Abstract
Objective
We explored the association between female gender and long COVID syndrome, defined as persistence of physical and/or psychological symptoms for more than 4 weeks after recovery from acute COVID-19 disease. The secondary aim was to identify predictors of long COVID syndrome by multivariable logistic regression analysis.
Methods
This was a single-centre prospective cohort study conducted at San Paolo Hospital in Milan, Italy. We enrolled adult patients who were evaluated at the post-COVID outpatient service of our Infectious Diseases Unit between 15 April 2020 and 15 December 2020. Participants were individuals who had clinically recovered from COVID-19 and in whom virological clearance had occurred. Previous infection by SARS-CoV-2 was microbiologically documented by positivity using a reverse-transcriptase polymerase chain reaction (RT-PCR) assay of nasopharyngeal swab. All enrolled patients underwent blood tests and a comprehensive medical examination at follow-up. Individuals were interviewed about resolved and persisting symptoms and were asked to fill in two questionnaires to allow assessment of the Hospital Anxiety and Depression symptoms (HADS) score and of the Impact of Event Scale–Revised (IES-R) score.
Results
A total of 377 patients were enrolled in the study. The median time from symtpom onset to virological clerance was 44 (37–53) days. A diagnosis of long COVID syndrome was made in 260/377 (69%) patients. The most common reported symptoms were fatigue (149/377, 39.5%), exertional dyspnoea (109/377, 28.9%), musculoskeletal pain (80/377, 21.2%) and “brain fog” (76/377, 20.2%). Anxiety symptoms were ascertained in 71/377 (18.8%) individuals, whereas 40/377 (10.6%) patients presented symptoms of depression. Post-traumatic stress disorder (defined by a pathological IES-R score) was diagnosed in one-third of patients (85/275, 31%). Female gender was independently associated with long COVID syndrome at multivariable analysis (AOR 3.3 vs. males, 95% CI 1.8–6.2, p < 0.0001). Advanced age (adjusted (A)OR 1.03 for 10 years older, 95% CI 1.01–1.05, p 0.01) and active smoking (AOR 0.19 for former smokers vs. active smokers, 95% CI 0.06–0.62, p 0.002) were also associated with a higher risk of long COVID, while no association was found between severity of disease and long COVID (AOR 0.67 for continuous positive airway pressure (CPAP)/non-invasive mechanical ventilation (NIMV)/orotracheal intubation (OTI) vs. no 02 therapy, 95% CI 0.29–1.55, p 0.85).
Discussion
Factors that were found to be associated with a higher risk of developing “long COVID” syndrome were female gender, older age and active smoking, but not severity of the acute disease. Individuals affected by SARS-CoV-2 infection with the aforementioned features should be early identified and involved in follow-up programmes.
Keywords: COVID-19, Gender, Long COVID, Post acute sequelae SARS COV-2 infection (PASC), Post COVID-19 condition, Post-COVID syndrome
Introduction
Over half of individuals affected by COVID-19 report persisting symptoms after recovery from acute illness. These symptoms can endure up to 6–7 months and even longer [[1], [2], [3], [4], [5]]. Since the persistence of symptoms represents a health concern, this phenomenon was given the name of “long COVID” syndrome. In the UK, the National Institute for Health and Care Excellence (NICE) defined long COVID as the persistence of symptoms for more than 4 weeks after the onset of acute illness [[6], [7], [8]]. Ongoing physical symptoms include fatigue, muscle weakness, shortness of breath or cough, joint or chest pain, as well as difficulty in concentration and memory disorders; the last two symptoms re-appear in a broader state of diminished mental capacity that may be described as “brain fog” [1,4,[9], [10], [11]]. As far as psychological manifestations are concerned, the most frequent are post-traumatic stress disorder (PTSD), depression, anxiety, obsessive–compulsive traits and insomnia [1,[12], [13], [14], [15]]. Previous studies have not distinctly identified predictors of long COVID syndrome; factors found to have a correlation with long COVID so far were increased length of acute illness, longer time from symptom onset to virological clearance and comorbidities [11,16]. Increased COVID-19 severity and female gender as predicting factors are instead not equivocal findings [1,4,5,8,13,[17], [18], [19], [20]]. Nevertheless, what emerges from preliminary data is the negative impact of long COVID on quality of life and its effects on patient work ability and social relationships [1,4,19]. Given the extent of such phenomenon, healthcare public resources will be utilized to face the issue even when the COVID-19 emergency status will end. It is therefore necessary to better understand the characteristics of long COVID, its predictors and possible long-term sequelae. Furthermore, the likelihood of presenting ongoing symptoms might differ according to several factors (e.g. socioeconomic status or cultural background) that should therefore be identified.
We thus investigated the incidence of physical and/or psychological symptoms characterising the “long COVID” syndrome in female gender and we studied the possible predictors of long COVID.
Materials and methods
Study design and ethics
This was a single-centre prospective cohort study conducted at San Paolo Hospital in Milan, Italy. Informed consent from all participants was obtained. The study was approved by the Ethics Committee Area 1, Milan (2020/ST/049 and 2020/ST/049_BIS, 11 March 2020).
Study population
We enrolled adult patients who were evaluated at the post-COVID outpatient clinic, which had been set up in April 2020, in our Infectious Diseases Unit between 15 April 2020 and 15 December 2020. Participants were patients who had been hospitalized for COVID-19 at San Paolo and San Carlo Hospitals in Milan and had been discharged between 1 March 2020 and 1 November 2020. Previous infection by SARS-CoV-2 was microbiologically documented by positivity of a reverse-transcriptase polymerase chain reaction (RT-PCR) assay for a nasopharyngeal swab. At the time of hospital discharge, patients were given an appointment for a follow-up visit in the outpatient clinic.
Not included in the study were patients who died during hospitalization or after discharge, those who were not given a follow-up appointment because they declined to participate or were unable to reach the hospital, and those who missed their appointment and were therefore lost to follow-up.
Patients who had been hospitalized for other medical issues and had a positive SARS-CoV-2 nasopharyngeal swab but remained asymptomatic for COVID-19 were also excluded from the study.
Follow-up implied
-
•
repetition of nasopharyngeal swab for patients who were discharged with the indication to self-isolate since their upper respiratory swab was still positive at the time of discharge;
-
•
blood tests and comprehensive medical visit at 1–3 months and later at 6 months after virological clearance (namely a negative nasopharyngeal swab).
This study reports data about the first follow-up assessment at 1–3 months after virological clearance.
A trained nurse was in charge of taking blood samples, whereas a team of Infectious Diseases specialists and clinical psychologists were involved in visiting and interviewing participants. Patients were asked to self-report a detailed list of symptoms and signs, specifying whether they had resolved or were still ongoing. Moreover, they were invited to fill in two questionnaires: the Hospital Anxiety and Depression Symptoms (HADS) [21] and the Impact of Event Scale–Revised (IES-R) [22]. A comprehensive physical examination was performed by trained Infectious Diseases specialists. Clinical psychologists revised the questionnaires and identified symptoms of anxiety or depression and PTSD by evaluating HADS and IES-R scores respectively.
Aims of the study
The primary aim of the study was to assess the incidence of long COVID syndrome in females. The secondary aim was to identify predictors of long COVID by multivariable logistic regression analysis.
Study procedures
Data of the acute phase of illness were collected. Disease severity was defined by the highest level of respiratory support required during hospitalization (no oxygen therapy required, low- or high-flow oxygen therapy, continuous positive airway pressure (CPAP), non-invasive mechanical ventilation (NIMV) and orotracheal intubation (OTI)). Treatments for COVID-19 included antiviral agents (remdesivir (RDV) or HIV protease inhibitors), hydroxychloroquine alone or in association with azithromycin, corticosteroids and immunomodulatory drugs (IL-6 receptor antagonists and JAK-STAT inhibitors). At follow-up, all patients underwent routine blood tests and a medical evaluation. All patients were asked about the following signs and symptoms: anosmia, dysgeusia, fever, gastrointestinal symptoms, rest or exertional dyspnoea, fatigue, musculoskeletal pain, muscle weakness, “brain fog” manifestations (namely difficulties in attention or concentration and memory disorders). HADS [21] was intended to measure anxiety and depression symptoms, whereas IES-R was used as a screening tool of PTSD [22]. A total HADS score higher than 8 denoted considerable symptoms of anxiety and depression, while a IES-R score above 33 was interpreted as highly suggestive for PTSD. Long COVID was defined as the persistence of ≥1 physical and/or psychological symptoms at follow-up [7].
Statistical analyses
Data are presented as median and interquartile range (IQR) for quantitative parameters and absolute numbers and percentages for categorical variables. A Venn diagram was used to show possible correlations among physical and psychological symptoms characterizing long COVID. Comparison between the group of patients diagnosed with long COVID and that of patients who did not and between females and males was investigated by Mann–Whitney test and chi-squared or Fisher's exact test. The association between female gender and long COVID was analysed by fitting a multivariable logistic regression analysis, adjusting for possible confounders (age, severity of disease, length of hospital stay [LOS], comorbidities, body mass index [BMI], smoking and time to virological clearance). Missing data were handled by the missing-indicator method. p values have been adjusted by Bonferroni's correction. Statistical analyses were performed with STATA software, version 14.0.
Results
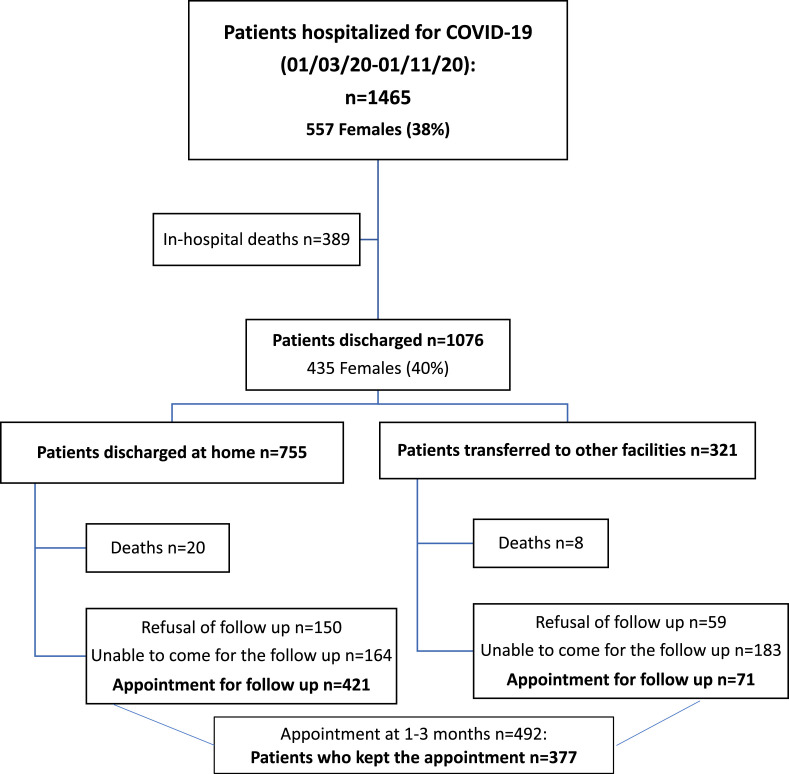
Fig. 1 depicts the study flowchart. A total of 492 patients discharged from San Paolo and San Carlo Hospitals in the aforementioned time period were given an appointment for a follow-up evaluation at the post-COVID outpatient clinic. In total 115/492 (23%) patients missed their visit, thus the study includes a total of 377/492 individuals, namely 77% of those who were given an appointment. Demographic and clinical characteristics of patients who missed the follow up appointment are presented in the Table S1.
Fig. 1.
Study flow chart.
The follow-up examination was done at a median of 102 (IQR 86–126) days from acute symptom onset, a median of 79 (IQR 69–102) days from clinical recovery and a median of 56 (IQR 47–74) days from virological clearance. Baseline demographic and clinical features of the enrolled patients are shown in Table 1 . Females accounted for 137/377 (36%) of all patients; median age was 57 (IQR 49–68) years. The most frequent acute symptoms were fever (280/377, 74%), cough (198/377, 53%), dyspnoea (161/377, 43%) and fatigue (59/377, 16%). In total, 346/377 (92%) presented interstitial pneumonia at chest X-ray or CT scan at the time of hospital admission. One-third of patients received CPAP or NIMV (115/377, 31%) during hospitalization, whereas only 30/377 (8%) patients underwent OTI.
Table 1.
Demographic and clinical characteristics of enrolled patients according to “long COVID” syndrome
| Parameters | Total population (N 377) | No long COVID (N 117) | Long COVID (N 260) | p |
|---|---|---|---|---|
| Age, years | 57 (49–68) | 56 (42–64) | 58 (51–69) | 0.01 |
| Gender, males | 240 (63.7) | 92 (78.6) | 148 (56.9) | <0.0001 |
| Comorbidities | 164 (43.5) | 45 (38.5) | 119 (45.8) | 0.241 |
| Smoking: | 0.046 | |||
| Never | 216 (57.3) | 71 (60.7) | 145 (55.8) | |
| Ex-smokers | 20 (5.3) | 9 (7.7) | 11 (4.2) | |
| Active-smokers | 131 (34.7) | 30 (25.7) | 101 (38.8) | |
| Unknown | 10 (2.7) | 7 (5.9) | 3 (1.2) | |
| Ethnicity: | 0.579 | |||
| White/Caucasian | 252 (66.8) | 82 (70.1) | 170 (65.4) | |
| Arabic | 16 (4.2) | 6 (5.1) | 10 (3.8) | |
| Black | 2 (0.5) | 0 | 2 (0.8) | |
| Asian | 7 (1.9) | 0 | 7 (2.7) | |
| Hispanic | 33 (8.7) | 9 (7.7) | 24 (9.2) | |
| Unknown | 67 (17.8) | 20 (17.1) | 47 (18.1) | |
| During hospitalization | ||||
| Symptoms at admission: | ||||
| Fever | 280 (74.3) | 96 (82.1) | 184 (70.8) | 0.023 |
| Cough | 198 (52.5) | 67 (57.3) | 131 (50.4) | 0.360 |
| Sore throat | 7 (1.9) | 3 (2.6) | 4 (1.5) | 0.501 |
| Chest pain | 16 (4.2) | 6 (5.1) | 10 (3.8) | 0.593 |
| Myalgia-arthralgia | 28 (7.4) | 10 (8.5) | 18 (6.9) | 0.422 |
| Fatigue | 59 (15.6) | 20 (17.1) | 39 (15) | 0.732 |
| Headache | 11 (2.9) | 4 (3.4) | 7 (2.7) | 0.754 |
| Dyspnoea | 161 (42.7) | 46 (39.3) | 115 (44.2) | 0.458 |
| Vomiting/nausea | 25 (6.6) | 9 (7.7) | 16 (6.2) | 0.526 |
| Anosmia/dysgeusia | 24 (6.4) | 9 (7.7) | 15 (5.8) | 0.478 |
| Pneumonia | 346 (91.8) | 108 (92.3) | 238 (91.5) | 0.372 |
| Oxygen therapy: | 0.879 | |||
| No O2 therapy | 35 (9.3) | 13 (11.1) | 22 (8.4) | |
| Low/high flows systems | 197 (52.2) | 60 (51.3) | 137 (52.7) | |
| CPAP, NIV | 115 (30.6) | 35 (29.9) | 80 (30.8) | |
| OTI | 30 (7.9) | 9 (7.7) | 21 (8.1) | |
| Length of hospital days (LOS) | 11 (6–20) | 10 (6–16) | 12 (7–21) | 0.232 |
| Treatments for COVID-19: | ||||
| Antivirals (RDV or PI) | 92 (22.4) | 30 (25.6) | 62 (23.8) | 0.746 |
| HQC ± azithromycin | 260 (68.9) | 83 (70.9) | 177 (68.1) | 0.661 |
| Steroid | 42 (11.1) | 14 (11.9) | 28 (10.8) | 0.798 |
| Immunomodulators | 28 (7.4) | 11 (9.4) | 17 (6.5) | 0.334 |
| Obesity: | 0.021 | |||
| BMI <30 | 227 (60.2) | 79 (67.5) | 148 (56.9) | |
| BMI ≥30 | 136 (36.1) | 31 (26.5) | 105 (40.4) | |
| Unknown | 14 (3.7) | 7 (6) | 7 (2.7) | |
| Follow-up visit | ||||
| Time since clinical recovery, days | 79 (69–102) | 81 (69–108) | 79 (69–97) | 0.599 |
| Time since virological clearance, days | 56 (47–74) | 56 (46–79) | 56 (47–74) | 0.981 |
| Time since symptoms onset, days | 102 (86–126) | 101 (85–131) | 103 (86–124) | 0.664 |
| Time from symptoms onset to virological clearance, days | 44 (37–53) | 43 (35–50) | 44 (38–55) | 0.081 |
| Blood exams at follow-up | ||||
| WBC, × 103/mm3 | 6.07 (5.11–7.07) | 5.89 (5.19–7.22) | 6.08 (5.1–6.99) | 0.770 |
| Hb, g/dL | 14.1 (13.2–15) | 14.6 (13.7–15.5) | 13.9 (12.8–14.8) | <0.0001 |
| PLT, × 103/mm3 | 243 (203–281) | 237 (208–272) | 246 (202–288) | 0.325 |
| Lymphocytes, cell/mm3 | 2.2 (1.81–2.6) | 2.24 (1.87–2.7) | 2.18 (1.79–2.57) | 0.274 |
| Creatinine, mg/dL | 0.8 (0.67–0.9) | 0.8 (0.7–1) | 0.8 (0.6–0.9) | 0.129 |
| GOT, UI/L | 27 (24–31) | 25 (21–30) | 26 (23–31) | 0.198 |
| GPT, UI/L | 21 (19–31) | 20 (16–29) | 20 (16–27) | 0.571 |
| LDH, UI/L | 196 (177–213) | 188 (173–209) | 196 (180–219) | 0.247 |
| CRP, mg/L | 5 (5–5.2) | 5 (5–5.1) | 5 (5–5.1) | 0.484 |
| D dimer, mg/L | 120 (81–199) | 110 (78–177) | 126 (87–204) | 0.04 |
CPAP, continuous positive airway pressure; OTI, orotracheal intubation; NIV, non-invasive ventilation. PI, protease inhibitor (darunavir/cobicistat, darunavir/ritonavir, lopinavir/ritonavir); RDV, remdesivir; HQC, hydroxychloroquine; WBC, white blood cell; Hb, haemoglobin; PLT, platelet count; CRP, C-reactive protein; GOT, serum glutamic oxalacetic transaminase; GPT, serum glutamic pyruvic transaminase; LDH, lactate dehydrogenase.
Mann Whitney and Chi-square for comparison between patients diagnosed with long COVID and patients without long COVID.
Women were less frequently active smokers than men. Furthermore, overall patients of female gender presented a milder form of disease, estimated as a lower proportion of OTI and a shorter LOS (Table 2 ). Blood tests at follow-up showed no significant alterations: C-reactive protein (CRP) and complete blood count (CBC) were normal for all patients, while D-dimer was still out of range in 28/377 (7.4%) patients without however any correlation with organ dysfunction (median pathological D-dimer was 399, IQR 310.7–579.2 ng/mL). In all 28 patients with elevated D-dimer at follow-up, levels were lower than the acute phase of illness; however, two of these patients presented persisting dyspnoea and therefore underwent chest CT scan with contrast medium that excluded pulmonary thromboembolism.
Table 2.
Comparison between males and females
| Parameters | Females (N 137) | Males (N 240) | p |
|---|---|---|---|
| Age, yearsa | 58 (50–68) | 57 (49–67) | 0.931 |
| Comorbidities | 57 (41.6) | 107 (44.6) | 0.672 |
| Smoking: | 0.008 | ||
| Never | 94 (68.6) | 122 (50.8) | |
| Ex-smokers | 10 (7.3) | 10 (4.2) | |
| Active smokers | 31 (22.6) | 100 (41.7) | |
| Unknown | 2 (1.5) | 8 (3.3) | |
| Ethnicity: | 0.691 | ||
| White/Caucasian | 90 (65.6) | 162 (67.5) | |
| Arabic | 6 (4.4) | 10 (4.2) | |
| Black | 2 (1.5) | 0 | |
| Asian | 2 (1.5) | 5 (2.1) | |
| Hispanic | 12 (8.8) | 21 (8.7) | |
| Unknown | 25 (18.2) | 42 (17.5) | |
| During hospitalization | |||
| Symptoms at admission: | |||
| Fever | 96 (70.1) | 184 (76.7) | 0.215 |
| Cough | 61 (44.5) | 137 (57.1) | 0.02 |
| Sore throat | 3 (2.2) | 4 (1.7) | 0.91 |
| Chest pain | 10 (7.3) | 6 (2.5) | 0.03 |
| Myalgia-arthralgia | 16 (11.7) | 12 (5) | 0.392 |
| Fatigue | 21 (15.4) | 38 (15.8) | 0.914 |
| Headache | 4 (2.9) | 7 (2.9) | 0.999 |
| Dyspnoea | 54 (39.4) | 107 (44.6) | 0.452 |
| Vomiting/nausea | 11 (8.1) | 14 (5.8) | 0.49 |
| Anosmia/dysgeusia | 12 (8.8) | 12 (5) | 0.237 |
| Pneumonia | 126 (91.9) | 220 (91.7) | 0.771 |
| Oxygen therapy: | 0.009 | ||
| No O2 therapy | 18 (13.1) | 17 (7.1) | |
| Low/high flows systems | 79 (57.7) | 118 (49.2) | |
| CPAP, NIV | 37 (27) | 78 (32.5) | |
| OTI | 3 (2.2) | 27 (11.2) | |
| Length of hospital days (LOS)a | 10 (6–16) | 12 (7–21) | 0.033 |
| Treatments for COVID-19: | |||
| Antivirals (RDV or PI) | 33 (24.1) | 59 (24.5) | 0.892 |
| HQC ± azithromycin | 90 (65.7) | 170 (70.8) | 0.356 |
| Steroid | 13 (9.5) | 29 (12.1) | 0.576 |
| Immunomodulating therapies | 8 (5.8) | 20 (8.3) | 0.351 |
| BMI, categories: | 0.003 | ||
| <18.5 | 5 (3.6) | 0 | |
| 18.5–24.99 | 31 (22.6) | 45 (18.7) | |
| 25-29.99 | 39 (28.5) | 107 (44.6) | |
| ≥30 | 56 (40.9) | 80 (33.3) | |
| Unknown | 6 (4.38) | 8 (3.3) | |
| Follow-up visit | |||
| Time since virological clearance, daysa | 59 (49–77) | 55 (46–72) | 0.05 |
| Time from symptoms onset to virological clearance, daysa | 45 (38–55) | 44 (37–52) | 0.471 |
Antivirals include: darunavir/cobicistat, darunavir/ritonavir, lopinavir/ritonavir, remdesivir. CPAP, continuous positive airway pressure; OTI, orotracheal intubation; NIV, non-invasive ventilation; HQC, hydroxychloroquine.
Quantitative variables are presented as median, (Interquartile Range); categorical variables are presented as absolute numbers (%).
Long COVID was observed in 260/377 (69%) patients; interestingly, 112/137 (81.7%) females presented long COVID syndrome. Within long COVID patients, 97/260 (37.3%) participants had only one persisting symptom, 84/260 (32.3%) had two persisting symptoms and 79/260 (30.4%) had three or more persisting symptoms. More specifically, a significant number of patients (142/260, 55%) reported ongoing physical symptoms only, 100/260 (38%) complained both physical and psychological symptoms, while 18/260 (7%) presented psychological distress solely at follow-up (Fig. S1a). Physical and psychological manifestations were similarly represented in both genders (Fig. S1b,c).
The most common physical symptoms characterising long COVID were fatigue (149/377, 39.5%), exertional dyspnoea (109/377, 28.9%), musculoskeletal pain (80/377, 21.2%) and “brain fog” (76/377, 20.2%) (Table 3 : physical symptoms).
Table 3.
Physical and psychological symptoms at follow-up
| Parameter | Study population N = 377 |
Males N = 240 |
Females N = 137 |
p |
|---|---|---|---|---|
| Physical symptoms | ||||
| Anosmia | 0.017 | |||
| No, ever | 179 (47.5%) | 127 (52.9%) | 52 (37.9%) | |
| Ongoing | 18 (4.8%) | 8 (3.3%) | 10 (7.3%) | |
| Resolved | 174 (46.2%) | 104 (43.4%) | 70 (51.1%) | |
| Unknown | 6 (1.6%) | 1 (0.4%) | 5 (3.6%) | |
| Dysgeusia | 0.005 | |||
| No, ever | 167 (44.3%) | 120 (50%) | 47 (34.3%) | |
| Ongoing | 20 (5.3%) | 8 (3.3%) | 12 (8.7%) | |
| Resolved | 184 (48.8%) | 111 (46.2%) | 73 (53.3%) | |
| Unknown | 6 (1.6%) | 1 (0.4%) | 5 (3.6%) | |
| GI symptoms | <0.001 | |||
| No, ever | 201 (53.3%) | 143 (59.6%) | 58 (42.3%) | |
| Ongoing | 6 (1.6%) | 1 (0.4%) | 5 (3.7%) | |
| Resolved | 162 (43%) | 91 (37.9%) | 71 (51.8%) | |
| Unknown | 8 (2.1%) | 5 (2.1%) | 3 (2.2%) | |
| Fever | 0.966 | |||
| No, ever | 22 (5.8%) | 14 (5.9%) | 8 (5.8%) | |
| Ongoing | 0 | 0 | 0 | |
| Resolved | 348 (92.3%) | 223 (92.9%) | 125 (91.2%) | |
| Unknown | 7 (1.9%) | 3 (1.2%) | 4 (2.9%) | |
| Joint pain or myalgia | ||||
| No, ever | 215 (57%) | 156 (65%) | 59 (43.1%) | |
| Ongoing | 80 (21.2%) | 30 (12.5%) | 50 (36.4%) | |
| Resolved | 74 (19.6%) | 50 (20.8%) | 24 (17.5%) | |
| Unknown | 8 (2.1%) | 4 (1.7%) | 4 (2.9%) | |
| Rest dyspnoea | 0.548 | |||
| No, ever | 103 (27.3%) | 70 (29.2%) | 33 (24.1%) | |
| Ongoing | 24 (6.4%) | 16 (6.7%) | 8 (5.8%) | |
| Resolved | 242 (64.2%) | 150 (62.5%) | 92 (67.1%) | |
| Unknown | 8 (2.1%) | 4 (1.6%) | 4 (2.9%) | |
| Exertional dyspnoea | 0.036 | |||
| No, ever | 87 (23.1%) | 61 (25.5%) | 26 (19%) | |
| Ongoing | 109 (28.9%) | 59 (24.6%) | 50 (36.5%) | |
| Resolved | 175 (46.4%) | 117 (48.7%) | 58 (42.3%) | |
| Unknown | 6 (1.6%) | 3 (1.2%) | 3 (2.2%) | |
| Fatigue | <0.001 | |||
| No, ever | 67 (17.8%) | 50 (20.9%) | 17 (12.5%) | |
| Ongoing | 149 (39.5%) | 74 (30.8%) | 75 (54.7%) | |
| Resolved | 154 (40.8%) | 113 (47.1%) | 41 (29.9%) | |
| Unknown | 7 (1.9%) | 3 (1.2%) | 4 (2.9%) | |
| Brain fog | <0.001 | |||
| No, ever | 274 (72.7%) | 190 (79.2%) | 84 (61.3%) | |
| Ongoing | 76 (20.2%) | 35 (14.6%) | 41 (29.9%) | |
| Resolved | 13 (3.4%) | 6 (2.5%) | 7 (5.1%) | |
| Unknown | 14 (3.7%) | 9 (3.7%) | 5 (3.7%) | |
| Other | 0.003 | |||
| No, ever | 300 (79.5%) | 198 (82.6%) | 102 (74.5%) | |
| Ongoing | 58 (15.4%) | 28 (11.7%) | 30 (21.9%) | |
| Resolved | 19 (5%) | 14 (5.7%) | 5 (3.6%) | |
| Psychological symptoms and PTSD | ||||
| IES-R, PTSD | 0.002 | |||
| Normal | 190 (50.4%) | 133 (55.4%) | 57 (41.6%) | |
| Pathological | 85 (22.5%) | 43 (17.9%) | 42 (30.6%) | |
| Unknown | 102 (27.1%) | 64 (26.7%) | 38 (27.8%) | |
| HADS, depression symptoms | 0.009 | |||
| Normal | 299 (79.3%) | 198 (82.5%) | 101 (73.7%) | |
| Pathological | 40 (10.6%) | 18 (7.5%) | 22 (16.1%) | |
| Unknown | 38 (10.1%) | 24 (10%) | 14 (10.2%) | |
| HADS, anxiety symptoms | <0.001 | |||
| Normal | 268 (71.1%) | 185 (77.1%) | 83 (60.6%) | |
| Pathological | 71 (18.8%) | 31 (12.9%) | 40 (29.2%) | |
| Unknown | 38 (10.1%) | 24 (10%) | 14 (10.2%) | |
Parameters are presented as absolute numbers (%).
GI, gastrointestinal symptoms; IES-R, impact of event scale - revised for diagnosis of post-traumatic stress disorders; HADS, Hospital Anxiety and Depression Scale for diagnosis of anxiety and depression symptoms; other, chest pain; headache; constipation; tinnitus; insomnia; palpitations; NSTEMI, cough; sore throat.
Chi-square or Fisher's exact test for comparison between males and females.
As far as psychological sequelae are concerned, manifestations of anxiety were the most frequent (71/377, 18.8%), while depression symptoms were present in 40/377, 10.6% of patients (although 38 patients did not complete HADS questionnaire being non-Italian speakers) (Table 3: psychological symptoms and PTSD). IES-R was available in an unselected sub-group of patients (275/377, 73% patients): in one-third (85/275, 31%) of cases the IES-R score resulted pathological (according to the aforementioned definition), possibly suggesting the presence of PTSD (Table 3: psychological symptoms and PTSD). Interestingly, women were characterized by a higher proportion of most physical symptoms and all psychological symptoms than men (Table 3).
At multivariable logistic regression analysis, female gender was associated with a threefold higher risk of long COVID, also after adjustment for age, severity of illness, LOS, comorbidities, smoking, BMI and time from symptoms onset to virological clearance (Table 4 ). Other independent predictors of long COVID were advanced age and active smoking. Severity of disease, LOS and time to virological clearance were not associated with long COVID at univariable and multivariable analyses (Table 4).
Table 4.
Factors associated with long COVID syndrome by fitting univariable and multivariable logistic regression analyses
| Parameters | OR (95%CI) | p | AOR (95%CI) | p |
|---|---|---|---|---|
| Gender: | ||||
| Male | 1 | 1 | ||
| Female | 2.78 (1.68–4.62) | <0.0001 | 3.32 (1.78–6.17) | <0.0001 |
| Age, 10 years older | 1.03 (1.01–1.04) | 0.001 | 1.03 (1.01–1.05) | 0.01 |
| O2 therapy: | ||||
| No O2 | 1 | 1 | ||
| O2 therapy low–high flows | 0.67 (0.38–1.19) | 0.17 | 0.39 (0.19–0.82) | 0.44 |
| CPAP/NIV/IOT | 0.97 (0.55–1.71) | 0.91 | 0.67 (0.29–1.55) | 0.85 |
| LOS, each day more | 1.01 (0.99–1.03) | 0.28 | 0.998 (0.97–1.03) | 0.92 |
| Comorbidities: | ||||
| No | 1 | 1 | ||
| Yes | 1.35 (0.86–2.11) | 0.19 | 1.05 (0.597–1.84) | 0.87 |
| Smoking: | ||||
| Active | 1 | 1 | ||
| Unknown | 0.13 (0.03–0.52) | 0.004 | 0.16 (0.04–0.75) | 0.31 |
| Never | 0.61 (0.37–0.997) | 0.05 | 0.56 (0.31–1.01) | 0.41 |
| Former | 0.36 (0.14–0.96) | 0.04 | 0.19 (0.06–0.62) | 0.002 |
| BMI: | ||||
| ≥30 | 1 | 1 | ||
| Unknown | 0.29 (0.096–0.91) | 0.03 | 0.13 (0.30–0.53) | 0.03 |
| <30 | 0.55 (2.27–5.06) | 0.02 | 0.55 (0.31–0.98) | 0.28 |
| Time from symptoms onset to virological clearance, each day more | 1.01 (0.99–1.02) | 0.64 | 0.99 (0.98–1.01) | 0.47 |
Each variable is mutually adjusted.
Outcome, long COVID syndrome. AOR, adjusted odds ratio; CPAP, continuous positive airway pressure; OTI, orotracheal intubation; NIV, non-invasive ventilation; LOS, length of hospital stay; BMI, body mass index.
Discussion
The American Center for Disease Control (CDC) and the British NICE have identified symptoms that could persist for weeks or even months after recovery from COVID-19. How long these symptoms could endure for, possible risk factors for their persistence and the predisposing patient features are all aspects to be further elucidated [[6], [7], [8],17]. Our study reveals that:
-
1.
The most common symptoms characterizing long COVID were both physical and psychological.
-
2.
Females had a 3-fold higher risk of being diagnosed with long COVID.
-
3.
Severity of disease and time to virological resolution were not associated with long COVID.
The high incidence of long COVID within our cohort was similar to that previously reported: more than half of patients reported symptoms lasting more than 2 months after symptom onset [4,5,16,18,19]. Some symptoms are also commonly seen in other viral illnesses and both psychical and psychological sequelae have been described in MERS and SARS [12,[23], [24], [25], [26], [27], [28], [29]].
Literature data are not equivocal about the association between females and long COVID: some preliminary studies have shown an increased prevalence of fatigue [24] or other symptoms among women [1,5,13,16], while in other studies no gender association was found [4,[18], [19], [20]]. Differences in ethnicity, living country and possibly socio-economic status might explain such contrasting results. Hormones may play a role in perpetuating the hyperinflammatory status of the acute phase even after recovery [30,31] and a stronger IgG antibodies production in females in the early phase of disease has been reported; this could turn out in a more favourable outcome in women [32], but might play a role as well in perpetuating disease manifestations. Furthermore, we might hypothesise that women are in general more attentive to their body and related distress.
Advanced age was associated with ongoing fatigue and musculoskeletal pain, or impairment in pulmonary functions, reflecting a decline in organ function and a slower ability to recover [1,16]. As far as our finding that reported a weak association between obesity and long COVID is concerned, this was not confirmed in a previous study that however focused on persisting fatigue solely [24]. Increased inflammation, defective adaptative immune responses, endothelial dysfunction and coagulation-related disorders are all phenomena that have been well described in obesity and might represent a plausible explanation to the link we observed between high BMI and long COVID [33]. Finally, the relationship between smoking and long COVID has not been previously explored; the most frequent symptom reported by smokers was however shortness of breath, which possibly reflects an underlying pulmonary dysfunction.
We were expecting to find an association between severity of disease and residual symptoms, as reported by previous studies [1]. However, long COVID has been previously described also in not hospitalised patients diagnosed with a mild, self-limiting disease [6,[16], [17], [18],24].
Our study has some limitations: a possible selection bias due to losses to follow-up; the limited sample size for patients with severe disease and females; the lack in validated tools to assess dyspnoea and fatigue and in information about the presence of the symptoms before the onset of acute infection. The possible association between female gender and long COVID should be confirmed in larger populations and by means of a longer follow-up. The evaluation of our patients at six months still needs to be completed and this will certainly allow us to collect further data and build stronger evidence.
In conclusion, in our setting long COVID was a frequent long-term complication of COVID-19 and was diagnosed more frequently in women than in men. It was also commonly seen in patients who recovered from a mild disease form. Follow-up outpatient services are therefore needed in order to manage this syndrome and to better understand the possible association between symptoms and residual organ impairment, and their impact on patient quality of life.
Author contributions
F. Bai wrote the manuscript. D. Tomasoni created the dataset, D. Tomasoni and F. Bai performed statistical analyses. F. Bai, D. Tomasoni, C. Falcinella, D. Barbanotti, R. Castoldi, G. Mulè, M. Augello, D. Mondatore, M. Allegrini, A. Cona, D. Tesoro, G. Tagliaferri, O. Viganò, E. Suardi, C. Tincati, B. Varisco and T. Beringheli followed up patients, contributed to acquisition of data and revised the article. A. Tavelli and S. Terzoni contributed to analyses and interpretation of data. G. Marchetti and A. d’Arminio Monforte contributed to the design of the study and revised the article. C. Luridiana, K. Piscopo, E Vegni performed psychological evaluations and revised the final version of the manuscript. All the authors have read and approved the final version of the paper.
Transparency declaration
Authors do not have conflicts of interests to declare for this work. Prof G. Marchetti and Prof. A. d’Arminio Monforte report grants from Gilead, ViiV and Jannsen outside this research. Funding: No financial support was obtained for this study.
Acknowledgements
Preliminary data were presented as oral communication at the 31st European Congress of Clinical Microbiology and Infectious Diseases (ECCMID), 9–12 July 2021. We are grateful to all patients and their families for their kind participation in the study. We would like to acknowledge all the staff of the Clinic of Infectious Diseases, Department of Health Sciences, San Paolo Hospital, ASST Santi Paolo e Carlo, University of Milan, Italy, for the precious help in the study.
Editor: A. Huttner
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2021.11.002.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Multimedia component 1
Multimedia component 2
Multimedia component 3
References
- 1.Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Editorial Long COVID: let patients help define long-lasting COVID symptoms. Nature. 2020;586:170. doi: 10.1038/d41586-020-02796-2. [DOI] [PubMed] [Google Scholar]
- 3.Davis A.E., Assaf G.S., McCorkell L., Wei H., Low RJ., Re’em Y., et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi: 10.1016/j.eclinm.2021.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carfì A., Bernabei R., Landi F. Gemelli against COVID-19 post-acute care study group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venturelli S., Benatti S.V., Casati M., Binda F., Zuglian G., Imeri G., et al. Surviving COVID-19 in Bergamo Province: a post-acute outpatient re-evaluation. Epidemiol Infect. 2021:1–25. doi: 10.1017/S0950268821000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Venkatesan P. NICE guideline on long COVID. Lancet Respir Med. 2021;9:129. doi: 10.1016/S2213-2600(21)00031-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah W., Hillman T., Playford E.D., Hishmeh L. Managing the long term effects of COVID-19: summary of NICE, SIGN, and RCGP rapid guideline. BMJ. 2021;372:n136. doi: 10.1136/bmj.n136. [DOI] [PubMed] [Google Scholar]
- 8.Sivan M., Taylor S. NICE guideline on long COVID. BMJ. 2020;371:m4938. doi: 10.1136/bmj.m4938. [DOI] [PubMed] [Google Scholar]
- 9.Mahase E. COVID-19: what do we know about "long COVID. BMJ. 2020;370:m2815. doi: 10.1136/bmj.m2815. [DOI] [PubMed] [Google Scholar]
- 10.Mandal S., Barnett J., Brill S.E., Brown J.S., Denneny E.K., Hare S.S., et al. Long-COVID’: a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax. 2021;76:396–398. doi: 10.1136/thoraxjnl-2020-215818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carvalho-Schneider C., Laurent E., Lemaignen A., Beaufils E., Bourbao-Tournois C., Laribi S., et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin Microbiol Infect. 2020;27:258–263. doi: 10.1016/j.cmi.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tomasoni D., Bai F., Castoldi R., Barbanotti D., Falcinella C., Mulè G., et al. Anxiety and depression symptoms after virological clearance of COVID-19: a cross-sectional study in Milan, Italy. J Med Virol. 2020;93:1175–1179. doi: 10.1002/jmv.26459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazza M.G., De Lorenzo R., Conte C., Poletti S., Vai B., Bollettini I., et al. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Troyer E.A., Kohn J.N., Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 2020;87:34–39. doi: 10.1016/j.bbi.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaseda E.T., Levine A.J. Post-traumatic stress disorder: a differential diagnostic consideration for COVID-19 survivors. Clin Neuropsychol. 2020;34:1498–1514. doi: 10.1080/13854046.2020.1811894. [DOI] [PubMed] [Google Scholar]
- 16.Xiong Q., XuM, Li J., Liu Y., Zhang J., Xu Y., et al. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin Microbiol Infect. 2021;27:89–95. doi: 10.1016/j.cmi.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention Long-term effects of COVID-19. 2020. https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects.html Available at: [PubMed]
- 18.Moreno-Pérez O., Merino E., Leon-Ramirez J.M., Andres M., Ramos J.M., Arenas-Jiménez J., et al. Post-acute COVID-19 Syndrome. Incidence and risk factors: a Mediterranean cohort study. J Infect. 2021;82:378–383. doi: 10.1016/j.jinf.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chopra V., Flanders S.A., O’Malley M., Malani A.N., Prescott H.C. Sixty-day outcomes among patients hospitalized with COVID-19. Ann Intern Med. 2020;174:576–578. doi: 10.7326/M20-5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daher A., Balfanz P., Cornelissen C., Muller A., Bergs I., Marx N., et al. Follow up of patients with severe coronavirus disease 2019 (COVID-19): pulmonary and extrapulmonary disease sequelae. Respir Med. 2020;174:106197. doi: 10.1016/j.rmed.2020.106197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zigmond A.S., Snaith R.P. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 22.Mak I.W., Chu C.M., Pan P.C., Yiu M.G., Chan V.L. Long-term psychiatric morbidities among SARS survivors. Gen Hosp Psychiatry. 2009;31:318–326. doi: 10.1016/j.genhosppsych.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmed H., Patel K., Greenwood D.C., Halpin S., Lewthwaite P., Salawu A., et al. Long-term clinical outcomes in survivors of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus outbreaks after hospitalisation or ICU admission: a systematic review and meta-analysis. J Rehabil Med. 2020;52 doi: 10.2340/16501977-2694. [DOI] [PubMed] [Google Scholar]
- 24.Townsend L., Dyer A.H., Jones K., Dunne J., Mooney A., Gaffney F., et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS One. 2020;15 doi: 10.1371/journal.pone.0240784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moldofsky H., Patcai J. Chronic widespread musculoskeletal pain, fatigue, depression and disordered sleep in chronic post-SARS syndrome; a case-controlled study. BMC Neurol. 2011;11:37. doi: 10.1186/1471-2377-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hickie I., Davenport T., Wakefield D., Vollmer-Conna U., Cameron B., Vernon S.D., et al. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. BMJ. 2006;333:575. doi: 10.1136/bmj.38933.585764.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee S.H., Shin H.S., Park H.Y., Kim J.L., Lee J.J., Lee H., et al. Depression as a mediator of chronic fatigue and post-traumatic stress symptoms in Middle East respiratory syndrome survivors. Psychiatry Investig. 2019;16:59–64. doi: 10.30773/pi.2018.10.22.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vindegaard N., Benros M.E. COVID-19 pandemic and mental health consequences: systematic review of the current evidence. Brain Behav Immun. 2020;89:531–542. doi: 10.1016/j.bbi.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murata S., Rezeppa T., Thoma B., Marengo L., Krancevich K., Chiyka E., et al. The psychiatric sequelae of the COVID-19 pandemic in adolescents, adults, and health care workers. Depress Anxiety. 2020;38:233–246. doi: 10.1002/da.23120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohamed M.S., Moulin T.C., Schiöth H.B. Sex differences in COVID-19: the role of androgens in disease severity and progression. Endocrine. 2021;71:3–8. doi: 10.1007/s12020-020-02536-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bienvenu L.A., Noonan J., Wang X., Peter K. Higher mortality of COVID-19 in males: sex differences in immune response and cardiovascular comorbidities. Cardiovasc Res. 2020;116:2197–2206. doi: 10.1093/cvr/cvaa284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeng F., Dai C., Cai P., Wang J., Xu L., Li J., et al. A comparison study of SARS-CoV-2 IgG antibody between male and female COVID-19 patients: a possible reason underlying different outcome between sex. J Med Virol. 2020;92:2050–2054. doi: 10.1002/jmv.25989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drucker D.J. Diabetes, obesity, metabolism, and SARS-CoV-2 infection: the end of the beginning. Cell Metab. 2021;33:479–498. doi: 10.1016/j.cmet.2021.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multimedia component 1
Multimedia component 2
Multimedia component 3