Abstract
Drug therapy of immune-mediated inflammatory arthropathies is not always satisfactory, and there is a risk of adverse events. Granulocyte and monocyte/macrophage apheresis (GMA) is a non-pharmacological therapeutic option that is beneficial and very well tolerated. GMA involves passing blood through a column with cellulose acetate beads to remove increased and activated myeloid lineage cells and improve the cytokine profile. The technique reduces pain and inflammation. We present four clinical reports that illustrate the clinical uses of GMA with the medical device Adacolumn® in patients with different backgrounds and immune-mediated inflammatory arthritis. The results were positive, and no adverse events were reported.
Keywords: disease-modifying anti-rheumatic drugs, leukapheresis, non-steroidal anti-inflammatory agents, pain, psoriatic arthritis, rheumatoid arthritis, tumour necrosis factor inhibitors
Introduction
Immune-mediated inflammatory diseases (IMIDs), specifically immune-mediated inflammatory arthropathies (IMIAs), share two common features: they are characterized by an inappropriate or excessive immune response that is related to cytokine imbalance,1 and they can improve with similar treatments.2 Rheumatoid arthritis (RA) is the most common type of IMIA, with a prevalence of 0.2–1.2% in various countries3 (0.82% in Spain).4 Psoriatic arthritis (PsA) is recorded in up to 30% of patients with psoriasis.5 Arthritis is also a common extraintestinal complication of inflammatory bowel disease, affecting up to 30% of patients with ulcerative colitis (UC).6
RA was long considered a chronic and disabling disease but advances in its management can now modify the course of the disease.7 However, no causal treatment is currently available.8 Traditional treatment of RA and other IMIAs involves non-steroidal anti-inflammatory drugs (NSAIDs), corticosteroids, disease-modifying anti-rheumatic drugs (DMARDs, also named conventional synthetic or csDMARDs) and biological agents (also known as biological DMARDs or bDMARDs). According to the European League Against Rheumatism organization, RA treatment should be initiated with a combination of csDMARDs and corticosteroids, followed by bDMARDs.9 Low-dose methotrexate (MTX) is the main csDMARD; it is effective but its use leads to various adverse events, with nausea/vomiting as the most common.10
Biological agents that target inflammation-related cytokines have notably improved the prognosis of RA, especially the anti-TNF agents alone or in combination with MTX.11 However, anti-TNF agents are not devoid of adverse events such as an increase of the risk of infections, neurological and cardiac diseases,11 or malignancies, which can be serious.12 Although the association between biological agents and cancer has been questioned,13,14 it cannot be totally ruled out.14 Furthermore, up to 40% of patients treated with an anti-TNF agent discontinue therapy due to insufficient efficacy or adverse events.15
Sustained remission is the main objective, especially in RA, though it is rarely achieved in clinical practice.7 Current treatments do not always achieve disease control or remission but can induce adverse events of variable severity.16
Some patients with RA are refractory or have contraindications to these agents and up to 50% of patients treated with csDMARDs or bDMARDs must withdraw therapy after 12–18 months because of adverse events or lack of efficacy.17,18 Furthermore, up to 30% of patients do not respond to any of the current drug therapies.9 Another concern is that adherence to anti-TNF agents is not high, with less than 50% of patients continuing the same treatment at 5 years and requiring a switch to another form of treatment.19
An important issue is the health-related quality of life (HRQoL), which is impaired in patients with IMIDs. According to a study of 530 patients with various IMIDs, those presenting with IMIAs, mainly RA or PsA, had a low HRQoL, especially in the mobility domain of EQ-5D-5L. In patients with IMIDs, the factors contributing to the risk of low HRQoL included female sex, rheumatological IMIDs and current use of biological agents.2
Overall, new therapeutic strategies are needed in RA.8 Immune cell-based therapies, such as the granulocyte and monocyte/macrophage apheresis (GMA), are another approach for the treatment of IMIAs. Because of its different mechanism of action, GMA could provide results in patients who are refractory or add synergistic effects in those with partial responses or loss of response when used in combination as demonstrated in patients with inflammatory bowel disease.20–23
Methods
Adacolumn* is a Class IIb medical device for GMA. It is licensed in Europe for the treatment of inflammatory bowel disease (both Crohn disease and UC), active RA with symptoms resistant to conventional drugs, ocular Behçet disease, systemic lupus erythematosus, or pustular psoriasis.24 GMA is an extracorporeal treatment in which the patient’s blood passes through a 335-mL column with 220 g of 2-mm cellulose acetate beads. Blood immunoglobulin G (IgG) or complement iC3b is linked to the bead surface. Myeloid lineage cells express Fcγ receptors or complement receptors. IgG binds to the Fcγ receptor and iC3b to the complement receptor. Consequently, the beads retain the elevated and potentially activated myeloid lineage leukocytes, namely granulocytes (65%) and activated monocytes (35%, with 55% macrophages).25,26 Finally, blood is reinfused.
Figure 1 shows the mode of action of GMA.27 The various immunomodulatory effects of GMA include the reduction of plasma pro-inflammatory cytokine levels, modification of leukocyte morphology with changes in surface receptors that decreases the infiltration of the activated cells not retained by the column in the inflamed tissue, induction of regulatory T and B cells and myeloid-derived suppressor cells, as well as the down-regulation of pro-inflammatory effector T cells, probably because some apoptotic cells pass through the outflow line from the apheresis column into the bloodstream.28 Apoptotic cells have been shown to exert immunomodulatory properties that can be beneficial to patients with RA.29 In rabbits with ovalbumin-induced arthritis, apoptotic cells induced B cell function, thus improving chronic intestinal inflammation.30 In short, GMA not only decreases activated neutrophils and monocytes in the blood but also increases levels of anti-inflammatory cytokines (IL-10, IL-1ra, HGF) and reduces levels of pro-inflammatory cytokines (TNF, IL-6, IL-8, IL-1β).25,31 However, despite the depletion of myeloid lineage cells, GMA does not induce immunosuppression because immature or inactive cells from the bone marrow replace the cells that have been removed. Therefore, GMA is not associated with an increased risk of infections or tumours as can occur with immunosuppressive drugs.25
Figure 1.
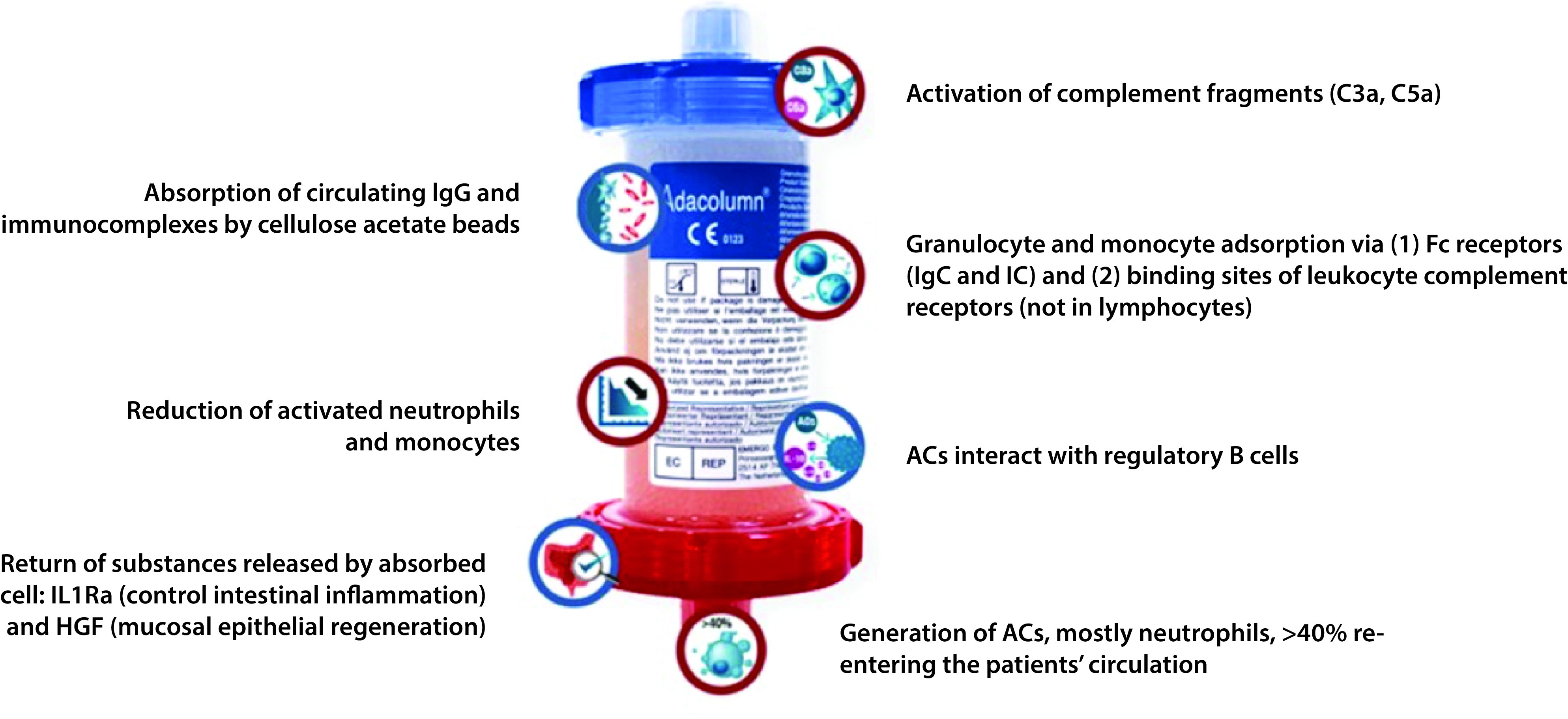
Mode of action of GMA.27 Reproduced with permission from www.adacyte.com.
AC, apoptotic cell; C, complement; Fc, fragment crystallizable region; HGF, hepatocyte growth factor; Ig, immunoglobulin; IC, immune complex.
GMA is initiated with 1 or 2 sessions a week for 5–10 weeks until 8–10 sessions (induction treatment) followed by 1 or 2 sessions monthly if necessary in the more refractory cases (maintenance treatment).24 Each session lasts a minimum of 60 minutes, though more time (90–120 minutes) or greater processed blood volumes achieve better results.
We present four case reports of patients with IMIAs treated with GMA in the rheumatology department of a teaching hospital. Signed patient consent was not required because we de-identified all data presented in this article.
Case series
Case 1: GMA to reduce NSAID intake
Case 1 involves a 48-year-old woman with a medical history of PsA and Behçet disease. She was also diagnosed with heart disease, hypertension and chronic bronchitis. Because of previous intolerance to MTX and dermatological toxicity with secukinumab, she was treated with golimumab 100 mg per month and leflunomide 20 mg daily. However, the arthritis affecting her wrists and ankles and the inflammatory low back pain were very intense, even at night, with a score of 7 on the visual analogue scale (VAS; 0–10). Daily intake of ibuprofen reached 1800 mg.
In November 2019, she agreed to undergo GMA in addition to her usual treatment (golimumab, leflunomide and ibuprofen). Treatment was initiated with ten sessions instead of the usual six, with two sessions per week for 5 weeks. Joint pain improved and NSAID intake was reduced or even suppressed occasionally. Subsequent maintenance treatment was with one session monthly. Golimumab and leflunomide were continued during GMA.
In October 2020, whilst the patient was being treated with golimumab, leflunomide and monthly GMA, she reported pain affecting the costovertebral joints, left hip and lower back. She was then treated with ibuprofen 1300 mg daily, prednisone 2.5 mg daily, golimumab, leflunomide and two sessions of GMA per month until March 2021. At the last visit, she had no joint stiffness and no pain at night and occasionally took ibuprofen 600 mg/d. However, her veins had become fragile due to repeated puncture. Her rheumatologist decided to suspend GMA, albeit with the intention of reintroducing it if symptoms worsened. Currently, she is receiving golimumab and leflunomide. The GMA results were considered very satisfactory.
Case 2: GMA as adjuvant therapy to DMARDs and biological agents
Case 2 involved a 39-year-old man with UC treated with vedolizumab 300 mg every 8 weeks. He was referred from the digestive diseases department with arthritis in the knees, hands and elbows as well as severe pain (VAS 10) and all-day joint stiffness. MTX was initiated with fast dose escalation up to 25 mg weekly and his pain improved (VAS 5).
GMA was started in September 2020 besides vedolizumab and MTX, beginning with two sessions per week for 5 weeks and then one session per month as maintenance therapy. MTX was then withdrawn, though joint symptoms and signs worsened after 3 months. Therefore, MTX was reinitiated at the previous dose and the patient’s condition improved. The effect of GMA was considered positive. Currently, the patient is asymptomatic and is receiving one GMA session per month, vedolizumab 300 mg every 8 weeks, MTX 25 mg weekly and folic acid 5 mg weekly.
Case 3: GMA as low-risk therapy in a patient with poor response to multiple drugs
The patient was a 41-year-old man with axial and peripheral spondyloarthritis associated with UC. He had a background of intolerance to infliximab and adalimumab and he was receiving vedolizumab 300 mg every 8 weeks, salazopyrine 2 g/d and azathioprine 150 mg/d. However, the level of faecal calprotectin was 24 μg/g and arthritis was poorly controlled. The patient had extreme pain (VAS 10) in the neck, shoulders, elbows, wrists, knees and some interphalangeal joints. He also had morning stiffness for 3 hours. Markers of inflammation were increased, with ERS of 67 mm/h and C-reactive protein (CRP) of 3.81 mg/L.
The patient refused MTX because he was planning to have a baby. GMA was proposed in addition to vedolizumab, salazopyrin and azathioprine. He received two sessions per week (ten sessions per month in January and February 2020). After that, he had two sessions per month from March to June 2020. Neck pain was reduced (VAS 5), morning stiffness vanished, and wrists and interphalangeal joints were free of arthritis. Laboratory tests showed ERS of 7 mm/h and CRP of 1.58 mg/L. GMA was then administered at one session per month. However, arthritis worsened and the patient needed intra-articular corticosteroids in his left knee. Moreover, inflammatory markers increased (ERS of 19 mm/h and CRP of 2 mg/L). Therefore, in December 2020, the patient again received two GMA sessions per month. Evolution has been very satisfactory and, to date, he continues his treatment with GMA besides his usual drug therapy.
Case 4: GMA as temporary therapy
The patient was a 60-year-old woman with RA treated with a combination of adalimumab 40 mg every other week and MTX 25 mg weekly plus folic acid. As she presented recurrent dental implant infection, DMARDs were discontinued and the patient only received NSAIDs and tramadol. All the implants were extracted because her gums did not heal.
She was referred to our rheumatology department. She presented with arthritis in wrists, metacarpophalangeal joints of both hands and knees as well as neck and shoulder pain. ERS was 31 m/h. She then received ten sessions of GMA (two sessions per week) plus ibuprofen 1800 mg/d, omeprazole 20 mg/d and prednisone 5 mg/d. This treatment improved her wrist arthritis and neck and shoulder pain. As her gums have completely healed, her usual treatment with adalimumab 40 mg every other week, MTX 25 mg weekly and folic acid plus prednisone was reinitiated, in addition to one GMA session per month. The patient says that her arthritis and pain have improved.
Discussion
The first studies of GMA in patients with inflammatory arthritis showed the efficacy and safety of the device.32–36 Results in animal models also supported the use of GMA.36,37 These early studies were performed in Japan, where GMA was developed. Subsequent studies38–40 included both Japanese and Caucasian patients and the results were also satisfactory. However, no randomized controlled trials of GMA have been performed, probably because the requirements for approval of medical devices are not as strict as those for drugs. Another possible reason could be that the still small number of candidates prevents the performance of a proper randomized controlled trial. Even so, it would be interesting to compare GMA with next-generation biological agents for the treatment of IMIAs.
The four case reports illustrate situations where GMA can be appropriate in the framework of IMIAs. Case 1 shows a patient with intense pain and very high NSAID intake despite receiving golimumab and leflunomide. GMA not only improved pain but also made it possible to reduce NSAID intake. The patient was unable to tolerate MTX or secukinumab. GMA obviated the need for another drug and improves the patient’s overall condition. Case 2 is an example of GMA as an adjuvant therapy. The patient was already receiving vedolizumab and MTX but his pain and stiffness persisted. GMA improved the symptoms, though better results were achieved with the combination of vedolizumab, MTX and GMA. Case 3 describes a patient with intolerance to some anti-TNF agents and poor response to multiple drugs. Furthermore, the patient was planning to have a baby and MTX was not appropriate. Results of GMA were very satisfactory, with improvement of pain and morning stiffness. Because of the retrospective nature of this case series, not all data on inflammatory markers were available. However, it would be interesting to assess the evolution of inflammatory and biological markers after GMA sessions as it has been studied in patients with UC treated with GMA.41,42 Finally, in case 4, GMA was used as a temporary treatment to also solve extra-articular manifestations related to the inflammatory condition. The patient had had repeated infections and gum healing problems. However, as RA symptoms improved during GMA treatment, the patient continues to receive it in addition to her usual DMARD therapy.
GMA can also be useful in other various situations, such as that of a young woman with RA who desires to become pregnant and must therefore avoid certain drugs. Another example would be GMA as temporary treatment whilst waiting for the effect of a biological agent with a slow mechanism of action.
In our opinion, GMA is useful in IMIAs, especially in the following scenarios, all of which involve active disease:
- Frail patients (GMA as effective and safe therapy, without inducing immunosuppression)
- Patients treated with DMARDs and biological agents (GMA as adjuvant therapy)
- Patients who desire pregnancy
- Patients who have recently initiated treatment with a biological agent and have not achieved complete response (GMA as temporary therapy)
- Patients with adverse events caused by previous drugs (GMA as low-risk treatment)
GMA is well tolerated and, as expected, no adverse events were recorded in the four case reports. However, the state of the patient’s veins can prevent or limit GMA, as in case 1. This is a potential limitation of GMA. Another potential disadvantage of GMA could be its cost. However, to date, there have been no studies on the cost-effectiveness of GMA in the management of IMIAs. In patients with moderate-to-severe UC, GMA reduces the costs associated with hospitalizations, surgery, drug therapy and outpatient care/medical visits compared with conventional treatment.43,44 It is also related to an increase in quality-adjusted life years.43 Moreover, compared with infliximab for the treatment of moderate-to-severe UC in patients who are either steroid dependent steroid resistant, GMA is a cost-saving strategy.45 In addition, reducing the frequency of hospitalizations and surgery can decrease indirect and intangible costs and may also be associated with an increase in patient productivity and HRQoL.44 We can hypothesize that, in IMIAs, GMA could also have benefits beyond improvement in pain and inflammation, with effects such as higher mobility and productivity and better HRQoL. Although we did not evaluate HRQoL in the four cases presented, we consider that study of the relationship between GMA and HRQoL in patients with IMIAs could enhance the use of this procedure.
Conclusion
In conclusion, GMA is effective and safe for the management of IMIAs. Our findings support the use of GMA in clinical practice.
Acknowledgements
Writing and editorial assistance was provided by Content Ed Net (Madrid, Spain).
Footnotes
Adacolumn®, JIMRO Co. Ltd., Takasaki, Japan/Adacyte Therapeutics, Sant Cugat del Vallés, Barcelona, Spain.
Contributions: All authors have contributed significantly to the conception, design, or acquisition of data, or analysis and interpretation of data. All authors have participated in drafting, reviewing and/or revising the manuscript and have approved its submission. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: The authors declare that they have no conflicts of interest relevant to this manuscript. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2021/10/dic.2021-8-5-COI.pdf
Funding declaration: Writing and editorial assistance was funded by Adacyte.
Correct attribution: Copyright © 2021 Carro Martínez AV, Montolio Chiva L, Robustillo Villarino M. https://doi.org/10.7573/dic.2021-8-5. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Provenance: Submitted; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editorial office editorial@drugsincontext.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
References
- 1.Williams J, Meyers JA. Immune-Mediated Inflammatory Disorders (I.M.I.D.s): the economic and clinical costs. Am J Manag Care. 2002;8(21):S668–S681. [PubMed] [Google Scholar]
- 2.Spierings J, Sloeserwij A, Vianen ME, et al. Health-related quality of life in patients with immune mediated inflammatory diseases: a cross-sectional, multidisciplinary study. Clin Immunol. 2020;214:108392. doi: 10.1016/j.clim.2020.108392. [DOI] [PubMed] [Google Scholar]
- 3.Alamanos Y, Voulgari PV, Drosos AA. Incidence and prevalence of rheumatoid arthritis, based on the 1987 American College of Rheumatology Criteria: a systematic review. Semin Arthritis Rheum. 2006;36(3):182–188. doi: 10.1016/j.semarthrit.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Silva-Fernández L, Macía-Villa C, Seoane-Mato D, et al. The prevalence of rheumatoid arthritis in Spain. Sci Rep. 2020;10:21551. doi: 10.1038/s41598-020-76511-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med. 2017;376(10):957–970. doi: 10.1056/NEJMra1505557. [DOI] [PubMed] [Google Scholar]
- 6.Smith MW. Ulcerative Colitis and Joint Pain. [Accessed June 22, 2021]. https://www.webmd.com/ibd-crohns-disease/ulcerative-colitis/ulcerative-colitis-joint-pain.
- 7.Ajeganova S, Huizinga T. Sustained remission in rheumatoid arthritis: latest evidence and clinical considerations. Ther Adv Musculoskelet Dis. 2017;9(10):249–262. doi: 10.1177/1759720X17720366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saas P, Bonnefoy F, Toussirot E, Perruche S. Harnessing apoptotic cell clearance to treat autoimmune arthritis. Front Immunol. 2017;8:1191. doi: 10.3389/fimmu.2017.01191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smolen JS, Landewé RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):S685–S699. doi: 10.1136/annrheumdis-2019-216655. [DOI] [PubMed] [Google Scholar]
- 10.Mazaud C, Fardet L. Relative risk of and determinants for adverse events of methotrexate prescribed at a low dose: a systematic review and meta-analysis of randomized placebo-controlled trials. Br J Dermatol. 2017;177(4):978–986. doi: 10.1111/bjd.15377. [DOI] [PubMed] [Google Scholar]
- 11.Atzeni F, Nucera V, Gerratana E, et al. Concerns about the safety of anti-TNF agents when treating rheumatic diseases. Expert Opin Drug Saf. 2020;19(6):695–705. doi: 10.1080/14740338.2020.1763299. [DOI] [PubMed] [Google Scholar]
- 12.De Camargo MC, Barros BCA, Fulone I, et al. Adverse events in patients with rheumatoid arthritis and psoriatic arthritis receiving long-term biological agents in a real-life setting. Front Pharmacol. 2019;10:965. doi: 10.3389/fphar.2019.00965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mercer LK, Regierer AC, Mariette X, et al. Spectrum of lymphomas across different drug treatment groups in rheumatoid arthritis: a European registries collaborative project. Ann Rheum Dis. 2017;76(12):2025–2030. doi: 10.1136/annrheumdis-2017-211623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wadström H, Frisell T, Askling J. Malignant neoplasms in patients with rheumatoid arthritis treated with tumor necrosis factor inhibitors, tocilizumab, abatacept, or rituximab in clinical practice: a nationwide cohort study from Sweden. JAMA Intern Med. 2017;177(11):1605–1612. doi: 10.1001/jamainternmed.2017.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubbert-Roth A, Szabó MZ, Kedves M, Nagy G, Atzeni F, Sarzi-Puttini P. Failure of anti-TNF treatment in patients with rheumatoid arthritis: the pros and cons of the early use of alternative biological agents. Autoimmun Rev. 2019;18(12):102398. doi: 10.1016/j.autrev.2019.102398. [DOI] [PubMed] [Google Scholar]
- 16.Shams S, Martinez JM, Dawson JRD, et al. The therapeutic landscape of rheumatoid arthritis: current state and future directions. Front Pharmacol. 2021;12:680043. doi: 10.3389/fphar.2021.680043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aletaha D, Stamm T, Kapral T, et al. Survival and effectiveness of leflunomide compared with methotrexate and sulfasalazine in rheumatoid arthritis: a matched observational study. Ann Rheum Dis. 2003;62(10):944–951. doi: 10.1136/ARD.62.10.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frisell T, Dehlin M, Di Giuseppe D, Feltelius N, Turesson C, Askling J. Comparative effectiveness of abatacept, rituximab, tocilizumab and TNFi biologics in RA: results from the nationwide Swedish register. Rheumatology. 2019;58(8):1367–1377. doi: 10.1093/RHEUMATOLOGY/KEY433. [DOI] [PubMed] [Google Scholar]
- 19.Simard JF, Arkema EV, Sundström A, et al. Ten years with biologics: to whom do data on effectiveness and safety apply? Rheumatology. 2011;50(1):204–213. doi: 10.1093/rheumatology/keq326. [DOI] [PubMed] [Google Scholar]
- 20.Yokoyama Y, Kamikozuru K, Watanabe K, Nakamura S. Inflammatory bowel disease patients experiencing a loss of response to infliximab regain long-term response after undergoing granulocyte/monocyte apheresis: a case series. Cytokine. 2018;103:25–28. doi: 10.1016/J.CYTO.2017.12.030. [DOI] [PubMed] [Google Scholar]
- 21.Rodríguez-Lago I, Benítez J, Sempere L, et al. The combination of granulocyte-monocyte apheresis and vedolizumab: a new treatment option for ulcerative colitis? J Clin Apher. 2019;34(6):680–685. doi: 10.1002/JCA.21746. [DOI] [PubMed] [Google Scholar]
- 22.Rodríguez–Lago I, Sempere L, Gutiérrez A, et al. Granulocyte–monocyte apheresis: an alternative combination therapy after loss of response to anti-TNF agents in ulcerative colitis. Scand J Gastroenterol. 2019;54(4):459–464. doi: 10.1080/00365521.2019.1600715. [DOI] [PubMed] [Google Scholar]
- 23.Yokoyama Y, Sawada K, Aoyama N, et al. Efficacy of granulocyte and monocyte adsorptive apheresis in patients with inflammatory bowel disease showing lost response to infliximab. J Crohns Colitis. 2020;14(9):1264–1273. doi: 10.1093/ECCO-JCC/JJAA051. [DOI] [PubMed] [Google Scholar]
- 24.Adacolumn, a granulocyte and monocyte/macrophage adsorption apheresis device. [Accessed May 24, 2021]. https://www.adacyte.com/wp-content/uploads/2021/02/adacolumn-instructions-for-use.pdf.
- 25.Hanai H, Takeda Y, Eberhardson M, et al. The mode of actions of the Adacolumn therapeutic leucocytapheresis in patients with inflammatory bowel disease: a concise review. Clin Exp Immunol. 2011;163(1):50–58. doi: 10.1111/j.1365-2249.2010.04279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adacyte Therapeutics. Adacolumn. Adamonitor SC. Reference Manual. Sant Cugat del Vallés; Barcelona, España;: 2021. [Google Scholar]
- 27.Adacolumn: mode of action. [Accessed October 13, 2021]. https://www.adacyte.com/product/gma-in-inflammatory-bowel-disease-ibd/
- 28.Kanekura T. Clinical and immunological effects of adsorptive myeloid lineage leukocyte apheresis in patients with immune disorders. J Dermatol. 2018;45(8):943–950. doi: 10.1111/1346-8138.14471. [DOI] [PubMed] [Google Scholar]
- 29.Toussirot E, Bonnefoy F, Vauchy C, Perruche S, Saas P. Mini-review: the administration of apoptotic cells for treating rheumatoid arthritis: current knowledge and clinical perspectives. Front Immunol. 2021;12:630170. doi: 10.3389/FIMMU.2021.630170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ansary MMU, Ishihara S, Oka A, et al. Apoptotic cells ameliorate chronic intestinal inflammation by enhancing regulatory B-cell function. Inflamm Bowel Dis. 2014;20(12):2308–2320. doi: 10.1097/MIB.0000000000000240. [DOI] [PubMed] [Google Scholar]
- 31.Saniabadi AR, Hanai H, Takeuchi K, et al. Adacolumn, an adsorptive carrier based granulocyte and monocyte apheresis device for the treatment of inflammatory and refractory diseases associated with leukocytes. Ther Apher. 2003;7(1):48–59. doi: 10.1046/j.1526-0968.2003.00012.x. [DOI] [PubMed] [Google Scholar]
- 32.Fujimori J, Yoshino S, Koiwa M, et al. Improvement in rheumatoid arthritis following application of an extracorporeal granulotrap column, G-l. Rheumatol Int. 1996;15(5):175–180. doi: 10.1007/bf00290518. [DOI] [PubMed] [Google Scholar]
- 33.Ohara M, Saniabadi AR, Kokuma S, et al. Granulocytapheresis in the treatment of patients with rheumatoid arthritis. Artif Organs. 1997;21(9):989–994. doi: 10.1111/j.1525-1594.1997.tb00513.x. [DOI] [PubMed] [Google Scholar]
- 34.Kashiwagi N, Hirata I, Kasukawa R. A role for granulocyte and monocyte apheresis in the treatment of rheumatoid arthritis. Ther Apher. 1998;2(2):134–141. doi: 10.1111/j.1744-9987.1998.tb00091.x. [DOI] [PubMed] [Google Scholar]
- 35.Mori S, Nagashima M, Yoshida K, et al. Granulocyte adsorptive apheresis for leg ulcers complicated by rheumatoid arthritis: A report on three successfully treated cases. Int J Dermatol. 2004;43(10):732–735. doi: 10.1111/j.1365-4632.2004.01986.x. [DOI] [PubMed] [Google Scholar]
- 36.Kyogoku M. Clinical and basic studies on the G-1 column, a new extracorporeal therapeutic device effective in controlling rheumatoid arthritis. Inflamm Res. 1998;47(Suppl 3):S166–S176. doi: 10.1007/s000110050311. [DOI] [PubMed] [Google Scholar]
- 37.Kashiwagi N, Nakano M, Saniabadi AR, Adachi M, Yoshikawa T. Anti-inflammatory effect of granulocyte and monocyte adsorption apheresis in a rabbit model of immune arthritis. Inflammation. 2002;26(4):199–205. doi: 10.1023/A:1016523914161. [DOI] [PubMed] [Google Scholar]
- 38.Saniabadi AR, Hanai H, Suzuki Y, et al. Adacolumn for selective leukocytapheresis as a non-pharmacological treatment for patients with disorders of the immune system: an adjunct or an alternative to drug therapy? J Clin Apher. 2005;20(3):171–184. doi: 10.1002/jca.20046. [DOI] [PubMed] [Google Scholar]
- 39.Sanmartí R, Marsal S, Valverde J, et al. Adsorptive granulocyte/monocyte apheresis for the treatment of refractory rheumatoid arthritis: an open pilot multicentre trial. Rheumatology. 2005;44(9):1140–1144. doi: 10.1093/rheumatology/keh701. [DOI] [PubMed] [Google Scholar]
- 40.Bazzichi L, Giuliano T, Rossi A, et al. Partial remission of refractory RA after adacolumn cytapheresis: a case report. Rheumatol Int. 2008;28(3):295–297. doi: 10.1007/s00296-007-0427-1. [DOI] [PubMed] [Google Scholar]
- 41.Shimoyama T, Yamamoto T, Umegae S, Matsumoto K. Faecal calprotectin level for assessing endoscopic activity and predicting future clinical course in patients with moderately active ulcerative colitis undergoing granulomonocytapheresis: a prospective cohort study. BMC Gastroenterol. 2018;18(1):120. doi: 10.1186/S12876-018-0853-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamamoto T, Tanaka T, Yokoyama T, et al. Efficacy of granulocyte and monocyte apheresis for antibiotic-refractory pouchitis after proctocolectomy for ulcerative colitis: an open-label, prospective, multicentre study. Therap Adv Gastroenterol. 2017;10(2):199–206. doi: 10.1177/1756283X16679348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Persson U, Borg S, Hjelmgren J, Ljung T, Riis L, Østergaard Thomsen O. PG18. Economic evaluation of Adacolumn apheresis for treatment of patients with moderate to severe Crohn’s disease or ulcerative colitis. Value Health. 2005;8(6):A120–A121. [Google Scholar]
- 44.Panés J, Guilera M, Ginard D, et al. Treatment cost of ulcerative colitis. Is apheresis with Adacolumn® cost-effective? Dig Liver Dis. 2007;39(7):617–625. doi: 10.1016/j.dld.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 45.Ravasio R, De Silvestro G, Saggioro A, Sturniolo GC, Vernia P. Economic assessment of granulocyte and monocyte adsorption apheresis (Adacolumn®) vs. a biologic agent in the treatment of ulcerative colitis in patients with dependence or resistance to corticosteroids in Italy. G Ital di Heal Technol Assess. 2013;6(2–3):55–64. doi: 10.1007/s40269-013-0008-y. [DOI] [Google Scholar]

