Abstract
Despite continued research, pancreatic ductal adenocarcinoma (PDAC) remains one of the main causes of cancer death. Interest is growing in the role of the tumour suppressors breast cancer 1 (BRCA1) and BRCA2—typically associated with breast and ovarian cancer—in the pathogenesis of PDAC. Indeed, both germline and sporadic mutations in BRCA1/2 have been found to play a role in the development of PDAC. However, data regarding BRCA1/2-mutant PDAC are lacking. In this review, we aim to outline the specific landscape of BRCA-mutant PDAC, focusing on heritability, clinical features, differences between BRCA1 and 2 mutations and between germline and sporadic alterations, as well as established therapeutic strategies and those that are still under evaluation.
Subject terms: Gastrointestinal cancer, Cancer genetics
Background
Pancreatic ductal adenocarcinoma (PDAC) represents the most common form of pancreatic cancer. It is the seventh leading cause of cancer death worldwide, with an estimated 9% 5-year survival rate [1, 2]. It usually arises in elderly patients, with a mean age at onset of 71 years for men and 75 years for women [1, 2]. The mainstay of PDAC treatment is chemotherapy; however, this malignancy still has a poor prognosis, and research efforts are thus focused on identifying new therapeutic targets and strategies in addition to investigating the genomic landscape of this genetically and biologically heterogeneous tumour type [2–9]. The vast majority (>80%) of PDAC cases have a sporadic origin, whereas only a small proportion (<10%) result from inherited germline mutations [10–12]. KRAS (90%), CDKN2A (90%), TP53 (70%), SMAD4 (55%), chromatin (20%), DNA repair (17%), cell-cycle regulators (15%), WNT (10%), Robo/slit pathway (5%), Notch signalling (5%) have been identified as the main molecular pathways and genes involved in PDAC development [2, 13].
The genes encoding breast cancer 1 and 2—BRCA1 and BRCA2—play a crucial role in the response to DNA damage, by mediating the repair of DNA double-strand breaks (DSBs) via homologous recombination (HR) [14]. BRCA1/2-deficient cells that lack HR activity accumulate DSBs, resulting in genomic instability and an increased predisposition to malignant transformation and progression [15, 16]. Germline BRCA1/2 mutations are found in approximately 5–10% of cases of familial PDAC and approximately 3% of cases of apparently sporadic PDAC [17], and, after breast cancer and ovarian cancer, PDAC has been reported to be the third most common cancer associated with these mutations [2, 18]. Histologically, although the majority of pancreatic cancers associated with germline and somatic BRCA mutations comprise PDAC, both mutation types have also been reported in acinar cell carcinomas of the pancreas, which are much rarer [19]. Epidemiology studies and a study examining loss of BRCA2 heterozygosity in PDAC tissue suggested a reliable link between BRCA2 carriers and an increased PDAC risk (relative risk in 222 BRCA2-mutant families assessed by Moran et al.: 4.1, 95% confidence interval [CI], 1.9–7.8; standardised incidence ratio in 459 BRCA2-mutant patients evaluated by Mersch et al.: 21.7, 95% CI, 13.1–34.0; P < 0.001); however, this association is not as clearly defined for BRCA1 carriers [20–25]. Indeed, in BRCA2-mutant patients, the relative risk is ~3–4-fold higher (3.51, 95% CI 1.87–6.58) [21]. For BRCA1 carriers, the relative risk is estimated to be two-fold higher (2.26, 95% CI 1.26–4.06), but some studies have failed to identify any significant association between BRCA1 mutations and PDAC, suggesting a low penetrance for this malignancy [14, 15, 20].
In this review, we aim to outline the specific landscape of BRCA-mutant PDAC, focusing on heritability, clinical features, and differences between BRCA1 and BRCA2 mutations and between germline and sporadic alterations. We also discuss established therapeutic strategies based on the increased sensitivity of BRCA-mutant cells to platinum-based drugs and PARPi, as well as alternative approaches that are under evaluation.
Mutant BRCA versus wild-type BRCA in PDAC
PDAC caused by mutations in BRCA1/2 seems to be different to PDAC occurring in the general population. Generally, PDAC patients belonging to families with known BRCA1/2 mutations are a decade younger [26]. In BRCA1-mutant families, the mean age at PDAC diagnosis is 62.9 (standard deviation 12.0) with a median age of 59 (range 45–80) in males and 68 (range 38–87) in females (male:female ratio=2.00), whereas in BRCA2-mutant families, the mean age at diagnosis is 62.9 (standard deviation 11.7), with a median age of 67 years (range 39–78) in males and 59 (range 46–81) in females (male:female ratio = 1.11) [27]. Differences in patient survival and molecular landscape between BRCA-mutant and wild-type PDAC have also been investigated.
BRCA mutations and patient survival
Most BRCA alterations in PDAC are frame-shifting indels, stop-gain mutations and splice-site mutations; single-nucleotide substitutions are rare [28]. BRCA1/2 mutations can be classified as definitely pathogenic; likely pathogenic; uncertain, likely not pathogenic or of little clinical significance; not pathogenic; or of no clinical significance [29]. The results of genetic tests and the analysis of tumour signatures, which play a key role in the detection of different variants, thus give an important indication of the pathogenicity and the implications of a detected mutation. However, the rarity of the diagnosis of BRCA1/2-mutant PDAC, compounded by the infrequent nature of genetic testing, has led to the publication of only a few studies assessing the impact of BRCA mutations on the survival of patients, with controversial results [26, 30–33]. A retrospective, single-institution, case-control study demonstrated that the presence of a germline BRCA1/2 mutation in patients with resected, sporadic PDAC was independently associated with inferior overall survival (OS) and inferior disease-free survival compared with matched BRCA-wild-type patients [17]. Takeuchi et al. [34] analysed the presence of mutations in the entire coding region of the BRCA pathway genes (BRCA1, BRCA2 and partner and localiser of BRCA2 (PALB2) in 42 surgically resected PDAC tumours, and assessed their correlation with clinical-pathological features. Thirteen rare germline mutations were identified in the BRCA pathway genes and their functional effect was examined using online prediction programs, including ClinVar. One frameshift mutation (BRCA2S2148fs) was considered to be pathogenic, seven as being of uncertain significance or having conflicting interpretations of pathogenicity, and three as benign; ‘no information’ on the predicted effect of the other mutations was available. BRCA2R18H and BRCA2G2044V were apparently enriched in tumour cells. However, in contrast with the case-control study outlined above, patients with potentially deleterious mutations (such as pathogenic, conflicting, uncertain) in BRCA pathway genes, or those with no information, had a significantly better prognosis than those without mutations or with benign mutations as assessed by ClinVar (5-year OS was 68.6% versus 19.2%, respectively; P = 0.031 by log-rank test) [34]. Since BRCA1/2 has a crucial tumour suppressor role, the better prognosis observed in patients with deleterious mutations of these genes might appear unexpected and it is indeed not exactly understood. Most patients received adjuvant chemotherapy with S-1 and gemcitabine (only some patients were treated with cisplatin) and no significant differences in chemotherapy administration were reported among the cohorts, thus excluding a key prognostic role of chemotherapy in patients with genomic instability. Notably, these data should be considered with caution due to the retrospective nature of this study and the small sample size, which prevent from drawing definitive conclusions. Further prospective and larger studies are needed to explore the prognostic role of BRCA1/2 deleterious mutations.
The effect of BRCA mutations on the molecular landscape of PDAC
Little is known about the molecular differences that might exist between PDAC patients with mutated BRCA1/2 and those with wild-type BRCA1/2. An immunohistochemistry and next-generation sequencing evaluation of 2818 PDAC samples identified BRCA1 mutations in 1.3% and BRCA2 mutations in 3.1% of samples; concomitant mutations in PALB2 were not reported. The mutational profile of PDAC samples from BRCA-mutant patients was significantly different from that of patients with wild-type BRCA1/2 PDAC. Indeed, mutations in TP53 and CDKN2A were less frequent in BRCA-mutant PDAC samples than in BRCA-wild-type PDAC samples, whereas mutations in APC, SETD2, FLCN, ERBB3, SUFU, WT1 and KMT2A were more common. Notably, these differences in the mutational profile might reflect the pathways and mediators involved in carcinogenesis in BRCA1/2-mutant and wild-type PDAC, thus suggesting the existence of potential differences in this process between mutant and wild-type tumours. Moreover, 4.8% of BRCA-mutant PDAC samples showed microsatellite instability high/deficient mismatch repair (MSI-H/dMMR) status versus 1.2% of wild-type BRCA1/2 samples; the tumour mutational burden was higher in the BRCA-mutant PDAC samples compared with the wild-type BRCA1/2 samples irrespective of microsatellite status [35]. Further studies are required to assess the real impact of the presence or absence of BRCA mutations on the molecular landscape of PDAC, which is likely to impinge on patient prognosis and have implications for treatment.
BRCA mutations and heritability in PDAC
Among the genes that are considered to be involved in PDAC susceptibility, BRCA1, BRCA2, PALB2 and CKDN2A appear to account for the majority of known genetic causes of hereditary PDAC [18, 36–39].
BRCA1/2 mutations and family history
Familial pancreatic cancer (FPC) is a term that can be applied to families with at least two first-degree relatives with PDAC who do not fulfil the criteria for other familial cancer syndromes, and is responsible for 10% of cases of PDAC [36–41]. Data on the genetic basis of FPC largely arise from the observed increase in the risk of pancreatic cancer in patients with hereditary cancer syndromes. Many of the studies included only patients with mutated BRCA2; in patients with FPC, the prevalence of BRCA2 mutations was found to be 3–17% in the case of ≥3 relatives with PDAC history, whereas, in unselected patients, the prevalence of BRCA2 mutations has been reported to be 5–7% [23, 42, 43]. These prevalence data show an increase of detected BRCA2 mutations when a selection of patients according to PDAC family history is applied.
The PACGENE study [44] found that the 8% of probands who have a first-degree relative with PDAC—unselected for hereditary cancer syndrome patterns or genetic mutational status—harbour a deleterious mutation in one of four genes: BRCA1, BRCA2, PALB2 and CDKN2A. Probands with relatives other than first-degree relatives with PDAC might also carry a deleterious mutation in the same four genes, although with significantly less probability. The researchers confirmed that these four genes together harbour approximately 5–10% of deleterious mutations in FPC. Overall, any proband with a family history of PDAC has a 6.7% probability of carrying a deleterious mutation in one of these genes. Mutations in BRCA2 and CDKN2A were detected more often than those in BRCA1 and PALB2, consistent with the published literature [42, 43, 45, 46]. Furthermore, the authors also found a younger age of onset of PDAC among probands with a mutation in one of the four genes. A specific age at which to define early-onset pancreatic cancer has not been identified yet, although a cut off of 50 years old has been considered in previous studies of FPC [47, 48]. Having a member of the family with a young-onset pancreatic cancer confers an added risk in FPC kindreds; this finding might help tailoring the clinical/genetic counselling and screening proposal to families with high risk of developing PDAC [48]. Interestingly, the number of family members affected by PDAC did not correlate with the probability of detecting deleterious mutations [44].
BRCA1/2 mutations and ethnicity
It is important to indicate that population ethnicity influences the prevalence rates of BRCA1/2 mutations and should be taken in consideration when interpreting the literature. In particular, one of the most studied populations is represented by the Ashkenazi Jews (AJs), who have become the subject of many genetic studies of FPC and other related BRCA-mutant cancer syndromes. In the AJ population, up to 21% of patients with PDAC harbour a BRCA1/2 mutation [49, 50]. AJs are known to have founder mutations in BRCA1 (185_186delAG and 5382insC in 0.4% and 0.1%, respectively, of AJ women) and BRCA2 (6174delT in 0.6% of Ashkenazi women) that underlie hereditary breast cancer [22, 45, 46]. These founder mutations might also contribute to PDAC predisposition; however, few prospective studies are available and most data derive from clinical database reviews. Ferrone et al. identified that, of 187 Jewish patients who underwent surgery for PDAC, 5.5% of patients with AJ ancestry (unselected for family history of cancer) harboured one of the three common AJ founder mutations (185_186delAG, 5382insC or 6174delT); in AJ families with a history of breast cancer and PDAC, 14.2% were found to carry a mutation in BRCA1 or BRCA2, with nearly equal distribution between the two genes [30, 51]. Salo-Mullen et al. reported that BRCA2 was the most common gene found to be altered in a series of patients with PDAC who presented for clinical cancer genetics evaluation, accounting for 54% of all identified pathogenic mutations; 85.5% of patients had either a personal history of a second malignancy or at least one first or second degree relative with a history of breast cancer, ovarian cancer, colorectal cancer or PDAC [52]. Overall prevalence of BRCA1/2 mutations in AJ patients is reported to be 2.5%, that is approximately five times higher than prevalence in the general population [53, 54].
In a large prospective analysis, Holter et al. reported the prevalence of germline BRCA1/2 mutations in a cohort of unselected patients with incident PDAC diagnosis. Clinical and family histories of these patients were assessed in an attempt to determine predictive factors for genetic testing. Germline BRCA mutations were identified in 4.6% of the patients: 1% had a BRCA1 mutation and 3.6% had a BRCA2 mutation. Notably, 12.1% of AJ patients were BRCA-mutant, compared with 3.7% of non-AJ patients [11]. The absence of a significant family history in the majority of patients with an established deleterious germline mutation might be the result of incomplete penetrance, rather than a de novo germline mutation, which has not been well studied in PDAC [55].
Smith et al. [56] recommend reflex testing for germline mutations in BRCA1, BRCA2, PALB2 and ATM for patients with French–Canadian/AJ ancestry, and full gene sequencing of patients with incident pancreatic cancer ≤50 years or with family history criteria. Reflex testing is also recommended by the National Comprehensive Cancer Network in the Genetic/Familial High-Risk Assessment [57].
Genetic testing
Regardless of the ethnicity, the identification of BRCA mutations allows family members to receive genetic testing and counselling [58]. Genetic counselling is recommended for patients harbouring a pathogenic mutation and for those with a positive family history of cancer, especially PDAC, regardless of mutation status [57, 59, 60]. The American Society of Clinical Oncology and National Comprehensive Cancer Network guidelines recommend germline testing using comprehensive multigene panels for hereditary cancer syndromes that are associated with an increased risk of PDAC for all PDAC patients, irrespective of family history. Indeed, test panels allow individuals to be simultaneously assessed for mutations in multiple genes associated with cancer (generally ATM, BRCA1, BRCA2, CDKN2A, PALB2, STK11, MLH1, MSH2, MSH6 and PMS2) [61]. The use of this approach has confirmed the presence of actionable BRCA1/2 pathogenic, or likely pathogenic, variants (0–3% for BRCA1 and 1–6% for BRCA2, respectively) in unselected PDAC population, thus suggesting potential therapeutic implications [57, 62].
Evidence suggests that screening in high-risk subjects is associated with down-staging of incident tumours, although larger studies are needed to confirm the long-term survival benefit. PDAC screening can be considered for first-degree relatives of individuals with FPC and/or individuals with a family history of PDAC who harbour pathogenic germline variants in genes associated with PDAC susceptibility, after extensive discussion on potential risks/benefits and limitations of surveillance, and should be performed at high-volume centres of expertise. Surveillance could be performed using contrast-enhanced pancreas magnetic resonance imaging/magnetic resonance cholangiopancreatography and/or endoscopic ultrasonography [57, 59, 60]. Furthermore, as many of the germline mutations associated with an increased risk of PDAC are also associated with highly penetrant hereditary cancer syndromes (e.g. Lynch syndrome, hereditary breast cancer and ovarian cancer), and consequently with a higher risk of other cancers, effective strategies for the prevention and screening of these tumours should also be offered [59].
Germline BRCA mutations and somatic BRCA mutations in PDAC
It is well known that BRCA mutations can be germline or somatic [49]. Somatic BRCA1/2 mutations are responsible for cases of sporadic PDAC, are detectable only in the tumour tissue and have been reported in 1–4% cases of PDAC [35].
‘Apparent’ sporadic PDAC
Sporadic PDAC that harbours somatic BRCA mutations and ‘apparently’ sporadic PDAC with germline BRCA mutations comprise two different entities. Sindo et al. investigated the prevalence of deleterious germline BRCA1/2 mutations in apparently sporadic PDAC—that is, cases of PDAC with no significant family history of cancer—by sequencing 32 genes, including known pancreatic cancer susceptibility genes, from the DNA of normal (i.e. non-tumour) tissue obtained from 854 patients with PDAC. Thirty-three of these patients presented with a deleterious germline mutation: 12 involved the BRCA2 gene and three involved the BRCA1 gene [55]. It has also been reported in different studies that a percentage of non-selected patients with PDAC harboured a germline BRCA1/2 mutation [11, 23]. Table 1 shows the occurrence of germline BRCA mutations in apparently sporadic PDAC [11, 55, 63–66]. Another study revealed a downregulation of BRCA1 expression at the RNA level in patients with chronic pancreatitis, and downregulation at both the RNA and protein levels in sporadic PDAC, demonstrating a correlation between BRCA1 expression and PDAC development [67]. These data highlight the importance of extending the criteria used to determine the appropriateness of gene testing beyond the existence of a significant family history in order to avoid missing known deleterious pancreatic cancer susceptibility gene mutations that could potentially be targeted.
Table 1.
Prevalence of germline BRCA1/2 mutations in apparently sporadic pancreatic cancer.
| Authors/year | Total no. of PDAC patients | BRCA1/2 mutations no. patients (%) | Reference |
|---|---|---|---|
| Holter et al., 2015 | 306 |
BRCA1: 3 (0.98%) BRCA2: 11 (3.59%) |
[11] |
| Shindo et al., 2017 | 854 |
BRCA1: 3 (0.35%) BRCA2: 12 (1.4%) |
[55] |
| Grant et al., 2015 | 290 |
BRCA1: 1 (0.34%) BRCA2: 2 (0.68%) |
[63] |
| Hu et al., 2018 | 2999 |
BRCA1: 18 (0.6%) BRCA2: 57 (1.9%) |
[64] |
| Brand et al., 2018 | 298 |
BRCA1: 4 (1.34%) BRCA2: 4 (1.34%) |
[65] |
| Yurgelun et al., 2019 | 289 |
BRCA1: 3 (1.03%) BRCA2: 4 (1.38%) |
[66] |
PDAC pancreatic ductal adenocarcinoma.
BRCA1/2 mutations and treatment potential
The identification of a germline BRCA1 or BRCA2 mutation is often used to stratify patients for treatment with platinum-based therapy or poly-ADP ribose polymerase (PARP) inhibitors (PARPi, see below). However, heterogeneous responses are often seen using this approach, prompting Wang et al. to develop a predictive and prognostic model of germline BRCA-mutant PDAC in the preclinical setting. In the case of homologous repair deficiency (HRD), tumour polyploidy and a basal-like transcriptomic subtype were independent predictors of poorer survival; HRD genomic hallmarks were crucial for sensitivity to platinum and PARPi, whereas tumour polyploidy predicted resistance [68].
Although no specific data are available regarding somatic BRCA-mutant PDAC and platinum-based treatment, a new classification based on whole-genome sequencing indicates that most somatic BRCA-mutant and germline BRCA-mutant PDACs seem to be part of the unstable subtype, characterised by the presence of genomic instability, with an apparent increased response to platinum-derived therapies both in patients and in patient-derived xenografts, suggesting that no difference in treatment response exists between somatic and germline mutations [69–71]. Different studies have investigated or are still studying the effect of PARPi in BRCA-mutant PDAC [72–74]. However, only a few studies have considered both germline and somatic mutations. Among them, a Phase II multicentre study evaluated the efficacy and safety of rucaparib in 19 patients with germline/somatic BRCA1/2-mutant locally advanced or metastatic PDAC. Three patients had an objective response; of these, two harboured somatic BRCA2 mutations [73]. A Phase II open label study will evaluate the effectiveness, safety, and anti-tumour activity of rucaparib in patients with advanced germline/somatic BRCA1/2-mutant or PALB2-mutant PDAC (NCT03140670) [75]. The prevalence of these mutations and their association with improved clinical outcomes are also the tertiary objectives in a Phase II randomised study in which the primary objective will be the efficacy of modified 5-fluorouracil, irinotecan, levofolinic acid (mFOLFIRI) and veliparib compared to a control arm of FOLFIRI in patients with metastatic PDAC (NCT02890355). Very few studies focus on both germline and somatic mutations, since most consider only germline alterations. Furthermore, no comparative studies between PDAC with germline mutations versus PDAC harbouring somatic mutations assessing potential differences with respect to clinical tumour behaviour, response to treatment and clinical outcomes are available.
BRCA-mutant PDAC and platinum-based therapy
Platinum-based chemotherapies exert their cytotoxic effect by binding directly to DNA, causing crosslinking of DNA strands and inducing DNA DSBs, which cannot be effectively repaired in the presence of BRCA mutations [76]. An enhanced sensitivity to platinum-based chemotherapies was first demonstrated in BRCA1/2-mutant breast cancer and ovarian cancer [77, 78]. Accordingly, the potential benefit of these has been evaluated in PDAC patients who harbour BRCA1/2 or PALB2 germline mutations, both in the early and advanced setting (Table 2).
Table 2.
Main clinical studies of platinum-based therapy in BRCA-mutant pancreatic ductal adenocarcinoma.
| Authors/year | Study design | No. of patients | Disease stage | Results in BRCA1/2-WT patients | Results in BRCA1/2-mutant patients | P | Reference |
|---|---|---|---|---|---|---|---|
| Golan et al., 2014 | Retrospective analysis | 43 | III–IV | mOS 13 months | mOS 15 months | 0.77 | [26] |
| Reiss et al., 2018 | Retrospective Case-control | 70 | III–IV | mOS 15.5 months | mOS 20.1 months | 0.002 | [79] |
| Golan et al., 2017 | Retrospective case-control | 74 | I–II | mOS 43.8 months | mOS 44.4 months | 0.775 | [32] |
| Yu et al., 2019 | Retrospective case-control | 96 | I–II | mOS 23.2 months | mOS 46.6 months | 0.0156 | [80] |
| Wattenberg et al., 2020 | Retrospective case-control | 78 | III–IV | ORR 21% | ORR 58% | 0.0022 | [81] |
| O Reilly et al., 2020 | Phase II, randomised | 50 | III–IV | – | RR 74.1% cisplatin–gemcitabine (arm A) versus 65.2% cisplatin–gemcitabine plus veliparib (arm B) | 0.55 | [82] |
WT wild type, mOS median overall survival, m months, ORR objective response rate, RR response rate.
Focus on stage I–II disease
Golan et al. [32] assessed the predictive and prognostic impact of pathogenic germline BRCA/PALB2 mutations in patients with resected PDAC in a retrospective case-control, multi-institution study. Globally, 25 patients with resected BRCA1/2-mutant PDAC were matched to 49 wild-type control subjects. No statistically significant differences in median OS (mOS), the primary endpoint (37.06 versus 38.77 months, P = 0.838), or median disease-free survival (14.3 versus 12.0 months, P = 0.303) were observed between cases and controls. However, when patients who were treated with platinum chemotherapy in the perioperative setting were analysed, a non-significant trend towards improved disease-free survival was observed among BRCA-mutant patients (n = 10) as compared with controls (n = 7) (39.1 versus 12.4 months, P = 0.255); no difference in OS was reported (43.8 months for BRCA-mutant patients versus 44.4 months for controls; P = 0.775) [32].
Another retrospective case-control study [80] analysed 32 patients with germline BRCA/PALB2 mutations and resected PDAC matched to 64 mutation-negative patients. In each group, 11 patients received perioperative platinum chemotherapy (n = 13 5-fluorouracil, oxaliplatin, irinotecan, levofolinic acid [FOLFIRINOX], n = 4 5-fluorouracil, oxaliplatin, levofolinic acid [FOLFOX], n = 1 capecitabine, oxaliplatin, levofolinic acid, n = 1 carboplatin/gemcitabine, n = 2 cisplatin/gemcitabine, n = 1 oxaliplatin/gemcitabine). The mOS (primary endpoint) was 46.6 months in the BRCA-mutant group compared to 23.2 months in the BRCA-wild-type group (hazard ratio [HR] 0.49; P = 0.0156). In this population, a survival advantage in patients with BRCA-mutant PDAC compared to those with wild-type PDAC, rather surprisingly since mutation involving tumour suppressors are expected to be related to worse survival. However, due to the retrospective nature of the study, these findings should be considered with caution. The subgroup analysis of patients treated perioperatively with platinum showed that the mOS was not reached in the mutant group, versus 23.1 months in the BRCA-wild-type group (HR 0.12; P = 0.0193). Finally, when BRCA-mutant patients were evaluated by platinum exposure, a trend toward an improvement in mOS was observed among those who received perioperative platinum (n = 11) versus patients who did not (n = 15) (HR 0.52; P = 0.0421) [80].
Focus on stage III–IV disease
In 2011, Lowery et al. [83] reported partial (n = 4) or complete (n = 1) radiological responses in five out of six BRCA-mutant PDAC patients treated with platinum-based first-line chemotherapy. This favourable finding was supported by the publication in the same year of a complete pathological response of a case report treated with cisplatin-based therapy [84]. In 2014, Golan et al. reported the results of their retrospective analysis of BRCA1/2-mutant PDAC patients. Among the 58 eligible patients, mOS was not reached for patients with stage I/II disease (n = 15) after 60 months but was 12 months for those with stage III/IV (n = 43) disease. Twenty-two out of these 43 patients with stage III/IV disease received platinum-based therapies: 18 were treated with cisplatin/gemcitabine, 3 with FOLFIRINOX and 1 with oxaliplatin/gemcitabine. These patients showed a statistically significant improvement in OS (22 versus 9 months, respectively; P = 0.039) compared with those patients who didn’t receive platinum (n = 21). No statistically significant differences in survival outcomes were found between the BRCA1-mutant subgroup and the BRCA2-mutant one (mOS 15 versus 13 months, respectively; P = 0.77) [26]. Reiss et al. later performed a retrospective case-control study on 29 BRCA/PALB2-mutant patients with advanced PDAC patients who were matched to 58 control subjects (non-carriers or untested). A statistically significant benefit in OS (primary endpoint) was observed in the mutation-positive patients compared to the matched controls (mOS: 21.8 versus 8.1 months, respectively. HR 0.35; P < 0.001). When patients who received platinum-based chemotherapy were analysed (n = 18), a statistically significant benefit in OS was observed in the mutation-positive group compared with control patients (mOS at a median follow-up of 20.1 months: not reached versus 15.5 months, respectively; HR 0.25; P = 0.002), whereas no significant survival differences were reported in patients who were not treated with platinum (HR 0.54; P = 0.12). Subgroup analysis comparing oxaliplatin to cisplatin did not show any difference between the different regimens administered (i.e. FOLFIRINOX, FOLFOX, cisplatin/gemcitabine, other not specified platinum therapies) [79].
Wattenberg et al. [81] evaluated the impact of platinum-based therapies in terms of objective response in a 2020 retrospective, case-control analysis. The authors analysed the effect of platinum-based therapies in 26 patients with germline mutations in BRCA1/2 or PALB2 and 52 matched control patients with advanced PDAC; FOLFIRINOX was the most commonly used regimen in the control group, whereas a significantly higher number of mutation-positive patients received cisplatin/gemcitabine. The primary endpoints were objective response rate (ORR) and real-world progression-free survival (rwPFS). The ORR was significantly higher in mutation-positive patients treated with platinum-based therapies compared with the control group (58% versus 21%; P = 0.0022). No significant difference in ORR was observed among the different platinum-based regimens in the mutant patients (P = 0.814). Conversely, all objective responses in the control group occurred in patients treated with FOLFIRINOX. Notably, mutation-positive patients who received first-line platinum therapy had a significantly better ORR than matched control patients (68% versus 29%; P = 0.007) and a numerically higher ORR when compared to mutation-positive patients who received platinum in the second-line setting (68% versus 20%; P = 0.0507). Moreover, mutation-positive patients had a significantly longer rwPFS than control patients (median rwPFS 10.1 versus 6.9 months, HR 0.43; P = 0.0068), and those mutation-positive patients who received first-line platinum had a longer rwPFS (21.1 months) than control patients (7.9 months; P = 0.0046) and mutation-positive patients treated with platinum therapy in the second- or later lines (2.5 months; P = 0.0001) [81].
Because of their retrospective nature, small sample size, frequent lack of a control group and heterogeneity of platinum therapies, none of these studies was able to demonstrate the superiority of one platinum-based regimen over another in BRCA/PALB2-mutant patients. However, despite the limitations of these studies, the results from all of them suggest that patients with germline BRCA/PALB2-mutant PDAC represent a small, but clinically significant, subset who might benefit from cytotoxic therapies, such as platinum-based chemotherapy, in the presence of defective HR. To date, unfortunately, no data are available to assume that these observations might be extended to PDAC patients with somatic alterations in these genes.
PARP inhibitors in BRCA-mutant PDAC
PARP enzymes, which polymerise ADP-ribose units, play a key role in the repair of single-stranded DNA breaks through the base-excision repair system. PARP-1 is crucial in this process and represents the main target of PARPi in BRCA1/2-mutant tumours, on the basis of the mechanism of synthetic lethality [85], a phenomenon in which the combination of two gene perturbations leads to cell death, whilst the perturbation of either of two genes individually has no detrimental effect. In the absence of functional BRCA1/2- or PALB2-encoded proteins, PARP-1 is overexpressed in order to compensate for their reduced activity [86]. PARPi bind to the catalytic site of PARP enzymes, blocking their activity and simultaneously trapping them inside the DNA, which leads to the collapse of the replication forks. Thus, PARPi leads to a significant accumulation of DSBs, resulting in cell death [85, 87].
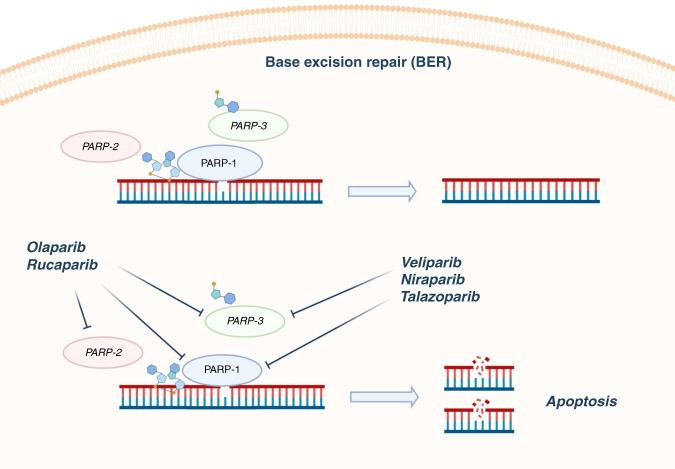
Various PARPi have been developed (Fig. 1). Olaparib and rucaparib target PARP-1, PARP-2 and PARP-3, whereas veliparib, niraparib and talazoparib target PARP-1 and PARP-3. Each PARPi shows different catalytic inhibition and PARP trapping potency—the ability to trap PARP–DNA complexes. Notably, the PARP trapping potency, rather than the IC50, seems to be the main driver for cytotoxicity. Therefore, the different PARP trapping capacities of the PARPi might be quite relevant in influencing the capability to induce the cell death [85, 88].
Fig. 1. PARP inhibitors in BRCA1/2-mutant pancreatic cancer.
The figure shows the PARP inhibitors that have been evaluated in pancreatic ductal adenocarcinoma, together with their mechanism of action. PARP enzymes are crucial in the homologous recombination system, particularly in base-excision repair. By binding to the catalytic site of PARPs, PARP inhibitors block their activity and trap the enzymes inside the DNA. PARPi lead to a significant accumulation of DNA double-strand breaks, resulting in cell death through the phenomenon of synthetic lethality when BRCA1/2 is deficient. Olaparib and rucaparib target PARP-1, PARP-2 and PARP-3, whereas veliparib, niraparib and talazoparib target PARP-1 and PARP-3. PARP poly-ADP ribose polymerase.
The first preclinical studies that showed the high lethality of PARPi in cancer cells harbouring BRCA mutations were published in 2005 [86, 89]. Subsequent Phase I studies investigated the optimal dose of these drugs and demonstrated their efficacy in BRCA-mutant solid tumours. These Phase I/Ib studies with PARPi were performed on advanced solid tumours; some focused mainly on breast cancer and ovarian cancer, but all of them showed good results in terms of ORR [90–92]. Further Phase II studies with different PARPi have been conducted on advanced solid tumours with germline BRCA1/2 mutations, including PDAC. Some trials have focused on PARPi monotherapy, others on PARPi maintenance therapy and on a combination of PARPi and chemotherapy (Table 3).
Table 3.
Published clinical trials of PARP inhibitors in pancreatic cancer.
| Treatment strategy | Drugs | Phase | Setting | No. of patients | Prior platinum | Results | Reference |
|---|---|---|---|---|---|---|---|
| PARPi monotherapy for advanced disease | Olaparib | II | Recurrent gBRCA1/2-mutant PDAC after gemcitabine | 25 | 65% |
• Primary endpoint ORR: 22% • Secondary endpoints • SD at 8 weeks: 35% ▪ DOR: 134 days ▪ PFS: 4.6 months ▪ OS: 9.8 months • Safety ▪ Any grade AEs: 74% fatigue, 48% nausea, 40% anaemia ▪ grade ≥3 AEs: 17.4% anaemia, 13% fatigue |
[72] |
| Veliparib | II | Locally advanced/metastatic gBRCA1/2 or PALB2-mutant PDAC, 1–2 prior lines | 16 | 88%, of which 64.3% platinum resistant |
• Primary endpoint ORR: 0% ▪ Secondary endpoints ▪ PFS: 1.7 months ▪ OS: 3.1 months • Safety ▪ grade ≥3 AEs: 25% fatigue, 19% hyperbilirubinemia |
[93] | |
| Rucaparib | II | Locally advanced/metastatic gBRCA1/2 and sBRCA1/2-mutant PDAC, 1–2 prior lines | 19 | 78.9%, of which 42.1% platinum resistant |
• Primary endpoint ORR: 15.8% • Safety ▪ any grade AEs: 63.2% nausea, 47.4% anaemia ▪ grade ≥3 AEs: 31.6% anaemia, 15.8% fatigue, 15.8% ascites |
[94] | |
| PARPi + chemotherapy | Veliparib+ mFOLFIRI vs FOLFIRI | II | Metastatic PDAC, 1 prior line | 123 | Not specified |
• Primary endpoint OS: 5.1 vs 5.9 months (HR 1.3, 95% CI 0.9–2.0, P = 0.21) ▪ Secondary endpoint PFS: 2.1 vs 2.9 months (HR 1.5, 95% CI 1.0–2.2, P = 0.05) • Safety ▪ grade ≥3 AEs: neutropenia (33% vs 20%), fatigue (19% vs 4%), nausea (11% vs 4%) |
[95] |
| Veliparib+ mFOLFOX6 | I/II |
Metastatic DDR-mutant PDAC and/or family history suggestive of breast or ovarian cancer syndrome; Phase II: 2 cohorts 1) untreated patients; 2) previously treated patients |
I: 31; II: 33(15 untreated, 18 pretreated) |
24.5% |
• Primary endpoint ORR 26% all patients, 58% platinum-naive, FH + , DDR + patients (12) • Secondary endpoints ▪ PFS: 3.7 months ▪ OS: 8.5 months • Safety ▪ grade ≥3 AEs: 16% myelosuppression, 6% nausea/vomiting |
[96] | |
| cisplatin–gemcitabine (arm A) vs cisplatin–gemcitabine + veliparib (arm B) | II | Locally advanced or metastatic gBRCA/PALB2-mutant PDAC, untreated patients | 50 | No previous platinum treatment allowed |
• Primary endpoint RR: 74% arm A vs 65.2% arm B P = 0.55 • Secondary endpoints ▪ PFS: 10.1 months arm A (95% CI, 6.7–11.5) vs 9.7 months arm B (95% CI, 4.2–13.6), P = 0.73) ▪ OS: 15.5 months arm A (95% CI, 12.2–24.3) vs 16.4 months arm B (95% CI, 11.7–23.4 months; P = 0.6). • Safety ▪ grade ≥3 AEs: neutropenia (48% arm A vs 30% arm B), thrombocytopenia (55% arm A vs 9% arm B), anaemia (52% arm A vs 35% arm B) |
[82] | |
| PARPi as maintenance | Olaparib vs placebo | III | Metastatic gBRCA1/2-mutant PDAC not progressing during first-line platinum-based chemotherapy | 154 | Platinum sensitive |
• Primary endpoint PFS: 7.4 months olaparib vs 3.8 months; HR 0.53; 95% CI, 0.35–0.82; P = 0.004 • Secondary endpoints ▪ OS: 18.9 months olaparib vs 18.1 months; HR 0.91; 95% CI, 0.56–1.46; P = 0.68 • Safety ▪ Any grade AEs: fatigue or asthenia 60% olaparib vs 35% placebo, nausea 45% olaparib vs 23% placebo ▪ grade ≥3 AEs: anemia 11% olaparib vs 3% placebo, fatigue or asthenia 5% olaparib vs 2% placebo |
[74] |
| Rucaparib | II | Metastatic g/sBRCA1/2-mutant PDAC not progressing following at least 4 months of first-line platinum-based chemotherapy | 24 | Platinum sensitive |
• Primary endpoint PFS: 9.1 months • Secondary endpoints ▪ ORR: 36.8% • Safety ▪ Any grade: nausea (grade 1: 41.6%; grade 2: 4.2%), dysgeusia (grade 1: 33.3%), fatigue (grade 1: 25%) |
[75] |
PARPi poly-ADP ribose polymerase inhibitors, gBRCA1/2 germline BRCA1/2, sBRCA1/2 somatic BRCA1/2, PDAC pancreatic ductal adenocarcinoma, ORR objective response rate, SD stable disease, DOR duration of response, PFS progression-free survival, OS overall survival, AEs adverse events, PALB2 partner and localizer of BRCA2, mFOLFIRI modified 5-fluorouracil, irinotecan, levofolinic acid, FOLFIRI 5-fluorouracil, irinotecan, levofolinic acid, mFOLFOX6 modified 5-fluorouracil, oxaliplatin, levofolinic acid 6, DDR, DNA damage repair, RR response rate.
PARPi monotherapy
A single arm, Phase II study by Kaufman et al. [72] included a cohort of patients with germline BRCA1/2-mutant advanced PDAC progressing after gemcitabine (65% pretreated with a prior platinum-based regimen) who received olaparib. The primary endpoint, the ORR, was 22%. In terms of secondary endpoints, 35% reached stable disease at >8 weeks; the median duration of response was 134 days; PFS was 4.6 months; and OS was 9.8 months. The most frequent adverse events of any grade were fatigue (74%), nausea (48%), vomiting and anaemia (40%).
Lowery et al. [93] conducted a Phase II trial of veliparib on patients with stage III/IV PDAC and known germline mutations in BRCA1/2 or PALB2 who had been pretreated with 1–2 lines. The primary endpoint was ORR; secondary endpoints included PFS, duration of response, OS and safety. Veliparib showed a good tolerability profile, but no confirmed response was observed, although four (25%) patients remained on the study with stable disease for ≥4 months.
The RUCAPANC trial, by Shrofft et al. [94], assessed the safety and efficacy of rucaparib in advanced or metastatic PDAC patients with germline or somatic BRCA1/2 mutations. The study enrolled 19 patients, including 16 with a germline BRCA mutation and 3 with a somatic BRCA mutation. An ORR of 15.8% (3 of 19) was reached, and the disease control rate was 31.6% (6 of 19) in the overall population and 44.4% (4 of 9) in patients who received a previous chemotherapy regimen. As per protocol, enrolment was stopped due to insufficient ORR among the first 15 patients.
PARPi as maintenance treatment
The Phase III, randomised, double-blind study of Golan et al. [74] aimed to evaluate the efficacy of olaparib as maintenance therapy in patients affected by metastatic germline BRCA1/2-mutant PDAC not progressing after first-line platinum-based treatment. A total of 154 patients was randomly assigned (3:2 ratio) to receive olaparib tablets (300 mg twice daily; n = 92) or placebo (n = 62). The primary endpoint was PFS. The study met its primary endpoint: median PFS was higher in the olaparib arm compared to the placebo arm (7.4 months versus 3.8 months; HR 0.53; 95% CI 0.35–0.82; P = 0.004). At the interim analysis, no difference between olaparib and placebo groups in mOS, calculated at a data maturity of 46% (18.9 versus 18.1 months; HR 0.91; 95% CI 0.56–1.46; P = 0.68), was observed. The incidence of grade 3 or higher adverse events was 40% in the olaparib group compared with 23% in the placebo group. No significant difference in health-related quality of life was reported between the groups.
Another single arm, Phase II clinical trial of maintenance rucaparib in patients with advanced PDAC and germline or somatic BRCA or PALB2 mutation, whose cancer had not progressed following at least 4 months of platinum-based chemotherapy (NCT03140670), was conducted by Binder et al. [75]. The primary endpoint was PFS. Globally, 13 patients with a germline BRCA2 mutation, 3 with a germline BRCA1 mutation, 2 with a germline PALB2 mutation, and 1 with a somatic BRCA2 mutation were enrolled. Median PFS was 9.1 months from the start of rucaparib therapy; ORR was 36.8% (six partial responses; one complete response). The disease control rate was 89.5% for at least 8 weeks. Two patients (10.5%) demonstrated progressive disease at a first follow-up scan 2 months after the start of treatment; eight received rucaparib for >6 months and two patients for >1 year (13 months and 15 months, respectively). The seven responding patients included those with germline BRCA2 mutations (n = 4), germline PALB2 mutations (n = 2) and a somatic BRCA2 mutation (n = 1). The authors concluded that rucaparib maintenance shows encouraging disease control with an acceptable safety profile (the most common adverse events being nausea (grade 1, 41.6%; grade 2, 4.2%), dysgeusia (grade 1, 33.3%) and fatigue (grade 1, 25%)).
PARPi plus chemotherapy
Veliparib was evaluated in combination with FOLFIRI versus FOLFIRI alone in a Phase II study of patients with metastatic PDAC, of whom 11 (9%) had HRD, including four germline mutations (in BRCA1, BRCA2, ATM) and seven somatic mutations (in BRCA2, PALB2, ATM, CDK12) [95]. An additional 24 patients (20%) had germline mutations (n = 11, e.g. in FANC, BLM, SLX4, CHEK2) or somatic mutations (n = 13, e.g. in FANC, BLM, POLD1, RIF1, MSH2, MSH6) in other DNA repair genes that are not classified as featuring in HRD. A planned interim futility analysis at 35% of expected PFS events showed that the combination of veliparib and FOLFIRI was unlikely to be superior to FOLFIRI; moreover, an increased toxicity was observed (most common grade 3/4 adverse events: neutropenia (33% versus 20%), fatigue (19% versus 4%) and nausea (11% versus 4%)) [92].
In the Phase II trial by Pishvaian et al. [96], patients were preselected to receive veliparib plus modified FOLFOX6 if they had either a pathogenic germline or somatic HRD mutation (in BRCA1/2, PALB2, ATM), and/or a family history suggestive of breast cancer or ovarian cancer syndrome. The primary objective was ORR, whereas key secondary endpoints were PFS and OS. The treatment combination was well tolerated and showed promising efficacy, especially in platinum-naive patients who had a positive family history and/or harboured HRD mutations, for which the ORR was 58%.
A multicentre, randomised, prospective, Phase II trial showed promising activity of the combination of cisplatin and gemcitabine in patients with advanced or metastatic germline BRCA/PALB2-mutant PDAC [82]. In this study, 50 patients were treated with cisplatin and gemcitabine plus or minus veliparib (arms A and B, respectively). The response rate, the primary endpoint of the study, was high in both groups (74% arm A versus 65.2% arm B); the addition of veliparib did not improve the response (P = 0.55). Interestingly, the 2- and 3-year survival rates of the entire cohort observed in this study are the longest reported in any randomised trial in PDAC (30.6% and 17.8%, respectively). More grade 3–4 haematologic adverse events and dose reductions were observed in arm A than in arm B. However, although the addition of veliparib did not improve the response rate, this study supports the effectiveness of cisplatin plus gemcitabine for the treatment of patients with advanced germline BRCA/PALB2-mutant PDAC, thus suggesting this regimen as a standard approach in this patient population [82].
PARPi: overcoming hurdles
Unfortunately, the emergence of PARPi resistance is not uncommon. The mechanisms of resistance so far explored include restoration of HR, stabilisation of the replication forks, alternative mRNA splicing, reduced PARP-1 trapping, P-glycoprotein-mediated drug efflux, cell-cycle control alterations, changes in miRNA expression patterns and dysregulation of signalling pathways [97]. Restoration of HR is the most studied event, and can result from genetic and epigenetic phenomena. The development of secondary, reversion mutations that lead to the restoration of BRCA expression seems to be the main underlying mechanism and has been described in various patients with BRCA2-mutant PDAC who developed resistance to PARPi with or without platinum [98–100]. The loss of BRCA1 promoter methylation might also restore the expression of functional BRCA1 to levels similar to those seen in HR-proficient tumours [98].
The occurrence of PARPi resistance reflects the complexity and heterogeneity of BRCA1/2-mutant PDAC and requires further studies to fully understand the underlying mechanisms and to explore potential therapeutic strategies to overcome this issue.
Current trials are investigating the safety and efficacy of PARPi as a single agent and in combination with other drugs, both in the early and in the metastatic setting (Table 4). In particular, the combination of PARPi and immunotherapy is one of the latest approaches to treatment. PARP inhibition might cause BRCA-mutant tumours to become sensitive to immunotherapy not only by enhancing tumour immunogenicity through an increase in tumour antigen burden but also by increasing the expression of the immune checkpoint protein programmed death-ligand 1 in tumour tissue through the ATM–ATR–CHK1 pathway [101]. Data from these trials will hopefully provide new information on the management of patients with BRCA-mutant PDAC.
Table 4.
Ongoing clinical trials of PARP inhibitors in pancreatic cancer.
| Phase | Treatment arms | Setting | Treatment strategy | NCT number |
|---|---|---|---|---|
| I | NMS-03305293 | Advanced/metastatic, relapsed/refractory solid tumours | Single agent, after progression on standard treatment | NCT04182516 |
| I/II | Nanoliposomal Irinotecan + leucovorin + fluorouracil + rucaparib | Previously treated metastatic pancreatic, colorectal, gastroesophageal, biliary cancer | In combination with chemotherapy (as part of initial treatment) | NCT03337087 |
| II | rucaparib | Solid tumours with deleterious mutations in homologous recombination repair genes | Initial treatment | NCT04171700 |
| II | rucaparib | BRCA1-, BRCA2- or PALB2-mutant pancreatic cancer | Maintenance in BRCA1-, BRCA2- or PALB2-mutant pancreatic cancer without evidence of progression on platinum-based therapy | NCT03140670 |
| II | niraparib | Unresectable and metastatic pancreatic cancer with deficiencies in HR DNA repair | Single agent after ≥2 lines | NCT03601923 |
| II | niraparib | Metastatic pancreatic cancer after previous chemotherapy | Single agent after ≥2 lines | NCT03553004 |
| II | niraparib + nivolumab versus niraparib + ipilimumab | Inoperable PDAC | Maintenance in inoperable PDAC and stability on platinum-based chemotherapy for ≥16 weeks without evidence of progressive disease | NCT 03404960 |
PDAC pancreatic ductal adenocarcinoma, HR homologous repair.
Conclusions
BRCA1/2-mutant PDAC represents a type of PC with specific disease features that are still to be fully understood. BRCA1/2-mutant PDAC represents the largest molecular subgroup of pancreatic cancer, and BRCA1/2 genes alterations are the most explored ‘targetable’ mutations in prospective interventional clinical trials. In the era of precision medicine, this aspect appears crucial in PDAC, where the vast majority of efforts using agents against other molecular targets have provided disappointing results [2]. The discovery of the involvement of BRCA1/2 in PDAC development should lead to more attention being focused on the family history to identify which patients and relatives should be considered for genetic counselling.
No specific randomised trials have been conducted to investigate potential differences between germline and somatic mutations in BRCA1/2; most trials have focused on germline alterations and only a few patients with somatic BRCA-mutant PDAC have been enrolled. Owing to the biological rationale of HR mechanisms in which BRCA1/2 are involved, the BRCA1/2 PDAC landscape requires further research with traditional cytotoxic agents, especially platinum-based chemotherapy, and at the same time opens a new chapter on innovative therapeutic strategies—namely, PARPi as a single agent and in combination with other drugs. Unfortunately, no gain in OS has been reported with PARPi monotherapy and patients develop resistance [67]. Further research is urgently needed in order to improve PDAC survival outcomes. In this respect, an assessment of the association between PARPi and immunotherapy seems promising. Many questions remain unanswered and the lack of biomarkers to improve the treatment choice and clinical outcomes presents a challenge. Thus, the identification of predictive markers is crucial. The concept of ‘BRCAness’ has emerged to describe the high‐grade genomic instability present in non‐BRCA‐mutated cancers that resembles tumours originating from germline BRCA‐mutated carriers, and represents a phenotype of defective HR to which somatic mutations in different HR genes, such as BRCA1/2, ATM, PALB2, CHEK1, RAD51, and FANCA, can contribute. BRCAness is under evaluation as a biomarker for DNA-damaging agents and PARPi, but its measures and its predictive role still require further investigation [49, 102]. Germline mutations that are involved with BRCA1/2 and the HR pathway (e.g. ATM, PALB2, ATR, RAD 51, CHEK2, FANCA and BRIP1) have also been considered as potential predictive biomarkers for the same treatment strategies. Currently, clinical trials are assessing PARP inhibitors in patients with PDAC harbouring PALB2, ATM, CHEK2 germline mutations. On the other hand, the role of other germline mutations in the HRD pathway remains to be determined [49]. In the era of precision medicine, further studies are needed to identify predictive biomarkers in order to apply a better selection of patients with BRCA-mutant PDAC with the aim to offer them a tailored treatment.
Author contributions
EL proposed the manuscript topic, conceived, wrote, revised and co-ordinated the manuscript. PZ wrote and co-ordinated the manuscript. DS wrote the introduction, the sections “Mutant BRCA versus wild-type BRCA in PDAC” and “BRCA mutations and heritability in PDAC”. MD wrote the section “Germline BRCA mutations and somatic BRCA mutations in PDAC” and conceived Table 1. AP wrote the section “PARP inhibitors in BRCA-mutant PDAC” and conceived Fig. 1 and Tables 3 and 4. ST wrote the section “BRCA-mutant PDAC and platinum-based therapy” and conceived Table 2. SC wrote the section “BRCA-mutant PDAC and platinum-based therapy”. NL helped write the introduction and formatting the manuscript. SM helped write the section “Mutant BRCA versus wild-type BRCA in PDAC”. M. Persano helped write the section “BRCA mutations and heritability in PDAC”. MM helped write the section “BRCA-mutant PDAC and platinum-based therapy”. CD helped write the section “PARP inhibitors in BRCA-mutant PDAC”. LD reviewed the manuscript. VP reviewed the manuscript. M. Puzzoni reviewed the manuscript. MS conceived, reviewed and supervised the manuscript, had full access to the data in the study and final responsibility for the decision to submit for publication. All Authors have conceived and/or designed the work that led to the submission, acquired data, and/or played an important role in interpreting the results, drafted or revised the manuscript, approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Thus, they have all contributed to the work described sufficiently to be named as authors. All authors are aware of the submission of the revised manuscript and agree to it.
Funding information
The authors received no specific funding for this work.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent to publish
Not applicable.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Lai E, Puzzoni M, Ziranu P, Pretta A, Impera V, Mariani S, et al. New therapeutic targets in pancreatic cancer. Cancer Treat Rev. 2019;81:101926. doi: 10.1016/j.ctrv.2019.101926. [DOI] [PubMed] [Google Scholar]
- 3.Brunetti O, Luchini C, Argentiero A, Tommasi S, Mangia A, Aprile G, et al. The Italian rare pancreatic exocrine cancer initiative. Tumori. 2019;105:353–8. doi: 10.1177/0300891619839461. [DOI] [PubMed] [Google Scholar]
- 4.Brunetti O, Aprile G, Marchetti P, Vasile E, Casadei Gardini A, Scartozzi M, et al. Systemic chemotherapy for advanced rare pancreatic histotype tumors: a retrospective multicenter analysis. Pancreas. 2018;47:759–71. doi: 10.1097/MPA.0000000000001063. [DOI] [PubMed] [Google Scholar]
- 5.Aprile G, Negri FV, Giuliani F, DeCarlo E, Melisi D, Simionato F, et al. Second-line chemotherapy for advanced pancreatic cancer: which is the best option? Crit Rev Oncol Hematol. 2017;115:1–12. doi: 10.1016/j.critrevonc.2017.03.025. [DOI] [PubMed] [Google Scholar]
- 6.Silvestris N, Brunetti O, Vasile E, Cellini F, Cataldo I, Pusceddu V, et al. Multimodal treatment of resectable pancreatic ductal adenocarcinoma. Crit Rev Oncol Hematol. 2017;115:1–12. doi: 10.1016/j.critrevonc.2017.03.025. [DOI] [PubMed] [Google Scholar]
- 7.Pierantoni C, Pagliacci A, Scartozzi M, Berardi R, Bianconi M, Cascinu S. Pancreatic cancer: progress in cancer therapy. Crit Rev Oncol Hematol. 2008;67:27–38. doi: 10.1016/j.critrevonc.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Cascinu S, Scartozzi M, Carbonari G, Pierantoni C, Verdecchia L, Mariani C, et al. COX-2 and NF-KB overexpression is common in pancreatic cancer but does not predict for COX-2 inhibitors activity in combination with gemcitabine and oxaliplatin. Am J Clin Oncol. 2007;30:526–30. doi: 10.1097/COC.0b013e318054675c. [DOI] [PubMed] [Google Scholar]
- 9.Berardi R, Verdecchia L, Scartozzi M, Cascinu S. New frontiers in the neoadjuvant therapy of pancreatic adenocarcinoma: apart from therapeutical protocols. JOP. 2005;13:118–21. [PubMed] [Google Scholar]
- 10.Ghiorzo P. Genetic predisposition to pancreatic cancer. World J Gastroenterol. 2014;20:10778–89. doi: 10.3748/wjg.v20.i31.10778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holter S, Borgida A, Dodd A, Grant R, Semotiuk K, Hedley D, et al. Germline BRCA mutations in a large clinic-based cohort of patients with pancreatic adenocarcinoma. J Clin Oncol. 2015;33:3124–9. doi: 10.1200/JCO.2014.59.7401. [DOI] [PubMed] [Google Scholar]
- 12.Friedenson BBRCA1 and BRCA2 pathways and the risk of cancers other than breast or ovarian. Med Gen Med. 2005;7:60. [PMC free article] [PubMed] [Google Scholar]
- 13.Cai Y, Li X, Shen P, Zhang D. CCAT2 is an oncogenic long non-coding RNA in pancreatic ductal adenocarcinoma. Biol Res. 2018;51:1–9. doi: 10.1186/s40659-017-0149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Venkitaraman AR. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell. 2002;108:171–82. doi: 10.1016/S0092-8674(02)00615-3. [DOI] [PubMed] [Google Scholar]
- 15.Tutt A, Ashworth A. The relationship between the roles of BRCA genes in DNA repair and cancer predisposition. Trends Mol Med. 2002;8:571–6. doi: 10.1016/S1471-4914(02)02434-6. [DOI] [PubMed] [Google Scholar]
- 16.Walsh CS. Two decades beyond BRCA1/2: homologous recombination, hereditary cancer risk and a target for ovarian cancer therapy. Gynecol Oncol. 2015;137:343–50. doi: 10.1016/j.ygyno.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 17.Blair AB, Groot VP, Gemenetzis G, Wei J, Cameron JL, Weiss MJ, et al. BRCA1/BRCA2 germline mutation carriers and sporadic pancreatic ductal adenocarcinoma. J Am Coll Surg. 2018;226:630–637.e1. doi: 10.1016/j.jamcollsurg.2017.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lal G, Liu G, Schmocker B, Kaurah P, Ozcelik H, Narod SA, et al. Inherited predisposition to pancreatic adenocarcinoma: role of family history and germ-line p16, BRCA1, and BRCA2 mutations. Cancer Res. 2000;60:409–16. [PubMed] [Google Scholar]
- 19.Furukawa T, Sakamoto H, Takeuchi S, Ameri M, Kuboki Y, Yamamoto T, et al. Whole exome sequencing reveals recurrent mutations in BRCA2 and FAT genes in acinar cell carcinomas of the pancreas. Sci Rep. 2015;5:8829. doi: 10.1038/srep08829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson D, Easton DF. Cancer incidence in BRCA1 mutation carriers. J Natl Cancer Inst. 2002;94:1358–65. doi: 10.1093/jnci/94.18.1358. [DOI] [PubMed] [Google Scholar]
- 21.The Breast Cancer Linkage Consortium. Cancer risks in BRCA2 mutation carriers. J Natl Cancer Inst. 1999;91:1310–6. doi: 10.1093/jnci/91.15.1310. [DOI] [PubMed] [Google Scholar]
- 22.Van Asperen CJ, Brohet RM, Meijers-Heijboer EJ, Hoogerbrugge N, Verhoef S, Vasen HF, et al. Netherlands Collaborative Group on Hereditary Breast Cancer (HEBON). Cancer risks in BRCA2 families: estimates for sites other than breast and ovary. J Med Genet. 2005;42:711–9. doi: 10.1136/jmg.2004.028829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goggins M, Schutte M, Lu J, Moskaluk CA, Weinstein CL, Petersen GM, et al. Germline BRCA2 gene mutations in patients with apparently sporadic pancreatic carcinomas. Cancer Res. 1996;56:5360–4. [PubMed] [Google Scholar]
- 24.Moran A, O’Hara C, Khan S, Shack L, Woodward E, Maheret ER. Risk of cancer other than breast or ovarian in individuals with BRCA1 and BRCA2 mutations. Fam Cancer. 2012;11:235–42. doi: 10.1007/s10689-011-9506-2. [DOI] [PubMed] [Google Scholar]
- 25.Mersch J, Jackson MA, Park M, Nebgen D, Peterson SK, Singletary C, et al. Cancers associated with BRCA1 and BRCA2 mutations other than breast and ovarian. Cancer. 2015;121:269–75. doi: 10.1002/cncr.29041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Golan T, Kanji ZS, Epelbaum R, Devaud N, Dagan E, Holter S, et al. Overall survival and clinical characteristics of pancreatic cancer in BRCA mutation carriers. Br J Cancer. 2014;111:1132–8. doi: 10.1038/bjc.2014.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim DH, Crawford B, Ziegler J, Beattie MS. Prevalence and characteristics of pancreatic cancer in families with BRCA1 and BRCA2 mutations. Fam Cancer. 2009;8:153–8. doi: 10.1007/s10689-008-9220-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang D, Guan R, Tao Q, Liu S, Yu M, Li X. A novel somatic BRCA2 point mutation in a metastatic pancreatic cancer patient: a case report. BMC Med Genomics. 2021;14:6. doi: 10.1186/s12920-020-00850-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plon SE, Eccles DM, Easton D, Foulkes WD, Genuardi M, Greenblatt MS. Sequence variant classification and reporting: recommendations for improving the interpretation of cancer susceptibility genetic test results. Hum Mutat. 2008;29:1282–91. doi: 10.1002/humu.20880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrone CR, Levine DA, Tang LH, Allen PJ, Jarnagin W, Brennan MF, et al. BRCA germline mutations in Jewish patients with pancreatic adenocarcinoma. J Clin Oncol. 2009;27:433–8. doi: 10.1200/JCO.2008.18.5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu Y, Zhai K, Ke J, Li J, Gong Y, Yang Y, et al. BRCA1 missense polymorphisms are associated with poor prognosis of pancreatic cancer patients in a Chinese population. Oncotarget. 2017;8:36033–9. doi: 10.18632/oncotarget.16422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Golan T, Sella T, O'reilly EM, Katz MH, Epelbaum R, Kelsen DP, et al. Overall survival and clinical characteristics of BRCA mutation carriers with stage I/II pancreatic cancer. Br J Cancer. 2017;116:697–702. doi: 10.1038/bjc.2017.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iqbal J, Ragone A, Lubinski J, Lynch HT, Moller P, Ghadirian P, et al. The incidence of pancreatic cancer in BRCA1 and BRCA2 mutation carriers. Br J Cancer. 2012;107:2005–9. doi: 10.1038/bjc.2012.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takeuchi S, Doi M, Ikari N, Yamamoto M, Furukawa T. Mutations in BRCA1, BRCA2, and PALB2, and a panel of 50 cancer-associated genes in pancreatic ductal adenocarcinoma. Sci Rep. 2018;8:8105. doi: 10.1038/s41598-018-26526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seeber A, Zimmer K, Kocher F, Puccini A, Xiu J, Nabhan C, et al. Molecular characteristics of BRCA1/2 and 12 PALB2 mutations in pancreatic ductal adenocarcinoma. ESMO Open. 2020;5:e000942. doi: 10.1136/esmoopen-2020-000942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi C, Hruban RH, Klein AP. Familial pancreatic cancer. Arch Pathol Lab Med. 2009;133:365–74. doi: 10.5858/133.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones S, Hruban RH, Kamiyama M, Borges M, Zhang X, Parsons DW, et al. Exomic sequencing identifies PALB2 as a pancreatic susceptibility gene. Science. 2009;324:217. doi: 10.1126/science.1171202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McWilliams RR, Wieben ED, Rabe KG, Pedersen KS, Wu Y, Sicotte H, et al. Prevalence of CDKN2A mutations in pancreatic cancer patients: implication for genetic counseling. Eur J Human Genet. 2011;19:472–8. doi: 10.1038/ejhg.2010.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klein AP. Genetic susceptibility to pancreatic cancer. Mol Carcinog. 2012;51:1–24. doi: 10.1002/mc.20855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hahn SA, Greenhalf B, Ellis I, Sina-Frey M, Rieder H, Korte B, et al. BRCA2 germline mutations in familial pancreatic carcinoma. J Natl Cancer Inst. 2003;95:214–221. doi: 10.1093/jnci/95.3.214. [DOI] [PubMed] [Google Scholar]
- 41.Lowenfels AB, Maisonneuve P. Epidemiologic and etiologic factors of pancreatic cancer. Hematol Oncol Clin North Am. 2002;16:11–16. doi: 10.1016/S0889-8588(01)00003-X. [DOI] [PubMed] [Google Scholar]
- 42.Murphy KM, Brune KA, Griffin C, Sollenberger JE, Petersen GM, Bansal R, et al. Evaluation of candidate genes MAP2K4, MADH4, ACVR1B, and BRCA2 in familial pancreatic cancer: deleterious BRCA2 mutations in 17% Cancer Res. 2002;62:3789–93. [PubMed] [Google Scholar]
- 43.Couch FJ, Johnson MR, Rabe KG, Brune K, De Andrade M, Goggins M, et al. The prevalence of BRCA2 mutations in familial pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:342–6. doi: 10.1158/1055-9965.EPI-06-0783. [DOI] [PubMed] [Google Scholar]
- 44.Zhen DB, Rabe KG, Gallinger S, Syngal S, Schwartz AG, Goggins MG, et al. BRCA1, BRCA2, PALB2, and CDKN2A mutations in familial pancreatic cancer: a PACGENE study. Genet Med. 2015;17:569–77. doi: 10.1038/gim.2014.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harinck F, Kluijt I, van Mil SE, Waisfisz Q, van Os TA, Aalfs CM, et al. Routine testing for PALB2 mutations in familial pancreatic cancer families and breast cancer families with pancreatic cancer is not indicated. Eur J Hum Genet. 2012;20:577–9. doi: 10.1038/ejhg.2011.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grant RC, Al-Sukhni W, Borgida AE, Holter S, Kanji ZS, McPherson T, et al. Exome sequencing identifies nonsegregating nonsense ATM and PALB2 variants in familial pancreatic cancer. Hum Genomics. 2013;7:11. doi: 10.1186/1479-7364-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okur V, Chung WK. The impact of hereditary cancer gene panels on clinical care and lessons learned. Cold Spring Harb Mol Case Stud. 2017;3:a002154. doi: 10.1101/mcs.a002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brune KA, Lau B, Palmisano E, Canto M, Goggins MG, Hruban RH, et al. Importance of age of onset in pancreatic cancer kindreds. J Natl Cancer Inst. 2010;102:119–26. doi: 10.1093/jnci/djp466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wong W, Raufi AG, Safyan RA, Bates SE, Manji GA. BRCA mutations in pancreas cancer: spectrum, current management, challenges and future prospects. Cancer Manag Res. 2020;12:2731–42. doi: 10.2147/CMAR.S211151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Metcalfe KA, Poll A, Royer R, Llacuachaqui M, Tulman A, Sun P, et al. Screening for founder mutations in BRCA1 and BRCA2 in unselected Jewish women. J Clin Oncol. 2010;28:387–91. doi: 10.1200/JCO.2009.25.0712. [DOI] [PubMed] [Google Scholar]
- 51.Stadler ZK, Salo-Mullen E, Patil SM, Pietanza MC, Vijai J, Saloustros E, et al. Prevalence of BRCA1 and BRCA2 mutations in Ashkenazi Jewish families with breast and pancreatic cancer. Cancer. 2011;118:493–9. doi: 10.1002/cncr.26191. [DOI] [PubMed] [Google Scholar]
- 52.Salo-Mullen EE, O'reilly EM, Kelsen DP, Ashraf AM, Lowery MA, Yu KH, et al. Identification of germline genetic mutations in patients with pancreatic cancer. Cancer. 2015;121:4382–8. doi: 10.1002/cncr.29664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kirchhoff T, Satagopan JM, Kauff ND, Huang H, Kolachana P, Palmer C, et al. Frequency of BRCA1 and BRCA2 Mutations in Unselected Ashkenazi Jewish Patients With Colorectal Cancer. J Natl Cancer Inst. 2004;96:68–70. doi: 10.1093/jnci/djh006. [DOI] [PubMed] [Google Scholar]
- 54.Warner E, Foulkes W, Goodwin P, Meschino W, Blondal J, Paterson C, et al. Prevalence and penetrance of BRCA1 and BRCA2 gene mutations in unselected Ashkenazi Jewish women with breast cancer. J Natl Cancer Inst. 1999;91:1241–7. doi: 10.1093/jnci/91.14.1241. [DOI] [PubMed] [Google Scholar]
- 55.Shindo K, Yu J, Suenaga M, Fesharakizadeh S, Cho C, Macgregor-Das A, et al. Deleterious germline mutations in patients with apparently sporadic pancreatic adenocarcinoma. J Clin Oncol. 2017;35:3382–90. doi: 10.1200/JCO.2017.72.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith AL, Wong C, Cuggia A, Borgida A, Holter S, Hall A, et al. Reflex testing for germline BRCA1,BRCA2, PALB2, and ATM mutations in pancreatic cancer: mutation prevalence and clinical outcomes from two canadian research registries. JCO Precision Oncol. 2018;2:1–16. doi: 10.1200/PO.17.00098. [DOI] [PubMed] [Google Scholar]
- 57.Daly MB, Pal T, Berry MP, Buys SS, Dickson P, Domcheck SM, et al. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:77–102. [DOI] [PubMed]
- 58.Syngal S, Brand RE, Church JM, Giardiello FM, Hampel HL, Burt RW, et al. ACG clinical guideline: genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol. 2015;110:223–63. doi: 10.1038/ajg.2014.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stoffel EM, McKernin SE, Brand R, Canto M, Goggins M, Moravek C, et al. Evaluating susceptibility to pancreatic cancer: ASCO provisional clinical opinion. J Clin Oncol. 2019;37:153–64. doi: 10.1200/JCO.18.01489. [DOI] [PubMed] [Google Scholar]
- 60.Tempero MA, Malafa MP, Al-Hawary M, Behrman SW, Benson AB, Cardin DB, et al. NCCN Clinical Practice Guidelines in Oncology: Pancreatic Adenocarcinoma. Version 1.2021. December 10, 2020.
- 61.Abeliovich D, Kaduri L, Lerer I, Weinberg N, Amir G, Sagi M, et al. The founder mutations 185delAG and 5382insC in BRCA1 and 6174delT in BRCA2 appear in 60% of ovarian cancer and 30% of early-onset breast cancer patients among Ashkenazi women. Am J Hum Genet. 1997;60:505–14. [PMC free article] [PubMed] [Google Scholar]
- 62.Pilarski R. The role of BRCA testing in hereditary pancreatic and prostate cancer families. Am Soc Clin Oncol Educ Book. 2019;39:79–86. doi: 10.1200/EDBK_238977. [DOI] [PubMed] [Google Scholar]
- 63.Grant RC, Selander I, Connor AA, Selvarajah S, Borgida A, Briollais L, et al. Prevalence of germline mutations in cancer predisposition genes in patients with pancreatic cancer. Gastroenterology. 2015;148:556–64. doi: 10.1053/j.gastro.2014.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hu C, Hart SN, Polley EC, Gnanaolivu R, Shimelis H, Lee KY, et al. Association between inherited germline mutations in cancer predisposition genes and risk of pancreatic cancer. J Am Med Assoc. 2018;319:2401–9. doi: 10.1001/jama.2018.6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brand R, Borazanci E, Speare V, Dudley B, Karloski E, Peters MLB, et al. Prospective study of germline genetic testing in incident cases of pancreatic adenocarcinoma. Cancer. 2018;124:3520–7. doi: 10.1002/cncr.31628. [DOI] [PubMed] [Google Scholar]
- 66.Yurgelun MB, Chittenden AB, Morales-Oyarvide V, Rubinson DA, Dunne RF, Kozak MM, et al. Germline cancer susceptibility gene variants, somatic second hits, and survival outcomes in patients with resected pancreatic cancer. Genet Med. 2019;21:213–23. doi: 10.1038/s41436-018-0009-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beger C, Ramadani M, Meyer S, Leder G, Krüger M, Welte K, et al. Down-regulation of BRCA1 in chronic pancreatitis and sporadic pancreatic adenocarcinoma. Clin Cancer Res. 2004;10:3780–7. doi: 10.1158/1078-0432.CCR-0992-3. [DOI] [PubMed] [Google Scholar]
- 68.Wang Y, Park JYP, Pacis A, Denroche RE, Jang GH, Zhang A. A preclinical trial and molecularly-annotated patient cohort identify predictive biomarkers in homologous recombination deficient pancreatic cancer. Clin Cancer Res. 2020;26:5462–76. doi: 10.1158/1078-0432.CCR-20-1439. [DOI] [PubMed] [Google Scholar]
- 69.Waddell N, Pajic M, Patch AM, Chang DK, Kassahn KS, Bailey P, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518:495–501. doi: 10.1038/nature14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531:47–52. doi: 10.1038/nature16965. [DOI] [PubMed] [Google Scholar]
- 71.Golan T, Stossel C, Atias D, Buzhor E, Halperin S, Cohen K, et al. Recapitulating the clinical scenario of BRCA-associated pancreatic cancer in pre-clinical models. Int J Cancer. 2018;143:179–83. doi: 10.1002/ijc.31292. [DOI] [PubMed] [Google Scholar]
- 72.Kaufman B, Shapira-Frommer R, Schmutzler RK, Audeh MW, Friedlander M, Balmaña J, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33:244–50. doi: 10.1200/JCO.2014.56.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.De-Bono J, Ramanathan RK, Mina L, Chugh R, Glaspy J, Rafii S, et al. Phase I, dose-escalation, two-part trial of the PARP inhibitor talazoparib in patients with advanced germline BRCA1/2 mutations and selected sporadic cancers. Cancer Discov. 2017;7:620–9. doi: 10.1158/2159-8290.CD-16-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Golan T, Hammel P, Reni M, Van-Cutsem E, Macarulla T, Hall MJ, et al. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic. Cancer N Engl J Med. 2019;381:317–27. doi: 10.1056/NEJMoa1903387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Binder KAR, Mick R, O’Hara M, Teitelbaum U, Karasic T, Schneider C, et al. A phase II, single arm study of maintenance rucaparib in patients with platinum-sensitive advanced pancreatic cancer and a pathogenic germline or somatic mutation in BRCA1, BRCA2 or PALB2. Cancer Res. 2019;79 (Suppl 13):(abstract CT234). [DOI] [PubMed]
- 76.Pihlak R, Valle JW, Mc-Namara MG. Germline mutations in pancreatic cancer and potential new therapeutic options. Oncotarget. 2017;8:73240–57. doi: 10.18632/oncotarget.17291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tutt A, Tovey H, Cheang MCU, Kernaghan S, Kilburn L, Gazinska P. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT Trial. Nat Med. 2018;24:628–37. doi: 10.1038/s41591-018-0009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bolton KL, Chenevix-Trench G, Goh C, Sadetzki S, Ramus SJ, Karlan BY, et al. EMBRACE; kConFab Investigators; Cancer Genome Atlas Research Network. Association between BRCA1 and BRCA2 mutations and survival in women with invasive epithelial ovarian cancer. J Am Med Assoc. 2012;307:382–90. doi: 10.1001/jama.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reiss KA, Yu S, Judy R, Symecko H, Nathanson KL, Domchek SM. Retrospective survival analysis of patients with advanced pancreatic ductal adenocarcinoma and germline BRCA or PALB2 mutations. JCO Precision Oncol. 2018;2:1–9. doi: 10.1200/PO.17.00152. [DOI] [PubMed] [Google Scholar]
- 80.Yu S, Agarwal P, Mamtani R, Symecko H, Spielman K, O’Hara M, et al. Retrospective survival analysis of patients with resected pancreatic ductal adenocarcinoma and a germline BRCA or PALB2 mutation. JCO Precision Oncol. 2019;3:1–11. doi: 10.1200/PO.18.00271. [DOI] [PubMed] [Google Scholar]
- 81.Wattenberg MM, Asch D, Yu S, O'dwyer PJ, Domchek SM, Nathanson KL, et al. Platinum response characteristics of patients with pancreatic ductal adenocarcinoma and a germline BRCA1, BRCA2 or PALB2 mutation. Br J Cancer. 2020;122:333–9. doi: 10.1038/s41416-019-0582-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.O'reilly EM, Lee JW, Zalupski M, Capanu M, Park J, Golan T, et al. Randomized, multicenter, phase II trial of gemcitabine and cisplatin with or without veliparib in patients with pancreas adenocarcinoma and a germline BRCA/PALB2 mutation. J Clin Oncol. 2020;38:1378–88. doi: 10.1200/JCO.19.02931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lowery MA, Kelsen DP, Stadler ZK, Yu KH, Janjigian YY, Ludwig E, et al. An emerging entity: pancreatic adenocarcinoma associated with a known BRCA mutation: clinical descriptors, treatment implications, and future directions. Oncologist. 2011;16:1397–402. doi: 10.1634/theoncologist.2011-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sonnenblick A, Kadouri L, Appelbaum L, Peretz T, Sagi M, Goldberg Y, et al. Complete remission, in BRCA2 mutation carrier with metastatic pancreatic adenocarcinoma, treated with cisplatin based therapy. Cancer Biol Ther. 2011;12:165–8. doi: 10.4161/cbt.12.3.16292. [DOI] [PubMed] [Google Scholar]
- 85.Gupta M, Iyer R, Fountzilas C. Poly(ADP-ribose) polymerase inhibitors in pancreatic cancer: a new treatment paradigms and future implications. Cancers. 2019;11:1980. doi: 10.3390/cancers11121980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, et al. T: specific killing of BRCA2‑deficient tumours with inhibitors of poly(ADP‑ribose) polymerase. Nature. 2005;434:913–917. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 87.Lord CJ, Ashworth A. PARP inhibitors: synthetic lethality in the clinic. Science. 2017;355:1152–8. doi: 10.1126/science.aam7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Min A, Im SA. PARP inhibitors as therapeutics: beyond modulation of PARylation. Cancers. 2020;12:394. doi: 10.3390/cancers12020394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–21. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 90.Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, et al. Inhibition of poly (ADP-ri-bose) polymerase in tumors from BRCA mutation carriers. New Engl J Med. 2009;361:123–34. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 91.Tutt A, Robson M, Garber JE, Domchek SM, Audeh MW, Weitzel JN, et al. Oral poly(ADP- ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof of concept trial. Lancet. 2010;376:235–44. doi: 10.1016/S0140-6736(10)60892-6. [DOI] [PubMed] [Google Scholar]
- 92.Audeh MW, Carmichael J, Penson RT, Friedlander M, Powell B, Bell-McGuinn KM, et al. Oral poly (ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof of-concept trial. Lancet. 2010;376:245–51. doi: 10.1016/S0140-6736(10)60893-8. [DOI] [PubMed] [Google Scholar]
- 93.Lowery MA, Kelsen DP, Capanu M, Smith SC, Lee JW, Stadler ZK, et al. Phase II trial of veliparib in patients with previously treated BRCA-mutated pancreas ductal adenocarcinoma. Eur J Cancer. 2018;89:19–26. doi: 10.1016/j.ejca.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shroff RT, Hendifar A, McWilliams RR, Geva R, Epelbaum R, Rolfe L, et al. Rucaparib monotherapy in patients with pancreatic cancer and a known deleterious BRCA mutation. JCO Precis Oncol. 2018 doi: 10.1200/PO.17.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chiorean EG, Guthrie KA, Philip PA, Swisher EM, Jalikis F, Pishvaian MJ, et al. Randomized phase II study of second-line modified FOLFIRI with PARP inhibitor ABT-888 (Veliparib) (NSC-737664) versus FOLFIRI in metastatic pancreatic cancer (mPC): SWOG S1513. J Clin Oncol. 2019;37:4014. [DOI] [PMC free article] [PubMed]
- 96.Pishvaian MJ, Wang H, Parenti S, He AR, Hwang JJ, Ley L, et al. Final report of a phase I/II study of veliparib (Vel) in combination with 5-FU and oxaliplatin (FOLFOX) in patients (pts) with metastatic pancreatic cancer (mPDAC) J Clin Oncol. 2019;37:4014–4014. doi: 10.1200/JCO.2019.37.15_suppl.4014. [DOI] [Google Scholar]
- 97.Kim DS, Camacho CV, Kraus WL. Alternate therapeutic pathways for PARP inhibitors and potential mechanisms of resistance. Exp Mol Med. 2021;53:42–51. doi: 10.1038/s12276-021-00557-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pishvaian MJ, Biankin AV, Bailey P, Chang DK, Laheru D, Wolfgang CL, et al. BRCA2 secondary mutation-mediated resistance to platinum and PARP inhibitor-based therapy in pancreatic cancer. Br J Cancer. 2017;116:1021–6. doi: 10.1038/bjc.2017.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tao H, Liu S, Huang D, Han X, Wu X, Shao YW, et al. Acquired multiple secondary BRCA2 mutations upon PARPi resistance in a metastatic pancreatic cancer patient harboring a BRCA2 germline mutation. Am J Transl Res. 2020;12:612–7. [PMC free article] [PubMed] [Google Scholar]
- 100.Tobalina L, Armenia J, Irving E, O’Connor MJ, Forment JV. A meta-analysis of reversion mutations in BRCA genes identifies signatures of DNA end-joining repair mechanisms driving therapy resistance. Ann Oncol. 2020;32:103–12. doi: 10.1016/j.annonc.2020.10.470. [DOI] [PubMed] [Google Scholar]
- 101.Kasi A, Al-Jumayli M, Park R, Baranda J, Sun W. Update on the role of poly (ADP-ribose) polymerase inhibitors in the DNA repair-deficient pancreatic cancers: a narrative review. J Pancreat Cancer. 2020;6:107–15. doi: 10.1089/pancan.2020.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hu Y, Guo M. Synthetic lethality strategies: beyond BRCA1/2 mutations in pancreatic cancer. Cancer Sci. 2020;111:3111–21. doi: 10.1111/cas.14565. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.