PURPOSE
Cholangiocarcinomas (CCA) are a group of heterogeneous tumors arising from the biliary epithelia. Significant sequencing efforts have provided further insights into the molecular mechanisms of this disease including fibroblast growth factor receptor (FGFR) alterations, which occurs in approximately 15%-20% of intrahepatic CCAs. Herein, we describe the FGFR inhibitor (FGFRi)-associated treatment toxicity and cancer-specific outcomes from a multicenter single-institution cohort.
METHODS
This is a retrospective study of patients with CCA and known FGFR alterations treated with FGFRi. We describe the toxicity and efficacy in patients treated at Mayo Clinic between January 2010 and December 2020.
RESULTS
Our group identified 61 patients with advanced or metastatic CCA, 19 males (31%) and 42 females (69%), harboring FGFR alterations who received FGFRi. The most common grade 1 or higher adverse events for all patients included fatigue (92%), AST elevations (78%), anemia (80%), decreased platelet count (63%), and hyperphosphatemia (74%). Median progression-free survival on FGFRi was 5.8 months for all patients (95% CI, 4.9 to 9.0). Females had significantly longer progression-free survival at 6.9 months (95% CI, 5.2 to 11.8) on FGFRi compared with males at 4.9 months (95% CI, 2.8 to not estimable; P = .038).
CONCLUSION
FGFRi are well tolerated with clinical efficacy. With the recent approval of FGFRi by the US Food and Drug Administration and ongoing clinical trials for new FGFRi, understanding outcomes and toxicity associated with these medications is important for precision oncology.
INTRODUCTION
Cholangiocarcinoma (CCA) is an aggressive and rare epithelial malignancy of the biliary tract. Recent comprehensive sequencing efforts have identified actionable alterations in patients with CCA,1 including the genes encoding fibroblast growth factor receptors (FGFRs). FGFRs are tyrosine kinases that play a crucial role in cell proliferation, differentiation, migration, and survival.2 FGFR2 fusions or gene rearrangements are identified in 15%-20% of intrahepatic cholangiocarcinoma.3-5 Inhibition of FGFR signaling in CCA has demonstrated significant antitumor activity.5-10 Pemigatinib is the first FGFR inhibitor (FGFRi) to receive accelerated approval by the US Food and Drug Administration for patients with advanced or metastatic CCA harboring FGFR2 fusion or rearrangements. The approval indication requires progression on at least one prior line of therapy.8,11,12 Infigratinib is the second FGFRi approved for use in the same setting. As a class, common toxicities associated with FGFRi include hyperphosphatemia, fatigue, stomatitis, alopecia, blurry vision, and palmar-plantar erythrodysesthesia.5,13-15
CONTEXT
Key Objective
Two fibroblast growth factor receptor inhibitors are now approved for use in patients with fibroblast growth factor receptor alterations in cholangiocarcinoma. Toxicity and efficacy of individual drugs have been reported in their respective trials. Our study is the first to assess the toxicity and efficacy of this class of inhibitors in a real-world setting.
Knowledge Generated
As a class, common side effects included fatigue, elevation in liver enzymes, decreased platelets, and hyperphosphatemia. Duration of benefit is longer in females compared with males.
Relevance
FGFR inhibitors as a class have tolerable toxicity with clear clinical benefit in the second line and beyond. Early recognition of these side effects and interventions will mitigate these effects for improved outcome in precision oncology.
Herein, we described the characteristics, toxicity, and treatment outcomes among patients with CCA harboring FGFR alterations treated with different FGFRi from a multicenter single-institution experience.
METHODS
Study Population
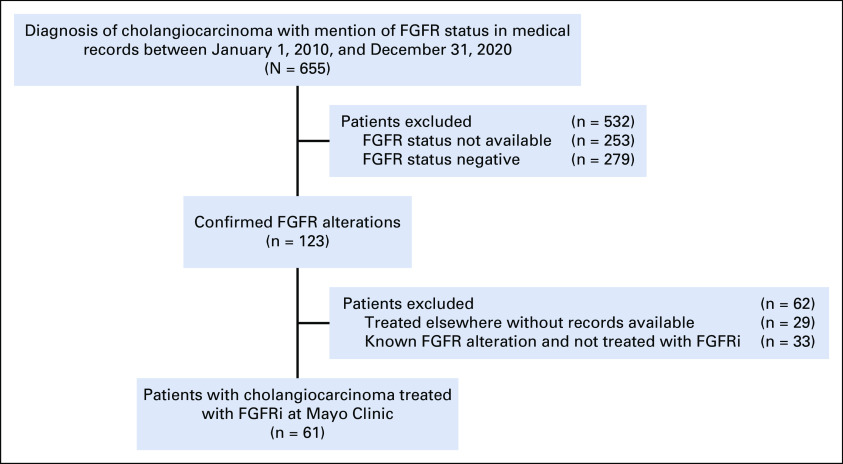
We conducted a retrospective study of patients with pathologic confirmed diagnosis of CCA treated at the Mayo Clinic Enterprise (Rochester, Arizona, and Florida) between January 1, 2010, and December 31, 2020. The study was reviewed and approved by the Mayo Clinic institutional review board. Patients and their clinical data were identified and obtained via a database using key search terms. The 61 identified patients had FGFR alterations obtained from clinical genomic reports including FoundationOne, TEMPUS, Guardant 360 (FoundationOne; Foundation Medicine, Cambridge, MA; TEMPUS, Chicago, IL; Guardant Health, Redwood City, CA), and internal clinical laboratory improvement amendments-validated fluorescence in situ hybridization break apart assay.16
Demographic characteristics including body mass index, body surface area, clinical history, diagnosis and tumor location, tumor stage and grade at diagnosis, systemic treatments received including FGFRi, and adverse events were recorded. Incidence of the following toxicities regardless of treatment attribution was collected: hyperphosphatemia, peripheral neuropathy, alopecia, paronychia, dry eye, blurry vision, palmar-plantar erythrodysesthesia, fatigue, and mucositis, and abnormalities of lipase, AST, ALT, alkaline phosphatase, total bilirubin, hemoglobin, platelet count, and WBC count. These toxicities were collected as they are commonly attributed to FGFRi use. Toxicity was graded according to the Common Terminology Criteria for Adverse Events version 5.0.17 Computed tomography and/or magnetic resonance imaging scans were used to assess for tumor response by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 criteria.
End Points
The primary outcome was toxicity; the highest grade experienced for each adverse event was used for analysis. High-grade toxicities were defined as a toxicity grade 3 or higher. Secondary outcomes were overall survival (OS) and progression-free survival (PFS). OS is defined as time from initiation of FGFRi until death, because of any cause. PFS is defined as time from initiation of FGFRi until disease progression, per RECIST 1.1. On average, scans were performed every 2 months while on FGFRi treatment. If the drug was discontinued because of toxicity and the patient had not progressed before the next treatment, the patient was censored at the last disease assessment before next line of therapy. Carbohydrate Antigen 19-9 (CA 19-9) was collected within two months of starting FGFRi therapy (baseline) and during FGFRi therapy. Best CA 19-9 response was defined as the lowest value during FGFRi therapy. Objective response was defined as the composite of complete response and partial response, per RECIST 1.1. Disease control was defined as the composite of complete response, partial response, and stable disease, per RECIST 1.1. Objective response rate and disease control rate (DCR) were calculated as proportion of patients experiencing objective response or disease control, respectively.
Statistical Analysis
Continuous variables were presented as medians with range, whereas categorical variables were expressed as count and percentages. The distribution of time-to-event end points was estimated by Kaplan-Meier curves.18 OS and PFS comparisons across sex categories were tested using log-rank test.19 Multivariable Cox proportional hazard model was used to assess the association between sex and PFS while adjusting for potential confounders, age (at FGFRi initiation), FGFR mutation type (fusion or rearrangement v other alterations), and stage (I or II v III or IV) and prior lines of therapy (1 v more than 1). A P value < .05 was considered statistically significant. All statistical analyses were performed using JMP 14.1 (SAS Institute, Cary, NC) and R version 3.6.2 (Vienna, Austria).
RESULTS
Demographics and Clinical Characteristics
Of 655 patients with CCA identified in our study, 123 (19%) had an identified FGFR alteration as demonstrated by next-generation sequencing or by fluorescence in situ hybridization assay. The remaining 532 patients were excluded from analysis because of absence of FGFR alteration or not having these results available on chart review. Of the 123 patients with an identified FGFR alteration, 61 (50%) patients were treated with an FGFRi at the Mayo Clinic, of which 19 (31%) were male and 42 (69%) were female. A CONSORT diagram is shown in Appendix Figure A1.
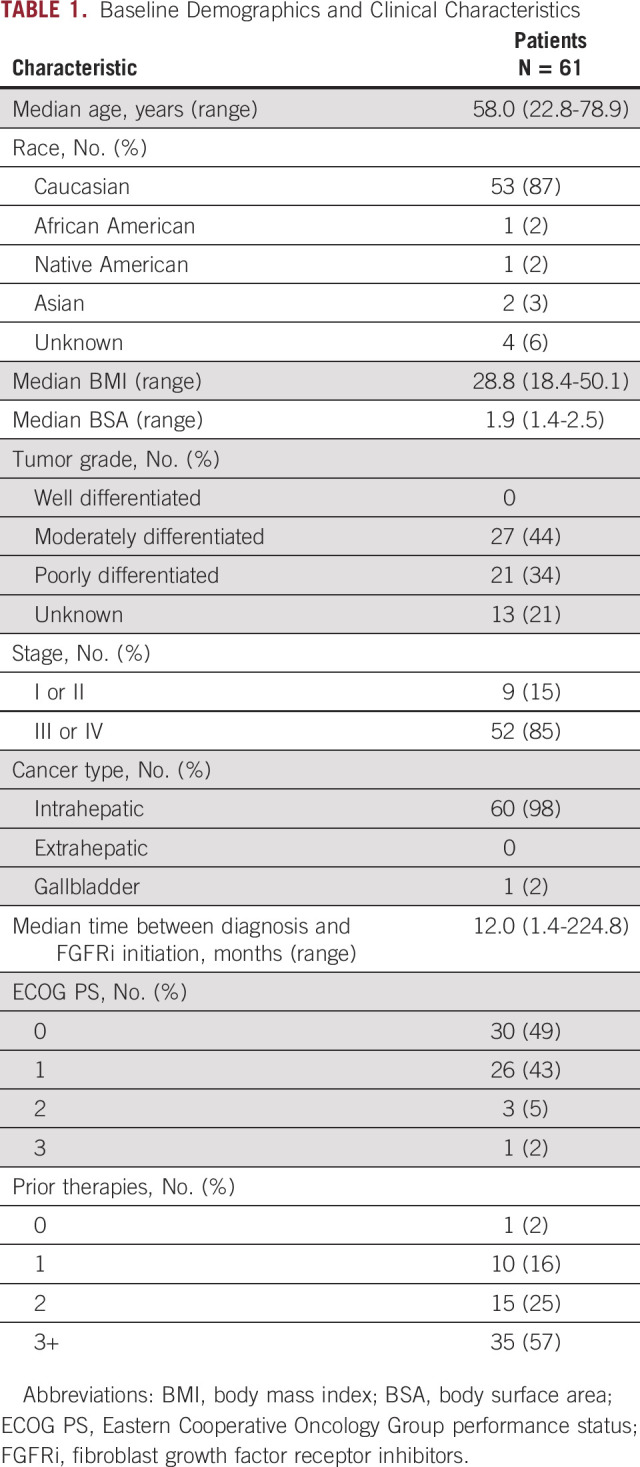
The median age of patients treated with an FGFRi was 58.0 years (range, 22.8-78.9 years). Fifty-three (87%) patients were Caucasian. Most patients had advanced disease (n = 52, 85%) and predominantly had intrahepatic cholangiocarcinoma (n = 60, 98%). Twenty-one (44%) patients had poorly differentiated histology. The median time between FGFR status identification and initiation of FGFRi therapy was 3.2 months (0.0-23.3 months). For all patients, the median time from diagnosis to initiation of FGFRi therapy was 12.0 months (1.4-224.8 months). Overall, the median follow-up time among patients who are alive was 2 years.
The median CA 19-9 (normal < 35 U/mL) at the time of FGFRi initiation was 64 U/mL (3-22,680 U/mL). The best median CA 19-9 response during FGFRi therapy was 62 U/mL (3-23,431 U/mL). Baseline patient demographics and clinical characteristics are summarized in Table 1.
TABLE 1.
Baseline Demographics and Clinical Characteristics
FGFR Inhibitors' and Mutations' Descriptions
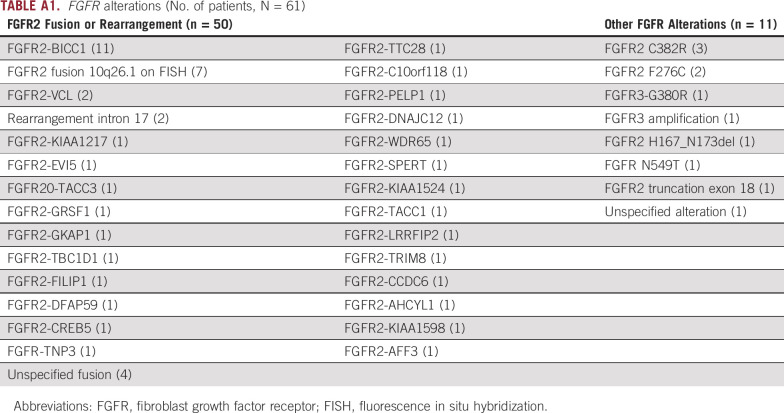
The 61 patients in our cohort were treated with FGFRi including ponatinib, pemigatinib, futibatinib, derazantinib, pazopanib, and infigratinib. A total of six patients received a second FGFRi during their treatment course. Appendix Table A1 showed the FGFR alterations that were identified in patients included in our study. Fifty-six patients (92%) had the exact alteration identified. The most common FGFR genetic alteration seen in our study was the FGFR2-BICC1 fusion (n = 11, 18%). Fifty (82%) patients had an FGFR2 fusion or rearrangement and 11 (18%) patients had other FGFR alterations. Appendix Table A2 demonstrates other clinically significant mutations identified on next-generation sequencing in all patients included in the study. Other clinically relevant mutations included BAP1 in 21 (34%), TP53 mutations in four (7%), and CDKN2A/B mutations in 13 (26%) patients. Thirty-five (57%) patients had microsatellite stability information available on next generation sequencing reports and all were microsatellite-stable (MSI-stable). Tumor mutational burden (TMB) status was available for 37 (61%) patients with a median TMB of 2.2 m/MB (0-13).
Toxicity
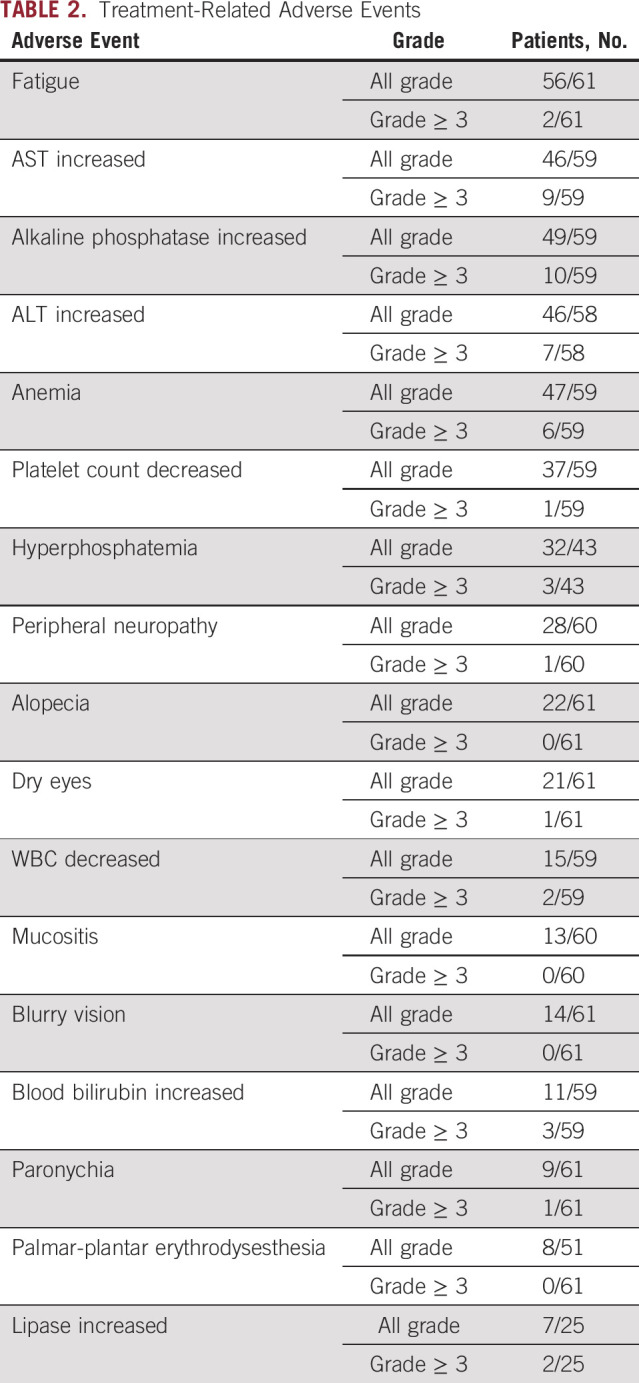
All patients in this study experienced at least one grade 1 or worse adverse event. The most common grade 1 or higher adverse events, regardless of attribution, for all patients included fatigue (92%), elevation of AST (78%), anemia (80%), decreased platelet count (63%), and hyperphosphatemia (74%; Table 2). Five (8%) patients required podiatry or dermatology appointments for paronychia. Ten (16%) patients were recommended a low-phosphorus diet and four (7%) patients met with a nutritionist related to their hyperphosphatemia. Fifteen (25%) patients were seen by an ophthalmologist for eye symptoms including dry eyes and/or blurry vision. Thirty-seven (61%) of patients had a documented eye examination before commencing treatment with an FGFRi. The most common grade 3 or higher events, irrespective of cause, included liver enzyme alterations (n = 9, 15%) and anemia (n = 6, 10%). Adverse event information is summarized in Table 2.
TABLE 2.
Treatment-Related Adverse Events
The main reason for discontinuing an FGFRi was progression of disease in 39 (63%) patients. At the time of this manuscript preparation, 16 (26%) patients were still undergoing treatment with an FGFRi. Five patients (8%) discontinued therapy because of side effects including cytopenias, cognitive changes, severe back pain, and persistently elevated liver enzymes. Significant fatigue, myalgias, hyperphosphatemia, cytopenias, elevated liver enzymes, back pain or abdominal pain, rash, and blurry vision led to dose reduction in 15 patients (25%).
Efficacy
Median PFS for patients treated with an FGFRi was 5.8 months (95% CI, 4.9 to 9.0; Fig 1A). Median OS from time of FGFRi initiation was 15.3 months (95% CI, 11.8 to 24.0; Fig 1B). At the time of this analysis, 34 (55%) patients had died from any cause. Median OS from time of diagnosis was 35.7 months (95% CI, 26.2 to 66.1; Appendix Fig A2). Of note, six patients received a second FGFRi therapy, and the median treatment duration for these patients on the second FGFRi was 4.0 months (range, 1.6-7.9 months).
FIG 1.
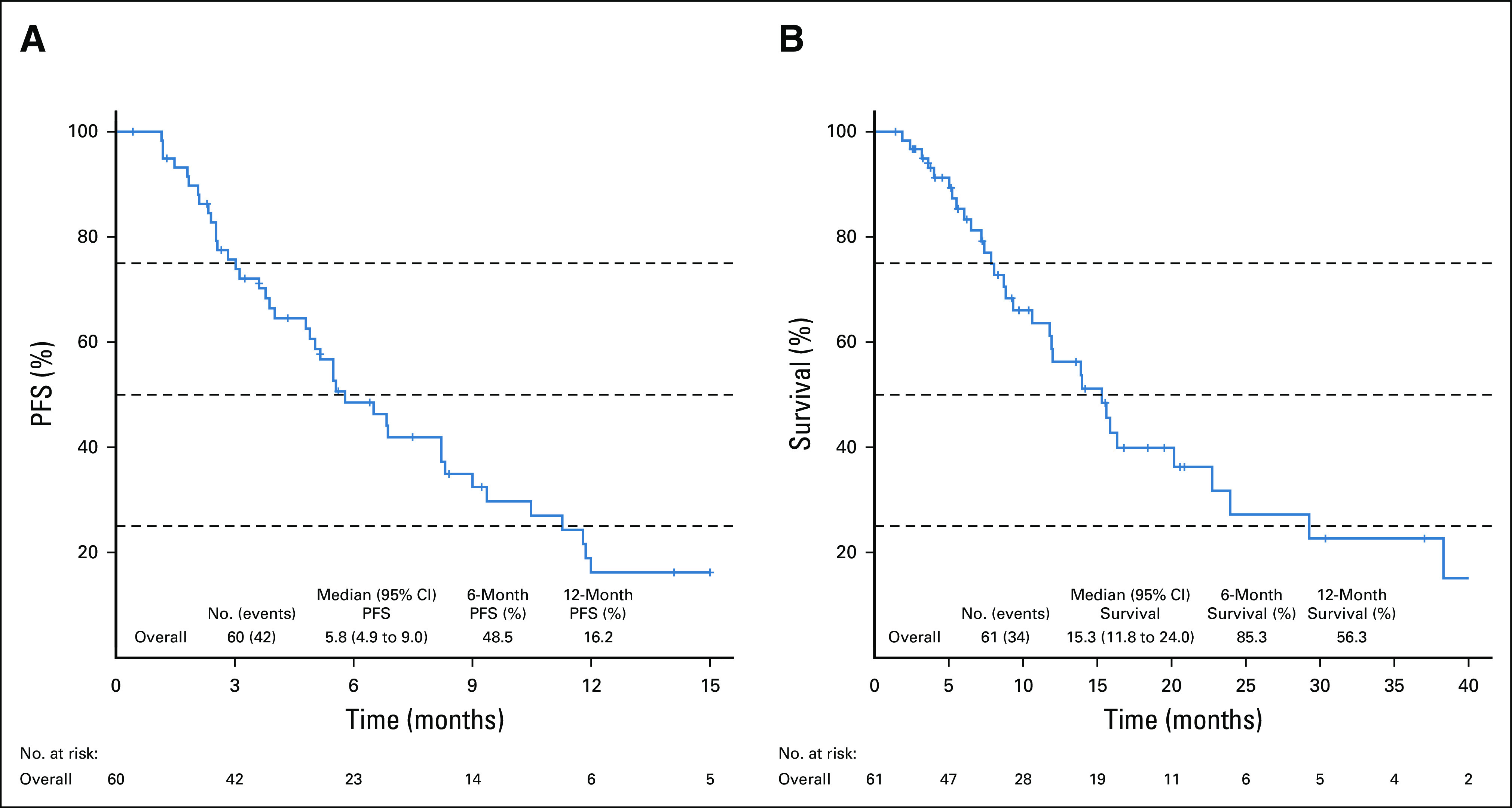
Survival curves for patients treated with an FGFRi. (A) PFS for patients treated with an FGFRi. (B) Overall survival for patients treated with an FGFRi. FGFRi, fibroblast growth factor receptor inhibitors; PFS, progression-free survival.
The objective response rate for the entire cohort of 61 patients was 13% (95% CI, 6.0 to 24.9). The DCR for all patients was 78% (95% CI, 65.3 to 87.7). Response rates to treatment with FGFRi are summarized in Table 3. Treatment duration, best response, treatment received, and mutation type are depicted in Figure 2. Best percent change from baseline in tumor measurement, treatment received, and mutation type are depicted in Figure 3.
TABLE 3.
Response to Treatment
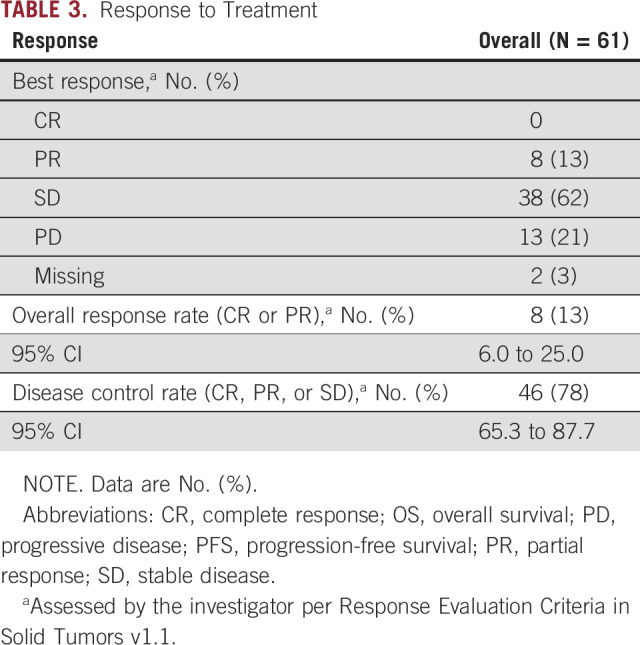
FIG 2.
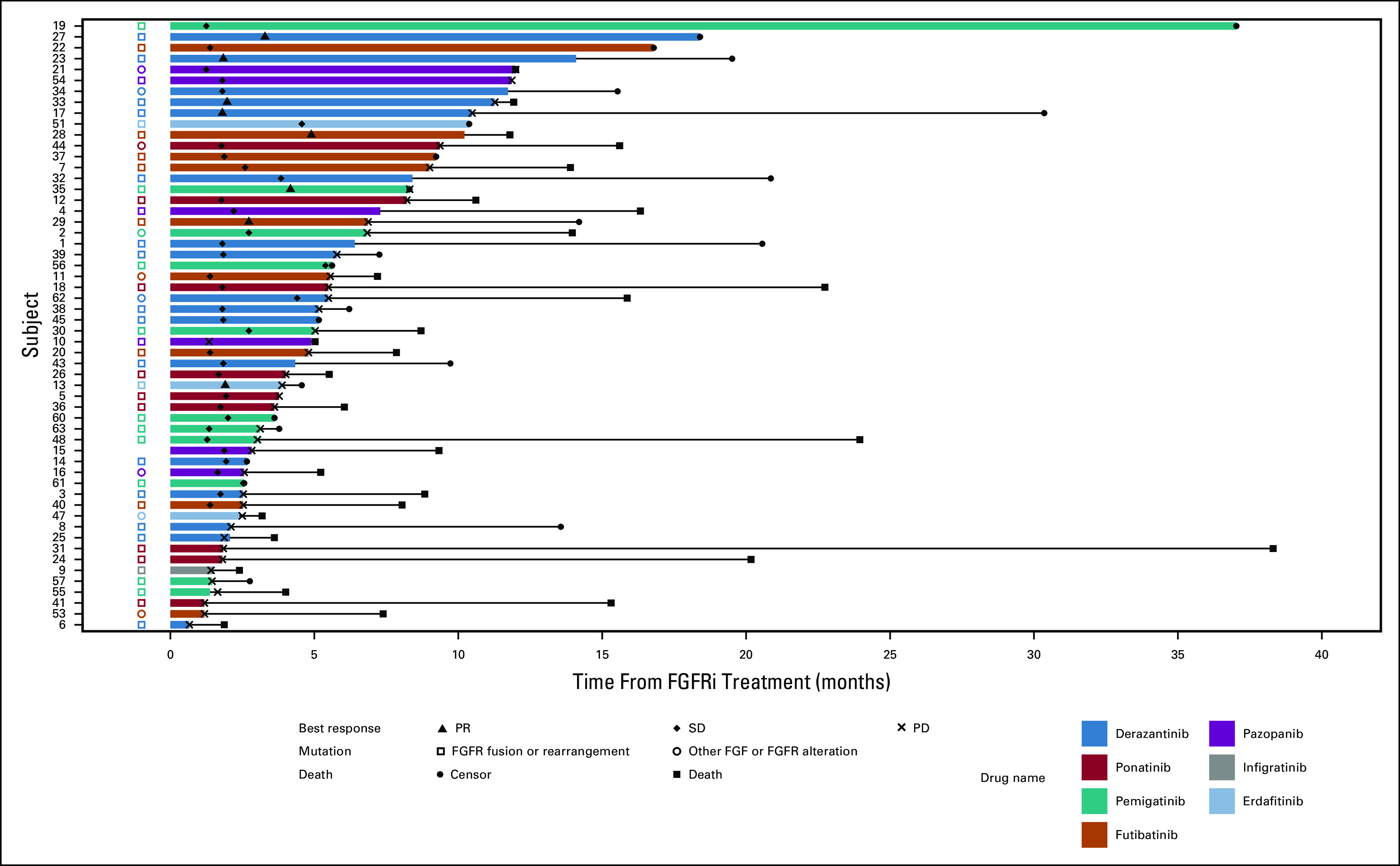
Swimmer plot of PFS of patients treated with FGFRi. FGFR, fibroblast growth factor receptor; FGFRi, fibroblast growth factor receptor inhibitors; PD, progressive disease; PFS, progression-free survival; PR, partial response; SD, stable disease.
FIG 3.
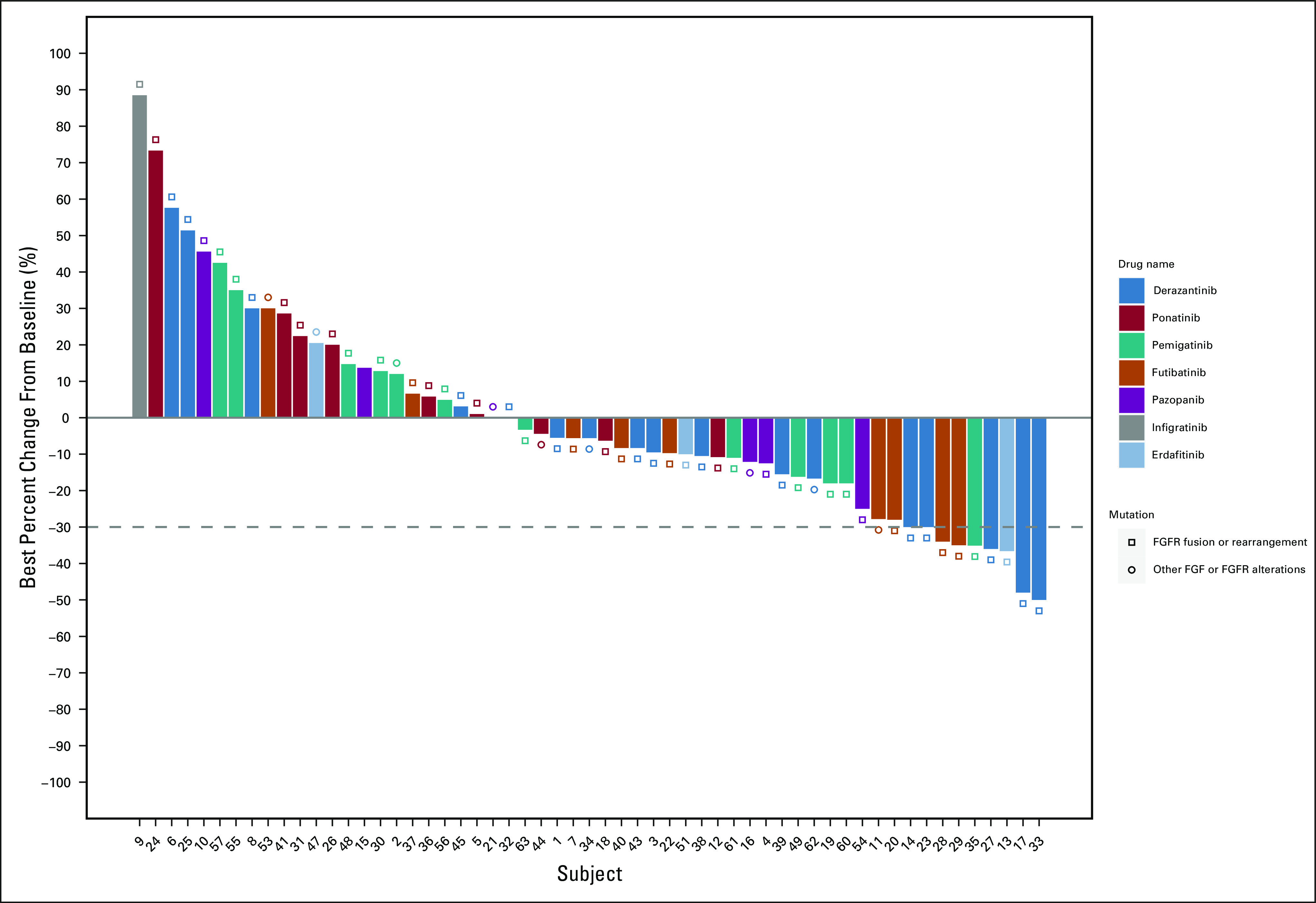
Waterfall plot of response rates in patients treated with FGFRi. FGFR, fibroblast growth factor receptor; FGFRi, fibroblast growth factor receptor inhibitors.
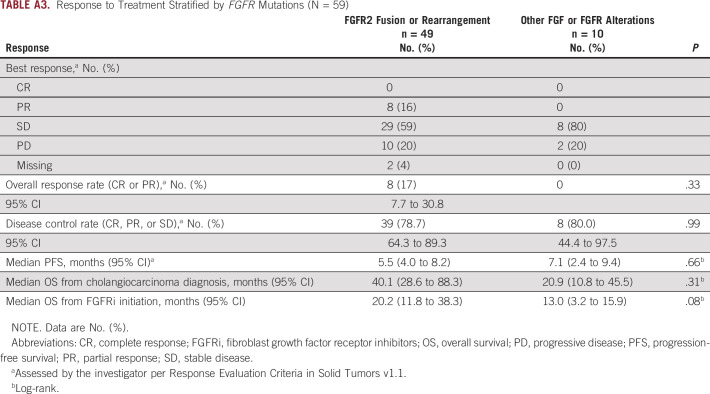
Median OS from time of FGFRi initiation for patients with FGFR2 fusion or rearrangement was 20.2 months (95% CI, 11.8 to 38.3) compared to patients with other FGFR alterations at 13.0 months (95% CI, 3.2 to 15.9; P = .08; Appendix Table A3).
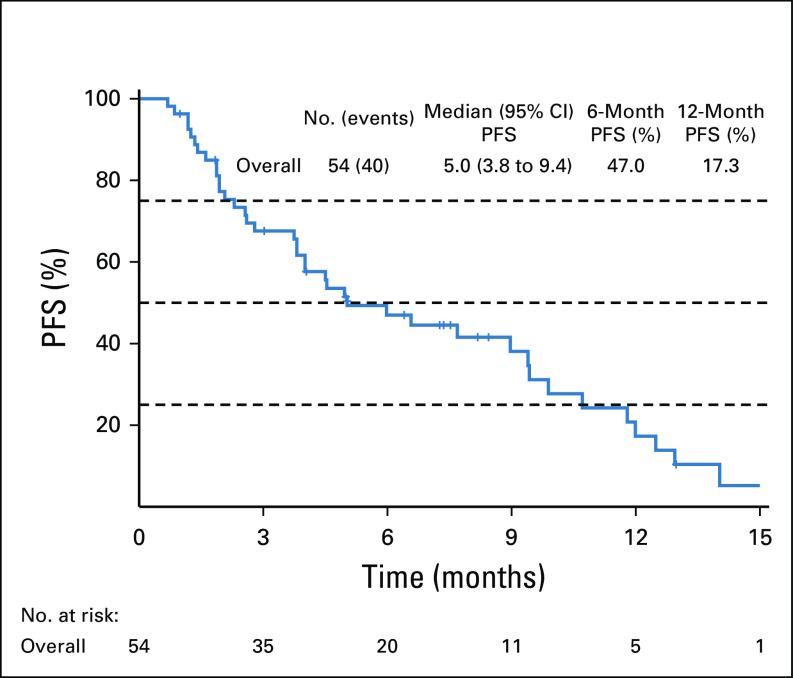
We also assessed PFS for first-line therapy treatment for all patients. Fifty-four (89%) patients received gemcitabine and cisplatin as first-line chemotherapy. The overall median PFS for the whole cohort was 5.0 months (95% CI, 3.8 to 9.4; Appendix Fig A3).
Females had significantly longer PFS at 6.9 months (95% CI, 5.2 to 11.8) on FGFRi compared with males at 4.9 months (95% CI, 2.8 to not estimable; P = .038). There was no difference in OS from time of FGFRi initiation between males and females (Appendix Fig A4). Female sex remained protective with a hazard ratio of 0.48 (95% CI, 0.24 to 0.94, P = .03) in a multivariable Cox regression model.
DISCUSSION
Genomic profiling has already altered the treatment paradigm of biliary tract cancers, specifically CCA, which is enriched in several actionable mutations. Our current study describes a large multicenter single-institution experience with FGFRi in patients with CCA. We identified 61 patients with advanced or metastatic CCA with an FGFR alteration treated with FGFRi. The most common grade 1 or higher adverse events for all patients included fatigue (92%), AST (78%), anemia (80%), decreased platelet count (63%), hyperphosphatemia (74%), alopecia (36%), and dry eyes (34%). This is comparable to adverse event rates in the published literature.5,20 Paronychia occurred in 15% of patients in this study and has been reported in 7% of patients treated with infigratinib and 24% of patients treated with erdafitinib.5,7,20 The observed difference in toxicity is likely explained by the use of different FGFRi in this study. These agents are well tolerated with 5 (8.2%) patients discontinuing therapy because of toxicity. Further assessment on quality of life compared with systemic chemotherapy is warranted.
In our cohort, the median PFS in all patients treated with an FGFRi was 5.8 months with a DCR of 78%. OS for patients treated with an FGFRi, regardless of FGFR fusion or mutation, was 15.3 months. These findings are consistent with others.5,21 The response rate is lower compared with the individual therapy reported and likely reflects the selective and nonselective nature of targets.7,11,22-24 Pazopanib is a nonselective tyrosine kinase inhibitor, whereas futibatinib is a third-generation, irreversible FGFR tyrosine kinase inhibitor.25 Because of the limitation of sample size, our study is not adequate to compare efficacy among individual therapy.
Of the patients included in our cohort, a disproportionate number are females. FGFR alterations have been observed at a higher frequency in females compared with males in CCA.3,7,11,26 Females had longer duration of response to therapy (6.9 v 4.9 months, P = .038); Multivariate analysis showed a hazard ratio of 0.48 (95% CI, 0.24 to 0.94; P = .03), adjusting for age, stage, prior one or more therapy, and FGFR status. OS was not statistically significant (20.2 months v 10.6 months, P = .16). There was no significant difference between choice of FGFRi agent between males and females. The sex-specific differences in outcome in patients with CCA have been observed previously.27,28 However, these observations did not include genomic subtyping of CCA (eg, FGFR2). Our study is unique from these prior reports in that we analyzed patients with only FGFR alterations treated with FGFRi. In fact, sex-specific differences in outcome have been described in many different solid malignancies including head and neck cancers.29,30 Independent of circulating sex hormones, these differences have been linked to sexual differentiation, a process involving genetic and epigenetic mechanisms,31 in addition to tumor behavior, tumor kinetics, comorbidities or delayed diagnosis,32 and sex-based molecular signatures.33 The relationship between sex, molecular patterns, response to treatment as well as toxicity is unknown with FGFRi in CCA and warrants further prospective studies.
Previously, the ABC-02 trial has reported a median PFS for first-line therapy with gemcitabine and cisplatin of 8.0 months.34 In our cohort, the median PFS for patients treated with first-line platinum-based therapy was 5.0 months. A recent study has also demonstrated that patients treated with FGFRi therapy had shorter PFS on first-line therapy.35 In another study comparing patients with CCA with and without FGFR mutations, the authors found that first-line therapy PFS was 6.2 months in patients with FGFR mutations.36 These data suggest that patients with FGFR mutations may have more indolent disease course and is more resistant to cytotoxic chemotherapy. The role of using FGFRi in the front-line setting is being investigated in several clinical trials (NCT04093362, NCT03773302, NCT03656536). We eagerly await the results from these trials, which will likely change the standard of care for these patients.
This study has several limitations including the small sample size and its retrospective nature. Data set regarding labs is incomplete and not every patient had all labs performed to assess for toxicities related to FGFRi. We were able to collect more than 90% of the needed information. The data collection also introduced potential selection bias since only patients who survived long enough to receive FGFRi would be included in this analysis. However, the results from our study reflect similar findings reported in the literature.3
In summary, this was a large multicenter single-institution cohort study assessing the toxicity and outcomes among patients with CCA treated with FGFRi. These data reflect the real-world experience at a tertiary cancer center. FGFRi clearly demonstrated clinical benefit with tolerable toxicity profile. As this class of drugs is increasingly used in the clinic, understanding of the toxicity and efficacy will be important in precision oncology.
Appendix
FIG A1.
CONSORT diagram depictingpatient disposition. FGFR, fibroblast growth factor receptor; FGFRi, fibroblast growth factor receptor inhibitors.
FIG A2.
Overall survival from time of diagnosis for patients treated with an FGFRi. FGFRi, fibroblast growth factor receptor inhibitors.
FIG A3.
PFS for all patients treated with gemcitabine and cisplatin as first-line therapy. PFS, progression-free survival
FIG A4.
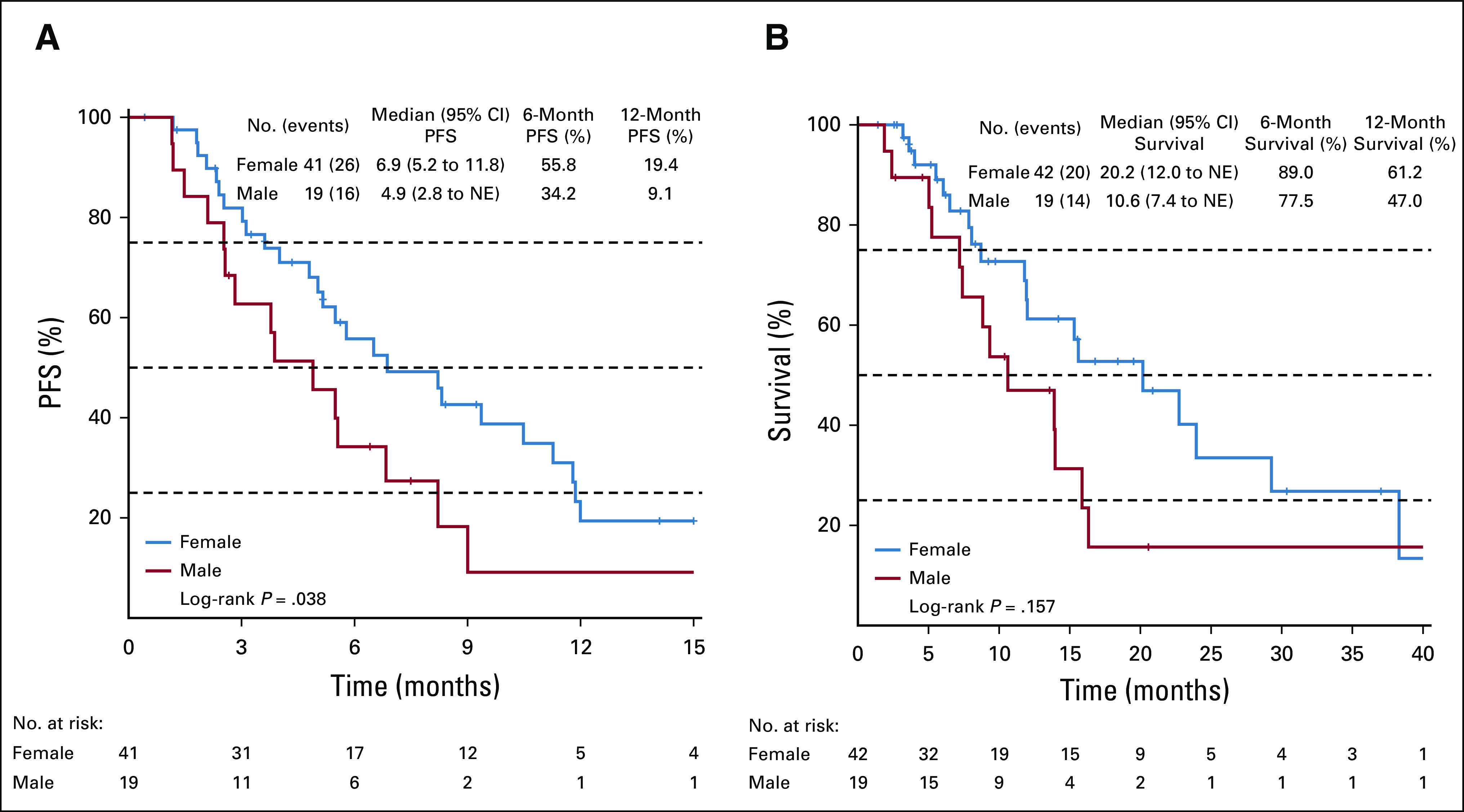
Survival curves for patients treated with an FGFRi stratified by sex. (A) PFS for patients treated with an FGFRi. (B) Overall survival for patients treated with an FGFRi. FGFRi, fibroblast growth factor receptor inhibitors; NE, not estimable; PFS, progression-free survival.
TABLE A1.
FGFR alterations (No. of patients, N = 61)
TABLE A2.
Other Clinically Significant Mutations on next generation sequencing (No. of patients, N = 63)
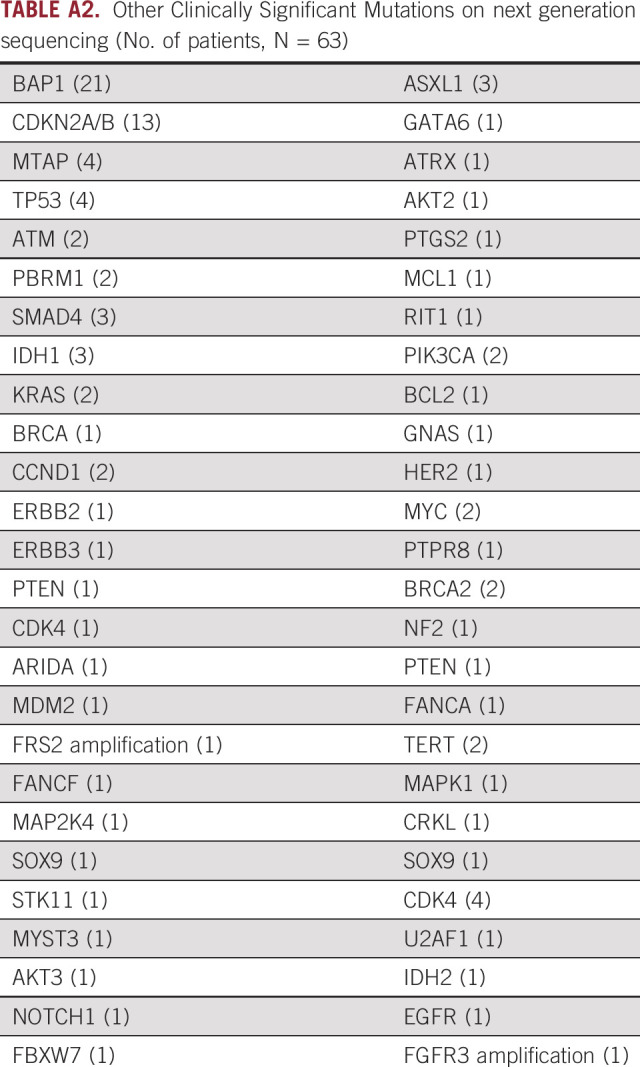
TABLE A3.
Response to Treatment Stratified by FGFR Mutations (N = 59)
Amit Mahipal
Honoraria: Eisai
Consulting or Advisory Role: QED Therapeutics
Research Funding: Taiho Pharmaceutical
Joseph J. Larson
Employment: Amazing You Therapy LLC
Leadership: Amazing You Therapy LLC
Stock and Other Ownership Interests: Amazing You Therapy LLC
Kabir Mody
Stock and Other Ownership Interests: CytoDyn, Oncotherapeutics
Consulting or Advisory Role: Celgene, Genentech/Roche, Merrimack, Eisai, AstraZeneca, Vicus Therapeutics, Ipsen
Research Funding: FibroGen, Senhwa Biosciences, ARIAD, TRACON Pharma, MedImmune, Agios, ArQule, Taiho Pharmaceutical, Gritstone Oncology, Incyte, Merck, Vyriad, Turnstone Bio, AstraZeneca, Basilea
Zhaohui Jin
Consulting or Advisory Role: Novartis, QED Therapeutics
Joleen Hubbard
Consulting or Advisory Role: Bayer, Taiho Oncology
Research Funding: Boston Biomedical, Senhwa Biosciences, Bayer, Merck, Taiho Pharmaceutical, Treos Bio, eFFECTOR Therapeutics, Hutchison MediPharma, Seattle Genetics, Trovagene, Translational Research in Oncology, Incyte
Thorvardur Halfdanarson
Consulting or Advisory Role: Lexicon, Ipsen, Advanced Accelerator Applications, Curium Pharma, ScioScientific, TERUMO
Research Funding: Ipsen, Agios, Thermo Fisher Scientific, Basilea, Turnstone Bio, Advanced Accelerator Applications, Novartis
Aminah Jatoi
Research Funding: AstraZeneca
Robert R. McWilliams
Stock and Other Ownership Interests: Zentalis
Consulting or Advisory Role: Merrimack, Newlink Genetics, Zentalis
Research Funding: Newlink Genetics, Merck, Bristol Myers Squibb, GlaxoSmithKline/Tesaro
Wen Wee Ma
Consulting or Advisory Role: Wellstat Therapeutics
Research Funding: Merrimack, Athenex, Bristol Myers Squibb, Ipsen, Actuate Therapeutics, Sun Pharma, BioMed Valley Discoveries
Sumera Ilyas
Consulting or Advisory Role: AstraZeneca
Rory Smoot
Consulting or Advisory Role: AstraZeneca
Lewis Roberts
Consulting or Advisory Role: Bayer, GRAIL, RedHill Biopharma, TAVEC, Exact Sciences, QED Therapeutics, Biocompatibles
Speakers' Bureau: Bayer
Research Funding: ARIAD, BTG, Exact Sciences, Gilead Sciences, Wako Diagnostics, Glycotest, RedHill Biopharma
Patents, Royalties, Other Intellectual Property: US Patent No. 9,469,877: Materials and Methods for Diagnosis, Prognosis, Monitoring of Recurrence and Assessment of Therapeutic/Prophylactic Treatment of Pancreaticobiliary Cancer, Five Prime Therapeutics. Royalties
Travel, Accommodations, Expenses: Gilead Sciences
Gregory Gores
Honoraria: Sagimet
Mitesh Borad
Stock and Other Ownership Interests: Gilead Sciences, AVEO, Intercept Pharmaceuticals, Spectrum Pharmaceuticals
Consulting or Advisory Role: G1 Therapeutics, Fujifilm, Agios, Insys Therapeutics, Novartis, ArQule, Celgene, Inspyr Therapeutics, Halozyme, Pieris Pharmaceuticals, Taiho Pharmaceutical, Immunovative Therapies, Exelixis, Lynx Group, Genentech, Western Oncolytics, Klus Pharma, De Novo Pharmaceuticals, Merck, Imvax
Research Funding: Boston Biomedical, miRNA Therapeutics, Senhwa Biosciences, MedImmune, BiolineRx, Agios, Halozyme, Celgene, Threshold Pharmaceuticals, Toray Industries, Dicerna, Sillajen, Eisai, Taiho Pharmaceutical, EMD Serono, Isis Pharmaceuticals, Incyte, Sun Biopharma, ARIAD, ImClone Systems, QED Therapeutics, Puma Biotechnology, Adaptimmune, Merck Serono, RedHill Biopharma, Basilea
Travel, Accommodations, Expenses: ArQule, Celgene, AstraZeneca
Tanios S. Bekaii-Saab
Consulting or Advisory Role: Amgen, Ipsen, Lilly, Bayer, Roche/Genentech, AbbVie, Incyte, Immuneering, Seattle Genetics, Pfizer, Boehringer Ingelheim, Janssen, Eisai, Daiichi Sankyo/UCB Japan, AstraZeneca, Exact Sciences, Natera, Treos Bio, Celularity, SOBI, BeiGene, Foundation Medicine
Patents, Royalties, Other Intellectual Property: Patent WO/2018/183488, Patent WO/2019/055687
Other Relationship: Exelixis, Merck, AstraZeneca, Lilly, Pancreatic Cancer Action Network
Nguyen H. Tran
Honoraria: QED Therapeutics
Consulting or Advisory Role: QED Therapeutics
No other potential conflicts of interest were reported.
SUPPORT
Supported by the National Institutes of Health Grants: P30CA15083 (Mayo Clinic Comprehensive Cancer Center grant).
AUTHOR CONTRIBUTIONS
Conception and design: Jennifer J. Gile, Amit Mahipal, Wen Wee Ma, Mitesh Borad, Nguyen H. Tran
Provision of study materials or patients: Thorvardur Halfdanarson, Steven R. Alberts, Wen Wee Ma, Lewis Roberts, Tanios S. Bekaii-Saab
Collection and assembly of data: Jennifer J. Gile, Thorvardur Halfdanarson, Steven R. Alberts, Mitesh Borad, Nguyen H. Tran
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by the authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Amit Mahipal
Honoraria: Eisai
Consulting or Advisory Role: QED Therapeutics
Research Funding: Taiho Pharmaceutical
Joseph J. Larson
Employment: Amazing You Therapy LLC
Leadership: Amazing You Therapy LLC
Stock and Other Ownership Interests: Amazing You Therapy LLC
Kabir Mody
Stock and Other Ownership Interests: CytoDyn, Oncotherapeutics
Consulting or Advisory Role: Celgene, Genentech/Roche, Merrimack, Eisai, AstraZeneca, Vicus Therapeutics, Ipsen
Research Funding: FibroGen, Senhwa Biosciences, ARIAD, TRACON Pharma, MedImmune, Agios, ArQule, Taiho Pharmaceutical, Gritstone Oncology, Incyte, Merck, Vyriad, Turnstone Bio, AstraZeneca, Basilea
Zhaohui Jin
Consulting or Advisory Role: Novartis, QED Therapeutics
Joleen Hubbard
Consulting or Advisory Role: Bayer, Taiho Oncology
Research Funding: Boston Biomedical, Senhwa Biosciences, Bayer, Merck, Taiho Pharmaceutical, Treos Bio, eFFECTOR Therapeutics, Hutchison MediPharma, Seattle Genetics, Trovagene, Translational Research in Oncology, Incyte
Thorvardur Halfdanarson
Consulting or Advisory Role: Lexicon, Ipsen, Advanced Accelerator Applications, Curium Pharma, ScioScientific, TERUMO
Research Funding: Ipsen, Agios, Thermo Fisher Scientific, Basilea, Turnstone Bio, Advanced Accelerator Applications, Novartis
Aminah Jatoi
Research Funding: AstraZeneca
Robert R. McWilliams
Stock and Other Ownership Interests: Zentalis
Consulting or Advisory Role: Merrimack, Newlink Genetics, Zentalis
Research Funding: Newlink Genetics, Merck, Bristol Myers Squibb, GlaxoSmithKline/Tesaro
Wen Wee Ma
Consulting or Advisory Role: Wellstat Therapeutics
Research Funding: Merrimack, Athenex, Bristol Myers Squibb, Ipsen, Actuate Therapeutics, Sun Pharma, BioMed Valley Discoveries
Sumera Ilyas
Consulting or Advisory Role: AstraZeneca
Rory Smoot
Consulting or Advisory Role: AstraZeneca
Lewis Roberts
Consulting or Advisory Role: Bayer, GRAIL, RedHill Biopharma, TAVEC, Exact Sciences, QED Therapeutics, Biocompatibles
Speakers' Bureau: Bayer
Research Funding: ARIAD, BTG, Exact Sciences, Gilead Sciences, Wako Diagnostics, Glycotest, RedHill Biopharma
Patents, Royalties, Other Intellectual Property: US Patent No. 9,469,877: Materials and Methods for Diagnosis, Prognosis, Monitoring of Recurrence and Assessment of Therapeutic/Prophylactic Treatment of Pancreaticobiliary Cancer, Five Prime Therapeutics. Royalties
Travel, Accommodations, Expenses: Gilead Sciences
Gregory Gores
Honoraria: Sagimet
Mitesh Borad
Stock and Other Ownership Interests: Gilead Sciences, AVEO, Intercept Pharmaceuticals, Spectrum Pharmaceuticals
Consulting or Advisory Role: G1 Therapeutics, Fujifilm, Agios, Insys Therapeutics, Novartis, ArQule, Celgene, Inspyr Therapeutics, Halozyme, Pieris Pharmaceuticals, Taiho Pharmaceutical, Immunovative Therapies, Exelixis, Lynx Group, Genentech, Western Oncolytics, Klus Pharma, De Novo Pharmaceuticals, Merck, Imvax
Research Funding: Boston Biomedical, miRNA Therapeutics, Senhwa Biosciences, MedImmune, BiolineRx, Agios, Halozyme, Celgene, Threshold Pharmaceuticals, Toray Industries, Dicerna, Sillajen, Eisai, Taiho Pharmaceutical, EMD Serono, Isis Pharmaceuticals, Incyte, Sun Biopharma, ARIAD, ImClone Systems, QED Therapeutics, Puma Biotechnology, Adaptimmune, Merck Serono, RedHill Biopharma, Basilea
Travel, Accommodations, Expenses: ArQule, Celgene, AstraZeneca
Tanios S. Bekaii-Saab
Consulting or Advisory Role: Amgen, Ipsen, Lilly, Bayer, Roche/Genentech, AbbVie, Incyte, Immuneering, Seattle Genetics, Pfizer, Boehringer Ingelheim, Janssen, Eisai, Daiichi Sankyo/UCB Japan, AstraZeneca, Exact Sciences, Natera, Treos Bio, Celularity, SOBI, BeiGene, Foundation Medicine
Patents, Royalties, Other Intellectual Property: Patent WO/2018/183488, Patent WO/2019/055687
Other Relationship: Exelixis, Merck, AstraZeneca, Lilly, Pancreatic Cancer Action Network
Nguyen H. Tran
Honoraria: QED Therapeutics
Consulting or Advisory Role: QED Therapeutics
No other potential conflicts of interest were reported.
REFERENCES
- 1.Lowery MA, Ptashkin R, Jordan E, et al. : Comprehensive molecular profiling of intrahepatic and extrahepatic cholangiocarcinomas: Potential targets for intervention. Clin Cancer Res 24:4154-4161, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dai S, Zhou Z, Chen Z, et al. : Fibroblast growth factor receptors (FGFRs): Structures and small molecule inhibitors. Cells 8:614, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jain A, Borad MJ, Kelley RK, et al. : Cholangiocarcinoma with FGFR genetic aberrations: A unique clinical phenotype. JCO Precis Oncol 2:1-12, 2018 [DOI] [PubMed] [Google Scholar]
- 4.Borad MJ, Gores GJ, Roberts LR: Fibroblast growth factor receptor 2 fusions as a target for treating cholangiocarcinoma. Curr Opin Gastroenterol 31:264-268, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Javle M, Lowery M, Shroff RT, et al. : Phase II study of BGJ398 in patients with FGFR-altered advanced cholangiocarcinoma. J Clin Oncol 36:276-282, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papadopoulos KP, El-Rayes BF, Tolcher AW, et al. : A phase 1 study of ARQ 087, an oral pan-FGFR inhibitor in patients with advanced solid tumours. Br J Cancer 117:1592-1599, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazzaferro V, El-Rayes BF, Droz Dit Busset M, et al. : Derazantinib (ARQ 087) in advanced or inoperable FGFR2 gene fusion-positive intrahepatic cholangiocarcinoma. Br J Cancer 120:165-171, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoy SM: Pemigatinib: First approval. Drugs 80:923-929, 2020 [DOI] [PubMed] [Google Scholar]
- 9.Makawita S, Abou-Alfa GK, Roychowdhury S, et al. : Infigratinib in patients with advanced cholangiocarcinoma with FGFR2 gene fusions/translocations: The PROOF 301 trial. Future Oncol 16:2375-2384, 2020 [DOI] [PubMed] [Google Scholar]
- 10.Mahipal A, Tella SH, Kommalapati A, et al. : FGFR2 genomic aberrations: Achilles heel in the management of advanced cholangiocarcinoma. Cancer Treat Rev 78:1-7, 2019 [DOI] [PubMed] [Google Scholar]
- 11.Abou-Alfa GK, Sahai V, Hollebecque A, et al. : Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol 21:671-684, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahipal A, Tella SH, Kommalapati A, et al. : Prevention and treatment of FGFR inhibitor-associated toxicities. Crit Rev Oncol Hematol 155:103091, 2020 [DOI] [PubMed] [Google Scholar]
- 13.Chae YK, Ranganath K, Hammerman PS, et al. : Inhibition of the fibroblast growth factor receptor (FGFR) pathway: The current landscape and barriers to clinical application. Oncotarget 8:16052-16074, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shin E, Lim DH, Han J, et al. : Markedly increased ocular side effect causing severe vision deterioration after chemotherapy using new or investigational epidermal or fibroblast growth factor receptor inhibitors. BMC Ophthalmol 20:19, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saka H, Kitagawa C, Kogure Y, et al. : Safety, tolerability and pharmacokinetics of the fibroblast growth factor receptor inhibitor AZD4547 in Japanese patients with advanced solid tumours: A phase I study. Invest New Drugs 35:451-462, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borad MJ, Champion MD, Egan JB, et al. : Integrated genomic characterization reveals novel, therapeutically relevant drug targets in FGFR and EGFR pathways in sporadic intrahepatic cholangiocarcinoma. PLoS Genet 10:e1004135, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trotti A, Colevas AD, Setser A, et al. : CTCAE v3.0: Development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 13:176-181, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Kaplan EL, Meier P: Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457-481, 1958 [Google Scholar]
- 19.Mantel N: Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 50:163-170, 1966 [PubMed] [Google Scholar]
- 20.Lacouture ME, Sibaud V, Anadkat MJ, et al. : Dermatologic adverse events associated with selective fibroblast growth factor receptor inhibitors: Overview, prevention, and management guidelines. Oncologist 26:e316-e326, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, Xing X, Li Q, et al. : Targeting the FGFR signaling pathway in cholangiocarcinoma: Promise or delusion? Ther Adv Med Oncol 12:1758835920940948, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banales JM, Marin JJG, Lamarca A, et al. : Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol 17:557-588, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cleary JM, Iyer G, Oh D-Y, et al. : Final results from the phase I study expansion cohort of the selective FGFR inhibitor Debio 1,347 in patients with solid tumors harboring an FGFR gene fusion. J Clin Oncol 38, 2020. (suppl 15; abstr 3603) [Google Scholar]
- 24.Javle MM, Roychowdhury S, Kelley RK, et al. : Final results from a phase II study of infigratinib (BGJ398), an FGFR-selective tyrosine kinase inhibitor, in patients with previously treated advanced cholangiocarcinoma harboring an FGFR2 gene fusion or rearrangement. J Clin Oncol 39, 2021. (suppl 3; abstr 265) [Google Scholar]
- 25.Goyal L, Meric-Bernstam F, Hollebecque A, et al. : FOENIX-CCA2: A phase II, open-label, multicenter study of futibatinib in patients (pts) with intrahepatic cholangiocarcinoma (iCCA) harboring FGFR2 gene fusions or other rearrangements. J Clin Oncol 38, 2020. (suppl 15; abstr 108) [Google Scholar]
- 26.Lamarca A, Barriuso J, McNamara MG, et al. : Molecular targeted therapies: Ready for “prime time” in biliary tract cancer. J Hepatol 73:170-185, 2020 [DOI] [PubMed] [Google Scholar]
- 27.Kim BW, Oh CM, Choi HY, et al. : Incidence and overall survival of biliary tract cancers in South Korea from 2006 to 2015: Using the National Health Information Database. Gut Liver 13:104-113, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mody K, Antwi SO, Hodge DO, et al. : A SEER-based multi-ethnic picture of advanced intrahepatic cholangiocarcinoma in the United States pre- and post-the advent of gemcitabine/cisplatin. J Gastrointest Oncol 9:1063-1073, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cook MB, McGlynn KA, Devesa SS, et al. : Sex disparities in cancer mortality and survival. Cancer Epidemiol Biomarkers Prev 20:1629-1637, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim HI, Lim H, Moon A: Sex differences in cancer: Epidemiology, genetics and therapy. Biomol Ther (Seoul) 26:335-342, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubin JB, Lagas JS, Broestl L, et al. : Sex differences in cancer mechanisms. Biol Sex Differ 11:17, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dorak MT, Karpuzoglu E: Gender differences in cancer susceptibility: An inadequately addressed issue. Front Genet 3:268, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuan Y, Liu L, Chen H, et al. : Comprehensive characterization of molecular differences in cancer between male and female patients. Cancer Cell 29:711-722, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valle J, Wasan H, Palmer DH, et al. : Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 362:1273-1281, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Boileve A, Baiev I, Dinicola C, et al. : Clinical and molecular features of patients with cholangiocarcinoma harboring FGFR genetic alterations. J Clin Oncol 37, 2019. (suppl 15; abstr 4084) [Google Scholar]
- 36.Abou-Alfa GK, Bibeau K, Schultz N, et al. : Effect of FGFR2 alterations on survival in patients receiving systemic chemotherapy for intrahepatic cholangiocarcinoma. J Clin Oncol 39, 2021. (suppl 3; abstr 303) [DOI] [PMC free article] [PubMed] [Google Scholar]