ABSTRACT
The objective of this systematic literature review was to evaluate the efficacy of probiotic, prebiotic, and synbiotic interventions compared with control on improving growth outcomes of children living in low- and middle-income countries (LMICs). Probiotics had a beneficial effect on ≥1 of the growth outcomes in 5 out of the 11 included studies. Of these, 3 studies were conducted in undernourished children, 1 in healthy children, and 1 in children without a described health status. No effect of prebiotics on growth outcomes was seen in the 4 included studies. Synbiotics had a beneficial effect on growth outcomes in 3 out of 4 studies. Although a limited number of studies with high heterogeneity indicate that probiotics and synbiotics may have the potential to improve the growth of both undernourished and healthy children living in LMICs, more research is needed to confirm the observed effects. This review was registered at www.crd.york.ac.uk/prospero/ as CRD42020212998.
Keywords: child, growth, LMICs, nutrition, prebiotics, probiotics, synbiotics
Probiotics and synbiotics may improve the growth of children living in LMICs, but no firm conclusions can be made owing to the small number of studies present with high heterogeneity.
Introduction
Child undernutrition is a major public health issue worldwide (1). In 2019, an estimated 144 million children < 5 y old were stunted and 47 million were wasted (1). Prevention of delayed growth in childhood is critical, because it can have both immediate and long-term consequences for health and developmental potential that are difficult to reverse (2). In 2012, the World Health Assembly established Global Nutrition 2025 targets, of which one aims to achieve a 40% reduction in the number of stunted children < 5 y of age by 2025 (3). Stunting is difficult to address, because it is related to many factors including socioeconomic status, dietary intake of mother and child, infections, and the environment (4). In addition, the gut microbiota—the bacteria, archaea, and fungi living in the digestive tract—play a role in the regulation of the energy harvesting from nutrients, growth hormone signaling, colonization resistance, immune tolerance against pathogens, and other pathways associated with healthy child growth (5). Infants’ gut microbiota are typically colonized by facultative anaerobes, followed by obligate anaerobes including Bifidobacterium, Bacteroides, and Clostridium during the first 6 mo of life. The diversity in microbiota is narrow and dominated by species involved in human milk oligosaccharide (HMO) metabolism in breastfed infants (6). In children from 6 mo to 2 y of age, the introduction of solid foods and exposure to environmental microbes trigger the expansion of the gut microbiome's diversity. Because the gut microbiota drives a weaning reaction critical for immune maturity and tolerance later in life, it is hypothesized that gut microbiome maturation partially regulates growth during this time period and dysregulation of the gut microbiome may contribute to childhood stunting (5). Impaired development of the gut microbiota causes stunted children to have an immature gut microbiota as compared with nonstunted children of the same age. Gut immaturity is measured by a lower microbiota-for-age z score (MAZ), characterized by a lower α-diversity of the gut microbiota, and disproportionally higher concentrations of Proteobacteria (7). The evidence for a causal role of the gut microbiota in delayed growth is increasing (5). An immature and less diverse microbiota, which is often present in children with delayed growth, may reduce or prevent the success of nutritional interventions aiming to promote growth (8). Therefore, in recent decades the scientific focus has shifted to interventions targeting the gut microbiota such as probiotics, prebiotics, and synbiotics because they may have the potential to address stunting effectively (5).
A probiotic contains live microorganisms which, when administered in adequate amounts, confer a health benefit on the host (9). Probiotics are strain-specific and can result in different health benefits (10). They may improve child growth through modulation of the gut microbiota and immune system, inhibition of pathogen growth, prevention of infections, lowering diarrhea incidence, improvement of the absorption of energy, and improvement of the absorption of several micronutrients (11–14).
A prebiotic is a substrate that is selectively utilized by host microorganisms conferring a health benefit (15). Prebiotics promote the growth of ≥1 specific bacteria, mainly bifidobacteria and lactobacilli, leading to among other things the production of SCFAs which inhibit pathogen growth (16, 17). In addition, prebiotics enhance the uptake of micronutrients, delay gastric emptying, improve gut barrier function, and modulate the immune system (16, 17).
A synbiotic is a mixture comprising live microorganisms and substrates selectively utilized by host microorganisms that confers a health benefit on the host (18). The beneficial effects of prebiotics and probiotics taken separately may be enhanced further if combined (19).
Most previous reviews evaluating the effect of nutrition interventions on growth outcomes in children in low-and middle-income countries (LMICs) concerned several (combinations of) micronutrients and education (2, 20), but did not include probiotic, prebiotic, and synbiotic interventions. In addition, they did not include any gut microbiota outcomes (2, 21), even though the gut microbiota is important in growth retardation (5). Other reviews on probiotics, prebiotics, and synbiotics focused mostly on infants < 1 y old without making a distinction between developed and developing countries (22–24). To our knowledge, the review of Onubi et al. (12) is the only review investigating the effect of probiotics on growth in children older than 1 y without specific disease conditions while making a distinction between developed and developing countries. However, they did not focus exclusively on LMICs and more recent studies have since been published. Onubi et al. (12) observed a benefit of dietary intake of probiotics on weight and height gain in undernourished children in developing countries and a possible benefit in well-nourished children. In LMICs, the potential of probiotics might be larger than in developed countries owing to the environment and other related factors of the participating children. Further, the implications of these adversities can go beyond physical outcomes to other areas of child functioning and also need to be considered in evaluating treatment efficacy. Therefore, the objective of this review was to systematically evaluate the effects of probiotic, prebiotic, and synbiotic interventions in comparison with standard care or control on several physical growth outcomes of children aged 6–59 mo living in LMICs. The findings of this review will help to develop effective and durable interventions for children in LMICs by targeting the gut microbiome, which can potentially improve the efficacy of nutrition-based treatments (25–27).
Methods
The study was registered at the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42020212998). For the reporting of this systematic review the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) were used (28). Supplemental Table 1 shows the PRISMA checklist.
Inclusion and exclusion criteria
Parallel controlled trials, including cluster-randomized trials, involving children aged 6–59 mo—although studies with a wider age range were included—in LMICs as classified by the World Bank (29) published up to 30 September, 2020 in the English language were included. The interventions comprised probiotics, prebiotics, or synbiotics as defined by the International Scientific Association for Probiotics and Prebiotics (ISAPP) (9, 15, 18). Prebiotics other than oligosaccharides such as PUFAs were included, because they are recognized as prebiotics by the ISAPP (15). Microbiota-directed complementary foods (MDCFs) were included in the search scope as well, but no trials meeting the inclusion and exclusion criteria were found. All the studies reported ≥1 of the following outcomes: height, weight, height-for-age z score (HAZ), weight-for-age z score (WAZ), weight-for-height z score (WHZ), stunting, underweight, or wasting. Stunting, underweight, and wasting were respectively defined as an HAZ, WAZ, or WHZ < −2SD from the median of the WHO Child Growth Standards (30). Only interventions with a duration of ≥3 mo were included to ensure that possible effects on growth were measurable. A shorter period can be misleading for the weight outcome of children aged >1 y (31). For the comparison with the intervention, we included studies providing standard care, a control such as a placebo, or no intervention.
Search strategy
Relevant articles were identified using PubMed, Scopus, and the Cochrane Library. A list of relevant Medical Subject Headings words and keywords was generated and reviewed by all authors. Supplemental Tables 2–10 show the search queries for the databases. Additional articles were identified with hand searching using other reviews, citations, reference lists, and Google Scholar.
Data extraction
The identified articles were exported to an Endnote library and duplicates were removed. If there were multiple reports of a primary study, the most complete description of the data with the longest follow-up was used. After title and abstract screening, the full text of articles was assessed for eligibility using the inclusion and exclusion criteria. Data on participants, study characteristics, interventions, outcomes, and results were extracted from the final subset of eligible studies. If outcome data were lacking in the articles, authors were contacted to request additional information. The screening and extraction were performed in duplicate by 2 reviewers independently, and any discrepancies were resolved through discussion with a third author where necessary.
Outcome variables
Height, weight, HAZ, WAZ, WHZ, stunting, underweight, and wasting were the primary outcome variables. The mean ± SD changes in height, weight, HAZ, WAZ, and WHZ were extracted. If they were not presented, medians [IQRs] or the baseline and final means ± SDs were extracted. For stunting, underweight, and wasting the proportions [n (%)] were extracted. If present, the risk ratios, prevalence ratios, or ORs with 95% CIs were extracted as well. Where available, secondary outcome variables were extracted including markers of gut health and microbiota, such as MAZ, α-diversity, IgA, and the lactulose:mannitol ratio.
Quality assessment
The quality of the individual trials was evaluated using the Cochrane Risk of Bias tool. Random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias were judged as “high risk,” “unclear,” or “low risk.” The certainty of the evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach. By evaluating the risk of bias, imprecision, inconsistency, indirectness, and publication bias, the certainty of the evidence was judged as “very low,” “low,” “moderate,” or “high” (32). When no summary effect measure was obtained, the GRADE approach for rating the certainty in evidence in the absence of a single estimate of effect was used (33). The quality assessment was performed in duplicate and any discrepancies were resolved through discussion with a third author where necessary.
Data analysis
When available, missing values were extracted from graphs using the online application WebPlotDigitizer version 4.3 [done on 1 occasion (34)]. Where medians [IQRs] were reported, they were converted to means ± SDs according to the formulas outlined in the Cochrane Handbook (35). The mean differences (MDs) were calculated by subtracting the baseline mean from the final mean. The related SDs were imputed according to the equations by Follmann et al. (36). For these SD imputations, we assumed correlation coefficients of 0.995 and 0.990 for weight and height, respectively (37). For HAZ and WAZ of the intervention group we assumed correlation coefficients of 0.91 and 0.86, respectively, and 0.89 and 0.82 for the HAZ and WAZ of the control group, respectively (38). The correction factor for WHZ was estimated as the mean of the correction factors for HAZ and WAZ.
Owing to the lack of comparability of the study populations and the probiotic, prebiotic, and synbiotic interventions, no summary effect measures were computed. Nevertheless, a forest plot without overall effect size was compiled for illustrative purposes, for which the standardized mean differences (SMDs) with 95% CIs were computed for each study separately using Hedges’ g (bias-corrected SMD) and the inverse variance method. To reduce the probability of a type 1 error and to avoid multiple counting of the control group in trials with multiple intervention groups and a single control group, the sample size of the control group was divided equally between the number of intervention groups, while retaining the mean change and its SD (39). All analyses were conducted with R version 4.0.3 (R Core Team) using the packages “meta” and “forest plot.”
Results
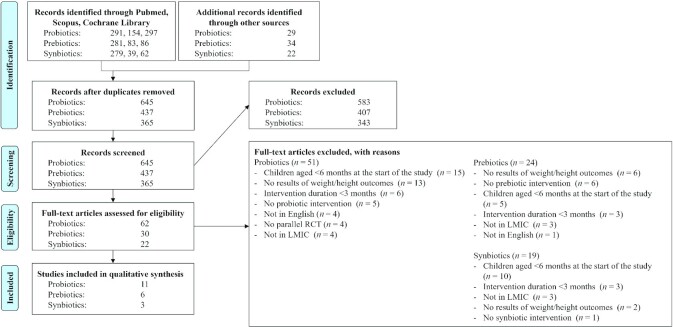
Figure 1 shows the PRISMA flow diagram of the inclusion of studies. A total of 20 articles, all published in the last 2 decades, were included in this systematic review.
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 flow diagram for probiotics, prebiotics, and synbiotics. LMIC, low- and middle-income country; RCT, randomized controlled trials.
Probiotics
Eleven studies with probiotics met the inclusion criteria (34, 40–49). Table 1 shows the study characteristics. In total, the trials analyzed the data of 5776 children. An equal number of 4 studies included healthy children (40, 42, 44, 49) and undernourished children (41, 43, 45, 46). In 3 studies the health status was not well described (34, 47, 48). The interventions included different species, mostly from the Lactobacillus genus (40–48), with a milk drink as the predominant vehicle of delivery (34, 40, 45, 47–49). The administered dosage ranged from 5 × 107 to 1.61 × 1010 CFU/d. The control group received most often a placebo (34, 40–42, 44, 47–49) but an isocaloric replacement (46) or nothing (43, 45) was also used.
TABLE 1.
Characteristics of all intervention studies with probiotics included in the review
| Authors | Age | Country | Health status | Children receiving antibiotics | Intervention duration | Probiotic intervention | Control | Vehicle | Growth as primary or secondary outcome |
|---|---|---|---|---|---|---|---|---|---|
| Agustina et al. (40) | 1–6 y | Indonesia | Healthy | Excluded | 6 mo | 5 × 108 CFU L. casei CRL 431 per day (n = 120); 5 × 108 CFU L. reuteri DSM 17938 per day (n = 124) | Placebo (n = 126) | Straws with oil in low-lactose milk | Secondary |
| Grenov et al. (41) | 6–59 mo | Uganda | Severely acutely malnourished | Included | From hospitalization to 8 or 12 wk after discharge | 5 × 109 CFU B. animalis ssp. lactis and 5 × 109 CFU L. rhamnosus GG per day (n = 138) | Placebo (n = 142) | Sachet with maltodextrin | Secondary |
| Hemalatha et al. (42) | 2–5 y | India | Healthy | Excluded | 9 mo | 2–5 × 109 CFU L. paracasei Lpc-37 (ATCC SD5275) per day (n = 105); 2–5 × 109 CFU B. animalis ssp. lactis HN019 (AGAL NM97/09513) per day (n = 113) | Placebo (n = 108) | Milk | Secondary |
| Kara et al. (43) | 0.5–5 y | Turkey | Stunted/underweight and hospitalized | Excluded | 3 mo | 109 CFU L. rhamnosus GG per day (n = 38) | Received nothing additional (n = 33) | Drops | Secondary |
| Kusumo et al. (44) | 2–24 mo | Indonesia | Healthy | Excluded | 90 d | 1.61 × 1010 CFU L. plantarum IS-10506 per day (n = 9); 1.61 × 1010 CFU L. plantarum IS-10506 per day + zinc (n = 10) | Placebo (n = 11); placebo + zinc (n = 8) | Powder with maltodextrin | Secondary |
| Mai et al. (45) | 3–5 y | Vietnam | Nutrient-deprived | Excluded | 12 wk | 6.5 × 109 CFU L. casei strain Shirota per day (n = 510) | Received nothing additional (n = 493) | Fermented milk | Secondary |
| Nopchinda et al. (34) | 6–25 mo | Thailand | Not described | Not described | 6 mo | 1.5 × 109 CFU B. animalis ssp. lactis per day (n = 36); 7.5 × 108 CFU B. animalis ssp. lactis + 7.5 × 108 CFU S. thermophilus per day (n = 23) | Placebo (n = 25) | Milk formula | Primary |
| Saran et al. (46) | 2–5 y | India | Generally undernourished and stunted | Not described | 6 mo | 5 × 107 CFU L. acidophilus per day (n = 50) | 2 biscuits as isocaloric replacement (n = 50) | Curd | Primary |
| Silva et al. (47) | 2–5 y | Brazil | Not described | Not described | 101 class days | 108 CFU L. acidophilus 5 d/wk (n = 69) | Placebo (n = 39) | Milk drink | Secondary |
| Sur et al. (48) | 1–5 y | India | Not described | Not described | 12 wk | 6.5 × 109 CFU L. casei strain Shirota per day (n = 1654) | Placebo (n = 1663) | Nutrient drink | Secondary |
| Surono et al. (49) | 15–54 mo | Indonesia | Healthy | Excluded | 90 d | 2.31 × 108 CFU E. faecium IS-27526 per day (n = 39) | Placebo (n = 40) | UHT low-fat milk | Secondary |
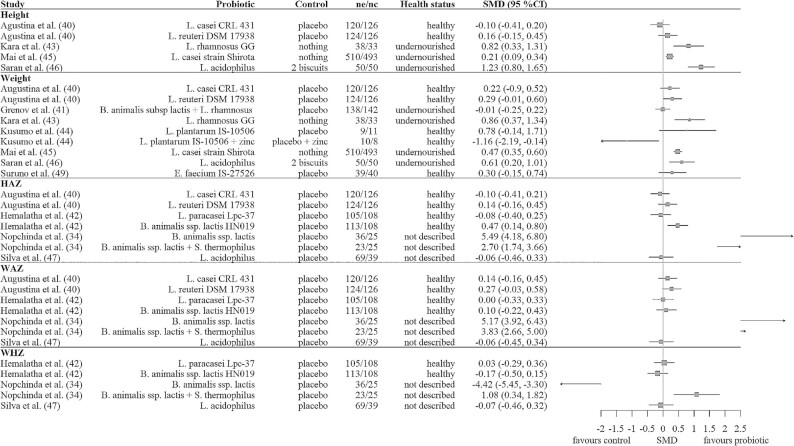
Figure 2 shows the calculated SMDs with 95% CIs for the probiotic studies. Five out of the 11 studies showed a beneficial effect of the probiotic on ≥1 of the growth outcomes. Of the 4 studies including healthy children, only 1 showed beneficial effects. For the studies including undernourished children, this was 3 out of 4. Of the 3 studies in which the health status was not described, 1 showed beneficial effects. Increments in height and weight were found for the probiotics Lactobacillus rhamnosus GG, Lactobacillus casei strain Shirota, and Lactobacillus acidophilus in undernourished children as compared with no treatment or an isocaloric replacement with 2 biscuits. Three studies measured underweight, stunting, or wasting. No significant changes were observed in underweight or stunting after L. casei strain Shirota or Lactobacillus reuteri DSM 17938 supplementation (40), in wasting after Bifidobacterium animalis ssp. lactis and L. rhamnosus GG supplementation (41), or in underweight after L. casei strain Shirota supplementation (48).
FIGURE 2.
Forest plot of the SMDs with 95% CIs for probiotic studies. No summary SMD is calculated owing to heterogeneity and the small number of studies. HAZ, height-for-age z score; nc, number of children in the control group; ne, number of children in the experimental probiotic group; SMD, standardized mean difference; WAZ, weight-for-age z score; WHZ, weight-for-height z score.
Analysis of the microbiota composition was described in 2 of the included studies. In the study of Hemalatha et al. (42) which included healthy children, the overall counts of total bacteria, lactobacilli, and bifidobacteria in the stool samples were similar in the groups receiving Lactobacillus paracasei Lpc-37, B. animalis ssp. lactis HN019, and control before and after the intervention. However, after 6 mo of supplementation, L. paracasei Lpc-37 was significantly higher in those supplemented with L. paracasei Lpc-37, and B. animalis ssp. lactis HN019 was significantly higher in those supplemented with B. animalis ssp. lactis HN019, than in the other groups. Nevertheless, the latter was no longer observed at the end of supplementation (9 mo) when B. animalis ssp. lactis HN019 was found to be similar in all 3 groups. After 9 mo, fecal IgA was significantly decreased in the intervention group receiving B. animalis ssp. lactis HN019 compared with the placebo group. Fecal IgA was not significantly changed in the intervention group receiving L. paracasei Lpc-37 compared with the control group (42).
Sur et al. (48) detected no differences in bacterial, viral, and parasitic agents between the probiotic and control groups of children with diarrhea, except for Aeromonas spp. and Cryptosporidium spp., which were significantly higher in the control group.
Kusumo et al. (44) measured a significantly higher fecal secretory IgA in the placebo group than in the experimental groups receiving Lactobacillus plantarum IS-10506. Kara et al. (43) and Surono et al. (49) observed no significant differences in the change of fecal IgA between the control and probiotic groups receiving either L. rhamnosus GG or Enterococcus faecium IS-27526.
Prebiotics
Six studies with prebiotics met the inclusion criteria (38, 50–54). Table 2 shows the study characteristics. In total, the trials comprised 1207 children. All included children were healthy, except for those in the study of Jones et al. (51) in which undernourished, on-site outpatients were included. In 4 of the studies, oligosaccharides were used as the prebiotic, namely fructo-oligosaccharides (FOSs), galacto-oligosaccharides (GOSs), or a combination of GOSs and polydextrose. The oligosaccharide dosages ranged from 0.5 to 7.5 g/d (38, 52–54). The other 2 studies used n–3 long-chain (LC)-PUFAs from fish oil and α-linolenic acid (18:3n–3) from flaxseed oil as a prebiotic with a dosage of 79–285 mg/d (50, 51). All control groups received a placebo.
TABLE 2.
Characteristics of all intervention studies with prebiotics included in the review1
| Authors | Age | Country | Health status | Children receiving antibiotics | Intervention duration | Prebiotic intervention | Control | Vehicle | Growth as a primary or secondary outcome |
|---|---|---|---|---|---|---|---|---|---|
| Argaw et al. (50) | 6–12 mo | Ethiopia | Healthy | Excluded | 12 mo | 500 mg n–3 LC-PUFA (fish oil) consisting of 285 mg EPA + 215 mg DHA (n = 90) | Placebo (n = 90) | Supplement with 19 micronutrients | Primary |
| Duggan et al. (38) | 6–12 mo | Peru | Healthy | Excluded | 6 mo | 0.5 g OF/d (n = 118); 0.5 g OF + 1 mg Zn/d (n = 157) | Placebo (n = 120); placebo + 1 mg Zn/d (n = 152) | Cereal | Secondary |
| Jones et al. (51) | 6–60 mo | Kenya | Malnourished, on-site outpatients | Not described | 84 d | ALA (flaxseed oil) (n = 20); ALA (flaxseed oil) + 214 mg n–3 LC-PUFA (fish oil) consisting of 79 mg EPA + 135 mg DHA (n = 20); ALA (flaxseed oil) + 214 mg n–3 LC-PUFA (fish oil) consisting of 79 mg EPA + 135 mg DHA ( n = 20) | Placebo (n = 20); placebo (n = 20); placebo + ALA (flaxseed oil) (n = 20) | Ready-to-use therapeutic food | Secondary |
| Nakamura et al. (52) | 25–59 mo | Bangladesh | Healthy | Excluded | 6 mo | 2 g FOSs/d (n = 64) | Placebo (n = 69) | Isotonic solution | Primary |
| Paganini et al. (53) | 6.5–9.5 mo | Kenya | Healthy | Excluded | 4 mo | 7.5 g GOSs + 5 g Fe/d (n = 48) | Placebo + 5 g Fe (n = 49) | Micronutrient powder | Secondary |
| Ribeiro et al. (54) | 9–48 mo | Brazil | Healthy | Included | 108 d | 1 g GOSs + 1 g PDX/d (n = 63) | Placebo (n = 67) | Cow milk–based follow-on formula | Secondary |
1ALA, α-linolenic acid; FOS, fructo-oligosaccharide; GOS, galacto-oligosaccharide; LC-PUFA, long-chain PUFA; OF, oligofructose; PDX, polydextrose.
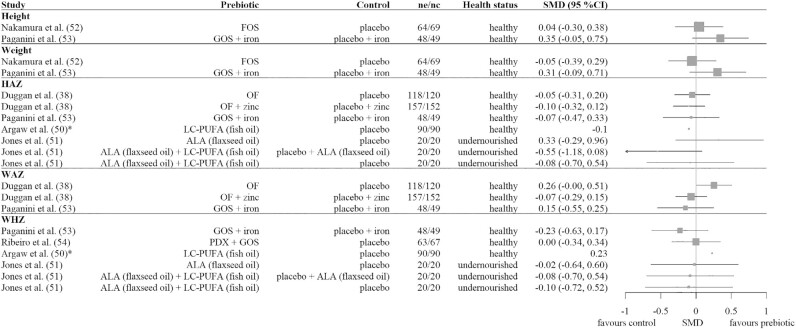
Figure 3 shows the SMDs with 95% CIs for the growth outcomes of the prebiotic studies. No significant effects of the prebiotics were seen. Paganini et al. (53) reported as well that they did not observe significant differences in the change of underweight, wasting, or stunting between the groups. Argaw et al. (50) did not report any measure of variance but described that they found no significant effect on HAZ and a small but significant positive effect on WHZ. The change in the number of stunted and wasted children was 15 (16.6%) and −2 (−2.2%) in the intervention group compared with 12 (14.4%) and 1 (1.11%) in the control group, respectively.
FIGURE 3.
Forest plot of the SMDs with 95% CIs for prebiotic studies. No summary SMD is calculated owing to heterogeneity and the small number of studies. *Mean difference because no SD was given. ALA, α-linolenic acid; FOS, fructo-oligosaccharide; GOS, galacto-oligosaccharide; HAZ, height-for-age z score; LC-PUFA, long-chain PUFA; nc, number of children in the control group; ne, number of children in the experimental prebiotic group; OF, oligofructose; PDX, polydextrose; SMD, standardized mean difference; WAZ, weight-for-age z score; WHZ, weight-for-height z score.
Analysis of the microbiota was only performed by Paganini et al. (53). They observed that in the GOS + iron group there were higher abundances of bifidobacteria and lactobacilli, significantly lower abundances of Clostridiales, and significantly lower abundances of virulence and toxin genes (VTGs) of pathogens than in the iron group (53).
Synbiotics
Four synbiotic studies met the inclusion criteria (55–58). Table 3 shows the study characteristics. In total, the trials analyzed the data of 1098 children. Two studies included healthy children (56, 57), 1 study included children with failure to thrive (defined as either WAZ < 5th percentile or WHZ < 10th percentile) (55), and 1 study did not describe the health status of participating children (58). All studies had a placebo as a control. The intervention and control treatments were mostly provided as milk-based drinks (56–58), except for Famouri et al. (55) who used a starch powder.
TABLE 3.
Characteristics of all intervention studies with synbiotics included in the review1
| Authors | Age | Country | Health status | Children receiving antibiotics | Intervention duration | Synbiotic intervention | Control | Vehicle | Growth as a primary or secondary outcome |
|---|---|---|---|---|---|---|---|---|---|
| Famouri et al. (55) | 12–54 mo | Iran | Failure to thrive | Not described | 6 mo | 1.5 × 108 spore B. coagulans + 100 mg FOSs (n = 42) | Placebo (n = 42) | Starch powder | Primary |
| Firmansyah et al. (56) | 12 mo | Indonesia | Healthy | Excluded | 4 mo | 5.8 × 108 CFU B. longum BL999 + 1.2 × 109 CFU L. rhamonosus LPR + 2.5 g linoleic acid + 0.3 mg linolenic acid + 13.8 mg AA + 13.2 mg DHA + 0.6 g inulin + 1.4 g FOSs/d (n = 199) | Placebo (n = 194) | Cow milk–based drink | Primary |
| Kosuwon et al. (57) | 1–3 y | Thailand | Healthy | Included (68% received antibiotics since birth) | 12 wk | 1.1 × 1010 CFU Bifidobacterium breve M-16V + 5.4 g scGOSs + 0.6 g lcFOSs/d (n = 60) | Placebo (n = 59) | Young child formula | Secondary |
| Sazawal et al. (58) | 1–4 y | India | Not described | Included | 1 y | 1.6 × 107 CFU Bifidobacterium lactis HN019 + 2.4 g prebiotic oligosaccharides per day (n = 257) | Placebo (n = 245) | Milk powder | Secondary |
1AA, arachidonic acid; FOS, fructo-oligosaccharide; lcFOS, long-chain fructo-oligosaccharide; scGOS, short-chain galacto-oligosaccharide.
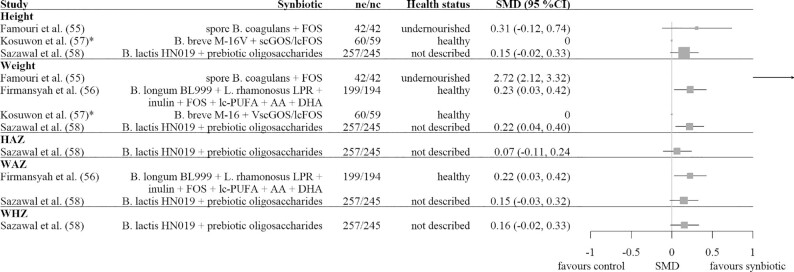
Figure 4 shows the SMDs with 95% CIs for the outcomes of the 4 synbiotic studies. In 3 out of 4 studies, the synbiotic beneficially affected ≥1 of the growth outcomes. The strongest effects were observed for weight and WAZ in the studies of Firmansyah et al. (56) using Bifidobacterium longum BL999 + L. rhamnosus LPR + inulin + FOSs + LC-PUFAs + arachidonic acid (20:4n–6) + DHA (22:6n–3), and of Sazawal et al. (58) using Bifidobacterium lactis HN019 + prebiotic oligosaccharides. In addition to the outcomes shown in Figure 4, Firmansyah et al. (56) found no significant treatment effect on height gain, but they did not report the quantitative data.
FIGURE 4.
Forest plot of the SMDs with 95% CIs for synbiotic studies. No summary SMD is calculated owing to heterogeneity and the small number of studies. For Firmansyah et al. (56) the least-squares mean was used. *Mean difference because no SD was given. AA, arachidonic acid; FOS, fructo-oligosaccharide; HAZ, height-for-age z score; nc, number of children in the control group; ne, number of children in the experimental synbiotic group; scGOS/lcFOS, short-chain galacto-oligosaccharide/long-chain fructo-oligosaccharide; SMD, standardized mean difference; WAZ, weight-for-age z score; WHZ, weight-for-height z score.
Analysis of the microbiota was only performed by Kosuwon et al. (57). They showed that the change in proportion and absolute counts of bifidobacteria were significantly higher in the synbiotic group receiving Bifidobacterium breve M-16 with short-chain (sc)GOSs/long chain (lc)FOSs than in the control group. No significant changes were seen for bacterial members belonging to the Eubacterium rectale–Clostridium coccoides, Clostridium histolyticum, Clostridium lituseburense, Bacteroides–Prevotella, Lactobacillus–Enterococcus, or Enterobacteriacaeae groups. The secretory IgA concentration increased in the synbiotic group and slightly dropped in the control group during the 12 wk of treatment, but these changes were not statistically different.
Quality and certainty of the evidence
Supplemental Tables 11–13 show the quality assessment of the individual studies as conducted with the Cochrane Risk of Bias tool. Supplemental Table 14 presents the certainty of evidence as assessed with GRADE. The certainty of the evidence for probiotics was judged as very low owing to methodological limitations of the studies, inconsistency of the results, and imprecision. The certainty of the evidence for prebiotics was judged as low owing to methodological limitations of the studies and imprecision. The certainty of the evidence for synbiotics was judged as moderate owing to imprecision.
Discussion
In this review, 5 out of the 11 probiotic studies showed a beneficial effect of probiotics on ≥1 of the growth outcomes as compared with control, of which 1 out of 4 studies were in healthy children and 3 out of 4 studies were in undernourished children. Of the 3 studies in which the health status was not described, 1 showed beneficial effects. No significant effects of prebiotics on any of the growth outcomes were seen. Synbiotics appeared more beneficial than probiotics and prebiotics alone, with 3 out of 4 studies showing a beneficial effect of the synbiotics on the growth outcomes. More specifically, the probiotic strains L. rhamnosus GG and L. casei strain Shirota showed consistent beneficial effects on the growth outcomes. In addition, the species L. acidophilus, B. animalis ssp. lactis, and Bacillus coagulans showed overall beneficial effects in the probiotic and synbiotic studies. Furthermore, the prebiotics GOSs, oligosaccharides (including oligofructose), and the combination of linolenic acid, linoleic acid, arachidonic acid, DHA, inulin, and FOSs showed beneficial effects on the growth of children living in LMICs.
Probiotics
The 11 studies gave little evidence that probiotics affect the growth of children when compared with control. A higher percentage of studies including undernourished children showed an effect than of studies including healthy children. The certainty of the evidence for probiotics was judged as low owing to methodological limitations of the studies, inconsistency of the results, and imprecision.
A limitation of the probiotic analysis was that antibiotic usage was not taken into account owing to the small number of included studies, yet this could affect the outcomes. Antibiotics reduce gut colonization and hence the efficacy of probiotics (59). Probiotics may be more effective in undernourished children than in healthy children, because they may restore their dysbiotic microbiota, affecting growth (60, 61). Notably, the study by Nopchinda et al. (34) had large SMDs and although B. animalis ssp. lactis + Streptococcus thermophilus increased HAZ and WHZ, it decreased WAZ. Moreover, the control group had a decreased MD in HAZ, but increased the MD in WAZ and WHZ after 6 mo of intervention. One would expect an increase in weight before an increase in height (62). This, together with the small sample size of the study, raises questions about the quality of the study.
Compared with the review of Onubi et al. (12), we included 6 more recent studies with an intervention duration of ≥3 mo, whereas Onubi et al. (12) included studies with a duration of ≥14 d and located in either developing or developed countries. In addition, Onubi et al. (12) included 4 synbiotic studies and 1 study with yogurt (12), which were excluded in our probiotic analysis. The systematic review of Onubi et al. (12) observed a benefit of probiotics on weight and height gain in undernourished children in developing countries and a possible benefit in well-nourished children. Likewise, our review observed predominantly a beneficial effect of probiotics on the growth outcomes in undernourished children. This could be explained by the microbiota-modulating effect of probiotics. Probiotics may ameliorate dysbiotic microbiota in undernourished children, thereby affecting growth (60, 61). However, in this review, undernourished children often received nothing or an isocaloric replacement as control, whereas healthy children received a placebo as control. For the interventions in undernourished children, differences in macronutrient or micronutrient composition between the intervention and placebo might be responsible for unequal effects on growth. Nevertheless, Onubi et al. (12) included studies in which also other components, like prebiotics, next to the probiotic differed between the intervention and control groups. Because Onubi et al. (12) did observe the same distinction in the effect of probiotics on growth between healthy and undernourished children, it is most likely that the beneficial effect of probiotics in undernourished children and lack of effect in healthy children is at least partially explained by the children's health status, which is also theoretically supported.
Prebiotics
The 6 identified studies in the current review did not show an effect of prebiotics on any of the growth outcomes of children living in LMICs. The certainty of the evidence for prebiotics was judged as low owing to methodological limitations of the studies and imprecision.
To date, no reviews are available concerning the effects of prebiotics on the growth of children living in LMICs. Most research is performed on healthy infants living in high-income countries. In contrast to our findings, in the meta-analysis by Mugambi et al. (23), prebiotics significantly increased weight gain of full-term healthy infants aged 0–1 y as compared with control (MD: 0.97 g/d; 95% CI: 0.24, 1.70 g/d; P = 0.01), but did not affect height gain (MD: 0.01 cm/wk; 95% CI: −0.01, 0.04 cm/wk; P = 0.34). Mugambi et al. (23) found that prebiotics increased the abundance of bifidobacteria nonsignificantly (MD: 0.92; 95% CI: −0.03, 1.86; P = 0.06), whereas no changes in the abundance of lactobacilli and pathogens were found. In line with our findings, the systematic review by Skórka et al. (63) observed no growth effects of formula supplemented with prebiotics in healthy term infants.
The lack of effect found in our study may be explained by the small number of included trials, by lack of power because the growth outcomes were mostly secondary outcomes based on small sample sizes, and because only healthy children were included. Furthermore, the doses of 0.5–2 g of prebiotics used by Duggan et al. (38), Nakamura et al. (52), and Ribeiro et al. (54) may have been too low to induce an effect, because most prebiotics require an oral dosage of ≥3 g/d to induce an effect (15, 64). For inulin, an amount of even 8 g/d is needed (65). A lower dosage may not ensure the selective effect of the prebiotic in the gut and thus its beneficial effects on physical growth. For example, studies in human adults show no bifidogenic effects for 2.5 g GOSs (66) or 2.5 g short-chain FOSs (67), but do show bifidogenic and thus prebiotic effects for 5 g GOSs (66) and 5 g short-chain FOSs (67). Paganini et al. (53) showed that 7.5 g GOSs + iron beneficially changed the microbiota of healthy children living in LMICs as compared with iron only. The effect of prebiotics in healthy children might be different than in undernourished children, because undernourished children have immature, less diverse, and dysbiotic gut microbiota (5). No evidence for the effect of prebiotics in undernourished children from a well-powered study is present.
Synbiotics
The 4 included studies showed overall a beneficial effect of synbiotics on growth outcomes, especially weight. The certainty of the evidence for synbiotics was judged as moderate owing to imprecision.
Previous reviews investigating the effect of synbiotics on the growth of children included studies from developed countries only. The reviews by Mugambi et al. (23) and the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) both included the same 3 European synbiotic studies in which combinations of bifidobacteria and/or lactobacilli with GOSs and/or FOSs were investigated. In contrast to our findings, these studies did not show an increase in height or weight of full-term infants (23, 68). This difference in outcome compared with our study could be explained by the fact that our review only included studies in LMICs, where the potential of synbiotics might be larger than in developed countries owing to the environment and other related factors.
Synbiotics are developed such that they can work in a synergistic manner, in which the prebiotics are specifically and preferentially fermented by the probiotic. This increases the opportunity for the probiotic to adhere and grow in the gut (69). In theory, this could especially be true for undernourished children who have dysbiotic gut microbiota and increased colonization potential. Kosuwon et al. (57) did not include an scGOS/lcFOS arm alone nor a B. breve M-16V arm alone, and could therefore not conclude whether the effect was attributable to either of the 2 components or a combinatorial effect. However, in the study of Chua et al. (70), the same synbiotic was used as by Kosuwon et al. (57), and they observed that the synbiotic caused earlier colonization of the gut microbiota by bifidobacteria in infants with a compromised microbiota at birth than when the prebiotic alone was administered to the infants. Nevertheless, because there was no probiotic arm alone, no conclusion could be made as to whether the effect was only due to the probiotic, or whether the effect was due to a synergistic effect of both the probiotic and prebiotic. Similarly, the other 3 included synbiotic studies in our review were not designed to investigate whether the synbiotics work complementarily or synergistically. Moreover, they did not give a theoretical underpinning of the complementary or synergistic properties of their synbiotics (55, 56, 58).
Noteworthy is that Famouri et al. (55) used a very low dosage of FOSs (0.1 g/d), whereas a daily dose of 2.5–10 g prebiotics is suggested to be required to exert beneficial effects (71). So, B. coagulans is most likely the major contributor to the beneficial effect on growth demonstrated in their study including children with failure to thrive.
The gut microbiome
Only 5 probiotic studies, 1 prebiotic study, and 1 synbiotic study included markers of gut health and microbiota. Of these studies, only 2 observed a clear beneficial effect on the markers of gut health and microbiota. Kusumo et al. (44) measured a significantly higher fecal secretory IgA in the placebo group than in the experimental groups receiving L. plantarum IS-10506 and Paganini et al. (53) observed that in the GOSs + iron group there were higher abundances of bifidobacteria and lactobacilli, significantly lower abundances of Clostridiales, and significantly lower abundances of VTGs of pathogens than in the iron group. The 5 other studies might lack effects because they were mostly performed in healthy children. Whereas healthy children have developed a healthy microbiota, undernourished children have an immature, less diverse, and dysbiotic gut microbiota which might be modified using gut microbiota–targeted nutritional interventions (5).
Increased attention has been paid to the colonization and development of the intestinal microbiota and the genetic, biological, and environmental influences. However, there are still many unknowns regarding what characterizes a healthy intestinal microbiome and the underlying mechanisms involved, especially within LMIC contexts. Referential phenotypes of “mature” compared with “immature” microbiota should be developed to better understand the pathogenesis of the intestinal microbiota (25). This is needed to determine the extent of the benefits of the gut microbiota–targeted nutritional interventions.
Strengths, limitations, and further research
This is the first review that we know of to analyze and give a comprehensive overview of the available research on several nutrition-based gut-targeted interventions in children 6–59 mo old living in LMICs. It encompasses food-based, published, randomized, controlled intervention studies with a duration of ≥3 mo, multiple growth outcomes, and gut microbial outcomes, which give valuable insights on the mechanisms for the effects of the interventions.
Overall, the evidence was too limited to draw firm conclusions about the effect of gut-targeted interventions on growth outcomes in children living in LMICs, because only a small number of studies per intervention type was obtained and the included studies were heterogeneous in study populations and treatments. Owing to the small number of studies, no subgroup analysis based on antibiotic use or health status could be conducted. The dose, duration, and composition of probiotics, prebiotics, and synbiotics are likely to be specific for several factors such as host microbiota, medication, habitual diet, and possibly yet-to-be-identified host genetic factors (18). This partially explained the low certainty of the evidence. The certainty of the evidence for probiotics, prebiotics, and synbiotics was very low to moderate, mostly owing to imprecision and methodological limitations of the studies. Moreover, there could be potential publication bias because only published articles were included.
More research is required to further investigate the efficacy of gut-targeted nutritional interventions on growth in children living in LMICs. Especially synbiotics showed potential. In such studies, gut microbiota composition should be analyzed to obtain a better insight into the mechanisms. When doing so, the effects of antibiotic use on the relation between gut-targeted nutritional interventions and growth should be explored.
Furthermore, it would be of interest to investigate the role of the initial health status of the children on the effects of the gut-targeted interventions, because in our review effects of probiotics were primarily seen in undernourished children, whereas synbiotics had positive effects in both undernourished and healthy children. Moreover, attention should be paid to other community and societal factors such as health care, education, society and culture, and agriculture and food systems. These factors have an impact on growth and development of children (72) and may influence the effectiveness of gut-targeted nutritional interventions.
The potential of new and promising upcoming nutritional interventions such as MDCFs (73) and postbiotics (74) should be investigated. MDCF is complementary food made of locally available food ingredients that can stimulate the proliferation of growth-promoting members of the gut microbiota (75). Recently Chen et al. (76) showed beneficial effects of an MDCF prototype on several growth outcomes as compared with ready-to-use supplementary food in Bangladeshi children with moderate acute malnutrition aged between 12 and 18 mo. Postbiotics are preparations of inanimate microorganisms and/or their components that confer a health benefit on the host (77). As a next step, it would be of interest to include promising gut-targeted interventions in combination with proven nutrition or water, sanitation, and hygiene (WASH) interventions (78–80) to assess whether these have additional or synergistic effects on growth outcomes.
Conclusion
This review indicated that, to date, overall no firm conclusions can be made about the effect of probiotics, prebiotics, and synbiotics on the growth of children living in LMICs, owing to high heterogeneity, the limited number of studies present, and the low certainty of the evidence. Although promising effects of specific probiotic strains and synbiotics on improving growth in both healthy and undernourished children were demonstrated in approximately half of the studies, more large-scale, well-designed controlled trials are needed to confirm the effect of these gut-targeted interventions. Specific attention should be paid to the role of the microbiota, antibiotic use, and the initial nutritional and health status of the children.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—LAJH, SP, AG, and AE: conducted the research; LAJH: analyzed the data and wrote the paper; AE: had primary responsibility for the final content; and all authors: designed the research and edited, read, and approved the final manuscript.
Notes
Supported by Unilever. The funder was involved in the design, analysis, and interpretation of the data.
Author disclosures: SP, AG, and AE are employees of Unilever. All other authors report no conflicts of interest.
Supplemental Tables 1–14 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/cdn/.
Abbreviations used: FOS, fructo-oligosaccharide; GOS, galacto-oligosaccharide; GRADE, Grading of Recommendations Assessment, Development and Evaluation; HAZ, height-for-age z score; ISAPP, International Scientific Association for Probiotics and Prebiotics; lcFOS, long-chain fructo-oligosaccharide; LC-PUFA, long-chain PUFA; LMIC, low- and middle-income country; MAZ, microbiota-for-age z score; MD, mean difference; MDCF, microbiota-directed complementary food; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; scGOS, short-chain galacto-oligosaccharide; SMD, standardized mean difference; VTG, virulence and toxin gene; WAZ, weight-for-age z score; WHZ, weight-for-height z score.
Contributor Information
Lise AJ Heuven, Division of Human Nutrition and Health, Wageningen University & Research, Wageningen, Netherlands; Unilever Foods Innovation Centre, Wageningen, Netherlands.
Simone Pyle, Unilever Foods Innovation Centre, Wageningen, Netherlands.
Arno Greyling, Unilever Foods Innovation Centre, Wageningen, Netherlands.
Alida Melse-Boonstra, Division of Human Nutrition and Health, Wageningen University & Research, Wageningen, Netherlands.
Ans Eilander, Email: ans.eilander@unilever.com, Unilever Foods Innovation Centre, Wageningen, Netherlands.
References
- 1. WHO . Child growth. [Internet]. Geneva, Switzerland: World Health Organization; 2021; [cited 20 January, 2021]. Available from: https://www.who.int/health-topics/child-growth. [Google Scholar]
- 2. Park JJ, Harari O, Siden E, Dron L, Zannat N-E, Singer J, Lester RT, Thorlund K, Mills EJ. Interventions to improve linear growth during complementary feeding period for children aged 6-24 months living in low- and middle-income countries: a systematic review and network meta-analysis. Gates Open Res. 2020;3:1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. WHO . Global targets 2025: to improve maternal, infant and young child nutrition. [Internet]. Geneva, Switzerland: World Health Organization; 2014 [cited 20 September, 2020]. Available from: https://www.who.int/teams/nutrition-and-food-safety/global-targets-2025. [Google Scholar]
- 4. WHO . Reducing stunting in children: equity considerations for achieving the Global Nutrition Targets 2025. [Internet]. Geneva, Switzerland: World Health Organization; 2018; [cited 20 September, 2020]. Available from: https://apps.who.int/iris/bitstream/handle/10665/260202/9789241513647-eng.pdf?sequence=1&isAllowed=y. [Google Scholar]
- 5. Robertson RC. The gut microbiome in child malnutrition. Nestle Nutr Inst Workshop Ser. 2020;93:133–44. [DOI] [PubMed] [Google Scholar]
- 6. Robertson RC, Manges AR, Finlay BB, Prendergast AJ. The human microbiome and child growth – first 1000 days and beyond. Trends Microbiol. 2019;27(2):131–47. [DOI] [PubMed] [Google Scholar]
- 7. Mirzaei MK, Khan MAA, Ghosh P, Taranu ZE, Taguer M, Ru J, Chowdhury R, Kabir MM, Deng L, Mondal Det al. Bacteriophages isolated from stunted children can regulate gut bacterial communities in an age-specific manner. Cell Host Microbe. 2020;27(2):199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aakko J, Grześkowiak Ł, Asukas T, Päivänsäde E, Lehto K-M, Fan Y-M, Mangani C, Maleta K, Ashorn P, Salminen S. Lipid-based nutrient supplements do not affect gut Bifidobacterium microbiota in Malawian infants: a randomized trial. J Pediatr Gastroenterol Nutr. 2017;64(4):610–15. [DOI] [PubMed] [Google Scholar]
- 9. Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen Set al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506–14. [DOI] [PubMed] [Google Scholar]
- 10. Ouwehand AC, Salminen S, Isolauri E. Probiotics: an overview of beneficial effects. Antonie Van Leeuwenhoek. 2002;82(1/4):279–89. [PubMed] [Google Scholar]
- 11. Bermudez-Brito M, Plaza-Díaz J, Muñoz-Quezada S, Gómez-Llorente C, Gil A. Probiotic mechanisms of action. Ann Nutr Metab. 2012;61(2):160–74. [DOI] [PubMed] [Google Scholar]
- 12. Onubi OJ, Poobalan AS, Dineen B, Marais D, McNeill G. Effects of probiotics on child growth: a systematic review. J Health Popul Nutr. 2015;34:8–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Angelakis E, Merhej V, Raoult D. Related actions of probiotics and antibiotics on gut microbiota and weight modification. Lancet Infect Dis. 2013;13(10):889–99. [DOI] [PubMed] [Google Scholar]
- 14. Solis B, Samartin S, Gomez S, Nova E, de la Rosa B, Marcos A. Probiotics as a help in children suffering from malnutrition and diarrhoea. Eur J Clin Nutr. 2002;56(S3):S57–9. [DOI] [PubMed] [Google Scholar]
- 15. Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Scott K, Stanton C, Swanson KS, Cani PDet al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14(8):491–502. [DOI] [PubMed] [Google Scholar]
- 16. Pekmez CT, Dragsted LO, Brahe LK. Gut microbiota alterations and dietary modulation in childhood malnutrition – the role of short chain fatty acids. Clin Nutr. 2019;38(2):615–30. [DOI] [PubMed] [Google Scholar]
- 17. Slavin J. Fiber and prebiotics: mechanisms and health benefits. Nutrients. 2013;5(4):1417–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Swanson KS, Gibson GR, Hutkins R, Reimer RA, Reid G, Verbeke K, Scott KP, Holscher HD, Azad MB, Delzenne NMet al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat Rev Gastroenterol Hepatol. 2020;17(11):687–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Markowiak P, Śliżewska K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients. 2017;9(9):1021–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goudet SM, Bogin BA, Madise NJ, Griffiths PL. Nutritional interventions for preventing stunting in children (birth to 59 months) living in urban slums in low- and middle-income countries (LMIC). Cochrane Database Syst Rev. 2019;6(6):CD011695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Panjwani A, Heidkamp R. Complementary feeding interventions have a small but significant impact on linear and ponderal growth of children in low- and middle-income countries: a systematic review and meta-analysis. J Nutr. 2017;147(11):2169S–78S. [DOI] [PubMed] [Google Scholar]
- 22. Dror T, Dickstein Y, Dubourg G, Paul M. Microbiota manipulation for weight change. Microb Pathog. 2017;106:146–61. [DOI] [PubMed] [Google Scholar]
- 23. Mugambi MN, Musekiwa A, Lombard M, Young T, Blaauw R. Synbiotics, probiotics or prebiotics in infant formula for full term infants: a systematic review. Nutr J. 2012;11:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Szajewska H, Chmielewska A. Growth of infants fed formula supplemented with Bifidobacteriumlactis Bb12 or Lactobacillus GG: a systematic review of randomized controlled trials. BMC Pediatr. 2013;13:185–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Andrews K, Gonzalez A. Contextual risk factors impacting the colonization and development of the intestinal microbiota: implications for children in low- and middle-income countries. Dev Psychobiol. 2019;61(5):714–28. [DOI] [PubMed] [Google Scholar]
- 26. Grantham-McGregor SM, Fernald L, Kagawa R, Walker S. Effects of integrated child development and nutrition interventions on child development and nutritional status. Ann N Y Acad Sci. 2014;1308(1):11–32. [DOI] [PubMed] [Google Scholar]
- 27. O'Mahony SM, Clarke G, Dinan T, Cryan J. Early-life adversity and brain development: is the microbiome a missing piece of the puzzle?. Neuroscience. 2017;342:37–54. [DOI] [PubMed] [Google Scholar]
- 28. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. World Bank . World Bank country and lending groups. [Internet]. Washington (DC): The World Bank; 2020; [cited 3 September, 2020]. Available from: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups. [Google Scholar]
- 30. De Onis M, Onyango A, Borghi E, Siyam A, Pinol A, Garza C, Martines J, Martorell R, Victora C, Bhan Met al. WHO child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. [Internet]. Geneva, Switzerland: World Health Organization; 2006; [cited 21 September, 2020]. Available from: https://www.who.int/publications/i/item/924154693X. [Google Scholar]
- 31. Royal College of Paediatrics and Child Health (RCPCH) . Plotting and assessing infants and toddlers up to age 4 years: UK-WHO Growth Charts. [Internet]. London, United Kingdom: RCPCH; 2009; [cited 12 November, 2020]. Available from: https://www.rcpch.ac.uk/sites/default/files/Plotting_toddlers.pdf. [Google Scholar]
- 32. Schünemann H, Brożek J, Guyatt G, Oxman Aeditors. The GRADE handbook. [Internet]. London, United Kingdom: The Cochrane Collaboration; 2013; [cited 12 November, 2020]. Available from:https://gdt.gradepro.org/app/handbook/handbook.html. [Google Scholar]
- 33. Murad MH, Mustafa RA, Schünemann HJ, Sultan S, Santesso N. Rating the certainty in evidence in the absence of a single estimate of effect. Evid Based Med. 2017;22(3):85–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nopchinda S, Varavithya W, Phuapradit P, Sangchai R, Suthutvoravut U, Chantraruksa V, Haschke F. Effect of Bifidobacterium Bb12 with or without Streptococcus thermophilus supplemented formula on nutritional status. J Med Assoc Thai. 2002;85(S4):S1225–31. [PubMed] [Google Scholar]
- 35. Higgins JPT, Li T, Deeks, J. Choosing effect measures and computing estimates of effect. [Internet]. In: Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, Welch Veditors. Cochrane handbook for systematic reviews of interventions, version 6.1. Cochrane; 2020; [cited 12 November, 2020]. Available from: https://training.cochrane.org/handbook/current/chapter-06. [Google Scholar]
- 36. Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol. 1992;45(7):769–73. [DOI] [PubMed] [Google Scholar]
- 37. Leppik A, Jürimäe T, Jürimäe J. Reproducibility of anthropometric measurements in children: a longitudinal study. Anthropol Anz. 2004;62(1):79–91. [PubMed] [Google Scholar]
- 38. Duggan C, Penny ME, Hibberd P, Gil A, Huapaya A, Cooper A, Coletta F, Emenhiser C, Kleinman RE. Oligofructose-supplemented infant cereal: 2 randomized, blinded, community-based trials in Peruvian infants. Am J Clin Nutr. 2003;77(4):937–42. [DOI] [PubMed] [Google Scholar]
- 39. Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, Welch V editors. Cochrane handbook for systematic reviews of interventions version 6.1. [Internet]. Cochrane; 2020; [cited 12 November, 2020]. Available from: https://training.cochrane.org/handbook/current. [Google Scholar]
- 40. Agustina R, Bovee-Oudenhoven IMJ, Lukito W, Fahmida U, van de Rest O, Zimmermann MB, Firmansyah A, Wulanti R, Albers R, van den Heuvel EGHMet al. Probiotics Lactobacillusreuteri DSM 17938 and Lactobacilluscasei CRL 431 modestly increase growth, but not iron and zinc status, among Indonesian children aged 1–6 years. J Nutr. 2013;143(7):1184–93. [DOI] [PubMed] [Google Scholar]
- 41. Grenov B, Namusoke H, Lanyero B, Nabukeera-Barungi N, Ritz C, Mølgaard C, Friis H, Michaelsen KF. Effect of probiotics on diarrhea in children with severe acute malnutrition: a randomized controlled study in Uganda. J Pediatr Gastroenterol Nutr. 2017;64(3):396–403. [DOI] [PubMed] [Google Scholar]
- 42. Hemalatha R, Ouwehand A, Forssten S, Geddan J, Mamidi R, Bhaskar V, Radhakrishna K. A community-based randomized double blind controlled trial of Lactobacillusparacasei and Bifidobacteriumlactis on reducing risk for diarrhea and fever in preschool children in an urban slum in India. Eur J Nutr Food Saf. 2014;4(4):325–41. [Google Scholar]
- 43. Kara S, Volkan B, Erten I. Lactobacillus rhamnosus GG can protect malnourished children. Benef Microbes. 2019;10(3):237–44. [DOI] [PubMed] [Google Scholar]
- 44. Kusumo P, Bela B, Wibowo H, Munasir Z, Surono I. Lactobacillus plantarum IS-10506 supplementation increases faecal sIgA and immune response in children younger than two years. Benef Microbes. 2019;10(3):245–52. [DOI] [PubMed] [Google Scholar]
- 45. Mai TT, Thu PT, Hang HT, Trang TTT, Yui S, Shigehisa A, Tien VT, Dung TV, Nga PB, Hung NTet al. Efficacy of probiotics on digestive disorders and acute respiratory infections: a controlled clinical trial in young Vietnamese children. Eur J Clin Nutr. 2021;75(3):513–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Saran S, Gopalan S, Krishna TP. Use of fermented foods to combat stunting and failure to thrive. Nutrition. 2002;18(5):393–6. [DOI] [PubMed] [Google Scholar]
- 47. Silva MR, Dias G, Ferreira CL, Franceschini SC, Costa NM. Growth of preschool children was improved when fed an iron-fortified fermented milk beverage supplemented with Lactobacillusacidophilus. Nutr Res. 2008;28(4):226–32. [DOI] [PubMed] [Google Scholar]
- 48. Sur D, Manna B, Niyogi S, Ramamurthy T, Palit A, Nomoto K, Takahashi T, Shima T, Tsuji H, Kurakawa Tet al. Role of probiotic in preventing acute diarrhoea in children: a community-based, randomized, double-blind placebo-controlled field trial in an urban slum. Epidemiol Infect. 2011;139(6):919–26. [DOI] [PubMed] [Google Scholar]
- 49. Surono IS, Koestomo FP, Novitasari N, Zakaria FR. Novel probiotic Enterococcusfaecium IS-27526 supplementation increased total salivary sIgA level and bodyweight of pre-school children: a pilot study. Anaerobe. 2011;17(6):496–500. [DOI] [PubMed] [Google Scholar]
- 50. Argaw A, Wondafrash M, Bouckaert KP, Kolsteren P, Lachat C, Belachew T, De Meulenaer B, Huybregts L. Effects of n–3 long-chain PUFA supplementation to lactating mothers and their breastfed children on child growth and morbidity: a 2× 2 factorial randomized controlled trial in rural Ethiopia. Am J Clin Nutr. 2018;107(3):454–64. [DOI] [PubMed] [Google Scholar]
- 51. Jones KD, Ali R, Khasira MA, Odera D, West AL, Koster G, Akomo P, Talbert AW, Goss VM, Ngari Met al. Ready-to-use therapeutic food with elevated n-3 polyunsaturated fatty acid content, with or without fish oil, to treat severe acute malnutrition: a randomized controlled trial. BMC Med. 2015;13(1):93–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nakamura S, Sarker SA, Wahed MA, Wagatsuma Y, Oku T, Moji K. Prebiotic effect of daily fructooligosaccharide intake on weight gain and reduction of acute diarrhea among children in a Bangladesh urban slum: a randomized double-masked placebo-controlled study. Trop Med Health. 2006;34(3):125–31. [Google Scholar]
- 53. Paganini D, Uyoga MA, Kortman GA, Cercamondi CI, Moretti D, Barth-Jaeggi T, Schwab C, Boekhorst J, Timmerman HM, Lacroix Cet al. Prebiotic galacto-oligosaccharides mitigate the adverse effects of iron fortification on the gut microbiome: a randomised controlled study in Kenyan infants. Gut. 2017;66(11):1956–67. [DOI] [PubMed] [Google Scholar]
- 54. Ribeiro TC, Costa-Ribeiro H Jr, Almeida PS, Pontes MV, Leite ME, Filadelfo LR, Khoury JC, Bean JA, Mitmesser SH, Vanderhoof JAet al. Stool pattern changes in toddlers consuming a follow-on formula supplemented with polydextrose and galactooligosaccharides. J Pediatr Gastroenterol Nutr. 2012;54(2):288–90. [DOI] [PubMed] [Google Scholar]
- 55. Famouri F, Khoshdel A, Golshani A, Kheiri S, Saneian H, Kelishadi R. Effects of synbiotics on treatment of children with failure to thrive: a triple blind placebo-controlled trial. J Res Med Sci. 2014;19(11):1046–50. [PMC free article] [PubMed] [Google Scholar]
- 56. Firmansyah A, Dwipoerwantoro PG, Kadim M, Alatas S, Conus N, Lestarina L, Bouisset F, Steenhout P. Improved growth of toddlers fed a milk containing synbiotics. Asia Pac J Clin Nutr. 2011;20(1):69–76. [PubMed] [Google Scholar]
- 57. Kosuwon P, Lao-Araya M, Uthaisangsook S, Lay C, Bindels J, Knol J, Chatchatee P. A synbiotic mixture of scGOS/lcFOS and Bifidobacteriumbreve M-16V increases faecal Bifidobacterium in healthy young children. Benef Microbes. 2018;9(4):541–52. [DOI] [PubMed] [Google Scholar]
- 58. Sazawal S, Dhingra U, Hiremath G, Sarkar A, Dhingra P, Dutta A, Menon VP, Black RE. Effects of Bifidobacteriumlactis HN019 and prebiotic oligosaccharide added to milk on iron status, anemia, and growth among children 1 to 4 years old. J Pediatr Gastroenterol Nutr. 2010;51(3):341–6. [DOI] [PubMed] [Google Scholar]
- 59. Kerac M, Bunn J, Seal A, Thindwa M, Tomkins A, Sadler K, Bahwere P, Collins S. Probiotics and prebiotics for severe acute malnutrition (PRONUT study): a double-blind efficacy randomised controlled trial in Malawi. Lancet. 2009;374(9684):136–44. [DOI] [PubMed] [Google Scholar]
- 60. Castro-Mejía JL, O'Ferrall S, Krych Ł, O'Mahony E, Namusoke H, Lanyero B, Kot W, Nabukeera-Barungi N, Michaelsen KF, Mølgaard Cet al. Restitution of gut microbiota in Ugandan children administered with probiotics (Lactobacillus rhamnosus GG and Bifidobacteriumanimalis subsp. lactis BB-12) during treatment for severe acute malnutrition. Gut Microbes. 2020;11(4):855–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Edwards PTK, Kashyap PC, Preidis GA. Microbiota on biotics: probiotics, prebiotics, and synbiotics to optimize growth and metabolism. Am J Physiol Gastrointest Liver Physiol. 2020;319(3):G382–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Richard SA, Black RE, Checkley W. Revisiting the relationship of weight and height in early childhood. Adv Nutr. 2012;3(2):250–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Skórka A, Pieścik-Lech M, Kołodziej M, Szajewska H. Infant formulae supplemented with prebiotics: are they better than unsupplemented formulae? An updated systematic review. Br J Nutr. 2018;119(7):810–25. [DOI] [PubMed] [Google Scholar]
- 64. Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, Rowland I, Wolvers D, Watzl B, Szajewska H, Stahl B. Prebiotic effects: metabolic and health benefits. Br J Nutr. 2010;104(S2):S1–S63. [DOI] [PubMed] [Google Scholar]
- 65. Douglas LC, Sanders ME. Probiotics and prebiotics in dietetics practice. J Am Diet Assoc. 2008;108(3):510–21. [DOI] [PubMed] [Google Scholar]
- 66. Davis L, Martinez I, Walter J, Hutkins R. A dose dependent impact of prebiotic galactooligosaccharides on the intestinal microbiota of healthy adults. Int J Food Microbiol. 2010;144(2):285–92. [DOI] [PubMed] [Google Scholar]
- 67. Bouhnik Y, Vahedi K, Achour L, Attar A, Salfati J, Pochart P, Marteau P, Flourié B, Bornet F, Rambaud J-C. Short-chain fructo-oligosaccharide administration dose-dependently increases fecal bifidobacteria in healthy humans. J Nutr. 1999;129(1):113–16. [DOI] [PubMed] [Google Scholar]
- 68. Braegger C, Chmielewska A, Decsi T, Kolacek S, Mihatsch W, Moreno L, Piescik M, Puntis J, Shamir R, Szajewska Het al. Supplementation of infant formula with probiotics and/or prebiotics: a systematic review and comment by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2011;52(2):238–50. [DOI] [PubMed] [Google Scholar]
- 69. Kok CR, Quintero DFG, Niyirora C, Rose D, Li A, Hutkins R. An in vitro enrichment strategy for formulating synergistic synbiotics. Appl Environ Microbiol. 2019;85(16):e01073–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chua MC, Ben-Amor K, Lay C, Goh AEN, Chiang WC, Rao R, Chew C, Chaithongwongwatthana S, Khemapech N, Knol Jet al. Effect of synbiotic on the gut microbiota of cesarean delivered infants: a randomized, double-blind, multicenter study. J Pediatr Gastroenterol Nutr. 2017;65(1):102–6. [DOI] [PubMed] [Google Scholar]
- 71. Davani-Davari D, Negahdaripour M, Karimzadeh I, Seifan M, Mohkam M, Masoumi SJ, Berenjian A, Ghasemi Y. Prebiotics: definition, types, sources, mechanisms, and clinical applications. Foods. 2019;8(3):92–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Stewart CP, Iannotti L, Dewey KG, Michaelsen KF, Onyango AW. Contextualising complementary feeding in a broader framework for stunting prevention. Matern Child Nutr. 2013;9(S2):27–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gehrig JL, Venkatesh S, Chang H-W, Hibberd MC, Kung VL, Cheng J, Chen RY, Subramanian S, Cowardin CA, Meier MFet al. Effects of microbiota-directed foods in gnotobiotic animals and undernourished children. Science. 2019;365(6449):eaau4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hernández-Granados MJ, Franco-Robles E. Postbiotics in human health: possible new functional ingredients?. Food Res Int. 2020;137:109660. [DOI] [PubMed] [Google Scholar]
- 75. Mostafa I, Nahar NN, Islam MM, Huq S, Mustafa M, Barratt M, Gordon JI, Ahmed T. Proof-of-concept study of the efficacy of a microbiota-directed complementary food formulation (MDCF) for treating moderate acute malnutrition. BMC Public Health. 2020;20(1):242–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chen RY, Mostafa I, Hibberd MC, Das S, Mahfuz M, Naila NN, Islam MM, Huq S, Alam MA, Zaman MUet al. A microbiota-directed food intervention for undernourished children. N Engl J Med. 2021;384(16):1517–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Salminen S, Collado MC, Endo A, Hill C, Lebeer S, Quigley EMM, Sanders ME, Shamir R, Swann JR, Szajewska Het al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat Rev Gastroenterol Hepatol. 2021;18(9):649–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Luby SP, Rahman M, Arnold BF, Unicomb L, Ashraf S, Winch PJ, Stewart CP, Begum F, Hussain F, Benjamin-Chung Jet al. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhoea and child growth in rural Bangladesh: a cluster randomised controlled trial. Lancet Glob Health. 2018;6(3):e302–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Pickering AJ, Null C, Winch PJ, Mangwadu G, Arnold BF, Prendergast AJ, Njenga SM, Rahman M, Ntozini R, Benjamin-Chung Jet al. The WASH Benefits and SHINE trials: interpretation of WASH intervention effects on linear growth and diarrhoea. Lancet Glob Health. 2019;7(8):e1139–46. [DOI] [PubMed] [Google Scholar]
- 80. Victora CG, Christian P, Vidaletti LP, Gatica-Domínguez G, Menon P, Black RE. Revisiting maternal and child undernutrition in low-income and middle-income countries: variable progress towards an unfinished agenda. Lancet. 2021;397(10282):1388–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.