Abstract
Background
As of September 17, 2021, coronavirus disease 2019 (COVID-19) has infected more than 226 million people in a worldwide pandemic, with conservative estimates suggesting that there are more than 204 million convalescent patients with COVID-19. Previous studies have indicated that patients in the recovery phase exhibit decreased function of multiple organs. In China, traditional Chinese medicine (TCM) treatment is recommended in the rehabilitation period of COVID-19; however, the safety and efficacy of such treatment remain to be confirmed.
Aim of study
The present study aimed to evaluate the efficacy and safety of Bufei Huoxue (BFHX) in restoring the functional status and exercise tolerance of patients recovering from COVID-19.
Methods
A total of 131 patients in the rehabilitation period of COVID-19 infection were randomly divided into a Bufei Huoxue (BFHX) group (n = 66) and a placebo group (n = 65). BFHX or placebo was given orally three times a day (1.4 g/dose) for 90 days. The primary outcomes was to evaluate improvements in exercise tolerance and imaging manifestations on chest computed tomography (CT).
Results
After the exclusion of two patients who withdrew prior to receiving any medications, 129 patients were recruited, including 64 patients in the BFHX group and 65 patients in the placebo group. After 3 months of treatment, the BFHX group exhibited greater attenuation of pneumonia lesions on chest CT than the placebo group (P0.05). Improvements in 6-min walk distance (6MWD) relative to baseline were also significantly better in the BFHX group than in the placebo group (P0.01). Scores on the Fatigue Assessment Inventory (FAI) were lower in the BFHX group than in the placebo group (P0.05). Although the rate of adverse events was higher in the BFHX group than in the placebo group (9.38% vs. 4.62%), the difference was not significant (P0.3241).
Conclusions
BFHX may exert strong rehabilitative effects on physiological activity in patients recovering from COVID-19, which may in turn attenuate symptoms of fatigue and improve exercise tolerance.
Keywords: COVID-19 convalescence, Randomised controlled trial, Bufei Huoxue capsules, Chinese Materia Medica
Graphical abstract
Abbreviations
- 6MWD
6-min walk distance
- BFHX
Bufei Huoxue capsules
- BMI
body mass index
- CI
confidence interval
- COVID-19
coronavirus disease 2019
- FAI
Fatigue Assessment Inventory
- FAS
full analysis set
- IL6
interleukin 6
- ITT
intention-to-treat
- LOCF
last observation carried forward
- MAPK8
mitogen-activated protein kinase 8
- NCOA2
nuclear receptor coactivator 2
- PPS
per protocol set
- SS
safety set
- PTGS
post-transcriptional gene silencing
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- SGRQ
St George's respiratory questionnaire
- TCM
traditional Chinese medicine
1. Introduction
Since December 2019, a novel coronavirus known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread worldwide. As of September 17th, 2021, the number of coronavirus disease 2019 (COVID-19) cases continues to increase, with 226,236,577 confirmed cases, 4,654,548 deaths, and 204,397,158 recovered individuals across 185 countries and regions (COVID-19 coronavirus pandemic, 2021). Due to worldwide efforts targeted toward rapid vaccine development, there are currently several vaccines against COVID-19 on the market, offering hope for curbing the spread of the virus. Studies are now providing greater insight into the long-term effects of COVID-19 on the human body. Researchers have reported that 63% of discharged patients still exhibit lung injury on computed tomography (CT) images 6 months after the onset of disease (Shaw et al., 2021; Sonnweber, 2020). One research study from China observed that 76% of COVID-19 survivors still had at least one clinical symptom—which commonly included fatigue, myasthenia (63%), and sleeping disorders (26%)—6 months after acute infection (Huang et al., 2021). A prospective UK cohort study from medRxiv also noted that 70% of patients with COVID-19 were not fully recovered after discharge (Rachael et al., 2021). In the meantime, Chinese researchers and doctors have investigated the convalescence of patients with COVID-19 at an earlier timepoint for better clinical outcomes (General Office of the National Health Commission, 2020). In view of this situation, Current guidelines for the diagnosis and treatment of COVID-19 (i.e., the fourth edition of COVID-19 diagnosis and treatment guidelines issued by the National Health Commission of the People's Republic of China) recommend the use of traditional Chinese medicine (TCM) during the rehabilitation period. It's been proven that the application of TCM can shorten the hospital stay of the patients who were infected by coronavirus (Huang et al., 2020). For thousands of years, TCM has been to treat infectious diseases. In TCM, patients are evaluated and treated based on its special theories and aetiologies. The prescriptions of TCM typically consists of a complex combination of components according to TCM signs (Wu et al., 2008). From TCM's point of view, the principal pathogenesis of viral pneumonia is the damage of Qi by evil toxin (also considered as pro-inflammatory factors). The COVID-19 patients are more common in the manifestations of Qi deficiency and blood stasis, for which reason they are prone to have fatigue and decreased exercise endurance. Replenishing and restoring pulmonary Qi are important approach for rehabilitation of COVID-19 (Wu et al., 2021).
The Bufei Huoxue capsule (BFHX) is a widely known Chinese patent medicine that is typically used to promote blood circulation and improve lung and kidney function in patients with cardiopulmonary diseases (Wu et al., 2012). BFHX consists of three ingredients from TCM: Astragali radix, Paeoniae radix rubra, and Psoraleae fructus (The Official Product Information for Bufei Huoxue Capsule). Astragali radix exerts broad-spectrum effects on the human body by strengthening the immune system, increasing tolerance against hypoxia, regulating organ function, and preventing microbial infection (Yao et al., 2003; Hu et al., 2018; Hong et al., 2017; Luo et al., 2020). Paeoniae radix rubra exerts therapeutic effects by improving microcirculation, reducing the viscosity of serum and plasma, and clearing excessive “heat” and “cold” from the blood (Liu et al., 2000; Luo et al., 2002). Psoraleae fructus plays a role in strengthening myocardial function, dilating the coronary arteries, and increasing blood flow (Zhao, 2002; Qu et al., 2019; Zhang et al., 2020). The polysalen polysaccharides in psoralen can also significantly enhance immunity (Zhang et al., 2017). The incorporation of these three Chinese medicinal herbs in BFHX may help to improve lung function and microcirculation, in addition to providing anti-microbial, anti-endotoxin, and anti-hypoxia benefits (Xie et al., 2019; Ma et al., 2016; Guo and Zhang, 2007; Yang et al., 2005).
Recently, some studies based on network pharmacology have indicated that BFHX exhibits therapeutic effects in those recovering from viral pneumonia by supressing inflammatory pathways (Rao et al., 2020; Guo et al., 2020). As a respiratory infectious disease, COVID-19 is considered related to qi deficiency in the lung and spleen; thus, proponents of TCM have argued that BFHX may exert a therapeutic effect in patients recovering from COVID-19. Accordingly, BFHX has been integrated into clinical guidelines in several provinces of China for the recovery management of COVID-19 (Beijing Administration of Traditional Chinese Medicine, 2020; Guizhou Administration of Traditional Chinese Medicine, 2020; Health Commission of Anhui Province, 2020). Multiple clinical trials have demonstrated that BFHX exerts beneficial effects in patients with lung diseases including chronic obstructive pulmonary disease, asthma, and pulmonary tuberculosis. These effects may be due to reductions in acute exacerbation, alleviation of inflammatory responses in the airway, and reversal of immunologic disorders (Bai et al., 2016; Yu et al., 2019; Xu et al., 2018), which have been cited as common clinical symptoms and pathogenetic mechanisms in COVID-19. Therefore, to provide evidence-based data for the use of BFHX in COVID-19 convalescence, the present randomised, double-blind, placebo-controlled clinical trial aimed to evaluate the efficacy and safety of BFHX in restoring the functional status and exercise tolerance of patients recovering from COVID-19.
2. Materials and methods
This was a multicentre, randomised, double-blind, placebo-controlled study (Chen et al., 2021). All patients were hospitalised with COVID-19 at five hospitals in China. Te study was approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University (registration number: 2020–87).
Written informed consent was obtained from all study participants. This study was registered with the China Clinical Trial Registration Centre (registration number: ChiCTR2000032573).
2.1. Inclusion and exclusion criteria
We enrolled hospitalised patients who met the following criteria: (1) age18 years; (2) diagnosis of COVID-19 in accordance with “The Diagnosis and Treatment Scheme of COVID-19 (7th Trial Version)”; (3) patient condition meeting the discharge standards stipulated in “The Diagnosis and Treatment Scheme of COVID-19 (7th Trial Version)” after treatment; (4) qi deficiency in the lung and spleen based on “The Diagnosis and Treatment Scheme of COVID-19 (7th Trial Version)”; (5) patients and their dependents agreeing to participate in the study and providing written informed consent.
Exclusion criteria were as follows: (1) known or suspected allergy to the components of BFHX; (2) acute infections caused by other viruses or bacteria; (3) abnormal liver and kidney function tests (alanine aminotransferase [ALT], aspartate aminotransferase [AST], and serum creatinine [SCr]1.5 times the upper limit of normal); (4) participation in other clinical drug trials; (5) pregnancy, lactation, or other conditions preventing adoption of effective contraceptive measures during the trial period; (6) severe comorbid liver disease (such as liver tumours or various types of hepatitis), history of drug-induced liver injury, or current history of/potential for the use of drugs with the potential to damage the liver during the study period (e.g., immunosuppressive agents such as cyclosporine and tacrolimus; anti-tuberculosis drugs such as rifampicin and isoniazid; chemotherapy drugs such as cyclophosphamide, methotrexate, and azathioprine; restorative medicinal herbs for liver damage such as polygonum multiflorum, tusanqi, and tripterygium wilfordii); and (7) any other circumstances under which the investigators considered the patient unsuitable for participation in the study.
2.2. Calculation of sample size
Based on previous research, the 6-min walk distance (6MWD) was selected as the primary efficacy indicator for estimating the sample size (Brooks et al., 2003; Fujimoto et al., 2017). Assuming that the 6MWD in the BFHX-treated group would be 60 m more than that in the control group on average, the sample size of each group was estimated as 31 (Std = 60; α = 0.05; β = 0.10) using PASS (Version 11.0.7). We increased the sample size to 65 in each group in consideration of study risk and bias.
2.3. Study medication
BFHX (Chinese medicine Z20030063, Guangdong Lei Yun Shang Pharmaceutical Co., Ltd. (Yunfu, Guangdong Province, China); batch number 022001; specifications: 0.35 g per capsule) was obtained in the form of hard capsules containing fine brown particles/powder that is slightly fragrant, sour, and bitter. BFHX is composed of three herbs: Psoraleae fructus (Buguzhi) (40%), Astragali radix (Huangqi) (40%), and Paeoniae radix rubra (Chishao) (20%). The placebo capsule (Guangdong Lei Yun Shang Pharmaceutical Co., Ltd.; batch number 012007; specifications: 0.35 g capsules) was made of starch, caramel, and tartrazine, and its smell, colour, shape, and packaging were the same as those for the BFHX capsule. The drug quality standards conform to the regulations of the Chinese Pharmacopoeia (Chinese Pharmacopoeia Commission, 2015). The chemical construction and quality of BFHX capsules were assessed by high performance liquid chromatography (HPLC). The fingerprint of BFHX can be seen in supplemental figures and tables (Tang et al., 2018).
2.4. Intervention and efficacy evaluation
A list of 160-case random sequences was generated by computer using a randomisation scheme based on the stratified block random method. Data analysis was performed independently by professional statisticians to guarantee that all enrolled participants were evenly allocated to the BFHX or control groups. Then, 80 participants from each group were randomly selected. The allocation code was individually sealed in an opaque envelope. In this trial, all participants and researchers involved in drug distribution, outcome evaluation, and data analysis were blinded to the allocation. Urgent unblinding, under the principal investigators' permission, was permitted in instances of severe adverse events or other unpredictable situations. Every participant was offered rehabilitation options ranging from oxygen to aerosol inhalation, respiratory training, and sports exercises, depending on the actual treatment needs. Eligible patients received BFHX capsules or placebo capsules at an oral dose of 1.4 g (four capsules) thrice daily for 90 days concomitantly with rehabilitation therapy.
All patients underwent monthly follow-up for 3 months. Basic information, clinical symptoms, vital signs, medication status, and adverse events were recorded to evaluate the participants' exercise tolerance and degree of symptom improvement. The primary indicators included improvements in 6MWD and findings on chest computed tomography (CT) images. Secondary efficacy measurements consisted of Chinese medicine syndrome scores, St. George's Respiratory Questionnaire (SGRQ) score, Borg-Dyspnea Scale scores, and Fatigue Assessment Inventory (FAI) scores. Additionally, any side effects or adverse events during the 3-month follow-up period were recorded. Quantification of lung lesions on CT was performed using an intelligent Evaluation System of Chest CT for COVID-19 (YT-CT-Lung, YITU Healthcare Technology Co., Ltd., China), which combines a fully convolutional network with adaptive thresholding and morphological operations for the segmentation of lungs and pneumonia lesions. The automatic lesion delineation in each case was confirmed or modified based on the consensus of two chest radiologists (Liu et al., 2020).
2.5. Safety monitoring
In this study, several indices were monitored to assess safety: (1) vital signs including body temperature, heart rate, respiratory rate, and blood pressure; (2) laboratory examination results including complete blood count (white blood cells, red blood cells, haemoglobin, platelets, neutrophils, and lymphocytes); clinical urine test results (leukocytes, blood, protein, glucose); liver function (AST, ALT, total bilirubin, γ-glutamyl transpeptidase, and alkaline phosphatase levels); and renal function (blood urea nitrogen and SCr); and (3) the incidence, timing, severity, and duration of adverse events.
2.6. Statistical analysis
Based on the intention-to-treat (ITT) principle, all participants taking medicine after randomisation were included in the statistical analysis. Efficacy and safety analyses were performed using the ITT datasets. Per-protocol datasets were selected from the ITT population for efficacy analysis.
Descriptive statistics including counts, percentages, means, and standard deviations were calculated for categorical and continuous outcomes, respectively. Means and standard deviations were reported for the primary and secondary indicators. For continuous outcomes, a two-tailed Student's t-test and Wilcoxon–Mann–Whitney test were used to test for differences between groups for normally and non-normally distributed variables, respectively. Fisher's exact test was used to analyse categorical outcomes. The centre effect was considered using the generalised linear model. Two-sided tests were set at a significance level of 0.05. We did not consider type I error correction because of the exploratory nature of the research. SAS version 9.4 (SAS Institute Inc.) was used for all analyses.
Sensitivity analysis of the primary indicators was conducted using the imputed data of the last observation carried forward (LOCF). For participants who prematurely withdrew from the trial, the outcomes of the last visit were used as the final outcome.
3. Results
3.1. Patient sample and characteristics
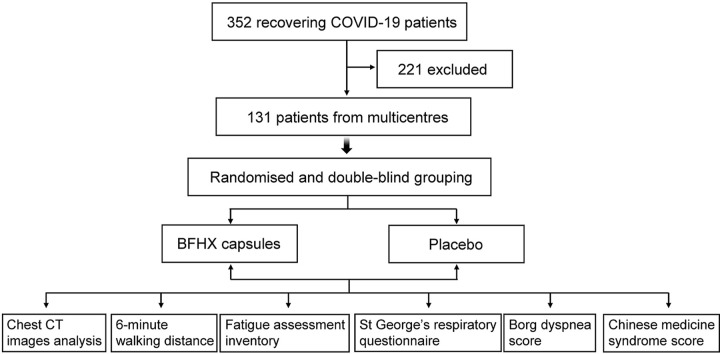
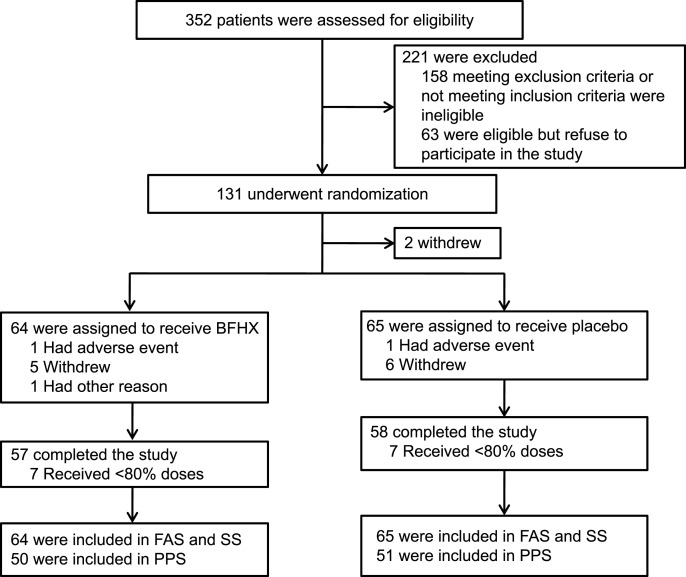
From May 4 to June 21, 2020, 352 patients discharged following COVID-19 treatment at five subcentre university hospitals in China were screened, and 131 patients who were eligible for the trial were recruited randomly. All participants were randomly divided into the BFHX (n66) and placebo (control, n65) groups using a ratio of 1:1. Two patients did not take the test drug after randomisation. A total of 129 patients were eventually enrolled in this study, and 115 patients completed the study (Fig. 1 ). Patient characteristics are shown in Table 1 . Both groups were comparable in terms of demographic characteristics, severity of illness, and time of discharge (P 0.05). There were also no differences in the mainstay of treatment or concomitant medications.
Fig. 1.
Study flow chart.
Table 1.
Comparison of patient characteristics at baseline.
| Term | BFHX (N = 64) | Placebo (N = 65) | P-value a |
|---|---|---|---|
| Male (%) | 31 (48.44) | 29 (44.62) | 0.7254 |
| Age (years, ) | 54.16 ± 12.11 | 52.51 ± 12.31 | 0.4448 |
| Age group (years, %) | 0.7810 | ||
| 18–40 | 6 (9.38) | 8 (12.31) | |
| 41–64 | 47 (73.44) | 44 (67.69) | |
| 65–75 | 8 (12.50) | 11 (16.92) | |
| >75 | 3 (4.69) | 2 (3.08) | |
| Height (cm) | 164.80 ± 7.21 | 163.94 ± 7.65 | 0.5163 |
| Weight (kg) | 68.08 ± 11.78 | 65.92 ± 9.80 | 0.2603 |
| BMI (kg/m2) | 24.995 ± 3.536 | 24.474 ± 2.894 | 0.3607 |
| Han nationality (%) | 64 (100.00) | 65 (100.00) | – |
| Manual labour (%) | 12 (18.75) | 9 (13.85) | 0.4835 |
| Marriage | 0.3800 | ||
| Married | 58 (90.63) | 62 (95.38) | |
| Unmarried | 5 (7.81) | 3 (4.62) | |
| Other | 1 (1.56) | 0 (0.00) | |
| Severe/critical patients (%) | 13 (20.31) | 7 (10.77) | 0.1516 |
| Time from confirmation to randomisation (day) | 131.2 ± 14.0 | 130.7 ± 14.6 | 0.8254 |
| Time from discharge to randomisation (day) | 94.3 ± 17.0 | 94.3 ± 20.5 | 0.9845 |
| Comorbidities (N, %) | |||
| Rheumatic diseases | 1 (1.56) | 0 (0.00) | 0.4961 |
| Respiratory diseases | 2 (3.13) | 1 (1.54) | 0.6191 |
| Urinary system diseases | 1 (1.56) | 1 (1.54) | 1.0000 |
| Endocrine and metabolic system diseases | 11 (17.19) | 10 (15.38) | 0.8155 |
| Nervous system diseases | 1 (1.56) | 0 (0.00) | 0.4961 |
| Digestive system diseases | 1 (1.56) | 6 (9.23) | 0.1148 |
| Cardiovascular diseases | 12 (18.75) | 13 (20.00) | 1.0000 |
| Ophthalmic Diseases | 1 (1.56) | 0 (0.00) | 0.4961 |
| Classification of concomitant medication (N, %) | |||
| Digestive and metabolic system | 10 (15.63) | 5 (7.69) | 0.1807 |
| Cardiovascular system | 9 (14.06) | 10 (15.38) | 1.0000 |
| Chinese medicine/Chinese patent medicine | 3 (4.69) | 1 (1.54) | 0.3652 |
| Blood system | 2 (3.13) | 5 (7.69) | 0.4401 |
| Endocrine system | 1 (1.56) | 1 (1.54) | 1.0000 |
| Respiratory system | 1 (1.56) | 0 (0.00) | 0.4961 |
| Motor system | 1 (1.56) | 0 (0.00) | 0.4961 |
| Nervous system | 1 (1.56) | 0 (0.00) | 0.4961 |
| Antibiotics | 1 (1.56) | 0 (0.00) | 0.4961 |
| Combined | 1 (1.56) | 0 (0.00) | 0.4961 |
P-values were calculated for continuous outcomes with t-tests for the change from baseline to the last visit after three months of treatment; Fisher’ s exact test was performed for categorical outcomes.
3.2. Primary indicators
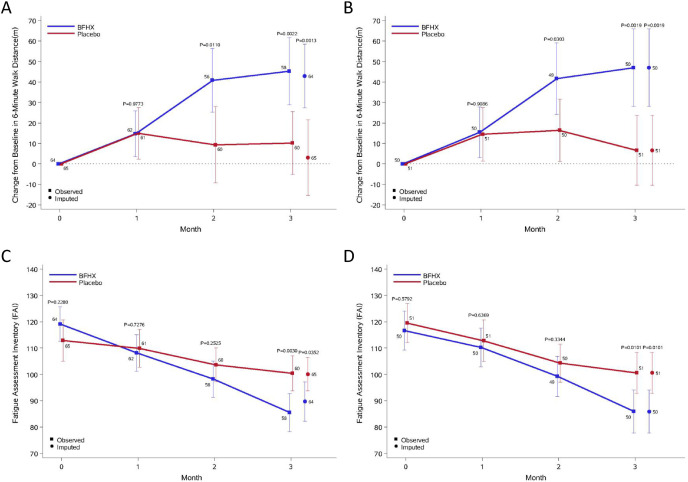
After undergoing therapy for 3 months, patients in the BFHX group exhibited significant decreases in the volume of total lung lesions (P 0.0243), volume of ground-glass opacities (P 0.0444), volume of consolidations (P 0.0188), and the percentage of consolidations in the whole lung (P 0.0428) on chest CT when compared with the placebo group (Table 2 ). Improvements in pneumonia as reflected by chest CT were observed in 15.09% and 7.84% of patients in the BFHX and placebo groups, respectively (Table 3 ). More than 80% of patients in the two groups remained in stable condition. Notably, six patients in the placebo group developed worsening of pneumonia, whereas none in the BFHX group presented with deterioration (Table 3). Improvements in 6MWD were significantly better in the BFHX-treatment group than in the control group (FAS: 40.8 ± 57.9 m vs. 9.3 ± 72.0 m; 95% CI: 7.4–55.6 m; P 0.0110). This effect was also observed after 3 months of treatment (FAS: 45.3 ± 62.4 m vs. 10.1 ± 59.4 m; 95% CI: 12.9–57.3 m; P 0.0022.) (Fig. 2 A and B).
Table 2.
Changes in primary and secondary indicators from baseline to after three months of treatment a
| Indicator |
BFHX (N = 64) b |
Placebo (N = 65) b |
Least Squares Means Differences (95% CI) |
P-valueb |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of patientsc | Baseline | After 3 months of treatment | Change | No. of patientsc | Baseline | 3 months after treatment | Change | |||
| Primary indicator | ||||||||||
| Chest CT Indexes | ||||||||||
| Volume of total lung lesions (cm³) | 53 | 31.5 ± 120.5 | 14.3 ± 32.1 | -19.2 ± 96.4 | 51 | 15.1 ± 39.4 | 29.2 ± 75.4 | 15.1 ± 47.5 | -34.0 (-63.5 to -4.6) | 0.0243 |
| Volume of the ground-glass opacities (cm³) | 53 | 29.3 ± 115.4 | 13.2 ± 30.6 | -18.3 ± 92.0 | 51 | 14.2 ± 38.2 | 23.9 ± 71.4 | 10.5 ± 42.4 | -32.6 (-60.7 to -4.5) | 0.0444 |
| Volume of the consolidations (cm³) | 53 | 1.8 ± 34.4 | 1.1 ± 2.3 | -0.9 ± 4.7 | 51 | 1.0 ± 1.6 | 2.0 ± 4.1 | 1.1 ± 3.4 | -1.4 (-2.6 to -0.1) | 0.0188 |
| 6-Min Walk Distance (m) | 58 | 427.3 ± 73.6 | 475.6 ± 63.7 | 45.3 ± 62.4 | 60 | 435.0 ± 73.3 | 445.5 ± 69.1 | 10.1 ± 59.4 | 34.2 (11.7–56.8) | 0.0022 |
| Secondary indicator | ||||||||||
| Fatigue Assessment Inventory | 58 | 119.1 ± 26.2 | 85.5 ± 27.6 | -31.2 ± 27.0 | 60 | 112.9 ± 31.6 | 100.4 ± 25.7 | -12.5 ± 36.1 | -17.8 (-29.5 to -6.2) | 0.0019 |
| Total SGRQ | 58 | 16.0 ± 12.1 | 3.2 ± 2.9 | -12.0 ± 10.9 | 60 | 14.0 ± 10.1 | 4.5 ± 4.2 | -9.1 ± 8.6 | -2.4 (-5.8 to 1.0) | 0.1148 |
| Part 1 SGRQ | 58 | 4.0 ± 4.3 | 0.5 ± 0.9 | -3.2 ± 4.0 | 60 | 3.8 ± 4.0 | 0.5 ± 1.1 | -3.4 ± 4.1 | 0.3 (-1.2 to 1.7) | 0.8310 |
| Part 2 SGRQ | 58 | 9.3 ± 8.0 | 1.4 ± 2.4 | -7.5 ± 7.1 | 60 | 7.5 ± 6.5 | 2.3 ± 3.3 | -4.9 ± 4.9 | -2.2 (-4.4 to -0.1) | 0.0234 |
| Borg Dyspnea score | 58 | 2.1 ± 1.3 | 0.7 ± 1.2 | -1.3 ± 0.9 | 60 | 2.1 ± 1.2 | 0.9 ± 1.4 | -1.2 ± 1.3 | -0.1 (-0.5 to 0.2) | 0.4801 |
| Chinese medicine symptom complex score | 58 | 4.3 ± 2.5 | 1.1 ± 1.7 | -3.1 ± 2.6 | 60 | 4.5 ± 2.8 | 0.9 ± 1.2 | -3.4 ± 2.4 | 0.4 (-0.4 to 1.3) | 0.4723 |
‡ P-values were calculated for continuous outcomes with t-tests for the change from baseline to the last visit after three months of treatment; Fisher’ s exact test was performed for categorical outcomes.
Data are presented as the means ± standard deviations. The changes from baseline to the end of three months of treatment were arithmetic. N lrb% is the number of patients and percentage. The least squares mean difference was calculated by analysing the generalised linear regression model with site as a confounder.
Total patients are allocated to the intention-to-treat population.
No. of patients observed at end of three months of treatment.
Table 3.
Improvement of CT severity of pneumonia N (%).
| Level | BFHX (N = 64) | Placebo (N = 65) | P-value |
|---|---|---|---|
| Improved | 8 (15.09) | 4 (7.84) | 0.0238 |
| Stable | 45 (84.91) | 41 (80.39) | |
| Deteriorative | 0 (0) | 6 (11.76) | |
| Total | 53 (100) | 51 (100) |
P-value was calculated with Fisher’ s exact test.
Fig. 2.
Mean changes in 6-min walking distance and Fatigue Assessment Inventory results relative to baseline after three months of treatment in the placebo and BFHX Groups.
A. Mean changes from baseline in 6-min walk distance in the BFHX and control groups in the full dataset. B. Mean changes from baseline in 6-min walk distance in the BFHX and control groups in the full dataset in the per-protocol dataset. C. Fatigue Assessment Inventory scores in the BFHX and control groups in the full dataset. D. Fatigue Assessment Inventory scores in the BFHX and control groups in the per-protocol dataset. Data are presented as medians with 95% confidence intervals (95% CI). The last observation carried forward was imputed in the case of death or clinical worsening without a termination visit or measurement at the termination visit.
BFHX: Bufei Huoxue.
3.3. Secondary indicators
No overall significant difference was found in the SGRQ scores between the two groups (P 0.1148). While there was no difference in the first part of the SGRQ score (P 0.8310), the two groups exhibited significant differences in the second part of the SGRQ score (P 0.0234). No statistical differences in Borg-Dyspnea Scale (P 0.4801) or TCM syndrome (P 0.4723) scores were observed (Table 2). However, FAI scores (reflecting symptom recovery) were significantly lower in the BFHX group than in the control group (FAS: 85.5 ± 27.6 vs. 100.4 ± 25.7; P 0.0030) (Fig. 2C and D).
3.4. Safety
The most common adverse event was elevated ALT and AST levels. Laboratory tests for other aberrant values were rarely performed. After drug withdrawal and symptomatic treatment, the abnormal test indices returned to normal within two weeks in patients experiencing adverse events (AEs). There was no significant difference in the incidence of adverse events between groups (P 0.3241), and no serious AEs occurred during this study (Table 4 ).
Table 4.
Comparison of adverse events N (%).
| Adverse | BFHX | Placebo | P-value |
|---|---|---|---|
| Total (%) | 6 (9.38) | 3 (4.62) | 0.3241 |
| Abnormal liver function (%) | 4 (6.25) | 2 (3.08) | 0.4401 |
| Liver injury (%) | 1 (1.56) | 0 (0.00) | 0.4961 |
| Diarrhoea (%) | 1 (1.56) | 0 (0.00) | 0.4961 |
| Excessive menstruation (%) | 0 (0.00) | 1 (1.54) | 1.0000 |
P-value was calculated with Fisher’ s exact test.
4. Discussion
In attempting to provide in-depth insight into the damage that SARS-CoV-2 inflicts on the human body, researchers have increasingly recognised that many patients who have recovered from COVID-19 continue to live with lingering symptoms that compromise their overall quality of life (Wang et al., 2020). A clinical study found that patients with COVID-19 who had been discharged still experienced fatigue (13%), palpitations (10%), dyspnoea (9%), cough (6%), lower limb oedema (1%), chest pain (1%), and haemoptysis (0.2%) 3 months later. The incidence rates of fatigue, palpitations, and dyspnoea are also significantly higher in critically ill patients with COVID-19 than in non-critically ill patients (Qin et al., 2021). Fatigue or myasthenia, sleep difficulties, and anxiety or depression were also common among COVID-19 survivors 6 months after acute infection (Qin et al., 2021). Critically ill patients with a prolonged inpatient stay have been the main target population of long-term recovery intervention, as they are prone to develop more severe chest CT imaging manifestations and pulmonary diffusion capacity damage. Studies have reported that 63% of COVID-19 survivors experience pulmonary sequelae, including impairments in lung function and pulmonary vessel lesions (Shaw et al., 2021; Sonnweber et al., 2020; Huang et al., 2021). Thus, it is necessary to carry out interventions during the recovery period of COVID-19 to ensure that survivors can return to their previous life, occupation, and functional status.
In this study, we aimed to evaluate the efficacy and safety of BFHX in restoring the functional status and exercise tolerance of patients recovering from COVID-19 infection. Although a few patients in both groups received other rehabilitation interventions (aerobic exercise, breathing exercises, Baduanjin, and psychological therapy), there were no significant differences in these interventions between the two groups (P > 0.05), eliminating the confounding effect of other rehabilitation efforts. Our findings indicated that treatment with BFHX for 3 months resulted in an improvement in the 6MWD and imaging manifestations on chest CT. Three months of treatment also helped reduce fatigue and improve exercise tolerance.
Previous studies have reported that the major CT findings of COVID-19 include bilateral ground-glass opacities, consolidation, and peripheral and diffuse distribution (Liu et al., 2020). As BFHX was associated with better attenuation of COVID-19 manifestations on chest CT than placebo in the convalescent period, our findings suggest that BFHX promotes the absorption of lung lesions and prevents deterioration of pneumonia following COVID-19 infection.
Improvements in scores on the second part of the SGRQ, which assesses exercise capacity, were also higher in the BFHX group than in the control group. In addition, there was no statistically significant difference in the incidence of AEs between the two groups, indicating that BFHX represents a potential therapy with a favourable safety profile for the treatment of COVID-19 during convalescence.
Based on current experiences with COVID-19 treatment, TCM has already been incorporated into the Chinese rehabilitation guidelines for COVID-19, which has been beneficial for the immunity of patients recovering from COVID-19 (General Office of the National Health Commission, 2020). BFHX consists of psoralen, paeoniflorin, isopsoralen, verbasil glucoside, pentagalloyl glucose, verbasil isoflavones, tonic osteostatin, and isopsoralen (Tang et al., 2018). Previous clinical studies have shown that BFHX suppresses inflammatory injury of the lung, ameliorates lung function and pulmonary vascular haemodynamics, and through these mechanisms, relieves cough and chronic obstructive pulmonary disease (Xie et al., 2019). A network pharmacology study demonstrated that BFHX can also exert a therapeutic effect during the rehabilitation period of COVID-19 by targeting multiple cytokines and signalling pathways such as interleukin 6 (IL6), mitogen-activated protein kinase 8 (MAPK8), post-transcriptional gene silencing 1 (PTGS1), PTGS2, and nuclear receptor coactivator 2 (NCOA2) (Guo et al., 2020). These findings may provide a pharmacological basis for understanding improvements in symptoms following treatment with BFHX capsules in patients recovering from COVID-19.
There are a few limitations to this study. First, more objective evaluations are required to verify whether BFHX improves symptoms by ameliorating pulmonary diffusion function. In addition, a larger sample size is required to thoroughly verify the therapeutic effects of BFHX for all symptoms.
5. Conclusions
Neglecting rehabilitation intervention for patients with COVID-19 may result in prolonged recovery time and irreversible sequelae, which greatly deteriorate a patient's quality of life. The results of this multicentre, double-blind, randomised controlled trial suggest that BFHX reduces symptoms of fatigue and improves exercise tolerance in patients recovering from COVID-19. We believe that administration of BFHX to convalescent patients with fatigue, residual lung damage, and impaired exercise tolerance after discharge from the hospital will vastly minimise these symptoms and promote patient recovery following COVID-19 infection.
CRediT authorship contribution statement
Yuqin Chen: Conceptualization, Methodology, Writing – original draft. Chunli Liu: Investigation, Resources, Methodology. Tingping Wang: Investigation, Resources. Jingjing Qi: Investigation, Resources. Xiaoqing Jia: Investigation, Resources. Xiansheng Zeng: Investigation, Resources. Jianling Bai: Formal analysis, Data curation, Visualization. Wenju Lu: Methodology, Project administration. Yu Deng: Formal analysis, Data curation. Bihua Zhong: Investigation. Wenjun He: Investigation. Yue Xing: Writing – original draft. Zhan Lian: Investigation. Haohao Zhou: Investigation. Junping Yan: Investigation. Xuejiao Yang: Investigation. Hao Yu: Investigation. Jiawei Zhou: Investigation. Dansha Zhou: Writing – review & editing. Lixia Qiu: Formal analysis, Visualization. Nanshan Zhong: Writing – review & editing, Project administration, Funding acquisition. Jian Wang: Conceptualization, Writing – review & editing, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jep.2021.114830.
Conflicts of interest
The authors have declared no conflicts of interest. Guangdong Leiyunshang Pharmaceutical Co., Ltd. provided the medications for the study. However, they did not participate in research design, data collection, data analysis, data interpretation, or manuscript writing.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University. All participants provided written informed consent.
Data availability and consent to participate
The authors declare that the data supporting the findings of this study are available from the corresponding authors upon reasonable request. Information that could compromise the privacy of research participants is not available.
Funding
This study was financially supported by grants from the Guangzhou Institute of Respiratory Health for Key Research of Independent Project (Grant No. ZNSA-2020013); Guangdong-Hong Kong-Macao Joint Laboratory for respiratory infectious desease independent project (Grant No. GHMJLRID-Z-202110) and Guangdong Lei Yun Shang Pharmaceutical Co., Ltd., who were not involved in the study, including the planning, execution, data management, statistical analysis, evaluation, or write-up.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Bai S.R., Wu Y., Wang Y., et al. Effect of bailing capsules combined with Bufei Huoxue capsules on pulmonary rehabilitation in patients with chronic obstructive pulmonary disease at stable stage of lung-kidney-qi deficiency syndrome. Chin. J. Exp. Trad. Med. Form. 2016;22:182–186. (in Chinese with English abstract) [Google Scholar]
- Beijing Administration of Traditional Chinese Medicine . 2020. Suggestions on TCM rehabilitation guidance of New Coronavirus Pneumonia in Convalescent Stage in Beijing (Trial)http://zyj.beijing.gov.cn/sy/tzgg/202003/t20200314_1706179.html [Google Scholar]
- Brooks D., Solway S., Gibbons W.J. ATS statement on six-minute walk test. Am. J. Respir. Crit. Care Med. 2003;167:1287. doi: 10.1164/ajrccm.167.9.950. [DOI] [PubMed] [Google Scholar]
- Chen Y.Q., He W.J., Lu W.J., et al. Bufei huoxue capsules in the management of convalescent COVID-19 infection: study protocol for a multicenter, double-blind, and randomized controlled trial. Pulm. Circ. 2021;11(3) doi: 10.1177/20458940211032125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COVID-19 coronavirus pandemic 2021. https://www.worldometers.info/coronavirus/
- Fujimoto Y., Oki Y., Kaneko M., et al. Usefulness of the desaturation-distance ratio from the six-minute walk test for patients with COPD. Int. J. Chronic Obstr. Pulm. Dis. 2017;12:2669–2675. doi: 10.2147/COPD.S143477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- General Office of the National Health Commission . 2020. Notice on issuing a new coronavirus pneumonia prevention and control plan (Trial Version 7)http://www.nhc.gov.cn/cms-search/xxgk/getManuscriptXxgk.htm?id=318683cbfaee4191aee29cd774b19d8d [Google Scholar]
- Guizhou Administration of Traditional Chinese Medicine . second ed. 2020. Guizhou Province COVID-19 Prevention and Treatment of Traditional Chinese Medicine Reference Program.http://atcm.guizhou.gov.cn/xwzx/zyyw/202002/t20200219_50116162.html [Google Scholar]
- Guo H., Zhang H.W. Clinical study of the herbal capsules of nourishing lung and activating blood circulation to treat chronic pulmonary cardiac disease. Chin. Gen. Pract. 2007;10:667–668. (in Chinese with English abstract) [Google Scholar]
- Guo S., Wu W.X., Xie H., Li Q., Wang H.B., Duan J.A. Molecular mechanism of Bufei Huoxue Capsule on COVID-2019 based on network pharmacology and molecular docking. Chin. Tradit. Herb. Drugs. 2020;51:2307–2316. (in Chinese with English abstract) [Google Scholar]
- Health Commission of Anhui Province . 2020. Expert Consensus on Traditional Chinese Medicine Treatment of New Coronavirus Pneumonia in Anhui Province.http://wjw.ah.gov.cn/xwzx/gzdt/51945681.html [Google Scholar]
- Hong Y., Liu X.H., Chen Y.L., et al. Pharmacodynamical study about immunoenhancement effect of Radix Astragali on mice. J. Zhejiang Norm. Univ. Nat. Sci. 2017;2:201–205. (in Chinese with English abstract) [Google Scholar]
- Hu X., Shang Y.M., Xing B.C. Clinical observation on 82 cases of initial pulmonary tuberculosis by adjuvant treatment of Bufei Huoxue Capsule. J. Tradit. Chin. Med. 2018;59:1671–1673. 1684 (in Chinese with English abstract) [Google Scholar]
- Huang Z., Fu F., Ye H., Gao H., Tan B., Wang R., Lin N., Qin L., Chen W. Chinese herbal Huo-Gu Formula for treatment of steroid-associated osteonecrosis of femoral head: a 14-years follow-up of convalescent SARS patients. J. Orthopaed. Transl. 2020;23:122–131. doi: 10.1016/j.jot.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Huang L., Wang Y., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Wang J., Yang J. Study on activating blood and eliminating stasis of total paeony glycoside (TPG) Zhong Yao Cai. 2000;23:557–560. (in Chinese with English abstract) [PubMed] [Google Scholar]
- Liu F., Zhang Q., Huang C., et al. CT quantification of pneumonia lesions in early days predicts progression to severe illness in a cohort of COVID-19 patients. Theranostics. 2020;10:5613–5622. doi: 10.7150/thno.45985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H.P., Li J.K., Jiao Y. Effect of acupoint injecting with Radix Paeonia Rubra injection on nail fold microcirculation. Chin. Naturop. 2002;10:23. (in Chinese with English abstract) [Google Scholar]
- Luo Z.Y., Qing D.G., Sun Y., Xu X.Q., Zhang J., Wang Z.R. Activation of macrophage of Phenylethanol Glycosides Extracted from Cistanche tubulosa and its synergistic effect with Angelica sinensis and Astragalus propinquus in regulating immunity. Sci. Technol. Food. Ind. 2020;41:311–316. (in Chinese with English abstract) [Google Scholar]
- Ma Z.G., Yu J., Li Y., Cao H.R. The effects of invigorating lung and activating blood circulation capsule on inflammatory factors and immunologic function of patients with bronchial asthma at remission stage. Lab. Med. Clin. 2016;13:2746–2748. (in Chinese with English abstract) [Google Scholar]
- Qin W., Chen S., Zhang Y., et al. Diffusion capacity abnormalities for carbon monoxide in patients with COVID-19 at three-month follow-up. Eur. Respir. J. 2021 doi: 10.1183/13993003.03677-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu J.T., Wang J.L., Chai S.W., Liu F. Study on the vasodilatory effect mechanism of psoralen and bakuchiol. Chin. Pharm. 2019;30:3364–3368. (in Chinese with English abstract) [Google Scholar]
- Rachael A.E., Hamish M., Ewen H., et al. Physical, cognitive and mental health impacts of COVID-19 following hospitalisation: a multi-centre prospective cohort study. medRxiv. 2021 doi: 10.1101/2021.03.22.21254057. [DOI] [Google Scholar]
- Rao H.Y., Zhan P., Li P.B., Guan M.Y., Su W.W. Discovery of the active ingredients and the mechanism of action for the effects of Bufei Huoxue capsule on pneumonia recovery based on network pharmacology methods. Acta Sci. Nat. Univ. Sunyatseni. 2020;60:42–49. (in Chinese with English abstract) [Google Scholar]
- Shaw B., Daskareh M., Gholamrezanezhad A. The lingering manifestations of COVID-19 during and after convalescence: update on long-term pulmonary consequences of coronavirus disease 2019 (COVID-19) Radiol. Med. 2021;126:40–46. doi: 10.1007/s11547-020-01295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnweber T., Sahanic S., Pizzini A., et al. Cardiopulmonary recovery after COVID-19 - an observational prospective multi-center trial. Eur. Respir. J. 2020;57 doi: 10.1183/13993003.03481-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q., Zhu X.X., Zheng Y.Y., Guan M.Y., Peng W., Su W.W. HPLC fingerprint of Bufei Huoxue capsules. Cent. S. Pharm. 2018;16:1200–1204. (in Chinese with English abstract) [Google Scholar]
- The Official Product Information for Bufei Huoxue Capsule [http://www.lys.cn/productsandintroductions/info.aspx?itemid=55&Lcid=20]. Last accessed on Sep 15th, 2020.
- Wang F., Kream R.M., Stefano G.B. Long-term respiratory and neurological sequelae of COVID-19. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020;26 doi: 10.12659/MSM.928996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T., Yang X., Zeng X., Poole P. Traditional Chinese medicine in the treatment of acute respiratory tract infections. Respir. Med. 2008;102(8):1093–1098. doi: 10.1016/j.rmed.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Xie Z., Di P. Clinical effect of Bufei Huoxue capsules. Chin. Pract. Med. 2012;7:148–149. [Google Scholar]
- Wu X.Q., Zhang W.N., Hao M.Z., Liu X.P., Xiao J., Wang T.F., Dong Y.Z., Zhao J. How Chinese herbal medicine prevents epidemics: from ancient pestilences to COVID-19 pandemic. Am. J. Chin. Med. 2021;49(5):1017–1044. doi: 10.1142/S0192415X2150049X. [DOI] [PubMed] [Google Scholar]
- Xie M., Luo Y.N., Li N., Jing F.R., Shi Z.H. Therapeutic effects of Bufeihuoxue capsule combined with Chuankezhi injection on chronic obstructive pulmonary disease at stable period. Heb. Med. J. 2019;41:981–984. (in Chinese with English abstract) [Google Scholar]
- Xu D., Jia X.Y., Hu M. Effect of astragalus polysaccharide on growth performance and immunity of weaned lamb. Chin. Feed. 2018;12:56–59. (in Chinese with English abstract) [Google Scholar]
- Yang B., Meng L.M., Lu H., Wang S.H., Wang P. A randomized and comparative control clinical study of the herbal capsules for nourishing lung and activating blood circulation for the treatment of chronic pulmonary cardiac disease. Chin. J. New Drugs. 2005;14:1192–1195. (in Chinese with English abstract) [Google Scholar]
- Yao L., Yao D. Improving the body's immunity and delaying aging—on the new medicinal uses of ginkgo biloba, berries, and astragalus. J. Pract. Chin. Mod. Med. 2003;16:1649. (in Chinese with English abstract) [Google Scholar]
- Yu X.Y., Geng L.M., Hou Y.Y. Effects of comprehensive external therapy combined with Bufei Huoxue Capsule on inflammatory factors and clinical efficacy of patients with chronic bronchial asthma at chronic duration stage. Chin. J. New Drugs. 2019;28:1094–1098. (in Chinese with English abstract) [Google Scholar]
- Zhang D.X., Qu J.T., Dou Y.T., Liu S.W., Wang J.L. Research progress on immune regulation of psoralen and its active ingredients. Curr. Immunol. 2017;37:80–83. (in Chinese with English abstract) [Google Scholar]
- Zhang D., Qu J., Dou Y., Liu S., Wang J. Study on mechanism of vasodilatory effect of isopsoralen. Liaoning J. Trad. Chin. Med. 2020;47:189–192. (in Chinese with English abstract) [Google Scholar]
- Zhao Z. Psoralen increases white blood cells, improves myocardial ischemia, lowers blood lipids. J. Tradit. Chin. Med. 2002;43:413. (in Chinese with English abstract) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting the findings of this study are available from the corresponding authors upon reasonable request. Information that could compromise the privacy of research participants is not available.