Abstract
Background
High-risk features, such as T4 disease, bowel obstruction, poorly/undifferentiated histology, lymphovascular, perineural invasion, and <12 lymph nodes sampled, indicate poor prognosis and define high-risk stage II disease in proficient mismatch repair stage II colon cancer (CC). The prognostic role of high-risk features in dMMR/MSI-H stage II CC is unknown. Similarly, the role of adjuvant therapy in high-risk stage II CC with dMMR/MSI-H (≥1 high-risk feature) has not been studied in prospective trials. The aim of this analysis of the National Cancer Database is to evaluate the prognostic value of high-risk features in stage II dMMR/MSI-H CC.
Methods
Univariate (UVA) and multivariate (MVA) Cox proportional hazards (Cox-PH) models were built to assess the association between clinical and demographic characteristics and overall survival. Kaplan–Meier survival curves were generated with log-rank tests to evaluate the association between adjuvant chemotherapy in high-risk and low-risk cohorts separately.
Results
A total of 2,293 stage II CC patients have dMMR/MSI-H; of those, 29.5% (n = 676) had high-risk features. The high-risk dMMR/MSI-H patients had worse overall survival [5-year survival and 95%CI, 73.2% (67.3–78.1%) vs. 80.3% (76.7–83.5%), p = 0.0001]. In patients with stage II dMMR/MSI-H CC, the high-risk features were associated with shorter overall survival (OS) along with male sex, positive carcinoembryonic antigen, Charlson–Deyo score >1, and older age. Adjuvant chemotherapy administration was associated with better OS, regardless of the high-risk features in dMMR/MSI-H (log-rank test, p = 0.001) or not (p = 0.0006). When stratified by age, the benefit of chemotherapy was evident only in patients age ≥65 with high-risk features.
Conclusion
High-risk features are prognostic in the setting of dMMR/MSI-H stage II CC. Adjuvant chemotherapy may improve survival specifically in patients ≥65 years and with high-risk features.
Keywords: high risk, stage II, adjuvant chemotherapy, colon, cancer
Introduction
Colorectal cancer is the third most common cancer and the second leading cause of cancer-related mortality in the United States. It is estimated that 104,270 new cases of colon cancer (CC) will be diagnosed in 2021 in the US (1). Approximately 28% of patients with CC have stage II disease at presentation (2). The risk stratification of patients with stage II CC is dependent on molecular and clinicopathologic features. A prognostic role of high-risk features, such as T4 disease, bowel obstruction, poorly/undifferentiated histology, lymphovascular, perineural invasion, and <12 lymph nodes sampled, is well established, and high-risk features increase the risk of cancer recurrence and the benefit from adjuvant therapy in patients with microsatellite stable disease stage II CC (3–5). A subgroup of high-risk patients with stage II CC with T4 disease may have a statistically inferior survival compared to those with stage IIIa tumors (6). Adjuvant chemotherapy improves progression-free survival (PFS) and overall survival (OS) in stages II and III CC (7, 8). The benefit of adjuvant therapy in stage II CC is relatively small, and as such, it is not routinely administered (9). Patient preferences, treatment-related toxicities, and the risk characteristics of the tumor are considered in treatment decisions regarding adjuvant therapy.
Approximately 15% of colorectal cancers (CRCs) are dMMR/MSI-H, and patients with dMMR/MSI-H colon cancer are more likely to have a stage II disease (10). Mismatch repair (MMR) proteins are nuclear enzymes that bind to areas of abnormal DNA and repair base–base mismatch during cellular proliferation and division (11). Defects in DNA mismatch repair genes (MLH1, MSH2, MSH6, and PMS2) can lead to insertion or deletion of repeating nucleotide sequences in a process known as microsatellite instability (MSI). One third of these dMMR/MSI-H CC cases are inherited, known as Lynch syndrome carriers, and the rest are sporadic. MLH1 is considered the most commonly affected in the sporadic cases, which is more common in older patients and associated with BRAF V600E mutation (12). The microsatellite instability status of a tumor impacts the prognosis and benefit to adjuvant chemotherapy in patients with stage II CC (10–12). Multiple retrospective studies have shown that stage II patients with dMMR/MSI-H CC have a reduced metastatic potential and a more favorable prognosis compared to those with proficient mismatch repair (pMMR) tumors (13–16). In addition, previous retrospective studies of stages II and III colon cancer patients, analyzing data from randomized adjuvant therapy clinical trials, showed that stage II colon cancer patients with dMMR/MSI-H status did not benefit from adjuvant 5-FU-based chemotherapy (3, 10, 12, 17). Furthermore, Sargent et al. showed a decrease in overall survival (hazard ratio, 2.95; 95%CI, 1.02 to 8.54; p = 0.04) in dMMR/MSI-H stage II patients who were treated with single-agent 5FU compared to surgery alone (10). Whether high-risk features are prognostic in patients with dMMR/MSI-H stage II CC is not well established. The role of adjuvant chemotherapy in patients with high-risk dMMR/MSI-H stage II CC is not well defined. This study aimed to evaluate the prognostic value of high-risk features in dMMR/MSI-H stage II CC and their impact on adjuvant chemotherapy in high-risk stage II CC with dMMR/MSI-H.
Materials and Methods
Patient Selection
The National Cancer Database (NCDB) is a large cancer directory that represents approximately 70% of all newly diagnosed cancers in the US. The inclusion criteria for this study included the following: International Classification of Diseases for Oncology, third edition, morphological codes (8020, 8140, 8144, 8210, 8211, 8480, 8481, and 8490) and topography codes (C18.0-9), in participant user data files between the years 2010 and 2013. MSI status information was not available for patients diagnosed before 2010. The primary outcome was the overall survival of patients with dMMR/MSI-H stage II with high-risk features.
Eligibility Criteria
Patient information was independently reviewed by two of the authors for the eligibility criteria. The patients were deemed eligible if they have dMMR/MSI-H stage II CC. Patients with mixed adeno-squamous histology, rectosigmoid location, and rectal cancer were excluded. Patients who received radiation therapy before or after surgery were excluded. Patients who received chemotherapy prior to surgery were excluded, as this may impact the pathologic stage at resection. High-risk features were defined as the following: <12 lymph nodes examined, lymphovascular invasion (LVI), positive surgical margin, pT4 tumor. No data were available for obstruction or perforation at diagnosis. Poor or undifferentiated histology was not included as a high-risk feature, as it is a good prognostic factor in dMMR/MSI-H stage II CC (18). High-risk stage II CC was defined as having at least one high-risk feature. Institutional approval and informed consent were not required for this study since the patient information in the database is completely de-identified, and the database is legally accessible to the public.
Data Extraction and Statistical Analysis
The patient-specific covariates included were date of diagnosis, date of death, age, gender, race, tumor site, histology, insurance status, stage, presence of metastatic disease, co-morbid medical conditions, location of treatment, and treatment regimen (single or multi-agent chemotherapy). The treatment and clinical outcomes included overall survival rate. All data were checked for internal consistency.
Analysis
All patients in the analysis had dMMR/MSI-H stage II colon cancer. Univariate and multivariate analyses were conducted to identify the factors associated with patient outcome (OS). The clinical and demographic characteristics of the patients were summarized using descriptive statistics as appropriate for variable type and distribution (chi-square test for categorical variables and ANOVA for numerical variables). Univariate and multivariate Cox-PH models were built to assess the association between patient characteristics and survival. Backward selection with an alpha level of removal of.05 was used in the multivariate analysis. The Kaplan–Meier survival curves were generated with log-rank tests to evaluate the association between adjuvant chemotherapy in high-risk and low-risk cohorts separately. All analyses were performed with a significance level of 0.05 (two-sided) with SAS Statistical Package, v9.4 (SAS institute, Inc., Cary, North Carolina).
Results
Of the 249,571 patients with stage II colorectal cancer diagnosed between 2010 and 2013 in the NCDB database, 6,426 patients were determined to have dMMR/MSI-H status, and 2,293 met the inclusion criteria of the study ( Figure 1 ). Females accounted for 58.2% of patients; 87.4% were Caucasian. The median age was 69 years (range, 21–90 years old). The most common tumor location was the ascending colon (32.5%), followed by the cecum (27.1%) and the transverse colon (13.1%). The sigmoid and the descending colon accounted for 9.7 and 5.0% of cases, respectively ( Table 1 ).
Figure 1.
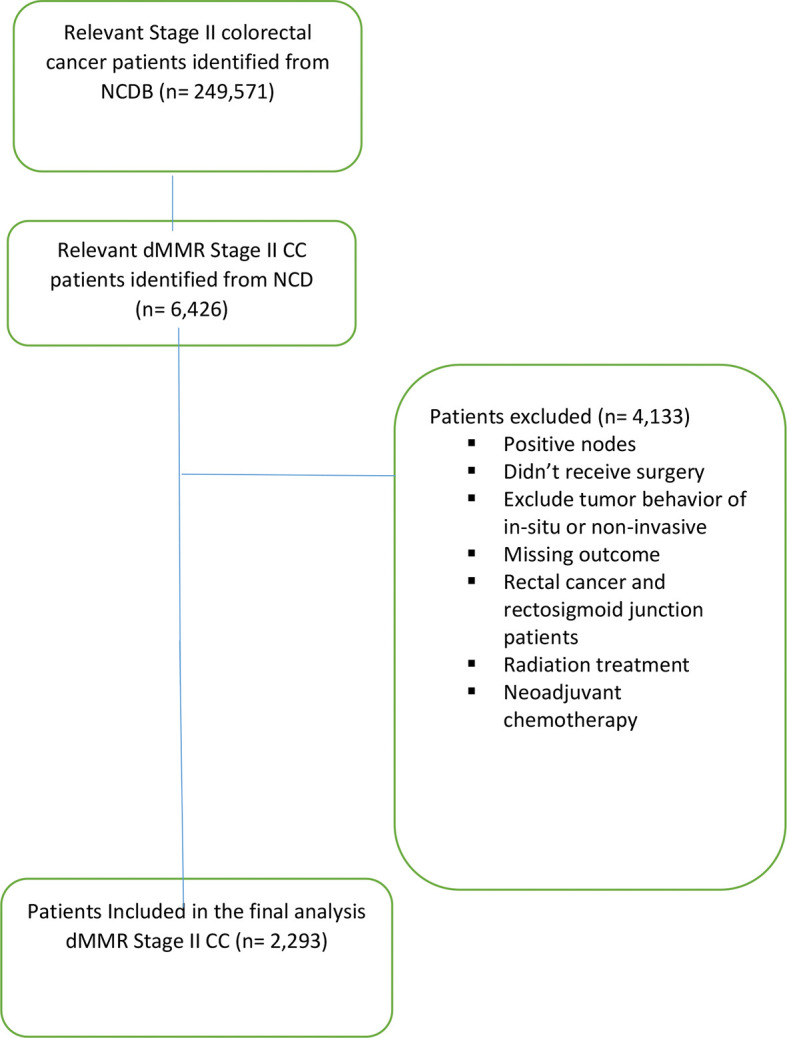
Consort diagram outlining the study selection.
Table 1.
Demographics and clinical characteristics of patients.
| Variable | N (%) |
|---|---|
| Total | 2,293 |
| Gender | |
| Male | 958 (41.8) |
| Female | 1,335 (58.2) |
| Age | |
| Median | 69 (21–90) |
| STD | 14.78 |
| Race | |
| White | 2,004 (87.4) |
| African American | 188 (8.2) |
| Others | 101 (4.4) |
| Year of diagnosis | |
| 2010 | 413 (18.0) |
| 2011 | 566 (24.7) |
| 2012 | 633 (27.6) |
| 2013 | 681 (29.7) |
| Charlson–Deyo score | |
| 0 | 1,540 (67.2) |
| 1 | 537 (23.4) |
| 2+ | 216 (9.4) |
| Site | |
| Appendix | 8 (0.4) |
| Cecum | 621 (27.1) |
| Ascending colon | 746 (32.5) |
| Hepatic flexure | 143 (6.2) |
| Transverse colon | 301 (13.1) |
| Splenic flexure | 80 (3.5) |
| Descending colon | 115 (5.0) |
| Sigmoid colon | 222 (9.7) |
| Overlapping | 36 (1.6) |
| Not otherwise specified | 21 (0.9) |
| Surgery at primary site | |
| Partial colectomy | 487 (21.2) |
| Subtotal colectomy/hemicolectomy | 1,722 (75.1) |
| Total colectomy | 42 (1.8) |
| Total proctocolectomy | 10 (0.4) |
| Unknown | 32 (1.4) |
| Grade | |
| Well differentiated/moderately differentiated | 1,540 (67.2) |
| Poorly differentiated/undifferentiated | 753 (32.8) |
| Pathological T stage | |
| T3 | 1,957 (85.3) |
| T4 | 321 (14.0) |
| Risk group per study | |
| High risk | 676 (29.5) |
| Not high risk | 1,617 (70.5) |
| High-risk features a | |
| T4 | 321 (14.0) |
| LVI | 329 (14.3) |
| <12 lymph node removed | 89 (3.9) |
| Positive margin | 69 (3.0) |
| Systemic/surgery sequence | |
| No systemic therapy | 1,862 (81.2) |
| Systemic therapy after surgery | 431 (18.8) |
| Chemotherapy | |
| No chemotherapy | 1,862 (81.2) |
| Single-agent chemotherapy | 108 (4.7) |
| Multiagent chemotherapy | 292 (12.7) |
| Chemotherapy type and number of agents not documented | 31 (1.4) |
High-risk features not mutually exclusive.
In the entire cohort, 29.5% (n = 676) of patients were deemed to have a high-risk stage II CC. Positive margins, LVI, and less than 12 lymph nodes examined were observed as 3.0, 14.3, and 3.9%, respectively. pT4 was present in 14.0% of patients ( Table 1 ). Of the high-risk patients, 36.1% (n = 244) received adjuvant chemotherapy, of whom 72.1% (n = 176) received multiagent therapy and 23.4% (n = 57) received single-agent chemotherapy, and 4.5% (n = 11) received an unknown number of agents.
On univariate analysis, high-risk status, pT4A/B tumor (pT3 as reference), pathological stage IIB/C (pathological stage IIA as reference), <12 lymph nodes (≥12 lymph nodes as reference), positive margins (negative margins as reference), Charlson–Deyo score >1 (0 as reference), and elder age (continuous scale) at diagnosis were associated with worse overall survival ( Table 2 ). On multivariate analysis, male sex, positive surgical margin (negative margin as reference), Charlson–Deyo score >1 (0 as reference), high-risk disease, and older age at diagnosis were associated with worse OS ( Table 2 ).
Table 2.
Overall survival (OS) by mismatch repair and treatment status in univariate and multivariate analysis.
| Univariate OS | p-values | Multivariate OS | p-values | |||
|---|---|---|---|---|---|---|
| HR | 95%CI | HR | 95%CI | |||
| Age | 1.05 (1.04–1.06) | <0.001 | 1.04 (1.03–1.06) | <.001 | ||
| Male gender | 1.02 (0.82–1.28) | 0.843 | 1.36 (1.07–1.71) | 0.004 | ||
| High risk | 1.56 (1.24–1.97) | <0.001 | 1.71 (1.35–2.17) | <0.001 | ||
| Adjuvant chemotherapy | 0.52 (0.36–0.73) | <0.001 | 0.66 (0.44–0.98) | 0.040 | ||
| Pathological T4A a | 1.72 (1.19–2.49) | 0.004 | ||||
| Pathological T4B a | 2.19 (1.53–3.14) | <0.001 | ||||
| Pathologic stage IIB a | 1.63 (1.13–2.35) | 0.009 | ||||
| Pathologic stage IIC a | 2.27 (1.58–3.25) | <.001 | ||||
| LVI a | 1.34 (1.00–1.81) | 0.248 | ||||
| <12 LNS a | 2.06 (1.35–3.15) | <.001 | ||||
| Positive surgical margin a | 2.79 (1.79–4.34) | <.001 | ||||
| Charlson–Deyo score 1 | 1.71 (1.31–2.22) | <.001 | 1.45 (1.11–1.90) | <.001 | ||
| Charlson–Deyo score 2+ | 3.89 (2.93–5.17) | <.001 | 2.72 (2.03–3.65) | <.001 | ||
Removed from the multivariate model to avoid collinearity with high risk.
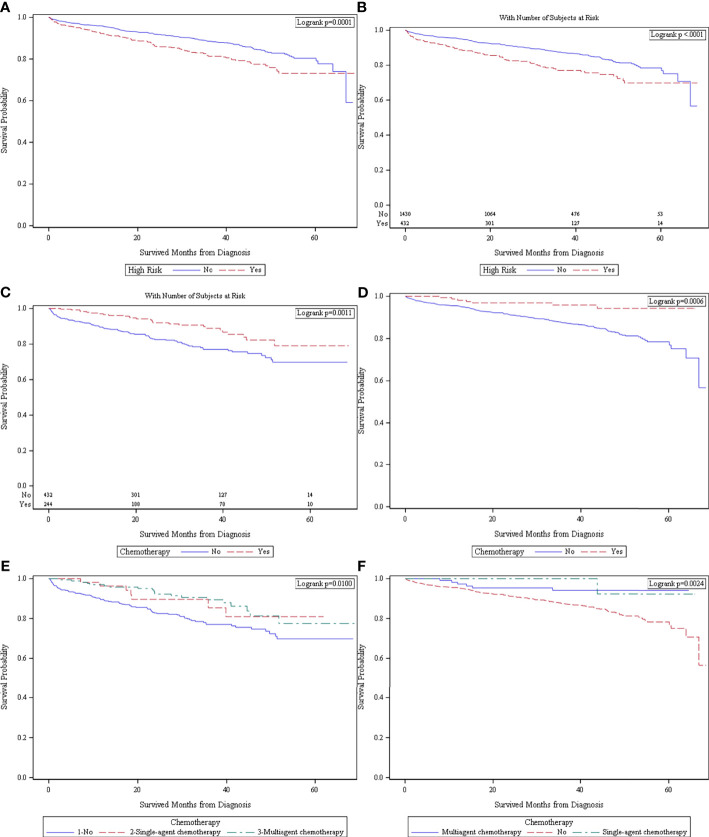
High-risk dMMR/MSI-H patients had worse OS compared to non-high-risk dMMR/MSI-H patients in the entire cohort when not stratified by status of adjuvant chemotherapy administration [5-year survival and 95%CI: 73.2% (67.3–78.1%) vs. 80.3% (76.7–83.5%), p = 0.0001, Figure 2A ]. Median survival is not reachable in our cohort since none of the cohorts had more than 50% of patients who died at the end of follow-up; hence, 5-year survival was provided. In patients who received no adjuvant chemotherapy, the high-risk dMMR/MSI-H patients had worse OS [5-year survival and 95%CI: 69.8% (62.6–75.9%) vs. 78.4% (74.3–81.9%), p < 0.0001, Figure 2B ].
Figure 2.
(A) Survival in high-risk (n = 676) versus no-high-risk (n = 1,617) patients in the entire cohort (n = 2,293) when not stratified by chemotherapy status. (B) Survival in patients who received no adjuvant chemotherapy (n = 1,862) in high-risk (n = 432) and no-high-risk (n = 1,430) patients. (C) Survival in high-risk patients (n = 676) who received adjuvant chemotherapy (n = 244) versus no adjuvant chemotherapy (n = 432). (D) Survival in patients with no high-risk features (n = 1,617) who received adjuvant chemotherapy (n = 187) versus no adjuvant chemotherapy (n = 1,430). (E) Survival in patients with high risk (n = 676) who received no adjuvant chemotherapy (n = 432), single-agent chemotherapy (n = 57), and multiagent chemotherapy (n = 176). (F) Survival in patients with no high-risk features who received no chemotherapy (n = 1,430), single-agent chemotherapy (n = 51), and multiagent chemotherapy (n = 116).
High-risk dMMR/MSI-H patients who received adjuvant chemotherapy had better OS compared to those who had no chemotherapy [5-year survival and 95%CI: 78.0% (66.4–86.0%) vs. 69.8% (62.6–75.9%), p = 0.0011, Figure 2C ]. In patients with no high-risk features, patients who received adjuvant chemotherapy had better OS [5-year survival and 95%CI: 94.3% (87.6–99.4%) vs. 78.4% (74.3–81.9%), p = 0.0006, Figure 2D ]. In the patient groups by single/multi-agent adjuvant chemotherapy, single-agent and multi-agent chemotherapy patients demonstrated similar OS, which were both better than those with no chemotherapy. This finding is consistent in high-risk-feature (p = 0.01, Figure 2E ) and no-high-risk feature patients (p = 0.0024, Figure 2F ).
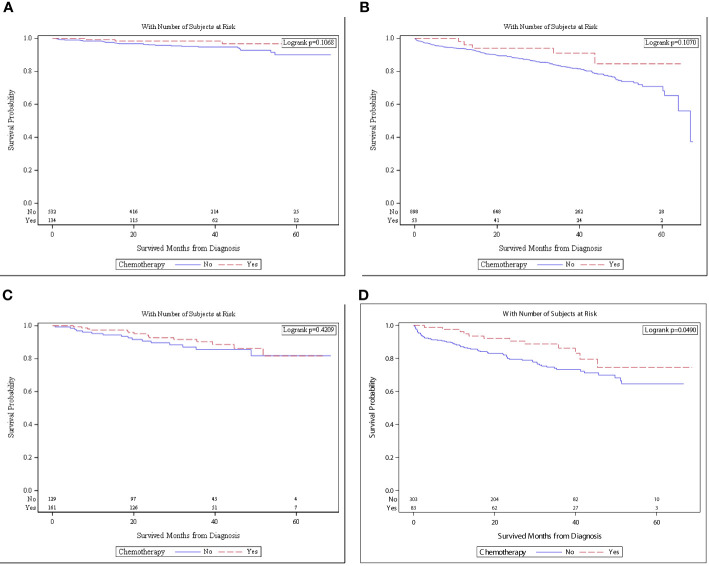
When patients with no high-risk features were stratified by age, chemotherapy was no longer associated with better OS. The overall survival in patients with no high-risk features and aged <65 was not different with chemotherapy versus no chemotherapy [5-year OS and 95%CI: 96.7% (89.4–99.0%) vs. 90.0%: (83.9–93.9%), p = 0.1068, Figure 3A ]. The overall survival in the same cohort aged ≥65 years was not different with chemotherapy versus with no chemotherapy [5-year survival and 95%CI: 96.1% (62.5–94.2%) and 70.9% (65.1–75.9%), p = 0.1070, Figure 3B ].
Figure 3.
(A) Survival in patients with no high risk and <65 of age who received chemotherapy (n = 134) versus no chemotherapy (n = 532). (B) Survival in patients with no high risk and ≥65 of age who received chemotherapy (n = 53) versus no chemotherapy (n = 153). (C) Survival in patients with high risk and <65 of age who received chemotherapy (n = 161) versus no chemotherapy (n = 129). (D) Survival in patients with high risk and ≥65 of age who received chemotherapy (n = 83) versus no chemotherapy (n = 303).
The overall survival in patients with high-risk features was stratified by age. OS in patients aged <65 who received chemotherapy was not different than those of patients who did not receive chemotherapy [5-year OS and 95%CI: 81.7% (67.0–90.3%) vs. 81.8% (69.4–89.5%), p = 0.4209, Figure 3C ]. The overall survival in the same cohort aged ≥65 years was superior with chemotherapy versus with no chemotherapy [5-year OS and 95%CI: 74.5% (56.2–86.1%) vs. 64.6% (55.5–72.3%), p = 0.0490, Figure 3D ].
Discussion
Prior reports and guidelines established the prognostic value of high-risk features in molecularly unspecified stage II CRC (9, 19–21). However, the therapeutic and prognostic implications of dMMR/MSI-H with high-risk clinicopathologic features have not been adequately studied. This study demonstrates that high-risk features are also prognostic in patients with stage II dMMR/MSI-H. This is the largest published study to establish the prognostic impact of high-risk features in dMMR/MSI-H stage II CRC.
The prognostic role of high-risk features in stage II dMMR/MSI-H setting raises the question regarding the role of adjuvant therapy in this group of patients. In this study, we observed a significant overall survival benefit in patients with high-risk dMMR/MSI-H stage II CC who received adjuvant chemotherapy compared with those who received surgery only. The benefit was associated with both multiagent and single-agent adjuvant chemotherapy. This is a novel finding that has a potential impact on clinical practice in the absence of data from clinical trials. The survival benefit persisted in patients >65 years when stratified by age. Sporadic dMMR/MSI-H is known to be associated with older age at diagnosis compared to germline dMMR/MSI-H (17, 22). In this study, patients with no high-risk features and dMMR/MSI-H stage II colon cancer also benefited from chemotherapy; however, age was an important confounding factor. Survival benefit was not evident in the low-risk group when stratified by age. We speculate that patients in the older age group of high-risk dMMR/MSI-H stage II CC of this study may have had a sporadic dMMR/MSI-H disease and may have derived more benefit from adjuvant chemotherapy compared to the younger population, which possibly may have had a higher rate of germline dMMR/MSI-H. It should be noted that no data were available in NCDB for BRAF status, MLH1 methylation status, and germline testing of family history.
Several prior studies, including retrospective analysis and observational reports, have attempted to address the role of adjuvant chemotherapy in stage II CC with high-risk features; however, these studies did not include the dMMR//MSI-H status (3, 23). Data about the role of adjuvant chemotherapy in high-risk dMMR/MSI-H stage II CC is limited. Tougeron et al. reported the clinical outcomes of stage II and III dMMR/MSI-H CC patients treated between 2000 and 2011 in a multicenter retrospective French study (24). Sixty percent (n = 149) of the patients were deemed to have high-risk factors, and 22% (n = 33) of the high-risk patients were treated with adjuvant chemotherapy. The high-risk features included pT4, VELIPI criteria (vascular emboli, lymphatic invasion, or perineural invasion), poor/undifferentiated histology, less than eight lymph nodes examined, tumor perforation, and initial bowel obstruction. Patients who were treated with adjuvant FOLFOX and not the single agent 5-FU showed a trend for longer disease-free survival compared to surgery alone. Although it included specific chemotherapy regimen information, the number of patients in that study was much smaller than in the present study.
Control for variables that could have influenced the results, such as histopathologic features, tumor grade, age, and performance status, was performed. Significant limitations still exist in this analysis, and these include retrospective design, lack of randomization, and no individualized patient data regarding the specifics of chemotherapy or follow-up. The reasons why adjuvant chemotherapy was not administered is unknown. The precise chemotherapy agents administered were not available. The sporadic versus germline mutational status is unknown, and the prevalence of other genomic alterations is similarly unknown. The overall survival is not cancer specific in this study, as NCDB includes only all-cause overall survival. In addition, the high-risk features did not include obstruction or perforation, as there was no data available in the database. Tumor perforation was shown to be associated with interperitoneal tumor dissemination (25), which raises the concern of whether these patients actually have stage II disease. The reported incidence of perforation and obstruction in stage II CC is less than 10% (26–28), and the impact of tumor cell spillage on recurrence and survival increases the risks substantially and may contribute to an increase in the benefit of adjuvant therapy.
Conclusion
The prognostic value of high-risk features in dMMR/MSI-H stage II CC is confirmed. The prognostic value of high-risk features should be considered in adjuvant therapy discussions. Adjuvant chemotherapy may be associated with better OS in high-risk dMMR/MSI-H stage II CC patients, but significant limitations exist, including the retrospective nature of the data set. In the absence of randomized trials, the benefits and the risks of adjuvant therapy should be discussed with the patients with high-risk dMMR/MSI-H stage II CC. Further research needs to be done in the low-risk dMMR/MSI-H stage II CC to confirm the lack of benefit from adjuvant chemotherapy.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author Contributions
AM and MA participated in collecting data, writing the manuscript, and editing the manuscript. MD participated in collecting data, writing the manuscript, and editing the manuscript. MB participated in the analysis of data and editing the manuscript. RJ, PP, CW, OA, WS, TG, and GB participated in editing the manuscript. BE-R participated in mentoring the whole project and editing the manuscript. All authors read and approved the final manuscript.
Funding
The research reported in this publication was supported in part by the Winship Research Informatics Shared Resource of Winship Cancer Institute of Emory University and National Institutes of Health/National Cancer Institute under award number P30CA138292.
Author Disclaimer
The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed or the conclusions drawn from these data by the investigator.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Part of the data presented in this study was presented at the 2018 ASCO Annual Meeting in Chicago, IL, United States. The data used in the study are derived from a de-identified National Cancer Database (NCDB) file. The NCDB is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society.
Abbreviations
dMMR, deficient mismatch repair; pMMR, proficient mismatch repair; MSI-H, microsatellite high; MSS, microsatellite stable.
References
- 1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA: A Cancer J Clin (2021) 71(1):7–33. doi: 10.3322/caac.21654 [DOI] [PubMed] [Google Scholar]
- 2. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer Statistics, 2009. CA Cancer J Clin (2009) 59(4):225–49. doi: 10.3322/caac.20006 [DOI] [PubMed] [Google Scholar]
- 3. Verhoeff SR, van Erning FN, Lemmens VE, de Wilt JH, Pruijt JF. Adjuvant Chemotherapy Is Not Associated With Improved Survival for All High-Risk Factors in Stage II Colon Cancer. Int J Cancer (2016) 139(1):187–93. doi: 10.1002/ijc.30053 [DOI] [PubMed] [Google Scholar]
- 4. Casadaban L, Rauscher G, Aklilu M, Villenes D, Freels S, Maker AJ. Adjuvant Chemotherapy is Associated With Improved Survival in Patients With Stage II Colon Cancer. Cancer (2016) 122(21):3277–87. doi: 10.1002/cncr.30181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Andre T, de Gramont A, Vernerey D, Chibaudel B, Bonnetain F, Tijeras-Raballand A, et al. Adjuvant Fluorouracil, Leucovorin, and Oxaliplatin in Stage II to III Colon Cancer: Updated 10-Year Survival and Outcomes According to BRAF Mutation and Mismatch Repair Status of the MOSAIC Study. J Clin Oncol (2015) 33(35):4176–87. doi: 10.1200/JCO.2015.63.4238 [DOI] [PubMed] [Google Scholar]
- 6. Bastos DA, Ribeiro SC, de Freitas D, Hoff PM. Combination Therapy in High-Risk Stage II or Stage III Colon Cancer: Current Practice and Future Prospects. Ther Adv Med Oncol (2010) 2(4):261–72. doi: 10.1177/1758834010367905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wolmark N, Rockette H, Mamounas E, Jones J, Wieand S, Wickerham DL, et al. Clinical Trial to Assess the Relative Efficacy of Fluorouracil and Leucovorin, Fluorouracil and Levamisole, and Fluorouracil, Leucovorin, and Levamisole in Patients With Dukes’ B and C Carcinoma of the Colon: Results From National Surgical Adjuvant Breast and Bowel Project C-04. J Clin Oncol (1999) 17(11):3553–9. doi: 10.1200/JCO.1999.17.11.3553 [DOI] [PubMed] [Google Scholar]
- 8. Wolmark N, Rockette H, Fisher B, Wickerham DL, Redmond C, Fisher ER, et al. The Benefit of Leucovorin-Modulated Fluorouracil as Postoperative Adjuvant Therapy for Primary Colon Cancer: Results From National Surgical Adjuvant Breast and Bowel Project Protocol C-03. J Clin Oncol (1993) 11(10):1879–87. doi: 10.1200/JCO.1993.11.10.1879 [DOI] [PubMed] [Google Scholar]
- 9. NCCN . Clinical Practice Guidelines in Oncology. Colon Cancer (2019). [DOI] [PubMed] [Google Scholar]
- 10. Sargent DJ, Marsoni S, Monges G, Thibodeau SN, Labianca R, Hamilton SR, et al. Defective Mismatch Repair as a Predictive Marker for Lack of Efficacy of Fluorouracil-Based Adjuvant Therapy in Colon Cancer. J Clin Oncol (2010) 28(20):3219–26. doi: 10.1200/JCO.2009.27.1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kheirelseid EA, Miller N, Chang KH, Curran C, Hennessey E, Sheehan M, et al. Mismatch Repair Protein Expression in Colorectal Cancer. J Gastrointest Oncol (2013) 4(4):397–408. doi: 10.3978/j.issn.2078-6891.2013.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kawakami H, Zaanan A, Sinicrope FA. Implications of Mismatch Repair-Deficient Status on Management of Early Stage Colorectal Cancer. J Gastrointest Oncol (2015) 6(6):676–84. doi: 10.3978/j.issn.2078-6891.2015.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sinicrope FA, Yang ZJ. Prognostic and Predictive Impact of DNA Mismatch Repair in the Management of Colorectal Cancer. Future Oncol (2011) 7(3):467–74. doi: 10.2217/fon.11.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang CM, Lv JF, Gong L, Yu LY, Chen XP, Zhou HH, et al. Role of Deficient Mismatch Repair in the Personalized Management of Colorectal Cancer. Int J Environ Res Public Health (2016) 13(9). doi: 10.3390/ijerph13090892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sinicrope FA. DNA Mismatch Repair and Adjuvant Chemotherapy in Sporadic Colon Cancer. Nat Rev Clin Oncol (2010) 7(3):174–7. doi: 10.1038/nrclinonc.2009.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Popat S, Hubner R, Houlston RS. Systematic Review of Microsatellite Instability and Colorectal Cancer Prognosis. J Clin Oncol (2005) 23(3):609–18. doi: 10.1200/JCO.2005.01.086 [DOI] [PubMed] [Google Scholar]
- 17. Sinicrope FA, Foster NR, Thibodeau SN, Marsoni S, Monges G, Labianca R, et al. DNA Mismatch Repair Status and Colon Cancer Recurrence and Survival in Clinical Trials of 5-Fluorouracil-Based Adjuvant Therapy. J Natl Cancer Inst (2011) 103(11):863–75. doi: 10.1093/jnci/djr153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Henson DE, Albores-Saavedra J, Corle D. Carcinoma of the Gallbladder. Histologic Types, Stage of Disease, Grade, and Survival Rates. Cancer (1992) 70(6):1493–7. doi: [DOI] [PubMed] [Google Scholar]
- 19. Quah HM, Chou JF, Gonen M, Shia J, Schrag D, Landmann RG, et al. Identification of Patients With High-Risk Stage II Colon Cancer for Adjuvant Therapy. Dis Colon Rectum (2008) 51(5):503–7. doi: 10.1007/s10350-008-9246-z [DOI] [PubMed] [Google Scholar]
- 20. Benson AB, 3rd, Schrag D, Somerfield MR, Cohen AM, Figueredo AT, Flynn PJ, et al. American Society of Clinical Oncology Recommendations on Adjuvant Chemotherapy for Stage II Colon Cancer. J Clin Oncol (2004) 22(16):3408–19. doi: 10.1200/JCO.2004.05.063 [DOI] [PubMed] [Google Scholar]
- 21. Schmoll HJ, Van Cutsem E, Stein A, Valentini V, Glimelius B, Haustermans K, et al. ESMO Consensus Guidelines for Management of Patients With Colon and Rectal Cancer. A Personalized Approach to Clinical Decision Making. Ann Oncol (2012) 23(10):2479–516. doi: 10.1093/annonc/mds236 [DOI] [PubMed] [Google Scholar]
- 22. Cohen R, Buhard O, Cervera P, Hain E, Dumont S, Bardier A, et al. Clinical and Molecular Characterisation of Hereditary and Sporadic Metastatic Colorectal Cancers Harbouring Microsatellite Instability/DNA Mismatch Repair Deficiency. Eur J Cancer (2017) 86:266–74. doi: 10.1016/j.ejca.2017.09.022 [DOI] [PubMed] [Google Scholar]
- 23. O’Connor ES, Greenblatt DY, LoConte NK, Gangnon RE, Liou J-I, Heise CP, et al. Adjuvant Chemotherapy for Stage II Colon Cancer With Poor Prognostic Features. J Clin Oncol (2011) 29(25):3381–8. doi: 10.1200/JCO.2010.34.3426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tougeron D, Mouillet G, Trouilloud I, Lecomte T, Coriat R, Aparicio T, et al. Efficacy of Adjuvant Chemotherapy in Colon Cancer With Microsatellite Instability: A Large Multicenter AGEO Study. J Natl Cancer Inst (2016) 108(7). doi: 10.1093/jnci/djv438 [DOI] [PubMed] [Google Scholar]
- 25. Koppe MJ, Boerman OC, Oyen WJG, Bleichrodt RP. Peritoneal Carcinomatosis of Colorectal Origin: Incidence and Current Treatment Strategies. Ann Surg (2006) 243(2):212–22. doi: 10.1097/01.sla.0000197702.46394.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Badia JM, Sitges-Serra A, Pla J, Martí Ragué J, Roqueta F, Sitges-Creus A. Perforation of Colonic Neoplasms. A Review of 36 Cases. Int J Colorectal Dis (1987) 2(4):187–9. doi: 10.1007/BF01649502 [DOI] [PubMed] [Google Scholar]
- 27. Mandava N, Kumar S, Pizzi WF, Aprile IJ. Perforated Colorectal Carcinomas. Am J Surg (1996) 172(3):236–8. doi: 10.1016/S0002-9610(96)00164-X [DOI] [PubMed] [Google Scholar]
- 28. Lee IK, Sung NY, Lee YS, Lee SC, Kang WK, Cho HM, et al. The Survival Rate and Prognostic Factors in 26 Perforated Colorectal Cancer Patients. Int J Colorectal Dis (2007) 22(5):467–73. doi: 10.1007/s00384-006-0184-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.