Abstract
Fasciolosis is a zoonotic parasitic disease caused by the trematode Fasciola hepatica. The proteases are essential for the survival of parasites. The present study was aimed to determine serine proteases activities in miracidia of F. hepatica and evaluate the effects of pH and different inhibitors on the serine proteases activities. Adult F. hepatica helminths were removed from naturally infected livers of the slaughtered cattle and crushed. The eggs were incubated at 28.00 ˚C for 16 days. The released miracidia were homogenized and total proteolytic activity of the extract of miracidia at different pH values were evaluated. Serine proteases activities were determined using specific substrates. The inhibitory effects of chemical and herbal inhibitors on the enzymes were also assessed. The extract of miracidia hydrolyzed azocasein with optimum activity at pH 8.00. The optimum pH effect on serine proteases activities was found at alkaline pH. Phenylmethylsulfonyl fluoride and Bowman-Birk inhibitors inhibited and decreased the proteases activities in the miracidia extract. It was concluded that there were proteases activities in miracidia of F. hepatica which were inhibited by chemical and herbal inhibitors.
Key Words: Fasciola hepatica, Miracidia, Serine proteases
Introduction
Fascioliasis is a cosmopolitan disease caused by Fasciola hepatica in both humans and animals.1 The World Health Organization recognizes fascioliasis as a major food-borne disease, with up to 17 million human cases in 61 countries with 180 million at risk.2,3 Fascioliasis is also a common parasitic infection in livestock causing significant losses in growth and production with annual economic losses of more than 3 billion US$ worldwide.2,4
Proteolysis is a crucial activity for physiological operations and facilitate important activities of parasites like invasion, egg hatching, excystment, nutrition, helminths fecundity, immune evasion and modulation of the host or fluke physiology.5-12 This makes proteases key targets for vaccine and drug production.13,14 The largest family of proteases is serine proteases which are important in penetration and tissue migration of a wide range of helminths larvae and are expressed in newly excysted larvae, both immature and mature flukes.15,16 Most earlier studies on proteases in Fasciola and Schistosoma species were, respectively, focused on cysteine and aspartic proteases, and serine proteases.17-20
In 1947, the possible role of proteases inhibitors in plant protection revealed that the insect larvae were unable to develop normally on soybean products. Trypsin inhibitors were expressed in soybeans and were toxic to the larvae of flour beetle.21 So far, many proteases inhibitors with activities against insects are reported.22,23 The role of serine proteases inhibitors as defensive compounds against predators was particularly well established.24 Environmental factors like pH are critical for life cycle of F. hepatica, specifically miracidia penetration into the aquatic snails, i.e. lymnaeid snails. Dalton et al. noted that serine proteases with “trypsin-like” activities from secretions of cercariae of S. mansoni were involved in host invasion.25 Accordingly, chemical and herbal inhibitors of serine proteases may probably be useful to inhibit miracidia penetration into the body of snails. Thus, the present study was carried out to determine serine proteases activities in miracidia of F. hepatica and to assess the effects of pH and different inhibitors on the serine proteases activities.
Materials and Methods
Helminths sampling and eggs collection. Adult Fasciola was isolated from naturally infected livers of the slaughtered cattle. The livers were cut into small pieces and adult Fasciola helminths were removed. They were washed several times in 0.01 M phosphate buffer saline (PBS; pH = 7.20) (Merck, Darmstadt, Germany) and stained using Asetokarmin (Shahrazma Co., Tehran, Iran). Fasciola species were examined under light microscope at 100× magnification and identified using key identification described by Soulsby.26 The identified helminths, F. hepatica were microscopically examined for the presence of eggs and crushed in a mortar containing 10.00 mL of distilled water and sieved to gather the eggs.1 The eggs were washed several times using 0.086% Ringer’s solution (Samen Co., Mashhad, Iran) and centrifuged at 445 g for 5 min. The supernatant was discarded and the precipitated eggs were washed three times. The collected eggs were incubated at 24.00 ˚C for 16 days. On the last day, they were exposed to the light with density of 100 Watts for 6 hr to stimulate miracidia release as previously described.1
Total protein and enzymes assay. To access total cytosolic proteins, miracidia were ground in liquid nitrogen eight times and then centrifuged at 16,000 g for 10 min at 4.00 ˚C. The supernatant was collected as the extract of miracidia and its protein content was determined using Bradford method.27 The pH optimum of proteases activities was determined and general proteolytic activities was also assessed using azocasein substrate.28 The reaction mixture including azocasein (Sigma-Aldrich, Taufkirchen, Germany) 2.00% (30.00 µL), universal buffer (90.00 µL, containing acetate, phosphate, borate (Merck, Darmstadt, Germany) 50.00 mM at pH 2.00-12.00) and miracidia extract (15.00 µL) was incubated at 36.00 ˚C for 60 min. Protein digestion was stopped using trichloroacetic acid (Merck) 30.00% (30.00 µL, v/v). To precipitate non-hydrolyzed azocasein, reaction mixture was incubated at 4.00 ˚C for 1 hr. The supernatant was collected and signal was measured by absorbance (450 nm) on ELISA plate reader (Nowin-gostar, Tehran, Iran).
Serine proteases assays. Serine proteases, i.e. trypsin and chymotrypsin were evaluated by adding miracidia extract (10.00 µL) and substrate solution 1.00 mM (10.00 µL) to universal buffer (85.00 µL; pH 2.00 - 12.00) and light absorbance was measured in wavelength of 405 nm on ELISA plate reader (Nowingostar). The substrates of BApNA (1.00 mM; Nα-benzoyl-DL-arginine 4-nitroanilide; Sigma-Aldrich) and SAAPFpNA (1.00 mM; N-succinyl-alanine-alanine-prolin-phenylalaninc-p-nitroanilide; Sigma-Aldrich) were, respectively, applied to do trypsin and chymotrypsin assays.29-31
Serine proteases, chemical and herbal inhibitors assays. The chemical inhibitors of serine proteases, i.e. phenylmethylsulfonyl fluoride (PMSF; 5.00 mM; Sigma-Aldrich), pepstatin (10.00 µM; Sigma-Aldrich) and ethylenediaminetetraacetic acid (EDTA; 10.00 mM, Sigma-Aldrich) was separately added to the extract of miracidia (15.00 µL), azocasein 2.00% (30.00 µL; Sigma-Aldrich) and universal buffer (90.00 µL) in parallel with control group. The mixture was incubated at 36.00 ˚C for 60 min and trichloroacetic acid 30.00% (30.00 µL; Merck) was added to stop protein digestion. Non-hydrolyzed azocasein was precipitated at 4.00 ˚C for 1 hr. The supernatant was collected and signal was measured by absorbance (450 nm) on ELISA plate reader (Nowingostar).
The herbal inhibitor, soybean Bowman-Birk inhibitor (SBBI; Sigma-Aldrich) activity was determined following described method with minor modification.32 Briefly, miracidia extract of F. hepatica (50.00 µg mL-1) as treatment groups along with SBBI (500 µg mL-1; Sigma-Aldrich) and control group were incubated at room temperature for 2 hr and centrifuged.33 Sediments were homogenized several times by grinding in liquid nitrogen and centrifuged at 16,000 g for 10 min at 4.00 ˚C. The supernatant was collected and serine proteases activities were measured using a mixture composed of the substrates (5.00 µL) and extract (10.00 µL) which added to universal buffer (85.00 µL; pH = 2.00 - 12.00) and signal was measured by absorbance (450 nm) on ELISA plate reader (Nowingostar).
Results
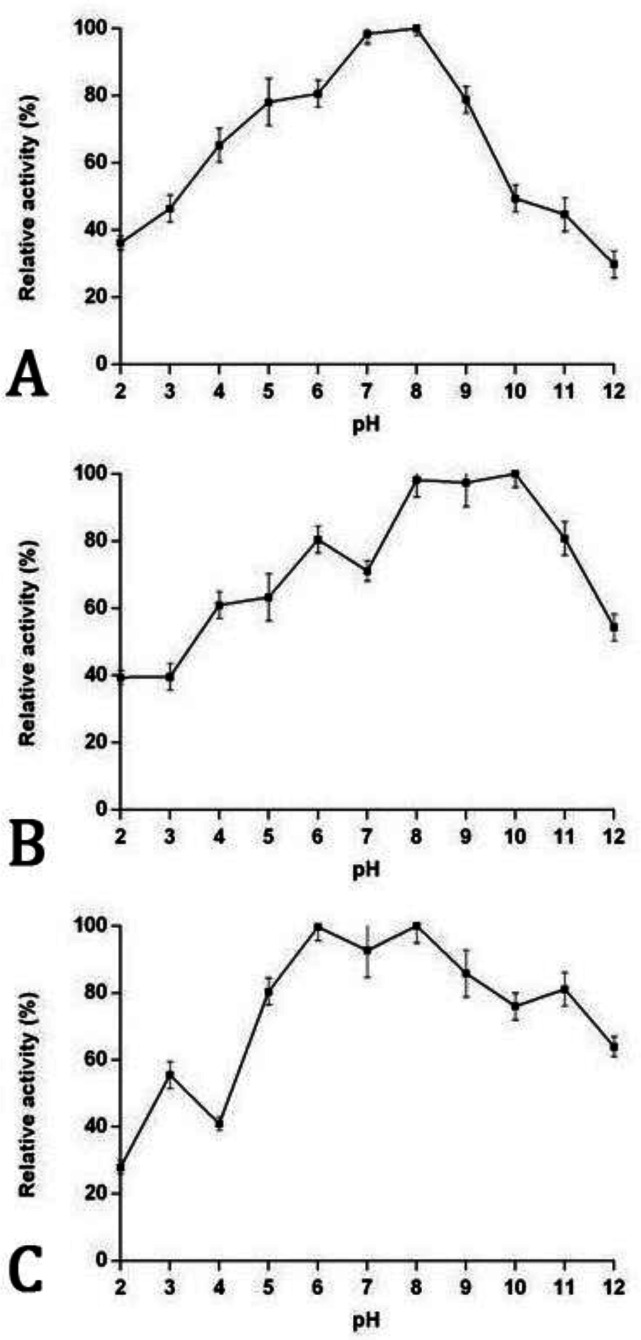
Serine proteases activities. The proteolytic activity and pH effects are shown in Figure 1A. The total protein content of miracidia was 0.47 ± 0.08 mg mL-1. The azocasein hydrolysis exhibited proteolytic activity in a wide range of pH 2.00 (36.00%) to pH 12.00 (29.00%). The maximum proteolytic activity in the extract of miracidia was at pH 8.00. The highest activity of the enzyme was approximately preserved at pH 7.00 (98.49%).
Fig. 1.
The proteolytic activity (A), trypsin activity (B), and chymotrypsin activity (C) of the extract of miracidia of Fasciola hepatica in different pH values using azocasein and SAAPFpNA
Serine proteases assay. Data pertaining to the enzymes responsible for hydrolysis of the substrate is tabulated in Table 1. The miracidia extract demonstrated the maximum activity toward BApNA at pH 10.00 (pH = 6.00 - 11.00) and 98.20% activity at pH 8.00 (Fig. 1B). The miracidia extract also had the highest activity toward SAAPFpNA at pH 8.00 (pH = 6.00 - 11.00) and 99.73% activity at pH 6.00 (Fig. 1C).
Table 1.
The effect of protease inhibitors on the extract of miracidia of Fasciola hepatica protease activity
| Type of target protease | Inhibitor | Concentration (mM) | Residual activity (%) |
|---|---|---|---|
| Serine | PMSF | 5.00 | 28.20 |
| Aspartic | Pepstatin | 10.00 | 59.30 |
| Metallo | EDTA | 10.00 | 79.80 |
PMSF: Phenylmethylsulfonyl fluoride; EDTA: Ethylenediaminetetraacetic acid.
The chemical and herbal inhibitors assays. The chemical inhibitors effects on serine proteases were 71.80% for PMSF, 40.70% for pepstatin, and 20.20% for EDTA. Of those, PMSF demonstrated the highest inhibitory effect with 70.00% reduction in serine proteases activities. The inhibitory effect of herbal inhibitor, SBBI on serine proteases activities in the extract was decreased and revealed effective inhibitory action against examined serine proteases toward BApNA (43.10% in 50.00 µg mL-1 and 50.20% in 500µg mL-1) and SAAPFpNA (53.00% in 50.00 µg mL-1 and 60.10% in 500 µg mL-1), (Table 2). The inhibitory effect of SBBI on serine proteases was found to be more effective in 500 µg mL-1. Both concentrations of SBBI, 50.00 µg mL-1 and 500 µg mL-1 had more inhibitory effect on chymotrypsin activity than trypsin activity.
Table 2.
The effects of soybean Bowman-Birk inhibitor (SBBI) on the extract of miracidia of Fasciola hepatica protease activity
| SBBI | Relative activity (%) | |
|---|---|---|
| BApNA (1.00 mM) | SAAPFpNA (1.00 mM) | |
| 50.00 µg mL -1 | 43.10 | 53.00 |
| 500 µg mL -1 | 50.20 | 60.10 |
BApNA: Nα-benzoyl-DL-arginine 4-nitroanilide, and SAAPFpNA: N-succinyl-alanine-alanine-prolin-phenylalaninc-p- nitroanilide.
Discussion
Many parasites penetrate into host tissues through proteolytic enzymes secretion which play an important role in migration of miracidia of F. hepatica in the intermediate hosts.34 In the current study, the extract of miracidia of F. hepatica hydrolyzed azocasein in different levels of pH and serine proteases activities. The optimum pH for serine proteases was in agreement with findings related to Leishmania amazonensis and Eimeria tenella.35,36 Geiger and Fritz demonstrated trypsin activity in larvae stage of Chrysomya bezziana was at pH 5.00 - 7.00.37 Johnston et al. noted trypsin-like and chymotrypsin enzymes in Heliothis virescens hydrolyzed synthetic substrates.38 Serine proteases activities are also reported from F. gigantica at pH 7.50.39 While Dalton and Heffernan demonstrated that all released proteases from F. hepatica were cysteine proteases.6
In the present study, serine proteases activities in miracidia of F. hepatica were inhibited with PMSF and pepstatin. In other studies, serine proteases were inhibited with inhibitors.31,38 In earlier studies, chemical inhibitors like thiol also inhibited proteases activities from F. hepatica, F. gigantica, S. mansoni, H. virescens, and Paragonymus westemani.38,40-45 Rege et al. reported serine proteases from adult stage of F. hepatica which were inhibited with leupeptin and PMSF in accordance with Dalton and Heffernan.6,46 Herbal inhibitors were competitive inhibitors of proteinases with a similar mode of action.47 Many studies were focused on the effects of herbal inhibitors, i.e. SBBI and soybean trypsin inhibitor (SBTI) in larval stages of parasites.48,49 In the present work, SBBI and PMSF inhibited proteases activities of the miracidia extract which indicated trypsin and chymo-trypsin activities. In another study, cysteine proteinases from adult stage of F. hepatica were sensitive to the inhibitors.7 Wijffels et al. reported that maximum proteolytic activity in adult stage of F. gigantica was at pH 4.00 - 6.00 which inhibited with chemical inhibitors, i.e. PMSF and pepstatin.7 Broadway and Duffey demonstrated the effects of purified SBTI and potato inhibitor II on growth and digestive physiology of larvae of H. zea and Spodoptera exigua.50
The present work was the first report of proteases activities in miracidia extract of F. hepatica at different pH levels. The optimal pH probably could be used to alter pH for reducing miracidium penetration into intermediate host snails. It was also demonstrated that the chemical and herbal inhibitors of serine proteases might be useful for interfering with the penetration into the tissues of snails. Therefore, further studies are recommended to do investigation on bioassay and effectiveness of SBBI for determination of its role in restriction of miracidia penetration into the intermediate hosts.
Conflict of interests
The authors declare that there is no conflict of interests.
References
- 1.Yakhchali M, Bahramnejad K. Inhibition effect of pH on the hatchability of Fasciola miracidia under laboratory conditions. Iran J Parasitol. 2016;11(1):30–34. [PMC free article] [PubMed] [Google Scholar]
- 2.Mas-Coma S. Epidemiology of fascioliasis in human endemic areas. J Helminthol. 2005;79(3):207–216. doi: 10.1079/joh2005296. [DOI] [PubMed] [Google Scholar]
- 3.McManus DP, Dalton JP. Vaccines against the zoonotic trematodes Schistosoma japonicum, Fasciola hepatica and Fasciola gigantica. Parasitology. 2006:133 Suppl: S43–S61. doi: 10.1017/S0031182006001806. [DOI] [PubMed] [Google Scholar]
- 4.Piedrafita D, Spithill TW, Smith RE, et al. Improving animal and human health through understanding liver fluke immunology. Parasite Immunol. 2010;32(8):572–581. doi: 10.1111/j.1365-3024.2010.01223.x. [DOI] [PubMed] [Google Scholar]
- 5.Rawlings ND, Barrett AJ, Finn R. Twenty years of the MEROPS database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res. 2016;44(D1):D343–D350. doi: 10.1093/nar/gkv1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalton JP, Heffernan M. Thiol proteases released in vitro by Fasciola hepatica. Mol Biochem Parasitol. 1989;35(2):161–166. doi: 10.1016/0166-6851(89)90118-7. [DOI] [PubMed] [Google Scholar]
- 7.Wijffels GL, Salvatore L, Dosen M, et al. Vaccination of sheep with purified cysteine proteinases of Fasciola hepatica decreases worm fecundity. Exp Parasitol. 1994;78(2):132–148. doi: 10.1006/expr.1994.1014. [DOI] [PubMed] [Google Scholar]
- 8.Cocude C, Pierrot C, Cêtre C, et al. Identification of a developmentally regulated Schistosoma mansoni serine protease homologous to mouse plasma kallikrein and human factor I. Parasitology. 1999;118(Pt 4):389–396. doi: 10.1017/s0031182098003874. [DOI] [PubMed] [Google Scholar]
- 9.Da'dara A, Skelly PJ. Manipulation of vascular function by blood flukes? Blood Rev. 2011;25(4):175–179. doi: 10.1016/j.blre.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horn M, Fajtová P, Rojo Arreola L, et al. Trypsin- and Chymotrypsin-like serine proteases in Schistosoma mansoni-- 'the undiscovered country'. PLoS Negl Trop Dis. 2014;8(3):e2766. doi: 10.1371/journal.pntd.0002766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fajtová P, Ŝtefanic S, Hradilek M, et al. Prolyl oligopeptidase from the blood fluke Schistosoma mansoni: from functional analysis to anti-schistosomal inhibitors. PLoS Negl Trop Dis. 2015;9(6):e0003827. doi: 10.1371/journal.pntd.0003827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dvořák J, Fajtová P, Ulrychová L, et al. Excretion/ secretion products from Schistosoma mansoni adults, eggs and schistosomula have unique peptidase specificity profiles. Biochimie. 2016;122:99–109. doi: 10.1016/j.biochi.2015.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jílková A, Řezáćova P, Lepŝík M, et al. Structural basis for inhibition of cathepsin B drug target from the human blood fluke, Schistosoma mansoni. J Biol Chem. 2011;286(41):35770–35781. doi: 10.1074/jbc.M111.271304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Molina-Hernández V, Mulcahy G, Pérez J, et al. Fasciola hepatica vaccine: we may not be there yet but we're on the right road. Vet Parasitol. 2015;208(1-2):101–111. doi: 10.1016/j.vetpar.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Y, Wen YJ, Cai YN, et al. Serine proteases of parasitic helminths. Korean J Parasitol. 2015;53(1):1–11. doi: 10.3347/kjp.2015.53.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sajid M, McKerrow JH. Cysteine proteases of parasitic organisms. Mol Biochem Parasitol. 2002;120(1):1–21. doi: 10.1016/s0166-6851(01)00438-8. [DOI] [PubMed] [Google Scholar]
- 17.Robinson MW, Dalton JP, Donnelly S. Helminth pathogen cathepsin proteases: it's a family affair. Trends Biochem Sci. 2008;33(12):601–608. doi: 10.1016/j.tibs.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Kasný M, Mikes L, Hampl V, et al. Chapter 4. Peptidases of trematodes. Adv Parasitol. 2009;69:205–297. doi: 10.1016/S0065-308X(09)69004-7. [DOI] [PubMed] [Google Scholar]
- 19.Mc Veigh P, Maule AG, Dalton JP, et al. Fasciola hepatica virulence-associated cysteine peptidases: a systems biology perspective. Microbes Infect. 2012;14(4):301–310. doi: 10.1016/j.micinf.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 20.Ingram JR, Rafi SB, Eroy-Reveles AA, et al. Investigation of the proteolytic functions of an expanded cercarial elastase gene family in Schistosoma mansoni. PLoS Negl Trop Dis. 2012;6(4):e1589. doi: 10.1371/journal.pntd.0001589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lipke H, Fraenkel GS, Liener IE. Growth inhibitors Effects of soybean inhibitors on growth of Tribolium confusum. J. Agric. Food Chem. 1954;2:410–414. [Google Scholar]
- 22.Pannetier C, Giband M, Couzi P, et al. Introduction of new traits into cotton through genetic engineering: insect resistance as example. Euphytica. 1997;96(1):163–166. [Google Scholar]
- 23.Koiwa H, Shade RE, Zhu-Salzman K, et al. Phage display selection can differentiate insecticidal activity of soybean cystatins. Plant J. 1998;14(3):371–379. doi: 10.1046/j.1365-313x.1998.00119.x. [DOI] [PubMed] [Google Scholar]
- 24.Huber R, Carrell RW. Implications of the three-dimensional structure of Alpha 1-antitrypsin for structure and function of serpins. Biochemistry. 1989;28(23):8951–8966. doi: 10.1021/bi00449a001. [DOI] [PubMed] [Google Scholar]
- 25.Dalton JP, Clough KA, Jones MK, et al. The cysteine proteinases of Schistosoma mansoni cercariae. Parasitology. 1997;114(Pt 2):105–112. doi: 10.1017/s003118209600830x. [DOI] [PubMed] [Google Scholar]
- 26.Soulsby EJL. Helminths, arthropods and protozoa of domesticated animals. 7th ed. London, UK: Baillière Tindall, 1982:800–809. [Google Scholar]
- 27.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 28.Elpidina EN, Vinokurov KS, Gromenko VA, et al. Compartmentalization of proteinases and Amylasesin Nauphoeta cinerea. midgut. Arch Insect Biochem Physiol. 2001;48(4):206–216. doi: 10.1002/arch.10000. [DOI] [PubMed] [Google Scholar]
- 29.Erlanger BF, Kokowsky N, Cohen W. The preparation and properties of two new chromogenic substrates of trypsin. Arch Biochem Biophys. 1961;95:271–278. doi: 10.1016/0003-9861(61)90145-x. [DOI] [PubMed] [Google Scholar]
- 30.Erlanger BF, Cooper AG, Bendich AJ. On the hetero-geneity of three-times-crystallized α-Chymotrypsin. Biochemistry. 1964;3(12):1880–1883. doi: 10.1021/bi00900a015. [DOI] [PubMed] [Google Scholar]
- 31.Da Silva-Lopez RE, Giovanni-De-Simone S. Leishmania (Leishmania) amazonensis: purification and characterization of a promastigote serine protease. Exp Parasitol. 2004;107(3-4):173–182. doi: 10.1016/j.exppara.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Kakade ΜL, Simons Ν, Liener ΙΕ. An evaluation of natural vs synthetic substrates for measuring the antitrypsin activities of soybean samples. Cereal Chem. 1969;46:518–526. [Google Scholar]
- 33.Jiraungkoorskul W, Sahaphong S, Tansatit T, et al. Eurytrema pancreaticum: the in vitro effect of praziquantel and triclabendazole on the adult fluke. Exp Parasitol. 2005;111(3):172–177. doi: 10.1016/j.exppara.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 34.McKerrow JH, Jones P, Sage H, et al. Proteinases from invasive larvae of the trematode parasite Schistosorna rnansoni degrade connective-tissue and basement-membrane macromolecules. Biochem J. 1985;231(1):47–51. doi: 10.1042/bj2310047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ribeiro de Andrade A, Santoro MM, Melo NM, et al. Leishmania (Leishmania) amazonensis: purification and characterization of a soluble serine oligo-peptidase from promastigotes. Exp Parasitol. 1998;89(2):153–160. doi: 10.1006/expr.1997.4269. [DOI] [PubMed] [Google Scholar]
- 36.Michalski WP, Crooks JK, Prowse SJ. Purification and characterization of a serine-type protease from Eimeria tenella oocytes. Int J Parasitol. 1994;24(2):189–195. doi: 10.1016/0020-7519(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 37.Geiger R, Fritz H. Trypsin. In: Bergmeyer HU, editor. Methods of enzymatic analysis. Vol 5. Weinheim, Germany: Verlag-Chemie; 1984. pp. 119–129. [Google Scholar]
- 38.Johnston K, Lee MJ, Brough C, et al. Protease activities in the larval midgut of Heliothis virescens: Evidence for trypsin and chymotrypsin-like enzymes. Insect Biochem Mol Biol. 1995;25(3):375–383. [Google Scholar]
- 39.Mohamed SA, Fahmy AS, Mohamed TM, et al. Proteases in egg, miracidium and adult of Fasciola gigantica, Characterization of serine and cysteine proteases from adult. Comp Biochem Physiol B Biochem Mol Biol. 2005;142(2):192–200. doi: 10.1016/j.cbpc.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 40.Fagbemi BO, Hillyer GV. The purification and characterization of a cysteine protease of Fasciola gigantica adult worms. Vet Parasitol. 1992;43(3-4):223–232. doi: 10.1016/0304-4017(92)90163-4. [DOI] [PubMed] [Google Scholar]
- 41.Yamakami K, Hamajima F. A neutral thiol protease secreted from newly excysted metacercariae of trematode parasite Paragonimus westemani: purification and characterization. Comp. Biochem Physiol B. 1990;95(4):755–758. doi: 10.1016/0305-0491(90)90312-h. [DOI] [PubMed] [Google Scholar]
- 42.Ghoneim H, Klinkert MQ. Biochemical properties of purified cathepsin B from Schistosoma mansoni. Int J Parasitol. 1995;25(12):1515–1519. doi: 10.1016/0020-7519(95)00079-8. [DOI] [PubMed] [Google Scholar]
- 43.Auriault C, Pierce R, Cesari IM, et al. Neutral protease activities at different developmental stages of Schisto-soma mansoni in mammalian hosts. Comp Biochem Physiol B Biochem Mol Biol. 1982;72(3):377–384. doi: 10.1016/0305-0491(82)90215-2. [DOI] [PubMed] [Google Scholar]
- 44.Smith AM, Dowd AJ, Heffernan M, et al. Fasciola hepatica: a secreted cathepsin L-like proteinase cleaves host immunoglobulin. Int J Parasitol. 1993;23(8):977–983. doi: 10.1016/0020-7519(93)90117-h. [DOI] [PubMed] [Google Scholar]
- 45.Harmsen MM, Cornelissen JBWJ, Buijs HECM, et al. Identification of a novel Fasciola hepatica cathepsin L protease containing protective epitopes within the propeptide. Int J Parasitol. 2004;34(6):675–382. doi: 10.1016/j.ijpara.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 46.Rege NN, Nazareth HM, Isaac A, et al. Immuno-therapeutic modulation of intraperitoneal adhesions by Asparagus racemosus. J Postgrad Med. 1989;35(4):199–203. [PubMed] [Google Scholar]
- 47.Laskowski Jr M, Kato I. Protein inhibitors of proteinases. Annu Rev Biochem. 1980;49:593–626. doi: 10.1146/annurev.bi.49.070180.003113. [DOI] [PubMed] [Google Scholar]
- 48.Hines ME, Nielsen SS, Shade RE, et al. The effect of two proteinase inhibitors, E-64 and the Bowman-Birk inhibitor, on the developmental time and mortality of Acanthoscelides obtectus. Entomol Exp Appl. 1990;57(3):201–207. [Google Scholar]
- 49.Johnston KA, Lee MJ, Gatehouse JA, et al. The partial purification and characterisation of serine protease activity in midgut of larval Helicoverpa armigera. Insect Biochem Mol Biol. 1991;21(4):389–397. [Google Scholar]
- 50.Broadway RM, Duffey SS. The effect of dietary protein on the growth and digestive physiology of larval Heliothis zea and Spodoptera exigua. J Insect Physiol. 1986;32(8):673–680. [Google Scholar]