Abstract
Background and Objectives
To assess the utility of EEG-fMRI for epilepsy surgery, we evaluated surgical outcome in relation to the resection of the most significant EEG-fMRI response.
Methods
Patients with postoperative neuroimaging and follow-up of at least 1 year were included. In EEG-fMRI responses, we defined as primary the cluster with the highest absolute t value located in the cortex and evaluated 3 levels of confidence for the results. The threshold for low confidence was t ≥ 3.1 (p < 0.005); the one for medium confidence corresponded to correction for multiple comparisons with a false discovery rate of 0.05; and a result reached high confidence when the primary cluster was much more significant than the next highest cluster. Concordance with the resection was determined by comparison to postoperative neuroimaging.
Results
We evaluated 106 epilepsy surgeries in 84 patients. An increasing association between concordance and surgical outcome with higher levels of confidence was demonstrated. If the peak response was not resected, the surgical outcome was likely to be poor: for the high confidence level, no patient had a good outcome; for the medium and low levels, only 18% and 28% had a good outcome. The positive predictive value remained low for all confidence levels, indicating that removing the maximum cluster did not ensure seizure freedom.
Discussion
Resection of the primary EEG-fMRI cluster, especially in high confidence cases, is necessary to obtain a good outcome but not sufficient.
Classification of Evidence
This study provides Class II evidence that failure to resect the primary EEG-fMRI cluster is associated with poorer epilepsy surgery outcomes.
The simultaneous recording of EEG and fMRI has provided critical insights into epilepsy research.1 Empowering fMRI analysis with the specificity of EEG to epileptic activity has created a powerful tool for the study of brain regions involved in focal2,3 and generalized epilepsies.4 Interictal EEG-fMRI studies have proved useful in the presurgical evaluation of patients with focal epilepsy,5-8 with the aim of localizing the spike onset zone,3 a marker of the epileptogenic zone.
Identifying which part of the EEG-fMRI response needs to be resected has been approached by studying the peak of the blood oxygen level–dependent (BOLD) contrast.6,8-11 These studies, based on small patient groups, suggest that resection of the maximal response correlates with good seizure outcome.
Concordance of the peak response to the seizure onset zone identified with stereo-EEG (SEEG) was explored12: there was 90% probability of concordance of the most significant BOLD cluster to the seizure onset zone if the second most significant cluster was much less significant than the first, that is, when the most significant cluster stood out from the rest of the response. This indicates that all peak BOLD responses to interictal epileptic spikes do not have the same predictive power.
To determine the utility of EEG-fMRI in a clinical setting, we assessed the relation of surgical outcome with the resection of the most significant BOLD cluster while using different levels of significance to separate results that are really trustworthy from when they are simply indicative of the region to be resected.
Methods
Study Population
We included all patients with epilepsy who underwent EEG-fMRI between 2006 and 2019, were subsequently operated on, and had a follow-up of at least 1 year and postoperative neuroimaging. Surgical outcomes were classified according to the Engel system.13 The quality of imaging should be sufficient to identify the resection cavity. Ideally, we opted for 1-mm-resolution T1 MRI scans obtained a few months after the resection. If not available, any magnetic resonance (MR) sequence with <5-mm slice separation or a CT scan was accepted, provided that coregistration with the anatomic T1 acquired during EEG-fMRI scanning could be performed.
Standard Protocol Approvals, Registrations, and Patient Consents
This study was approved by the Montreal Neurological Institute and Hospital Research Ethics Board. All patients gave written informed consent.
EEG-fMRI Acquisition and Analysis
EEG-fMRI acquisition was described in previous studies.6,12,14 Briefly, EEG was recorded with BrainAmp MR amplifiers (Brain Products GmbH, Gilching, Germany) simultaneously with fMRI using synchronization of EEG and MR clocks in our 3T scanner (Siemens Trio before 2017, Siemens Prisma thereafter; Munich, Germany), with 25 MR-compatible electrodes placed on the scalp using the 10-20 (reference FCz) system and electrodes from the 10-10 system (F9, T9, P9, F10, T10, and P10). Foam pillows were used to immobilize the head, and sandbags immobilized the cables. Images were collected in runs of 200 frames (6 minutes) for a scan time of 60 to 90 minutes with patients at rest. Helium pump and ventilation were on. T2*-weighted echo planar imaging sequences were used with the following settings: until July 2008: repetition time 1.75 seconds, echo time 30 milliseconds, 64 × 64 matrix, 25 slices voxel 5 × 5 × 5 mm, and flip angle 90°; from July 2008: repetition time 1.9 seconds, echo time 25 milliseconds, 64 × 64 matrix, 33 slices, voxel 3.7 × 3.7 × 3.7 mm, and flip angle 90°.
BrainVision Analyzer was used to remove MR and ballistocardiogram artifact from the EEG with average artifact subtraction. Experienced neurophysiologists labeled the interictal epileptiform discharges (IEDs) recorded during the scan, taking into account the usual morphology known from the patient's routine or long-term monitoring EEG. IEDs with the same spatial distribution (of similar or different morphologies) were grouped, whereas IEDs with different spatial distributions (for example, independent bilateral temporal spikes) were considered different IED types.
The EEGs of the scans have been analyzed by several neurophysiologists for different projects. For this study, the IED labels already existing in our database were reused. In case of multiple sets of markers having different approaches for IED grouping for the same study, we opted for the one in which the IED types best matched the routine EEG reports; if there were several, we opted for the one done closer to the time of surgery planning. If some IED types occurred rarely while 1 type was clearly dominant, only the latter was analyzed.
All scans were preprocessed and analyzed with our latest pipeline and tools. There are many different approaches to analyzing the functional images, and there is no consensus on the preprocessing steps or the integration methods15 of EEG-informed fMRI. Our preprocessing pipeline generally followed the main recommendations of methodologic reviews15 and included slice time correction, estimation of motion parameters and in-run motion correction, between-runs motion correction, and smoothing. The slice time correction was performed with fractional-delay all-pass filters on the basis of noncausal maximally flat IIR filters.16 The motion correction was performed with minctools; all frames in a run were aligned to the sixth frame; and the smoothing used a 3-dimensional 5-mm gaussian kernel. For movements >1 mm, the corresponding frames were censored; that is, the rows of the corresponding time point were removed from the design matrix.17 A template was coregistered to the individual’s MR to produce an automatic brain segmentation and mask (excluding everything outside the brain and including gray matter, white matter, and subcortical structures, as well as the ventricles).
The result of analyzing an EEG-fMRI study is a statistical map of BOLD changes correlated to the IEDs, referred to as a t map. To derive this, we used an event-related design with fMRIstat18 as described in previous studies.14 Briefly, 4 regressors were modeled using the convolution of the timing and duration of each IED with 4 hemodynamic response functions (HRFs) peaking at 3, 5, 7, and 9 seconds,19 while 6 motion parameters, 4 temporal trends, and the global signal were modeled as confounds. At each voxel, the most significant t value from the 4 t maps created with the 4 HRFs was used to create the final single combined t map.
Levels of Significance
For each IED type analyzed, we considered both positive (activation) and negative (deactivation) BOLD responses because the latter were proved to be equally important in localizing the epileptogenic zone.20-22 We defined the primary cluster as the cluster with the highest absolute t value at a peak located in the cerebral cortex, with the exception of negative BOLD responses in the default mode network,23 that is, usually symmetric responses involving the posterior cingulate, precuneus, inferior parietal, and medial prefrontal cortices because these have no value regarding the localization of epileptic activity.24,25
Cluster significance was assessed at 3 levels of confidence (low, medium, high). The low confidence level was defined as a response with 5 contiguous voxels with a t value ≥3.1, corresponding to p < 0.005 for the combined analysis using the 4 HRFs.19 The medium confidence level was defined by t values higher than the threshold corresponding to corrected whole-brain topologic false discovery rate (FDR) of 0.05.26 For the high confidence level, we used the criterion previously described for detecting clusters with >90% probability of indicating the seizure onset zone defined by SEEG.12 These are primary clusters that are highly significant, while the next significant cluster is much less significant or absent. Specifically, the cluster was classified as having high probability of corresponding to the seizure onset zone when the equation |t1| × 0 .025 + (|t1| − |t2|) × 0.080 > 0.30212 was fulfilled, where t1 refers to the t value of the peak of the primary cluster and t2 to the cluster with the next highest peak, with |t2| ≥ 3.1. In qualitative terms, this equation creates, in the plane defined by t1 and t1 − t2, a fraction of this plane where t1 is high, or t1 − t2 is high, or a combination of both is sufficiently high.
Comparison to Resected Area
Preoperative anatomic scans were coregistered to functional images and postoperative scans were subsequently coregistered to anatomic image, using a linear transformation with 6 df. Verification of these transformations was done by visual evaluation of the intersection between the cavity and the median functional image. In this visual evaluation, any large sagging around the cavity was taken into account. Results of fMRI analysis were superimposed on the preoperative anatomic scan and compared to the postoperative one. The t maps were visually examined and classified as in previous studies6 into one of the following categories: concordant when the peak, that is, the voxel with the maximum absolute t value, of the primary cluster was inside the resection cavity; partially concordant when the peak of the primary cluster was outside but close to the resection (within 2 cm of resection borders) and part of the primary cluster was included in the resection area; partially discordant when no part of the primary cluster was included in the resection but a significant cluster other than the primary one was included; and discordant when no significant cluster was included in the resection.
Using a binary classification for prediction of surgical outcome, the concordant and partially concordant studies would predict a good outcome, while discordant and partially discordant studies would predict a poor outcome. Examples for these categories are given in Figures 1–3.
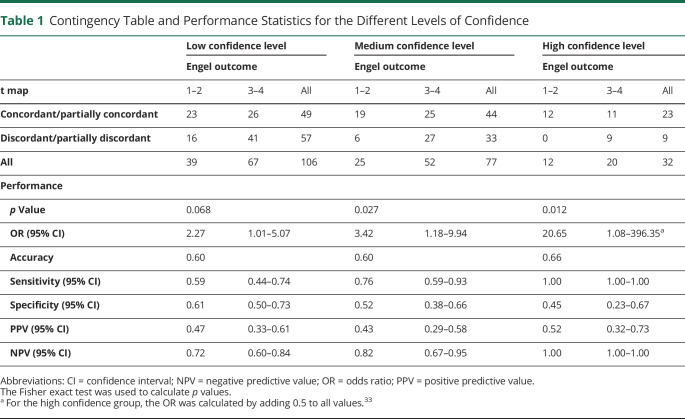
Figure 1. Example of a Concordant Study.
In this bilateral temporal case, left temporal interictal epileptiform discharges (n = 32) consisting of spikes with maximum amplitude at F7, T3,T9, and P9 followed by a slow wave (A) resulted in an activation cluster in the left anterior temporal lobe. Preoperative and postoperative MRIs. (B.a) The primary cluster with a peak value of t = 7.39 was included in the resection. (B.b) The next most significant cluster with a peak value of t = 6.38 was an activation cluster in the left fusiform gyrus, which was not resected. These t values are above false discovery rate level (4.72). This case fulfilled our first 2 levels of confidence but did not fulfill the criterion for high confidence results because the t value of the second most significant cluster was close to the first. This example would be predicted as a good outcome case with medium confidence, but this patient belongs to the poor outcome group (Engel 3).
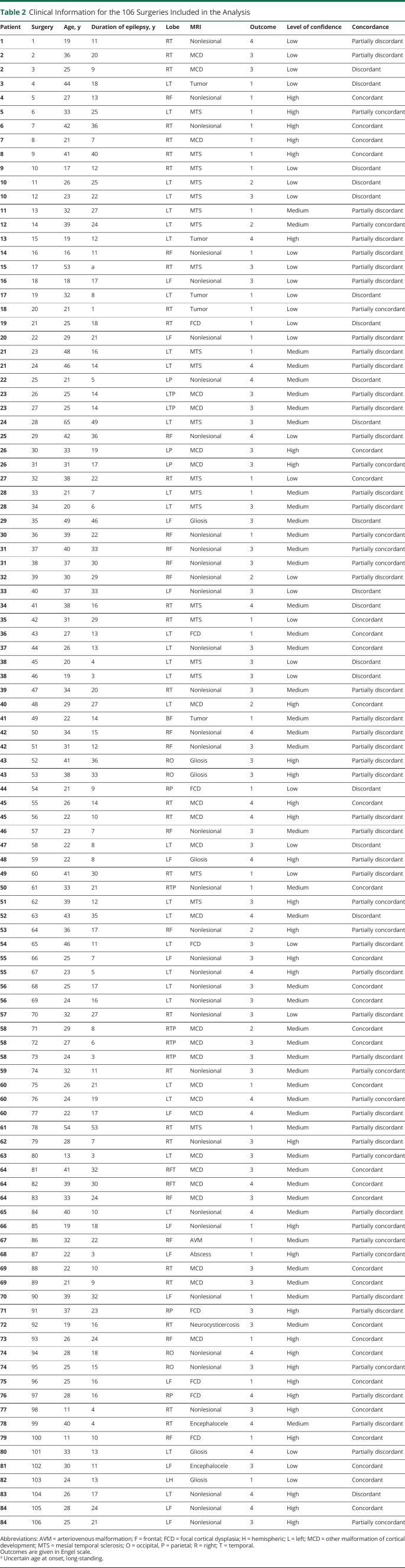
Figure 2. Example of a Partially Concordant Study.
Right posterior quadrant interictal epileptiform discharges (n = 223) with a maximum at T6P10O2 (A) produced a deactivation cluster in the right posterior temporal lobe. Preoperative and postoperative MRIs. (B.a) Part of the primary cluster was included in the resection, but the peak t = −7.23 was not. (B.b) The second most significant cluster with peak t = −5.94 was on the left posterior temporal lobe. The values were above the false discovery rate level of 5.30 but did not fulfill the criterion for high confidence results. This example would be predicted as a good outcome case with medium confidence. This patient remained seizure-free for 3 years, but seizures eventually recurred (Engel class 3). A second surgery extended the first resection, and the patient is again seizure-free but with a follow-up of 8 months.
Figure 3. Two Examples of Partially Discordant Studies.
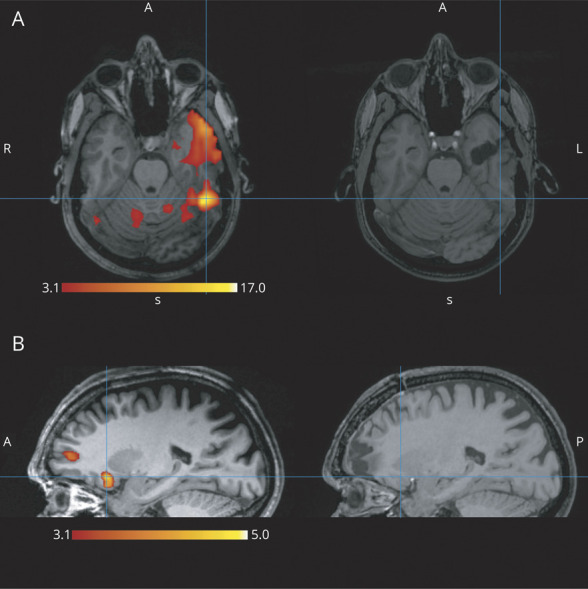
(A) The peak (t = 17.41) of the primary cluster in the left fusiform gyrus was outside the resection. The resection includes part of the same cluster in the anterior temporal lobe, but because the peak is >2 cm from the resection borders, it is not considered partially concordant. In this case, the second most significant cluster had peak t = 6.20 (not shown), so it fulfills the criterion for high confidence results and would be predicted as a poor outcome case with high confidence. The actual outcome was indeed Engel class 4. (B) The primary cluster with peak t = 4.99 in the right orbitofrontal area is not included in the resection. There is another activation cluster (peak t = 3.88) in the resection cavity at the frontal pole; therefore, the results are partially discordant. These clusters are all below the false discovery rate (5.00) level, resulting in a low confidence study. In this case, the prediction would be poor outcome with low confidence. The patient improved, having only rare nocturnal events and no diurnal ones (Engel class 2).
At map without any t value above the low confidence threshold was considered not significant. A t map exhibiting widespread patterns like the ones seen in generalized epilepsies or a result only in the default mode network was classified as nonlocalizing. Ictal t maps and results from IEDs contralateral to the resection were not included. Callosotomies were excluded; radiofrequency thermocoagulations of epileptic foci were included if the treated area was clearly identifiable in postoperative imaging.
Surgical Outcome
Good outcome was defined as Engel classes 1 (free of disabling seizures) and 2 (rare disabling seizures), whereas poor outcome consisted of Engel classes 3 (worthwhile improvement) and 4 (no worthwhile improvement).13
Data Availability
Raw data files supporting the findings of this study are available from 2 authors (N.v.E. and J.G.) on reasonable request and approval by the ethics boards of the corresponding institutions.
Classification of Evidence
For the main research question regarding the relation of epilepsy surgery outcome to the resection of the primary BOLD cluster, the study is rated Class II because of the retrospective design.
Results
Patients and Surgery Characteristics
EEG-fMRI is formally a research procedure, and only a small fraction of surgical candidates investigated at our hospital are scanned. There is no systematic patient selection, but the most complex cases tend to be referred. We identified 147 patients who were operated on after an EEG-fMRI scan. Some patients were operated on more than once, with extension of their previous resection, and some were investigated with multiple scans, so we examined a total of 184 scans and 199 surgeries and performed the analysis per surgery.
After application of the inclusion criteria for follow-up and postoperative imaging and exclusion of 2 patients with callosotomies, 128 surgeries remained, with 134 scans from 104 patients. Two scans were excluded because they were done with a different setup (256-channel EEG cap for a HD-EEG-fMRI project), 2 were interrupted before functional image acquisition (1 panic attack, 1 seizure), and 19 (15%) were not active (no IEDs during fMRI study). The remaining 111 scans corresponded to 87 patients and 109 surgeries (17 patients had >1 scan, maximum 3 scans; 19 patients had >1 surgery, maximum 3 surgeries).
The analysis of the 111 scans produced 125 t maps (77 patients had 1 t map coming from 1 event type, 8 patients had 2 t maps from 2 event types, and 2 patients had 3 t maps from 3 event types). For each surgery, the t map with the highest t value was selected and then compared to postoperative imaging. Note that it is possible for a t map to be compared to 2 subsequent surgeries because it could be discordant to the first but concordant to the second. Nonlocalizing t maps, as defined in the Methods, were excluded from further evaluation. This resulted in 106 t maps being compared to 106 surgeries from 84 patients. Patient selection is summarized in Figure 4.
Figure 4. Patient Selection Flowchart.
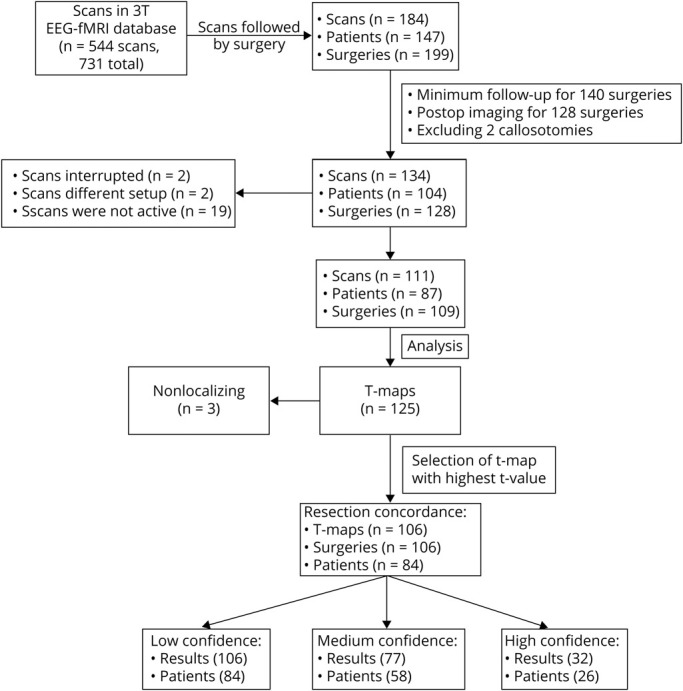
Of the 199 surgeries, 59 were excluded due to inadequate follow-up: 29 with no follow-up in our center, 15 lost to follow-up within a year, 4 with a second surgery within a year, and 11 recently operated on and not yet having reached a year of follow-up. Twelve did not have adequate postoperative imaging, and 2 were callosotomies.
The study included 84 patients (44 female) with a median age at surgery of 28 years (range 11–65 years) and median duration of epilepsy of 16 years (range 1–53 years). The surgery targeted the temporal lobe in 54% of patients, frontal in 27%, parietal in 6%, occipital in 4%, and multiple lobes in 9%. About one-third were nonlesional (36%); 20% had MR evidence of mesiotemporal sclerosis, 9% focal cortical dysplasia, 17% other malformations of cortical development, 6% tumors, and 12% other lesions.
Surgical Outcome and Concordance Between Resection and BOLD Response
All 106 surgeries had a corresponding t map reaching the low level of significance (t values >3.1); 77 (58 patients) had a result reaching the medium level (threshold at FDR); and 32 (26 patients) had a result reaching the high level.
Figure 1 demonstrates an example of a concordant study. The t map revealed an activation in the left anterior temporal lobe. The primary cluster with a peak value of t = 7.39 was included in the resection. The next most significant cluster with a peak value of t = 6.38 was an activation in the left fusiform gyrus, which was not resected. These t values were above FDR level (4.72). This case fulfilled our first 2 levels of confidence but did not fulfill the criterion for high confidence because the t value of the second most significant cluster was close to the first.
An example of a partially concordant study is described in Figure 2. The primary cluster was a deactivation in the right posterior temporal lobe. Only part of the primary cluster was included in the resection, and the peak value of t = −7.23 was not. The second most significant cluster with peak value of t = −5.94 was on the left posterior temporal lobe. The values were above the FDR level of 5.30 but did not fulfill the criterion for high confidence.
Two examples of partially discordant studies are shown in Figure 3. In the first, the peak value (t = 17.41) of the primary cluster in the left fusiform gyrus was outside the resection. Although the resection included part of the same cluster in the anterior temporal lobe, the peak was >2 cm from the resection borders, so it was considered partially disconcordant. The second most significant cluster had a much lower peak value of t = 6.20 (not shown), so this study fulfilled the criterion for high confidence. The second example had a primary cluster with a peak value of t = 4.99 in the right orbitofrontal area, which was not included in the resection. Another activation cluster (peak t = 3.88) was included in the resection cavity at the frontal pole. These clusters were all below the FDR (5.00) level, and the patient belonged to the low confidence group.
More than one-third of the surgeries had a good outcome (39 of 106, 37%). To relate the surgical outcome to the resection of the peak of the primary BOLD cluster, a binary classification was used by merging concordant and partially concordant categories, as well as partially discordant with discordant, to create contingency matrices (Table 1). The medium (t > FDR) and high (fulfilling the provided criterion12) confidence t maps have contingency matrices that are significant at a 5% level for nonrandom associations (p = 0.027 and 0.012, Fisher exact test), whereas for the low confidence t maps (t > 3.1), the contingency matrix does not reach statistical significance (p = 0.068). For the high confidence level, there is no case of a discordant t map to the resection cavity that had a good outcome, giving a negative predictive value of 100%. For the medium confidence level, the negative predictive value remains high but drops to 82% and for the low confidence level to 72%. On the other hand, the positive predictive value is low: for the high confidence level, 12 concordant cases had a good outcome and 11 had a poor outcome (52% positive predictive value); for the medium level, the positive predictive value drops to 43% and to 47% for the low level. The odds ratios (ratio of chance of good outcome when the primary peak is included in the resection vs chance when it is not) (95% confidence interval) for the 3 levels of confidence are, from high to low, 20.65 (1.08–396.35), 3.42 (1.18–9.94), and 2.27 (1.01–5.07).
Table 1.
Contingency Table and Performance Statistics for the Different Levels of Confidence
The exploratory subgroup analysis is available from OSF: https://osf.io/962hs.
In summary, if the peak of the primary BOLD cluster of the EEG-fMRI results was not resected, a poor outcome is expected: for the high confidence level, no patient had good outcome; for the medium confidence level, only 18% had good outcome; and for the low confidence level, only 28% had good outcome. The resection is necessary but not sufficient: the positive predictive value remains low (43%–52%) for all the levels of confidence (approximately half of the patients in whom the maximum BOLD is resected are not seizure-free).
Determining High Level of Confidence
Our definition of a high confidence level was based on the criterion described in a previous study comparing EEG-fMRI clusters to SEEG.12 The results of our study fulfilling this criterion are presented in Figure 5. As a secondary analysis, we examined in our patient group whether we can modify this equation while keeping a high negative predictive value to be able to make predictions with high confidence in a larger proportion of patients. With a 100% negative predictive value, that is, completely separating the t maps in the discordant–good outcome category, we selected the inequality |t1| × 0. 0813 + (|t1| − |t2|) × 0.6135 > 1 for further evaluation because it includes as many cases as possible (Figure 5). With this new criterion, 51 surgeries are now included as high confidence results, with a negative predictive value of 100% and positive predictive value of 44% (Fisher exact test, p = 0.0019, odds ratio 24.95, 95% confidence interval 1.39–448.89). This equation would therefore increase the number of patients in the high confidence group from 32 to 51 while retaining similar predictive values.
Figure 5. Criterion for High Confidence Results.
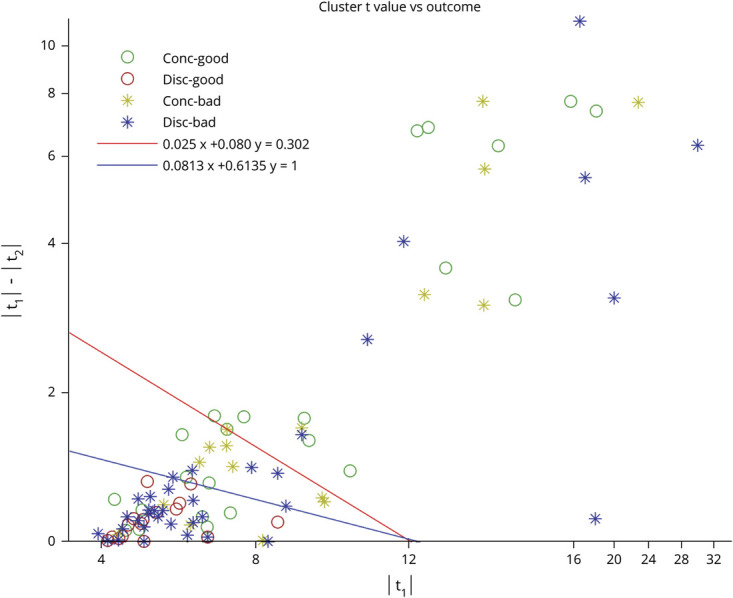
Every surgery is positioned in the plane determined by the peak t values of the primary cluster (x-axis) and the difference between primary and second most significant cluster (y-axis). Different colors and symbols represent outcomes. Every t map at the right of the red line belongs to the high confidence group according to the criterion from Khoo et al.12; t maps at the left of the red line do not belong to the high confidence group. Blue line represents the modified criterion, which is determined by the equation |t1| × 0 0.0813 + (|t1|−|t2|) × 0.6135 > 1. There is no surgery with good outcome for a discordant map on the right of the blue line; that is, for high confidence cases (right of the blue line), if the primary peak was not resected (discordant map), we did not have a good outcome. Note that the axes are not linear for x > 16 and y > 4. Conc = concordance.
Classification of Evidence
This is a retrospective study with clearly defined inclusion and exclusion criteria; the surgical outcome was determined objectively without knowledge of the EEG-fMRI result and thus fulfills Class II criteria for rating diagnostic accuracy studies.
Discussion
Our main finding is the very strong negative predictive value of EEG-fMRI results in epilepsy surgery. With high confidence results, no patient had a good outcome if the primary cluster was not resected; and for the medium and low confidence results, only 18% and 28% of the patients had a good outcome. This is a very strong indicator that resection of the primary cluster is a necessary, though not sufficient, condition for a good outcome: necessary because sparing the primary cluster is associated with poor outcome (to achieve good outcome, it must be resected) and not sufficient because resecting the primary cluster alone does not predict a good outcome (approximately half of the cases in which the primary cluster was resected had a poor outcome). This can be translated into clinical practice: EEG-fMRI can be used to complement or direct other techniques because the primary cluster should not be overlooked during epilepsy surgery, but only as a part of a comprehensive protocol because it cannot on its own predict a good outcome.
The clinical application of this result is tempered by the restricted number of patients in the high and medium confidence groups. We provided a modified equation to define high confidence in EEG-fMRI results, which would have to be confirmed in the future. This new criterion allows more patients to have high confidence results while keeping the negative predictive value at 100%. For instance, 51 of 106 surgeries in our study would be reclassified as high confidence results. If this is confirmed, it greatly increases the yield of EEG-fMRI studies when we consider only high confidence results as clinically informative.
The clinical value of EEG-fMRI in epilepsy surgery has been explored before, but only a few studies have examined the surgical outcome and concordance of EEG-fMRI results to the resection.6,9-11,27 Direct comparisons are difficult due to the small numbers of patients in these studies and the differences in the definition of concordance and in what is considered good outcome. Methodologic differences in the statistical analysis of EEG-fMRI also exist between groups. Our definition of good outcome included Engel 1 and 2 categories. This is a point of variability when comparing with studies that similarly transform the Engel or International League Against Epilepsy outcome scales to binary classifications. In this study, the reasoning was that the clinical usefulness of the EEG-fMRI scan should be demonstrated with Engel 1 to 2 outcomes because surgical outcome depends on many parameters, and we should not expect that EEG-fMRI scans alone would be able to predict complete seizure freedom as reflected in Engel 1.
We suggested in 2 smaller studies that resection of the peak of EEG-fMRI results has a high negative predictive value6,27 for the outcome in epilepsy surgery (some patients from these 2 studies are included in the current study). Another group of researchers focusing on 30 patients with temporal lobe epilepsy11 also reported a high (78%) negative predictive value of EEG-fMRI.
On the other hand, lower negative predictive values can be extracted from the data reported in 2 studies focusing on multimodal presurgical evaluation.28,29 Though not their focus, a separate evaluation of EEG-fMRI results was reported. In the first study, a pediatric population,28 11 of 20 patients belonged to the discordant with good outcome group, i.e., cases with good outcome in which the EEG-fMRI result was not resected, with a negative predictive value of 31%. In the other study comparing concordance of different noninvasive techniques to SEEG and surgical outcome,27 the number of cases in the similar discordant with good outcome group was also high (11 of 26). This, however, was not a direct comparison with the resection, so it is not directly comparable to our study. Only 3 of 26 patients in that study had outcome worse than Engel 2 and would fit our poor outcome definition; this is very different from our cohort. Moreover, in all of these studies, the authors did not provide information on the t values of their EEG-fMRI results or a method to evaluate the level of confidence in the result, which is not surprising given the relatively small number of patients (subsets of these are too small to be meaningful). Our data suggest, however, that in scans with high confidence results, the clinical utility is much higher than these earlier studies report.
Taking into account the findings published previously using scalp EEG-fMRI5,6,8,9,11 and intracranial EEG-fMRI,30 our large study can be used to clarify the role of EEG-fMRI in prospective presurgical workup. On high confidence EEG-fMRI results, if the primary BOLD cluster is discordant with the presumed epileptogenic zone and is not planned to be included in the resection, there is high chance of poor postsurgical outcome; hence, the surgical hypothesis needs to be reviewed and revised or investigated further with intracranial implantation.
Our series contains a high number of poor outcome results, indicating that complicated cases were included. In our center, referrals for EEG-fMRI are biased toward complicated cases in which the standard workup could not conclude with a hypothesis for a surgical target or an alternative hypothesis could not be excluded. We have also chosen to include cases in which radiofrequency thermocoagulation was performed; these are known to have low chances of seizure freedom.31 Indeed, the 4 included surgeries (30, 31, 89, and 101 in Table 2) have poor outcome. Nevertheless, the bias toward the complex cases does not alter the validity of our results. A less complex case would imply simple localization with standard presurgical evaluation. We cannot assess what to expect from EEG-fMRI in cases not regularly studied by our group. In other words, we cannot be certain whether less complex cases would provide low, medium, or high confidence results. On the other hand, one would expect that less complex cases would have better surgical outcomes, whereas more complex cases would have worse surgical outcomes. With this in mind, we may have underestimated the positive predictive value of EEG-fMRI because, even with good localization of a plausible surgical target, the outcome may be worse in complex cases. There is no reason to believe that in less complex cases a high confidence BOLD result could be ignored from surgical planning.
Table 2.
Clinical Information for the 106 Surgeries Included in the Analysis
Our focus was the primary cluster of the EEG-fMRI result, even though EEG-fMRI studies often result in multiple activation clusters. This was selected for convenience because the peak is an easy and unambiguous measure to identify and localize. Although it may not convey other information available in the t map that could be important, our data suggest that it is a useful clinical indicator and should not be ignored in planning surgery. The approach of defining the level of confidence in the result using the relationship of the t values of the primary and secondary clusters also enhances this measure. It is possible that in results not reaching high confidence, another indicator of the t map could be more informative.
Although we classified the resulting t maps using the partially concordant and partially discordant categories, these were merged with the fully concordant and discordant groups for statistical analysis. This reflects the decision to focus on the primary cluster. In both concordant and partially concordant t maps, at least part of the primary cluster was resected, whereas in partially discordant and discordant t maps, no part of the primary cluster was resected.
We can only speculate on why resection of the peak of the primary cluster is not sufficient for good outcome. This simple localization, ignoring the extent of the response and the other clusters, does not define the borders of the epileptogenic area. This is a limitation of many methods localizing the epileptogenic zone. We also have to consider difficult cases in which the seizure onset zone was indeed removed and a secondary area of epileptogenicity takes over after surgery.
We chose to study surgeries, thus including some patients more than once. This decision was based on the idea that each subsequent resection may have different concordance with a prior EEG-fMRI scan and the fact that some patients had a new scan between surgeries. This is also reflected in the poor outcome results; each subsequent resection implies poor outcome for the previous one. Regarding the patients with multiple scans, reproducibility of EEG-fMRI has been studied before,32 but this was not an aim of this study. In any case, only 1 t map was compared to each resection.
In some patients, particularly in recent years, results of EEG-fMRI were taken into consideration when surgery was performed. This does not alter the validity of our study; it may simply increase the number of patients in whom BOLD maximum and resection coincide.15
Finally, although this study was not designed to estimate the yield of EEG-fMRI, mainly due to the selection of patients who were known to have sufficiently active interictal activity for an EEG-fMRI study and were subsequently operated on, only 15% of the studies that fulfilled the criteria for inclusion were not active.
We described a measure for the level of confidence in EEG-fMRI results and compared the surgical outcomes when the resection included or did not include the primary cluster. For the high level of confidence, we found no patient with a good outcome when the primary cluster was not resected. Although the outcome of epilepsy surgery depends on many different factors, resection of the peak of the primary cluster of the EEG-fMRI study has a high negative predictive value and should be taken into account during the presurgical evaluation.
Acknowledgment
The authors thank the coordinator of the EEG technicians, Lorraine Allard, and the 3T MR technologists, Ron Lopez, David Costa, and Louise Marcotte, for their support and professionalism. They also thank Dr. Birgit Frauscher for her comments on the style of the manuscript.
Glossary
- BOLD
blood oxygen level–dependent
- FDR
false discovery rate
- HRF
hemodynamic response function
- IED
interictal epileptiform discharge
- MR
magnetic resonance
- SEEG
stereo-EEG
Appendix. Authors

Footnotes
Class of Evidence: NPub.org/coe
Study Funding
This work was supported by the Canadian Institutes of Health Research (FDN 143208). Dr. Khoo is funded by a Grant-in-Aid for Scientific Research (18H06261, 19K21353, 20K09368) from the Ministry of Education, Culture, Sports, Science and Technology of Japan and a grant from the National Institute of Information and Communications Technology of Japan, and was supported by Mark Rayport and Shirley Ferguson Rayport fellowship in epilepsy surgery and the Preston Robb fellowship of the Montreal Neurological Institute (Canada), a research fellowship of the Uehara Memorial Foundation (Japan). She received a sponsored award from the Japanese Epilepsy Society, support from the American Epilepsy Society Fellows program, and travel bursary from the International League Against Epilepsy.
Disclosure
A.M. Koupparis and N. von Ellenrieder report no disclosures relevant to the manuscript. H.M. Khoo is funded by Grant-in-Aid for Scientific Research (18H06261, 19K21353, 20K09368) from the Ministry of Education, Culture, Sports, Science and Technology of Japan and a grant by the National Institute of Information and Communications Technology of Japan, and was supported by Mark Rayport and Shirley Ferguson Rayport fellowship in epilepsy surgery and the Preston Robb fellowship of the Montreal Neurological Institute (Canada), a research fellowship of the Uehara Memorial Foundation (Japan). She received a sponsored award from the Japanese Epilepsy Society, support from the American Epilepsy Society Fellows program, and travel bursary from the International League Against Epilepsy. N. Zazubovits, D.K. Nguyen, J.A. Hall, R.W.R. Dudley, and F. Dubeau report no disclosures relevant to the manuscript. J. Gotman is funded by the Canadian Institutes of Health Research (FDN 143208). Go to Neurology.org/N for full disclosures.
References
- 1.Moeller F, Siniatchkin M, Gotman J. Simultaneous EEG and fMRI Recordings (EEG–fMRI). In: Ulmer S, Jansen O, eds. fMRI. Springer International Publishing; 2020:175-191. Accessed May 30, 2020. link.springer.com/10.1007/978-3-030-41874-8_13 [Google Scholar]
- 2.Bénar C-G, Grova C, Kobayashi E, et al. EEG–fMRI of epileptic spikes: concordance with EEG source localization and intracranial EEG. NeuroImage. 2006;30(4):1161-1170. [DOI] [PubMed] [Google Scholar]
- 3.Khoo HM, von Ellenrieder N, Zazubovits N, He D, Dubeau F, Gotman J. The spike onset zone: the region where epileptic spikes start and from where they propagate. Neurology. 2018;91(7):e666-e674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aghakhani Y, Bagshaw AP, Bénar CG, et al. fMRI activation during spike and wave discharges in idiopathic generalized epilepsy. Brain. 2004;127(pt 5):1127-1144. [DOI] [PubMed] [Google Scholar]
- 5.Zijlmans M, Huiskamp G, Hersevoort M, Seppenwoolde JH, van Huffelen AC, Leijten FS. EEG-fMRI in the preoperative work-up for epilepsy surgery. Brain. 2007;130(pt 9):2343-2353. [DOI] [PubMed] [Google Scholar]
- 6.An D, Fahoum F, Hall J, Olivier A, Gotman J, Dubeau F. Electroencephalography/functional magnetic resonance imaging responses help predict surgical outcome in focal epilepsy. Epilepsia. 2013;54(12):2184-2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Markoula S, Chaudhary UJ, Perani S, et al. The impact of mapping interictal discharges using EEG-fMRI on the epilepsy presurgical clinical decision making process: a prospective study. Seizure. 2018;61:30-37. [DOI] [PubMed] [Google Scholar]
- 8.Kowalczyk MA, Omidvarnia A, Abbott DF, Tailby C, Vaughan DN, Jackson GD. Clinical benefit of presurgical EEG-fMRI in difficult-to-localize focal epilepsy: a single-institution retrospective review. Epilepsia. 2020;61(1):49-60. [DOI] [PubMed] [Google Scholar]
- 9.Thornton R, Laufs H, Rodionov R, et al. EEG correlated functional MRI and postoperative outcome in focal epilepsy. J Neurol Neurosurg Psychiatry. 2010;81(8):922-927. [DOI] [PubMed] [Google Scholar]
- 10.Thornton R, Vulliemoz S, Rodionov R, et al. Epileptic networks in focal cortical dysplasia revealed using electroencephalography–functional magnetic resonance imaging. Ann Neurol. 2011;70(5):822-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coan AC, Chaudhary UJ, Grouiller F, et al. EEG-fMRI in the presurgical evaluation of temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 2016;87(6):642-649. [DOI] [PubMed] [Google Scholar]
- 12.Khoo HM, Hao Y, von Ellenrieder N, et al. The hemodynamic response to interictal epileptic discharges localizes the seizure-onset zone. Epilepsia. 2017;58(5):811-823. [DOI] [PubMed] [Google Scholar]
- 13.Engel J, Van Ness P, Rasmussen T, Ojemann L. Outcome with respect to epileptic seizures. Surg Treat Epilepsies. 1993:609-621. [Google Scholar]
- 14.Yamazoe T, von Ellenrieder N, Khoo HM, et al. Widespread interictal epileptic discharge more likely than focal discharges to unveil the seizure onset zone in EEG-fMRI. Clin Neurophysiol. 2019;130(4):429-438. [DOI] [PubMed] [Google Scholar]
- 15.Abreu R, Leal A, Figueiredo P. EEG-informed fMRI: a review of data analysis methods. Front Hum Neurosci. 2018;12:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jahani Yekta MM. Wideband maximally flat fractional-delay allpass filters. Electron Lett. 2010;46:722. [Google Scholar]
- 17.Power JD, Schlaggar BL, Petersen SE. Recent progress and outstanding issues in motion correction in resting state fMRI. NeuroImage. 2015;105:536-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Worsley KJ, Liao CH, Aston J, et al. A general statistical analysis for fMRI data. NeuroImage. 2002;15(1):1-15. [DOI] [PubMed] [Google Scholar]
- 19.Bagshaw AP, Aghakhani Y, Bénar CG, et al. EEG-fMRI of focal epileptic spikes: analysis with multiple haemodynamic functions and comparison with gadolinium-enhanced MR angiograms. Hum Brain Mapp. 2004;22(3):179-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi E, Bagshaw AP, Grova C, Dubeau F, Gotman J. Negative BOLD responses to epileptic spikes. Hum Brain Mapp. 2006;27(6):488-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pittau F, Fahoum F, Zelmann R, Dubeau F, Gotman J. Negative BOLD response to interictal epileptic discharges in focal epilepsy. Brain Topogr. 2013;26(4):627-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rathakrishnan R, Moeller F, Levan P, Dubeau F, Gotman J. BOLD signal changes preceding negative responses in EEG-fMRI in patients with focal epilepsy. Epilepsia. 2010;51(9):1837-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fahoum F, Zelmann R, Tyvaert L, Dubeau F, Gotman J. Epileptic discharges affect the default mode network--FMRI and intracerebral EEG evidence. PLoS One. 2013;8(6):e68038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laufs H, Hamandi K, Salek-Haddadi A, Kleinschmidt AK, Duncan JS, Lemieux L. Temporal lobe interictal epileptic discharges affect cerebral activity in “default mode” brain regions. Hum Brain Mapp. 2007;28(10):1023-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995;57(1):289-300. [Google Scholar]
- 27.Pittau F, Ferri L, Fahoum F, Dubeau F, Gotman J. Contributions of EEG-fMRI to assessing the epileptogenicity of focal cortical dysplasia. Front Comput Neurosci. 2017;11:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Centeno M, Tierney TM, Perani S, et al. Combined electroencephalography–functional magnetic resonance imaging and electrical source imaging improves localization of pediatric focal epilepsy. Ann Neurol. 2017;82(2):278-287. [DOI] [PubMed] [Google Scholar]
- 29.Rossi Sebastiano D, Tassi L, Duran D, et al. Identifying the epileptogenic zone by four non-invasive imaging techniques versus stereo-EEG in MRI-negative pre-surgery epilepsy patients. Clin Neurophysiol. 2020;131:1815-1823. [DOI] [PubMed] [Google Scholar]
- 30.Chaudhary U, Carmichael D, Rodionov R, et al. Mapping the irritative zone using simultaneous intracranial EEG-fMRI and comparison with postsurgical outcome: 043. Epilepsia. 2012:1-212. [Google Scholar]
- 31.Bourdillon P, Isnard J, Catenoix H, et al. Stereo electroencephalography–guided radiofrequency thermocoagulation (SEEG-guided RF-TC) in drug-resistant focal epilepsy: results from a 10-year experience. Epilepsia. 2017;58(1):85-93. [DOI] [PubMed] [Google Scholar]
- 32.Gholipour T, Moeller F, Pittau F, Dubeau F, Gotman J. Reproducibility of interictal EEG-fMRI results in patients with epilepsy. Epilepsia. 2011;52(3):433-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higgins JPT, Li T, Deeks JJ. Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JPT, Thomas J, Cumpston T, et al., eds. Cochrane Handbook for Systematic Reviews of Interventions Version 60. Cochrane; 2019. Accessed June 30, 2020. training.cochrane.org/handbook. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data files supporting the findings of this study are available from 2 authors (N.v.E. and J.G.) on reasonable request and approval by the ethics boards of the corresponding institutions.