Abstract
Objective:
To investigate the applications of robot-assisted surgery and its effect on surgical outcomes in orthopaedic trauma patients.
Data Sources:
A search was performed in PubMed and Embase for articles in English, Dutch, German, or French, without restrictions on follow-up times, study size, or year of publication.
Study Selection:
Studies were included if they investigated patients undergoing robot-assisted fracture fixation surgery for orthopaedic trauma.
Data Extraction:
Outcomes studied were operating time, fluoroscopy time/frequency, complications, functional outcomes, intraoperative blood loss, fracture healing, and screw placement accuracy. Critical appraisal was done by using the Methodological Index for Non-Randomized Studies.
Data Synthesis:
Narrative review.
Conclusions:
A total of 3832 hits were identified with the search and 8 studies were included with a combined total of 437 included patients, 3 retrospective cohort studies, 2 prospective cohort studies, 1 cohort study not otherwise specified, 1 case series, and 1 randomized controlled trial. Four studies investigated pelvic ring fractures, 3 studies investigated femur fractures, and 1 study investigated scaphoid fractures. Seven investigated percutaneous screw fixation and 1 studied intramedullary nail fixation. One robotic system was used across all studies, the TiRobot, and all procedures were performed in China. The limited evidence suggests that that robot-assisted orthopaedic trauma surgery may reduce operating time, use of fluoroscopy, intraoperative blood loss, and improve screw placement accuracy, but the overall quality of evidence was low with a high risk of bias. Robot-assisted fracture fixation does not appear to lead to better functional outcomes for the patient.
Level of evidence: III
Keywords: fracture fixation, orthopaedic trauma, robot-assisted, systematic review, trauma surgery
1. Introduction
Robotic surgery techniques are emerging in many specialties, such as surgical oncology, urology, endocrine surgery, and cardiac surgery.[1–4] There are many advantages to the use of surgical robots. In general surgery, they improve dexterity and hand-eye coordination, they can provide the surgeon with a more ergonomic position and make surgical approaches possible that were previously thought technically impossible.[1,5] Other advantages include a wider range of motion, better three-dimensional (3D) visualization compared with laparoscopic procedures, and the ability to perform telesurgery that minimizes radiation exposure.[1,6] Most surgical robots cost between $1 and $2,5 million, making the required initial investment one of the major obstacles for the widespread implementation of robotic surgery.[3,7] Other disadvantages are the loss of haptic sensation, size of the machines, and the required trained staff in the operating theater.[1] It is possible that these disadvantages will improve over time, as is often the case with technological advance.[1]
For the purpose of this review, the difference between robot-assisted surgery and computer-assisted surgical navigation should be clarified. Computer-assisted surgical navigation comprises any type of computer-based procedure that uses advanced technology such as 3D imaging or augmented reality in planning performing surgical procedures. Robotic surgery involves the use of an advanced surgical robot, where the surgeon may or may not be present at the operating table. A surgical robot is a computerized system that can assist with surgical navigation, often with an arm capable of performing certain surgical tasks with the help of instruments attached to the arm such as a guidance sleeve. Robots may be controlled by the surgeon or partially autonomous, and sometimes the surgeon does not have to be present in the operation theatre at all (i.e., telesurgical procedures). It should be noted that most robotic systems are designed to be compatible with computer-assisted navigation systems.[2,6,8] In summary, robots in surgery are used for assistance in surgical navigation, but not all computer-assisted surgical navigation systems are robots.
In orthopaedics, a few review studies have been done investigating the application and efficacy of robotics.[9–12] Robots have been extensively used in spine surgery for the placement of pedicle screws and have been shown to give better outcomes then conventional techniques.[9,10] Robots have also been used in hip arthroplasty and total knee arthroplasty, but there is no conclusive evidence that robots are superior to the conventional technique, since surgery times are much longer, costs are high, and complication rates are higher in robotic surgery groups.[9,10] Although these review papers also claim to investigate trauma, their primary focus was elective orthopaedic surgery.[9–11] This review investigating robot-assisted fracture fixation in orthopaedic trauma surgery provides an overview of the current applications in traumatology. The aim of this study is to investigate the application of robot-assisted surgery and its effect on surgical outcomes in orthopaedic trauma patients.
2. Methods
This systematic review was written in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses PRISMA guidelines.[13] The study protocol was registered in the international prospective register of systematic reviews (PROSPERO ID CRD42020167808). This was a literature review, hence no patients were included in this study. The study was deemed exempt from Institutional Review Board and Animal Use Committee Review.
2.1. Literature search and study selection
A systematic search was done in PubMed and EMBASE on July 26, 2019 using search terms and synonyms for orthopaedics, trauma, fracture, and robotics. A professional medical librarian helped build the search syntax (Supplement 1, http://links.lww.com/OTAI/A20). No filters were applied for the search. Authors HJS and DH independently assessed title and abstracts for eligibility. Full-text screening was done when at least one of the authors deemed a study eligible. Disagreements between authors were solved by consensus. For all included studies, forward and backward citation tracking was done to identify any possible additional studies. Screening was done using the Rayyan application by Qatar Computing Research Institute, Doha, Qatar.[14]
2.2. Eligibility criteria
Studies were included if they met the inclusion criteria: the studied population was made up of trauma patients undergoing surgery for orthopaedic trauma (i.e., traumatic fractures of the appendicular skeleton or pelvis, nonpathological). Surgical procedures studied were robot-assisted fixation or partially robot-assisted fixation of the fracture. Study designs were either randomized controlled trails, observational cohort studies (comparative and noncomparative), case-series, or retrospective cohort studies. Studied outcome was at least one of the following; operation time, fluoroscopy time, fluoroscopy frequency, postoperative mortality, postoperative complications, postoperative physical performance and functional outcomes, patient-reported outcomes, ergonomic outcomes for the surgeon, perioperative blood loss (mL), fracture healing, and screw placement accuracy for percutaneous interventions (Gras-Marintschev, Hamelinck, or Liebergal classification).[15–17] Full text was available in English, Dutch, French, or German. Papers studying computer-assisted surgical navigation without the use of a robot were excluded. There were no restrictions regarding follow-up times, study size, patient age, or year of publication.
2.3. Data extraction, quality assessment, and data synthesis
Data was extracted by author HJS. In addition to the outcomes specified in the inclusion criteria, the following general study information was collected; first author name, year of publication, country, study design, mean/median age, percentage of female participants, sample size, fracture type, and length of follow-up. The level of evidence for each study was determined by using the guidelines of the American Academy of Orthopaedic Surgeons.[18] Data on surgical procedures were extracted; type of procedure, type of robot used, robot planning time (i.e., time required to set up system and complete surgical planning by the robot), duration of surgical procedure, fluoroscopy time (i.e., total time in seconds that the fluoroscope was emitting ionizing radiation), and fluoroscopy frequency (i.e., the number of times that fluoroscopy imaging was used intraoperatively).
For quality assessment, the studies were rated using the Methodological Index for Non-Randomized Studies.[19] The principal approach to data synthesis was a narrative review of the results. A meta-analysis was not performed due to the heterogeneity of the results.
3. Results
3.1. Study selection
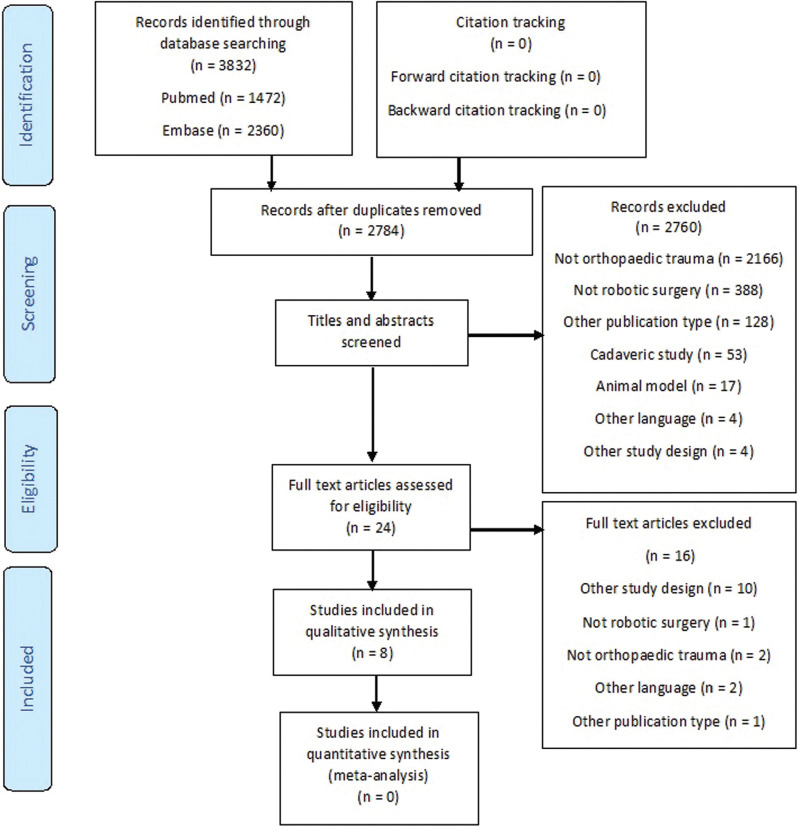
A total of 3832 hits were identified with the search (Fig. 1). After removal of duplicates and title and abstract screening, 24 full-text articles were reviewed, 8 of which were included in this systematic review.[20–27] After forward and backward citation tracking of the selected articles, no additional studies were identified that met the inclusion criteria.
Figure 1.
PRISMA flow chart. This figure shows the study selection process at each stage.
3.2. Study characteristics
All studies originated from China and were published between 2017 and 2019 (Table 1). Sample size ranged between 10[25] and 91[26] patients with a combined total of 437 patients. The aim of the identified studies was to report the application and initial results of robot-assisted fracture fixation. Mean/median age ranged between 31[25] and 76[21] years (unweighted average 48 years) and there were between 0%[25] and 62%[20] female patients (weighed average 45%). The same robotic system was used across all included studies, the TiRobot (TINAVI, China).[20–27] There were 6 cohort studies,[20,21,23,24,26,27] 1 case series,[25] and 1 randomized controlled trail.[22] Four studies investigated pelvic ring fractures,[22–24,26] 3 studies investigated proximal femur fractures,[20,21,27] and 1 study investigated nondisplaced scaphoid fractures.[25] Two surgical procedures were described; 7 studies investigated percutaneous screw fixation,[20,22–27] and 1 study investigated intramedullary nailing.[21] Six studies compared robot-assisted surgery to a cohort of patients undergoing conventional surgery.[20–23,26,27]
Table 1.
Study characteristics
| Author year | Study design | Type of fracture studied | Surgical procedure | Lvl | Mean/median age (in years) | Female (%) | Sample size | Length of follow-up (mo) |
|---|---|---|---|---|---|---|---|---|
| Tao Long 2019 | Prospective cohort | Posterior pelvic ring fractures | Percutaneous screw fixation | III | Mean 36 ± 8 | 42% | Total: 91 participantsRobot group: 66 screwsConventional group 43 screws | 8–32 |
| Hua-shui Liu 2019 | Retrospective cohort | Anterior and posterior pelvic ring fractures | Percutaneous screw fixation | III | Mean 40.2 ± 13.6 | 34% | Total: 86 participantsRobot group: 86Conventional group: N/A | 3–6 |
| Jun-Qiang Wang 2017 | RCT | Posterior pelvic ring fractures | Percutaneous screw fixation | II | Robot group median 43.0 IQR (35–52)Conventional group median 36.0 IQR (25.0–47.0)P value .340 | 40% | Total: 45 participantsRobot group: 23Conventional group: 22 | N/A |
| Hua-shui Liu 2018 | Cohort | Unstable pelvic ring fractures | Percutaneous screw fixation | III | Robot group mean 37.4 ± 6.6Control group mean 39.8 ± 7.1P value .270 | 38% | Total: 45 participantsRobot group: 24Conventional group: 21 | 3 |
| Hai Lan 2019 | Retrospective cohort | Intertrochanteric femur fractures | Intramedullary nail fixation | III | Mean 76 | 51% | Total: 51 participantsRobot group: 25Conventional group: 26 | 12–24 |
| Sheng-jun Duan 2019 | Prospective cohort | Femoral neck fractures | Percutaneous screw fixation | III | Robot group mean 61.7 ± 5.2Conventional group mean 62.1 ± 4.1P value .727 | 59% | Total: 49 participantsRobot group: 26Conventional group: 23 | N/A |
| Meng He 2019 | Retrospective cohort | Femoral neck fractures | Percutaneous screw fixation | III | Robot group mean 56 (range 39–82)Conventional group 56.2 (range 30–84)Distribution not specified, P value not reported | 62% | Total: 60 participantsRobot group: 30Conventional group: 30 | 12–24 |
| Bo Liu 2019 | Case series | Nondisplaced scaphoid fractures | Percutaneous screw fixation | IV | Mean 31 (range 27–56) | 0% | Total: 10 participantsRobot group: 10Conventional group: N/A | 6–8 |
3.3. Study quality and risk of bias assessment
The authors identified 1 level II study,[22] 6 level III studies,[20,21,23,24,26,27] and 1 level IV study.[25] The overall risk of bias was considered high across all studies (Table 2). The mean Methodological Index for Non-Randomized Studies score was 14.4 (range 8[25]–19[22,23]).[20,21,23–27] None of the studies reported a sample size calculation and were likely underpowered.[20–27] Since all but one study lacked a detailed description of outcome measurement collection, studies were prone to data collection bias. Except for the study by Liu et al,[23] it was not reported whether patients were included consecutively, resulting in a high risk of selection bias.[20–22,24–27] Although He et al[20] claimed to conduct a randomized trial, the study design was a retrospective cohort study, and no explanation was given as to how randomization was achieved; therefore, the authors classified this study as a retrospective cohort.
Table 2.
Study quality and risk of bias assessment
| MINORS 1–7 | MINORS 8–12 for comparative studies | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||||
| Author | Design | Stated aim | Inclusion of consecutive patients | Prospective data collection | Appropriate endpoint | Unbiased evaluation of endpoints | Follow-up period appropriate | Loss to follow-up <5% | Control group with golden standard intervention | Contemporary groups | Baseline equivalance of groups | Prospective calculation of sample size | Statistical analysis suitable for study design | Total score | Maximum score |
| Tao Long, 2019 | Prospective cohort | 2 | 0 | 2 | 2 | 0 | 2 | 0 | 2 | 2 | 2 | 0 | 2 | 16 | 24 |
| Hua-shui Liu, 2019 | Retrospective cohort study | 2 | 0 | 1 | 2 | 1 | 2 | 1 | N/A | N/A | N/A | N/A | N/A | 9 | 14 |
| Hua-shui Liu, 2018 | Cohort study | 2 | 2 | 0 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 0 | 19 | 24 | |
| Hai Lan, 2019 | Retrospective cohort study | 2 | 0 | 1 | 2 | 1 | 1 | 2 | 2 | 2 | 2 | 0 | 2 | 17 | 24 |
| Sheng-jun Duan, 2019 | Prospective cohort | 2 | 0 | 1 | 2 | 0 | 2 | 0 | 2 | 2 | 2 | 0 | 2 | 15 | 24 |
| Meng He, 2019 | Retrospective cohort study | 1 | 0 | 1 | 2 | 0 | 2 | 0 | 2 | 2 | 1 | 0 | 1 | 12 | 24 |
| Bo Liu, 2019 | Case series | 2 | 0 | 0 | 2 | 0 | 2 | 2 | N/A | N/A | N/A | N/A | N/A | 8 | 14 |
| Jun-Qiang Wang, 2017 | RCT | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 0 | 2 | 19 | 24 |
3.4. Outcomes
The studies reported the following outcomes: robot planning time, operating time, fluoroscopy time/frequency, screw placement accuracy, intraoperative blood loss, postoperative physical performance and functional outcomes, and wound/fracture healing time (Tables 3 and 4).[20–27]
Table 3.
Surgical outcomes
| Author Year | Outcomes studied | Operating time (min) | Robot planning time (min) | Fluoroscopy frequency | Fluoroscopy time (min/s) | Screw placement |
|---|---|---|---|---|---|---|
| Tao Long 2019 | Operating time (min), planning time (min), fluoroscopy frequency, fluoroscopy time (min), length of incision, intraoperative blood loss (mL), anesthesia time (min), wound healing and fracture results, fracture reduction (Matta standard), Majeed function | Robot group: 33.25 ± 6.46conventional group: 63.55 ± 6.62P < .001 | 6.71 ± 4.19 | Robot group: 8.49 ± 2.37Conventional group: 18.67 ± 4.18P < .001 | Robot group 5.88 ± 1.29 (min)Conventional group: 11.05 ± 2.98 (min)P < .001 | N/A |
| Hua-shui Liu 2019 | Operating time (min), fluoroscopy frequency, fluoroscopy time (sec), screw placement accuracy, incision length, blood loss, facture healing time, Majeed score | Robot group: 175 ± 32.6 | N/A | 29.1 ± 10.5 per screw | 6.1 ± 0.2 (s) per screw | Positioning error 2.31 ± 1.03 mmAngular error 2.24 ± 1.32° |
| Jun-Qiang Wang 2017 | Operating time after reduction of the pelvis, robot planning time, fluoroscopy time after reduction pelvic (sec), screw placement accuracy (GrasMarintschev), number of guidewire attempts | Robot group: median 150.0IQR (75–230)Conventional group: median 104.0IQR (60.0–154.0)P = .158 | Median 7.8 | N/A | Robot group:median 6.0 IQR 6.0–9.0 (sec)Conventional group:median 36.0 IQR 21.5–48.0 (s)P < .001 | Robot group:23 excellent, 0 good, 0 poorConventional group:16 excellent, 5 good, 1 poorP = 0.009 |
| Hua-shui Liu 2018 | Operating time, fluoroscopy frequency, total number of drills, intraoperative blood loss, fracture healing, Majeed score, activities of daily living, pain, gait, walking distance, standing, presence of nerve damage | Robot group: 65.4 ± 10.9Conventional group: 86.7 ± 14.7P < .01 | N/A | Robot group: 29.2 ± 7.6Conventional group: 52.3 ± 12.4P < .001 | N/A | N/A |
| Hai Lan 2019 | Operating time, fluoroscopy frequency, total number of drills, intraoperative bleeding, fracture healing, Harris hip score | Robot group: 65.44 ± 8.01Conventional group: 77.50 ± 16.64P = .002 | N/A | Robot group: 10.28 ± 0.61Conventional group: 13.23 ± 1.75P < .001 | N/A | N/A |
| Sheng-jun Duan 2019 | Operating time, fluoroscopy frequency, screw placement accuracy (Hamelinck), total number of drills, intraoperative bleeding, fracture healing, Harris hip score | Robot group: 77.3 ± 9.3Conventional group: 79 ± 9.8P = .547 | Included in total operation time for robot group | Robot group: 28.6 ± 9.6Conventional group: 46.7 ± 12.4P < .001 | N/A | Screw parrelellism (points) robot group: 24.0 ± 0.6 conventional group 21.5 ± 1.2 (P < .001)Triangular area (mm2) robot group 72.0 ± 6.7 conventional group 53.8 ± 10.4 (P < .001) |
| Meng He 2019 | Robot planning time, fluoroscopy time, total number of drills, screw placement (Liebergal), Harris hip score | N/A | 2.8 | N/A | Robot group: 5.65 (sec) per screwConventional group: 14.14 (sec) per screwP < .01 | Anteroposterior dispersion (%) for robot group 35.13; for conventional group 85.29 (P value < 0.01)Lateral dispesion (%) for robot navigation group 70.08; for conventional group 58.29 (P value < .01)Anteroposterior screw shaft angle (°) for robot group 1.08; for conventional group 1.2 (P value .438)Lateral screw shaft angle (°) for robot group 1.25; for conventional group 1.82 (P value .028) |
| Bo Liu 2019 | Operating time, fracture healing time, Mayo wrist score | 40 (range 27–56) | Included in total operation time for robot group | N/A | N/A | N/A |
Table 4.
Patient outcomes
| Author Year | Intraoperative blood loss (mL) robot group | Intraoperative blood loss (mL) conventional group | P value | Fracture-healing time robot group (mo) | Fracture-healing result conventional group (mo) | P value | Functional outcomes | P value |
|---|---|---|---|---|---|---|---|---|
| Tao Long 2019 | 33.89 ± 16.4 (15–80) | 43.04 ± 12.34 (30–80) | <.001 | 4.61 ± 0.68 (3.5–6.3) | 4.56 ± 0.78 (3.4–6.2) | .53 | Majeed functionRobot group 49 excellent, 2 good, 5 fair, 0 poorConventional group 30 excellent, 2 good, 3 fair, 0 poor | NS |
| Hua-shui Liu 2019 | 35.2 ± 3.6 (5–50) | N/A | N/A | 3 mo | N/A | N/A | Majeed functionRobot group: 57 cases excellent, 26 good, 3 fair | N/A |
| Jun-Qiang Wang 2017 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Hua-shui Liu 2018 | 35.0 ± 7.2 | 46.2 ± 9.3 | <.001 | 4.3 ± 0.7 | 4.5 ± 1.1 | .45 | Majeed function (score)Robot group: 86.4 ± 7.2Conventional group: 84.3 ± 10.3 | .43 |
| Hai Lan 2019 | 90.80 ± 14.98 | 118 ± 32.21 | <.001 | Fracture healing rate100% at follow-up | Fracture healing rate100% at follow-up | NS | Harris hip scoreRobot group: 86.68 ± 6.23Conventional group: 82.69 ± 6.85 | .034 |
| Sheng-jun Duan 2019 | 9.5 ± 6.8 | 41.3 ± 12.4 | <.001 | 4.6 ± 1.9 | 5.3 ± 2.1 | .223 | Harris hip scoreRobot group: 88.3 ± 4.4Conventional group: 87.6 ± 3.9 | .559 |
| Meng He 2019 | N/A | N/A | N/A | N/A | N/A | N/A | Harris hip scoreRobot group: 85.20Conventional group: 83.45 | NS |
| Bo Liu 2019 | N/A | N/A | N/A | mean 8 wks (range 7–10) | N/A | Mayo wrist score robot group: 96 (85–100) | N/A |
3.4.1. Robot planning time
Three studies reported planning time for the robot, ranging between 2.8 and 7.8 minutes (Table 3).[22,26,27] Long et al[26] reported a mean robot planning time of 6.71 minutes with a standard deviation of 4.19 for percutaneous sacroiliac screw placement for posterior pelvic ring fractures. Wang et al[22] reported a median planning time of 7.8 minutes for percutaneous sacroiliac screw placement for posterior pelvic ring fractures. He et al[20] reported a mean planning time of 2.8 minutes for percutaneous screw placement in femoral neck fractures. Two studies included robot planning time in the calculation of operating time.[25,27]
3.4.2. Operating time
Three studies found a statistically significant reduction in operating time (Table 3).[21,23,26] Long et al[26] reported a mean operating time of 33.25 minutes (± 6.46) for the robot-assisted surgery group for percutaneous screw fixation of posterior pelvic ring fractures, compared with 63.55 minutes (± 6.62) in the conventional surgery group (P < .001). Liu et al[23] reported a mean operating time of 65.4 minutes (± 10.9) in the robot-assisted surgery group for percutaneous screw fixation of unstable pelvic ring fractures compared to 86.7 (± 14.7) in the conventional surgery group (P < .01). Lan et al[21] reported a mean operating time of 65.44 minutes (± 8.01) for robot-assisted intramedullary nailing of intertrochanteric femur fractures, compared with 77.50 minutes (± 16.64) in the conventional surgery group (P = .002). Two studies reported an increase in operating time, but this increase was not statistically significant in either study. [22,27]
3.4.3. Fluoroscopy frequency
Two studies investigated fluoroscopy frequency in percutaneous screw fixation for pelvic ring fractures (Table 3). Long et al[26] reported a mean in the robot-assisted surgery group of 8.49 (± 2.37), compared with 18.67 (± 4.18) in the conventional surgery group (P < .001). Liu et al[23] reported a mean of 29.2 (± 7.7) in the robot-assisted surgery group versus 52.3 (± 12.4) in the conventional surgery cohort (P < .001). Lan et al[21] found a mean frequency of 10.28 (± 0.61) in the robot-assisted group for intramedullary nailing of intertrochanteric femur fractures, compared with 13.23 (± 1.75) in the conventional surgery cohort (P < .001). For percutaneous screw fixation of femoral neck fractures, Duan et al[27] reported a frequency of 28.6 (± 9.6) in the robot-assisted surgery group versus 46.7 (± 12.4) in the conventional surgery group (P < .001).
3.4.4. Fluoroscopy time
For pelvic ring fractures, Long et al[26] reported an average of 5.88 minutes (± 1.29) in the Robot group versus 11.05 (±2.98) in the conventional group (P < .001), and Wang et al[22] reported a median of 6.0 (IQR 6.0–9.0) seconds versus 36 (IQR 21.5–48.0) in the conventional group (P < .001) (Table 3). It was not specified whether this was the total intraoperative fluoroscopy time or time per screw. For percutaneous screw fixation of femoral neck fractures, He et al[20] reported an average of 5.65 seconds per screw, compared to 14.14 seconds in the conventional surgery group.
3.4.5. Screw placement accuracy
Liu et al[24] reported a positioning error of 2.31 ± 1.03 mm and an angular error of 2.24 ± 1.32 degrees for robot-assisted insertion of percutaneous screws for pelvis ring fractures (Table 3). Wang et al used the Gras-Marintschev classification to measure screw placement for posterior pelvic ring fractures and reported superior screw placement in the robot group (P = .009).[15,22] Duan et al used the Hamelinck classification in their study investigating percutaneous pinning of femoral neck fractures to assess screw parallelism (points) and triangular area (mm2) and showed a statistically significant difference in favor of the robot-assisted group.[16,27] He et al used a method described by Liebergal et al to measure anteroposterior dispersion, lateral dispersion, anteroposterior screw shaft angle, and lateral screw shaft angle for percutaneous pinning of femoral neck fractures and found a statistically significant difference in favor of the robot group.[17,20] The authors of this review postulate that screw placement is difficult to measure, and outcomes such as parallelism and triangular area may not be measures that are clinically important. For example, one could argue that if a satisfactory reduction was accomplished with no nerve injury, that the accuracy is 100%.
3.4.6. Intraoperative blood loss
For pelvic fractures, Long et al reported a mean intraoperative blood loss of 33.89 mL (±16.4) for the robot group, versus 43.04 mL (± 12.34) in the conventional cohort (P < .001),[26] and Liu et al reported a loss of 35.0 mL (± 7.2) for the robot group and 46.2 mL (± 9.3) for the conventional group (P < .001) (Table 4).[23] Lan et al[21] reported a loss of 98.8 mL (± 14.98) in the robot group and 118 mL (± 32.31) in the conventional surgery group for the intramedullary nailing of intertrochanteric femur fractures (P < .001). Duan et al[27], reported a loss of 9.5 mL (± 6.8) in the robot group and 41.3 mL (± 12.4) in the conventional surgery group (P < .001) for percutaneous screw fixation of femoral neck fractures. No consensus exists on the definition of clinically important intraoperative blood loss, and the Standardised Endpoints for Perioperative Medicine collaborative is currently conducting a review to reach consensus on this matter.[28] The authors of this review do not consider the blood loss as reported in the included papers of this review to be clinically important.
3.4.7. Postoperative physical performance and functional outcomes
For pelvic ring fractures, 3 studies reported patient outcomes after a follow-up period using the Majeed score (Table 4).[23,24,26,29] The overall outcomes were excellent or good in both the robot and conventional surgery group, and there were no statistically significant differences between groups.[23,24,26] For hip fractures, the Harris Hip Score was used to measure functional outcome in 3 studies investigating hip fractures.[20,21,27,30] Lan et al found a statistically significant difference in favor of the robot group; the mean Harris Hip Score was 86.68 (± 6.23) in the robot group and 82.69 (± 6.85) in the conventional group (P = .034); however, this difference is not clinically significant.[21,31] The other 2 studies by He et al and Duan et al reported no significant difference between the robot-assisted surgery group and the conventional control group.[20,27] Liu et al reported a mean Mayo wrist score of 96 (range 85–100) for scaphoid fractures treated with robot-assisted percutaneous screw fixation at follow-up.[25,32]
3.4.8. Fracture-healing time
Six studies reported fracture-healing times,[21,23–27] 4 of which made a comparison with a conventional surgery control group, none of these studies found a significant difference in fracture-healing time between groups (Table 4).[21,23,26,27]
4. Discussion
This review identified 8 studies that reported on the application of robot-assisted surgery in orthopaedic trauma.[20–27] The overall quality of evidence was considered low with a high risk of bias.
4.1. The robotic system
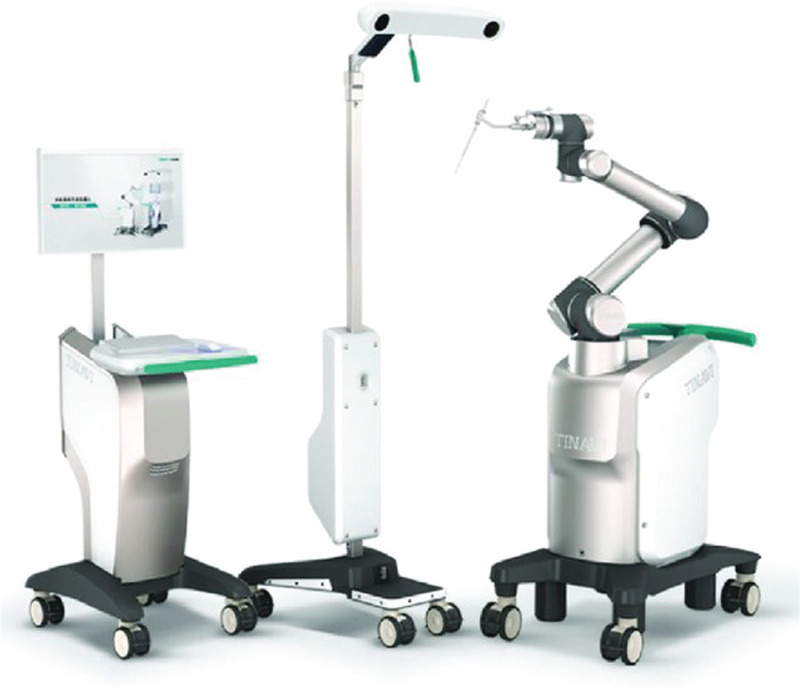
Only 1 robotic system was identified in this review, the TiRobot (TINAVI Medical Technologies, Bejing, China).[33] The TiRobot is a robotic surgical guidance system that uses an intelligent algorithm to calculate guidance wire and screw trajectories, using a combination of 3D imaging reconstructed from radiography and optical real-time guidance and navigation. It does not perform other surgical tasks besides navigation and guidance.
This robotic system consists of 3 parts (Fig. 2).[34] First, a robotic arm with 6 degrees of freedom, which can hold surgical tools and guide screw insertion. It is designed for maximum reach and a small footprint in the operating theatre and can be operated both automatically and manually. Second, an optical tracking station that uses an infrared stereo camera and 1 or more reference frames that are attached to the patient to help guide the positioning of the robotic arm. Third, an integrated navigation and planning station, which uses intraoperative fluoroscopy images made with a 3D C-arm (Siemens Medical Solutions, Erlangen, Germany).[33,35]
Figure 2.
The TiRobot. The TiRobot consists of a planning station, optical tracker, and a robotic arm to assist with surgical guidance. Figure previously published under a creative commons licence.[34]
The authors of this paper assume that the TiRobot system is not available outside of China, but were unable to confirm this with the producer. On their website, DePuy Synthes (Johnson & Johnson Medical Devices, Shanghai, China) announced their future collaboration with TINAVI.[36] This might indicate that steps are being taken to introduce this technology to the rest of the world. Other orthopaedic robotic systems, such as the Robodoc and MAKO, are being used in arthroplasty, but not in orthopaedic trauma surgery.[9–11]
4.2. Interpretation of results
4.2.1. Operation time and robot planning time
It was unclear for most studies whether robot planning time was included in the calculation of total operating time. Studies that made a comparison between a robot group and a conventional group reported operating times of approximately 1 or 2 hours in both groups. The (unweighted) pooled reduction was 21 minutes, which seems low, considering the cost of robotic systems and the required time investment for training surgeons and other OR-personnel.
4.2.2. Fluoroscopy time and frequency
The occupational health hazard that results from radiation in orthopaedic trauma surgery is often underestimated.[37,38] The overall evidence found in this review suggests that robot-assisted surgery can help reduce the total amount of radiation exposure for both the surgeon and the patient. The TiRobot still requires the surgeon to be present at the operating table. This review did not identify papers describing robotic systems that could be completely controlled remotely, a feature that could potentially eliminate radiation exposure for the surgeon entirely.
4.2.3. Screw placement accuracy
Robot-assisted procedures showed more accurate percutaneous screw placement across all papers that studied this outcome. Although accurate screw placement is of vital importance in percutaneous fixation, it remains unclear whether this improved accuracy is clinically important. Nevertheless, more accurate screw placement may be an important advantage of this technique. For example, insertion of sacroiliac screws is a relatively uncommon procedure. It is a difficult procedure with a steep learning curve that carries with it the risk of iatrogenic injury of neurovascular structures with aberrant screw placement.[39–41]
4.2.4. Intraoperative blood loss
All studies with conventional surgery as a control group found statistically significant less intraoperative blood loss in the robot group (P < .001).[21,23,26,27] Overall, intraoperative blood loss was low in both the robot-assisted surgery groups (90 mL or less) and control groups (118 mL or less). The biggest reduction of intraoperative blood loss (32 mL) was found in the study by Duan et al,[27] but this reduction is likely not clinically significant.
4.2.5. Postoperative physical performance and functional outcomes
Functional outcomes between robot-assisted procedures and conventional surgery were comparable, although most studies were likely underpowered to detect significant differences. Lan et al[21] found that Harris Hip Score in the robot-assisted group was 4 points higher on average after intramedullary nailing for intertrochanteric fractures. However, the Harris Hip Score has a minimally clinically important difference of 8 points, and therefore this statistically significant difference is not clinically significant.[31]
4.2.6. Fracture healing
Robot-assisted surgery did not affect fracture healing time in the included studies. The authors speculate that it is unlikely that the use of a robot significantly affects fracture healing, and that this outcome may not be the most relevant for future investigations.
4.2.7. Strengths and limitations
This study has several limitations. First, because this is a relatively new development in the field of traumatology, only 8 studies met the inclusion criteria for this review. The heterogeneity of the studies made it difficult to summarize the evidence in a clear and concise manner and precluded us from performing a meta-analysis. Second, papers published in Mandarin were excluded. The authors identified several papers that were written in Mandarin. These papers had no English abstract and were unavailable in full text. It is possible that this has led to selection bias. Third, the overall quality of included studies was low with a high risk of bias.
This is the first systematic review to describe the applications of robot-assisted fracture fixation surgery in orthopaedic trauma surgery and its effect on surgical and patient outcomes. This review shows that the clinical application of robot-assisted fracture fixation surgery has only recently emerged in traumatology and so far only in China. More importantly, this review identified pitfalls and limitations of current investigations and made recommendations for future research in this new field of orthopaedic trauma surgery.
4.2.8. Future perspectives and recommendations for future studies
Although extensively studied in arthroplasty, there are few publications reporting the clinical application of robot-assisted surgery in orthopaedic trauma.[9,10] As shown in this review, advantages of robot-assisted trauma surgery may include reduced operating time, improved percutaneous screw placement accuracy, lower blood loss, and lower radiation exposure to both surgeon and patient. The purpose of the system is to assist with surgical guidance, but there are a few drawbacks that should be pointed out. First, the improvement of outcomes seems low considering the required investment to purchase the equipment and train personnel. Second, there is some question about the clinical relevance of the improvement of outcomes. The main advantage of the technology appears to be in increasing the accuracy of percutaneous screws. This may have a role in reducing the risk of neurovascular injury in percutaneous pelvic fracture surgery—particularly when performed by low volume surgeons. Third, this technology is new, and is currently only available in China. Fourth, as shown in this review, there is very little high-quality research in this field from which reliable conclusions can be drawn. Ergonomics might be another possible advantage of robot-assisted surgery, which may improve ergonomics for the surgeon.[5,42] Unfortunately, none of the studies included in this review reported ergonomic outcomes for the surgeon, and the authors recommend that these outcomes are included in future investigations. It remains to be seen whether robot-assisted fracture fixation will be the future in orthopaedic trauma, or a solution to a nonexistent problem. The authors of this study also recommend that outcomes in future studies should be clearly defined (e.g., specify whether robot planning time is included in total operation time), and include outcomes relevant to the surgeon and/or the patient.
5. Conclusion
The emergence of robot-assisted orthopaedic trauma surgery is a new development in orthopaedic trauma. There is limited evidence that suggests that robot-assisted orthopaedic trauma surgery may reduce operating time, use of fluoroscopy, intraoperative blood loss, and improve screw placement accuracy. However, for most studies it was unclear how outcomes were measured, and there is some question about the clinical relevance of the marginally improved outcomes. There is currently no conclusive evidence that robot-assisted fixation in orthopaedic trauma surgery leads to better functional outcomes for either the patient or the surgeon. More high-quality research is needed in this field.
References
- 1.Lanfranco AR, Castellanos AE, Desai JP, Meyers WC. Robotic surgery: a current perspective. Ann Surg. 2004;239:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mack MJ. Minimally invasive and robotic surgery. J Am Med Assoc. 2001;285:568–572. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed K, Ibrahim A, Wang TT, et al. Assessing the cost effectiveness of robotics in urological surgery—a systematic review. BJU Int. 2012;110:1544–1556. [DOI] [PubMed] [Google Scholar]
- 4.Ruurda JP, van der Sluis PC, van der Horst S, van Hilllegersberg R. Robot-assisted minimally invasive esophagectomy for esophageal cancer: a systematic review. J Surg Oncol. 2015;112:257–265. [DOI] [PubMed] [Google Scholar]
- 5.Van Der Schatte Olivier RH, Van’t Hullenaar CDP, Ruurda JP, Broeders IAMJ. Ergonomics, user comfort, and performance in standard and robot-assisted laparoscopic surgery. Surg Endosc. 2009;23:1365–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ballantyne GH. Robotic surgery, telerobotic surgery, telepresence, and telementoring: review of early clinical results. Surg Endosc Other Interv Tech. 2002;16:1389–1402. [DOI] [PubMed] [Google Scholar]
- 7.Turchetti G, Palla I, Pierotti F, Cuschieri A. Economic evaluation of da Vinci-assisted robotic surgery: a systematic review. Surg Endosc. 2012;26:598–606. [DOI] [PubMed] [Google Scholar]
- 8.Sugano N. Computer-assisted orthopaedic surgery and robotic surgery in total hip arthroplasty. Clin Orthop Surg. 2013;5:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karuppiah K, Sinha J. Robotics in trauma and orthopaedics. Ann R Coll Surg Engl. 2018;100:8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karthik K, Colegate-Stone T, Dasgupta P, Tavakkolizadeh A, Sinha J. Robotic surgery in trauma and orthopaedics: a systematic review. Bone Jt J. 2015;97-B:292–299. [DOI] [PubMed] [Google Scholar]
- 11.Janipireddy SB, Saeed ZA, Saeed MZ. Role of robotics in trauma and orthopaedics. Int J Res Med Sci. 2017;5:3268. [Google Scholar]
- 12.Jacofsky DJ, Allen M. Robotics in arthroplasty: a comprehensive review. J Arthroplasty. 2016;31:2353–2363. [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG, Group TP. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gras F, Marintschev I, Wilharm A, Klos K, Mückley T, Hofmann GO. 2D-fluoroscopic navigated percutaneous screw fixation of pelvic ring injuries—a case series. BMC Musculoskelet Disord. 2010;11:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamelinck HKM, Haagmans M, Snoeren MM, Biert J, Van Vugt AB, Frölke JPM. Safety of computer-assisted surgery for cannulated hip screws. Clin Orthop Relat Res. 2007;455:241–245. [DOI] [PubMed] [Google Scholar]
- 17.Liebergall M, Ben-David D, Weil Y, Peyser A, Mosheiff R. Computerized navigation for the internal fixation of femoral neck fractures. J Bone Jt Surg. 2006;88:1748. [DOI] [PubMed] [Google Scholar]
- 18. Okike K. Evidence-Based Orthopaedics: Levels of Evidence and Guidelines in Orthopaedic Surgery. In: Orthopaedic Knowledge Update 10, American Academy of Orthopaedic Surgeons. 2011; 157–165. [Google Scholar]
- 19.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712–716. [DOI] [PubMed] [Google Scholar]
- 20.He M, Han W, Zhao CP, et al. Evaluation of a bi-planar robot navigation system for insertion of cannulated screws in femoral neck fractures. Orthop Surg. 2019;11:373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lan H, Tan Z, Li KN, Gao JH, Liu TH. Intramedullary nail fixation assisted by orthopaedic robot navigation for intertrochanteric fractures in elderly patients. Orthop Surg. 2019;11:255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang JQ, Wang Y, Feng Y, et al. Percutaneous sacroiliac screw placement: a prospective randomized comparison of robot-assisted navigation procedures with a conventional technique. Chin Med J (Engl). 2017;130:2527–2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu HS, Duan SJ, Liu SD, Jia FS, Zhu LM, Liu MC. Robot-assisted percutaneous screw placement combined with pelvic internal fixator for minimally invasive treatment of unstable pelvic ring fractures. Int J Med Robot Comput Assist Surg. 2018;14:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu HS, Duan SJ, Xin FZ, Zhang Z, Wang XG, Liu SD. Robot-assisted minimally-invasive internal fixation of pelvic ring injuries: a single-center experience. Orthop Surg. 2019;11:42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu B, Wu F, Chen S, Jiang X, Tian W. Robot-assisted percutaneous scaphoid fracture fixation: a report of ten patients. J Hand Surg Eur Vol. 2019;44:685–691. [DOI] [PubMed] [Google Scholar]
- 26.Long T, Li KN, Gao JH, et al. Comparative study of percutaneous sacroiliac screw with or without tirobot assistance for treating pelvic posterior ring fractures. Orthop Surg. 2019;11:386–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu HS, Wu WC, Wu WC, et al. Robot-assisted percutaneous cannulated screw fixation of femoral neck fractures: preliminary clinical results. Orthop Surg. 2019;11:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bartoszko J, Vorobeichik L, Jayarajah M, et al. Defining clinically important perioperative blood loss and transfusion for the Standardised Endpoints for Perioperative Medicine (StEP) collaborative: a protocol for a scoping review. BMJ Open. 2017;7:e016743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Majeed SA. Grading the outcome of pelvic fractures. J Bone Joint Surg Br. 1989;71-B:304–306. [DOI] [PubMed] [Google Scholar]
- 30.Harris William H. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty: an end-result study using a new method of result evaluation. J Bone Jt Surg. 1969;51:737–755. [PubMed] [Google Scholar]
- 31.Çelik D, Çoban Ö, Kılıçoğlu Ö. Minimal clinically important difference of commonly used hip-, knee-, foot-, and ankle-specific questionnaires: a systematic review. J Clin Epidemiol. 2019;113:44–57. [DOI] [PubMed] [Google Scholar]
- 32.Cooney WP, Bussey R, Dobyns JH, et al. Difficult wrist fractures. Perilunate fracture-dislocations of the wrist. Clin Orthop Relat Res. 1987;136–147. [PubMed] [Google Scholar]
- 33. TINAVI Intelligent Medical Solutions. TiRobot Introduction. https://www.tinavi.com/index.php?m=content&c=index&a=lists&catid=9. Published 2020. [Google Scholar]
- 34.Feng S, Tian W, Wei Y. Clinical effects of oblique lateral interbody fusion by conventional open versus percutaneous robot-assisted minimally invasive pedicle screw placement in elderly patients. Orthop Surg. 2020;12:86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tian W, Wang H, Liu YJ. Robot-assisted anterior odontoid screw fixation: a case report. Orthop Surg. 2016;8:400–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Johnson&Johnson Medical Devices Companies. Johnson & Johnson Medical Shanghai and TINAVI Sign Strategic Collaboration to Expand Digital Surgery Footprint for Spine and Trauma Surgery. Available at: https://www.jnjmedicaldevices.com/en-US/news-events/johnson-johnson-medical-shanghai-and-tinavi-sign-strategic-collaboration-expand-digital. Published 2020. in press. [Google Scholar]
- 37.Mastrangelo G, Fedeli U, Fadda E, Giovanazzi A, Scoizzato L, Saia B. Increased cancer risk among surgeons in an orthopaedic hospital. Occup Med (Lond). 2005;55:498–500. [DOI] [PubMed] [Google Scholar]
- 38.Hafez MA, Smith RM, Matthews SJ, Kalap G, Sherman KP. Radiation exposure to the hands of orthopaedic surgeons: are we underestimating the risk? Arch Orthop Trauma Surg. 2005;125:330–335. [DOI] [PubMed] [Google Scholar]
- 39.Chip Routt ML, Simonian PT, Mills WJ. Iliosacral screw fixation: early complications of the percutaneous technique. J Orthop Trauma. 1997;11:584–589. [DOI] [PubMed] [Google Scholar]
- 40.Rush R, Ginsberg HJ, Jenkinson R, Whyne CM. Beyond the operating room: a simulator for sacroiliac screw insertion. Surg Innov. 2008;15:321–323. [DOI] [PubMed] [Google Scholar]
- 41.Riehl J, Widmaier J. A simulator model for sacroiliac screw placement. J Surg Educ. 2012;69:282–285. [DOI] [PubMed] [Google Scholar]
- 42.Berguer R, Smith W. An ergonomic comparison of robotic and laparoscopic technique: the influence of surgeon experience and task complexity. J Surg Res. 2006;134:87–92. [DOI] [PubMed] [Google Scholar]