Abstract
Hypercoagulability and the need for prioritizing coagulation markers for prognostic abilities have been highlighted in COVID-19. We aimed to quantify the associations of D-dimer with disease progression in patients with COVID-19. This systematic review and meta-analysis was registered with PROSPERO, CRD42020186661.We included 113 studies in our systematic review, of which 100 records (n = 38,310) with D-dimer data) were considered for meta-analysis. Across 68 unadjusted (n = 26,960) and 39 adjusted studies (n = 15,653) reporting initial D-dimer, a significant association was found in patients with higher D-dimer for the risk of overall disease progression (unadjusted odds ratio (uOR) 3.15; adjusted odds ratio (aOR) 1.64). The time-to-event outcomes were pooled across 19 unadjusted (n = 9743) and 21 adjusted studies (n = 13,287); a strong association was found in patients with higher D-dimers for the risk of overall disease progression (unadjusted hazard ratio (uHR) 1.41; adjusted hazard ratio (aHR) 1.10). The prognostic use of higher D-dimer was found to be promising for predicting overall disease progression (studies 68, area under curve 0.75) in COVID-19. Our study showed that higher D-dimer levels provide prognostic information useful for clinicians to early assess COVID-19 patients at risk for disease progression and mortality outcomes. This study, recommends rapid assessment of D-dimer for predicting adverse outcomes in COVID-19.
Subject terms: Biochemistry, Biomarkers, Diseases, Risk factors
Introduction
Ever since the emergence of COVID-19 in December, 2019, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread rapidly across the globe1. With wide spectrum of symptoms, about 20–26% of patients with COVID-19 pneumonia become severe or critically ill, requiring hospitalization for respiratory support. With poor prognosis, the mortality rates vary from 26 to 61.5%1–6.
Early identification of patients at risk for disease progression is a major concern among clinicians, for developing management strategies in order to prevent mortality outcomes. Therefore, identification of better predictors of prognosis is of great clinical significance, and the need for prioritizing coagulation markers for prognostic abilities has been highlighted5–7. Several researchers have paid much attention to D-dimer, reporting its significant raise in severe cases and non-survivors, as compared to non-severe patients and survivors5–27. It has proposed that, as a marker of coagulation, increased D-dimer reflect hypercoagulability and thrombotic burden, guiding clinicians for using anticoagulation in COVID-19 patients27–29. Several studies have reported an increased D-dimer in positive relationship to disease severity, composite outcomes and high mortality events in COVID-1930–55. However, these individual studies are limited by sample size and reported different clinical outcomes based on unadjusted and/or adjusted models. Therefore, the available evidence on the prognostic information pertaining to D-dimer requires validation through meta-analysis. Further, the available reviews are of questionable quality (usually pooling of median and inter quartile ranges reported in included studies) or involve meta-analysis with less number of studies56–60.
In this study, we present a comprehensive meta-analysis to explore the prognostic use of D-dimer by the analysis of unadjusted and adjusted risk estimates (odds ratios) for disease severity, composite outcomes and mortality events. We also report the association of increased D-dimer with time-event-outcomes (unadjusted and adjusted hazards ratios) in COVID-19 patients. Further, the prognostic information of D-dimer was pooled for obtaining sensitivity, specificity, diagnostic odds ratio (DOR), and the area under curve (AUC) values for predicting COVID-19 disease progression.
Methods
Search strategy and selection criteria
The search for relevant literature was primarily conducted in PubMed, and then in the other databases such as Science Direct, Springer Author Mapper, Google Scholar, Scopus and Web of Science. The literature search was conducted using the keywords “COVID-19”, “nCoV-2019”, “nCoV”, “SARS-CoV-2”, “Novel Coronavirus”, “Severe Acute Respirator Syndrome Coronavirus-2” in combination with “Coagulation Dysfunction” and “D-dimer”. Considering the rapid growth in the COVID-19 research, the relevant databases (“LitCovid”, “CDC”, “WHO” and “NIH”), Coronavirus resource directories of major publishers (ELSEVIER, The Lancet, Springer and WILEY) and major journals (BMJ, NEJM, JAMA and the Major Respiratory Medicine journals) were searched. Additionally, the bibliographies of published articles were manually searched for potential literature. Two of the authors (SRV and PK) designed the search strategy for literature retrieval, and the other authors reviewed and verified the search strategy and retrieved literature. No filters/limits were applied during literature search. We followed PRISMA guidelines, and the protocol of this study was registered with PROSPERO, CRD42020186661.
Eligible studies had to report D-dimer results in COVID-19 patients. The eligible studies reported the direct effect sizes in the form of odds ratios of D-dimer or number of events in the form of 2 × 2 table (for predicting disease severity) and/or time-to-event outcomes in the form of hazards ratios of D-dimer for predicting deaths in COVID-19. Additionally, studies reporting direct ROC data or 2 × 2 tables were also eligible. The article types such as reviews, opinions, editorials, case-reports and studies not reporting D-dimer in association with COVID-19 severity and mortality were excluded. Three (SRV, PK, ND) independently screened (titles and abstracts) and reviewed the studies for their eligibility and any disagreements were resolved upon discussion with another author (SV) for consensus.
Data extraction and definitions
The same authors (SRV, ND) involved in the literature search performed data extraction independently using the previously agreed-upon data extraction forms (MS word and colour coded-excel sheets). Any discrepancies were resolved via consensus with the other author (PK). The data extractions included: study author; country; study duration; age; male; female; outcomes (severity/mortality/CEP); number of higher D-dimer events (2 × 2 table data) in the severe versus non-severe and non-survival versus survival groups of COVID-19 patients; unadjusted and adjusted odds ratios and hazards ratios (with 95% CI levels) of D-dimer in relationship to COVID-19 disease severity and mortality; medication details; and percentages of comorbidities such as COPD, CVD, diabetes, hypertension.
COVID-19 diagnosis and severity definitions were according to the WHO interim guidance and/or the National health commission of China guidelines61,62. Severe COVID-19 was classified as having ARDS, oxygen saturation of ≤ 93%, need for ICU care or mechanical ventilation. The composite end point was defined as need for ICU care or mechanical ventilation or deaths. Mortality outcome was differentiated between non-survivals (deaths) versus survival (alive/discharged/recovered) COVID-19 patients. The time-to-event was defined as the time from hospitalization to ICU admission or death.
Quality assessment and Statistical analysis
Two authors (SRV, ND) independently assessed the quality of eligible studies using the QUIPS tool63. Any disagreements were resolved by discussion with the Professor level third reviewer (SV). The quality assessment domains include: study participation; study attrition; prognostic factor measurement; outcome; confounding; statistical analysis and reporting. Based on these domains, the included studies were rated for risk of bias as ‘low’, ‘moderate’ or ‘high’.
The unadjusted and adjusted odds ratios and hazards ratios with 95% CI limits reported to describe the relationship of D-dimer with disease progression: severity; CEP; and mortality outcomes in COVID-19 patients were used. The data reported in 2 × 2 table format were used for obtaining unadjusted odds ratios. Considering the possible heterogeneity, random effects meta-analyses were conducted for conservative pooled-effect sizes for overall disease progression (severity + CEP + mortality) and sub-group analysis based on the individual ‘outcome type’ and ‘country’. Heterogeneity was assessed using the I-square statistic. The meta-regression and a one-study leave-out sensitivity analyses were performed to study the influence of certain variables and individual studies. Publication bias was tested using the funnel-plot asymmetry followed by Begg’s correlation and Egger’s regression tests. In case of a significant publication bias, Duval and Tweedie’s trim and fill method was used for obtaining adjusted values. We used Review Manager (Version 5.4) and Comprehensive Meta-analysis (Version 3) for the analysis of odds ratios and hazards ratios.
Further, meta-analysis of diagnostic test accuracy was conducted using relevant direct data and data from 2 × 2 tables to obtain pooled sensitivity, pooled specificity, positive- and negative-likelihood ratios, diagnostic odds ratios and AUC values using the random-effects DerSimonian-Laird method. The summary of receiver operating characteristic curves (SROC) was constructed with Moses linear model. Heterogeneity due to threshold and non-threshold effects was assessed by Spearman correlation analysis and the Cochran Q method with inconsistency (I2) test, respectively. These analyses were done using Meta-DiSc software (version 1.4).
Results
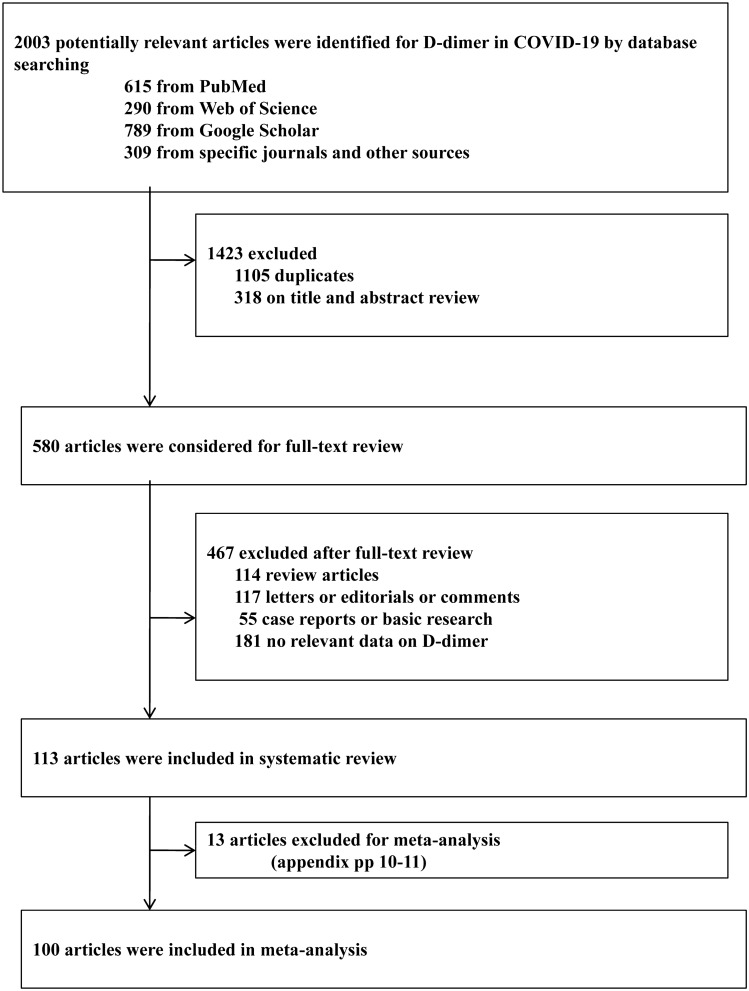
We identified a total of 2003 records by literature searching. Based on the initial screening of titles and abstracts, and after the removal of duplicates, 580 articles were considered further. Following the full-text review, 467 articles were removed, and the remaining 113 studies were included in systematic review (Fig. 1)4–55,64–124. Of these, 100 articles were finally included for meta-analysis (n = 38,310 with available D-dimer data)4–55,64–111. Across the included studies, the D-dimer levels were considered as ‘initial’ when measured within 24–48 h or the first measurement upon hospitalization. Whereas, the D-dimer levels were considered to be ‘dynamic’, for peak or longitudinal changes between different days, after initial assessment7,37,40,43,45,55,64,86,88. The adjusted factors vary across included studies (includes; age, sex, comorbidities, treatments and other lab variables) for obtaining independent effect sizes as adjusted odds and hazard ratios.
Figure 1.
Study selection.
As we searched the literature for relevant studies of any design, majority of the included studies were retrospective cohorts, all included adult patients aged > 18 years (range 41–73 years), with the male and female % ranging from 36–91 to 8–64%, respectively. The proportions of any comorbidity across included studies range from 23.1 to 79.7%, with COPD (1–34%), diabetes (4.6–98.5%), and hypertension (14.2–79%). The proportions of deaths and recovery across reported studies were 1.4–74.07%, and 2.94–96.6%, respectively. Disease severity was compared between non-severe (mild-moderate) and severe to critical groups or requiring for ICU care or mechanical ventilation. Composite end point was defined as need for ICU care, mechanical ventilation and deaths. Time-to-event was defined as the time from hospital admission until the event or censoring. All studies were published in 2020: seventy-eight from China, 14 from USA, 10 from Italy, 4 from Spain, 3 from France, 2 from Turkey, one each from India, Iran and Europe. The criteria by WHO interim guidance or the national commission of China guidelines or laboratory confirmation by real-time polymerase chain reaction (RT-PCR) were used for COVID-19 across the studies. The main characteristics of the included (Appendix Table 1) and excluded studies (Appendix Table 2) and QUIPS assessments (Appendix Table 3) were presented in the Appendix (pp. 1–19).
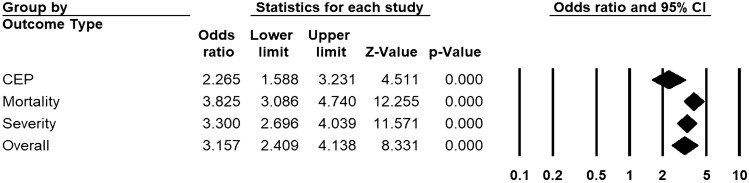
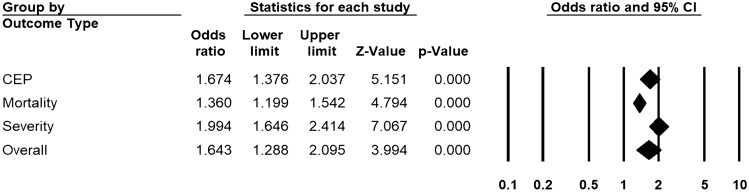
Across 68 unadjusted (n = 26,960) and 39 adjusted studies (n = 15,653) reporting initial D-dimer, a strong relationship was found in patients with higher D-dimers for the risk of overall disease progression (uOR 3.15, 95% CI 2.41 to 4.14, I2 = 92.3%, Fig. 2; aOR 1.64, 95% CI 1.29 to 2.10, I2 = 83.3%, Fig. 3). This pooled estimate corrected for publication bias, using trim and fill method, still showed a significant relationship (uOR 2.44, 95% CI 2.16 to 2.77; aOR 1.40, 95% CI 1.25 to 2.52). By sub-group analysis based on the outcome type, this association was found to be significant for disease severity (unadjusted studies 40, n = 15,358, uOR 3.30, 95% CI 2.67 to 4.04; adjusted studies 11, n = 4759, aOR 1.99, 95% CI 1.64 to 2.41), mortality (unadjusted studies 40, n = 15,613, uOR 3.82, 95% CI 3.08 to 4.74; adjusted studies 22, n = 9989, aOR 1.36, 95% CI 1.19 to 1.54), and CEP (unadjusted studies 11, n = 7004, uOR 2.26, 95% CI 1.58 to 3.23; adjusted studies 9, n = 4102, aOR 1.67, 95% CI 1.37 to 2.03) (Figs. 2 and 3, and Appendix pp. 20–23 for individual forest plots).
Figure 2.
Pooled estimate of unadjusted odds ratios for the association of D-dimer with disease progression in patients with COVID-19.
Figure 3.
Pooled estimate of adjusted odds ratios for the association of D-dimer with disease progression in patients with COVID-19.
Across 11 unadjusted (n = 4702) and 7 adjusted observations on dynamic/peak D-dimer (n = 2063), a significant association was found in patients with higher D-dimers for the risk of overall disease progression (uOR 2.31, 95% CI 1.38 to 3.85, I2 = 93.9%; aOR 1.51, 95% CI 1.15 to 1.97, I2 = 87.8%). The strength of this association remained to be significant for mortality outcome (unadjusted studies 7, uOR 3.13, 95% CI 1.98 to 4.92; adjusted studies 3, aOR 1.51, 95% CI 1.01 to 2.27), (Appendix p. 24).
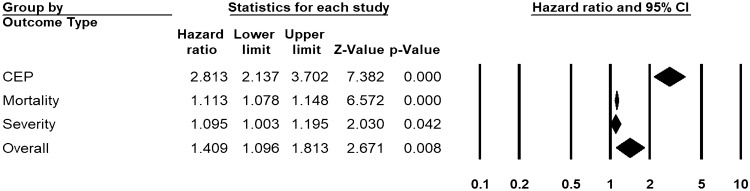
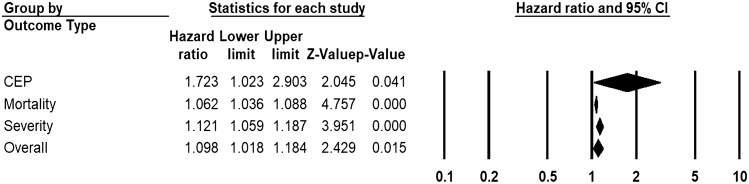
Across 19 unadjusted (n = 9743) and 21 adjusted studies (n = 13,287) reporting time-to-event estimates, a strong association was found in patients with higher D-dimers for the risk of overall disease progression (uHR 1.41, 95% CI 1.10 to 1.81, I2 = 95.3%, Fig. 4; aHR 1.10, 95% CI 1.02 to 1.20, I2 = 91.4%, Fig. 5). This pooled estimate corrected for publication bias, using trim and fill method, still showed a significant relationship (uHR 1.08, 95% CI 1.04 to 1.12; aHR 1.07, 95% CI 1.04 to 1.09). By sub-group analysis based on the outcome type, this association was found to be significant for disease severity (unadjusted studies 3, n = 1621, uHR 3.30, 95% CI 2.67 to 4.04; adjusted studies 3, n = 1645, aHR 1.10, 95% CI 1.003 to 1.195), mortality (unadjusted studies 13, n = 8834, uHR 1.11, 95% CI 1.07 to 1.15; adjusted studies 15, n = 11,586, aHR 1.06, 95% CI 1.03 to 1.09), and CEP (unadjusted studies 3, n = 796, uHR 2.81, 95% CI 2.14 to 3.70; adjusted studies 2, n = 1121, aHR 1.72, 95% CI 1.02 to 2.90) (Figs. 4 and 5, and Appendix p. 25).
Figure 4.
Pooled estimate of unadjusted hazard ratios for the association of D-dimer with disease progression in patients with COVID-19.
Figure 5.
Pooled estimate of adjusted hazard ratios for the association of D-dimer with disease progression in patients with COVID-19.
The higher D-dimers were found to be useful in predicting overall disease progression (studies 68, sensitivity 0.59, specificity 0.62, DOR 4.92, AUC 0.75), severity (studies 32, sensitivity 0.55, specificity 0.56, DOR 3.49, AUC 0.69), mortality (studies 32, sensitivity 0.64, specificity 0.66, DOR 7.20, AUC 0.79), and CEP outcomes (studies 4, sensitivity 0.75, specificity 0.55, DOR 4.70, AUC 0.93) (Table 1).
Table 1.
Pooled estimates of sensitivity, specificity, diagnostic odds ratio, and AUC values of D-dimer for predicting disease progression.
| Severity | Mortality | CEP | Overall-disease progression | |
|---|---|---|---|---|
| Pooled sensitivity | ||||
| n | 32 | 32 | 4 | 68 |
| I-Square | 96.8 | 90.8 | 50.6 | 95.2 |
| Pooled Sensitivity | 0.55 | 0.64 | 0.75 | 0.59 |
| CI | 0.54–0.57 | 0.62–0.66 | 0.69–0.80 | 0.57–0.60 |
| Pooled specificity | ||||
| n | 32 | 32 | 4 | 68 |
| I-Square | 95.8 | 98.7 | 92.0 | 98.0 |
| Pooled Specificity | 0.56 | 0.66 | 0.55 | 0.62 |
| CI | 0.55–0.57 | 0.65–0.67 | 0.52–0.59 | 0.61–0.63 |
| Pooled PLR | ||||
| n | 32 | 32 | 4 | 68 |
| I-Square | 86.6 | 96.8 | 87.7 | 93.8 |
| Pooled PLR | 1.69 | 2.67 | 1.95 | 2.12 |
| CI | 1.51–1.89 | 2.19–3.26 | 1.36–2.80 | 1.91–2.35 |
| Pooled NLR | ||||
| n | 32 | 32 | 4 | 68 |
| I-Square | 89.2 | 67.3 | 58.2 | 85.1 |
| Pooled NLR | 0.54 | 0.48 | 0.47 | 0.50 |
| CI | 0.46–0.63 | 0.43–0.54 | 0.31–0.69 | 0.45–0.55 |
| Pooled DOR | ||||
| n | 32 | 32 | 4 | 68 |
| I-Square | 83.8 | 82.7 | 81 | 84.9 |
| Pooled DOR | 3.49 | 7.20 | 4.70 | 4.92 |
| CI | 2.66–4.58 | 5.23–9.90 | 2.01–10.97 | 4.00–6.06 |
| Cochran-Q | 190.92 | 179.32 | 15.75 | 444.52 |
| p | 0.00 | 0.00 | 0.001 | 0.00 |
| Threshold effect | ||||
| Spearman’s correlation | 0.55 | 0.61 | − 0.20 | 0.49 |
| p | 0.001 | 0.00 | 0.80 | 0.00 |
| SROC | ||||
| AUC (SE) | 0.69 (0.02) | 0.79 (0.02) | 0.95 (0.12) | 0.75 (0.01) |
| CI | 0.67–0.72 | 0.77–0.81 | 0.80–1.05 | 0.73–0.77 |
AUC = area under curve. CEP = composite end points. CI = confidence interval. DOR = diagnostic odds ratio. NLR = negative likelihood ratio. PLR = positive likelihood ratio. SROC = summary receiver operating curves.
By meta-regression (data not shown), the models comprised of age and sex (model 1), comorbidities (model 2), deaths and recovery % (model 3) could be potential contributors of heterogeneity. By sub-group analysis based on the variable ‘country’; studies presenting wider CIs of ORs (one each from ‘India’ and ‘Iran’, and 7 from ‘Italy’). Similarly, one multi-country study from ‘Europe’ presenting wider CIs of HR could be a possible source of heterogeneity. However, we did a random-effects meta-analysis that assigns weight to each included study by incorporating between-studies variance. We further analysed the robustness of our findings by sensitivity analysis, revealing that no particular observation could significantly affect any of the pooled estimates. The country-wide subgroup analyses were shown in Appendix (pp. 26–27).
Discussion
The findings of this meta-analysis provide the best comprehensive evidence that higher D-dimer was associated with disease severity and CEP in COVID-19. The mortality outcome was significantly associated with higher D-dimer levels. Importantly, our analysis suggests that D-dimer (initial and dynamic) is an independent prognostic marker as evidenced by the unadjusted and adjusted-OR estimates for disease progression. Our analysis also suggests that higher D-dimer (initial) exhibits strong and independent association with time-to-event outcome estimates (unadjusted and adjusted-HRs). Further, our findings indicate that higher D-dimer exhibit good predictive abilities as a prognostic marker of disease severity (AUC 0.69), CEP (0.93) and mortality outcomes (0.79).
In accordance with previous evidence, our results on the association of higher D-dimer with disease progression in COVID-19 support that severe patients are at higher risk of hypercoagulability34,38,97,98. Also, a large body of evidence shows that, the non-surviving COVID-19 exhibit significantly higher D-dimer levels, reflective of hypercoagulability status4,5,13–15,64–66. These results suggest that higher D-dimer levels in COVID-19 patients might indicate coagulopathy and thrombotic risk. Several mechanisms explain higher D-dimer and hypercoagulability in COVID-195,6,125–127. Critically ill patients present with more severe hypoxia and lung injury. Severe and critical COVID-19 patients are presented with higher PAI-1 levels leading to impaired fibrinolytic and thrombus dissolution systems5,125. Hypoxaemia induced vasoconstriction leading to reduced blood flow and vascular occlusion, endothelial dysfunction, inflammation, major comorbidities such as hypertension and diabetes, old age, and prolonged bed rest are among the other factors126. Severe and critical COVID-19 patients are usually complicated by other comorbidities, organ dysfunctions and disseminated intravascular coagulation (DIC). Thrombotic and haemorrhagic events were common complications in non-survivors. As a marker of coagulation, and an important component of DIC, increased D-dimer is associated with the mortality outcome5,6,127.
A body of evidence suggests a correlation between markers of inflammation and coagulopathy10,29, cytokine storm lead to thrombus formation through platelet activation37,128. Higher D-dimer levels in COVID-19 patients suggestive of higher risk for disease progression may also indicate higher risk for thrombotic events. D-dimer has been reported to be an important prognostic factor for abnormal DLCO. For patients with raised D-dimer, pulmonary rehabilitation is recommended even in the absence of severe respiratory symptoms. The same study reported radiographic and physiological abnormalities in high proportion of patients 3 months after their discharge129. Hanif et al.37 found that none of the patients on anticoagulation showed thrombotic complications, highlighting the potential for early anticoagulation. Evidence shows that anticoagulation treatments were promising in reversing the procoagulant pattern130. Several studies have reported anticoagulation strategies for critical COVID-19 patients131. Recent studies show improved outcomes with anticoagulation in COVID-19 patients37,54,79,93,132,133. There is a need for further well controlled randomized trials to determine the clinical effectiveness.
Of note, hypercoagulability has been reported to occur at the early stages of COVID-195,37, and procoagulant state is evidenced with micro- and macro-thrombi in autopsy studies134. Therefore, it is important to identify coagulation parameters for classifying high risk individuals for earliest possible intervention of coagulopathy. Varied D-dimer measurements with disease progression could provide predictive information to clinicians for early recognition of COVID-19 patients at risk for developing outcomes. This would also assist the concerned clinicians to develop anticoagulation therapeutic strategies, at the earliest to prevent disease progression to mortality. Our results suggest that D-dimer is a useful predictive marker for the severity of disease, CEP and mortality in COVID-19. This study can come handy for the clinicians to select high risk patients for their early management and save medical resources for the growing number of cases.
Our study has some strengths and limitations. Though we report the association of dynamic D-dimer levels with disease progression, we found only 11 and 7 observations for uOR and aOR, respectively. Therefore, further studies monitoring the dynamic profiles of D-dimer are still needed. The strong associations of initial D-dimer level with overall disease progression, severity, CEP, and mortality outcomes in COVID-19 patients (using large sample sizes), were similar between uOR and aOR estimates. Of note, we also report strong relationship of initial D-dimer with time-to-event outcomes, significant in both uHR and aHR estimates. The predictive abilities of initial D-dimer were promising with good DOR and AUC estimates for COVID-19 disease progression. The important limitation is the heterogeneity among the observational studies with retrospective design, which is inevitable in the meta-analysis of prognostic studies. Though we used random-effects model, assigning weight to each included study by incorporating between-studies variance, the clinical heterogeneity could not be completely ruled-out. The degree and diversity of severity, different comorbidities and treatment options, sample sizes and adjusted variables might have affected the clinical course and outcomes estimates. Owing to the retrospective nature of the studies, it is possible that D-dimer levels might have influenced anticoagulant treatment decisions, and vice versa. Even though participants with available D-dimer data were only included in the respective analysis of included studies, missing participants could potentially have introduced some bias. As most of the studies retrospectively extracted D-dimer data from the medical records of the patients during the hospital admissions, clear information on the measurement methods was not available in the included studies with varied D-dimer cut-offs across the studies. As there is no information as to why these cut-offs varied across studies, this study highlighting the use of D-dimer measurements for predicting disease severity, support the ISTH guidelines recommending the need for accurate D-dimer reporting in COVID-19135. Nevertheless, this study is the largest to comprehensively Meta-analyze D-dimer as a prognostic marker in association with disease progression in large sample size of COVID-19 patients. This study provides reliable evidence based on rigorous statistical analysis of unadjusted and adjusted estimates for outcomes risk along with the pooled diagnostic accuracy indices. Owing to this promising evidence on D-dimer, its further use in combination with other markers could be investigated further in well controlled studies.
Implications for practice
Our study identified D-dimer as a promising prognostic factor for predicting disease severity, composite and mortality outcomes in COVID-19. The associations were significant in unadjusted and adjusted models. Further, higher D-dimer levels in patients with COVID-19 were associated with time-to-event hazard ratios. Our study supports dynamic monitoring of D-dimer levels for early risk assessment by the clinicians involved in the management of COVID-19 patients using anticoagulation strategies. This supports the use of D-dimer as a prognostic marker for early identification of covid-19 patients at high risk for adverse outcomes.
Implications for future research
Owing to the evidence of inflammation and hyper coagulation in COVID-19136,137, the measurements of a routine coagulation marker, D-dimer provide reliable predictive information on disease progression. However, as different cut-offs were used across studies, there is a need to establish one universal cut-off point for predicting disease progression in COVID-19. Further, well controlled prospective studies investigating multi-marker strategies along with D-dimer could provide much useful information for predicting disease progression in COVID-19.
Conclusion
In this meta-analysis, we identified all reported prognostic information on D-dimer for disease progression and mortality events in patients with COVID-19. Our results suggest that higher D-dimer levels in COVID-19 patients are significantly associated with disease progression. Higher D-dimer levels, indicative of hypercoagulability, are found to predict disease severity, composite outcomes and mortality events in both unadjusted and adjusted models of odds ratios and time-to-events hazards ratios. Further, the increased D-dimer could provide promising prognostic information for predicting disease progression in COVID-19. This study, recommends for rapid assessment of this coagulation marker, also support the ISTH guidelines for accurate D-dimer reporting in COVID-19. Owing to the growing number of coronavirus cases, our study provides valuable information for clinicians to early assess COVID-19 patients at risk for disease progression and mortality outcomes. Further prospective randomized studies are needed to confirm the results of this meta-analysis.
Supplementary Information
Acknowledgements
Dr. Varikasuvu SR, specially acknowledges “Bhairavi Sisters” (Sahasra&Aagneya) for the time I could not give them during this work.
Author contributions
S.R.V. and N.D. conceived the study. S.R.V., N.D. and P.K. conducted literature search. S.R.V. and S.A. analysed the data. M.M. assisted in literature search and analysis. S.R.V. wrote the manuscript. N.D., S.V. and P.G. supervised and critically revised the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-01462-5.
References
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle Region—Case series. N. Engl. J. Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liao D, Zhou F, Luo L, et al. Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: A retrospective cohort study. Lancet Haematol. 2020;7:e671–e678. doi: 10.1016/S2352-3026(20)30217-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li C, Hu B, Zhang Z, et al. D-dimer Triage for COVID-19. Acad. Emerg. Med. 2020;27:612–613. doi: 10.1111/acem.14037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aloisio E, Chibireva M, Serafini L, et al. A comprehensive appraisal of laboratory biochemistry tests as major predictors of COVID-19 severity. Arch. Pathol. Lab. Med. 2020 doi: 10.5858/arpa.2020-0389-sa. [DOI] [PubMed] [Google Scholar]
- 9.Aloisio E, Serafini L, Chibireva M, Dolci A, Panteghini M. Hypoalbuminemia and elevated D-dimer in COVID-19 patients: A call for result harmonization. Clin. Chem. Lab. Med. 2020 doi: 10.1515/cclm-2020-1038. [DOI] [PubMed] [Google Scholar]
- 10.Al-Samkari H, Karp Leaf RS, Dzik WH, et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136:489–500. doi: 10.1182/blood.2020006520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ayanian S, Reyes J, Lynn L, Teufel K. The association between biomarkers and clinical outcomes in novel coronavirus pneumonia in a US cohort. Biomark. Med. 2020 doi: 10.2217/bmm-2020-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bao C, Tao X, Cui W, et al. SARS-CoV-2 induced thrombocytopenia as an important biomarker significantly correlated with abnormal coagulation function, increased intravascular blood clot risk and mortality in COVID-19 patients. Exp. Hematol. Oncol. 2020 doi: 10.1186/s40164-020-00172-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baycan OF, Barman HA, Atici A, et al. Evaluation of biventricular function in patients with COVID-19 using speckle tracking echocardiography. Int. J. Cardiovasc. Imaging. 2020;37(1):135–144. doi: 10.1007/s10554-020-01968-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berger JS, Kunichoff D, Adhikari S, et al. Prevalence and outcomes of D-dimer elevation in hospitalized patients with COVID-19. Arterioscler. Thromb. Vasc. Biol. 2020;40(10):2539–2547. doi: 10.1161/ATVBAHA.120.314872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhadade R, Harde M, deSouza R, et al. Appraisal of critically Ill COVID-19 patients at a dedicated COVID hospital. J. Assoc. Phys. India. 2020;68:14–19. [PubMed] [Google Scholar]
- 16.Bi X, Su Z, Yan H, et al. Prediction of severe illness due to COVID-19 based on an analysis of initial Fibrinogen to Albumin Ratio and Platelet count. Platelets. 2020;31:674–679. doi: 10.1080/09537104.2020.1760230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bossini N, Alberici F, Delbarba E, et al. Kidney transplant patients with SARS-CoV-2 infection: The Brescia Renal COVID task force experience. Am. J. Transplant. 2020;20(11):3019–3029. doi: 10.1111/ajt.16176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cariou B, Hadjadj S, Wargny M, et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: The CORONADO study. Diabetologia. 2020;63:1500–1515. doi: 10.1007/s00125-020-05180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cecconi M, Piovani D, Brunetta E, et al. Early predictors of clinical deterioration in a cohort of 239 patients hospitalized for Covid-19 infection in Lombardy, Italy. J. Clin. Med. 2020;9:1548. doi: 10.3390/jcm9051548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cen Y, Chen X, Shen Y, et al. Risk factors for disease progression in patients with mild to moderate coronavirus disease 2019—A multi-centre observational study. Clin. Microbiol. Infect. 2020;26:1242–1247. doi: 10.1016/j.cmi.2020.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen L, Yu J, He W, et al. Risk factors for death in 1859 subjects with COVID-19. Leukemia. 2020;34:2173–2183. doi: 10.1038/s41375-020-0911-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen LY, Chu HK, Bai T, et al. Liver damage at admission is an independent prognostic factor for COVID-19. J. Dig. Dis. 2020 doi: 10.1111/1751-2980.12925. [DOI] [PubMed] [Google Scholar]
- 23.Chen R, Sang L, Jiang M, et al. Longitudinal hematologic and immunologic variations associated with the progression of COVID-19 patients in China. J. Allergy Clin. Immunol. 2020;146:89–100. doi: 10.1016/j.jaci.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ. 2020 doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng A, Hu L, Wang Y, et al. Diagnostic performance of initial blood urea nitrogen combined with D-dimer levels for predicting in-hospital mortality in COVID-19 patients. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.106110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chilimuri S, Sun H, Alemam A, et al. Predictors of mortality in adults admitted with COVID-19: Retrospective cohort study from New York City. West J. Emerg. Med. 2020;21:779–784. doi: 10.5811/westjem.2020.6.47919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coppelli A, Giannarelli R, Aragona M, et al. Hyperglycemia at hospital admission is associated with severity of the prognosis in patients hospitalized for COVID-19: The Pisa COVID-19 study. Diabetes Care. 2020;43(10):2345–2348. doi: 10.2337/dc20-1380. [DOI] [PubMed] [Google Scholar]
- 28.Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: A prospective cohort study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naymagon L, Zubizarreta N, Feld J, et al. Admission D-dimer levels, D-dimer trends, and outcomes in COVID-19. Thromb. Res. 2020 doi: 10.1016/j.thromres.2020.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Micco P, Russo V, Carannante N, et al. Clotting factors in COVID-19: Epidemiological association and prognostic values in different clinical presentations in an Italian Cohort. J. Clin. Med. 2020;9:1371. doi: 10.3390/jcm9051371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dong Y, Zhou H, Li M, et al. A novel simple scoring model for predicting severity of patients with SARS-CoV-2 infection. Transbound. Emerg. Dis. 2020;67(6):2823–2829. doi: 10.1111/tbed.13651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duan J, Wang X, Chi J, et al. Correlation between the variables collected at admission and progression to severe cases during hospitalization among patients with COVID-19 in Chongqing. J. Med. Virol. 2020;92(11):2616–2622. doi: 10.1002/jmv.26082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng X, Li P, Ma L, et al. Clinical characteristics and short-term outcomes of severe patients with COVID-19 in Wuhan, China. Front. Med. 2020;7:491. doi: 10.3389/fmed.2020.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao Y, Li T, Han M, et al. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J. Med. Virol. 2020;92:791–796. doi: 10.1002/jmv.25770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.GhaffariRahbar M, Nafar M, Khoshdel A, et al. Low rate of COVID-19 pneumonia in kidney transplant recipients—A battle between infection and immune response? Transpl. Infect. Dis. 2020 doi: 10.1111/tid.13406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gormez S, Ekicibasi E, Degirmencioglu A, et al. Association between renin-angiotensin-aldosterone system inhibitor treatment, neutrophil-lymphocyte ratio, D-Dimer and clinical severity of COVID-19 in hospitalized patients: A multicenter, observational study. J. Hum. Hypertens. 2020;35:1–10. doi: 10.1038/s41371-020-00405-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanif A, Khan S, Mantri N, et al. Thrombotic complications and anticoagulation in COVID-19 pneumonia: A New York City hospital experience. Ann. Hematol. 2020;99:2323–2328. doi: 10.1007/s00277-020-04216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harmouch F, Shah K, Hippen JT, Kumar A, Goel H. Is it all in the heart? Myocardial injury as major predictor of mortality among hospitalized COVID-19 patients. J. Med. Virol. 2020;93(2):973–982. doi: 10.1002/jmv.26347. [DOI] [PubMed] [Google Scholar]
- 39.Huang H, Zhang M, Chen C, et al. Clinical Characteristics of COVID-19 in patients with pre-existing ILD: A retrospective study in a single center in Wuhan, China. J. Med. Virol. 2020 doi: 10.1002/jmv.26174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li C, Jiang J, Wang F, et al. Longitudinal correlation of biomarkers of cardiac injury, inflammation, and coagulation to outcome in hospitalized COVID-19 patients. J. Mol. Cell Cardiol. 2020;147:74. doi: 10.1016/j.yjmcc.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li G, Deng Q, Feng J, Li F, Xiong N, He Q. Clinical characteristics of diabetic patients with COVID-19. J. Diabetes Res. 2020;2020:1–5. doi: 10.1155/2020/1652403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li K, Li K, Chen D, et al. Predictors of fatality including radiographic findings in adults with COVID-19. Respir. Res. 2020 doi: 10.1186/s12931-020-01411-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Q, Cao Y, Chen L, et al. Hematological features of persons with COVID-19. Leukemia. 2020;34:2163–2172. doi: 10.1038/s41375-020-0910-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li T, Lu L, Zhang W, et al. Clinical characteristics of 312 hospitalized older patients with COVID-19 in Wuhan, China. Arch. Gerontol. Geriatr. 2020;91:104185. doi: 10.1016/j.archger.2020.104185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Y, Zhao K, Wei H, et al. Dynamic relationship between D-dimer and COVID-19 severity. Br. J. Haematol. 2020;190:e24–e27. doi: 10.1111/bjh.16797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Y, Han X, Alwalid O, et al. Baseline characteristics and risk factors for short-term outcomes in 132 COVID-19 patients with diabetes in Wuhan China: A retrospective study. Diabetes Res. Clin. Pract. 2020;166:108299. doi: 10.1016/j.diabres.2020.108299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu F, Zhang Q, Huang C, et al. CT quantification of pneumonia lesions in early days predicts progression to severe illness in a cohort of COVID-19 patients. Theranostics. 2020;10:5613–5622. doi: 10.7150/thno.45985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu J, Han P, Wu J, Gong J, Tian D. Prevalence and predictive value of hypocalcemia in severe COVID-19 patients. J. Infect. Public Health. 2020;13:1224. doi: 10.1016/j.jiph.2020.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu J, Zhang S, Wu Z, et al. Clinical outcomes of COVID-19 in Wuhan, China: A large cohort study. Ann. Intensive Care. 2020 doi: 10.1186/s13613-020-00706-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Q, Chen H, Li J, et al. Fasting blood glucose predicts the occurrence of critical illness in COVID-19 patients: A multicenter retrospective cohort study. J. Infect. 2020;81:e20–e23. doi: 10.1016/j.jinf.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu Q, Song NC, Zheng ZK, Li JS, Li SK. Laboratory findings and a combined multifactorial approach to predict death in critically ill patients with COVID-19: A retrospective study. Epidemiol. Infect. 2020 doi: 10.1017/S0950268820001442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu S, Zhang Q, Wang W, et al. Hyperglycemia is a strong predictor of poor prognosis in COVID-19. Diabetes Res. Clin. Pract. 2020;167:108338. doi: 10.1016/j.diabres.2020.108338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu T, Zhang J, Yang Y, et al. The role of interleukin-6 in monitoring severe case of coronavirus disease 2019. EMBO Mol. Med. 2020 doi: 10.15252/emmm.202012421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu X, Li Z, Liu S, et al. Potential therapeutic effects of dipyridamole in the severely ill patients with COVID-19. Acta Pharm. Sin. B. 2020;10:1205–1215. doi: 10.1016/j.apsb.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Long H, Nie L, Xiang X, et al. D-dimer and prothrombin time are the significant indicators of severe COVID-19 and poor prognosis. Biomed. Res. Int. 2020 doi: 10.1155/2020/6159720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alnor A, Sandberg MB, Gils C, Vinholt PJ. Laboratory tests and outcome for patients with coronavirus disease 2019: A systematic review and meta-analysis. J. Appl. Lab. Med. 2020;5:1038–1049. doi: 10.1093/jalm/jfaa098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paliogiannis P, Mangoni AA, Dettori P, et al. D-dimer concentrations and COVID-19 severity: A systematic review and meta-analysis. Front. Public Health. 2020;8:432. doi: 10.3389/fpubh.2020.00432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ghayda RA, Lee J, Lee JY, et al. Correlations of clinical and laboratory characteristics of COVID-19: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health. 2020;17:5026. doi: 10.3390/ijerph17145026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ghahramani S, Tabrizi R, Lankarani KB, et al. Laboratory features of severe vs. non-severe COVID-19 patients in Asian populations: A systematic review and meta-analysis. Eur. J. Med. Res. 2020;25:30. doi: 10.1186/s40001-020-00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Soraya GV, Ulhaq ZS. Crucial laboratory parameters in COVID-19 diagnosis and prognosis: An updated meta-analysis. Med. Clin. (Barc.) 2020;155:143–151. doi: 10.1016/j.medcli.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance. January 28, 2020. https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf.
- 62.National Health Commission & National Administration of Traditional Chinese Medicine Diagnosis and treatment protocol for novel coronavirus pneumonia (trial version 7) Chin. Med. J. 2020;133:1087–1095. doi: 10.1097/CM9.0000000000000819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grooten WJA, Tseli E, Äng BO, et al. Elaborating on the assessment of the risk of bias in prognostic studies in pain rehabilitation using QUIPS—Aspects of interrater agreement. Diagn. Progn. Res. 2019;3:5. doi: 10.1186/s41512-019-0050-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maeda T, Obata R, Rizk DOD, Kuno T. The association of interleukin-6 value, interleukin inhibitors, and outcomes of patients with COVID-19 in New York City. J. Med. Virol. 2020;93(1):463–471. doi: 10.1002/jmv.26365. [DOI] [PubMed] [Google Scholar]
- 65.Mikami T, Miyashita H, Yamada T, et al. Risk factors for mortality in patients with COVID-19 in New York City. J. Gen. Intern. Med. 2020;36:1–10. doi: 10.1007/s11606-020-05983-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moreno-Pérez O, Andres M, Leon-Ramirez JM, et al. Experience with tocilizumab in severe COVID-19 pneumonia after 80 days of follow-up: A retrospective cohort study. J. Autoimmun. 2020;114:102523. doi: 10.1016/j.jaut.2020.102523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pan F, Yang L, Li Y, et al. Factors associated with death outcome in patients with severe coronavirus disease-19 (Covid-19): A case-control study. Int. J. Med. Sci. 2020;17:1281–1292. doi: 10.7150/ijms.46614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Paranjpe I, Russak A, De Freitas JK, et al. Clinical characteristics of hospitalized Covid-19 patients in New York City. medRxiv. 2020 doi: 10.1101/2020.04.19.20062117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: Prospective cohort study. BMJ. 2020 doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qin W, Hu BZ, Zhang Z, et al. Clinical characteristics and death risk factors of severe COVID-19. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:648–653. doi: 10.3760/cma.j.cn112147-20200320-00380. [DOI] [PubMed] [Google Scholar]
- 71.Laguna-Goya R, Utrero-Rico A, Talayero P, et al. IL-6-based mortality risk model for hospitalized patients with COVID-19. J. Allergy Clin. Immunol. 2020 doi: 10.1016/j.jaci.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sardu C, Marfella R, Maggi P, et al. Implications of AB0 blood group in hypertensive patients with covid-19. BMC Cardiovasc. Disord. 2020 doi: 10.1186/s12872-020-01658-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sciascia S, Aprà F, Baffa A, et al. Pilot prospective open, single-arm multicentre study on off-label use of tocilizumab in patients with severe COVID-19. Clin. Exp. Rheumatol. 2020;38:529–532. [PubMed] [Google Scholar]
- 74.Shang Y, Liu T, Wei Y, et al. Scoring systems for predicting mortality for severe patients with COVID-19. EClinicalMedicine. 2020 doi: 10.1016/j.eclinm.2020.100426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sisó-Almirall A, Kostov B, Mas-Heredia M, et al. Prognostic factors in Spanish COVID-19 patients: A case series from Barcelona. PLoS ONE. 2020;15:e0237960. doi: 10.1371/journal.pone.0237960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smadja DM, Guerin CL, Chocron R, et al. Angiopoietin-2 as a marker of endothelial activation is a good predictor factor for intensive care unit admission of COVID-19 patients. Angiogenesis. 2020;1:1. doi: 10.1007/s10456-020-09730-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Somers EC, Eschenauer GA, Troost JP, et al. Tocilizumab for treatment of mechanically ventilated patients with COVID-19. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sun Y, Dong Y, Wang L, et al. Characteristics and prognostic factors of disease severity in patients with COVID-19: The Beijing experience. J. Autoimmun. 2020;112:102473. doi: 10.1016/j.jaut.2020.102473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tao Z, Xu J, Chen W, et al. Anaemia is associated with severe illness in COVID-19: A retrospective cohort study. J. Med. Virol. 2020;93(3):1478–1488. doi: 10.1002/jmv.26444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tian J, Yuan X, Xiao J, et al. Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: A multicentre, retrospective, cohort study. Lancet Oncol. 2020;21:893–903. doi: 10.1016/S1470-2045(20)30309-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Volo T, Stritoni P, Battel I, et al. Elective tracheostomy during COVID-19 outbreak: To whom, when, how? Early experience from Venice, Italy. Eur. Arch. Oto-Rhino-Laryngol. 2020 doi: 10.1007/s00405-020-06190-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang F, Hou H, Luo Y, et al. The laboratory tests and host immunity of COVID-19 patients with different severity of illness. JCI Insight. 2020 doi: 10.1172/JCI.INSIGHT.137799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang F, Hou H, Wang T, et al. Establishing a model for predicting the outcome of COVID-19 based on combination of laboratory tests. Travel Med. Infect. Dis. 2020 doi: 10.1016/j.tmaid.2020.101782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang K, Zuo P, Liu Y, et al. Clinical and laboratory predictors of in-hospital mortality in patients with coronavirus disease-2019: A cohort study in Wuhan, China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang M, Zhu Q, Fu J, Liu L, Xiao M, Du Y. Differences of inflammatory and non-inflammatory indicators in Coronavirus disease-19 (COVID-19) with different severity. Infect. Genet. Evol. 2020;85:104511. doi: 10.1016/j.meegid.2020.104511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang T, Tang C, Chen R, et al. Clinical features of coronavirus disease 2019 patients with mechanical ventilation. Crit. Care Med. 2020 doi: 10.1097/CCM.0000000000004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang Y, Zhou Y, Yang Z, Xia D, Hu Y, Geng S. Clinical characteristics of patients with severe pneumonia caused by the SARS-CoV-2 in Wuhan, China. Respiration. 2020;99:1–9. doi: 10.1159/000500727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Watanabe M, Caruso D, Tuccinardi D, et al. Visceral fat shows the strongest association with the need of intensive Care in Patients with COVID-19. Metabolism. 2020;111:154319. doi: 10.1016/j.metabol.2020.154319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wendel Garcia PD, Fumeaux T, Guerci P, et al. Prognostic factors associated with mortality risk and disease progression in 639 critically ill patients with COVID-19 in Europe: Initial report of the international RISC-19-ICU prospective observational cohort. EClinicalMedicine. 2020 doi: 10.1016/j.eclinm.2020.100449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xia P, Wen Y, Duan Y, et al. Clinicopathological features and outcomes of acute kidney injury in critically ill COVID-19 with prolonged disease course: A retrospective cohort. J. Am. Soc. Nephrol. 2020;31:2205–2221. doi: 10.1681/ASN.2020040426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xie J, Wu W, Li S, et al. Clinical characteristics and outcomes of critically ill patients with novel coronavirus infectious disease (COVID-19) in China: A retrospective multicenter study. Intensive Care Med. 2020;46(10):1863–1872. doi: 10.1007/s00134-020-06211-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xiong B, Liu T, Luo P, et al. Prominent hypercoagulability associated with inflammatory state among cancer patients with SARS-CoV-2 infection. Front. Oncol. 2020;10:1345. doi: 10.3389/fonc.2020.01345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang AP, Li HM, Tao WQ, et al. Infection with SARS-CoV-2 causes abnormal laboratory results of multiple organs in patients. Aging (Albany, NY) 2020;12:10059–10069. doi: 10.18632/aging.103255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang Q, Zhou Y, Wang X, et al. Effect of hypertension on outcomes of adult inpatients with COVID-19 in Wuhan, China: A propensity score-matching analysis. Respir. Res. 2020;21:172. doi: 10.1186/s12931-020-01435-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yao Q, Wang P, Wang X, et al. A retrospective study of risk factors for severe acute respiratory syndrome coronavirus 2 infections in hospitalized adult patients. Polish Arch. Intern. Med. 2020;130:390–399. doi: 10.20452/pamw.15312. [DOI] [PubMed] [Google Scholar]
- 97.Yao Y, Cao J, Wang Q, et al. D-dimer as a biomarker for disease severity and mortality in COVID-19 patients: A case control study. J. Intensive Care. 2020;8:49. doi: 10.1186/s40560-020-00466-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yu C, Lei Q, Li W, Wang X, Li W, Liu W. Epidemiological and clinical characteristics of 1663 hospitalized patients infected with COVID-19 in Wuhan, China: A single-center experience. J. Infect. Public Health. 2020;13:1202. doi: 10.1016/j.jiph.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yu HH, Qin C, Chen M, Wang W, Tian DS. D-dimer level is associated with the severity of COVID-19. Thromb. Res. 2020;195:219–225. doi: 10.1016/j.thromres.2020.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zeng D-X, Xu J-L, Mao Q-X, et al. Association of Padua prediction score with in-hospital prognosis in COVID-19 patients. QJM. 2020 doi: 10.1093/qjmed/hcaa224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zeng Z, Ma Y, Zeng H, et al. Simple nomogram based on initial laboratory data for predicting the probability of ICU transfer of COVID-19 patients: Multicenter retrospective study. J. Med. Virol. 2020 doi: 10.1002/jmv.26244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhan T, Liu M, Tang Y, et al. Retrospective analysis of clinical characteristics of 405 patients with COVID-19. J. Int. Med. Res. 2020;48:1–10. doi: 10.1177/0300060520949039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang G, Zhang J, Wang B, Zhu X, Wang Q, Qiu S. Analysis of clinical characteristics and laboratory findings of 95 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A retrospective analysis. Respir. Res. 2020 doi: 10.1186/s12931-020-01338-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang J, Dong X, Cao Y, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy Eur. J. Allergy Clin. Immunol. 2020;75:1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 105.Zhang J, Cao Y, Tan G, et al. Clinical, radiological and laboratory characteristics and risk factors for severity and mortality of 289 hospitalized COVID-19 patients. Allergy. 2020 doi: 10.1111/all.14496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang J, Liu P, Wang M, et al. The clinical data from 19 critically ill patients with coronavirus disease 2019: a single-centered, retrospective, observational study. J. Public Health. 2020 doi: 10.1007/s10389-020-01291-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang L, Yan X, Fan Q, et al. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J. Thromb. Haemost. 2020;18:1324–1329. doi: 10.1111/jth.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang SX, Li J, Zhou P, et al. The analysis of clinical characteristics of 34 novel coronavirus pneumonia cases in Ningxia Hui autonomous region. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:431–436. doi: 10.3760/cma.j.cn112147-20200219-00121. [DOI] [PubMed] [Google Scholar]
- 109.Zhao J, Gao HY, Feng ZY, Wu QJ. A retrospective analysis of the clinical and epidemiological characteristics of COVID-19 patients in Henan Provincial People’s Hospital, Zhengzhou, China. Front. Med. 2020;7:286. doi: 10.3389/fmed.2020.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhong ZF, Huang J, Yang X, et al. Epidemiological and clinical characteristics of COVID-19 patients in Hengyang, Hunan Province, China. World J. Clin. Cases. 2020;8:2554–2565. doi: 10.12998/wjcc.v8.i12.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zou Y, Guo H, Zhang Y, et al. Analysis of coagulation parameters in patients with COVID-19 in Shanghai, China. Biosci. Trends. 2020 doi: 10.5582/bst.2020.03086. [DOI] [PubMed] [Google Scholar]
- 112.Chen Q, Xu L, Dai Y, et al. Cardiovascular manifestations in severe and critical patients with COVID-19. Clin. Cardiol. 2020;43:796–802. doi: 10.1002/clc.23384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Francone M, Iafrate F, Masci GM, et al. Chest CT score in COVID-19 patients: correlation with disease severity and short-term prognosis. Eur. Radiol. 2020;30(12):6808–6817. doi: 10.1007/s00330-020-07033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gavin W, Campbell E, Zaidi S-A, et al. Clinical characteristics, outcomes and prognosticators in adult patients hospitalized with COVID-19. Am. J. Infect. Control. 2020;49(2):158–165. doi: 10.1016/j.ajic.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Guo T, Shen Q, Guo W, et al. Clinical characteristics of elderly patients with COVID-19 in Hunan Province, China: A multicentre, retrospective study. Gerontology. 2020;66:1–9. doi: 10.1159/000508734. [DOI] [PubMed] [Google Scholar]
- 116.Huang D, Wang T, Chen Z, Yang H, Yao R, Liang Z. A novel risk score to predict diagnosis with coronavirus disease 2019 (COVID-19) in suspected patients: A retrospective, multicenter, and observational study. J. Med. Virol. 2020;92(11):2709–2717. doi: 10.1002/jmv.26143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li X, Wang L, Yan S, et al. Clinical characteristics of 25 death cases with COVID-19: A retrospective review of medical records in a Single Medical Center, Wuhan, China. Int. J. Infect. Dis. 2020;94:128–132. doi: 10.1016/j.ijid.2020.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ma S, Lai X, Chen Z, Tu S, Qin K. Clinical characteristics of critically ill patients co-infected with SARS-CoV-2 and the influenza virus in Wuhan, China. Int. J. Infect. Dis. 2020;96:683–687. doi: 10.1016/j.ijid.2020.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Martín-Rojas RM, Pérez-Rus G, Delgado-Pinos VE, et al. COVID-19 coagulopathy: An in-depth analysis of the coagulation system. Eur. J. Haematol. 2020 doi: 10.1111/ejh.13501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wu Y, Hou B, Liu J, Chen Y, Zhong P. Risk factors associated with long-term hospitalization in patients with COVID-19: A single-centered, retrospective study. Front. Med. 2020;7:315. doi: 10.3389/fmed.2020.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ye W, Chen G, Li X, et al. Dynamic changes of D-dimer and neutrophil-lymphocyte count ratio as prognostic biomarkers in COVID-19. Respir. Res. 2020;21:169. doi: 10.1186/s12931-020-01428-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang B, Zhou X, Qiu Y, et al. Clinical characteristics of 82 cases of death from COVID-19. PLoS ONE. 2020 doi: 10.1371/journal.pone.0235458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhou Y, Zhang Z, Tian J, Xiong S. Risk factors associated with disease progression in a cohort of patients infected with the 2019 novel coronavirus. Ann. Palliat. Med. 2020;9:428–436. doi: 10.21037/apm.2020.03.26. [DOI] [PubMed] [Google Scholar]
- 124.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wu YP, Wei R, Liu ZH, et al. Analysis of thrombotic factors in severe acute respiratory syndrome (SARS) patients. Thromb. Haemost. 2006;96:100–101. doi: 10.1160/TH06-04-0219. [DOI] [PubMed] [Google Scholar]
- 126.Grimmer B, Kuebler WM. The endothelium in hypoxic pulmonary vasoconstriction. J. Appl. Physiol. 2017;123:1635–1646. doi: 10.1152/japplphysiol.00120.2017. [DOI] [PubMed] [Google Scholar]
- 127.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhao YM, Shang YM, Song WB, et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020;25:100463. doi: 10.1016/j.eclinm.2020.100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Iba T, Levy JH, Warkentin TE, et al. Diagnosis and management of sepsis-induced coagulopathy and disseminated intravascular coagulation. J. Thromb. Haemost. 2019;17:1989–1994. doi: 10.1111/jth.14578. [DOI] [PubMed] [Google Scholar]
- 131.Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Paranjpe I, Fuster V, Lala A, et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients With COVID-19. J. Am. Coll. Cardiol. 2020;76:122–124. doi: 10.1016/j.jacc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang P, Qu Y, Tu J, et al. Applicability of bedside ultrasonography for the diagnosis of deep venous thrombosis in patients with COVID-19 and treatment with low molecular weight heparin. J. Clin. Ultrasound. 2020 doi: 10.1002/jcu.22898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wichmann D, Sperhake JP, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID-19: A prospective cohort study. Ann. Intern. Med. 2020;173:268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Thachil J, Longstaff C, Favaloro EJ, et al. The need for accurate D-dimer reporting in COVID-19: Communication from the ISTH SSC on fibrinolysis. J. Thromb. Haemost. 2020;18:2408–2411. doi: 10.1111/jth.14956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Varikasuvu SR, Varshney S, Dutt N. Markers of coagulation dysfunction and inflammation in diabetic and non-diabetic COVID-19. J. Thromb. Thrombolysis. 2021;51:941–946. doi: 10.1007/s11239-020-02270-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hariyanto TI, Japar KV, Kwenandar F, et al. Inflammatory and hematologic markers as predictors of severe outcomes in COVID-19 infection: A systematic review and meta-analysis. Am. J. Emerg. Med. 2021;41:110–119. doi: 10.1016/j.ajem.2020.12.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.