Abstract
Coronavirus disease-19 (COVID-19) is caused by the newly discovered coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). While the lung remains the primary target site of COVID-19 injury, damage to myocardium, and other organs also contribute to the morbidity and mortality of this disease. There is also increasing demand to visualize viral components within tissue specimens. Here we discuss the cardiac autopsy findings of 12 intensive care unit (ICU) naïve and PCR-positive COVID-19 cases using a combination of histological, Immunohistochemical/immunofluorescent and molecular techniques. We performed SARS-CoV-2 qRT-PCR on fresh tissue from all cases; RNA-ISH and IHC for SARS-CoV-2 were performed on selected cases using FFPE tissue from heart. Eight of these patients also had positive post-mortem serology for SARS-CoV-2. Histopathologic changes in the coronary vessels and inflammation of the myocardium as well as in the endocardium were documented which support the reports of a cardiac component to the viral infection. As in the pulmonary reports, widespread platelet and fibrin thrombi were also identified in the cardiac tissue. In keeping with vaccine-induced activation of virus-specific CD4+ and CD8+ T cells, and release of cytokines such as interferon-gamma (IFNγ), we observed similar immune cellular distribution and cytokines in these patients. Immunohistochemical and immunofluorescent localisation for the viral Spike (S-protein) protein and the nucleocapsid protein (NP) were performed; presence of these aggregates may possibly contribute to cardiac ischemia and even remodelling.
Keywords: PCR, COVID-19, autopsy, multiplex, serology
Introduction
SARS-CoV-2 (Covid 19), a novel corona virus was first implicated as the cause of a rapidly spreading infectious upper respiratory illness in late 2019 (Zhu et al., 2020) resulting in an exponential increase in global infections (WHO, 2020). There is much to be done as thenatural history of this disease has yet to be elucidated and whilst there has been an emphasis on pulmonary findings, there are now increasing reports that Covid-19 may also affect the cardiovascular and other organ systems (Babapoor-Farrokhran et al., 2020; Barton et al., 2020). In fact, Yang et al. reported death of a small number of patients who died within a short period of time after admission, in other words, sudden death (Yang et al., 2021). Moreover, sudden cardiac arrest and death had been reported as early as 2020 despite improvement of general condition and constitutional symptoms (Shirazi et al., 2021).
Much about the pathogenesis of SARS-CoV-2 and the heart remains unknown (Siripanthong et al., 2020). Angiotensin (AT) converting enzyme 2 (ACE2), known as the cellular receptor for SARS-CoV-2 is ubiquitously expressed with the highest levels detected in the cardiovascular system (cardiomyocytes, cardiac fibroblasts, vascular smooth muscle cells and endothelial cells) as well as intestine, kidneys and lungs (Grifoni et al., 2020; Le Bert et al., 2020). We discuss the cardiac (and vascular) pathology seen in twelve cases of sudden death in patients who were also Covid-19 positive. We document possible evolution of the disease with little or no medical intervention in a study of these autopsy cases. We also aim to document the cellular immune response observed in the COVID-19 patients.
Methods
In our series of twelve male autopsy cases, ten cases were sudden unexplained deaths. Case 1 had presented to his physician with anosmia, had a swab taken and was sent home where he was found collapsed the next day before the test results were known. The remaining 9 cases had a variety of complains including chest pain, epigastric pain or discomfort. Case 10 was last heard complaining of chest pain but was found dead 2 days later. Of these only two cases had known premorbid illnesses on record. Case 8 had a history of hypertension whilst case 11, who was unemployed, is the only case with documented premorbid conditions of poorly controlled diabetes and hypertension. He was being managed by his physician for 2 weeks of fever and cough before being tested positive for Covid-19. He collapsed at home a day later.
Two cases were unnatural deaths, having fallen from height (case 2 was admitted to hospital for
observation after 5 days of fever and a positive test, whilst case 9 was admitted in a facility for
well and asymptomatic Covid-19 patients.
All subjects, except for case 11, worked in the construction industry. None of the 12 cases.
Presented with severe respiratory symptoms nor required supplemental oxygen. In cases where clinical history was not available, we have taken the date of the first positive PCR test as the most probable start point of COVID-19 infection and have stratified the patients accordingly.
All twelve cases were referred to Health Sciences Authority for autopsy (mean age = 44.1 year range:27–69 years) (Table 1) under the Second Schedule of the Coroners Act Cap 63A (Revised Edition 2012 Singapore Statutes). All autopsies were carried out either in biosafety level (BL)-BSL3 or BSL4 autopsy facilities. All subjects were male.
TABLE 1.
Patient characteristics and autopsy findings.
| case No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gender | M | M | M | M | M | M | M | M | M | M | M | M |
| AGE | 32 | 46 | 47 | 53 | 30 | 41 | 41 | 48 | 27 | 42 | 69 | 59 |
| BMI | 25.28 | 19.05 | 20.62 | 26.67 | 16.11 | 27.1 | 21.87 | 18.94 | 22.41 | 18.31 | 21.48 | 31.7 |
| Ante mortem test (PCR) | YES | YES | NA | NA | NA | YES | NEG | YES | YES | YES | YES | YES |
| TEST TO DEATH INTERVAL (DAYS) | 6 HRS | 4 DAYS | NA | NA | NA | 42 DAYS | NA | 38 DAYS | 19 DAYS | 47–48 DAYS | 1 DAY | 41 DAYS |
| PRIOR MEDICAL CONDITIONS | NIL | NIL | NIL | NIL | NIL | Not known | Not known | Not known | NIL | Not known | DM TYPE 2. HYPT. HYPOTHYROID. OBESE. CKD | Nil |
| CIRCUMSTANCES OF DEATH | SUDDEN DEATH | Fell from Height | SUDDEN DEATH | SUDDEN DEATH | SUDDEN DEATH | SUDDEN DEATH | SUDDEN DEATH | SUDDEN DEATH | Fell from height | SUDDEN DEATH, DECOMPOSED | ARI; SUDDEN DEATH | SUDDEN DEATH |
| HEART WT (gm) | 345 | 235 | 419 | 410 | 245 | 404 | 245 | 340 | 222 | 244 | 463 | 398 |
| HEART GENERAL DESCRIPTION | SOFT AND FLABBY. | SOFT AND FLABBY. | SOFT AND FLABBY | TRANSMURAL RUPTURE, ANTERIOR-ANTEROSEPTAL WALL, JUNCTION OF UPPER 2/3 AND DISTAL 1/3 LV. | SOFT AND FLABBY | SOFT AND FLABBY, FIBRINOUS ADHESIONS OVER RA, RV ENDOCARDIUM | GROSSLY NORMAL | GROSSLY NORMAL | GROSSLY NORMAL | DECOMPOSED | HEART ENLARGED | HEART ENLARGED |
| CORONARY arteries | LCA: PINPOINT RESIDUAL LUMEN | NORMAL. | LCA ATHEROSCLEROTIC WITH 50% OCCLUSION OF LAD. | LAD 10–25%. DARK RED THROMBUS, TOTAL OCCLUSION | LAD- ORGANISING THROMBUS. | MILD ATHEROSCLEROSIS | ||||||
| LAD 10–25% | PROX LAD 75%- | LCA 25/LAD 90/LCX 75/RCA PINPOINT | NAD | LAD 10–25 | LCA 10 LAD 90-FIRST DIAGONAL PINPOINT | LCA 50 LAD25 | ||||||
| SEROLOGY (IgM + IgG) | CLOTTED | CLOTTED | POSITIVE; COI 2.22 | POSITIVE; COI 2.38 | CLOTTED | POSITIVE; COI 89.8 | POSITIVE; COI 9.91 | POSITIVE; COI 114 | POSITIVE; COI21.5 | UNSUITABLE FOR ANALYSIS | NO; COI 0.534 | POSITIVE; COI:8.73 |
| Swab (Nasal) | DETECTED | DETECTED | PRESUMPTIVE POSITIVE | NOT DETECTED | DETECTED | NOT DETECTED | NEGATIVE | NEGATIVE | NEGATIVE | DETECTED | DETECTED | NEGATIVE |
| Swab (PNS) | DETECTED | DETECTED | DETECTED | DETECTED | PRESUMPTIVE POSITIVE | NOT DETECTED | DETECTED | DETECTED | DETECTED | DETECTED | DETECTED | NEGATIVE |
| Swab (Tracheal) | DETECTED | DETECTED | PRESUMPTIVE POSITIVE | NOT DETECTED | DETECTED | NOT DETECTED | NEGATIVE | NEGATIVE | DETECTED | NEGATIVE | DETECTED | NEGATIVE |
| Swab (Ileal) | DETECTED | DETECTED | NOT DETECTED | INCONCLUSIVE | DETECTED | DETECTED | NEGATIVE | NEGATIVE | NEGATIVE | DETECTED. STRONG POSITIVE | PRESUMPTIVE POSITIVE | NEGATIVE |
| Swab (CNS) | DETECTED | DETECTED | NOT DETECTED | NOT DETECTED | NOT DETECTED | NOT DETECTED | NEGATIVE | NEGATIVE | NEGATIVE | NEGATIVE | DETECTED | NEGATIVE |
LCA; Left coronary artery, LAD: Left anterior descending artery, PROX LAD: Proximal Left anterior descending artery, LCX: Left circumflex artery, RCA: right coronary artery.
Results
Vasculature
The findings are stratified with respect to known or estimated duration of symptoms. The earlier group comprised 5 cases with symptom duration ranging between <12 h and 5 days. In 4 of these, we observed complete occlusion of the coronary artery by fresh fibrin thrombi; the epicardial coronary vessels showed pre-existing mild atherosclerosis (Figure 1A). In a fifth patient (case 2), we observed that the patency of the arterial lumen was compromised by apposition of the endothelium (Figure 1B).
FIGURE 1.

(A) Coronary vessel with fresh fibrin thrombus, moderate inflammation, mild fibrosis (H&E, x200). (B) apposition of vascular endothelium (H&E, x200). (C) Perivascular inflammation around epicardial vessel (H&E, x200). (D) CD4-positive T-cells in perivascular tissue, 200x. (E) Perivascular inflammation extending to adipose tissue and involving peripheral nerve (H&E, x200).
An inflammatory infiltrate was seen around epicardial vessels of varying diameter, predominantly in a perivascular location, extending into the outer layers of the vessel wall and outwards into the pericardial adipose tissue (Figure 1C, patient 3, Table 2). On cross sections, the interface of the more intense inflammatory infiltrates imbued a stellate appearance. The inflammatory cells were predominantly lymphoid in nature, particularly CD4-positive (IHC) with scanty CD8-positive T-cells and CD20-positive B cells (Figure 1D). In some cases, the latter two subtypes of lymphocytes were virtually absent. Except for two cases where the eosinophilic infiltrate was heavy, eosinophils and monocytes were also noted in smaller amounts. Involvement of nerves by chronic inflammation were also seen Figure 1E.
TABLE 2.
Microscopic findings; key: Light yellow <7 days; Pink >7 days.
| case no | 1 | 2 | 3 | 4 | 5 | 7 | 11 | 9 | 6 | 8 | 10 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Interval from ante-mortem PCR to autopsy | 6 h | 4 days | NA | NA | NA | NA | 1 day | 19 | 42 days | 38 days | 47–48 days | 41 days |
| Epicardial vessel lumen | occluded | occluded (lumen closed by apposition) | occluded | occluded | occluded | 80% | occluded atherosclerotic with inflammatory infiltrates | myointimal thickening | myointimal thickening | myointimal thickening | myointimal thickening | myointimal thickening |
| Fresh thrombus | yes | no | yes | yes | yes | no | no | no | no | no | no | no |
| Fibrin thrombi | yes | yes | yes | yes | yes | Yes | yes | yes | Yes | yes | Autolytic changes | yes |
| Perivascular inflammation | Yes 3 + mixed inflammatory cells | yes 1 + lymphocytic infiltration | yes 2 + lymphocytic infiltration | Yes 1 + lymphocytic infiltration | Yes 2 + mixed inflammatory cells | yes | Lymphoid aggregates | 1 + lymphocytic infiltration | 1 + lymphocytic infiltration | Lymphoid aggregates | Autolytic changes | Lymphoid aggregates |
| Myocardium | ||||||||||||
| inflammatory infiltrate | 2 + lymphocytic | no | 3 + lymphocytes and eosinophils | 1 + Lymphocytes | 3 + lymphocytes and eosinophils | 2 + lymphocytes | scanty lymphocytes | 1 + LYMPHOCYTES | 2 + lymphocytes | 1 + lymphocytes | Autolytic changes | 1 + lymphocytes, eosinophils |
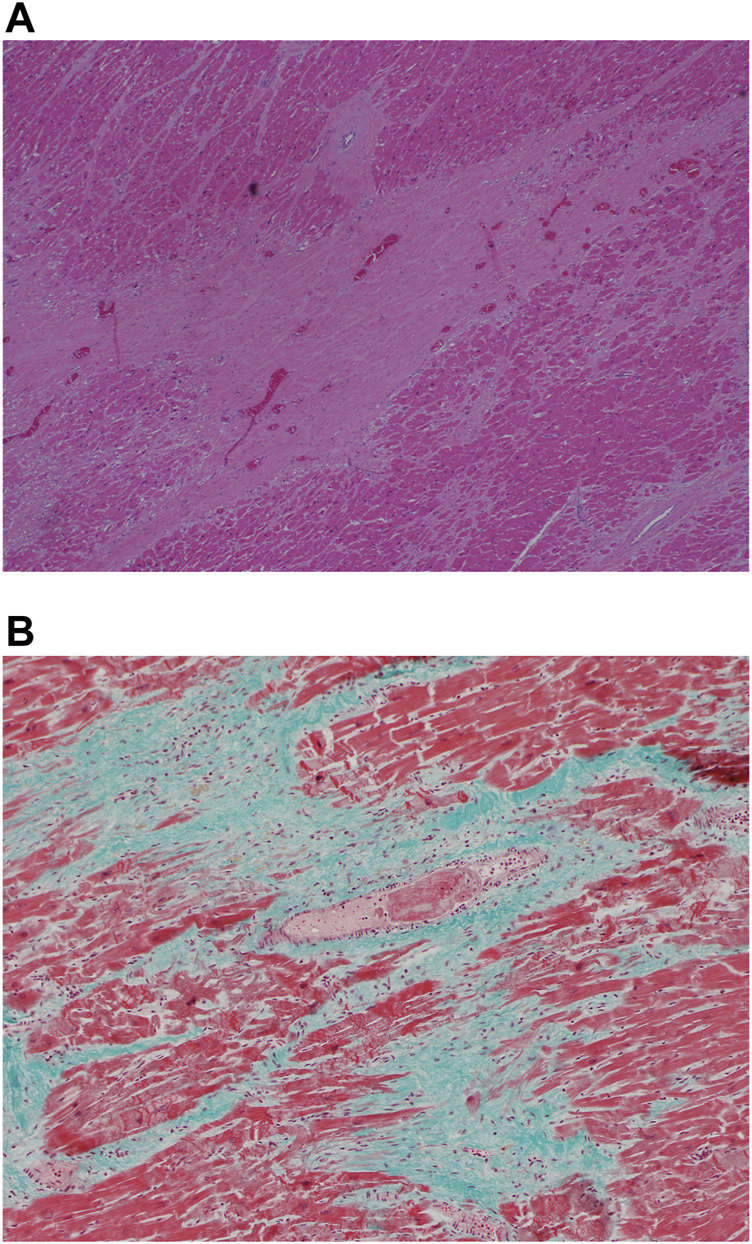
In cases where symptoms persisted for more than 14 days, the inflammatory infiltrate was less prominent. The coronary vessels showed medial and intimal hypertrophy with focal dilatation, outpouching or tortuosity of vessels giving an irregular appearance (Figure 2A, case 5 A fine perivascular fibrosis was seen around both thin and thick-walled vessels of varying diameters including capillary vessels. The pericardial adipose tissue was involved in the inflammation in all cases, irrespective of duration of symptoms (Figure 2B).
FIGURE 2.

(A) Remodeling of myocardial vesels showing myointimal hypertrophy/thickening with perivascular lymphoid aggregates (H&E, x200). (B) Perivascular inflammation involving epicardial vessel (H&E, x200).
Myocardium
The perivascular inflammatory infiltrate described previously was observed to follow the vessels into the myocardium and could be seen extending along the longitudinal axis of the vessels. In some cases, the inflammatory infiltrates were seen around the perivascular spaces, within the wall and lumen. Again, the lymphoid cells were predominantly of CD4 lineage with virtually no CD8-positive T-cells or CD20-positive B-cells. Obliteration of the vascular lumen by a proliferation of spindled myofibroblasts was seen in one case (Figure 3A, case 5).
FIGURE 3.
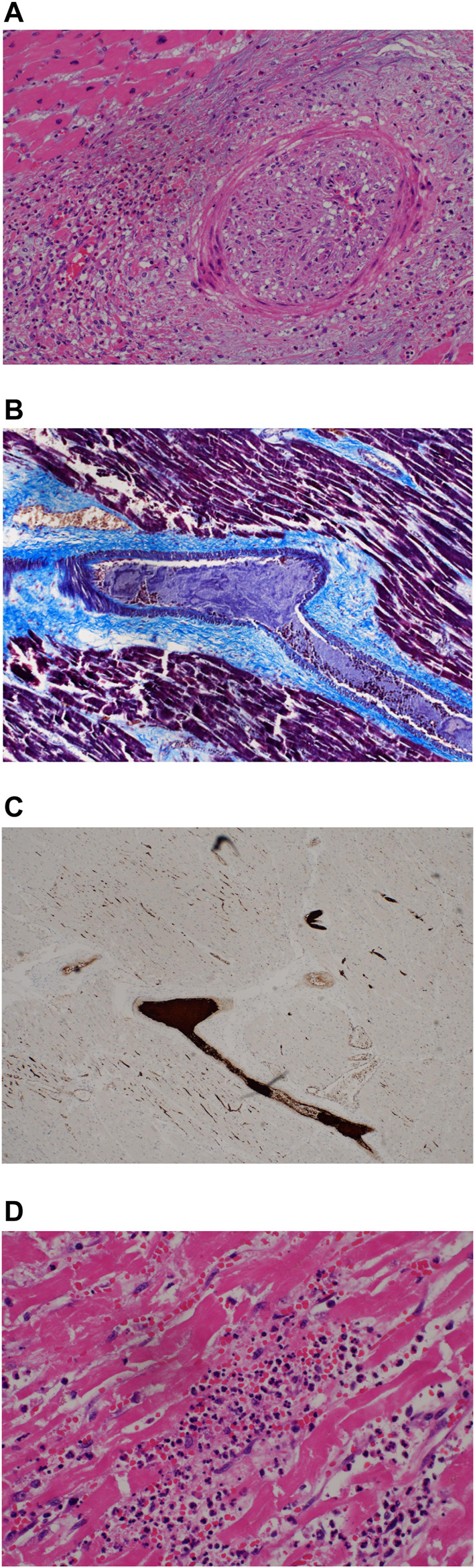
(A) Occlusion of coronary artery lumen by proliferation of spindled cells (H&E, x200). (B) Fibrin thrombi. MSB stain (200x). (C) Platelet thrombi, CD61 immunohistochemical stain (200x).
Extensive fibrin and platelet thrombi were noted in the myocardial vessels as well as in the myocardial microvasculature. Mature fibrin thrombi were observed with MSB stains (Figure 3B); intravascular platelet aggregates were demonstrated by CD61 antibody (Figure 3C).
Myocarditis as evidenced by chronic inflammation and myocyte necrosiswas marked in 4 of the 5 earlier cases but could be seen in patchy fashion even in the later cases (Figure 4A, case 3) The inflammatory cells comprised a mixed population of lymphoid cells, neutrophils, eosinophils and monocytes; eosinophils were prominent in case 3,. Granulation tissue reaction with presence of reactive stromal cells was demonstrated in 2 of the earlier cases whilst a patchy stellate myocardial fibrosis was noted in the later cases (more than 7 days) (Figure 4B,C, case 8). This fibrosis could also be seen sweeping along the long axis of the myocardial vessels appearing to mirror the inflammatory infiltrate in the earlier cases. Within the fibrous tissue, thin-walled vessels were noted. It is unclear if these vessels represent residual vasculature or neovascularization. (Figure 4D, case 8).
FIGURE 4.
(A) Myocarditis with myocyte necrosis (H&E, x200). (B) Stellate myocardial fibrosis (H&E, 200x). (C) Masson trichrome stain confirming fibrosis (200x).
Endocardium
Focal infiltration of the endocardial lining by mononuclear cells was noted in the earlier cases (Figure 5A–C, case 5).
FIGURE 5.

(A) Endocardial fresh blood clot (H&E, 200x). (B) Endocardial organizing thrombus (H&E, 200x). (C) Endocarditis (H&E, 200x).
RNAscope in-Situ Hybridization
RNAscope assay following the manufacturer’s protocols was applied to five cases. Weak positive signals were detected within myocytes in three cases (cases 2,3 and 5). Co-localization of SARS-CoV-2 with its entry receptor ACE2 and serine protease TMPRSS2 (type II transmembrane serine protease) in different cell types, using RNAscope in situ hybridization, was found in various compartments of the heart, such as endothelial smooth muscle, myocardium, fibroblastic and inflammatory cells (Figure 6).
FIGURE 6.
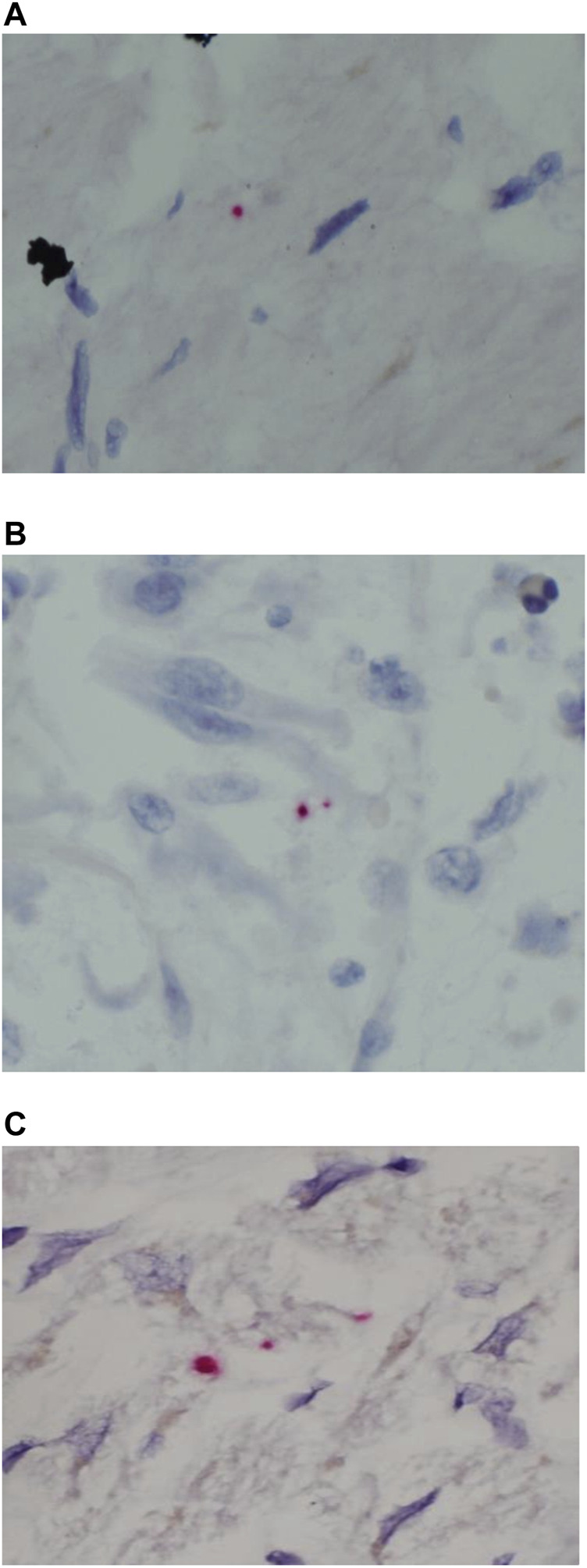
SARS-CoV-2 ISH in myocardium from the autopsy of patients who died secondary to COVID-19 infection. (A–C) Positive reactions for the probes directed against SARS-CoV-2 S-protein (red dot) original magnifications ×400).
Immunohistochemistry
Detection of viral NP in myocardium was attempted using immunohistochemistry and identified in two cases including one case with coexisting myocardial viral S protein on RNAscope assay. The second case was 38 days post-covid-19 infection and viral NP-protein was detected within the vascular lumen as well as the immediate perivascular space (Figure 7, case 8).
FIGURE 7.
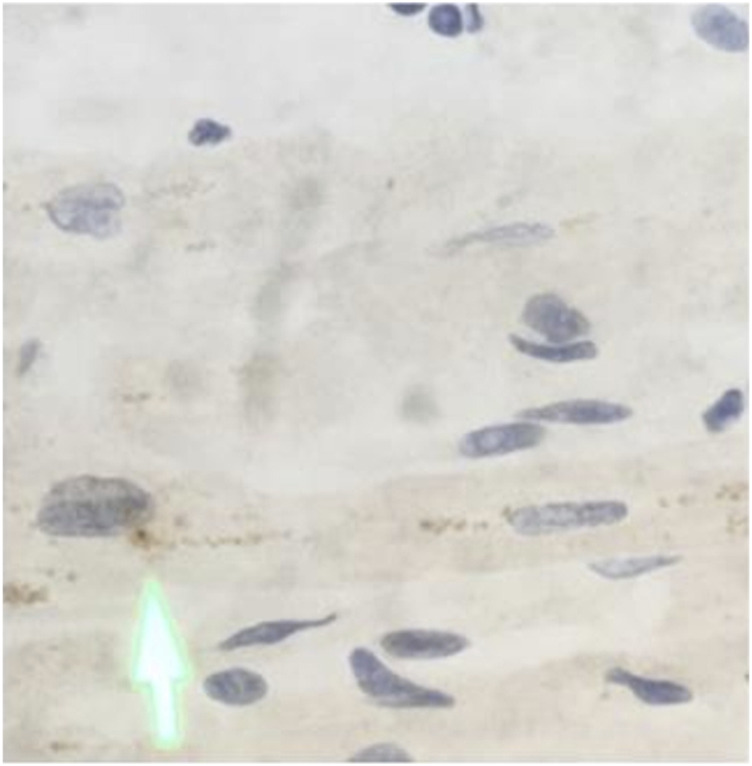
Immunohistochemical analysis in myocardium from the autopsy of patient who died secondary to COVID-19 infection (A) SARS-CoV-2 is positive (arrow) in the myocardial tissue, original magnifications ×400.
mIHC/IF
We investigated the presence of the SARS-COV2 NP protein (Shirazi et al., 2021; Siripanthong et al., 2020; Le Bert et al., 2020; Grifoni et al., 2020) and the associated immune microenvironment by using multiplex IHC/IF technique (Ni et al., 2020; Thieme et al., 2020; Stack et al., 2014; Abel et al., 2014; Lovisa et al., 2015; Garnelo et al., 2015; Yeong et al., 2017; Garnelo et al., 2017; Esbona et al., 2016; Mlecnik et al., 2016; Nghiem et al., 2016; Feng et al., 2016; Lim et al., 2018). The SARS-COV2 NP protein was found predominantly near the perivascular regions colocalizing with receptors of SARS-COV2, ie ACE2 and TMPRSS2 (Yeong et al., 2019; Lam et al., 2019) (Figure 3). Interestingly, the pathogenic cytokines such as IL-6 (Xu et al., 2020a; Hoffmann et al., 2020; Qin et al., 2020; Wang et al., 2020) and IL-1β (Huang et al., 2020a; Xu et al., 2020a; Diao et al., 2020; Shi et al., 2020) were also detected in close proximity in the background of fibrosis highlighted by the expression of Collagen I and III (Liao et al., 2020; Wen et al., 2020).. (Figure 8)
FIGURE 8.
Representative images of heart tissue stained using multiplex immunohistochemistry/immunofluorescence (mIHC/IF) [CD3 (green), ACE2/TMPRSS2 (yellow), Virus NP (red), IL6 (magenta), Collagen1+3 (orange), IL1B (cyan), DAPI (blue)] (Magnification, 400X).
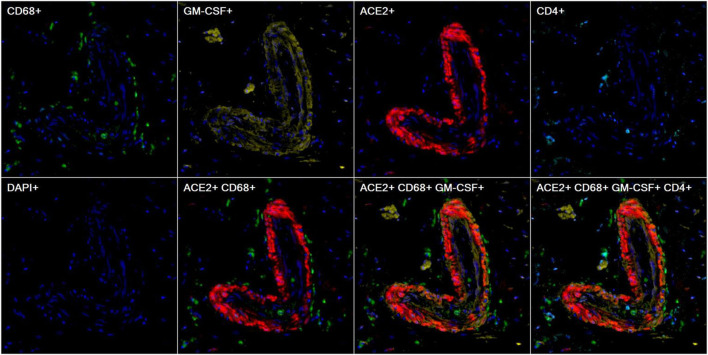
We further demonstrated that some of the ACE2+ cells were macrophages which are in line with previous reports (Delpino and Quarleri, 2020; George et al., 2020). Colocalization of the pathogenic cytokine GM-CSF (Diao et al., 2020) as well as surrounding T-cells were also observed in the proximity (Figure 9, case 8).
FIGURE 9.
Representative images of heart tissue stained using multiplex immunohistochemistry/immunofluorescence (mIHC/IF) [CD68 (green), GM-CSF (yellow), ACE2 (red), CD4 (cyan), DAPI (blue)] (Magnification, 200X).
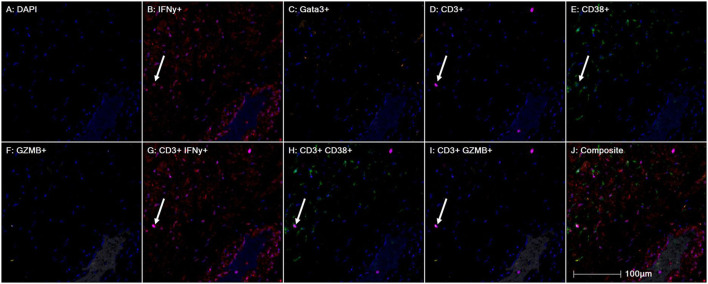
Furthermore, we interrogated the immune phenotype of the T-cells present in the peri-vascular regions whereby Th2 cells, highlighted by absence of GATA3 nuclear stain, were virtually absent (Robinson et al., 2020). However, few T-cells demonstrated a Th-1 cell-like phenotype which expressed pathogenic cytokines such as interferon-gamma (Zheng and Flavell, 1997; Xu et al., 2020a; Diao et al., 2020; Weiskopf et al., 2020; Yao et al., 2020). Some of the T-cells expressed granzme-B signifying the effector function of cytotoxic T cells (Thevarajan et al., 2020; WHO, 2020; Zheng et al., 2020), as well as CD38 which has been widely reported as one of the SARS-COV2-specific T-cell marker (Huang et al., 2020a; Xu et al., 2020b; Craver et al., 2020; Thevarajan et al., 2020; Wang et al., 2020) (Figure 10, case 8).
FIGURE 10.
Representative images of heart tissue stained using multiplex immunohistochemistry/immunofluorescence (mIHC/IF) Interferon gamma (red), GATA3 (orange), CD3 (magenta), CD38 (green), Granzyme B (yellow) (Magnification, 200X).
SARS-CoV-Ab Serology
COI values for 7 of the 8 patients were clearly positive ranging from 2.22 to 114; it was 0.534 in the remaining patient. The range in 362 prepandemic male (age: mean, SD - 43.3, 14.5 years) sera on this assay was 0.066–0.373 (data not shown).
Discussion
We document 12 autopsy cases with cardiac changes. Four of these cases had histologic evidence of myocarditis with marked predominantly lymphocytic and some eosinophilic infiltrates. The presence of SARS NP protein in a perivascular location, the proximity of T-cells and cytokine GM-CSF bum IHC/IF appears to corroborate changes observed at the light miscroscopy. Whether these changes were purely due to an underlying ischemic heart disease or whether the process was exacerbated by the viral infection is unclear.
Myocardial cells are a potential target of SARS-CoV-2, and myocarditis has been reported in a limited series in China, where 7% of deaths were attributed to myocardial damage with circulatory failure without a clear, definite diagnosis of myocarditis (Cheung et al., 2020). Others have described fulminant myocarditis in the setting of high viral load, with autopsy findings of myocardial inflammatory infiltrate, but without evidence of myocardial COVID-19 disease (Robinson et al., 2020). Although much of the published literature has focussed on the pulmonary changes, findings from our autopsy series demonstrate that significant cardiac pathology may be associated with COVID-19 infection. It has already been postulated that in addition to coronary plaque destabilization and hypoxia, the possible mechanisms of COVID-19-related myocardial injury could be direct damage to the cardiomyocytes, chronic inflammation, myocardial interstitial fibrosis, interferon-mediated immune response and exaggerated cytokine response by T-cells (Babapoor-Farrokhran et al., 2020).
The records of the Forensic Department, Singapore show that in 2018, a total of 1,414 cases were autopsied and 18 were certified as myocarditis. In 2019 there were 1,262 cases of which 23 were certified as myocarditis. 131 cases were certified as Coronary heart disease in 2018 and 108 in 2019. A small autopsy series of four non-COVID-19 deceased patients during the same period did not reveal any significant myocardial inflammation (Supplementary Table S2).
In SARS autopsies (Chong et al., 2004) we reported pulmonary thromboembolic, deep vein thrombosis, and marantic valvular vegetations with widespread intravascular fibrin thrombi. As the SARS patients had a history of admission to intensive care units (ICU), it could be argued that these changes were related to ICU support and therapy. However, our current cases are all ICU-naïve.
SARS-CoV-2 is known to use the ACE2 receptor as a channel for entrance into the cell as receptors have been reported to be present in cardiac smooth muscle, vascular smooth muscle and endothelial cells as well as pneumocytes and enterocytes (Dandekar and Perlman, 2005; Yajima and Knowlton, 2009). It is possible that the virus enters these cells including the cardiac myocytes and that the immediate innate cytokine response may cause the initial myocardial damage early in the infection and that the arrival of the T-lymphocytes would further intensify this. Our studies have been able to demonstrate viral signals within the cardiac myocytes using both ISH and mIF assays. The latter has also demonstrated increased cytokine and interferon activity within the myocardium in perivascular locations co-locating with SARS-CoV-2specific T cells as well as macrophages. We note that in staining for the ACE2 receptor, the distribution of these receptors is not uniform throughout the cardiac and vascular samples. This underlying variation may underscore the heterogenous distribution of cardiac injury seen in our cases.
Yajima and Knowlton (2009); Wick et al. (2004) Immunohistochemical findings in our cases suggest the presence of virus within the vessel lumen and wall as well as within immediate perivascular space together with the presence of activated lymphocytes and cytokine activity (Figure 4). Macrophage/monocytes are known to be able to transport Corona viruses and studies have also suggested that T-lymphocytes may be similarly infected (Wick et al., 2004; Yajima and Knowlton, 2009). Hence whether this vascular inflammation is a direct reaction to the existence of the virus within the endothelium or a combination of dysregulated T cell, cytokine and humoral response remains to be seen. Consequently, it is tempting to propose that the observed perivascular and intravascular inflammation modulates the vascular integrity leading to vascular remodelling in the long-term.
The cytokine and inflammatory cell activity within the lumen are interesting as this suggests injury to the endothelial lining. Our cases show widespread platelet and fibrin thrombi within myocardial vessels and the myocardial microvasculature which appears to persist even in cases with a prolonged disease. The presence of possible NP protein signals within the lumen of the vessel may also suggest persistence of viral presence in circulating monocytes as another stimulus for the microembolic phenomena (Figure 4).
One of our cases also showed marked fibroblastic activity within intramyocardial vessels, suggesting possibly immune-mediated injury to endothelial lining possibly contributing to a stenosing lesion (Thieme et al., 2020). In recent years, pro-fibrotic role of the innate immune system has become apparent. Early events of fibrosis comprise inflammatory changes (Wick et al., 2010), including recruitment of mononuclear inflammatory infiltrates. Although, the initial events in activation of host defence mechanisms are still largely unknown, It has been proposed that viral myocarditis may have several phases from predisposition or susceptibility of the cardiac myocyte to infection, entry and active viral replication in the myocyte, persistence of the viral genome without detectable replication and remodelling without detectable viral genome (Varga et al., 2020).
Using mIF methods, we have demonstrated presence of IL-1B and IL6 activity within collagen fibers in the regions of myocardial fibrosis, both of which have been known to play a profibrotic role (Huang et al., 2020a; Patil et al., 2020). It is possible that profibrotic cytokines and mediators released during myocarditis phase in susceptible individuals activate fibroblasts and stimulate fibroblast differentiation leading to subsequent cardiac remodelling. One further interesting point is the persistent epicardial adipose tissue injury seen in all cases manifested by small aggregates of CD4+ T-lymphocytes around small thin-walled vessels and edematous tissue in the early cases to patchy aggregates of lymphocytes and contraction of the epicardial layer. As this epicardial adipose tissue has been postulated to be active in secretion of endocrine and paracrine substances, this persistent inflammation at this location may further compound vascular injury (Huang et al., 2020b).
Interestingly, use for SARS-CoV2 antibody titer has not been reported before for forensic purposes. The Roche assay detects total antibodies to the nucleocapsid protein. The results are reported as a cut-off index (COI); COI >1.4 are considered positive. This suggests that the COI of 0.534 may represent a subclinical antibody titer. Our findings suggest a possible forensic application for the Roche serology test.
Although direct SARS‐CoV‐2-induced myocardial is a consideration, COVID‐19‐associated cardiac damage is widely attributed to exaggerated immune response. One of the early reports describing myocardial inflammation in SARS‐CoV‐2 infection reported fulminant myocarditis with elevated IL‐6 levels along with other cardiac injury markers (Wong et al., 2004). Various cohort‐based studies also showed an increased cytokine production during COVID‐19 infection, and cytokine storm in these patients was found to be associated with the disease severity and patient survival (Molenkamp et al., 2020). The immune response in SARS patients is mainly mediated through the Th1‐cell activity as opposed to SARS‐CoV‐2 infection, where an imbalance between both Th1 and Th2 activity was found to support the inflammatory surge (Peiris et al., 2003; Corman et al., 2021). Overall, evidence from the published studies and ours implies that the SARS‐CoV‐2‐induced inflammatory response may be a possible cause of cardiac damage in patients and could be targeted for therapeutic interventions. The robust elicitation of IFNγ-producing CD4+ T cells in our studies indicates that a cellular immune response with potential anti-viral properties mirrors the strong neutralizing antibody and cytokine response seen in vaccine trials (Sahin et al., 2020). More recently, Bearse et al demonstrated that cardiac infection with SARS-CoV-2 was common among patients succumbing to COVID-19 infection. This study also showed that SARS-CoV-2-associated cardiac infection was associated with more cardiac inflammation and electrocardiographic changes (Bearse et al., 2021). Nonbiologic immunosuppression is associated with lower incidences of myocarditis and cardiac infection by SARS-CoV-2. In our series none of the patients received COVID-19 specific treatment.
Decrease of eosinophils was a critical event described in sudden deaths, which is consistent with previous report that eosinophils may predict the outcome of COVID-19 progression (Babapoor-Farrokhran et al., 2020). Patients with high percentage of neutrophils or neutrophils count had an increased risk of sudden death, probably due to cytokine storm activated by neutrophils (Wu et al., 2020). Unfortunately we did not have corroborating evidence to monitor immune cell counts or inflammatory biomarkers.
Exposure and susceptibility to COVID-19 are partly influenced by occupation and working environment. Migrant workers as in our cohort constituted a significant proportion of the workforce in sectors that have remained active throughout the crisis, such as construction work, logistics and deliveries. Several confounding factors such as inability to work in isolation, lack of access to private transportation, close physical proximity with coworkers and in some instances lack of adequate protective equipment render these workers particularly susceptible.
Although our study only examined male patients, the observed myocarditis is still concordant with other studies. In one study, the overall risk of patients with COVID-19 was nearly 16 times the risk for myocarditis compared with patients who did not have COVID-19. Patients with myocarditis were more commonly male (59.3 versus 41.7%). Despite the limitations, the observed myocardial lesions in our cohort may still be concordant with other studies (Boehmer et al., 2021).
Despite limited existing evidence, our study may provide relevant clues to associate sudden death of COVID-19 patients and potential risk factors. However, several limitations should be considered in our study: 1) it was a retrospective, single-center study and we were not able to conduct all radiographic or laboratory examinations in our subjects 2) interpretation of our findings might be limited by the small sample size 3) data collected for each patient may not be uniform as they represented different disease stages, which might lead to bias in clinical characteristics. Finally, as this is a descriptive/observational study, further mechanistic explanation needs to be clarified. Despite these limitations, our study demonstrated some insights into the characteristics of COVID-19 patients potentially at risk of sudden death. This would help physicians to effectively triage patients with particularly poor prognosis on admission to reduce the fatality rate.
Conclusion
Understanding the pathogenesis of COVID-19 infection is vital to the proper management of this disease. Conventional autopsy studies combined with state-of-the art molecular techniques are an integral part of this process. Here we highlight the incidence of increased cardiac and vascular events in COVID-19-infection which may underlie inflammatory syndromes (Wong et al., 2004) and raise the possibility for long term complications of potentially cardiotropic and persistent virus. Either persistent viraemia or migration of infected immune cells from the extracardiac locations likely occurs in COVID‐19 patients which may exacerbate underlying ischemic myocardial injury. The association of COVID19 NP protein with endothelial cells, cardiac smooth muscle cells warrants further investigation particularly in COVID19 recovering patients. The possible contribution to susceptibility of cardiac complications by gender, nutrition, genetics or viral mutation should also be considered.
Methodology
Real-Time Polymerase Chain Reaction
The inoculated swabs were tested at Singapore General Hospital Molecular Laboratory either using the automated Roche Cobas 6,800 System (Roche Molecular Systems, Inc., Branchburg, NJ) cobas SARS CoV-2 test, a dual-target (E gene and ORF1) qualitative real-time RT-PCR assay, or using our in-house developed SARS CoV-2 RT-PCR assay targeting the SARS-CoV-2 E-gene region (modified from the protocol published by Corman et al. (2021).
Multiplex Immunohistochemistry/Immunofluorescence
mIHC/IF was performed using an Opal Multiplex fIHC kit (Akoya Bioscience, Menlo Park, California, USA), as previously described by our group and in other studies (Abel et al., 2014; Stack et al., 2014; Garnelo et al., 2015; Lovisa et al., 2015; Esbona et al., 2016; Garnelo et al., 2017; Yeong et al., 2017; Grifoni et al., 2020; Le Bert et al., 2020; Ni et al., 2020; Siripanthong et al., 2020; Thieme et al., 2020; Shirazi et al., 2021). Slides were labelled with primary antibodies, followed by appropriate secondary antibodies (see Supplementary Table S1). Particularly for this panel, we followed the detailed protocol that our group previous reported as protocol manuscript (Nghiem et al., 2016) and hereby briefly described.
FFPE tissue sections were cut onto Bond Plus slides (Leica Biosystems Richmond) and heated at 60°C for 20 min (Feng et al., 2016). Tissue slides were then subjected to deparaffinisation, rehydration and heat-induced epitope retrieval (HIER) using a Leica Bond Max autostainer (Leica Biosystems Melbourne), prior to endogenous peroxidase blocking (Leica Biosystems Newcastle). Slides were incubated with primary antibodies followed by application of polymeric HRP-conjugated secondary antibodies (Leica Biosystems Newcastle). An appropriate Opal fluorophore-conjugated Tyramide signal amplification (TSA) (Akoya Bioscience, Menlo Park, California, United States) was then added at 1:100 dilution. Slides were rinsed with washing buffer after each step. Following TSA deposition, slides were again subjected to HIER to strip the tissue-bound primary/secondary antibody complexes and ready for labelling of the next marker. These steps were repeated until all six markers were labelled and finally added with spectral DAPI (Akoya Bioscience, Menlo Park, California, United States) at 1:10 dilution. Slides were mounted in ProLong Diamond Anti-fade Mountant (Molecular Probes, Life Technologies, United States) and cured in the dark at room temperature for 24 h. Images (viable tumour regions were selected by pathologists) were acquired for each case using a Vectra three pathology imaging system microscope (Akoya Bioscience, Menlo Park, California, United States) then analysed and scored by pathologist with inForm software (version 2.4.2; Akoya Bioscience, Menlo Park, California, United States) (Zheng and Flavell, 1997; Weiskopf et al., 2020) as well as HALO TM (Indica Labs) (Xu et al., 2020a; Hoffmann et al., 2020).
RNA in-Situ Hybridization
For RNAscope RNA-ISH (Advanced Cell Diagnostics) analysis of EBNA1, standard RNAscope manufacturer’s protocols were followed using the RNAscope H2O2 and protease pretreatment kit (ACD, reference# 322,381), RNAscope Target retrieval buffer (ACD, reference# 322,000), and appropriate positive and negative RNA probes for controls.
Immunohistochemistry
IHC was performed on the FFPE tissue samples as previously described (Mlecnik et al., 2016; Qin et al., 2020; Wang et al., 2020). Tissue sections (4 μm thick) were labelled with antibodies targeting SARS-CoV-2 NP, as listed in Supplementary Table S1. Appropriate controls were included. To evaluate the antibody-labelled sections, images were captured using an IntelliSite Ultra-Fast Scanner (Philips, Eindhoven, Netherlands).
SARS-CoV-Ab Serology
Post-mortem arterial blood from all 8 COVID19 RT-PCR positive patients was tested SARS-CoV-Ab was measured on the Cobas e801 immunoassay analyzer (Roche). This assay measures total antibodies directed against the nucleocapsid protein. The assay has a specificity of 99.9% (714/715) and a sensitivity of 48.2% within the first week after positive RT-PCR results (n = 189) rising to 97.1% 14 days post-PCR diagnosis (n = 70). COI>1.0 are considered positive. for SARS-CoV-2 antibodies on the Roche Cobas e801 analyzer as per the manufacturer’s instructions. The performance of this Roche assay has been evaluated and verified recently (Lau et al., 2020).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
SingHealth CIRB Committee waived the requirement for ethical approval and written informed consent for participants in this study because this application involves the use of de-identified slides that cannot be readily identified in accordance with the national legislation and the institutional requirements.
Author Contributions
KC: Optimization, validation and reporting RT-PCR assay TA: Optimization, validation and reporting serology assay JY: Optimization, validation and reporting of multiplex immunoassays PC: Conducting autopsy and reviewing histology slides JI and PYC: Reviewing histology slides.
All authors whose names appear on the submission.
1) made substantial contributions to the conception of the work, acquisition, analysis, or interpretation of data.
2) drafted the work or revised it critically for important intellectual content
3) approved the version to be published and
4) agree to be accountable for all aspects of the work
Funding
This publication was funded by SingHealth Duke-NUS Pathology Academic Clinical Program (ACP).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmolb.2021.658932/full#supplementary-material
References
- Abel E. J., Bauman T. M., Weiker M., Shi F., Downs T. M., Jarrard D. F., et al. (2014). Analysis and Validation of Tissue Biomarkers for Renal Cell Carcinoma Using Automated High-Throughput Evaluation of Protein Expression. Hum. Pathol. 45 (5), 1092–1099. 10.1016/j.humpath.2014.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babapoor-Farrokhran S., GillWalker D. J., Rasekhi R. T., Bozorgnia B., Amanullah A. (2020). Myocardial Injury and COVID-19: Possible Mechanisms. Life Sci. 253, 117723. 10.1016/j.lfs.2020.117723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton L. M., Duval E. J., Stroberg E., Ghosh S., Mukhopadhyay S. (2020). COVID-19 Autopsies, Oklahoma, USA. Am. J. Clin. Pathol. 153, 725–733. 10.1093/ajcp/aqaa062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearse M., Hung Y. P., Krauson A. J., Bonanno L., Boyraz B., Harris C. K., et al. (2021). Factors Associated with Myocardial SARS-CoV-2 Infection, Myocarditis, and Cardiac Inflammation in Patients with COVID-19. Mod. Pathol. 34, 1345–1357. 10.1038/s41379-021-00790-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehmer T. K., Kompaniyets L., Lavery A. M., Hsu J., Ko J. Y., Yusuf H., et al. (2021). Association between COVID-19 and Myocarditis Using Hospital-Based Administrative Data - United States, March 2020-January 2021. MMWR Morb. Mortal. Wkly. Rep. 70, 1228–1232. 10.15585/mmwr.mm7035e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung E. W., Zachariah P., Gorelik M., Boneparth A., Kernie S. G., Orange J. S., et al. (2020). Multisystem Inflammatory Syndrome Related to COVID-19 in Previously Healthy Children and Adolescents in New York City. JAMA 324, 294–296. 10.1001/jama.2020.10374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong P. Y., Chui P., Ling A. E., Franks T. J., Tai D. Y. H., Leo Y. S., et al. (2004). Analysis of Deaths during the Severe Acute Respiratory Syndrome (SARS) Epidemic in Singapore: Challenges in Determining a SARS Diagnosis. Arch. Pathol. Lab. Med. 128 (2), 195–204. 10.5858/2004-128-195-aoddts [DOI] [PubMed] [Google Scholar]
- Corman V. M., Landt O., Kaiser M. (2021). The Cytokine Storm and COVID-19. J. Med. Virol. 25 (1), 250–256. 10.1002/jmv.26232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craver R., Huber S., Sandomirsky M., McKenna D., Schieffelin J., Finger L. (2020). Fatal Eosinophilic Myocarditis in a Healthy 17-Year-Old Male with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2c). Fetal Pediatr. Pathol. 39, 263–268. 10.1080/15513815.2020.1761491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandekar A. A., Perlman S. (2005). Immunopathogenesis of Coronavirus Infections: Implications for SARS. Nat. Rev. Immunol. 5 (12), 917–927. 10.1038/nri1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpino M. V., Quarleri J. (2020). SARS-CoV-2 Pathogenesis: Imbalance in the Renin-Angiotensin System Favors Lung Fibrosis. Front. Cel. Infect. Microbiol. 10, 340. 10.3389/fcimb.2020.00340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L., et al. (2020). Reduction and Functional Exhaustion of T Cells in Patients with Coronavirus Disease 2019 (COVID-19). Front. Immunol. 11, 827. 10.3389/fimmu.2020.00827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esbona K., Inman D., Saha S., Jeffery J., Schedin P., Wilke L., et al. (2016). COX-2 Modulates Mammary Tumor Progression in Response to Collagen Density. Breast Cancer Res. 18 (1), 35. 10.1186/s13058-016-0695-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z., Jensen S. M., Messenheimer D. J., Farhad M., Neuberger M., Bifulco C. B., et al. (2016). Multispectral Imaging of T and B Cells in Murine Spleen and Tumor. J. Immunol. 196 (9), 3943–3950. 10.4049/jimmunol.1502635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnelo M., Tan A., Her Z., Yeong J., Lim C. J., Chen J., et al. (2015). Interaction between Tumour-Infiltrating B Cells and T Cells Controls the Progression of Hepatocellular Carcinoma. Gut 66 (310814), 342–351. 10.1136/gutjnl-2015-310814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnelo M., Tan A., Her Z., Yeong J., Lim C. J., Chen J., et al. (2017). Interaction between Tumour-Infiltrating B Cells and T Cells Controls the Progression of Hepatocellular Carcinoma. Gut 66 (2), 342–351. 10.1136/gutjnl-2015-310814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George P. M., Wells A. U., Jenkins R. G. (2020). Pulmonary Fibrosis and COVID-19: The Potential Role for Antifibrotic Therapy. Lancet Respir. Med. 8, 807–815. 10.1016/S2213-2600(20)30225-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifoni A., Weiskopf D., Ramirez S. I., Mateus J., Dan J. M., Moderbacher C. R., et al. (2020). Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 181, 1489–1501. 10.1016/j.cell.2020.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., et al. (2020). SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 181 (2), 271–280. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. (2020a). Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. The Lancet 395 (10223), 497–506. 10.1016/s0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H.-I., Lin J.-Y., Chiang H.-C., Huang P.-N., Lin Q.-D., Shih S.-R. (2020b). Exosomes Facilitate Transmission of Enterovirus A71 from Human Intestinal Epithelial Cells. J. Infect. Dis. 222 (3), 456–469. 10.1093/infdis/jiaa174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam J. H., Ng H. H. M., Lim C. J., Sim X. N., Malavasi F., Li H., et al. (2019). Expression of CD38 on Macrophages Predicts Improved Prognosis in Hepatocellular Carcinoma. Front. Immunol. 10, 2093. 10.3389/fimmu.2019.02093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau C. S., Hoo S. P., Yew S. F., Ong S. K., Lum L. T., Heng P. Y., et al. (2020). Evaluation of an Electrochemiluminescent SARS-CoV-2 Antibody Assay. J. Appl. Lab. Med. 5, 1313–1323. 10.1093/jalm/jfaa134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bert N., Tan A. T., Kunasegaran K., Tham C. Y. L., Hafezi M., Chia A., et al. (2020). SARS-CoV-2-specific T Cell Immunity in Cases of COVID-19 and SARS, and Uninfected Controls. Nature 584, 457–462. 10.1038/s41586-020-2550-z [DOI] [PubMed] [Google Scholar]
- Liao M., Liu Y., Yuan J., Wen Y., Xu G., Zhao J., et al. (2020). The Landscape of Lung Bronchoalveolar Immune Cells in COVID-19 Revealed by Single-Cell RNA Sequencing. Nat. Med. 26, 842–844. 10.1101/2020.02.23.20026690 [DOI] [PubMed] [Google Scholar]
- Lim J. C. T., Yeong J. P. S., Lim C. J., Ong C. C. H., Wong S. C., Chew V. S. P., et al. (2018). An Automated Staining Protocol for Seven-Colour Immunofluorescence of Human Tissue Sections for Diagnostic and Prognostic Use. Pathology 50 (3), 333–341. 10.1016/j.pathol.2017.11.087 [DOI] [PubMed] [Google Scholar]
- Lovisa S., LeBleu V. S., Tampe B., Sugimoto H., Vadnagara K., Carstens J. L., et al. (2015). Epithelial-to-Mesenchymal Transition Induces Cell Cycle Arrest and Parenchymal Damage in Renal Fibrosis. Nat. Med. 21 (9), 998–1009. 10.1038/nm.3902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlecnik B., Bindea G., Kirilovsky A., Angell H. K., Obenauf A. C., Tosolini M., et al. (2016). The Tumor Microenvironment and Immunoscore Are Critical Determinants of Dissemination to Distant Metastasis. Sci. Transl. Med. 8 (327), 327ra26. 10.1126/scitranslmed.aad6352 [DOI] [PubMed] [Google Scholar]
- Molenkamp R., Meijer A., Chu D. K., Bleicker T., Brünink S., Schneider J., et al. (2020). Detection of 2019 Novel Coronavirus (2019-nCoV) by Real-Time RT-PCR. Euro Surveill. 25 (3), 2000045. 10.2807/1560-7917.ES.2020.25.3.2000045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nghiem P. T., Bhatia S., Lipson E. J., Kudchadkar R. R., Miller N. J., Annamalai L., et al. (2016). PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. N. Engl. J. Med. 374 (26), 2542–2552. 10.1056/nejmoa1603702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni L., Ye F., Cheng M.-L., Feng Y., Deng Y.-Q., Zhao H., et al. (2020). Detection of SARS-CoV-2-specific Humoral and Cellular Immunity in COVID-19 Convalescent Individuals. Immunity 52, 971–977. 10.1016/j.immuni.2020.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil M., Singh S., Henderson J., Krishnamurthy P. (2020). Mechanisms of COVID‐19‐induced Cardiovascular Disease: Is Sepsis or Exosome the Missing Link?. J. Cel Physiol 236, 3366–3382. 10.1002/jcp.30109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris J., Lai S., Poon L., Guan Y., Yam L., Lim W., et al. (2003). Coronavirus as a Possible Cause of Severe Acute Respiratory Syndrome. The Lancet 361, 1319–1325. 10.1016/s0140-6736(03)13077-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., et al. (2020). Dysregulation of Immune Response in Patients with Coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 71, 762–768. 10.1093/cid/ciaa248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson E. L., Alkass K., Bergmann O., Maguire J. J., Roderick H. L., Davenport A. P. (2020). Genes Encoding ACE2, TMPRSS2 and Related Proteins Mediating SARS-CoV-2 Viral Entry Are Upregulated with Age in Human Cardiomyocytes. J. Mol. Cell Cardiol. 147, 88–91. 10.1016/j.yjmcc.2020.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin U., Muik A., Derhovanessian E., Vogler I., Kranz L. M., Vormehr M., et al. (2020). COVID-19 Vaccine BNT162b1 Elicits Human Antibody and TH1 T Cell Responses. Nature 586, 594–599. 10.1038/s41586-020-2814-7 [DOI] [PubMed] [Google Scholar]
- Shi Y., Tan M., Chen X., Liu Y., Huang J., Ou J., et al. (2020). Immunopathological Characteristics of Coronavirus Disease 2019 Cases in Guangzhou, China. medRxiv 160, 261–268. 10.1101/2020.03.12.20034736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirazi S., Mami S., Mohtadi N., Ghaysouri A., Tavan H., Nazari A., et al. (2021). Sudden Cardiac Death in COVID-19 Patients, a Report of Three Cases. Future Cardiol. 17 (1), 113–118. 10.2217/fca-2020-0082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siripanthong B., Nazarian S., Muser D., Deo R., Santangeli P., Khanji M. Y., et al. (2020). Recognizing COVID-19-Related Myocarditis: The Possible Pathophysiology and Proposed Guideline for Diagnosis and Management. Heart Rhythm 17, 1463–1471. 10.1016/j.hrthm.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack E. C., Wang C., Roman K. A., Hoyt C. C. (2014). Multiplexed Immunohistochemistry, Imaging, and Quantitation: a Review, with an Assessment of Tyramide Signal Amplification, Multispectral Imaging and Multiplex Analysis. Methods 70 (1), 46–58. 10.1016/j.ymeth.2014.08.016 [DOI] [PubMed] [Google Scholar]
- Thevarajan I., Nguyen T. H. O., Koutsakos M., Druce J., Caly L., van de Sandt C. E., et al. (2020). Breadth of Concomitant Immune Responses Prior to Patient Recovery: A Case Report of Non-Severe COVID-19. Nat. Med. 26 (4), 453–455. 10.1038/s41591-020-0819-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thieme C. J., Anft M., Paniskaki K., Blazquez-Navarro A., Doevelaar A., Seibert F. S., et al. (2020). The SARS-CoV-2 T-Cell Immunity Is Directed against the Spike, Membrane, and Nucleocapsid Protein and Associated with COVID 19 Severity. Cell Rep. Med. 1 (6), 10092. 10.1101/2020.05.13.20100636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga Z., Flammer A. J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A. S., et al. (2020). Endothelial Cell Infection and Endotheliitis in COVID-19. The Lancet 395, 1417–1418. 10.1016/s0140-6736(20)30937-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Xie J., Zhao L., Fei X., Zhang H., Tan Y., et al. (2020). Alveolar Macrophage Dysfunction and Cytokine Storm in the Pathogenesis of Two Severe COVID-19 Patients. EBioMedicine 57, 102833. 10.1016/j.ebiom.2020.102833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskopf D., Schmitz K., Raadsen M., Grifoni A., Okba N., Endeman H., et al. (2020). Phenotype of SARS-CoV-2-specific T-Cells in COVID-19 Patients with Acute Respiratory Distress Syndrome. Sci Immunol. 5 (48), eabd2071. 10.1126/sciimmunol.abd2071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen W., Su W., Tang H., Le W., Zhang X., Zheng Y., et al. (2020). Immune Cell Profiling of COVID-19 Patients in the Recovery Stage by Single-Cell Sequencing. Cell Discov 6 (1), 31. 10.1038/s41421-020-0168-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2020). Available at: www.who.int/emergencies/diseases/novel-coronavirus-2-19/interactive-timeline#event-71 accessioned (Accessed Aug 20, 2020).
- Wick G., Backovic A., Rabensteiner E., Plank N., Schwentner C., Sgonc R. (2010). The Immunology of Fibrosis: Innate and Adaptive Responses. Trends Immunol. 31 (3), 110–119. 10.1016/j.it.2009.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick G., Knoflach M., Xu Q. (2004). Autoimmune and Inflammatory Mechanisms in Atherosclerosis. Annu. Rev. Immunol. 22, 361–403. 10.1146/annurev.immunol.22.012703.104644 [DOI] [PubMed] [Google Scholar]
- Wong C. K., Lam C. W. K., Wu A. K. L., Ip W. K., Lee N. L. S., Chan I. H. S., et al. (2004). Plasma Inflammatory Cytokines and Chemokines in Severe Acute Respiratory Syndrome. Clin. Exp. Immunol. 136 (1), 95–103. 10.1111/j.1365-2249.2004.02415.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Chen X., Cai Y., Xia J. a., Zhou X., Xu S., et al. (2020). Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 180, 934–943. 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Chen P., Wang J., Feng J., Zhou H., Li X., et al. (2020). Evolution of the Novel Coronavirus from the Ongoing Wuhan Outbreak and Modeling of its Spike Protein for Risk of Human Transmission. Sci. China Life Sci. 63 (1674-7305), 457–460. 10.1007/s11427-020-1637-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., et al. (2020). Pathological Findings of COVID-19 Associated with Acute Respiratory Distress Syndrome. Lancet Respir. Med. 8 (4), 420–422. 10.1016/s2213-2600(20)30076-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima T., Knowlton K. U. (2009). Viral Myocarditis. Circulation 119, 2615–2624. 10.1161/circulationaha.108.766022 [DOI] [PubMed] [Google Scholar]
- Yang N., Tian K., Jin M., Zhang X., Zhang F., Shi X., et al. (2021). Sudden Death of COVID-19 Patients in Wuhan, China: A Retrospective Cohort Study. J. Glob. Health 11, 05006. 10.7189/jogh.11.05006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X. H., Li T. Y., He Z. C., Ping Y. F., Liu H. W., Yu S. C., et al. (2020). A Pathological Report of Three COVID-19 Cases by Minimal Invasive Autopsies. Zhonghua Bing Li Xue Za Zhi 49 (5), 411–417. 10.3760/cma.j.cn112151-20200312-00193 [DOI] [PubMed] [Google Scholar]
- Yeong J., Lim J. C. T., Lee B., Li H., Ong C. C. H., Thike A. A., et al. (2019). Prognostic Value of CD8 + PD-1+ Immune Infiltrates and PDCD1 Gene Expression in Triple Negative Breast Cancer. J. Immunotherapy Cancer 7 (1), 34. 10.1186/s40425-019-0499-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeong J., Thike A. A., Lim J. C. T., Lee B., Li H., Wong S.-C., et al. (2017). Higher Densities of Foxp3+ Regulatory T Cells Are Associated with Better Prognosis in Triple-Negative Breast Cancer. Breast Cancer Res. Treat. 163 (1), 21–35. 10.1007/s10549-017-4161-4 [DOI] [PubMed] [Google Scholar]
- Zheng M., Gao Y., Wang G., Song G., Liu S., Sun D., et al. (2020). Functional Exhaustion of Antiviral Lymphocytes in COVID-19 Patients. Cell Mol Immunol 17, 533–535. 10.1038/s41423-020-0402-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W.-P., Flavell R. A. (1997). The Transcription Factor GATA-3 Is Necessary and Sufficient for Th2 Cytokine Gene Expression in CD4 T Cells. Cell 89 (4), 587–596. 10.1016/s0092-8674(00)80240-8 [DOI] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. (2020). A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 382, 727–733. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.