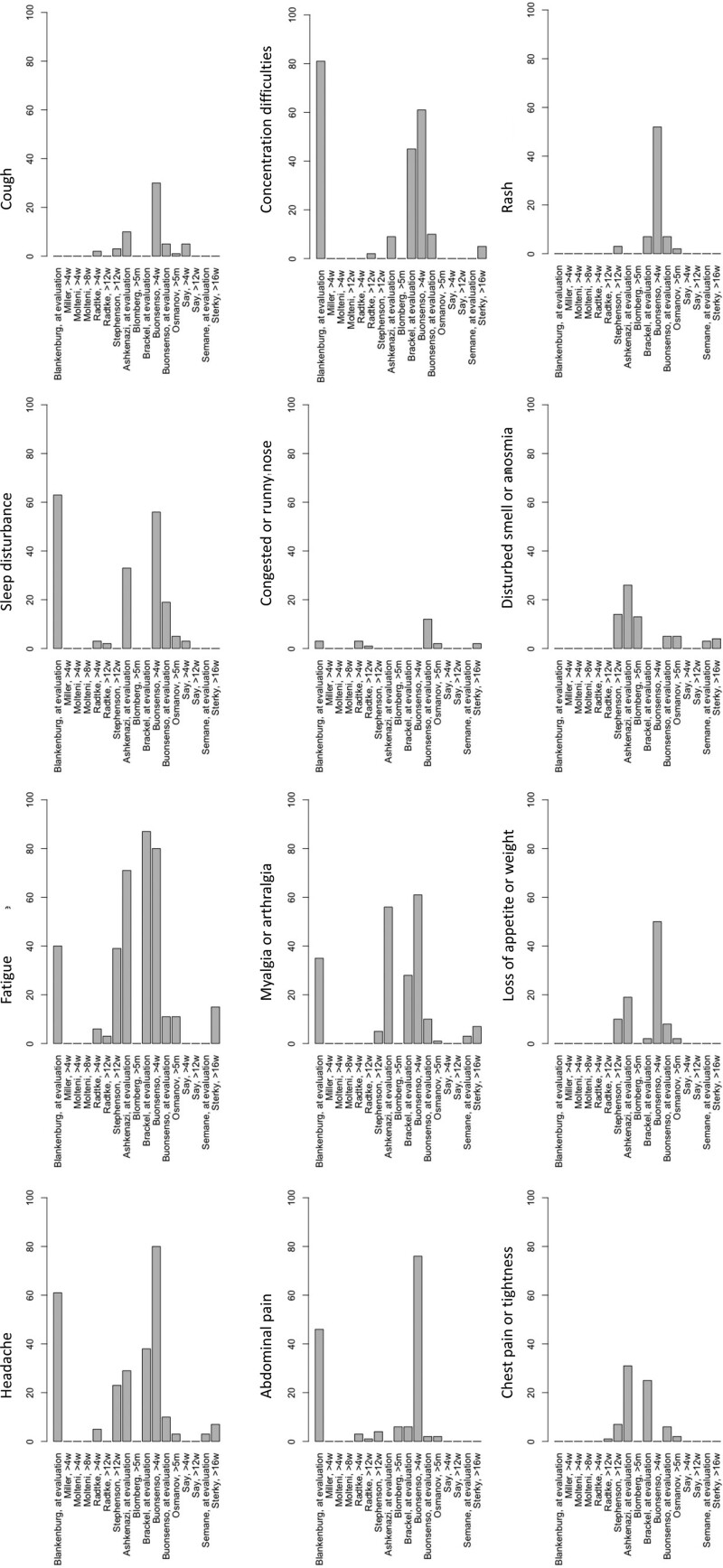
The prevalence of long COVID symptoms varied considerably between studies from 4 to 66%.26,33–38,40–42 There was also a large variation in the reported frequency of persistent symptoms. The most common reported symptoms were headache (3 to 80%), fatigue (3 to 87%), sleep disturbance (2 to 63%), concentration difficulties (2 to 81%), abdominal pain (1 to 76%), myalgia or arthralgia (1 to 61%), congested or runny nose (1 to 12%), cough (1 to 30%), chest tightness or pain (1 to 31%), loss of appetite or weight (2 to 50%), disturbed smell or anosmia (3 to 26%), and rash (2 to 52%) (Fig. 2; Table, Supplementary Digital Content 1, http://links.lww.com/INF/E531).26,30–42 Four studies reported a much higher prevalence of symptoms compared with the other studies.26,30–32 Of these studies, 3 were done at arbitrary timepoints after a SARS-CoV-2 infection.26,30,32 Six studies reported a positive correlation between increasing age,30,35–37,39,40 3 between female sex30,36,37 and 1 each between allergic diseases40 or worse pre-infection physical and mental health37 and the prevalence of persisting symptoms.40 Furthermore, one study found an association between longer hospitalization and more severe persistent symptoms, and between PIMS-TS and a higher prevalence of persistent symptoms.38
FIGURE 2.
Most common reported persistent symptoms (%) after SARS-CoV-2 infection in children and adolescents (for studies in which a symptom was not reported bars are set at 0, except for Say, >12w when all children were asymptomatic).
A control group was included in only 5 of the 14 studies. These 5 studies reported symptoms in children and adolescents without evidence of SARS-CoV-2 infection as a comparison group.30,34–37 Three of these studies found persistent symptoms to be more prevalent in children and adolescents with evidence of a SARS-CoV-2 infection.35–37
STRENGTHS AND LIMITATIONS OF STUDIES
The strengths and limitations of the studies are summarized in Table 2. Almost all the studies to date on long COVID in children and adolescents have major limitations.
The first major limitation is the lack of a clear case definition meaning studies have used variable inclusion criteria and follow-up times. Some studies included children with self-reported SARS-CoV-2 infection without laboratory confirmation.31,32 In addition to the heterogeneity in inclusion criteria, studies followed up children at arbitrary time points and the method of assessment varied. Most studies relied on self- or parent-reported symptoms from questionnaires without clinical assessment and objective parameters, such as lung function testing or imaging.26,30–32,34–38,40 Using apps or online questionnaires is likely to select participants from higher socio-economic background, who have a lower risk of poor outcomes following SARS-CoV-2 infection.51
A second major limitation is the lack of a control group in the majority of studies. In the absence of a control group, it is impossible to distinguish symptoms of long COVID from symptoms attributable to the pandemic, such as lockdown measures (school closures, deprivation of seeing friends or being unable to do sports and other activities) or seeing family and friends suffering or even dying from COVID. The results from the studies to date suggest that infection-associated symptoms are not necessarily more common or severe than pandemic-associated symptoms.30,34 The prevalence of symptoms consistent with long COVID, including psychosomatic symptoms, have been considerably higher in children and adolescents since the start of the pandemic, and lockdown measures have been shown to have negative effects on the well-being and mental health of children and adolescents.52,53 While lockdown measures, including school closures decrease SARS-CoV-2 transmission and prevent late manifestations of COVID, these actions restrict social contact, self-determination and education, and therefore amplify pandemic-associated symptoms.
A third important limitation is selection bias as many studies had a low response rate (13% in a recent study).37 As those with persisting symptoms might be more likely to respond to surveys, this can lead to a substantial overestimation of the prevalence of long COVID. Also, as children and adolescents with mild symptoms might not seek testing, selection and misclassification bias could also lead to an overestimate.
Another limitation is that almost all studies include a wide range of age groups. It is likely that the incidence and characteristics of long COVID vary between adolescents and younger children. As the risks and benefits of COVID vaccines differs between these age groups, more studies are needed that provide age-specific data. Furthermore, none of the studies investigated the impact of initial disease severity on the risk of long COVID. Finally, all studies are likely to have been done before the delta variant becoming dominant, which may have a different risk of long COVID.
Adding to the confusion has been the use of the term long COVID to encompass those with objective complications of COVID (such as pulmonary fibrosis or myocardial dysfunction), those with mental health problems,21,22 and those with more subjective and nonspecific symptoms reminiscent of postviral chronic fatigue syndrome or myalgic encephalomyelitis. A separation of postintensive care syndrome, postviral fatigue syndrome, and long-term COVID syndrome has been suggested for the adult population and could be adopted for children.54
CONCLUSIONS
In summary, the evidence for long COVID in children and adolescents is limited, and all studies to date have substantial limitations or do not show a difference between children who had been infected by SARS-CoV-2 and those who were not. The absence of a control group in the majority of studies makes it difficult to separate symptoms attributable to long COVID from pandemic-associated symptoms.30,34,36
In light of the large number of children and adolescents infected with SARS-CoV-2, the impact of even a low prevalence of persisting symptoms will be considerable. However, in the majority of studies, symptoms did not persist longer than 12 weeks.33–35,41 Consistent with this, 1 study that did find a difference between cases and controls in persisting symptoms (at 4 weeks post COVID) reported that by 8 weeks, most symptoms had resolved, suggesting long COVID might be less of a concern in children and adolescents than in adults.35 Interestingly in one study, more than half of adolescents in the uninfected control group reported symptoms at 12 weeks despite only 8% reporting symptoms at the time of testing for SARS-CoV-2.37
The relative scarcity of studies of long COVID and the limitations of those reported to date mean the true incidence of this syndrome in children and adolescents remains uncertain. The impact of age, disease severity and duration, virus strain, and other factors on the risk of long COVID in this age group also remains to be determined.
In light of the importance of long COVID in the risk-benefit equation for policy decisions on COVID vaccines for children and adolescents, further studies to accurately determine the risk of long COVID are urgently needed.55 These should include rigorous control groups, including children with other infections and those admitted to hospital or intensive care for other reasons. Longitudinal cohort studies should include regular testing for SARS-CoV-2 to confirm infection, meticulous capture of symptoms, follow-up times that are both consistent and sufficiently long to account for intermittent symptoms, and recording of preexisting medical conditions. More research to identify underlying immunological mechanisms of long COVID is also needed.
Supplementary Material
Footnotes
The authors have no funding or conflicts of interest to disclose.
P.Z. drafted the initial article. L.P. and N.C. contributed to the writing and critical revision of the article, and all authors approved the final article as submitted.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com)
REFERENCES
- 1.Götzinger F, Santiago-García B, Noguera-Julián A, et al. ; ptbnet COVID-19 Study Group. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020;4:653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zimmermann P, Curtis N. Coronavirus infections in children including COVID-19: an overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children. Pediatr Infect Dis J. 2020;39:355–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castagnoli R, Votto M, Licari A, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr. 2020;174:882–889. [DOI] [PubMed] [Google Scholar]
- 4.Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109:1088–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zimmermann P, Curtis N. Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections [published online ahead of print December 1, 2020]. Arch Dis Child doi: 10.1136/archdischild-2020-320338. [DOI] [PubMed] [Google Scholar]
- 6.Zimmermann P, Curtis N. COVID-19 in children, pregnancy and neonates: a review of epidemiologic and clinical features. Pediatr Infect Dis J. 2020;39:469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uka A, Buettcher M, Bernhard-Stirnemann S, et al. Factors associated with hospital and intensive care admission in paediatric SARS-CoV-2 infection: a prospective nationwide observational cohort study. Eur J Pediatr. In review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ward JL, Harwood R, Smith C, et al. Risk factors for intensive care admission and death amongst children and young people admitted to hospital with COVID-19 and PIMS-TS in England during the first pandemic year. medRxiv. 2021. doi:10.1101/2021.07.01.21259785 [Google Scholar]
- 9.WHO. COVID-19 detailed surveillance data dashboard. Available at: https://covid19.who.int. Accessed July 27, 2021.
- 10.Beck A, Gandhi M. Adjudicating reasons for hospitalization reveals that severe illness from COVID-19 in children is rare. Hosp Pediatr. 2021;11:e159–e160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Viner RM, Whittaker E. Kawasaki-like disease: emerging complication during the COVID-19 pandemic. Lancet. 2020;395:1741–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreira A. Kawasaki disease linked to COVID-19 in children. Nat Rev Immunol. 2020;20:407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toubiana J, Poirault C, Corsia A, et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ. 2020;369:m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riphagen S, Gomez X, Gonzalez-Martinez C, et al. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levin M. Childhood multisystem inflammatory syndrome—a new challenge in the pandemic. N Engl J Med. 2020;383:393–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moraleda C, Serna-Pascual M, Soriano-Arandes A, et al. ; EPICO-AEP Working Group. Multi-inflammatory syndrome in children related to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in Spain. Clin Infect Dis. 2021;72:e397–e401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheung EW, Zachariah P, Gorelik M, et al. Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York City. JAMA. 2020;324:294–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whittaker E, Bamford A, Kenny J, et al. ; PIMS-TS Study Group and EUCLIDS and PERFORM Consortia. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Payne AB, Gilani Z, Godfred-Cato S, et al. Incidence of multisystem inflammatory syndrome in children among US persons infected with SARS-CoV-2. JAMA Network Open. 2021;4:e2116420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bliddal S, Banasik K, Pedersen OB, et al. Acute and persistent symptoms in non-hospitalized PCR-confirmed COVID-19 patients. Sci Rep. 2021;11:13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mandal S, Barnett J, Brill SE, et al. ; ARC Study Group. ‘Long-COVID’: a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax. 2021;76:396–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang L, Yao Q, Gu X, et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. The Lancet. 2021;398:747–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Office for National Statistics. Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK. Available at https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/1july2021. Accessed July 1, 2021.
- 26.Buonsenso D, Munblit D, De Rose C, et al. Preliminary evidence on long COVID in children. Acta Paediatr. 2021;110:2208–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 Long-term effects of COVID-19: a systematic review and meta-analysis. medRxiv. 2021. doi:10.1038/s41598-021-95565-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sigfrid L, Drake TM, Pauley E, et al. Long Covid in adults discharged from UK hospitals after Covid-19: a prospective, multicentre cohort study using the ISARIC WHO clinical characterisation protocol. medRxiv. 2021. doi: 10.1016/j.lanepe.2021.100186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Munblit D, Bobkova P, Spiridonova E, et al. Risk factors for long-term consequences of COVID-19 in hospitalised adults in Moscow using the ISARIC global follow-up protocol: StopCOVID cohort study. medRxiv. 2021. doi:10.1101/2021.02.17.21251895 [Google Scholar]
- 30.Blankenburg J, Wekenborg MK, Reichert J, et al. Mental health of Adolescents in the pandemic: long-COVID19 or long-pandemic syndrome? medRxiv. 2021. doi:10.1101/2021.05.11.21257037. [Google Scholar]
- 31.Buonsenso D, Espuny Pujol F, Munblit D, Mcfarland S, Simpson F. Clinical characteristics, activity levels and mental health problems in children with long COVID: a survey of 510 children. 2021:2021030271. doi: 10.20944/preprints202103.0271.v1. [DOI] [PMC free article] [PubMed]
- 32.Brackel CLH, Lap CR, Buddingh EP, et al. Pediatric long-COVID: an overlooked phenomenon? Pediatr Pulmonol. 2021;56:2495–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blomberg B, Mohn KG, Brokstad KA, et al. Long COVID in a prospective cohort of home-isolated patients [published online ahead of print June 23, 2021]. Nat Med doi: 10.1038/s41591-021-01433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Radtke T, Ulyte A, Puhan MA, Kriemler S. Long-term symptoms after SARS-CoV-2 infection in children and adolescents [published online ahead of print July 15, 2021]. JAMA doi: 10.1001/jama.2021.11880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Molteni E, Sudre CH, Canas LS, et al. Illness duration and symptom profile in symptomatic UK school-aged children tested for SARS-CoV-2 [published online ahead of print August 3, 2021]. Lancet Child Adolesc Health doi: 10.1016/S2352-4642(21)00198-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller F, Nguyen V, Navaratnam AMD, et al. Prevalence of persistent symptoms in children during the COVID-19 pandemic: evidence from a household cohort study in England and Wales. medRxiv. 2021. doi: 10.1101/2021.05.28.21257602. [DOI] [PMC free article] [PubMed]
- 37.Stephenson T, Pinto Pereira S, Shafran R, et al. Long COVID - the physical and mental health of children and non-hospitalised young people 3 months after SARS-CoV-2 infection; a national matched cohort study (The CLoCk) Study. Nature Portfolio. 2021. (in review). [Google Scholar]
- 38.Sterky E, Olsson-Åkefeldt S, Hertting O, et al. Persistent symptoms in Swedish children after hospitalisation due to COVID-19. Acta Paediatr. 2021;110:2578–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ashkenazi-Hoffnung L, Shmueli E, Ehrlich S, et al. Long COVID in children: observations from a designated pediatric clinic [published online ahead of print August 5, 2021]. Pediatr Infect Dis J doi: 10.1097/INF.0000000000003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Osmanov IM, Spiridonova E, Bobkova P, et al. Risk factors for long covid in previously hospitalised children using the ISARIC Global follow-up protocol: a prospective cohort study [published online ahead of print July 1, 2021]. Eur Respir J doi: 10.1183/13993003.01341-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Say D, Crawford N, McNab S, et al. Post-acute COVID-19 outcomes in children with mild and asymptomatic disease. Lancet Child Adolesc Health. 2021;5:e22–e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smane L, Stars I, Pucuka Z, et al. Persistent clinical features in paediatric patients after SARS-CoV-2 virological recovery: a retrospective population-based cohort study from a single centre in Latvia. BMJ Paediatr Open. 2020;4:e000905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nogueira López J, Grasa C, Calvo C, et al. Long-term symptoms of COVID-19 in children. Acta Paediatr. 2021;110:2282–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ludvigsson JF. Case report and systematic review suggest that children may experience similar long-term effects to adults after clinical COVID-19. Acta Paediatr. 2021;110:914–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Denina M, Pruccoli G, Scolfaro C, et al. Sequelae of COVID-19 in hospitalized children: a 4-months follow-up. Pediatr Infect Dis J. 2020;39:e458–e459. [DOI] [PubMed] [Google Scholar]
- 46.Petersen MS, Kristiansen MF, Hanusson KD, et al. Long COVID in the Faroe Islands - a longitudinal study among non-hospitalized patients [published online ahead of print November 30, 2020]. Clin Infect Dis doi: 10.1093/cid/ciaa1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Isoldi S, Mallardo S, Marcellino A, et al. The comprehensive clinic, laboratory, and instrumental evaluation of children with COVID-19: a 6-months prospective study. J Med Virol. 2021;93:3122–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bottino I, Patria MF, Milani GP, et al. Can asymptomatic or non-severe SARS-CoV-2 infection cause medium-term pulmonary sequelae in children? Front Pediatr. 2021;9:621019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walsh-Messinger J, Manis H, Vrabec A, et al. The kids are not alright: a preliminary report of post-COVID syndrome in university students. J Am Coll Health. 2021:1–7. [DOI] [PMC free article] [PubMed]
- 50.Chevinsky JR, Tao G, Lavery AM, et al. Late conditions diagnosed 1-4 months following an initial coronavirus disease 2019 (COVID-19) encounter: a matched-cohort study using inpatient and outpatient administrative data-United States, 1 March-30 June 2020. Clin Infect Dis. 2021;73:S5–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Varsavsky T, Graham MS, Canas LS, et al. Detecting COVID-19 infection hotspots in England using large-scale self-reported data from a mobile application: a prospective, observational study. Lancet Public Health. 2021;6:e21–e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ravens-Sieberer U, Kaman A, Otto C, et al. Mental health and quality of life in children and adolescents during the COVID-19 pandemic-results of the copsy study. Dtsch Arztebl Int. 2020;117:828–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Figueiredo CS, Sandre PC, Portugal LCL, et al. COVID-19 pandemic impact on children and adolescents’ mental health: biological, environmental, and social factors. Prog Neuropsychopharmacol Biol Psychiatry. 2021;106:110171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shepherd C. The ME Association. Post Covid-19 Fatigue, Post/long COVID-19 syndromes and post-Covid ME/CFS. 2020. Available at: https://meassociation.org.uk/wp-content/uploads/Post-Covid-Fatigue-Syndrome-and-MECFS-September-2020.pdf?fbclid=IwAR3Kiu6wkWZnVMRFMULC6UauJCs3OMj395CnwXVOUr63eJDjQEhcGtffIJI. Accessed August 5, 2021.
- 55.Munblit D, Sigfrid L, Warner JO. Setting priorities to address research gaps in long-term COVID-19 outcomes in children [published online ahead of print August 2, 2021]. JAMA Pediatr doi: 10.1001/jamapediatrics.2021.2281. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.