Abstract
Objective
On evaluating the guidelines from previous studies, we found no randomized controlled trials on the use of beta-blockers for heart failure (HF) that employed as evidence for heart rate targets of 60 or 70 beats/min. In this study, we aimed to assess the target heart rate in patients with HF treated with beta-blockers.
Methods
We used the keywords, “heart failure” and “beta-blocker” to search PubMed, Ovid, EMBASE, and Cochrane from 1966 to June 2021. Two authors independently reviewed the results of the search strategy and selected all the studies that reported the effect of beta-blockers on all-cause mortality in patients with HFrEF. We conducted analyses using Review Manager, version 5.0 and Stata version 12.0. Risk of bias was assessed regarding randomization, allocation sequence concealment, blinding, incomplete outcome data, and other biases. Sensitivity analysis was carried out to compare the results of fixed effect model with the results of random effect.
Results
No clinical trial supported the optimal heart rate of 60 beats/min. Risk ratio (RR) and 95% confidence interval (CI) were 0.77 (0.71, 0.83) and 0.86 (0.76, 0.97) in the subgroup with a baseline heart rate >80 beats/min and subgroup with baseline of ≤80 beats/min, respectively. RR and 95% CI were 0.92 (0.82, 1.02) and 0.77 (0.65, 0.92) in 2 subgroups with heart rate controlled ≥70 beats/min and 60–70 beats/min, respectively. Accumulated to MOCHA 1 trial (heart rate controlled 70 beats/min), there was no significant difference in mortality between the experimental group and the control group (RR=0.91, 95% CI 0.82–1.02). Accumulated to SENIORS trial (heart rate controlled 68.8 beats/min), there was a difference in mortality between the experimental and the control groups (RR=0.90, 95% CI 0.82–0.99).
Conclusion
The main effect of beta-blockers in the treatment of HF is achieved by lowering heart rate. The use of beta-blockers did not benefit in people with HFrEF whose heart rate was 77 beats/min before they started the treatment regimen. In patients with HFrEF, the purpose of beta-blockers is to control the heart rate to 65–70 beats min.
Keywords: beta-blocker, heart rate, heart failure, death, ejection fraction, meta-analysis
Introduction
Beta-blockers are the cornerstone of treatment for heart failure with reduced ejection fraction (HFrEF) (1, 2). Current guidelines (3–5) recommend that patients with stable, symptomatic HF [New York Heart Association (NYHA) class II–IV] should start using beta blockade as early as possible and eventually continue to use it at the maximum tolerable dose. However, there are no specific targets for the use of beta blockers.
Heart rate is an independent risk factor for HF (6). An observational study involving 112,680 people showed that people in the general population with heart rate controlled at approximately 65 beats/min have the lowest total mortality rates and cardiovascular mortality rates (7). An observational study of 145,211 patients with HF reported a J-shaped relationship between hospital mortality and heart rate. They found that the mortality rate was the lowest among those with a heart rate of 70–75 beats/min (8). Both the American College of Cardiology/American Heart Association (3, 9) and European Society of Cardiology (5) guidelines recommend that patients with a heart rate higher than 70 beats/min after beta-blocker use should consider using ivabradine. This suggests that the heart rate should be controlled at about 70 beats/min with beta blockers. However, so far, no randomized controlled trials of beta blockers for HF were used as evidence for heart rate targets of 60 or 70 beats/min.
This systematic review of randomized controlled trials of beta-blockers in patients with HFrEF was conducted to assess the target heart rate of patients with HF treated with beta blockers.
Methods
We searched PubMed, Ovid, EMBASE, and Cochrane from 1966 to June 2021. No language restrictions were applied, and only human studies, clinical trials, randomized and controlled trials’ publications were considered. “Heart failure” and “beta-blocker” were used as keywords. In addition, we searched recent meta-analyses or reviews of beta-blocker in heart failure and HF guidelines.
Selection and data abstraction
Two authors independently reviewed the results and selected studies that reported the effect of beta-blockers on all-cause mortality in patients with HFrEF. Studies were excluded if they did not report death at the end of the follow-up, used beta-blockers for one month or less, or enrolled less than 50 patients. Trials were excluded if there was no difference in the heart rate between the two groups at the end of the trial.
Two authors independently extracted all outcome data with subsequent discussion of any discrepancies. The outcomes from each study were extracted in intention-to-treat categories rather than per-protocol categories (that is, all outcomes were analyzed by randomization group to avoid bias from excluding patients who dropped out, were withdrawn, or did not adhere to treatment).
Statistical analysis
A meta-analyses and subgroup analysis were conducted using Review Manager, version 5.0 (The Cochrane Collaboration, Copenhagen, Denmark). We did cumulative analyses using Stata version 12.0. Owing to the relatively common outcome of interest, we calculated risk ratios (RRs) and 95% confidence interval (CI). We assessed and quantified statistical heterogeneity for each outcome of interest using the Cochran Q test and the I2 statistic, respectively. The I2 statistic quantifies the percentage of statistical heterogeneity due to between-study variability. By convention, values ≤25%, 25% to 50%, and ≥50% are considered to have low, moderate, and high amounts of heterogeneity, respectively. If the heterogeneity was high, the statistical method chose the random effect model.
Results
Study selection and evaluation
Among the 8 citations that we identified in our search, 106 were potentially eligible for inclusion. Consequently, 84 were excluded after a detailed review (Fig. 1).
Figure 1.

Study flow diagram
Studies included in the systematic review
Table 1 shows the features from 22 (10–31) randomized trials. Three trials (13, 17, 28) reported outcome data in subgroups (each of these subgroups is reported as a separate row in Table 1). Therefore, a total of 27 trials or subgroups were included in the statistical analysis. The randomization scheme was used in all the experiments, and loss of follow-up and withdrawal were reported. The other experiments were carried out with a double blind design scheme, except Anderson1985, MDC1993, CIBIS1994, BEST2001, CAPRICORN2001, CARMEN2004. According to the Jadad scoring scale, all included trials were high-quality studies.
Table 1.
Characteristics of inclusion trials (Continue)
| Study | Sample size, n beta-blocker vs. control | Mean age, year beta-blocker vs. control | Men, % beta-blocker vs. control | Objects of study | Average follow-up time | Beta-blocker therapy, final dose, mg/day | Baseline heart rate in treatment group, beats/min | Baseline heart rate in control group, beats/min | Baseline systolic pressure in treatment group, mm Hg | Baseline mean LVEF in treatment group | Heart rate in follow-up treatment group | Heart rate in follow-up control group |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anderson, 1985 (10) | 25 vs. 25 | 50±15 vs. 51±13 | 56 vs. 76 | Diagnosis of idiopathic dilated cardiomyopathy made under an approved protocol, LVEF <0.40 | 19 months | Metoprolol, 61 | 85 | 85 | — | 0.29±0.1 | 75±12 | 84±21 |
| MDC, 1993 (11) | 194 vs. 189 | 49±12 vs.49±12 | 75 vs. 70 | Idiopathic dilated cardiomyopathy LVEF <0.40 | 12 months | Metoprolol, 108±51 | 91±18 | 90±17 | 118±17 | 0.22±0.08 | — | — |
| Fisher, 1994 (12) | 25 vs. 25 | 63±8 vs. 63±10 | 100 vs. 92 | Chronic heart failure and coronary artery disease LVEF ≤0.40 | 6 months | Metoprolol, 87 ± 25 | 82±12 | 86±12 | 117±25 | 0.22±0.08 | — | — |
| Bristow I, 1994 (13) low-dose | 38 vs. 34 | 55±2 vs. 52±2 | 68 vs. 59 | Subjects were required to have heart failure symptoms of at least 1 month’s duration, LVEF <0.40, | 12 weeks | Bucindolol, 12.5 | 86±2 | 87±2 | 114±3 | 0.247±0.013 | Decreased 6.0±1.2 | Increased 1.2±2.6 |
| Bristow II, 1994 (13) medium-dose | 32 vs. 34 | 56±2 vs. 52±2 | 56 vs. 59 | 12 weeks | Bucindolol, 50 | 87±2 | 87±2 | 122+4 | 0.241±0.012 | Decreased 5.0±1.4 | Increased 1.2±2.6 | |
| Bristow III, 1994 (13) high-dose | 35 vs. 34 | 56±1 vs. 52±2 | 60 vs. 59 | 12 weeks | Bucindolol 200 | 88±2 | 87±2 | 117±3 | 0.232±0.011 | Decreased 5.0±1.4 | Increased 1.2±2.6 | |
| CIBIS, 1994 (14) | 320 vs. 321 | 60.1±1.2 vs. 59.2±1.1 | 82.5 vs. 83 | Chronic heart failure of various etiologies, LVEF <0.40 | 1.9±0.1 years | Bisoprolol, 3.8±0.2 | 82.8±1.5 | 82.5±1.6 | 127.7±1.7 | 0.250±0.009 | Decreased 15.7±1.7 | Unchanged |
| Olsen, 1995 (15) | 36 vs. 24 | 54±2 vs. 50±3 | 94 vs. 92 | Stable heart failure caused by ischemic or idiopathic dilated cardiomyopathy, LVEF <0.35 | 3 months | Carvedilol, 50 weight <75 kg; 100 weight >75 kg | 87±3 | 83±3 | —— | 0.20 ± 0.0l | 67±3 | 84±3 |
| PRESCISE, 1996 (16) | 133 vs. 145 | 59.3±11.8 vs. 61.2±11.8 | 74 vs. 73 | LVEF ≤0.35 | 6 months | Carvedilol, 28±13 | 85±12 | 83±12 | 117±18 | 0.22±0.07 | Decreased 16.3 | Decreased 1.9 |
| MOCHA I, 1996 (17) low-dose | 83 vs. 84 | 58±11 vs. 60±11 | 74 vs. 76 | Symptomatic heart failure from ischemic or non-ischemic dilated cardiomyopathy, LVEF ≤0.35 | 6 months | Carvedilol 12.5 | 86±15 | 83±16 | 115±19 | 0.23±0.08 | 70±21 | 80±12 |
| MOCHAII, 1996 (17) medium-dose | 89 vs. 84 | 60±13 vs. 60±11 | 76 vs. 76 | Carvedilol, 25 | 80±13 | 83±16 | 113±16 | 0.23±0.08 | 68±12 | 80±12 | ||
| MOCHA III, 1996 (17) high-dose | 89 vs. 84 | 60±13 vs. 60±11 | 78 vs. 76 | Carvedilol, 50 | 84±17 | 83±16 | 117±18 | 0.23±0.08 | 67±13 | 80±12 | ||
| Cohn, 1997 (18) | 70 vs. 35 | 59.7±13.9 vs. 60.6±11.6 | 54 vs. 66 | symptomatic, advanced heart failure, LVEF ≤0.35 | 6 months | Carvedilol, 50 | 85 | 79 | 117 | 0.22±0.08 | 68 | 81 |
| ANZ, 1997 (19) | 207 vs. 208 | 67 | 80 | chronic stable heart failure due to ischemic heart disease, LVEF <0.45 | 6 months | Carvedilol, 47 mg | —— | —— | —— | —— | —— | —— |
| CIBIS II, 1999 (20) | 1327 vs. 1320 | 61 vs. 61 | 81 vs. 80 | symptomatic heart failure, LVEF ≤0.35 | 1.3 years | Bisoprolol, 5.0–10.0 | 81.0±15.5 | 79.9±14.5 | 129.2±10.2 | 0.275±0.06 | —— | —— |
| RESOLVD, 2000 (21) | 214 vs. 212 | 62±12 vs. 61±11 | 79 vs. 80 | LVEF <0.40 | 17 weeks | Metoprolol CR 156±70 | —— | —— | —— | 0.28±0.11 | —— | —— |
| Strum, 2000 (22) | 51 vs. 49 | 51±11 vs. 52±10 | 86 vs. 90 | LVEF ≤0.25 | 2 years | Atenolol, 125 mg | 89±15 | 91±15 | 115±18 | 0.17±0.05 | —— | —— |
| BEST, 2001 (23) | 1354 vs. 1354 | 60±12.6 vs. 60±12.3 | 77 vs. 79 | NYHA class III or IV heart failure that was due to primary or secondary dilated cardiomyopathy LVEF≤0.35 | 3 years | Bucindolol 50 weight <75 kg 100 weight >75 kg | 82±13.4 | 81±13.1 | 117±18.2 | 0.23±0.074 | Decreased 8.6±13.9 | Decreased 2.1±13.4 |
| CAPRICORN, 2001 (24) | 975 vs. 984 | 63 vs. 63 | 73 vs. 74 | AMI LVEF ≤0.4 | 2.5 years | Carvedilol, 12.5–50 mg | 77.3± 11.4 | 77.2±11.3 | 121.6 | 0.329 | —— | —— |
| PACKER, 2001 (25) | 1156 vs. 1133 | 63.4±11.5 vs. 63.2±11.4 | 79 vs. 80 | severe chronic heart failure LVEF <0.25 | 10.4 months | Carvedilol, 37 mg | 83±13 | 83±12 | 123±19 | 0.199±0.040 | —— | —— |
| MERIT-HF, 2002, (26) | 1806 vs. 1845 | 63.7 vs. 63.6 | 77.6 vs. 77.2 | Symptomatic chronic heart failure, NYHA class II or IV, LVEF ≤0.40 | 2.4 years | Metoprolol, 192/76 | 83 | 83 | 130 | 0.28 | 67 | Decreased 0.28 |
| CHRISTMAS, 2003 (27) | 193 vs. 194 | 62±9 vs. 63±9 | 90 vs. 90 | Stable chronic heart failure owing to coronary artery disease, NYHA class I–III, LVEF <0.40 | 6 months | Carvedilol, 12.5–100 | 77±11 | 78±13 | 127 | 0.30 | 65±13 | 81±13 |
| CARMEN I, 2004 (28) | 191 vs. 190 | 61.9 vs. 62.9 | 77 vs. 84 | Stable mild CHF, LVEF <0.40 | 18 months | Carvedilol, 47.9 | 77±10.5 | 78±10.9 | 129 | —— | 70–75 | 75–80 |
| CARMEN II, 2004 (28) | 191 vs. 190 | 62.1 vs. 62.9 | 81 vs. 84 | Carvedilol, 48.7 | 78±10.9 | 78±10.9 | 131 | —— | 70–75 | 75–80 | ||
| CIBIS III, 2005 (29) | 505 vs. 505 | 72.4±5.8 vs. 72.5±5.7 | 65.9 vs. 70.5 | NYHA class II or III, LVEF ≤0.35 | 1.22 years | Bisoprolol, 5–10 | 78.8±13.8 | 79.5±13.2 | 134.5±17.0 | 0.288±0.048 | 66.7±11.8 | 67.5±12.7 |
| ENECA, 2005 (30) | 134 vs. 126 | 71.97±5.02 vs. 72.19±5.20 | 70.15 vs. 76.98 | NYHA class II–IV, LVEF ≤0.35 | 2 months | Nebivolol, 7.4 | 76.90±10.88 | 75.29±9.96 | 134.64±16.57 | 0.2541±0.0709 | 67.08±9.21 | 75.00±9.62 |
| SENIORS, 2005 (31) | 1067 vs. 1061 | 76.1±4.8 vs. 76.1±4.6 | 61.6 vs. 64.7 | A clinical history of chronic heart failure, LVEF ≤0.35 | 12 months | Nebivolol, 7.7±3.6 | 79.2±13.6 | 78.9±13.7 | 138.6 | 0.36±0.13 | 68.8±12.5 | 77.4±13.5 |
LVEF - left ventricular ejection fraction; NYHA - New York Heart Association
Data synthesis
Only 19 out of 27 trials or subgroups described end-point heart rate. Except for the CARMEN trial, which controlled the heart rate within the range 70–75 beats/min, the remaining reports described specific heart rate at the end of the trial (Fig. 2). The lowest end-point heart rate was reported in the CHRISTMAS trial, which controlled the heart rate at 65 beats/min. No clinical trial controlled the heart rate at 60 beats/min. Heart rate was controlled at 65–70 beats/min in 12 trials, 70–80 beats/min in 5 trials, and above 80 beats/min in 3 trials. Only MERIT-HF and MOCHA III trials showed that beta-blockers reduced mortality in patients with HF in the 19 trials. The heart rate was controlled at 67 beats/min in both trials.
Figure 2.

Endpoint heart rate of included trials
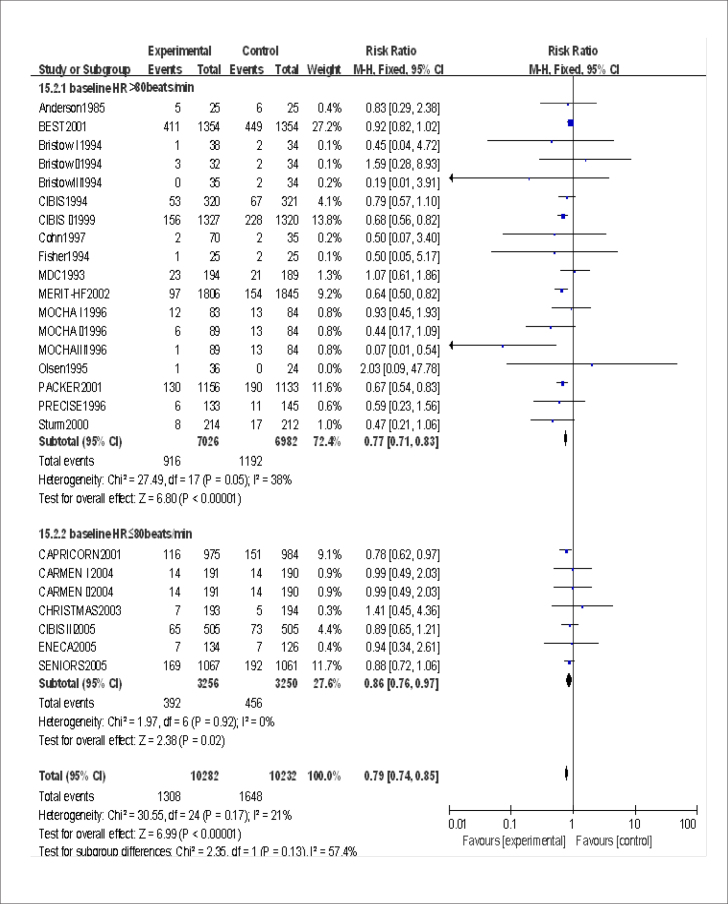
A total 25/27 trials or subgroups provided baseline heart rate. The lowest baseline average heart rate was 76.90 beats/min in 25 clinical trials or subgroups, and the highest was 91 beats/min. Subgroup analysis was based on the baseline heart rate level (Fig. 3). RR and 95% CI were 0.77 (0.71, 0.83) and 0.86 (0.76, 0.97) in the subgroup with baseline heart rate >80 beats/min and subgroup with baseline ≤80 beats/min, respectively. The use of beta-blockers in the treatment of HF in people with a baseline heart rate >80 beats/min and ≤80 beats/min was beneficial. However, the benefits of beta-blockers decreased in people with heart rate lower than 80 beats/min. It is still unclear whether the benefits of beta-blocker therapy for HF are likely to disappear with a further reduction in baseline heart rate. The lowest baseline heart rate (approximately 77 beats/min) was reported in ENCA, CAPRICORN, CHRISTMAS, and CARMEN I. A meta-analysis of the four trials (Fig. 4) showed that RR and 95% CI were 0.82 (0.67, 1.00), indicating no significant difference in the mortality rate between the beta-blockers and control groups. The sensitivity analysis using the random-effect model yielded significantly similar results.
Figure 3.
Assessment of the effect of beta-blockers on mortality by subgroup analysis grouped according to baseline heart rate in the experimental group
Figure 4.
Effect of beta-blockers on mortality in population with a baseline heart rate of 77 beats/min
Furthermore, subgroup analysis was performed according to the heart rate at the end of the trial (Fig. 5). The heterogeneity was low, and the fixed-effect model was used. RR and 95% CI were 0.92 (0.82, 1.02) and 0.77 (0.65, 0.92) in two subgroups with heart rate control ≥70 beats/min and 60–70 beats/min, respectively. These data suggested no significant difference in mortality of patients with HF who used beta-blockers and controlled their heart rate above 70 beats/min compared with placebo therapy. Controlling heart rate at 60–70 beats/min can significantly reduce mortality. We can infer that the benefit of beta-blockers in the treatment of HF mainly occurs through the reduction of heart rate. Beta-blockers are beneficial only when used to reduce heart rate below 70 beats/min. The sensitivity analysis using the random-effect model yielded significantly similar results.
Figure 5.
Assessment of the effect of beta-blockers on mortality by subgroup analysis grouped according to end-stage heart rate in the experimental group
The cumulative meta-analysis was performed according to the end-point heart rate from high to low (Fig. 6). Accumulated to MOCHA I trial, there was no significant difference in mortality between the experimental group and the control group (RR=0.91, 95% CI 0.82–1.02). Accumulated to SENIORS trial (heart rate controlled 68.8 beats/min), there was a difference in mortality between the experimental and the control groups (RR=0.90, 95% CI 0.82–0.99). The end-point heart rate of MOCHA I trial was 70 beats/min and that of SENIORS trial was 68.8 beats/min. The results showed no significant differences in mortality between placebo and beta-blockers in controlling the heart rate to 70 beats/min. The mortality rate was reduced when the heart rate was lowered to 68.8 beats/min by beta-blockers compared with that of the control group. This outcome was consistent with the results of subgroup analysis.
Figure 6.
Cumulative meta-analysis based on end-point heart rate
In 27 trials or subgroups, only CIBIS II, MERIT-HF, and MOCHA III showed that the use of beta-blockers reduced the mortality in patients with HFrEF, whereas there was no significant difference in the mortality between the experimental and the control groups in other 24 trials or subgroups. The heterogeneity of the inclusion test was low, and the fixed effect model was adopted. Our meta-analysis (Fig. 7) showed that beta-blocker therapy reduced the mortality in patients with HFrEF (RR=0.79, 95% CI 0.74–0.84).
Figure 7.
Forest flop for reducing mortality using beta-blockers
Discussion
Heart rate is an independent risk factor for HF (6). The resting heart rate has been identified as a particular modifying risk factor for HFrEF (32). Previous evidence (33, 34) suggests that the higher reduction in the heart rate resulted in a better overall prognosis in patients with HF. Therefore, recent guidelines (3, 5) recommend stricter heart rate control with a target of 60 or 70 beats/min. However, there is no sufficient basis for setting these heart rate targets. Observational studies (7) have shown that for the general population, the total mortality and cardiovascular mortality rates were the lowest in people with heart rate of approximately 65 beats/min. For patients with HFrEF, the mortality rate was the lowest when the heart rate was between 70 and 75 beats/min (8). All these results suggest that for patients with HF, heart rate is clearly related to mortality. Not the lower the better, but there is a heart rate range to make mortality the lowest. Subgroup analysis according to baseline heart rate showed that there was no significant benefit from beta-blockers in the population with baseline heart rate of 77 beats/min. In addition, the cumulative meta-analysis showed statistical differences until the end-stage heart rate was below 70 beats/min. The RR values gradually decreased along with the decrease of heart rate, but the decrease range became smaller and smaller. Accumulated to CHRISTMAS trial, RR value was higher than before, which may be related to the sample size of the test itself. It may also be that when the heart rate is controlled to 65 beats/min, the heart rate further decreases without more benefit or even the benefit begins to decrease. This needs further trial confirmation.
Beta-blockers reduce morbidity and mortality in patients with HFrEF (1). Nonetheless, it remain unclear whether the key mechanisms underpinning their benefits are protection of adrenergic receptors from heightened sympathetic activity or reduction in heart rate. It is also uncertain whether the efficacy of beta-blocker is related to dose, reduction in heart rate, or the achieved heart rate (35, 36). Whether clinicians should strive to achieve a target heart rate or a target dose of beta-blocker remains unanswered.
A large retrospective clinical (37) study involving 1,669 patients suggested that the use of beta-blockers to achieve the target dose or target heart rate (50–70 beats per minute) had similar benefits and that controlling the heart rate after reaching the target dose was still beneficial. The new premise was that the aim of using beta blockers is not to achieve the maximum tolerable dose, but to control heart rate (38, 39).
The SHIFT (32) trial is the first trial to specifically test the effect of isolated heart-rate reduction on outcomes in a population with HF. Treatment with ivabradine was associated with an average reduction in heart rate of 15 bpm from a baseline value of 80 bpm, which was largely maintained throughout the course of the study. In the SHIFT population, patients with heart rates higher than the median were at increased risk of an event and received greater event-reducing benefit from ivabradine than did those with heart rates lower than the median. This is consistent with our conclusion.
The relationship between dose and efficacy of beta-blockers was not evaluated in this paper. However, our subgroup analysis confirmed that the use of beta blockers did not reduce mortality in patients with baseline heart rate of 77 beats/min. In the SHIFT (32) trial, 3,181 (56%) patients on beta blockers were treated with at least 50% of the target doses, and 1,488 (26%) were at target doses. The results showed that the use of ivabradine on this basis benefited by lowering the heart rate, which suggests that the dose of the Beytagh blocker is not critical. Compared with placebo treatment, there was no significant difference in mortality among those using beta blockers that controlled heart rate over 70 beats/min. Controlling heart rate at 60–70 beats/min can significantly reduce mortality. The cumulative meta-analysis also showed that there was no significant difference in the mortality between placebo and beta-blocker groups when controlling the heart rate above 70 beats/min. The use of beta-blockers lowered the heart rate to 68.8 beats/min and reduced the mortality compared with that in the control groups. Our findings suggest that beta-blockers can reduce mortality in the treatment of HF depending on the specific heart rate.
Study limitations
This was a meta-analysis. Background therapy of the included trials would have changed since these trials were conducted. In addition, the heart rate was not measured in a standardized fashion. Moreover, different patient study groups and different beta-blockers were used in different trials, which is a major reason for heterogeneity. The degree of heterogeneity is also assessed. A certain degree of heterogeneity does not affect the stability of the results.
Our analysis plan specified that only mortality should be analyzed as an outcome. The benefits of beta-blockers may manifest as improved symptoms, shortened hospitalization times and duration, reduced heart-related events, and so forth. These benefits are not analyzable in this paper.
Conclusion
The main benefit of beta-blockers in the treatment of HF is achieved by lowering heart rate. Patients with HFrEF whose heart rate is approximately 70 beats/min have the lowest mortality rate. In addition, the use of beta-blockers did not significantly benefit patients with HFrEF whose heart rate was 77 beats/min before the use of beta-blockers. In patients with HFrEF with a higher heart rate, the administration of beta-blockers to control heart rate to 70 beats/min can significantly reduce mortality. Further reduction of heart rate to 65 beats/min may not increase the benefit.
HIGHLIGHTS.
This meta-analysis assesses target heart rate (HR) in patients with heart failure (HF) with reduced ejection fraction (HFrEF) treated with beta-blockers.
This meta-analysis confirms a clear and specific relationship between HR and beta blockers in the treatment of HF.
The use of beta-blockers did not significantly benefit people with HFrEF whose HR was 77 beats/min before the use of beta-blockers.
Acknowledgments
We would like to thank Hui Li, MD and Haibo Xu, MD, for their valuable input.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Author contributions: Concept – J.Z., W.C.; Design – X.G., W.C.; Supervision – J.Z.; Fundings – None; Materials – Y.Z., H.H., J.Y.; Data collection &/or processing – X.G., Y.Z., H.H.; Analysis &/or interpretation – X.G., Y.Z., H.H.; Literature search – J.Y.; Writing – X.G.; Critical review – J.Z., W.C.
References
- 1.Brophy JM, Joseph L, Rouleau JL. Beta-blockers in congestive heart failure. A Bayesian meta-analysis. Ann Intern Med. 2001;134:550–60. doi: 10.7326/0003-4819-134-7-200104030-00008. [DOI] [PubMed] [Google Scholar]
- 2.Lee HY, Baek SH. Optimal Use of Beta-Blockers for Congestive Heart Failure. Circ J. 2016;80:565–71. doi: 10.1253/circj.CJ-16-0101. [DOI] [PubMed] [Google Scholar]
- 3.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Colvin MM, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70:776–803. doi: 10.1016/j.jacc.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 4.Yancy CW, Januzzi JL, Jr, Allen LA, Butler J, Davis LL, Fonarow GC, et al. 2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: Answers to 10 Pivotal Issues About Heart Failure With Reduced Ejection Fraction: A Report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2018;71:201–30. doi: 10.1016/j.jacc.2017.11.025. [DOI] [PubMed] [Google Scholar]
- 5.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. Authors/Task Force Members; Document Reviewers. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 6.Vazir A, Claggett B, Jhund P, Castagno D, Skali H, Yusuf S, et al. Prognostic importance of temporal changes in resting heart rate in heart failure patients: an analysis of the CHARM program. Eur Heart J. 2015;36:669–75. doi: 10.1093/eurheartj/ehu401. [DOI] [PubMed] [Google Scholar]
- 7.Woodward M, Webster R, Murakami Y, Barzi F, Lam TH, Fang X, et al. from the Asia Pacific Cohort Studies Collaboration. The association between resting heart rate, cardiovascular disease and mortality: evidence from 112,680 men and women in 12 cohorts. Eur J Prev Cardiol. 2014;21:719–26. doi: 10.1177/2047487312452501. [DOI] [PubMed] [Google Scholar]
- 8.Bui AL, Grau-Sepulveda MV, Hernandez AF, Peterson ED, Yancy CW, Bhatt DL, et al. Admission heart rate and in-hospital outcomes in patients hospitalized for heart failure in sinus rhythm and in atrial fibrillation. Am Heart J. 2013;165:567–74. doi: 10.1016/j.ahj.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Writing Committee Members; Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, et al. American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 10.Anderson JL, Lutz JR, Gilbert EM, Sorensen SG, Yanowitz FG, Menlove RL, et al. A randomized trial of low-dose beta-blockade therapy for idiopathic dilated cardiomyopathy. Am J Cardiol. 1985;55:471–5. doi: 10.1016/0002-9149(85)90396-0. [DOI] [PubMed] [Google Scholar]
- 11.Waagstein F, Bristow MR, Swedberg K, Camerini F, Fowler MB, Silver MA, et al. Beneficial effects of metoprolol in idiopathic dilated cardiomyopathy. Metoprolol in Dilated Cardiomyopathy (MDC) Trial Study Group. Lancet. 1993;342:1441–6. doi: 10.1016/0140-6736(93)92930-R. [DOI] [PubMed] [Google Scholar]
- 12.Fisher ML, Gottlieb SS, Plotnick GD, Greenberg NL, Patten RD, Bennett SK, et al. Beneficial effects of metoprolol in heart failure associated with coronary artery disease: a randomized trial. J Am Coll Cardiol. 1994;23:943–50. doi: 10.1016/0735-1097(94)90641-6. [DOI] [PubMed] [Google Scholar]
- 13.Bristow MR, O’Connell JB, Gilbert EM, French WJ, Leatherman G, Kantrowitz NE, et al. Dose-response of chronic beta-blocker treatment in heart failure from either idiopathic dilated or ischemic cardiomyopathy. Bucindolol Investigators. Circulation. 1994;89:1632–42. doi: 10.1161/01.CIR.89.4.1632. [DOI] [PubMed] [Google Scholar]
- 14.A randomized trial of beta-blockade in heart failure. The Cardiac Insufficiency Bisoprolol Study (CIBIS) CIBIS Investigators and Committees. Circulation. 1994;90:1765–73. doi: 10.1161/01.cir.90.4.1765. [DOI] [PubMed] [Google Scholar]
- 15.Olsen SL, Gilbert EM, Renlund DG, Taylor DO, Yanowitz FD, Bristow MR. Carvedilol improves left ventricular function and symptoms in chronic heart failure: a double-blind randomized study. J Am Coll Cardiol. 1995;25:1225–31. doi: 10.1016/0735-1097(95)00012-S. [DOI] [PubMed] [Google Scholar]
- 16.Packer M, Colucci WS, Sackner-Bernstein JD, Liang CS, Goldscher DA, Freeman I, et al. Double-blind, placebo-controlled study of the effects of carvedilol in patients with moderate to severe heart failure. The PRECISE Trial. Prospective Randomized Evaluation of Carvedilol on Symptoms and Exercise. Circulation. 1996;94:2793–9. doi: 10.1161/01.CIR.94.11.2793. [DOI] [PubMed] [Google Scholar]
- 17.Bristow MR, Gilbert EM, Abraham WT, Adams KF, Fowler MB, Hershberger RE, et al. Carvedilol produces dose-related improvements in left ventricular function and survival in subjects with chronic heart failure. MOCHA Investigators. Circulation. 1996;94:2807–16. doi: 10.1161/01.CIR.94.11.2807. [DOI] [PubMed] [Google Scholar]
- 18.Cohn JN, Fowler MB, Bristow MR, Colucci WS, Gilbert EM, Kinhal V, et al. Safety and efficacy of carvedilol in severe heart failure. The U.S. Carvedilol Heart Failure Study Group. J Card Fail. 1997;3:173–9. doi: 10.1016/S1071-9164(97)90013-0. [DOI] [PubMed] [Google Scholar]
- 19.Randomised placebo-controlled trial of carvedilol in patients with congestive heart failure due to ischaemic heart disease. Australia/New Zealand Heart Failure Research Collaborative Group. Lancet. 1997;349:375–80. [PubMed] [Google Scholar]
- 20.The Cardiac Insufficiency Bisoprolol Study II: (CIBIS-II): a randomised trial. Lancet. 1999;353:9–13. doi: 10.1016/S0140-6736(98)11181-9. [DOI] [PubMed] [Google Scholar]
- 21.Effects of metoprolol CR in patients with ischemic and dilated cardiomyopathy: the randomized evaluation of strategies for left ventricular dysfunction pilot study. Circulation. 2000;101:378–84. doi: 10.1161/01.CIR.101.4.378. [DOI] [PubMed] [Google Scholar]
- 22.Sturm B, Pacher R, Strametz-Juranek J, Berger R, Frey B, Stanek B. Effect of beta 1 blockade with atenolol on progression of heart failure in patients pretreated with high-dose enalapril. Eur J Heart Fail. 2000;2:407–12. doi: 10.1016/S1388-9842(00)00120-3. [DOI] [PubMed] [Google Scholar]
- 23.Beta-Blocker Evaluation of Survival Trial Investigators. Eichhorn EJ, Domanski MJ, Krause-Steinrauf H, Bristow MR, Lavori PW. A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med. 2001;344:1659–67. doi: 10.1056/NEJM200105313442202. [DOI] [PubMed] [Google Scholar]
- 24.Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet. 2001;357:1385–90. doi: 10.1016/S0140-6736(00)04560-8. [DOI] [PubMed] [Google Scholar]
- 25.Packer M, Coats AJ, Fowler MB, Katus HA, Krum H, Mohacsi P, et al. Carvedilol Prospective Randomized Cumulative Survival Study Group. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344:1651–8. doi: 10.1056/NEJM200105313442201. [DOI] [PubMed] [Google Scholar]
- 26.Wikstrand J, Hjalmarson A, Waagstein F, Fagerberg B, Goldstein S, Kjekshus J, et al. MERIT-HF Study Group. Dose of metoprolol CR/XL and clinical outcomes in patients with heart failure: analysis of the experience in metoprolol CR/XL randomized intervention trial in chronic heart failure (MERIT-HF) J Am Coll Cardiol. 2002;40:491–8. doi: 10.1016/S0735-1097(02)01970-8. [DOI] [PubMed] [Google Scholar]
- 27.Cleland JG, Pennell DJ, Ray SG, Coats AJ, Macfarlane PW, Murray GD, et al. Carvedilol hibernating reversible ischaemia trial: marker of success investigators. Myocardial viability as a determinant of the ejection fraction response to carvedilol in patients with heart failure (CHRISTMAS trial): randomised controlled trial. Lancet. 2003;362:14–21. doi: 10.1016/S0140-6736(03)13801-9. [DOI] [PubMed] [Google Scholar]
- 28.Komajda M, Lutiger B, Madeira H, Thygesen K, Bobbio M, Hildebrandt P, et al. CARMEN investigators and co-ordinators. Tolerability of carvedilol and ACE-Inhibition in mild heart failure. Results of CARMEN (Carvedilol ACE-Inhibitor Remodelling Mild CHF EvaluatioN) Eur J Heart Fail. 2004;6:467–75. doi: 10.1016/j.ejheart.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 29.Willenheimer R, van Veldhuisen DJ, Silke B, Erdmann E, Follath F, Krum H, et al. CIBIS III Investigators. Effect on survival and hospitalization of initiating treatment for chronic heart failure with bisoprolol followed by enalapril, as compared with the opposite sequence: results of the randomized Cardiac Insufficiency Bisoprolol Study (CIBIS) III. Circulation. 2005;112:2426–35. doi: 10.1161/CIRCULATIONAHA.105.582320. [DOI] [PubMed] [Google Scholar]
- 30.Edes I, Gasior Z, Wita K. Effects of nebivolol on left ventricular function in elderly patients with chronic heart failure: results of the ENECA study. Eur J Heart Fail. 2005;7:631–9. doi: 10.1016/j.ejheart.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 31.Flather MD, Shibata MC, Coats AJ, Van Veldhuisen DJ, Parkhomenko A, Borbola J, et al. SENIORS Investigators. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS) Eur Heart J. 2005;26:215–25. doi: 10.1093/eurheartj/ehi115. [DOI] [PubMed] [Google Scholar]
- 32.Swedberg K, Komajda M, Böhm M, Borer JS, Ford I, Dubost-Brama A, et al. SHIFT Investigators. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875–85. doi: 10.1016/S0140-6736(10)61198-1. [DOI] [PubMed] [Google Scholar]
- 33.Huang RL, Listerman J, Goring J, Giesberg C, Nading MA, Butler J. Beta-blocker therapy for heart failure: should the therapeutic target be dose or heart rate reduction? Congest Heart Fail. 2006;12:206–10. doi: 10.1111/j.1527-5299.2006.05477.x. [DOI] [PubMed] [Google Scholar]
- 34.McAlister FA, Wiebe N, Ezekowitz JA, Leung AA, Armstrong PW. Meta-analysis: beta-blocker dose, heart rate reduction, and death in patients with heart failure. Ann Intern Med. 2009;150:784–94. doi: 10.7326/0003-4819-150-11-200906020-00006. [DOI] [PubMed] [Google Scholar]
- 35.Böhm M, Perez AC, Jhund PS, Reil JC, Komajda M, Zile MR, et al. I-Preserve Committees and Investigators. Relationship between heart rate and mortality and morbidity in the irbesartan patients with heart failure and preserved systolic function trial (I-Preserve) Eur J Heart Fail. 2014;16:778–87. doi: 10.1002/ejhf.85. [DOI] [PubMed] [Google Scholar]
- 36.Kato N, Kinugawa K, Teruhiko I, Hironori M, Hisataka M, Toshiro I, et al. Differential impacts of achieved heart rate and achieved dose of β-blocker on clinical outcomes in heart failure with and without atrial fibrillation. Int J Cardiol. 2014;173:331–3. doi: 10.1016/j.ijcard.2014.03.050. [DOI] [PubMed] [Google Scholar]
- 37.Corletto A, Fröhlich H, Täger T, Hochadel M, Zahn R, Kilkowski C, et al. Beta blockers and chronic heart failure patients: prognostic impact of a dose targeted beta blocker therapy vs. heart rate targeted strategy. Clin Res Cardiol. 2018;107:1040–9. doi: 10.1007/s00392-018-1277-4. [DOI] [PubMed] [Google Scholar]
- 38.Gelbrich G, Edelmann F, Inkrot S, Lainscak M, Apostolovic S, Neskovic AN, et al. CIBIS-ELD investigators. Is target dose the treatment target? Uptitrating beta-blockers for heart failure in the elderly. Int J Cardiol. 2012;155:160–6. doi: 10.1016/j.ijcard.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 39.Flannery G, Gehrig-Mills R, Billah B, Krum H. Analysis of randomized controlled trials on the effect of magnitude of heart rate reduction on clinical outcomes in patients with systolic chronic heart failure receiving beta-blockers. Am J Cardiol. 2008;101:865–9. doi: 10.1016/j.amjcard.2007.11.023. [DOI] [PubMed] [Google Scholar]