Abstract
The present trial aims to evaluate a supplementation of the olive leaf extract (OLE) in adjunct with a weight loss diet on anthropometric indices, glycemic indices, lipid profile, as well as the level of adipokines, and free fatty acid in obese women. We carried out an 8-week randomized, placebo-controlled, double-blind, parallel-group, clinical trial. The participants were randomly stratified according to age and they were assigned to one of the two study groups: Standard weight loss diet (estimated daily energy requirements minus 500 kcal) + OLE supplementation (n = 35) in intervention group or Standard weight loss diet (estimated daily energy requirements minus 500 kcal) + placebo (n = 35) in placebo group. The study groups were homogeneous regarding the baseline age, height, weight, body mass index (BMI), waist circumferences, married status, and physical activity levels (p > 0.05). The results of analysis of covariance presented significant decreases in BMI, fat mass, and body weight in the OLE group compared to those in the placebo group (p < 0.05). At the end of the study, the serum levels of fasting blood sugar, insulin, low-density lipoprotein cholesterol, total cholesterol, leptin, fatty free acid, and homeostasis model assessment–insulin resistance significantly decreased, and serum levels of high-density lipoprotein cholesterol and adiponectin elevated in the intervention group (p < 0.05). Based on results it seems that the addition of OLE to a hypocaloric diet for 8-week compared with a hypocaloric diet alone may be more effective in modifying obesity and metabolic risk factors.
Trial Registration
Iranian Registry of Clinical Trials Identifier: IRCT20190129042552N2
Keywords: Obesity, Weight loss, Reducing diet, Olive leaf extract, Randomized controlled trial
INTRODUCTION
Obesity is defined as the excessive accumulation of fat in the body (body mass index [BMI] ≥ 30 kg/m2) and is associated with dysregulation of glucose and lipoprotein metabolism [1]. Obesity raises the risk of chronic disorders such as some cancers, cardiovascular disease, and type 2 diabetes mellitus [2]. According to the report of the World Health Organization (WHO) in 2016, more than 1.9 billion adults were overweight and 650 million were obese (13% of the world's adult population) [3]. Existing approaches for handling and treatment of obesity consist of weight loss diet, sport, behavioral changes, pharmacotherapy, and surgery [4]. The efficacy of each of these approaches is often inconclusive [5]. Anti-obesity drugs have adverse side effects such as headaches, vomiting, and myocardial infarctions [6]. However, natural products as the traditional source of medicinal compounds could be developed as complimentary safe and effective approaches for obesity [5,7,8].
The leaves of the olive plant (Olea europaea L.), family: Oleaceae, have been applied for centuries in traditional medicine to prevent and cure many illnesses such as wounds [9], fever [10], diabetes [11], gout [12], atherosclerosis [13], and hypertension [14]. Moreover, anti-inflammatory, antioxidant, anti-tumor, antiviral, and antimicrobial properties of olive leaf extract (OLE) were also reported [15,16] in the European and Mediterranean countries [17,18]. Its bioactive compounds such as oleuropein (most abundant biophenol), verbascoside, luteolin, rutin, catechin, and hydroxytyrosol (in lower quantities) may be responsible for the biologic activities [19,20].
Several OLE components have been revealed to represent beneficial effects against obesity both in vitro and in vivo. An animal study demonstrated that the oleuropein-supplemented high fat diet (HFD) for 10 weeks exerted protective effects against hepatic steatosis by decreasing the expression of some hepatic genes involved in the oxidative stress, detoxification of lipid peroxidation products, and inflammation [21]. Moreover, Hsu et al. [22] studied the anti-obesity effect of rutin for 8 weeks in HFD fed mice. They observed rutin as a supplement at a dose of 50 mg/kg significantly decreased body and adipose tissue weights, oxidative stress, serum insulin, lipid profiles, and leptin, as well as hepatic triacylglycerol and cholesterol levels [22]. Caffeic acid supplementation at 0.02% weight dose in HFD-fed mice displayed anti-obesity properties by improving body weight and visceral fat mass, blood lipid profile, and obesity-related hormones such as insulin and leptin [23]. Recently, Fki et al. [7] investigated and compared the hypolipidemic and hepatoprotective protective effects of oleuropein and hydroxytyrosol-rich OLE at 16 mg/kg in HFD fed rats. Oleuropein and hydroxytyrosol similarly decreased body weight and white adipose tissue accumulation by inhibiting the excess increase in the number and/or size of adipocytes and improved the lipid metabolism by elevating the antioxidant system capacity and preventing the expression of the proteins implicated in inflammation and liver injury [7]. In a study by Shen et al. [20], the addition of OLE to HFD in mice significantly reduced body weight gain, visceral fat-pad weights, and plasma lipid levels by downregulating the expression of genes involved in adipogenesis and increasing the mRNA expression of genes involved in mitochondrial biogenesis in the visceral adipose tissue.
The effect of supplementation with olive leaf extract on glucose homeostasis was investigated[24]. Forty-six overweight individuals underwent olive leaf extract supplementation (51 mg oleuropein and 66.9 mg hydroxytyrosol) for 12 weeks. The results of this study showed that supplementation with olive leaf extract improved insulin sensitivity by 15%. However, in this study, food intake was not properly controlled, and a restricted calorie diet was not applied. In a clinical trial examined the effect of consuming 3 cups of olive leaf tea a day on lipid metabolism in 110 non-diabetic and non-obese individuals for 12 weeks. The results showed that after the intervention, low-density lipoprotein cholesterol (LDL-C) and triglycerides (TGs) and fasting glucose levels decreased while body weight, waist circumference (WC) and insulin levels did not change. In this study, not only the exact dose of polyphenols received by individuals is not known, but also the main weight loss intervention, i.e., calorie-restricted diet was not considered and individuals were in the range of normal BMI or overweight [25].
Although several animal studies have evaluated the OLE and its bioactive constituents' effects against diet-induced obesity, the effect of OLE supplementation in obesity along with a weight loss diet has not been reported in the clinical trial. Since the present study aims to investigate a supplementation of the 250 mg OLE in adjunct with a weight loss diet for 8 weeks on weight loss, body composition, glycemic indices, lipid profile, as well as serum level of adiponectin, leptin, and free fatty acid in obese women.
MATERIALS AND METHODS
Study design
This study was a randomized, placebo-controlled, double-blind, parallel-group, clinical trial. This clinical trial was held at the Nutritional Research Center, Ahvaz Jundishapur University to investigate the efficacy of the 250 mg OLE in adjunct with weight loss diet for 8 weeks in obese women. This study was conducted only on females due to the more homogeneity of samples and also double prevalence of obesity among Iranian female in comparison with men [3]. This study, approved by the Medical Ethics Committee of Ahvaz Jundishapur University, is in accordance with the Declaration of Helsinki (approval number: IR.AJUMS.REC.1399.146) Then it was registered in the Iranian Registry of Clinical Trials (IRCT registration number: IRCT20190129042552N2.
Recruitment of study subjects
Premenopausal women volunteers recruited through electronic and paper media advertisements and the inclusion criteria were engaged in this trial after receiving their informed written consent. The following inclusion criteria were applied; BMI range of 30 to 40 kg/m2, the age of 18 years and older, absence of food allergies and menopause, having no medical disorders including diabetes, hepatic, cancer, renal, thyroid, and gastrointestinal disorders, no surgery for weight loss, no weight loss over the past 6 months, taking no herbs and drugs reducing appetite and weight as well as nutritional supplements. The subjects with any of the following criteria were excluded: unwilling to continue, no consumption of supplements exceed 10% of total administered supplements, become pregnant during the study, changes in physical activity, and changes in the dietary pattern during the study period.
Sample size calculation
The sample size was estimated as 32 subjects (in each group) based on a study by Lockyer et al. [26] and reduction in cholesterol level in response to OLE intervention (α = 0.05, β = 0.2, μ1 = 0.47; μ2 = 0.44; S1 = −0.33; S2 = 0.01) based on this formula:
By considering the 10% sample loss, finally, 35 subjects were recruited for each group.
Randomization and blinding
Using a permuted block randomization procedure by Random Allocation Software (RAS), the participants were randomly stratified according to age. Later, a random number was used for bottle naming (OLE or placebo). Blinding was done by sealing the bottles and their similarity. Treatment allocation was also blinded for investigator and participants.
Intervention process and compliance
They were allocated to 1 of the 2 following groups: 1) Intervention group: Standard weight loss diet + OLE supplementation (n = 35); 2) Placebo group: Standard weight loss diet + Placebo (n = 35).
Energy needs were considered by Mifflin Jeor St. equation: BMR (kcal/day) = 10 × Weight (kg) + 6.25 × Height (cm) − 5 × Age (year) − 161 (kcal/day) [27], and each subject of study in both groups followed a hypocaloric diet of 500 kcal below estimated energy requirements. In both study groups, the proportion of macronutrients were 55%, 30%, 15% for carbohydrate, fat, and protein; respectively [28]. OLE or placebo capsules were consumed twice daily, one capsule after lunch and one capsule after dinner. The placebo capsules had the same weight, taste, and flavor as the OLE capsules. To check compliance, participants were requested to record the date and time of capsules consumption and they were contacted every three days by a dietitian through call or SMS [29]. Moreover, the participants were requested not to alter their physical activity and dietary pattern during the 8 weeks' intervention period. Dietary intake was evaluated by a 3-day food record (2 weekdays and 1 weekend day) at the baseline and week 8.
Ingredients and nutrition information of OLE extract
OLE and placebo capsules were supplied by Shari Pharmacy Co. (Tehran, Iran). Each 125 mg OLE capsule comprised 50 mg oleuropein, other biophenol compounds are oleuropein verbascoside, luteolin, rutin, catechin, and hydroxytyrosol. Each placebo capsule comprised 125 mg starch.
Anthropometric analyses
At the baseline and the end of the intervention, body weight was evaluated using Seca scale (seca 630; seca GmbH, Hamburg, Germany) with an accuracy of 100 g. At the baseline, height was measured using Seca stadiometer (seca 700; seca GmbH) with an accuracy of 0.5 cm. BMI was computed by the following formula: body weight (kg)/height (m)2. WC was evaluated at the end of normal expiration in a standing position by a tape with an accuracy of 0.1 cm above the iliac crest, just below the lowest rib margin [29]. To measure fat and fat-free mass percentage, a direct segmental multi-frequency bioelectrical impedance method (Tanita BC-418; Tanita Corp., Tokyo, Japan) was used.
Dietary analyses
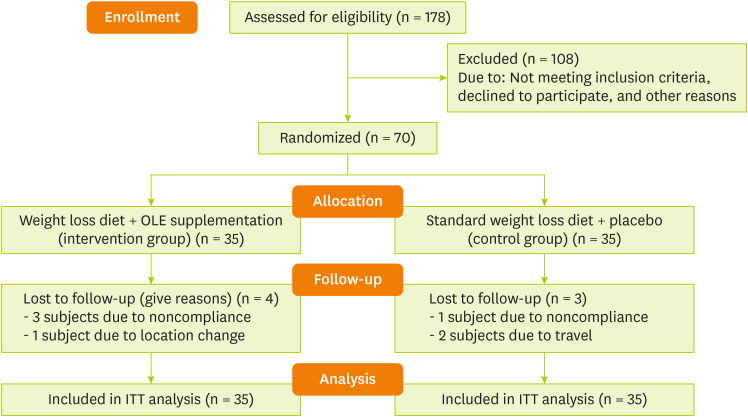
Total energy, macronutrients, and some micronutrients intakes were estimated by Nut IV software (Hearst Corporation, San Bruno, CA, USA). The demographic and physical activity data were obtained through demographic and International Physical Activity Questionnaire (IPAQ) questionnaires, respectively. The Persian translation of the short form IPAQ has been confirmed by Dashti et al. [30] (Cronbach's alpha = 0.7 and test-retest reliability coefficient = 0.9). The CONSORT flow diagram of the present study is exhibited in Figure 1.
Figure 1. CONSORT flow diagram.
OLE, olive leaf extract; ITT, intention-to-treat.
Biochemical analysis
At the baseline and end of the study, 10 mL of venous blood samples (in regular tubes) were obtained after 12 hours of overnight fasting and centrifuged at 1,500 rpm at 4°C for 15–20 minutes and were then stored at −80°C until biochemical analysis. Enzyme-linked immunosorbent assay kits were used to measure insulin (Monobind Inc., Düsseldorf, Germany), leptin (LDN, Nordhorn, Germany), total adiponectin (ZellBio GmbH, Lonsee, Germany), and FFA (Eastbiopharm Co. Ltd., Hangzhou, China) concentration.
TG, high-density lipoprotein cholesterol (HDL-C), LDL-C, total cholesterol (TC), and fasting blood glucose (FBG) were measured using the enzymatic method with kits from Pars-Azmoon (Tehran, Iran). Then, the Visceral Adiposity Index (VAI) based on the following formula [29,30]:
Homeostasis model assessment-insulin resistance (HOMA-IR) was measured by the following formula: fasting glucose (mg/dL) × fasting insulin (μu/mL)/405.
Statistical analysis
IBM SPSS statistics software, version 24) (IBM Corp., Armonk, NY, USA) was applied to conduct all statistical analyses. Kolmogorov-Smirnov test was used to confirm the normality of the variables. To compare parametric continuous data between and within the groups, an independent sample t-test and paired sample t-test were employed, respectively. To evaluate the differences between the two groups at the post-intervention stage, an analysis of covariance (ANCOVA) test was applied. A p value less than 0.05 was regarded to be statistically significant. Intention-to-treat (ITT) analysis was conducted as it was suggested for analysis results in clinical trials.
RESULTS
Four participants in the intervention group (three subjects due to noncompliance and one subject due to location change) and three participants in the placebo group (2 subjects due to travel and one subject due to noncompliance), were lost to follow. But, all the statistical analyses were done using the intention-to-treat principle (placebo group, n = 35 and OLE group, n = 35). The study groups were homogenous regarding the baseline age, height, weight, BMI, WC, married status, and physical activity levels (p > 0.05) (Table 1).
Table 1. Baseline characteristics of study participants.
| Variable | All subjects | Intervention group (n = 35) | Control group (n = 35) | p value* | |
|---|---|---|---|---|---|
| Age (years) | 35.3 ± 9.8 | 35.7 ± 9.1 | 34.9 ± 10.6 | 0.75 | |
| Weight (kg) | 89.3 ± 10.0 | 89.7 ± 10.5 | 88.9 ± 9.7 | 0.72 | |
| Height (cm) | 159.9 ± 5.9 | 160.1 ± 5.9 | 159.7 ± 6.1 | 0.79 | |
| BMI (kg/m2) | 34.8 ± 3.2 | 34.97 ± 3.4 | 34.7 ± 3.1 | 0.82 | |
| WC (cm) | 107.8 ± 8.2 | 108.4 ± 8.1 | 107.2 ± 8.3 | 0.57 | |
| Married status | 0.45 | ||||
| Married | 46 (65.7) | 25 (71.4) | 21 (60) | ||
| Unmarried | 24 (34.3) | 10 (28.5) | 14 (40) | ||
Values are expressed as mean ± standard deviation or number (%).
BMI, body mass index; WC, waist circumference.
*Independent t-test for numeric variables and Pearson's chi-square test for categorical variables.
Dietary intake
At the baseline, there were no significant differences in dietary intake variables between the study groups except for vitamin E (p = 0.025) and vitamin A intake (p = 0.011) (Table 2).
Table 2. Dietary intakes of the study participants at baseline and end of the intervention.
| Variable | Intervention group (n = 35) | Placebo group (n = 35) | p value* | |
|---|---|---|---|---|
| Energy (kcal/day) | ||||
| Baseline | 1,902.0 ± 642.9 | 1,730.2 ± 548.2 | 0.23 | |
| End | 1,306.8 ± 376.6 | 1,264.3 ± 322.5 | 0.62 | |
| p value† | 0.00 | 0.00 | ||
| Carbohydrate (g) | ||||
| Baseline | 226.1 ± 79.1 | 226.4 ± 72.6 | 0.98 | |
| End | 153.2 ± 57.6 | 158.7 ± 39.1 | 0.65 | |
| p value† | 0.00 | 0.00 | ||
| Protein (g) | ||||
| Baseline | 74.0 ± 33.9 | 68.8 ± 20.6 | 0.45 | |
| End | 51.6 ± 21.6 | 47.2 ± 16.7 | 0.36 | |
| p value† | 0.001 | 0.00 | ||
| Fat (g) | ||||
| Baseline | 81.0 ± 38.7 | 67.9 ± 22.2 | 0.08 | |
| End | 55.6 ± 17.9 | 50.6 ± 21.1 | 0.32 | |
| p value† | 0.003 | 0.003 | ||
| SFA (g) | ||||
| Baseline | 18.5 ± 8.9 | 18.2 ± 6.1 | 0.86 | |
| End | 12.9 ± 5.8 | 9.6 ± 5.1 | 0.63 | |
| p value† | 0.00 | 0.005 | ||
| MUFA (g) | ||||
| Baseline | 23.9 ± 14.7 | 22.4 ± 8.3 | 0.61 | |
| End | 16.2 ± 7.3 | 13.5 ± 6.4 | 0.12 | |
| p value† | 0.00 | 0.005 | ||
| PUFA (g) | ||||
| Baseline | 28.6 ± 19.5 | 21.3 ± 9.4 | 0.06 | |
| End | 17.5 ± 9.8 | 14.5 ± 9.5 | 0.23 | |
| p value† | 0.013 | 0.004 | ||
| Dietary fiber (g) | ||||
| Baseline | 9.5 ± 4.1 | 11.8 ± 5.3 | 0.052 | |
| End | 6.3 ± 4.8 | 8.3 ± 3.9 | 0.069 | |
| p value† | 0.002 | 0.014 | ||
| Vitamin A (µg) | ||||
| Baseline | 1,937.6 ± 2,463.3 | 752.0 ± 597.0 | 0.011 | |
| End | 1,181.2 ± 1,992.9 | 1,417.7 ± 1,787.6 | 0.62 | |
| p value† | 0.28 | 0.028 | ||
| Vitamin C (mg) | ||||
| Baseline | 40.3 ± 36.3 | 49.8 ± 37.1 | 0.29 | |
| End | 30.5 ± 25.4 | 43.2 ± 26.4 | 0.06 | |
| p value† | 0.073 | 0.307 | ||
| Vitamin E (mg) | ||||
| Baseline | 32.7 ± 28.6 | 20.5 ± 12.3 | 0.025 | |
| End | 18.7 ± 14.1 | 16.9 ± 15.8 | 0.62 | |
| p value† | 0.017 | 0.115 | ||
Values are expressed as means ± standard deviation. Bold-faced p value < 0.05 was considered as significant.
SFA, saturated fatty acid; PUFA, polyunsaturated fatty acid; MUFA, monounsaturated fatty acid.
*Independent t-test between the two groups at pre-and post-intervention; †Paired t-test.
In both groups, compared to the baseline, energy, protein, carbohydrates, total fat, polyunsaturated fatty acid (PUFA), monounsaturated fatty acid (MUFA), saturated fatty acid (SFA), and fiber intakes reduced significantly (p < 0.05). In the OLE group, vitamin E intake decreased significantly (p = 0.017). In the placebo group, compared to the baseline, vitamin A intake increased (p = 0.028). However, there were no significant differences in dietary intake variables between the study groups at the end of the study (p > 0.05).
Anthropometric indices
The mean scores of subjects' anthropometric indices are illustrated in Table 3. At the baseline, there were no significant differences in anthropometric indices between the study groups (p > 0.05). In the OLE group, compared to baseline all the anthropometric indices were significantly reduced (p < 0.05). In the placebo group, compared to baseline, body weight, BMI, fat mass, and WC decreased significantly (p < 0.05). The results of ANCOVA presented significant decreases in BMI, body weight, and fat mass in the OLE group compared to those in the placebo group (p < 0.05), but regarding muscle mass, WC and VAI no significant differences was observed between the study groups (p > 0.05).
Table 3. Anthropometric indices of participants at baseline and end of the intervention.
| Variable | Intervention group (n = 35) | Placebo group (n = 35) | p value* | p value† | |
|---|---|---|---|---|---|
| Body weight (kg) | |||||
| Before | 88.5 ± 9.9 | 87.8 ± 9.5 | 0.72 | ||
| After | 84.4 ± 9.8 | 84.9 ± 9.9 | 0.81 | 0.01 | |
| p value‡ | 0.00 | 0.00 | |||
| Difference | −4.1 ± 1.8 | −2.8 ± 1.9 | 0.01 | 0.015 | |
| BMI (kg/m2) | |||||
| Before | 34.6 ± 3.2 | 34.6 ± 3.2 | 0.82 | ||
| After | 33.0 ± 3.2 | 33.5 ± 3.2 | 0.57 | 0.016 | |
| p value‡ | 0.00 | 0.00 | |||
| Difference | −1.6 ± 0.7 | −1.1 ± 0.8 | 0.017 | 0.016 | |
| Fat mass (kg) | |||||
| Before | 36.3 ± 6.6 | 35.7 ± 7.3 | 0.56 | ||
| After | 33.7 ± 7.2 | 34.4 ± 7.7 | 0.71 | 0.002 | |
| p value‡ | 0.00 | 0.00 | |||
| Difference | −2.6 ± 1.7 | −1.3 ± 1.6 | 0.003 | 0.002 | |
| Muscle mass (kg) | |||||
| Before | 49.4 ± 4.2 | 49.5 ± 4.7 | 0.90 | ||
| After | 48.7 ± 4.6 | 49.0 ± 4.9 | 0.78 | 0.35 | |
| p value‡ | 0.08 | 0.12 | |||
| Difference | −0.7 ± 1.5 | −0.5 ± 1.9 | 0.54 | 0.35 | |
| WC (cm) | |||||
| Before | 107.6 ± 7.0 | 107.2 ± 8.6 | 0.85 | ||
| After | 103.1 ± 7.6 | 104.4 ± 9.5 | 0.53 | 0.42 | |
| p value‡ | 0.00 | 0.00 | |||
| Difference | −4.4 ± 3.6 | −2.7 ± 3.9 | 0.08 | 0.42 | |
| VAI | |||||
| Baseline | 2.5 ± 0.7 | 2.7 ± 1.2 | 0.44 | ||
| End | 2.3 ± 0.7 | 2.5 ± 0.9 | 0.19 | 0.81 | |
| p value‡ | 0.001 | 0.207 | |||
| Difference | −0.3 ± 0.3 | −0.2 ± 0.5 | 0.46 | 0.53 | |
| Physical activity (MET-min/week) | |||||
| Before | 2,203.0 ± 664.6 | 2,377.4 ± 1,141.4 | 0.43 | ||
| After | 2,366.2 ± 400.1 | 2,601.10 ± 719.6 | 0.13 | 0.68 | |
| p value‡ | 0.29 | 0.23 | |||
| Difference | 154.29 ± 630.3 | −98.71 ± 472.4 | 0.095 | 0.25 | |
BMI, body mass index; WC, waist circumference; VAI, Visceral Adiposity Index.
*Independent t-test between the 2 groups at pre-and post-intervention; †Analysis of covariance (adjusted for age, physical activity, dietary intake of energy, macronutrients, antioxidant vitamins such as vitamins A, C, and E, and corresponding baseline value); ‡Paired t-test.
Biochemical measurements
As shown in Table 4, at the baseline, no significant difference was observed between the OLE and placebo groups regarding the biochemical measurements (p > 0.05).
Table 4. Biochemical parameter values of the study groups at baseline and at the end of the intervention.
| Variable | Intervention group (n = 31) | Placebo group (n = 32) | p value* | p value† | |
|---|---|---|---|---|---|
| FBS (mg/dL) | |||||
| Baseline | 86.6 ± 11.9 | 88.4 ± 17.7 | 0.64 | ||
| End | 82.3 ± 13.4 | 87.9 ± 18.3 | 0.17 | 0.00 | |
| p value‡ | 0.001 | 0.512 | |||
| Difference | −4.3 ± 8.1 | −0.5 ± 7.9 | 0.06 | 0.02 | |
| TG (mg/dL) | |||||
| Baseline | 122.0 ± 30.5 | 124.5 ± 40.5 | 0.78 | ||
| End | 117.0 ± 33.8 | 122.5 ± 37.8 | 0.55 | 0.73 | |
| p value‡ | 0.073 | 0.53 | |||
| Difference | −5.0 ± 14.9 | −2.0 ± 18.7 | 0.50 | 0.73 | |
| TC (mg/dL) | |||||
| Baseline | 164.0 ± 20.6 | 158.5 ± 25.9 | 0.35 | ||
| End | 153.5 ± 16.1 | 156.0 ± 23.1 | 0.62 | 0.043 | |
| p value‡ | 0.00 | 0.00 | |||
| Difference | −10.5 ± 13.9 | −2.5 ± 13.9 | 0.025 | 0.039 | |
| HDL-C (mg/dL) | |||||
| Baseline | 42.3 ± 5.9 | 40.9 ± 6.7 | 0.38 | ||
| End | 44.6 ± 6.3 | 42.1 ± 6.2 | 0.12 | 0.32 | |
| p value‡ | 0.00 | 0.00 | |||
| Difference | 2.3 ± 1.9 | 1.2 ± 2.9 | 0.083 | 0.32 | |
| LDL-C (mg/dL) | |||||
| Baseline | 97.3 ± 19.4 | 92.6 ± 22.9 | 0.38 | ||
| End | 85.4 ± 16.2 | 89.3 ± 19.8 | 0.39 | 0.016 | |
| p value‡ | 0.020 | 0.038 | |||
| Difference | −11.8 ± 13.9 | −3.2 ± 14.1 | 0.01 | 0.016 | |
| Leptin (µg/L) | |||||
| Baseline | 53.28 ± 13.41 | 58.6 ± 17.0 | 0.17 | ||
| End | 42.8 ± 9.9 | 54.8 ± 12.9 | 0.003 | 0.014 | |
| p value‡ | 0.038 | 0.011 | |||
| Difference | −10.5 ± 12.6 | −3.7 ± 17.0 | 0.07 | 0.016 | |
| Adiponectin (ng/mL) | |||||
| Baseline | 7.5 ± 2.9 | 7.0 ± 2.4 | 0.45 | ||
| End | 9.4 ± 2.9 | 7.4 ± 2.7 | 0.004 | 0.041 | |
| p value‡ | 0.02 | 0.312 | |||
| Difference | 2.0 ± 3.1 | 0.4 ± 3.2 | 0.049 | 0.04 | |
| Insulin (μIU/mL) | |||||
| Baseline | 12.0 ± 8.7 | 12.6 ± 8.3 | 0.76 | ||
| End | 8.9 ± 6.5 | 10.7 ± 8.0 | 0.33 | 0.34 | |
| p value‡ | 0.004 | 0.055 | |||
| Difference | −3.1 ± 5.5 | −1.9 ± 5.4 | 0.40 | 0.76 | |
| HOMA-IR | |||||
| Baseline | 2.6 ± 2.1 | 3.0 ± 2.7 | 0.53 | ||
| End | 1.8 ± 1.4 | 2.5 ± 2.6 | 0.18 | 0.11 | |
| p value‡ | 0.003 | 0.044 | |||
| Difference | −0.7 ± 1.3 | −0.4 ± 1.2 | 0.32 | 0.66 | |
| FFA (µg/mL) | |||||
| Baseline | 246.7 ± 13.9 | 259.5 ± 18.7 | 0.22 | ||
| End | 193.2 ± 10.1 | 227.30 ± 16.4 | 0.00 | 0.00 | |
| p value‡ | 0.00 | 0.00 | |||
| Difference | −71.47 ± 3.7 | −32.26 ± 2.3 | < 0.001 | < 0.001 | |
Values are expressed as means ± standard deviation. Bold-faced p values < 0.05 was considered as significant.
FBS, fasting blood sugar; TG, triglyceride; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; HOMA-IR, homeostatic model assessment-insulin resistance; FFA, free fatty acids; ANCOVA, analysis of covariance.
*Independent t-test; †Covariance (ANCOVA) (adjusted for age, physical activity, dietary intake of energy, macronutrients, antioxidant vitamins such as vitamins A, C, and E, BMI, and corresponding baseline value); ‡Paired t-test.
At the end of the study, the blood concentration of FBS, TC, LDL-C, leptin, insulin, FFA, and HOMA-IR significantly decreased and serum levels of adiponectin and HDL-C elevated in the OLE group (p < 0.05). In the placebo group, at the end of the study, serum levels of TC, leptin, HOMA-IR, and FFA significantly decreased, and HDL-C elevated compared to baseline (p < 0.05). The mean difference in the FBS (p = 0.02), TC (p = 0.01), LDL (p = 0.004), leptin (p = 0.014), adiponectin (p = 0.041), and FFA (p < 0.001) between the 2 OLE and placebo groups after OLE supplementation was significant (tested by ANCOVA after adjusting for covariates).
DISCUSSION
The findings of this 8-weeks clinical trial showed that oral supplementation of OLE with a weight-loss diet in obese women resulted in significant reduction in body weight, BMI, fat mass, and serum levels of FBS, TC, LDL-C, leptin, and FFA, and significant increase in adiponectin serum level.
Positive energy balance under constant conditions increases the accumulation of fat and body weight and thus induces obesity [25]. Studies have shown that weight loss interventions (diet, supplements, etc.) can improve metabolic parameters such as glycemic status and lipid profile and reduce the mortality rate in obese adults by reducing only 5% to 10% of body weight [31,32]. Agreeing to the findings of this clinical trial, a cohort study showed the incidence of obesity was lower in individuals who consumed olive oil [33]. Various animal studies have shown that the use of OLE or oleuropein supplement has reduced body weight, fat mass, and expression of genes involved in adipogenesis and lipogenesis in obese mice on a high-fat diet [34,35,36]. Contrary to the results of the present study, a clinical trial by Araki et al. showed that consumption of 330 mL of olive leaf tea three times per day for 12 weeks in pre-diabetic individuals with BMI range between 23 to 29 kg/m2 had no significant effect on weight loss and WC. Nevertheless, a significant reduction was seen in WC when the analysis was limited to individuals with abdominal obesity [37]. According to the results, it can be said that the difference in BMI and intervention on obese people in this study (BMI ≥ 30 kg/m2) is one of the important reasons for the difference in findings. On the other hand, the type of intervention and the use of diet along with supplementation can also be another possible reason for better results in this study. In another crossover trial by de Bock et al. [38], it was found that the use of supplement containing OLE for 12 weeks had no a significant effect on body composition in middle-aged overweight men. Here again, it can be said that intervention on overweight people and lack of high BMI in these people, the use of supplement without diet and difference in the composition of the 2 supplements are important possible reasons for the lack of conclusion. In this study, there was also a small reduction in fat-free mass. However, this reduction in fat-free mass was not clinically significant compared to weight loss. Physiologically, 75% of the weight loss that occurs during a low-calorie diet is related to fat mass, and only 25% is related to fat-free mass. Therefore, it is possible to prevent excessive degradation of fat-free mass by using olive oil along with a weight-loss diet. A similar study by Rezaei et al. [39] reported that the use of olive oil (20 g/day) in combination with a weight loss diet (−500 kcal/day) for 12 weeks could prevent from significant decrease in fat free mass.
According to recent evidence, one possible mechanism is that olive leaf compounds improve obesity by reducing the accumulation of intracellular fat in the cell line of 3T3-L1 pre-adipocyte [23,40]. Another effect of OLE on obesity is to inhibit the adipogenesis process by inhibiting the conversion of pre-adipocyte to mature adipocyte (differentiation) [41]. In this process of differentiation, CCAAT-enhancer-binding proteins and peroxisome proliferator-activated receptor gamma (PPAR-γ) play a key role as transcription factors [42]. Therefore, OLE shows its anti-obesity effect by reducing the gene expression of these two transcription factors [34]. It has been shown that changes in the composition of the gut microbiota such as reduction of bacterial diversity and upsetting the balance between beneficial and harmful bacteria (decrease in Bacteroidetes and increase in Firmicutes) occur in obese individuals and the number of harmful bacteria increases in them [43]. Also, an animal study showed that germ-free mice extracted more energy from food and their body fat mass was significantly higher when were colonized with a microbiota from obese mice compared to when they were colonized with a microbiota from lean mice [44]. So, OLE maintains the balance of microbiota, reduces intestinal permeability, and in this way can also affect obesity [8,45].
Obesity is associated with hyperglycemia, disturbance in lipid metabolism, and increase of insulin resistance [8]. Olive leaves are rich in many phenolic compounds, one of the most abundant of these compounds is oleuropein) approximately 80%) [46]. Studies have shown that OLE improves glucose metabolism [37,47]. In line with the findings of the present study, a clinical trial by Wainstein et al. [48] described that consumption of OLE (one tablet of 500 mg/day) for 14 weeks resulted in improvement in fasting serum levels of insulin and HbA1c in type 2 diabetes mellitus patients. In the study of Wainstein, the supplementation dose and duration of intervention were higher. But in the present study, it seems that the weight loss diet could help supplementation in improving metabolic parameters. In another study, supplementation with OLE demonstrated a 28% improvement in pancreatic B-cell responsiveness and a 15% improvement in insulin sensitivity in middle-aged overweight men [38]. On the contrary, Rezaei et al. [39] showed that the supplementation with olive oil (20 g/day) in combination with a weight loss diet (−500 kcal/day) for 12 weeks could not induce a significant reduction in the serum levels of FBG, insulin, and HOMA-IR in patients with non-alcoholic fatty liver disease. Initial normal levels of data, different type of intervention (use of olive oil instead of extract) and different target population can be the reasons for different results in the two studies.
One of the mechanisms proposed for the hypoglycemic effects of OLE is the prevention of glucose uptake and starch digestion and also stimulation of the synthesis of hepatic glycogen. Another anti-glycemic effect is inhibition of pancreatic α-amylase activity [49]. Additionally, plasma levels of insulin-like growth factor-binding protein 1 (IGFBP-1) and insulin-like growth factor-binding protein 2 (IGFBP-2) are increased by OLE [49]. There is an inverse relationship between serum levels of insulin and IGFBP-1 concentrations. Also, increase of IGFBP-2 concentration leads to improve insulin sensitivity and prevent obesity [50].
Hypolipidemic effects of OLE have been reported in mice were fed a high-fat diet [8,35] and in subjects with hypercholesterolemia [51]. In line with the findings of this study, Lockyer et al. [24] found 6 weeks receiving phenolic-rich OLE containing 136 mg of oleuropein significantly decreased the serum levels of TG, TC, and LDL-C in prehypertensive male. In another study, daily supplementation with 100 mg oleuropein for 12 months resulted in a significant decrease in serum levels of LDL-C and TC in postmenopausal women, but TG and HDL-C were not significantly changed compared to the control group [52 ]. Normal baseline levels of TG and HDL-C and different design study could be the possible reasons for the difference in results. Contrary to that observed in this clinical trial, de Bock et al. [38] reported supplementation with OLE had no significant effect on lipid profile in middle-aged overweight men. Relatively normal levels of lipid profile, different target population, different type of intervention and different dose of supplementation are possible causes of insignificance. One of the mechanisms of effect on the lipid profile is that OLE reduces the activity of the hydroxymethylglutaryl-CoA reductase enzyme (rate-limiting enzyme of cholesterol synthesis) and inhibits the production of cholesterol. OLE also reduces the synthesis of Sterol regulatory element-binding transcription factor 1 and PPAR-γ and through it can prevent the increase of serum fatty acids and the synthesis of triglycerides [25].
Obesity has been shown to be associated with high levels of serum free fatty acids and impaired secretion of various adipokines such as leptin and adiponectin from adipose tissue [53]. Leptin is involved in the regulation of food intake and energy expenditure [54]. Studies have shown that serum levels of leptin increase in obese individuals and leading to the induction of insulin resistance. On the other hand, serum levels of adiponectin (role in inducing insulin sensitivity and improving lipid profile) reduce in these individuals [55,56 ]. Therefore, considering the relationship between leptin and adiponectin adipokines with metabolic factors, it can probably be said that the improvement of obesity through various interventions with an effect on these two adipokines can play a positive role in improving metabolic parameters. In confirmation of this subject, the study by Hotta et al. [57] showed that weight loss increased plasma levels of adiponectin in diabetic patients. Also, an inverse relationship was seen between adiponectin levels with glucose levels and fasting plasma insulin [57]. Similar to the beneficial effects of the OLE in this study, it was shown that supplementation with OLE significantly decreased serum levels of free fatty acids and leptin and increased serum levels of adiponectin in obese mice fed a high-fat diet [34]. Also, Mediterranean dietary patterns (rich in virgin olive oil) have been independently and positively associated with plasma levels of adiponectin and the oleic acid (monounsaturated fatty acid) in olive oil can affect the gene expression of leptin and adiponectin [58].
Therefore, it can be suggested one possible mechanism is that OLE improves glycemic status, lipid profile, and serum levels of free fatty acids by reduction of body weight, increasing serum levels of adiponectin, and decrease of serum leptin. Also, the use of a weight loss diet along with supplementation may help to enhance these effects.
To the best of the authors' knowledge, this is the first study evaluating the effects of OLE supplementation with a weight loss diet in obese women. Also, reporting the mean changes and presenting the results in the two forms of the crude model (without adjustment for confounding factors) and adjusted model (with eliminating the effect of confounding factors) increased the accuracy of the results. One of the limitations of this trial was the selection of only 2 groups. Therefore, it is suggested to design other studies with 4 study groups in the future (group 1, weight loss diet with placebo; group 2, weight loss diet with the supplement; group 3, placebo; group 4, supplement), also trials with more intervention time could be suggested for future studies.
CONCLUSION
In conclusion, based on results it seems that 8-week OLE supplementation along with a weight loss diet compared with a weight-loss diet alone may be more effective in improving obesity and metabolic risk factors.
ACKNOWLEDGEMENTS
This study was a part of the Ph.D. thesis of Forough Shayesteh. The authors express thanks to the Nutrition and Metabolic Disorders Research Center, and Research Center for Diabetes, and all the individuals who participated in this study.
Footnotes
Funding: This research was supported by grants from Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran (NRC-9901).
Conflict of Interests: The authors declare that they have no competing interests.
References
- 1.Atawia RT, Bunch KL, Toque HA, Caldwell RB, Caldwell RW. Mechanisms of obesity-induced metabolic and vascular dysfunctions. Front Biosci (Landmark Ed) 2019;24:890–934. doi: 10.2741/4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castaner O, Goday A, Park YM, Lee SH, Magkos F, Shiow STE, Schröder H. The gut microbiome profile in obesity: a systematic review. Int J Endocrinol. 2018;2018:4095789. doi: 10.1155/2018/4095789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390:2627–2642. doi: 10.1016/S0140-6736(17)32129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson WG, Cook DA, Clark MM, Bardia A, Levine JA. Treatment of obesity. Mayo Clin Proc. 2007;82:93–101. doi: 10.4065/82.1.93. [DOI] [PubMed] [Google Scholar]
- 5.Alamout MM, Rahmanian M, Aghamohammadi V, Mohammadi E, Nasiri K. Effectiveness of mindfulness based cognitive therapy on weight loss, improvement of hypertension and attentional bias to eating cues in overweight people. Int J Nurs Sci. 2019;7:35–40. doi: 10.1016/j.ijnss.2019.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bray GA, Tartaglia LA. Medicinal strategies in the treatment of obesity. Nature. 2000;404:672–677. doi: 10.1038/35007544. [DOI] [PubMed] [Google Scholar]
- 7.Fki I, Sayadi S, Mahmoudi A, Daoued I, Marrekchi R, Ghorbel H. Comparative study on beneficial effects of hydroxytyrosol- and oleuropein-rich olive leaf extracts on high-fat diet-induced lipid metabolism disturbance and liver injury in rats. Biomed Res Int. 2020;2020:1315202. doi: 10.1155/2020/1315202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vezza T, Rodríguez-Nogales A, Algieri F, Garrido-Mesa J, Romero M, Sánchez M, Toral M, Martín-García B, Gómez-Caravaca AM, Arráez-Román D, Segura-Carretero A, Micol V, García F, Utrilla MP, Duarte J, Rodríguez-Cabezas ME, Gálvez J. The metabolic and vascular protective effects of olive (Olea europaea L.) leaf extract in diet-induced obesity in mice are related to the amelioration of gut microbiota dysbiosis and to its immunomodulatory properties. Pharmacol Res. 2019;150:104487. doi: 10.1016/j.phrs.2019.104487. [DOI] [PubMed] [Google Scholar]
- 9.Koca U, Süntar I, Akkol EK, Yilmazer D, Alper M. Wound repair potential of Olea europaea L. leaf extracts revealed by in vivo experimental models and comparative evaluation of the extracts' antioxidant activity. J Med Food. 2011;14:140–146. doi: 10.1089/jmf.2010.0039. [DOI] [PubMed] [Google Scholar]
- 10.Lee OH, Lee BY, Lee J, Lee HB, Son JY, Park CS, Shetty K, Kim YC. Assessment of phenolics-enriched extract and fractions of olive leaves and their antioxidant activities. Bioresour Technol. 2009;100:6107–6113. doi: 10.1016/j.biortech.2009.06.059. [DOI] [PubMed] [Google Scholar]
- 11.Soliman GA, Saeedan AS, Abdel-Rahman RF, Ogaly HA, Abd-Elsalam RM, Abdel-Kader MS. Olive leaves extract attenuates type II diabetes mellitus-induced testicular damage in rats: molecular and biochemical study. Saudi Pharm J. 2019;27:326–340. doi: 10.1016/j.jsps.2018.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flemmig J, Kuchta K, Arnhold J, Rauwald HW. Olea europaea leaf (Ph.Eur.) extract as well as several of its isolated phenolics inhibit the gout-related enzyme xanthine oxidase. Phytomedicine. 2011;18:561–566. doi: 10.1016/j.phymed.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 13.Efentakis P, Iliodromitis EK, Mikros E, Papachristodoulou A, Dagres N, Skaltsounis AL, Andreadou I. Effects of the olive tree leaf constituents on myocardial oxidative damage and atherosclerosis. Planta Med. 2015;81:648–654. doi: 10.1055/s-0035-1546017. [DOI] [PubMed] [Google Scholar]
- 14.Susalit E, Agus N, Effendi I, Tjandrawinata RR, Nofiarny D, Perrinjaquet-Moccetti T, Verbruggen M. Olive (Olea europaea) leaf extract effective in patients with stage-1 hypertension: comparison with Captopril. Phytomedicine. 2011;18:251–258. doi: 10.1016/j.phymed.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 15.Zari TA, Al-Attar AM. Therapeutic effects of olive leaves extract on rats treated with a sublethal concentration of carbendazim. Eur Rev Med Pharmacol Sci. 2011;15:413–426. [PubMed] [Google Scholar]
- 16.Al-Attar AM, Alsalmi FA. Effect of Olea europaea leaves extract on streptozotocin induced diabetes in male albino rats. Saudi J Biol Sci. 2019;26:118–128. doi: 10.1016/j.sjbs.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salah MB, Abdelmelek H, Abderraba M. Study of phenolic composition and biological activities assessment of olive leaves from different varieties grown in Tunisia. Med Chem. 2012;2:107–111. [Google Scholar]
- 18.Tabera J, Guinda A, Ruiz-Rodríguez A, Señoráns FJ, Ibáñez E, Albi T, Reglero G. Countercurrent supercritical fluid extraction and fractionation of high-added-value compounds from a hexane extract of olive leaves. J Agric Food Chem. 2004;52:4774–4779. doi: 10.1021/jf049881+. [DOI] [PubMed] [Google Scholar]
- 19.Japón-Luján R, Luque-Rodríguez JM, Luque de Castro MD. Dynamic ultrasound-assisted extraction of oleuropein and related biophenols from olive leaves. J Chromatogr A. 2006;1108:76–82. doi: 10.1016/j.chroma.2005.12.106. [DOI] [PubMed] [Google Scholar]
- 20.Shen Y, Song SJ, Keum N, Park T. Olive leaf extract attenuates obesity in high-fat diet-fed mice by modulating the expression of molecules involved in adipogenesis and thermogenesis. Evid Based Complement Alternat Med. 2014;2014:971890. doi: 10.1155/2014/971890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim Y, Choi Y, Park T. Hepatoprotective effect of oleuropein in mice: mechanisms uncovered by gene expression profiling. Biotechnol J. 2010;5:950–960. doi: 10.1002/biot.201000068. [DOI] [PubMed] [Google Scholar]
- 22.Hsu CL, Wu CH, Huang SL, Yen GC. Phenolic compounds rutin and o-coumaric acid ameliorate obesity induced by high-fat diet in rats. J Agric Food Chem. 2009;57:425–431. doi: 10.1021/jf802715t. [DOI] [PubMed] [Google Scholar]
- 23.Cho AS, Jeon SM, Kim MJ, Yeo J, Seo KI, Choi MS, Lee MK. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem Toxicol. 2010;48:937–943. doi: 10.1016/j.fct.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Roriz AK, Passos LC, de Oliveira CC, Eickemberg M, Moreira PA, Sampaio LR. Evaluation of the accuracy of anthropometric clinical indicators of visceral fat in adults and elderly. PLoS One. 2014;9:e103499. doi: 10.1371/journal.pone.0103499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lean ME. Management of obesity and overweight. Medicine. 2019;47:175–183. [Google Scholar]
- 26.Lockyer S, Rowland I, Spencer JP, Yaqoob P, Stonehouse W. Impact of phenolic-rich olive leaf extract on blood pressure, plasma lipids and inflammatory markers: a randomised controlled trial. Eur J Nutr. 2017;56:1421–1432. doi: 10.1007/s00394-016-1188-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51:241–247. doi: 10.1093/ajcn/51.2.241. [DOI] [PubMed] [Google Scholar]
- 28.Haidari F, Aghamohammadi V, Mohammadshahi M, Ahmadi-Angali K. Effect of whey protein supplementation on levels of endocannabinoids and some of metabolic risk factors in obese women on a weight-loss diet: a study protocol for a randomized controlled trial. Nutr J. 2017;16:70. doi: 10.1186/s12937-017-0294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haidari F, Aghamohammadi V, Mohammadshahi M, Ahmadi-Angali K, Asghari-Jafarabadi M. Whey protein supplementation reducing fasting levels of anandamide and 2-AG without weight loss in pre-menopausal women with obesity on a weight-loss diet. Trials. 2020;21:657. doi: 10.1186/s13063-020-04586-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dashti S, Su TT, Esfehani AJ, Esfehani RJ. Effect of physical activity level on emotional status of Iranian women. World Appl Sci J. 2014;30:852–857. [Google Scholar]
- 31.Wing RR, Lang W, Wadden TA, Safford M, Knowler WC, Bertoni AG, Hill JO, Brancati FL, Peters A, Wagenknecht L, Look AHEAD Research Group Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481–1486. doi: 10.2337/dc10-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma C, Avenell A, Bolland M, Hudson J, Stewart F, Robertson C, Sharma P, Fraser C, MacLennan G. Effects of weight loss interventions for adults who are obese on mortality, cardiovascular disease, and cancer: systematic review and meta-analysis. BMJ. 2017;359:j4849. doi: 10.1136/bmj.j4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soriguer F, Almaraz MC, Ruiz-de-Adana MS, Esteva I, Linares F, García-Almeida JM, Morcillo S, García-Escobar E, Olveira-Fuster G, Rojo-Martínez G. Incidence of obesity is lower in persons who consume olive oil. Eur J Clin Nutr. 2009;63:1371–1374. doi: 10.1038/ejcn.2009.65. [DOI] [PubMed] [Google Scholar]
- 34.Jung YC, Kim HW, Min BK, Cho JY, Son HJ, Lee JY, Kim JY, Kwon SB, Li Q, Lee HW. Inhibitory effect of olive leaf extract on obesity in high-fat diet-induced mice. In Vivo. 2019;33:707–715. doi: 10.21873/invivo.11529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Stelt I, Hoek-van den Hil EF, Swarts HJ, Vervoort JJ, Hoving L, Skaltsounis L, Lemonakis N, Andreadou I, van Schothorst EM, Keijer J. Nutraceutical oleuropein supplementation prevents high fat diet-induced adiposity in mice. J Funct Foods. 2015;14:702–715. [Google Scholar]
- 36.Hadrich F, Garcia M, Maalej A, Moldes M, Isoda H, Feve B, Sayadi S. Oleuropein activated AMPK and induced insulin sensitivity in C2C12 muscle cells. Life Sci. 2016;151:167–173. doi: 10.1016/j.lfs.2016.02.027. [DOI] [PubMed] [Google Scholar]
- 37.Araki R, Fujie K, Yuine N, Watabe Y, Nakata Y, Suzuki H, Isoda H, Hashimoto K. Olive leaf tea is beneficial for lipid metabolism in adults with prediabetes: an exploratory randomized controlled trial. Nutr Res. 2019;67:60–66. doi: 10.1016/j.nutres.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 38.de Bock M, Derraik JG, Brennan CM, Biggs JB, Morgan PE, Hodgkinson SC, Hofman PL, Cutfield WS. Olive (Olea europaea L.) leaf polyphenols improve insulin sensitivity in middle-aged overweight men: a randomized, placebo-controlled, crossover trial. PLoS One. 2013;8:e57622. doi: 10.1371/journal.pone.0057622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rezaei S, Akhlaghi M, Sasani MR, Barati Boldaji R. Olive oil lessened fatty liver severity independent of cardiometabolic correction in patients with non-alcoholic fatty liver disease: a randomized clinical trial. Nutrition. 2019;57:154–161. doi: 10.1016/j.nut.2018.02.021. [DOI] [PubMed] [Google Scholar]
- 40.Drira R, Chen S, Sakamoto K. Oleuropein and hydroxytyrosol inhibit adipocyte differentiation in 3 T3-L1 cells. Life Sci. 2011;89:708–716. doi: 10.1016/j.lfs.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 41.Siersbæk R, Nielsen R, Mandrup S. Transcriptional networks and chromatin remodeling controlling adipogenesis. Trends Endocrinol Metab. 2012;23:56–64. doi: 10.1016/j.tem.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 42.Matsubara T, Takakura N, Urata M, Muramatsu Y, Tsuboi M, Yasuda K, Addison WN, Zhang M, Matsuo K, Nakatomi C, Shigeyama-Tada Y, Kaneuji T, Nakamichi A, Kokabu S. Geranylgeraniol induces PPARγ expression and enhances the biological effects of a PPARγ agonist in adipocyte lineage cells. In Vivo. 2018;32:1339–1344. doi: 10.21873/invivo.11384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davis CD. The gut microbiome and its role in obesity. Nutr Today. 2016;51:167–174. doi: 10.1097/NT.0000000000000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 45.Bouter KE, van Raalte DH, Groen AK, Nieuwdorp M. Role of the gut microbiome in the pathogenesis of obesity and obesity-related metabolic dysfunction. Gastroenterology. 2017;152:1671–1678. doi: 10.1053/j.gastro.2016.12.048. [DOI] [PubMed] [Google Scholar]
- 46.Park S, Choi Y, Um SJ, Yoon SK, Park T. Oleuropein attenuates hepatic steatosis induced by high-fat diet in mice. J Hepatol. 2011;54:984–993. doi: 10.1016/j.jhep.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 47.Jemai H, El Feki A, Sayadi S. Antidiabetic and antioxidant effects of hydroxytyrosol and oleuropein from olive leaves in alloxan-diabetic rats. J Agric Food Chem. 2009;57:8798–8804. doi: 10.1021/jf901280r. [DOI] [PubMed] [Google Scholar]
- 48.Wainstein J, Ganz T, Boaz M, Bar Dayan Y, Dolev E, Kerem Z, Madar Z. Olive leaf extract as a hypoglycemic agent in both human diabetic subjects and in rats. J Med Food. 2012;15:605–610. doi: 10.1089/jmf.2011.0243. [DOI] [PubMed] [Google Scholar]
- 49.Komaki E, Yamaguchi S, Maru I, Kinoshita M, Kakehi K, Ohta Y, Tsukada Y. Identification of anti-α-amylase components from olive leaf extracts. Food Sci Technol Res. 2003;9:35–39. [Google Scholar]
- 50.Heald AH, Cruickshank JK, Riste LK, Cade JE, Anderson S, Greenhalgh A, Sampayo J, Taylor W, Fraser W, White A, Gibson JM. Close relation of fasting insulin-like growth factor binding protein-1 (IGFBP-1) with glucose tolerance and cardiovascular risk in two populations. Diabetologia. 2001;44:333–339. doi: 10.1007/s001250051623. [DOI] [PubMed] [Google Scholar]
- 51.Fonolla J, Diaz-Ropero P, de la Fuente E, Quintela J. MS358. One-month consumption of an olive leaf extract enhances cardiovascular status in hypercholesterolemic subjects. Atheroscler Suppl. 2010;11:182. [Google Scholar]
- 52.Filip R, Possemiers S, Heyerick A, Pinheiro I, Raszewski G, Davicco MJ, Coxam V. Twelve-month consumption of a polyphenol extract from olive (Olea europaea) in a double blind, randomized trial increases serum total osteocalcin levels and improves serum lipid profiles in postmenopausal women with osteopenia. J Nutr Health Aging. 2015;19:77–86. doi: 10.1007/s12603-014-0480-x. [DOI] [PubMed] [Google Scholar]
- 53.Zaki M, Hussein J, Ibrahim AM, Youness ER. Circulating plasma free fatty acids, insulin resistance and metabolic markers in obese women. Biomed Pharmacol J. 2020;13:1595–1600. [Google Scholar]
- 54.Jafari-Vayghan H, Tarighat-Esfanjani A, Jafarabadi MA, Ebrahimi-Mameghani M, Ghadimi SS, Lalezadeh Z. Association between dietary patterns and serum leptin-to-adiponectin ratio in apparently healthy adults. J Am Coll Nutr. 2015;34:49–55. doi: 10.1080/07315724.2014.880389. [DOI] [PubMed] [Google Scholar]
- 55.Ghadge AA, Khaire AA. Leptin as a predictive marker for metabolic syndrome. Cytokine. 2019;121:154735. doi: 10.1016/j.cyto.2019.154735. [DOI] [PubMed] [Google Scholar]
- 56.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 57.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K, Nishida M, Kihara S, Sakai N, Nakajima T, Hasegawa K, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Hanafusa T, Matsuzawa Y. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 58.Scoditti E, Massaro M, Carluccio MA, Pellegrino M, Wabitsch M, Calabriso N, Storelli C, De Caterina R. Additive regulation of adiponectin expression by the Mediterranean diet olive oil components oleic acid and hydroxytyrosol in human adipocytes. PLoS One. 2015;10:e0128218. doi: 10.1371/journal.pone.0128218. [DOI] [PMC free article] [PubMed] [Google Scholar]