Abstract
Purpose:
CD19-redirected chimeric antigen receptor (CAR.CD19) T cells promote clinical responses in patients with relapsed/refractory B cell non-Hodgkin lymphomas (NHL) and chronic lymphocytic leukemia (CLL). However, patients showing sustained clinical responses after CAR.CD19-T treatment show increased infection risk due to compromised B-lymphocyte recovery. Mature B-cell-derived malignancies express monoclonal immunoglobulins bearing either κ- or λ-light-chains. We initially constructed CAR-T targeting the κ-light-chain (CAR.κ) and established a clinical study with it. We then modified the CAR molecule and then developed CAR-T targeting the λ-light chain (CAR.λ) and explored its antitumor activity.
Experimental Design:
Using Igλ+ lymphoma cell lines and patient-derived Igλ+ CLL cells, we evaluated the in vitro tumor cytotoxicity and cytokine profiles of CAR.λ. We also assessed the in vivo efficacy of CAR.λ in xenograft Igλ+ lymphoma models including a patient derived xenograft (PDX) of mantle cell lymphoma, and the effects of λ- or κ-light-chain specific CAR-T on normal B-lymphocytes in a humanized murine model.
Results:
CAR.λ demonstrated antitumor effects against Igλ+ lymphoma cells and patient-derived CLL cells in vitro, and in vivo in xenograft and PDX Igλ+ lymphoma murine models. Antitumor activity of CAR.λ was superimposable to CAR.CD19. Furthermore, we demonstrated in the humanized murine model that λ- or κ-light-chain specific CAR-T cells only depleted the corresponding targeted light-chain expressing normal B cells, while sparing the reciprocal light-chain carrying B cells.
Conclusions:
Adoptive transfer of CAR.λ and CAR.κ-T cells represents a useful and alternative modality to CAR.CD19-T cells in treating mature B-cell malignancies with minimal impact on humoral immunity.
Introduction
Chimeric antigen receptors (CARs) are synthetic molecules coupling highly specific antigen-binding properties of monoclonal antibodies to the T cell signaling and co-stimulatory domains, leading to lytic and proliferative capacities of CAR-T cells. Infusion of CAR-T cells targeting CD19 (CAR.CD19) has shown great potential in the treatment of B-cell-derived non-Hodgkin lymphomas (B-NHL) and chronic lymphocytic leukemia (CLL) (1–5). Notably, a significant number of patients who received CAR.CD19-T cells exhibited durable complete responses, with sub-analyses showing benefit in both older and younger patients, leading to the conclusion that CAR.CD19-T cells could be a very valuable treatment in patients with refractory and/or heavily pretreated lymphomas (3,4).
Effective long-term disease control achieved with CAR.CD19-T cells is, however, frequently associated with B-cell depletion and resultant hypogammaglobulinemia (6). While administration of polyclonal immunoglobulins (Igs) can ameliorate the side effect of hypogammaglobulinemia, this treatment is expensive. In addition, prolonged B-cell aplasia, combined with progressive T-cell dysfunction caused by previous chemotherapies, can predispose patients to life-threatening opportunistic infections. Recent analyses of patients receiving CAR.CD19-T cells show a significant number of patients developing hypogammaglobulinemia lasting many months with an elevated risk of sinopulmonary infections requiring inpatient care in up to 20% of patients (7,8). In addition, disease relapse due to the loss of the targeted CD19 epitope in patients receiving CAR.CD19-T cells has also been reported (9,10), urging for the identification of additional targets for CAR-T cells in B-cell malignancies.
Mature B-lymphocytes express monoclonal immunoglobulins (Igs) that contain either the κ- or λ-light chains but not both simultaneously, and κ- or λ-light chain expression remains clonally restricted in malignant B cells in B-NHL and CLL patients. Thus, CAR-T cells targeting the clonally restricted light chain expressed by the lymphoma cells should spare normal B cells expressing the reciprocal light chain, thereby lessening the negative impact on a patient’s humoral immunity. Multiple studies using flow cytometry demonstrated 92 – 98% positive expression of surface immunoglobulins (SIg) with light chain clonality across all subtypes of B-NHL (11–13). The most commonly associated B-NHL subtype with λ-light chain expression is mantle cell lymphoma (MCL), which has a λ:κ expression ratio of approximately 2:1 (14–16). With more than one-third of all B-NHL, and greater than two-thirds of MCL, expressing the λ-light chain monoclonally, a λ-light chain-targeting CAR-T cell approach would serve a major and critical need in treating relapsed/refractory B-NHL patients. We previously reported preclinical results targeting the κ-light chain in B-NHL with CAR-T cells, which led to a clinical trial demonstrating treatment feasibility, safety and achievement of clinical responses (17,18). We report here that targeting of the λ-light chain of immunoglobulins by CAR-T cells allows for the achievement of antitumor effects equivalent to CAR.CD19-T cells. Furthermore, in a humanized murine model we demonstrated that targeting the immunoglobulin light chains allowed sparing of normal B-lymphocytes expressing the reciprocal light chain.
Materials and Methods:
Cell lines and cell culture tumor cells.
Daudi, BV173, and Maver-1 were obtained from the American Type Culture Collection (ATCC; Rockville, MD). SP53 was kindly provided by Dr. Amin Hesham (M.D. Anderson Cancer Center, Houston, TX). All cells were maintained in culture with RPMI 1640 medium (Gibco-BRL, Gaithersburg, MD) containing 10% heat-inactivated fetal calf serum (FCS), 2 mM glutamine, 100 IU/mL penicillin, and 100 mg/mL streptomycin (all from BioWhittaker, Walkersville, MD). Cells were maintained in a humidified atmosphere containing 5% CO2 at 37°C.Tumor cells were routinely assessed for phenotypic identify and for the absence of mycoplasma contamination.
Human samples of B-CLL.
Samples were obtained from peripheral blood of treatment-naïve B-CLL patients through the Tissue Processing Facility at University North Carolina-Chapel (UNC). T lymphocytes were isolated from these samples using CD3 microbead antibody (Miltenyi Biotec, Bergisch Gladbach, Germany). CLL-derived T-lymphocytes were then activated and genetically modified to generate autologous CAR-T cells (see “Transduction and Expansion of Human T cells”), while tumor cells were stored to be subsequently used as a target in co-culture experiments (see “Co-culture Assays”). The protocol for collection of peripheral blood from patients with B-CLL was approved by the institutional review board (IRB) and ethic committee at UNC (Chapel Hill, NC).
Cloning of the single-chain antibody and generation of CARs.
We cloned VH and VL variable regions of the antibody targeting the λ-light chain of human immunoglobulins from the murine hybridoma HP6054 (ATCC) and generated a scFv. The scFv sequence was cloned in frame with the human CD8α hinge and transmembrane domain and with the CD28 co-stimulatory endodomain and the intracytoplasmic CD3ζ chain of the TCR/CD3 complex in the SFG retroviral backbone, as previously established (19). An additional retroviral vector was constructed to label tumor cells, which encoded the firefly luciferase gene (FFluc). This vector was used for stable transduction of tumor cell lines (17). After transduction, cells were selected in puromycin (Sigma, St Louis, MO), grown in culture, and used to inoculate SCID mice for in vivo experiments. Retroviral supernatants were prepared as previously described (17,20). Briefly, 293T cells were transfected with 3 plasmids (retroviral transfer vector, Peg-Pam encoding gag-pol, and RDF encoding the RD114 envelope), using GeneJuice transfection reagent (Novagen). Supernatants were collected at 48 and 72 hours for transduction of T cells.
Transduction and expansion of human T cells.
Buffy coats from healthy donors were obtained through the Gulf Coast Regional Blood Center, Houston, TX. Peripheral blood mononuclear cells (PBMCs) were isolated with Lymphoprep density separation (Fresenius Kabi Norge) and activated 1 μg/mL α-CD3 (Miltenyi Biotec) and 1 μg/mL α-CD28 (BD Biosciences) mAb coated plates. Forty-eight hours later, T lymphocytes were transduced with retroviral supernatants using retronectin-coated plates (Takara Bio), and expanded in complete medium (45% RPMI-1640 and 45% Click’s medium (Irvine Scientific), 10% FBS (Hyclone), 2 mM GlutaMAX, 100 IU/mL of penicillin and 100 μg/mL of streptomycin) with IL-7 (10 ng/mL; PeproTech) and IL-15 (5 ng/mL; PeproTech). Seven to twelve days later, cells were collected for in vitro or in vivo experiments, as previously described (18, 20).
Immunophenotyping.
Phycoerythrin (PE)-conjugated, fluorescein isothiocyanate (FITC)-conjugated, allophycocyanin (APC)-conjugated, brilliant violet 510 and 650-conjugated, allophycocyanin-cyanine7 tandem (APC-Cy7)-conjugated, and peridinin chlorophyll protein (PerCP)-conjugated CD3, CD4, CD8, and CD45 monoclonal antibodies were used to stain T lymphocytes, whereas CD19, CD20, anti-Igκ, and anti-Igλ antibodies were used to stain tumor cells. All antibodies were from Becton Dickinson. To detect the expression of CARs, T lymphocytes were stained with a monoclonal antibody conjugated with the Alexa Fluor 647 fluorophore provided by Jackson ImmunoResearch (West Grove, PA) recognizing the antigen binding fragment (Fab) component of mouse IgG that is part of the CAR construct. Cells were analyzed by fluorescence-activated cell sorting FACSCanto (Becton Dickinson). A flow cytometry assay was also used to measure the proliferative capacity of CAR-T cells against various tumor cell lines. Briefly, 2 × 107 T lymphocytes were incubated for 10 minutes at room temperature with 1.5 μM carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes Europe, Leiden, The Netherlands). These cells were cocultured either alone or with tumor cell lines (Daudi or SP53) at a 10:1 effector-to-target (E:T) ratio for 4 to 5 days at 37°C in 5% CO2. After incubation, CFSE-labeled cells were counted by FACScan gating on viable T cells.
Coculture assays.
CD19+ BV173, CD19+Igκ+ Daudi, and either CD19+Igλ+ SP53 or Maver-1 tumor cells were seeded into 24-well plates at 5 × 105 cells/well and non-transduced or CAR-T cells were added at different effector to target (E:T) ratios (E:T = 1:5 or 1:10). After 24 hours, supernatants were collected for IFNγ and IL-2 measurement. Five days later, cells were collected and stained with CD3 and CD19 antibodies for flow cytometry analysis (FACSCanto; Becton Dickinson). For assessing CAR-T cell cytotoxicity against primary human tumors, human B-CLL samples were obtained. Non-transduced or irrelevant CAR-T cells serving as negative control and CAR.λ-T cells derived from the patients’ PBMCs were plated and cultured with the B-CLL cells in an autologous manner at an effector to tumor ratio of 1:10. Supernatants were collected at 24 hours for cytokine measurement. After 48 hours, all cells were collected, stained with CD3 and CD19 antibodies, and then analyzed by flow cytometry (FACSCanto; Becton Dickinson).
Cytokine measurements.
Cytokines in culture supernatants and plasma were measured using enzyme-linked immunosorbent assay (ELISA) following manufacturer’s instructions (R&D Systems). Data were collected and analyzed using the Lumina-200 System and the Bio-Plex Manager 6.1 software (Bio-Rad).
In vivo studies in a xenograft NSG murine model.
CAR.κ-T cells were tested in NSG (NOD-scid IL2Rgnull) mice injected intravenously with 2 × 106 CD19+Igκ+ Daudi tumor cell line labeled with the Firefly luciferase gene (Daudi-FFLuc). Four days later, mice received control T cells or CAR.CD19 or CAR.κ-T cells. CAR.λ-T cells were tested in NSG mice injected intravenously with 2 × 106 CD19+Igλ+ Maver-1 tumor cell line labeled with the Firefly luciferase gene (Maver-FFLuc). Four days later, mice received control T cells or CAR.CD19, CAR.κ or CAR.λ-T cells. Tumor growth was monitored weekly by injecting mice intraperitoneally (i.p.) with D-luciferin (150 mg/kg, Xenolight, PerkinElmer). Photon emission was then analyzed within 20 minutes of D-luciferin injection, using the Xenogen-IVIS Imaging System as previously validated (17,19).
Patient-derived xenograft murine model.
A patient-derived, Igλ+ mantle cell lymphoma (MCL) cell line was purchased from a public repository (Dana Farber Cancer Institute) to create a patient-derived xenograft (PDX) murine model. NSG mice were injected in the flank with 3 × 106 tumor cells mixed with 100 μL of Matrigel matrix (Corning). After tumor engraftment defined as 250 mm3 on size measurement with calipers, mice were treated with either non-transduced, CAR.CD19, or CAR.λ-T cells intravenously. Tumor sizes were measured weekly for up to 60 days, or until the length of tumor reached 15 mm at which time mice were euthanized.
In vivo studies in a humanized murine model.
To assess the effect of CAR-T cells on normal human B-lymphocytes in vivo, NSG mice were first irradiated at 2.8 Gy to ablate their native bone marrow (19). Human umbilical cord blood-derived CD34+ hematopoietic stem cells were injected intravenously into the mice 2 – 3 three hours after irradiation. Human cell engraftment, and specifically B lymphocyte reconstitution, was then measured through serial blood measurements over 8 – 10 weeks. Once cell engraftment was achieved, B and T lymphocytes were quantified by flow cytometry. Mice with T cell engraftment or no B cell engraftment was sacrificed. The remaining mice were infused intravenously with either non-transduced T cells or CAR.CD19, CAR.κ, or CAR.λ-T cells. Mice were bled 7 days post-CAR-T-cell infusion to measure CD19+Igκ+ and CD19+Igλ+ B cells through flow cytometry.
Statistical analysis.
Data were presented with their means unless indicated otherwise. Statistical analyses were performed using GraphPad Prism software. Two-tailed unpaired t-test, one-way ANOVA, two-way ANOVA, Kruskal-Wallis multiple comparisons test, and log-rank Mantel-Cox tests were used. Bonferroni’s correction for multiple comparisons was used to calculate adjusted p values when appropriate. The exact p values were shown in the figures and/or their legends; ns, not significant. The specific statistical test used for each figure was described in the corresponding figure legend.
Results
CAR.λ-T cells selectively eliminate human Igλ+ lymphoma tumor cell lines and Igλ+ primary CLL cells.
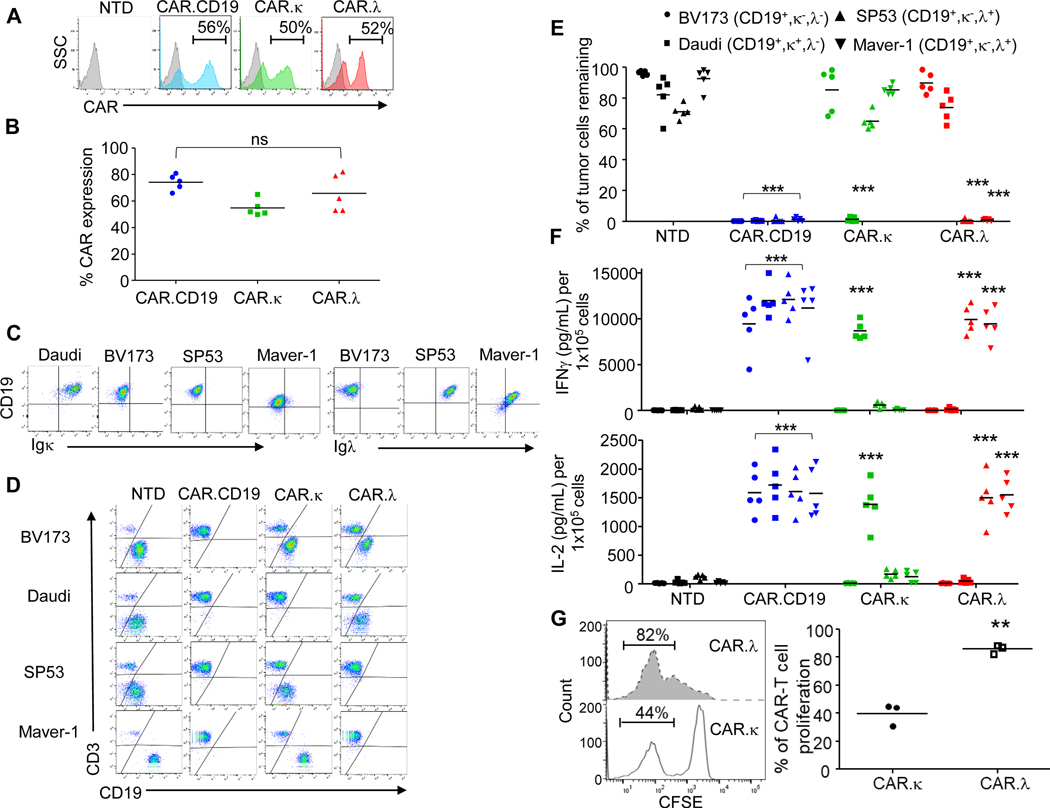
We have previously described a κ-light chain-targeting CAR construct (CAR.κ) in which we used a long spacer region (CH2-CH3 region and hinge of IgG1) and the transmembrane and signaling domains of CD28 along with the CD3ζ chain (17,18). Based on emerging data demonstrating the impact of the length and type of hinge and transmembrane domains on CAR functionality (21,22), we investigated if alternative CAR formats may affect the antitumor activity of the CAR.κ-T cells. In particular, we modified the spacer and transmembrane domains by using the stalk (hinge and transmembrane domain) of the human CD8α (Figure S1A), which is used in the CAR.CD19 construct encoding the 4–1BB endodomain recently approved by the FDA for the treatment of B-cell malignancies, and fused it with the endodomains containing the CD28 signaling domains and the CD3ζ chain (19,23,24). The new design of the CAR.κ displayed improved in vivo antitumor activity, which was superimposable to that of CAR.CD19-T cells (Figure S1B-F). Based on this new evidence, we constructed the CARs targeting the human immunoglobulin λ-light chain (CAR.λ) maintaining the same vector design. T lymphocytes obtained from five healthy donors were transduced using retroviral vectors expressing the CAR.CD19, CAR.κ or CAR.λ, while non-transduced (NTD) T cells were used as a negative control. As shown in Figure 1A, B, all CARs were equally expressed in T cells. CAR-T cells and NTD cells were then co-cultured with CD19+ B cell-derived tumor lines expressing either κ- or λ-light chains or no light chain expression (Figure 1C). Both CAR.λ and CAR.κ-T cells specifically recognized their targeted tumor cell lines Maver-1/SP53 and Daudi, respectively, while sparing the reciprocal light-chain expressing tumor cells. NTD cells did not display any antitumor activity, while CAR.CD19-T cells eliminated all three cell lines indiscriminately (Figure 1D, E). IFNγ and IL-2 detected in the supernatants collected from the co-culture assays correlated with the observed antitumor effects (Figure 1F). These results were reproducible at effector to tumor (E:T) ratios of 1:5 and 1:10. We also analyzed CAR-T cell proliferative capacity in response to their specific targets using a CFSE staining assay. CAR.λ-T cells showed significant proliferation when plated with Maver-1 cells, while conversely CAR.κ-T cells exhibited much less proliferation when exposed to the same tumor target, further demonstrating the selective tumor recognition of the CAR constructs (Figure 1G).
Figure 1. CAR.λ-T cells target Igλ+ lymphoma cell lines in vitro.
(A) Representative flow cytometry plots showing the expression of CAR.CD19, CAR.κ, and CAR.λ in T lymphocytes obtained from healthy donors. NTD indicates non-transduced control T cells. (B) Summary of the transduction efficiency in 5 healthy donors; ns = not significant; one-way ANOVA. (C) Representative flow cytometry plots illustrating the expression of CD19, Igκ and Igλ in the human B cell lymphoma cell lines Daudi, BV173, Maver-1 and SP53. (D) Representative flow cytometry plots illustrating the co-culture of NTD, CAR.CD19, CAR.κ, and CAR.λ-T cells. Cells were collected at day 5 of culture and tumor cells and T cells quantified by CD19 and CD3 expression, respectively. (E) Compiled data of the co-coculture experiments illustrated in (D); *** p-value < 0.001, two-way ANOVA, (n = 5). (F) Measurement of IFN-γ (upper panel) and IL-2 (lower panel) in the supernatants collected at 24 hours in the co-culture experiments illustrated in (D) *** p-value < 0.001, two-way ANOVA, (n = 5). (G) Representative flow cytometry plots (left panel) and compiled data for 3 donors (right panel) showing the CFSE dilution assay of CAR.λ-T cells co-cultured with irradiated Igλ+ tumor cell lines as compared to CAR.κ-T cells. ** p-value < 0.01, two-tailed t-test, (n = 3).
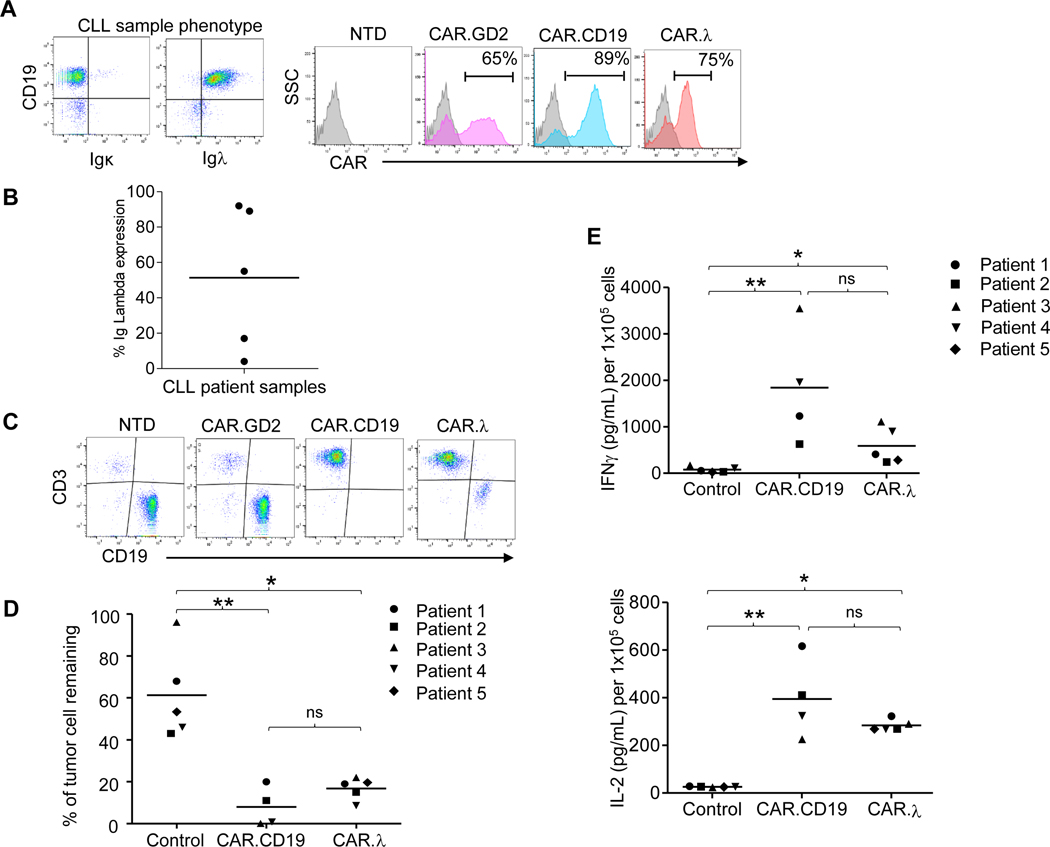
To assess the antitumor effects of CAR.λ-T cells against primary human tumor samples in an autologous setting, we used samples from five treatment-naïve B-CLL patients expressing the λ-light chain. CD3+ T lymphocytes were isolated from peripheral blood samples and engineered to express CAR.CD19, CAR.λ, or a CAR targeting an unrelated antigen (CAR.GD2) (25) (Figure 2A). Upon in vitro expansion, CAR-T cells were plated with their respective autologous B-CLL cells expressing different levels of Igλ (Figure 2B) at an E:T of 1:10 and co-cultured for 48 hours. While control T cells did not significantly affect B-CLL cells, both CAR.CD19 and CAR.λ-T cells displayed comparable ability to eliminate leukemic cells in culture (Figure 2C,D). Supernatants collected at 24 hours from the co-culture assays demonstrated elevated IFNγ and IL-2 cytokine production for both CAR.CD19 and CAR.λ-T cells, while control T cells did not display cytokine production (Figure 2E). Overall, these data show that CAR.λ-T cells specifically target Igλ-light chain expressing human tumors and exert similar antitumor activities compared to CAR.CD19.
Figure 2. Cytotoxic activity of autologous CAR.λ-T cells against B-CLL cells in vitro.
(A) Representative flow cytometry plots showing restricted Igλ expression in B-CLL cells and CAR expression in autologous T cells derived from CLL samples transduced to express CAR.GD2 (negative control), CAR.λ or CAR.CD19. (B) Summary of the Igλ expression levels in five CLL tumor samples. (C) Representative plots of coculture of B-CLL cells with autologous CAR-T cells. Tumor cells remaining were measured after 48 hours of co-culture by flow cytometry. (D) Summary of cocultures from all patient donors. Each symbol denotes a patient donor, while horizontal line depicts the mean. ** p-value < 0.01, * p-value < 0.05, when compared to control group, comparison between CAR.CD19 and CAR.λ was not significant, Kruskal-Wallis multiple comparisons test. (E) Measurement of IFN-γ (upper panel) and IL-2 (lower panel) released in the supernatants collected at 24 hours in co-culture illustrated in panel (A). ** p-value < 0.01, * p-value < 0.05, comparison between CAR.CD19 and CAR.λ was not significant, Kruskal-Wallis multiple comparisons test.
Antitumor activity of CAR.λ-T cells in vivo is equal to CAR.CD19-T cells.
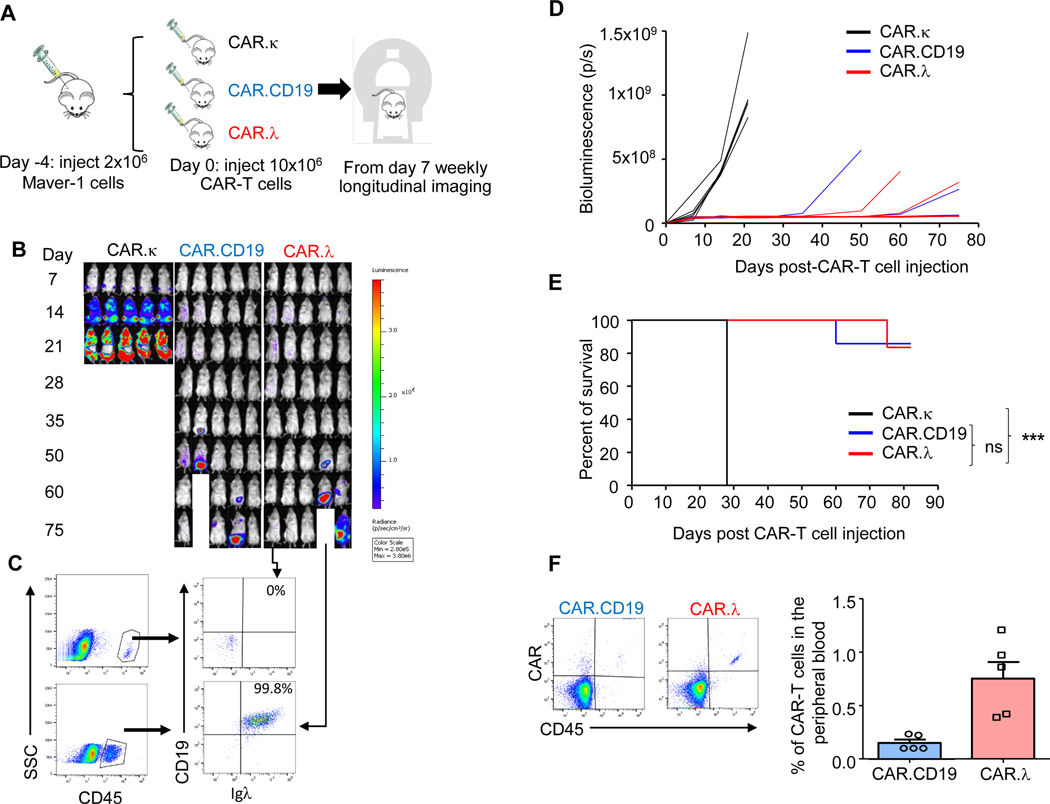
We established a xenograft lymphoma model in NSG (NOD-scid IL2Rgnull) mice and used a bioluminescence system to track tumor growth in vivo. T cells expressing CAR.CD19, CAR.κ, or CAR.λ were inoculated intravenously in CD19+Igλ+ lymphoma-bearing mice (Figure 3A). In mice treated with CAR.κ-T cells, the lymphoma grew unchecked and mice needed to be sacrificed after three weeks. In contrast, CAR.λ-T cells demonstrated substantial tumor control and showed equivalent efficacy to CAR.CD19-T cells (Figure 3B-D). Survival curves also exhibited equivalency between CAR.λ and CAR.CD19-T cells (Figure 3E and S2-S3). Mice treated with CAR.λ and CAR.CD19-T cells were bled 30 days post-CAR-T cell injection to examine the persistence of the CAR-T cells. Both groups showed presence of CAR-T cells in the peripheral blood (Figure 3F). When mice treated with CAR.λ-T cells were sacrificed to analyze for the presence of lymphoma at day 80, no tumor cells were detected by flow cytometry analysis in blood, bone marrow, liver, and spleen in mice without bioluminescence signal, while CD19+Igλ+ tumor cells were detected in mice showing bioluminescence signal (Figure 3C and S4).
Figure 3. CAR.λ and CAR.CD19-T cells show equivalent antitumor activity against Igλ+ lymphoma in vivo.
(A) Schema of the lymphoma xenograft model. NSG mice bearing firefly luciferase (FFluc) labelled Igλ+ Maver-1 tumor cells were treated with CAR.κ (negative control), CAR.CD19 (positive control) or CAR.λ-T cells. (B) Representative IVIS bioluminescence imaging showing tumor growth of different groups. (C) Mice treated with CAR.λ-T cells were sacrificed at day 75 and analyzed for presence of tumor. In mice with no detectable BLI signal, flow cytometry confirmed the lack of tumor cells in bone marrow, spleen and liver. In contrast, in mice with BLI signal, flow cytometry confirmed the presence of CD19+ tumor cells that retained Igλ expression. Flow plots shown here are of bone marrow samples. (D) Graph summary of tumor BLI kinetics of all treated mice described in (B) (n = 5). (E) Kaplan-Meier survival curves of treated mice described in (B). *** p-value < 0.001 (log-rank Mantel-Cox test) when comparing CAR.λ to CAR.κ-T cells (n = 5). (F) Representative flow cytometry plots (left panel) and quantification (right panel) of CAR-T cells detected in the peripheral blood of the mice treated as described in (B) at day 30.
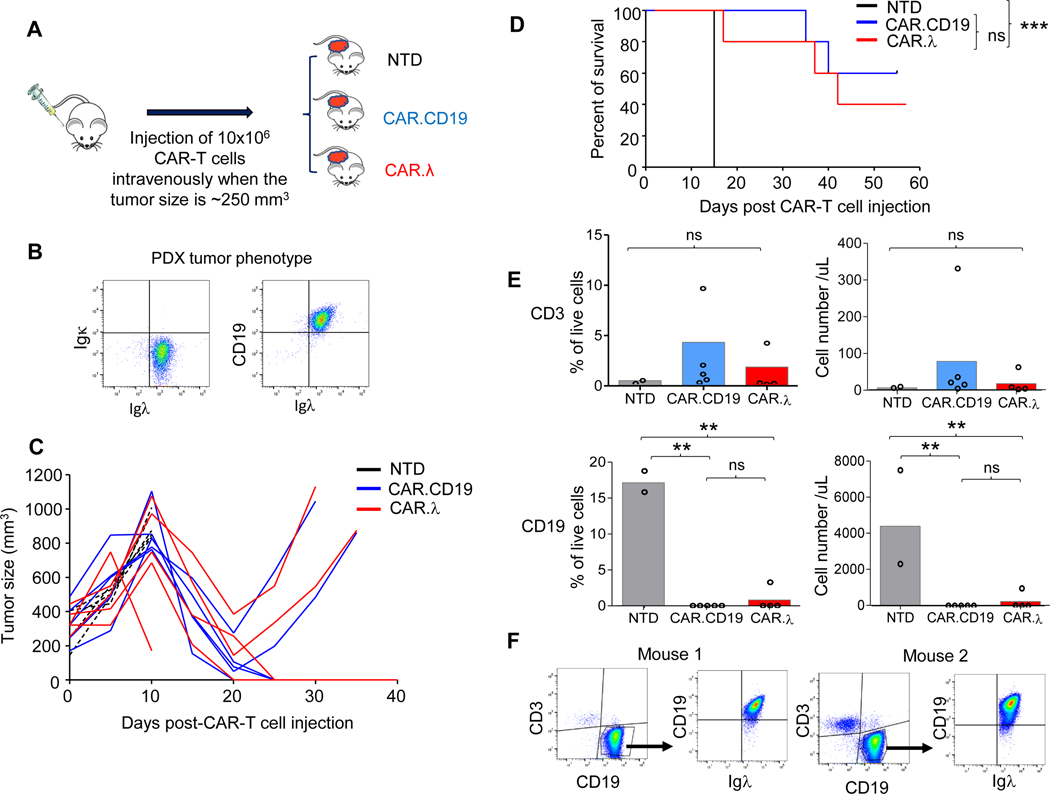
To further demonstrate the clinical relevance of the proposed CAR-T cells, we implemented a patient-derived xenograft (PDX) mouse model of Igλ+ MCL to test CAR.λ-T cells in vivo. NSG mice were inoculated in the flank with a CD19+Igλ+ MCL PDX, and tumor engrafted for 2–3 weeks (Figure 4A-B). After tumor engraftment, mice were injected intravenously with either NTD, CAR.CD19, or CAR.λ-T cells and then had their tumors measured prospectively. CAR.CD19 and CAR.λ-T cells demonstrated similar control of tumor growth (Figure 4C). Survival curves also demonstrated equivalency between the two treatment groups (Figure 4D). To assess CAR-T cell persistence, mice were bled at day 30 post-CAR-T cell injection to check for persistence as well as for presence of tumor. Despite the location of the tumor in the flank, flow cytometry analysis showed the presence of circulating tumor cells in mice treated with NTD cells (prior to euthanization), while mice treated with CAR.CD19 and CAR.λ-T cells showed very low or no detectable circulating tumor cells, while T cells were also equally detectable in the peripheral blood (Figure 4E). Two mice that had tumor recurrence in the CAR.λ-T cell treatment group, were sacrificed and had their tumors harvested. Flow cytometry analysis demonstrated continued Igλ expression by tumor cells indicating that tumor growth in these mice may not be due to antigen loss (Figure 4F). These results were reproducible when this PDX murine model was repeated with CAR-T cells from another healthy donor (Figure S5). Overall, these data show that both CAR.λ and CAR.CD19-T cells control MCL PDX in vivo.
Figure 4. CAR.λ and CAR.CD19-T cells demonstrate equivalent antitumor effects in a patient derived xenograft (PDX) MCL model.
(A) Schema of PDX model in which MCL cells derived from a patient were injected in the flank of NSG mice. After tumor engraftment (tumor size of 250 mm3), mice were injected intravenously with either NTD, CAR.CD19, or CAR.λ-T cells. (B) Flow cytometry plots showing CD19 and Igλ expression in PDX cells. (C) Kinetics of tumor growth in all treated mice described in (A). (D) Kaplan-Meier survival curves of mice treated as described in (A). *** p-value < 0.001, log-rank Mantel-Cox test (n = 5). (E) CD3+ (upper panels) and CD19+ (lower panels) cells in the peripheral blood of treated mice described in (A) (n = 5). ** p-value <0.01, one way ANOVA. (F) Representative flow cytometry plots showing the expression of CD19 and Igλ in PDX cells that recurred in two mice treated with CAR.λ-T cells showing no loss of the Igλ.
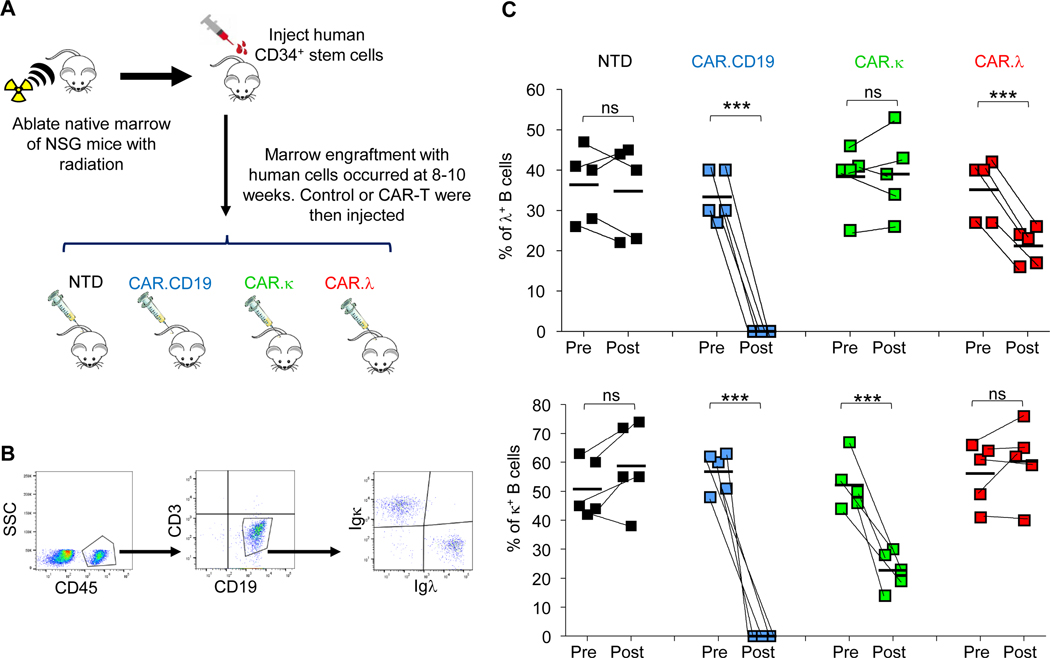
Immunoglobulin light chain-specific CAR-T cells spare normal B-lymphocytes expressing the reciprocal light chain in a humanized murine model.
To further characterize and evaluate the selectivity of our CAR constructs, and also to approximate conditions which are closer to the human immune system, we generated a humanized murine model (Figure 5A). These mice showed engraftment of human CD19+ B lymphocytes that expressed either the Igκ or Igλ, recapitulating the repertoire of human B cells (Figure 5B). Engrafted mice were then injected intravenously with either non-transduced (NTD), CAR.CD19, CAR.κ, or CAR.λ-T cells. The levels of the CD19+Igκ+ and CD19+Igλ+ B cells were measured in the peripheral blood seven days post-CAR-T cell infusion (Figure 5C). Mice infused with control NTD cells did not show any modification in B cell subset composition, while mice treated with CAR.CD19-T cells showed full depletion of B cells. Mice treated with either CAR.κ-T cells or CAR.λ-T cells demonstrated a significant reduction only of the appropriately targeted CD19+Igκ+ or CD19+Igλ+ B cells, respectively, while sparing CD19+Igλ+ or CD19+Igκ+ B cells, respectively. Overall, these data reinforce the target selectivity on normal B lymphocytes of the proposed immunoglobulin light-chain specific CAR-T cells.
Figure 5. CAR.λ and CAR.κ-T cells show selective depletion of normal B lymphocytes in a humanized murine model.
(A) Schema of the humanized murine model. (B) Representative flow analysis of peripheral blood from the humanized mice showing engraftment of CD19+Igκ+ and CD19+Igλ+ B-lymphocytes 8 weeks after the injection of CD34+ hematopoietic stem cells. (C) Modifications of CD19+Igλ+ (upper panel) and CD19+Igκ+ (lower panel) B cells in the peripheral blood of humanized mice at day 7 after treatment with CAR-T cells. Each individual line connecting two points represents an individual mouse (n = 5). *** p-value < 0.001, two-way ANOVA, when comparing levels of B cells pre-injection and post-injection of CAR-T cells.
Discussion
Despite clinical efficacy of CAR.CD19-T cells in patients with B-NHL and B-CLL, increased infection risk due to B cell aplasia and hypogammaglobulinemia remains a concern. Taking advantage of the clonally restricted expression of κ- and λ-light chains of human immunoglobulins on normal and malignant B cells, our proposed approach to target selectively the immunoglobulin light chains will spare a compartment of normal B cells while targeting tumor cells. This approach was initially translated into a phase I clinical study that demonstrated feasibility, safety and antitumor activity in patients with B cell malignancies infused with autologous CAR.κ-T cells (18). In this clinical trial patients were infused with CAR.κ-T cells without prior lymphodepleting chemotherapy, and clinical activity was observed without interference of soluble immunoglobulins as anticipated by our preclinical evaluation (17,18). Here we further extended the proposed strategy by generating CAR.λ-T cells.
Based on our previous preclinical and clinical experience (17,18), we first refined the structure of the backbone of the CAR cassette by modifications of the hinge and transmembrane regions and by adding these modifications we achieved increased antitumor activity in vivo. This novel design of the CAR.κ is currently under clinical investigation in B-cell lymphoid malignancies in which CAR.κ-T cells are infused in lymphodepleted patients (ClinicalTrials.gov Identifier: NCT04223765). Using the same CAR design, CAR.λ-T cells demonstrated effective in vitro cytotoxicity towards Igλ+ lymphoma/leukemia cells, while sparing the Igκ+ expressing cells. In our xenograft murine model, CAR.λ-T cells displayed equivalent antitumor activity as CAR.CD19-T cells without showing escape of lgλ- tumor cells, though this finding does not preclude the possibility of Igλ antigen loss from ever occurring. This equivalency in tumor eradication compared to CAR.CD19-T cells was also seen in vitro with patient-derived CLL cells and in vivo with a PDX xenograft model of MCL. Additionally, we implemented a humanized murine model showing reconstituted normal Igκ− and Igλ-restricted normal B cells, and demonstrated the selective B cell depletion of CAR.λ and CAR.κ-T cells, while CAR.CD19-T cells depleted all normal B cells.
It has been observed that about two-thirds of patients receiving CAR.CD19-T cells develop hypogammaglobulinemia. In one long-term study with a median follow-up of 28 months, 86 patients showed persistent hypogammaglobulinemia (IgG<400 mg/dL) and infectious events. Starting 90 days post-CAR.CD19-T cell infusion, 20% and 5% of patients needed inpatient or intensive care for infections, respectively, with the majority of the infections being sinopulmonary (8). A second study showed nearly half of patients receiving CAR.CD19-T cells exhibiting hypogammaglobulinemia (IgG<400 mg/dL) 61 to 90 days after CAR-T cell infusion, with 14% developing infections (26). B-cell aplasia due to on-target, off-tumor toxicity is an established effect of CAR.CD19-T cells. About half of patients treated with CAR.CD19-T cells show detectable B cells in the peripheral blood at 12 months post-CAR-T cell infusion (27). However, there is evidence for delayed CD4 count recovery requiring more than 12 months after CAR.CD19-T cells, with one of the studies noting herpes zoster infections and Pneumocysitis jiroveci pneumonia in a significant proportion of patients (28,29). These findings would suggest that even with detectable B lymphocyte recovery, their overall immune reconstitution is compromised and vulnerable to significant infection events. It remains to be clinically demonstrated if selective elimination of normal B cells expressing the targeted light chain of the Igs, with sparing of the reciprocal light chain-expressing B cells, provides better preserved immunity.
The majority of NHL are mature B cell derived with diffuse large cell lymphomas, follicular lymphomas, CLL/small lymphocytic lymphoma, and marginal zone lymphomas comprising over 60% of NHL. Therefore, there would be significant utility for immunoglobulin light-chain targeting CAR-T cells in NHL. Furthermore, since 20–30% of patients treated with CAR.CD19-T cells who relapse show loss of the CD19 epitope on their lymphoma cells, CARs redirected to the immunoglobulin light chain may be used in patients relapsed after CAR.CD19-T cell treatment (30).
Alternative targets to CD19 in B-cell malignancies are actively being investigated. The receptor tyrosine kinase‐like orphan receptor 1 (ROR1) is expressed at high levels in MCL and CLL, with analyses implicating higher ROR1 levels with accelerated disease progression and negative survival prognosis in CLL (31,32). ROR1-targeting CAR-T cells displayed promising antitumor effects in preclinical studies against CLL and MCL (33). However normal B cell precursors in the bone marrow also express ROR1, as do large areas of the gastrointestinal tract, so ROR1-specific CAR-T cells may not only negatively impact the B cell compartment, but also could have significant on-target but off-tumor toxicity (34). CD79b is a normal component of the multimeric B cell receptor complex, and is reliably expressed in many B cell malignancies (35). Targeting CD79b with antibody-drug conjugates like polatuzumab vedotin is already part of the treatment repertoire in B-NHL, and CAR-T cells against CD79b showed effective tumor control in preclinical models (36). CD20 is also a well-known target in B-NHL and CD20-specific CAR-T cells have been clinically tested (37). However, both CD79b and CD20 share the same issue as targeting CD19 in being uniformly expressed in normal B cells. Our approach targeting the immunoglobulin light chains could lend an advantage in this respect, since our data shows significant sparing of non-malignant B cells expressing the non-targeted light chain. Despite our demonstration of efficacy with our CAR.λ, there are factors which could potentially impact the translational efficacy of any CAR-T approach in B-NHL patients and which cannot be recapitulated fully within preclinical models. In particular, the xenograft tumor models do not recapitulate the complexity of the human diseases. However, our proposed experimental models are similar to those used to validate the antitumor activity of CAR.CD19-T cells which proved to be effective in clinical studies stimulating the clinical translation of preclinical findings.
In summary, our data suggests that a strategy targeting the λ-light chain as well as the κ-light chain of human immunoglobulins with CAR-T cells could represent a valuable and important modality in treating all mature B-NHL and B-CLL patients. Since the humoral immune response against antigens is typically polyclonal, the sparing of a subset of reciprocal κ-light chain expressing, non-malignant B cells could lessen deleterious effects on humoral immunity function.
Supplementary Material
Statement of Translational Relevance.
Adoptive transfer of chimeric antigen receptor T lymphocytes (CAR-T) directed at the CD19 antigen is a proven therapeutic option for relapsed/refractory B-cell Non-Hodgkin Lymphoma (B-NHL). Targeting this antigen, however, does not distinguish between normal and malignant B cells and causes profound B-cell aplasia and agammaglobulinemia with increased infection risk since CD19.CAR-T can persist long-term. Many B-NHL subtypes express surface immunoglobulin that is clonally restricted to either the kappa (Ig-κ) or lambda (Ig-λ) light chains. This research demonstrates targeting either Ig-κ or Ig-λ individually with CAR-T can eradicate malignant B cells expressing the targeted light chain as effectively as CD19.CAR-T, but also spare B lymphocytes expressing the reciprocal light chain, which could consequently reduce the impairment of humoral immunity in CAR-T recipients and potentially improve upon the existing CAR-T approaches for B-NHL.
Acknowledgments
This work was supported by R01 CA193140 (GD), T32 CA211058 (RR), and Lymphoma Research Foundation Clinical Investigator Career Development Grant 610516 (RR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of Interest Statement:
GD is a member of the scientific advisory board of Bellicum Pharmaceuticals and Catamaran. GD and BS are paid consultants for Tessa Therapeutics, GD reports receiving commercial research grants from Cell Medica and Bluebird Bio. No potential conflicts of interest were disclosed by the other authors.
Reference List
- 1.Hirayama AV, Gauthier J, Hay KA, Voutsinas JM, Wu Q, Gooley T, et al. The response to lymphodepletion impacts PFS in patients with aggressive non-Hodgkin lymphoma treated with CD19 CAR T cells. Blood 2019;133(17):1876–87 doi 10.1182/blood-2018-11-887067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frey NV, Gill S, Hexner EO, Schuster S, Nasta S, Loren A, et al. Long-Term Outcomes From a Randomized Dose Optimization Study of Chimeric Antigen Receptor Modified T Cells in Relapsed Chronic Lymphocytic Leukemia. J Clin Oncol 2020;38(25):2862–71 doi 10.1200/JCO.19.03237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med 2017;377(26):2531–44 doi 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med 2019;380(1):45–56 doi 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- 5.Kochenderfer JN, Dudley ME, Kassim SH, Somerville RP, Carpenter RO, Stetler-Stevenson M, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol 2015;33(6):540–9 doi 10.1200/jco.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maude SL, Teachey DT, Porter DL, Grupp SA. CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Blood 2015;125(26):4017–23 doi 10.1182/blood-2014-12-580068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobson CA, Hunter B, Armand P, Kamihara Y, Ritz J, Rodig SJ, et al. Axicabtagene Ciloleucel in the Real World: Outcomes and Predictors of Response, Resistance and Toxicity. Blood 2018;132(Supplement 1):92- doi 10.1182/blood-2018-99-117199. [DOI] [Google Scholar]
- 8.Cordeiro A, Bezerra ED, Hirayama AV, Hill JA, Wu QV, Voutsinas J, et al. Late Events after Treatment with CD19-Targeted Chimeric Antigen Receptor Modified T Cells. Biol Blood Marrow Transplant 2020;26(1):26–33 doi 10.1016/j.bbmt.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shalabi H, Kraft IL, Wang HW, Yuan CM, Yates B, Delbrook C, et al. Sequential loss of tumor surface antigens following chimeric antigen receptor T-cell therapies in diffuse large B-cell lymphoma. Haematologica 2018;103(5):e215–e8 doi 10.3324/haematol.2017.183459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sotillo E, Barrett DM, Black KL, Bagashev A, Oldridge D, Wu G, et al. Convergence of Acquired Mutations and Alternative Splicing of CD19 Enables Resistance to CART-19 Immunotherapy. Cancer Discov 2015;5(12):1282–95 doi 10.1158/2159-8290.CD-15-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li S, Eshleman JR, Borowitz MJ. Lack of surface immunoglobulin light chain expression by flow cytometric immunophenotyping can help diagnose peripheral B-cell lymphoma. Am J Clin Pathol 2002;118(2):229–34 doi 10.1309/57G0-1BNF-KB9R-L4HN. [DOI] [PubMed] [Google Scholar]
- 12.Liendo C, Danieu L, Al-Katib A, Koziner B. Phenotypic analysis by flow cytometry of surface immunoglobulin light chains and B and T cell antigens in lymph nodes involved with non-Hodgkin’s lymphoma. Am J Med 1985;79(4):445–54 doi 10.1016/0002-9343(85)90031-2. [DOI] [PubMed] [Google Scholar]
- 13.Kaleem Z, Zehnbauer BA, White G, Zutter MM. Lack of expression of surface immunoglobulin light chains in B-cell non-Hodgkin lymphomas. Am J Clin Pathol 2000;113(3):399–405 doi 10.1309/28ED-MM0T-DT3B-MT4P. [DOI] [PubMed] [Google Scholar]
- 14.Bertoni F, Ponzoni M. The cellular origin of mantle cell lymphoma. Int J Biochem Cell Biol 2007;39(10):1747–53 doi 10.1016/j.biocel.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 15.Bertoni F, Zucca E, Genini D, Cazzaniga G, Roggero E, Ghielmini M, et al. Immunoglobulin light chain kappa deletion rearrangement as a marker of clonality in mantle cell lymphoma. Leuk Lymphoma 1999;36(1–2):147–50 doi 10.3109/10428199909145958. [DOI] [PubMed] [Google Scholar]
- 16.Schraders M, Oeschger S, Kluin PM, Hebeda K, Schuuring E, Groenen PJ, et al. Hypermutation in mantle cell lymphoma does not indicate a clinical or biological subentity. Mod Pathol 2009;22(3):416–25 doi 10.1038/modpathol.2008.199. [DOI] [PubMed] [Google Scholar]
- 17.Vera J, Savoldo B, Vigouroux S, Biagi E, Pule M, Rossig C, et al. T lymphocytes redirected against the kappa light chain of human immunoglobulin efficiently kill mature B lymphocyte-derived malignant cells. Blood 2006;108(12):3890–7 doi 10.1182/blood-2006-04-017061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramos CA, Savoldo B, Torrano V, Ballard B, Zhang H, Dakhova O, et al. Clinical responses with T lymphocytes targeting malignancy-associated kappa light chains. J Clin Invest 2016;126(7):2588–96 doi 10.1172/JCI86000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diaconu I, Ballard B, Zhang M, Chen Y, West J, Dotti G, et al. Inducible Caspase-9 Selectively Modulates the Toxicities of CD19-Specific Chimeric Antigen Receptor-Modified T Cells. Mol Ther 2017;25(3):580–92 doi 10.1016/j.ymthe.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun C, Shou P, Du H, Hirabayashi K, Chen Y, Herring LE, et al. THEMIS-SHP1 Recruitment by 4–1BB Tunes LCK-Mediated Priming of Chimeric Antigen Receptor-Redirected T Cells. Cancer cell 2020;37(2):216–25 e6 doi 10.1016/j.ccell.2019.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hudecek M, Lupo-Stanghellini MT, Kosasih PL, Sommermeyer D, Jensen MC, Rader C, et al. Receptor affinity and extracellular domain modifications affect tumor recognition by ROR1-specific chimeric antigen receptor T cells. Clinical cancer research : an official journal of the American Association for Cancer Research 2013;19(12):3153–64 doi 10.1158/1078-0432.CCR-13-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geldres C, Savoldo B, Dotti G. Chimeric antigen receptor-redirected T cells return to the bench. Semin Immunol 2016;28(1):3–9 doi 10.1016/j.smim.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014;371(16):1507–17 doi 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imai C, Mihara K, Andreansky M, Nicholson IC, Pui CH, Geiger TL, et al. Chimeric receptors with 4–1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia 2004;18(4):676–84 doi 10.1038/sj.leu.2403302. [DOI] [PubMed] [Google Scholar]
- 25.Chen Y, Sun C, Landoni E, Metelitsa L, Dotti G, Savoldo B. Eradication of Neuroblastoma by T Cells Redirected with an Optimized GD2-Specific Chimeric Antigen Receptor and Interleukin-15. Clinical cancer research : an official journal of the American Association for Cancer Research 2019;25(9):2915–24 doi 10.1158/1078-0432.CCR-18-1811. [DOI] [PubMed] [Google Scholar]
- 26.Hill JA, Li D, Hay KA, Green ML, Cherian S, Chen X, et al. Infectious complications of CD19-targeted chimeric antigen receptor-modified T-cell immunotherapy. Blood 2018;131(1):121–30 doi 10.1182/blood-2017-07-793760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol 2019;20(1):31–42 doi 10.1016/S1470-2045(18)30864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Logue JM, Zucchetti E, Bachmeier CA, Krivenko GS, Larson V, Ninh D, et al. Immune reconstitution and associated infections following axicabtagene ciloleucel in relapsed or refractory large B-cell lymphoma. Haematologica 2020. doi 10.3324/haematol.2019.238634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strati P, Varma A, Adkins S, Nastoupil LJ, Westin J, Hagemeister FB, et al. Hematopoietic recovery and immune reconstitution after axicabtagene ciloleucel in patients with large B-cell lymphoma. Haematologica 2020. doi 10.3324/haematol.2020.254045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spiegel JY, Dahiya S, Jain MD, Nastoupil LJ, Ghobadi A, Lin Y, et al. Outcomes in large B-cell lymphoma progressing after axicabtagene ciloleucel (Axi-cel): Results from the U.S. Lymphoma CAR-T Consortium. Journal of Clinical Oncology 2019;37(15_suppl):7517- doi 10.1200/JCO.2019.37.15_suppl.7517. [DOI] [Google Scholar]
- 31.Baskar S, Kwong KY, Hofer T, Levy JM, Kennedy MG, Lee E, et al. Unique cell surface expression of receptor tyrosine kinase ROR1 in human B-cell chronic lymphocytic leukemia. Clinical cancer research : an official journal of the American Association for Cancer Research 2008;14(2):396–404 doi 10.1158/1078-0432.CCR-07-1823. [DOI] [PubMed] [Google Scholar]
- 32.Barna G, Mihalik R, Timar B, Tombol J, Csende Z, Sebestyen A, et al. ROR1 expression is not a unique marker of CLL. Hematol Oncol 2011;29(1):17–21 doi 10.1002/hon.948. [DOI] [PubMed] [Google Scholar]
- 33.Hudecek M, Schmitt TM, Baskar S, Lupo-Stanghellini MT, Nishida T, Yamamoto TN, et al. The B-cell tumor-associated antigen ROR1 can be targeted with T cells modified to express a ROR1-specific chimeric antigen receptor. Blood 2010;116(22):4532–41 doi 10.1182/blood-2010-05-283309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balakrishnan A, Goodpaster T, Randolph-Habecker J, Hoffstrom BG, Jalikis FG, Koch LK, et al. Analysis of ROR1 Protein Expression in Human Cancer and Normal Tissues. Clinical cancer research : an official journal of the American Association for Cancer Research 2017;23(12):3061–71 doi 10.1158/1078-0432.CCR-16-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cabezudo E, Carrara P, Morilla R, Matutes E. Quantitative analysis of CD79b, CD5 and CD19 in mature B-cell lymphoproliferative disorders. Haematologica 1999;84(5):413–8. [PubMed] [Google Scholar]
- 36.Sehn LH, Herrera AF, Flowers CR, Kamdar MK, McMillan A, Hertzberg M, et al. Polatuzumab Vedotin in Relapsed or Refractory Diffuse Large B-Cell Lymphoma. J Clin Oncol 2020;38(2):155–65 doi 10.1200/JCO.19.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Till BG, Jensen MC, Wang J, Qian X, Gopal AK, Maloney DG, et al. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4–1BB domains: pilot clinical trial results. Blood 2012;119(17):3940–50 doi 10.1182/blood-2011-10-387969. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.