Abstract
The purpose of this study was to investigate the effect of two aflatoxins (AFs) sources for experimental induction of aflatoxicosis in ducklings. Dietary supplementation of aflatoxin-contaminated grounded rice grains previously was cultured by Aspergillus parasiticus and dietary supplementation of aflatoxin methanolic extract of contaminated rice grains. A total number of 18 four-day-old ducklings were treated with varying sources of AFs. Treatments included: A: Control (basal diet without AFs), B: Contaminated feed with 0.20 mg kg-1 AFs (ground rice grains), C: Contaminated feed with 0.20 mg kg-1 AFs (methanol extract of contaminated rice grains). Results showed a significant decrease in serum levels of total protein, albumin, glucose, triglyceride, cholesterol, HDL, LDL and creatinine. The serum’s hepatic enzymes levels were not affected in AFs-treated groups but lactate dehydrogenase (LDH) concentration increased by the two AFs sources. The relative weight of the liver and spleen was significantly increased in AFs-fed groups. Histopathological liver examination showed the vacuolar degeneration with small and large lipid droplets in hepatocytes in the AFs- contaminated groups. Dietary AFs resulted in a significant decrease of villus height, villus width and villus surface area of the small intestine compared to the control group. In conclusion, the results showed that the induction of experimental aflatoxicosis via two investigated AFs sources had slight differences concerning the studied parameters. It seems the group consumed ground rice grains indicated slightly fewer aflatoxicosis symptoms than the methanolic extract.
Key Words: Aflatoxin, Ducklings, Intestinal morphology, Viscera organs
Introduction
Mycotoxins are secondary metabolites of fungal and aflatoxins are the most thoroughly studied mycotoxins, which were identified as a serious livestock problem in the 1950's. However, it was only in 1960 during the studies in the United Kingdom which was called turkey “x” disease causative agent. Aspergillus flavus and Aspergillus parasiticus were recognized as the organisms accountable for the expansion of the toxin in the feed.1 The sensitivity to AFs varies according to species, dose, gender, age and nutritional status of the animal.2 Oral administrations of AFs or aflatoxin B1 (AFB1) alone in the diet as a contaminated-rice powder,3-5 dissolved in olive oil by stomach tube,6,7 or in drinking water by oral gavage8 caused liver tumors (hepatocellular tumors) in all species tested. Many studies on AFs toxicity have been conducted; however, there are few researches on the efficiency of different sources and methods of application in vivo in animal models. Researchers investigated the production of AFs on the agricultural commodities including rice, wheat, corn, soybeans and sorghum, and found that rice grain was the best substrate.9 Recently, extraction methods have been developed for the separation and purification of active compounds from a solid matrix and increases their access and influence compared to the raw substrate.10 There are wide varieties of solvents used for the extraction of mycotoxins. The most common solvents used for the extraction of mycotoxins from the matrix such as herbal medicines are acetonitrile, methanol and chloroform.11 Therefore, it was assumed in the current study that due to increased access to these active compounds, AFs extracted from a raw medium such as rice grain can effectively induce aflatoxicosis in a short time by describing the serum biochemical and intestinal morphological indices in an experimental animal model.
Materials and Methods
Experimental birds and diets. The animal use protocol was approved by the Institutional Animal Care of Tarbiat Modares University, Tehran, Iran (82/2067-March, 07, 2016). A total number of 18 four-day- old Pekin ducklings with an average initial body weight of 62.50 ± 5.00 g were obtained from a local hatchery (Moghadam Farm, Qom, Iran). The ducklings were then weighed, labeled, and randomly assigned to 18 battery cages (200 cm2 floor spaces per bird) equipped with nipple waterers in an environmentally controlled room. The two weeks experiment consisted of three treatments with six individual birds in a completely randomized experimental design. All diets were formulated to meet or exceed the National Research Council requirements for ducks from 4 to 18 days of age (Table 1).12 Dietary treatments included: A: Control (basal diet without AFs), B: Contaminated feed with 0.20 mg kg-1 AFs (contaminated rice grains powder) C: Contaminated feed with 0.20 mg kg-1 AFs (methanol extract of contaminated rice grains). Feed and water were provided ad libitum during the period of study (4 to 18-day post-hatch). Ducklings were monitored daily for any health problems.
Table 1.
Ingredient composition and proximate analysis of diet.
| Ingredients | Quantity (%) |
|---|---|
| Corn | 56.29 |
| Soybean meal | 39.05 |
| Soy oil | 1.69 |
| MCP | 1.19 |
| CaCO3 | 0.88 |
| Common salt | 0.34 |
| DL-Methionine | 0.06 |
| Vitamin and mineral premix* | 0.50 |
| Total | 100 |
| Nutrients composition (%) | |
| Metabolizable energy (kcal kg -1 ) | 2,900 |
| Crude Protein | 22.00 |
| Calcium | 0.65 |
| Available phosphorus | 0.40 |
| Methionine | 0.40 |
| Methionine + cystine | 0.76 |
| Lysine | 1.19 |
| Threonine | 0.83 |
| Sodium | 0.15 |
*Provided per kg of diet, Vitamin A: 8,000 IU, Vitamin D3: 1,200 IU, Vitamin E: 3.00 IU, Vitamin K3: 2.00 mg, Riboflavin: 8.00 mg, Nicotinic acid: 10.00 mg, Pantothenic acid: 150 mg, Copper: 2.00 mg, Iodine: 1.20 mg, Cobalt: 0.20 mg, Selenium: 0.10 mg.
Aflatoxins production. The AFs were produced via fermentation of rice by Aspergillus parasiticus PTCC-5286. The sterile substrate, placed in Erlenmeyer flasks, was inoculated with 2.00 mL of an aqueous suspension of the mold containing 107 spores mL-1. Cultures were allowed to grow for 7 days at 25.00 ˚C in darkness. On the seventh day, the Erlenmeyer flasks were autoclaved, and the culture material was dried for 48 hr at 40.00 ˚C in a forced-air oven and then ground to a fine powder.13 The AFs levels in the ground culture material were measured by HPLC method.14 The contaminated rice powder (raw substrate containing AFs) was incorporated into the basal diet to provide 0.20 mg AFs kg-1 of feed as the source 1, and the AFs extracted with methanol was sprayed on the basal diet to provide 0.20 mg AFs kg-1 of feed as source 2.
Sample collection. At the end of the experiment, ducklings were fasted for 12 hr. The body weight of each bird was recorded, and a 5.00-mL blood sample was collected from jugular vein in a test tube without anticoagulant. Blood samples were centrifuged at 2,500 g for 10 min, and the serum was separated and stored in 1.50 mL centrifuge tubes at −20.00 ˚C until analysis. After bleeding, birds were euthanized by decapitation for organ weight measurement, including heart, liver, spleen, kidney and bursa of Fabricius and their relative weights were calculated as (organ weight/live body weight) × 100.
Serum biochemistry and enzyme activity. Six replicate serum samples per treatment (18 samples in total) were analyzed for albumin, globulin, total protein, glucose, uric acid, creatinine, triglyceride, cholesterol, HDL, LDL, alanine aminotransferase (ALT), alkaline phos-phatase (ALP), aspartate aminotransferase (AST), lactate dehydrogenase (LDH). The analyses of the serum samples were performed by spectrophotometric methods using commercially available kits (Pars Azmun, Tehran, Iran).
Intestinal morphology. The digestive tract was carefully excised and sections were removed from the jejunum, the samples were flushed with physiological saline and plunged in 10.00% formalin, processed by the standard paraffin sectioning, stained by Hematoxylin-Eosin (H&E) and examined under a light microscope. Villus length was measured from the top of the villus to the upper part of the lamina propria, crypt depth was measured from the base upwards to the region of transition between the crypt and villus, villus width was measured at the widest area of each villus15 and villus surface area was calculated using the formula:
Area (mm 2 ) = 2π× (VW/2) ×VL
where, VW is the villus width (µm) and VL is the villus length (µm).16 Reported values were means of 10 villi from each bird, six birds per treatment.
Liver histopathology. Parts of livers were fixed in 10.00% neutral buffered formalin and the fixed tissues were trimmed, embedded in paraffin, and stained with H&E for histopathological examination by light microscope.
Statistical analysis. All data were analyzed using the GLM procedure using SAS software (version 9.1; SAS Institute, Cary, USA) for completely randomized design. The means were compared by Duncan's multiple range tests (p ≤ 0.05).
Results
The results of HPLC analysis to assess the concentration of AFs in rice powder samples are presented in Table 2. The relative weights of heart, gizzard, bursa of Fabricius and proventriculus were not significantly affected by treatments (p > 0.05), (Table 3). The ducklings fed AFs contaminating diets by either source had higher relative weight of the spleen compared to control group (p ≤ 0.05), however, there was no significant difference between the AFs contaminated groups with various sources. Further-more, the AFs contaminate diets by the methanol extraction had higher relative weights of the liver compared to other experimental groups (p ≤ 0.05).
Table 2.
HPLC analysis for rice powder samples
| Aflatoxins | Concentration ( mg kg -1 ) | Limit | LOQ |
|---|---|---|---|
| AFB 1 | 14.25 | < 5.00 | 0.40 |
| AFB 2 | 1.04 | ND | 0.08 |
| AFG 1 | ND | ND | 0.40 |
| AFG 2 | ND | ND | 0.08 |
| Total | 15.29 | < 3000 | - |
LOQ: Limit of quantification, ND: Not detected.
Table 3.
Effects of treatments on weight of viscera and jejunum morphology in ducklings (18-days old).
| Parameter | Treatments | p -value | ||
|---|---|---|---|---|
| A | B | C | ||
| - | AFs (from raw rice grain powder) | AFs (from methanol extract) | ||
| Bursa of Fabricius (g per 100 g of BW) | 0.13 ± 0.04 | 0.10 ± 0.04 | 0.11 ± 0.03 | 0.36 |
| Liver (g per 100 g of BW) | 5.29 ± 1.15b | 5.62 ± 0.70ab | 7.40 ± 1.40a | 0.04 |
| Spleen (g per 100 g of BW) | 0.20 ± 0.06b | 0.33 ± 0.02a | 0.32 ± 0.07a | 0.02 |
| Heart (g per 100 g of BW) | 0.93 ± 0.16 | 0.86 ± 0.14 | 0.88 ± 0.14 | 0.77 |
| Gizzard (g per 100 g of BW) | 6.65 ± 0.94 | 5.79 ± 1.46 | 6.56 ± 0.75 | 0.51 |
| Villus height (µm) | 767.80 ± 118.65a | 663.30 ± 93.47b | 548.30 ± 127.63 c | 0.001 |
| Villus width (µm) | 108.50 ± 22.13 | 109.30 ± 30.11 | 101.60 ± 21.18 | 0.50 |
| Crypt depth (µm) | 5.30 ± 1.42b | 6.90 ± 1.35a | 5.50 ± 1.03b | 0. 008 |
| Goblet cell density (No. per 100 µm) | 5.10 ± 1.46 | 4.40 ± 0.74 | 4.60 ± 0.92 | 0. 17 |
| Villus surface area (mm 2 ) | 0.25 ± 0.07a | 0.22 ± 0.06a | 0.17 ± 0.05b | 0.066 |
ab Means with the same superscript in the same row for each variable showing no significant difference (p ≤ 0.05).
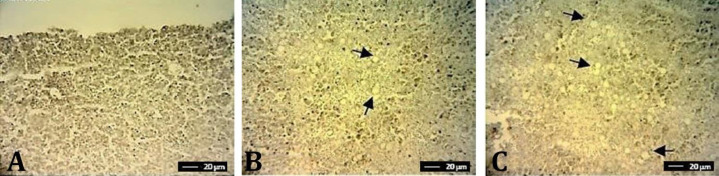
Morphometric studies showed that feeding ducklings with AFs contaminated diets by both sources resulted in a significant decrease in jejunum villus height and villus surface area compared to the control group (p ≤ 0.05). Jejunum villus width and goblet cell counts were not significantly affected by treatments (p > 0.05). Feeding 0.20 mg kg-1 AFs in the form of contaminated rice powder and methanol extract resulted in the reduction of serum total protein, albumin, glucose, triglyceride, cholesterol, HDL, LDL, and creatinine (p ≤ 0.05). However, triglyceride and creatinine levels were not significantly different between the groups of methanol extract and control. There was no difference in ALT, AST, and ALP levels among the groups, however, LDH level was affected significantly by different dietary treatments (p ≤ 0.05), (Table 4). The group received contaminated rice powder showed a significant increase in comparison with the other dietary treatments (p ≤ 0.05). Histopathological examinations disclosed accumulation of large fat droplets replacing the nucleus and resulting in cell swelling (hydropic degeneration) in hepatocytes of duckling given dietary aflatoxin by the two AFs sources. In the control group, the hepatic tissue structures were normal and showed no sign of liver injury as judged by light microscopic observations (Fig. 1).
Table 4.
Effects of treatments on serum biochemical parameters and hepatic enzymes activity (18 days old)
| Parameter | Treatments | p -value | ||
|---|---|---|---|---|
| A | B | C | ||
| - | AFs (from raw rice grain powder) | AFs (from methanol extract) | ||
| Protein (g dL -1 ) Total | 4.80 ± 0.11a | 4.49 ± 0.26ab | 4.42 ± 0.34 b | 0.05 |
| Albumin (g dL -1 ) | 1.86 ± 0.05a | 1.55 ± 0.07b | 1.61 ± 0.06b | 0.001 |
| Globulin (g dL -1 ) | 2.94 ± 0.09 | 2.87 ± 0.28 | 2.88 ± 0.20 | 0. 81 |
| Triglyceride (mg dL -1 ) | 155.16 ± 8.67a | 132.59 ± 12.71b | 146.55 ± 14.25ab | 0.01 |
| Cholesterol (mg dL -1 ) | 181.47 ± 14.76a | 135.45 ± 10.91b | 125.70 ± 8.24b | 0.04 |
| HDL (mg dL -1 ) | 47.39 ± 2.92a | 40.72 ± 4.33b | 41.56 ± 4.87b | 0.03 |
| LDL (mg dL -1 ) | 134.08 ± 12.81a | 95.22 ± 7.22b | 84.14 ± 4.35c | 0.001 |
| Glucose (mg dL -1 ) | 139.88 ± 7.98a | 132.44 ± 11.75ab | 121.85 ± 13.63b | 0.04 |
| Uric acid (mg dL -1 ) | 5.66 ± 0.36b | 6.38 ± 0.53a | 6.13 ± 0.35ab | 0.03 |
| Alanine aminotransferase (IU L -1 ) | 46.64 ± 18.26 | 53.60 ± 19.50 | 49.18 ± 26.66 | 0.94 |
| Aspartate aminotransferase (IU L -1 ) | 48.74 ± 13.89 | 59.66 ± 26.80 | 70.14 ± 5.55 | 0.15 |
| Lactate dehydrogenase (IU L -1 ) | 780.40 ± 212.04b | 1375.60 ± 391.04a | 1012.90 ± 244.82b | 0.01 |
| Alkaline phosphatase (IU L -1 ) | 167.41 ± 39.78 | 191.92 ± 58.46 | 153.93 ± 14.58 | 0.30 |
ab Means with the same superscript in the same row for each variable showing no significant difference (p ≤ 0.05).
Fig. 1.
Photomicrographs (optical microscopy) of Hematoxylin and Eosin-stained ducklings liver sections from different treatments A) Normal histological appearance of the liver in the control group, B) Vacuolar degeneration with small and large fat droplets (black arrows) in ducklings given dietary AFs at concentration of 0.20 ppm via methanol extract and , via rice powder (C)
Discussion
Aflatoxins have been reported to affect the various body organs and increase the size of liver, kidney, gizzard and spleen and decrease the weight of bursa of Fabricius and thymus.17 The addition of AFs in the diet of broilers showed a significant increase in relative weights of liver, kidney, gizzard and spleen.18,19 Regardless of the atrophy of the bursa of Fabricius and thymus glands, the apparent alteration of splenic function is also of diagnostic significance and implies the alteration in the birds with aflatoxicosis.17 The liver is intended the target organ for AFs because it is the organ where most AFs are converted to the reactive 8,9-epoxide form, which is found to bind DNA and proteins, damaging the liver cell and increasing liver weight.20,21 Our results were in agreement with previous reports that AFB1 reported to cause pallor discoloration and enlargement of the liver in broiler and ducklings.22,23
The gastrointestinal tract is the principal route of entry of mycotoxin- contaminated diet also the main path of excretion aflatoxin metabolites from the bile.24 A larger small intestine volume or surface area (the surface area is further increased by villi and microvilli) presumably allows more area over which these nutrients can be digested and absorbed and also the villus surface area is an important factor for gastrointestinal absorption.25 According to results of the present study absorptive surface jejunum was declined by consumption of AFs sources.
Intestinal epithelial cells can be exposed to high concentrations of toxins by ingestion of aflatoxins-contaminated feed and direct intestinal injury can be applied by the biological action of mycotoxins.26 Epithelium cells lining the small intestine have a high turnover, as it is necessary to hold the natural balance. Investigation showed that intestinal morphology (intestinal crypt depth) and the specific activity of intestinal disaccharidase and maltase were also altered by AFB1 ingestion.27 Broilers fed diets contaminated with 0.50 mg deoxynivalenol kg-1 had shorter and thinner villi which resulted in lighter small intestines compared to birds fed control diets.28
According to the results obtained in the current study ducklings exposed to two various sources of AFs showed a decline in the jejunum villus height and subsequently a decrease in the villus surface area. The small intestine provides the main way for excretion of xenobiotics.29 Enzymatic biotransformation in intestinal mucosal cells has the potential to remove these generally hydrophobic xenobiotics by directly making easy their repulsion to the intestinal lumen, or by conjugation with subsequent excretion.28 However, the biotransformation could also potentially activate some xenobiotics such as AFs with toxic effects on the organism. One of the important structures on the apical surface of small intestines epithelial cells is microvilli.29 Investigators reported that intestine function and growth performance of animals are associated with the morphology of the villi and crypts.30 The involvement of microvilli has been established in various functions such as secretion, mechanotransduction, absorption, and cellular adhesion. On the other hand, epithelial cell mitochondrion plays important roles not only in producing ATP but in controlling apoptosis and contributing to the calcium homeostasis process of cells.31 Damage to the mitochondria and microvilli by aflatoxins can cause dysfunction of these structures resulting in functional disorders of absorptive cells in the small intestine because it can induce reactive oxygen species to cause oxidative stress by damaging cells and DNA and induce genetic alterations leading to DNA damage and mitochondrial permeability alterations.32
Aflatoxicosis is related to serum biochemical changes involving decreased total protein, albumin, cholesterol and triglyceride values33 which are the results of impaired carbohydrate and lipid metabolism.34
The liver is the primary site for protein synthesis, enzymatic metabolism, detoxification protective functions, AFs activation and their toxicity.35 The AFB1 adducts with biomolecules cause damage to liver cells that have a negative effect on metabolic functions of the liver during AFB1 exposure. This is represented by AFB1 that reduced total serum protein levels, as the liver is responsible for the production of most proteins and aflatoxicosis negatively affects albumin, globulin, cholesterol, and triglyceride levels in serum.33,36 Protein content may be decreased because AFB1-DNA adducts prevent transcription or translation and AFB1-lysine adducts result in protein degradation or excretion. AFB1-exposed chickens exhibited a reduction in hepatic fatty acid synthesis which could be accountable for the lower production of serum cholesterol and triglycerides.1 The results of this study in regards to reduction in serum total protein, albumin, cholesterol, and triglyceride were in agreement with previous results on broilers34 and ducks.37
Aflatoxin has a marked effect on hepatic enzyme activities that reflect liver damage and leakage of enzymes in the bloodstream.36 Previous researchers showed that consuming aflatoxin increased the concentration of liver enzymes especially AST, ALT and LDH in broilers34,38 and ducks.36 In the present study, the increase in LDH activity due to aflatoxins consumption by two sources was found to be significant. It has been reported that LDH level may be elevated due to cell necrosis in many diseases.39
Liver is the major target organ in terms of the pathological effects of the mycotoxin.15 AFs can induce reactive oxygen species generation leading to oxidative stress and liver injury.40 Hepatocytes control hepatic biochemical and metabolic functions in the liver, including triglyceride metabolism.41 Disorder of the release in hepatic triglyceride to the plasma due to aflatoxicosis resulted in the accumulation of large lipid droplets in liver tissue and changes in plasma lipid profile.42 In the present study, significant changes were observed in histopathology in birds fed AFs contaminated diets by two sources, although no changes were observed in control group that were in agreement with previous investigations.43-45
In conclusion, the current study demonstrated that feeding ducklings with AFs-contaminated diets by the two applied sources from 4 to 18 day of age-induced deleterious effects on organ development, intestinal morphology and biochemical parameters. According to the results obtained from the analysis of target parameters it seemed that diets contaminated with two sources exerted harmful effects, although in the methanol extract consumer group was slightly higher. A possible hypothesis for these observations was that the methanolic extract could provide quick access to effective compounds such as AFs compared to raw rice grain powder.
Acknowledgments
We would like to thank the animal caretakers at the Department of Poultry Science (Tarbiat Modares University, Tehran, Iran) for the help provided in handling the animals involved in this study.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Monson SM, Coulombe RA, Reed KM. Aflatoxicosis: Lessons from toxicity and responses to aflatoxin B1 in poultry. Agriculture. 2015;5(3):742–777. [Google Scholar]
- 2.Girish C, Smith T. Impact of feed-borne mycotoxins on avian cell-mediated and humoral immune responses. World Mycotoxin J. 2008;1(2):105–121. [Google Scholar]
- 3.Zhao J, Shirley RB, Dibner JD, et al. Comparison of hydrated sodium calcium aluminosilicate and yeast cell wall on counteracting aflatoxicosis in broiler chicks. Poult Sci. 2010;89(10):2147–2156. doi: 10.3382/ps.2009-00608. [DOI] [PubMed] [Google Scholar]
- 4.Rosa AP, Uttpatel R, Santurio JM, et al. Performance of broilers derived from breeder hens fed with diets containing AFs and EGM as adsorbent. R Bras Zootec. 2012;41(2):347–352. [Google Scholar]
- 5.Kraieski AL, Hayashi RM, Sanches A, et al. Effect of aflatoxin experimental ingestion and Eimeira vaccine challenges on intestinal histopathology and immune cellular dynamic of broilers: applying an intestinal health index. Poult Sci. 2017;96(5):1078–1087. doi: 10.3382/ps/pew397. [DOI] [PubMed] [Google Scholar]
- 6.Hussain S, Khan MZ, Khan A, et al. Toxico-pathological effects in rats induced by concurrent exposure to aflatoxin and cypermethrin. Toxicon. 2009;53(1):33–41. doi: 10.1016/j.toxicon.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 7.El-Mahalaway AM. Protective effect of curcumin against experimentally induced aflatoxicosis on the renal cortex of adult male albino rats: a histological and immunohisochemical study. Int J Clin Exp Pathol. 2015;8(6):6019–6030. [PMC free article] [PubMed] [Google Scholar]
- 8.Buss P, Caviezel M, Lutz WK. Linear dose-response relationship for DNA adducts in rat liver from chronic exposure to aflatoxin B1. Carcinogenesis. 1990;11(12):2133–2135. doi: 10.1093/carcin/11.12.2133. [DOI] [PubMed] [Google Scholar]
- 9.Ma H, Zhang N, Sun L, et al. Effects of different substrates and oils on aflatoxin B1 production by Aspergillus parasiticus. Eur Food Res Technol. 2015;240(3):627–634. [Google Scholar]
- 10.Huie CW. A review of modern sample-preparation techniques for the extraction and analysis of medicinal plants. Anal Bioanal Chem. 2002;373(1-2):23–30. doi: 10.1007/s00216-002-1265-3. [DOI] [PubMed] [Google Scholar]
- 11.Zhang L, Dou X-W, Zhang C, et al. A review of current methods for analysis of mycotoxins in herbal medicines. Toxins (Basel) 2018;10(2) doi: 10.3390/toxins10020065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Research Council. Nutrient requirements of poultry. Washington, USA: National Academy of Science Press; 1994. pp. 42–43. [Google Scholar]
- 13.Shotwell OL, Hesseltine CW, Stubblefield RD. Production of aflatoxin on rice. Appl Microbiol. 1966;14(3):425–428. doi: 10.1128/am.14.3.425-428.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yazdanpanah H, Zarghi A, Shafaati AR, et al. Analysis of aflatoxin b1 in Iranian foods using HPLC and a monolithic column and estimation of its dietary intake. Iran J Pharm Res. 2013;12(Suppl):83–89. [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X, Murdoch R, Zhang Q, et al. Effects of dietary protein concentration on performance and nutrient digestibility in Pekin ducks during aflatoxicosis. Poult Sci. 2016;95(4):834–841. doi: 10.3382/ps/pev378. [DOI] [PubMed] [Google Scholar]
- 16.Galarza-Seeber R, Latorre JD, Bielke LR, et al. Leaky gut and mycotoxins: Aflatoxin B1 does not increase gut permeability in broiler chickens. Front Vet Sci. 2016;3:10. doi: 10.3389/fvets.2016.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manafi M. Counteracting effect of high grade sodium bentonite during aflatoxicosis in broilers. J Agric Sci Technol. 2012;14(3):539–547. [Google Scholar]
- 18.Girish CK, Devegowda G. Evaluation of modified gluco-mannan (Mycosorb®) and hydrated sodium calcium aluminosilicate to ameliorate the individual and combined toxicity of aflatoxin and T-2 toxin in broiler chickens. In proceedings: 16th Australian Poultry Science Symposium. Sydney, Australia. 2004:126–129. [Google Scholar]
- 19.Gowda NKS, Ledoux DR, Rottinghaus GE, et al. Efficacy of turmeric (Curcuma longa), containing a known level of curcumin, and a hydrated sodium calcium alumino-silicate to ameliorate the adverse effects of aflatoxin in broiler chicks. Poult Sci. 2008;87(6):1125–1130. doi: 10.3382/ps.2007-00313. [DOI] [PubMed] [Google Scholar]
- 20.Pasha TN, Farooq MU, Khattak FM, et al. Effectiveness of sodium bentonite and two commercial products as aflatoxin absorbents in diets for broiler chickens. Anim Feed Sci Tech. 2007;132(1-2):103–110. [Google Scholar]
- 21.Solis-Cruz B, Hernandez-Patlan D, Petrone VM, et al. Evaluation of cellulosic polymers and curcumin to reduce aflatoxin B1 toxic effects on performance, biochemical, and immunological parameters of broiler chickens. Toxins (Basel) 2019;11(2):121. doi: 10.3390/toxins11020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hussain Z, Khan MZ, Saleemi MK, et al. Clinicopatho-logical effects of prolonged intoxication of aflatoxin B1 in broiler chicken. Pak Vet J. 2016;36(4):477–481. [Google Scholar]
- 23.Arak H, Karimi Torshizi MA, Hedayati M, et al. Comparative evaluation of aflatoxin and mineral binding activity of molecular imprinted polymer designed for dummy template using in vitro and in vivo models. Toxicon. 2019;166:66–75. doi: 10.1016/j.toxicon.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Bbosa GS, Kitya D, Lubega A, et al. Review of the biological and health effects of aflatoxins on body organs and body systems. In: Razzaghi-Abyaneh M (Ed). Aflatoxins: recent advances and future prospects. London, UK: Intech Open Ltd. 2013:239–265. [Google Scholar]
- 25.Lavin SR, Karasov WH, Ives AR, et al. Morphometrics of the avian small intestine compared with that of nonflying mammals: a phylogenetic approach. Physiol Biochem Zool. 2008;81(5):526–550. doi: 10.1086/590395. [DOI] [PubMed] [Google Scholar]
- 26.Peng X, Zhang S, Fang J, et al. Protective roles of sodium selenite against aflatoxin B1-induced apoptosis of jejunum in broilers. Int J Environ Res Public Health. 2014;11(12):13130–13143. doi: 10.3390/ijerph111213130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Applegate TJ, Schatzmayr G, Prickel K, et al. Effect of aflatoxin culture on intestinal function and nutrient loss in laying hens. Poult Sci. 2009;88(6):1235–1241. doi: 10.3382/ps.2008-00494. [DOI] [PubMed] [Google Scholar]
- 28.Awad WA, Böhm J, Razzazi-Fazeli E, et al. Effects of feeding deoxynivalenol contaminated wheat on growth performance, organ weights, and histological parameters of the intestine of broiler chickens. J Anim Physiol Anim Nutr (Berl) 2006;90(1-2):32–37. doi: 10.1111/j.1439-0396.2005.00616.x. [DOI] [PubMed] [Google Scholar]
- 29.de Santa Barbara P, van den Brink GR, Roberts DJ. Development and differentiation of the intestinal epithelium. Cell Mol Life Sci. 2003;60(7):1322–1332. doi: 10.1007/s00018-003-2289-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang JF, Song XM, Huang X, et al. Effects of alfalfa meal on growth performance and gastrointestinal tract development of growing ducks. Asian-Australas J Anim Sci. 2012;25(10):1445–1450. doi: 10.5713/ajas.2012.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang F, Zuo Z, Chen K, et al. Histopathological injuries, ultrastructural changes, and depressed TLR expression in the small intestine of broiler chickens with aflatoxin B1. Toxins (Basel) 2018;10(4):131. doi: 10.3390/toxins10040131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alassane-Kpembi I, Pinton P, Oswald IP. Effects of mycotoxins on the intestine. Toxins (Basel) 2019;11(3):159. doi: 10.3390/toxins11030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siloto EV, Oliveira EFA, Sartori JR, et al. Lipid metabolism of commercial layers fed diets containing aflatoxin, fumonisin, and a binder. Poult Sci. 2013;92(8):2077–2083. doi: 10.3382/ps.2012-02777. [DOI] [PubMed] [Google Scholar]
- 34.Azizpour A, Moghadam N. Effects of yeast gluco-mannan and sodium bentonite on the toxicity of afla-toxin in broilers. Braz J Poult Sci. 2015;17(spe):7–13. [Google Scholar]
- 35.Rawal S, Kim JE, Coulombe Jr R. Aflatoxin B1 in poultry: toxicology, metabolism and prevention. Res Vet Sci. 2010;89(3):325–331. doi: 10.1016/j.rvsc.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 36.Chen X, Horn N, Cotter PF, et al. Growth, serum biochemistry, complement activity, and liver gene expression responses of Pekin ducklings to graded levels of cultured aflatoxin B1. Poult Sci. 2014;93(8):2028–2036. doi: 10.3382/ps.2014-03904. [DOI] [PubMed] [Google Scholar]
- 37.Rattanasinthuphong K, Tengjaroenkul B, Tengjaro-enkul U, et al. Efficacy of mycosorbents to ameliorate the adverse effects of natural aflatoxin contamination in the diets of Cherry Valley ducks. Livest Res Rural Dev. 2017;29:3. [Google Scholar]
- 38.Barati M, Chamani M, Mousavi SN, et al. Effect of commercial toxin binder, native probiotic strains, cell wall yeast and aluminosilicate in diets contaminated with aflatoxin, on the expression of GOT2, CYP450 1A5 genes and serum concentrations of liver enzymes in broiler chickens. Kafkas Univ Vet Fak Derg. 2017;23(6):953–960. [Google Scholar]
- 39.Kaki S, Moeini MM, Cheraghi J. Effects of zeolite and mycosorb on serum biochemical and hematological parameters of broilers chicken aflatoxicosis. J. Blood Lymph. 2012;2:105 –7831. [Google Scholar]
- 40.Mary VS, Theumer MG, Arias SL, et al. Reactive oxygen species sources and biomolecular oxidative damage induced by aflatoxin B1 and fumonisin B1 in rat spleen mononuclear cells. Toxicology. 2012;302(2-3):299–307. doi: 10.1016/j.tox.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 41.Alves‐Bezerra M, Cohen DE. Triglyceride metabolism in the liver. Compr Physiol. 2011;8(1):1–8. doi: 10.1002/cphy.c170012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rotimi OA, Rotimi SO, Duru CU, et al. Acute aflatoxin B1–Induced hepatotoxicity alters gene expression and disrupts lipid and lipoprotein metabolism in rats. Toxicol Rep. 2017;4:408–414. doi: 10.1016/j.toxrep.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumar R, Balachandran C. Histopathological changes in broiler chickens fed aflatoxin and cyclopiazonic acid. Vet Arh. 2009;79(1):31–40. [Google Scholar]
- 44.Colakoglu F, Donmez HH. Effects of aflatoxin on liver and protective effectiveness of esterified glucomannan in Merino rams. Sci World J. 2012:2012–462925. doi: 10.1100/2012/462925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gholami-Ahangaran M, Rangsaz N, Azizi S. Evaluation of turmeric (Curcuma longa) effect on biochemical and pathological parameters of liver and kidney in chicken aflatoxicosis. Pharm Biol. 2016;54(5):780–787. doi: 10.3109/13880209.2015.1080731. [DOI] [PubMed] [Google Scholar]