Abstract
Gastrointestinal motility disorders can occur as either increased or decreased movements. Studies have shown that herbal ingredients such as essential oils can modify the increase and decrease of gastrointestinal movements of ruminants. Cinnamaldehyde at room temperature is an oily yellow liquid which is obtained from the steam distillation of the oil of cinnamon bark. It bears carminative activity and gastrointestinal, antimicrobial, and vasodilatory effects. This study examined the effects of cinnamaldehyde on the contraction of circular smooth muscles of abomasal fundus and the antrum, duodenum, and ileum of healthy cows using an in vitro approach. The results indicated that cinnamaldehyde had relaxant effects on the basal tonus and contractions caused by barium chloride (BaCl2) and carbachol (CCh) in these tissues dependent upon concentration and the origin of the smooth muscle. These effects were more prominent in the ileal smooth muscle preparations than in other tissues. This substance in the smooth muscle preparations of the abomasal fundus not only had no significant effect on the basal tonus, but also significantly increased the contractions caused by barium chloride at low concentrations. Study of the mechanism of action showed that, similar to verapamil, cinnamaldehyde applied its relaxation effect by blocking the calcium channels. The results showed that cinnamaldehyde possessed a spasmolytic effect mediated through blockage of the calcium channels, which may provide a pharmacological base to its medicinal use for diarrhea and spasms.
Key Words: Calcium channels, Cinnamaldehyde, Contraction, Cow, Smooth muscle
Introduction
The gastrointestinal disorders of ruminants can be divided into four main categories: Disruption in move-ment, secretion, digestion, and absorption. Gastrointestinal motility disorders may be seen in two forms: Increased and decreased movements.1 An increase in irritability in a particular segment of the intestine increases its motor activity and disrupts the natural downward gradient of the movements leading to the passage of the contents from the esophagus to the rectum. With this irritability, not only does the slope or passage speed of the contents to the rectum increases, but the increased activity of a stimulated part may be so high that it causes a reversed slope to the mouth and creates smoky waves toward the mouth as well.1 The increased gastrointestinal movements followed by the spasm of the smooth muscle of cow intestine cause abdominal pain.2 Also, increased intestinal movements and reduced intestinal transfer time may lead to dyspepsia and malabsorption and diarrhea due to insufficient time for digestion and absorption of consumed food.3,4 Therefore, the use of a spasmolytic drug seems necessary in such cases to reduce gastrointestinal movements.
Given the increasing tendency to use medicinal herbs in modern animal husbandry to produce organic products, medicinal plants have become the focus of attention as one of the most important alternative substances for traditional chemicals, especially antibiotics.5 Studies have shown that herbal ingredients such as essential oils, tannins, and saponins can increase or decrease the gastrointestinal movements of ruminants.6
Essential oils are considered more among the herbal ingredients than the rest of the materials. A research showed that some essential oils increased digestive secretions, reduced the number of pathogenic bacteria, and boosted the immune system.7
Cinnamaldehyde (3-phenylprop-2-enal), at room temperature is an oily yellow liquid with a boiling point of 246 ˚C. Cinnamon gets its flavor and taste from the essential oil that constitutes 1.00% to 4.00% of the bark of the Cinamomum zeylanicum tree. This oil contains various compounds, however, the highest frequency (65.00% to 75.00% of the oil) belongs to cinnamaldehyde. Cinnamaldehyde is obtained from the steam distillation, an especially efficient process, of the oil of cinnamon bark.8
Cinnamaldehyde bears carminative activity and reduces contractions of the smooth muscles in the ileum and trachea of Indian guinea pigs as well as the stomach, ileum, and colon of dogs.9 It also reduces gastric movements in rats, intestinal movements in mice, and the occurrence of stress-induced ulcers in mice.10
Cinnamaldehyde also bears antioxidant properties, the ability to cause apoptosis in cancer cells, glucolipid-reducing effects in diabetic animals, antimicrobial effects on pathogenic bacteria in animal and human food, and rumen-moderating fermentation in heifers. Moreover, it reduces the concentrations of serum glycerol and total glyceride and increases mean daily growth in lambs.11-15
The current study investigated the in vitro effects of cinnamaldehyde on basal tonus and contractions caused by spasmogens such as barium chloride (BaCl2) and carbachol chloride (C.ch) in the circular smooth muscle preparations of bovine abomasal fundus and the antrum, duodenum, and ileum and also aimed to determine its mechanism of action.
Materials and Methods
Chemicals and incubation medium. Cinnamaldehyde, acetylcholine chloride (Ach), verapamil hydrochloride, and CCh were purchased from Sigma Chemicals Co. (St. Louis, USA). Calcium chloride, potassium chloride, sodium chloride, glucose, magnesium sulfate, sodium dihydrogen phosphate, magnesium chloride, sodium bicarbonate, BaCl2, and dimethyl sulfoxide (DMSO) were obtained from Merck (Darmstadt, Germany).
Compositions (mM) of bathing solutions. 1) Krebs solution for abomasal and duodenal preparations: NaCl (118), KCl (4.75), MgSO4 (1.20), NaH2PO4 (1.20), CaCl2 (2.50), NaHCO3 (25.00), and glucose (11.50). 2) Tyrode’s solution for ileal preparations: NaCl (136.90), KCl (2.70), CaCl2 (1.80), NaHCO3 (11.90), MgCl2 (1.10), NaH2 PO4 (0.40), and glucose (5.60). 3) K+-rich and Ca++-free Tyrode’s solution (depolarization solution) for determining the calcium antagonist activity of cinnamaldehyde: NaC (91.04), KCl (50.00), MgCl2 (1.05), NaHCO3 (11.90), NaH2 PO4 (0.42), glucose (5.55), and EDTA (0.10) mM.
Gastrointestinal tissue collection and preparation. Tissue samples were collected from routinely slaughtered Holstein dairy cows with no previous history of gastro- intestinal disorders. The abomasum, duodenum, and ileum were removed within 20 to 30 min after stunning. Full-thickness specimens were harvested from the fundus and antrum of the abomasum, duodenum, and ileum by dissecting a rectangular piece of tissue from the location. Tissue specimens were immediately rinsed with cooled (4.00 ˚C) Krebs solution (for the fundus, antrum, and duodenum) and Tyrode’s solution (for the ileum). Specimens were stored at 1.00 L of cooled (4.00 ˚C) Krebs (for the fundus, antrum, and duodenum) and Tyrode’s (for the ileum) solution that had been oxygenated (95.00% O2 and 5.00% CO2) for 2 hr and transported from the slaughterhouse to the laboratory within 60 min. Upon arrival at the laboratory, whole pieces of tissue were placed in a Petri dish filled with Krebs or Tyrode’s solution, compatible with tissue sample, at room temperature. The mucosa was carefully removed from the muscle layers, and muscle strips (5.00 × 20.00 mm) were cut parallel to the circular muscle fibers.
Registration of smooth muscle activity. The muscle strips were mounted in separated 25-mL chambers, maintained at 37.00 ˚C in Krebs or Tyrode’s solution, and gassed continuously with a mixture of 95.00% O2 and 5.00% CO2. One end of each strip was fixed to the bottom of the chamber, and the other end was attached to an isometric muscle transducer (model TRI 202P; PanLab, Barcelona, Spain) coupled to a bridge amplifier (ML224; AD Instruments, Castle Hill, Australia) and data acquisition PowerLab system (ML870; AD Instruments) using Labchart Software (version 8.0; AD instruments).
Design of experiments. Specimens were allowed to equilibrate in the organ bath for 1 hr and muscle tension was preset to 2.00 g in two steps (1.00 g each) at 10 min intervals. During this time, the chamber solution was replaced every 15 min with fresh solution. All specimens were tested for functional viability prior to and after all experiments by adding 10.00 µM acetylcholine to the organ bath. In evaluating the effects of cinnamaldehyde on the smooth muscle preparations, including the circular smooth muscle of the abomasal fundus, abomasal antrum, duodenum, and ileum, the tissue isolated from each part was studied in the three groups, each containing eight tissue samples. In the first group, the effect of cumulative concentrations of cinnamaldehyde was examined on the basal tonus. When the tissue sample reached an equilibrium point in the tissue bath and followed a fixed baseline, the incremental concentrations of cinnam-aldehyde (0.001 to 10.00 M) were added cumulatively to the bath. In the second group, after the tissue samples reached equilibrium, they first underwent contraction under the influence of barium chloride (3.00 mM). Then the incremental concentrations of the studied substance (0.001 to 10.00 M) were added cumulatively to the bath. In the third group, the tissue samples were initially contracted under the influence of carbachol (CCh; 1.00 µM), and then, the incremental concentrations of cinnamaldehyde (0.001 to 10.00 M) were added cumulatively to the bath. To prepare various concentrations of cinnamaldehyde, this substance was first dissolved in a solution of DMSO 5.00% (as solvent). Then, concentrations of 0.001, 0.003, 0.01, 0.03, 0.10, 0.30, 1.00, 3.00, and 10.00 molar were prepared.
Determination of calcium antagonist activity. To confirm the calcium antagonist activity of cinnamaldehyde, the smooth muscle preparations from the ileum were studied in four groups, each containing six tissue samples. In the first group, cinnamaldehyde with a concentration of 3 molar; in the second group, cinnamaldehyde with a concentration of 10.00 molar; and in the third and fourth groups, verapamil with concentrations of 0.10 µM and 0.30 µM, respectively, were used according to the method described below to incubate the tissue samples and obtain the response curve to calcium concentrations. The tissue samples were allowed to stabilize in normal Tyrode’s solution, which was then replaced with Ca++-free Tyrode’s solution containing EDTA (0.10 mM) for 30 min in order to remove calcium from the tissues. This solution was further replaced with K+-rich and Ca++-free Tyrode’s solution. Following an incubation period of 30 min, control concentration response curves (CRCs) of Ca++ were obtained. When the control CRCs of Ca++ were found to be superimposable (usually after two cycles), the tissues were pretreated with cinnamaldehyde or verapamil for 10 min to test the possible calcium channel blocking effect.16
Statistical analysis. Visual inspection of boxplots was used to explore outliers and extremes values. The Shapiro-Wilks test and inspection of histograms and residual plots were used to examine the assumption of normality and homogeneity of variation. Statistical analysis of data was conducted using two-way repeated measures analysis of variance followed by Bonferroni test for multiple comparisons, if required. The results are presented as mean ± standard error. A value of p < 0.05 was considered significant. Statistical analyses were carried out using SPSS (version 25.0; IBM Corp., Armonk, USA).
Results
The results showed that various concentrations of cinnamaldehyde significantly decreased the basal and stimulated contractions in the smooth muscle pre-parations of the bovine digestive tract. This effect was more prominent in the ileal smooth muscle preparations than in other parts. In addition, this substance in the smooth muscle preparations of the abomasal fundus not only did not have a major effect on the basal tonus, but also significantly increased the contractions created by barium chloride at low concentrations. The study of the mechanism of action revealed that, like verapamil, cinnamaldehyde applied its relaxation effect through blocking the calcium channels. The cinnamaldehyde solvent (i.e. DMSO) had no significant effect on the contraction of smooth muscle isolates. At all stages of the tests, the effects created by cinnamaldehyde were eliminated after the tissue was rinsed. At the end of the test, the tissues showed a normal reaction to acetylcholine, indicating no tissue damage due to the presence of this substance.
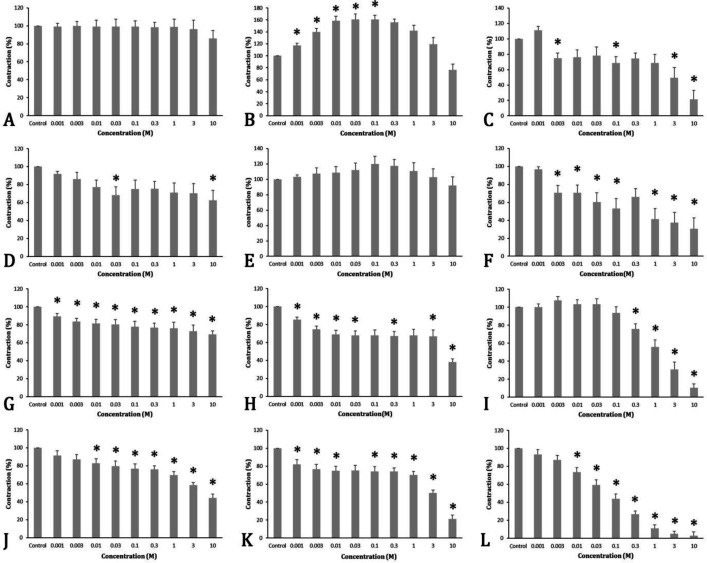
Cinnamaldehyde effects on the contractions of smooth muscles of the fundus . Examining the effects of various concentrations of cinnamaldehyde on the basal tonus in the smooth muscle of the fundus indicated that none of the concentrations had a significant effect. How-ever, they significantly increased the contractions caused by barium chloride at concentrations of 0.001 to 0.10 molar in the studied tissue. They also significantly reduced the contractions caused by CCh at concentrations of 0.003, 0.10, 3.00, and 10.00 molar (p < 0.05), (Fig. 1A-1C).
Fig. 1.
The effect of various concentrations of cinnamaldehyde for: Contraction in the circular smooth muscles of the bovine abomasal fundus (A-C). Contraction in the circular smooth muscles of bovine abomasal antrum (D-F). Contraction in the circular smooth muscles of bovine duodenum (G-I). Contraction in the circular smooth muscles of bovine ileum (J-L).
A, D, G, and J: Basal tonus (Base), B, E, H, and K: Barium chloride (BaCl2) induced, and C, F, I, and L: Carbachol-induced (CCh). The values indicate the mean and SEM. * indicates a significant difference compared to the control group (p < 0.05).
Cinnamaldehyde effect on the contractions of the smooth muscles of the antrum. The cumulative addition of various concentrations of cinnamaldehyde caused a significant reduction in the basal tonus at concentrations of 0.03 and 10.00 molar in the smooth muscles of the antrum. However, none of the concentrations had a significant effect on the contractions caused by barium chloride, and the contractions induced by CCh in the mentioned tissue were significantly reduced at concentrations of 0.003, 0.01, 0.03, 0.10, 1.00, 3.00, and 10.00 molar (p < 0.05), (Fig. 1D-1F).
Cinnamaldehyde effect on the contractions of the smooth muscles of the duodenum. The cumulative concentrations of cinnamaldehyde caused a significant reduction of basal tonus in the smooth muscles of the duodenum at concentrations of 0.001 to 10.00 molar. They also significantly reduced the contractions induced by barium chloride at concentrations of 0.001, 0.003, 0.01, 0.03, 0.30, 3.00, and 10.00 molar and the contractions induced by CCh at concentrations from 0.30 to 10.00 molar (p < 0.05), (Fig. 1G-1I).
Cinnamaldehyde effect on the contraction of the smooth muscles of the ileum. The cumulative addition of various concentrations of cinnamaldehyde caused a significant reduction in the basal tonus at concentrations of 0.01 to 10.00 molar in the smooth muscles of the ileum and significantly reduced the contractions induced by barium chloride at concentrations of 0.001 to 0.01 and 0.10 to 10.00 molar. It also caused a significant reduction in CCh-induced contractions at concentrations of 0.01 to 10.00 molar (p < 0.05), (Fig. 1J-1L).
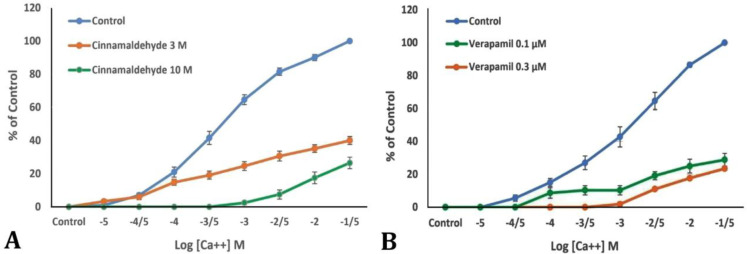
Blocking effect on calcium channels. Examination of the tissue responses to various concentrations of calcium before and after incubation with cinnamaldehyde revealed that the tissue response to various concentrations of calcium were decreased after treatment with the mentioned substance compared to before. The results were also compared to those from verapamil-treated samples, the standard of calcium channels blockers and found to be similar (Fig. 2).
Fig. 2.
Control concentration response curves (CRCs) of Ca++ in bovine ileum in the absence and presence of increasing concentrations of A) cinnamaldehyde and B) verapamil. The values expressed are mean ± SEM, (n = 6)
Discussion
In the present study, the effects of cinnamaldehyde on the contraction of smooth muscle preparations of different parts of the bovine digestive tract were examined. Tissue samples from different parts of the gastrointestinal tract were used in order to identify differences in the responses of smooth muscles from different origins to cinnam-aldehyde. The main finding revealed that cinnamaldehyde reduced the basal tonus and stimulated contractions of the smooth muscles of the digestive tract with dependence on concentration and the origin of the smooth muscle, and it bears spasmolytic effects which were more prominent in the smooth muscles of the ileum than in other parts. In the smooth muscle preparations of the abomasal fundus, cinnamaldehyde not only did not have a significant effect on the basal tonus, but it also significantly increased the contractions caused by barium chloride at low concentrations. Its probable mechanism was also determined. It was found that cinnamaldehyde relaxes the contractions by blocking the calcium channels.
The findings regarding the cinnamaldehyde relaxation effects in this study were consistent with the findings from previous studies on this substance. The study of cinnamaldehyde effects on the coronary arteries of pigs showed that cinnamaldehyde caused coronary artery relaxation concentration-dependently and inhibited the contractions induced by 40.00 mM, KCl Ca++ in an environment free of Ca++. Therefore, cinnamaldehyde had a vasodilatory effect.17
In another study, the effect of cinnamaldehyde on rat aorta was investigated, and the results showed that cinnamaldehyde caused relaxation of the aorta contracted by phenylephrine dose-dependently. In tissue affected by cinnamaldehyde, the contractions induced by angiotensin II, 5 hydroxytryptamine, dopamine, endothelin-1, and phenylephrine were significantly inhibited. It has also been shown that this vasodilator exerts its effect by inhibiting the release of Ca or preventing its influence.18
Examining the effect of cinnamaldehyde on the gastrointestinal tract of mice, guinea pigs, and rats revealed that cinnamaldehyde slightly reduced the basal tonus in the ileum of guinea pigs and mice and inhibited contractions caused by acetylcholine and histamine in the ileum of the examined animals, similar to papaverine. It also had an inhibitory effect on the spontaneous contractions of the stomach in rats.10
An in vitro study on the rat uterus showed that cinnam-aldehyde concentration-dependently led to a significant reduction in contractions of the uterus and contractions induced by oxytocin and KCl. It also significantly reduced contractions caused by the activation of L-type Ca++ channels by Bay K8644. Therefore, the main mechanism of cinnamaldehyde could be the inhibition of L-type Ca++ channels, which prevents the penetration of calcium.19
In the present study, an important point among the results was the presence of a slight relaxation effect of cinnamaldehyde on the contraction of the smooth muscles isolates of the abomasum, especially the fundus part, which even increased the contractions induced by barium chloride at low concentrations.
The review of previous studies on the effects of herbal essential oils on gastrointestinal movements suggested that herbal essential oils had an inhibitory effect on the movements of the abomasum. A study showed the inhibitory effects of the essential oil of bunium persicum on the contractions of the smooth muscles of sheep rumen and abomasum.20
Contrary to the studies discussed above, it seems that this substance can be used to reduce movements in cases of diarrhea and spasmodic colic with a minimal effect on the movements of the abomasum and regardless of the incidence of movement diseases of the abomasum.
In the present study, the study of the mechanism of action determined that when the ileal tissue was affected by various concentrations of calcium in a calcium-free and potassium-rich environment before and after incubation with cinnamaldehyde, the tissue response to various concentrations of calcium after treatment with the mentioned substance was decreased, which was similar to the response of tissue treated with verapamil, considered as the standard for calcium channels blockers.
Like other muscular cells, the contractility in smooth muscles is regulated by the concentration of intracellular cytoplasmic calcium, which activates the contraction elements.21
Increased intracellular calcium occurs through extracellular calcium penetration or through the release of it from intracellular reserves in the sarcoplasmic network. The most important route of calcium entering the cell is through the L-type voltage-dependent Ca++ channels (VDCs).22 Therefore, when a tissue is affected by various concentrations of calcium in a calcium-free and potassium-rich environment (depolarization solution), contractile effects are expected to occur in that tissue because of calcium entering the cells through the calcium channels.23
In the current study, before treating the target tissue with cinnamaldehyde or verapamil, a strong contractile response was observed in the tissue when different concentrations of calcium were added. After exposing the tissue sample to the mentioned substances, the contractile response created by adding various concentrations of calcium was weaker than in the previous stage, i.e. before treatment with cinnamaldehyde or verapamil.
These results suggested that cinnamaldehyde, similar to verapamil, had a calcium channel blocking effect, which provided a pharmacological basis for its antispasmodic and anti-diarrheal effects, like the blockers of calcium channels that are considered useful as anti-diarrhea and anti-spasm agents.
Acknowledgments
This study was supported by the Faculty of Veterinary Medicine, Urmia University. The authors would like to thank Dr. Esmaeal Tamaddonfard for his valuable contributions.
Conflict of interest
There are no conflicts of interest of any kind.
References
- 1.Constable PD, Hinchclif KW, Done SH, et al. Veterinary medicine: A textbook of the diseases of cattle, horses, sheep, pigs and goats. 11th ed. Pennsylvania, USA: Saunders ; 2017. pp. 176–178. [Google Scholar]
- 2.Fecteau G, Desrochers A, Francoz D, et al. Diagnostic approach to the acute abdomen. Vet Clin North Am Food Anim Pract. 2018;34(1):19–33. doi: 10.1016/j.cvfa.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Navarre CB, Roussel AJ. Gastrointestinal motility and disease in large animals. J Vet Intern Med. 1996;10(2):51–59. doi: 10.1111/j.1939-1676.1996.tb02027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heller MC, Chigerwe M. Diagnosis and treatment of infectious enteritis in neonatal and juvenile ruminants. Vet Clin North Am Food Anim Pract. 2018;34(1):101–117. doi: 10.1016/j.cvfa.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ugbogu EA, Elghandour MMMY, Ikpeazu VO, et al. The potential impacts of dietary plant natural products on the sustainable mitigation of methane emission from livestock farming. J Clean Prod. 2019;213:915–925. [Google Scholar]
- 6.Mendel M, Chłopecka M, Dziekan N, et al. Phytogenic feed additives as potential gut contractility modifiers-A review. Anim Feed Sci Technol. 2017;230:30–46. [Google Scholar]
- 7.Brenes A, Roura E. Essential oils in poultry nutrition: Main effects and modes of action. Anim Feed Sci Technol. 2010;(1-2):1–14. 158. [Google Scholar]
- 8.Burnham PM. Molecule of the month Cinnamaldehyde, The smell and flavour of cinnamon. [Accessed July 10, 2021]. Available at: http://www.chm.bris.ac.uk/motm/cinnamaldehyde/cinnh.htm.
- 9.Wynn S, Fougere B. Veterinary herbal medicine. 1st ed. Philadelphia, USA: Mosby. 2006:513–514. [Google Scholar]
- 10.Harada M, Yano S. Pharmacological studies on Chinese cinnamon Effects of cinnamaldehyde on the cardiovascular and digestive systems. Chem Pharm Bull (Tokyo) 1975;(5):941–947. doi: 10.1248/cpb.23.941. 23. [DOI] [PubMed] [Google Scholar]
- 11.Friedman M. Chemistry, antimicrobial mechanisms, and antibiotic activities of cinnamaldehyde against pathogenic bacteria in animal feeds and human foods. J Agric Food Chem. 2017;(48):10406–10423. doi: 10.1021/acs.jafc.7b04344. 65. [DOI] [PubMed] [Google Scholar]
- 12.Hong S-H, Ismail IA, Kang S-M, et al. Cinnamaldehydes in cancer chemotherapy. Phytother Res. 2016;(5):754–767. doi: 10.1002/ptr.5592. 30. [DOI] [PubMed] [Google Scholar]
- 13.Chaves AV, Stanford K, Dugan MER, et al. Effects of cinnamaldehyde, garlic and juniper berry essential oils on rumen fermentation, blood metabolites, growth performance, and carcass characteristics of growing lambs. Livest Sci. 2008;(2-3):215–224. 117. [Google Scholar]
- 14.Babu PS, Prabuseenivasan S, Ignacimuthu S. Cinnamaldehyde--a potential antidiabetic agent. Phytomedicine. 2007;(1):15–22. doi: 10.1016/j.phymed.2006.11.005. 14. [DOI] [PubMed] [Google Scholar]
- 15.Cardozo PW, Calsamiglia S, Ferret A, et al. Effects of alfalfa extract, anise, capsicum, and a mixture of cinnamaldehyde and a eugenol on ruminal fermentation and protein degradation in beef heifers fed a high-concentrate diet. J Anim Sci. 2006;(10):2801–2808. doi: 10.2527/jas.2005-593. 84. [DOI] [PubMed] [Google Scholar]
- 16.Farre AJ, Colombo M, Fort M, et al. Differential effects of various Ca2+ antagonists. Gen Pharmacol. 1991;(1):177–181. doi: 10.1016/0306-3623(91)90331-y. 22. [DOI] [PubMed] [Google Scholar]
- 17.Raffai G, Kim B, Park S, et al. Cinnamaldehyde and cinnamaldehyde-containing micelles induce relaxation of isolated porcine coronary arteries: role of nitric oxide and calcium. Int J Nanomedicine. 2014;9:2557–2566. doi: 10.2147/IJN.S56578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xue YL, Shi HX, Murad F, et al. Vasodilatory effects of cinnamaldehyde and its mechanism of action in the rat aorta. Vasc Health Risk Manag. 2011;7:273–280. doi: 10.2147/VHRM.S15429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alotaibi M. The effect of cinnamon extract on isolated rat uterine strips. Reprod Biol. 2016;(1):27–33. doi: 10.1016/j.repbio.2015.12.001. 16. [DOI] [PubMed] [Google Scholar]
- 20.Jalilzadeh-Amin G, Maham M, Dalir-Naghadeh B, et al. Effects of bunium persicum (Boiss ) essential oil on the contractile responses of smooth muscle (an in vitro study) Vet Res Forum. 2011;(2):87–96. 2. [Google Scholar]
- 21.Yu J, Bose R. Calcium channels in smooth muscle. Gastroenterology. 1991;(5 Pt 1):1448–1460. 100. [PubMed] [Google Scholar]
- 22.Lyford GL, Farrugia G. Ion channels in gastrointestinal smooth muscle and interstitial cells of Cajal. Curr Opin Pharmacol. 2003;(6):583–587. doi: 10.1016/j.coph.2003.06.010. 3. [DOI] [PubMed] [Google Scholar]
- 23.Evans ED, Mangel AW. Depolarization-stimulated contractility of gastrointestinal smooth muscle in calcium-free solution. ISRN Gastroenterol. 2011;2011:692528. doi: 10.5402/2011/692528. [DOI] [PMC free article] [PubMed] [Google Scholar]