Abstract
Background
In Korea, the first community outbreak of coronavirus disease 2019 (COVID-19) occurred in Daegu on February 18, 2020. This study was performed to investigate the prevalence of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) antibodies in healthcare workers (HCWs) at 6 major hospitals in Daegu.
Methods
Blood specimens of 2,935 HCWs at 6 major hospitals in Daegu from January 2021 to February 2021 were collected. Every specimen was tested for antibody against SARS-CoV-2 using both Elecsys Anti-SARS-CoV-2 electrochemiluminescence immunoassay (Roche Diagnostics, Rotkreuz, Switzerland) and R-FIND COVID-19 IgG/M/A enzyme-linked immunosorbent assay kit (SG medical Inc., Seoul, Korea) as screening tests. If 1 or more of these screening test results was positive, 2 additional antibody tests were performed using Abbott Anti-SARS-CoV-2 IgG assay (Abbott, Abbott Park, IL, USA) and cPass SARS-CoV-2 Neutralization Antibody Detection Kit (GenScript USA Inc., Piscataway, NJ, USA). If 2 or more of the total 4 test results were positive, it was determined as positive for the antibody against SARS-CoV-2.
Results
According to the criteria of SARS-CoV-2 antibody positivity determination, 12 subjects were determined as positive. The overall positive rate of the SARS-CoV-2 antibody was 0.41% (12/2,935). Of the 12 subjects determined as positive, 7 were diagnosed with COVID-19, and the remaining 5 were nondiagnosed cases of COVID-19.
Conclusion
In early 2021, the overall seroprevalence of SARS-CoV-2 antibody among HCW located in Daegu was 0.41%, and 0.17% excluding COVID-19 confirmed subjects. These results were not particularly high compared with the general public and were much lower than HCWs in other countries.
Keywords: COVID-19, SARS-CoV-2 Antibody, Healthcare Worker, Neutralizing Antibody
Graphical Abstract
INTRODUCTION
In Korea, the first coronavirus disease 2019 (COVID-19) patient was detected on January 20, 2020.1 The first community outbreak occurred in Daegu, starting with patient 31 on February 18, and soon spread across the country.1 With a maximum of 741 new patients per day in the city as of February 29, 2020, the cumulative number of patients had surpassed 6,000 by the end of March 2020.2 Such a sudden outbreak in the early stages of the pandemic without sufficient preparation caused great confusion and exposed healthcare workers (HCWs) to a number of risks as well.
Although vaccination began for HCWs from the end of February 2021, the prevalence of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) antibody in HCWs has not been conducted properly. This study was performed to investigate the prevalence of SARS-CoV-2 antibody in HCWs at 6 major hospitals in Daegu and was intended to be used as a key indicator in infection control and vaccine policies for HCWs.
METHODS
Subjects
Blood specimens of 2,935 HCWs were collected at 6 major hospitals in Daegu (Kyungpook National University Hospital, Daegu Fatima Hospital, Yeungnam University Medical Center, Keimyung University Dongsan Medical Center, Kyungpook National University Chilgok Hospital and Daegu Catholic University Medical Center), Korea from January 18, 2021 to February 26, 2021. All subjects participated voluntarily.
Criteria of SARS-CoV-2 antibody positivity determination
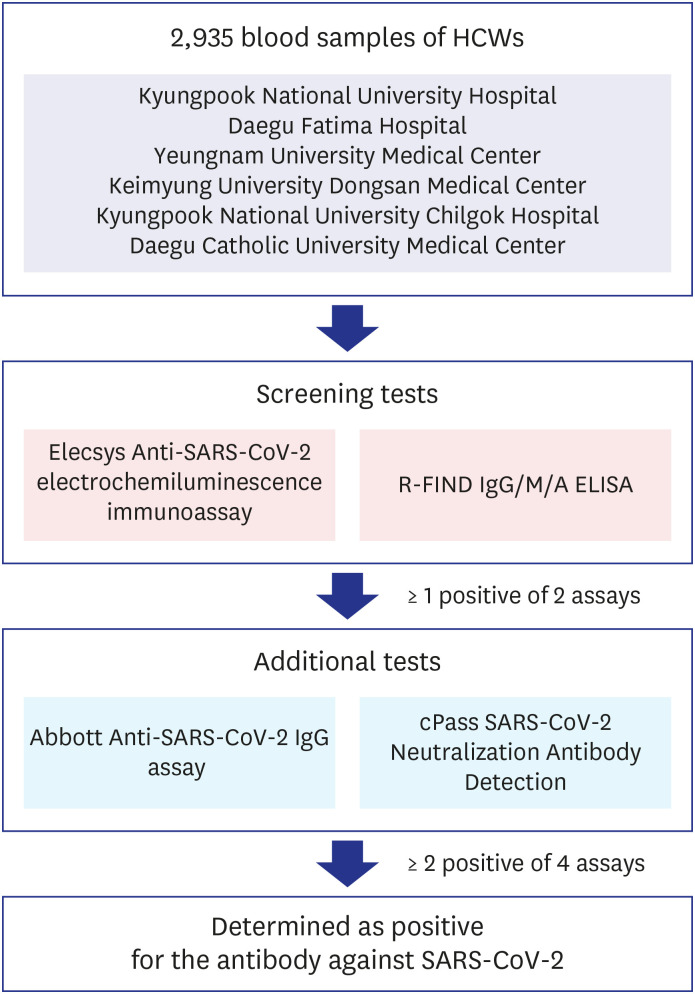
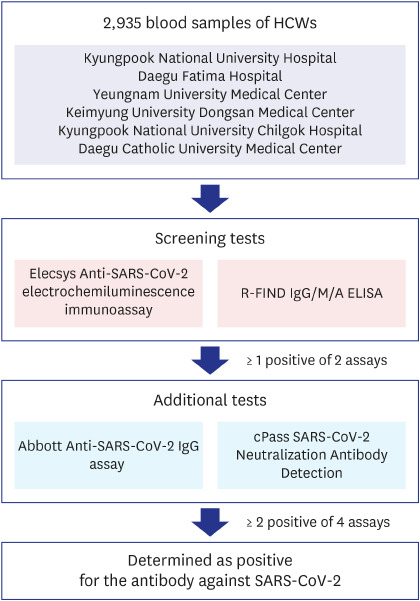
Every specimen was tested for antibody against SARS-CoV-2 using both Elecsys Anti-SARS-CoV-2 electrochemiluminescence immunoassay (Roche Diagnostics, Rotkreuz, Switzerland) and R-FIND COVID-19 IgG/M/A enzyme-linked immunosorbent assay (ELISA) kit (SG medical Inc., Seoul, Korea) as screening tests. If 1 or more of these screening test results was positive, 2 additional antibody tests were performed using Abbott Anti-SARS-CoV-2 IgG assay (Abbott, Abbott Park, IL, USA) and cPass SARS-CoV-2 Neutralization Antibody Detection Kit (GenScript USA Inc., Piscataway, NJ, USA). If 2 or more of the total 4 test results were positive, it was determined as positive for the antibody against SARS-CoV-2 (Fig. 1). All of these antibody tests were performed at the Diagnostic Immunologic Center of Seegene Medical Foundation.
Fig. 1. Criteria of SARS-CoV-2 antibody positivity determination. Total 2,935 blood samples of HCWs were tested using 2 antibody assays simultaneously as screening tests. If 1 of these screening results was positive, 2 additional antibody tests were performed. If 2 or more of the total 4 test results were positive, it was determined as positive for the antibody against SARS-CoV-2.
HCW = healthcare worker, SARS-CoV-2 = severe acute respiratory syndrome coronavirus-2, ELISA = enzyme-linked immunosorbent assay.
Elecsys Anti-SARS-CoV-2 electrochemiluminescence immunoassay
The Elecsys Anti-SARS-CoV-2 electrochemiluminescence immunoassay is intended to qualitatively detect antibodies (including IgG) against SARS-CoV-2 in human serum using cobas e 801 analyzer (Roche Diagnostics). The immunoassay uses the principle of the double-antigen sandwich method representing nucleocapsid antigens to determine SARS-CoV-2 antibodies. We performed the Elecsys Anti-SARS-CoV-2 immunoassay according to the manufacturer's instructions in the present study. The assay results were interpreted as nonreactive/negative (cutoff index < 1) and reactive/positive (cutoff index ≥ 1).
R-FIND IgG/M/A ELISA
R-FIND COVID-19 IgG/M/A ELISA Kit is an ELISA for detecting and qualitative determination of IgG, IgM, and IgA antibodies to the nuclear capsid protein (NP) of SARS-CoV-2. Samples were incubated in microwells coated with NP protein, and anti-NP antibodies in samples were captured by pre-coated NP. Then, horseradish peroxidase (HRP)-conjugated anti-human IgG/M/A is added. HRP conjugated anti-human IgG/M/A binds to the antibodies previously bound to NP. Optical density (OD) was measured at 450 nm, and the average value of OD of the negative control (Avg. of NC) was calculated. Next, the cutoffs were calculated using the following formulas: positive cutoff = 1.1 × (Avg. of NC + 0.25), and negative cutoff = 0.9 × (Avg. of NC + 0.25). The assay results were determined as positive (sample OD ≥ positive cutoff), negative (sample OD ≤ negative cutoff), and borderline (positive cutoff > sample OD > negative cutoff). When the result was borderline, the test was repeated with aliquot sample.
Abbott anti-SARS-CoV-2 IgG assay
Abbott anti-SARS-CoV-2 IgG assay is a chemiluminescent microparticle immunoassay detecting IgG antibodies against the SARS-CoV-2 NP using an Architect i2000 instrument (Abbott). The assay reports a ratio of sample absorbance to calibrator absorbance using an assay-specific calibrator. The result was interpreted as a positive (index ≥ 1.4) or negative (index < 1.4) using an index value (ratio over the threshold value).
cPass SARS-CoV-2 neutralization antibody detection kit
The cPass SARS-CoV-2 Neutralization antibody detection kit is a blocking ELISA for qualitative and direct detection of neutralizing antibodies to SARS-CoV-2. This assay was designed to mimic the virus–host interaction by direct protein–protein interaction using the purified receptor-binding domain (RBD) and the human ACE2 receptor protein (hACE2). The protein–protein interaction between HRP conjugated recombinant SARS-CoV-2 RBD fragment (HRP-RBD) and hACE2 is blocked by neutralizing antibodies against SARS-CoV-2 RBD. Samples were incubated with the HRP-RBD, and circulating neutralization antibodies were bound with HRP-RBD. Then, the mixture was added to the plate coated with the hACE2 protein, and unbounded HRP-RBD was captured by pre-coated hACE2 protein. Remain neutralization antibodies HRP-RBD complexes in the supernatant were removed during washing. OD was measured at 450 nm. The interpretation of the result was determined by signal inhibition rate (= [1 − OD value of sample/OD value of negative control] × 100%). The assay results were interpreted as follows: < 20% signal inhibition for samples that were nonreactive/negative for neutralizing antibodies for SARS-CoV-2; ≥ 20% signal inhibition for samples that were reactive/positive for neutralizing antibodies for SARS-CoV-2.
Ethics statement
The present study protocol was reviewed and approved by the Daegu Joint Institutional Review Board (approval No. 2020-12-001). All subjects submitted informed consent when they were enrolled.
RESULTS
Demographics of the subjects
Demographics and clinical information of the subjects are shown in Table 1. The 20s and 30s accounted for 56.69%, and the females accounted for 77.24% of the total 2,935 subjects. Nurses were the most common with 43.78% occurrence among the occupational groups. HCWs in contact with COVID-19-confirmed patients or specimens were 25.08% and 6.81%, respectively. Ten subjects (0.34%) diagnosed with COVID-19 were included, and most of the subjects (76.80%) had no polymerase chain reaction (PCR) test or quarantine experience.
Table 1. General information of subjects (n = 2,935).
| Variables | No. (%) | |
|---|---|---|
| Age, yr | ||
| 20–29 | 826 (28.14) | |
| 30–39 | 838 (28.55) | |
| 40–49 | 561 (19.11) | |
| 50–59 | 541 (18.43) | |
| 60–69 | 145 (4.94) | |
| ≥ 70 | 2 (0.07) | |
| No response | 22 (0.75) | |
| Sex | ||
| Female | 2,267 (77.24) | |
| Male | 646 (22.01) | |
| No response | 22 (0.75) | |
| Profession | ||
| Medical doctor | 194 (6.61) | |
| Nurse | 1,285 (43.78) | |
| Medical technician | 456 (15.54) | |
| Clerical position | 920 (31.35) | |
| No response | 80 (2.73) | |
| COVID-19 diagnosis and quarantine experience | ||
| PCR (+)/quarantine | 10 (0.34) | |
| PCR (−)/quarantine | 559 (19.01) | |
| PCR not tested/quarantine | 34 (1.16) | |
| PCR not tested/no quarantine | 2,253 (76.80) | |
| No response | 79 (2.69) | |
| Scope of work | ||
| Contact with COVID-19 patients | 736 (25.08) | |
| Sample handling of COVID-19 patients | 200 (6.81) | |
| Contact with non-COVID-19 patients | 1,104 (37.61) | |
| Sample handling of non-COVID-19 patients | 157 (5.35) | |
| No contact of patients and samples | 712 (24.26) | |
| No response | 26 (0.89) | |
COVID-19 = coronavirus disease 2019, PCR = polymerase chain reaction.
Prevalence of SARS-CoV-2 antibody in subjects
According to the criteria of SARS-CoV-2 antibody positivity determination, 12 subjects were determined as positive (Fig. 1). The overall positive rate of the SARS-CoV-2 antibody was 0.41% (12/2,935) (Table 2). Seven out of those 12 positive subjects were diagnosed with COVID-19, and the remaining 5 were nondiagnosed cases of COVID-19.
Table 2. Prevalence of SARS-CoV-2 antibody in subjects.
| Variables | No. of cases | No. of positive cases | Positive cases for the antibody against SARS-CoV-2a | ||||
|---|---|---|---|---|---|---|---|
| Screening tests | Additional tests | ||||||
| Roche Elecsys Anti-SARS-CoV-2 assay | SG Medical R-FIND COVID-19 IgG/M/A ELISA | Abbott Anti-SARS-CoV-2 IgG assay | GenScript cPass SARS-CoV-2 Neutralization Antibody assay | ||||
| HCWs diagnosed COVID-19 with SARS-CoV-2 PCR | 10 (0.34) | 9 (0.31) | 2 (0.07) | 5 | 6 | 7 (0.24) | |
| Hospital A | 2 | 1 | 0 | 0 | 1 | 1 [0.20] | |
| Hospital B | 1 | 1 | 1 | 1 | 1 | 1 [0.20] | |
| Hospital C | 1 | 1 | 1 | 1 | 1 | 1 [0.20] | |
| Hospital D | 0 | 0 | 0 | 0 | 0 | 0 [0.00] | |
| Hospital E | 5 | 5 | 0 | 3 | 3 | 4 [0.78] | |
| Hospital F | 1 | 1 | 0 | 0 | 0 | 0 [0.00] | |
| HCWs other than diagnosed COVID-19 | 2,925 (99.66) | 2 (0.07) | 56 (1.91) | 4 | 2 | 5 (0.17) | |
| Hospital A | 501 | 0 | 3 | 0 | 0 | 0 [0.00] | |
| Hospital B | 506 | 0 | 17 | 2 | 0 | 2 [0.40] | |
| Hospital C | 504 | 0 | 9 | 0 | 0 | 0 [0.00] | |
| Hospital D | 501 | 1 | 7 | 0 | 1 | 1 [0.20] | |
| Hospital E | 510 | 1 | 12 | 2 | 1 | 2 [0.39] | |
| Hospital F | 403 | 0 | 8 | 0 | 0 | 0 [0.00] | |
| Total | 2,935 (100) | 11 (0.38) | 58 (1.98) | 9 | 8 | 12 (0.41) | |
(): percentage of total 2,935 samples; []: percentage of total samples of each institute.
HCW = healthcare worker, COVID-19 = coronavirus disease 2019, SARS-CoV-2 = severe acute respiratory syndrome coronavirus-2, PCR = polymerase chain reaction, ELISA = enzyme-linked immunosorbent assay.
aIf 2 or more of the test results were positive, it was determined as positive for the antibody against SARS-CoV-2.
SARS-CoV-2 antibody test results of HCWs diagnosed as COVID-19 with SARS-CoV-2 PCR
Of the 10 subjects diagnosed with COVID-19, 7 were finally determined to be SARS-CoV-2 antibody positive, 2 were positive for all 4 antibody tests, 1 was positive for 3 antibody tests, and 4 were positive for 2 tests. Of the remaining 3 COVID-19 confirmed cases, 2 were positive for one antibody test and 1 was negative for all tests (Table 3).
Table 3. SARS-CoV-2 antibody test results of HCWs diagnosed COVID-19 with SARS-CoV-2 PCR.
| Age/sex | Roche Elecsys Anti-SARS-CoV-2 assay | SG Medical R-FIND COVID-19 IgG/M/A ELISA | Abbott Anti-SARS-CoV-2 IgG assay | GenScript cPass SARS-CoV-2 Neutralization Antibody assay | Duration from SARS-CoV-2 PCR confirmation to the antibody test, wks | Profession | Scope of work | Final result of the antibody against SARS-CoV-2a | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Index | Result | OD | Index | Result | Index | Result | Neutralization rate (%) | Result | |||||
| 49/F | 205 | + | 0.650 | 1.83 | + | 5.38 | + | 95.1 | + | 38 | Nurse | Contact with COVID-19 patients | + |
| 61/M | 5.59 | + | 2.126 | 5.45 | + | 10.14 | + | 62.6 | + | 3 | Medical doctor | Contact with COVID-19 patients | + |
| 27/F | 186 | + | 0.256 | 0.73 | − | 3.17 | + | 52.8 | + | 45 | Nurse | Contact with COVID-19 patients | + |
| 27/F | 33.1 | + | 0.311 | 0.89 | − | 1.51 | + | 18.8 | − | 43 | Nurse | Contact with COVID-19 patients | + |
| 32/F | 7.59 | + | 0.081 | 0.22 | − | 0.23 | − | 45.2 | + | 44 | Nurse | Contact with COVID-19 patients | + |
| 38/F | 80.8 | + | 0.184 | 0.51 | − | 0.23 | − | 45.2 | + | 44 | Nurse | Contact with COVID-19 patients | + |
| 49/M | 20.8 | + | 0.289 | 0.88 | − | 0.49 | − | 23.1 | + | 46 | Clerical position | No contact of patients and samples | + |
| 25/F | 79.2 | + | 0.217 | 0.62 | − | 0.96 | − | 19.7 | − | 43 | Nurse | Contact with COVID-19 patients | − |
| 28/M | 24.2 | + | 0.085 | 0.25 | − | 0.37 | − | 19.1 | − | N/A | Clerical position | No contact of patients and samples | − |
| 51/M | 0.31 | − | 0.152 | 0.46 | − | 0.16 | − | −14.4 | − | 45 | Clerical position | Contact with COVID-19 patients | − |
HCW = healthcare worker, COVID-19 = coronavirus disease 2019, SARS-CoV-2 = severe acute respiratory syndrome coronavirus-2, ELISA = enzyme-linked immunosorbent assay, PCR = polymerase chain reaction, N/A = not available, OD = optical density.
aIf 2 or more of the test results were positive, it was determined as positive for the antibody against SARS-CoV-2.
Confirmed positive cases for the antibody against SARS-CoV-2 among HCWs other than diagnosed with COVID-19
Of the remaining HCWs, excluding the subjects diagnosed with COVID-19, 5 were finally determined to be positive for the SARS-CoV-2 antibody. One was positive for all 4 antibody tests and 4 were positive for 2 tests. Two of them were subjected to a SARS-CoV-2 PCR test who came in contact with a COVID-19 patient at work and experienced quarantine. The remaining 3 subjects had no experience of PCR testing or quarantine. All 5 were not suspicious of coming in contact with a COVID-19 patient outside the workplace nor had they experienced any symptoms requiring treatment.
DISCUSSION
In this study, the overall positive rate of SARS-CoV-2 antibody in HCWs was found to be 0.41% (12/2,935), and 0.17% (5/2,925) excluding 10 COVID-19 confirmed subjects. These findings are similar to the result of a previous study, which reported 0.3% (1/309) seropositivity for SARS-CoV-2 among HCWs designated to confirmed COVID-19 patient.3
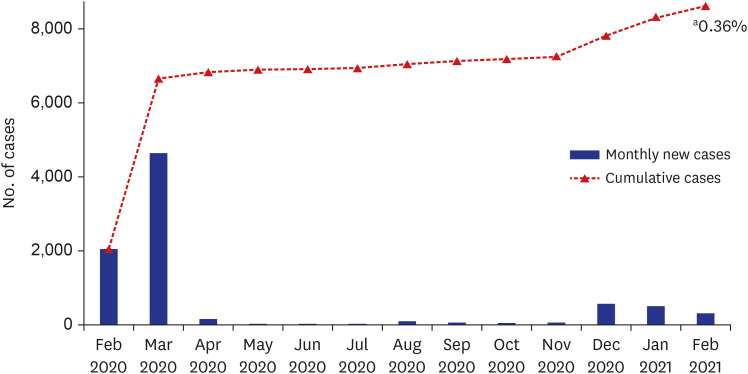
In Daegu, the first large outbreak caused more than 6,000 cases in February and March 2020, and since then, the total cumulative number of cases had been 8,623 until February 2021.4 Based on the population of 2,401,110 at the end of December 2020, the COVID-19 incidence rate in February 2021 in Daegu was 0.36% (8,623/2,401,110) (Fig. 2). In this study, there were 10 confirmed cases of COVID-19, which was 0.34% of the total subjects, and it was found that there was little difference with the COVID-19 incidence rate in the general population of Daegu. Hospital E had the highest number of SARS-CoV-2 antibody-positive HCWs. However, it was thought that there was little difference between institutions except for COVID-19 confirmed cases (Table 2).
Fig. 2. Trend of COVID-19 confirmed cases in Daegu.
COVID-19 = coronavirus disease 2019.
aCOVID-19 incidence rate in Daegu area in February 2021 ( ).
).
According to the results announced by Korean Disease Control and Prevention Agency, the positive rates of SARS-CoV-2 antibody among the 3rd participants of National Health Nutrition Survey (15 cities and provinces nationwide, from August 14 to October 31, 2020) and military camps (residents of Yungun training center, from September to October) were 0.22% (3/1,379) and 0.36% (25/6,859), respectively.1 And the positive rates of antibodies in nondiagnosed participants, excluding those with previously confirmed cases, were 0.07% (1/1,377) and 0.22% (15/6,849), respectively. Therefore, the prevalence of SARS-CoV-2 antibody is not particularly high in HCWs compared with the general public. The subject hospitals were in charge of hospitalization and treatment for COVID-19 confirmed patients during the early pandemic, so it seemed that they performed prevention for infection successfully even in situations where the risk of exposure was particularly high.
A recent meta-analysis of 49 studies involving a total of 127,480 subjects documented that the overall seroprevalence for HCWs worldwide was 8.7% (95% confidence interval 6.7–10.9%), ranging from 0% to 45.3% among studies. North America (12.7%) was higher than Europe (8.5%), Africa (8.2%), or Asia (4%).5 Thus, the results of the present study revealed that seroprevalence of HCWs in Daegu, Korea was much lower than those of the other countries, although there are many factors to consider such as different degree of contact with the COVID-19 patients, different assay kits used, timing of implementation, or other variable circumstances.
The neutralizing antibody to SARS-CoV-2 in individuals with COVID-19 experience was reported to have stable antibody titers for several months after infection.6 Another study documented that the neutralizing antibody against SARS-CoV-2 among infected HCWs declined rapidly and might even be lost from 2 months after disease onset.7 The neutralizing antibody was detected at 66.7% (8/12) among the antibody-positive participants. Moreover, 3 of the 12 COVID-19 confirmed subjects had neutralization rates of 18.8%, 19.7%, and 19.1%, respectively, which were very close to the cutoff value (20%). Those results were determined as negative in this case, but it would be appropriate to consider as positive with a slight decrease in titer.
A previous report demonstrated that the rates of antibody positivity according to commercial immunoassays were high (69.0–91.4%) at 8 months after infection, even in asymptomatic or mildly symptomatic participants.8 Most of the confirmed cases in this study were infected during the initial outbreak. Therefore, it was shown that the titer of antibody against SARS-CoV-2 including neutralizing antibody were maintained for >10 months, and it seemed to persist for > 1 year (Table 2).
Even in the subjects without symptoms other than confirmed COVID-19, 5 cases were found to be positive for the SARS-CoV-2 antibody (Table 4). It might be seen that asymptomatic infections existed among the HCWs. Some of them did not have any contact with COVID-19 patients, and the route of infection was unclear. Antibodies were reported to be detected even among asymptomatic individuals without a history of COVID-19 or close contact with them.9 Therefore, it is suggested that HCWs without symptoms or contact history will need to strictly follow the infection prevention policy.
Table 4. Confirmed cases of positive for the antibody against SARS-CoV-2 among HCWs other than diagnosed COVID-19.
| Age/sex | Roche Elecsys Anti-SARS-CoV-2 assay | SG Medical R-FIND COVID-19 IgG/M/A ELISA | Abbott Anti-SARS-CoV-2 IgG assay | GenScript cPass SARS-CoV-2 Neutralization Antibody assay | Profession | COVID-19 diagnosis and quarantine experience | Scope of work | Suspected contact with a COVID-19 patient outside the workplace | COVID-19–related symptoms that require treatment | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Index | Result | OD | Index | Result | Index | Result | Neutralization rate (%) | Result | ||||||
| 58/F | 0.07 | − | 0.450 | 1.34 | + | 2.61 | + | 13.5 | − | Clerical position | PCR not tested/no quarantine | No contact of patients and samples | No | No |
| 52/M | 0.07 | − | 1.283 | 3.81 | + | 6.52 | + | 12.6 | − | Medical technician | PCR not tested/no quarantine | Contact with non-COVID-19 patients | No | No |
| 29/F | 2.95 | + | 0.167 | 0.47 | − | 0.16 | − | 31.6 | + | Clerical position | PCR not tested/no quarantine | Contact with non-COVID-19 patients | No | No |
| 51/F | 94.5 | + | 0.733 | 2.04 | + | 2.38 | + | 25.8 | + | Clerical position | PCR (−)/quarantine | Contact with COVID-19 patients | No | No |
| 27/F | 0.07 | − | 0.586 | 1.69 | + | 3.18 | + | −7.6 | − | Nurse | PCR (−)/quarantine | Contact with COVID-19 patients | No | No |
HCW = healthcare worker, COVID-19 = coronavirus disease 2019, SARS-CoV-2 = severe acute respiratory syndrome coronavirus-2, ELISA = enzyme-linked immunosorbent assay, PCR = polymerase chain reaction, OD = optical density.
As the SARS-CoV-2 antibody immunoassays may show different results, serological assessment by a single assay requires caution in interpretation and monitoring.10 A meta-analysis reported sensitivity of 96.6–99.7% for chemiluminescent immunoassays, 84.3% for ELISAs, and 66.6% for lateral flow immunoassays, respectively, citing heterogeneity among different serologic test kits.11 Park et al.12 recommended using two or more immunoassays to increase the positive predictive value when the prevalence of COVID-19 is low. Accordingly, we used two immunoassay kits as screening test tools, including an ELISA and an ECLIA kit, to exclude false-positive or false-negative results. Positive results were additionally confirmed using another 2 immunoassay kits, including a neutralization antibody assay. The discrepant results among the kits were also found in this study. Only 3 out of 12 seropositive subjects tested positive in all 4 immunoassay kits. More research data are needed regarding differences in sensitivity, specificity, and results between antibody detection kits.
In conclusion, this study reported that overall seroprevalence of SARS-CoV-2 antibody among HCWs in Daegu, Korea was 0.41%, and 0.17% excluding COVID-19 confirmed subjects. Those results were not particularly high compared with the general public and much lower than HCWs in other countries. Because there are infections that are asymptomatic and have no contact history, it is necessary to follow the policy for the infection prevention among HCWs. In addition, because most HCWs do not have antibodies, vaccination is needed to protect the HCWs and the hospital visitors who will come into contact with them.
ACKNOWLEDGMENTS
The authors thank all healthcare workers (HCWs) in Daegu, Korea for their hard work during the COVID-19 pandemic and voluntary participation in this study.
Footnotes
Funding: This work was supported by Seegene Medical Foundation in 2020.
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Suh JS, Kim YK, Minn D.
- Data curation: Kim YK, Chang SH, Minn D.
- Formal analysis: Suh JS, Minn D, Kim YK, Chang SH.
- Investigation: Song DY, Lee CH, Ryoo NH, Song KE, Jeon CH, Kim YK.
- Methodology: Suh JS, Minn D, Kim YK.
- Validation: Chang SH, Minn D.
- Writing - original draft: Chang SH, Kim YK, Minn D.
- Writing - review & editing: Kim YK, Minn D, Song DY, Lee CH, Ryoo NH, Jeon CH, Song KE, Suh JS, Chang SH.
References
- 1.Ministry of Health and Welfare (KR) Coronavirus disease-19, Republic of Korea. [Updated 2021]. [Accessed March 12, 2021]. http://ncov.mohw.go.kr .
- 2.Kim SW, Lee KS, Kim K, Lee JJ, Kim JY Daegu Medical Association. A brief telephone severity scoring system and therapeutic living centers solved acute hospital-bed shortage during the COVID-19 outbreak in Daegu, Korea. J Korean Med Sci. 2020;35(15):e152. doi: 10.3346/jkms.2020.35.e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ko JH, Lee JY, Kim HA, Kang SJ, Baek JY, Park SJ, et al. Serologic evaluation of healthcare workers caring for COVID-19 patients in the Republic of Korea. Front Microbiol. 2020;11:587613. doi: 10.3389/fmicb.2020.587613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daegu Metropolitan City Hall. Daegu Metropolitan City COVID-19 status. [Updated 2021]. [Accessed July 28, 2021]. http://covid19.daegu.go.kr/00937420.html .
- 5.Galanis P, Vraka I, Fragkou D, Bilali A, Kaitelidou D. Seroprevalence of SARS-CoV-2 antibodies and associated factors in healthcare workers: a systematic review and meta-analysis. J Hosp Infect. 2021;108:120–134. doi: 10.1016/j.jhin.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee SM, Kim IS, Lim S, Lee SJ, Kim WJ, Shin KH, et al. Comparison of serologic response of hospitalized COVID-19 patients using 8 immunoassays. J Korean Med Sci. 2021;36(9):e64. doi: 10.3346/jkms.2021.36.e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lisboa Bastos M, Tavaziva G, Abidi SK, Campbell JR, Haraoui LP, Johnston JC, et al. Diagnostic accuracy of serological tests for covid-19: systematic review and meta-analysis. BMJ. 2020;370:m2516. doi: 10.1136/bmj.m2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wajnberg A, Amanat F, Firpo A, Altman DR, Bailey MJ, Mansour M, et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370(6521):1227–1230. doi: 10.1126/science.abd7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song SK, Lee DH, Nam JH, Kim KT, Do JS, Kang DW, et al. IgG seroprevalence of COVID-19 among individuals without a history of the coronavirus disease infection in Daegu, Korea. J Korean Med Sci. 2020;35(29):e269. doi: 10.3346/jkms.2020.35.e269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marot S, Malet I, Leducq V, Zafilaza K, Sterlin D, Planas D, et al. Rapid decline of neutralizing antibodies against SARS-CoV-2 among infected healthcare workers. Nat Commun. 2021;12(1):844. doi: 10.1038/s41467-021-21111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choe PG, Kim KH, Kang CK, Suh HJ, Kang E, Lee SY, et al. Antibody responses 8 months after asymptomatic or mild SARS-CoV-2 infection. Emerg Infect Dis. 2021;27(3):928–931. doi: 10.3201/eid2703.204543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park Y, Hong KH, Lee SK, Hyun J, Oh EJ, Lee J, et al. Performance comparison of five SARS-CoV-2 antibody assays for seroprevalence studies. Ann Lab Med. 2022;42(1):71–78. doi: 10.3343/alm.2022.42.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]