Supplemental Digital Content is available in the text.
Keywords: coronavirus disease 2019, intensive care unit, mechanical ventilation, severe acute respiratory syndrome coronavirus 2
IMPORTANCE:
Factors associated with mortality in coronavirus disease 2019 patients on invasive mechanical ventilation are still not fully elucidated.
OBJECTIVES:
To identify patient-level parameters, readily available at the bedside, associated with the risk of in-hospital mortality within 28 days from commencement of invasive mechanical ventilation or coronavirus disease 2019.
DESIGN, SETTING, AND PARTICIPANTS:
Prospective observational cohort study by the global Coronavirus Disease 2019 Critical Care Consortium. Patients with laboratory-confirmed coronavirus disease 2019 requiring invasive mechanical ventilation from February 2, 2020, to May 15, 2021.
MAIN OUTCOMES AND MEASURES:
Patient characteristics and clinical data were assessed upon ICU admission, the commencement of invasive mechanical ventilation and for 28 days thereafter. We primarily aimed to identify time-independent and time-dependent risk factors for 28-day invasive mechanical ventilation mortality.
RESULTS:
One-thousand five-hundred eighty-seven patients were included in the survival analysis; 588 patients died in hospital within 28 days of commencing invasive mechanical ventilation (37%). Cox-regression analysis identified associations between the hazard of 28-day invasive mechanical ventilation mortality with age (hazard ratio, 1.26 per 10-yr increase in age; 95% CI, 1.16–1.37; p < 0.001), positive end-expiratory pressure upon commencement of invasive mechanical ventilation (hazard ratio, 0.81 per 5 cm H2O increase; 95% CI, 0.67–0.97; p = 0.02). Time-dependent parameters associated with 28-day invasive mechanical ventilation mortality were serum creatinine (hazard ratio, 1.28 per doubling; 95% CI, 1.15–1.41; p < 0.001), lactate (hazard ratio, 1.22 per doubling; 95% CI, 1.11–1.34; p < 0.001), Paco2 (hazard ratio, 1.63 per doubling; 95% CI, 1.19–2.25; p < 0.001), pH (hazard ratio, 0.89 per 0.1 increase; 95% CI, 0.8–14; p = 0.041), Pao2/Fio2 (hazard ratio, 0.58 per doubling; 95% CI, 0.52–0.66; p < 0.001), and mean arterial pressure (hazard ratio, 0.92 per 10 mm Hg increase; 95% CI, 0.88–0.97; p = 0.003).
CONCLUSIONS AND RELEVANCE:
This international study suggests that in patients with coronavirus disease 2019 on invasive mechanical ventilation, older age and clinically relevant variables monitored at baseline or sequentially during the course of invasive mechanical ventilation are associated with 28-day invasive mechanical ventilation mortality hazard. Further investigation is warranted to validate any causative roles these parameters might play in influencing clinical outcomes.
In 2020, outbreaks due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) were reported globally (1). Among patients with coronavirus disease 2019 (COVID-19), a subset developed critical illness, and up to 17% of those severe patients received invasive mechanical ventilation (IMV) (2–5). Reports on critically ill patients have been limited to small cohorts (3, 6), single-center reports (7), mixed populations with and without need of IMV (2, 4, 8, 9), and single-country studies (10). Early studies have revealed substantial variability in mortality rates—ranging from 30% (2) to 80% (2, 4, 8).
Studies (2, 10–12) that have focused on COVID-19 patients on IMV have identified a variety of demographic and clinical characteristics associated with mortality. In COVID-19 patients requiring IMV, routinely measured parameters could be of significant prognostic value. The pattern and value of their clinical trajectory have been investigated in a recent study (9) in a mixed Italian population, but corroboration of these findings in international populations on IMV remains to be elucidated. Ignoring changes in biochemical parameters over time when estimating associations with hospital outcomes is likely to produce biased estimates (13). For clinicians wanting to use model outputs for prognostic purposes, the presence of such biases will have implications for identifying patients at high risk of mortality.
In early January 2020, the COVID-19 Critical Care Consortium (COVID-19–CCC) was founded to provide a global perspective on the management of critically ill COVID-19 patients and resulting outcomes to overcome many of the limitations of single-center and single-nation studies. In this analysis, we present an inclusive characterization of mechanically ventilated patients to identify baseline and longitudinal factors associated with in-hospital mortality assessed over the first 28 days after the commencement of IMV (28-d IMV mortality).
MATERIALS AND METHODS
Study Design and Setting
We analyzed COVID-19–CCC study (14) dataset (Trial registration: ACTRN12620000421932), which is a prospective international, multicenter, observational study in 377 hospitals spanning 53 countries. The study protocol was approved by the Alfred Hospital Ethics Committee, Melbourne, Australia (Project: 62066, Local reference: 108/20). Participating hospitals obtained local ethics committee approval, and a waiver of informed consent was granted in all cases. De-identified patient data were collected and stored via the Research Electronic Data Capture electronic data capture tool, hosted at the University of Oxford, Oxford, United Kingdom; University College Dublin, Dublin, Ireland; and Monash University, Melbourne, Victoria, Australia.
Participants
Patients admitted to a COVID-19–CCC ICUs, from February 2, 2021, to May 15, 2021, with laboratory-confirmed (real-time polymerase chain reaction) diagnosis of SARS-CoV-2 infection and requiring IMV for any cause were enrolled. Patients under the age of 15 years and those admitted to the ICU for reasons not related to an acute SARS-CoV-2 infection were excluded. Given our interest in examining associations between routinely tested parameters while in the ICU, we further identified the subset of patients with available longitudinal data over the course of ICU admission.
Variables, Data Sources, Measurements, and Definitions
After enrollment, data on demographics, comorbidities, clinical symptoms, and laboratory results were collected by clinical/research staff in all participating ICUs and recorded in an electronic case report form (14, 15). Details of respiratory and hemodynamic support, physiologic variables, and laboratory results were collected daily up to 28 days from commencement of IMV. When multiple results for the same test were available for a single given day, the worst daily value was recorded preferentially. The duration of IMV and ICU stay also were recorded. In this article, analysis of daily data was restricted to the first 28 days following the initiation of IMV. Copies of case report forms detailing all variables can be found with the published study protocol (14).
Primary Outcome
The primary outcome was 28-day IMV mortality. We hypothesized that time-independent factors and temporal trends of continuous parameters, frequently assessed in patients on IMV, could influence the expected risk of 28-day IMV mortality. Given that some patients did not have final disposition at the time of database lock, those who were discharged alive from the hospital within 28 days were censored on the date of hospital discharge; patients transferred within 28 days to another healthcare facility were censored on the date of transfer; patients whose outcome was not finalized on day 28 were censored at the last known date of daily data collection.
Secondary Outcomes
Variable associations with the hazard of being discharged alive from the hospital were modeled to account for the competing risk. We also describe the overall duration of IMV, hospital stay duration, tracheostomy use, and the occurrence of complications on IMV.
Statistical Analysis
Further details about the statistical analysis are reported in the Supplementary Digital Content (http://links.lww.com/CCX/A834). In addition, variable transformations are detailed in Table 1 (Supplementary Digital Content, http://links.lww.com/CCX/A852). Descriptive statistics included patient demographics, comorbidities, admission signs and symptoms, clinical signs at IMV commencement, and ICU management. Continuous variables were summarized as medians with interquartile ranges. Categorical variables were summarized as frequencies with percentages. Data completeness per variable was also reported in all tables.
For the subset of patients with daily (longitudinal) data collected on clinical parameters, we first examined temporal trends over the first 28 days from commencement of IMV. Data were presented visually as unadjusted means and 95% CIs and not clustered per survival or discharge outcome. The resulting outputs allowed us to assess changes in clinical parameters during IMV, to inform the formulation of time-to-event models for estimating the hazards of mortality and discharge.
We performed time-to-event analysis to examine associations between critical variables measured on or before the commencement of IMV (time-independent) and variables assessed over time on the hazards of mortality and discharge (28-d IMV discharge) up to 28 days from commencement of IMV (13). Mortality and discharge were considered as competing events. Associations with each outcome were estimated using cause-specific Cox proportional hazard models. Models included fixed effects for age, sex, body mass index, cardiac arrest before IMV, and comorbidities reported at hospital admission (diabetes, hypertension, chronic cardiac disease, chronic pulmonary disease), selected based on previous evidence approximately (2, 7, 10). For each patient, we included daily observations where all time-dependent variables were observed on the same day. Tidal volume and positive end-expiratory pressure (PEEP), measured upon commencement of IMV, were also included. Unlike other daily parameters, we considered baseline values for tidal volume and PEEP, as these variables are specific to time spent on IMV. Log-2 transformations were applied to serum creatinine, lactate, Paco2, and Pao2:Fio2 to resolve right-skewness in variables prior to inclusion as independent variables in each Cox model; a 1 unit increase in transformed variables therefore corresponded to a doubling in value on the original scale. The remaining variables were mean centered and appropriate scaled to improve interpretation of the estimated effect (Table 1, Supplementary Digital Content, http://links.lww.com/CCX/A852). The baseline hazard function was modeled on the calendar time scale stratified by geographic region (Africa, Asia, Australia/New Zealand, Europe, Latin America and the Caribbean, Northern America) to account for nonproportional effects (16).
Missing data on time-independent covariates, excluding cardiac arrest before IMV, were assumed to be missing at random. Values were imputed with Multiple Imputation using Chained Equations (MICE) (16). MICE is an iterative algorithm that applies a series of linked regression models to impute missing values for each covariate, conditional on values for remaining variables. Models are fitted to multiple independent runs of the MICE algorithm; results across multiple runs are combined to produce a result. For time-dependent variables, follow-up intervals were constructed using all available daily observations per patient in line with a model specification for time-to-event analyses (13). Final model results were pooled following ten independent rounds of MICE and model fitting.
All analyses were conducted using R Version 4.0.1 or higher (The R Foundation for Statistical Computing, Institute for Statistics and Mathematics, Vienna, Austria).
RESULTS
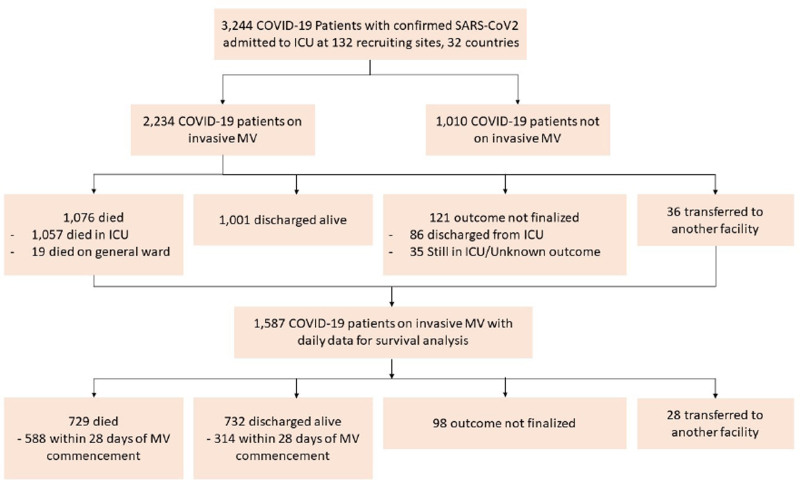
A total of 3,244 COVID-19 patients, enrolled at 132 collaborating sites across 32 countries, were screened for final analysis (Fig. 1). Among those patients, 2,234 (69%) received IMV and were included in the analysis, while 1,010 patients (31%) who never received IMV were excluded.
Figure 1.
Flow of patient enrollment by the censor date of December 29, 2020. COVID-19 = coronavirus disease 2019, MV = mechanical ventilation, SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
Patient Characteristics
In the studied cohort, the median age (IQR) was 59 years (49–68 yr), and patients were predominantly White (41%) and from Northern America (30%) (Table 2, Supplementary Digital Content, http://links.lww.com/CCX/A852). Hypertension, obesity, smoking, and diabetes were the most common comorbidities. The median time from onset of symptom to ICU admission was 8 days (IQR, 5–12 d), as was time from symptom onset to commencement of IMV (median, 8 d; IQR, 5–12 d). IMV was initiated upon ICU admission for 63% of the patients. At the time of IMV commencement (Table 1), median (IQR) creatinine and lactate were 1.0 mg/dL (0.7–1.4 mg/dL) and 1.4 mmol/L (1.0–2.1 mmol/L), respectively. Patients were severely hypoxemic, and their median (IQR) Pao2/Fio2 was 107 mm Hg (74–148 mm Hg), pH was 7.35 (7.28–7.42), and Paco2 was 43.8 mm Hg (36.0–53.2 mm Hg). Patients presented with respiratory system compliance of 33 mL/cm H2O (17–33) and were ventilated using PEEP of 12 cm H2O (2, 9–12). Vasopressors were required in 54% of the patients. Common treatment strategies over the first 28 days included antibiotics (95%), neuromuscular blocking agents (74%), prone positioning (51%), corticosteroids (52%), and antivirals (49%) (Table 2).
TABLE 1.
Clinical Characteristics Upon Commencement of Invasive Mechanical Ventilation
| Characteristic | All Mechanically Ventilated Patients (n = 2,234) | Patients Included in Survival Analysis (n = 1,587) |
|---|---|---|
| Clinical signs and laboratory findings within 24 hr from commencement of IMV | ||
| WBC count, 103/µL: n; median (IQR) | 1,504; 10.7 (7.2–14.9) | 1,167; 10.6 (7.1–14.7) |
| Lymphocyte count, 103/µL: n; median (IQR) | 1,114; 0.8 (0.5–1.2) | 890; 0.8 (0.5–1.1) |
| Neutrophils: lymphocyte ratio: n; median (IQR) | 1,037; 11 (6–19) | 832; 11 (6–19) |
| Temperature, °C: n; median (IQR) | 1,096; 37 (36–38) | 855; 37 (36–38) |
| Creatinine, mg/dL: n; median (IQR) | 1,536; 1.0 (0.7–1.4) | 1,205; 0.9 (0.7–1.4) |
| C-reactive protein level, mg/dL: n; median (IQR) | 967; 86 (16–178) | 819; 90 (18–183) |
| d-dimer, µg/mL: n; median (IQR) | 623; 1.6 (0.8–3.9) | 495; 1.4 (0.7–3.7) |
| Lactate, mmol/L: n; median (IQR) | 1,286; 1.4 (1.0–2.1) | 1,143; 1.4 (1.0–2.1) |
| Ferritin; ng/mL: n; median (IQR) | 492; 2.7 (1.4–4.5) | 398; 2.7 (1.5–5.4) |
| Interleukin-6; ng/L: n; median (IQR) | 167; 95 (32–295) | 156; 103 (33–313) |
| Gas exchange and level of support within 24 hr from commencement of IMV | ||
| pH: n; median (IQR) | 1,593; 7.35 (7.28–7.42) | 1,260; 7.35 (7.28–7.43) |
| Fio2, mm Hg: n; median (IQR) | 1,581; 0.80 (0.60–1.00) | 1,254; 0.80 (0.60–1.00) |
| Pao2/Fio2, mm Hg: n; median (IQR) | 1,460; 107.07 (74.00–158.05) | 1,211; 108.33 (74.85–157.50) |
| Paco2, mm Hg: n; median (IQR) | 1,577; 43.80 (36.00–53.20) | 1,245; 44.20 (36.90–53.60) |
| Static respiratory system compliance, mL/cm H2O: n; median (IQR) | 663; 33 (25–42) | 571; 33 (26–42) |
| Plateau pressure, cm H2O: n; median (IQR) | 875; 25 (21–28) | 755; 25 (21–28) |
| Driving pressure, cm H2O: n; median (IQR) | 872; 12 (10–15) | 753; 12 (10–15) |
| Respiratory rate, breaths/min: n; median (IQR) | 1,364; 22 (19–28) | 1,023; 22 (18–27) |
| Positive end-expiratory pressure level, cm H2O: n; median (IQR) | 1,392; 12 (10–14) | 1,066; 12 (10–14) |
| Minute ventilation, L/min: n; median (IQR) | 1,033; 9 (8–11) | 797; 9 (8–11) |
| Ventilatory ratio: n; median (IQR) | 836; 0.73 (0.59–0.92) | 675; 0.74 (0.60–0.93) |
| Heart rate, beats/min: n; median (IQR) | 1,384; 98 (78–116) | 1,019; 96 (76–114) |
| Mean arterial pressure, mm Hg: n; median (IQR) | 1,679; 75 (64–89) | 1,279; 74 (64–88) |
| Vasopressor/inotropic support, n (%) | 939/1,726 (54) | 732/1,300 (56) |
| Tracheostomy, n (%) | 27/1,745 (2) | 22/1,316 (2) |
IMV = invasive mechanical ventilation, IQR = interquartile range.
Twenty-eight-day ventilator-free day (VFD) was calculated as following: VFDs = 0 if subject dies within 28 d of mechanical ventilation; VFDs = of –x if successfully liberated from ventilation × days after initiation; VFDs = 0 if the subject is mechanically ventilated for > 28 d.
Static respiratory system compliance was calculated as: tidal volume (mL)/(static airway plateau pressure–positive end-expiratory pressure [cm H2O]).
Percentages are calculated for nonmissing data.
TABLE 2.
ICU Clinical Management Within the First 28 Days of ICU Admission
| Characteristic | All Mechanically Ventilated Patients (n = 2,234) | Patients Included in Survival Analysis (n = 1,587) |
|---|---|---|
| Antibiotics, n (%) | 2,098/2,203 (95) | 1,514/1,565 (97) |
| Any antiviral, n (%) | 890/1,822 (49) | 591/1,286 (46) |
| Remdesivir, n (%) | 281/1,205 (23) | 177/867 (20) |
| Corticosteroids, n (%) | 816/1,573 (52) | 596/1,138 (52) |
| Continuous renal replacement therapy, n (%) | 329/2,148 (15) | 231/1,545 (15) |
| Vasoactive drugs, n (%) | 1,263/2,118 (60) | 913/1,527 (60) |
| Cardiac assist devices, n (%) | 111/2,141 (5) | 91/1,532 (6) |
| Extracorporeal membrane oxygenation, n (%) | 499/2,202 (23) | 350/1,577 (22) |
| Prone positioning, n (%) | 1,129/2,204 (51) | 927/1,577 (59) |
| Use of inhaled nitric oxide, n (%) | 251/2,203 (11) | 198/1,576 (13) |
| Use of neuromuscular blockade, n (%) | 1,627/2,198 (74) | 1,275/1,575 (81) |
| Recruitment maneuvers, n (%) | 541/2,042 (26) | 483/1,472 (33) |
| Tracheostomy inserted, n (%) | 366/2,166 (17) | 304/1,576 (19) |
| 28-d ventilator-free day, d: n; median (IQR) | 2,082; 0 (0–13) | 1,471; 0 (0–13) |
| Days from ICU admission to death: n; median (IQR) | 1,076; 13 (5–23) | 729; 14 (7–24) |
| Days from IMV commencement to death: n; median (IQR) | 1,076; 12 (6–23) | 729; 13 (7–24) |
| Duration of ICU stay (died), d: n; median (IQR) | 1,076; 13 (5–23) | 729; 14 (6–23) |
| Duration of ICU stay (discharged), d: n; median (IQR) | 1,001; 20 (12–34) | 732; 20 (13–34) |
| Days from hospital admission to IMV commencement: n; median (IQR) | 2,231; 0 (0–3) | 1,585; 0 (0–3) |
| Days from ICU admission to IMV commencement: n; median (IQR) | 2,234; 0 (0–0) | 1,587; 0 (0–0) |
| Days from first reported symptom to IMV commencement: n; median (IQR) | 2,169; 8 (5–12) | 1,545; 8 (5–12) |
| Commenced IMV on ICU admission, n (%) | 1,413/2,234 (63) | 1,039/1,587 (65) |
IMV = invasive mechanical ventilation, IQR = interquartile range.
Twenty-eight-day ventilator-free day (VFD) was calculated as following: VFDs = 0 if subject dies within 28 d of mechanical ventilation; VFDs = of –x if successfully liberated from ventilation × days after initiation; VFDs = 0 if the subject is mechanically ventilated for > 28 d.
Percentages are calculated for nonmissing data.
In 1,587 patients with complete daily assessment, 588 patients died in hospital within 28 days of commencing IMV (37%), 28 patients (1.8%) were transferred to another hospital. At the study end date, outcomes of 98 patients (6.2%) who were censored at their last known follow-up date were unknown, based on daily data collection. Among patients who died, the median time to death was 14 days (IQR, 6–23 d) from ICU admission. For patients with a reported cause of death (n = 675), respiratory failure was the most common (n = 268; 40%), while other causes included multiple organ failure (n = 233; 35%), septic shock (n = 65; 10%), cardiac failure (n = 37; 5%), cerebrovascular accident (n = 19; 3%), hemorrhagic shock (n = 6; 1%), or other causes (n = 47; 7%).
Dynamics of Daily Clinical Parameters
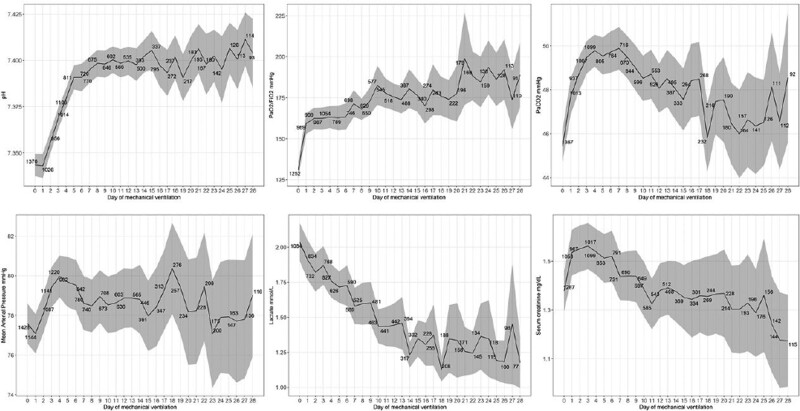
Daily averages for clinical variables, including arterial blood gases, are depicted in Figure 2. There was a clear improvement in the dynamics of Pao2/Fio2 (from 131.1 ± 2.5 mm Hg upon start IMV to 173.9 ± 9.1 mm Hg at 28 d), pH (from 7.34 ± 0.003 to 7.40 ± 0.01), serum creatinine (from 1.38 ± 0.04 to 1.17 ± 0.01 mg/dL), and lactate (from 2.03 ± 0.07 to 1.18 ± 0.09 mmol/L). Differently, trajectories of Paco2 and mean arterial pressure (MAP) were more convoluted, with early worsening during the first days of IMV and delayed improvement. Figure 1 (Supplementary Digital Content, http://links.lww.com/CCX/A852) shows ventilatory modes throughout the study period. Controlled modes were predominantly used during the first 2 weeks of IMV. Stratification of clinical variables and ventilatory settings for patients with known final outcome within the first 28 days of IMV are reported separately in Figures 2 and 3 (Supplementary Digital Content, http://links.lww.com/CCX/A852). There was an apparent discrepancy between survivors and nonsurvivors in the applied Fio2, while tidal volume and PEEP were similar throughout the assessment period.
Figure 2.
Dynamics of time-dependent parameters included in survival analysis. Average daily parameters collected during the first 28 d following commencement of mechanical ventilation. Data are reported as unadjusted means and 95% CIs.
Primary Outcome
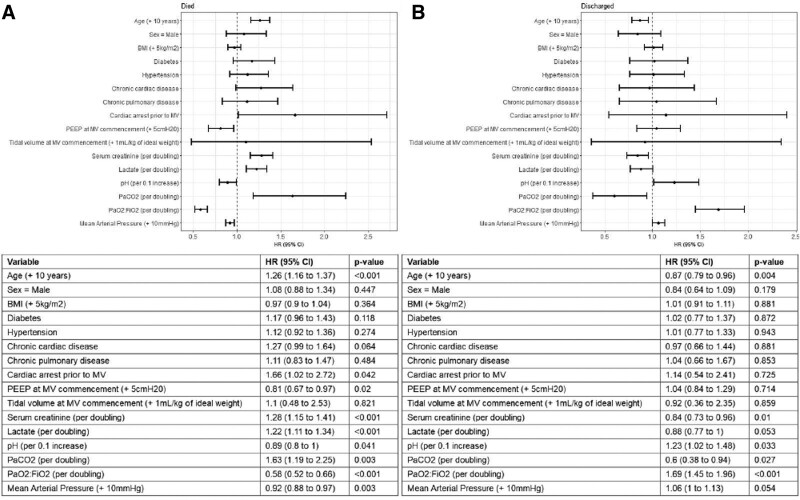
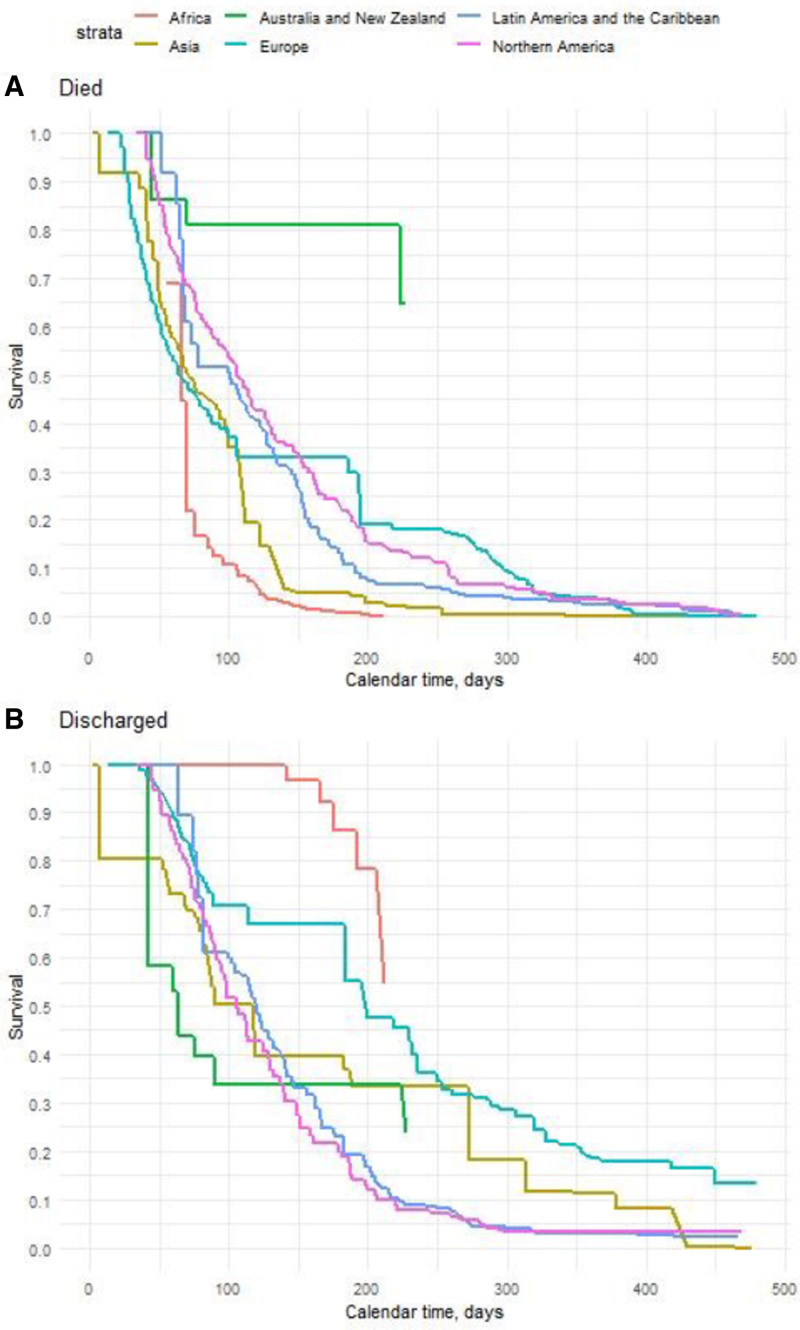
Time-to-event analysis (Fig. 3A) identified age (hazard ratio [HR], 1.26 per 10-yr increase in age; 95% CI, 1.16–1.37; p < 0.001) and PEEP upon commencement of IMV (HR, 0.81 per 5 cm H2O increase; 95% CI, 0.67–0.97; p = 0.02) as statistically significant associations with of the hazard of 28-day IMV mortality. Among time-dependent variables, an increase in serum creatinine (HR, 1.28 per doubling; 95% CI, 1.15–1.41; p < 0.001), lactate (HR, 1.22 per doubling; 95% CI, 1.11–1.34; p < 0.001), and Paco2 (HR, 1.63 per doubling; 95% CI, 1.19–2.25; p = 0.003) increased the hazards of 28-day IMV mortality. Conversely, an increase in pH (HR, 0.89 per 0.1 increase; 95% CI, 0.78–1; p = 0.041), Pao2/Fio2 (HR, 0.58 per doubling; 95% CI, 0.52–0.66; p < 0.001), and MAP (HR, 0.92 per 10 mm Hg increase; 95% CI, 0.88–0.97; p < 0.003) decreased the hazards of 28-day IMV mortality. Figure 4A depicts variability in the stratified baseline survival function for the death, by geographic regions.
Figure 3.
Association of clinical variables with major outcomes. Cause-specific Cox proportional hazards modeling to estimate the hazards of death (A) or discharge (B), up to 28 d following commencement of mechanical ventilation (MV). The model considered time-independent variables: age, sex, body mass index (BMI), diabetes, hypertension, chronic cardiac disease, chronic pulmonary disease, cardiac arrest before invasive MV, highest positive end-expiratory pressure (PEEP) within 24 hr from commencing of MV (mean centered at 10 cm H2O and scaled by 5 cm H2O), and tidal volume upon commencement of MV. The model also included time-dependent covariates: serum creatinine, serum lactate, pH, Paco2, Pao2/Fio2, and mean arterial pressure. Covariate effects are presented as hazard ratios (HRs) and 95% CI.
Figure 4.
Geographical differences of major outcomes. Baseline survival curves by region (Africa, Asia, Australia/New Zealand, Europe, Latin America, and the Caribbean, Northern America) for in-hospital mortality (A) and discharge alive from the hospital (B). Baseline stratification in cause-specific Cox models accounted for nonproportional effects attributable to geographic region. Baseline hazards were modeling on the calendar time scale, with independent right-censoring applied to outcomes censored up to 28 d from commencement of mechanical ventilation.
Secondary Outcomes
Estimated HRs for the variables as mentioned above and the hazard of discharge are reported in Figure 3B. Results indicated that older age (HR, 0.87 per 10-yr increase in age; 95% CI, 0.79–0.96; p = 0.004), increased creatinine (HR, 0.84 per doubling; 95% CI, 0.73–0.96; p = 0.01), and Paco2 (HR, 0.6 per doubling; 95% CI, 0.38–0.94; p = 0.027) decreased the hazards of 28-day IMV discharge, while higher Pao2/Fio2 (HR, 1.69 per doubling; 95% CI, 1.45–1.96; p < 0.001) increased the hazard. Similar to mortality, stratification of the baseline survival function for the hazards of discharge revealed nonproportional effects between geographic regions (Fig. 4B).
Tracheostomy was carried out in 304 of 1,576 patients (19%) included in the survival analysis (Table 2). During hospitalization, the most common complications in the mechanically ventilated population were cardiac arrhythmia (23%), pleural effusion (19%), and cardiac arrest (21%) (Table 3, Supplementary Digital Content, http://links.lww.com/CCX/A852). The median duration of IMV was 14 days (IQR, 7–25 d). Among patients in whom final disposition was available within 28 days of IMV, the median time from ICU to death was 14 days (IQR, 7–22 d). Among patients known to be discharged alive from the hospital, the median duration of ICU admission was 20 days (IQR, 13–34 d).
DISCUSSION
The present international multicenter cohort study from six continents constitutes the most extensive epidemiological investigation of IMV patients with COVID-19. The study enabled delineation of the clinical course during the first 28 days, corroborating age and accounting for longitudinal changes in pH, Pao2/Fio2, MAP, Paco2, lactate, and creatinine when assessing associations with mortality.
Reported mortality rates in mechanical ventilated COVID-19 patients have varied (2, 4, 8, 12, 34). Those findings have been potentially biased by highly variable censor dates to define death, and substantial percentages of patients still requiring ICU care at the chosen censor date (2, 4, 6–8, 17, 24, 35–37). In our study, among 1,587 ventilated patients, we reported an overall 28-day IMV mortality of 37%, similar to rates from the Netherlands (10) and Spain (7) and lower than figures from United Kingdom (18). Given the observational nature of our study, extrapolations on the mortality figures can only be speculative. Nevertheless, our findings should be interpreted in the context of the enrollment period since early dismal survival might have been counterbalanced by lower mortality rates later in the pandemic. In addition, in comparison with previous single-country observational studies (19, 20), corticosteroids were used in fewer patients, due to either differences in practice among geographical regions or inclusion of data acquired early in the pandemic.
In line with previous reports (8, 10, 12, 21, 22) that found older populations at the highest risk of mortality, age shared a positive association with the hazard of 28-day IMV mortality. Of note, we report a slightly younger population than previous investigations (7, 21, 23, 24), possibly because, in 2020, IMV was primarily reserved for younger patients in some of the geographical regions comprising our network. Irrespectively, it is still not fully elucidated why COVID-19 is more lethal in older adults, and several theories that detail potential risks associated with age-related changes to the immune cells, inflammasome activity, epigenome, and characteristic comorbidities have emerged (25). Among the other time-independent factors, only baseline PEEP was found to reduce mortality hazards in our analyses, in contrast with previous evidence associating higher PEEP with mortality (21). These findings emphasize the challenges in setting the optimal PEEP in COVID-19 patients (26), particularly in light of early controversial reports on heterogeneous static respiratory system compliance in infected patients (27). Nevertheless, potential dissimilarities in ventilatory management across different geographical regions should be considered to cautiously infer from these results.
We found that several clinical variables increased the risk of 28-day IMV mortality. To the best of our knowledge, our report is the first that applied Cox proportional hazards modeling of 28-day IMV mortality to assess the impact of time-dependent clinical variables, considering the competing risk of ICU discharge. Indeed, previous investigations (7, 8, 10, 18, 21, 28) focused on risk factors for mortality appraised at a fixed time point, limiting inferences on variables that dynamically change during IMV. Zanella et al (9) specifically focused on temporal trends of clinical parameters and mortality, but in a mixed Italian population of COVID-19 patients, requiring in 21% of the cases noninvasive ventilation. Conversely, our investigation focused on patients on IMV and by incorporating geographic region stratification in modeling, we found substantial differences in survival across the globe, in line with the most recent reports from low-middle-income countries (29). As expected, the severity of hypoxemia during IMV was strongly associated with both mortality and delayed discharge. In this cohort, patients presented a considerable improvement in Pao2/Fio2 during the first 24 hours of IMV, similarly to previous evidence (7, 9), and potentially related to the prompt pronation, neuromuscular blocking agents, and high PEEP after commencement of IMV. However, the data also emphasize potential long-term respiratory dysfunction since moderate hypoxemia persisted throughout the assessment period. The study also identified the association of Paco2 with the hazard of death, which could be related to higher lung disease severity, resulting dead space, and variations in ventilatory strategies. Furthermore, an increase in Paco2 during the initial days of IMV was evident in this cohort, possibly related to the initial hypercatabolic state, inadequate ventilatory management, micro or macrovascular pulmonary thrombosis (30–32), or simply to respiratory fatigue, given that on average, patients were intubated 8 days from symptom onset. Previous studies (8–10, 18, 21) failed to corroborate serum lactate and MAP as risk factors for mortality in COVID-19 patients on IMV. In a large critically ill population from the United Kingdom, lactate within 24 hours from ICU admission was an early predictor of mortality (18). However, the evolution of such parameter in this population was unknown, and only 59% required IMV. Cytokine storm and septic shock in COVID-19 are linked with hemodynamic instability and lactic acidosis, and multiple organ failure in the most severe cases. Thus, our findings imply that trends in hemodynamic impairment could be valuable in risk stratification. However, heterogeneities in local management of vasopressors, which were administered in 56% of our studied population, could have also played a role in mortality risk. In contrast with previous findings (9, 10), temporal change in pH was associated with mortality risk. On average, pH normalization was achieved within 1 week. It is uncertain whether low pH was driven by refractory hypercapnia, metabolic disturbance, or a mixed acid-base disorder. Importantly, interdependence of aforementioned variables should also be considered. Indeed, pH and Paco2 coupling may have been present during refractory hypercapnia, and similarly, pH and lactate correlation could have been the result of sustained acidemia during severe hypotensive states or other metabolic disturbances. Finally, the association of serum creatinine with the hazards of death is important because most studied patients did not have chronic kidney disease before hospitalization. Further investigation of the multifactorial etiology of renal impairment in COVID-19 is urgently needed, as the angiotensin-converting enzyme 2 receptor is critical for SARS-CoV-2 cell entry and widely expressed in the kidneys (33, 38), but IMV and septic shock might have also contributed to kidney injury.
In efforts to inform the field as to characteristics of COVID-19 infection, early publications limited to small-patient-series or single-country experiences have appeared. These articles reported conflicting findings related to center-specific patient populations, resource availability differences, and patient management strategy variations. The current study overcomes some of these limitations by providing a detailed global analysis of demographics and comorbidities associated with mortality and, for the first time, account for the dynamics of a clinically relevant subset of commonly tested variables associated with hazards of 28-day IMV. Further analyses of the COVID-19–CCC dataset are focusing on the impact of treatments on mortality, specifically in subpopulations admitted to ICUs after the early phase of the pandemic. Limitations of the current report include that our model should not be used for prediction at an individual level due to the lack of validation in different and larger cohorts. Admission to ICU, indication for IMV were not standardized across countries and could have depended on local practices. In this cohort, several patients received IMV in 2020; thus, they may not reflect the current scenarios of ICU ventilatory management across the globe—to which subsequent reports can be compared. Further, many of the early pandemic centers were resource limited, which may have adversely impacted the noted outcomes. Approximately 50% of the patients received corticosteroids. Consequently, any extrapolation of our findings to patients receiving corticosteroids must be performed with caution (39, 40). Given that the analyses selectively focused on a subset of variables, other unmeasured factors could have biased our inferences about mortality risks. In addition, the 28-day follow-up could have biased results toward early mortality. Irrespectively, we precisely aimed at identifying key associations affecting mortality during the period of IMV, which in COVID-19 patients is approximately 10 days (10, 34, 41). Last, approximately 20% of the analyzed patients received extracorporeal membrane oxygenation, which could have interfered on the association between arterial blood gas analysis parameters and mortality risk.
CONCLUSIONS
This study represents the most extensive and comprehensive international cohort analyses of patient characteristics associated with mortality in COVID-19 patients requiring IMV. Age and commonly tested parameters in COVID-19 patients on IMV, including pH, blood gases, MAP, serum lactate, and creatinine, were associated with increased mortality hazard. These original findings offer new avenues for research efforts for the early identification of the patients most at risk and in need of altering clinical management strategies.
ACKNOWLEDGMENTS
We recognize the crucial importance of the International Severe Acute Respiratory and Emerging Infection Consortium and Short Period Incidence Study of Severe Acute Respiratory Infection networks in developing and expanding the global Coronavirus Disease 2019 Critical Care Consortium (COVID-19–CCC). We thank the generous support we received from the Extracorporeal Life Support Organization and the International Extracorporeal Membrane Oxygenation Network. We greatly acknowledge Adrian Barnett for his invaluable contribution to the analysis plan and statistical insights. We greatly acknowledge Bailey Schneider (University of Michigan and University of Queensland) for her input and contribution to the critical revision of the article. We owe Li Wenliang, MD from the Wuhan Central Hospital, an eternal debt of gratitude for reminding the world that doctors should never be censored during a pandemic. Finally, we acknowledge all members of the COVID-19–CCC and various collaborators.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Drs. Li Bassi and Suen equally contributed to this work.
Supported, in part, by grant from the University of Queensland, The Wesley Medical Research, The Prince Charles Hospital Foundation, The Health Research Board of Ireland; Biomedicine International Training Research Programme for Excellent Clinician-Scientists; The European Union Research and Innovation Programme (Horizon 2020); and La Caixa Foundation.
Dr. Li Bassi received research support from Fisher & Paykel outside the submitted work. Dr. Dalton consults with Innovative Extracorporeal Membrane Oxygenation Concepts, Abiomed, and Instrumentation Laboratory, which does not affect the current work. Dr. Brodie receives research support from ALung Technologies, and he has been on the medical advisory boards for Baxter, Abiomed, Xenios, and Hemovent. Dr. Fan reports personal fees from ALung Technologies, Baxter, Fresenius Medical Care, Getinge, and MC3 Cardiopulmonary outside the submitted work. Dr. Laffey reports consulting fees from Baxter and Cala Medical, both outside the submitted work. Dr. Nichol is supported by a Health Research Board of Ireland Award (CTN-2014-012). Dr. Fraser receives research support from Fisher & Paykel outside the submitted work. Dr. Grasselli reports personal fees from Draeger Medical, Biotest, Getinge, Fisher & Paykel, and Merck Sharp & Dohme outside the submitted work. Dr. Hodgson is funded by the National Health and Medical Research Council Grant. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Contributor Information
Collaborators: Tala Al-Dabbous, Huda Alfoudri, Mohammed Shamsah, Subbarao Elapavaluru, Ashley Berg, Christina Horn, Stephan Schroll, Jorge Velazco, Ludmyla Ploskanych, Wanda Fikes, Dan Meyer, Ashley Ehlers, Maysoon Shalabi-McGuire, Trent Witt, Lorenzo Grazioli, Luca Lorini, E. Wilson Grandin, Jose Nunez, Tiago Reyes, Diarmuid O’Briain, Stephanie Hunter, Mahesh Ramanan, Julia Affleck, Hemanth Hurkadli Veerendra, Sumeet Rai, Josie Russell-Brown, Mary Nourse, Mark Joseph, Brook Mitchell, Martha Tenzer, Carilion Clinic, Ryuzo Abe, Hwa Jin Cho, In Seok Jeong, Nicolas Brozzi, Omar Mehkri, Sudhir Krishnan, Abhijit Duggal, Stuart Houltham, Jerónimo Graf, Roderigo Diaz, Camila Delgado, Joyce González, Maria Soledad Sanchez, Diego Fernando Bautista Rincón, Angela Maria Marulanda Yanten, Melissa Bustamante Duque, Daniel Brodie, Desy Rusmawatiningtyas, Maria Callahan, M. Azhari Taufik, Elizabeth Yasmin Wardoyo, Margaretha Gunawan, Nurindah S Trisnaningrum, Vera Irawany, Muhammad Rayhan, Mauro Panigada, Antonia Pesenti, Alberto Zanella, Michela Leone, Giacomo Grasselli, Silvia Coppola, Sebastiano Colombo, Massimo Antonelli, Simone Carelli, Domenico L. Grieco, Motohiro Asaki, Kota Hoshino, Leonardo Salazar, Laura Duarte, John Laffey, Bairbre McNicholas, David Cosgrave, Joseph McCaffrey, Allison Bone, Yusuff Hakeem, James Winearls, Mandy Tallott, David Thomson, Christel Arnold-Day, Jerome Cupido, Zainap Fanie, Malcom Miller, Lisa Seymore, Dawid van Straaten, Ali Ait Hssain, Jeffrey Aliudin, Al-Reem Alqahtani, Khoulod Mohamed, Ahmed Mohamed, Darwin Tan, Joy Villanueva, Ahmed Zaqout, Ethan Kurtzman, Arben Ademi, Ana Dobrita, Khadija El Aoudi, Juliet Segura, Gezy Giwangkancana, Shinichiro Ohshimo, Koji Hoshino, Saito Hitoshi, Javier Osatnik, Anne Joosten, Antoni Torres, Minlan Yang, Ana Motos, Carlos Luna, Francisco Arancibia, Virginie Williams, Alexandre Noel, Nestor Luque, Trieu Huynh Trung, Sophie Yacoub, Marina Fantini, Ruth Noemi Jorge García, Enrique Chicote Alvarez, Anna Greti, Adrian Ceccato, Angel Sanchez, Ana Loza Vazquez, Ferran Roche-Campo, Diego Franch-Llasat, Divina Tuazon, Marcelo Amato, Luciana Cassimiro, Flavio Pola, Francis Ribeiro, Guilherme Fonseca, Heidi Dalton, Mehul Desai, Erik Osborn, Hala Deeb, Antonio Arcadipane, Gennaro Martucci, Giovanna Panarello, Chiara Vitiello, Claudia Bianco, Giovanna Occhipinti, Matteo Rossetti, Raffaele Cuffaro, Sung-Min Cho Johns, Hiroaki Shimizu, Naoki Moriyama, Jae-Burm Kim, Nobuya Kitamura, Alyaa Elhazmi, Abdullah Al-Hudaib, Johannes Gebauer, Toshiki Yokoyama, Abdulrahman Al-Fares, Sarah Buabbas, Esam Alamad, Fatma Alawadhi, Kalthoum Alawadi, Hiro Tanaka, Satoru Hashimoto, Masaki Yamazaki, Tak-Hyuck Oh, Mark Epler, Cathleen Forney, Louise Kruse, Jared Feister, Joelle Williamson, Katherine Grobengieser, Eric Gnall, Sasha Golden, Mara Caroline, Timothy Shapiro, Colleen Karaj, Lisa Thome, Lynn Sher, Mark Vanderland, Mary Welch, Sherry McDermott, Matthew Brain, Sarah Mineall, Dai Kimura, Luca Brazzi, Gabriele Sales, Tawnya Ogston, Dave Nagpal, Karlee Fischer, Roberto Lorusso, Mariano Esperatti, Diarmuid O’Briain, Edmund G. Carton, Ayan Sen, Amanda Palacios, Deborah Rainey, Gordan Samoukoviv, Josie Campisi, Emily Neumann, Cassandra Seefeldt, Lucia Durham, Octavio Falcucci, Amanda Emmrich, Jennifer Guy, Carling Johns, Nina Buchtele, Michael Schwameis, Stephanie-Susanne Stecher, Delila Singh, Michaela Barnikel, Lukas Arenz, Akram Zaaqoq, Lan Anh Galloway, Caitlin Merley, Marc Csete, Luisa Quesada, Isabela Saba, Daisuke Kasugai, Hiroaki Hiraiwa, Taku Tanaka, Eva Marwali, Yoel Purnama, Santi Rahayu Dewayanti, Ardiyan, Dafsah Arifa Juzar, Debby Siagian, Kita Jakarta, Yih-Sharng Chen, Mark Ogino, Indrek Ratsep, Getter Oigus, Kristo Erikson, Andra-Maris Post, Lauri Enneveer, Piret Sillaots, Frank Manetta, Effe Mihelis, Iam Claire Sarmiento, Mangala Narasimhan, Michael Varrone, Mamoru Komats, S. Veena Satyapriya, Amar Bhatt, Nahush A. Mokadam, Alberto Uribe, Alicia Gonzalez, Haixia Shi, Johnny McKeown, Joshua Pasek, Juan Fiorda, Marco Echeverria, Rita Moreno, Bishoy Zakhary, Marco Cavana, Alberto Cucino, Giuseppe Foti, Marco Giani, Vincenzo Russotto, Davide Chiumello, Valentina Castagna, Andrea Dell’Amore, Paolo Navalesi, Hoi-Ping Shum, Alain Vuysteke, Asad Usman, Andrew Acker, Benjamin Smood, Blake Mergler, Federico Sertic, Madhu Subramanian, Alexandra Sperry, Nicolas Rizer, Erlina Burhan, Menaldi Rasmin, Ernita Akmal, Faya Sitompul, Navy Lolong, Bhat Naivedh, Simon Erickson, Peter Barrett, David Dean, Julia Daugherty, Antonio Loforte, Irfan Khan, Mohammed Abraar Quraishi, Olivia DeSantis, Dominic So, Darshana Kandamby, Jose M. Mandei, Hans Natanael, Eka YudhaLantang, Anastasia Lantang, Surya Oto Wijaya, Anna Jung, George Ng, Wing Yiu Ng, Pauline Yeung Ng, Alexis Tabah, Megan Ratcliffe, Maree Duroux, Shingo Adachi, Shota Nakao, Pablo Blanco, Ana Prieto, Jesús Sánchez, Meghan Nicholson, Warwick Butt, Alyssa Serratore, Carmel Delzoppo, Pierre Janin, Elizabeth Yarad, Richard Totaro, Jennifer Coles, Bambang Pujo, Robert Balk, Andy Vissing, Esha Kapania, James Hays, Samuel Fox, Garrett Yantosh, Pavel Mishin, Saptadi Yuliarto, Kohar Hari Santoso, Susanthy Djajalaksana, Arie Zainul Fatoni, Masahiro Fukuda, Keibun Liu, Paolo Pelosi, Denise Battaglini, Diego Bastos, Sérgio Gaião, Jessica Buchner, Young-Jae Cho, Su Hwan Lee, Pranya Sakiyalak, Prompak Nitayavardhana, Tamara Seitz, Rakesh Arora, David Kent, Swapnil Parwar, Andrew Cheng, Jennene Miller, Shigeki Fujitani, Naoki Shimizu, Jai Madhok, Clark Owyang, Hergen Buscher, Claire Reynolds, Olavi Maasikas, Aleksan Beljantsev, Vladislav Mihnovits, Takako Akimoto, Mariko Aizawa, Kanako Horibe, Ryota Onodera, Carol Hodgson, Aidan Burrell, Meredith Young, Kiran Shekar, Niki McGuinness, Lacey Irvine, Brigid Flynn, Kazuhiro Sugiyama, Keiki Shimizu, Eddy Fan, Kathleen Exconde, Shingo Ichiba, Leslie Lussier, Gösta Lotz, Lars Maier, Esther Dreier, Neurinda Permata Kusumastuti, Colin McCloskey, Al-Awwab Dabaliz, Tarek B Elshazly, Josiah Smith, Konstanty S. Szuldrzynski, Piotr Bielański, Keith Wille, Srinivas Murthy, Ken Kuljit S. Parhar, Kirsten M. Fiest, Cassidy Codan, Anmol Shahid, Mohamed Fayed, Timothy Evans, Rebekah Garcia, Ashley Gutierrez, Hiroaki Shimizu, Tae Song, Rebecca Rose, Suzanne Bennett, Denise Richardson, Lovkesh Arora, Kristina Rappapport, Kristina Rudolph, Zita Sibenaller, Lori Stout, Alicia Walter, Daniel Herr, Nazli Vedadi, Robert Bartlett, Antonio Pesenti, Shaun Thompson, Lace Sindt, Sean Rajnic, Cale Ewald, Julie Hoffman, Matthew Griffee, Anna Ciullo, Yuri Kida, Ricard Ferrer Roca, JordI Riera, Sofia Contreras, Cynthia Alegre, Christy Kay, Irene Fischer, Elizabeth Renner, Hayato Taniguci, John Fraser, Gianluigi Li Bassi, Jacky Suen, Adrian Barnett, Nicole White, Kristen Gibbons, Simon Forsyth, Amanda Corley, India Pearse, Samuel Hinton, Gabriella Abbate, Halah Hassan, Silver Heinsar, Varun A Karnik, Katrina Ki, Hollier F. O’Neill, Nchafatso Obonyo, Janice D. Reid, Kei Sato, Kiran Shekar, Aapeli Vuorinen, Karin S. Wildi, Emily S. Wood, Stephanie Yerkovich, James Lee, Daniel Plotkin, Laura Merson, Emma Hartley, Bastian Lubis, Takanari Ikeyama, Balu Bhaskar, Jae-Seung Jung, Shay McGuinness, Glenn Eastwood, Sandra Rossi Marta, Fabio Guarracino, Fabio Guarracino, Stacy Gerle, Emily Coxon, Bruno Claro, Gonzo Gonzalez-Stawinski, Daniel Loverde, Namrata Patil, Vieri Parrini, Angela McBride, Kathryn Negaard, Angela Ratsch, Ahmad Abdelaziz, Juan David Uribe, Adriano Peris, Mark Sanders, Dominic Emerson, Muhammad Kamal, Pedro Povoa, Roland Francis, Ali Cherif, Sunimol Joseph, Matteo Di Nardo, Micheal Heard, Kimberly Kyle, Ray A Blackwell, Michael Piagnerelli, Patrick Biston, Hye Won Jeong, Reanna Smith, Yogi Prawira, Giorgia Montrucchio, Nadeem Rahman, Vivek Kakar, Michael Piagnerelli, Josefa Valenzuela Sarrazin, Arturo Huerta Garcia, Nahikari Salterain, Bart Meyns, Marsha Moreno, Rajat Walia, Amit Mehta, Annette Schweda, Moh Supriatna, Bhirowo Yudo, Cenk Kirakli, Melissa Williams, Kyung Hoon Kim, Alexandra Assad, Estefania Giraldo, Wojtek Karolak, Martin Balik, Elizabeth Pocock, Evan Gajkowski, Kanamoto Masafumi, Nicholas Barrett, Yoshihiro Takeyama, Sunghoon Park, Faizan Amin, Fina Meilyana Andriyani, Serhii Sudakevych, Angela Ratsch, Magdalena Vera, Rodrigo Cornejo, Patrícia Schwarz, Ana Carolina Mardini, Ary Serpa Neto, Andrea Villoldo, Alexandre Siciliano Colafranceschi, Alejandro Ubeda, Juan Granjean, Lívia Maria Garcia Melro, Giovana Fioravante Romualdo, Diego Gaia, Helmgton Souza, Diego Bastos, Filomena Galas, Rafael Máñez Mendiluce, Alejandra Sosa, Ignacio Martinez, Hiroshi Kurosawa, Juan Salgado, Beate Hugi-Mayr, Eric Charbonneau, Vitor Salvatore Barzilai, Veronica Monteiro, Rodrigo Ribeiro de Souza, Michael Harper, Hiroyuki Suzuki, Celina Adams, Jorge Brieva, George Nyale, Faisal Saleem Eltatar, Jihan Fatani, Husam Baeissa, Ayman AL Masri, Ahmed Rabie, Mok Yee Hui, Masahiro Yamane, Hanna Jung, Ayorinde Mojisola, Margaret, Newell Nacpil, Katja Ruck, Rhonda Bakken, Claire Jara, Tim Felton, Lorenzo Berra, Bobby Shah, Arpan Chakraborty, Monika Cardona, Gerry Capatos, Bindu Akkanti, Abiodun Orija, Harsh Jain, Asami Ito, Brahim Housni, Sennen Low, Koji Iihara, Joselito Chavez, Kollengode Ramanathan, Gustavo Zabert, Krubin Naidoo, Ian Seppelt, Marlice VanDyk, Sarah MacDonald, Shingo Ichiba, Randy McGregor, Teka Siebenaler, Hannah Flynn, Julia Garcia-Diaz, Catherine Harmon, Kristi Lofton, Toshiyuki Aokage, Kazuaki Shigemitsu, Andrea Moscatelli, Giuseppe Fiorentino, Matthias Baumgaertel, Serge Eddy Mba, Jana Assy, Amelya Hutahaean, Holly Roush, Kay A Sichting, Francesco Alessandri, Debra Burns, Ahmed Rabie, Gavin Salt, Carl P. Garabedian, Jonathan Millar, Malcolm Sim, Adrian Mattke, Danny McAuley, Jawad Tadili, Tim Frenzel, Yaron Bar-Lavie, Aaron Blandino Ortiz, Jackie Stone, Alexis Tabah, Antony Attokaran, Michael Farquharson, Brij Patel, Derek Gunning, Kenneth Baillie, Pia Watson, Kenji Tamai, Gede Ketut Sajinadiyasa, Dyah Kanyawati, Marcello Salgado, Assad Sassine, Bhirowo Yudo, Scott McCaul, Bongjin Lee, Sang Min Lee, Arnon Afek, Yoshiaki Iwashita, Laveena Munshi, Bambang Pujo Semedi, Neurinda Permata, Kusumastuti, Jack Metiva, Nicole Van Belle, Daniel Marino, Ignacio Martin-Loeches, Hergen Buscher, Lenny Ivatt, Chia Yew Woon, Hyun Mi Kang, Timothy Smith, Erskine James, Nawar Al-Rawas, Tomoyuki Endo, Yudai Iwasaki, Kenny Chan King-Chung, Vadim Gudzenko, Beate Hugi-Mayr, Fabio Taccone, Fajar Perdhana, Yoan Lamarche, Joao Miguel Ribeiro, Nikola Bradic, Klaartje Van den Bossche, Oude Lansink, Gurmeet Singh, Gerdy Debeuckelaere, Henry T. Stelfox, Cassia Yi, Jennifer Elia, Shu Fang, Thomas Tribble, Shyam Shankar, Paolo Navalesi, Raj Padmanabhan, Bill Hallinan, Luca Paoletti, Yolanda Leyva, Tatuma Fykuda, Jenelle Badulak, Jillian Koch, Amy Hackman, Lisa Janowaik, Deb Hernandez, Jennifer Osofsky, Katia Donadello, Aizah Lawang, Josh Fine, Benjamin Davidson, and Andres Oswaldo Razo Vazquez
REFERENCES
- 1.Johns Hopkins Coronavirus Resource Center. COVID-19 Map. Available at: https://coronavirus.jhu.edu/map.html. Accessed June 7, 2020
- 2.Grasselli G, Zangrillo A, Zanella A, et al. ; COVID-19 Lombardy ICU Network. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020; 323:1574–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan WJ, Ni ZY, Hu Y, et al. ; China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020; 382:1708–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richardson S, Hirsch JS, Narasimhan M, et al. ; the Northwell COVID-19 Research Consortium. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020; 323:2052–2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karagiannidis C, Mostert C, Hentschker C, et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: An observational study. Lancet Respir Med. 2020; 8:853–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020; 395:1054–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrando C, Suarez-Sipmann F, Mellado-Artigas R, et al. ; COVID-19 Spanish ICU Network. Clinical features, ventilatory management, and outcome of ARDS caused by COVID-19 are similar to other causes of ARDS. Intensive Care Med. 2020; 46:2200–2211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Docherty AB, Harrison EM, Green CA, et al. ; ISARIC4C investigators. Features of 20 133 UK patients in hospital with Covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Prospective observational cohort study. BMJ. 2020; 369:m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zanella A, Florio G, Antonelli M, et al. Time course of risk factors associated with mortality of 1260 critically ill patients with COVID-19 admitted to 24 Italian intensive care units. Intensive Care Med. 2021; 47:995–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Botta M, Tsonas AM, Pillay J, et al. Ventilation management and clinical outcomes in invasively ventilated patients with COVID-19 (PRoVENT-COVID): A national, multicentre, observational cohort study. Lancet Respir. 2020; 9:139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grasselli G, Scaravilli V, Tubiolo D, et al. Quality of life and lung function in survivors of extracorporeal membrane oxygenation for acute respiratory distress syndrome. Anesthesiology. 2019; 130:572–580 [DOI] [PubMed] [Google Scholar]
- 12.Lim ZJ, Subramaniam A, Ponnapa Reddy M, et al. Case fatality rates for patients with COVID-19 requiring invasive mechanical ventilation. A meta-analysis. Am J Respir Crit Care Med. 2021; 203:54–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolkewitz M, Lambert J, von Cube M, et al. Statistical analysis of clinical COVID-19 data: A concise overview of lessons learned, common errors and how to avoid them. Clin Epidemiol. 2020; 12:925–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Bassi G, Suen J, Barnett AG, et al. ; COVID-19 Critical Care Consortium Investigators. Design and rationale of the COVID-19 Critical Care Consortium international, multicentre, observational study. BMJ Open. 2020; 10:e041417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.COVID-19 Critical Care Consortium. ECMOCARD. Available at: https://www.elso.org/COVID19/ECMOCARD.aspx. Accessed June 2, 2020
- 16.Wolkewitz M, Cooper BS, Palomar-Martinez M, et al. Multiple time scales in modeling the incidence of infections acquired in intensive care units. BMC Med Res Methodol. 2016; 16:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med. 2020; 8:475–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrando-Vivas P, Doidge J, Thomas K, et al. Prognostic factors for 30-day mortality in critically ill patients with coronavirus disease 2019: An observational cohort study. Crit Care Med. 2020; 49: 102–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Backer DD, Azoulay E, Vincent JL. Corticosteroids in severe COVID-19: A critical view of the evidence. Crit Care. 2020; 24:627–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monedero P, Gea A, Castro P, et al. Early corticosteroids are associated with lower mortality in critically ill patients with COVID-19: A cohort study. Crit Care. 2021; 25:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grasselli G, Greco M, Zanella A, et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020; 180:1345–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020; 584:430–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Botta M, Tsonas AM, Pillay J, et al. ; PRoVENT-COVID Collaborative Group. Ventilation management and clinical outcomes in invasively ventilated patients with COVID-19 (PRoVENT-COVID): A national, multicentre, observational cohort study. Lancet Respir Med. 2021; 9:139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: A prospective cohort study. Lancet. 2020; 395:1763–1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mueller AL, McNamara MS, Sinclair DA. Why does COVID-19 disproportionately affect older people? Aging (Albany NY). 2020; 12:9959–9981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Bassi G, Suen JY, Dalton HJ, et al. ; COVID-19 Critical Care Consortium. An appraisal of respiratory system compliance in mechanically ventilated Covid-19 patients. Crit Care. 2021; 25:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gattinoni L, Chiumello D, Caironi P, et al. COVID-19 pneumonia: Different respiratory treatments for different phenotypes? Intensive Care Med. 2020; 46:1099–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bahl A, Van Baalen MN, Ortiz L, et al. Early predictors of in-hospital mortality in patients with COVID-19 in a large American cohort. Intern Emerg Med. 2020; 15:1485–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.African COVID-19 Critical Care Outcomes Study (ACCCOS) Investigators. Patient care and clinical outcomes for patients with COVID-19 infection admitted to African high-care or intensive care units (ACCCOS): A multicentre, prospective, observational cohort study. Lancet (London, England). 2021; 397:1885–1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020; 383:120–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iba T, Levy JH, Levi M, et al. Coagulopathy of coronavirus disease 2019. Crit Care Med. 2020; 48:1358–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miesbach W, Makris M. COVID-19: Coagulopathy, risk of thrombosis, and the rationale for anticoagulation. Clin Appl Thromb Hemost. 2020; 26:1076029620938149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Durvasula R, Wellington T, McNamara E, et al. COVID-19 and kidney failure in the acute care setting: Our experience from Seattle. Am J Kidney Dis. 2020; 76:4–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang R, Elhusseiny KM, Yeh YC, et al. COVID-19 ICU and mechanical ventilation patient characteristics and outcomes-a systematic review and meta-analysis. PLoS One. 2021; 16:e0246318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amit M, Sorkin A, Chen J, et al. Clinical course and outcomes of severe Covid-19: A national scale study. J Clin Med. 2020; 9:E2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Intensive Care National Audit and Research Centre. Reports. Available at: https://www.icnarc.org/Our-Audit/Audits/Cmp/Reports. Accessed October 24, 2020
- 37.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020; 323:1061–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Albini A, Noonan DM, Pelosi G, et al. The SARS-CoV-2 receptor, ACE-2, is expressed on many different cell types: Implications for ACE-inhibitor- and angiotensin II receptor blocker-based antihypertensive therapies-reply. Intern Emerg Med. 2020; 15:1583–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horby P, Lim WS, Emberson JR, et al. ; RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19 — preliminary report. N Engl J Med. 2020; 384:693–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.World Health Organization. Corticosteroids for COVID-19. Available at: https://www.who.int/publications/i/item/WHO-2019-nCoV-Corticosteroids-2020.1. Accessed January 6, 2021
- 41.Grasselli G, Cattaneo E, Florio G, et al. Mechanical ventilation parameters in critically ill COVID-19 patients: A scoping review. Crit Care. 2021; 25:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.