Background:
Adjuvant radiotherapy could be a necessary step in the oncological treatment for breast cancer. However, radiotherapy may have negative effects on implant-based immediate breast reconstruction. The aim of this study was to determine the impact of adjuvant radiation therapy on surgical results and patient-reported satisfaction outcomes in women undergoing immediate implant-based breast reconstruction.
Methods:
A systematic search in PubMed was conducted on September 2019 and updated on April 2021. The risk of bias of the included studies was assessed using the Newcastle-Ottawa Quality Assessment Form for Observational Studies. RevMan 5 was used for statistical analysis. We obtained relative risks to determine the complication incidence and mean differences for 2-year BREAST-Q scores.
Results:
Fourteen studies were included. A total of 11,958 implant-based immediate reconstructions were performed, 2311 received postmastectomy radiation therapy, and 9647 were considered as control group. Surgical complications, reoperation rates, and reconstruction failure were significantly higher among irradiated breasts. Significantly lower BREAST-Q scores were reported by irradiated women receiving radiotherapy.
Conclusions:
This systematic review and meta-analysis combines reconstruction complication rates with aesthetic and patient-reported satisfaction outcomes. Adjuvant radiotherapy is consistently associated with greater complication rates and poorer aesthetic and satisfaction outcomes. The magnitude of association is significantly lower when the reconstruction is based on autologous tissues.
Takeaways
Question: Is radiotherapy a good option when a breast implant/expander is used for mammary reconstruction?
Findings: Surgical complications, re-operation rates, and reconstruction failure were significantly higher among irradiated breasts. Significantly lower BREAST-Q scores were reported by women who received radiotherapy.
Meaning: Adjuvant radiotherapy is consistently associated with higher complication rates and poorer aesthetic and satisfaction outcomes.
INTRODUCTION
Even if early-stage cancer detection and screening methods have reduced breast cancer-related mortality, mastectomy and its surgical variants are still one of the main valuable tools in treating breast cancer patients.1 Both a cancer diagnosis and oncological surgery have a great impact, physically and emotionally, on women’s lives. Nearly half of the women receiving a mastectomy refer to having a negative body image and poor social and sexual well-being.2 Breast reconstruction in its multiple shapes and forms greatly improves breast cancer patients’ quality of life after mastectomy, as reflected on patient-reported questionnaires such as BREAST-Q or RAND-36.3
Immediate breast reconstruction offers multiple advantages, including a single operation, reduced overall costs, and early breast mound restoration, resulting in higher patient-reported aesthetic and psychological outcomes when compared to a delayed reconstruction.4 A study by Razdan et al5 assessing breast reconstruction trends during the 2010 decade showed a shift toward immediate breast reconstruction in women with postmastectomy radiotherapy (PMRT) indication; besides, they also found that implant-based techniques prevail over autologous reconstruction in the PMRT group. This, as well as other similar studies, shows a trend shift that questions one of the main axioms in plastic reconstructive surgery: radiotherapy (RT) as a relative contraindication when planning a reconstruction using exclusively alloplastic materials.
This study aimed to gather and assess the most recent evidence to try to quantify the impact that radiotherapy has on implant-based immediate breast reconstruction. This research included the possible correlation between the incidence of postreconstructive complications and aesthetic and patient-reported satisfaction outcomes. The hypothesis is that, even if radiation techniques have evolved, RT is still detrimental enough to keep considering it as, at least, a relative contraindication for exclusively implant-based reconstruction.
METHODS
This meta-analysis was conducted according to the Cochrane Handbook for Systematic Reviews of Interventions6 and to the MOOSE guidelines, specifically designed for observational study–based systematic reviews.7 Following PRISMA recommendations,8 all decision-making criteria were set beforehand.
Eligibility Criteria
All trials reporting on immediate breast reconstruction based on the tissue expander/implant technique published 2014–2020 in Q1–Q2 medical journals were included. We excluded noncomparative studies and those where the comparison groups were other than PMRT versus non-PMRT. We excluded non-English, French, or Spanish-written articles. Animal model or experimental studies were excluded, as well as studies with a small sample size (N < 30).
Information Sources and Search Strategy
A systematic search in PubMed was first performed on September 27, 2019 and updated on April 3, 2021. The following search terms (medical subject headings) were used: “{[adjuvant radiotherapy] AND immediate AND [“mammaplasty” (MeSH Terms) OR BREAST RECONSTRUCTION (Text Word)]} AND breast implant.” The last 5-year filter was applied.
Study Selection and Data Extraction
The following data were extracted from each article: author, publication year, type of cohort, age, number of breasts in each group (PMRT/non-PMRT), RT protocol and timing, and length of follow-up. The surgical outcomes were classified, as Apte et al9 proposed, in early or late complications according to the timing of each event, less than 6 weeks postsurgery, or more than 6 weeks postoperative. Early complications included surgical site infection, mastectomy flap necrosis, seroma/hematoma, and implant extrusion. The assessed late complications include capsular contracture (III–IV), need for revision surgery, and reconstructive failure. Aesthetic and patient-reported satisfaction outcomes were assessed according to the BREAST-Q questionnaire on satisfaction with breast, satisfaction with the outcome, psychosocial well-being, and physical well-being.
Risk of Bias Assessment
The methodological quality of the included studies was assessed using the Newcastle-Ottawa Quality Assessment Form for Cohort Studies,10 specifically designed for nonrandomized cohort studies. This evaluation form is based on three bias domains: selection, comparability, and outcome. On the outcome domain, for the follow-up length adequacy, we considered as acceptable 2 years or more of follow-up, since there is robust evidence that 74% of RT-associated complications occur during the first 3 years postimmediate reconstruction surgery, mainly in the first two.11
Statistical Analysis
All statistical analyses were performed using RevMan 5.4.1 (2020)12 as statistical software. We obtained risk ratios (relative risk, RR) for surgical complication incidence and a mean difference for BREAST-Q scores. Data were pooled with fixed-effects meta-analysis to determine measures of association or mean differences, and 95% confidence intervals (CIs) for each comparison.
Heterogeneity was assessed using Cochran’s Q test (considering heterogeneous results to have P < 0.1) and I2 index. By default, fixed-effects meta-analysis was used when I2 was less than 30%; if I2 was 30% or more, random effects meta-analysis would have been used instead. Funnel plots were used to assess the risk of publication bias.
RESULTS
Study Characteristics
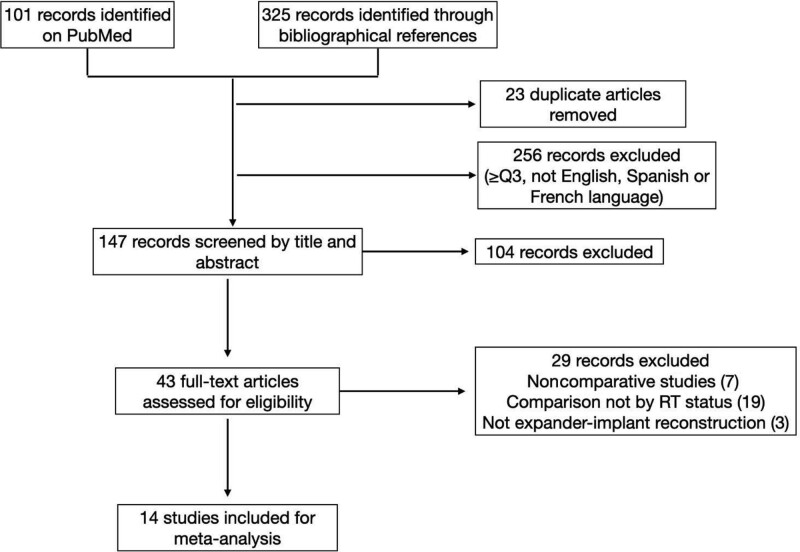
The search on PubMed resulted in 101 records (Fig. 1). After the removal of those meeting exclusion criteria, screening by title, abstract and full-text reading, nine studies were included for quantitative analysis. We also considered the bibliographical references of the included articles, which added 325 records. After the removal of duplicates and those meeting exclusion criteria, 147 articles were screened by title and abstract, 43 were considered for full-text assessment. Finally, 14 studies11,13–25 were included in this review, and 29 records were excluded.26–54 The main characteristics and results of the included studies are listed in Tables 1–3. Table 4 gathers the outcomes of the risk of bias assessment according to The Newcastle-Ottawa Scale.10
Fig. 1.
Study selection flow chart.
Table 1.
Characteristics of the Studies Included in the Systematic Review
| Study | Year | Type of Cohort | Age (Yrs) |
Non-RT | PMRT | Reconstruction Type* | RT Protocol | RT Timing | Follow-up(Yrs) |
|---|---|---|---|---|---|---|---|---|---|
| Cordeiro et al13 | 2014 | Prospective 1998–2010 |
46.9 | 1814 | 319 | Tissue expander/implant | 6 MV photons | RT on definitive implant, 4 wks after exchenge | 4.65 |
| Sbitany et al14 | 2014 | Prospective 2006–2012 |
45.4 | 727 | 113 | Tissue expander/implant | NR | NR | 1.94 |
| Chen et al15 | 2015 | Retrospective 2007–2013 |
50.27 54.88 |
30 | 38 8 |
Tissue expander/implant | NR | After complete expansion, before the exchange (38) Before tissue expander placement (8) |
NR |
| Cordeiro et al16 | 2015 | Prospective 2003–2012 | 46.7 | 1486 | 94 210 |
Tissue expander/implant Direct-to-implant |
6 MV/15 MV | 6 MV over implant, 15 MV over expander, depending on chemotherapy protocol | 3.57 |
| Reishet al17 | 2015 | Retrospective 2007–2012 | 46.95 | 517 | 45 | Tissue expander/implant or direct-to-implant depending on skin flap health | NR | Before/after tissue expander exchange depending on urgency or oncologist/surgeon preference | 1.8 |
| Seth et al18 | 2015 | Retrospective 1999–2008 |
48.6 50.9 |
879 51 |
248 23 |
Immediate tissue expander/implant Delayed tissue expander/implant |
NR | During expansion | NR |
| Muresan et al19 | 2017 | Retrospective 2010–2013 | 48.9 | 125 | 533 | Tissue expander/implant or direct-to-implant | 50–60 Gy, higher mean dose in supine vs prone position during RT | NR | 2.11 |
| Elswick et al20 | 2018 | Retrospective 2012–2016 | 48 | 39 | 54 | Tissue expander/implant | 50 Gy, 25 fractions | After complete expansion, before the exchange | 2.3 |
| Jagsi et al21 | 2018 | Prospective 2012–2015 |
NR | 1218 407 |
386 236 |
Tissue expander/implant autologous |
NR | NR | 2 |
| Smith et al22 | 2019 | Prospective 2025–2017 | 49 | 42 | 51 | Tissue expander/implant | 50 Gy in 25 fractions or 40 Gy in 15 fractions (hypofractionated group) | 6 wks after tissue expander location or 3–4 wks after chemotherapy | 1.33 |
| Zhang et al23 | 2019 | Retrospective 2001–2015 | 38† | 342 331 |
52 107 |
Tissue expander/implant autologous |
NR | NR | 4.8† |
| Lam et al11 | 2019 | Retrospective 1998–2010 | 47.01 | 324 | 118 | Tissue expander/implant | 50 Gy, 25 fractions | Over tissue expander after full expansion | 3.52 |
| Naoum et al24 | 2019 | Retrospective 1997–2017 | 49.3 | 603 462 220 |
236 171 122 |
Tissue expander/implant direct-to-implant Autologous |
NR | During expansion or after tissue expander exchange | 5.8† |
| Olinger et al25 | 2020 | Prospective 2012–2015 |
NR | 1093 88 |
316 13 |
Tissue expander/ implant direct-to-implant |
NR | NR | 2 |
*All expander-implant–based reconstructions are immediate unless otherwise specified.
†Median.
NR, not reported.
Table 3.
Two-year BREAST-Q Scores
| Study | Satisfaction with Breast | Satisfaction with Outcome | Psychosocial Well-being | Physical Well-being | |
|---|---|---|---|---|---|
| Cordeiro et al16 * | PMRT Non-RT |
56.2 64.1 |
68.4 73.5 |
71.1 76.4 |
72.5 78.5 |
| Jagsi et al21 † | PMRT Non-RT |
54.2 ± 19 65.4 ± 17.5 |
64.8 ± 22 71.3 ± 21.4 |
66.4 ± 19.2 75.2 ± 18.8 |
71.3 ± 14.1 77.6 ±14.1 |
| Olinger et al25 † | PMRT Non-RT |
54.5 ± 18.4 66.1 ± 17.3 |
64.3 ± 21.5 71.8 ± 21 |
*Adjusted median.
†Mean ± SD.
Table 4.
Newcastle-Ottawa Quality Assessment Form for Cohort Studies
| Study | Selection* | Comparability† | Outcome* | Total Score | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the Exposed Cohort | Selection of the Nonexposed Cohort | Ascertainment of Exposure | Outcome of Interest Not Present at Start of the Study | Comparability of Cohorts on the Basis of the Design or Analysis | Assessment of Outcome | Follow-up Long Enough | Adequacy of Follow-up | ||
| Cordeiro et al13 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Sbitany et al14 | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 1 | 8 |
| Chen et al15 | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 1 | 8 |
| Cordeiro et al16 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Reish et al17 | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 1 | 8 |
| Seth et al18 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 7 |
| Muresan et al19 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Elswick et al20 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Jagsi et al21 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 8 |
| Smith et al22 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 7 |
| Zhang et al23 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Lam et al11 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Naoum et al24 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| Olinger et al25 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 8 |
*Maximum score is 2
†Maximum score is 1
Table 2.
Early and Late Complication Incidence
| Study | N | Surgical Site Infection | Mastectomy Flap Necrosis | Seroma/Hematoma | Extrusion/Exposure | Capsular Contracture III–IV | Revision Surgery | Reconstructive Failure |
|---|---|---|---|---|---|---|---|---|
| Cordeiro et al13 | PMRT: 319 Non-RT: 1814 Total: 2133 |
29 (9.1%) 9 (0.5%) |
147 (46.1%) 116 (6.4%) |
6 (1.9%) 4 (0.2%) |
||||
| Sbitany et al14 | PMRT: 113 Non-RT: 727 Total: 840 |
25 (22.1%) 53 (7.3) |
17 (15%) 39 (5.3%) |
10 (8.8%) 56 (7.7%) |
12 (10.6%) 33 (4.5%) |
20 (17.7%) 37 (5.1%) |
||
| Chen et al15 | PMRT: 38 Non-RT: 30 Total: 68 |
17 (44.7%) 8 (26.6%) |
3 (7.9%) 3 (10.0%) |
5 (13.2%) 6 (20.0%) |
3 (7.9%) 1 (3.3%) |
11 (28.9%) 2 (6.6%) |
26 (68.4%) 14 (46.6%) |
|
| Cordeiro et al16 | PMRT: 210 Non-RT: 1486 Total: 1696 |
26 (12.4%) 68 (4.6%) |
||||||
| Reish et al17 | PMRT: 45 Non-RT: 517 Total: 562 |
3 (6.7%) 15 (2.9%) |
3 (6.7%) 28 (5.4%) |
1 (2.2%) 18 (3.5%) |
7 (15.6%) 12 (2.3%) |
4 (8.9%) 5 (0.9%) |
||
| Seth et al18 | PMRT: 248 Non-RT: 879 Total: 1127 |
20 (8.1%) 36 (4.1%) |
27 (10.9%) 67 (7.7%) |
16 (6.5%) 51 (5.8%) |
9 (3.6%) 8 (0.9%) |
35 (14.1%) 53 (6.0%) |
||
| Muresan et al19 | PMRT: 125 Non-RT: 533 Total: 658 |
19 (15.2%) 36 (6.8%) |
10 (8%) 38 (7.1%) |
6 (4.8%) 15 (2.8%) |
12 (9.6%) 13 (2.4%) |
8 (6.4%) 0 |
11 (8.8%) 4 (0.8%) |
|
| Elswick et al20 | PMRT: 54 Non-RT: 39 Total: 93 |
10 (18.5%) 3 (7.7%) |
1 (1.9%) 1 (2.6%) |
4 (7.4%) 3 (7.7%) |
1 (1.9%) 0 |
1 (1.9%) 0 |
16 (29.6%) 7 (17.9%) |
6 (11.1%) 1 (2.6%) |
| Jagsi et al21 | PMRT: 386 Non-RT: 1218 Total: 1604 |
47 (12.2%) 43 (3.5%) |
||||||
| Smith et al22 | PMRT: 51 Non-RT: 42 Total: 93 |
14 (27.5%) 1 (2.4%) |
2 (3.9%) 1 (2.4%) |
5 (9.8%) 1 (2.4%) |
1 (1.9%) 0 |
8 (15.7%) 2 (4.8%) |
||
| Zhang et al23 | PMRT: 52 Non-RT: 342 Total: 394 |
8 (15.4%) 23 (6.7%) |
||||||
| Lam et al11 | PMRT: 118 Non-RT: 324 Total: 442 |
5 /107 (4.7%) 11/450 (2.4%) |
16/107 (14.9%) 89/450 (19.7%) |
20/100 (20%) 24/316 (7.6%) |
||||
| Naoum et al24 | PMRT: 236 Non-RT: 603 Total: 839 |
37 (15.7%) 35 (5.8%) |
21 (8.9%) 31 (5.1%) |
12 (5.1%) 13 (2.2%) |
16 (6.8%) 4 (0.7%) |
36 (15.3%) 28 (4.6%) |
21 (9.1%) 17 (3.0%) |
|
| Olinger et al25 | PMRT: 316 Non-RT: 1093 Total: 1409 |
56 (17.0%) 48 (4.1%) |
Of a maximum of nine points, one study scored nine, 10 scored eight, and three scored seven points. As previously mentioned, follow-up of 2 years or more was considered adequate. Since two studies did not report the length of the follow-up they did not score for this item. All of the studies scored positively for the selection of the nonexposed cohort, ascertainment of the exposure, absence of the event of interest before the exposition, and assessment of the outcome since every complication or treatment required was recorded by healthcare professionals. Since two of the studies reported missing patients’ responses during the follow-up questionnaires, although they statistically adjusted and managed it, we decided to be conservative and did not give points for this item.
Early Complications
Surgical Site Infection
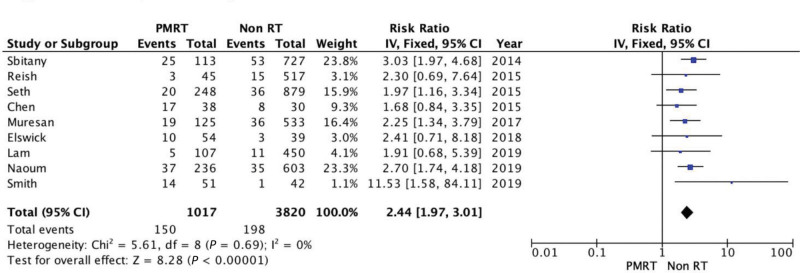
Nine studies assessed surgical site infection, yielding 4837 reconstruction procedures and 150/1017 cases in the PMRT group and 198/3820 in the non-RT group. PMRT was significantly associated with higher infection rates, resulting in a 2.44 RR (95% CI 1.97, 3.01; P < 0.00001). There was no evidence of significant heterogeneity (Chi square = 5.61; P = 0.69; I2 = 0%) (Fig. 2).
Fig. 2.
Forest plot for surgical site infections.
Mastectomy Skin Flap Necrosis
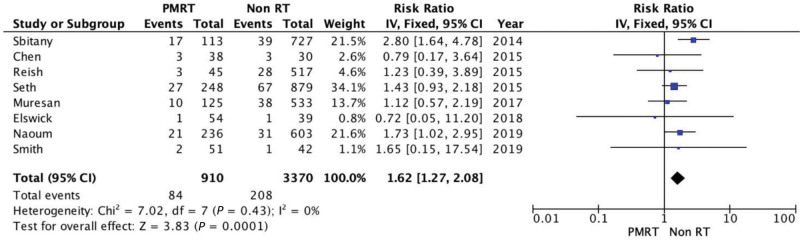
Mastectomy flap necrosis was assessed in eight of the selected studies. PMRT was significantly associated with a higher risk of skin necrosis (RR = 1.62; 95% CI = 1.27, 2.08). There was no evidence of significant heterogeneity (Chi square = 7.02; P = 0.43; I2 = 0%) (Fig. 3).
Fig. 3.
Forest plot for mastectomy flap necrosis.
Serohematoma
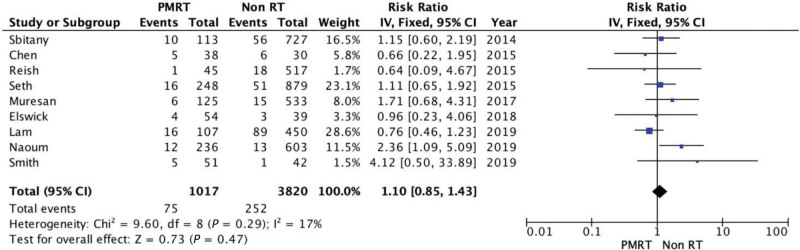
One study assessed the combined incidence of serohematoma, and eight reported the incidence of seroma and hematoma incidence. These results were managed jointly. The incidence of serohematoma was slightly higher among irradiated breasts. However, there was no significant difference between the two groups for this comparison (RR = 1.1; 95% CI = 0.85, 1.43). There was no evidence of significant heterogeneity (Chi square = 9.6; P = 0.29; I2 = 17%) (Fig. 4).
Fig. 4.
Forest plot for serohematoma.
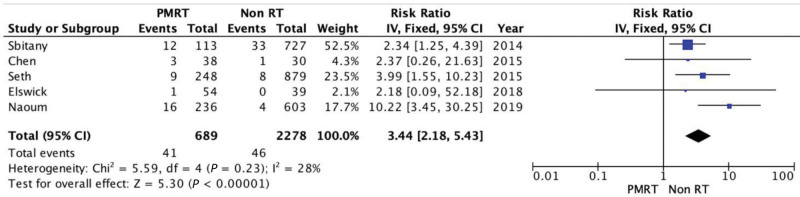
Implant Extrusion or Exposure
Five of the included studies reported data regarding implant extrusion. There was a higher extrusion rate in the PMRT group (5.95% versus 2.01%), RR of 3.44 (95% CI 2.18, 5.43). There was some evidence of not significant heterogeneity (Chi square = 5.59; P = 0.23; I2 = 28%) (Fig. 5).
Fig. 5.
Forest plot for implant extrusion or exposure.
Late Complications
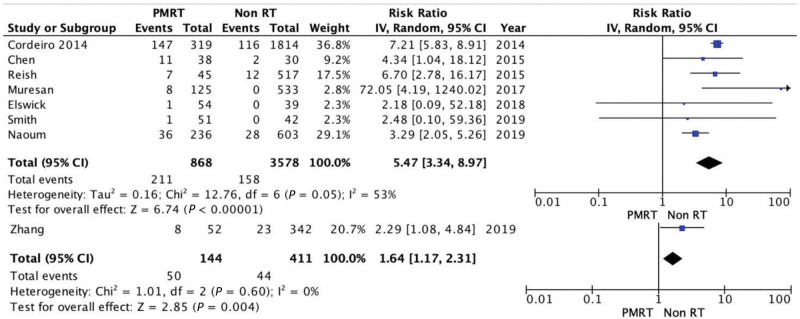
Capsular Contracture (III–IV)
Baker scale grade III–IV capsular contracture55 incidence was assessed in seven studies. There was a significantly higher incidence of capsular contracture in the PMRT group (24.31% versus 4.42%), with an RR of 5.47 and 95% CI of 3.34, 8.97. Since there was evidence of significant heterogeneity, random effects analysis was used for this comparison (Tau2 = 0.16; Chi square = 12.76; P = 0.05; I2 = 53%) (Fig. 6).
Fig. 6.
Forest plot for capsular contracture (III–IV).
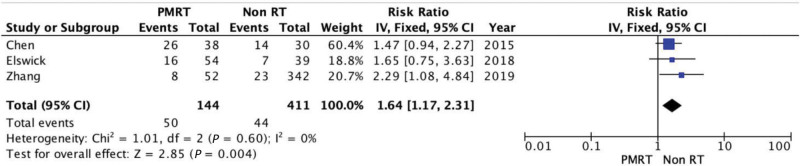
Revision Surgery
Revision surgery rates comprehend the unplanned return to the operating room due to acute complications or the consequences of previous events. The number of reoperations was significantly higher in the PMRT group (34.72%) versus non-RT group (10.70%): RR = 1.64; 95% CI = 1.17, 2.31. There was no evidence of significant heterogeneity (Chi square = 1.01; P = 0.60; I2 = 0%) (Fig. 7).
Fig. 7.
Forest plot for reintervention.
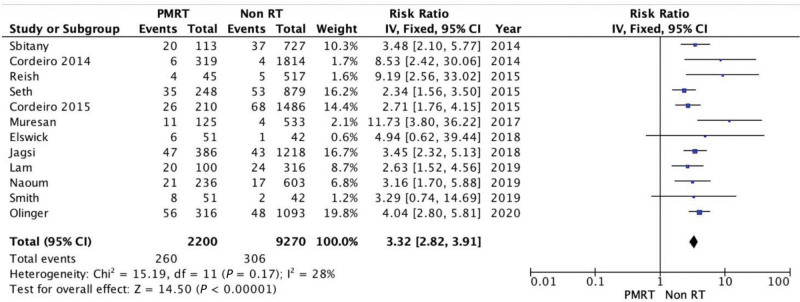
Reconstructive Failure
Reconstructive failure includes reconstruction failure, implant loss, or a reconstruction technique change to an autologous reconstruction. PMRT was associated with a significantly higher rate of reconstructive failure (RR = 3.32; 95% CI = 2.82, 3.91). There was no evidence of significant heterogeneity (Chi square = 15.19; P = 0.17; I2 = 28%) (Fig. 8).
Fig. 8.
Forest plot for reconstructive failure.
Aesthetic and Satisfaction Results
Four of the included studies include data regarding satisfaction with breasts at 2-years postreconstruction. Since Lam et al11 measured satisfaction in ordinal categories (poor, fair, good, excellent) and Cordeiro et al16 measured the BREAST-Q score using the adjusted median instead of the mean± SD, those studies could not be included for the quantitative analysis.
Satisfaction with Breast
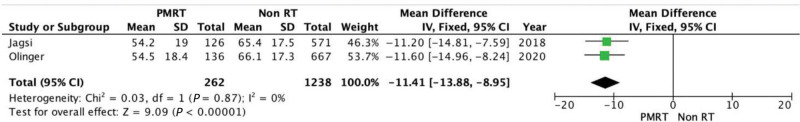
The difference between the two groups is statistically significant (P < 0.00001) in favor of the non-RT group. The mean difference is 11.41 (95% CI = −13.88, −8.95). There is no evidence of significant heterogeneity (Chi square = 0.03; P = 0.87; I2 = 0%) (Fig. 9).
Fig. 9.
Forest plot for satisfaction with breasts at 2 years.
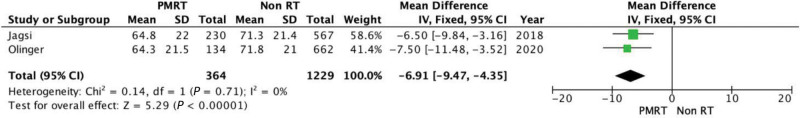
Satisfaction with Outcome
Satisfaction with outcome was assessed in two studies. There was statistically significant difference favoring non irradiated breasts (P < 0.00001). The mean difference is 6.91 (95% CI = −9.47, −4.35). There was no evidence of significant heterogeneity (Chi square = 0.14; P = 0.71; I2 = 0%] (Fig. 10).
Fig. 10.
Forest plot for satisfaction with outcomes at 2 years.
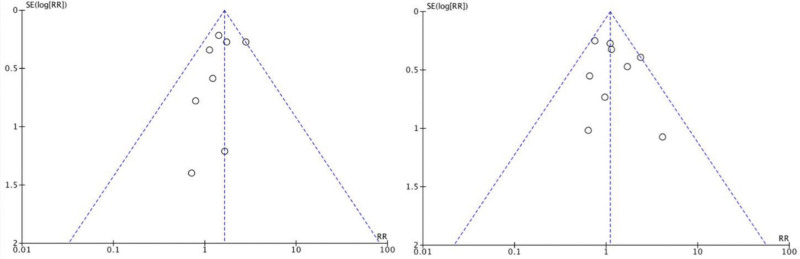
Risk of Publication Bias
Funnel plots for different comparisons were obtained for the risk of publication bias assessment (Fig. 11).
Fig. 11.
Funnel plot for risk of publication bias. Left, necrosi; right, serohematoma.
DISCUSSION
This systematic review and meta-analysis aimed to quantify the impact of adjuvant RT on implant-based breast reconstruction. This review showed consistent results regarding previous research33,56,57 since PMRT is significantly associated with a higher incidence of postoperative complications, higher reoperation and reconstructive failure rates, and poorer cosmetic and satisfaction outcomes.
Capsular contracture was the most frequent complication in irradiated breasts. Grade III–IV capsular contractures were considered clinically significant. Capsular contracture and, indirectly PMRT, do have a great impact on breast reconstruction results since they may require additional surgeries for correction or may increase the risk of reconstruction failure. Moreover, capsular contracture plays a great role in conditioning poorer cosmetic outcomes and lower patient satisfaction scores on the PMRT group. These results showing the association between PMRT and capsular contracture, besides obeying biological plausibility, are consistent with previous works.58,59
PMRT is also significantly associated with higher major complication incidence, and since major complications are defined as those requiring surgical intervention as part of the treatment, is necessarily associated with higher reoperation rates. Even if Chen et al15 also described this association between PMRT and higher complication rates, it was not statistically significant. This could be due to smaller sample size and lower statistical power compared to the rest of the included studies, in which this association was statistically significant. These results are, therefore, consistent with previously published literature.60–62 This increase of the RT attributable risk is statistically significant on implant-based immediate reconstructions but is not as pronounced on reconstruction based on autologous tissues. Zhang et al23 reported a rate of unplanned return to OR or the need for secondary surgeries for complication management. The reported rate was 10.3% on immediate autologous reconstruction for the PMRT group and 6.6% in the autologous control group; for implant-based reconstruction, the reoperation rate was 15.4% versus 6.7% in PMRT and control group, respectively. The incidence of major complications requiring surgical intervention is similar for both, alloplastic and autologous reconstruction in the absence of radiotherapy; therefore, the greater increase in major complication incidence and need for surgical revision could be attributed to the damaging effect of adjuvant RT. These higher rates of reoperation and secondary surgeries in women receiving adjuvant RT are consistent with the available evidence. Unukovych et al63 described a significant association between PMRT and a major need for surgical management of complications on implant-based immediate reconstructions with an OR of 5.2 (95% CI 1.9, 14.6, P = 0.002).
Regarding implant loss or reconstructive failure, the rate seems to be slightly lower compared to previous articles. This may be due to sophistication or improvement in radiotherapy techniques and a less aggressive surgical management of the mastectomy flap.44 Some authors suggest that better coverage of the implant may ensure reconstruction viability even if RT is applied. Nonetheless, a study assessing the use of acellular dermal matrix for implant coverage in irradiated immediate breast reconstruction showed no difference between acellular dermal matrix and the control group regarding complication rates.64 Several other factors may condition reconstruction outcomes or complication incidence and are seldom taken into account extensively in the reviewed literature. Age, body mass index, smoking status, and medical comorbidities such as hypertension or diabetes mellitus are considered independent risk factors for reconstruction-associated complications.65–67 A better understanding of these factors and the interaction between them would help to better determine the real and individualized risk of poorer reconstruction outcomes in each case.
The damaging effect of RT on the irradiated tissues and their vascular supply may compromise the feasibility, and surgical and aesthetic outcomes of the immediate breast reconstruction.33,68,69 PMRT is associated with poorer aesthetic outcomes when the immediate reconstruction is based on alloplastic materials but not when it is based on autologous techniques.21,25 Autologous reconstruction is also susceptible to RT-induced complications such as fat necrosis, atrophy, or fibrosis.33,70,71 However, RT attributable complication incidence is significantly lower with significantly better satisfaction and cosmetic outcomes.72 The differences found in the patient-reported BREAST-Q for satisfaction with breast and outcome at 2 years from the reconstruction increase in the long-term, resulting in progressively lower scores with the passage of time.73 Therefore, the patient’s life expectancy should be added to the previously mentioned list of factors that should be taken into account when assessing the most suitable reconstruction option for each woman.
The reconstruction cases assessed in this review were tissue expander/implant-based. However, the radiotherapy protocol and timing were different for each institution meaning that the stage of the reconstruction in which RT was applied may vary. Nonetheless, the results and associations found are consistent even with studies in which the RT was applied on the definitive implant.59 These poor outcomes occur when RT is applied on the tissue expander as much as on a definitive implant, meaning that the consequences of the RT on the irradiated breasts are consistently deleterious in every implant-based immediate reconstruction technique.
This systematic review has certain limitations. As an inherent limitation of systematic reviews and meta-analysis, the quality inferences made cannot exceed the quality of the studies they are based on. Hence, the methodological quality of the studies was confirmed based on the satisfactory scores in the Newcastle-Ottawa Quality Assessment Form for Cohort Studies.10 Hence, the strong association found in this review should not be dismissed alluding to the retrospective nature of the cohort studies that it is based on. Although the heterogeneity statistically assessed using Cochran’s Q test and I2 index was not significant, there was some kind of variability between studies that could not be extensively assessed. The variability between the published articles and previous research comprises different factors such as the type of mastectomy, RT timing and dosage of PMRT, immediate reconstruction technique (tissue expander/implant versus direct-to-implant), systemic chemotherapy, or patient-dependent factors or comorbidities. A better description and quantification of these factors would result in more rigorous research that would bring up more accurate conclusions. Even if randomized controlled trials could be carried out to solve the inherent biases when assessing retrospective cohorts and to verify these findings, these results are consistent with previous reviews and articles.56,57,74
CONCLUSIONS
This meta-analysis showed a significant association between adjuvant RT and a higher incidence of early complications (infection, necrosis, and implant extrusion) and late complications (capsular contracture and reoperation) with higher reconstructive failure or reconversion to autologous reconstruction rates when applied over implant-based immediate reconstruction. Moreover, PMRT was associated with poorer cosmetic outcomes and lower patient-reported satisfaction scores, both mid- and long-term. These results are consistent with previous reviews and articles. Furthermore, investigation of factors leading to poorer results would be needed to better understand the risk–benefit balance in each case for individualized counseling on which reconstructive method would most benefit each woman, short- and long-term.
Footnotes
Published online 5 November 2021.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. [DOI] [PubMed] [Google Scholar]
- 2.Lee C, Sunu C, Pignone M. Patient-reported outcomes of breast reconstruction after mastectomy: a systematic review. J Am Coll Surg. 2009;209:123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eltahir Y, Werners LLCH, Dreise MM, et al. Quality-of-life outcomes between mastectomy alone and breast reconstruction: comparison of patient-reported BREAST-Q and other health-related quality-of-life measures. Plast Reconstr Surg. 2013;132:201e–209e. [DOI] [PubMed] [Google Scholar]
- 4.Singh P, Hoffman K, Schaverien MV, et al. Neoadjuvant radiotherapy to facilitate immediate breast reconstruction: a systematic review and current clinical trials. Ann Surg Oncol. 2019;26:3312–3320. [DOI] [PubMed] [Google Scholar]
- 5.Razdan SN, Cordeiro PG, Albornoz CR, et al. National breast reconstruction utilization in the setting of postmastectomy radiotherapy. J Reconstr Microsurg. 2017;33:312–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Higgins J, Thomas J. (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2. (updated February 2021). Cochrane; 2021. Available at www.training.cochrane.org/handbook. Accessed September 2020. [Google Scholar]
- 7.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 8.Moher D, Liberati A, Tetzlaff J, et al. ; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Apte A, Walsh M, Balaji P, et al. Single stage immediate breast reconstruction with acellular dermal matrix and implant: defining the risks and outcomes of post-mastectomy radiotherapy. Surgeon. 2020;18:202–207. [DOI] [PubMed] [Google Scholar]
- 10.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed October 19, 2020.
- 11.Lam TC, Borotkanics R, Hsieh F, et al. Immediate two-stage prosthetic breast reconstruction failure: radiation is not the only culprit. Plast Reconstr Surg. 2018;141:1315–1324. [DOI] [PubMed] [Google Scholar]
- 12.Review Manager (RevMan) [Computer program]. Version 5.4.1, Cochrane Collaboration, 2020. Available at https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman/revman-non-cochrane-reviews. [Google Scholar]
- 13.Cordeiro PG, Albornoz CR, McCormick B, et al. The impact of postmastectomy radiotherapy on two-stage implant breast reconstruction: an analysis of long-term surgical outcomes, aesthetic results, and satisfaction over 13 years. Plast Reconstr Surg. 2014;134:588–595. [DOI] [PubMed] [Google Scholar]
- 14.Sbitany H, Wang F, Peled AW, et al. Immediate implant-based breast reconstruction following total skin-sparing mastectomy: defining the risk of preoperative and postoperative radiation therapy for surgical outcomes. Plast Reconstr Surg. 2014;134:396–404. [DOI] [PubMed] [Google Scholar]
- 15.Chen TA, Momeni A, Lee GK. Clinical outcomes in breast cancer expander-implant reconstructive patients with radiation therapy. J Plast Reconstr Aesthet Surg. 2016;69:14–22. [DOI] [PubMed] [Google Scholar]
- 16.Cordeiro PG, Albornoz CR, McCormick B, et al. What is the optimum timing of postmastectomy radiotherapy in two-stage prosthetic reconstruction: radiation to the tissue expander or permanent implant? Plast Reconstr Surg. 2015;135:1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reish RG, Lin A, Phillips NA, et al. Breast reconstruction outcomes after nipple-sparing mastectomy and radiation therapy. Plast Reconstr Surg. 2015;135:959–966. [DOI] [PubMed] [Google Scholar]
- 18.Seth AK, Silver HR, Hirsch EM, et al. Comparison of delayed and immediate tissue expander breast reconstruction in the setting of postmastectomy radiation therapy. Ann Plast Surg. 2015;75:503–507. [DOI] [PubMed] [Google Scholar]
- 19.Muresan H, Lam G, Cooper BT, et al. Impact of evolving radiation therapy techniques on implant-based breast reconstruction. Plast Reconstr Surg. 2017;139:1232e–1239e. [DOI] [PubMed] [Google Scholar]
- 20.Elswick SM, Harless CA, Bishop SN, et al. Prepectoral implant-based breast reconstruction with postmastectomy radiation therapy. Plast Reconstr Surg. 2018;142:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jagsi R, Momoh AO, Qi J, et al. Impact of radiotherapy on complications and patient-reported outcomes after breast reconstruction. J Natl Cancer Inst. 2018;110:157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith NL, Jethwa KR, Viehman JK, et al. Post-mastectomy intensity modulated proton therapy after immediate breast reconstruction: Initial report of reconstruction outcomes and predictors of complications. Radiother Oncol. 2019;140:76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang L, Jin K, Wang X, et al. The impact of radiotherapy on reoperation rates in patients undergoing mastectomy and breast reconstruction. Ann Surg Oncol. 2019;26:961–968. [DOI] [PubMed] [Google Scholar]
- 24.Naoum GE, Salama L, Niemierko A, et al. Single stage direct-to-implant breast reconstruction has lower complication rates than tissue expander and implant and comparable rates to autologous reconstruction in patients receiving postmastectomy radiation. Int J Radiat Oncol Biol Phys. 2020;106:514–524. [DOI] [PubMed] [Google Scholar]
- 25.Olinger TA, Berlin NL, Qi J, et al. Outcomes of immediate implant-based mastectomy reconstruction in women with previous breast radiotherapy. Plast Reconstr Surg. 2020;145:1029e–1036e. [DOI] [PubMed] [Google Scholar]
- 26.Reverberi C, Marinelli L, Campanella B, et al. Post-mastectomy immediate breast reconstruction and adjuvant radiotherapy: long term results of a mono institutional experience. Radiol Med. 2020;125:887–893. [DOI] [PubMed] [Google Scholar]
- 27.Ogita M, Nagura N, Kawamori J, et al. Risk factors for complications among breast cancer patients treated with post-mastectomy radiotherapy and immediate tissue-expander/permanent implant reconstruction: a retrospective cohort study. Breast Cancer. 2018;25:167–175. [DOI] [PubMed] [Google Scholar]
- 28.Naoum GE, Oladeru OT, Niemierko A, et al. Optimal breast reconstruction type for patients treated with neoadjuvant chemotherapy, mastectomy followed by radiation therapy. Breast Cancer Res Treat. 2020;183:127–136. [DOI] [PubMed] [Google Scholar]
- 29.Dave RV, Vucicevic A, Berrett E, et al. Risk factors for complications and implant loss after prepectoral implant-based immediate breast reconstruction: medium-term outcomes in a prospective cohort. Br J Surg. 2020;108:534–541. [DOI] [PubMed] [Google Scholar]
- 30.Sekiguchi K, Kawamori J, Yamauchi H. Breast reconstruction and postmastectomy radiotherapy: complications by type and timing and other problems in radiation oncology. Breast Cancer. 2017;24:511–520. [DOI] [PubMed] [Google Scholar]
- 31.See MS, Farhadi J. Radiation therapy and immediate breast reconstruction: novel approaches and evidence base for radiation effects on the reconstructed breast. Clin Plast Surg. 2018;45:13–24. [DOI] [PubMed] [Google Scholar]
- 32.Brennan ME, Flitcroft K, Warrier S, et al. Immediate expander/implant breast reconstruction followed by post-mastectomy radiotherapy for breast cancer: Aesthetic, surgical, satisfaction and quality of life outcomes in women with high-risk breast cancer. Breast. 2016;30:59–65. [DOI] [PubMed] [Google Scholar]
- 33.Jagsi R, Jiang J, Momoh AO, et al. Complications after mastectomy and immediate breast reconstruction for breast cancer: a claims-based analysis. Ann Surg. 2016;263:219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sue GR, Sun BJ, Lee GK. Complications after two-stage expander implant breast reconstruction requiring reoperation: a critical analysis of outcomes. Ann Plast Surg. 2018;80(5S Suppl 5):S292–S294. [DOI] [PubMed] [Google Scholar]
- 35.Sacotte R, Fine N, Kim JY, et al. Assessing long-term complications in patients undergoing immediate postmastectomy breast reconstruction and adjuvant radiation. Pract Radiat Oncol. 2017;7:e91–e97. [DOI] [PubMed] [Google Scholar]
- 36.Punglia RS, Ortiz Pimentel S, Cronin AM, et al. Patient-preferred outcomes measurement after post-mastectomy radiation therapy and immediate reconstruction. Breast J. 2020;26:319–321. [DOI] [PubMed] [Google Scholar]
- 37.Peled AW, Sears M, Wang F, et al. Complications after total skin-sparing mastectomy and expander-implant reconstruction: effects of radiation therapy on the stages of reconstruction. Ann Plast Surg. 2018;80:10–13. [DOI] [PubMed] [Google Scholar]
- 38.Razdan SN, Cordeiro PG, Albornoz CR, et al. Cost-effectiveness analysis of breast reconstruction options in the setting of postmastectomy radiotherapy using the BREAST-Q. Plast Reconstr Surg. 2016;137:510e–517e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Colwell AS, Smith BL. Complications after mastectomy and immediate breast reconstruction for breast cancer: how does the community compare? Ann Surg. 2016;263:228–229. [DOI] [PubMed] [Google Scholar]
- 40.Hirsch EM, Seth AK, Kim JYS, et al. Analysis of risk factors for complications in expander/implant breast reconstruction by stage of reconstruction. Plast Reconstr Surg. 2014;134:692e–699e. [DOI] [PubMed] [Google Scholar]
- 41.Mitchell MP, Wagner J, Butterworth J. Subcutaneous implant-based breast reconstruction, a modern challenge in postmastectomy radiation planning. Pract Radiat Oncol. 2018;8:153–156. [DOI] [PubMed] [Google Scholar]
- 42.Naoum GE, Salama L, Ho A, et al. The impact of chest wall boost on reconstruction complications and local control in patients treated for breast cancer. Int J Radiat Oncol Biol Phys. 2019;105:155–164. [DOI] [PubMed] [Google Scholar]
- 43.Ota D, Fukuuchi A, Iwahira Y, et al. Identification of complications in mastectomy with immediate reconstruction using tissue expanders and permanent implants for breast cancer patients. Breast Cancer. 2016;23:400–406. [DOI] [PubMed] [Google Scholar]
- 44.Roberts A, Baxter N, Camacho X, et al. Once is rarely enough: a population-based study of reoperations after postmastectomy breast reconstruction. Ann Surg Oncol. 2015;22:3302–3307. [DOI] [PubMed] [Google Scholar]
- 45.Wilkins EG, Hamill JB, Kim HM, et al. Complications in postmastectomy breast reconstruction: one-year outcomes of the Mastectomy Reconstruction Outcomes Consortium (MROC) study. Ann Surg. 2018;267:164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Colwell AS, Christensen JM. Nipple-sparing mastectomy and direct-to-implant breast reconstruction. Plast Reconstr Surg. 2017;140(5S Advances in Breast Reconstruction):44S–50S. [DOI] [PubMed] [Google Scholar]
- 47.Lam TC, Winch CJ. What would women choose when given a choice in breast reconstruction? Plast Reconstr Surg Glob Open. 2016;4:e1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pirro O, Mestak O, Vindigni V, et al. Comparison of patient-reported outcomes after implant versus autologous tissue breast reconstruction using the BREAST-Q. Plast Reconstr Surg Glob Open. 2017;5:e1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qureshi AA, Odom EB, Parikh RP, et al. Patient-reported outcomes of aesthetics and satisfaction in immediate breast reconstruction after nipple-sparing mastectomy with implants and fat grafting. Aesthet Surg J. 2017;37:999–1008. [DOI] [PubMed] [Google Scholar]
- 50.Santosa KB, Qi J, Kim HM, et al. Long-term patient-reported outcomes in postmastectomy breast reconstruction. JAMA Surg. 2018;153:891–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schnarrs RH, Carman CM, Tobin C, et al. Complication rates with human acellular dermal matrices: retrospective review of 211 consecutive breast reconstructions. Plast Reconstr Surg Glob Open. 2016;4:e1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sgarzani R, Negosanti L, Morselli PG, et al. Patient satisfaction and quality of life in DIEAP flap versus implant breast reconstruction. Surg Res Pract. 2015;2015:405163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woo A, Harless C, Jacobson SR. Revisiting an old place: single-surgeon experience on post-mastectomy subcutaneous implant-based breast reconstruction. Breast J. 2017;23:545–553. [DOI] [PubMed] [Google Scholar]
- 54.Zhu L, Mohan AT, Abdelsattar JM, et al. Comparison of subcutaneous versus submuscular expander placement in the first stage of immediate breast reconstruction. J Plast Reconstr Aesthet Surg. 2016;69:e77–e86. [DOI] [PubMed] [Google Scholar]
- 55.de Bakker E, van den Broek LJ, Ritt MJPF, et al. The histological composition of capsular contracture focussed on the inner layer of the capsule: an intra-donor Baker-I versus Baker-IV comparison. Aesthetic Plast Surg. 2018;42:1485–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pu Y, Mao TC, Zhang YM, et al. The role of postmastectomy radiation therapy in patients with immediate prosthetic breast reconstruction: a meta-analysis. Medicine (Baltimore). 2018;97:e9548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Momoh AO, Ahmed R, Kelley BP, et al. A systematic review of complications of implant-based breast reconstruction with prereconstruction and postreconstruction radiotherapy. Ann Surg Oncol. 2014;21:118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spear SL, Onyewu C. Staged breast reconstruction with saline-filled implants in the irradiated breast: recent trends and therapeutic implications. Plast Reconstr Surg. 2000;105:930–942. [DOI] [PubMed] [Google Scholar]
- 59.Cordeiro PG, Pusic AL, Disa JJ, et al. Irradiation after immediate tissue expander/implant breast reconstruction: outcomes, complications, aesthetic results, and satisfaction among 156 patients. Plast Reconstr Surg. 2004;113:877–881. [DOI] [PubMed] [Google Scholar]
- 60.Lee BT, A Adesiyun T, Colakoglu S, et al. Postmastectomy radiation therapy and breast reconstruction: an analysis of complications and patient satisfaction. Ann Plast Surg. 2010;64:679–683. [DOI] [PubMed] [Google Scholar]
- 61.Barry M, Kell MR. Radiotherapy and breast reconstruction: a meta-analysis. Breast Cancer Res Treat. 2011;127:15–22. [DOI] [PubMed] [Google Scholar]
- 62.Ascherman JA, Hanasono MM, Newman MI, et al. Implant reconstruction in breast cancer patients treated with radiation therapy. Plast Reconstr Surg. 2006;117:359–365. [DOI] [PubMed] [Google Scholar]
- 63.Unukovych D, Sandelin K, Wickman M, et al. Breast reconstruction in patients with personal and family history of breast cancer undergoing contralateral prophylactic mastectomy, a 10-year experience. Acta Oncol. 2012;51:934–941. [DOI] [PubMed] [Google Scholar]
- 64.Seth AK, Hirsch EM, Fine NA, et al. Utility of acellular dermis-assisted breast reconstruction in the setting of radiation: a comparative analysis. Plast Reconstr Surg. 2012;130:750–758. [DOI] [PubMed] [Google Scholar]
- 65.McCarthy CM, Mehrara BJ, Riedel E, et al. Predicting complications following expander/implant breast reconstruction: an outcomes analysis based on preoperative clinical risk. Plast Reconstr Surg. 2008;121:1886–1892. [DOI] [PubMed] [Google Scholar]
- 66.Goodwin SJ, McCarthy CM, Pusic AL, et al. Complications in smokers after postmastectomy tissue expander/implant breast reconstruction. Ann Plast Surg. 2005;55:16–19; discussion 19. [DOI] [PubMed] [Google Scholar]
- 67.Alderman AK, Wilkins EG, Kim HM, et al. Complications in postmastectomy breast reconstruction: two-year results of the Michigan Breast Reconstruction Outcome Study. Plast Reconstr Surg. 2002;109:2265–2274. [DOI] [PubMed] [Google Scholar]
- 68.Krueger EA, Wilkins EG, Strawderman M, et al. Complications and patient satisfaction following expander/implant breast reconstruction with and without radiotherapy. Int J Radiat Oncol Biol Phys. 2001;49:713–721. [DOI] [PubMed] [Google Scholar]
- 69.Contant CM, van Geel AN, van der Holt B, et al. Morbidity of immediate breast reconstruction (IBR) after mastectomy by a subpectorally placed silicone prosthesis: the adverse effect of radiotherapy. Eur Surg Oncol. 2000;26:344–350. [DOI] [PubMed] [Google Scholar]
- 70.Williams JK, Carlson GW, Bostwick J, III, et al. The effects of radiation treatment after TRAM flap breast reconstruction. Plast Reconstr Surg. 1997;100:1153–1160. [DOI] [PubMed] [Google Scholar]
- 71.Rogers NE, Allen RJ. Radiation effects on breast reconstruction with the deep inferior epigastric perforator flap. Plast Reconstr Surg. 2002;109:1919–1924; discussion 1925. [DOI] [PubMed] [Google Scholar]
- 72.Chawla AK, Kachnic LA, Taghian AG, et al. Radiotherapy and breast reconstruction: complications and cosmesis with TRAM versus tissue expander/implant. Int J Radiat Oncol Biol Phys. 2002;54:520–526. [DOI] [PubMed] [Google Scholar]
- 73.Hu ES, Pusic AL, Waljee JF, et al. Patient-reported aesthetic satisfaction with breast reconstruction during the long-term survivorship period. Plast Reconstr Surg. 2009;124:1–8. [DOI] [PubMed] [Google Scholar]
- 74.Magill LJ, Robertson FP, Jell G, et al. Determining the outcomes of post-mastectomy radiation therapy delivered to the definitive implant in patients undergoing one- and two-stage implant-based breast reconstruction: a systematic review and meta-analysis. J Plast Reconstr Aesthet Surg. 2017;70:1329–1335. [DOI] [PubMed] [Google Scholar]