Abstract
Maternal colonization with group B Streptococcus (GBS) is a risk factor for neonatal GBS disease. Whereas serotypes Ia, Ib, II, III, and V are prevalent in the United States, types VI and VIII predominate in Japan. Recently, a serotype VIII strain was detected among 114 clinical GBS isolates from a Boston, Mass., hospital.
Group B Streptococcus (GBS) is a prominent pathogen responsible for sepsis, pneumonia, and meningitis among newborns; urinary tract infection, endometritis, and chorioamnionitis among peripartum women; and primary bacteremia among the elderly (12). Although GBS is a component of the normal human gastrointestinal flora, vaginal and/or rectal colonization with GBS during pregnancy is a risk factor for neonatal GBS disease. Of the nine currently identified GBS serotypes, five (Ia, Ib, II, III, and V) are prevalent in the United States, with type V as the most recently emergent serotype (2, 11). GBS types VI and VIII predominate among pregnant Japanese women (4), and only a single isolate of type VIII has thus far been reported in the United States (2). GBS types IV and VII are encountered only rarely (2, 3). Distribution studies reveal population shifts of known GBS serotypes and the emergence of new types; these changes have implications for the formulation of a multivalent vaccine. In this study, we examined the present distribution of GBS serotypes in Boston, Mass., where the first serotype distribution study was performed over 35 years ago (1).
One hundred nine clinical samples obtained between January and March 1999 from the Brigham and Women's Hospital Clinical Microbiology Laboratory were identified as belonging to Lancefield's group B by a latex agglutination test (Streptex; Murex Diagnostics, Inc., Norcross, Ga.). GBS isolates were individually scored on blood agar plates and incubated overnight at 37°C. Included on each plate as positive controls were GBS strains of type Ia (O90), type Ib (H36B), type II (18RS21), type III (M781), and type V (CJB111). Each isolate was sequentially transferred from the blood agar plate onto five nitrocellulose membranes (Millipore Corporation, Bedford, Mass.) by gently placing the membranes over the colonies for 5 to 10 s. The membranes were then placed (sample side up) into an empty petri plate, and the samples were heat fixed by incubation at 60°C for 1 h. Membranes were blocked with 1% skim milk and rinsed thrice with 40 mM phosphate buffer containing 0.05% Tween 20 (PB/T). Rabbit serum specific to each GBS capsular polysaccharide (CPS)-tetanus toxoid conjugate vaccine, i.e., serotype Ia, Ib, II, III, V, VI, and VIII conjugates (8–10, 13, 14), served as the primary antibody and was used at a dilution at which cross-reactions with heterologous serotypes were minimal to none. The membranes were incubated with the primary antibody for 1 h at room temperature with gentle agitation and then washed thrice with PB/T. Goat anti-rabbit immunoglobulin G-alkaline phosphatase conjugate (Southern Biotechnology Associates, Birmingham, Ala.), diluted 1:3,000, was used as the secondary antibody; after its addition, the membranes were incubated for 1 h with gentle agitation at room temperature. Membranes were rinsed thrice with PB/T and developed by the addition of 5-bromo-4-chloro-3-indolylphosphate toluidinium–nitroblue tetrazolium phosphatase substrate (Kirkegaard and Perry Laboratories, Gaithersburg, Md.). The reaction was stopped by rinsing the membranes with water.
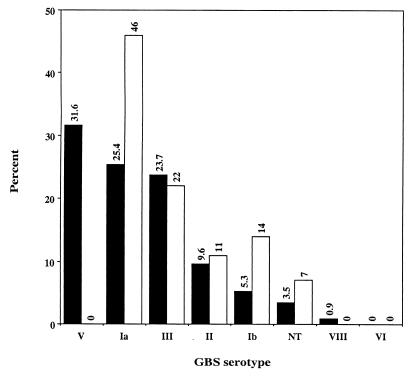
Ninety-four percent (103 of 109) of the GBS samples were cultured from females; and two isolates were obtained from newborns. Most (90.7%) of the isolates originated from the genitourinary tract of women. A total of 114 GBS isolates were obtained from the 109 samples, with dual serotypes in five samples (4.4%). GBS types Ia, III, and V accounted for 80% of the isolates (Fig. 1), and types Ib and II accounted for 15%. Surprisingly, one isolate was identified as type VIII—a serotype rare outside Japan (2). Only four isolates (3.5%) were nontypeable.
FIG. 1.
Comparison of group B streptococcal serotype distribution in Boston among 114 clinical isolates obtained in 1999 (black bars) and among 149 isolates obtained in 1962 to 1963 (white bars) (1).
Samples that reacted strongly with antibody to more than one serotype were again plated, and 10 to 15 individual colonies were grown on a blood agar plate, transferred to nitrocellulose membranes, and probed with specific antiserum to the serotypes present in the first screen. An interesting finding was that type V was isolated from each dual-serotype sample (Ia-V, n = 1; Ib-V, n = 2; III-V, n = 2). Moreover, type V was found in three of the four samples from males (type III was isolated from the fourth sample). Of the two isolates from babies, one was type Ia and the other was type III.
In the past, serotyping of GBS has required a tedious process of antigen extraction and precipitation reactions with adsorbed whole-cell antisera (5). Adsorption of whole-cell antisera was necessary to remove cross-reactivity with other GBS surface components, such as the group B carbohydrate and protein antigens. The availability of high-titered CPS-specific antisera to conjugate vaccines has resulted in a more direct and rapid method of serotyping while eliminating the need for extensive adsorptions to remove antibody to non-CPS surface antigens; the minor CPS cross-reactivity in some of the high-titered antisera has been reduced by adsorption with the interfering GBS serotype. High-titered CPS-specific sera also have allowed the detection of more than one serotype in a sample. In this study, each of five samples contained two serotypes, and type V was involved in each of these cases.
A study by Eickhoff and colleagues in Boston in 1964 revealed that collectively types Ia and III accounted for 68% of all cases of GBS infection (1), whereas in our study, serotypes Ia and III together accounted for 49% of the total (Fig. 1). Serotype V had not emerged in the early 1960s but accounted for 32% of the total isolates in our study (Fig. 1). Indeed, the overall distribution of GBS serotypes in our study was similar to those in current, and more extensively performed, epidemiological studies (2, 6). However, of the combined 614 invasive pediatric and adult isolates collected in those studies, only one was serotype VIII. The occurrence of GBS type VIII among our relatively small number of samples may signal future changes in serotype distribution in the United States. A multivalent GBS vaccine for use in the United States would currently include types Ia, Ib, II, III, and V (7); this formulation would provide coverage against 96% of the isolates in this study.
Acknowledgments
We thank Andrew B. Onderdonk and Dennis L. Kasper for advice and critical review of the manuscript and Julie McCoy and Jaylyn Olivo for editorial assistance.
This work was supported by an award from the William F. Milton Fund to L.C.P.
REFERENCES
- 1.Eickhoff T C, Klein J O, Daly A K, Ingall D, Finland M. Neonatal sepsis and other infections due to group B beta-hemolytic streptococci. N Engl J Med. 1964;271:1221–1228. doi: 10.1056/NEJM196412102712401. [DOI] [PubMed] [Google Scholar]
- 2.Harrison L H, Elliott J A, Dwyer D M, Libonati J P, Ferrieri P, Billmann L, Schuchat A. Serotype distribution of invasive group B streptococcal isolates in Maryland: implications for vaccine formulation. J Infect Dis. 1998;177:998–1002. doi: 10.1086/515260. [DOI] [PubMed] [Google Scholar]
- 3.Kvam A I, Efstratiou A, Bevanger L, Cookson B D, Marticorena I F, George R C, Maeland J A. Distribution of serovariants of group B streptococci in isolates from England and Norway. J Med Microbiol. 1995;42:246–250. doi: 10.1099/00222615-42-4-246. [DOI] [PubMed] [Google Scholar]
- 4.Lachenauer C S, Kasper D L, Shimada J, Ichiman Y, Ohtsuka H, Kaku M, Paoletti L C, Ferrieri P, Madoff L C. Serotypes VI and VIII predominate among group B streptococci isolated from pregnant Japanese women. J Infect Dis. 1999;179:1030–1033. doi: 10.1086/314666. [DOI] [PubMed] [Google Scholar]
- 5.Lancefield R C. A serological differentiation of specific types of bovine hemolytic streptococci (group B) J Exp Med. 1934;59:441–458. doi: 10.1084/jem.59.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin F Y, Clemens J D, Azimi P H, Regan J A, Weisman L E, Philips J B, 3rd, Rhoads G G, Clark P, Brenner R A, Ferrieri P. Capsular polysaccharide types of group B streptococcal isolates from neonates with early-onset systemic infection. J Infect Dis. 1998;177:790–792. doi: 10.1086/517810. [DOI] [PubMed] [Google Scholar]
- 7.Paoletti L C, Baker C J, Kasper D L. The First Annual Conference on Vaccine Research, Washington, D.C. 1998. Neonatal group B streptococcal disease: progress towards a multivalent maternal vaccine, abstr. P16; p. 43. [Google Scholar]
- 8.Paoletti L C, Pinel J, Johnson K D, Reinap B, Ross R A, Kasper D L. Synthesis and preclinical evaluation of glycoconjugate vaccines against group B Streptococcus types VI and VIII. J Infect Dis. 1999;180:892–895. doi: 10.1086/314955. [DOI] [PubMed] [Google Scholar]
- 9.Paoletti L C, Wessels M R, Michon F, DiFabio J, Jennings H J, Kasper D L. Group B Streptococcus type II polysaccharide-tetanus toxoid conjugate vaccine. Infect Immun. 1992;60:4009–4014. doi: 10.1128/iai.60.10.4009-4014.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paoletti L C, Wessels M R, Rodewald A K, Shroff A A, Jennings H J, Kasper D L. Neonatal mouse protection against infection with multiple group B streptococcal (GBS) serotypes by maternal immunization with a tetravalent GBS polysaccharide-tetanus toxoid conjugate vaccine. Infect Immun. 1994;62:3236–3243. doi: 10.1128/iai.62.8.3236-3243.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rench M A, Baker C J. Neonatal sepsis caused by a new group B streptococcal serotype. J Pediatr. 1993;122:638–640. doi: 10.1016/s0022-3476(05)83554-1. [DOI] [PubMed] [Google Scholar]
- 12.Schuchat A. Epidemiology of group B streptococcal disease in the United States: shifting paradigms. Clin Microbiol Rev. 1998;11:497–513. doi: 10.1128/cmr.11.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wessels M R, Paoletti L C, Kasper D L, DiFabio J L, Michon F, Holme K, Jennings H J. Immunogenicity in animals of a polysaccharide-protein conjugate vaccine against type III group B Streptococcus. J Clin Investig. 1990;86:1428–1433. doi: 10.1172/JCI114858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wessels M R, Paoletti L C, Rodewald A K, Michon F, DiFabio J, Jennings H J, Kasper D L. Stimulation of protective antibodies against type Ia and Ib group B streptococci by a type Ia polysaccharide-tetanus toxoid conjugate vaccine. Infect Immun. 1993;61:4760–4766. doi: 10.1128/iai.61.11.4760-4766.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]