Abstract
Background
Prostate cancer (Pca) is the most common cancer type among males worldwide. Dysregulation of Ca2+ signaling plays important roles during Pca progression. However, there is lack of information about the role of endolysosomal Ca2+ -permeable channels in Pca progression.
Methods
The expression pattern of MCOLN2 was studied by immunohistochemistry and western blot. Cell viability assay, transwell assay and in vivo tumorigenesis were performed to evaluate the functional role of MCOLN2. Downstream targets of MCOLN2 were investigated by cytokine array, enzyme-linked immunosorbent assay, Ca2+ release experiments and luciferase reporter assays.
Results
We report that MCOLN2 expression is significantly elevated in Pca tissues, and associated with poor prognosis. Overexpression of MCOLN2 promoted Pca cells proliferation, migration and invasion. Importantly, knockdown of MCOLN2 inhibited Pca xenograft tumor growth and bone lesion development in vivo. In addition, MCOLN2 promoted the production and release of IL-1β. Moreover, luciferase reporter assay and western blot revealed that MCOLN2 promoted Pca development by regulating the IL-1β/NF-κB pathway.
Conclusion
In summary, MCOLN2 is crucially involved in Pca progression. Mechanistically, MCOLN2 regulates Pca progression via IL-1β/NF-κB pathway. Our study highlights an intriguing possibility of targeting MCOLN2 as potential therapeutic strategy in Pca treatment.
Subject terms: Prostate cancer, Oncogenes
Background
Prostate cancer (Pca) is the second most common cause of cancer deaths among males in developed countries [1]. Androgen deprivation therapy is the first-line therapy for Pca; however, it is associated with significant adverse effects and inevitably becomes ineffective once androgen-independent prostate tumor cells begin to grow. Eventually the cancer progresses to metastatic castration-resistant Pca, resulting in death [2–4]. Metastatic lesions of Pca are incurable [5]. In spite of the high incidence and mortality, the etiology of Pca remains unknown [6]. Therefore, identification of new molecular targets for Pca therapies is urgently needed for early diagnosis and better treatments for patients with Pca [3].
Transient receptor potential (TRP) channels are a group of cation channels that are expressed in the plasma membrane and the membrane of intracellular organelles such as endo- and sarcoplasmic reticulum, lysosomes and endosomes [7]. Most TRP channels are Ca2+-permeable, thus they play an important role in the maintenance of cellular Ca2+ homeostasis [7]. Dysregulation of these channels may distort Ca2+ signaling, thereafter promoting pathophysiological cancer hallmarks, such as cancer cell survival, proliferation and invasion [8–10]. In this regard, several TRP isoforms that are located on the plasma membrane thus mediating extracellular Ca2+ entry, have been shown to be involved in the proliferation, apoptosis and/or migration of Pca cells [8, 11].
Unlike most other TRP family members, the mucolipin subfamily of TRP channels, including Mucolipin-1, -2 and -3 (MCOLN1, -2 and -3), is exclusively located in the endolysosomal membrane [12]. Activity of MCOLN channels causes endolysosomal Ca2+ release, which is accompanied by cytosolic Ca2+ rise [12]. Functionally, MCOLN-mediated Ca2+ signaling plays a pivotal role in vesicular trafficking events during autophagy and lysosomal exocytosis [13]. Among the mucolipin subfamily, MCOLN1 is the best studied one. Recent studies have found that MCOLN1 can enhance tumor growth and invasion in a variety of cancer types, including triple-negative breast cancer, melanoma and head-and-neck tumor [14–16]. Unlike MCOLIN1, MCOLN2 is a poorly characterised channel and expressed mainly in lymphoid and myeloid tissues [17], where MCOLN2 participates in innate immunity-defense against various pathogens including virus and bacteria [18, 19]. There is currently no report about involvement of any MCOLN channels in Pca.
Given the increasing appreciation of the link between inflammation and cancer, the role of inflammatory cytokines, especially IL-1β, in the pathogenesis of cancer has been extensively investigated [20, 21]. Fibrosarcoma cells that are genetically modified to constitutively secret mature IL-1β displayed an increased growth, invasiveness and angiogenesis [20, 21]. In several human cancers including Pca, local IL-1β expression by the malignant cells or the microenvironment has been associated with aggressive tumor growth and poor prognosis [22–24]. These evidences demonstrate a pro-tumorigenic role of IL-1β. Interestingly, substantial amount of evidence also suggests an important role of cytosolic Ca2 signaling and lysosomal Ca2+ release in promoting IL-1β production and release in leukocytes [25–27]. However, such kind of connection between Ca2+ and IL-1β has never been explored in Pca cells. It is unclear whether endolysosomal Ca2+ channels in general and MCOLN2 in particular can promote IL-1β production and release in Pca progression.
In the present study, we identified the first endolysosomal Ca2+-permeable channel MCOLN2 that is crucially involved in Pca progression. We showed that MCOLN2 was upregulated in prostate tumor tissues and correlated with poor prognosis. Overexpression of MCOLN2 promoted tumor growth, migration and invasion. Mechanistically, we demonstrated that MCOLN2 acted through IL-1β/NF-κB pathway to exert its pro-tumorigenic effect to promote Pca progression.
Methods
Bioinformatics analysis
Meta-analysis of the expression pattern of MCOLN family in Pca in five independent microarray datasets (Each of the datasets has all MCOLN family member expression data) was performed in Oncomine website (https://www.oncomine.org). The datasets (GSE17951 and GSE46602) analysed in this study were downloaded from the Gene Expression Omnibus (GEO). The gene expression data and survival information for Pca were originated from TCGA and analyzed by The Human Protein Atlas website (https://www.proteinatlas.org/).
Plasmids, antibodies and reagents
pCMV5-flag-MCOLN2 and pLKO-tet-on-MCOLN2 shRNA were constructed by our lab. GFP-RelA was obtained from addgene (#23255). The antibodies used were listed as follows: human anti-MCOLN2 antibody (1:1000, #ACC-082, Alomone labs) or (1:1000, #sc-393538, Santa Cruz), anti-Vimentin (1:1000, #5741, CST), anti-ZO-1 (1:1000, #8193, CST), anti-E-Cadherin (1:1000, # 3195, CST), anti-Slug (1:500, #9585, CST), anti-NF-κB p65 (RelA) (1:1000, #8242, CST), anti-Phospho-NF-κB p65 (p-RelA) (1:1000, #3033, CST), anti-IκB-α (1:1000, #4814, CST), anti-Phospho- IκB-α (1:1000, #2859, CST), anti-Ki-67 (1:1000, A2094, ABclonal), anti-LAMP-1 (1:1000, MA1-164, Invitrogen), anti-RAB7 (1:1000, ab50533, Abcam), anti-GAPDH (1:1000, #97166, CST). Neutralised IL-1β antibody (500 ng/ml, AF-201-SP) and recombinant human IL-1β (201-LB-005) were from R&D Systems. Glycyl-L-phenylalanine-beta-naphthylamide (GPN) (145914, Abcam), ML-SA1 (SML0627, Sigma–Aldrich), Ionomycin (I3909, Sigma–Aldrich), BAPTA-AM (A1076, Sigma–Aldrich) were used in culture cells.
Cell culture
293FT cell lines, human Pca cell lines (PC-3, DU145, LNCaP) and benign hyperplastic prostatic epithelial cell line (BPH-1) were purchased from the American Type Culture Collection (ATCC). Cells were cultured in Dulbecco’s modified Eagle’s media (DMEM) or Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 10%(v/v) fetal bovine serum (FBS) and 100 Units/mL of penicillin, and 100 µg/mL of streptomycin. These cell lines were checked for mycoplasma presence routinely.
siRNA transfection
Exponentially growing untreated PC-3 and DU145 cells were plated in six-well plate for 24 h before transfection. When cells were grown at 60–70% confluence, 30 pmol of specific siRNA targeting the mRNA of MCOLN2 or scrambled siRNA (NC-si) with Lipofectamine RNAiMax reagent (ThermoFisher Scientific, #13778150) were added to conduct siRNA transfection. After treatment for at least 48 h, the interference efficiency was confirmed by RT-PCR and western blotting, and the cells were harvested for further assays. The siRNA oligonucleotides were synthesised from GenePharma (Shanghai, China) and the detailed sequences of siRNAs used in this study were listed in Supplementary Table 1.
Human tissue array and scoring
Immunohistochemistry staining for MCOLN2 was performed on human Pca tissue microarray specimens (PR8011b, US Biomax, USA), using the MCOLN2 antibody (1:400, Alomone labs). The percentage of MCOLN2-positive cancer cells and the staining intensity grade (0–3) were determined. The cancer cell staining index, a factor of the staining percentage and intensity, was obtained for each tissue section.
RNA extraction and RT-PCR
Total RNA was extracted using TRIzol (Invitrogen). The cDNA was synthesised from 2 µg of total RNA using M-MLV reverse transcriptase (Thermo Fisher Scientific) for quantitative RT-PCR. The primer sequence of target genes and reference gene GAPDH were listed in Supplementary Table 2.
Western blots
Total protein was extracted from cells using cell lysis buffer containing PMSF (Beyotime). The samples were separated by 8–12% SDS-polyacrylamide gel electrophoresis, followed by transferring to PVDF membrane. After blocking in PBS containing 5% bovine serum albumin, the membrane was incubated with primary antibody at 4 °C overnight, followed by incubation with a peroxidase-linked secondary antibody (Thermo Fisher Scientific) at room temperature for 1 h. The signals were detected by X-ray film after incubating with western blotting Luminol reagent (GE Health care).
Immunocytochemistry
The cells grown on coverslips were washed with PBS twice, fixed in 4% paraformaldehyde (Sigma–Aldrich, USA) in PBS, permeabilised with 0.1% Triton X-100 (Sigma–Aldrich, USA), and then blocked with 5% bovine serum albumin (Thermo Fisher Scientific) in PBS for 1 h at room temperature. The cells were incubated with primary antibodies at 4 °C overnight, followed by incubation with fluorescence-conjugated secondary antibodies for 90 min at room temperature in the dark. Images were acquired using a confocal microscope.
Cell viability assay
Cell growth was determined by cell counting kit-8 (CCK8) (#c0038, Beyotime). Cells at a density of 2 × 103 per well were grown in the 96-well plates in 0.1 ml full medium at 37 °C for different period of times. Each treatment had five duplicated wells, and CCK8 assays were repeated three time according to the manufacturer’s protocol [28].
Colony-formation assay
Briefly, single-cell suspension was plated at a density of 500 cells per well in six-well plate. The culture medium was replaced every 3 days. Two weeks later, formed clones were fixed and stained with 0.5% crystal violet, and the colonies with more than fifty cells were counted under a microscope.
Migration and invasion assays
Cells were plated into the upper chamber (without fetal bovine serum) with 8 µm pore (BD Falcon, Franklin Lakes, NJ, USA) at a density of 5 × 104 per well. Chambers without or with Matrigel matrix were inserted into matching 24-well plate containing 20% FBS for migration or invasion assays. After migrated or invasive cells adhering to the lower surface of chamber were fixed and stained with 0.5% crystal violet and counted under microscope.
Wound-healing assay
Wound-healing assays were performed following the procedure described elsewhere (https://ibidi.com/culture-inserts/25-25-culture-inserts-2-well-for-self-insertion.html). A two-well culture insert (#80209, ibidi, WI, USA) was placed on the culture surface. A cell suspension was added to both reservoirs and allowed to attach. Remove the two-well culture insert after plating cells for 12 h to create a cell-free gap of ~500 µm, 10 fields were randomly selected in each well to record the gap area following fixing and staining with crystal violet at appropriated time to analyze cell migration.
Lentivirus production and cell transduction
HEK293FT cells were seeded in 10 cm dishes. The cells with 60–80% confluence were co-transfected with pLKO-Tet-On (Novartis), 7.5 µg psPAX2 (Addgene, 12260) and 7.5 µg pMD2.G (Addgene, 12259) using a commercial lentivirus package kit (Biowit, Shenzhen, China) according to manufacturer’s instruction. The supernatants were harvested at 48 and 72 h, centrifuged at 400 × g for 10 min, and then passed through 0.45 µm filters. The collected virus was stored at −80 °C. The shRNA sequences of MCOLN2 were listed in Supplementary Table 3.
Pca cells were seeded in six-well plate. When the cells reached 40% confluence, lentiviral conditional medium was added. After 48 h, the cells were treated with 2 µg/ml puromycin (Thermo Fisher Scientific) for 4 days to select positively infected cells. And the MCOLN2 shRNA expression was induced by 100 ng/ml doxycycline (Sigma–Aldrich) for at least 48 h. MCOLN2 knockdown cells were confirmed with qPCR and western blots.
[Ca2+]i measurement
Ca2+ measurement was performed as described in our previous report [29]. Briefly, DU145 and PC-3 cells were plated on the coverslips for 48 h before measurements. The cells were loaded with fluo-4/AM (Invitrogen, 5 µM) and 0.02% Pluronic F-127 (Invitrogen) for 30 min in dark at 37 °C. The coverslip was gently rinsed twice with Ca2+ free-PSS in mM: 140 NaCl, 5 KCl, 1 MgCl2, 10 glucose, 0.2 EGTA, 5 HEPES, pH 7.4 and placed in a bath chamber for measurement. Four micromolar ionomycin was applied to the cells to induce Ca2+ release from the intracellular Ca2+ stores.
Fluo-4 ratio (F1/F0) was used to monitor changes in intracellular [Ca2+]i upon stimulation by using a confocal microscope (Olympus FV1000, Japan).
ELISA (enzyme-linked immunosorbent assay) analysis of IL-1β
Secreted IL-1β was detected by ELISA using human IL-1β ELISA kit (R&D systems, USA) according to the manufacturer’s instruction. Briefly, the cells were plated in six-well plate and cultured with free FBS medium for 24 h. After treatment, the supernatant from the cultured cells was collected by centrifugation and applied to ELISA experiments immediately. The final amount of interested protein from each group was normalised to the cell number.
Cytokine assay
PC-3 cells were plated in six-well plates and transfected with NC-si or siMCOLN2 for 48 h. Cell lysates were collected and subjected to human cytokine antibody array (Abcam, ab133997) according to the manufacturer’s instructions. Briefly, the arrays were first blocked with blocking buffer, then incubated with 1 ml diluted cell lysate protein at 4 °C overnight, followed by incubation with Biotin-conjugated anticytokines cocktail at 4 °C overnight. The membranes were washed and then incubated with 2 ml HRP-conjugated streptavidin at 4 °C overnight, followed by chemiluminescence detection using a CCD camera with the imaging system. Quantitative array analysis was performed using Image J.
Luciferase reporter assay
Pca cells were plated at 50–60% confluence in 24-well plates. 24 h later, cells were transiently transfected with 1 µg total DNA using Lipofectamine 2000 Transfection Reagent (ThermoFisher). The pGMNF-κB-Luc was transfected into cells with one of the following siRNAs or plasmids: scrambled siRNA or Control, siMCOLN2 or MCOLN2. Co-transfection of Renilla luciferase under the control of the SV40 early enhancer/promoter region (p SV40-RL, Promega) was used as a control. After 48 h, the cells were collected and subjected to a Dual Luciferase Reporter Assay System (E1910, Promega, USA). Firefly luciferase activity was normalised to Renilla luciferase activity for each sample. All transfections were performed at least three times, in triplicate.
In vivo xenograft assay
Animal experiments were approved by the Animal Experimentation Ethics Committee of The Chinese University of Hong Kong, performed in compliance with the guide for the care and used of laboratory animals (National Institutes of Health Publication, 8th edition, updated 2011). Four- to five-weeks-old male nude mice were used in this study.
5 × 106 transfected DU145 cells/100 µl of PBS per mouse were injected subcutaneously in the lower back of the nude mice. Tumor length and width were measured every 3 days until the end of the experiment. Three weeks later, mice were sacrificed by CO2 inhalation, and tumors were isolated and measured. Tumor volumes were calculated: Volume = Length × Width2/2. For intratibial injections, the mice were anaesthetised with isoflurane and one million transfected PC-3 cells in 10 µl of PBS were injected into the left tibia of each mouse. Mice were imaged using Bruker SkyScan 1278 microCT for bone lesion area. Mice that never developed lesions were considered technical injection failures and were excluded from the studies. Four weeks later, mice were sacrificed by CO2 inhalation, and the bone lesion areas were measured by Image J.
For both cases, doxycycline (30 µg/gram of body weight per mouse) was administered by intraperitoneal injection every two days to induce the knockdown of MCOLN2 expression and PBS was as vehicle control.
Statistical analysis
All the data were presented as means ± S.D (at least three biological replicates or three independent experiments). Statistical analysis was performed using GraphPad Prism (GraphPad software, San Diego, California, USA) or SPSS V.16.0. Survival analysis was carried out using the Kaplan–Meier method, and the log-rank test was used to compare the survival curves. Student’s t-test or one-way ANOVA was used for comparison between groups. Tests of normality were passed, although for experiments with small sample sizes, the ability to test for normality is limited. Variances were similar between all the statistically compared groups. P ≤ 0.05 was considered statistically significant.
For animal studies, A priori sample size calculations were not performed. The mice were randomly divided into two groups and all the experiments were performed in a completely blinded manner.
Results
MCOLN2 expression was increased during malignant transformation and predicted a poor prognosis in Pca
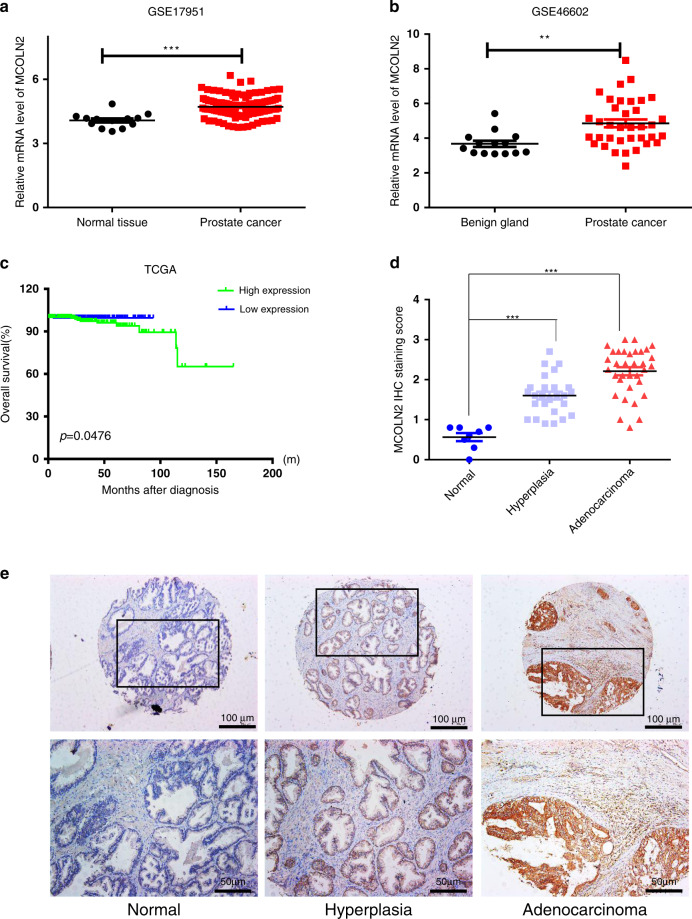
To assess the role of mucolipin subfamily of TRP channels (MCOLN1, 2 and 3) in Pca, we performed meta-analysis in five independent microarray datasets on Oncomine website [30–34]. The results showed that among three family members, MCOLN2 was significantly upregulated in Pca tissues compared to normal tissues (Fig. S1). MCOLN2 upregulation in Pca tissues was confirmed in another two independent GEO datasets (Fig. 1a, b). To test the prognostic value of MCOLN2, data derived from The Cancer Genome Atlas (TCGA) cohort with Pca clinical follow-up information was used for Kaplan–Meier analysis. Of note, Pca patients with higher MCOLN2 expression have significant poor prognosis compared to those with lower MCOLN2 expression (Fig. 1c). To further determine the protein expression pattern of MCOLN2 during Pca development, we performed immunohistochemical staining of MCOLN2 in a large-scale human Pca tissue microarray (TMA). The results showed that MCOLN2 expression was increased from human normal prostate tissues to prostate hyperplasia tissues to Pca tissues (Fig. 1d, e). These results suggest that MCOLN2 expression is increased during Pca development and correlated with poor prognosis.
Fig. 1. MCOLN2 expression was increased during malignant transformation and predicted a poor prognosis in Pca.
a, b Expression analysis of MCOLN2 in tumors and normal prostate tissue samples using two independent cohorts [GSE17951 (T = 109, N = 13); GSE46602 (T = 36, N = 14)] derived from Gene Expression Omnibus datasets. c Kaplan–Meier analysis (log-rank test) of the overall survival of patients with Pca based on MCOLN2 expression. Data were derived from The Cancer Genome Atlas (TCGA) cohort. The median expression value was used to define the high and low expression group. d Immunohistochemical analysis of MCOLN2 expression in human PCA tissue microarray. e Representative immunohistochemical staining images showing MCOLN2 expression from normal prostate tissue to hyperplasia tissue and prostate adenocarcinoma tissue. Scale bar: 100 µm or 50 µm. **p < 0.01, ***p < 0.001.
MCOLN2 promoted Pca cell proliferation in vitro and in vivo
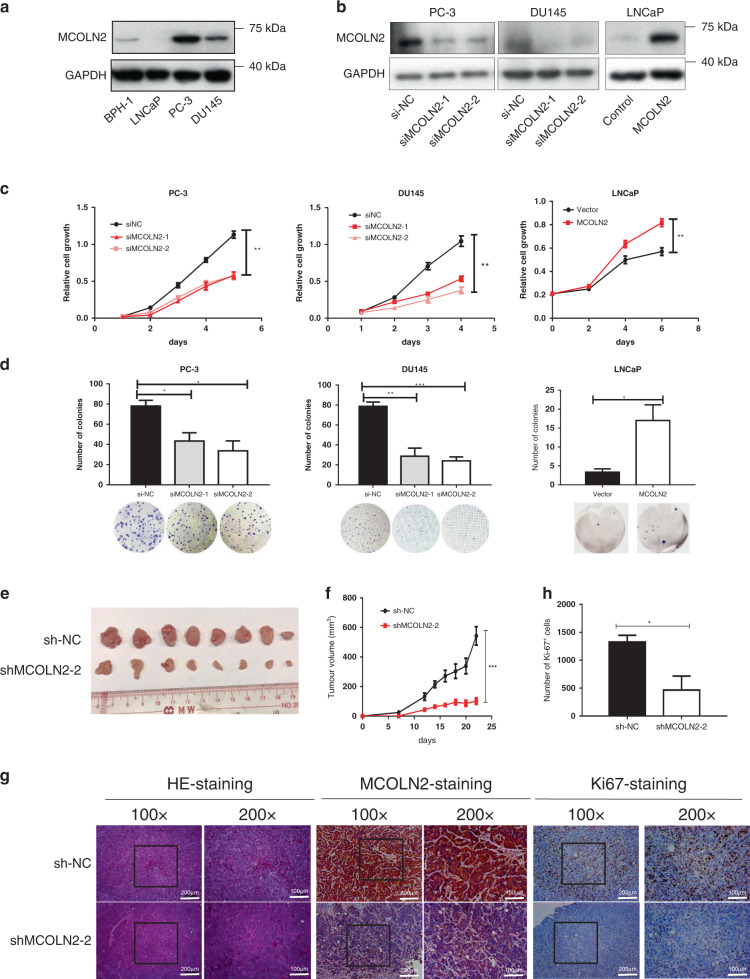
To investigate the role of MCOLN2 in progression of Pca, endogenous MCOLN2 expression was compared among different Pca cell lines (LNCaP, PC-3 and DU145) and benign prostate cell (BPH-1). The expression of MCOLN2 was higher in Pca cell PC-3 and DU145 than in BPH-1. LNCaP cells had the lowest expression of MCOLN2 (Fig. 2a). In order to evaluate the functional role of MCOLN2 in Pca, we knocked down MCOLN2 expression in PC-3 and DU145 but overexpressed MCOLN2 in LNCaP cells (Fig. 2b). The results showed that MCOLN2 knockdown with siRNA significantly inhibited the proliferation of PC-3 and DU145 cells after 3 days (Fig. 2c). Similar results were observed in doxycycline (Dox) inducible MCOLN2 stable knockdown PC-3 and DU145 cell lines (Fig. S2A, B). And exogenous overexpression of MCOLN2 significantly accelerated the proliferation of LNCaP cells (Fig. 2c). Furthermore, foci formation assay results also showed that MCOLN2 silencing significantly reduced the size and number of colonies formed by PC-3 and DU145 cells, whereas exogenous overexpression of MCOLN2 had opposite effect in LNCaP cells (Figs. 2d and Fig. S2C, D). These results indicated that MCOLN2 promoted Pca cell growth in vitro.
Fig. 2. MCOLN2 promoted proliferation of Pca cells in vitro and in vivo.
a Western blots detected endogenous MCOLN2 expression in human benign prostate cell (BPH-1) and Pca cells. b MCOLN2 expression in PC-3, DU145 and LNCap cells after knockdown or overexpression of MCOLN2. c, d Cell proliferation and foci formation analysis were performed after knockdown or overexpression of MCOLN2 in Pca cells. NC, negative control. Mean ± S.D. (n ≥ 3). *P < 0.05, **P < 0.01, ***P < 0.001. e–g MCOLN2 in PCa induced xenograft tumor growth. Nude mice were inoculated with DU145 cells carrying Dox-inducible shMCOLN2. e Tumor picture from mice inoculated with DU145 cells carrying Dox-inducible shMCOLN2. f Growth curve of tumor volume measured on indicated days. Mean ± S.D. (n = 8). g Pictures of the H&E staining (left), immunohistochemical staining for MCOLN2 (middle) and Ki-67 (right) in tumors. Scale bar: 100 µm or 200 µm. h Number of Ki-67-positive cells in control and MCOLN2 stable knockdown tumor groups. ShNC, negative control shRNA.
Next we investigated whether MCOLN2 affected the tumorigenesis of Pca cells in vivo. DU145 cells with Dox-inducible MCOLN2 stable knockdown were subcutaneously injected into BALB/c nude mice to generate xenograft tumors. Dox was given at the indicated timepoints. The results showed that MCOLN2 knockdown significantly inhibited tumor growth as early as at the first week and continually until the end of experiments (Fig. 2e, f). The tumors in MCOLN2 knockdown group were smaller in size and lower in weight than those in negative control (shNC) group (p < 0.01) (Fig. 2e and Fig. S2E). IHC staining confirmed that MCOLN2 expression and the percentage of Ki-67-positive proliferation cells were both lower in MCOLN2 knockdown (shMCOLN2-2) group compared to the shNC control group (Fig. 2g, h).
MCOLN2 promoted migration and invasion of Pca cells in vitro and bone lesion development in vivo
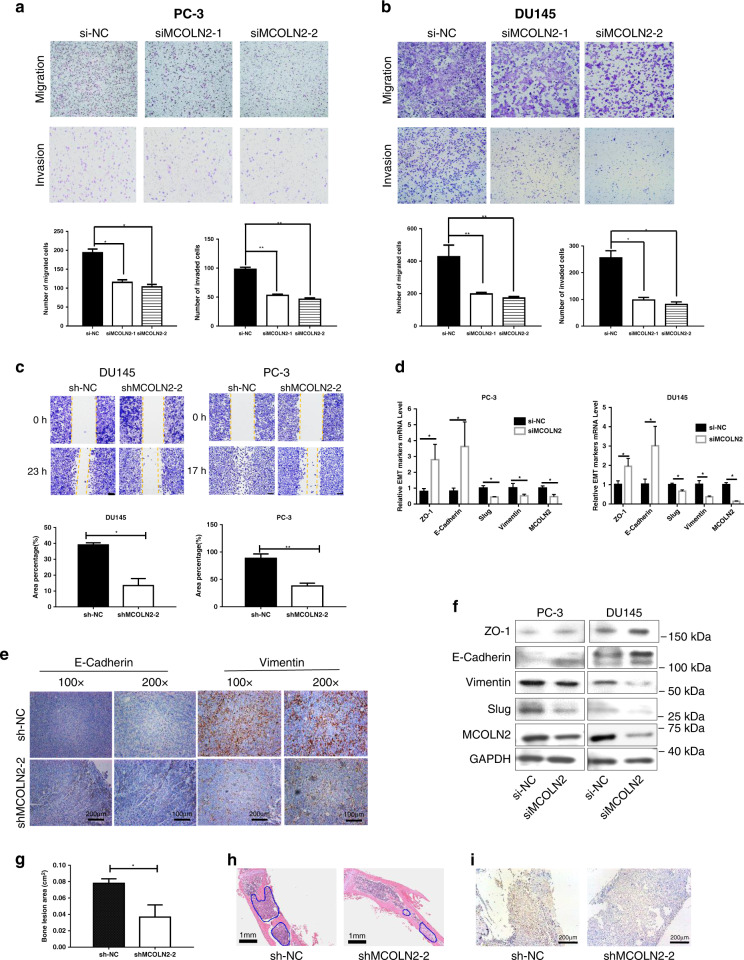
To investigate the role of MCOLN2 in the motility and invasion of Pca cells, transwell experiments were performed. MCOLN2 knockdown significantly impaired the migration and invasion ability of PC-3 (Fig. 3a) and DU145 cells (Fig. 3b), whereas MCOLN2 overexpression largely facilitated the motility of LNCaP cells (Fig. S3A). In wound-healing assays, the results showed that MCOLN2 silencing significantly reduced the wound closure of DU145 and PC-3 cells (Fig. 3c, Fig. S3B). Given that epithelial-mesenchymal transition (EMT) is a critical event involved in the process of tumor metastasis [35], the impact of MCOLN2 on the expression of several EMT markers was examined in DU145 and PC-3 cells after MCOLN2 knockdown. MCOLN2 knockdown significantly decreased the expression of Slug and Vimentin but increased the expression of ZO-1 and E-Cadherin at both mRNA (Fig. 3d) and protein levels (Fig. 3f). The effect of MCOLN2 knockdown on the expression of Vimentin and E-Cadherin proteins were further confirmed in the xenograft tumor tissues (Fig. 3e). These data strongly suggest that MCOLN2 promotes the migration and invasion of Pca cells.
Fig. 3. MCOLN2 promoted Pca cells migration, invasion and bone lesion developmengt through epithelial-mesenchymal transition (EMT).
a, b Effects of MCOLN2 knockdown on migration and invasion of PC-3 (a) and DU145 (b) cells evaluated by transwell assays. Mean ± S.D. (n = 3). c Knockdown of MCOLN2 expression on the migration ability of DU145 and PC-3 cells by wound-healing assay. Mean ± S.D. (n = 3). d Effects of MCOLN2 silencing on the mRNA abundance of selected EMT markers in PC-3 and DU145 cells. Mean ± S.D. (n = 3). e Representative images of IHC staining for E-cadherin and Vimentin in xenograft tissues. Scale bar: 100 μm (200×) or 200 μm (100×). f Effects of MCOLN2 silencing on the protein expression of selected EMT markers in PC-3 and DU145 cells. Mean ± S.D. (n = 3). g–i MCOLN2 in PCa induced bone lesion development. Nude mice were intratibially injected with PC-3 cells carrying Dox-inducible shMCOLN2. g Quantification of the bone lesion areas in PC-3 injected nude mice tibiae. Mean ± S.D. (n = 4). h Representative H&E staining of the tibiae. The blue outlines indicated the PC-3 tumor growth in the bone marrow. Scale bar: 1 mm. i Representative immunohistochemical staining of MCOLN2 in PC-3 tumor cells inside the mouse tibiae. Scale bar: 200 μm. *P < 0.05, **P < 0.01, ***P < 0.001.
Bone is the preferential site for PCa metastasis. To study the role of MCOLN2 in PCa induced bone lesion development, PC-3 cells with Dox-inducible MCOLN2 stable knockdown were intratibially injected into the nude mice and the area of bone lesion was measured. A significant decrease of bone lesion area was observed in Dox-induced MCOLN2 knockdown group (shMCOLN2-2) (Fig. 3g, Fig. S3C). The growth of PC-3 tumors in the tibiae with bone lesion was confirmed by H&E staining (Fig. 3h). IHC staining confirmed that MCOLN2 and Vimentin expressional levels were lower in shMCOLN2-2 group compared to the shNC control group (Fig. 3i and Fig. S3D).
Identification of IL-1β as the downstream signaling molecule of MCOLN2
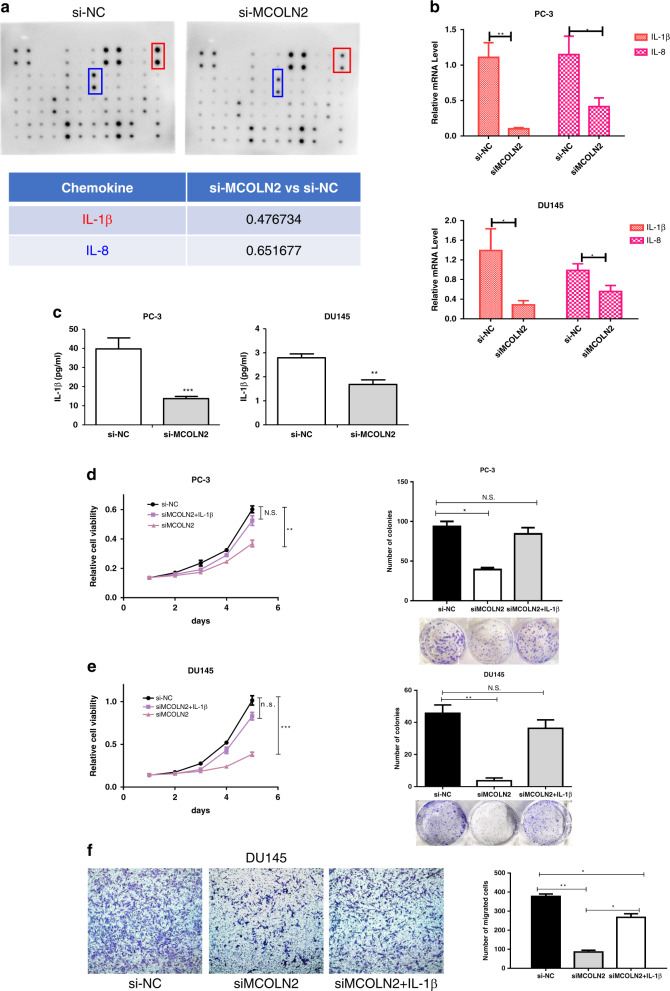
To identify the downstream mediators of MCOLN2 in Pca progression, we first compared the expression profiles of various chemokines and growth factors between negative control group (NC) and MCOLN2 knockdown group using cytokine array analysis. Two cytokines (IL-1β and IL-8) were significantly decreased in the siMCOLN2 cells compared with the control cells (Fig. 4a) and confirmed by real-time PCR (Fig. 4b). Real-time PCR results also showed that MCOLN2 overexpression caused an upregulation of IL-1β in LNCaP cells (Fig. S4A). We further quantified the secretion of IL-1β protein by enzyme-linked immunosorbent assay in the culture medium. Knockdown of MCOLN2 expression decreased IL-1β secretion from PC-3 and DU145 cells (Fig. 4c).
Fig. 4. Identification of IL-1β as the downstream target of MCOLN2.
a Cytokine array of the cell lysates from siNC and siMCOLN2 PC-3 cells. Relative signal intensity of indicated chemokines is calculated by image J in the table. b IL-1β and IL-8 mRNA levels were determined by qPCR in PC-3 and DU145 cells after MCOLN2 knockdown. c IL-1β protein level in the conditional medium (CM) from siNC and siMCOLN2 PC-3 and DU145 cells were detected by ELISA. d–f PC-3 and DU145 cells transfected with siNC or siMCOLN2 were added with the indicated 25 ng/ml recombinant human IL-1β protein for 2 days. The proliferation (d, e), colony formation (d, e) and migration (f) were examined by CCK8, foci formation and transwell assay. Mean ± S.D. (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001.
To explore whether MCOLN2 regulated Pca progression through or in part by modulating IL-1β expression and activity, we tried to rescue the inhibitory effect of MCOLN2 knockdown on Pca progression with IL-1β recombinant protein. Our results showed that re-introduction of IL-1β significantly increased the growth of PC-3 or DU145 cells to the level found in control cells, as determined by both CCK8 and foci formation assay (Fig. 4d, e). Furthermore, re-introduction of IL-1β also rescued the migration ability of DU145-siMCOLN2 cells (Fig. 4f). These results strongly suggest that alteration of IL-1β levels induced by MCOLN2 are indeed responsible for Pca progression.
MCOLN2 mediated endolysosomal Ca2+ release to regulate IL-1β production and secretion
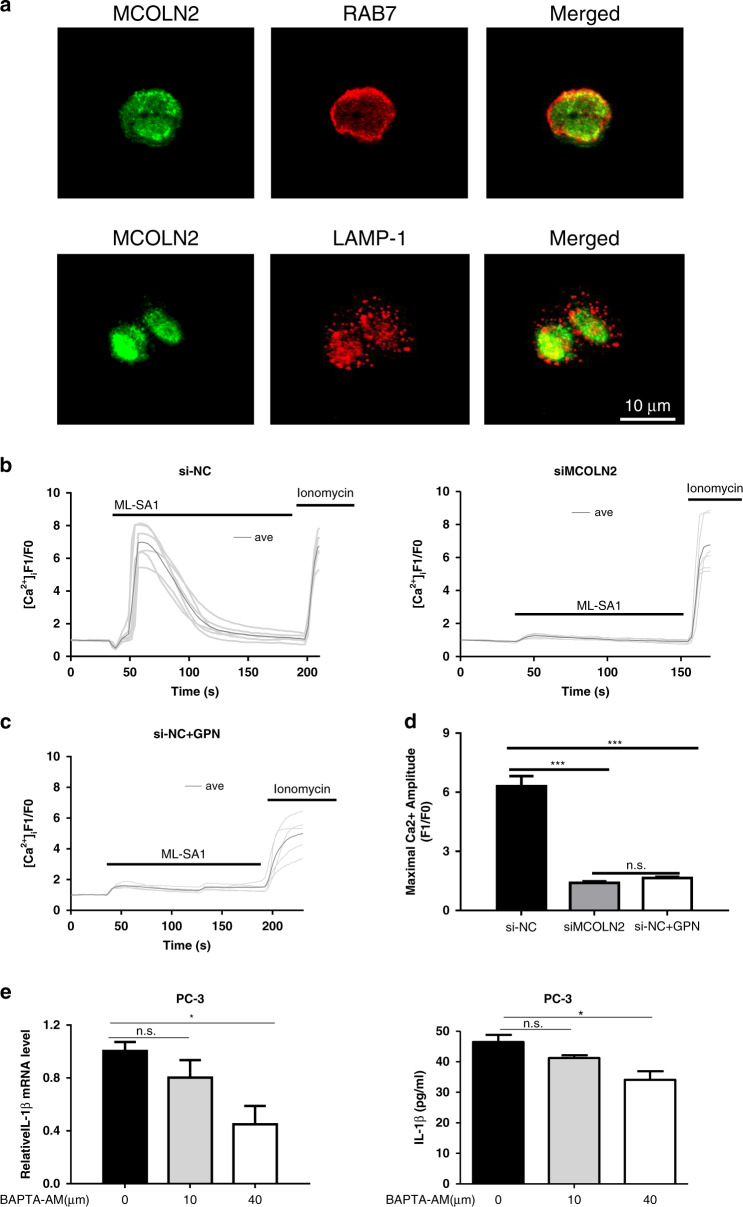
Subcellular localisation of MCOLN2 proteins was examined in PC-3 and DU145 cells. MCOLN2 displayed partial co-localisation with late endosome marker RAB7 and low level of co-localisation with lysosome marker LAMP-1 (Fig. 5a and Fig. S4B), confirming endolysosomal localisation of MCOLN2.
Fig. 5. MCOLN2 mediated endolysosomal Ca2+ release to regulate IL-1β production and release.
a Representative images of co-localisation of MCOLN2 proteins with late endosomal marker RAB7 (upper) and lysosomal marker LAMP-1 (bottom) in PC-3. The right most panels were the merged images. Scale bar = 10 μm. Representatives from three experiments. In b, c, the cells were bathed in a Ca2+-free physiological saline. b ML-SA1-induced cytosolic Ca2+ rise in PC-3 cells with or without MCOLN2 knockdown. Data were representative time course traces of cytosolic Ca2+ change in response to 20 µM ML-SA1 treatment. c Representative time course traces of cytosolic Ca2+ change in response to 20 µM ML-SA1 in PC-3 cells with GPN pretreatment. 200 µM GPN treatment was for 30 min in normal medium. d Summary data of the maximal Ca2+ rise in response to ML-SA1 induction as in b and c. Means ± S.D. (n = 6). e IL-1β mRNA and protein levels were detected by qPCR and ELISA after different doses of BAPTA-AM treatment in PC-3 cells. Means ± S.D. (n = 3). *P < 0.05, ***P < 0.001.
Next, we studied whether MCOLN2 could mediate endolysosomal Ca2+ release to induce cytosolic Ca2+ rise. Pca cells with or without MCOLN2 knockdown were loaded with a Ca2+-sensitive fluorescence dye Fluo-4/AM to monitor changes in cytosolic Ca2+ . The cells were bathed in Ca2+-free physiological solution and challenged with a membrane-permeable MCOLN2 activator ML-SA1 to induce intracellular Ca2+ release. The results showed that knockdown of MCOLN2 in PC-3 and DU145 cells reduced intracellular Ca2+ release in response to 20 µM ML-SA1 (Fig. 5b and Fig. S4C), confirming the role of MCOLN2 in intracellular Ca2+ release. To further dissect the source of ML-SA1-induced intracellular Ca2+ release, we used glycyl-L-phenylalanine-beta-naphthylamide (GPN) to disrupt endolysosomes, followed by ML-SA1 challenge. GPN treatment abolished the ML-SA1-induced intracellular Ca2+ release (Fig. 5c, d and Fig. S4D, E), confirming that MCOLN2 mediated endolysosomal Ca2+ release to induce cytosolic Ca2+ rise. We next investigated the role of cytosolic Ca2+ level in regulating IL-1β production and secretion. Chelation of cytosolic Ca2+ by BAPTA-AM suppressed the transcription and protein secretion of IL-1β in PC-3 (Fig. 5e). Taken together, our data suggest that MCOLN2-mediated cytosolic Ca2+ rise may regulate IL-1β expression and secretion in Pca cells.
MCOLN2 regulated IL-1β/NF-κB pathway
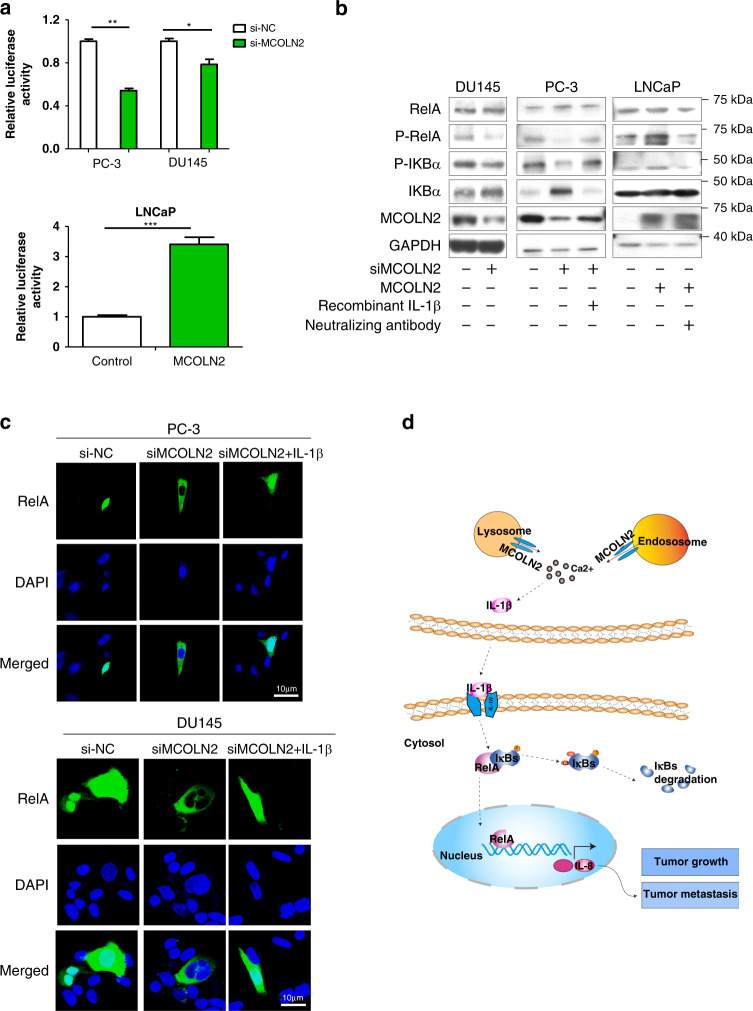
NF-κB is a Ca2+-activated transcriptional factor and activated by pro-inflammatory cytokines such as IL-1β [36, 37]. To determine the involvement of NF-κB, we first performed NF-κB luciferase reporter assay. The results showed that MCOLN2 knockdown significantly decreased NF-κB transcriptional activity in both PC-3 and DU145 cells (Fig. 6a). MCOLN2 overexpression in LNCaP cells, however, increased its activity (Fig. 6a). Furthermore, MCOLN2 knockdown reduced the phosphorylation level of RelA and NF-κB inhibitor alpha (IκBa) (Fig. 6b), and markedly decreased the translocation of RelA to nucleus (Fig. 6c and Fig. S5), all of which indicated a decreased NF-κB pathway activity. Importantly, this effect could be reversed by introducing IL-1β recombinant protein in MCOLN2 knockdown cells (Fig. 6b, c and Fig. S5) or by introducing IL-1β neutralising antibody in MCOLN2 overexpression cells (Fig. 6b). These results suggest that NF-κB is downstream of MCOLN2 and IL-1β. Collectively, these results suggest that MCOLN2 regulates IL-1β/NF-κB pathway.
Fig. 6. MCOLN2 regulated IL-1β/NF-κB pathway.
a NF-κB luciferase reporter assays were performed in PC-3 and DU145 cells with or without MCOLN2 knockdown (upper) and in LNCaP cells with or without MCOLN2 overexpression (bottom). Mean ± S.D. (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001. b NF-κB pathway and MCOLN2 expression were detected by western blot after Pca cells treated with the indicated treatment. c Immunofluorescent staining of exogenous RelA subcellular localisation in PC-3 (upper) and DU145 cells (bottom) with or without MCOLN2 knockdown and IL-1β treatment. The cells were overexpressed with RelA-GFP plasmids. Nuclei were counterstained with DAPI (pseudo-colored in blue). Scale bar, 10 µm. d Schematic diagram illustrated that MCOLN2 regulated IL-1β/NF-κB pathway to promote Pca progression. MCOLN2 mediates endolysosomal Ca2+ release, which induces cytosolic Ca2+ rise and IL-1β transcription and release. The released IL-1β from tumor cells can activate NF-κB pathway. Activation of NF-κB pathway induces IL-8 transcription and other downstream targets to promote tumor growth and metastasis.
Discussion
Several TRP channels have previously been reported to play a role in the regulation of proliferation, apoptosis and migration of Pca cells. For example, TRPV6, TRPC6 and TRPV2 promote Pca progression via stimulating Pca cell proliferation or enhancing migration and invasion. On the contrary, TRPM8 reduces Pca progression via stimulating apoptosis and preventing cell migration [8, 11]. However, the above-mentioned channels are mostly localised on the plasma membrane, mediating extracellular Ca2+ entry. In the present study, we explored the functional role of an endolysosomal TRP channel MCOLN2 in Pca. Western blot result showed that MCOLN2 expression was high in androgen-independent Pca cells PC-3 and DU145, but low in androgen-dependent Pca cells LNCaP. Immunohistochemistry staining showed that MCOLN2 expression was increased in hyperplasia and adenocarcinoma prostate tissues during malignant transformation. These data combined with bioinformatics analysis implied a potential role of MCOLN2 during Pca development. However, it still needs further study to investigate whether MCOLN2 expression is regulated by androgen receptor pathway. In vitro functional studies showed that knockdown of MCOLN2 expression in PC-3 and DU145 cells reduced the cell proliferation, migration and invasion, whereas overexpression of MCOLN2 in LNCaP cells had an opposite effect.
The role of MCOLN2 in Pca was further examined in animal models in vivo. Indeed, we found that MCOLN2 knockdown reduced the growth of Pca cells-formed prostate tumor xenografts in athymic nude mice by more than 80% and bone lesion development in athymic nude mice. Together, our in vitro and in vivo experiments from cultured human Pca cells to mouse xenograft model to patients’ samples all demonstrated a critical role of MCOLN2 in promoting Pca progression. Thus, we uncovered MCOLN2 as the first endolysosomal TRP channel that can promote Pca progression. The effectiveness of MCOLN2 knockdown on prostate tumor growth also highlights an intriguing possibility of targeting MCOLN2 as a potential therapeutic strategy in Pca treatment.
MCOLN family channels mediate endolysosomal Ca2+ release, which is accompanied by cytosolic Ca2+ rise [12]. In our study, ML-SA1-stimulated cytosolic Ca2+ rise was markedly reduced by MCOLN2 knockdown, and was abolished by GPN, an agent that disrupts acidic endolysosomes. These data confirmed MCOLN2 as an endolysosomal Ca2+ release channel in Pca cells. One recent study reported that endolysosomal Ca2+ release might facilitate endolysosomal exocytosis to induce IL-1β release in human monocytes [26]. Given the important functional role of inflammatory cytokine IL-1β in the pathogenesis of cancer, we next explored whether MCOLN2 could act through IL-1β signaling pathway to promote Pca progression. Indeed, our results showed that knockdown of MCOLN2 or chelation of cytosolic Ca2+ with BAPTA-AM could both reduce the production and release of IL-1β in PC-3 cells. Furthermore, exogenous application of IL-1β recombinant protein could rescue the MCOLN2 knockdown-induced reduction in cancer cell proliferation and migration. These results demonstrated that IL-1β situated downstream of MCOLN2, and that MCOLN2 acted through the production and release of inflammatory cytokine IL-1β to promote the proliferation and migration of Pca cells.
NF-κB is a Ca2+-activated transcriptional factor and could be stimulated by IL-1 family members [36]. Therefore, we next explored whether NF-κB could be a downstream signal of MCOLN2. The results showed that knockdown of MCOLN2 in PC-3 and DU145 cells reduced the level of phospho-p65 and phospho-IκBa, whereas overexpression of MCOLN2 in LNCaP cells had opposite effect. Furthermore, in the presence of IL-1β recombination proteins, MCOLN2 knockdown could no longer reduce the level of phospho-RelA and phospho-IκBa. Moreover, MCOLN2 knockdown reduced the nuclear translocation of RelA, the effect of which was reversed by exogenous application of IL-1β. Collectively, these data indicate that MCOLN2 regulate IL-1β production and release to activate the downstream NF-κB pathway and promote Pca progression. A schematic model is shown in Fig. 6d.
Several endolysosomal Ca2+-release channels have already been reported to be linked to cancer progression via different mechanisms. For example, MCOLN1 may modulate mTORC1 and lysosomal ATP release to promote the development of triple-negative breast cancer [14] and melanoma [15]. Two-pore channels (TPC), which are another group of lysosomal Ca2+-release channels, may enhance β1-integrin trafficking to alter migration and metastasis of lung cancer [12, 38]. In the present study, we demonstrated that MCOLN2 acts through IL-1β/NF-κB pathway to promote Pca progression, adding a novel mechanism through which endolysosomal Ca2+ channels can regulate cancer progression. Together, it is clear that endolysosomal Ca2+ channels may regulate cancer progression through different mechanisms depending on cancer types.
In summary, our study found that MCOLN2, which is upregulated in Pca and correlated with poor prognosis, can promote Pca growth and metastasis. Mechanistically, MCOLN2 mediates endolysosomal Ca2+ release, subsequently stimulates IL-1β/NF-κB pathway to promote Pca progression. The present study highlights an intriguing possibility of targeting MCOLN2 and/or its signaling axis as a potential therapeutic strategy.
Supplementary information
Acknowledgements
Not applicable.
Author contributions
HY, MX, ZM, CL and XM performed the experiments. HY, XM and XY conceived and designed the study, analyzed and interpreted the data. FC and LJ provided assistance in the study. HY, XM and XY wrote the paper with feedback from all authors. All authors read and approved the final manuscript.
Funding information
This work was supported by the grants from Hong Kong Research Grant Committee [AoE/M-05/12, 14100619, RIF/R4005-18F]; Hong Kong Health and Medical Research Fund 06170176; The National Natural Science Foundation of China (81702533); the Fundamental Research Funds for the Central Universities (19ykpy07) and the Natural Science Foundation of Guangdong Province (2019A1515010099;2021A1515012081).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics approval and consent to participate
All animal experiments were approved by the Animal Experimentation Ethics Committee of The Chinese University of Hong Kong, performed in compliance with the guide for the care and used of laboratory animals.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xiangqi Meng, Email: mengxq3@mail.sysu.edu.cn.
Xiaoqiang Yao, Email: yao2068@cuhk.edu.hk.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-021-01537-0.
References
- 1.Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145–64. doi: 10.3322/caac.21601. [DOI] [PubMed] [Google Scholar]
- 2.Horwich A, Parker C, Bangma C, Kataja V, Group EGW. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21:v129–33. [DOI] [PubMed]
- 3.Lang L, Shay C, Zhao X, Teng Y. Combined targeting of Arf1 and Ras potentiates anticancer activity for prostate cancer therapeutics. J Exp Clin Cancer Res. 2017;36:112. doi: 10.1186/s13046-017-0583-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li P, Yang R, Gao WQ. Contributions of epithelial-mesenchymal transition and cancer stem cells to the development of castration resistance of prostate cancer. Mol Cancer. 2014;13:55. doi: 10.1186/1476-4598-13-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Body JJ, Casimiro S, Costa L. Targeting bone metastases in prostate cancer: improving clinical outcome. Nat Rev Urol. 2015;12:340–56. doi: 10.1038/nrurol.2015.90. [DOI] [PubMed] [Google Scholar]
- 6.Hsing AW, Chokkalingam AP. Prostate cancer epidemiology. Front Biosci. 2006;11:1388–413. doi: 10.2741/1891. [DOI] [PubMed] [Google Scholar]
- 7.Van Haute C, De Ridder D, Nilius B. TRP channels in human prostate. ScientificWorldJournal. 2010;10:1597–611. doi: 10.1100/tsw.2010.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shapovalov G, Ritaine A, Skryma R, Prevarskaya N. Role of TRP ion channels in cancer and tumorigenesis. Semin Immunopathol. 2016;38:357–69. doi: 10.1007/s00281-015-0525-1. [DOI] [PubMed] [Google Scholar]
- 9.Zhang X, Zhang L, Lin B, Chai X, Li R, Liao Y, et al. Phospholipid phosphatase 4 promotes proliferation and tumorigenesis, and activates Ca(2+)-permeable Cationic Channel in lung carcinoma cells. Mol Cancer. 2017;16:147. doi: 10.1186/s12943-017-0717-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prevarskaya N, Skryma R, Bidaux G, Flourakis M, Shuba Y. Ion channels in death and differentiation of prostate cancer cells. Cell Death Differ. 2007;14:1295–304. doi: 10.1038/sj.cdd.4402162. [DOI] [PubMed] [Google Scholar]
- 11.Gkika D, Prevarskaya N. TRP channels in prostate cancer: the good, the bad and the ugly? Asian J Androl. 2011;13:673–6. doi: 10.1038/aja.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alharbi AF, Parrington J. Endolysosomal Ca(2+) signaling in cancer: the role of TPC2, from tumorigenesis to metastasis. Front Cell Dev Biol. 2019;7:302. [DOI] [PMC free article] [PubMed]
- 13.Li P, Gu M, Xu H. Lysosomal ion channels as decoders of cellular signals. Trends Biochem Sci. 2019;44:110–24. doi: 10.1016/j.tibs.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu M, Almasi S, Yang Y, Yan C, Sterea AM, Rizvi Syeda AK, et al. The lysosomal TRPML1 channel regulates triple negative breast cancer development by promoting mTORC1 and purinergic signaling pathways. Cell Calcium. 2019;79:80–8. doi: 10.1016/j.ceca.2019.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kasitinon SY, Eskiocak U, Martin M, Bezwada D, Khivansara V, Tasdogan A, et al. TRPML1 promotes protein homeostasis in melanoma cells by negatively regulating MAPK and mTORC1 signaling. Cell Rep. 2019;28:2293–305 e9. doi: 10.1016/j.celrep.2019.07.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jung J, Cho KJ, Naji AK, Clemons KN, Wong CO, Villanueva M, et al. HRAS-driven cancer cells are vulnerable to TRPML1 inhibition. EMBO Rep. 2019;20:4. doi: 10.15252/embr.201846685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuajungco MP, Silva J, Habibi A, Valadez JA. The mucolipin-2 (TRPML2) ion channel: a tissue-specific protein crucial to normal cell function. Pflug Arch. 2016;468:177–92. doi: 10.1007/s00424-015-1732-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun L, Hua Y, Vergarajauregui S, Diab HI, Puertollano R. Novel role of TRPML2 in the regulation of the innate immune response. J Immunol. 2015;195:4922–32. doi: 10.4049/jimmunol.1500163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sterea AM, Almasi S, El, Hiani Y. The hidden potential of lysosomal ion channels: a new era of oncogenes. Cell Calcium. 2018;72:91–103. doi: 10.1016/j.ceca.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Baker KJ, Houston A, Brint E. IL-1 family members in cancer; two sides to every story. Front Immunol. 2019;10:1197. doi: 10.3389/fimmu.2019.01197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krelin Y, Voronov E, Dotan S, Elkabets M, Reich E, Fogel M, et al. Interleukin-1beta-driven inflammation promotes the development and invasiveness of chemical carcinogen-induced tumors. Cancer Res. 2007;67:1062–71. doi: 10.1158/0008-5472.CAN-06-2956. [DOI] [PubMed] [Google Scholar]
- 22.Dinarello CA. Therapeutic strategies to reduce IL-1 activity in treating local and systemic inflammation. Curr Opin Pharmacol. 2004;4:378–85. doi: 10.1016/j.coph.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Apte RN, Krelin Y, Song X, Dotan S, Recih E, Elkabets M, et al. Effects of micro-environment- and malignant cell-derived interleukin-1 in carcinogenesis, tumour invasiveness and tumour-host interactions. Eur J Cancer. 2006;42:751–9. doi: 10.1016/j.ejca.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Liu Q, Russell MR, Shahriari K, Jernigan DL, Lioni MI, Garcia FU, et al. Interleukin-1beta promotes skeletal colonization and progression of metastatic prostate cancer cells with neuroendocrine features. Cancer Res. 2013;73:3297–305. doi: 10.1158/0008-5472.CAN-12-3970. [DOI] [PubMed] [Google Scholar]
- 25.Gudipaty L, Munetz J, Verhoef PA, Dubyak GR. Essential role for Ca2+ in regulation of IL-1beta secretion by P2X7 nucleotide receptor in monocytes, macrophages, and HEK-293 cells. Am J Physiol Cell Physiol. 2003;285:C286–99. doi: 10.1152/ajpcell.00070.2003. [DOI] [PubMed] [Google Scholar]
- 26.Tseng HHL, Vong CT, Kwan YW, Lee SM, Hoi MPM. Lysosomal Ca(2+) signaling regulates high glucose-mediated interleukin-1beta secretion via transcription factor EB in human monocytic cells. Front Immunol. 2017;8:1161. doi: 10.3389/fimmu.2017.01161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng Q, Tan Q, Ren Y, Reinach PS, Li L, Ge C, et al. Hyperosmotic stress-induced TRPM2 channel activation stimulates NLRP3 inflammasome activity in primary human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2018;59:3259–68. doi: 10.1167/iovs.18-23965. [DOI] [PubMed] [Google Scholar]
- 28.Iliopoulos D, Fabbri M, Druck T, Qin HR, Han SY, Huebner K. Inhibition of breast cancer cell growth in vitro and in vivo: effect of restoration of Wwox expression. Clin Cancer Res. 2007;13:268–74. doi: 10.1158/1078-0432.CCR-06-2038. [DOI] [PubMed] [Google Scholar]
- 29.Zhu Y, Xie M, Meng Z, Leung LK, Chan FL, Hu X, et al. Knockdown of TM9SF4 boosts ER stress to trigger cell death of chemoresistant breast cancer cells. Oncogene. 2019;38:5778–91. doi: 10.1038/s41388-019-0846-y. [DOI] [PubMed] [Google Scholar]
- 30.Arredouani MS, Lu B, Bhasin M, Eljanne M, Yue W, Mosquera JM, et al. Identification of the transcription factor single-minded homologue 2 as a potential biomarker and immunotherapy target in prostate cancer. Clin Cancer Res. 2009;15:5794–802. doi: 10.1158/1078-0432.CCR-09-0911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grasso CS, Wu YM, Robinson DR, Cao X, Dhanasekaran SM, Khan AP, et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012;487:239–43. doi: 10.1038/nature11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18:11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vanaja DK, Cheville JC, Iturria SJ, Young CY. Transcriptional silencing of zinc finger protein 185 identified by expression profiling is associated with prostate cancer progression. Cancer Res. 2003;63:3877–82. [PubMed] [Google Scholar]
- 34.Varambally S, Yu J, Laxman B, Rhodes DR, Mehra R, Tomlins SA, et al. Integrative genomic and proteomic analysis of prostate cancer reveals signatures of metastatic progression. Cancer Cell. 2005;8:393–406. doi: 10.1016/j.ccr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 35.Davis FM, Azimi I, Faville RA, Peters AA, Jalink K, Putney JW, Jr., et al. Induction of epithelial-mesenchymal transition (EMT) in breast cancer cells is calcium signal dependent. Oncogene. 2014;33:2307–16. doi: 10.1038/onc.2013.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.See V, Rajala NK, Spiller DG, White MR. Calcium-dependent regulation of the cell cycle via a novel MAPK–NF-kappaB pathway in Swiss 3T3 cells. J Cell Biol. 2004;166:661–72. doi: 10.1083/jcb.200402136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yao J, Zhao L, Zhao Q, Zhao Y, Sun Y, Zhang Y, et al. NF-kappaB and Nrf2 signaling pathways contribute to wogonin-mediated inhibition of inflammation-associated colorectal carcinogenesis. Cell Death Dis. 2014;5:e1283. doi: 10.1038/cddis.2014.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen ON, Grimm C, Schneider LS, Chao YK, Atzberger C, Bartel K, et al. Two-pore channel function is crucial for the migration of invasive cancer cells. Cancer Res. 2017;77:1427–38. doi: 10.1158/0008-5472.CAN-16-0852. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.