Abstract
Background
The COVID-19 epidemic raises important questions about the efficacy of vaccines for people treated with ocrelizumab, an anti-CD20 therapy. Ocrelizumab has been shown to reduce the humoral response to SARS-CoV-2 infection and vaccination, but the T-cell response to vaccination has not been fully characterized. We sought to provide data regarding B and T-cell mediated responses to SARS-CoV-2 vaccination in ocrelizumab-treated patients, and to determine what variables correlate with vaccine immunogenicity. We hypothesized that patients without a humoral response to SARS-CoV-2 vaccination would still have intact T-cell responses.
Methods
We conducted a prospective, observational, single center cohort study of patients with MS treated with either ocrelizumab or natalizumab as a comparator between March 2, 2021, and July 1, 2021. Eligible patients were age 18 to 55 and had no known prior infection with, or vaccination against, SARS-CoV-2. Patients with prior use of immunosuppressive or chemotherapeutic agents, or treatment with any anti-CD20 therapy other than ocrelizumab within 12 months of enrollment, were excluded. The Roche Elecsys anti-SARS-CoV-2 S immunoassay was performed prior to and 3–4 weeks post vaccination to evaluate the antibody response to SARS-CoV-2 spike IgG. The Adaptive Biotechnologies T-Detect COVID Test was performed to evaluate the adaptive T-cell immune response to SARS-CoV-2 in OCR-treated patients with no detectable antibodies. Data were analyzed using descriptive statistics, Fisher's exact test, and Wilcoxon rank sum.
Results
Forty-eight patients were enrolled in the study, 69% treated with ocrelizumab and 31% treated with natalizumab. Eighteen percent of ocrelizumab and 100% of natalizumab patients had a positive antibody response. In ocrelizumab-treated patients, there was no correlation between age, sex, BMI, total number of infusions, immunoglobulin G, CD19, or absolute lymphocyte count and antibody response. There was a trend suggesting that a longer interval between the last infusion and vaccination increased the likelihood of producing antibodies (P = 0.062). All ocrelizumab patients with negative antibody responses had positive T-cell responses.
Conclusions
Treatment with ocrelizumab substantially impaired the humoral response to SAR-CoV-2 vaccination but did not impair T-cell responses. Further research is needed to determine if the T-cell response to SARS-CoV-2 vaccination is sufficient to prevent infection or reduce severity of COVID in patients who did not produce antibodies.
Keyword: SARS-CoV-2, Immunogenicity, Multiple sclerosis, COVID-19, Natalizumab, Ocrelizumab, Coronavirus vaccine
1. Introduction
Clinicians caring for individuals with multiple sclerosis (MS) have faced significant challenges during the coronavirus (COVID-19) pandemic. Disease modifying therapies (DMTs), and specifically, anti-CD20 therapies, can increase susceptibility to infection and impair the response to vaccines. (Hauser et al., 2017; Bar-Or et al., 2020) COVID vaccines have been shown to dramatically reduce the risk of SARS-CoV-2 infection, (Polack et al., 2020; Baden et al., 2021; Sadoff et al., 2021) but their efficacy in patients on anti-CD20 therapy is unproven, complicating decisions about MS treatment and about the timing of both anti-CD20 infusions and vaccinations.
Both B-cell mediated humoral and T-cell mediated cellular responses are involved in vaccine immunogenicity. Anti-CD20 therapies reduce the population of both naïve and memory B-cells and thus can impair the humoral vaccine response. (Hauser et al., 2017; Montalban et al., 2017) Ocrelizumab (OCR), an anti-CD20 monoclonal antibody, is a highly effective treatment for MS, (Hauser et al., 2017; Montalban et al., 2017) but has been shown to cause an attenuated humoral response to vaccines. (Bar-Or et al., 2020) However, in the case of the flu vaccine, seroprotective antibody levels were still achieved. (Bar-Or et al., 2020) In OCR-treated patients, several studies have shown an impaired humoral response yet preserved T-cell response following SARS-CoV-2 infection or vaccination with Pfizer-BioNTech COVID-19 (BNT162b2) or Moderna Tx, Inc (mRNA-1273). (Kister et al., 2021; A Achiron et al., 2021; Sormani et al., 2021; Apostolidis et al., 2021; Iannetta et al., 2021) In contrast, natalizumab (NTZ), an α4-integrin monoclonal antibody, is unlikely to impact vaccine efficacy. (Kaufman et al., 2014)
In this study, we evaluated the humoral response to SARS-CoV-2 vaccines in adult MS patients treated with OCR, using NTZ as a real-world comparator. We then assessed the T-cell response for those OCR-treated patients who did not produce detectable antibodies. We screened for the presence of SARS-CoV-2 antibodies prior to vaccination to ensure that humoral immunity was the result of vaccination rather than preexisting antibodies.
2. Materials and methods
2.1. Study design
We conducted a prospective, single center, observational cohort study of multiple sclerosis patients at the Elliot Lewis Center for Multiple Sclerosis Care (MA, USA) between March 2, 2021, and July 1, 2021. The study received approval from the Advarra institutional review board (MOD00928529), and research was conducted in accordance with the principles expressed in the Declaration of Helsinki. Patients were informed about the objectives of the study, and written informed consent was obtained prior to enrollment in accordance with U.S. law and good clinical practice.
2.2. Study participants
Participants were multiple sclerosis patients who met revised 2017 McDonald criteria, (Thompson et al., 2018) ages 18 to 55 years, with an EDSS of 0 to 5.5, who were treated with either OCR or NTZ for a minimum of 6 months, and who intended to receive one of the available COVID-19 vaccines. Patients could be vaccinated at any point during their OCR or NTZ dosing cycles. Exclusionary criteria included previous infection with COVID-19, a positive test for SARS-Cov-2 spike protein antibodies prior to vaccination, prior vaccination against COVID-19, prior use of immunosuppressive or chemotherapy treatment, prior treatment with a B-cell depleting therapy other than OCR within 12 months of enrollment, use of systemic corticosteroid therapy within 12 weeks of screening, and pregnancy.
2.3. Demographic factors
Demographic information collected included sex, age, weight, height, smoking history, MS subtype, disease duration, time since last relapse, EDSS, and type and duration of previous MS treatment. Initiation date of the current MS treatment, date of the most recent infusion prior to enrollment, and the most recent laboratory values (CD19, CD4, absolute lymphocyte count [ALC], immunoglobulin G [IgG]) at the OCR infusion prior to enrollment, were also recorded.
2.4. Assessments
The Roche Elecsys Anti-SARS-CoV-2 S immunoassay was performed per the manufacturer's regulations using the cobas e602 analyzer (Elecsys 2020) to identify individuals with an antibody response to SARS-CoV-2. This was done prior to the first COVID-19 vaccination dose and 3–4 weeks following the final vaccination dose.
The Adaptive Biotechnologies T-Detect COVID Test was performed per the manufacturer's regulations using the Kingfisher Flex 711 system and the MagMax DNA Multi-sample Ultra 2.0 reagent kits (Emergency Use Authorization (EUA) 2021) to identify individuals with an adaptive T-cell immune response to SARS-CoV-2. This assay utilized a multiplex polymerase chain reaction to detect rearranged T-cell receptor gene sequences isolated from whole blood samples. A positive or negative result was determined using a pre-specified threshold for TCR-beta sequences using a COVID-specific algorithm. The sensitivity was 92.11% and the specificity was 98.7%. This assay was performed 3–4 weeks following final vaccination dose in individuals without a detectable antibody response.
2.5. Definition of study end points
The primary endpoint of this study was the production of SARS-CoV-2 spike protein antibodies 4 weeks after completion of vaccination. Antibody test results were defined as either positive (≥0.80 U/mL) or negative (<0.80 U/mL) as per the manufacturer's guidelines.
The presence of SARS-CoV-2 specific T-cells was assessed in OCR-treated patients with a negative antibody response after vaccination. Demographic and clinical factors including weight, BMI, age, race, baseline level of disability, MS disease subtype, duration of disease, duration of treatment, time from prior relapse, and smoking status were assessed as potential predictors of vaccine response.
2.6. Statistical analysis
Data were analyzed using JMP Pro 16.0.0. (SAS Institute Inc 2021) Descriptive statistics are presented as medians and ranges for continuous variables and as frequencies (%) for categorical variables. Fisher's exact test was used to compare categorical data. Wilcoxon rank sum was used to compare nonparametric continuous data.
3. RESULTS
Forty-eight participants were included in our study. The median age was 42.5 years (range 25–56), and the median disease duration was l2 years (range 1–33). (Katz et al., 2021) Thirty-five participants (72.9%) were female. Thirty-three (68.8%) were treated with OCR, and 15 (31.3%) were treated with NTZ. The demographic and clinical variables of the 48 study participants, broken down by treatment, are presented in Table 1 .
Table 1.
Demographic and clinical characteristics of patients treated with ocrelizumab or natalizumab.
| No. (%) | ||
| OCR | NTZ | |
| Characteristics | N = 33 | N = 15 |
| Age, median (range), years | 44 (28–56) | 42 (25–53) |
| Female | 22 (67) | 13 (87) |
| MS Diagnosis | ||
| Relapsing MS | 28 (85) | 15 (100) |
| Progressive MS | 5 (15) | 0 (0) |
| Disease duration, median (range), years | 12 (1–33) | 12 (3–21) |
| EDSS, median (range) | 2 (0–3.5) | 1.5 (0–3) |
| BMI, mean (range), kg/m2 | 27.20 (19.7–42.2) | 23.2 (19.3–31.1) |
| Time between last infusion and first vaccine dose, median (range), days | 86 (11–222) | 16 (0–36) |
| Vaccine Type | ||
| Pfizer | 25 (76) | 8 (53) |
| Moderna | 7 (21) | 6 (40) |
| Johnson & Johnson | 1 (3) | 1 (7) |
| Positive SARS-CoV-2 spike protein antibodies | 6 (18) | 15 (100) |
| Positive SARS-CoV-2 T-cell response a | 27 (100) | NA |
Abbreviations: OCR, ocrelizumab; NTZ, natalizumab; MS, multiple sclerosis; EDSS, expanded disability status score; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; NA, not applicable.
SARS-CoV-2 T-cell responses were only measured in the 27 OCR subjects that did not produce detectable antibodies.
The majority of patients (68.8%) received the Pfizer-BioNTech BNT162b2 vaccine, 14.6% received the ModernaTX, Inc. mRNA-1273 vaccine, and 4.2% received Johnson & Johnson's Janssen JNJ-78,436,735 vaccine.
3.1. SARS-CoV-2 spike IGG and adaptive T-cell responses
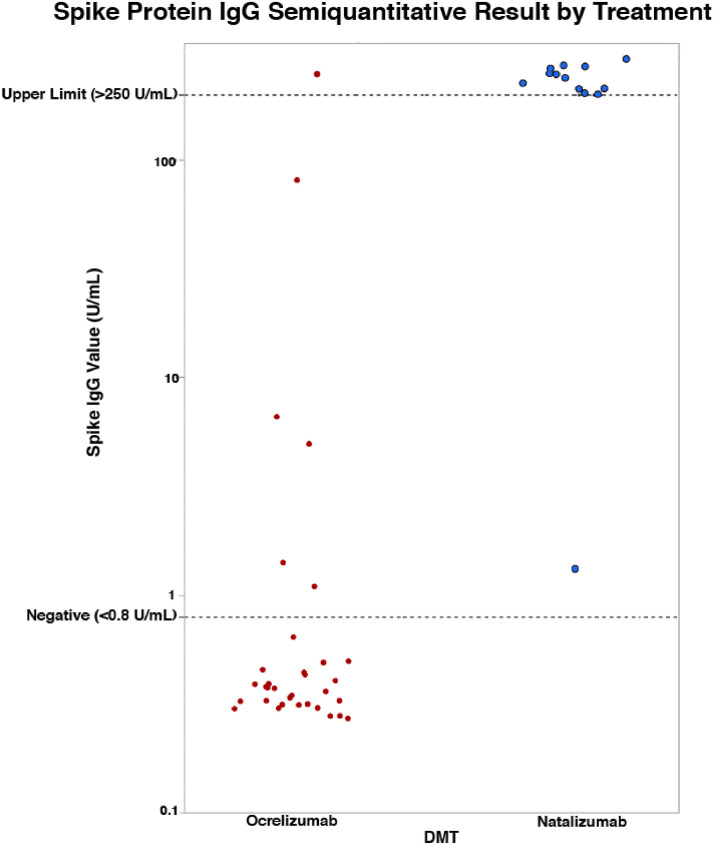
Six (18.2%) of the OCR-treated patients had a positive antibody response. All 15 of the NTZ-treated patients produced a positive response. Of the six OCR-treated patients who produced antibodies, only one had a result that exceeded the upper limit of 250 U/mL, whereas all but one (93.3%) of the NTZ-treated patients exceeded the upper limit (see Fig. 1 ).
Fig. 1.
SARS-CoV-2 spike protein IgG: ocrelizumab vs. natalizumab
Abbreviations: IgG, immunoglobulin G; DMT, disease modifying treatment.
The demographic and clinical characteristics of OCR-treated patients stratified by antibody response are presented in Table 2 .
Table 2.
Demographic and clinical characteristics of ocrelizumab-treated patients stratified by antibody response to SARS-CoV-2 vaccination.
| Spike IgG Negative | Spike IgG Positive | P value | |
| Characteristics | N = 27 | N = 6 | |
| Age, median (range) | 45 (28–56) | 41 (28–45) | 0.182 a |
| Female, no. (%) | 20 (74) | 2 (33) | 0.146 a |
| BMI>30, no. (%) | 12 (44) | 2 (33) | 1 b |
| Number of infusion cycles prior to vaccination, median (range) | 6 (2–11) | 4.5 (2–7) | 0.268 b |
| Time between last infusion and vaccination, median (range) | 75 (11–222) | 161 (16–191) | 0.065 a |
| ALC, median (range) | 1170 (503–2821) | 1143 (936–1981) | 0.575 a |
| IgG, median (range) | 1007 (521–1562) | 1208 (644–1538) | 0.183 a |
| Absolute CD19, median (range) | 0 (0–96) | 0.5 (0–50) | 0.289 a |
| Absolute CD4, median (range) | 679 (238–1582) | 694 (461–1107) | 0.591 a |
| Vaccine Type, no. (%) | |||
| Pfizer | 20 (74) | 5 (83) | Reference |
| Moderna | 6 (22) | 1 (17) | 1 b |
| Johnson & Johnson | 1 (4) | 0 (0) | 1 b |
Abbreviations: IgG, immunoglobulin G; ALC, absolute lymphocyte count; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared).
Wilcoxon/Kruskal-Wallis Tests was used to determine the P value.
Fisher exact test was used to determine the P value.
There was no correlation between antibody response and age, sex, BMI, vaccine type, number of infusion cycles, and IgG, CD19, or ALC. There was a trend suggesting that a longer interval between the last infusion and vaccination increased the likelihood of producing antibodies (P = 0.062).
Of the OCR-treated patients that had a negative antibody response, all patients (N = 27) had a positive adaptive T-cell response.
4. Discussion
Anti-CD20 therapy has proven to be effective for relapsing and primary progressive forms of MS, (Hauser et al., 2017; Montalban et al., 2017) but the potential impact on SARS-Cov-2 vaccine efficacy has led to difficult treatment dilemmas during the COVID-19 pandemic. Our study provides confirmatory data regarding the humoral and cellular responses to SARS-CoV-2 spike protein vaccination in OCR-treated patients. (Sormani et al., 2021; Apostolidis et al., 2021)
We found that (80%) of MS patients treated with OCR had no measurable antibody production following COVID vaccination, similar to that seen in a prior study. (Sormani et al., 2021; Apostolidis et al., 2021; A Achiron et al., 2021) This finding is not unexpected in patients treated with anti-CD20 therapies but is disappointing, as a previous study demonstrated an attenuated, but significant, response to flu vaccination in patients treated with OCR. (Bar-Or et al., 2020) In the same study, antibody production was found to be weakest in response to novel antigens. (Bar-Or et al., 2020) We postulate that the mRNA and DNA vaccines might have less cross-reactivity with previously encountered non-SARS coronaviruses because they expose the immune system to a single, highly specific antigen, and thus might produce a less robust immune response. In contrast, antibodies were detected in all patients treated with natalizumab, an expected outcome since it has no known impact on B-cell function.
There was a trend suggesting that a longer interval between the last infusion and vaccination increased the likelihood of producing antibodies. This corroborates findings from other studies that evaluated antibodies in OCR-treated patients after receiving mRNA vaccines. (Sormani et al., 2021; Apostolidis et al., 2021) Similarly, for rituximab-treated patients, there was a greater likelihood of producing antibodies after flu vaccination in patients 6 to 10 months after infusion compared with 4 to 8 weeks after infusion. (Westra et al., 2014) In our study, we found no other identifiable factors associated with the ability to mount a humoral response. In the individuals that did produce a measurable antibody response, the reported titers from the semiquantitative test were substantially lower than those seen in the natalizumab group.
Although the presence of antibodies in response to vaccination has been presumed to be an indicator of protective immunity, little is known about the antibody levels required to prevent or reduce the severity of infection. A NEJM editorial relating to rituximab noted that the FDA advised against checking post-vaccination antibody levels, as “positive titers may not reflect protection and negative titers may not indicate susceptibility” and that “T-cell responses … may provide some degree of protection, especially against severe disease”. (Kaul, 2021)
Importantly, all seronegative OCR patients produced a T-cell response to vaccination. This again corroborates findings from another study. (Apostolidis et al., 2021) Presumably, the presence of SARS-Cov-2 specific T-cells should afford some degree of immunologic protection. (Kaul, 2021) It has been proposed that T-cells may be as important as neutralizing antibodies in fighting and preventing severe COVID-19 infection. (Rydyznski Moderbacher et al., 2020) Rydyznski Moderbacher et al. demonstrated that SARS-CoV-2-specific CD4+ or CD8+ T-cell responses were associated with lower COVID-19 severity. (Rydyznski Moderbacher et al., 2020) In another study with patients previously infected with SARS, specific memory CD8 T-cells were detected up to six years after infection, suggesting that vaccines have the potential to provide long-lasting cellular immunity. (Tang et al., 2011)
One limitation of our study was the small sample size. As a result, we were unable to draw meaningful conclusions about factors contributing to immunogenicity, such as vaccine formulation, the number and temporal relationship of OCR infusions to vaccine administration, or demographic factors such as age and BMI. It has been demonstrated that a large percentage of OCR-treated patients have an inadequate humoral response to COVID infection; therefore, a negative antibody test prior to vaccination could not exclude prior COVID-19 infection. (Kister et al., 2021) The lack of testing for T-cell function in both seropositive OCR and NTZ patients was another limitation of the study. Additionally, the homogeneity of our population may affect generalizability of our findings as our patients were almost exclusively white. We excluded older patients with multiple comorbidities to avoid immuno-senescence and other health conditions as confounders. However, it would be reasonable to expect that compared to our study population, older patients with other underlying health conditions would have a similar or worse immunogenic response to vaccination.
Many questions remain regarding SARS-CoV-2 vaccination and anti-CD20 therapy. Whether age, BMI, and timing of OCR in relationship to vaccination affects immunogenicity remains unknown. Furthermore, in patients who did not produce antibodies in response to vaccination, it is not clear whether a T-cell response is sufficient to prevent infection or reduce severity of COVID.
Clinical decision making based on antibody production or T-cell response to SARS-Cov-2 vaccination remains challenging given the status of current research. Our study suggests that vaccine administration prior to initiation of anti-CD20 therapies is appropriate, if clinically feasible. Because of the current uncertainty surrounding vaccine efficacy, discontinuation of anti-CD20 therapies should be considered for high-risk populations, or for patients in whom benefit is questionable. It is vital to have a better understanding of the immunologic and clinical consequences of MS therapies, particularly in the current COVID pandemic.
Data statement
The dataset generated during the current study is available in the Mendeley Data repository (DOI: 10.17632/xvd8g9gb6t.1).
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
J.D. Katz: Conceptualization, Methodology, Investigation, Formal analysis, Writing – original draft, Visualization, Writing – review & editing. A.J. Bouley: Conceptualization, Methodology, Investigation, Formal analysis, Writing – original draft, Visualization, Writing – review & editing. R.M. Jungquist: Investigation, Data curation, Formal analysis, Writing – original draft, Visualization, Writing – review & editing. E.A. Douglas: Investigation, Data curation, Formal analysis, Writing – original draft, Visualization, Writing – review & editing. I.L. O'Shea: Investigation, Data curation, Formal analysis, Writing – original draft, Visualization, Writing – review & editing. E.S. Lathi: Conceptualization, Methodology, Investigation, Formal analysis, Writing – original draft, Visualization, Writing – review & editing.
Declaration of Competing Interest
The Authors declare that there are no conflicts of interest.
Acknowledgment
The Authors have no acknowledgments to declare.
References
- Hauser S.L., Bar-Or A., Comi G., et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N. Engl. J. Med. 2017 doi: 10.1056/NEJMoa1601277. [DOI] [PubMed] [Google Scholar]
- Bar-Or A., Calkwood J.C., Chognot C., et al. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis: the VELOCE study. Neurology. 2020 doi: 10.1212/WNL.0000000000010380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baden L.R., El Sahly H.M., Essink B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoff J., Le Gars M., Shukarev G., et al. Interim results of a phase 1–2a trial of Ad26.COV2.S Covid-19 vaccine. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2034201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalban X., Hauser S.L., Kappos L., et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N. Engl. J. Med. 2017 doi: 10.1056/NEJMoa1606468. [DOI] [PubMed] [Google Scholar]
- Kister I., Krogsgaard M., Mulligan M.J., et al. 73rd Congress of the American Academy of Neurology. Roche and Genentech; 2021. Preliminary results of ongoing, prospective study of antibody and T-cell responses to SARS-CoV-2 in patients with MS on ocrelizumab or other disease-modifying therapies. Apr 17-22. [Google Scholar]
- Achiron A., Mandel M., Dreyer-Alster S., et al. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. therapeutic advances in neurological disorders 2021. DOI: 10.1177/17562864211012835. [DOI] [PMC free article] [PubMed]
- Sormani M.P., Inglese M., Schiavetti I., et al. Effect of SARS-CoV-2 mRNA vaccination in MS patients treated with disease modifying therapies. EBioMedicine. 2021 doi: 10.1016/j.ebiom.2021.103581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolidis S.A., Kakara M., Painter M.M., et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat. Med. 2021 doi: 10.1038/s41591-021-01507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannetta M., Landi D., Cola G., et al. T-cell responses to SARS-CoV-2 in multiple sclerosis patients treated with ocrelizumab healed from COVID-19 with absent or low anti-spike antibody titers. Mult Scler Relat Disord. 2021 doi: 10.1016/j.msard.2021.103157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman M., Pardo G., Rossman H., et al. Natalizumab treatment shows no clinically meaningful effects on immunization responses in patients with relapsing-remitting multiple sclerosis. J. Neurol. Sci. 2014 doi: 10.1016/j.jns.2014.03.035. [DOI] [PubMed] [Google Scholar]
- Thompson A.J., Banwell B.L., Barkhof F., et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018 doi: 10.1016/S1474-4422(17)30470-2. [DOI] [PubMed] [Google Scholar]
- Elecsys Anti-SARS-CoV-2 S [Package Insert] 2020.
- Emergency Use Authorization (EUA) Summary T-Detect COVID Test 2021.
- SAS Institute Inc. JMP Pro; 16. 2021.
- Katz J., Bouley A., Jungquist R., et al. SARS-CoV-2 Vaccination Immune Response 2021. </Dataset>.
- Achiron A., Dolev M., Menascu S., et al. COVID-19 vaccination in patients with multiple sclerosis: what we have learnt by February 2021. Mult. Scler. 2021 doi: 10.1177/13524585211003476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westra J., van Assen S., Wilting K.R., et al. Rituximab impairs immunoglobulin (Ig)M and IgG (subclass) responses after influenza vaccination in rheumatoid arthritis patients: rituximab impairs IgM and IgG influenza response. Clin. Exp. Immunol. 2014 doi: 10.1111/cei.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul D. How should we advise our immunocompromised patients after COVID-19 vaccination? N Engl. J. Med. J. Watch. 2021 [Google Scholar]
- Rydyznski Moderbacher C., Ramirez S.I., Dan J.M., et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020 doi: 10.1016/j.cell.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang F., Quan Y., Xin Z., et al. Lack of peripheral memory B cell responses in recovered patients with severe acute respiratory syndrome: a six-year follow-up study. J. Immunol. 2011 doi: 10.4049/jimmunol.0903490. [DOI] [PubMed] [Google Scholar]