Abstract
Severe acute respiratory syndrome (SARS) is a highly contagious viral respiratory illness. This illness is spurred on by a coronavirus known as SARS-associated coronavirus (SARS-CoV). SARS was first detected in Asia in late February 2003. The genome of this virus is very similar to the SARS-CoV-2. Therefore, the study of SARS-CoV disease and the identification of effective drugs to treat this disease can be new clues for the treatment of SARS-Cov-2. This study aimed to discover novel potential drugs for SARS-CoV disease in order to treating SARS-Cov-2 disease based on a novel systems biology approach. To this end, gene co-expression network analysis was applied. First, the gene co-expression network was reconstructed for 1441 genes, and then two gene modules were discovered as significant modules. Next, a list of miRNAs and transcription factors that target gene co-expression modules' genes were gathered from the valid databases, and two sub-networks formed of transcription factors and miRNAs were established. Afterward, the list of the drugs targeting obtained sub-networks' genes was retrieved from the DGIDb database, and two drug-gene and drug-TF interaction networks were reconstructed. Finally, after conducting different network analyses, we proposed five drugs, including FLUOROURACIL, CISPLATIN, SIROLIMUS, CYCLOPHOSPHAMIDE, and METHYLDOPA, as candidate drugs for SARS-CoV-2 coronavirus treatment. Moreover, ten miRNAs including miR-193b, miR-192, miR-215, miR-34a, miR-16, miR-16, miR-92a, miR-30a, miR-7, and miR-26b were found to be significant miRNAs in treating SARS-CoV-2 coronavirus.
Subject terms: Computational models, Gene ontology, Microarrays, Drug screening
Introduction
Coronaviruses infect both animals and humans, causing intestinal and respiratory infections1,2. Severe acute respiratory syndrome (SARS) is a coronavirus-associated respiratory disease that was originally discovered in China in February 20033. Ten years after SARS coronavirus, Middle East respiratory syndrome (MERS) coronavirus was broke out in Middle Eastern countries2,4,5. SARS-CoV and MERS uses angiotensin-converting enzyme 2 (ACE2) and dipeptidyl peptidase 4(DPP4) as a receptor, respectively2. Additionally, in the autumn of 2019, the coronavirus SARS-CoV-2 broke out in the Chinese city of Wuhan4,6. Given that, there is a high similarity between SARS-CoV and SARS-CoV-2, the spread speed of SARS-CoV-2 is faster than SARS-CoV4. SARS-CoV-2 like SARS-CoV utilize the host cell ACE2 receptor4. ACE2 is a membrane receptor on the surface of many cell types and tissues including the lungs, heart, blood vessels, kidney, liver, and gastrointestinal2,4. SARS-CoV, SARS-CoV-2, and MERS all have similar genetic characteristics, and SARS-CoV-2 is very similar to SARS-CoV7. Due to the high genetic similarity of SARS-CoV and SARS-CoV-2, the results of the study on SARS-CoV can be a clue to the treatment of SARS-CoV-2. Due to the disease's significance and high death rate, early detection and treatment are essential. The medicinal drugs used to treat coronavirus infections are only intended to be used temporarily. Besides, clinical trials on medications and vaccines that are efficient in curing diseases take quite a long time. Furthermore, handling SARS viruses in vivo is often challenging and risky. However, the knowledge obtained by sequencing their genes, proteins, or RNA is simple and easy to manage through artificial intelligence 8. Additionally, miRNA-mRNA data sources have progressed considerably as prospective techniques for gaining a better understanding of potential SARS-CoV therapies, allowing network science and computational systems biology to become feasible9. According to these data, many scientists seek to identify the involved host genes and proteins in diseases to find a new therapy.
Recent research has identified a set of antiviral genes, such as ISG15, IFIH1, MX1, OAS1-3, IRF7, IRF9, and STAT1 expressed by host cells, which could be used as a new therapeutic target against coronavirus due to their response to viral infection10–18. In addition to therapeutic gene targets, miRNAs can also be used to suppress the viral genome due to their ability to regulate gene expression. MiRNAs are small non-coding RNA molecules that prevent mRNAs from being translated19,20. Therefore, miRNA-based therapy could be proposed for SARS-CoV treatment21,22. On the other hand, the role of pathologic processes in miRNAs, such as inflammatory responses and viral infection, has been recently verified21,23,24.
Drug repurposing (DR) is a strategy for identifying new therapeutic uses for approved or investigational drugs25,26. This approach is also referred to as drug reprofiling, drug re‑tasking, drug repositioning, drug therapeutic and drug recyclining26. It is an efficient approach for the development or discovery of drug molecules with new therapeutic indications25. Generally, the process of drug repurposing consists of three steps26: 1. identification of a candidate molecule for a particular indication. 2. mechanistic evaluation of the drug effect in preclinical models. 3. evaluation of efficacy in phase clinical trials II. Of these three steps, step 1 is crucial, and it is here that modern approaches to hypothesising may be most useful26. These systematic approaches can be divided into experimental and computational approaches26. Some of the computational approaches are: molecular docking, signature machiing, genetic association, network mapping, retrospective clinical analysis and novel data sources26. As well as, among the experimental methods, Binding assays to identify relevant target interactions and Phenotypic screening approaches can be mentioned26.
Recently, different articles based on network approaches have been published for drug repurposing27. SAveRUNNER28 is a network-based drug repurposing algorithm, which predicts drug-disease assosiations using network-based similarity measure. This algorithm provided as a freely available R-code29. SAveRUNNER is also been used as a drug repurposing tool for amyotrophic lateral sclerosis (ALS) disease30. Pasquale and colleaqes31, examined three different network-based approaches and identified 399 repurposable drugs for COVID-19 using SAveRUNNER algorithm. Another algorithm based on artificial intelligence, network diffusion, and network proximity introduced as a drug repurposing method for SARS-CoV-2 disease32. We also recently introduced a protein–protein interaction network approach in order to propose candidate drugs to treatment of SARS-CoV-2 disease21.
Tasnimul and colleague, recently introduced a network-based method for identifying and repurposing drugs for the treatment of SARS-CoV-2 disease33. In this method, differentially expressed genes between Idiopathic pulmonary fibrosis (IPF) and SARS-CoV-2 samples were compared and finally, some IPF drugs were proposed as candidate drugs to treat SARS-CoV-2 disease. Yadi et al.34 proposed a novel network-based drug repurposing methodology based on human interactome and protein–protein interaction networks. This method quntify the interplay between the drug targets and HCoV–host interactome in the human protein–protein interaction network. In this study, 16 potential drugs was introduced to treat SARS-CoV-2 disease. In another study, Hangyu and colleaque35 developed a machine-learning -based method to predict virus-host interactions at both organism and protein levels for SARS-Cov-2 disease. In this method, a multi-layer virus-host interaction network was constructed. CoVex36, an interactive online platform for SARS-CoV-2, introduced by Sepideh and colleaque. This platform, integrates human protein–protein interactions, virus-human protein interactions and drug-target interactions. Zhihao and colleaque37 constructed a autophagy interaction network based on competitive endogenous RNA(ceRNA) in SARS-CoV-2 infection. In this study, hsa-miR-4772–5p, hsa-miR- 192–5p, hsa-miR-652–3p, hsa-miR- 192–5p, hsa-miR-340–3p, CCR2 and TP53INP2 introduced as potential biomarkers in predicting changes in mild SARS-CoV-2 infection. In comparison to the mentioned network-based methods, in our study, the gene co-expression network is used. In addition to the gene co-expression network, regulatory interactions including miRNA-Gene, TF-Gene, and TF-miRNA have also been used in our study. Moreover, drug-gene and drug-TF interaction networks have been studied and investigated in this study. As well as, some of the genes that regulate more miRNAs, are also introduced as effective miRNAs in SARS-Cov disease. Changes in the expression of these genes can affect the expression of target miRNAs.
The current research aimed to discover the genes and miRNAs involved in SARS-CoV disease and repurpose candidate drugs for this diease in order to treating SARS-CoV-2 coronavirus based on a co-expression network analysis. In this regard, this study used a co-expression network analysis to identify potential drugs for the treatment of SARS-CoV. The methodology we used in this study is an entirely novel method based on gene module identification.
The technique first entails identifying a list of genes (human genes) expressed differentially in healthy and SARSCoV-infected samples. After obtaining differentially expressed genes between healthy and SARSCoV-infected samples, the co-expression network is reconstructed in STRING online tool38. Then, two significant gene modules are discovered from the gene co-expression network. Afterward, a list of miRNAs and transcription factor genes that have a regulatory impact on modules' genes are collected from a valid database (TRRUST v239 and miRWalk v240), and different network analyses are done on these biomolecules. Finally, the list of drugs that target modules' genes are gathered from the (DGIdb)41 database, and then two drug-gene interaction networks are reconstructed. The workflow diagram of this study is demonstrated in Fig. 1. As shown in this figure, the method's output is some candidate drugs for the treatment of SARS coronavirus. In this study, FLUOROURACIL, CISPLATIN, SIROLIMUS, CYCLOPHOSPHAMIDE, and METHYLDOPA are the key drugs reported for treating SARS-CoV-2 coronavirus. Moreover, hsa-miR-193b, hsa-miR-192, hsa-miR-215, hsa-miR-34a, hsa-miR-16, hsa-miR-16, hsa-miR-92a, hsa-miR-30a, hsa-miR-7, and hsa-miR-26b are candidate miRNAs, which are significant in the treatment of SARS-CoV-2 disease.
Figure 1.
The overall workflow of the proposed method. In this method, a network-based approach is applied to drug repurposing for coronavirus disease treatment. (a) At first, a transcriptome profile for healthy (control) and SARS-CoV-infected samples were taken from the GEO database with the accession number GSE1739. (b) Then, after identifying differentially expressed genes in the control and disease groups, the gene co-expression network is reconstructed, and two significant gene modules are discovered from the co-expression network. (c) Next, for every gene module, the TF-miRNA-TG network is reconstructed independently. The information of TFs-miRNAs, TFs-TGs, and miRNAs-TGs regulations are taken from the TransmiR42, TRRUST39, and miRWalk40 databases, respectively. (d) Afterward, Drug-gene and Drug-TF networks are reconstructed for TF-miRNA-TG networks independently. (e) Finally, 19 drugs are proposed as candidate drugs for coronavirus treatment.
Result
Gene co-expression network analysis and gene modulation.
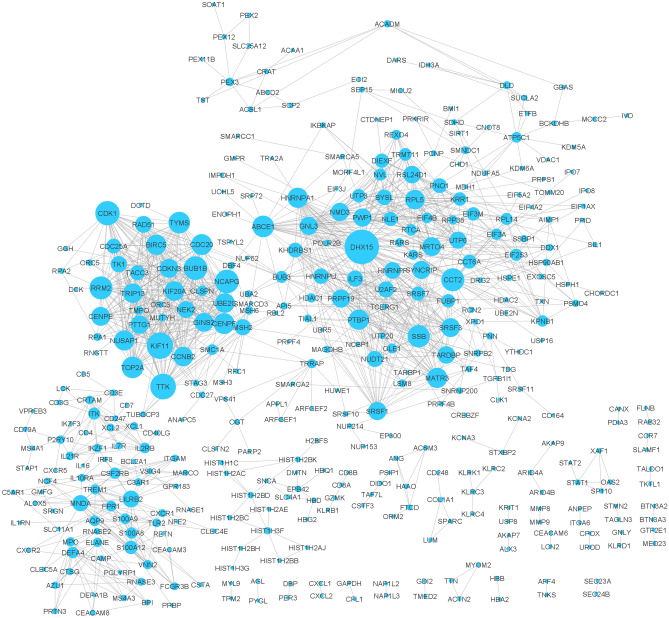
First 1441 differentially expressed genes between normal and SARS infected groups with p-values less than 0.05 were assumed as primary genes. Then, using this primary gene list, the gene co-expression network was reconstructed in the STRING database. In this network 1050 genes out of 1441 primary genes were disconnected. Therefore, these disconnected genes were removed from the network and a network with 391 genes was obtained. Figure 2 shows the co-expression network for these genes. Supplementary file S6 contains more information on the topological characteristics of this network.
Figure 2.
Gene co-expression network for the primary genes (disconnected genes were removed from the network). The size of the nodes indicates its degree. There are 391 nodes and 1273 edges in this network. DHX15 is the highest degree node in this network.
After analyzing the network in Cytoscape software43, generally, 391 nodes and 1273 edges were observed in the reconstructed co-expression network. After clustering the gene co-expression network, we discovered two significant modules (Module A and Module B) in the co-expression network. The list of genes for these two modules is reported in Table 1.
Table 1.
Gene names of modules A and B.
| Module name | Gene names |
|---|---|
| Module A | TOP2A (24), TTK (24), NUSAP1(24), UBE2C(24), CENPF(24), KIF20A(24), CDK1(24), CDC20(24), BIRC5(24), TRIP13(23), NCAPG(24), GINS2(23), KIF11(24), CENPE(22), BUB1B(24), CCNB2(24), CDC25A(17), TACC3(17), RAD51(20), TYMS(22), CDKN3(22), RRM2(24), TK1(19), NEK2(19), PTTG1(22) |
| Module B | SRSF1(16), SYNCRIP(14), HNRNPU(16), DHX15(29), SSB(19), MATR3(17), GNL3(13), NUDT21(16), U2AF2(17), HNRNPF(17), SRSF7(15), SRSF3(17), KHDRBS1(14), FUBP1(13), PRPF19(14), BYSL(12), ILF3(17), NLE1(12), UTP6(12), RSL24D1(12), MRTO4(13), DIEXF(12), PTBP1(17), UTP3(12), PWP1(12), PNO1(12), KRR1(12), NMD3(12), HNRNPA1(17), TARDBP(17) |
Numbers inside the parentheses represent the genes degree.
Transcription factors, miRNAs, and target genes interaction network
At first two TF-miRNA-TG sub-networks named TF-miRNA-TG_A and TF-miRNA-TG_B were reconstructed for gene modules A and B, respectively. As demonstrated in previous section, two significant gene modules were considered for more analysis. To do this, the data of TFs-TGs, TFs-miRNAs, and miRNAs-TGs regulatory interactions were retrieved from the TRRUST, TransmiR, and miRWalk databases, respectively. These two sub-networks are shown in Figure S1 (See supplementary file S6). The information for TFs and TGs Interactions are activation, repression, and unknown. In addition, regulatory interactions information for TFs and TGs are: activation, activation(feedback), regulation, regulation(feedback), autoregulatory negative feedback, loop(feedback), repression, repression(feedback), auto-regulatory feedback circuit, and activation (negative regulatory loop). This information for TF-miRNA-TG_A and TF-miRNA-TG_B sub-networks (see supplementary file S7) are reported in supplementary file S2. Moreover, the topological properties for these sub-networks was reported in supplementary file S6.
In order to analyze the TFs, which regulate more Genes and miRNAs in the TF-miRNA-TG_A and TF-miRNA-TG_B networks, the high degree TF nodes were selected and reported as significant TFs. To do this, among TF nodes in the TF-miRNA-TG_A and TF-miRNA-TG_B sub-networks, the TFs with a degree of 10 and higher were selected (Table 2). The number of TGs and target miRNAs for every TF are listed in Table 2 as well.
Table 2.
High degree nodes of the TFs in the TF-miRNA-TG_A and B sub-networks.
| Module name | TF | Number of TGs | Number of target miRNAs |
|---|---|---|---|
| TF-miRNA-TG_A | MYC | 3 | 91 |
| TF-miRNA-TG_B | TP53 | 5 | 89 |
| MYC | 1 | 91 | |
| NFKB1 | 1 | 67 | |
| RELA | 1 | 59 | |
| STAT3 | 1 | 53 | |
| MYCN | 1 | 52 | |
| SP1 | 4 | 38 | |
| ESR1 | 1 | 30 | |
| E2F3 | 1 | 20 | |
| KLF4 | 1 | 18 | |
| CTNNB1 | 1 | 16 | |
| DNMT1 | 1 | 15 | |
| NANOG | 1 | 14 | |
| TP73 | 2 | 12 | |
| LEF1 | 1 | 12 | |
| MYB | 1 | 10 | |
| FOXO3 | 1 | 9 |
In addition to the TFs, a list of miRNAs that regulate more genes in the TF-miRNA-TG_A and TF-miRNA-TG_B networks were selected and reported as key miRNAs. To this end, for every TF-miRNA-TG network, five miRNAs with high degrees were assumed. These miRNAs for TF-miRNA-TG_A were hsa-miR-193b, hsa-miR-192, hsa-miR-215, hsa-miR-34a, and hsa-miR-16. For TF-miRNA-TG_B network, the selected miRNAs were hsa-miR-16, hsa-miR-92a, hsa-miR-30a, hsa-miR-7, and hsa-miR-26b. Among these miRNAs, hsa-miR-16 regulates more genes in both subnetworks. The list of these miRNAs, along with their degree and target genes, are reported in Table 3.
Table 3.
High degree nodes of the miRNAs in the TF-miRNA-TG_A and B sub-networks.
| miRNA | Degree | Target genes |
|---|---|---|
| TF-miRNA-TG_A | ||
| hsa-miR-193b | 13 | RRM2, RAD51, CDC25A, CDK1, CDC20, NCAPG, KIF11, TYMS, UBE2C, TACC3, GINS2, TRIP13, BUB1B |
| hsa-miR-192 | 10 | RAD51, CDC25A, CDC20, CENPF, CDKN3, KIF20A, TTK, TRIP13, BUB1B, CENPE |
| hsa-miR-215 | 9 | RAD51, CDC20, CENPF, CDKN3, KIF20A, TTK, TRIP13, BUB1B, CENPE |
| hsa-miR-34a | 7 | RRM2, CDC25A, BIRC5, CDC20, NCAPG, KIF11, TYMS, hsa-miR-34a |
| hsa-miR-16 | 7 | CDC25A, BIRC5, CDK1, CDC20, NCAPG, UBE2C, CENPF |
| TF-miRNA-TG_B | ||
| hsa-miR-16 | 8 | HNRNPF, HNRNPA1, SRSF1, NLE1, NMD3, DIEXF, UTP3, BYSL |
| hsa-miR-92a | 7 | HNRNPF, PTBP1, PNO1, NLE1, NMD3, KHDRBS1, U2AF2 |
| hsa-miR-30a | 6 | HNRNPA1, HRNPU, SRSF7, UTP6, PTBP1, PRPF19 |
| hsa-miR-7 | 4 | HNRNPU, MATR3, SRSF1, hsa-miR-7, ILF3 |
| hsa-miR-26b | 4 | MATR3, SYNCRIP, NMD3, GNL3 |
Enrichment analysis of genes
Gene ontology was performed for the module A and B gene lists, separately. The results for module A gene list show that they significantly enriched in mitotic cell cycle process, nuclear division, and mitotic nuclear division biological processes. Moreover, the gene list of module B significantly enriched in RNA processing, mRNA metabolic process, and RNA metabolic process biological processes. Then, a pathway enrichment analysis was done for modules A and B gene lists separately. The results showed that the module A and B genes, significantly enriched in Resolution of Sister Chromatid Cohesion and mRNA Splicing—Major Pathway pathways, respectively. More details of the GO and pathway enrichment analyses are reported in supplementary file S3.
Enrichment analysis of miRNAs
In order to check miRNA family for the TF-miRNA-TG_A and TF-miRNA-TG_B sub-networks, at first the list of miRNAs are imported into the TAM online tool. Then, the obtained result is reported in supplementary file S4. As reported in this file, both sub-networks significantly enriched in the let-7 and mir-17 families.
Drug-Gene interaction network
After gathering drug-gene interactions for TF-miRNA-TG_A and TF-miRNA-TG_B genes, the drug-gene interaction network was reconstructed and is demonstrated in Fig. 3. As shown in this network, some drugs have a high degree, which means that these drugs target and regulate more genes. Therefore, high-degree drugs were selected and reported as significant, as they regulate more genes in module_A and module_B. In this regard, the drugs with a degree of 3 or higher were selected and are reported in Table 4. As reported, FLUOROURACIL, EPIRUBICIN, and FOSTAMATINIB are effective drugs and targeted 4, 3, and 3 genes, respectively.
Figure 3.
The drug-gene interaction network for TF-miRNA-TG_A and TF-miRNA-TG_B sub-networks. Blue circles show TGs, and pink octagons show drugs. The size of the octagon nodes indicates its degree. The high degree drug is FLUOROURACIL with 4 target genes.
Table 4.
High degree drugs in the drug-gene network.
| Drug | Degree | Target genes |
|---|---|---|
| FLUOROURACIL | 4 | BIRC5, GNL3, TYMS, TOP2A |
| EPIRUBICIN | 3 | BIRC5, GNL3, TOP2A |
| FOSTAMATINIB | 3 | NEK2, CDK1, TTK |
Of all the drugs, only those with degree 3 or higher have been reported.
In addition to the Drug-gene interaction network, the drug-TF network was reconstructed to discover the drugs that target the transcription factors of the TF-miRNA-TG_A and TF-miRNA-TG_B sub-networks. This network is shown in Fig. 4. In this network, the drugs with a degree of 4 and higher were selected and reported as significant drugs. More details of the effective drugs are reported in Table 5. As the table shows, CISPLATIN, SIROLIMUS, and CYCLOPHOSPHAMIDE drugs targeted 11, 6, and 5 TFS, respectively, and the others targeted 4 TFs. The complete information of Drug-Gene and Drug-TF interactions are provided in supplementary file S5. Additionally, supplemental file S6 has detailed information on the network topological characteristics of the Drug-Gene and Drug-TF networks.
Figure 4.
Drug-TF interaction network for TF-miRNA-TG_A and TF-miRNA-TG_B sub-networks. Green triangle shapes show genes, and pink octagon shapes show drugs. The size of the octagon nodes indicates its degree. The high degree drug is CISPLATIN with 11 target genes.
Table 5.
High degree drugs in the Drug-TF network.
| Drug | Degree | Target TFs |
|---|---|---|
| CISPLATIN | 11 | BRCA2, DNMT1, E2F1, EHMT2, ESR1, MYC, MYCN, RB1, TP53, TP73 |
| SIROLIMUS | 6 | APC, RB1, RBL2, TCF7L2, TP53, WT1 |
| CYCLOPHOSPHAMIDE | 5 | BRCA2, CTNNB1, EHMT2, MYCN, TP53 |
| METHYLDOPA | 5 | EHMT2, ESR1, HDAC1, TP53, TP73 |
| VORINOSTAT | 4 | HDAC1, MYC, RB1, TP53 |
| OLAPARIB | 4 | BRCA2, ESR1, MYC, TP53 |
| MITOXANTRONE | 4 | DNMT1, EHMT2, NFKB1, TP53 |
| FLUOROURACIL | 4 | APC, E2F1, MYB, TP53 |
| EVEROLIMUS | 4 | BRCA2, CTNNB1, ESR1, RB1 |
| PACLITAXEL | 4 | BRCA2, E2F1, MYB, TP53 |
| DAUNORUBICIN | 4 | EHMT2, HDAC1, TP53, WT1 |
| ZINC CHLORIDE | 4 | ESR1, HDAC1, TP53, TP73 |
| METHOTREXATE | 4 | E2F1, EHMT2, RB1, TP53 |
| CARBOPLATIN | 4 | BRCA2, ETS2, TP53, TP73 |
| BORTEZOMIB | 4 | E2F1, NFKB1, RB1, TP53 |
| NICLOSAMIDE | 4 | APC, EHMT2, STAT3, TP53 |
| ETOPOSIDE | 4 | BRCA2, E2F1, MYCN, TP53 |
Of all the drugs, only those with degree 4 or higher have been reported.
Recently, the potential effect of 6710 drugs as SARS-CoV-2 inhibitors is tested in vitro and in vivo44. The results of this report show that some of the drugs proposed in this article (see Tables 4 and 5) have an inhibitory effect on SARS-CoV-2. According to this report, OLAPARIB, NICLOSAMID and METHOTREXATE have a very weak, weak, and strong effects on SARS-CoV-2, respectively. As well as, DAUNORUBICIN and BORTEZOMIB have a cytotoxic effect on this disease.
GSEA and candidate drugs validation
To evaluate the proposed drugs, the Connectivity Map(CMAP) analysis was utilized. To do this, from the Drug-Gene and Drug-TF networks, the drugs with a degree of 4 or above were selected and imported to Enrichr CMAP database. Then, the impact of these drugs on target genes was evaluated. Enrichr CMAP database contains CMAP-up and CMAP-down datasets. In Tables 3 and 4, from among the 19 repurposed drugs for SARS-CoV disease, only nine drugs, including SIROLIMUS, METHYLDOPA, VORINOSTAT, PACLITAXEL, DAUNORUBICIN, METHOTREXATE, NICLOSAMIDE, and ETOPOSIDE, were validated by CMAP analysis. Table 6 shows the validated drugs together with the corresponding unregulated or downregulated target genes.
Table 6.
The validated candidate drugs by CMAP analysis.
| Drug name | Gene names (↓:Downregulated and ↑: upregulated) |
|---|---|
| SIROLIMUS | TYMS (↑), NLE1 (↑), CDC25A (↑), BYSL (↓), UTP3 (↓), GNL3 (↓), MRTO4 (↓), UTP6 (↓), KRR1 (↓), CDC25A (↓) |
| METHYLDOPA | NMD3 (↓) |
| VORINOSTAT | CCNB2 (↓) |
| PACLITAXEL | KHDRBS1 (↑) |
| DAUNORUBICIN | BUB1B (↓), CENPE (↓), NMD3 (↓) |
| METHOTREXATE | UBE2C (↓), CDC20 (↓), BUB1B (↓), KIF20A (↓), PTTG1 (↓), CDKN3 (↓), KIF11 (↓), TACC3 (↓), TOP2A (↓), TTK (↓), CENPF (↓), CCNB2 (↓) |
| NICLOSAMIDE | HNRNPA1 (↑) GINS2 (↓), BYSL (↓), RRM2 (↓) |
| ETOPOSIDE | UBE2C (↓), CDC20 (↓), KIF20A (↓), NCAPG (↓), PTTG1 (↓), PNO1 (↓), NEK2 (↓), CENPF (↓), TOP2A (↓), KIF11 (↓), CDKN3 (↓), TACC3 (↓), TTK (↓) |
Discussion
In this article, a network-based approach was applied to discover therapeutic drugs for SARS-CoV-2 disease. Due to the high genetic similarity of SARS-CoV and SARS-CoV-2, the results of the study on SARS-CoV can be a clue to the treatment of SARS-CoV-2. To this end, at first, differentially expressed significant genes (P-value < 0.05) in healthy and SARS-CoV infected samples were selected, then the gene co-expression network was reconstructed in STRING database for the filtered genes.
After reconstructing the gene co-expression network, we discovered two significant gene modules using ClusterViz plugin in Cytoscape software. These two obtained gene co-expression modules (Module A and Module B) contained 25 and 30 genes, respectively. In the next step, a list of TFs and miRNAs which regulate these module's genes were gathered from the TRRUST and miRWalk2.0 databases, respectively. Moreover, the regulation information of the TFs and miRNAs were obtained from the TransmiR database. After collecting the TFs, miRNAs, and TGs regulation information, the two sub-networks of TF-miRNA-TG_A and TF-miRNA-TG_B were reconstructed. TF-miRNA-TG_A contains 347 miRNAs, 25 TGs, and 57 TFs, and TF-miRNA-TG_B contains 116 miRNAs, 25 TGs, and 4 TFs.
To analyze the biological processes and pathways in which Module A and Module B are involved, Gene Ontology (GO) and pathway enrichment analyses were done using a DAVID tool. The GO enrichment analysis showed that the genes in Module A significantly enriched in mitotic cell cycle process, nuclear division, and mitotic nuclear division biological processes. In Module B, the genes significantly enriched in RNA processing, mRNA metabolic process, and RNA metabolic process terms. The Reactome pathway enrichment analysis showed that modules A and B enriched in Resolution of Sister Chromatid Cohesion and mRNA Splicing—Major Pathway terms, respectively (see supplementary file S3). To analyze the TF-miRNA-TG_A and TF-miRNA-TG_B sub-network's miRNAs, the TAM online tool was utilized. Using this tool, we identified miRNAs families for these sub-networks. The results showed that the miRNAs of both sub-networks significantly enriched in let-7 and mir-17 families. Some other significant miRNAs families are reported in the supplementary file S4.
Given that the transcription factors have considerable roles in the gene expression process, the TFs of both TF-miRNA-TG_A and TF-miRNA-TG_B sub-networks were studied and evaluated. To this end, from any TF-miRNA-TG sub-networks, the TFs with a degree of 10 and above were selected and reported. The high-degree TFs were MYC, TP53, NFKB1, RELA, STAT3, MYCN, SP1, ESR1, E2F3, KLF4, CTNNB1, DNMT1, NANOG, TP73, LEF1, MYB, and FOXO3. These 17 transcription factor genes can be evaluated in further studies on SARS-CoV-2 coronavirus disease.
Hub miRNAs in TF-miRNA-TG_A and TF-miRNA-TG_B sub-networks are essential and crucial, as they regulate more genes of the subnetworks at a post-translational regulation level and can impact biological processes. Therefore, for each TF-miRNA-TG subnetwork, five high degree miRNAs were selected and reported as significant miRNAs. Based on the findings, hsa-miR-193b, hsa-miR-192, hsa-miR-215, hsa-miR-34a, and hsa-miR-16 were significant miRNAs for TF-miRNA-TG_A subnetwork, and hsa-miR-16, hsa-miR-92a, hsa-miR-30a, hsa-miR-7, and hsa-miR-26b were found to be significant miRNAs for TF-miRNA-TG_B subnetwork as well. Among these miRNAs, hsa-miR-16 regulates more genes in both subnetworks. According to the literature, some of these miRNAs have been studied in SARS coronavirus.
Kyung Hee Choi et al.45 found that has-miR-193 is a dual-strand tumor suppressor and a novel therapeutic target for lung cancer. In another study, Huajun Hu et al.46 reported that this miRNA is a tumor suppressor in Non-small cell lung cancer (NSCLC). Liang Sun et al.47 concluded that hsa-miR-193b regulates the RAB22A oncogene, inhibits breast cancer growth, and may have significant implications for cancer therapy. In addition, this miRNA regulates breast cancer cell migration and vasculogenic mimicry by DDAH148. Moreover, it could function as a tumor-suppressive miRNA in breast cancer49 and inhibits breast cancer metastasis50.
Martyna Filipska et al.51 found that hsa-miR-192-5p has a functional role in squamous cell lung cancer cells. In Peng Zou et al.52 reported that this miRNA suppresses the progression of lung cancer bone metastasis by targeting TRIM44. Moreover, this miRNA has been introduced as a prognostic marker for NSCLC participants53. Importantly, hsa-miR-192 induces Cisplatin-resistance, inhibits cell apoptosis in lung cancer54 and the proliferation, migration, and invasion of osteosarcoma cells, and promotes apoptosis55. Xiaopan et al.56 reported that hsa-miR-215 suppresses proliferation and migration of non-small cell lung cancer cells(NSCLC). This miRNA is downregulated in NSCLC tissues and may play a key role in the development of NSCLC. The lower expression of has-miR-215 in NSCLC is negatively associated with lymphatic metastasis and TNM staging57. This miRNA targets ZEB2 in human non-small cell lung cancer and functions as a tumor suppressor58. Ariana Centa et al.59 expressed that has-miR-34a-5p is identified as the regulator of mRNA targets involved in endothelial, inflammatory signaling pathways, and viral diseases. Furthermore, in the present study, the expression of this miRNA was significantly down-regulated in the COVID-19 patients compared to the Controls. Also, Martin Hart et al.60, in their systems biology, analysis identified miR-34a as strongly associated with pathogenesis. In another study, Rieko Aida et al.61 reported that apigenin might induce apoptosis by down-regulating SNAI1 through miR-34a-5p up-regulation in A549 cells. Woo Ryung Kim et al.62 reported that hsa-miR-16-5p is commonly bound to SARS-CoV, MERS-CoV, and SARS-CoV-2. In Zofia Wicik et al.63 showed that this miRNA could regulate ACE2 networks. Moreover, this miRNA can link the pathogenesis of HIV-1 and malaria64–66. Similarly, Jianghong Wei et al.67 found that overexpression of miR-16 inhibited the growth and metastasis of the DMS-53 lung cancer cells. Alireza Paniri et al.68 reported that hsa-miR-26b-5p strongly targets ACE2 and have an important effect on SARS coronavirus. Like has-miR-16-5p, has-mir-26b-5p can regulate ACE2 networks as well63. Moreover, this paper reported that has-miR-26b-5p may plays a significant role in the pathogenesis of HF in COVID-19 patients. The effect of this miRNA in SARS coronavirus was studied by Laura Teodori and her colleagues69. Moreover, Yang Gao et al.70 reported that this miRNA plays an important role in tumor suppression in lung cancer. According to M Xia et al., this miRNA could suppress lung cancer cells' proliferation, migration, and invasion. Min Jiang et al.71 reported that has-miR-92a family could be ideal biomarkers for cancer diagnosis and prognosis. Also, our study revealed that the expression of has-mir-92a was upregulated in lung squamous cell carcinoma (LUSC). Besides, this miRNA could promote growth, metastasis, and chemoresistance in NSCLC cells72. This miRNA was thus introduced as a plasma biomarker for small cell lung cancer73. Jianhua Gong et al.74 revealed that the has-miR-92a up-regulation could significantly induce proliferation and inhibit apoptosis of lung cancer cells. Jianjie Zhu et al.75 revealed that the upregulation of has-mir-30-5p in lung cancer cell lines inhibited cell proliferation in vitro and in vivo. This miRNA suppresses lung cancer progression by targeting SIRT176. Also, the lack of its expression promotes the growth of lung cancer cells by targeting MEF2D. Moreover, Xiaowei Quan et al.77 and Ruixue Tang et al.78 revealed that miR-30a-5p expression is downregulated in NSCLC. In addition, the increase in miR-30a-5p level could enhance Bax protein level and decrease Bcl-2 protein level77. In the field of pharmaceutical research, Xiaojie Xu et al.79 reported that miR-30a-5p enhances paclitaxel sensitivity in non-small cell lung cancer through targeting BCL-2 expression. Haiping Xiao et al.80 believed that has-miR-7-5p suppresses tumor metastasis of NSCLC by targeting NOVA2. Plus, Kenneth Lundstrom81 revealed that Rotavirus (RV) miR-7 can inhibit rotavirus replication by targeting the RV nonstructural protein 5. In another study, it was found that has-miR-7 could repress fibrogenesis of lung fibroblasts induced by TGF-β182. In addition, Xiaofei Zhang et al.83 reported that the overexpressed CDR1as functions as an oncogene to promote the tumor progression via miR-7 in non-small-cell lung cancer.
From the perspective of pharmacological studies, our finding shows that 470 drugs target TF and non-TF genes in both TF-miRNA-TG_A and TF-miRNA-TG_B subnetworks. Of 470 drugs, 62 drugs target both TF and non-TF genes, 95 drugs target non-TF genes, and 436 drugs target TF genes. From among the 470 obtained drugs, the drugs which target more genes were selected and discussed. In the drug-gene network, the drugs with a degree of 3 or above were selected and reported as potential and effective drugs for treating patients infected with SARS coronavirus. These drugs, including FLUOROURACIL, EPIRUBICIN, and FOSTAMATINIB, target at least three genes in the drug-gene network. Also, the drugs with a degree of 4 or above were selected and reported for the drug-TF network. These high-degree drugs were CISPLATIN, SIROLIMUS, CYCLOPHOSPHAMIDE, METHYLDOPA, VORINOSTAT, OLAPARIB, MITOXANTRONE, FLUOROURACIL, EVEROLIMUS, PACLITAXEL, DAUNORUBICIN, ZINC CHLORIDE, METHOTREXATE, CARBOPLATIN, BORTEZOMIB, NICLOSAMIDE, and ETOPOSIDE. Of the reported drugs, CISPLATIN targets 11 Transcription factor genes and may have a crucial impact on SARS coronavirus disease. We found that some of these drugs have been studied in SARS-CoV and SARS-CoV-2 coronaviruses, and others can be assumed as candidate drugs for SARS-CoV-2 coronavirus disease therapeutic.
Shamim I. Ahmad, in his recent study, revealed that FLUOROURACIL, in combination with deoxyribose and deoxyribonucleosides, can be a therapeutic option for SARS coronavirus84. EPIRUBICIN, VAPREOTIDA, and SAQUINAVIR have been proposed as key drugs in SARS coronavirus treatment85. Also, Strich et al.86 introduced the FOSTAMATINIB as a potential therapeutic for COVID-19. Moreover, FOSTAMATINIB has the potential to treat serious outcomes of coronavirus COVID-19, including acute lung injury (ALI) and acute respiratory distress syndrome (ARDS)87,88. In addition, several studies evaluated and showed the impact of FOSTAMATINIB on SARS coronavirus89–92. The mTOR signaling plays a crucial role in MERS-CoV infection93. In this regard, Yadi Zhou et al.34 observed that the SIROLIMUS is an inhibitor of mTOR with both antifungal and antineoplastic properties. In addition, this drug has been presented as a viral protein expression blocker94. Swaroop Revannasiddaiaha et al.95 showed that CYCLOPHOSPHAMIDE had a potential role in mitigation of acute respiratory distress syndrome among patients with SARS-CoV-2. Moreover, Brocato et al.96 , Othenin-Girard et al.97, Corso et al.98, Schaecher et al.99, and Revannasiddaiah et al.95 evaluated and showed the impact of CYCLOPHOSPHAMIDE on SARS coronavirus disease with different approaches.
Al-Rashedi et al.100 noted that the OLAPARIB is a potential drug for treating patients infected with SARS-COV-2. MITOXANTRONE has also been introduced as potential inhibitors of SARS-CoV-2 Mpro101. Safavi et al.102 showed that the METHOTREXATE silence the immune activation in patients with COVID-19. Also, Sujoy Khan et al.103 proposed this drug as a potential drug for treating patients infected with COVID-19. Additionally, this drug has a protective effect on SARS-CoV-2 infection via downregulating ACE2104. In this study, another drug that we have reported as a potential drug for SARS coronavirus was NICLOSAMIDE. This drug has previously been reported as an antiviral agent against COVID-19105,106. Other studies have reported the potential of this drug in treating patients infected with COVID-19107,108. Different studies have been undertaken on the effect of ETOPOSIDE on SARS coronavirus disease109–112. In our previous research based on protein–protein-network analysis, we proposed PACLITAXEL, CARBOPLATIN, BORTEZOMIB, VORINOSTAT, and DAUNORUBICIN as potential drugs for SARS-CoV-2 coronavirus treatment21. In this study, PACLITAXEL was introduced as the most potent therapeutic candidate drug. In previous research, rare studies examined the effect of this drug on SARS-CoV-2 disease.
In conclusion, based on our results, these 19 drugs can be assumed as candidate therapeutic drugs for SARS-CoV-2 coronavirus. Moreover, along with some other drugs, nine miRNAs were proposed as candidate miRNAs, which may play an important role in treating SARS-CoV-2 disease. These candidate miRNAs include hsa-miR-193b, hsa-miR-192, hsa-miR-215, hsa-miR-34a,hsa-miR-16, hsa-miR-92a, hsa-miR-30a, hsa-miR-7, and hsa-miR-26b.
Conclusion
In this study, focusing on the gene expression profile of SARS-CoV samples, an attempt was made to identify effective drugs for the treatment of this disease with a gene co-expression network-based approach. Given that the genomes of SARS-CoV and SARS-CoV-2 are very similar, it is expected that the drugs introduced to treat SARS-CoV coronavirus would also be effective in treating SARS-CoV-2 disease. Current research aimed to discover novel potential drugs for SARS-CoV disease in order to treating SARS-CoV-2 coronavirus based on a co-expression network analysis. To this end, at first, significant DEGs in normal and SARS-CoV infected samples were selected and then the gene co-expression network was reconstructed and two gene modules were discovered as significant modules. Then, two significant gene modules were discovered from the reconstructed co-expression network. Next, for the obtained modules, two sub-networks named TF-miRNA-TG_A and TF-miRNA-TG_B were drawn. Afterward, the list of the drugs targeting TF-miRNA-TG_A and TF-miRNA-TG_B sub-networks' genes was extracted, and two drug-gene and drug-TF interaction networks were drawn. Eventually, five drugs including FLUOROURACIL, CISPLATIN, SIROLIMUS, CYCLOPHOSPHAMIDE, and METHYLDOPA are proposed as poteintial drugs for SARS-CoV-2 coronavirus treatment. As well as, ten miRNAs including miR-193b, miR-192, miR-215, miR-34a, miR-16, miR-16, miR-92a, miR-30a, miR-7, and miR-26b were found to be significant miRNAs in treating SARS-CoV-2 coronavirus.
Methods
Dataset and preprocessing
The gene expression data used in this work were downloaded from the NCBI Gene Expression Omnibus (GEO) database with the accession number GSE1739. This data contains gene expression profiles of normal and Severe Acute Respiratory Syndrome (SARS) infected patients' blood samples. To assign probes to gene IDs, the annotation file published by Affymetrix was used.
In this article, a network-based approach was applied to discover therapeutic drugs for SARS-CoV-2 disease. To this end, at first, differentially expressed significant genes (p_value < 0.05) in healthy and SARS-CoV infected samples were selected, and then the gene co-expression network was reconstructed in STRING database for the filtered genes.
Network reconstruction and module extraction
At first, differentially expressed genes were extracted for the normal and SARS infected groups. In order to calculate the differentially expressed genes, adjusted p_value was calculated using Benjamini & Hochberg false discovery rate method. Then, 1441 genes with p-values less than 0.05 were assumed as primary genes, which were then used in network reconstruction. The list of mentioned primary genes is reported in Supplementary file S1. Afterward, the gene co-expression network is reconstructed by primary genes in STRING database. In this web tool, the minimum required interaction score parameter is adjusted to 0.04.
In order to analyze the reconstructed co-expression network, the Cytoscape software43 version 3.8.2 was used. ClusterViz113 plugin was used to identify gene modules (highly interconnected regions) in the co-expression network. ClusterViz is a Cytoscpae plugin, which discovers modules in a biological network using various clustering algorithms. This plugin contains three commonly used clustering algorithms, including FAG-EC, EAGLE, and MCODE.
In this research, we have applied all of the algorithms and the results did not have significant difference, so we have decided to select one of them. Therefore, the MCODE (Molecular Complex Detection) algorithm was used to find the gene co-expression modules. MCODE is a graph theoretic clustering algorithm for discovering strongly connected regions in a given network113. This algorithm selects the seed nodes and expand them based on the density of the cluster and density of the local neighborhood113. The MCODE algorithm was performed with the following parameters: Degree threshold = 2, NodeScore Threshold = 0.2, K-Core Threshold = 2, and Maxdepth = 100.
Transcription factors, miRNAs, and target genes interaction network
To investigate the effect of Transcription Factors (TF) and microRNAs (miRNA) on target genes (TG), the TF-miRNA-TG sub-networks were reconstructed for gene modules.
Transcription factors (TF) are proteins that regulate the rate of transcription of genetic information from DNA to messenger RNA114. miRNAs are small non-coding RNAs that function in RNA silencing and post-transcriptional gene regulation23,115. Both TFs and miRNAs regulate gene expression116. To get regulatory interactions information of TFs-TGs, the TRRUST online database was utilized. This database contains 8,444 and 6,552 TF-target regulatory relationships of 800 human TFs and 828 mouse TFs, respectively. In addition to TFs-TGs regulatory information, TFs-miRNAs regulatory interactions are essential. This information is obtained from the TransmiR V242 database. TransmiR v2.0 incorporates 3,730 TF-miRNA regulatory interactions, covering 623 TFs and 785 miRNAs for 19 organisms. In this study, the information of miRNAs-TGs regulatory interactions was retrieved from the miRWalk 2.0 database. This database contains both validated and predicted interactions. In this study, only experimentally validated miRNAs–TGs interactions were considered.
In order to analyze the reconstructed co-expression network, the Cytoscape software was used. After analyzing the network, generally, 391 nodes and 1273 edges were observed in the reconstructed co-expression network. ClusterViz113 plugin was used to identify gene modules (highly interconnected regions) in the co-expression network. ClusterViz is a Cytoscpae plugin, which discovers modules in a biological network using various clustering algorithms. This plugin contains three commonly used clustering algorithms, including FAG-EC, EAGLE, and MCODE. The MCODE (Molecular Complex Detection) algorithm was used to find the gene co-expression modules in this study. The MCODE algorithm was performed with the following parameters: Degree threshold = 2, NodeScore Threshold = 0.2, K-Core Threshold = 2, and Maxdepth = 100. After clustering the gene co-expression network, we discovered two significant modules (Module A and Module B) in the co-expression network.
Enrichment analysis
The Database for Annotation, Visualization, and Integrated Discovery (DAVID ) v6.8117,118 was used for the enrichment analysis of the genes. The gene ontology and pathway enrichment analysis were done for obtained gene modules.
To identify miRNAs family, the TAM online tool119 was applied. To do this, all the miRNAs in TF-miRNA-TG sub-networks were imported to the TAM tool separately, and then significant miRNA families were identified.
Drug-Gene interaction network
To identify the drugs that target TF-miRNA-TG_A and TF-miRNA-TG_B TF and non-TF genes, the Drug Gene Interaction Database (DGIdb)41 was used. This database retrieves drug-gene interaction information from 24 other related databases. In this study, to identify drug-genes interaction, only approved drugs were used.
GSEA and candidate drugs validation
In order to evaluate the proposed drugs for SARS-CoV-2 disease, the GSEA was performed by querying the Enrichr database120. To this end, the Enrichr database was utilized to perform the Connectivity Map(CMAP) analysis121.
Supplementary Information
Author contributions
H.M.G. wrote the main manuscript. H.M.G and A.R. performed the analyses. H.M.G. and E.S. reconstructed the networks. H.M.G., A.R. and F.F.A. interpreted the results and wrote the manuscript. H.M.G., R.A.I. analyzed the results. All authors reviewed the manuscript.
Funding
This work was supported by the Islamic Azad University, Tabriz branch.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-01410-3.
References
- 1.Masters, P. & Perlman, S. (Lippincott Williams & Wilkins, 2013).
- 2.Cui J, Li F, Shi Z-L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McBride R, Fielding BC. The role of severe acute respiratory syndrome (SARS)-coronavirus accessory proteins in virus pathogenesis. Viruses. 2012;4:2902–2923. doi: 10.3390/v4112902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rabaan AA, et al. SARS-CoV-2, SARS-CoV, and MERS-COV: a comparative overview. Infez Med. 2020;28:174–184. [PubMed] [Google Scholar]
- 5.Zaki AM, Van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 6.El-Hachem N, et al. Integrative transcriptome analyses empower the anti-COVID-19 drug arsenal. Iscience. 2020;23:101697. doi: 10.1016/j.isci.2020.101697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu N, et al. A novel coronavirus from patients with pneumonia in China. New England J. Med. 2020;2:1056. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MotieGhader H, Gharaghani S, Masoudi-Sobhanzadeh Y, Masoudi-Nejad A. Sequential and mixed genetic algorithm and learning automata (SGALA, MGALA) for feature selection in QSAR. Iran. J. Pharm. Res. IJPR. 2017;16:533. [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Q, Coumoul X, Grandjean P, Barouki R, Audouze K. Endocrine disrupting chemicals and COVID-19 relationships: A computational systems biology approach. Environ. Int. 2020;12:106232. doi: 10.1016/j.envint.2020.106232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei, J. et al. Genome-wide CRISPR screen reveals host genes that regulate SARS-CoV-2 infection. Biorxiv (2020).
- 11.Chakraborty C, et al. Consider TLR5 for new therapeutic development against COVID-19. J. Med. Virol. 2020;2:1069. doi: 10.1002/jmv.25997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma G, Hanania NA, Shim YM. The aging immune system and its relationship to the development of chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2009;6:573–580. doi: 10.1513/pats.200904-022RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saha A, et al. Tocilizumab: a therapeutic option for the treatment of cytokine storm syndrome in COVID-19. Arch. Med. Res. 2020;51:595–597. doi: 10.1016/j.arcmed.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhattacharya M, et al. Development of epitope-based peptide vaccine against novel coronavirus 2019 (SARS-COV-2): Immunoinformatics approach. J. Med. Virol. 2020;92:618–631. doi: 10.1002/jmv.25736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blanco-Melo, D. et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 181, 1036–1045. e1039 (2020). [DOI] [PMC free article] [PubMed]
- 16.Zhou, Z. et al. Overly exuberant innate immune response to SARS-CoV-2 infection. (2020).
- 17.Xiong Y, et al. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerging microbes & infections. 2020;9:761–770. doi: 10.1080/22221751.2020.1747363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prasad K, et al. Targeting hub genes and pathways of innate immune response in COVID-19: a network biology perspective. Int. J. Biol. Macromol. 2020;163:1–8. doi: 10.1016/j.ijbiomac.2020.06.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zakeri NSS, Pashazadeh S, MotieGhader H. Gene biomarker discovery at different stages of Alzheimer using gene co-expression network approach. Sci. Rep. 2020;10:1–13. doi: 10.1038/s41598-020-69553-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adhami M, MotieGhader H, Haghdoost AA, Afshar RM, Sadeghi B. Gene co-expression network approach for predicting prognostic microRNA biomarkers in different subtypes of breast cancer. Genomics. 2020;112:135–143. doi: 10.1016/j.ygeno.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 21.Adhami M, Sadeghi B, Rezapour A, Haghdoost AA, MotieGhader H. Repurposing novel therapeutic candidate drugs for coronavirus disease-19 based on protein-protein interaction network analysis. BMC Biotechnol. 2021;21:1–11. doi: 10.1186/s12896-021-00680-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sardar, R., Satish, D., Birla, S. & Gupta, D. Comparative analyses of SAR-CoV2 genomes from different geographical locations and other coronavirus family genomes reveals unique features potentially consequential to host-virus interaction and pathogenesis. BioRxiv (2020). [DOI] [PMC free article] [PubMed]
- 23.Motieghader H, Kouhsar M, Najafi A, Sadeghi B, Masoudi-Nejad A. mRNA–miRNA bipartite network reconstruction to predict prognostic module biomarkers in colorectal cancer stage differentiation. Mol. BioSyst. 2017;13:2168–2180. doi: 10.1039/C7MB00400A. [DOI] [PubMed] [Google Scholar]
- 24.MotieGhader H, Masoudi-Sobhanzadeh Y, Ashtiani SH, Masoudi-Nejad A. mRNA and microRNA selection for breast cancer molecular subtype stratification using meta-heuristic based algorithms. Genomics. 2020;112:3207–3217. doi: 10.1016/j.ygeno.2020.06.014. [DOI] [PubMed] [Google Scholar]
- 25.Rudrapal, M., Khairnar, J. & Jadhav, G. Drug repurposing (DR): an emerging approach in drug discovery. Drug Repurposing Hypothesis Mol. Asp. Ther. Appl (2020).
- 26.Pushpakom S, et al. Drug repurposing: progress, challenges and recommendations. Nat. Rev. Drug Discovery. 2019;18:41–58. doi: 10.1038/nrd.2018.168. [DOI] [PubMed] [Google Scholar]
- 27.Conte, F. et al. A paradigm shift in medicine: A comprehensive review of network-based approaches. Biochimica et Biophysica Acta (BBA)-Gene Regulatory Mechanisms 1863, 194416 (2020). [DOI] [PubMed]
- 28.Fiscon, G., Conte, F., Farina, L. & Paci, P. SAveRUNNER: a network-based algorithm for drug repurposing and its application to COVID-19. PLoS Comput. Biol. 17, e1008686 (2021). [DOI] [PMC free article] [PubMed]
- 29.Fiscon G, Paci P. SAveRUNNER: an R-based tool for drug repurposing. BMC Bioinf. 2021;22:1–10. doi: 10.1186/s12859-021-04076-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fiscon, G., Conte, F., Amadio, S., Volonté, C. & Paci, P. Drug repurposing: a network-based approach to amyotrophic lateral sclerosis. Neurotherapeutics, 1–14 (2021). [DOI] [PMC free article] [PubMed]
- 31.Sibilio, P. et al.. In silico drug repurposing in COVID-19: a network-based analysis. Biomed. Pharmacother. 142, 111954 (2021). [DOI] [PMC free article] [PubMed]
- 32.Gysi, D.M. et al.. Network medicine framework for identifying drug-repurposing opportunities for COVID-19. Proc. Natl. Acad. Sci. 118 (2021). [DOI] [PMC free article] [PubMed]
- 33.Taz TA, et al. Network-based identification genetic effect of SARS-CoV-2 infections to Idiopathic pulmonary fibrosis (IPF) patients. Brief. Bioinf. 2021;22:1254–1266. doi: 10.1093/bib/bbaa235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Y, et al. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov. 2020;6:1–18. doi: 10.1038/s41421-020-0153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Du, H., Chen, F., Liu, H. & Hong, P. Network-based virus-host interaction prediction with application to SARS-CoV-2. Patterns 2, 100242 (2021). [DOI] [PMC free article] [PubMed]
- 36.Sadegh S, et al. Exploring the SARS-CoV-2 virus-host-drug interactome for drug repurposing. Nat. Commun. 2020;11:1–9. doi: 10.1038/s41467-020-17189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen, Z. et al. Construction of an autophagy interaction network based on competitive endogenous RNA reveals the key pathways and central genes of SARS-CoV-2 infection in vivo. Microb. Pathogenesis, 105051 (2021). [DOI] [PMC free article] [PubMed]
- 38.Jensen LJ, et al. STRING 8—a global view on proteins and their functional interactions in 630 organisms. Nucleic Acids Res. 2009;37:D412–D416. doi: 10.1093/nar/gkn760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han H, et al. TRRUST v2: an expanded reference database of human and mouse transcriptional regulatory interactions. Nucleic Acids Res. 2018;46:D380–D386. doi: 10.1093/nar/gkx1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dweep, H. & Gretz, N. miRWalk2.0: a comprehensive atlas of microRNA-target interactions. Nat. Methods 12, 697–697 (2015). [DOI] [PubMed]
- 41.Freshour, S.L. et al.. Integration of the Drug–Gene Interaction Database (DGIdb 4.0) with open crowdsource efforts. Nucleic Acids Res. 49, D1144-D1151 (2021). [DOI] [PMC free article] [PubMed]
- 42.Wang J, Lu M, Qiu C, Cui Q. TransmiR: a transcription factor–microRNA regulation database. Nucleic Acids Res. 2010;38:D119–D122. doi: 10.1093/nar/gkp803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shannon P, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patten, J. et al.. Multidose evaluation of 6,710 drug repurposing library identifies potent SARS-CoV-2 infection inhibitors In Vitro and In Vivo. bioRxiv (2021).
- 45.Choi, K.H., Shin, C.H., Lee, W.J., Ji, H. & Kim, H.H. Dual-strand tumor suppressor miR-193b-3p and-5p inhibit malignant phenotypes of lung cancer by suppressing their common targets. Biosci. Rep. 39 (2019). [DOI] [PMC free article] [PubMed]
- 46.Hu H, Li S, Liu J, Ni B. MicroRNA-193b modulates proliferation, migration, and invasion of non-small cell lung cancer cells. Acta Biochim Biophys. Sin. 2012;44:424–430. doi: 10.1093/abbs/gms018. [DOI] [PubMed] [Google Scholar]
- 47.Sun L, et al. Regulation of RAB22A by mir-193b inhibits breast cancer growth and metastasis mediated by exosomes. Int. J. Oncol. 2018;53:2705–2714. doi: 10.3892/ijo.2018.4571. [DOI] [PubMed] [Google Scholar]
- 48.Hulin J-A, et al. MiR-193b regulates breast cancer cell migration and vasculogenic mimicry by targeting dimethylarginine dimethylaminohydrolase 1. Sci. Rep. 2017;7:1–15. doi: 10.1038/s41598-017-14454-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu S, et al. CD44v6 targeted by miR-193b-5p in the coding region modulates the migration and invasion of breast cancer cells. J. Cancer. 2020;11:260. doi: 10.7150/jca.35067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hashemi ZS, Moghadam MF, Farokhimanesh S, Rajabibazl M, Sadroddiny E. Inhibition of breast cancer metastasis by co-transfection of miR-31/193b-mimics. Iran. J. Basic Med. Sci. 2018;21:427. doi: 10.22038/IJBMS.2018.26614.6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Filipska M, et al. MiR-192 and miR-662 enhance chemoresistance and invasiveness of squamous cell lung carcinoma. Lung Cancer. 2018;118:111–118. doi: 10.1016/j.lungcan.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 52.Zou P, et al. miR-192-5p suppresses the progression of lung cancer bone metastasis by targeting TRIM44. Sci. Rep. 2019;9:1–9. doi: 10.1038/s41598-019-56018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang T, Li W, Li H, Li W. Dysregulation of exosomal miR-192 and miR-194 expression in lung adenocarcinoma patients. Saudi J. Biol. Sci. 2021;28:1561–1568. doi: 10.1016/j.sjbs.2021.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang, F. et al.. MiR-192 confers cisplatin resistance by targeting Bim in lung cancer. Zhongguo fei ai za zhi 17 (2014). [DOI] [PMC free article] [PubMed]
- 55.Shang G, et al. MicroRNA-192 inhibits the proliferation, migration and invasion of osteosarcoma cells and promotes apoptosis by targeting matrix metalloproteinase-11. Oncol. Lett. 2018;15:7265–7272. doi: 10.3892/ol.2018.8239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cai X, et al. miR-215 suppresses proliferation and migration of non-small cell lung cancer cells. Oncol. Lett. 2017;13:2349–2353. doi: 10.3892/ol.2017.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yao Y, Shen H, Zhou Y, Yang Z, Hu T. MicroRNA-215 suppresses the proliferation, migration and invasion of non-small cell lung carcinoma cells through the downregulation of matrix metalloproteinase-16 expression. Exp. Ther. Med. 2018;15:3239–3246. doi: 10.3892/etm.2018.5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hou Y, et al. miR-215 functions as a tumor suppressor and directly targets ZEB2 in human non-small cell lung cancer. Oncol. Lett. 2015;10:1985–1992. doi: 10.3892/ol.2015.3587. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 59.Centa A, et al. Deregulated miRNA expression is associated with endothelial dysfunction in post-mortem lung biopsies of COVID-19 patients. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021;320:L405–L412. doi: 10.1152/ajplung.00457.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hart M, et al. Identification of miR-34a-target interactions by a combined network based and experimental approach. Oncotarget. 2016;7:34288. doi: 10.18632/oncotarget.9103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aida R, et al. miR-34a-5p might have an important role for inducing apoptosis by down-regulation of SNAI1 in apigenin-treated lung cancer cells. Mol. Biol. Rep. 2021;48:2291–2297. doi: 10.1007/s11033-021-06255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim WR, et al. Expression Analyses of MicroRNAs in Hamster Lung Tissues Infected by SARS-CoV-2. Mol. Cells. 2020;43:953. doi: 10.14348/molcells.2020.0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wicik Z, et al. ACE2 interaction networks in COVID-19: a physiological framework for prediction of outcome in patients with cardiovascular risk factors. J. Clin. Med. 2020;9:3743. doi: 10.3390/jcm9113743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mariconti M, et al. Correction to: Role of microRNAs in host defense against Echinococcus granulosus infection: a preliminary assessment. Immunol. Res. 2019;67:98–98. doi: 10.1007/s12026-018-9060-1. [DOI] [PubMed] [Google Scholar]
- 65.Biswas S, Haleyurgirisetty M, Lee S, Hewlett I, Devadas K. Development and validation of plasma miRNA biomarker signature panel for the detection of early HIV-1 infection. EBioMedicine. 2019;43:307–316. doi: 10.1016/j.ebiom.2019.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ketprasit N, et al. The characterization of extracellular vesicles-derived microRNAs in Thai malaria patients. Malar. J. 2020;19:1–14. doi: 10.1186/s12936-020-03360-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wei, J. et al. MicroRNA-16 inhibits the proliferation and metastasis of human lung cancer cells by modulating the expression of YAP1. J. BU ON. Off. J. Balkan Union Oncol. 25, 862–868 (2020). [PubMed]
- 68.Paniri, A., Hosseini, M.M., Moballegh-Eslam, M. & Akhavan-Niaki, H. Comprehensive in silico identification of impacts of ACE2 SNPs on COVID-19 susceptibility in different populations. Gene Rep. 22, 100979 (2021). [DOI] [PMC free article] [PubMed]
- 69.Teodori, L. et al.. MicroRNAs bioinformatics analyses identifying HDAC pathway as a putative target for existing anti‐COVID‐19 therapeutics. Front. Pharmacol. 11 (2020). [DOI] [PMC free article] [PubMed]
- 70.Gao, Y. & Yang, F. MiR-26b regulates invasion and migration of lung cancer cells through targeting hENT1 depending on RhoA/ROCK-1 pathway. Zhong nan da xue xue bao. Yi xue ban Journal of Central South University. Medical Sciences 42, 755–761 (2017). [DOI] [PubMed]
- 71.Jiang M, Li X, Quan X, Li X, Zhou B. MiR-92a family: a novel diagnostic biomarker and potential therapeutic target in human cancers. Front. Mol. Biosci. 2019;6:98. doi: 10.3389/fmolb.2019.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ren P, Gong F, Zhang Y, Jiang J, Zhang H. MicroRNA-92a promotes growth, metastasis, and chemoresistance in non-small cell lung cancer cells by targeting PTEN. Tumor. Biol. 2016;37:3215–3225. doi: 10.1007/s13277-015-4150-3. [DOI] [PubMed] [Google Scholar]
- 73.Yu Y, et al. Plasma miR-92a-2 as a biomarker for small cell lung cancer. Cancer Biomark. 2017;18:319–327. doi: 10.3233/CBM-160254. [DOI] [PubMed] [Google Scholar]
- 74.Gong J, et al. The relationship between miR-17-5p, miR-92a, and let-7b expression with non-small cell lung cancer targeted drug resistance. J. Buon. 2017;22:454–461. [PubMed] [Google Scholar]
- 75.Zhu J, et al. CD73/NT5E is a target of miR-30a-5p and plays an important role in the pathogenesis of non-small cell lung cancer. Mol. Cancer. 2017;16:1–15. doi: 10.1186/s12943-017-0591-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guan Y, Rao Z, Chen C. miR-30a suppresses lung cancer progression by targeting SIRT1. Oncotarget. 2018;9:4924. doi: 10.18632/oncotarget.23529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Quan X, Li X, Yin Z, Ren Y, Zhou B. p53/miR-30a-5p/SOX4 feedback loop mediates cellular proliferation, apoptosis, and migration of non-small-cell lung cancer. J. Cell. Physiol. 2019;234:22884–22895. doi: 10.1002/jcp.28851. [DOI] [PubMed] [Google Scholar]
- 78.Tang R, et al. Downregulation of MiR-30a is associated with poor prognosis in lung cancer. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2015;21:2514. doi: 10.12659/MSM.894372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xu X, et al. miR-30a-5p enhances paclitaxel sensitivity in non-small cell lung cancer through targeting BCL-2 expression. J. Mol. Med. 2017;95:861–871. doi: 10.1007/s00109-017-1539-z. [DOI] [PubMed] [Google Scholar]
- 80.Xiao H. MiR-7-5p suppresses tumor metastasis of non-small cell lung cancer by targeting NOVA2. Cell. Mol. Biol. Lett. 2019;24:1–13. doi: 10.1186/s11658-019-0188-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lundstrom, K. (Multidisciplinary Digital Publishing Institute, 2020).
- 82.Yao W, et al. The CDR1as/miR-7/TGFBR2 axis modulates EMT in silica-induced pulmonary fibrosis. Toxicol. Sci. 2018;166:465–478. doi: 10.1093/toxsci/kfy221. [DOI] [PubMed] [Google Scholar]
- 83.Zhang X, Yang D, Wei Y. Overexpressed CDR1as functions as an oncogene to promote the tumor progression via miR-7 in non-small-cell lung cancer. Onco. Targets. Ther. 2018;11:3979. doi: 10.2147/OTT.S158316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ahmad, S.I. 5-Fluorouracil in combination with deoxyribonucleosides and deoxyribose as possible therapeutic options for the Coronavirus, COVID-19 infection. Med. Hypotheses 142, 109754 (2020). [DOI] [PMC free article] [PubMed]
- 85.Khan, M.A. et al.. Comparative molecular investigation of the potential inhibitors against SARS-CoV-2 main protease: a molecular docking study. J. Biomol. Struct. Dyn. 1–7 (2020). [DOI] [PMC free article] [PubMed]
- 86.Strich JR, et al. Fostamatinib inhibits neutrophils extracellular traps induced by COVID-19 patient plasma: a potential therapeutic. J. Infect. Dis. 2021;223:981–984. doi: 10.1093/infdis/jiaa789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tabassum, N., Zhang, H. & Stebbing, J. Repurposing fostamatinib to combat SARS-CoV-2-induced acute lung injury. Cell Rep. Med. 1, 100145 (2020). [DOI] [PMC free article] [PubMed]
- 88.Malimova, M. et al. A high content screen for mucin-1-reducing compounds identifies fostamatinib as a candidate for rapid repurposing for acute lung injury during the COVID-19 pandemic. bioRxiv (2020). [DOI] [PMC free article] [PubMed]
- 89.Apostolidis, S.A. et al. Signaling through FcγRIIA and the C5a-C5aR pathway mediates platelet hyperactivation in COVID-19. bioRxiv (2021). [DOI] [PMC free article] [PubMed]
- 90.Liu D-Y, et al. Drug repurposing for COVID-19 treatment by integrating network pharmacology and transcriptomics. Pharmaceutics. 2021;13:545. doi: 10.3390/pharmaceutics13040545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hoepel, W. et al. High titers and low fucosylation of early human anti–SARS-CoV-2 IgG promote inflammation by alveolar macrophages. Sci. Transl. Med. 13 (2021). [DOI] [PMC free article] [PubMed]
- 92.Vergis, N. et al. Multi-arm Trial of Inflammatory Signal Inhibitors (MATIS) for hospitalised patients with mild or moderate COVID-19 pneumonia: a structured summary of a study protocol for a randomised controlled trial. Trials (2021). [DOI] [PMC free article] [PubMed]
- 93.Kindrachuk J, et al. Antiviral potential of ERK/MAPK and PI3K/AKT/mTOR signaling modulation for Middle East respiratory syndrome coronavirus infection as identified by temporal kinome analysis. Antimicrob. Agents Chemother. 2015;59:1088–1099. doi: 10.1128/AAC.03659-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tabari, M.A.K., Khoshhal, H., Tafazoli, A., Khandan, M. & Bagheri, A. Applying computer simulations in battling with COVID-19, using pre-analyzed molecular and chemical data to face the pandemic. Inf. Med. Unlocked 21, 100458 (2020). [DOI] [PMC free article] [PubMed]
- 95.Revannasiddaiah, S., Devadas, S.K., Palassery, R., Pant, N.K. & Maka, V.V. A potential role for cyclophosphamide in the mitigation of acute respiratory distress syndrome among patients with SARS-CoV-2. Medi. Hypotheses 144, 109850 (2020). [DOI] [PMC free article] [PubMed]
- 96.Brocato RL, et al. Disruption of adaptive immunity enhances disease in SARS-CoV-2-infected Syrian hamsters. J. Virol. 2020;94:e01683–e1620. doi: 10.1128/JVI.01683-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Othenin-Girard, A. et al. Multisystem inflammatory syndrome with refractory cardiogenic shock due to acute myocarditis and mononeuritis multiplex after SARS-CoV-2 infection in an adult. Swiss Med. Weekly 150 (2020). [DOI] [PubMed]
- 98.Corso, C.R., de Oliveira, N.M.T. & Maria-Ferreira, D. Susceptibility to SARS-CoV-2 infection in patients undergoing chemotherapy and radiation therapy. J. Infect. Public Health (2021). [DOI] [PMC free article] [PubMed]
- 99.Schaecher SR, et al. An immunosuppressed Syrian golden hamster model for SARS-CoV infection. Virology. 2008;380:312–321. doi: 10.1016/j.virol.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Al-Rashedi, N.A., Munahi, M.G. & AH ALObaidi, L. Prediction of potential inhibitors against SARS-CoV-2 endoribonuclease: RNA immunity sensing. J. Biomol. Struct. Dyn. 1–14 (2020). [DOI] [PMC free article] [PubMed]
- 101.Lokhande, K.B., Doiphode, S., Vyas, R. & Swamy, K.V. Molecular docking and simulation studies on SARS-CoV-2 Mpro reveals Mitoxantrone, Leucovorin, Birinapant, and Dynasore as potent drugs against COVID-19. J. Biomol. Struct. Dyn. 1–12 (2020). [DOI] [PMC free article] [PubMed]
- 102.Safavi, F. & Nath, A. Silencing of immune activation with methotrexate in patients with COVID-19. J. Neurol. Sci. 415 (2020). [DOI] [PMC free article] [PubMed]
- 103.Durairajb, S.K.S. JAK Inhibition with Methotrexate as Treatment for COVID-19 Is a Double-Edged Sword. (2020). [DOI] [PMC free article] [PubMed]
- 104.Schälter F, et al. Does methotrexate influence COVID-19 infection? Case series and mechanistic data. Arthritis Res. Ther. 2021;23:1–6. doi: 10.1186/s13075-021-02464-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pindiprolu, S.K.S. & Pindiprolu, S.H. Plausible mechanisms of Niclosamide as an antiviral agent against COVID-19. Medi. Hypotheses 140, 109765 (2020). [DOI] [PMC free article] [PubMed]
- 106.Backer, V. et al. A randomized, double-blind, placebo-controlled phase 1 trial of inhaled and intranasal niclosamide: a broad spectrum antiviral candidate for treatment of COVID-19. Lancet Reg. Health-Europe 4, 100084 (2021). [DOI] [PMC free article] [PubMed]
- 107.Yu S, et al. Niclosamide-clay intercalate coated with nonionic polymer for enhanced bioavailability toward COVID-19 treatment. Polymers. 2021;13:1044. doi: 10.3390/polym13071044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kunzelmann, K. Getting hands on a drug for Covid-19: Inhaled and Intranasal Niclosamide. Lancet Reg. Health–Europe 4 (2021). [DOI] [PMC free article] [PubMed]
- 109.Hamizi, K., Aouidane, S. & Belaaloui, G. Etoposide-based therapy for severe forms of COVID-19. Medi. Hypotheses 142, 109826 (2020). [DOI] [PMC free article] [PubMed]
- 110.Takami A. Possible role of low-dose etoposide therapy for hemophagocytic lymphohistiocytosis by COVID-19. Int. J. Hematol. 2020;112:122–124. doi: 10.1007/s12185-020-02888-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.MB, M., LV, M. & FR, M. Etoposide treatment adjunctive to immunosuppressants for critically ill COVID-19 patients. J. Infect. (2020). [DOI] [PMC free article] [PubMed]
- 112.Lovetrue, B. The AI-discovered aetiology of COVID-19 and rationale of the irinotecan+ etoposide combination therapy for critically ill COVID-19 patients. Med. Hypotheses 144, 110180 (2020). [DOI] [PMC free article] [PubMed]
- 113.Wang J, et al. ClusterViz: a cytoscape APP for cluster analysis of biological network. IEEE/ACM Trans. Comput. Biol. Bioinf. 2014;12:815–822. doi: 10.1109/TCBB.2014.2361348. [DOI] [PubMed] [Google Scholar]
- 114.Chen, X., Peng, H. & Yin, Z. (Hindawi, 2016).
- 115.Li M-H, Fu S-B, Xiao H-S. Genome-wide analysis of microRNA and mRNA expression signatures in cancer. Acta Pharmacol. Sin. 2015;36:1200–1211. doi: 10.1038/aps.2015.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mullany LE, et al. MicroRNA-transcription factor interactions and their combined effect on target gene expression in colon cancer cases. Genes Chromosom. Cancer. 2018;57:192–202. doi: 10.1002/gcc.22520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009;4:44. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 118.Huang DW, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li, J. et al. TAM 2.0: tool for MicroRNA set analysis. Nucleic Acids Res. 46, W180-W185 (2018). [DOI] [PMC free article] [PubMed]
- 120.Chen EY, et al. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinf. 2013;14:1–14. doi: 10.1186/1471-2105-14-S18-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lamb, J. et al.. The Connectivity Map: using gene-expression signatures to connect small molecules, genes, and disease. Science 313, 1929–1935 (2006). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.