Abstract
Aims
We studied the extent/area of electrical pulmonary vein isolation (PVI) after either pulsed field ablation (PFA) using a pentaspline catheter or thermal ablation technologies.
Methods and results
In a clinical trial (NCT03714178), paroxysmal atrial fibrillation (PAF) patients underwent PVI with a multi-electrode pentaspline PFA catheter using a biphasic waveform, and after 75 days, detailed voltage maps were created during protocol-specified remapping studies. Comparative voltage mapping data were retrospectively collected from consecutive PAF patients who (i) underwent PVI using thermal energy, (ii) underwent reablation for recurrence, and (iii) had durably isolated PVs. The left and right PV antral isolation areas and non-ablated posterior wall were quantified. There were 20 patients with durable PVI in the PFA cohort, and 39 in the thermal ablation cohort [29 radiofrequency ablation (RFA), 6 cryoballoon, and 4 visually guided laser balloon]. Pulsed field ablation patients were younger with shorter follow-up. Left atrial diameter and ventricular systolic function were preserved in both cohorts. There was no significant difference between the PFA and thermal ablation cohorts in either the left- and right-sided PV isolation areas, or the non-ablated posterior wall area. The right superior PV isolation area was smaller with PFA than RFA, but this disappeared after propensity score matching. Notch-like normal voltage areas were seen at the posterior aspect of the carina in the balloon sub-cohort, but not the PFA or RFA cohorts.
Conclusion
Catheter-based PVI with the pentaspline PFA catheter creates chronic PV antral isolation areas as encompassing as thermal energy ablation.
Keywords: Pulsed field ablation, Pulmonary vein isolation, Atrial fibrillation, Electroporation, Level of isolation
What’s new?
After an initial pulmonary vein isolation (PVI) procedure using either pulsed field ablation (PFA) or thermal ablation [with point-by-point radiofrequency ablation (RFA) catheters, cryoballoon, or laser balloon], remapping procedures revealed that the extent of the isolated area of each PV antrum was not significantly different between the PFA and thermal ablation cohorts.
Qualitatively, there were notch-like normal voltage areas at the posterior carina in the Balloon cohort, but not in the PFA or RFA cohorts.
The right superior PVI area was smaller with PFA than RFA, but this disappeared after propensity score matching.
After propensity matching, PFA created a larger isolation area at the left inferior PV than RFA.
Introduction
Pulsed field ablation (PFA) has garnered substantial attention because of its unique tissue-preferential, non-thermal mechanism of cardiac ablation.1–4 Pre-clinical experiments have demonstrated that catheter-based PFA can create durable atrial lesions, with histological evidence of consistent transmurality and contiguity.5,6 This has led to the successful clinical use of a multi-electrode pentaspline PFA catheter in patients with atrial fibrillation (AF), with excellent lesion durability.7,8 In contrast to thermal energy approaches for AF ablation, PFA appears to have a reduced dependence on tissue contact because the electrical field acts on a volume, irrespective of whether this includes blood or tissue. Cardiac tissue within this field undergoes irreversible electroporation (IRE) when the field strength exceeds the tissue’s IRE threshold.
Since larger pulmonary vein isolation (PVI) areas are thought to result in lower AF recurrence rates, during AF ablation, circumferential PVI typically incorporates attempts to include the PV antrum.9 Studies with cryoballoon ablation have revealed that the area of PVI is wide and antral,10,11 but thermal balloon ablation catheters are highly reliant on tissue contact, so the PVI area is dependent upon the left atrial and PV anatomy. On the other hand, focal radiofrequency ablation (RFA) catheters allow the operator to design lines regardless of LA-PV anatomy, potentially encompassing a larger PVI area.12
Herein, we compare the atrial-PV tissue incorporated into the PVI lesion set—based on extent and morphology—after PFA using the pentaspline catheter to thermal energy ablation, including RFA and the balloon technologies (cryothermy and laser ablation). To avoid the reversible injury that might be present in the acute, immediate post-ablation period (e.g. oedema, Haemorrhage, reversible electroporation, etc.), we studied a population of patients who had previously undergone PVI with one of these ablation technologies, subsequently underwent high-density electroanatomical mapping, and were found to have durable PVI. That is, we compared the level of electrical isolation after PFA vs. thermal ablation, when each ablation modality had been used optimally to achieve durable PVI.
Methods
Data source
This study retrospectively employed two data sources: (i) PFA cohort: clinical data from the PEFCAT trial (Safety and Feasibility Study of the FARAPULSE Endocardial Ablation System to Treat Paroxysmal Atrial Fibrillation; NCT03714178), and (ii) Thermal cohort: clinical data from patients treated with thermal energy at the Mount Sinai Hospital. PEFCAT was a single-arm feasibility study of PFA conducted at 2 European centres, one of which sources the data analysed herein (Homolka Hospital, Prague, Czech Republic). The study’s sponsor, Farapulse Inc. (Menlo Park, CA, USA), is also the manufacturer of the PFA system. All patients provided signed informed consent before the procedure. The research reported in this paper adhered to the Helsinki Declaration as revised in 2013; ethical committee approval was obtained.
Study population
At the time of the analysis, PEFCAT had enrolled 50 patients at Homolka Hospital with symptomatic paroxysmal atrial fibrillation (PAF) resistant to antiarrhythmic medications, a left ventricular ejection fraction >40%, and left atrial (LA) anteroposterior dimension <5.0 cm. There were no exclusions for PV anatomy. From this 50-patient cohort, 45 underwent protocol pre-specified invasive PV reassessment at ∼75 days after the index procedure.
Regarding the thermal ablation cohort, 204 consecutive patients with PAF underwent redo ablation for AF recurrence between April 2015 to August 2020. All patients were referred to the Icahn School of Medicine at Mount Sinai for electrophysiological evaluation and catheter ablation. We excluded patients with (i) any PV reconnections, (ii) prior additional ablation such as roof line ablation, LA posterior wall ablation, or ablation of complex fractionated atrial electrograms, and (iii) previous multiple AF ablation sessions.
We compared the PVI areas: (i) of the PFA cohort with the thermal ablation cohort, (ii) between the PFA, RFA, and Balloon ablation cohorts, and (iii) between the PFA and RFA cohorts after propensity score matching of patient characteristics.
Index procedures—pulsed field ablation
Ablation procedures were performed as previously described.7 In brief, after femoral venous access, a 12 Fr over-the-wire multi-electrode pentaspline PFA catheter (Farawave, Farapulse Inc.) was advanced through a 13 Fr deflectable sheath to the left atrium via transseptal puncture. The PVI workflow was performed under moderate sedation, including propofol. Typically, the catheter was advanced over a guidewire such that the splines achieved circumferential contact/proximity at the PV antra. The catheter was rotated between applications to ensure circumferential PV ostial and antral coverage. The therapeutic waveform is structured as a hierarchical set of microsecond-scale pulses emitted in bipolar fashion between electrodes, with ablation delivery synchronized to five successive pacing stimuli. Generator output ranged from 1800 to 2000 V per application. After ablation, a circular mapping catheter (Lasso, Biosense Webster, Irvine, CA, USA) assessed electrical PV activity, followed by post-ablation voltage mapping using a multi-electrode mapping catheter and mapping system (PentaRay and CARTO3, Biosense Webster, Irvine, CA, USA).
Patients underwent invasive reassessment electrophysiological mapping at ∼75 days after the index ablation procedure. During this repeat procedure, the durability of PV isolation was assessed using the same multi-electrode mapping catheter and voltage amplitude mapping was performed.
Thermal energy ablation (radiofrequency ablation, visually guided laser balloon, and cryoballoon)
Patients underwent RFA under general anaesthesia with double transseptal puncture. Electroanatomic LA-PV maps were created using either the EnSite NavX (Abbott, St. Paul, MN, USA) or CARTO3 (Biosense Webster, Irvine, CA, USA) navigation system. Circumferential PVI was performed using either an externally irrigated contact force sensing catheter (ThermoCool Smarttouch, Biosense Webster, or TactiCath, Abbott) or a non-force sensing catheter (Thermocool catheter, Biosense Webster; Figure 1). In all procedures, a deflectable sheath (Agilis, Abbott) was used to enhance catheter stability, and single interrupted point-by-point ablation lesions were delivered in power control mode. Either oesophageal temperature monitoring (15 of 29 patients) or mechanical oesophageal deviation (14 of 29) was used in all patients. Isoproterenol-induced or adenosine-induced latent PV reconnection and non-PV triggers were also targeted if present.
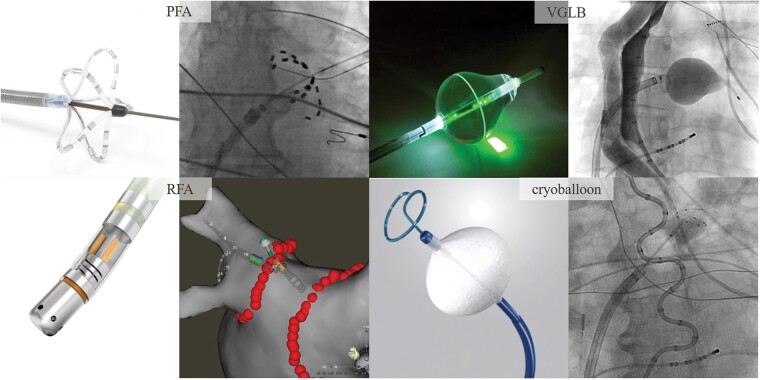
Figure 1.
Catheter ablation technologies. PFA: The multispline PFA catheter is positioned at the ostia of each vein to deliver a therapeutic waveform. RFA: Point-by-point ablation lesions are delivered to achieve circumferential PVI. VGLB: Under endoscopic visual guidance, ablation lesions are delivered in a circumferential, contiguous, and overlapping manner around the vein. Cryoballoon: With the balloon apposed against the ostia of each vein, cryoapplications are delivered. PFA, pulsed field ablation; RFA, radiofrequency ablation; VGLB, visually guided laser balloon.
Visually guided laser balloon (VGLB; HeartLight, CardioFocus, Marlborough, MA, USA) ablation was performed as previously described.13 Briefly, following a transseptal puncture, a 12-Fr deflectable sheath was positioned in the LA. The VGLB catheter was advanced through the sheath and inflated at the ostium of the target PV (Figure 1). Under visual guidance, ablation lesions were delivered in a circumferential, contiguous, and overlapping manner around the PV. After placement of the initial anatomically-guided encircling lesion set, a circular mapping catheter was used to assess for electrical PVI. If the PV was not isolated, the VGLB catheter was used to deliver additional lesions to the area of electrical breakthrough.
Cryoballoon ablation was performed under general anaesthesia. After transseptal puncture, the 12-Fr deflectable catheter was then positioned over-the-wire into the LA, through which the 28-mm cryoballoon (Arctic Front Advance; Medtronic plc, Dublin, Ireland) was advanced. The J wire or a spiral mapping catheter (Achieve; Medtronic plc) was positioned in the targeted pulmonary vein and the cryoballoon positioned at the PV ostium. The balloon was inflated and venography was performed to confirm PV occlusion (Figure 1). Two applications were administered per vein with targeted ablation times of 4 min each. The pulldown technique was used, as needed, to close inferior leaks. Phrenic nerve pacing from within the superior vena cave was performed to ensure nerve integrity. The oesophageal temperature was monitored throughout ablation and was terminated when the temperature decreased to <20°C.
Patients who underwent redo ablation for clinical recurrence after thermal ablation, either RFA or balloon ablation, were included in this analysis provided they demonstrated durable PVI at the time of repeat procedure. During the repeat procedure, the durability of PV isolation was confirmed using a multi-electrode catheter and a high-density electroanatomical voltage amplitude map was created.
Voltage mapping and measurements in the chronic phase
The three-dimensional geometry of the LA and PVs was reconstructed using either the EnSite NavX (St. Jude Medical, St. Paul, MN, USA) or CARTO (Biosense Webster, Irvine, CA, USA) navigation system. High-density bipolar voltage mapping was performed using a multispline mapping catheter (PentaRay, Biosense Webster; or HD grid, Abbott; or Inquiry AFocus, St. Jude Medical). For the purpose of comparison, a peak-to-peak bipolar electrogram amplitude <0.5 mV was defined as the low-voltage threshold for all maps in accordance with previous studies.12,14 The PV ostium was identified as the point of maximal inflection between the PV wall and LA wall, and the PV antrum was defined as the region proximal to the PV ostium excluding the PVs. In patients with a common PV, we defined the second branch of common trunk as PV ostium as described in previous study.9 The LA posterior wall surface area was defined as the area bordered by the PV lesions and two lines connecting the most superior- and inferior-most aspects of the circumferential ablation lines, respectively. The surface areas of the isolated left- and right-sided PV antra and non-ablated posterior wall were quantified (Supplementary material online, Figure S1). The CARTO and Ensite systems automatically calculated the surface area and the distance from manually selected points.
Statistical analysis
Values of categorical variables are reported as numbers and percentages, and values of continuous variables are reported as mean with standard deviation (SD) or median with interquartile range (IQR). Test of normality was conducted by Kolmogorov-Smirnov or Shapiro-Wilk. Continuous variables were compared using Student’s t-test or Mann-Whitney U test, and categorical variables were compared using Fisher's exact or chi-square tests, as appropriate. Propensity scores were estimated for patients who underwent PFA or RFA using a logistic regression model, and the following parameters were matched: sex, age, left ventricular ejection fraction, and left atrial anteroposterior diameter. The patients were matched on a 1:1 basis using a nearest neighbour algorithm without replacement and a calliper width equal to 0.2 of the standard deviations (SDs) of the propensity scores. A P-value <0.05 was considered significant. All analyses were performed using SPSS version 24.0 (IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
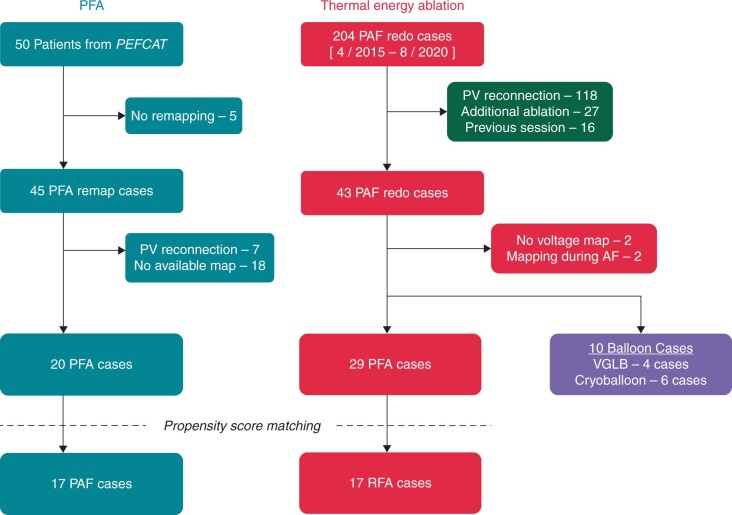
In the PFA cohort, among a total of 45 patients undergoing invasive PV reassessment at a protocol-mandated time point of 75 days after the index ablation procedure, we retrospectively identified 20 patients with durable PVI and available high-quality maps (Figure 2). In the 204-patient thermal ablation cohort, we excluded 161 patients meeting the exclusion criteria; two additional patients each were excluded for either no available voltage maps, or for having only voltage mapping performed during AF. Accordingly, there were 39 patients in the thermal ablation cohort—29 patients treated with RFA, and 10 patients treated with balloon technologies (4 VGLB and 6 cryoballoon). Two patients (1 RFA and 1 cryoballoon) underwent index procedures at another hospital. Two patients from RFA cohort received extra-PV ablation—one with superior vena cava isolation and one with ablation of a non-PV trigger at the interatrial septum.
Figure 2.
Patient selection flow chart. AF, atrial fibrillation; PAF, paroxysmal atrial fibrillation; PFA, pulsed field ablation; PV, pulmonary vein; RFA, radiofrequency ablation; VGLB, visually guided laser balloon.
Patient characteristics are summarized in Table 1. The average age of the PFA cohort was younger than the thermal ablation cohort. Other patient characteristics were similar between cohorts, and typical of patients referred for PAF ablation: predominantly male, overall preserved left ventricular ejection fraction, and mildly dilated LA. As the patients in the thermal energy ablation cohort had redo procedures only after the AF recurrence, the Follow-up period for these patients was longer than for patients in the PFA cohort.
Table 1.
Baseline characteristics
| Thermal energy ablation |
|||||
|---|---|---|---|---|---|
| PFA n = 20 | Total n = 39 | RFA n = 29 | Balloon ablation n = 10 (Cryo n = 6, VGLB n = 4) | P-value* | |
| Age, years ± SD | 56.9 ± 11.0 | 66.1 ± 9.3 | 66.4 ± 8.4 | 65.1 ± 12.1 | 0.001 |
| Male | 15 (75.0) | 25 (64.1) | 19 (65.5) | 6 (60.0) | 0.40 |
| LA diameter, mm ± SD | 41.7 ± 5.0 | 41.1 ± 6.0 (n = 32) | 41.4 ± 6.4 (n = 27) | 39.6 ± 3.8 (n = 5) | 0.39 |
| LVEF, % ± SD | 63.6 ± 3.7 | 60.8 ± 7.5 (n = 35) | 60.9 ± 7.8 (n = 28) | 60.6 ± 6.7 (n = 7) | 0.07 |
| Hypertension | 13 (65.0) | 24 (61.5) | 20 (69.0) | 4 (40.0) | 0.80 |
| Diabetes | 2 (10.0) | 5 (12.8) | 3 (10.3) | 2 (20.0) | 1 |
| Stroke or TIA | 1 (5.0) | 2 (5.1) | 2 (6.9) | 0 (0) | 1 |
| CAD (MI/CABG) | 0 (0.0) | 2 (5.1) | 2 (6.9) | 0 (0) | 0.54 |
| Left common PV | 2 (10.0) | 4 (10.3) | 3 (10.3) | 1 (10) | 1 |
| Redo procedure date, day (IQR) | 84 (69–90) | 758 (319–1287) (n = 37) | 708 (310–1326) (n = 28) | 821 (316–1622) (n = 9) | <0.001 |
CABG, coronary artery bypass grafting; CAD, coronary artery disease; LA, left atrium; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PFA, pulsed field ablation; PV, pulmonary vein; RFA, radiofrequency ablation; VGLB, visually guided laser balloon.
Data shown as number (%) unless otherwise specified.
P-value compares PFA (n = 20) vs. thermal energy ablation (n = 39).
Mapping data and quantitative analysis
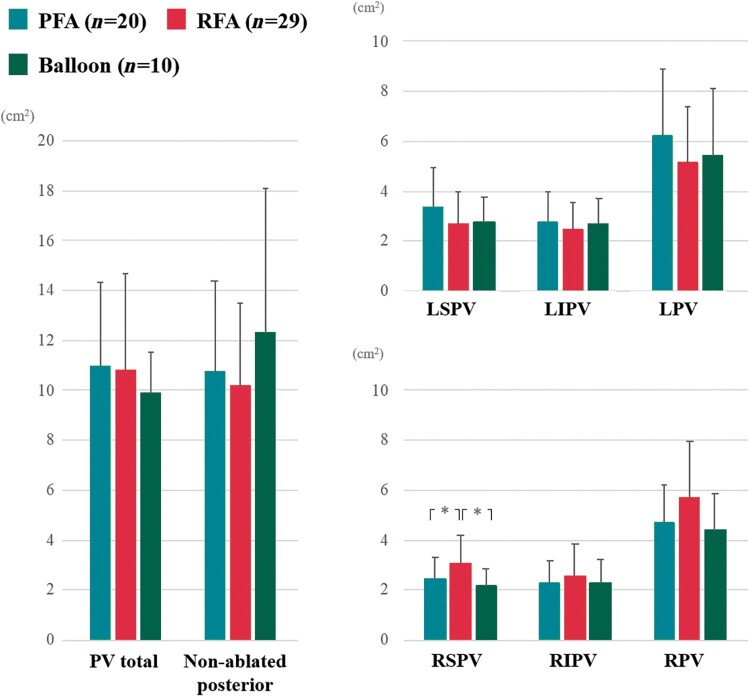
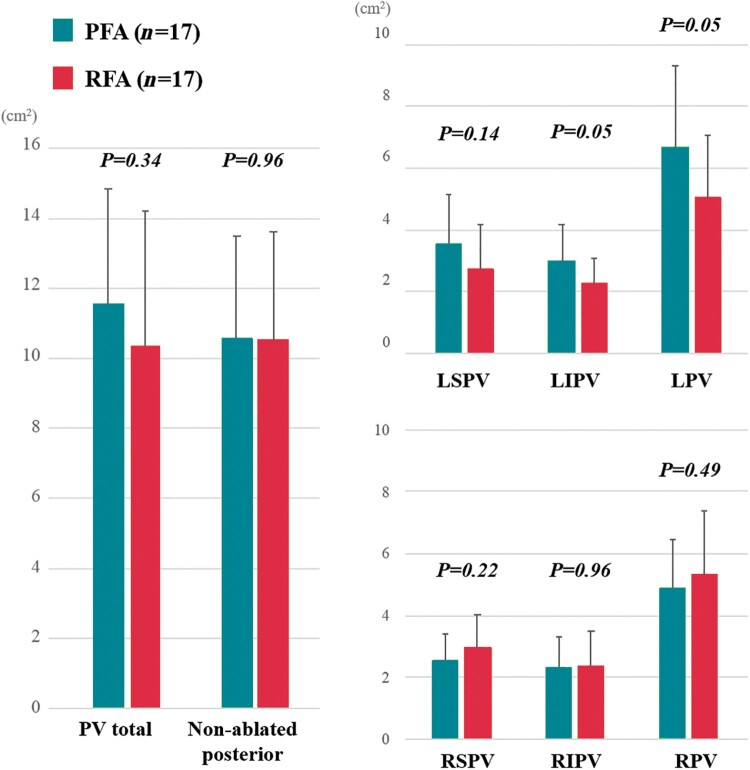
The number of electrograms acquired with the mapping catheter was 874 (520–1228) in the PFA cohort and 940 (463–2276) in the thermal ablation cohort (P = 0.16). Voltage maps were created during mostly sinus rhythm (55 of 59; 93.2%), otherwise during atypical atrial flutter (4 of 59; 6.8%). The isolated area of each PV antrum was not significantly different between the PFA and thermal cohorts (Table 2 and Figure 3). The total ablation area and non-ablated area on the posterior wall were also similar between cohorts (11.0 ± 3.4 vs. 10.6 ± 3.4cm2; P = 0.70 and 10.7 ± 3.7 vs. 10.7 ± 4.1cm2; P = 1.00). The total LAPW area was also identical between cohorts (P = 0.79).
Table 2.
Comparison of isolation areas between PFA and thermal ablation
| PFA n = 20 | Thermal energy n = 39 | P-value | |
|---|---|---|---|
| LPV total, cm2 ± SD | 6.2 ± 2.7 | 5.2 ± 2.1 | 0.12 |
| LSPV, cm2 ± SD | 3.4 ± 1.6 | 2.7 ± 1.2 | 0.09 |
| LIPV, cm2 ± SD | 2.8 ± 1.2 | 2.5 ± 1.1 | 0.43 |
| RPV total, cm2 ± SD | 4.7 ± 1.5 | 5.4 ± 2.1 | 0.24 |
| RSPV, cm2 ± SD | 2.4 ± 0.9 | 2.9 ± 1.1 | 0.14 |
| RIPV, cm2 ± SD | 2.3 ± 0.9 | 2.5 ± 1.2 | 0.47 |
| PV total, cm2 ± SD | 11.0 ± 3.4 | 10.6 ± 3.4 | 0.70 |
| Non-ablated area, cm2 ± SD | 10.7 ± 3.7 | 10.7 ± 4.1 | 1.00 |
| Total PW area, cm2 ± SD | 21.7 ± 5.4 | 21.3 ± 4.8 | 0.79 |
| Sinus rhythm during mapping, n (%) | 19 (95.0%) | 36 (92.3%) | 1 |
| No. of points, n (IQR) | 874 (520–1228) | 940 (463–2276) | 0.164 |
IQR, interquartile range; LAPW, left atrial posterior wall; LIPV, left inferior pulmonary vein; LPV, left pulmonary vein; LSPV, left superior pulmonary vein; RIPV, right inferior pulmonary vein; RPV, right pulmonary vein; RSPV, right superior pulmonary vein; PW, posterior wall.
Figure 3.
Pulsed field ablation (PFA) vs. thermal energy ablation. The isolated area of each PV antrum and non-ablated posterior LA area were not significantly different between the cohorts. LIPV, left inferior pulmonary vein; LPV, left pulmonary vein; LSPV, left superior pulmonary vein; RIPV, right inferior pulmonary vein; RPV, right pulmonary vein; RSPV, right superior pulmonary vein.
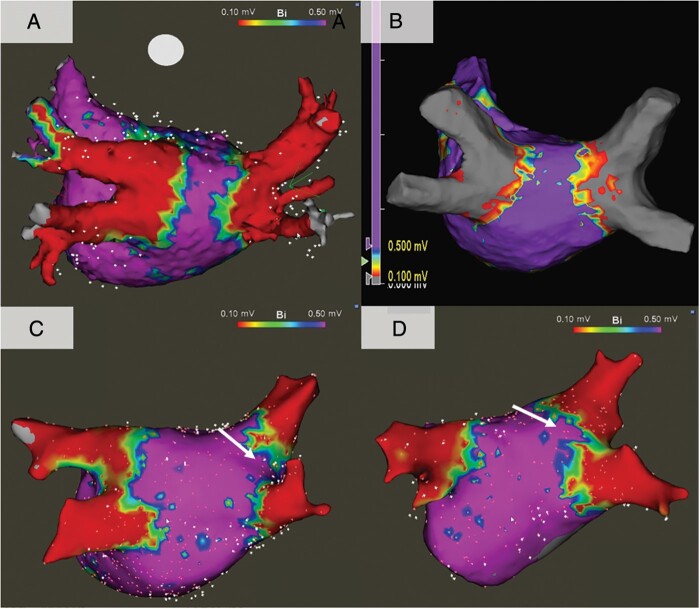
Representative examples of lesion sets produced by PFA, RFA and Balloon ablation are shown in Figure 4. Qualitatively, there were notch-like normal voltage areas at the posterior side of carina in the Balloon cohort, but not in either the PFA or RFA cohorts. The total ablation area and non-ablated area on the posterior wall were similar among cohorts. The isolation area of the right superior PV in the PFA cohort was smaller than that of the RFA cohort but was identical to the Balloon ablation cohort (Figure 5 and Supplementary material online, Table S1). Similarly, RSPV isolation area was significantly smaller in the Balloon ablation cohort than the RFA cohort. The isolation areas of each other PV antra were not significantly different between the cohorts.
Figure 4.
Representative voltage maps. (A) Pulsed field ablation, (B) radiofrequency ablation, (C) cryoballoon ablation, and (D) visually guided laser balloon. In the balloon sub-cohorts, there were notch-like areas of normal voltage at the carinal areas (white arrows).
Figure 5.
Pulsed field ablation (PFA) vs. radiofrequency ablation (RFA) vs. balloon ablation. The total ablation area and non-ablated area on the posterior LA wall were similar among the cohorts. The isolation area of the RSPV in the PFA cohort was smaller than that of RFA cohort, but similar to the balloon ablation cohort. LIPV, left inferior pulmonary vein; LPV, left pulmonary vein; LSPV, left superior pulmonary vein; RIPV, right inferior pulmonary vein; RPV, right pulmonary vein; RSPV, right superior pulmonary vein. *P<0.05
Patients characteristics between the propensity score-matched PFA (n = 17) and RFA (n = 17) cohorts are summarized in Supplementary material online, Table S2. After propensity matching, the significant difference in the RSPV isolation area between the cohorts disappeared. On the other hand, patients in PFA cohort had larger isolation areas for the left inferior PV than patients in the RFA cohort (Figure 6).
Figure 6.
Pulsed field ablation (PFA) vs. radiofrequency ablation (RFA) after propensity matching. After the propensity scores matching, there was no significant difference in the RSPV isolation areas between the cohorts. Patients in the PFA cohort had a larger isolation area in at the left inferior PV than patients in the RFA cohort. LIPV, left inferior pulmonary vein; LPV, left pulmonary vein; LSPV, left superior pulmonary vein; RIPV, right inferior pulmonary vein; RPV, right pulmonary vein; RSPV, right superior pulmonary vein.
Discussion
To the best of our knowledge, this study is the first to compare the extent and morphology of the area of electrical PVI incorporated into PFA vs. thermal energy ablation. These data demonstrated that catheter-based PVI with the multi-electrode pentaspline PFA catheter creates a PV antral isolation area as encompassing as thermal energy ablation.
PFA is an emerging technology in electrophysiological field which is capable of creating cardiac lesions with non-thermal energy. As part of its mechanism of action, it is believed that by applying an electrical field across the cell membrane lipid bilayer, there is formation of aqueous pores in the cell membrane with subsequent necrotic cell death.2 When used on atrial tissue, PFA using the pentaspline catheter can create durable lesions without inducing thermal injury,7 and is unique amongst energy sources in that it is capable of sparing collateral structures such as the phrenic nerve and oesophagus.5,15 Another unique feature of PFA is reduced contact dependence for enacting tissue ablation. Indeed, detailed computational simulations suggest that despite gaps of 2 mm between the PFA catheter and atrial tissue, adequate lesions can still be generated.16
In several previous studies, balloon ablation systems have proven capable of creating wide antral lesions in the chronic phase;17,18 indeed, our balloon cohort had a similar degree of an isolated area in the LA.18 On the other hand, another previous study described that the PVI areas during the chronic phase after balloon ablation using the 2nd generation Cryoballoon were smaller than the expected isolation area during circumferential RF ablation.12 In our study, while there was no significant difference between the PFA and thermal cohorts, the isolation area of the RFA cohort was also larger than that of the Balloon cohort. Furthermore, there was a notch at the posterior carinal area between the PVs in the Balloon ablation cohort, indicating that the balloon was located somewhat more distal to the PVs, especially at the left side. Those notches were not seen in the PFA or RFA cohorts.
The total isolation area after PFA more closely resembled the RFA cohort. After propensity matching, PFA created a larger isolation area at the left inferior PV than RFA. This might be related to the influence of the electrical field which does not require tissue contact, or to the fact that in the flower pose, the PFA multispline catheter can considerably extend the level of isolation. Indeed, using this catheter in a series of persistent AF patients, we previously demonstrated that by employing the flower pose, the entire posterior LA wall between the PVs can be ablated.19 Regarding the RSPV, the isolation areas in the PFA and balloon cohorts were smaller than that in the RFA cohort, suggesting that they may ablate somewhat more distal aspects of the is PV. However, the significance between RFA and PFA disappeared after propensity matching.
The criteria for low voltage may vary according to the rhythm during mapping. In a recent study, a cut-off value of 0.38 mV in atrial flutter had a good correlation with the value of 0.5 mV in sinus rhythm.20 Accordingly, it is conceivable that a larger low-voltage area might be identified using a cut-off value of <0.5 mV if the chamber was mapped during atrial flutter. But even after excluding the 4 patients mapped during atrial flutter in this analysis (1 roof-dependent flutter after PFA, 1 cavo-tricuspid isthmus-dependent flutter after laser balloon ablation, and 2 mitral isthmus-dependent flutters after RFA), the ablated area metrics were the same (Supplementary material online, Table S3).
Study limitations
This was a retrospective study and the various cohorts were derived from different sources; while we did perform propensity matching, the data is not randomized and raises the possibility of unknown confounders. Two patients (1 RFA and 1 Cryoablation) from the thermal energy ablation cohort underwent index procedures at outside hospitals, so the details of the initial procedures from these patients were not available. The volume of the LA and each PV diameter were not available. Although there were no clinical instances of PV stenosis, cardiac CT imaging between initial and redo procedures to assess for PV stenosis was performed in only 7 of 39 patients (17.9%) in the thermal energy cohort. The time between initial ablation and remapping was significantly longer in the thermal energy cohort than the PFA group—although there is little evidence that the level of PV isolation should differ further over time. Although we could adjust the factors which may have an effect on the low-voltage area such as sex, age, LA diameter, and EF, we could not adjust the follow-up date. Finally, the size of the electrical field may be varied by a variety of factors including the catheter shape, electrode configuration, pulse train composition, amplitude, duration, frequency, and lesion deployment strategy. Thus, the results of this study must be considered limited to this specific pentaspline PFA catheter, and even then, only for this biphasic/bipolar waveform employed herein.
Conclusions
Catheter-based PVI with the pentaspline PFA catheter facilitates wide PV antral isolation areas as inclusive as that created by standard thermal energy ablation strategies, including point-by-point radiofrequency ablation, cryoballoon ablation, and laser balloon ablation.
Supplementary material
Supplementary material is available at Europace online.
Funding
The PFA trials were supported by the manufacturer of the pulse field ablation system, Farapulse Inc.
Conflict of interest: V.Y.R. owns stock in Farapulse and Manual Surgical Sciences; and has served as a consultant for Farapulse, Cardiofocus, Medtronic, Abbott, Biosense Webster, and Boston Scientific. V.Y.R. also has conflicts with other medical companies unrelated to this manuscript—a full list is provided in the Supplementary material online. P.N. has received grant support from Farapulse, Cardiofocus, Biosense-Webster and Abbott. S.R.D. owns stock in Farapulse and Manual Surgical Sciences and serves as a consultant to Cardiofocus and has received grant support from Biosense-Webster. J.S.K. serves as a consultant to, and has received grant support from Farapulse. J.S.K. also has conflicts with other companies not related to this article (see Supplementary material online). All remaining authors have declared no conflicts of interest.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
Supplementary Material
References
- 1. Davalos RV, Mir LM, Rubinsky B.. Tissue ablation with irreversible electroporation. Ann Biomed Eng 2005;33:223–31. [DOI] [PubMed] [Google Scholar]
- 2. Kotnik T, Kramar P, Pucihar G, Miklavčič D, Tarek M.. Cell membrane electroporation - Part 1: The phenomenon. IEEE Electr Insul Mag 2012;28:14–23. [Google Scholar]
- 3. Reddy VY, Koruth J, Jais P, Petru J, Timko F, Skalsky I. et al. Ablation of atrial fibrillation with pulsed electric fields: an ultra-rapid, tissue-selective modality for cardiac ablation. JACC Clin Electrophysiol 2018;4:987–95. [DOI] [PubMed] [Google Scholar]
- 4. Koruth JS, Kuroki K, Iwasawa J, Viswanathan R, Buck ED, Donskoy E. et al. Endocardial ventricular pulsed field ablation : a proof-of-concept preclinical evaluation. Europace 2020;22:434–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Koruth J, Kuroki K, Iwasawa J, Enomoto Y, Viswanathan R, Brose R. et al. Preclinical evaluation of pulsed field ablation: electrophysiological and histological assessment of thoracic vein isolation. Circ Arrhythm Electrophysiol 2019;12:e007781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Howard B, Haines DE, Verma A, Packer D, Kirchhof N, Barka N. et al. Reduction in pulmonary vein stenosis and collateral damage with pulsed field ablation compared to radiofrequency ablation in a canine model. Circ Arrhythm Electrophysiol 2020;13:e008337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reddy VY, Neuzil P, Koruth JS, Petru J, Funosako M, Cochet H. et al. Pulsed field ablation for pulmonary vein isolation in atrial fibrillation. J Am Coll Cardiol 2019;74:315–26. [DOI] [PubMed] [Google Scholar]
- 8. Reddy VY, Dukkipati SR, Neuzil P, Pulsed field ablation of paroxysmal atrial fibrillation: one-year outcomes of IMPULSE, PEFCAT & PEFCAT II.JACC Clin Electrophysiol 2021. [DOI] [PubMed] [Google Scholar]
- 9. Kiuchi K, Kircher S, Watanabe N, Gaspar T, Rolf S, Arya A. et al. Quantitative analysis of isolation area and rhythm outcome in patients with paroxysmal atrial fibrillation after circumferential pulmonary vein antrum isolation using the pace-and-ablate technique. Circ Arrhythm Electrophysiol 2012;5:667–75. [DOI] [PubMed] [Google Scholar]
- 10. Reddy VY, Neuzil P, d’Avila A, Laragy M, Malchano ZJ, Kralovec S. et al. Balloon catheter ablation to treat paroxysmal atrial fibrillation: what is the level of pulmonary venous isolation? Heart Rhythm 2008;5:353–60. [DOI] [PubMed] [Google Scholar]
- 11. Kenigsberg DN, Martin N, Lim HW, Kowalski M, Ellenbogen KA.. Quantification of the cryoablation zone demarcated by pre- and postprocedural electroanatomic mapping in patients with atrial fibrillation using the 28-mm second-generation cryoballoon. Heart Rhythm 2015;12:283–90. [DOI] [PubMed] [Google Scholar]
- 12. Miyazaki S, Taniguchi H, Hachiya H, Nakamura H, Takagi T, Iwasawa J. et al. Quantitative analysis of the isolation area during the chronic phase after a 28-mm second-generation cryoballoon ablation demarcated by high-resolution electroanatomic mapping. Circ Arrhythmia Electrophysiol 2016;9:e003879. [DOI] [PubMed] [Google Scholar]
- 13. Dukkipati SR, Cuoco F, Kutinsky I, Aryana A, Bahnson TD, Lakkireddy D. et al. Pulmonary vein isolation using the visually guided laser balloon a prospective, multicenter, and randomized comparison to standard radiofrequency ablation. J Am Coll Cardiol 2015;66:1350–60. [DOI] [PubMed] [Google Scholar]
- 14. Anter E, Tschabrunn CM, Josephson ME.. High-resolution mapping of scar-related atrial arrhythmias using smaller electrodes with closer interelectrode spacing. Circ Arrhythm Electrophysiol 2015;8:537–45. [DOI] [PubMed] [Google Scholar]
- 15. Loh P, Groen MHA, Neven K, Kassenberg W, Wittkampf FHM, Loh P.. Pulmonary vein isolation with single pulse irreversible electroporation : a first in human study in 10 patients with atrial fibrillation. Circ Arrhythm Electrophysiol 2020;13:e008192. [DOI] [PubMed] [Google Scholar]
- 16. Ramirez FD, Reddy VY, Viswanathan R, Hocini M, Jaïs P.. Emerging technologies for pulmonary vein isolation. Circ Res 2020;127:170–83. [DOI] [PubMed] [Google Scholar]
- 17. Nagashima K, Okumura Y, Watanabe I, Nakahara S, Hori Y, Iso K. et al. Hot balloon versus cryoballoon ablation for atrial fibrillation: lesion characteristics and middle-term outcomes. Circ Arrhythm Electrophysiol 2018;11:e005861. [DOI] [PubMed] [Google Scholar]
- 18. Nanbu T, Yotsukura A, Suzuki G, Ishidoya Y, Sano F, Yoshida I. et al. Important factors in left atrial posterior wall isolation using 28-mm cryoballoon ablation for persistent atrial fibrillation—block line or isolation area? J Cardiovasc Electrophysiol 2020;31:119–27. [DOI] [PubMed] [Google Scholar]
- 19. Reddy VY, Anic A, Koruth J, Petru J, Funasako M, Minami K. et al. Pulsed field ablation in patients with persistent atrial fibrillation. J Am Coll Cardiol 2020;76:1068–80. [DOI] [PubMed] [Google Scholar]
- 20. Rodríguez-Mañero M, Valderrábano M, Baluja A, Kreidieh O, Martínez-Sande JL, García-Seara J. et al. Validating left atrial low voltage areas during atrial fibrillation and atrial flutter using multielectrode automated electroanatomic mapping. JACC Clin Electrophysiol 2018;4:1541–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.