Abstract
Background
Intensity modulated radiotherapy (IMRT) has the perceived advantage of function preservation by reduction of toxicities in the treatment of laryngo-pharyngeal malignancies. The aim of the study was to assess changes in dysphagia from baseline (i.e. prior to start of treatment) at three and six months post treatment in patients with laryngo-pharyngeal malignancies treated with radical radiotherapy ± chemotherapy. Functional assessment of other structures involved in swallowing was also studied.
Materials and methods
40 patients were sampled consecutively. 33 were available for final analysis. Dysphagia, laryngeal edema, xerostomia and voice of patients were assessed at baseline and at three and six months after treatment. Radiation was delivered with simultaneous integrated boost (SIB) using volumetric modulated radiation therapy (VMAT). Concurrent chemotherapy was three weekly cisplatin 100 mg/m2.
Results
Proportion of patients with dysphagia rose significantly from 45.5% before the start of treatment to 57.6% at three months and 60.6% at six months post treatment (p = 0.019). 67% patients received chemotherapy and addition of chemotherapy had a significant correlation with dysphagia (p = 0.05, r = −0.336). Severity of dysphagia at three and six months correlated significantly with the mean dose received by the superior constrictors (p = 0.003, r = 0.508 and p = 0.024, r = 0.391) and oral cavity (p = 0.001, r = 0.558 and p = 0.003, r = 0.501). There was a significant worsening in laryngeal edema at three and six months post treatment (p < 0.01) when compared to the pre-treatment examination findings with 60.6% of patients having grade two edema at six months. Significant fall in the mean spoken fundamental frequency from baseline was seen at 6 months (p = 0.04), mean fall was 21.3 Hz (95% CI: 1.5–41 Hz) with significant increase in roughness of voice post treatment (p = 0.01).
Conclusion
There was progressive worsening in dysphagia, laryngeal edema and voice in laryngo-pharyngeal malignancies post radical radiotherapy ± chemotherapy.
Keywords: dysphagia, radiotherapy, laryngeal function, simultaneous integrated boost, function preservation
Introduction
Chemoradiation has a central role in the treatment of locally advanced laryngo-pharyngeal malignancies with function preservation as one of the main advantages [1]. However, treatment related toxicities remain a major concern for patients receiving radical chemoradiation [2]. Modern techniques of radiation delivery like intensity modulated radiotherapy (IMRT) and volumetric modulated arc therapy (VMAT) have reduced treatment related toxicities [3, 4]. These techniques also allow for simultaneous integrated boost (SIB) or “dose painting” — delivery of higher dose per fraction to the tumour and standard dose per fraction to areas at intermediate and low risk [4, 5].
Despite the use of modern treatment delivery techniques, dysphagia remains one of the most distressing acute as well as late toxicity associated with radical radiation therapy in head and neck cancers [7]. Swallowing being a complex process has many structures playing important roles in its proper execution, such as the parotids, larynx, oral cavity and constrictor muscles [8]. Impaired functioning of each of these organs as reflected by the development of xerostomia, laryngeal edema etc. would also contribute to the changes in dysphagia after treatment [3, 9]. In this study, we aimed to evaluate the changes in dysphagia, laryngeal edema, xerostomia and voice after treatment with radical radiotherapy delivered by VMAT with SIB ± concurrent chemotherapy in laryngo-pharyngeal primaries and correlate the toxicities with doses received by OARs.
Materials and methods
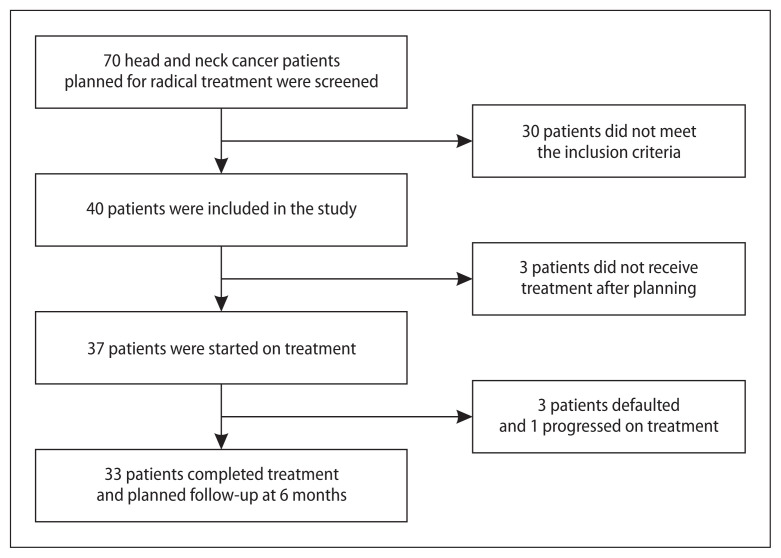
This prospective observational study included consecutive patients with pathologically confirmed squamous cell carcinomas of oropharynx, hypopharynx and larynx attending the radiation oncology outpatient department of our center from January 2017 to August 2018. Patient recruitment details are given in Figure 1. Primary treatment was planned with radical radiation or chemoradiation after discussion in a multidisciplinary tumour board consisting of head and neck surgery, radiation oncology, radiology and head and neck physiotherapy specialists. Patients with a poor performance status (Eastern Cooperative Oncology Group performance score ≥ 3), those who have previously undergone head and neck surgery, radiotherapy or systemic chemotherapy and those dependent on tracheostomy tubes were excluded. Staging was done according to the AJCC 7th edition manual. The study was approved by the Institute ethics committee. Written informed consent was given by all patients.
Figure 1.
Strobe diagram
Treatment
Patients were immobilized using a thermoplastic immobilization system. After Contrast enhanced CT Simulation, gross tumour delineation was done based on information from the simulation scans, endoscopic findings and review of diagnostic imaging. Treatment planning was done with the ECLIPSE planning system 10.0 (Varian Medical Systems, Palo Alto, CA). The prescription was 66 Gy in 30 fractions to the high risk (gross disease and nodes involved with five mm margins), 60 Gy in 30 fractions to the intermediate risk (nodal stations involved with one cm margin to the gross disease), and 54 Gy in 30 fractions to the low risk planning target volumes (PTV). Radiation was delivered as SIB using VMAT. Organs at risk (OAR), such as the spinal cord, brainstem, three pharyngeal constrictors, larynx, oral cavity including the minor salivary glands and parotids, were contoured according to standard published guidelines [10–12]. Constraints for OARs were largely based on Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC) and standard institutional protocols [13]. Plans were optimized such that 95% of PTV was covered by the prescription dose. During optimisation and plan evaluation, PTV coverage was not compromised at the cost of sparing an OAR. Offline image verification was done and No Action Level (NAL protocol) was followed for calculating setup errors. Once weekly cone beam CT (CBCT) was taken after correction of errors for treatment verification.
Concurrent chemotherapy administered was three weekly cisplatin 100 mg/m2. Patients were reviewed weekly during the course of radiation for assessment of toxicities.
Endpoints
The primary endpoint was to assess the change in the severity of dysphagia from baseline (i.e. prior to start of treatment) and at three and six months post treatment. The third and sixth month time points were chosen to represent acute and late dysphagia, respectively [14]. Any dysphagia interfering with normal food intake was taken as significant (grade one and above).
Secondary endpoints included the assessment of toxicities of other structures (eg. larynx, salivary glands) involved in the process of swallowing which included laryngeal edema, voice changes and xerostomia. Correlation of toxicities with doses received by different OARs was done. Mean doses were selected for correlation of toxicities as the OARs discussed were mostly parallel structures whose toxicities correlated well with mean doses [13]. Toxicities were graded by the European organization for research and treatment of cancer (EORTC)/Radiation Therapy Oncology Group (RTOG) Radiation morbidity scoring criteria [15].
Voice analysis was based on recordings which included a standard passage in vernacular and three sustained vowels (a, i and u) repeated thrice. Maximum phonation times for each vowel were noted. Sony F-V 120 unidirectional microphone was used for recording. Recorded samples were analyzed using the VAGMI version 8.1 voice processing software. Fundamental frequencies of the three vowels and mean spoken fundamental frequency from the recorded passage were obtained.
Perceptual assessment of voice was done by the GRBAS scale of the Japan society of Logopedics and Phoniatrics. Scores of zero, one, two, or three were given for each of the five voice qualities; Grade, Roughness, Breathiness, Asthenia, and Strain, where zero was normal and three was severe impairment [16]. Scoring was done by a trained speech language pathologist not privy to the patient status or voice sample information.
A questionnaire based evaluation of xerostomia at three time points was done [17]. Subjects rated eight symptoms on an 11-point ordinal Likert scale from zero to ten, with higher scores indicating greater discomfort due to dryness. Each item score was added, to get the final score ranging between zero and eighty. Higher scores represented greater levels of xerostomia. Weights of the patients were taken prior to the start of treatment and at three and six months after completion of treatment.
Statistical analysis
We hypothesised that there will be an increase in the proportion of patients with dysphagia from baseline post radical radiation ± chemotherapy at six months. The minimum expected proportion of patients reporting any degree of dysphagia was 0.77 [18]. Sample size was estimated to be 40 using the formula for estimating single proportion at 5% level of significance, 20% precision and 30% dropout. All statistical tests considered p < .05 as statistically significant.
SPSS version 19 was used for analysis. Non-parametric parameters were compared using the Friedman test. Continuous variables were analysed using ANOVA. Post hoc analysis with the Bonferroni correction was done when results were significant. Spearman’s correlation coefficient was used to identify the correlation of grades of toxicities with doses received to OARs.
Results
Baseline characteristics of the patients are given in Table 1. The mean (SD) cumulative dose of cisplatin received was 233 (68) mg. 72% of the patients received at least two cycles of chemotherapy. The mean (SD) duration of radiation course was 51 (11.0) days.
Table 1.
Principal characteristics of the patients (n = 33)
| Factor | Description |
|---|---|
| Age | |
| Age (years) | Mean = 60.6, SD = 8.9 |
| Sex | |
| Male | 91% (n = 30) |
| Female | 9% (n = 3) |
| Smoking | |
| Yes | 85% (n = 28) |
| No | 15% (n = 5) |
| T stage | |
| T1 | 9% (n = 3) |
| T2 | 9% (n = 3) |
| T3 | 39.4% (n = 13) |
| T4 | 42.5% (n = 14) |
| N stage | |
| N0 | 15% (n = 5) |
| N1 | 48.4% (n = 16) |
| N2 | 36.3% (n = 13) |
| Stagewise distibution of site | |
| Oropharynx: n, (stages 1/2/3/4) | 13, (1/1/3/8) |
| Hypopharnx: n, (stage 1/2/3/4) | 4, (0/0/2/2) |
| Larynx :n, (stage 1/2/3/4) | 16, (2/1/9/4) |
| Chemotherapy | |
| Yes | 67% (n = 22) |
| No | 33% (n = 11) |
SD — standard deviation
There was a significant correlation between tumour site and mean doses to the superior constrictors (p < 0.001, r = 0.613), oral cavity (p < 0.001, r = 0.852) and larynx (p < 0.001, r = 0.649). The site wise distribution of the doses received by OARs is given in Table 2.
Table 2.
Site wise distribution of doses to the organ at risks (OARs) (n = 33)
| OAR | Median of the mean dose to OARs in Gy (Q3–Q1) | ||
|---|---|---|---|
| Oropharynx | Hypopharynx | Larynx | |
| Superior constrictor | 67.3 (68.3–64.9) | 63.8 (67.3–57.8) | 55.6 (60.9–35.9) |
| Middle constrictor | 67.2 (68.1–58.4) | 67.9 (68.2–66.9) | 66.8 (67.7–64.6) |
| Inferior constrictor | 54.6 (60.4–51.9) | 67.8 (67.9–66.8) | 66.5 (67.7–64.3) |
| Larynx | 55.8 (61.7–52.5) | 67.9 (68.5–67.0) | 66.9 (67.9–65 ) |
| Oral cavity | 61.9 (64.7–55.4) | 38 (42.7–35.5) | 20.7 (34.2–16.5) |
| Parotids | 26.4 (32.2–23.4) | 31.5 (45.5–29.1) | 22.2 (25.3–20.2) |
Q1 — first quartile; Q3 — third quartile
Dysphagia
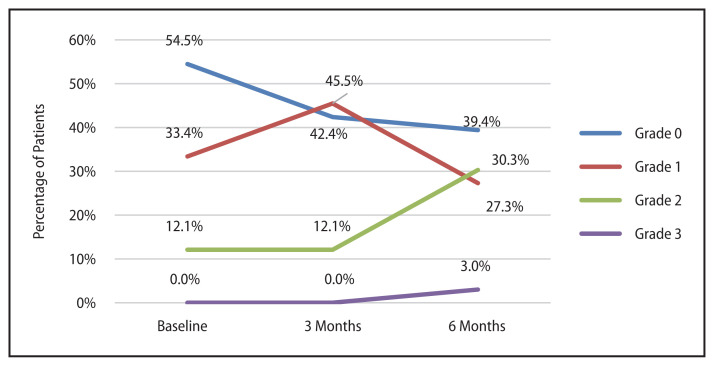
The proportion of patients with dysphagia at baseline was 45.5%. At three months post treatment it rose to 57.6% and at six months it became 60.6%. The change in severity of dysphagia with time (Fig. 2) was significant (p = 0.019). Post hoc analysis revealed the difference to be significant between baseline and six months post treatment (p = 0.036). 36% (n = 12) patients had a worsening of dysphagia, 9% (n = 3) had an improvement and 55% (n = 18) did not have any change in the severity of dysphagia at six months compared to baseline. There was a significant correlation between dysphagia at six months and the addition of chemotherapy (p = 0.05, r = −0.336). 41% of patients who received chemotherapy had a worsening of dysphagia at six months compared to 27% patients who did not receive chemotherapy.
Figure 2.
Change in severity of dysphagia with time
Mean dose received by the superior constrictors correlated significantly with dysphagia at three and six months post treatment. (p = 0.003, r = 0.508& p = 0.024, r = 0.391 respectively). All patients with ≥ grade two dysphagia at three and six months post treatment received mean dose > 50Gy to the superior constrictor. There was no correlation with the severity of dysphagia and mean dose to the middle and inferior constrictors.
Mean dose to the oral cavity correlated with the severity of dysphagia at three and six months (p = 0.001, r = 0.558 and p = 0.003, r = 0.501 respectively). Almost all patients with ≥ grade two dysphagia at six months received mean dose >35 Gy to the oral cavity.
Laryngeal edema
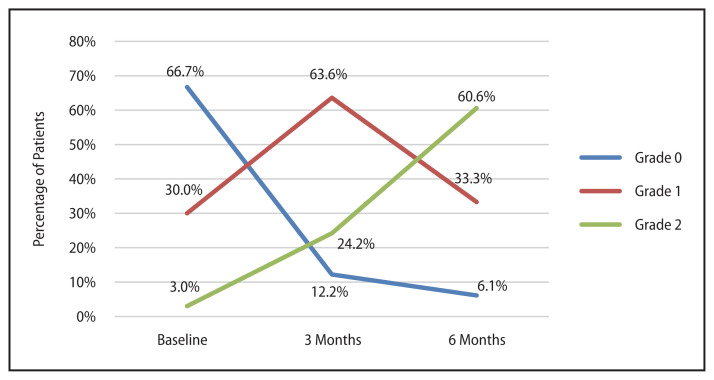
Proportion of patients presenting with grade two laryngeal edema rose from 3% at baseline to 24% at three months and 61% at six months (Fig. 3). The difference in grades of laryngeal edema from baseline were significant at three and six months (p < 0.001 for both). All patients developing grade two laryngeal edema at six months received mean laryngeal dose more than 55 Gy. There was no correlation with the severity of dysphagia and laryngeal edema post treatment.
Figure 3.
Change in severity of laryngeal edema with time
Voice changes
There was a significant fall in fundamental frequencies of the vowels a, i and u from baseline (Tab. 3). Post hoc analysis showed a significant difference in fundamental frequencies for a and i at three months post treatment when compared to the values at baseline (p < 0.001 and p = 0.016, respectively). Mean fall in fundamental frequency for a at three months was 15.49 Hz (95% CI: 8.5–22.4 Hz) and for i at three months it was 15.06 Hz (95% CI: 2.3–27.8 Hz). The fall in fundamental frequency for a was significant at six months as well when compared to the baseline (p = 0.008), with mean fall of 18.14 Hz (95% CI: 4–32 Hz).
Table 3.
Change in parameters studied with time (n = 33)
| Factor studied | Baseline | 3 months | 6 months | p–value |
|---|---|---|---|---|
| FF of vowel a in Hz, mean (SD) | 137.3 (34.2) | 121.8 (28.5) | 119.1 (26.5) | < 0.001 |
| FF of vowel i in Hz, mean (SD) | 149.9 (43.8) | 134.8 (30) | 132.9 (33.4) | 0.02 |
| FF of vowel u Hz, mean (SD) | 149.8(35.6) | 133.7 (26.5) | 136.5 (31.1) | 0.009 |
| MSFF in Hz, mean (SD) | 142.4 (43.5) | 135.1 (27.1) | 121.1 (23.2) | 0.017 |
| Xerostomia score, median (Q3–Q1) | 0 (5–0) | 25(50–3) | 14 (41–0) | < 0.001 |
| Weight in kg, mean (SD) | 55 (12.4) | 50.1 (12) | 49.9 (12) | < 0.001 |
FF — fundamental frequency; Q1 — first quartile; Q3 — third quartile; MSFF — mean spoken fundamental frequency; SD — standard deviation
There was a significant fall in the mean spoken fundamental frequency (Tab. 3). Post hoc analysis showed a significant change from baseline to six months (p = 0.04) with mean fall of 21.3 Hz (95% CI: 1.5–41Hz) and from three months to six months (p = 0.023), with mean fall of 14 Hz (95% CI: 1.5–26.5Hz).
Perceptual assessment of voice by GRBAS scaling revealed significant changes in the subjective parameter of roughness post treatment (p = 0.01). Post hoc analysis revealed a significant difference in roughness between three and six months (p = 0.003) with nearly 50% of patients having increased roughness. There was a non-significant increase in the maximum phonation times of the vowels at three months from baseline with decrease from three to six months.
Other toxicities
There was a worsening of xerostomia scores at three months post treatment with a significant improvement in nearly all cases at six months (Tab. 3, p < 0.001 for both). Xerostomia scores at three months correlated significantly with mean dose to the oral cavity and the parotids (p = 0.002, r = 0.531 and p = 0.047, r = 0.34, respectively). Three patients developed grade three dermatitis and two patients developed grade three oral mucositis during treatment. Addition of chemotherapy did not have any significant impact on mucositis.
More than 50% of patients had weight loss of ≥ five kg from the baseline at three and six months after treatment, (Tab. 3, p < 0.001 for both). Mean weight loss at six months was 5.03 kg with 95% CI (3–7 kg).
Discussion
Dysphagia remains a dreaded complication after radical chemoradiotherapy. The identification of dysphagia and aspiration related structures (DARS) by Eisbruch et al. [19] has been pivotal in limiting this toxicity. The results of the DARS study by Nutting et al. [20] clarified the advantage of limiting the mean doses to key swallowing structures like the pharyngeal constrictors with a significant reduction in patient reported swallowing difficulties in the dysphagia optimised IMRT (Do-IMRT) arm.
Changes in dysphagia with time for head and neck malignancies treated non-surgically have been evaluated in similar studies. Roe et al21 showed a significant decrease in the swallow performance at three months post treatment. Wilson et al.22 demonstrated 18% deterioration in MDADI (M.D. Anderson Dysphagia Inventory) scores three months post treatment The proportion of patients with ≥ grade two dysphagia rose from 0% at baseline to 41% at the end of three months in an identical setting by Mazzola et al. [14] as well.
However, the results of our study showed that dysphagia continued to worsen steadily from baseline at six months post treatment as well with the proportion of patients with ≥ grade two dysphagia reaching 33% at six months. This contrasts with the above studies which showed a plateauing of the swallowing difficulties with even a trend for improvement at six months when compared to three months [14, 21, 22]. The inability to meet the dose constraints of the OARs, inclusion of higher proportion of patients with dysphagia at the onset of treatment (45.5% vs. 0%) [14, 22], higher proportion of concurrent chemotherapy (67% vs. 54%) [14] and higher proportion of smokers in the study population (85% vs. 57%) [21, 23] could have contributed to the increase in proportion of patients with ≥ grade two dysphagia at six months. The fall in the weight of patients observed after completion of treatment also serves as a pointer to the progressive worsening of dysphagia that is observed three and six months after treatment [24].
Thus, in this study, treatment related toxicities continued to be a matter of concern with majority of patients having difficulty in the ability to take normal diet even at six months post treatment.
The severity of dysphagia post treatment correlated significantly with the dose to the superior constrictors. Nearly all patients with ≥ grade two dysphagia at six months had mean dose > 50 Gy to the superior constrictor. This validates the results of a recent metaanalysis which predicted higher occurrence of dysphagia with mean doses in excess of 50 Gy to the pharyngeal constrictor muscles [25]. The study by Mazola et al. [14] also estimated the odds ratio of developing dysphagia at six months to be 9.3 when the mean dose to the superior constrictors exceeded 50 Gy.
The tongue along with secretions from the minor salivary glands plays a key role in the oral preparatory phase and the oral phase of swallowing. Damage to these structures after treatment could be the reason for the correlation between dysphagia and xerostomia scores with mean doses to the oral cavity [26]. The observations made here regarding the occurrence of progressive worsening of dysphagia when mean doses to the oral cavity exceeded 35 Gy could also serve as an indicator for prescription of mean dose constraints to the oral cavity during treatment planning.
The role of DARS in producing dysphagia is also influenced by the site of the primary as is observed in our study by virtue of the doses received by the OARs [27]. The mean dose to the oral cavity was limited to less than 35 Gy in more than half of non-oropharyngeal primaries and in nearly half of laryngeal primaries, the mean dose to the superior constrictors was limited to less than 50 Gy. Thus, the site of the primary also has an important role in practical attainment of constraints to the different OARs.
The rising trend of laryngeal edema at three and six months post treatment in head and neck malignancies receiving radiotherapy is also seen in the study by Sanguineti et al. [28]. However the mean dose (SD) received by the larynx in our study was 62.6 (6.3) Gy which was well in excess of the recommended constraints (mean dose < 44 Gy) for laryngeal edema rates of less than 20%. Progressive edema of the larynx post treatment may result in long term adverse effects involving phonation, swallowing etc. [9, 25]. This, along with other factors like altered microcirculation, fibrosis and neuromuscular fold weakness could result in the fall in fundamental frequencies as observed [29].
Voice is a multidimensional construct forming an important part of a person’s identity. Fundamental frequency is an objective measure of the pitch of the voice [30]. The changes in voice as a result of development of laryngeal edema as well as the changes in pitch secondary to variations in the fundamental frequencies can change self-perception as well as how the person is perceived by others. Thus, it has the potential to affect the social, emotional and overall quality of life of the patient [31].
The changes in fundamental frequencies and subjective voice parameters in both non-laryngeal and laryngeal primaries receiving radical chemoradiation were assessed by Paleri et al. [29] and Karlssen et al. [30], respectively. The fall in frequencies and the worsening in subjective voice assessment seen in our study were common to those studies as well. The post treatment effects in vocal folds as a result of late effects of radiotherapy could be the prime reason for worsening of roughness observed here as described in the study by Karlssen et al. [33].
The progressive worsening of toxicities at six months post treatment could limit the perceived advantage of function preservation with upfront radical radiation ± chemotherapy. The results of the DARS study have clarified the impact of pharyngeal muscle sparing in improving dysphagia in patients with head and neck cancer [20]. Thus, treatment goals to limit the volume of key structures receiving doses in excess of recommended constraints need to be explored. This may include routine use of Do-IMRT, adaptive radiotherapy and induction chemotherapy with techniques of radiation delivery like SIB with VMAT in laryngopharyngeal tumours, especially in the locally advanced setting [33, 34].
Novelty
This study demonstrated significant continuing worsening of dysphagia from baseline at six months post radical radiation treatment along with progressive worsening of laryngeal edema and subjective and objective voice parameters. The identification of key structures like the oral cavity and the superior constrictors in limiting the occurrence of treatment related dysphagia with validation of the current recommendations for dose constraints have also been done.
Limitations
Registration of MRI with planning CT was not done for OAR delineation. Majority of the patients belonged to the locally advanced stage with large primaries and, hence, the constraints to the OARs could not be met. HPV status of oropharyngeal tumours was not assessed. The limited duration of follow up post treatment was a deterrent in identifying possible improvements in dysphagia with passage of time.
Conclusion
The observed long term worsening of dysphagia, laryngeal edema and voice from baseline at 6 months post treatment could necessitate the use of routine dysphagia optimised-IMRT and/or adaptive radiotherapy for function preservation. The improvements in functional outcomes with addition of induction chemotherapy to radical radiotherapy ± concurrent chemotherapy when compared to upfront chemoradiation, especially in locally advanced laryngo-pharyngeal tumours, also need to be evaluated further.
Acknowledgments
We would like to thank the Medical physicists and Radiation therapy technologists of our department for the valuable inputs for the study. We would also like to thank Mrs. Kalaivani Muthaiyan, Dr Pragna Sagar Rapole, Dr Chaithra M.S and Dr Francis V James for their support and encouragement.
Footnotes
Conflict of interest
None declared.
Funding
None declared.
References
- 1.Dandekar M, D’Cruz A. Organ preservation strategies: Review of literature and their applicability in developing nations. South Asian J Cancer. 2014;3(3):147–150. doi: 10.4103/2278-330X.136764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma A, Mohanti BK, Thakar A, et al. Concomitant chemoradiation versus radical radiotherapy in advanced squamous cell carcinoma of oropharynx and nasopharynx using weekly cisplatin: a phase II randomized trial. Ann Oncol. 2010;21(11):2272–2277. doi: 10.1093/annonc/mdq219. [DOI] [PubMed] [Google Scholar]
- 3.Nutting CM, Morden JP, Harrington KJ, et al. PARSP ORT trial management group. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSP ORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12(2):127–136. doi: 10.1016/S1470-2045(10)70290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franzese C, Fogliata A, Clerici E, et al. Toxicity profile and early clinical outcome for advanced head and neck cancer patients treated with simultaneous integrated boost and volumetric modulated arc therapy. Radiat Oncol. 2015;10:224. doi: 10.1186/s13014-015-0535-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franceschini D, Paiar F, Meattini I, et al. Simultaneous integrated boost-intensity-modulated radiotherapy in head and neck cancer. Laryngoscope. 2013;123(12):E97–103. doi: 10.1002/lary.24257. [DOI] [PubMed] [Google Scholar]
- 6.Chen D, Menon H, Verma V, et al. Results of a Phase 1/2 Trial of Chemoradiotherapy With Simultaneous Integrated Boost of Radiotherapy Dose in Unresectable Locally Advanced Esophageal Cancer. JAMA Oncol. 2019;5(11):1597–1604. doi: 10.1001/jamaoncol.2019.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hutchison AR, Cartmill B, Wall LR, et al. Dysphagia optimized radiotherapy to reduce swallowing dysfunction severity in patients undergoing treatment for head and neck cancer: A systematized scoping review. Head Neck. 2019;41(6):2024–2033. doi: 10.1002/hed.25688. [DOI] [PubMed] [Google Scholar]
- 8.Murphy BA, Gilbert J. Dysphagia in head and neck cancer patients treated with radiation: assessment, sequelae, and rehabilitation. Semin Radiat Oncol. 2009;19(1):35–42. doi: 10.1016/j.semradonc.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Rancati T, Schwarz M, Allen AM, et al. Radiation dose-volume effects in the larynx and pharynx. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S64–S69. doi: 10.1016/j.ijrobp.2009.03.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dirix P, Abbeel S, Vanstraelen B, et al. Dysphagia after chemoradiotherapy for head-and-neck squamous cell carcinoma: dose-effect relationships for the swallowing structures. Int J Radiat Oncol Biol Phys. 2009;75(2):385–392. doi: 10.1016/j.ijrobp.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 11.Barnhart MK, Cartmill B, Ward EC, et al. CT-based delineation of organs at risk in the head and neck region: DAHANCA, EORTC, GORTEC, HKNPCSG, NCIC CTG, NCRI, NRG Oncology and TROG consensus guidelines. Radiother Oncol. 2015;117(1):83–90. doi: 10.1016/j.radonc.2015.07.041. [DOI] [PubMed] [Google Scholar]
- 12.Sun Y, Yu XL, Luo W, et al. Recommendation for a contouring method and atlas of organs at risk in nasopharyngeal carcinoma patients receiving intensity-modulated radiotherapy. Radiother Oncol. 2014;110(3):390–397. doi: 10.1016/j.radonc.2013.10.035. [DOI] [PubMed] [Google Scholar]
- 13.Marks LB, Yorke ED, Jackson A, et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S10–S19. doi: 10.1016/j.ijrobp.2009.07.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mazzola R, Ricchetti F, Fiorentino A, et al. Dose-volume-related dysphagia after constrictor muscles definition in head and neck cancer intensity-modulated radiation treatment. Br J Radiol. 2014;87(1044):20140543. doi: 10.1259/bjr.20140543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31(5):1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 16.Carding PN, Wilson JA, MacKenzie K, et al. Measuring voice outcomes: state of the science review. J Laryngol Otol. 2009;123(8):823–829. doi: 10.1017/S0022215109005398. [DOI] [PubMed] [Google Scholar]
- 17.Eisbruch A, Kim H, Terrell J, et al. Xerostomia and its predictors following parotid-sparing irradiation of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;50(3):695–704. doi: 10.1016/s0360-3016(01)01512-7. [DOI] [PubMed] [Google Scholar]
- 18.Scorsetti M, Fogliata A, Castiglioni S, et al. Early clinical experience with volumetric modulated arc therapy in head and neck cancer patients. Radiat Oncol. 2010;5:93. doi: 10.1186/1748-717X-5-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisbruch A, Schwartz M, Rasch C, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys. 2004;60(5):1425–1439. doi: 10.1016/j.ijrobp.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 20.Nutting C, Rooney K, Foran B, et al. Results of a randomized phase III study of dysphagia-optimized intensity modulated radiotherapy (Do-IMRT) versus standard IMRT (S-IMRT) in head and neck cancer. J Clin Oncol. 2020;38(15 Suppl):6508–6508. doi: 10.1200/jco.2020.38.15_suppl.6508. [DOI] [Google Scholar]
- 21.Roe JWG, Drinnan MJ, Carding PN, et al. Patient-reported outcomes following parotid-sparing intensity-modulated radiotherapy for head and neck cancer. How important is dysphagia? Oral Oncol. 2014;50(12):1182–1187. doi: 10.1016/j.oraloncology.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 22.Wilson JA, Carding PN, Patterson JM. Dysphagia after nonsurgical head and neck cancer treatment: patients’ perspectives. Otolaryngol Head Neck Surg. 2011;145(5):767–771. doi: 10.1177/0194599811414506. [DOI] [PubMed] [Google Scholar]
- 23.Caglar HB, Tishler RB, Othus M, et al. Dose to larynx predicts for swallowing complications after intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72(4):1110–1118. doi: 10.1016/j.ijrobp.2008.02.048. [DOI] [PubMed] [Google Scholar]
- 24.Cacicedo J, Dal Pra A, Alongi F, et al. Impact of weight loss in patients with head and neck carcinoma undergoing radiotherapy: is it an underestimated phenomenon? A radiation oncologist’s perspective. Eur J Clin Nutr. 2015;69(7):757–760. doi: 10.1038/ejcn.2015.65. [DOI] [PubMed] [Google Scholar]
- 25.Charters EK, Bogaardt H, Freeman-Sanderson AL, et al. Systematic review and meta-analysis of the impact of dosimetry to dysphagia and aspiration related structures. Head Neck. 2019;41(6):1984–1998. doi: 10.1002/hed.25631. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz DL, Hutcheson K, Barringer D, et al. Candidate dosimetric predictors of long-term swallowing dysfunction after oropharyngeal intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78(5):1356–1365. doi: 10.1016/j.ijrobp.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vainshtein J, Eisbruch A. Function, muscles, and sparing by IMRT for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2013;85(3):577–578. doi: 10.1016/j.ijrobp.2012.08.040. [DOI] [PubMed] [Google Scholar]
- 28.Sanguineti G, Adapala P, Endres EJ, et al. Dosimetric predictors of laryngeal edema. Int J Radiat Oncol Biol Phys. 2007;68(3):741–749. doi: 10.1016/j.ijrobp.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 29.Meleca RJ, Dworkin JP, Kewson DT, et al. Functional outcomes following nonsurgical treatment for advanced-stage laryngeal carcinoma. Laryngoscope. 2003;113(4):720–728. doi: 10.1097/00005537-200304000-00025. [DOI] [PubMed] [Google Scholar]
- 30.Hoyt DJ, Lettinga JW, Leopold KA, et al. The effect of head and neck radiation therapy on voice quality. Laryngoscope. 1992;102(5):477–480. doi: 10.1288/00005537-199205000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Bibby JRL, Cotton SM, Perry A, et al. Voice outcomes after radiotherapy treatment for early glottic cancer: assessment using multidimensional tools. Head Neck. 2008;30(5):600–610. doi: 10.1002/hed.20750. [DOI] [PubMed] [Google Scholar]
- 32.Paleri V, Carding P, Chatterjee S, et al. Voice outcomes after concurrent chemoradiotherapy for advanced nonlaryngeal head and neck cancer: a prospective study. Head Neck. 2012;34(12):1747–1752. doi: 10.1002/hed.22003. [DOI] [PubMed] [Google Scholar]
- 33.Karlsson T, Bergström L, Ward E, et al. A prospective longitudinal study of voice characteristics and health-related quality of life outcomes following laryngeal cancer treatment with radiotherapy. Acta Oncol. 2016;55(6):693–699. doi: 10.3109/0284186X.2016.1150604. [DOI] [PubMed] [Google Scholar]
- 34.Ferrari D, Ghi MG, Franzese C, et al. The Slippery Role of Induction Chemotherapy in Head and Neck Cancer: Myth and Reality. Front Oncol. 2020;10:7. doi: 10.3389/fonc.2020.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petkar I, Rooney K, Roe JWG, et al. DARS : a phase III randomised multicentre study of dysphagia- optimised intensity- modulated radiotherapy (Do-IMRT) versus standard intensity- modulated radiotherapy (S-IMRT) in head and neck cancer. BMC Cancer. 2016;16(1):770. doi: 10.1186/s12885-016-2813-0. [DOI] [PMC free article] [PubMed] [Google Scholar]