 ABSTRACTS
ABSTRACTS
20th ISoP Annual Meeting
“Integrated pharmacovigilance for safer patients”
8–10 November 2021
Muscat, Oman
(Hybrid meeting)
The International Society of Pharmacovigilance (ISoP) aims to develop its activities on a worldwide basis towards supporting safer use of medicines in clinical practice.
ISoP encourages and supports the use of all types of information and methodologies in providing optimal drug treatment for patients. The Society is open to anyone interested in learning about better ways for patients to receive and use medicines safely, including academics, epidemiologists, medicines regulators, clinical pharmacologists, pharmaceutical industry representatives, practising clinicians, pharmacists and other healthcare professionals.
Countries where there are ISoP members:
From Argentina to Zimbabwe, in countries in Europe to Asia, across the Americas, and from Australia to Africa, we have members in all five continents.
“By becoming a member of ISoP, you will have the opportunity to share your knowledge and ideas and to contribute to improving pharmacovigilance activities worldwide.”
Dr Mira Harrison-Woolrych, President of the International Society of Pharmacovigilance
ISoP Membership incentives include:
Discounted online subscription to the Drug Safety journal
Face-to-face meetings and webinars
Annual conference and training courses
Involvement in ISoP’s Chapters and Special Interest Groups
Opportunities for networking and other professional collaborations
Regular newsletters and copies of the ISoP Star
For more information, please visit http://www.isoponline.org, the Society’s official website.
International Society of Pharmacovigilance
ISoP Secretariat Ltd
140 Emmanuel Road, London SW12 0HS, UK
Tel and Fax: +44 (0)20 3256 0027
administration@isoponline.org
ISoP 2021 Local Organising Committee
Chair: Mayada Alkhakany, ISoP Middle East Chapter (United Arab Emirates)
Co-Chair: Thamir Alshammari, ISoP Middle East Chapter (Saudi Arabia)
Sameh Al-Zubiedi, ISoP Middle East Chapter (Jordan)
Hussain Talib Al Ramimmy, Directorate General of Pharmaceutical Affairs & Drug Control, Ministry of Health (Oman)
Imad Eldin Mohamed Nour, Directorate General of Pharmaceutical Affairs & Drug Control, Ministry of Health (Oman)
Adil Saleh Al Balushi, Directorate General of Pharmaceutical Affairs & Drug Control, Ministry of Health (Oman)
Husam Salamat, ISoP Middle East Chapter (Jordan)
ISoP 2021 Scientific Committee
Chair: Sten Olsson, ISoP Past President (Sweden)
Co-Chair: Thamir Alshammari, ISoP Middle East Chapter (Saudi Arabia)
Andrew Bate, GlaxoSmithKline (UK)
Andrea Kuemmerle, Medicines for Malaria Venture (Switzerland)
Jean-Christophe Delumeau, Bayer (Singapore)
Comfort Ogar, Management Sciences for Health (Nigeria)
Shanthi Pal, WHO (Switzerland)
Manal Younus, Iraqi Pharmacovigilance Center (Iraq)
Abstract reviewers
Chair: Sten Olsson, ISoP Past President (Sweden)
Thamir Alshammari, ISoP Middle East Chapter (Saudi Arabia)
Andrew Bate, GlaxoSmithKline (UK)
Rebecca Chandler, Coalition for Epidemic Preparedness Innovations (Sweden)
Jean-Christophe Delumeau, Bayer (Singapore)
Andrea Kuemmerle, Medicines for Malaria Venture (Switzerland)
Birgitta Grundmark, Norwegian Medicines Agency (Sweden)
Mira Harrison-Woolrych, ISoP President (New Zealand)
Gianluca Trifirò, University of Verona (Italy)
Mónica Tarapués, Universidad Central del Ecuador (Ecuador)
Comfort Ogar, Management Sciences for Health (Nigeria)
Manal Younus, Iraqi Pharmacovigilance Center (Iraq)
Zhang Li, Dongfang Hospital, Beijing University of Chinese Medicine (China)
ISoP 2021 Poster Prize Committee
Chair: Thamir Alshammari, ISoP Middle East Chapter (Saudi Arabia)
Rebecca Chandler, Coalition for Epidemic Preparedness Innovations (Sweden)
Sameh Al-Zubiedi, ISoP Middle East Chapter (Jordan)
ISoP Executive Committee and Advisory Board 2019–2022
Mira Harrison-Woolrych, President (New Zealand)
Rebecca Chandler, Vice-President (Sweden)
Deirdre McCarthy, Secretary General (USA)
Jean-Christophe Delumeau, Treasurer (Singapore)
Board Members
Angela Caro (Colombia)
Jan Petracek (Czech Republic)
Mónica Tarapués (Ecuador)
Gianluca Trifirò (Italy)
Manal Younus (Iraq)
Zhang Li (People’s Republic of China)
Sten Olsson, Past-President (Sweden)
Disclaimer
ISoP requests a high standard of science is followed concerning publications and presentations at all its annual conferences and training courses. However, ISoP as a whole or its Advisory Board and Executive Committee (EC) or appointed Scientific Committees, or its members, do not take any responsibility for the completeness or correctness of data, or references given by authors in publications and presentations at ISoP scientific meetings.
It is not within the remit of ISoP or its advisory committees, to seek clarification or detailed information from authors about data in submitted abstracts. Moreover, it is not within the scope of ISoP and its committees to monitor compliance with any legal obligations, for example, reporting requirements or regulatory actions.
ORAL PRESENTATIONS
O-001 Oral Presentation: Nigeria is Making Progress Implementing the Active Drug Safety Monitoring and Management Scheme for New and Repurposed Antituberculosis Drugs
Y. Kambai Avong1, A. S. Abiodun2, B. Jatau3, A. T. Shuibu4, C. Elagbaje5, A. Opadeyi6, I. Ali2, B. Fraden2, L. Harmark7, G. A. Kayode8, E. Tiemersma9, A. Isah6, H. Tumwijukye10, R. Cutler11, L. Pont12, F. Cobelens13
1University of Technology Sydney Australia, Graduate School of Health, Sydney, Australia; 2National Agency for Food and Drug Administration and Control, Pharmacovigilance/Post-marketing Surveillance, Abuja, Nigeria; 3Institute of Human Virology Nigeria, Patient-Care and Treatment, Abuja, Nigeria; 4National Tuberculosis and Leprosy Control Programme, Pharmacy, Abuja, Nigeria; 5KNCV Tuberculosis Foundation Nigeria, TB Project, Abuja, Nigeria; 6University of Nigeria-Benin, Clinical Pharmacology and Therapeutics, Benin, Nigeria; 7LAREB, Pharmacovigilance, S-Hertogenbosch, The Netherlands; 8University of Bristol-United Kingdom, Clinical Research, Bristol, UK; 9KNCV Tuberculosis Foundation-Netherlands, Epidemiology, Amsterdam, Nigeria; 10Amsterdam Institute for Global Health and Development AIGHD-Amsterdam-Netherlands, Legal, Amsterdam, Nigeria; 11University of Technology, Discipline of Pharmacy-Graduate School of Health, Sydney, Australia; 12University of Technology Sydney, Pharmacy, Sydney, Australia; 13Amsterdam Institute for Global Health and Development AIGHD-Amsterdam-Netherlands, Global Health and Development, Amsterdam, The Netherlands
Background/Introduction: The implementation of the active drug safety monitoring and management scheme (aDSM) instituted by the World Health Organization (WHO) [1–3] has witnessed different levels of progress in different countries world-wide [4]. Broad based collaboration among stakeholders is critical for the success of the scheme. The primary stakeholders in Nigeria are the national drug regulatory body, NAFDAC (National Agency for Food and Drug Administration and Control) and the NTBLCP (National Tuberculosis and Leprosy Control Program). The PAVIA project (funded by EDCTP) strengthened collaboration between these stakeholders in Nigeria [5], which has a yearly estimate of 21,000 incidence of multidrug/rifampicin-resistant tuberculosis [6].
Objective/Aim: To ascertain the implementation of the aDSM scheme in an era of enhanced support from external collaborators, such as the PAVIA project by describing: (a) the total number of adverse drug reactions (ADRs) reported (b) proportions of ADRs associated with the new antituberculosis and repurposed drugs (c) characteristics of the ADRs and (d) the collaboration among the primary stakeholders.
Methods: Individual case safety reports (ICSR) submitted to the NAFDAC database by the NTLCP from 2017 to 2021 were extracted and analyzed. The new antituberculosis drugs requiring aDSM were bedaquiline and delamanid, combined with the repurposed drugs (capreomycin, clofazimine, cycloserine, ethambutol, kanamycin, levofloxacin, linezolid, moxifloxacin, and pyrazinamide). The ADRs submitted to the NAFDAC database by the NTBLCP and the characteristics of the ADRs were the basis for assessing the collaboration. Summary statistics was applied for the analysis.
Results: The NTBLCP submitted a total of 284 reports to the NAFDAC database: 251/284 (88%) were from the repurposed drugs and 33/284 (12%) from bedaquiline [29/33 (88%)] and delamanid [4/33 (12%)]. ADRs were most frequently reported for men [285/499 (57%)] and the age range, 18–44 years [353/510 (69.2%)]. Vomiting, hypokalemia, and arthralgia [69 (13.2%) vs 55 (10.5%) vs 47 (9.1%)] had the highest reporting frequency and gastrointestinal, ear and labyrinth and nervous system disorders were the frequently reported system organs affected by the ADRs [108 (20.6%) vs 102 (19.5%) vs 93 (17.7%)]. Prolonged hospitalization (18/3.4%) was the frequently reported indicator for classifying ADRs as serious. Overall, there were 10 (1.9%) fatalities.
Conclusion: The submission of ADRs reports to NAFDAC by the NTBLCP, evidenced by the reported ADRs and their characteristics suggests collaboration by our definition. Nigeria tends to be progressing implementing the aDSM scheme.
References/Further Sources of Information
World Health Organization. WHO treatment guidelines for drug-resistant tuberculosis, 2016 update Geneva: World Health Organization, 2016.
World Health Organization. Global tuberculosis report 2020. Geneva: World Health Organization; 2020.
World Health Organization. WHO consolidated guidelines on drug-resistant tuberculosis treatment. Geneva: World Health Organization; 2019.
World Health Organization. Active TB drug-safety monitoring and management (aDSM). WHO/HTM/TB/2015.28. Geneva: World Health Organization; 2015.
Nigeria Federal Ministry of Health. National guidelines for Clinical and Programmatic Management of Drug Resistant Tuberculosis in Nigeria. (PMDT guidelines—3rd edition) November 2020: Abuja: Ministry of Health.
Federal Ministry of Health. Nigerian National Pharmacovigilance Policy and Implementation Framework – First Revision, Abuja, June 2020.
O-002 Oral Presentation: Analyzing the U.S. Post-Marketing Safety Surveillance of COVID-19 Vaccines
O. Albalawi1, M. Alomran1, G. Alsagri1, T. Althunian1, T. Alshammari1,2
1Saudi Food and Drug Authority, Executive Directorate for Research and Studies, Riyadh, Saudi Arabia; 2Riyadh Elm University, College of Pharmacy, Riyadh, Saudi Arabia
Background/Introduction: Since December 2020, three COVID-19 vaccines have been authorized in the United States (U.S.) and were proceeded by large immunization programs.
Objective/Aim: The aim of this study was to characterize the U.S. post-marketing safety (PMS) profiles of these vaccines with an in-depth analysis of mortality data.
Methods: This was a retrospective database analysis study. Details of the U.S. PMS reports (15 December 2020 to 19 March 2021) of the three vaccines (Pfizer-BioNTech, Moderna, and Janssen Ad26.COV2.S) were retrieved from the U.S. Vaccine Adverse Event Reporting System (VAERS). A descriptive analysis was conducted to characterize the reported adverse events (AEs). A comparative (Pfizer-BioNTech vs. Moderna) analysis of mortality was conducted. The mean count ratio of death between the two vaccines was estimated using a negative binomial regression model adjusting for the measured confounders.
Results: A total of 44,451 AE reports were retrieved (corresponding to 0.05% of the vaccinated U.S. population at least one dose). The initial estimated reporting rates for AEs in the US were 37.5 cases 100,000 of all doses administered (15 December 2020 to 19 March 2021). The median age in the identified reports was 49 years, and more than two-third of patients were female (73.9%). The most frequently reported AEs (per total number of AE reports) were pain (35.3%), injection site reactions (27.0%), headache (18.6%), fatigue (13.0%), and pyrexia and dizziness (10%, respectively). Serious AEs were reported in only 6,514 (14.6%) of the reports with 4,108 hospitalizations. The total number of death reports was 1,919, and the median age at death was 80 years. Of the death cases, 38.4% (737) had a history of a cardiovascular disease, 16.3% had diabetes, 13% had a respiratory disease and 9.7% had renal disease. The mean count ratio of Moderna (n = 997) vs. Pfizer-BioNTech (n = 899) of 1.07 (95% confidence interval 0.86 to 1.33).
Conclusion: The vast majority of PMS AEs in the U.S. were non-serious, and the number of serious AEs is very low given the total number of vaccinated U.S. population. No differences in the mortality findings between Moderna and Pfizer-BioNTech were observed.
References/Further Sources of Information
Centers for Disease Control and Prevention (CDC). COVID Data Tracker [Internet]. 2021 [updated 2021; cited 28 MAR 2021]. Available from: https://covid.cdc.gov/covid-data-tracker/#datatracker-home.
The U.S. Food and Drug Administration. Pfizer-BioNTech COVID-19 Vaccine EUA Letter of Authorization. 2020;564:1–9. Available from: https://www.fda.gov/media/144412/download.
Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2020;403–16.
Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–15.
Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N Engl J Med. 2021;1–15.
Julianne Gee, Paige Marquez, John Su, Geoffrey M. Calvert, Ruiling Liu, Tanya Myers, Narayan Nair, Stacey Martin, Thomas Clark, Lauri Markowitz, Nicole Lindsey, Bicheng Zhang, Charles Licata, Amelia Jazwa, Mark Sotir, Tom Shimabukuro. First Month of COVID-19 Vaccine Safety Monitoring. MMWR Morb Mortal Wkly Rep. 2021;70(8):283–8.
Centers for Disease Control and Prevention (CDC). About The Vaccine Adverse Event Reporting System (VAERS) [Internet]. 2021 [Updated 2021 cited 2021 March 28]. Available from: https://wonder.cdc.gov/vaers.htm.
Tom T. Shimabukuro, MD, MPH, MBAa,*, Michael Nguyen, MDb, David Martin, MD, MPHb, and Frank DeStefano, MD M. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine. 2015;33(36):4398–405.
The U.S. Food and Drug Administration. What is a Serious Adverse Event? [Internet]. 2021[2016; cited year 28 Mar 2021]. Available from: https://www.fda.gov/safety/reporting-serious-problems-fda/what-serious-adverse-event.
Centers for Disease Control and Prevention (CDC). Trends in Number of COVID-19 Vaccinations in the US [Internet]. 2021 [Updated 2021 MAR; cited 23 MAR 2021]. Available from: https://covid.cdc.gov/covid-data-tracker/#vaccination-trend.
Our World in Data. Research, Statistics and Vaccinations, Coronavirus (COVID-19) [Internet]. 2021 [Updated 2021 MAR; cited 23 MAR 2021]. Available from: https://ourworldindata.org/covid-vaccinations.
O-003 Oral Presentation: Nonsteroidal Anti-Inflammatory Drugs Exposure in Complicated Acute Community-Acquired Bacterial Infections, a French Multicentre Case-Control Study
C. Deschanvres1, P. Le Turnier1, A. Le Gouge2, A. Legras3, T. Guimard4, E. Baux5, J. P. Talarmin6, O. Epaulard7, L. Bernard8, A. S. Lecompte1, J. Orain1, G. Veyrac9, A. P. Jonville-Bera10, B. Giraudeau11, N. Asseray1
1University Hospital, Infectious Disease Department, Nantes, France; 2University Hospital, Biostatistics Department, Tours, France; 3University Hospital, Intensive Care, Tours, France; 4Departmental Hospital, Infectious Disease Department, La Roche sur Yon, France; 5University Hospital, Infectious Disease Department, Nancy, France; 6Cornouaille Hospital, Infectious Disease Department, Quimper, France; 7University Hospital, Infectious Disease Department, Grenoble, France; 8University Hospital, Infectious Disease Department, Tours, France; 9University Hospital, Clinical Pharmacology Department, Nantes, France; 10University Hospital, Clinical Pharmacology Department, Tours, France; 11University Hospital, Biostatistics Department, Tours, France
Background/Introduction: Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) are widely consumed. Some studies suggest an association between NSAIDs exposure and more severe bacterial infections [1].
As a result of pharmacovigilance signal, the French National Agency for Medicines and Health product safety (ANSM) has emitted a national safety alert in January 2020 to avoid over the counter sale for NSAIDs. Since May 2019, European Medicines Agency has initiated a signal evaluation procedure on serious infectious complications associated with ibuprofen and ketoprofen.
Objective/Aim: To explore the association between NSAIDs use and the occurrence of complicated Acute Community-Acquired Bacterial Infections (ACABI).
Methods: In this multicentre case–control study, we compared cases of adult hospitalized for complicated ACABI (defined by one of the following criteria: > 2 infected sites, abscess > 3 cm, per-cutaneous drainage or septic surgery) with controls hospitalized for uncomplicated ACABI. Cases and controls were matched in a 1:1 ratio based on the primary infection site of the cases, age and co-morbidities. NSAID exposure was collected using a standardized questionnaire and was defined by systemic NSAID use in the 14 days prior to hospitalization. The exclusion criteria were immunosuppression, chronic exposure to NSAIDs, healthcare-associated infections and inability to be interviewed. Factors associated with complicated ACABI were analysed by conditional logistic regression using univariate and multivariate analysis.
Results: Between September 2016 and December 2018, 150 pairs were included. After matching verification criteria, 148 pairs were analysed. The main infection sites were intra-abdominal (28%, 42/148), skin and soft tissue (24%, 36/148) and ear/nose/throat (16%, 23/148). Inclusion criteria for cases were abscess formation (134/148, 91%), invasive procedure (110/148, 74%) and multiple infection sites (17%, 25/148). NSAID prior exposure was observed in 40% (59/148) of cases and 24% (36/148) of controls (p = 0.004). Ibuprofen was the most commonly used NSAID. Factors associated with complicated ACABI in multivariate analysis were an history of pain symptom (adjusted odds ratio [aOR] 4.0; 95% Confidence Interval [95 CI] 1.6–9.9), male gender (aOR 2.0; 95 CI 1.2–3.5), NSAIDs exposure (aOR 2.1; 95 CI 1.1–4.0) and a longer delay between symptoms onset and hospitalization (aOR 1.9; 95% CI 1.3–2.7). Complicated ACABI were also associated with increased hospitalization length of stay and billing.
Conclusion: We demonstrate here that NSAIDs exposure is associated with an increased risk of complicated ACABI. Patients and physicians should be advised not to use NSAIDS when symptoms suggestive of infection are present. These results support the current approach of European and French agencies.
References/Further Sources of Information
Le Turnier P, Boutoille D, Joyau C, Veyrac G, Asseray N. Bacterial infections and NSAIDs exposure? Seek septic complications. Eur J Intern Med. 2017 Jun;41:e33–e34.
O-004 Oral Presentation: Evaluation of the Need for Specific Quantitative Signal Detection Strategies for the Pharmacovigilance of Vaccines
F. Haguinet1, O. Mahaux1, V. Kara2, C. Bonal3, A. Bate2
1GSK-Wavre, Safety Innovation and Analytics, Wavre, Belgium; 2GSK-London, Safety Innovation and Analytics, London, UK; 3GSK-London, Shared Safety Sciences, London, UK
Background/Introduction: Methods for signal detection in spontaneous reports of adverse events are generally numerator-based, disproportionality analyses (DPA). In 2015, GSK implemented a signal detection strategy for GSK vaccines which included a stratified proportional reporting ratio (PRR) OR a test for unexpected time-to-onset (TTO) distribution (QSD) (1). The choice of stratified PRR rather than stratified multi-item gamma Poisson shrinker (MGPS) (2) was partly motivated by work of (3), which demonstrated the value of the PRR over MGPS for small size spontaneous reporting databases (SRD) with a few dominating products. In 2016, IMI-PROTECT recommended in general to avoid separation of vaccines and non-vaccines (4).
Objective/Aim: To evaluate the performance of a new QSD strategy for GSK vaccines considering recommendations from IMI-PROTECT using the entire SRD as background.
Methods: Data to 01-Mar-2021 were considered, comprising a random sample of 8 GSK vaccines, representing 319K cases among the 663K in vaccines SRD. Different strategies were investigated: stratified PRR, stratified MGPS and unexpected TTO distribution in vaccines data and the stratified MGPS in pooled vaccines and drugs data. Performance characteristics were evaluated with a test set made of adverse events listed in the labels. Stratification categorization was done by metric.
Results: Stratified PRR in vaccines data had 12% of Se and 19% of PPV. TTO analysis 17% of Se and 43% of PPV. Their concordances of alerts and TPs were 10 and 20%, respectively. When both were combined, Se was 24% and PPV was 26%. Stratified MGPS in vaccines data had 14% of Se and 24% of PPV. The same analysis with background extended to drugs had 33% of Se and 24% of PPV. The combination of stratified MGPS in all data with TTO analysis had 39% of Se and 25% of PPV and concordances with GSK vaccines QSD were 45% for all alerts and 58% for TPs.
Conclusion: Despite limitations including a test set based on the labels of 01-Mar-2021, this evaluation supports the value of conducting TTO analyses as a complement DPA for quantitative signal detection in the SRD of vaccines. Further aligning quantitative signal detection strategy across vaccines and drugs has practical benefits and these results are supportive of replacing ‘first pass’ PRR analysis using a background of other vaccines by MGPS analyses against the entire SRD (vaccines and drugs background).
Disclosure of Interest: All authors are employed by and hold shares in GSK. All authors have no non-financial relationships and activities.
References/Further Sources of Information
Van Holle L, Bauchau V. Signal detection on spontaneous reports of adverse events following immunisation: a comparison of the performance of a disproportionality-based algorithm and a time-to-onset-based algorithm. Pharmacoepidemiol Drug Saf. 2014;23(2):178–85.
Dumouchel W. Bayesian Data Mining in Large Frequency Tables, with an Application to the FDA Spontaneous Reporting System. Am Stat. 1999;53(3):177–90.
Van Holle L, Bauchau V. The upper bound to the Relative Reporting Ratio-a measure of the impact of the violation of hidden assumptions underlying some disproportionality methods used in signal detection. Pharmacoepidemiol Drug Saf. 2014;23(8):787–94.
Seabroke S, Candore G, Juhlin K, Quarcoo N, Wisniewski A, Arani R, et al. Performance of stratified and subgrouped disproportionality analyses in spontaneous databases. Drug Saf. 2016;39(4):355–64.
O-005 Oral Presentation: Accesses to Emergency Department and Hospitalizations in New Users of Biologic Therapies for Ulcerative Colitis in Tuscany: The MICHELANGELO Study
G. Valdiserra1, S. Tillati1, V. Lorenzoni2, R. Gini3, G. Turchetti2, S. Giometto1, C. Bartolini3, O. Paoletti3, I. Convertino1, S. Ferraro1, E. Cappello1, M. Fornai4, E. Lucenteforte1, M. Tuccori5
1University of Pisa, Department of Clinical and Experimental Medicine, Pisa, Italy; 2Scuola Superiore Sant’Anna, Institute of Management, Pisa, Italy; 3Tuscan Regional Healthcare Agency, Pharmacoepidemiology, Florence, Italy; 4University of Pisa-University Hospital of Pisa, Department of Clinical and Experimental Medicine-Unit of Adverse Drug Reactions Monitoring, Pisa, Italy; 5University Hospital of Pisa-University of Pisa, Unit of Adverse Drug Reactions Monitoring-Department of Clinical and Experimental Medicine, Pisa, Italy
Background/Introduction: Biologic treatments for ulcerative colitis (UC) (adalimumab, infliximab, golimumab, vedolizumab) are recommended when failure with traditional therapies occurred [1, 2]. Real-world studies may provide important effectiveness and safety information for the optimization of care.
Objective/Aim: The study aim is to describe the utilization of Regional Healthcare System (RHS) facilities of Tuscany (Italy), including Emergency Department (ED) admissions and hospitalizations for any causes, in new users of UC biologic therapies.
Methods: A descriptive, retrospective cohort study (EUPAS40896) was performed using Tuscan healthcare administrative databases, namely the hospital discharge registry, the ED admission records and the drug-reimbursement database. We created four drug-users cohorts with the following inclusion criteria: first supply of a biologic therapy (one cohort for each of the four drugs of interest) from January 2015 to December 2019; ≥ 18 years old; five years of history data (look-back period); at least one year of follow-up; UC diagnosis OR UC co-payment exemption code in the look-back or in the follow-up OR a gastroenterological visit in the year before the first supply. We described the number of patients with at least one ED access and one hospitalization for any cause, the time to the first ED access and to hospitalization for any cause, and the main causes of ED access and hospitalization.
Results: The new users of biologic therapies were: 239 adalimumab, 175 infliximab, 110 golimumab, 107 vedolizumab. In the one-year follow-up, patients with at least one ED access were: 85 (36%) adalimumab, 65 (37%) infliximab, 43 (43%) vedolizumab, 42 (38%) golimumab. The major causes of ED admission, not necessary drug-related, were symptoms, sings, and ill-defined conditions (31 adalimumab, 27 infliximab and 25 golimumab) and injury and poisoning (31 adalimumab and 19 vedolizumab). In the 1-year follow-up, patients with at least one hospitalization were: 61 (35%) infliximab, 54 (26%) adalimumab, 33 (30%) golimumab, and 31 (29%) vedolizumab. Infectious and parasitic diseases were the major causes observed. The mean time to the first ED access and to the first hospitalization ranged from 140 days (infliximab) to 176 days (adalimumab) and from 139 days (golimumab) to 166 days (vedolizumab), respectively.
Conclusion: The assessment of RHS showed that the occurrence of ED accesses and hospitalizations was almost similar among the new users of UC biologic therapies, while the time to the first ED and the first hospitalization modestly varied among the four cohorts.
References/Further Sources of Information
D’Amico F, Parigi TL, Fiorino G, Peyrin-Biroulet L, Danese S. Tofacitinib in the treatment of ulcerative colitis: efficacy and safety from clinical trials to real-world experience. Therap Adv Gastroenterol. 2019;12. https://doi.org/10.1177/1756284819848631.
Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017;389(10080):1756–70. https://doi.org/10.1016/S0140-6736(16)32126-2.
O-006 Oral Presentation: Machine Learning on Drug-Specific Data to Predict Small Molecule Teratogenicity
A. Challa1,2,3,4, A. Beam4,5, M. Shen3, T. Peryea3, R. Lavieri1, J. Goldstein6, E. Lippmann2, D. Aronoff7,8,9
1Vanderbilt University Medical Center, Vanderbilt Institute for Clinical and Translational Research, Nashville, USA; 2Vanderbilt University, Department of Chemical and Biomolecular Engineering, Nashville, USA; 3National Institutes of Health, National Center for Advancing Translational Sciences, Rockville, USA; 4Harvard Medical School, Department of Biomedical Informatics, Boston, USA; 5Harvard T.H. Chan School of Public Health, Department of Epidemiology, Boston, USA; 6Northwestern University, Department of Pathology, Chicago, USA; 7Vanderbilt University Medical Center, Division of Infectious Diseases-Department of Medicine, Nashville, USA; 8Vanderbilt University Medical Center, Department of Pathology-Microbiology and Immunology, Nashville, USA; 9Vanderbilt University Medical Center, Department of Obstetrics and Gynecology, Nashville, USA
Background/Introduction: Pregnant women are a vulnerable population, given the sensitivity of a developing fetus to chemical exposures. However, prescribing behavior for the expectant patient is guided on limited human data and conflicting cases of adverse outcomes due to the exclusion of pregnant populations from randomized, controlled trials. These factors increase risk for iatrogenic fetal toxicity and reduce quality of care for pregnant populations (1–3). We hypothesize that machine learning (ML) on structural, meta-structural, and in vitro bioactivity data inherent to prescriptible small molecules can systematically predict drug teratology and provide more standardized definitions of existing teratogenicity scores.
Objective/Aim: We developed a generalizable ML platform to predict teratogenic risk for drugs potentially prescriptible in pregnancy using clinical, chemical, and bioactivity data; this model may have eventual application to informing clinical decision support.
Methods: We extracted chemical structures of all molecules (N = 9,099) in the public-domain pharmacopeia DrugBank 5.1.0 (4) and all drug profiles (N = 652) from SafeFetus, a patient registry that maintains the largest publically-available database of structured teratogenicity scores (5). For molecules common to both sets (N = 616), we obtained efficacy, curve class, and IC50 data for all targets implicated in teratogenesis and screened through the Toxicology in the 21st Century program of the National Institutes of Health (6). Molecular stability, druglikeness, and mutagenicity predictions were calculated for all molecules in the Molecular Operating Environment. Following feature selection, descriptors were aligned by the t-Distributed Stochastic Neighbor Embedding (t-SNE) procedure to discover clustering relationships between molecular features and teratogenic risk. We then enabled a supervised ML model (gradient boosting machine (GBM) with five-fold cross-validation) to predict teratogenicity score from subsets of descriptors.
Results: From t-SNE, we identified three chemical moieties associated with marked increase in teratogenic risk. Our GBM predicts three clinically-redefined classes of teratogenicity with AUC = 0.78 and 64.7% predictive accuracy (SD = 3.0%). This accuracy is approximately double that of a blind control for the same task, suggesting successful modeling.
Conclusion: We present a first-in-kind application of computing to study and predict teratogenicity. Our model identifies structural characteristics that predispose a drug to increased teratogenicity, providing more standardized definitions of teratogenicity scores than are currently available. When further developed, this work could inform safer prescribing behavior in pregnancy.
References/Further Sources of Information
Garry VF, Truran P. Chapter 62—Teratogenicity. In: Gupta RC, editor. Reproductive and Developmental Toxicology (Second Edition) [Internet]. Academic Press; 2017 [cited 2019 Sep 17]. p. 1167–81. http://www.sciencedirect.com/science/article/pii/B9780128042397000627.
Cohen RL. Evaluation of the teratogenicity of drugs. Clin Pharmacol Ther. 1964 Jul 1;5(4):480–514.
van der Zande ISE, van der Graaf R, Oudijk MA, van Delden JJM. Vulnerability of pregnant women in clinical research. J Med Ethics. 2017;43(10):657–63.
Wishart DS, Knox C, Guo AC, Shrivastava S, Hassanali M, Stothard P, et al. DrugBank: a comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res. 2006 Jan 1;34(Database issue):D668–72.
Medication in Pregnancy and Breastfeeding | SafeFetus.com [Internet]. [cited 2019 Nov 19]. Available from: https://www.safefetus.com/.
Toxicology in the 21st Century (Tox21) [Internet]. National Center for Advancing Translational Sciences. 2017 [cited 2019 Sep 18]. https://ncats.nih.gov/tox21.
O-007 Oral Presentation: Preventing an Opioid Crisis in Europe: An Overview of Measures to Support Medicines Regulators
E. Monzon1, M. Abou Taam2, M. C. Pesquidou3, P. Bahri4, P. Balabanov4, L. de Fays5, C. A. Herrera6, A. Noor7, A. Saint-Raymond4, S. Straus8, A. Inoubli9
1Agence Nationale de Sécurité du Médicament et des Produits de Santé, Safety Management, Saint-Denis, France; 2Agence Nationale de Sécurité du Médicament et des Produits de Santé, Pain Control-Aneasthetics-Rheumatology-Addiction Drugs, Saint-Denis, France; 3Agence Nationale de Sécurité du Médicament et des Produits de Santé, European Strategy, Saint-Denis, France; 4European Medicines Agency, European Taskforce on Opioids, Amsterdam, The Netherlands; 5Federaal Agentschap voor Geneesmiddelen en Gezondheidsproducten-Agence Fédérale des Médicaments et des Produits de Santé, European Taskforce on Opioids, Brussels, Belgium; 6Organisation for Economic Co-operation and Development, European Taskforce on Opioids, Paris, France; 7European Monitoring Centre for Drugs and Drug Addiction, European Taskforce on Opioids, Lisbon, Portugal; 8College ter Beoordeling van Geneesmiddelen, European Taskforce on Opioids, Utrecht, The Netherlands; 9Agence Nationale de Sécurité du Médicament et des Produits de Santé, European Taskforce on Opioids, Saint-Denis, France
Background/Introduction: Opioid use disorders (OUD) typically include chronic, relapsing illness, associated with abuse and/or dependence [1]. OUD can involve misuse of prescribed opioid medications, use of diverted opioid medications, and/or use of illicitly obtained opioids.
One of its most serious risks is overdose, which can be fatal. In the US, an opioid crisis was declared in 2017 [2]. A task-force led by the European Medicine Agency (EMA) was created in 2019 with the goals to review and assess the current situation and prevent such a crisis in Europe. The main objectives of this task force are to exchange information on opioid use, work on crisis prevention and preparedness, and discuss available and possible health initiatives applied in the different health systems across Europe.
Objective/Aim: The first step of the task force was to compile all health measures in European countries used so far to prevent and minimize opioid-related risks with diversion, misuse, abuse, dependence, and overdose of opioids.
Methods: Data were collected from two important reports (from the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) [3] and the Organisation for Economic Co-operation and Development (OECD) [4]) and from three surveys (non-urgent requests for information within the EU regulatory pharmacovigilance system) between 2017 and 2018 (n (countries) = 31 including UK). An analytical framework was developed to categorize all collected measures.
Results: A large set of measures was retrieved from 25 member states and the UK. These measures were organized in four different categories: (a) health system policies and interventions; (b) information measures and research; (c) cooperation, customs and justice system; and (d) social measures. Besides, a map of institutional stakeholders across Europe was developed, highlighting the complexity of all actors’ responsibilities for opioids regulation and control in Europe.
Conclusion: This analysis showed that the possible measures to address an opioid crisis are not limited to medicines regulation but also rely on policy, communication and social measures.
New measures are being developed to address changing situations. Close collaboration is crucial, both at national and EU, and also international level, between health and regulatory authorities as well as other stakeholders, especially non-governmental bodies. Working in close contact with opioid users is key to address OUD. The outcomes of the next steps of the task force are also expected to further inform initiatives in Europe.
References/Further Sources of Information
UpToDate. Opioid use disorder: Epidemiology, pharmacology, clinical manifestations, course, screening, assessment, and diagnosis. [Internet]. [cited 2021 June 15]. https://www.uptodate.com/contents/opioid-use-disorder-epidemiology-pharmacology-clinical-manifestations-course-screening-assessment-and-diagnosis.
Affairs (ASPA) AS of P. What is the U.S. Opioid Epidemic? [Internet]. HHS.gov. https://plus.google.com/+HHS; 2017 [cited 2021 June 15]. https://www.hhs.gov/opioids/about-the-epidemic/index.html.
European Monitoring Centre for Drugs and Drug Addiction. European drug report 2019: trends and developments. [Internet]. LU: Publications Office; 2019 [cited 2021 May 24]. https://data.europa.eu/doi/10.2810/191370.
Addressing Problematic Opioid Use in OECD Countries | en | OECD [Internet]. [cited 2021 May 24]. https://www.oecd.org/health/addressing-problematic-opioid-use-in-oecd-countries-a18286f0-en.htm.
O-008 Oral Presentation: Presentation of Safety Risks Throughout the Product Lifecycle
C. Semenza1, S. Ramsden2
1TransCelerate Biopharma Inc., Communications, West Conshohocken, USA; 2Boehringer-Ingelheim, Pharmacovigilance Corporate, Ingelheim am Rhein, Germany
Background/Introduction: From first in human through post-authorization, the regulatory definitions of risks and guidelines related to the presentation of safety information vary across key safety documents and across Health Authorities. The TransCelerate Interpretation of Pharmacovigilance Guidance and Regulations Initiative’s Presentation of Safety Risks Throughout the Lifecycle Team designed a Framework with an outline of regulatory requirements and considerations for presenting risks in key required safety related regulatory documents throughout the lifecycle.
Objective/Aim: This framework supports a transparent approach to presenting risks and provides a starting point for the collaboration between companies and Health Authorities to protect and improve the health of trial subjects, patients and the public.
Methods: This framework was developed in accordance with specific Health Authority guidance documents applicable throughout the product lifecycle to support safety related regulatory documents such as safety sections of the Investigator’s Brochure, Development Safety Update Report, Periodic Safety Update Report/Periodic Benefit-Risk Evaluation Report and Risk Management Plan.
Results: The information was synthesized into a framework by the TransCelerate initiative team to review the key definitions of risk and provide clarity and assist with presenting risks across the lifecycle in order to meet health authority requirements.
Conclusion: The Presentation of Safety Risks Throughout the Product Lifecycle Framework reviews the key definitions of risk and provide clarity, in addition to assisting with how to handle presenting risks across the lifecycle in line with health authority requirements. An example of the benefits realized by a Sponsor company from leveraging this framework will also be shared.
References/Further Sources of Information
N/A.
O-009 Oral Presentation: Development of a Method to Assess the Clinical Quality of Pregnancy Pharmacovigilance Data—A Contribution from the ConcePTION Project
Y. Weetink1, K. Chamani1, E. van Puijenbroek1
1The Netherlands Pharmacovigilance Centre Lareb, Science and Research, ‘s-Hertogenbosch, The Netherlands
Background/Introduction: Clinically well-documented reports are more likely to lead to a reliable assessment of drug safety signals because they can provide more accurate arguments for assessing the causal association between medicinal products and adverse events [1, 2]. Currently, none of the existing tools are designed for the assessment of clinical quality of pregnancy data [1, 2]. To study the clinical quality of pregnancy pharmacovigilance data, a dedicated tool is developed. The method relies both on the relevance of information as well as its quality.
Objective/Aim: The aim of this study is to develop and validate a tool (PregDoc) to measure the clinical quality of pregnancy pharmacovigilance data.
Methods: Variables considered important for causality assessment of the medicinal product and the outcome/complication in pregnancy were initially brought forward by an expert panel. A survey was distributed among international experts to supplement the initial selection with additional variables. In a second survey, all variables were assessed for relevance. The final selection of variables was based on a focus group discussion.
In total 210 case reports regarding the use of medicinal products during pregnancy were extracted from several data sources (Teratology Information Services, pregnancy or drug registries, spontaneous reports from a national pharmacovigilance centre and a Marketing Authorisation Holder, and literature reports). Reports were anonymized and blinded for source. Clinical quality of all reports was scored as poor, moderate, good or excellent by means of (1) the PregDoc tool and (2) expert assessment based on global introspection (gold standard). 105 case reports were used for the validation of the tool in terms of weighing of the variables through a prediction model. The remaining 105 case reports were used to test the model. Performance will be expressed as sensitivity and specificity. The final results will be presented at the ISoP meeting.
Results: PregDoc consists of 22 variables divided over five domains: outcome, risk factors, exposure, child, and pregnancy/labour. The final clinical quality score is calculated by (number of variables present in the case report/number of relevant variables for this possible drug-outcome relation*100).
Conclusion: After the validation and testing of the PregDoc clinical quality assessment tool, it can be used for the comparison of the clinical quality of the different pregnancy data collection sources. This may facilitate the improvement of pregnancy pharmacovigilance data collection in general.
References/Further Sources of Information
Oosterhuis I, Rolfes L, Ekhart C, Muller-Hansma A, Härmark L. First experiences with a tool to measure the level of clinical information present in adverse drug reaction reports. Expert Opin Drug Saf. 2017;17(2):111–5.
Bergvall T, Noren GN, Lindquist M. vigiGrade: a tool to identify well-documented individual case reports and highlight systematic data quality issues. Drug Saf. 2014;37(1):65–77.
O-010 Oral Presentation: Knowledge, Attitude and Practice of Community Pharmacists on Safe Use of Medications During Pregnancy: A Cross-Sectional Study
E. Alghamdi1, R. Alshuhayb2, F. Alharbi1
1Saudi Food and Drug Authority, Drug Safety, Riyadh, Saudi Arabia; 2King Saud University, Pharmacy College, Riyadh, Saudi Arabia
Background/Introduction: Dispensing during pregnancy requires careful estimation of the risk/benefit ratio for the mother and her Baby [1, 2]. Community pharmacists (CPs) play a critical role in the safe use of medications during pregnancy. The Saudi government has established the Wasfaty system, which is an electronic system linking government hospitals and primary care clinics to community pharmacies to facilitate dispensing medications for out-patients [3–5].
Objective/Aim: To evaluate the CP’s knowledge, attitude, and practice (KAP) towards the safe use of medications during pregnancy in Saudi Arabia.
Methods: A cross-sectional web-based survey was conducted from March to June 2021, among CPs who were working in community pharmacies that have the Wasfaty service. The questionnaire includes Likert type questions to assess the KAP of CPs towards safe use of medications during pregnancy. Descriptive statistics were performed to represent the socio-demographic characteristics and KAP levels using SPSS Statistical Package (2015).
Results: 90 out of 260 (34.6%) of CPs were responded to the survey. The median age of respondents was 33 years (range 25–60). The majority of the participants were men (85:95.6%), dispensed ≤ 50 prescriptions per day, had only undergraduate qualifications, and had an average of 9 years of work experience.
Most of the CPs were aware of the teratogen definition (83.33%), and they were aware of the US FDA guidance on safe use medicine during pregnancy (84.44%). With respect to their knowledge of the teratogenic risk of 20 commonly known medications, 7.8% of CPs had the appropriate knowledge of ≥ 90% of the listed medications, while 11.11% had the appropriate knowledge of ≤ 60% of the listed medications. The majority of CPs had the appropriate knowledge of > 60 to < 90% of the listed medications during pregnancy.
Among all CPs, 60 % were confident when dispense drugs during pregnancy. 94.4% were taking extra caution when dispensing drugs during pregnancy. 50.6% of CPs thought that all OTC drugs are not safe in pregnant women.
Almost half of CPs were shown unsafe dispensing practices for pregnant women as shown in.
Conclusion: CPs have adequate knowledge of the safe use of medications during pregnancy. However, it has not been reflected in both their attitude and practice. Policies and procedures in community pharmacies should be established for dispensing medications during pregnancy. Training of CPs about medication counseling, dispensing medications during pregnancy should be performed on a regular-basis.
References/Further Sources of Information
Mulder B, Schuiling-Veninga NC, Morssink LP, et al. Risks versus benefits of medication use during pregnancy: What do women perceive? Pharmacoepidemiol Drug Saf. 2014;23(S1):313–14.
Sachdeva P, Patel BG, Patel BK. Drug use in pregnancy; a point to ponder! Indian J Pharm Sci. 2009;71(1):1.
Kingdom of Saudi Arabia Healthcare Overview 2018. https://www.colliers.com/-/media/files/emea/uae/case-studies/2018-overview/ksa-healthcare-overview-thepulse-8th-edition.pdf?la=en-gb.
Al-Arifi MN. Patients’ perception, views and satisfaction with pharmacists’ role as health care provider in community pharmacy setting at Riyadh, Saudi Arabia. Saudi Pharm J. 2012;20(4):323–30.
WASFATY. https://pp.wasfaty.sa/. Accessed 17 June 2021.
O-011 Oral Presentation: Should We Worry About Flare-Ups Following Vaccination in Systemic Lupus Patients? A Nationwide Case-Crossover Study Using the French Healthcare Database
L. Grimaldi1, Y. Hamon2, P. Attias3, T. Duchemin2, A. Buchard4, L. Abenhaim5, Y. Moride6
1Hospital Group Paris-Saclay-Assistance Publique-Hôpitaux de Paris-UFR des Sciences de la Santé-University Paris-Saclay-Paris-France, Department of Pharmacology, Paris, France; 2Re-Meds, Data Analysis, Paris, France; 3Re-Meds, Medical Department, Paris, France; 4Hôpitaux Universitaires de Genève, Psychiatrie Adulte, Geneva, Switzerland; 5London School of Hygiene and Tropical Medicine, Pharmacoepidemiology, London, UK; 6Rutgers-The State University of New Jersey, Pharmacoepidemiology, New Brunswick, USA
Background/Introduction: Vaccination is widely used in systemic lupus erythematosus (SLE) patients, who are at high risk of infection due to immunosuppression. The immunogenicity and safety of vaccines in SLE is debated [1, 2] but large-scale studies remain scarce.
Objective/Aim: To evaluate the impact of vaccination on SLE flare-ups in the real-world setting.
Methods: A case-crossover study using the French SNDS healthcare database was conducted. The SNDS covers around 99% of the population (> 66 million persons) from birth to death or emigration, making it one of the world's largest continuous and comprehensive claims database. Database records demographic data, pharmacy dispensing claims, outpatient healthcare encounters as well as hospital admissions with primary, secondary and associated ICD10 diagnoses.
A cohort of SLE patients was identified over an 11-year period (1st January 2008–31st December 2018). Cases were SLE patients who experienced a SLE flare-up during the study period, ascertained using either a new pharmacy dispensing claim for high-dose corticosteroid within 7 days, emergency room visit or hospitalization with SLE-related primary discharge diagnosis. Vaccine exposure in the 2 months prior to the flare-up date (risk window) was compared to prior exposure in up to 4 control time windows per patient—each control window was 2 months. Conditional logistic regression with Generalized Estimating Equation models were used to measure the association between vaccination and flare-up accounting for the occurrence of multiple flare-ups within an individual, healthcare use and seasonality. Stratifications by vaccine type (bacterial, viral, combination) or nature (antigenic, live attenuated), demographic characteristics, number of flare-ups, time since SLE diagnosis, and disease activity were performed.
Results: A total of 45,178 SLE patients with 148,839 flare-ups were identified. Patients’ mean age was 41.6 years [SD 16.4] and 86.2% were females. 69.2% were vaccinated at least once during the study period, distributed as follows: vaccine combinations (47.0%), flu (28.3%), pneumococcus (23.2%), hepatitis B (5.2%) and others (< 4%). The Odds Ratio (OR) for any vaccination was 1.01 [0.98–1.04]. ORs according to vaccine type were 1.04 [0.98–1.11], 1.0 [0.98–1.06] and 0.9 [0.89–1.01] for bacterial, viral and vaccine combinations, respectively. ORs according to vaccine nature were 1.03 [0.99–1.06] and 1.19 [0.95–1.49] for antigenic and live attenuated vaccines, respectively. Other stratifications yielded similar results.
Conclusion: From this large-scale population-based study, no association between vaccination and flare-ups in SLE was observed neither in the overall population or in any of the subpopulations investigated.
References/Further Sources of Information
Liao Z, Tang H, Xu X, Liang Y, Xiong Y, Ni J. Immunogenicity and safety of influenza vaccination in systemic lupus erythematosus patients compared with healthy controls: a meta-analysis. PLoS One. 2016 Feb 4;11(2):e0147856.
Vista ES, Crowe SR, Thompson LF, Air GM, Robertson JM, Guthridge JM, et al. Influenza vaccination can induce new-onset anticardiolipins but not β2-glycoprotein-I antibodies among patients with systemic lupus erythematosus. Lupus. 2012 Feb;21(2):168–74.
POSTERS
P001 Implementation of Risk Minimization Measures to Reduce the Risk of Tuberculosis Among Anti-Tumor Necrosis Factor Users
N. Al-Fadel1, A. Almutairi1, T. Aldhirgham2, L. Abu esba3, A. Alrwisan2, F. Alharbi1
1Saudi Food and Drug Authority, Drug Safety and Risk Management Department, Riyadh, Saudi Arabia; 2Saudi Food and Drug Authority, Research Department, Riyadh, Saudi Arabia; 3Ministry of the National Guard-Health Affairs, Pharmaceutical Care Services Department, Riyadh, Saudi Arabia
Background/Introduction: The Saudi Food and Drug Authority (SFDA) has implemented different risk minimization measures (RMMs) for anti-tumour necrosis factor (anti-TNF) alpha agents to minimize the risk of Tuberculosis (TB) reactivation such as patient alert card, prescriber guide and TB screening brochure [1].
Objective/Aim: To examine the status of RMMs implementation in patients newly received anti-TNF therapy.
Methods: We included patients who had at least one prescription for anti-TNF (infliximab or adalimumab), using electronic health records from King Abdulaziz Medical City in Riyadh between 2016 and 2019. The index date was first recorded anti-TNF prescription. Incident users of anti-TNF were divided into pre- and post-RMMs implementation groups depending on their first recorded prescription. RMMs were implemented in January 2016 and January 2017 for adalimumab and infliximab, respectively. RMMs implementation was operationalized as TB laboratory test (chest X-ray or QuantiFERON test) was done within one month prior to the index date. We calculated the proportion of patients who received TB tests for each drug. We also compared the unadjusted proportion of implementation of RMMs through chi-square test.
Results: A total of 388 anti-TNF users included in the study. In the pre-RMMs implementation period, 67 patients received infliximab, while no pre-RMMs data was available for adalimumab, as RMMs and adalimumab were introduced to the study site at the same time. The post-RMMs implementation period comprised 116 infliximab-exposed patients and 205 adalimumab-exposed patients. A total of 14 (20.9%) infliximab-exposed patients had an X-ray prior to treatment and 12 (17.9%) had a QuantiFERON test prior to treatment in pre-RMMs implementation phase. In the post-RMMs phase, the proportion of infliximab-exposed with an X-ray was 37.1%, and was 47.4% with QuantiFERON test before treatment. For adalimumab, 14.6% of patients had an X-ray and 21% had a QuantiFERON test prior to treatment. A pre-post RMMs implementation comparison for TB screening among infliximab users showed a significant increase in the rates of X-ray tests prior to treatment (20.9 % before RMMs to 37.1% after RMMs implementation, p = 0.035) and the rates of QuantiFERON tests prior to treatment (17.9 % before RMMs to 47.4 % after RMMs implementation, p < 0.001).
Conclusion: TB screening for infliximab and adalimumab was not optimal. However, we noted an improvement in TB screening after implementation of RMMs for infliximab. More data are imperative to assess the implementation of RMMs across various institutions.
References/Further Sources of Information
Saudi Food and Drug Authority. Risk minimization measures list. https://www.sfda.gov.sa/en/RMM. Accessed 21 June 2021.
P002 The Fundamental Value of Exposure Data for Pharmacovigilance: Review of Different Aggregation Levels of Exposure Data in Pharmaceutical Companies’ PSURs
A. Amiri1,2, J. Okai1,2, P. Bahri2, V. Macolić Šarinić2
1Utrecht University, Pharmaceutical Sciences, Utrecht, The Netherlands; 2European Medicines Agency, Human Medicines Division-Pharmacovigilance Office, Amsterdam, The Netherlands
Background/Introduction: Understanding medicines exposure is fundamental for signal detection, risk assessment and decision-making on regulatory action. As a denominator, exposure data place the number of reported cases of suspected adverse reactions in relation to the number of people exposed. Exposure data can also project the impact of regulatory action and evaluate the effectiveness of risk minimization measures (RMMs). Different exposure units and parameters are appropriate for different posologies, as discussed at ISoP before [1]. In line with the ICH-E2C(R2) standards [2], legal obligations in the European Union (EU) require marketing authorization holders (MAHs) to provide cumulative and interval exposure data from marketing experience in periodic safety update reports (PSURs), and EU good pharmacovigilance practice guidelines (EU-GVP) detail the requirements [3]. The need for high-quality exposure data justifies reviewing exposure data provided by MAHs.
Objective/Aim: To describe the different levels at which exposure data are aggregated in PSURs.
Methods: We reviewed the exposure data in 62 PSURs submitted by MAHs to the EU regulatory network between 2012 and 2020. The reviewed PSURs concerned nationally authorized products containing antineoplastic agents (ATC-code L01). The provided exposure data were categorized into three levels of aggregation: (1) worldwide; (2) EU; (3) EU Member States (MS). The categorization was analyzed using descriptive statistics.
Results: In total, 87% (N = 54) of MAHs reported exposure data at worldwide level, while 13% (N = 8) did not provide any data. 46% (N = 25) of MAHs that provided worldwide data supplemented them by EU and/or MS data: 24% of MAHs (N = 13) with exposure data at MS level, 15% (N = 8) at EU level and 7% (N = 4) at both MS and EU level. The exposure data were expressed in various units, namely as number of persons, person-time or defined daily dose (DDD).
Conclusion: These results show that the majority of MAHs reported exposure data only at worldwide level, thereby possibly ‘diluting’ the denominator—the absence of a local denominator may possibly hinder signal detection for case reports that could constitute a local safety concern if local cases were analyzed with local exposure data. This could delay the timely detection of safety signals in local contexts (co-morbidity, risk factors, medicines use) and negatively impact public and patient health. Besides, the absence of local exposure data (MS and/or EU level) could hinder the evaluation of implemented RMMs and understanding of the impact of regulatory action.
References/Further Sources of Information
Bahri P, Bégaud B, Moore N. ABSTRACTS: ISoP Annual Conference ‘Joining Forces for Managing Risks’ Liège, Belgium 11–13 October, 2006. Drug-Safety 29, 980 (2006). https://doi.org/10.2165/00002018-200629100-00077.
The European Medicines Agency. ICH guideline E2C (R2) on periodic benefit-risk evaluation report (PBRER) [Internet]. Ema.europa.eu. 2013 [cited 6 June 2021]. Available from: E2C (R2) Step 5 Periodic benefit-risk evaluation report (PBRER) (europa.eu).
The European Medicines Agency. Module VII—Periodic safety update report (Rev 1) [Internet]. Ema.europa.eu. 2013 [cited 6 June 2021]. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-good-pharmacovigilance-practices-gvp-module-vii-periodic-safety-update-report_en.pdf.
P003 Reporting Quality of Evaluations of Additional Risk Minimisation Measures by MAH: An Assessment of Studies Submitted in the European Union
J. Okai1,2, P. Bahri2, V. Macolić Šarinić2
1Utrecht University, Pharmaceutical Sciences, Utrecht, The Netherlands; 2European Medicines Agency, Human Medicines Division-Pharmacovigilance Office, Amsterdam, The Netherlands
Background/Introduction: High reporting quality of effectiveness evaluation studies for additional risk minimisation measures (aRMMs) is required for the regulatory ability to assess aRMMs and take evidence-based decisions. In the European Union (EU), aRMMs are imposed by regulatory agencies under legislation and Good Pharmacovigilance Practice guidelines (EU-GVP) to minimize serious risks associated with medicines, if safety concerns are insufficiently addressed with routine RMMs [1]. Experiences and concerns about low quality of evaluation study reports have led pharmacoepidemiology experts in 2017 to issue the Reporting recommendation Intended for pharmaceutical risk Minimisation Evaluation Studies (RIMES) as a reporting standard with 43 items in four domains: (1) key information; (2) intervention design; (3) program implementation; and (4) evaluation [2]. The 2021 revision of EU-GVP therefore recommends use of RIMES [3]. The adverse impact of low reporting quality of aRMM effectiveness evaluation studies on regulatory decision-making and pharmacovigilance overall justifies reviewing the reporting quality in the EU.
Objective/Aim: Describing the current reporting quality of aRMM effectiveness evaluation studies submitted by marketing authorisation holders (MAHs) and highlighting where improvements in reporting could be made.
Methods: aRMM effectiveness evaluation studies submitted to the EU regulatory network in 2019 and 2020 were retrieved from databases of the European Medicines Agency (EMA). The quality of the study reports was assessed using the RIMES. The categorised results were analysed using descriptive statistics and a threshold of 30% to identify frequent reporting gaps.
Results: 23 studies were appraised. The majority of the studies frequently described the author information and affiliations, study design, obtained results and report section headings were consistent with RIMES items. The used risk minimisation tools, prespecified threshold for determining intervention success, intervention demography and objectives were frequently reported (> 30%). On the other hand, information on the aRMM intervention design, testing and implementation were not frequently reported (< 30%). The extent of intervention integration, hypothesis and factors influencing program effectiveness were only sporadically reported.
Conclusion: Major reporting gaps were identified in the intervention design and program implementation domains. These gaps in reporting quality may delay or result in unjust regulatory decisions, as they may impair the evidence-base and understanding of aRMM effectiveness. Improvements of the reporting quality of aRMM effectiveness evaluation studies are warranted. The quality of study reports is expected to increase given that RIMES is now expected in EU-GVP, thereby improving the evidence-base for timely modifications of aRMMs in order to optimise patient safety.
References/Further Sources of Information
European Medicines Agency. Guideline on good pharmacovigilance practices (GVP) Module XVI—Risk minimisation measures: selection of tools and effectiveness indicators (Rev 3). [Internet]. Ema.europa.eu. 2021 [Cited 14 June 2021]. Available from: Guideline on good pharmacovigilance practices (GVP) - Module Risk Minimisation Measures (europa.eu).
Smith MY, Russell A, Bahri P, Mol PGM, Frise S, Freeman E, Morrato EH. The RIMES statement: a checklist to assess the quality of studies evaluating risk minimization programs for medicinal products. Drug Saf. 2018;41(4):389–401. https://doi.org/10.1007/s40264-017-0619-x.
European Medicines Agency. Guideline on good pharmacovigilance practices (GVP) Module XVI Addendum II—Methods for effectiveness evaluation. [Internet]. Ema.europa.eu. 2021 [Cited 14 June 2021]. Available from: Guideline on good pharmacovigilance practices (GVP) - Module XVI Addendum II - Evaluation (europa.eu).
P004 Cancer Treatments Associated with Melanoma Diagnosis in Claims Data: Results from an Exploratory Study
H. Kawabata1, X. Pan1, J. Zheng1, J. Zhang2, S. Dey3, S. Moinuddeen3, S. Pp3
1Bristol Myers Squibb, Integrated Oncology Epidemiology III, Lawrenceville, USA; 2Bristol Myers Squibb, MSA Solid Tumor Oncology, Lawrenceville, USA; 3Mu Sigma Business Solutions, Data Science Team, Bengaluru, India
Background/Introduction: While administrative claims data are useful for studies of cancer treatment outcomes, the identification of cancer patients can be challenging because of the reimbursement focus of claims. Algorithms using treatment claims to confirm cancer diagnoses have been developed, but they can be challenging because of numerous indications for many treatments including non-cancer ones. In this study, we looked for treatments associated with melanoma diagnoses in the following categories: chemotherapy, immune checkpoint inhibitors (ICIs), targeted therapy, radiotherapy, and surgery.
Objective/Aim: This study looks at the proximity of melanoma diagnoses to a selected set of frequently (in claims data) used cancer treatments as a way to identify those treatments that could be used to confirm melanoma diagnoses.
Methods: We used the administrative claims database PharMetrics Plus, which is large and covers the United States without significant imbalance by region. We selected patients with claims during the period January 2016 through December 2019 and searched for claims of cancer treatments in the categories of chemotherapy, ICIs, targeted therapy, radiotherapy, and surgery. For each claim involving one of these treatments, we counted the instances where a melanoma diagnosis (ICD-10-CM code: C43) was recorded 5 and 30 days prior to treatment, as well as for all of the patient’s medical history. We separately counted the instances where a melanoma diagnosis was recorded at any time in the study period.
Results: We examined 96 chemotherapy medications, 98 targeted therapy medications, 7 ICIs, 78 procedure codes for radiation therapies, 2751 procedure codes for surgery, and 37 procedure codes for stem cell transplant related to cancer. The results for notable treatments within the study categories are presented in Table 1. No treatments were tightly associated with melanoma, but the ICI treatments (nivolumab and pembrolizumab) are potentially useful because of their relatively large numbers. Treatments with indications for other conditions or other cancers (such as paclitaxel) may also be useful. Surgery, for example, has relatively large numbers (45,000 within 5 days and 55,000 in the patient’s history) and hence could be useful to confirm a diagnosis of melanoma using claims data.
Conclusion: Overall, surgery and ICI treatment categories are more often related to melanoma diagnosis in claims data.
References/Further Sources of Information
P005 Cancer Treatments Associated with Kidney Cancer Diagnosis in Claims Data: Results from an Exploratory Study
H. Kawabata1, X. Pan1, J. Zheng1, J. Zhang2, S. Pp3, S. Moinuddeen3, S. Dey3
1Bristol Myers Squibb, Integrated Oncology Epidemiology III, Lawrenceville, USA; 2Bristol Myers Squibb, MSA Solid Tumor Oncology, Lawrenceville, USA; 3Mu Sigma Business Solutions, Data Science Team, Bengaluru, India
Background/Introduction: The identification of cancer patients using administrative claims data can be challenging due to the focus of claims for reimbursement purposes. Algorithms using treatment claims to confirm cancer diagnoses have been developed but they can become complicated because a treatment may be used for numerous indications including non-cancer conditions. In this study, we look for treatments associated with kidney cancer diagnoses in the following categories: chemotherapy, immune checkpoint inhibitors (ICIs), targeted therapy, and radiotherapy.
Objective/Aim: This study aims to identify treatments that could be used to confirm renal cell carcinoma diagnoses with claims data by the proximity of kidney cancer diagnoses to a selected set of frequently used cancer treatments.
Methods: We used the administrative claims database PharMetrics Plus, which covers more than 165 million persons and is representative of the United States. We selected patients with claims during the period January 2016 through December 2019 and searched for claims of cancer treatments in the categories of chemotherapy, immune checkpoint inhibitors (ICI), targeted therapy, and radiotherapy. For each claim involving one of these treatments, we counted the instances where a kidney cancer diagnosis (ICD-10-CM codes: C64–C65) was recorded 5 and 30 days prior to treatment, as well as for all of the patient’s medical history. We separately counted the instances where a kidney cancer diagnosis was recorded at any time in the study period.
Results: We examined 96 chemotherapy medications, 98 targeted therapy medications, 7 ICIs, 78 procedure codes for radiation therapies, 2751 procedure codes for surgery, and 37 procedure codes for stem cell transplant related to cancer. The results for notable treatments within the study categories are presented in Table 1. The most frequent ICI treatments associated with kidney cancer diagnosis were ipilimumab and nivolumab. Of the chemotherapies and targeted therapies studied, those most highly associated with kidney cancer diagnosis were dactinomycin and temsirolimus, respectively. Approximately 1% of radiation treatment delivery was associated with kidney cancer diagnoses.
Conclusion: Based on this research, the most frequent cancer treatment medication category associated with kidney cancer diagnosis was ICIs, followed by chemotherapy medications.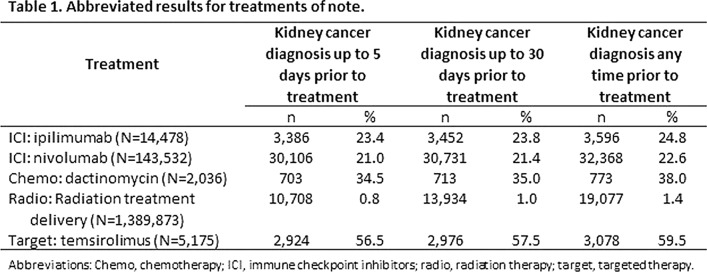
References/Further Sources of Information
P006 Cancer Treatments Associated with Lymphoma Diagnosis in Claims Data: Results from an Exploratory Study
S. Pp1, J. Zheng2, X. Pan2, H. Kawabata2, J. Zhang3, S. Moinuddeen1, S. Dey1
1Mu Sigma Business Solutions, Data Science Team, Bengaluru, India; 2Bristol Myers Squibb, Integrated Oncology Epidemiology III, Lawrenceville, USA; 3Bristol Myers Squibb, MSA Solid Tumor Oncology, Lawrenceville, USA
Background/Introduction: While administrative claims data are useful for studies of cancer treatment outcomes, the identification of cancer patients can be challenging because of the reimbursement focus of claims. Algorithms using treatment claims to confirm cancer diagnoses have been developed, but they can be challenging because of numerous indications for many treatments including non-cancer ones. In this study, we looked for treatments associated with lymphoma diagnoses in the following categories: chemotherapy, immune checkpoint inhibitors (ICIs), targeted therapy, radiotherapy, and surgery.
Objective/Aim: This study looks at the proximity of lymphoma diagnoses to a selected set of frequently (in claims data) used cancer treatments as a way to identify those treatments that could be used to confirm lymphoma diagnoses.
Methods: We used the administrative claims database PharMetrics Plus, which is large and covers the United States without significant imbalance by region. We selected patients with claims during the period January 2016 through December 2019 and searched for claims of cancer treatments in the categories of chemotherapy, ICIs, targeted therapy, and radiotherapy. For each claim involving one of these treatments, we counted the instances where a lymphoma diagnosis (ICD-10-CM codes: C81–C83) was recorded 5 and 30 days prior to treatment, as well as for all of the patient’s medical history. We separately counted the instances where a lymphoma diagnosis was recorded at any time in the study period.
Results: We examined 96 chemotherapy medications, 98 targeted therapy medications, 7 ICIs, 78 procedure codes for radiation therapies, 2751 procedure codes for surgery, and 37 procedure codes for stem cell transplant related to cancer. The results for notable treatments within the study categories are presented in Table 1. Chemotherapy treatments dacarbazine, vinblastine, and bendamustine showed reasonable associations with lymphoma diagnoses, identifying about 25,000 encounters each. The targeted treatment rituximab was associated with a larger number of encounters, 83,611 in the five-day window and 100,551 in the full patient history. The number of radiation treatments is understated here for technical reasons (specificity of coding) but still identify about 25,000 encounters. ICI treatments, used widely for other cancers, are not associated with lymphoma diagnoses in these data.
Conclusion: While these data show that chemotherapy treatments are useful for confirming lymphoma diagnoses in claims data, targeted and radiation therapies also deserve a close examination.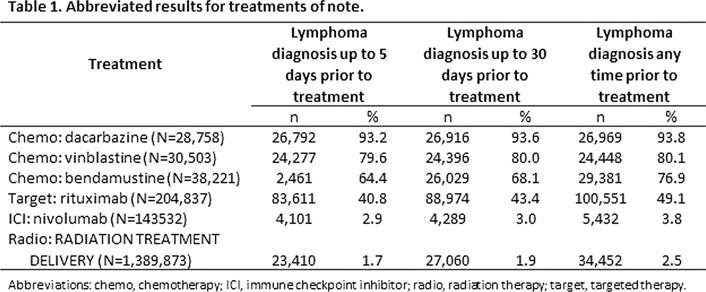
References/Further Sources of Information
P007 Cancer Treatments Associated with Liver Cancer Diagnosis in Claims Data: Results from an Exploratory Study
J. Zheng1, X. Pan1, H. Kawabata1, S. Pp2, S. Moinuddeen2, J. Zhang3, S. Dey2
1Bristol Myers Squibb, Integrated Oncology Epidemiology III, Lawrenceville, USA; 2Mu Sigma Business Solutions, Data Science Team, Bengaluru, India; 3Bristol Myers Squibb, MSA Solid Tumor Oncology, Lawrenceville, USA
Background/Introduction: While administrative claims data are useful for studies of cancer treatment outcomes, the identification of cancer patients can be challenging because of the reimbursement focus of claims. Algorithms using treatment claims to confirm cancer diagnoses have been developed, but they can be challenging because of numerous indications for many treatments including non-cancer ones. In this study, we looked for treatments associated with liver cancer diagnoses in the following categories: chemotherapy, immune checkpoint inhibitors (ICIs), targeted therapy, and radiotherapy.
Objective/Aim: This study looks at the proximity of liver cancer diagnoses to a selected set of frequently (in claims data) used cancer treatments as a way to identify those treatments that could be used to confirm liver cancer diagnoses.
Methods: We used the administrative claims database PharMetrics Plus, which is large and covers the United States without significant imbalance by region. We selected patients with claims during the period January 2016 through December 2019 and searched for claims of cancer treatments in the categories of chemotherapy, ICIs, targeted therapy, and radiotherapy. For each claim involving one of these treatments, we counted the instances where a liver cancer diagnosis (ICD-10-CM code: C22) was recorded 5 and 30 days prior to treatment, as well as for all of the patient’s medical history. We separately counted the instances where a liver cancer diagnosis was recorded at any time in the study period.
Results: We examined 96 chemotherapy medications, 98 targeted therapy medications, 7 ICIs, 78 procedure codes for radiation therapies, 2751 procedure codes for surgery, and 37 procedure codes for stem cell transplant related to cancer. The results for notable treatments within the study categories are presented in Table 1. None of these treatments are tightly associated with liver cancer diagnoses, the best being the targeted therapy ramucirumab. Increasing the time window to find cancer diagnoses improved the association significantly. But, other treatments with lower percentages—but with higher absolute numbers, such as fluorouracil—are better at identifying larger numbers of encounters.
Conclusion: There are no individual medications tightly associated with a liver cancer diagnosis in these data. Overall, the chemotherapy treatment category has a relatively higher association with liver cancer diagnosis.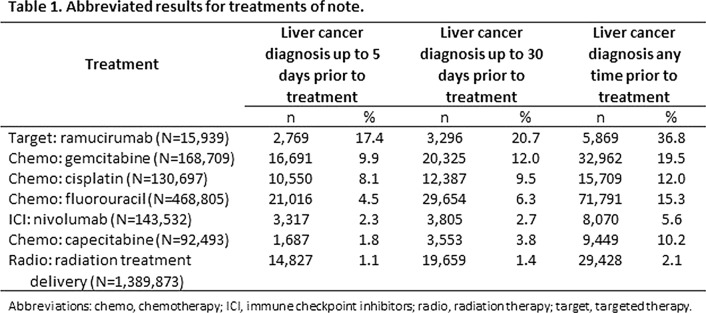
References/Further Sources of Information
P008 Cancer Treatments Associated with Lung Cancer Diagnosis in Claims Data: Results from an Exploratory Study
X. Pan1, H. Kawabata1, J. Zheng1, J. Zhang2, S. Moinuddeen3, S. Dey3, S. Pp3
1Bristol Myers Squibb, Integrated Oncology Epidemiology III, Lawrenceville, USA; 2Bristol Myers Squibb, MSA Solid Tumor Oncology, Lawrenceville, USA; 3Mu Sigma Business Solutions, Data Science Team, Bengaluru, India
Background/Introduction: While administrative claims data are useful for studies of cancer treatment outcomes, the identification of cancer patients can be challenging because of the reimbursement focus of claims. Algorithms using treatment claims to confirm cancer diagnoses have been developed, but they can be challenging because of numerous indications for many treatments including non-cancer ones. In this study, we looked for treatments associated with lung cancer diagnoses in the following categories: chemotherapy, immune checkpoint inhibitors (ICIs), targeted therapy, radiotherapy, and surgery.
Objective/Aim: This study looks at the proximity of lung cancer diagnoses to a selected set of frequently (in claims data) used cancer treatments as a way to identify those treatments that could be used to confirm lung cancer diagnoses.
Methods: We used the administrative claims database PharMetrics Plus, which is large and covers the United States without significant imbalance by region. We selected patients with claims during the period January 2016 through December 2019 and searched for claims of cancer treatments in the categories of chemotherapy, ICIs, targeted therapy, radiotherapy, and surgery. For each claim involving one of these treatments, we counted the instances where a lung cancer diagnosis (ICD-10-CM code: C34) was recorded 5 and 30 days prior to treatment, as well as for all of the patient’s medical history. We separately counted the instances where a lung cancer diagnosis was recorded at any time in the study period.
Results: We examined 96 chemotherapy medications, 98 targeted therapy medications, 7 ICIs, 78 procedure codes for radiation therapies, 2751 procedure codes for surgery, and 37 procedure codes for stem cell transplant related to cancer. The results for notable treatments within the study categories are presented in Table 1. Those treatments with indications primarily for lung cancer, such as durvalumab, were tightly associated (98%) with lung cancer. Treatments with indications for other cancers as well, such as nivolumab, were not as tightly associated (only 35.5%) with lung cancer. However, necitumumab was tightly associated with lung cancer (98%) but was not widely used (only 205 claims in our data). Such tight associations with low utilization are problematic for use as confirmatory evidence of lung cancer diagnosis.
Conclusion: Overall, ICI treatment and chemotherapy categories are highly associated with a diagnosis of lung cancer in claims data.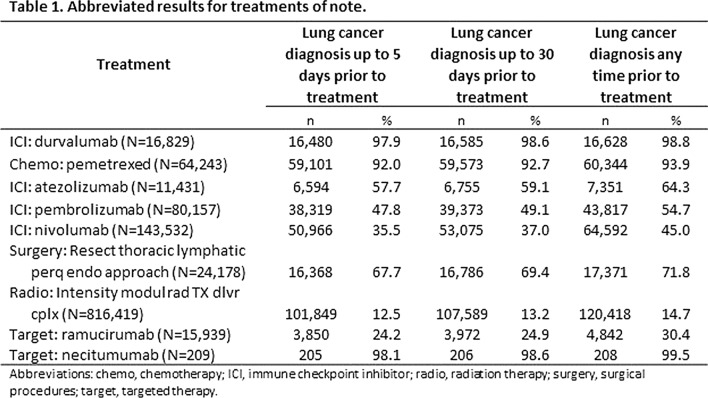
References/Further Sources of Information
P009 Associating Cancer Treatments and Diagnoses: A Claims Data Analysis
X. Pan1, J. Zheng1, H. Kawabata1, J. Zhang2, S. Pp3, S. Dey3, S. Moinuddeen3
1Bristol Myers Squibb, Integrated Oncology Epidemiology III, Lawrenceville, USA; 2Bristol Myers Squibb, MSA Solid Tumor Oncology, Lawrenceville, USA; 3Mu Sigma Business Solutions, Data Science Team, Bengaluru, India
Background/Introduction: The introduction of novel anti-cancer treatments has increased treatment choices and improved prognosis for patients. While administrative claims data are useful for the study treatment outcomes, because of the reimbursement focus of claims the identification of the specific cancers for which these treatments are being used is often challenging. Algorithms that use treatment claims to confirm cancer diagnoses are often used, but they can be inexact, as off-label use of many treatments is prevalent. In this study, we examine the cancer diagnoses associated with selected treatments.
Objective/Aim: This study examines the proximity of cancer diagnoses to a selected set of frequently used cancer treatments.
Methods: We used the administrative claims database PharMetrics Plus, which has the advantage of being large and covers the United States without significant imbalance by region. We selected patients with claims during the period January 2016 through December 2019 and searched for claims of cancer treatments in the categories of chemotherapy, immune checkpoint inhibitors (ICIs), targeted therapy, radiation, and surgery. For each claim that included one of the selected study treatments, we examined the period prior to the date of treatment as follows: up to 5 days, 30 days, and all of the patient’s history prior to the treatment. We counted the instances where a cancer diagnosis (ICD-10-CM codes: C00–C26, C30–C41, C43–C58, C60–C96) was recorded. We separately counted the instances where a cancer diagnosis was recorded at any time in the study period.
Results: We examined 96 chemotherapy medications, 98 targeted therapy medications, 7 ICIs, 78 procedure codes for radiation therapies, 2751 procedure codes for surgery, and 37 procedure codes for stem cell transplant related to cancer. The results for treatments of note within the five treatment categories are presented in Table 1. ICI treatments plus paclitaxel (chemotherapy) had a cancer diagnosis within 5 days prior to the treatment 99% of the time. In greater than 90% of the procedures, biopsy/removal of lymph nodes and radiation therapy had cancer diagnoses within 5 days prior. The diagnosis rates for other treatments varied in their association and increased as the time period prior to treatment increased. Methotrexate (chemotherapy) and bevacizumab (targeted therapy), with many indications, were problematic in confirming a cancer diagnosis.
Conclusion: Drug treatments and procedures with narrow indications are useful to confirm a cancer diagnosis; those with wider indications may be problematic.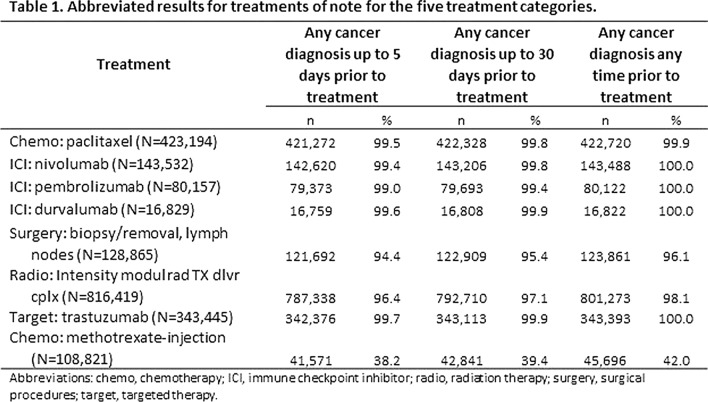
References/Further Sources of Information
P010 Cancer Treatments Associated with Colorectal Cancer Diagnoses in Claims Data: Results from an Exploratory Study
X. Pan1, H. Kawabata1, J. Zheng1, J. Zhang2, S. Pp3, S. Dey3, S. Moinuddeen3
1Bristol Myers Squibb, Integrated Oncology Epidemiology III, Lawrenceville, USA; 2Bristol Myers Squibb, MSA Solid Tumor Oncology, Lawrenceville, USA; 3Mu Sigma Business Solutions, Data Science Team, Bengaluru, India
Background/Introduction: While administrative claims data are useful for studies of cancer treatment outcomes, the identification of cancer patients can be challenging because of the reimbursement focus of claims. Algorithms using treatment claims to confirm cancer diagnoses have been developed, but they can be challenging because of numerous indications for many treatments including non-cancer ones. In this study, we looked for treatments associated with colorectal cancer (CRC) diagnoses in the following categories: chemotherapy, immune checkpoint inhibitors (ICIs), targeted therapy, and radiotherapy.
Objective/Aim: This study looks at the proximity of CRC diagnoses to a selected set of frequently (in claims data) used cancer treatments as a way to identify those treatments that could be used to confirm colorectal cancer diagnoses.
Methods: We used the administrative claims database PharMetrics Plus, which is large and covers the United States without significant imbalance by region. We selected patients with claims during the period January 2016 through December 2019 and searched for claims of cancer treatments in the categories of chemotherapy, ICIs, targeted therapy, and radiotherapy. For each claim involving one of these treatments, we counted the instances where a CRC diagnosis (ICD-10-CM code: C18) was recorded 5 and 30 days prior to treatment, as well as for all of the patient’s medical history. We separately counted the instances where a CRC diagnosis was recorded at any time in the study period.
Results: We examined 96 chemotherapy medications, 98 targeted therapy medications, 7 ICIs, 78 procedure codes for radiation therapies, 2751 procedure codes for surgery and 37 procedure codes for stem cell transplant related to cancer. The results for notable treatments within the study categories are presented in Table 1. The targeted treatment panitumumab was associated with CRC 92% of the time (for all patient history). ICIs were not associated with CRC diagnoses, likely due to their more frequent use in the treatment of other cancers. The chemotherapy treatments oxaliplatin and fluorouracil were associated with CRC only 49 and 50% of the time, respectively, but had high use (127,000 and 235,000, respectively, at the 5-day window) and could be used to confirm a large number of encounters.
Conclusion: While treatments that have tight associations with CRC may be useful to confirm the diagnosis, others with high utilization could also be associated, even if not as tightly.
References/Further Sources of Information
P011 Immunization Errors Related to COVID-19 Vaccines in Morocco: Surveillance and Risk Minimization Actions
L. Alj1, A. Tebaa1, N. Hermani2, R. Soulaymani Bencheikh1
1Centre Anti Poison et de Pharmacovigilance du Maroc, Ministry of Health, Rabat, Morocco; 2Direction Régionale de la Santé Casablanca Settat, Ministry of Health, Casablanca, Morocco
Background/Introduction: Immunization errors (IEs) are one of the types of adverse events following immunization (AEFI) considered as preventable. They result from errors in vaccine preparation, handling, storage or administration [1].
Objective/Aim: To describe the profile of IEs related to COVID-19 vaccines reported to the Centre Anti Poison et de Pharmacovigilance du Maroc (CAPM) and risk minimization actions (RMAs) taken proactively and reactively to prevent IEs occurrence.
Methods: We conducted a prospective analysis of IEs reported to the CAPM as part of the surveillance of AEFI related to COVID-19 vaccines. Data were collected using the AEFI reporting form, the electronic line listing and the hotlines established as part of the national COVID-19 immunization campaign launched on 28 January 2021. Immunization errors were classified according to their occurrence in the vaccine use process and their type using MedRRA. Investigations were performed to identify causes and contributing factors that lead to IEs, in the view of implementing RMAs.
Results: As of 11 June 2021, the total number of IEs was 56, with an incidence of 4 cases per million doses of vaccine administered. All IEs were reported by healthcare professionals (HCPs). They occurred at the administration stage (n = 51) and the preparation stage (n = 5). The administration of the wrong vaccine was the main type of IEs (n = 26), followed by the inappropriate schedule of vaccine administration (n = 20). Two patients presented harm and two vaccinators developed ophthalmic adverse reaction. Prior to the launching of the campaign, proactive RMAs were designed: (i) training of HCPs involved in the campaign on the process of maintaining the cold chain, preparation and administration of vaccines and the workflow of an immunization session, (ii) the exclusively use of one type of vaccine in the same vaccination site, (iii) the development of an information technology system for managing patient ’appointments to be vaccinated. The investigations performed revealed that organizational issues were the main cause behind IEs happening, particularly at vaccination sites that were strained to use both available vaccines. Reactive measures, implemented in collaboration with the National Immunization Committee, highlighted the importance of seeking for contraindications or reasons that would affect patient suitability for vaccination, and applying best practices for immunization session management.
Conclusion: The proper planning of proactive RMAs is key to reduce the likelihood of IEs. Moreover, immunization process vulnerabilities identified following IEs happening, should be tackled to prevent harm and maintain population ‘confidence in the national COVID-19 campaign.
References/Further Sources of Information
Covid-19 vaccines: safety surveillance manual. Geneva: World Health Organization; 2020. Licence: CC BY-NC-SA 3.0 IGO.
P012 Delayed Extensive Local Reactions: A Case Series to the mRNA-1273 Vaccine Against SARS-CoV-2
R. Ferreira Da Silva1,2,3, I. Ribeiro-Vaz1,2,3, A. M. Silva1,2,3, P. Guedes4, M. Morato5, J. Junqueira Polónia1,3,6
1Porto Pharmacovigilance Centre-Faculty of Medicine of The University of Porto, Porto, Portugal; 2Department of Community Medicine-Health Information and Decision-Faculty of Medicine of The University of Porto, Porto, Portugal; 3University of Porto, Center for Health Technology and Services Research, Porto, Portugal; 4Unidade Local de Saúde de Matosinhos, Unidade Local de Saúde de Matosinhos, Matosinhos, Portugal; 5LAQV/REQUIMTE-Laboratory of Pharmacology-Department of Drug Sciences-Faculty of Pharmacy of the University of Porto, Porto, Portugal; 6University of Porto, Department of Medicine-Faculty of Medicine of The University of Porto, Porto, Portugal
Background/Introduction: Adverse events following immunisation (AEFIs) are unpleasant/unintended medical occurrences (sign, symptom, laboratory finding, disease) observed after immunisation, with or without causal association with vaccine usage [1]. They are expected to occur acutely after the administration of an injectable, as is reported in the summary of product characteristics (SmPC) of the mRNA-1273 vaccine [2]. However, there are also some reports of delayed injection-site reactions as AEFIs of mRNA vaccines against SARS-CoV-2 [3–6]. The occurrence of large local reactions may impact adherence to vaccination, particularly to the second dose, compromising the efficacy of the vaccine.
Objective/Aim: To describe a case series of 15 patients with delayed large local reactions (dLLR) to the mRNA-1273 vaccine.
Methods: We analysed a case series of 15 patients presenting dLLR to the mRNA-1273 vaccine, which were reported to the Porto Pharmacovigilance Centre between May 21 and June 2021 by a physician at a COVID-19 vaccination centre. We analysed: (1) age and sex of the patient; (2) type of AEFIs—Preferred Term (PT) hierarchy level coding, according to the MedDRA (Medical Dictionary for Regulatory Activities); (3) time to onset after immunisation; and (4) therapeutic prescribed.
Results: Almost all the cases were observed in women (n = 14). The median age for all patients was 53 years (range 27–73). According to the PT, six types of AEFIs were reported: vaccination site erythema (n = 15), vaccination site warmth (n = 14), extensive swelling of vaccination limb (n = 9), vaccination site pain (n = 2), vaccination site pruritus (n = 1) and vaccination site oedema (n = 1). In ten patients, the reactogenic reaction included three AEFIs: erythema and warmth at the vaccination site with extensive swelling of the limb. The median time to onset was 7 days after the first dose (range 6–14). Seven patients received treatment for their symptoms: antihistamine (n = 7), corticosteroid (n = 3), NSAID (2), cryotherapy (n = 2) and antibiotic (n = 1); for eight patients the treatment remains unknown.
Conclusion: We report a series of dLLR after the mRNA-1273 vaccine, which probably represents an AEFI not yet described in the SmPC. The pathophysiological mechanism underlying this dLLR remains unclear, although the clinical criteria for these reactions are consistent with delayed-type hypersensitivity. Further insight on the occurrence of this clinical situation is crucial to further characterise the safety of this and other SARS-CoV-2 vaccines and to define strategies to inform the population in order to promote adherence to vaccination.
References/Further Sources of Information
ICH. International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). Medical Dictionary for Regulatory Activities. https://www.meddra.org/.
Agency EM. COVID-19 mRNA Vaccine Moderna (nucleoside modified): Summary of product characteristics 2021. https://www.ema.europa.eu/en/documents/product-information/covid-19-vaccine-moderna-epar-product-information_pt.pdf.
Blumenthal KG, Freeman EE, Saff RR, Robinson LB, Wolfson AR, Foreman RK, et al. Delayed Large Local Reactions to mRNA-1273 Vaccine against SARS-CoV-2. N Engl J Med. 2021;384(13):1273–7.
Baeck M, Marot L, Belkhir L. Delayed large local reactions to mRNA vaccines. N Engl J Med. 2021;384(24).
Grobusch MP, Schnyder J, Garcia-Garrido HM, Wisman JJ, Stijnis C, Goorhuis A, et al. Delayed large local reaction to the adenovirus-vectored (ChAdOx1) vaccine. Travel Med Infect Dis. 2021;43:102093.
Johnston MS, Galan A, Watsky KL, Little AJ. Delayed localised hypersensitivity reactions to the Moderna COVID-19 vaccine: a case series. JAMA Dermatol. 2021;157(6):716–20.
P013 Epidemiological Profile of Patients with Adverse Events Following COVID-19 Immunization at the University Hospital of Marrakesh
A. Habchane1, S. Khatem1, A. Alioua1, A. Takoui2, S. Zaoui1
1University Hospital Mohammed VI, Pharmacology-Toxicology, Marrakesh, Morocco; 2University Hospital Mohammed VI, General Direction, Marrakesh, Morocco
Background/Introduction: Vaccines against COVID-19 have been shown to be effective in reducing the severity, mortality, and spread of COVID-19 infections [1]. The safety of the vaccines currently used in Morocco has been well established [2], but like any other health products, they are likely to cause adverse events.
Objective/Aim: To describe the epidemiological and clinical characteristics of patients who presented with adverse events following immunization (AEFI) against COVID-19 vaccines at the University Hospital (UH) of Marrakesh.
Methods: Descriptive study including the reports of AEFI received at the pharmacovigilance unit of the Mohammed VI UH of Marrakesh from the vaccination unit of the UH and its different departments, over a period of 4 months and a half (February 1st–June 15th, 2021). The epidemiological and clinical characteristics of these patients were noted.
Results: A total of 62 reports were received, 80.65% of which came from the UH vaccination unit dedicated to its staff. Thus, the percentage of vaccinated staff having developed AEFIs is 2.6% (out of a total number of vaccinated personnel = 1930). The notifications received from the various departments that hospitalized cases of AEFI and from health centers represented 19.35%. There was clear female predominance in our series (72.58%). The most represented age groups were 18–34 years (35.48%) and 55–70 years (20.97%). However, age information was not included in 24.19% of the reports. Among our patients, 35.48% had a pathological history, with diabetes and hypertension being the mostly observed (36.36% and 31.82%, respectively) and 17.74% of them were on concomitant medications of which 22.73% and 13.64% were on antihypertensive and oral antidiabetic drugs, respectively. The most reported adverse events were general disorders and administration site conditions and nervous system disorders (30.00% and 24.29%, respectively) with different onset times [the most observed ones were 1–24 h (37.86%) and < 1 h (26.43%)]. The patient outcome was good in 91.37% of cases. All our patients were vaccinated with the AstraZeneca™ vaccine. The events occurred after the first dose in 87.10% of cases.
Conclusion: We have tried through this work to elucidate the epidemiological and clinical features of the patients who have developed AEFIs in our facility, in order to identify the affected patients’ backgrounds and to have an orientation on eventual risk factors.
References/Further Sources of Information
Rutkowski K, Mirakian R, Till S, Rutkowski R, Wagner A. Adverse reactions to COVID‐19 vaccines: a practical approach. Clin Exp Allergy. 2021 Jun;51(6):770–7.
Campagne de vaccination contre le coronavirus au Maroc [Internet]. [cited 2021 Jun 19]. http://www.liqahcorona.ma/fr.
P014 Steroid Stewardship as a Tool to Tackle Glucocorticoid Induced Hyperglycemia in COVID-19 Patients
A. Issac1, J. J. Kochuparambil1, J. Vayalikunnel1
1Mary Queen’s Mission Hospital, Department of Service Excellence and Quality, Kanjirappally, India
Background/Introduction: Hyperglycemia has been identified as an independent predictor of mortality in hospitalized patients with COVID-19. Administration of systemic corticosteroids can worsen the incidence of hyperglycemia in COVID-19 patients.
Objective/Aim: To characterize the incidence of Glucocorticoid Induced Hyperglycemia (GCIH) in COVID -19 patients and to evaluate the effectiveness of a Steroid Stewardship Program in countering this effect.
Methods: This prospective, interventional study included 100 COVID-19 confirmed patients admitted between March 12–May 12, 2021 who are categorized into B&C as per the guidelines of directorate of health services, Kerala, India. A five-item Steroid Appropriate Index (SAI) was prepared based on the onset of symptoms, weight of the patient, CT Thorax severity score, preferred route of administration and the underlying diseases for choosing the appropriate doing of the preferred corticosteroid. Demographic profile of these patients was collected and they were followed up till discharge/death to track the incidence of worsening of hyperglycemia or new onset of diabetes. The effectiveness of SAI was analyzed by comparing the data of treatment and clinical outcome among 100 COVID-19 confirmed patients admitted before and after implementation of the scale.
Results: Majority of the patients were males (57) and the mean age was 45.7 ± 16.6 years. The incidence of new onset of hyperglycemia i.e., GCIH was more among the retrospective cohort when compared with the SAI cohort (7% vs 26%, p < 0.001). The study also revealed that past history of diabetes (p < 0.001), age (p = 0.002), number of medicines used for treatment (p<0.001) are significantly associated with glucocorticoid induced hyperglycemia.
Conclusion: The appropriate selection of corticosteroids based of the real-world scenarios can decrease the incidence of adverse events associated. Implementation of steroid appropriate index and proper monitoring can be an aid for the same.
References/Further Sources of Information
Kow CS, Hasan SS. Corticosteroid-related in-hospital hyperglycemia: does it negate mortality benefits in coronavirus disease 2019? Clin Inf Dis. 2020.
P015 An Active Surveillance Study to Trace the Risk Factors Associated with Adverse Drug Reactions in COVID-19 Treatment
J. J. Kochuparambil1, A. Issac1, A. Nidha1, J. Vayalikunnel1
1Mary Queen’s Mission Hospital, Department of Service Excellence and Quality, Kanjirappally, India
Background/Introduction: Second wave of COVID-19 in India has been drastic in terms of the number of people affected directly and indirectly.
Objective/Aim: To characterize the adverse drug reactions (ADRs) and its the risk factors in COVID -19 treatment in a secondary care hospital in South India.
Methods: A prospective observational study was carried out on 327 patients admitted between April 1 and May 15, 2021 using an active in person surveillance system. The demographic characters of all the patients including the time of admission, comorbid diseases, length of stay, history of drug allergies and the number of medications used during hospitalization were collected. After identification of an adverse drug event, the causality, suspicious drugs and prognosis of ADRs were also recorded. Descriptive statistics and multivariate logistic regressions were carried out to analyze the risk factors of ADRs.
Results: Out of the 327 patients, 194 (59.3%) were men and the mean age was 45.7 ± 16.6 years. 122 ADRs were reported among 87 patients with an incidence rate of 26.6%. Most of the reactions were associated with dexamethasone (31.9%), remdesivir (18%) and favipiravir (14.7%) with dexamethasone induced hyperglycemia being most prominent. Majority of the suspected ADR cases were categorized to probable (61.8%) according to WHO-UMC causality assessment. When compared with the No ADRs group, the length of stay (p = 0.004), history of drug allergies (p < 0.001), number of drugs used in treatment (p < 0.001) were significantly higher in the ADRs group. Multivariable analysis revealed that length of stay (OR 2.03; 95% CI 1.02–3.98; P = 0.04), comorbidities (OR 2.08; 95% CI 1.05–4.18; P = 0.04), number of drugs used for treatment (OR 3.17; 95% CI 1.60–6.27; P = 0.001) and were independent risk factor for ADRs in the patients.
Conclusion: Active surveillance measures are important in case of all drugs used for COVID treatment so as to keep the living guidelines the most live one.
References/Further Sources of Information
Sun J, Deng X, Chen X, Huang J, Huang S, Li Y, Feng J, Liu J, He G. Incidence of adverse drug reactions in covid‐19 patients in China: an active monitoring study by Hospital Pharmacovigilance System. Clin Pharmacol Ther. 2020 Oct;108(4):791–7.
P016 Events Supposedly Attributed to Vaccine or Immunization of the Pfizer-BioNTech vaccine in front-line personnel of the National Institute of Cardiology
J. A. Maza1, Z. Albores Citlali2, I. Gutiérrez1, A. Pérez Barragán1, H. A. Martínez1, J. A. Cosio1, M. E. Jiménez Corona3, G. Rojas Velasco2
1Pharmacotherapeutic Follow-up Unit, Department of Clinical Pharmacology, Mexico City, Mexico; 2National Institute of Cardiology, Cardiovascular Intensive Care Unit, Mexico City, Mexico; 3National Institute of Cardiology, Epidemiology Department Chief, Mexico City, Mexico
Background/Introduction: COVID-19 has had a significant impact in the last year. Research and implementation of new vaccines against SARS-Cov-2 have given hope of preventing its spread. They have not been tested in the entire population, hence the importance of events presumably attributable to vaccination and immunization (ESAVI’s), the results will contribute to the biosafety of the vaccine, improvement in the effectiveness of pharmacovigilance of ESAVI’s, implementation of measures to combat it. The institutional pharmacovigilance center was fundamental to carry out this study.
Objective/Aim: Evaluation of events supposedly attributable to vaccination and immunization by Pfizer vaccine applied in front-line personnel of the National Institute of Cardiology (INC).
Methods: Retrospective observational passive pharmacovigilance study of a cohort of first-line health personnel, all of whom worked in the care of patients with COVID-19 in a tertiary level hospital. The data obtained were captured in a database submitted for validation to the pharmacovigilance department and were analyzed.
Results: The present study was conducted during the period December 2020 to March 2021, initial doses of Pfizer vaccine were administered to 1950 workers, reporting 59 events; 1570 second doses, of which 63 ESAVI’s were reported. Of the 127 reports obtained, 5 were discarded due to follow-up problems. The most frequent findings in relation to the total number of doses administered were headache (2.4%), pain/sensitivity at the site of application, asthenia and myalgia with 1.8% respectively, arthralgias (1.6%), fever and adynamia (1.4%).
Conclusion: The Pfizer vaccine presented a very low incidence of ESAVI’s in the first line personnel of the INC Ignacio Chavez. Symptoms appeared from 30 min to 4 h after application, 75% of the population that presented reaction in the first application, repeated in the subsequent application and it was observed that the persistence of symptoms was longer lasting. The most affected age group was 30–40 years old. Therefore, it is concluded that the analysis of ESAVI’s in all vaccines is of great importance to evaluate the safety of each one of them.
References/Further Sources of Information
P017 Corticosteroid Safety in COVID-19 Patients: A Pharmacovigilance Study
I. Gutierrez1, J. A. Maza1, M. Puerto1, A. Pérez1, F. Rosado1, K. Peredo1, A. Quiroz1, A. Murillo1, E. Berrios1, M. Gaxiola1, G. Rojas1
1Instituto Nacional de Cardiología Ignacio Chávez, Clinical Pharmacology, Ciudad de México, Mexico
Background/Introduction: The use of oral and intravenous corticosteroids as a treatment for SARS-CoV-2 infection has been shown to inhibit the exaggerated inflammatory response, reducing symptoms and days of hospitalization of patients. However, its use is controversial because not enough clinical studies have been made to verify the safety of the drugs.
Objective/Aim: To assess the safety profile of glucocorticoid treatment, at high and low doses, in suspected and/or confirmed patients with COVID-19 at the Ignacio Chávez National Institute of Cardiology, determining the most frequent side effects in patients, and assessing whether the administration of the drugs represents a greater benefit than the risk of presenting these effects.
Methods: 366 patients were evaluated and divided into 3 groups: use of methylprednisolone (155 mg average) every 24 h for 3 days, dexamethasone (6 mg) every 24 h for 10 days, and patients with no use of corticosteroids. 87 patients in the study where excluded for meeting exclusion criteria.
Hyperglycemia was evaluated in terms of its harshness and severity in relation to the maximum glucose level that the patients presented during the first 10 days after the start of treatment. Drug-associated hyperglycemia was not considered in patients admitted with hyperglycemic uncontrolled levels greater than 200 mg/dL
Results: We observed that the cumulative incidence of hyperglycemia in the group with high doses of corticosteroids (methylprednisolone 155 mg day average) is 70%, low doses of corticosteroids (Dexamethasone 6 mg/day) is 67% and that of the group without corticosteroids is 31%.
Based on the results or the 95% confidence interval calculation for estimating the risk of infections and hyperglycemia after the use of methylprednisolone and dexamethasone in the patients evaluated, it was observed that there is a risk of these effects occurring after the use of corticosteroids. However, it is not possible to define whether the use of any of the drugs represents a greater risk within this group of drugs, since the interval between the two groups of drugs is similar.
Conclusion: The use of glucocorticoids at high and low doses as a treatment for COVID-19 disease has been shown to be effective in reducing symptoms and days of hospitalization in these patients. However, based on the results obtained, it was concluded that the use of these drugs can cause changes in the basal glucose of patients, in addition to increasing the risk of contracting in-hospital infections due to immunosuppression secondary to the use of glucocorticoids.
References/Further Sources of Information
Singhal T. A review of coronavirus disease-2019 (COVID-19). Indian J Pediatr. 13 marzo 2020;87(4):281–6.
Solinas Cinzia, Perra Laura, Aiello Marco, et al. A critical evaluation of glucocorticoids in the management of severe COVID-19. Cytokine Growth Factor Rev. 24 junio 2020;54:8–23.
Qing Ye, Bili Wang, Jianhua Mao. The pathogenesis and treatment of the cytokine storm in COVID-19. J Infect. 24 marzo 2020;80:607–13.
Naserghandi A, Allameh S, Saffarpour R. All about COVID-19 in brief. New Microbes New Infect. 13 abril 2020;35(C):100678.
Abdin Shifaa M, Elgendy Sara M, Alyammahi Shatha K, et al. Tackling the cytokine storm in COVID-19, challenges and hopes. Life Sci. 11 julio 2020;257:118054.
Escuela Andaluza de Salud Pública. Tratamientos para el COVID-19: sospechas de reacciones adversas. 2020. https://cadime.es/noticias/destacados/121-destacados-covid-19/709-tratamientos-para-el-covid-19-sospechas-de-reacciones-adversas.html.
Agencia Española de Medicamentos y Productos Sanitarios. Sospechas de reacciones adversas notificadas con tratamientos utilizados en COVID-19. 23 octubre 2020. https://www.aemps.gob.es/laAEMPS/docs/reacciones-adversas-COVID-19-202007927.pdf.
RECOVERY dexamethasone. Randomised Evaluation of COVID-19 Therapy. https://www.recoverytrial.net/.
Centro de Información de Medicamentos. Ficha técnica: Dexametasona. Agencia Española de Medicamentos y Productos Sanitarios. https://cima.aemps.es/cima/dochtml/ft/81417/FT_81417.html.
Asociación española de pediatría: Comité de medicamentos. Pediamécum AEP: Dexametasona. Mayo 2016. https://www.aeped.es/comite-medicamentos/pediamecum/dexametasona.
Morieri Mario L, Fadini Gian P, Boscari Federico, et al. Hyperglycemia, glucocorticoid therapy and outcome of COVID-19. Diabetes Res Clin Pract. 2020;108449.
Ilias I, Zabuliene L. Hyperglycemia and the novel Covid-19 infection: possible pathophysiologic mechanisms. Med Hypotheses. junio 2020;139:109699.
Balanciano Giselle, Carrasco Gabriela, García Darío, et al. Uso de dexametasona en pacientes internados con COVID-19. Informe de evaluación de tecnología sanitaria. Red Argentina Pública de Evaluación de Tecnologías Sanitarias. agosto 2020.
Cain Derek W, Cidlowski John A. After 62 years of regulating immunity, dexamethasone meets COVID-19. Nat Rev Immunol. 2020;20(10):587–8.
García Milián, Ana Julia. Farmacovigilancia hospitalaria. Revista Cubana de Oftalmología. 30 junio 2016;29(4):1561–3070.
Romagnoli Stefano, Peris Adriano, De Gaudio Raffaele, et al. SARS-CoV-2 and COVID-19: from de bench to the beside. Physiol Rev. 1 octubre 2020;100(4):1455–66.
Twomey Julianne D, Luo Shen, Dean Alexis Q, et al. Covid-19 update: the race to therapeutic development. Drug Resistance Updates. 24 octubre 2020;53:100733.
Asselah Tarik, Durantel David, Pasmant Eric, et al. COVID-19: Discovery, diagnosis and drug development. J Hepatol. 2021. 8 octubre 2020;74:168–84.
Cascella Marco, Rajnik Michael, Cuomo Arturo, et al. Features, Evaluation, and Treatment of Coronavirus. StatPearls Publishing. 4 octubre 2020.
Herrera-Lasso Regás Valeria, Dordal Culla María T, Lleonart Bellfill Ramón. Reacciones adversas a fármacos utilizados en el tratamiento específico de la infección por SARS-CoV-2. Med Clin. 9 julio 2020;155(10):448–53.
Organización mundial de la salud. Corticoides para el tratamiento de la COVID-19. Organización Mundial de la Salud 2020. 2 septiembre 2020. https://apps.who.int/iris/handle/10665/334338.
Aikaterini Papamanoli, Yoo Jeanwoo, Prabhjot Grewal. High-dose methylprednisolone in nonintubated patients with severe COVID-19 pneumonia. Eur J Clin Investig. Febrero 2021. Septiembre 2020;51(2):e13458. https://doi.org/10.1111/eci.13458.
Lokesh Edara, Tarun Suvvari, Lakshmi Kutikuppala. High dose steroid therapy to prevent severe hypoxia in COVID-19 patients: a potential solution for low resource clinical setting. Cureus. 2020;12(12):e12330. https://doi.org/10.7759/cureus.12330.
P018 Unexpected Adverse Reactions Reported for COVID-19 Vaccines: A Descriptive Analysis of Spontaneous Reports Received by a Portuguese Regional Pharmacovigilance Unit
J. Abrantes1,2, I. Tavares1,2, D. Mendes1,2, A. Penedones1,2, C. Alves1,2,3, F. Batel-Marques1,2,3
1Pharmacovigilance Unit of Coimbra-UFC, Centre for Health Technology Assessment and Drug Research-CHAD-Association for Innovation and Biomedical Research on Light and Image-AIBILI, Coimbra, Portugal; 2Drug Safety and Effectiveness Research Network, DruSER.Net, Coimbra, Portugal; 3University of Coimbra, Laboratory of Social Pharmacy and Public Health-School of Pharmacy, Coimbra, Portugal
Background/Introduction: The approval of COVID-19 vaccines has been based on fast clinical development programs leading to limited knowledge on safety data [1]. Unexpected adverse drug reactions (ADRs), including rare ADRs or delayed ADRs from long-term exposure, may occur [2]. Moreover pre-marketing clinical trials do not have power to detect all ADRs, thereby highlighting the need to analyse post-marketing spontaneous reports (SRs).
Objective/Aim: To identify unexpected ADRs associated with COVID-19 vaccines spontaneously reported to the Regional Pharmacovigilance Unit of Coimbra in Portugal (UFC).
Methods: SRs of ADRs associated with COVID-19 vaccines received by UFC between 30/12/2020 and 30/04/2021 were included. ADRs were coded with MedDRA®, v.24 (System Organ Classification [SOC] and Preferred Term [PT]). ADRs were classified as “expected” or “unexpected” according to their description on the vaccines Summary of Product Characteristics (SmPC). Further, each unexpected ADR was classified as an important medical event (IME) according to the list from the European Medicines Agency (EMA).
Results: A total of 771 SRs, of which 262 SRs (34%) contained a total of 420 unexpected ADRs were received. For Comirnaty®, out of 287 unexpected ADRs, 26 (9.1%) were IME. The most frequently reported IMEs were angioedema (n = 2; 7.7%), bradycardia (n = 2; 7.7%), death (n = 2; 7.7%), deep vein thrombosis (n = 2; 7.7%), ischaemic stroke (n = 2; 7.7%), and seizure (n = 2; 7.7%). The remaining ADRs (acute kidney injury, cardio-respiratory arrest, cerebrovascular accident, corneal opacity, haemorrhagic stroke, hypertensive crisis, laryngeal oedema, loss of consciousness, pneumonia, pulmonary oedema, sepsis, status epilepticus, tongue paralysis and upper gastrointestinal haemorrhage), had one report each (n=14; 53.8%). Of the 121 unexpected ADRs reported for Vaxzevria®, 20 were IME (16.5%). The most frequently reported IMEs were ischaemic stroke (n = 5; 25.0%), and conjunctival haemorrhage (n = 3; 15.0%). The remaining ADRs (acute myocardial infarction, altered state of consciousness, angina pectoris, arrhythmia, death, guillain-Barre syndrome, hypothermia, mesenteric vein thrombosis, monoplegia, pericarditis, syncope and truncus coeliacus thrombosis), had one report each (n = 12; 60.0%). Twelve unexpected ADRs were reported for the Moderna COVID-19 vaccine®, of which 4 IMEs (33.3%), corresponding to cerebrovascular accident (n = 1; 25.0%), conjunctival haemorrhage (n = 1; 25.0%), death (n = 1; 25.0%) and retinal artery occlusion (n = 1; 25.0%).
Conclusion: A considerable proportion of SRs received by UFC contained unexpected ADRs, among which 11.9% were IMEs. These results highlight the value of post-marketing spontaneous reporting to identify unknown ADrs and to better characterize the safety profiles of newly approved COVID-19 vaccines, as well as their benefit/risk ratios.
References/Further Sources of Information
Petousis-Harris H. Assessing the safety of COVID-19 vaccines: a primer. Drug Saf. 2020;43(12):1205–102.
Mendes D, Rigueiro G, Silva RS, et al. Intensive safety monitoring program of antineoplastic medicines: a pilot study in a Portuguese oncology hospital. J Oncol Pharm Pract. 2020;26(1):133–40.
P019 The Relationship Between the Severity and Mortality of SARS-CoV-2 Infection and Ibuprofen: A Meta-Analysis
T. Oscanoa1, V. Palomino-Serna1, G. Orellana-Nuñez1, J. Pasache-Juarez1, C. Osccorima Gonzales1, A. Carvajal2
1Universidad Nacional Mayor de San Marcos-Facultad de Medicina-Lima-Perú., Pharmacology, Lima, Peru; 2Universidad de Valladolid, Pharmacology, Valladolid, Spain
Background/Introduction: Ibuprofen is a non-steroidal anti-inflammatory drug (NSAID); it is considered as relatively safe and is widely used in the world. However, at the beginning of the COVID-19 pandemic, ibuprofen was associated with an increase in severity or mortality of the infection [1–4].
Objective/Aim: To conduct a meta-analysis of the association between ibuprofen use and SARS-CoV-2 infection severity or mortality.
Methods: We searched PubMed, EMBASE, Google Scholar and the Cochrane Database of Systematic Reviews for observational studies published between January 2020 and May 2021. Studies were included if they contained data on ibuprofen use and SARS-CoV-2 infection severity or mortality. Information upon study design, location, year of publication, number of participants, sex, age at baseline, outcome and exposure definitions was gathered. The quality of studies included was assessed with the Newcastle–Ottawa Scale (NOS). The analysis was performed based on a random-effects model; the summary effect and its confidence interval were calculated.
Results: Eight observational studies comprising a total of 1785.730 participants were identified for inclusion (cohort, 5; case-control, 2; cross-sectional, 1). Mean age was 54.4 (SD 12.6) years-old and 50.2% were men. The mean NOS score of included studies was 7.7 (range 7–9). The studies were from Austria, Denmark (2), Israel, Saudi Arabia, UK (2) and USA. Patients exposed to ibuprofen while infected with SARS-CoV-2 had not higher severity or mortality; summary odds ratios were 0.81 (95% CI 0.58–1.12, p = 0.14) and 0.95 (95% CI 0.79–1.14, p = 0.42), respectively.
Conclusion: At present, the available evidence does not support the hypothesis of an increased SARS-CoV-2 risk associated with ibuprofen. However, more evidence needs to accumulate before this controversy can be resolved.
References/Further Sources of Information
Kragholm K, Gerds TA, Fosbøl E, Andersen MP, Phelps M, Butt JH, et al. Association between prescribed ibuprofen and severe COVID‐19 infection: a nationwide register‐based cohort study. Clin Transl Sci. 2020;13:1103–7. https://doi.org/10.1111/cts.12904.
Rinott E, Kozer E, Shapira Y, Bar-Haim A, Youngster I. Ibuprofen use and clinical outcomes in COVID-19 patients. Clin Microbiol Infect. 2020;26:1259.e5–1259.e7. https://doi.org/10.1016/j.cmi.2020.06.003.
Drake TM, Fairfield CJ, Pius R, Knight SR, Norman L, Girvan M, et al. Non-steroidal anti-inflammatory drug use and outcomes of COVID-19 in the ISARIC Clinical Characterisation Protocol UK cohort: a matched, prospective cohort study. Lancet Rheumatol. 2021;3:e498–506. https://doi.org/10.1016/S2665-9913(21)00104-1.
Sourij H, Aziz F, Bräuer A, Ciardi C, Clodi M, Fasching P, et al. COVID‐19 fatality prediction in people with diabetes and prediabetes using a simple score upon hospital admission. Diabetes Obes Metab. 2020;dom.14256. https://doi.org/10.1111/dom.14256.
P020 Safety Monitoring and Signal Detection for the Novel COVID-19 Vaccines on a Global Scale
A. Rudolph1, H. Taavola1, C. Rausch1, Y. Haasum1, D. L. Doan1, S. Hult1, Q. Y. Yue1
1Uppsala Monitoring Center, Research and Education-Pharmacovigilance Science, Uppsala, Sweden
Background/Introduction: Up to 14 June 2021, VigiBase, the World Health Organization (WHO) global database of individual case safety reports (ICSRs) [1], contained over 26 million reports from more than 140 member countries of the WHO Program for International Drug Monitoring (PIDM). VigiBase is managed by Uppsala Monitoring Center (UMC) on behalf of the WHO.
Since the end of November 2020, 1.1 million ICSRs for COVID-19 vaccines were entered in VigiBase accounting for 41% of all vaccine reports in the database. The large number of reports received in a short time requires semi-automated data handling and signal prioritization exceeding “normal” signal detection operations.
Objective/Aim: To monitor the safety of the COVID-19 vaccines from a global perspective.
Methods: Screenings of VigiBase are performed regularly. Statistically ranked drug-event combination lists that focus on a theme, e.g. low- and middle-income countries, emerging safety signals, or testing new methods are prepared. Multi-disciplinary teams then assess the combinations together. This is typically done during focused multi-day workshops. To complement this, general regular screenings are performed and hints from other sources (e.g., scientific literature, media reports, etc.) are followed.
Several statistical methods are used in the screening efforts. Disproportionality analysis compares the observed number of reports for a drug—event combination to the expected number based on the overall reporting in the database. vigiRank, [2] an algorithm combining five strength-of-evidence parameters into a score, provides a ranking of drug-event combinations. vigiGroup clustering [3] automatically groups reports with similar adverse event profiles in a data driven way. Its purpose is to uncover clinically coherent pictures that might otherwise evade detection. vigiPoint [4] is a tool to quickly explore differences in one set of reports compared to one or more reference sets. This enables exploration of various covariates. Features that are significantly and robustly different are highlighted for review.
Identified preliminary safety signals are subject to weekly prioritization. Points to consider for prioritizing a combination for in-depth assessment include e.g. multinational reporting, the reaction’s seriousness, etc.
Confirmed safety signals are shared with the WHO PIMD member states.
Results: Up to 14 June 2021, ten preliminary safety signals have been identified for in-depth assessment via different signal detection activities. Another 38 preliminary safety signals are being monitored.
Conclusion: VigiBase is the world’s largest database of ICSRs. Therefore, UMC is in a good position to monitor the COVID-19 vaccines’ safety maintaining the global perspective with the potential to find emerging safety signals earlier.
References/Further Sources of Information
Uppsala Monitoring Center. VigiBase. https://www.who-umc.org/vigibase/vigibase/. Accessed 21 June 2021.
Caster O, Juhlin K, Watson S, Norén GN. Improved statistical signal detection in pharmacovigilance by combining multiple strength-of-evidence aspects in vigiRank: retrospective evaluation against emerging safety signals. Drug Saf. 2014;37(8):617–28. https://doi.org/10.1007/s40264-014-0204-5.
Norén GN, Meldau E-L, Chandler RE. Consensus clustering for case series identification and adverse event profiles in pharmacovigilance. Submitted to Artif Intell Med. 2021.
Juhlin K, Star K, Norén GN. A method for data‐driven exploration to pinpoint key features in medical data and facilitate expert review. Pharmacoepidemiol Drug Saf. 2017;26(10):1256–65. https://doi.org/10.1002/pds.4285.
P021 Withdrawn
P022 Serious Adverse Events Following Immunization (SAE) with the Covid 19 Vaccine in Morocco: Pharmacovigilance Centre Database
H. Sefiani MD PhD1, G. Benabdallah1, A. Elrherbi MD1, A. Tebaa MD1, R. Soulaymani Bencheikh Pr1,2
1CAPM-Rabat WHO Collaborating Centre-Rcc, Pharmacovigilance, Rabat, Morocco; 2Faculté de médecine et de pharmacie de Rabat, Pharmacologie, Rabat, Morocco
Background/Introduction: Since the beginning of the national vaccination strategy development, The Moroccan pharmacovigilance centre was involved in the national vaccination campaign to ensure a successful covid 19 AEFIs surveillance.
Objective/Aim: This study is a descriptive analysis of (SAE) with covid 19 vaccines in Morocco during the vaccination campaign 2021
Methods: Data on adverse events following immunisation (AEFI) was collected since January 28, 2021 through the national passive and stimulated surveillance systems. The AEFIs including serious adverse events (SAEs) were classified using the MedDRA terminology. SAE were analyzed according to system organ class, seriousness criterias, patient characteristics, outcome, reporter designation and Covid 19 vaccine brand.
Results: In more than 8 Millions vaccines, 15 187 AEFI were reported (1.92 per 1000 immunizations), 181 cases reported at least one SAE (1.2% of total cases), which represents 0.03 cases per 1000 vaccines. The sex ratio is 1.02. 154 cases were reported with the Astra Zeneca (AZ) vaccine, which represents 1.4% out 10 930 cases AZ reported , and 25 cases with the Sinopharm vaccine, which represents 0.6% of the 3859 cases reported with this vaccine. The seriousness criteria was hospitalization in 62.5% of cases. The cases classified as severe reported an average of 3 AEFIs per case classified in the different MedDRA SOCs, represented mainly by neurological conditions (44.1%) and general disorders (28.9%). This study showed that the AEFI reporting rate after covid 19 vaccination was high. Mild systemic reactions accounted for the majority of reported AEFI, and fatal SAEs were rare
Conclusion: This study showed the importance of an efficient safety monitoring system which can detect and characterize SAE and set up the safety profile of this new covid 19 vaccines.
References/Further Sources of Information
Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet. 2000;356:1255–1259.
VigiBase Data. https://vigilyze.who-umc.org.
P023 Safety of ChAdOx1 nCoV-19 Vaccine: Analysis of Adverse Reactions of COVID-19 AstraZeneca Vaccine Observed in a University Hospital in Morocco
A. Tebaa1, B. Moukafih2, B. Leila3, R. Soulaymani-Bencheikh1, A. El kartouti4, S. Achour5
1Moroccan Anti Poison and Pharmacovigilance Center-Rabat 10000-Morocco; 2Central Pharmacy Department-CHU Hassan II-Fez-Morocco, Pharmacology Laboratory-Faculty of Medicine and Pharmacy of Fez-Sidi Mohammed Ben Abdellah University-Fez-Morocco, Fez, Morocco; 3Santé de travail-CHU Hassan II-Fez-Morocco, Epidemiology and Health Sciences Research Laboratory-Faculty of Medicine and Pharmacy of Fez-Sidi Mohammed Ben Abdellah University-Fez-Morocco, Fez, Morocco; 4Hospital Pharmacy Service-Moulay Ismaïl Military Hospital-Meknès-Morocco, Pharmacology Laboratory-Faculty of Medicine and Pharmacy of Fez-Sidi Mohammed Ben Abdellah University-Fez-Morocco, Fez, Morocco; 5Toxicology Department-CHU Hassan II-Fez-Morocco, Medical Center for Biomedical and Translational Research-Faculty of Medicine and Pharmacy of Fez-Sidi Mohammed Ben Abdellah University-Fez-Morocco, Fez, Morocco
Background/Introduction: Vaccination has played a crucial role in the improvement of global health [1]. The appearance of the strain of COVID-19 severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its impact on global health have made the development of effective and safe vaccines crucial for this new lethal strain [2]. The development of safe, effective, affordable vaccines against COVID-19 remains the cornerstone to mitigating this pandemic. Recent reports of thrombosis following AstraZeneca COVID-19 vaccine in young females (< 55 years-old) led to temporary suspension and urgent investigation by the European Medicines Agency (EMA) that concluded that vaccine benefits still outweigh its side effects (SEs).
Objective/Aim: Therefore, this study aims to provide early independent evidence on the vaccine SEs’ prevalence and their potential risk factors.
Methods: A cross-sectional survey-based study was carried out between February and April 2021 in at the university hospital of Fez in Morocco, for healthcare workers who recently received the AstraZeneca COVID-19 vaccine. The study used a questionnaire composed of sixteen multiple-choice items covering demographic variables, medical anamneses, and local, systemic, oral, and skin related SEs of the vaccine.
Results: Out of the forty included participants, 69.4% were females. Their mean age was 35.37 ± 32.31 (26–62) years-old, 11.7% had chronic illnesses and 17.4% were receiving medical treatments. Overall, 65.6% of the participants reported at least one SE. The most common local SE was injection site pain (61.8%), and the most common systemic SEs were fatigue (42.4%), muscle pain (38.8%), chills (45.1%), feeling unwell (21.3%), nausea (25.4%), and headache (34.5.3%). The vast majority (86.8%) resolved within 1–3 days, 62.1% who received symptomatic treatment, and the below 35 years-old group was the least affected age group. The SEs’ frequency was significantly higher in previously infected participants; Chronic illnesses and medical treatments were not associated with an increased risk of SE incidence and frequency. No blood disorder SEs were reported in our sample.
Conclusion: Further independent studies are highly required to evaluate the safety of the AstraZeneca vaccine and to explore whether gender or previous infection could be associated with the vaccine SEs
References/Further Sources of Information
Greenwood B. The contribution of vaccination to global health: past, present and future. Philos Trans R Soc Lond B Biol Sci. 2014;369:Article 20130433.
Padron-Regalado E. Vaccines for SARS-CoV-2: lessons from other coronavirus strains. Infect Dis Ther. 2020;9:255–74.
P024 Optimizing Signal Detection for Large Scale COVID-19 Vaccination
F. Van Hunsel1, I. Oosterhuis2, E. van Puijenbroek1,3
1Netherlands Pharmacovigilance Centre Lareb, Signal Detection, ‘s Hertogenbosch, The Netherlands; 2Netherlands Pharmacovigilance Centre Lareb, ADR Reporting, ‘s Hertogenbosch, The Netherlands; 3University of Groningen, Groningen Research Institute of Pharmacy-PharmacoTherapy-Epidemiology and Economics, Groningen, The Netherlands
Background/Introduction: Early 2021, the COVID-19 vaccination campaign in the Netherlands started. Four brands (Pfizer, Moderna, AstraZeneca and Janssen), based on two vaccine types (mRNA and viral vector) became available, each with limited real-world data on safety. The vast amount of COVID vaccines administered in a short time span necessitated the development of a dedicated signal detection approach to enable a near real-time signal detection at the national PV centre in the Netherlands (Lareb). Signal detection relied both on the spontaneous reporting system (SRS) as well as an Intensive Monitoring Study (LIM) for COVID vaccines, similar to yearly Influenza vaccine monitoring [1].
Objective/Aim: To describe the methods for vaccine safety monitoring during the COVID pandemic; both for the SRS and LIM.
Methods: We distinguished four different classes of safety signals, for which signal detection relied on either SRS of LIM data and was supported by different methods of (statistical) analysis (Table 1). The main driver of signal detection remains clinical assessment of cases by a team of dedicated vaccine experts at Lareb, assisted by a network of experts working in a clinical setting (Clinical Advisory Board (CAB).
On a daily basis cases of AESI were discussed in a signal detection meeting. When needed further analyses was conducted. The CAB was expanded with experts from vascular medicine, allergology, immunology, among others. A custom made electronic Reaction Monitoring Report (eRMR) for COVID vaccines was analyzed on a weekly basis. For the LIM Cohort multivariable logistic regression analysis were performed. For potential signals Observed-Over-Expected analysis were performed using background rates of the events in the population. Due to linkage with the COVID vaccination Information and Monitoring System (CIMS) of the RIVM a dedicated protocol was in place for near real-time monitoring for batch related problems.
Results: Until 25 May 2021 Lareb received and assessed 60,437 reports on Covid-19 vaccines, of which 1478 were deemed serious according to international criteria. The CAB aided in assessing reports and signals. Observed-Over-Expected analyses were hampered by a lack of complete data of given vaccinations in CIMS. The linkage however allowed for batch-analysis; batch-numbers for 79% of cases in Q1 were known.
Conclusion: During the national COVID vaccination campaign a large number of AEFI were reported that required the development of a variety of methods for the SRS and Intensive Monitoring studies and procedures to allow for timely signal detection. The experiences for signal detection gained may serve as a blueprint for future mass vaccination programmes.
References/Further Sources of Information
van Balveren-Slingerland L, Kant A, Härmark L. Web-based intensive monitoring of adverse events following influenza vaccination in general practice. Vaccine. 2015 May 5;33(19):2283–8.
Scholl J, van Hunsel F, Hilgersom W, van Puijenbroek EP. Vaxbatch: a stratified disproportionality analysis tool for batch-related AEFI monitoring in vaccines. pharmacoepidemiol Drug Saf. 2020;29(supl.3):501–2.
P025 Managing Individual Case Safety Reports (ICSRs) Following COVID-19 Vaccination Campaign
I. Oosterhuis1, L. Harmark1, F. Van Hunsel1, A. Kant1
1Netherlands Pharmacovigilance Centre Lareb, Reporting and Signal Detection, ‘s Hertogenbosch, The Netherlands
Background/Introduction: Early 2021, the COVID-19 vaccination campaign in the Netherlands started. The Netherlands Pharmacovigilance Centre Lareb had to deal with high volumes of ICSRs, to be processed and assessed in a short time span. This necessitated the development of a dedicated system to enable a near real-time vaccine monitoring.
Objective/Aim: To describe the development of an infrastructure which allows processing and assessment of large volumes of ISCRs during the COVID-19 vaccination campaign in the Netherlands.
Methods: A specific COVID vaccine web-based reporting form enabled collecting information on the vaccine administered, Adverse Events Following Immunisation (AEFIs) and other information needed for assessment and signal detection. Conditional questions were developed to collect high quality information on specific AEFIs. Preselected fields were mapped to corresponding ICH e2B(R3) fields in the ICSR management system. Where not known and with permission from the reporter, batch numbers were retrieved from the national vaccination registry (CIMS) maintained by RIVM.
A fully automatic process for reports enabled handling of the majority of common and known reported AEFIs. All other ICSRs were triaged daily. We arranged a “non-priority” team that handled reports on most common AEFIs and reports we selected as “non-priority”; and a ‘high-priority team’, consisting of medical doctors and pharmacists with vaccine-expertise, for assessing more complex and also serious reports. The latter team was also responsible for signal detection.
Results: Up to June 1st 2021, Lareb received 75.840 ICSRs. This is a reporting rate of approximately 1% of the total amounts of vaccines given in the Netherlands. Fully automatic processing was possible for 48.3% of all received ICSRs on daily basis, making these reports directly available for signal detection. After daily triage of all remaining incoming ICSRs, 4.7% is flagged as a ‘high priority’ report. The other 47.0% of the “non-priority” reports could be handled within a few minutes by the assessor. Batch-numbers were added from CIMS in 79% of the retrieval in Q1.
With this way of working, we managed processing all ICSRs for signal detection on a daily basis. 99,99% of the serious ICSRs was compliant with a 15 days’ timeframe for Eudravigilance. The nonserious reports were processed within five weeks (100% compliancy).
Conclusion: A new approach which is highly technical supportive enabled to process about 5.000 reports every week. This process can also be used to handle other vaccine ISCRs in the future.
References/Further Sources of Information
Van Puijenbroek E, Broos N, van Grootheest K. Monitoring adverse events of the vaccination campaign against influenza A (H1N1) in the Netherlands. Drug Saf. 2010 Dec 1;33(12):1097–108.
P026 Cohort Event Monitoring of COVID-19 Vaccine Reactogenicity in the Netherlands Using Patient Reported Outcomes
L. Rolfes1, L. Härmark1, A. Kant1, L. van Balveren1, F. Van Hunsel1
1Netherlands Pharmacovigilance Centre Lareb, Signal Detection, ‘s-Hertogenbosch, The Netherlands
Background/Introduction: The rapid deployment of COVID-19 vaccines has put the safety of these vaccines in the spot light. So far four COVID-19 vaccines are approved in Europe: mRNA vaccines from Pfizer and Moderna and viral vector vaccines from AstraZeneca and Janssen. Common Adverse Events following Immunization (AEFI) for these vaccines include manifestation of the inflammatory response such as pain, redness, swelling and pyrexia. To provide more insight in differences in vaccinated population in terms of age, gender, and medical background in relation to reactogenicity, data from the Dutch COVID-19 vaccine Cohort Event Monitoring were used.
Objective/Aim: To explore factors that are associated with the occurrence of COVID-19 vaccine reactogenicity after the first dose in the Netherlands.
Methods: A web-based prospective cohort design using patient reported outcomes was used [1]. Dutch participant, vaccinated with a COVID-19 vaccine, and registered for this study during February to May 2021 were included. A baseline questionnaire gives information on participant characteristics (age, gender, length, weight), comorbidities, concomitant medication, use of an antipyretic drug several hours before or after vaccination, and a history of experienced COVID-19 disease demonstrated by a positive test. The questionnaire about AEFI was sent 7 days after vaccination. Multivariable logistic regression analysis was used for analysis, with significance level of < 0.05.
Results: Out of 21.822 participants included, 11.542 (52.9%) experienced reactogenicity. Compared to the Pfizer vaccine, the highest odds ratio (OR) for developing reactogenicity was for the AstraZeneca vaccine (OR 4.18) followed by Moderna (OR 1.77), and Janssen (OR 1.74). Participants with a history of COVID-19 disease had a 2.3 increased odds for reactogenicity. Women had a 2.04 increased odds compared to men. Older participants experienced less reactogenicity. Compared to the age group < 50, the ORs for the age groups 50–60, 61–79, and ≥ 80 were 0.51, 0.26, and 0.16 respectively. Comorbidities that were associated with reactogenicity were cardiac disorders (OR 1.15), musculoskeletal disorders (OR 1.34), respiratory disorders (OR 1.20), and infections (1.88). A body mass index of 25.0–29.9 and over 30 was negatively associated with reactogenicity (OR 0.90 and OR 0.71 respectively).
Conclusion: This extensive study with over 21.000 participants demonstrated that, taken into account all factors in the model, the mRNA vaccine from Pfizer gave the least reactogenicity. A person with a history of COVID-19 disease, female sex, younger age, and those with cardiac, musculoskeletal, respiratory disorders or infections had an increased odds for experiencing reactogenicity.
References/Further Sources of Information
van Balveren-Slingerland L, Kant A, Härmark L. Web-based intensive monitoring of adverse events following influenza vaccination in general practice. Vaccine. 2015 May 5;33(19):2283–8.
P027 Spontaneously Reported Adverse Events Following Immunisation with Sputnik V Vaccine in Montenegro
V. Vukicevic1, M. Stankovic1, S. Vujovic1
1Institute for Medicines and Medical Devices, Centre for Medicines Postmarketing Surveillance, Podgorica, Montenegro
Background/Introduction: Sputnik V (Gam-Covid-Vac, Gamaleya Research Institute of Epidemiology and Microbiology) was the first vaccine against Covid-19 available in Montenegro [1]. The vaccine was not registered in Montenegro, but it was imported in line with the Government’s decision on medicines supply during the pandemic. In February and March 2021, 7000 doses of the Gam-Covid-Vac Component I and 7000 doses of the Gam-Covid-Vac Component II were imported.
Objective/Aim: To assess safety profile of the vaccine.
Methods: All Adverse Events Following Immunisation (AEFIs) related to Sputnik V received by CInMED until 15 June 2021 were coded using appropriate Lowest Level Term of the MedDRA (version 24.0) and analysed. Results are presented at MedDRA Preferred Term (PT) level. Follow-up information was requested as needed for the sake of clarification and obtaining data on the outcome. Descriptive statistics were used.
Results: In total, 218 reports describing 746 adverse reactions were received. Majority were reported by physicians (215; 98.6%). One case (0.5%) was reported by a pharmacist, and two by patients (0.9%). Only 7 (3.2%) reports were related to older patients (≥ 65 years), while in one case (0.5%) age was not reported. Four cases were assessed as Important Medical Events; no other serious cases were recorded. Out of the 218 reports, 188 described 636 reactions related to Gam- Covid-Vac Component I (27 reports per 1000 imported doses) and 30 described 110 reactions related to Gam-Covid-Vac Component II (4 reports per 1000 imported doses). Majority of reports concerning both Component I (147; 78.2%) and Component II (23; 76.7%) related to female patients. Most of the 188 reports received for Component I described reactions related to General disorders and administration site conditions (178; 94.7%); Musculoskeletal and connective tissue disorders (84; 44.7%), and Nervous system disorders (68; 36.2%). The most frequently reported reactions for Component I are presented in Table. Similar distribution was noted for Component II. The number of linked cases describing AEFIs reported for the same patient after Component I and Component II was 18.
Conclusion: No signal detected. No safety concerns raised based on spontaneously reported AEFIs. The most frequently reported AEFIs were in line with the available information on the safety profile of the Sputnik V vaccine [2, 3] and similar to those identified with other anti Covid-19 vaccines [4, 5]. Due to small number of doses only frequent AEFIs could be detected.
References/Further Sources of Information
Public Relations Service of the Government of Montenegro. 5,000 Sputnik V vaccines arrive in Montenegro. https://www.gov.me/en/article/5000-sputnik-v-vaccines-arrive-in-montenegro. Accessed 14 June 2021.
Logunov DY, Dolzhikova IV, Zubkova OV, Tukhvatullin AI, Shcheblyakov DV, Dzharullaeva AS, et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet. 2020;396:887–97.
Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397:671–81.
Summary of Product Characteristics for Comirnaty concentrate for dispersion for injection COVID-19 mRNA Vaccine (nucleoside modified). https://www.ema.europa.eu/en/documents/product-information/comirnaty-epar-product-information_en.pdf. Accessed 14 June 2021.
Summary of Product Characteristics for Vaxzevria suspension for injection COVID-19 Vaccine (ChAdOx1-S [recombinant]). https://www.ema.europa.eu/en/documents/product-information/vaxzevria-previously-covid-19-vaccine-astrazeneca-epar-product-information_en.pdf. Accessed 14 June 2021.
P028 Assessment of Drug-Herbal Interaction Project: SFDA Experience
W. Alghamdi1, M. Almagrabi1, N. Alfadel1, F. Alharbi1
1Saudi Food and Drug Authority, Pharmacovigilance Drug Safety and Risk Management, Riyadh, Saudi Arabia
Background/Introduction: Drug interaction is a clinical phenomenon where the therapeutic action could be either exaggerated, diminished or turned into a toxic effect due to the co-administration of another substance. Little is known about the interaction between conventional drugs and herbal medicines. In Saudi Arabia, the use of herbal medicine is highly prevalent, and people believe it is more safe, effective, and cheaper than conventional medicine [1–3]. The Saudi Food and Drug authority (SFDA) established the Drug-Herbal Interaction Project to assess drug-herbal interactions.
Objective/Aim: The aim of this project is to detect safety signals related to drug-herbal interactions and assess these signals based on scientific evidence to ensure safe use of medications.
Methods: A list of SFDA registered herbal products were selected and prioritized based on commonly used herbs in Saudi Arabia. Reported drug-herbal interactions were retrieved from the World Health Organization (WHO) global database of individual case safety reports (VigiBase), AdisInsight and Natural Medicines database. Serious drug-herbal Interaction case reports were gathered for analysis. We excluded the interactions of non-registered interacting drugs and labeled interactions in the drug and herbal label, using the U.S. Food and Drug Administration (FDA) prescribing information (PI), European medicines agency’s (EMA) Summary of Product Characteristics (SmPCs) and European herbal monograph. A systematic literature search for available evidence regarding potential drug-herbal interactions is then performed.
Results: A total of 11 herbal products were evaluated for potential drug interactions, including Turmeric, Ginkgo biloba, Myrrha, Echinacea, Liquorice, Fenugreek, Milk Thistle, Ginseng, Hibiscus, Nigella Sativa, and Senna. The initial screening of safety information yielded 247 potential drug-herbal interactions, and 104 met the inclusion criteria for further analysis. The analysis yielded 16 local label updates for herbal products (n = 6) and drug products (n = 10), where the interaction was listed in FDA PI, EMA SmPCs or European herbal monograph. A total of 28 potential drug-herbal interactions were referred for further comprehensive safety evaluation.
Conclusion: Pharmacovigilance is essential in providing safety information including drug-herbal interaction. The drug-herbal interaction project in SFDA successfully improved screening and identification of potential drug-herbal interactions.
References/Further Sources of Information
Chan K. An overview on safety issues of interactions between traditional herbal medicines and pharmaceutical medicines. Bol Latinoam y del Caribe Plantas Med y Aromat. 2008;7(6):312–31.
Li H, Deng J, Yue Z, Zhang Y, Sun H. Detecting drug-herbal interaction using a spontaneous reporting system database: an example with benzylpenicillin and qingkailing injection. Eur J Clin Pharmacol. 2015;71(9):1139–45.
Alkhamaiseh SI, Aljofan M. Prevalence of use and reported side effects of herbal medicine among adults in Saudi Arabia. Complement Ther Med. 2020;48:102255.
P029 Liquorice Interaction with Thiazide and Thiazide-Like Diuretics and the Potential Risk of Hypokalemia
M. Almaghrabi1, W. Alghamdi1, N. Alfadel1, F. Alharbi1
1Saudi Food and Drug Authority, Drug Safety and Risk Management, Riyadh, Saudi Arabia
Background/Introduction: Liquorice has been used in traditional medicine to treat cough, heartburn, skin lesions, liver diseases, and nowadays in food and beverages [1, 2]. However, consuming large amounts of liquorice may result in mineralocorticoid excess disorder and hypokalemia [3]. Therefore, the concomitant use of liquorice with substances having the same effects, such as thiazide and thiazide-like diuretics, may result in severe complications.
Objective/Aim: This review aims to assess the relatedness of the potential drug-herbal interaction between liquorice and thiazide and thiazide-like diuretics as part of the drug-herbal interaction assessment project initiated by the Saudi Food and Drug Authority.
Methods: This safety review utilized several sources. A systematic literature search was performed in Cochrane, PubMed, and Google Scholar. Also, a search was conducted in Adis Insight, and the Natural Medicines Database. The search included published work from inception to March 2021 for eligible English publications with the following keywords: (Liquorice OR Licorice OR Glycyrrhizam OR Liquiritia officinalis OR Glycyrrhiza glabra) AND (hypokalemia OR decreased potassium OR low potassium level OR potassium decreased) AND (Thiazide or indapamide or hydrochlorothiazide or HCTZ or chlorthalidone or metolazone or methyclothiazide or bendroflumethiazide or polythiazide or hydroflumethiazide). Moreover, a search in the World Health Organization (WHO) database (VigiBase) was conducted on March 2021 to retrieve all reported cases worldwide of potential drug-herbal interaction between liquorice and thiazide diuretics. The assessment was performed and guided by the Strength-of-Recommendation Taxonomy tool. The interaction rate was determined based on level of significance.
Results: We found six published case reports and one randomized clinical trial reported interaction of liquorice with thiazide and thiazide-like diuretics [2, 4–9]. Case-reports were reported with indapamide (n = 1), chlorthalidone (n = 1), bendrofluazide (n = 1), hydrochlorothiazide (n = 2) and unspecified (n = 1). These cases suggest a temporal pharmacodynamics interaction between thiazide and liquorice, resulting in hypokalemia. A randomized clinical trial showed that despite consuming a low dose of liquorice together with hydrochlorothiazide treatment, it was associated with a high risk of hypokalemia in healthy people. Moreover, we found one case of a potential interaction between liquorice and indapamide in VigiBase. The interaction resulted in hypokalemia and resolved upon withdrawal of liquorice and indapamide. The interaction severity was high and caused life-threatening reactions. The interaction rate was moderate and required avoiding the combination. Overall, the findings were consistent among the sources and the likelihood of occurrence was probable.
Conclusion: The available evidence showed a possible pharmacodynamics interaction between liquorice and thiazide and thiazide-like diuretics. Further assessment by well-designed pharmacoepidemiological studies is needed.
References/Further Sources of Information
Marisa R, Assessor D, Calapai G, Delbò M. Assessment Report on Glycyrrhiza Glabra L. and/or Glycyrrhiza Inflata Bat. and/or Glycyrrhiza Uralensis Fisch., Radix. http://www.ema.europa.eu. Accessed 9 Mar 2021.
Hukkanen J, Ukkola O, Savolainen MJ. Effects of low-dose liquorice alone or in combination with hydrochlorothiazide on the plasma potassium in healthy volunteers. Blood Press. 2009;18(4):192–5. https://doi.org/10.1080/08037050903072515.
Mamedov NA, Egamberdieva D. Phytochemical constituents and pharmacological effects of licorice: a review. In: Plant and human health: pharmacology and therapeutic uses, vol. 3. Springer International Publishing. 2019. p. 1–21. https://doi.org/10.1007/978-3-030-04408-4_1.
Heidemann HT, Kreuzfelder E. Hypokalemic rhabdomyolysis with myoglobinuria due to licorice ingestion and diuretic treatment. Klin Wochenschr. 1983;61(6):303–5. https://doi.org/10.1007/BF01497780.
Chataway SJS, Mumford CJ, Ironside JW. Self-induced myopathy. Postgrad Med J. 1997;73(863):593–4. https://doi.org/10.1136/pgmj.73.863.593.
Horwitz H, Woeien VA, Petersen LW, Jimenez-Solem E. Hypokalemia and rhabdomyolysis. J Pharmacol Pharmacother. 2015;6(2):98–9. https://doi.org/10.4103/0976-500X.155488.
Folkersen L, Knudsen NA, Teglbjaerg PS. [Licorice. A basis for precautions one more time!]. Ugeskr Laeger. 1996;158(51):7420–1. PubMed. https://pubmed.ncbi.nlm.nih.gov/9012062/. Accessed 7 Mar 2021.
Brouwers AJ, van der Meulen J. [’Licorice hypertension’ also caused by licorice tea]. Ned Tijdschr Geneeskd. 2001;145(15):744–7. PubMed. https://pubmed.ncbi.nlm.nih.gov/11332259/. Accessed 7 Mar 2021.
Conn JW, Rovner DR, Cohen EL. Licorice-induced pseudoaldosteronism: hypertension, hypokalemia, aldosteronopenia, and suppressed plasma renin activity. JAMA J Am Med Assoc. 1968;205(7):492–6. https://doi.org/10.1001/jama.1968.03140330034006.
P030 Nephrotoxicity of Cytotoxic Drugs
S. El Baraka1, J. M. Ouedraogo1, A. Cherif Chefchaouni1, H. Bechar1, M. J. Belahcen1, Y. Rahali1
1National Institute of Oncology, Pharmacy, Rabat, Morocco
Background/Introduction: A cytotoxic active principle is defined as a substance which destroys abnormally transformed cells.
The nephrotoxicity induced by different oncological therapies can manifest as acute or chronic renal failure, tubular dysfunction, proteinuria or even arterial hypertension.
Objective/Aim: Record and present cytotoxic drugs nephrotoxicities in National Institute of Oncology.
Methods: A retrospective study of pharmacovigilance reports of nephrotoxicity cases declared following chemotherapy at the National Institute of Oncology in Rabat, evaluation of their mechanisms and prevalence according to the treatment protocol.
Results: Disparity of clinical cases and incrimination of 5 main mechanisms of nephrotoxicity of cytotoxic drugs: Hemodynamic impairment, vascular involvement, tubulotoxic involvement, interstitial involvement and precipitation of crystals.
Among the 71 reported cases, predominance of cases of nephrotoxicity or cisplatin was part of the therapeutic protocol.
Conclusion: During chemotherapy treatments, renal function should be carefully monitored during and several months after treatment, by monitoring glomerular filtration and tubular function. Blood pressure, creatinemia and proteinuria, and glomerular filtration rate should be monitored in particular.
Depending on the biological results, the clinical context and the therapeutic protocol followed, a dosage adjustment and therapeutic education for patients should be considered.
References/Further Sources of Information
National Institute of Oncology Database; Moroccan PharmacoVigilance database.
P031 Monitoring of N-Nitrosodimethylamine Levels in the Active Substances and the Finished Products of Valsartan
H. Attjioui1, Y. Brik2, A. Tebaa3, S. Mousannif1, B. Meddah1
1Mohammed V University in Rabat, Faculty of Medicine and Pharmacy, Rabat, Morocco; 2Ministry of Health, Laboratoire National de Contrôle de Médicaments, Rabat, Morocco; 3Ministry of Health, Centre Antipoison et de Pharmacovigilance du Maroc, Rabat, Morocco
Background/Introduction: Since the beginning of the “Valsartan crisis” in July 2018. Many laboratories have developed some test methods to identify and quantify trace amounts of nitrosamines in sartans based on different analytical principles. As recommended by the EDQM/EMA.
Objective/Aim: To demonstrate that the levels of NDMA impurities in the active ingredient batches of valsartan and pharmaceutical products marketed in Morocco are within the limits established by EDQM and accepted by EMA.
Methods: We performed the analytical control of impurities using one of the validated methods described in the OMCL website, more precisely the method proposed by the ANSM using HPLC-UV applicable to the determination of NDMA in pharmaceutical substances and ARA-II pharmaceutical products. Before carrying out the quality controls of these products, we proceeded to verification the method, the parameters determined for the verification of the validated method were: specificity, the limit of detection, the limit of quantification, accuracy, and repeatability.
The test was carried out for the batches of the active ingredients of valsartan and pharmaceutical products of 10 pharmaceutical laboratories on the Moroccan market and is still before their expiry date.
Results: This method obtained a detection limit of 0.07 ppm and a quantification limit of 0.21 ppm for NDMA. The method was shown to be specific (no interference between peaks), accurate (recovery rate obtained is within the range [80–120%], and repeatable (RSD < 6%).
Two drug substances of valsartan out of 10 analyzed and their finished products were found contaminated by NDMA with very high levels. These two raw materials belong to the manufacturer Zeihgiang Huahi. The implications of our findings for the testing of the substances and drug products were discussed.
Conclusion: The method for determining the NDMA content of valsartan is validated and meets all acceptance criteria. It is therefore concluded that the method is suitable for its intended use. The test conducted on the active substances and finished products based on valsartan, is part of a drug quality control approach, the presence of NDMA in the active substances of valsartan of the manufacturer Zhejiang and the associated finished products justify the decision of the regulatory authority regarding the withdrawal of batch of finished products, having Zhejiang as a manufacturer of raw material.
References/Further Sources of Information
Determination of NDMA in valsartan active substances and finished products by HPLC/UV. French National Agency for Medicines and Health Products Safety Laboratory Controls Division. Method reference: 18A0399-02.
P032 Analysis of the Risk for Children to Prescribe Medicines Contraindicated or That are Age Dependent at Children’s Hospital
A. Tebaa1, S. Mousannif2, A. Meftah3, A. Cheikh4, M. Bouatia2
1Moroccan Pharmacovigilance Center, Pharmacovigilance Department, Rabat, Morocco; 2Mohammed V University-Faculty of Medicine and Pharmacy of Rabat, Pharmacy Department-Pediatric Hospital Rabat, Rabat, Morocco; 3Pediatric Hospital Rabat, Pharmacy Department, Rabat, Morocco; 4Cheikh Zaid Hospital, Pharmacy, Rabat, Morocco
Background/Introduction: Purely pediatric forms are not very frequent in therapy, which leads the pediatrician to prescribe either forms reserved for adults with dosages and routes of administration are unsuitable for the child or sometimes even contraindicated.
Objective/Aim: The aim of this study was to analyze the drugs (all galenic forms included) listed in the therapeutic booklet of our pediatric hospital and their prescription for children.
Methods: We analyzed all the drugs listed in the pediatric hospital therapeutic booklet. The relative data for each drug was searched by referring to the summaries of product characteristics (the galenic form of the drug, the pharmacotherapeutic class, the indications, the daily dose used, the route of administration and finally their prescription for children). Four categories have been defined: drugs indicated for children regardless of age and weight, those with age limits, those with weight limits and those which are contraindicated in children and reserved for adults.
Results: 224 medicines are listed in the Therapeutic booklet of our paediatric hospital, 65% are injectable, 19% are oral forms, dietetic products represent 6%, fluids for inhalation constitute 3% and 7% for the other forms.
63% of these medicines are indicated for children regardless of age and weight, 27% of drugs are age dependant and 10% are contraindicated in children and reserved for adults.
The distribution of drugs that are not suitable for children (age dependant and reserved for adults) according to ATC Anatomical Therapeutic Chemical is as follows: 25% belongs to the class L (Antineoplastic and immunomodulating agents) also class J (Antiinfective for systemic use) represents the same percentage of 25%, then class A (Alimentary tract and metabolism) represents 17%, and 10% for class N (Nervous system), 9% belongs to class B (Blood and blood forming organs), followed by class V (Various) with 6%,the other classes (M:Musculo-skeletal system, P:Antiparasitic products, R:Respiratory system, C:Cardiovascular system, H:Systemic hormonal preparations) represent a percentage which varies between 1 to 3%.
Conclusion: In our study the percentage of drugs that are reserved for adults and whose use is contraindicated for children represents 10%. This result should alert the prescriber and the hospital pharmacist to better manage the risk of inappropriate use of drugs for children already weakened by their illnesses.
References/Further Sources of Information
Diop BBF, Cheikh A, Mefetah H, Moujemmi B, Draoui M, Bouatia M. DI-084 The difficulty and risk of prescription medicines in children: analysis of drugs listed in therapeutic booklet for paediatric hospital. Eur J Hosp Pharm. 2017 Mar 1;24(Suppl 1):A151.
Fontan JE, Mille F, Brion F. L’administration des médicaments à l’enfant hospitalisé. Archives de Pédiatrie. 2004 Oct;11(10):1173–84.
P033 Impact of Iron Chelators on Transaminases Levels in Thalassemia Patients
A. Tebaa1, S. Mousannif2, A. Meftah3, A. Cheikh4, M. Bouatia2
1Moroccan Pharmacovigilance Center, Pharmacovigilance Department, Rabat, Morocco; 2Mohammed V University-Faculty of Medicine and Pharmacy of Rabat, Pharmacy Department-Pediatric Hospital Rabat, Rabat, Morocco; 3Pediatric Hospital Rabat, Pharmacy Department, Rabat, Morocco; 4Cheikh Zaid Hospital, Pharmacy, Rabat, Morocco
Background/Introduction: Thalassemic syndromes are genetic disorders, they cause a reduction in the synthesis of globin chains, alpha (alpha-thalassemias), beta (beta-thalassemias) which are very common in the Mediterranean Basin (1), Morocco ranks 10th in Mediterranean Sea region (2). The β-thalassemia are characterized by severe anemia requiring a transfusion and iron chelation therapy (3).
Objective/Aim: The aim of this study is to evaluate and analyze the side effects linked to iron chelators (Deferiprone, Deferasirox, Deferoxamine) on transaminases levels detected in thalassemia patients.
Methods: This is a retrospective, prospective and descriptive study carried out over a period from September 15, 2020 to March 15, 2021, relating to all patients with thalassemia followed in the Hémato-Oncology Department at the Children's Hospital of Rabat, with the exception of patients participating in clinical trials. Epidemiological, clinical, and biological data were collected from the medical records of patients and also by direct questioning of patients during consultations. Data processing was carried out using Excel © software, and statistical analysis using software SPAD.
Results: During the study period, 99 patients were followed in the hematology department. The sex ratio M/F was 1,02. The median age is 16 years with an interquartile [10; 21]. 35.6% of patients had an increase in transaminases levels, the cause of this increase: 46.2% is related to Deferasirox, 30.8% is linked to the combination of Deferasirox and Deferiprone, 19.2% is caused by Deferiprone, finally 3.8% is related to Deferoxamine. The variation in the rate of increase of Aspartate aminotransferase ASAT and Alanine aminotransferase ALAT compared to normal level is summarized in the Table 1. In 7.7% of cases the increase in the level of transaminases appeared within one month while in 92.3% of cases the elevation was observed within more than one month. In 11.5% of cases the increase in the level of transaminases lasted less than a month while in 88.5% of cases the analysis of the level of transaminases is not repeated. The measures taken in response to this increase are: 11.5% of patients stopped Deferasirox, 7.7% of cases stopped Deferiprone, 3.8% stopped Deferoxamine, while in 76.9% of cases no measure has been.
Table 1 The variation in the rate of increase of ASAT and ALAT compared to normal level
| ASAT | |
| 40 < ASAT < 68UI/L | 57.6% |
| 85 < ASAT < 136UI/L | 34.5% |
| ASAT > 374UI/L | 7.6% |
| ALAT | |
| 66 < ASAT < 165UI/L | 53.8% |
| 275 < ASAT < 385UI/L | 7.6% |
| ALAT Normal | 23% |
| ALAT unknown | 15.4% |
Conclusion: It seems essential to ensure a follow-up of thalassemia patients to prevent the occurrence of complications related to chelation treatment.
References/Further Sources of Information
Kontoghiorghes GJ, Kontoghiorghe CN. Efficacy and safety of iron-chelation therapy with deferoxamine, deferiprone, and deferasirox for the treatment of iron-loaded patients with non-transfusion-dependent thalassemia syndromes. Drug Des Devel Ther. 2016 Jan;465.
Zahir H, Chakour M, Mouhib H, Yahyaoui H, Ait Ameur M. Epidemiological, clinico-biological, therapeutic and evolutionary aspects of β-thalassemia in Morocco. Ann Biol Clin (Paris). 2019 Apr;77(2):169–73.
Viprakasit V, Ekwattanakit S. Clinical classification, screening and diagnosis for thalassemia. Hematol Oncol Clin N Am. 2018 Apr;32(2):193–211.
P034 Analysis of Herbal Products and Their Mechanisms for Causing ADRs in the Dutch Pharmacovigilance Database
D. van der Kooi1,2, S. van de Koppel1, H. Woerdenbag2, F. Van Hunsel1
1Netherlands Pharmacovigilance Centre Lareb, Reporting and Signal Detection, ‘s-Hertogenbosch, The Netherlands; 2University of Groningen, Pharmacy Department-Faculty of Science and Engineering, Groningen, The Netherlands
Background/Introduction: Although herbal products are often perceived as generally harmless by consumers, their use can lead to adverse drug reactions (ADRs) and interactions. In addition to registered drugs and vaccines, Pharmacovigilance Centre Lareb is responsible for collecting and analyzing reports on herbal products and informing regulatory stakeholders and the public on potential risks.
Objective/Aim: To analyze cases of herbal products reported to Lareb between 1991 and February 2021 and to investigate the mechanisms of action leading to ADRs.
Methods: Cases of herbal ADRs were collected from the national spontaneous reporting database. Products were either coded with the Dutch Drug Dictionary in case they were registered as a medicinal product, or for non-registered products a separate product module has been developed in which the ingredients, product group and brand name can be coded and searched. Products were categorized according to The Herbal Anatomical Therapeutic Chemical Classification System (HATC) [1]. ADRs were coded with the MedDRA® dictionary. Seriousness of ADRs was coded according to CIOMS criteria. Descriptive statistics in MS Excel were used for analysis.
Results: A total of 789 reports were analyzed which described a total of 823 herbal products labelled as suspect drug and a total of 1727. The products can be divided into four categories (Table 1). The 522 singular herbal products contained a total of 86 different herbal ingredients. Of these products, Monascus purpurea (Red Yeast Rice) (n = 119), Mentha piperita (n = 53), Hypericum perforatum (n = 34), Valeriana officinalis (n = 32) and Plantago ovata (n = 29) were most often reported. Product issues include contaminated, adulterated and counterfeit herbal products (n = 18). The most common product found illegal in herbal products was sibutramine (n = 8). The most commonly reported HATC categories were Alimentary tract and metabolism (n = 183), Nervous system (n = 173), Cardiovascular system (140), Genitourinary system and sex hormones (n = 107) and Respiratory system (n = 76). In total 18 Ayurvedic products and four Traditional Chinese Medicine products were reported.
Conclusion: Analysis of the herbal products found a variety of suspected herbal ingredients, with Red Yeast Rice being most often reported. More than 15% of reports described serious reactions. A small part of the products contained illegal ingredients such as sibutramine.
References/Further Sources of Information
The Herbal Anatomical Therapeutic Chemical Classification System: Uppsala Monitoring Centre. https://www.who-umc.org/whodrug/whodrug-portfolio/whodrug-global/herbal-atc/.
P035 Development and Implementation of Strategic Frameworks for Polypharmacy Management in Healthcare Organizations: A Scoping Review
S. Albalushi1, T. McIntosh2, A. Grant3, D. Stewart4, S. Cunningham2
1Ministry of Health-Oman/Robert Gordon University, UK, Pharmaceutical Care, Muscat, Oman; 2Robert Gordon University, School of Pharmacy and Life Sciences, Aberdeen, UK; 3Robert Gordon University, School of Nursing-Midwifery, Aberdeen, UK; 4Qatar University, College of Pharmacy, Doha, Qatar
Background/Introduction: ‘Polypharmacy management’ (PM) guidelines have been developed using multidisciplinary collaboration and patient involvement [1]. However, challenges and barriers exist to their successful implementation. The WHO recommends theory-based organisational change strategies for improved implementation [2].
Objective/Aim: To identify the current evidence base around the development and implementation of strategic frameworks for implementation of polypharmacy guidelines in healthcare organisations.
Methods: The six-stage of Arksey and O’Malley framework and the PRISMA extension for Scoping Reviews was used. Eligibility criteria were defined as Table 1. Databases included: Medline, IPA, CINAHL and Business Source Complete searched from inception to December 2020. MeSH (or similar) terms were used. First stage involved screening; titles and abstracts, then review of full text articles. A charting tool for the included studies was developed according to the aim and findings were used to collate extracted information. All steps involved independent checks by two of the review team and disagreements mediated by a third member of the team.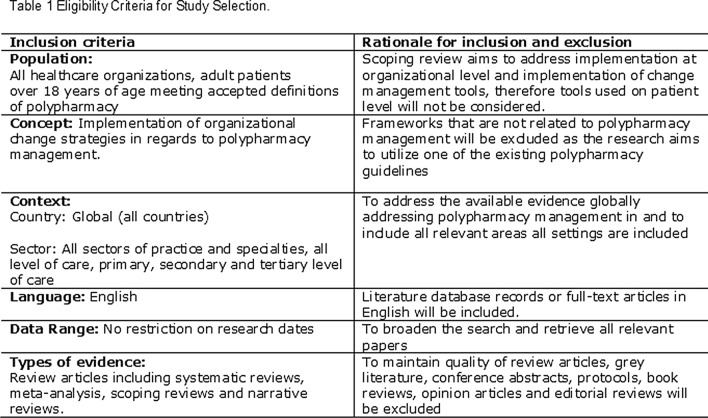
Results: Of the 702 records identified after removal of duplicates, 632 were removed after title/abstract screening and a further 63 removed after full text review. The 7 articles remaining fully met the eligibility criteria. These studies showed despite wide availability of polypharmacy guidelines in the West, particularly the European Union, there is limited evidence on the strategic development and implementation of polypharmacy management frameworks. The main characteristics of strategic approaches used included; Kotter’s eight step process for organizational change, theoretical domains framework to identify individual behavioural determinants, and a community-based medication management intervention. Reported barriers were; lack of data to create a sense of urgency, lack of a national plan for implementation, monitoring and evaluation, poor coordination of care, lack of time for staff, unclear allocation of tasks and responsibilities and lack of training. Organizational level facilitators included; government funding streams and strict regulatory environment, a national emphasis on quality of care for the elderly and presence of local evidence.
Conclusion: It is considered that despite the development of polypharmacy management guidelines there is a need for locally developed and consensus based organizational strategies for implementation of strategic frameworks. Further work is planned with a focus in Oman that will act as a case study to enhance understanding of the best approaches to achieve organizational implementation in the Middle East to impact for better patient care in relation to polypharmacy management.
References/Further Sources of Information
Mair A, Fernandez-Llimos F, Alonso A, Harrison C, Hurding S, Kempen T, Kinnear M, Michael N, McIntosh J, Wilson M, The Simpathy consortium. Polypharmacy Management by 2030: a patient safety challenge, 2nd edn. Coimbra: SIMPATHY Consortium. 2017.
World Health Organization. Medication safety in polypharmacy: technical report. World Health Organization. 2019. https://apps.who.int/iris/handle/10665/325454.
P036 Safety Profile of Cabazitaxel: A French Pharmacovigilance Database Analysis
C. Chinchilla1, M. N. Osmont1, A. Degremont1,2, E. Oger1,2, E. Polard1,2, L. M. Scailteux1,2
1Rennes University Hospital-Rennes-France, Pharmacovigilance-Pharmacoepidemiology and Drug Information Center, Rennes, France; 2Rennes University Hospital-Rennes-France, REPERES-Pharmacoepidemiology and Health Services Research, Rennes, France
Background/Introduction: Cabazitaxel is the last commercialized taxane, used as a second line treatment after docetaxel for metastatic castration-resistant prostate cancer. Market authorization was obtained in France in 2011 and few patients are exposed annually. Safety profile relies on clinical trials and small cohort studies, limiting the detection of rare and delayed adverse events.
Objective/Aim: To describe the real-life profile of adverse drug reactions (ADRs) observed with Cabazitaxel, based on cases registered in the French pharmacovigilance database to identify potential safety signals.
Methods: In this retrospective study, through the French pharmacovigilance database, we reviewed all cases of spontaneously reported ADRs involving Cabazitaxel as suspected or in interaction drug. Eligible cases were included from the marketing authorization date up to March 2021, classified by system organ class and severity. Data were compared with those of the risk management plan and with the literature.
Results: On a 10-year period, 87 cases of ADRs were registered in the French pharmacovigilance database. The mean age was 69, 77 years (SD ± 7,9) [LS1]. As expected, Cabazitaxel was mostly related with hematological toxicity (febrile neutropenia, anemia, thrombocytopenia) in almost 30% of cases (n = 25). Frequently, these ADRs occurred in association with serious and opportunistic infections, in some cases leading to death. We also found reported several cases of gastrointestinal toxicity (severe diarrhea and bleeding), hepatotoxicity, cardiotoxicity (including ischemic heart diseases), nephrotoxicity (haematuria) and pneumopathy. Other less common ADRs implicated were renal, ophthalmological, vascular and neurologic. Overall, 20 Cabazitaxel-related deaths were reported, mainly due to sepsis/infections; other causes of death included liver failure, multivisceral failure or gastrointestinal bleeding.
Conclusion: These results revealed known ADRs allowing to confirm an expected and monitored risk profile in the risk management plan for Cabazitaxel. Based on the analyzed data, no safety signals were highlighted.
References/Further Sources of Information
Corn PG, Agarwal N, Araujo JC, Sonpavde G. Taxane-based combination therapies for metastatic prostate cancer. European Urology Focus. May 2019;5(3):369–80.
Cornford P, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer. Part II-2020 update: treatment of relapsing and metastatic prostate cancer. Eur Urol. Feb 2021;79(2):263–82.
P037 Knowledge and Attitude About Ecopharmacovigilance Among Pharmacy Students in the University of the Basque Country (Spain). A Pilot Study
S. Domingo-Echaburu1, U. Lertxundi2, G. Orive3,4, U. Alzola-Elizondo5, Á. Sánchez-Pérez6,7
1Alto Deba Integrated Health Care Organization, Pharmacy Service, Arrasate-Mondragón, Spain; 2Bioaraba Health Research Institute-Osakidetza Basque Health Service-Araba Mental Health Network-Araba Psychiatric Hospital, Pharmacy Service, Vitoria-Gasteiz, Spain; 3School of Pharmacy-University of the Basque Country UPV/EHU, NanoBioCel Group-Laboratory of Pharmaceutics, Vitoria-Gasteiz, Spain; 4Bioaraba Health Research Institute, NanoBioCel Research Group, Vitoria-Gasteiz, Spain; 5BioCruces Health Research Institute, Primary Care Research Area of Bizkaia, Bilbao, Spain; 6BioCruces Health Research Institute, Dissemination and Implementation Sciences Research Group in Health Services, Bilbao, Spain; 7Subdirectorate of Healthcare, Primary Care Research Unit of Bizkaia, Bilbao, Spain
Background/Introduction: Drug pollution is an issue of growing concern [1]. However, healthcare professionals are likely unaware of the deleterious impacts on pharmaceuticals in the environment. Schools of Medicine, Pharmacy, and Nursing around the world generally overlook drug’s fate once excreted by patients [2]. One of the solutions proposed by the European Commission to deal with drug pollution is to increase awareness and education among health-care professionals [1]. However, only few studies are published including either physicians [3] and pharmacists [4]. As far as we are concerned, there are no studies in pharmacy students.
Objective/Aim: To evaluate the knowledge and attitude about ecopharmacovigilance among pharmacy students in the University of the Basque Country (located in Vitoria-Gasteiz, at the north of Spain).
Methods: We performed a pilot study, based on a self-administered on-line questionnaire with students from the last academic year of the pharmacy degree. The questionnaire consisted of 13 questions about knowledge, 14 about attitude and 3 other. The attitude scale was an agreement scale (being 0 “totally disagree” and 10 “totally agree”). The scale for knowledge questions was variable. Descriptive statistics were undertaken. The attitude scale will be further validated and be used for a larger study with students of all academic years. The study was approved by the Ethics Committee of the University of the Basque Country. The study was founded by Araba Mental Health Network and Fundación Vital.
Results: As the questionnaire was still opened by the 25th of June, preliminary data are shown. The questionnaire was sent to 186 students, 70 (37.6%) answered (71.4% women, mean age 23). 58 students (82.9%) did not know the concept “emerging pollutants”, and 52 participants (74.3%), had not heard about “One Health” concept. Only 7 participants (10%) knew about the Environmental Risk Assessment reports of the European Medicine Agency. The majority of students (n = 60; 85.7%) responded “do not to know/no answer” to the question about the most famous ecotoxicological disaster in Asian vultures caused by veterinary diclofenac. The median punctuation given to the education on the presence and effects of drugs in the environment in the pharmacy degree was 2.7 (± 2.3). The median degree of agreement with the following item from the attitude scale “the impact of pharmaceuticals in the environment is an important issue in Pharmacy” was 8.8 (± 1.4).
Conclusion: Preliminary data of this study show knowledge on ecopharmacovigilance is low among pharmacy students, but their attitude towards ecopharmacovigilance is positive.
References/Further Sources of Information
https://ec.europa.eu/environment/water/water-dangersub/pdf/strategic_approach_pharmaceuticals_env.PDF. Accessed 25 June 2021.
Lertxundi U, Domingo-Echaburu S, Orive G. It’s about time healthcare professionals and academics start thinking about drug pollution. Sustain Chem Pharm. 2020;16:100278.
Wang J, Li S, He B. Chinese physicians’ attitudes toward eco-directed sustainable prescribing from the perspective of ecopharmacovigilance: a cross-sectional study. BMJ Open. 2020;10:e035502.
Liu J, Wang J, Hu XM. Knowledge, perceptions, and practice of ecopharmacovigilance among pharmacy professionals in China. Environ Monit Assess. 2017;189(11):552.
P038 Prevention of GI Adverse Events in People with Epilepsy: Judging Lactose Intolerance with Anti-Epileptic Medications
H. Gandhi1
1Cadila Healthcare Ltd Zydus, Medical Affairs, Ahmedabad, India
Background/Introduction: Lactose intolerance is exceedingly common with global prevalence ranging from 50–80% in Hispanic population up to 100% in Asians [1–2]. Ingestion of pharmaceuticals containing lactose, particularly anti-epileptic drugs, not only increases the frequency of GI adverse events related to lactose intolerance (like abdominal pain, flatulence and diarrhea) but also compromises the efficacy and quality of life expected with such pharmacological treatments [3–5].
Objective/Aim: Several anti-epileptic drugs available in the pharmacies contain unspecified amounts of lactose. Objective of this study was to identify lactose-containing anti-epileptic medications so as to provide insights towards selection of the right anti-epileptic medications for PWE (people with epilepsy) and thus reduce GI adverse events associated with lactose intolerance.
Methods: This study identified the presence of lactose in the formulation of oral anti-epileptic drugs (reference and generic) based on the excipient list included in the summary of product characteristics (SmPC) approved in the EU [6]. Anti-epileptic drugs containing lactose were also mapped for the quantity of lactose in the formulation.
Results: We evaluated 9 anti-epileptic drugs for presence of lactose. Overall, four reference anti-epileptic drugs (including two newer anti-epileptics, perampanel and brivaracetam) contained lactose. Principles regarding the SmPC information for a generic require that the content of the generic’s summary of product characteristics (SmPC) should be in all relevant aspects consistent with that of the reference medicinal product. This does not account for differences in formulation especially when using different excipients. This study found at least 3 generic anti-epileptic drugs (levetiracetam, carbamazepine and oxcarbazepine) having lactose for which the reference products did not have lactose. However, no difference in the reported adverse events has been captured except for the mandatory inclusion of a statement of lactose intolerance in the SmPC. All these product (references or generic) contained lactose in the range 4–377 mg per tablet.
Conclusion: Anti-epileptic drugs frequently contain lactose in the formulation which might increase the frequency of GI adverse events. The cumulative risk of GI adverse events will increase if patients are prescribed more than one anti-epileptic drug containing lactose. The prescribing doctor may not recognize this since local regulations in most countries do not require listing on inactive excipients on the primary packaging.
References/Further Sources of Information
Mill et al. Managing acute pain in patients who report lactose intolerance: the safety of an old excipient re-examined. Ther Adv Drug Saf. 2018;9(5):227–35.
Dewiasty et al. Prevalence of lactose intolerance and nutrients intake in an older population regarded as lactase non-persistent. Clin Nutr ESPEN. 2021;43:317–21.
Eadala et al. Quantifying the 'hidden' lactose in drugs used for the treatment of gastrointestinal conditions. Aliment Pharmacol Ther. 2009;29(6):677–87.
Casellas et al. Perception of lactose intolerance impairs health-related quality of life. Eur J Clin Nutr. 2016;70:1068–72.
Yaman et al. Epileptic seizures associated with lactose intolerance in a child: a causal relationship? J Pediatr Neurol. 2012;10(2):151–4.
Electronic Medicine Compendium. https://www.medicines.org.uk/emc/. Accessed 11 June 2021.
P039 Dynamic Approach to Pharmacovigilance in the COVID-19 Era
N. Kapil1, M. Richardson1, D. Seekins1, J. Jokinen1, C. Kratt1, M. Harrison1, B. Dreyfus1, V. Lalchandani1, C. Bond1
1Bristol Myers Squibb, WorldWide Patient Safety, Princeton, USA
Background/Introduction: The clinical presentation and natural history of infection with the novel SARS-CoV-2 virus are relatively unknown, as are the risks associated with the infection and use of pharmaceutical agents.
Objective/Aim: Adapt existing pharmacovigilance capabilities to ensure appropriate evaluation of the benefit-risk profiles of specific therapies during the ongoing pandemic.
Methods: Bristol Myers Squibb (BMS) Epidemiology have created a tool to dynamically estimate background COVID-19 incidence rates by country, time-period, demographic categories, standards of care, and reporting practices. Local data were updated through the course of the pandemic with background rates re-estimated at regular intervals. Cases, severe cases, and deaths per 100,000 were rescaled to the age and geographic distribution of treated patients (in clinical programs or real-world use). Drug-specific Safety Management Teams used these background rates to identify potential COVID-19-related safety signals in investigational and marketed drugs. Real-world data sources were utilized to understand testing and treatment patterns.
The BMS Clinical Safety Program (CSP), a systematic data collection capability, reduces reporting bias and further enhances our understanding of adverse events of interest through the development and implementation of event-specific, targeted case report forms (CRFs) within BMS interventional clinical trials. The COVID-19 CSP CRFs collect patient-level COVID-19 data (i.e., risk factors, diagnosis, clinical presentation, progression, treatment, and outcomes).
Results: To date, we have not identified signals of increased risk related to COVID-19 infection that warrant additional action for any of our treatments. TriNetX COVID-19 Rapid Response Network data (February 2021) showed that among 250,000 patients with cancer, positivity rates were similar across immunotherapy (2.5%), chemotherapy (2.7%), and hormone therapy (2.5%). Explorys data showed a shift in median nivolumab treatment frequency from Q2W to Q3W among 1,114 patients with negative COVID-19 tests and to Q4W among 129 COVID-19-positive patients continuing treatment.
Conclusion: Individual case level findings and background incidence rates are mutually informative; remarkable or unexpected insights from one may prompt further analysis and validation from the other. Leveraging these complementary pharmacovigilance capabilities in parallel continues to provide a flexible and innovative means to identify potential safety signals, both quantitatively (epidemiology) and qualitatively (CSP), as the conditions of the pandemic evolve. The latest CSP adaptation has included incorporation of CRF data points focused on COVID-19 vaccination and its impact. Once sufficient CSP data are available, analysis will help us understand the impact of COVID-19 infections on the efficacy and safety of our investigational therapies and allow comparisons to real-world data.
References/Further Sources of Information
None.
P040 Impact of Tramadol Restriction Use in Iraq Health Sectors on Adverse Events Reports
S. Mohammed1, W. W. Mustafa1, M. Younis2
1AlRafidain University College, Faculty of Pharmacy, Baghdad, Iraq; 2Ministry of Health, Iraqi Pharmacovigilance Center, Baghdad, Iraq
Background/Introduction: Tramadol's analgesic power is 10 times less than morphine but is more likely to be safe than it. Iraqi Pharmacovigilance center received 184 adverse events reports related to tramadol intake. In this study, many reported were surveyed to assess the prevalence of these events in Iraqi patients across seven years of gathering reports and comparing them with global reports.
Objective/Aim: In this study, many reported were surveyed to assess the prevalence of these events in Iraqi patients across seven years of gathering reports and comparing them with global reports.
Methods: Descriptive assessment of Tramadol and its possible disorders during its use by searching in VigiBase data and their related Individual case safety report which is officially supported by WHO global database. All reported analyzed using Vigilyze data mining and comparing IC25 (which is used to evaluate strength of association between drug and ADR) between Iraq and worldwide records.
Results: A total of 184 cases were surveyed over 7 years of Iraqi Pharmacovigilance Center data collection for patients that use tramadol in different dosage forms. Of these, 32 cases have Hyperhidrosis and all of them appeared when tramadol used as monotherapy, 47 patients with vomiting and 67 with nausea. Some adverse effects appeared highly in Iraqi records comparing to that in globally recorded cases like Chest discomfort, Hyperhidrosis, Headache, Dyspnea and constipation. While other effects appeared lower than that globally reported like vomiting, hallucination, vertigo, respiratory depression and chills. No death appeared in all cases.
Conclusion: Due to the restriction for tramadol use by Ministry of Health and dispensing it in governmental sector in governmental hospitals only and under medical supervision, this lead to decrease the number of reported adverse effects and decrease the percentage of serious adverse events.
References/Further Sources of Information
WHO. Update Review Report on Tramadol. 36th ECDD. 2014.
Kenzie LP, Donald RJ, Margaret T. Abuse potential and pharmacological comparison of tramadol and morphine. Drug Alcohol Depend. 1991;27:7–17.
Hennies HH, Friderichs E, Schneider J. Receptor binding, analgesic and antitussive potency of tramadol and other selected opioids. Arzneim Forsch J Drug Res. 1988;38:877–80.
Paar WD, Frankus P, Dengler HJ. The metabolism of tramadol by human liver microsomes. Clin Investig. 1992;70:708–10.
Black PA, Cox SK, Macek M, Tieber A, Junge RE. Pharmacokinetics of tramadol hydrochloride and its metabolite O-desmethyltramadol in peafowl (Pavo cristatus). J Zoo Wildl Med. 2010;4:671–6.
Matthiesen T, Wohrmann T, Coogan TP, Uragg H. The experimental toxicology of tramadol: an overview. Toxicol Lett. 1998;95:63–71.
Dhillon S. Tramadol/paracetamol fixed-dose combination: a review of its use in the management of moderate to severe pain. Clin Drug Investig. 2010;30:711–38.
Kallen B, Reis M. Use of tramadol in early pregnancy and congenital malformation risk. Reprod Toxicol. 2015;58:246–51.
Muna Subedi, Shalini Bajaj, Maushmi S. Kumar, Mayur YC. An overview of tramadol and its usage in pain management and future perspective. Biomed Pharmacother. 2019;11:443–51.
Product Information. Ultram (tramadol). McNeil Pharmaceutical, Raritan, NJ. 2018.
P041 Adverse Effects of Hormonal Contraceptives Use in Teenage Girls: Systematic Review and Meta-Analysis
A. Rashed1, M. AbouDaya2, S. Tomlin3
1King’s College London, Institute of Pharmaceutical Science, London, UK; 2Barts Health NHS Trust, Pharmacy, London, UK; 3Great Ormond Street Hospital for Children NHS Foundation Trust, Pharmacy, London, UK
Background/Introduction: There is increased use of Hormonal Contraceptives (HCs) in female adolescents; during a period of growth, development, and hormonal changes, in western countries [1–3]. However, further investigation into long-term side effects is needed to better inform clinical guidelines on the use of HCs in teenage girls.
Objective/Aim: To summarise existing evidence on the possible adverse effects of HCs use in teenage girls.
Methods: A systematic and meta-analysis (PROSPERO registration number CRD42018117442) was conducted using the following data sources: Medline, EMBASE, CINAHL, BNI, and Cochrane Central Register of Controlled Trials. A literature search for all studies published in the English language between 2000–2019, reporting side effects of HCs in young females, 19 years of age or under was conducted. Two reviewers checked the quality of the studies and independently extracted data. Meta-analyses were performed, where possible, using the random-effects model.
Results: In total 52 studies were included. Meta-analyses were conducted on 15 of these studies, with a total of 6453 participants. HCs were shown to be associated with reduced bone development (spinal BMD mean difference − 0.39, 95% CI − 0.58 to − 0.20, P < 0.0001; femoral neck BMD mean difference − 0.25, 95% CI − 0.41 to − 0.09, P = 0.002; hip BMD mean difference − 0.34, 95% CI − 0.67 to 0.00, P = 0.05) and altered bleeding patterns (OR 3.17; 95% CI 1.31 to 7.64; P = 0.01) in female adolescents. The qualitative analysis further demonstrated the association of HCs with increased risk of breast cancer, depressive symptoms, and mood changes in young females.
Conclusion: Hormonal contraceptives were associated with significant adverse effects in female adolescents. With a rise in mental health illnesses in adolescents globally [3], the association of HCs use (exogenous hormones) with depression raises concerns and indicates that current practice may lead to a negative public health impact, particularly for teenage girls aged 12–15 year, who are still at a vulnerable age of hormonal and skeletal development.
References/Further Sources of Information
Rashed AN, Hsia Y, Wilton L, Ziller M, Kostev K, Tomlin S. Trends and patterns of hormonal contraceptive prescribing for adolescents in primary care in the UK. J Fam Plann Reprod Health Care. 2015; 41:216–22.
Ziller M, Rashed AN, Ziller V, Kostev K. The prescribing of contraceptives for adolescents in German gynecologic practices in 2007 and 2011: a retrospective database analysis. J Pediatr Adolesc Gynecol. 2013; 26:261–4.
Ott MA, Sucato GS, Committee on Adolescence. Contraception for adolescents. Pediatrics. 2014;134:1257–81.
Kessler RC, Angermeyer M, Anthony JC, Graaf RD, Demyttenaere K, Gasquet I, et al. Lifetime prevalence and age-of-onset distributions of mental disorders in the World Health Organization’s World Mental Health Survey Initiative. World Psychiatry. 2007;6:168–76.
P042 Infectious Risk After Ruxolitinib Exposure: French Pharmacovigilance Data
B. Azzouz1, S. Pinel2, G. Dorguin1, S. Martin1, A. Morel1, T. Trenque1
1Reims University Hospital, Regional Centre of Pharmacovigilance and Pharmacoepidemiology, Reims, France; 2Fernand-Widal Hospital, Regional Pharmacovigilance Center, Paris, France
Background/Introduction: Ruxolitinib is an oral inhibitor of Janus Kinases (JAK) JAK1 and JAK2, which is approved for myelofibrosis and polycythemia vera management. Ruxolitinib exerts immunosuppressive activity that may increase the risk of infectious complications (1).
Objective/Aim: We aimed to describe the infectious profile in patients treated with ruxolitinib.
Methods: We analyzed all spontaneous reports of infections and infestations with ruxolitinib recorded in the French Pharmacovigilance Database (FPVD) from 01/01/1985 to 14/04/2021.
Results: Fifty-six notifications were registered in the FPVD during the study period. Mean age was 68.5 ± 10 years (40–89 years), 33 men and 23 women. Ninety-five percent of notifications were serious (n = 53), mainly due to hospitalization or prolongation of existing hospitalization (n = 30). The most reported infections were: herpes viral infections n = 13 with herpes zoster n = 9 and Kaposi’s sarcoma n = 2, tuberculous infections n = 8, atypical mycobacterial infections n = 6, pneumocystis infections n = 3, septic shock n = 3, aspergillus infections n = 2, progressive multifocal leukoencephalopathy n = 2, pneumonia n = 2 and ocular toxoplasmosis n = 2. Mean time to onset was 166 days (1–1177 days) but was mainly less than 1 month. The action taken with ruxolitinib: drug discontinuation n = 19, decreased dose n = 10, unchanged dose n = 25, unknown n = 2. After drug withdrawal or dose reduction the outcome was: favorable n = 12, fatal n = 10, not resolved n = 5 and unknown n = 2. Twelve deaths were reported, three due to septic shock.
Conclusion: Physicians must be sensitized to the infectious risk with ruxolitinib. Anti-infectious prophylaxis should be proposed to patients at risk. Increased monitoring for suggestive signs or symptoms of infection is recommended, especially during the first month of exposure.
References/Further Sources of Information
Lussana F, Cattaneo M, Rambaldi A, Squizzato A. Ruxolitinib-associated infections: a systematic review and meta-analysis. Am J Hematol. 2018;93(3):339–47.
P043 Atorvastatin and Memory Impairment: Analysis of French Pharmacovigilance Data
E. Lepoix1, S. Martin1, B. Laoulaou1, A. Morel1, T. Trenque1, B. Azzouz1
1Reims University Hospital, Regional Centre of Pharmacovigilance and Pharmacoepidemiology, Reims, France
Background/Introduction: Statins are the first line treatment in isolated hypercholesterolemia, simvastatin and atorvastatin are recommended with a better cost-efficiency. Well-known adverse drug reactions (ADRs) of statins are muscular pain and rhabdomyolysis. Some published studies, based on drug safety databases, raise memory impairment risk after statin exposure [1, 2]. The Summary of Product characteristics of atorvastatin lists memory impairment among potential ADRs, frequency of occurrence ranges from 1/1000 to 1/100.
Objective/Aim: To determine whether atorvastatin exposure is a risk factor of memory impairment.
Methods: We analyzed spontaneous reports of memory impairment with atorvastatin recorded in the French Pharmacovigilance Database (FPVD) from 1st January 1985 to 31th December 2020. We performed a broad search using the following MedDRA High Level Terms: “Memory loss (excl. dementia)” and “Amnestic symptoms”. For each report, the following data were extracted: gender, age, seriousness, outcomes, time to onset and action taken with the suspected drug. The association between memory impairment and atorvastatin exposure was assessed using the case/non-case method.
Results: During the study period, 871,273 spontaneous reports were registered in the FPVD; 18 were memory impairment after atorvastatin exposure. Mean age was 58.6 ± 12.8 years old (range 40–86 years old), sex ratio was 1.6 (11 males, 7 females). In 67% of reports (n = 12), atorvastatin was the only suspected drug. Mean time to onset was 1447 days (range 1–7300 days). Atorvastatin was discontinued in 14 reports. The outcome after atorvastatin withdrawal was favourable in 12 reports. A positive rechallenge was performed in one case. The switch of atorvastatin by simvastatin led to symptoms recurrence. The reporting odds ratio was 1.57 (95% CI 0.99–2.50).
Conclusion: Atorvastatin can induce memory impairment, the outcome is favourable after its discontinuation. This risk is unknown to the medical community. Memory disorders can be caused by multiple ethiologies. Physicians should bear in mind the iatrogenic factor, especially atorvastatin exposure. The case/non-case using the FPVD did not support the hypothesis that atorvastatin is a potential risk factor of memory impairment. A study in a larger database, as the WHO pharmacovigilance database, is mandatory.
References/Further Sources of Information
Wagstaff R, Mitton MW, Arvik BM, et al. Statin-associated memory loss: analysis of 60 case reports and review of the literature. Pharmacotherapy. 2003;23(7):871–80.
King DS, Wilburn AJ, Wofford MR, et al. Cognitive impairment associated with atorvastatin and simvastatin. Pharmacotherapy. 2003;23(12):1663–7.
P044 Beta Blockers Overdose, Characteristics and Outcome: Analysis of French Pharmacovigilance Database
M. Jung1, B. Azzouz1, A. Morel1, S. Martin1, T. Trenque1
1CHU Reims, Pharmacovigilance Pharmacoepidemiology, Reims, France
Background/Introduction: Beta-blocker overdose is potentially life threatening due to the strong blood pressure-lowering and heart rate-lowering effects.
Objective/Aim: The objective of this study was to analyze the reports of overdose intentional or accidental for six beta-blockers commonly prescribed from a database of spontaneous reports.
Methods: The study was conducted using the French National Pharmacovigilance database (FPVD).
From 1985 until 31st December 2020, all reports coded with the preferred terms (PT) “overdose”, “accidental overdose” and “intentional overdose” associated with the following suspected drugs; beta blockers (acebutolol, atenolol, bisoprolol, nebivolol, propranolol and sotalol); were included in the study.
Results: There were 593 case reports referenced in the FPVD, 27 cases were excluded.
Most cases are an intentional overdose (n = 452). An accidental overdose was observed in six children under five years old, two children were hospitalized in intensive unit. The number of cases for each molecule is: propranolol: n = 221; bisoprolol: n = 168; atenolol: n = 89; acebutolol: n = 47; nebivolol: n = 43; sotalol: n = 25. The plasmatic concentration of the drug was determined in 228 cases and correlated with seriousness. The mean percentage of death is 6.2% of which a percentage of 8.2 for propranolol.
Conclusion: This study highlights the high proportion of accidental overdose in beta blockers overdose. In beta-blocker overdose, the mortality is low with adequate care. Determination of plasmatic concentration is still poorly carried and remains to be developed.
References/Further Sources of Information
No references.
P045 Non Icteric Cholestasis and Cytolytic Hepatitis: Temocillin-Related?
M. Dufosse 11, P. Boivin2, F. Adrien3, V. Gras1, K. Masmoudi1
1CHU Amiens-Picardie, Centre Régional de pharmacovigilance, Amiens, France; 2CHU Amiens-Picardie, Service de Réanimation cardio-thoracique, Amiens, France; 3CHU Amiens-Picardie, Pharmacie à Usage Intérieur, Amiens, France
Background/Introduction: Temocillin, a forgotten antibacterial agent has been revived for its activity against Extended-Spectrum Beta-Lactamases (ESBL) and AmpC-producing Enterobacteriaceae [1]. To date, known adverse effects of temocillin are diarrhoea, urticarial or erythematous rash, fever, arthralgia, myalgia, angioedema, anaphylaxis, phlebitis, thrombophlebitis and neurological disorders with convulsions in patients suffering from renal failure [2].
Objective/Aim: We here report the case of a patient presenting with a suspected temocillin-induced acute cytolytic hepatitis.
Methods: A 37-years-old male patient has been admitted in Intensive Care Unit (ICU) for coma, in a context of sepsis. His medical history includes an acute lymphoblastic leukaemia, treated by bone marrow transplant in 2006, causing a subcutaneous graft versus host disease. He had neither history of alcoholism nor any known underlying hepatic disease. He has been later diagnosed with pneumonia due to an ESBL Escherichia coli. His treatment consisted in cefotaxime and metronidazole during 6 days, then tigecycline and trimethoprim sulfamethoxazole during 14 days when a Streptomonas maltophilia was discovered in his broncho-alveolar lavage liquid. Finally, IV temocillin high dosage (6 g daily) was implemented. Before this treatment, his hepatic enzymes levels were normal, except for GGT level which was slightly above normal values (Table 1).
Results: On day 5 of temocillin treatment, a routine biological check showed a severe non-icteric cytolytic hepatitis and a cholestasis with ASAT and ALAT, 22 times, GGT 46 times and ALP 88 times upper normal values (Table 1) without any clinical manifestation, as total bilirubin levels were normal.
On day 7, temocillin is interrupted and replaced by ciprofloxacin. Hemodynamic, ischemic, infectious or auto-immune causes were excluded.
Viral assessments and abdominal ultrasonography were also negative. A hepatic biopsy was performed, which aspect was compatible with a drug-induced hepatotoxicity.
Two days after temocillin interruption, hepatic enzymes levels decreased dramatically (Table 1). A follow-up biological assessment performed 76 days after temocillin interruption found hepatic enzymes levels even lower.
Conclusion: Beta lactams may be associated with idiosyncratic drug-induced liver injury [3] but a literature review found no similar case of such liver toxicity associated with temocillin.
Our clinical report suggests that temocillin have a role in inducing hepatotoxicology, in view of the adverse drug reaction chronology and its rapid regression after temocillin withdrawal. However, our patient was treated with tramadol, which is well known for hepatic toxicity, for weeks before enzymes levels elevation.
Although this observation corresponds with temocillin pharmacokinetics (elimination half-life is approximately 5 h, and renal clearance takes about 24 h [4]), we can’t exclude tramadol’s role.
References/Further Sources of Information
Balakrishnan I, Awad-El-Kariem FM, Aali A, Kumari P, Mulla R, Tan B, et al. Temocillin use in England: clinical and microbiological efficacies in infections caused by extended-spectrum and/or derepressed AmpC β-lactamase-producing Enterobacteriaceae. J Antimicrob Chemother. 2011;66:2628–31. https://doi.org/10.1093/jac/dkr317.
Negaban 1 g, powder for solution for injection/infusion—Summary of Product Characteristics (SmPC)—(eMC) n.d. https://www.medicines.org.uk/emc/product/466/smpc. Accessed 26 July 2018.
Fontana RJ, Shakil AO, Greenson JK, Boyd I, Lee WM. Acute liver failure due to amoxicillin and amoxicillin/clavulanate. Dig Dis Sci. 2005;50:1785–90. https://doi.org/10.1007/s10620-005-2938-5.
Alexandre K, Fantin B. Pharmacokinetics and Pharmacodynamics of Temocillin. Clin Pharmacokinet. 2018;57:287–96. https://doi.org/10.1007/s40262-017-0584-7.
P046 Adverse Drug Reaction Rates in an Acute Hospital
C. Anton1, Z. Al-hilali2, K. Badyal1, R. Ferner1
1West Midlands Centre for Adverse Drug Reactions, Pharmacy, Birmingham, UK; 2University of Birmingham, School of Pharmacy, Birmingham, UK
Background/Introduction: Adverse drug reactions (ADRs) that are detected in a hospital setting (1, 2) are under-reported. Regulators encourage reports of adverse reactions sufficiently severe to cause or prolong hospital admission (3). Our hospital has recently introduced a new electronic patient record that allows electronic prescribing and medication administration.
Objective/Aim: To estimate the proportion of ADRs coded in the electronic patient record in a UK hospital trust caring for acutely ill patients, but not reported to the regulator.
Methods: Sandwell and West Birmingham Hospitals Trust is a 600 bedded acute trust in the UK. An electronic prescribing system (Cerner, North Kansas City, MO, USA) was introduced in September 2019. During the nine months January–September 2020, we identified occurrences in the electronic prescribing system where drug therapy had been stopped, and the reason given was “ADR” or “Drug allergy”. We also identified the adverse drug reactions submitted to the MHRA’s Yellow Card scheme from the hospital trust during the same period.
Results: We identified 220 instances where the prescriber had stopped a drug and cited the reason as ADR (n = 112) or drug allergy (n = 108). During the same period, 61 Yellow Card reports were sent from the trust. The demographics of the patients, and commonly listed drugs, for the two groups, are listed in the table.
Yellow Card reports are anonymised but contain sufficient demographic detail (patient age, sex) and details of the drugs prescribed to enable the two data sets to be compared. There were no reports in common indicating an under-reporting rate of 100%.
Conclusion: The result was initially surprising. The electronic prescribing system was new to the hospital and had not been implemented in some areas. During the study period there was no automatic reporting of ADR reports from the system to the regulator. It is possible that prescribers when stopping a drug thought that this action automatically reported on their behalf so did no go on to submit a report manually. The majority of the drugs reported on a Yellow Card were those used in an outpatient setting so these had not been captured in the electronic patient record. More detailed analysis of the reports in the system showed that the reason for stopping “ADR” had been probably selected in error being the first on the drop-down list. As there were no reports in common to both methods we could not use capture-recapture to estimate the rate in the hospital (4).
References/Further Sources of Information
Pirmohamed M, James S, Meakin S, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004;329:15–19.
Weiss AJ, Freemantle WJ, Heslin KC, Barrett ML. Adverse drug events in US hospitals 2010 versus 2014. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb234-Adverse-Drug-Events.pdf. Accessed 10/5/21.
Medicines and Healthcare products Regulatory Agency: The Yellow Card scheme: guidance for healthcare professionals, patients and the public. https://www.gov.uk/guidance/the-yellow-card-scheme-guidance-for-healthcare-professionals. Accessed 10/5/21.
Cox AR, Anton C, Goh CHF, Easter M, Langford NJ, Ferner RE. Adverse drug reactions in patients admitted to hospital identified by discharge ICD-10 codes and by spontaneous reports. Br J Clin Pharmacol. 2001;52:337–9.
P047 Drug Utilization Study of Antidiabetic Agents During 2012-2019 in Romania
C. Bucsa1, A. Farcas1, I. Cazacu2, C. Mogosan1, A. Rusu3
1Iuliu Hatieganu University of Medicine and Pharmacy, Drug Information Research Center, Cluj-Napoca, Romania; 2Iuliu Hatieganu University of Medicine and Pharmacy, Department of Pharmacology-Physiology and Pathophysiology-Faculty of Pharmacy, Cluj-Napoca, Romania; 3Iuliu Hatieganu University of Medicine and Pharmacy, Diabetes and Nutrition Diseases Department-Faculty of Medicine, Cluj-Napoca, Romania
Background/Introduction: Several new antidiabetic medicines (ADMs) were authorized during the last decades and this resulted in changes in diabetes therapy.
Objective/Aim: To analyze the market trends of ADMs in Romania during 2012–2019 and to describe the utilization of the new ADMs (glucagon-like peptide-1 receptor agonists [GLP-1], dipeptidyl peptidase-4 inhibitors [DPP-4] and sodium-glucose transport protein 2 inhibitors [SGLT-2]) during 2019.
Methods: We conducted a retrospective analysis of pharmacy claims data from IQVIA Romania during 2012–2019. For trends analysis, we used nationally extrapolated data (IQVIA proprietary algorithm with maximum 2% error) and for the detailed 2019 analysis, we used exact data retrieved from approximately 40% of pharmacies in Romania. We analyzed the reimbursed prescriptions with at least one ADM (all ATC10 codes) as defined daily doses (DDDs)/1000 inhabitants/day and number of prescriptions (for 2019).
Results: The total number of ADMs DDDs/1000/day increased by 54.3% between 2012 (48.1)–2019 (74.2) at national level. A comparison between the successive years showed a continuous increase in ADMs consumption (the highest of 9.2% between 2016–2017), except for 2013–2014 where a slight decrease was observed (0.7%). Metformin was the most prescribed molecule and its consumption increased constantly during 2012–2019 (14.0–23.7 DDDs/1000/day), also gaining in share of the non-insulin ADMs (39.8–4.5%). Sulphonylureas (SU) were the 2nd most prevalent ADM with increase in prescribing between 2012–2017 and slight fluctuations during 2017–2018–2019 (15.9–20.3 and 20.3–19.7–20.7 DDDs/1000/day, respectively), but continuous decrease of its share in the non-insulin ADMs during 2012–2019 (45.20–36.7%). Although a steady increase was observed for the new ADMs (GLP-1, DPP-4, and SGLT-2) throughout the study period, reaching a share of 10.7% of the non-insulin ADMs in 2019, the uptake of the new ADM was low. In 2019 the number of DDDs/1000 inhabitants/day was 2.03 for DDP-4i, 2.39 for GLP-1 RA and 1.6 for SGLT2i. Of the total number of antidiabetics in 2019, most were prescribed by physicians specialized in diabetes, nutrition and metabolic diseases (65.21%), followed by general practice (26.90%) and internal medicine (5.07%) physicians.
Conclusion: An overall trend of market increase was found for ADMs between 2012–2019, corresponding to the increase in the prevalence of diabetes in Romania [1]. As expected, an increased prescription of new ADMs was found during 2021–2019, but their uptake was still low. Surprisingly, SU showed a continuously increased prescription up until 2019.
References/Further Sources of Information
Mota M, Popa SG, Mota E, Mitrea A, Catrinoiu D, Cheta DM, et al. Prevalence of diabetes mellitus and prediabetes in the adult Romanian population: PREDATORR study. J Diabetes. 2016;8(3):336–44.
P048 Lactic Acidosis Induced by Short-Term Linezolid Use: A Case Report
A. Chaibi1, S. Hamza1,2, T. Dendane2, K. Abidi2
1University of Mohammed V Souissi 10000-Rabat-Morocco, Department of Clinical Pharmacy, Rabat, Morocco; 2University of Mohammed V Souissi 10000-Rabat-Morocco, Medical Intensive Care Unit-Ibn Sina University Hospital-10000 Rabat-Morocco, Rabat, Morocco
Background/Introduction: Linezolide is an antibiotic belonging to the oxazolidinone family. We report in this work a very rare observation of lactic acidosis induced by linezolide after 48 h. The various investigations carried out by the clinical pharmacist revealed that after the introduction of linezolid a sudden onset of lactic acidosis appeared.
Objective/Aim: In first stage demonstrated the implication of the linezolide in the lactic acidosis in short terms as well as the interest of the pharmacist clinician in the determination of the drug which is responsible and the conduct to hold.
Methods: Case Report.
Results: Observation: the patient was 86 years old and had a history of type 2 diabetes for 40 years under insulin, hypertension under treatment, prostate neoplasia under hormone therapy. Admitted to the emergency room for urinary sepsis and dementia syndrome. The patient was admitted to the intensive care unit because of the worsening of his general condition: anorexia, decompensation of his diabetes (blood sugar above 5) and a septic shock with a urinary starting point. The patient was put on Linezolid injection (600 mg × 2/day) and Meropenem injection (3 g/day). 2 days after the administration of linezolid, the patient presented a lactate level of 6.6 mmol/l knowing that his initial lactate level was 1.6 mmol/l. After analysis of the prescription and investigation by the clinical pharmacist, it was found that there was a link between linezolid and lactic acidosis. Linezolid was discontinued.
24 h after the lactate level dropped to 1.15 mmol/l. This sudden onset and rapid improvement of the lactate level allowed us to incriminate the linezolid even if the literature mentions the onset of lactic acidosis over the long term (4 weeks).
Conclusion: The presence of a drug expert pharmacist in an intensive care unit is important, as it allows the detection of all problems related to therapeutics.
References/Further Sources of Information
Protti A, Ronchi D, Bassi G, Fortunato F, Bordoni A, Rizzuti T, Fumagalli R. Changes in whole-body oxygen consumption and skeletal muscle mitochondria during linezolid-induced lactic acidosis. Cri Care Med. 2016;44(7):e579–e582. https://doi.org/10.1097/ccm.0000000000001478.
Mao Y, Dai D, Jin H, Wang Y. The risk factors of linezolid-induced lactic acidosis. Medicine. September 2018;97(36):e12114.https://doi.org/10.1097/MD.0000000000012114.
Lee YR, Powell N, Bonatti H, Sawyer RG, Barroso L, Pruett TL, Sifri CD, Volles D. Early development of lactic acidosis with short term linezolid treatment in a renal recipient. J Chemother. 2008 Dec;20(6):766–7. https://doi.org/10.1179/joc.2008.20.6.766. PMID: 19129081.
P049 Combination of Clopidogrel and Proton Pump Inhibitors: Assessment of Pharmaceutical Interventions in the Emergency Department of Ibn Sina Hospital
A. Chaibi1, Y. Boudina2, M. Alilou2
1Laboratory of Medicinal Chemistry-Clinical and Hospital Pharmacy, Faculty of Medicine and Pharmacy-Mohammed V University-Rabat, Rabat, Morocco; 2Emergency Department-Ibn Sina Hospital, Faculty of Medicine and Pharmacy of Rabat-Mohamed V University, Rabat, Morocco
Background/Introduction: Proton pump inhibitors (PPIs) are frequently associated with a dual antiplatelet therapy (Clopidogrel + Aspirin) especially in acute coronary syndrome to reduce the risk of gastrointestinal bleeding.
Proton pump inhibitors are inhibitors of CYP2C19, which is involved in the biotransformation of Clopidogrel (prodrug) into active metabolites, thus reducing the biological response to Clopidogrel (1). The Proton pump inhibitors-Clopidogrel interaction is a matter of debate, as several studies have shown that certain PPIs are associated with an increased risk of cardiovascular events when combined with Clopidogrel (2, 3).
Objective/Aim: Analysis of all prescriptions containing Clopidogrel and verification of the compliance of proton pump inhibitors prescribing with the guidelines and issuance of pharmaceutical interventions.
Methods: We performed a prospective observational study of prescriptions including Clopidogrel, analyzed by the clinical pharmacist in the Emergency Department of Ibn Sina Hospital from February 2021 to May 2021, with issuance of pharmaceutical interventions.
Results: The results showed that out of the total prescriptions analyzed (n = 56), 25% (n = 14) of them did not include a PPIs. And among the 75% (n = 42) with a PPI, 76% (n = 32) had Rabeprazole, 19% (n = 8) had Omeprazole, and 4.5% (n = 2) had Esomeprazole.
A total of 32 pharmaceutical interventions were performed for 39.3% (n = 22) of the prescriptions: 68.75% (n = 22) of co-prescribing Rabeprazole, 25% (n = 8) stopping Omeprazole, and 6.25% (n = 2) stopping Esomeprazole, and all of them was accepted by the prescribers.
Conclusion: This study shows that a significant number of pharmaceutical interventions were performed on prescriptions with Clopidogrel for the choice of the proton pump inhibitors as recommended on the guidelines, and that some prescribers were not aware of the impact of this interaction.
These results will allow to raise awareness among prescribers of the importance of proton pump inhibitors in dual antiplatelet therapy and the risk of drug interactions.
References/Further Sources of Information
Furuta T, Iwaki T, Umemura K. Influences of different proton pump inhibitors on the anti-platelet function of clopidogrel in relation to CYP2C19 genotypes. Br J Clin Pharmacol. 2010 Sep;70(3):383–92. https://doi.org/10.1111/j.1365-2125.2010.03717.x.
Juurlink DN, Gomes T, Ko DT, Szmitko PE, Austin PC, Tu JV, et al. A population-based study of the drug interaction between proton pump inhibitors and clopidogrel. CMAJ. March 31, 2009;180(7):713–8.
Gilard M, Arnaud B, Cornily JC, Le Gal G, Lacut K, Le Calvez G, Mansourati J, Mottier D, Abgrall JF, Boschat J. Influence of omeprazole on the antiplatelet action of clopidogrel associated with aspirin: the randomized, double-blind OCLA (Omeprazole CLopidogrel Aspirin) study. J Am Coll Cardiol. 2008 Jan 22;51(3):256–60. https://doi.org/10.1016/j.jacc.2007.06.064.
P050 Iatrogenic QT Allongation After Concomitant Use of Domperidone with Fluconazole and Ciprofloxacin: Case Report and Review of the Literature
C. Aicha1, H. Rahmouni1, A. Barkat2, M. A. Radouani2
1University of Mohamed V Souissi 10000-Rabat-Morocco, Department of Clinical Pharmacy, Rabat, Morocco; 2Children’s Hospital-Ibn Sina University Hospital Centre, Neonatology Department, Rabat, Morocco
Background/Introduction: Domperidone is a peripheral dopamine antagonist, with antiemetic and gastroprokinetic properties, used in pediatrics for the treatment of RGO associated with an acidity reducer, but it is considered an arrhythmogenic drug by his power to allongation the QT space and therefore risk of ventricular arrhythmia and torsade de pointe.
Objective/Aim: Identify the different torsadogenic drugs that can cause cardiac rhythm disorders in a neonatal resuscitation unit of the children's hospital of Rabat Morocco.
Methods: Description of a clinical case of a patient who presented with iatrogenic cardiac rhythm disturbances that was attributed after thorough analysis of the patient's prescription to the concomitant use of drugs with known QT prolongation effect.
Results: We describe a male infant aged 4 months and 5 days, former preterm infant of 36 SA. Hospitalized in the neonatal service for severe transient respiratory distress complicated by several nosocomial infections for whom anti-infective treatment with ciprofloxacin and fluconazole and anti-reflux treatment with domperidone and sodium alginate were prescribed. The patient presented with bradycardia rhythm disturbances, following which an ECG was performed and revealed a lengthening of the QT space whose etiology has been attributed to an iatrogenic cause. Following a thorough analysis of the patient's prescription by the pharmacist, three drugs have been incriminated as they have a known risk of torsade de pointes with QT prolongation: domperidone, ciprofloxacin and fluconazole. Prescribing the three molecules simultaneously increases the torsadogenic risk. In assessing the benefit-risk balance, the pharmacist suggested that domperidone be stopped. The patient's evolution was favourable with disappearance of cardiac rhythm disorders.
Conclusion: The value of having a pharmaceutical presence in the neonatology department for a detailed analysis of the prescription and to avoid any iatrogenic drug event.
References/Further Sources of Information
Rossi, M.; Giorgi, G. Domperidone and long QT syndrome. CDS. 2010;5(3):257–62. https://doi.org/10.2174/157488610791698334.
Morris AD, Chen J, Lau E, Poh J. Domperidone-associated QT interval prolongation in non-oncologic pediatric patients: a review of the literature. Can J Hosp Pharm. 2016;69(3):224–230.
Günlemez A, Babaoğlu A, Arısoy AE, Türker G, Gökalp AS. Effect of domperidone on the QTc interval in premature infants. J Perinatol. 2010;30(1):50–53. https://doi.org/10.1038/jp.2009.96.
P051 National Study on Suicidal Behavior After Initiation of Antidepressant Therapy in Older Adults with Previous Self-Harm Episode
K. Hedna1, M. Waern2
1University of Gothenburg, Institute of Neuroscience and Physiology-Center for Ageing and Health AgeCap, Gothenburg, Sweden; 2University of Gothenburg, Institute of Neuroscience and Physiology-Center for Ageing and Health AgeCap, Gothenburg, Sweden
Background/Introduction: The treatment of depression is considered a major preventive strategy of late-life suicide, and antidepressants (AD) are by far the most common type of depression treatment available for older adults today. Some mixed-age studies suggested a possible emergence or worsening of suicidal behaviour shortly after initiation of AD treatment (1, 2). Surprisingly, no study has investigated the recurrence of suicidal behaviour in older adults who initiated an AD after an episode of self-harm, although persons with a previous self-harm episode constitute a group with a particularly high risk of subsequent suicidal behaviour (3). Large population-based studies provide a useful context to examine recurrence of suicidal behaviour the general population with enough power and with limited risk of inclusion or lost to follow-up biases.
Objective/Aim: To assess the reoccurrence of suicidal behaviour among older adults (75+) who initiated antidepressant treatment after an episode of self-harm, and to identify associated risk factors.
Methods: We conducted a longitudinal national register-based retrospective cohort study of Swedish residents aged 75+ (N = 506) who initiated antidepressant treatment between January 1, 2006, and December 31, 2013, within a year after an episode of self-harm and followed for up to one year after initiation of the AD therapy. Multiple national registers were linked using the personal identity number (the Swedish Prescribed Drug Register, the National Patient Register, the Cause of Death Register, the Care and Social Services Register, the Longitudinal Integration Database for Health Insurance and Labor Market Studies and Total Population Register). Cox regression analysis was used to assess factors associated with the recurrence of fatal or non-fatal suicidal behaviour within a year after treatment initiation.
Results: Overall, 48 individuals (9.5%) had a new episode of suicidal behaviour: 39 (7.7%) repeated a non-fatal self-harm and 9 (1.8%) died by suicide within a year of antidepressant initiation. In total, 38 (79.2%) of these episodes occurred within 6 months after AD initiation. Male gender was associated with increased risk of repeated suicidal behaviour (Adjusted Hazard ratio (aHR) 1.97, 95% CI 1.06–3.68). An association of similar magnitude was found with concomitant use of hypnotics.
Conclusion: Four out five episodes of repetition of suicidal behaviour occurred within six months after the initiation of antidepressant treatment. Men had increased risk of repetition of suicidal behaviour. A potential increased risk may be also considered in users of hypnotics.
References/Further Sources of Information
Coupland C, Hill T, Morriss R, Arthur A, Moore M, Hippisley-Cox J. Antidepressant use and risk of suicide and attempted suicide or self harm in people aged 20 to 64: cohort study using a primary care database. BMJ. 2015;350:h517.
Stone M, Laughren T, Jones ML, Levenson M, Holland PC, Hughes A, et al. Risk of suicidality in clinical trials of antidepressants in adults: analysis of proprietary data submitted to US Food and Drug Administration. BMJ. 2009;339:b2880.
Morgan C, Webb RT, Carr MJ, Kontopantelis E, Chew-Graham CA, et al. Self-harm in a primary care cohort of older people: incidence, clinical management, and risk of suicide and other causes of death. Lancet Psychiatry. 2018;5(11):905–12.
P052 Measuring the Impact of Meprobamate Marketing Authorisation Withdrawal on Public Health in France and UK
S. Lane1, E. Lynn1, J. Slattery2, S. Shakir1
1Drug Safety Research Unit, Research, Southampton, UK; 2European Medicines Agency, Pharmacovigilance and Epidemiology, Amsterdam, The Netherlands
Background/Introduction: Impact of pharmacovigilance (PV) actions such as marketing authorisation (MA) withdrawal on public health is not normally measured quantitatively. Removing a medicine from market may significantly affect public health (PH) by avoiding adverse drug reactions (ADRs), which should be measured quantitatively if possible. Meprobamate products, marketed in 1955 and withdrawn in March 2012 due to cases of severe hypotension, demonstrate the model [1].
Objective/Aim: To identify a method to quantify impact of MA withdrawal of meprobamate in terms of morbidity and mortality changes.
Methods: Patients exposed to meprobamate were counted in quarterly periods for 12 months pre- and post-withdrawal of MA by sex and decade of age, in IQVIA Medical Research Data (IMRD)-France and IMRD-UK electronic health record databases; annual exposure was estimated. Reason for MA withdrawal (ADR of interest) was obtained from EMA records. Quantitative research addressing the ADR of interest was identified by systematic review, allowing an estimation of absolute risk in exposed patients. PH impact, expressed as an annual crude number of ADRs avoided as a result of PV action, was estimated by modelling product usage figures and ADR risk. This number of expected ADRs was scaled up to give an approximation for the source population. Background risk put results into context.
Results: At the time of meprobamate withdrawal, 7,036,949 patients were active in IMRD-France and 5,335,435 patients in IMRD-UK. Prevalence of meprobamate exposure during the 12 months before withdrawal was 13.0% and 1.1% respectively. Average number of patients exposed to meprobamate decreased by 86.8% in IMRD-France and by 15.2% in IMRD-UK populations after its withdrawal, but the number of exposed patients did not reach 0 within 12 months in either population. Risk of hypotension was estimated at 41.2% from published studies. Based on risk and usage estimates, 1558 new attributable cases of hypotension were expected amongst meprobamate-exposed IMRD-France and IMRD-UK patients per year. Therefore, withdrawal of meprobamate from market could prevent up to 1558 cases of severe hypotension annually throughout the IMRD-France and IMRD-UK populations if meprobamate exposure was eliminated.
Conclusion: This model quantifies the PH impact of MA withdrawal of meprobamate in terms of serious morbidity, assuming exposure was eliminated. This method can be applied to other products in a range of settings.
References/Further Sources of Information
European Medicines Agency. Assessment report for meprobamate-containing medicinal products for oral use. London: European Medicines Agency; 2012 30 March 2012. Contract No.: EMA/212617/2012.
P053 Measuring Public Health Impact of the Suspension of Diane-35 and its Generics in France
S. Lane1, E. Lynn1, J. Slattery2, S. Shakir1
1Drug Safety Research Unit, Research, Southampton, UK; 2European Medicines Agency, Pharmacovigilance and Epidemiology, Amsterdam, The Netherlands
Background/Introduction: Impact of pharmacovigilance (PV) actions such as suspension of marketing authorisation (MA) on public health is not often measured quantitatively, yet permanent or temporary removal of medicines from market will benefit public health (PH) through avoiding adverse drug reactions (ADRs). This should be measured quantitatively wherever possible. The oral contraceptive (OC) and acne treatment Diane-35 and its generics were suspended in France in 2013 due to concerns of thromboembolism; these products demonstrate use of the model.
Objective/Aim: To identify a method to quantify impact of MA suspension of Diane-35 and generics in terms of morbidity and mortality changes.
Methods: Patients exposed to these products were counted in quarterly periods for 12 months pre- and post-MA suspension in IQVIA Medical Research Data (IMRD)-France electronic health record database; prevalence of exposure was estimated. Reason for suspension (ADR of interest) was obtained from EMA records [1]. Quantitative research addressing the ADR of interest was identified by systematic review, allowing estimation of absolute risk in exposed patients. PH impact, expressed as annual crude number of ADRs avoided as a result of suspension, was estimated by modelling product utilisation figures and ADR risk.
Results: At the time of the suspension of these products in France, 7,365,526 patients were active in IMRD-France. Average prevalence of exposure to these products in the IMRD-France population during the 12 months prior to suspension was 9.4% (including males and females); average exposure decreased by 92.8% following suspension. Based on risk of thromboembolism in exposed patients calculated from published studies (53.9%), it was estimated that up to 1467 cases of thromboembolism could be avoided annually amongst the exposed IMRD-France population if these products remained unavailable.
Conclusion: Risk of thromboembolism in exposed patients and thus ADRs avoided may have been overestimated. As Diane-35 and generics were returned to market in France shortly following suspension [2]; estimated impact of this suspension is only applicable to the period during which these OCs were unavailable. However prevalent exposure of these products remained low during the 12 months following suspension, so it is expected that cases of thromboembolism were considerably reduced following their suspension. Assessment of risks associated with alternative contraceptives and with pregnancy was not in the scope of this project but should be considered in future impact models.
References/Further Sources of Information
European Medicines Agency starts safety review of Diane 35 and its generics [press release]. London 2013. https://www.ema.europa.eu/en/news/european-medicines-agency-starts-safety-review-diane-35-its-generics.
Arie S. European Commission orders France to lift ban on acne pill. BMJ. 2013;347:f4932.
P054 Immunological Mechanisms Involved in Peri-Infusional Adverse Drug Reactions to Paclitaxel
M. López-Gamboa1, J. C. Ceballos Villalva1
1INCAN, Pharmacovigilance, CdMx, Mexico
Background/Introduction: The recognition of drugs as foreign substances to the organism triggers a series of damages at different levels through autoimmune reactions and hypersensitivity responses. Paclitaxel is frequently the cause of adverse drug reactions (ADR). Due to the low water solubility of this drug, its co-administration with Cremophor EL is required. The possible mechanisms proposed for paclitaxel-associated peri-infusional reactions include both IgE-dependent and non-IgE-dependent mechanisms, with a major role for histamine preformed and stored in the granules of the mast cells for clinical effects after cell activation.
Objective/Aim: The aim was to characterize the immunological mechanism of paclitaxel peri-infusional ADR
Methods: An observational, descriptive and prospective study of peri-infusional ADR associated with paclitaxel reported from the outpatient chemotherapy service of the National Cancer Institute (INCan).
Results: 145 patients (age 19–75 years) were included; 138 were female and with ovarian cancer (33.10%) and breast cancer (30.34%). The population studied was 135 patients (defined as patients with ADR where paclitaxel was al list one of the suspected drugs), from which, 101 cases paclitaxel was the only suspected drug. 334 different ADR were clinically identified; 73.35% from paclitaxel, 25.45% from paclitaxel + taxane and 1.2% from paclitaxel + monoclonal antibody. It was observed that the first and second cycles presented the first and second place in frequency of ADR, respectively.
Even though the first and second cycles had a similar reaction profile, dyspnea, erythema and flushing also occurred in the second cycle, but with a lower frequency of occurrence. Since the literature explains as possible mechanisms of peri-infusional reactions the presence of IgE as a trigger for the degranulation of the mast cells, and on the other hand the direct action of drugs and excipients on the mast cell to trigger its degranulation, the analysis of our data suggests that the reactions observed in the first cycle would be due to a mechanism of direct action of paclitaxel or its excipients (mainly Cremophor EL) on the mast cells, since there is no history of previous exposure.
Conclusion: With this study we confirm that the highest frequency of adverse reactions to paclitaxel occur in the first and second cycle. Because the highest proportion is in the first cycle, the ADR could not be explained by the IgE mechanism, but however, it can be explained by the mechanism of direct damage of the drug or its excipients on the mast cells.
References/Further Sources of Information
Bonamichi R, Castells M. Diagnoses and management of drug hypersensitivity and anaphylaxis in cancer and chronic inflammatory diseases: reactions to taxanes and monoclonal antibodies. Clin Rev Allergy Immunol. 2018;54(3):375–85.
Brockow K. Chapter 3 Drug allergy: definitions and phenotypes. In: Khan D, Banerji A, editors. Drug allergy testing. USA: Elsevier; 2018. p. 19–26.
Giavina P, Patil S, Banerji A. Immediate hypersensitivity reaction to chemotherapeutic agents. J Allergy Clin Immunol Pract. 2017;5(3):593–9.
D’Errico S, Baldari B, Arcangeli M, Santurro A, Frati P, Fineschi V. Mast cells activation and high blood tryptase levels due to paclitaxel administration. Is Cremophor EL the culprit? Medicine. 2020;99:43.
Elhissi AMahmood R, Parveen I, Vali A, Najlah M, Phoenix D, et al. Chapter 18 Taxane anticancer formulations: changes and achievements. In: Ahmed W, Phoenix D, Jackson M, Charalambous C, editors. Advances in medical and surgery engineering. UK: Elsevier; 2020. p. 347–58.
Montañez M, Mayorga C, Bogas G, Barrionuevo E, Fernández R, Martin A, et al. Epidemiology, mechanisms, and diagnosis of drug-induced anaphylaxis. Front Immunol. 2017;8:1–10.
Zambernardi A, Label M. Reacciones cutáneas adversas a medicamentos: cómo identificar el desencadenante. Actas Dermosifiliogr. 2018;109(8):699–707.
P055 Guiding Principles for the Identification, Management, and Reporting of Safety Interests in Combination Therapies: Aggregate Documents
L. Lum1, J. Zhang2, N. Johnson2, R. Mentzer1, D. Seekins2
1Bristol Myers Squibb, Global Scientific and Regulatory Documentation, Princeton, USA; 2Bristol Myers Squibb, WorldWide Patient Safety, Princeton, USA
Background/Introduction: Multi-drug regimens (MDRs)—therapies that involve 2 or more drugs—are of increasing importance for drug development in many therapeutic settings. Compared with monotherapies, MDRs provide significant therapeutic advantages, but clinical development of MDRs presents additional challenges regarding safety characterization in combination. Guiding principles for MDRs are needed to identify safety issues, and to produce robust safety information for use by healthcare providers (HCPs) and health authorities (HAs).
Objective/Aim: The objective of the MDR therapy initiative is to ensure systematic and comprehensive safety review by developing guiding principles to identify, manage, and report safety interests for combination therapies. Given the variable nature of both the combinations being investigated as well as the conditions for which they are being investigated, the team is first focusing on providing a framework for considering the safety implications of MDR development in aggregate documents.
Methods: The aggregate documents sub-team reviewed regulations, company standard operating procedures, manuals, and model documents. The goal was to develop guiding principles to define, evaluate, and communicate safety issues that emerge throughout the span of clinical development for MDRs: (1) combination data from first-in-human studies to be included in the Investigator’s Brochure (IB); (2) advancing to Periodic Aggregate Safety Reports (e.g., the Developmental Safety Update Report [DSUR]); and (3) followed by the Periodic Benefit-Risk Evaluation Report (PBRER).
Results: The MDR therapy initiative team evaluated the findings and addressed key issues with interconnectivity among the topics of interest. The aggregate documents sub-team formulated the guidelines (Table 1) and updated model documents with clear communications of the guiding principles and detailed training for their implementation.
Conclusion: The MDR therapy working group implemented the adoption of practical and flexible guidance. These guiding principles address identification, management and reporting of safety interests for MDRs and produce a robust package of safety information on combination therapies for use by HCPs and HAs. These guiding principles regarding the presentation of combination therapy have been added to the Company’s model documents for the PBRER, DSUR, and IB to provide guidance on when and where to describe the safety data of MDRs.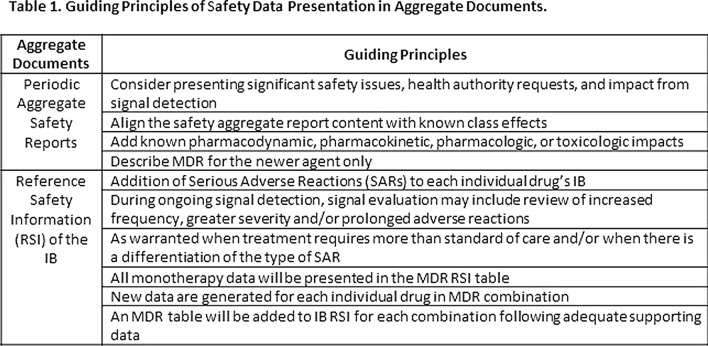
References/Further Sources of Information
ICH Guideline E2C (R2) on Periodic Benefit-risk Evaluation Report (PBRER). Step 5. EMA/CHMP/ICH/544553/1998. January 2013.
EMA Guideline on Good Pharmacovigilance Practices. Module VII –Periodic Safety Update Report. EMA/816292/2011. 22 June 2012.
ICH guideline E2F on Development Safety Update Report. Step 5. EMA/CHMP/ICH/309348/2008. September 2011.
EMA Guideline for good clinical practice E6(R2) EMA/CHMP/ICH/135/1995. 1 December 2016
Heads of Medicines Agencies (HMA) The Clinical Trial Facilitation Group (CTFG) Q&A document—Reference Safety Information. November 2017.
In memory of William O’Brien who led the company's MDR initiative.
P056 Adverse Reactions to Antituberculosis Drugs in Clinical Practice
O. Zhdanova1, G. Batischeva1, N. Perova1, E. Muzalevskaya1
1Voronezh State Medical University Named After N.N. Burdenko, Clinical Pharmacology, Voronezh, Russian Federation
Background/Introduction: Tuberculosis is a socially significant pathology. Antituberculosis therapy can last up to two years and consist of three or more etiotropic drugs, increasing risk of adverse reactions (ADR) [1, 2]. Additional risk factors for ADR are hepatitis, HIV, alcohol addiction [3]. Effective and safe antituberculosis therapy is essential to improve prognosis and life quality of a patient.
Objective/Aim: Analysis of ADR identified in clinical practice developed by patients during antituberculosis therapy.
Methods: Retrospective analysis of ADR cases of tuberculosis pharmacotherapy registered in Voronezh Regional Antituberculosis Centre in 2018–2020. The criterion for reaction severity was necessity of the drug withdrawal, introduction of additional therapy for ADR correction, hospitalization prolongation, a threat to life.
Results: In 2018–2020 390 cases of ADR were registered at Voronezh Regional Antituberculosis Centre, among which 282 cases (72.3%) were severe.
Most patients (74.6%) took 4 to 9 drugs simultaneously. 10–15 drugs were prescribed in 14.1% of the cases, more than 15 drugs—in 0.56% of the cases. All patients received drugs connected with tuberculosis and comorbidities treatment.
Antituberculosis drugs caused ADR in 77% of the cases: 17.7%—isoniazid, 18%—rifampicin, 23.9%—pyrazinamide, 17.3%—ethambutol.
Hepatotoxic reactions were most common—224 (57.4%) patients. Allergic reactions were observed in 45 (11.5%) patients, uric acid increase in blood—in 42 (10.7%), nephrotoxic effect—in 12 (3.8%), neurotoxicity—in 13 (3.3%), dyspeptic symptoms (nausea, vomiting, diarrhea)—in 13 (3.33%), and ototoxic effect (sensorineural hearing loss)—in 14 (3.59%) patients at simultaneous intake of aminoglycosides and fluoroquinolones. Cardiotoxic effect (prolonged QT interval) was observed in 11 (2.82%) patients at simultaneous intake of bedaquiline and levofloxacin, hematotoxic reactions—in 5 patients (1.28%), tendovaginitis due to fluoroquinolones treatment—in 1 patient.
ADR development required a reduction of antituberculosis drugs dosage in 11.3% of the cases or caused therapy interruption for 10–14 days in 88% of the patients.
All patients underwent detoxification with subsequent improvement of their condition. In 1 case a lethal outcome was registered due to a severe hepatotoxic reaction.
Conclusion: ADR to antituberculosis drugs were severe in 72.3% of the cases, led to hospitalization prolongation. Drug-induced liver injury prevailed (57.4%).
A significant factor of an adverse reaction is combination of drugs with unidirectional action (fluoroquinolones + aminoglycosides, bedaquiline + fluoroquinolones).
Adverse reactions are reversible in most patients. Effective pharmacological correction of antituberculosis drugs toxic effect is of great importance and allows to continue tuberculosis therapy [4].
References/Further Sources of Information
Sysoev PG. Adverse events of chemotherapy of tuberculosis/P.G. Sysoev, A.Y. Alexandrov, E.G. Miftahova//Synergy sciences. 2018;20:593–8. 2.
Effectiveness and safety of the bedaquiline-containing six-month chemotherapy regimens in pulmonary tuberculosis patients./Borisov S.E. [et al.]//Tuberculosis and socio-important diseases. 2015;3:30–49.
Markers of hepatotoxicity at different regimen of therapy in patients with tuberculosis/S.I. Makarova S.I. [et al.]//Bulletin of Novosibirsk state University. 2013;11(2):116–121.
Bueverov AO. Possibilities of treatment of medicinal liver lesions in the conditions of the need to continue taking hepatotoxic drugs/A.O. Bueverov//Lechaschi vrach. 2009;2:40–42. 3.
P057 Safety Signal of Dulaglutide and the Risk of Dysgeusia: Evidence From Pharmacovigilance Data
A. Alakeel1, M. Fouda1, R. Alamri1, A. Alomair1
1Saudi Food and Drug Authority, Pharmacovigilance Center, Riyadh, Saudi Arabia
Background/Introduction: Dulaglutide is glucagon-like peptide-1 (GLP-1) receptor agonist indicated to treat type 2 diabetes mellitus [1]. Dysgeusia is the persistent of unpleasant, abnormal, or altered taste sensation. It can be described as metallic taste and the disturbance time can be short or long term [2].
Objective/Aim: The aim of this review is to evaluate the risk of dysgeusia associated with the use of dulaglutide.
Methods: The Pharmacovigilance Center of Saudi Food and Drug Authority performed a comprehensive signal review using its national database as well as the World Health Organization (WHO) database, to retrieve related information for assessing the causality between dulaglutide and risk of dysgeusia [3]. We used the WHO criteria as standard for assessing the causality of the case-reports [4]. Literature review was done as well to find any supportive data.
Results: The number of retrieved cases for the combined drug/adverse drug reaction are 193 case-reports as of January 2021. The reviewers have selected and assessed the causality for the well-documented case-reports with completeness scores of 0.6 and above (18 cases); the value 1.0 indicated the highest score for best-written cases. Among the reviewed cases, 14 of them were assessable and about the half provides supportive association (1 probable, and 5 possible cases). The disproportionality of the observed and the expected reporting rate for drug/adverse drug reaction pair is estimated using information component (IC), a tool developed by WHO-UMC to measure the reporting ratio. The results of (IC = 0.5) revealed a positive statistical association for the drug/ADR combination [3]. In literature, we found one phase IV clinical study that examine dulaglutide and taste impaired (Dysgeusia). The ADR was found to affect 0.53% (174 out of 33,008), especially for people who are female, 60+ old and have been taking the drug for < 1 month. However, 25% of the people were taking metformin, a medication that causes dysgeusia as well [5]. Another study showed evidence on class effect of glucagonlike peptide-1 (glp-1) analogues (exenatide or liraglutide). Dysgeusia was mentioned as one of the post marketing ADRs [6].
Conclusion: The weighted cumulative evidences identified from the reported cases, data mining, and literature are sufficient to support a causal association between dulaglutide and the risk of dysgeusia. Health regulators and health care professionals must be aware of this potential risk and it is advisable to monitor any signs or symptoms in treated patients.
References/Further Sources of Information
Eli Lilly and Company. Product monograph of Dulaglutide (Trulicity)®; Retrieved from: http://pi.lilly.com/ca/trulicity-ca-pm.pdf. Accessed 1/12/2021.
Science Direct (2002). Dysgeusia; Retrieved from: https://www.sciencedirect.com/topics/pharmacology-toxicology-and-pharmaceutical-science/dysgeusia. Accessed 1/21/2021.
Uppsala Monitoring Center (UMC) (2020). Vigilyze database; Retrieved from: https://vigilyze.who-umc.org. Accessed 10/4/2020.
Uppsala Monitoring Center (UMC) (2020), The use of the WHO-UMC system for standardized case causality assessment; Retrieved from: https://www.who.int/medicines/areas/quality_safety/safety_efficacy/WHOcausality_assessment.pdf?ua=1. Accessed 23/7/2020.
Ehealthme (2020), Retrieved from: https://www.ehealthme.com/ds/trulicity/taste-impaired/. Accessed 1/21/2021.
Shared Care Protocol: Addition of a glucagonlike peptide-1 (GLP-1) analogues (exenatide or liraglutide) to patients already on insulin who have poorly controlled type 2 diabetes. Retrieved from: https://hertsvalleysccg.nhs.uk/application/files/7015/3633/4542/GLP-1_analogue_added_to_insulin_201207SCG.pdf. Accessed 1/21/2021.
P058 Pharmaceutical Interventions in an Intensive Care Unit of Covid-19 in Morocco
A. Chaibi1, O. Elqabissi2
1Hôpital Ibn Sina Rabat, Pharmacie clinique, Rabat, Morocco; 2Pharmacie Clinique Hôpital Ibn Sina Rabat, Rabat, Morocco
Objective/Aim: The objective of the study was to analyze pharmaceutical interventions and their impact in patients hospitalized in an intensive care unit of Covid-19 in a Moroccan university hospital center.
Methods: The study was initiated in an intensive care unit at Ibn Sina hospital in Rabat for a period of three months between November and February 2021. The prescriptions were analyzed and validated according to the methodology of the French Society of Clinical Pharmacy (SFPC). The clinical impact was evaluated according to the Hatoum scale.
Results: During the study period, corresponding to three months, 71 IP are identified with an acceptance rate of 99%. The sex ratio (M/F) was 1.7 in a favour of male predominance. The average age of our patients was 63 years. The main prescription problems encountered were overdose (34%), non-compliance with consensus (29%), under dosing (13%), and drug interaction (13%). Our interventions concerned dosage adjustment (52%), substitution/exchange (15%), therapeutic follow-up (12%), and drug withdrawal (8%). 98% of the interventions had a non-zero impact.
Conclusion: This study highlights the importance of the clinical pharmacist in the fight against drug-related harm especially in the context of the pandemic where vigilance is required.
References/Further Sources of Information
Mackenzie JS, Smith DW. COVID-19: New zoonosis caused by coronavirus from China: what we know and what we don’t know [published online ahead of print, March 17, 2020]. MicrobiolAust. 2020.
Anne-Laure Antoine, Yasmine Saichi, Tiphanie Fontaine, Virginie Lamand, Anne-Claire Cuquel. Covid-19: contribution of a hospital pharmacy to crisis management. 07/2020;6861(847):1–59.
C. Mabille, C. Joseph, J. Schmit, M. Belhout, A. Terrier-Lenglet. Pharmaceutical analysis of prescriptions for COVID patients. Med Infect Dis. , 21 August 2020;50(6).
P059 Retrospective Analysis of Adverse Drug Reactions in Bioequivalence Randomized Clinical Trials
C. Diaz-Tufinio1, D. F. Ruiz-Aguilar1, D. A. Durante-Salmerón1, J. A. Palma-Aguirre2
1Tecnologico de Monterrey, Escuela de Ingeniería y Ciencias, Mexico, Mexico; 2Axis Clinicals Latina, Clinical Unit, Mexico, Mexico
Background/Introduction: Bioequivalence studies test pharmacokinetic parameters between a reference product, whose patent has expired, vs. a test formulation containing the same active principle (AP) [1]. Although there is wide experience with these brand products in the market, it is important to monitor any adverse event arising from its consumption, not only in these randomized clinical trials (RCT), but throughout the real-world setting.
Objective/Aim: To analyze the adverse drug reactions (ADR) derived from bioequivalence trials conducted in Mexican population, studying them by therapeutic area and metabolizing enzymes of the active principles.
Methods: We analyzed retrospectively bioequivalence trials conducted between 2012 and October 2020 in the authorized third-party Axis Clinicals Latina in Mexico, with healthy volunteers. All protocols included were reviewed by an Institutional Review Board authorized by the National Commission of Bioethics (CONBIOETICA) [2], then submitted to the sanitary federal agency COFEPRIS for its approval, based on the current regulation [3], and uploaded to the National Registry of Clinical Trials [4]. The evaluation of ADR was performed through standardized algorithms, such as Naranjo algorithm, for assessing its severity, seriousness, and causality.
Results: Among 246 clinical trials with 290 individual analyses, 130 unique active principles (AP) comprising 13 therapeutic areas were studied. Out of 10,602 intention-to-treat volunteers, 10,125 completed the trials without any medical condition. Overall, 4285 ADR were registered, counting 2153 for the test products and 2085 for the reference formulations (47 ADR experienced prior to any drug administration). Regarding its seriousness, all ADR were non serious (level 1 of 2); about its severity, 73.5% resulted as moderate (level 2 of 3) and only 1.0% as severe (level 3 of 3).
Ranked by ADR rate (Number of ADR / subjects in each study), three AP showed a rate greater than 2 (Aripiprazole, Quetiapine and Mirtazapine), meaning several ADR experienced per subject. Then, 10 AP exhibited an ADR rate between 1–2. Four of them had rates between 0.8–1. Based on this ADR rate ranking, the top 5 of these 17 AP, as well as 13 out of these 17, belong to the neurology area, most of them being metabolized mainly by CYP2D6, CYP1A2 and CYP3A4.
Conclusion: With this work, we provide a cumulative descriptive analysis of the ADR in bioequivalence experiences, reporting the AP and its therapeutic areas with which greater rates of ADR are expected. The authors aim to boost the investigation of safety data in bioequivalence RCT.
References/Further Sources of Information
Midha KK, McKay G. Bioequivalence; its history, practice, and future. AAPS J. 2009 Oct 06;11(4):664–70.
CONBIOETICA (Comisión Nacional de Bioética). COMITÉS DE ÉTICA EN INVESTIGACIÓN. https://www.gob.mx/salud/conbioetica. Published 2019.
Comisión Federal para la Protección contra Riesgos Sanitarios (COFEPRIS). Norma Oficial Mexicana NOM-177-SSA1-2013. Diario Oficial de la Federación; 2013. http://www.dof.gob.mx/nota_detalle.php?codigo=5314833&fecha=20/09/2013
COFEPRIS. Consulta de ensayos clínicos registrados ante COFEPRIS. Registro Nacional de Estudios Clínicos (RNEC). http://siipris03.cofepris.gob.mx/Resoluciones/Consultas/ConWebRegEnsayosClinicos.asp.
P060 Persistent Sexual Dysfunction in SSRI Users: Scoping Review and Presentation of 93 Cases from The Netherlands
K. Chinchilla1, F. van Hunsel1, C. Ekhart1
1Netherlands Pharmacovigilance Centre Lareb, Signal Detection, ‘s-Hertogenbosch, The Netherlands
Background/Introduction: SSRIs are potent disruptors of sexual function. These effects can persist after the discontinuation of the SSRI, leading to Post SSRI Persistent Sexual Dysfunction (PSSD). This refers to a hypodynamic sexual state that can persist for decades carrying a high impact on the patient’s life (1, 2).
Objective/Aim: To map the characteristic factors related to PSSD through a literature review, and present the cases of PSSD received by the Netherlands pharmacovigilance centre Lareb.
Methods: A systematic literature review was performed following the PRISMA-ScR guidelines (3) to identify different aspects that describe PSSD. The search was conducted in PubMed and The Cochrane library using the keywords: SSRI prolonged sexual dysfunction, SSRI permanent sexual dysfunction, PSSD and Post SSRI sexual dysfunction. Articles published between 2000 and 2020 were included.
In addition, all reports submitted to Lareb between 1991 and 2021 with an SSRI as suspected drug and the persistence of the following ADRs: sexual dysfunction, erectile dysfunction, loss of libido, libido decreased, anorgasmia, ejaculation disorder or ejaculation failure were manually reviewed and analyzed.
Results: PSSD symptoms (Table 1) can emerge during treatment and endure or can appear after the withdrawal of the SSRI. There are no particular patient susceptibility characteristics and it can happen with all SSRIs. It is difficult to diagnose PSSD because sexual dysfunction can be seen as a sign of depression relapse and patients who express these sexual complaints frequently face lack of credibility and underestimation. The available treatment to alleviate the symptoms have little to no benefits and more research is needed to understand the mechanisms causing PSSD (1, 4, 5).
A total of 93 reports of PSSD were identified with the following ADRs: loss or decrease of libido (n = 56), erectile dysfunction (n = 26), ejaculation disorders (n = 6), anorgasmia (n = 7) and nonspecific ‘sexual dysfunction’ (n = 13) and the causative drugs were: paroxetine (n = 27), citalopram (n = 27), fluoxetine (n = 12), sertraline (n = 11), venlafaxine (n = 8), escitalopram (n = 8) and fluvoxamine (n = 2). The median time patients had not recovered was one year and four months after discontinuing their SSRI when they reported to Lareb.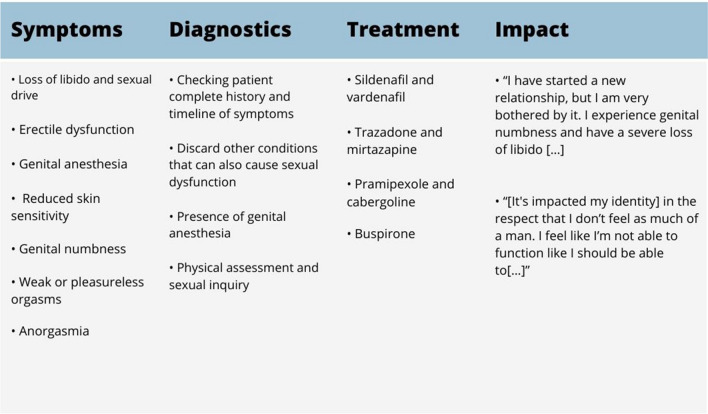
Conclusion: PSSD has physical and psychological implications. Recognition of PSSD among practitioners is necessary to ensure the best management and treatment possible for the patient.
References/Further Sources of Information
Healy D, Le Noury J, Mangin D. Enduring sexual dysfunction after treatment with antidepressants, 5α-reductase inhibitors and isotretinoin: 300 cases. Int J Risk Saf Med. 2018;29(3–4):125–34.
Stinson RD. The impact of persistent sexual side effects of selective serotonin reuptake inhibitors after discontinuing treatment: a qualitative study. Iowa Research Online University of Iowa; 2013.
Tricco A, Lillie i, Zarin s, O'Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):7.
Ben-Sheetrit J, Aizenberg D, Csoka AB, Weizman A, Hermesh H. Post-SSRI Sexual Dysfunction: Clinical Characterization and Preliminary Assessment of Contributory Factors and Dose-Response Relationship. J Clin Psychopharmacol. 2015;35(3):273–8.
Reisman Y. Sexual consequences of post-SSRI syndrome. Sex Med Rev. 2017;5(4):429–33.
P061 A Nationwide Study in Romania on the Use of Proton Pump Inhibitors Between 2012 and 2018
A. Farcas1, A. Rusu2, C. Mogosan1, C. Bucsa1
1“luliu Hatieganu” University of Medicine and Pharmacy Cluj-Napoca Romania, Drug Information Research Center, Cluj-Napoca, Romania; 2“Iuliu Hatieganu” University of Medicine and Pharmacy-Cluj-Napoca-Romania, Diabetes and Nutrition Diseases Department-, Cluj-Napoca, Romania
Background/Introduction: The use of proton pump inhibitors (PPIs) has grown every year in both western and eastern European countries leading to concerns regarding the progressive increase of the costs of therapy and greater potential harms for the patients when PPIs are misused.
Objective/Aim: As data in Romania is scarce, the objective of the present analysis was to describe the national trends in PPIs utilization during January 2012-December 2018 and prescription patterns during 2018.
Methods: We conducted a retrospective analysis of pharmacy sales data on PPIs provided by IQVIA Romania for the time of interest. IQVIA provided nationally extrapolated data (IQVIA proprietary algorithm with a maximum 2% error) which was used for trends analysis and data retrieved from approximately 4000 pharmacies (approx. 70% of the overall market) used for the 2018 prescription analysis. The data for utilization trends is expressed in defined daily dose (DDD) per 1000 inhabitants per day.
Results: Overall PPIs utilization increased from 29.53 DDD/1000/day in 2012 to 50.20 DDD/1000/day in 2018 (a 70% increase) with an increase of 23% in costs. A continuous increase in utilization in successive years was noticed for all PPIs (except lansoprazole). The highest increase was for pantoprazole (a 269% increase), followed by esomeprazole (a 191% increase), while the lansoprazole consumption decreased with 37%. Overall, the highest PPIs increase was noticed in-between 2015 and 2016 (13% increase). Throughout all years, the largest share of utilization among the class was for omeprazole, followed by pantoprazole, esomeprazole and lansoprazole. Among prescriptions from 2018, the standard 20 mg dose for omeprazole and 30 mg dose for lansoprazole were mostly prescribed (98% and 94%). For pantoprazole, the low 20 mg dose and the standard 40 mg dose were equally prescribed (52% and 48%). However, for esomeprazole the high 40 mg dose was recommended in 34% prescriptions, aside the standard 20 mg dose (55%) and the low 10 mg dose (11%).
Conclusion: The overall outpatients use of PPIs had a considerable 70% increase over the 7-years period analyzed. A further in-depth analysis of the appropriateness of use, including indication and therapy duration, is therefore warranted.
References/Further Sources of Information
N/A.
P062 Incidence of Adverse Drug Reactions in Hormonal Preparations Among Indian Patients: A Systematic Review and Meta-Analysis of Prospective Studies
P. Kharb1, V. Kapoor2, P. Salwan1
1SGT Medical College-Hospital and Research Institute-University Gurugram, Department of Pharmacology, Gurugram, India; 2Al Falah School of Medical Science and Research Centre, Department of Pharmacology, Faridabad, India
Background/Introduction: Adverse Drug Reactions (ADRs) are a well-recognized public health problem and have profound effects on the patients’ quality of life, as well as creating an increased burden on the healthcare system [1]. However, under-reporting of suspected ADRs by health professionals is a widespread problem in India [2]. Evidence-based systematic review and meta-analysis of adverse drug reactions in India is very sparsely reported.
Objective/Aim: To perform a comprehensive systematic review and meta-analysis of the prospective studies on adverse drug reactions (ADRs) from hormonal preparations among Indian patients including heterogeneity evaluation.
Methods: An electronic search from PubMed, IndMed/MedInd, Google Scholar and ProQuest from 1st January 2001 until July 2018 was conducted. All the prospective studies on ADRs related to hormonal preparations in Indian patients were included. A meta-analysis was performed by calculating incidence with their 95% confidence intervals (CIs), heterogeneity and risk of bias.
Results: A total of 25 prospective studies citations were included for the meta-analysis for monitoring adverse drug reactions in hormonal preparations published over 18-year period. The overall incidence of adverse drug reaction due to hormonal preparations reported from included studies was 0.46% [95% CI 0.19–0.84]. The level of heterogeneity between studies was high (I2 = 99.05% [95% CI 98.41–99.54]), hence this estimate has to be viewed with caution. Publication bias was detected for incidence estimates (Egger’s test: z = 4.450, p < 0.0001) using the funnel plot. The most common ADRs reported in this review were associated with the skin and subcutaneous tissue disorders, musculoskeletal disorders and endocrine disorders.
Conclusion: The present review highlights the incidence of ADRs and their characteristics in hormonal preparations among Indian patients. However, further studies are needed to monitor the adverse events in order to effectively report the incidence among Indian patients. There seems to be considerable potential for increasing the studies on assessing the ADRs by advocating the guided reporting indicators, overcoming one of the major limitation i.e. heterogeneity, owing to the wide variation in methodologies, thus reducing the risk of bias and guiding future research areas in this important area of concern.
References/Further Sources of Information
Gupta M, Kharb P, Kalaiselvan V, Shridhar M, Singh GN, Kshirsagar N, et al. Pharmacovigilance programme of India: the journey travelled and the way forward. WHO Drug Inf. 2018;32(1):10–7.
World Health Organization Safety of Medicines—A guide to detecting and reporting adverse drug reactions—why health professionals need to take action. Geneva: World Health Organization; 2002. 16p. Report Number: WHO/EDM/QSM/2002.2. Accessed from: http://apps.who.int/medicinedocs/en/d/Jh2992e/.
P063 Self-Medication of a Cancer Patient with Cresol
C. Nouibi1, H. Bechar2, A. Cherif Chefchaouni1, Y. Rahali1
1National Institute of Oncology Sidi Mohamed Ben Abdellah, Pharmacy, Rabat, Morocco; 2National Institute of Oncology Sidi Mohamed Ben Abdellah, Pharmacovigilance, Rabat, Morocco
Background/Introduction: Some non-medicinal products can be misused as an attempt to treat some chronic diseases that are difficult to cure, especially in a social environment and a cultural context that nourish these ideas and show off the virtues of certain substances to the detriment of the available therapeutic possibilities through medication. The use of such methods leads to a toxicity that can be serious, especially in the case of concomitant use, as was the case of our patient who consumed white marrubus, juniper oil, and a disinfectant: cresol.
Objective/Aim: We will be reporting the case of a patient who used some non-medicinal substances during the process of her treatment from breast cancer in the National Institute of Oncology in Rabat, Morocco.
Methods: A case report sent to the pharmacovigilance unit of the institute on March, 10th 2021.
Results: The patient was followed for breast cancer, treated by chemotherapy then radiotherapy. She used to take cresol at a rate of one spoonful per day during the first week and then half a glass during the second, in addition to juniper oil and white marrube, which required her hospitalization for monitoring since she presented signs of intoxication with gastritis, burns on the feet, hypersudation and obnubilation. The treatment was followed by the advice to immediately stop taking these substances and a raise of awareness about the danger of self-medication for the compliance of her treatment.
Conclusion: The favourable evolution of this patient's condition does not prevent the questioning of such practices dictated essentially by the weakened psychological factor especially with cancer patients who are particularly vulnerable.
References/Further Sources of Information
None.
P064 Hepatotoxicity During Treatment for Multidrug Resistant Tuberculosis
T. Oscanoa1,2,3, S. Moscol4, J. Luque3, S. Leon-Curiñaupa3, A. Carvajal5
1Universidad Nacional Mayor de San Marcos-Facultad de Medicina-Lima-Perú., Pharmacology, Lima, Peru; 2Hospital Almenara-ESSALUD, Geriatrics, Lima, Peru; 3Universidad de San Martín de Porres-Facultad de Medicina., Drug Safety Research Center., Lima, Peru; 4Hospital Almenara-ESSALUD, Pneumology, Lima, Peru; 5Universidad de Valladolid, Pharmacology, Valladolid, Spain
Background/Introduction: The treatment of tuberculosis faces two major challenges, drug resistance and adverse drug reactions (ADRs), among which drug-induced liver injury (DILI) is one of the most severe [1–3].
Objective/Aim: To describe the clinical characteristics of DILI in multidrug-resistant tuberculosis patients (MDR-TB).
Methods: This is a retrospective cases series. Clinical records of hospitalized patients diagnosed with MDR-TB and DILI at Almenara Hospital in Lima (Peru) from January 2014 to December 2018 were retrieved. The criteria of the DILI-Expert Working Group, for the diagnosis of DILI [4], and the RUCAM (Roussel Uclaf Causality Assessment Method) [5], for causality assessment were used.
Results: We reported 7 cases of MDR-TB and DILI, the mean age (standard deviation) was 39.1 (3.3) years. As an average, DILI appeared after 30.4 (27.7) days of starting treatment. Three (43%) patients presented jaundice. As for the pattern, in 4 (57%) was hepatocellular and in 3 (43%) cholestatic. In 4 patients, DILI were mild; it was moderate in 3. In all cases, the antimycobacterial pyrazinamide was involved (pyrazinamide solo, 4; pyrazinamide and ethionamide, 1; pyrazinamide, rifampicin, and isoniazid, 1; pyrazinamide and rifampicin, 1). The average hospital stay was 48.1 (48.7) days. The mean alkaline phosphatase (FA), alanine aminotransferase (ALT) and serum gamma-glutamyltranspeptidase (GGT) were 2.4 (1.1), 7.9 (7.1) and 5.6 (3.7) times the normal upper limit (NUL), respectively. The mean total bilirubin was 2.3 (2.1), range 0.5-6.4 mg / dl. As part of the patient discharge scheme, quinolones were administered to 7 patients (levofloxacin, 6; ofloxacin, 1) and in one patient amoxicillin acid/clavulanic acid was added.
Conclusion: DILI in MDR-TB patients seems to appear after the first month of treatment. The common injury pattern was hepatocellular and pyrazinamide was the antimycobacterial most frequently involved.
References/Further Sources of Information
Nathanson E, Gupta R, Huamani P, Leimane V, Pasechnikov AD, Tupasi TE, et al. Adverse events in the treatment of multidrug-resistant tuberculosis: results from the DOTS-Plus initiative. Int J Tuberc Lung Dis. 2004;8:1382–4.
Keshavjee S, Gelmanova IY, Shin SS, Mishustin SP, Andreev YG, Atwood S, et al. Hepatotoxicity during treatment for multidrug-resistant tuberculosis: occurrence, management and outcome. Int J Tuberc Lung Dis. 2012;16:596–603. https://doi.org/10.5588/ijtld.11.0591.
Lee SS, Lee CM, Kim TH, Kim JJ, Lee JM, Kim HJ, et al. Frequency and risk factors of drug-induced liver injury during treatment of multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2016;20:800–5. https://doi.org/10.5588/ijtld.15.0668.
Aithal GP, Watkins PB, Andrade RJ, Larrey D, Molokhia M, Takikawa H, et al. Case definition and phenotype standardization in drug-induced liver injury. Clin Pharmacol Ther. 2011;89:806–15. https://doi.org/10.1038/clpt.2011.58.
Danan G, Teschke R. RUCAM in drug and herb induced liver injury: the update. Int J Mol Sci. 2015;17:14. https://doi.org/10.3390/ijms17010014.
P065 Drug-Induced Vasculitis: A Report of 06 Cases
B. Moukafih1, A. Tebaa2, R. Soulaymani-Bencheikh2, A. El kartouti3, S. Achour4
1Central Pharmacy Department-CHU Hassan II-Fez-Morocco, Pharmacology Laboratory-Faculty of Medicine and Pharmacy of Fez-Sidi Mohammed Ben Abdellah University-Fez-Morocco, Fez, Morocco; 2Moroccan Anti Poison and Pharmacovigilance Center-Rabat 10000-Morocco, 3Hospital Pharmacy Service-Moulay Ismaïl Military Hospital-Meknès-Morocco, Pharmacology Laboratory-Faculty of Medicine and Pharmacy of Fez-Sidi Mohammed Ben Abdellah University-Fez-Morocco, Fez, Morocco; 4Toxicology Department-CHU Hassan II-Fez-Morocco, Medical Center for Biomedical and Translational Research-Faculty of Medicine and Pharmacy of Fez-Sidi Mohammed Ben Abdellah University-Fez-Morocco, Fez, Morocco
Background/Introduction: Cutaneous vasculitis is defined as inflammation of the skin blood vessels [1]. Histologically, the most common cutaneous manifestation of this clinical entity is leukocytoclastic vasculitis, which preferentially affects small vessels of the lower extremities and is clinically characterized by palpable purpuric lesions, sometimes with slight focal necrosis and ulceration [2].
Objective/Aim: Our study aimed to study the epidemiological, clinical, histopathological and evolutionary characteristics of drug-induced vasculitis from a series of cases and to specify the different drugs involved.
Methods: We conducted a retrospective study during the period from January 2020 to June 2021 from the cases notified to the regional pharmacovigilance center Fez, Morocco. The diagnosis was established according to the criteria proposed by the group of the American college of rheumatology (ACR).
Results: Our study included six cases of drug-induced vasculitis over a 10-year period, with a mean incidence of 1.3 new cases per year. Mean age of patients was 51.4 years. The mean delay from the treatment onset was 12.23 days with extremes ranging from 8 days to 6 weeks. Four had pure skin involvement. Association with other extracutaneous complaints was present in two cases. Cutaneous biopsy was performed in all patients showing a pathological pattern of leukocytoclastic vasculitis, associated with fibrinoid necrosis, extravasation of red blood cells and allergic capillaritis. The outcome was favourable for all patients. The offending drugs in our series were Penicillins (two cases), Allopurinol (two cases), phenobarbital (one case), and Ciprofloxacin (one case).
Conclusion: Clinicians should be aware of this serious adverse event because any continuation of treatment may be fatal. Anamnestic, clinical, biological and histopathological findings allow the early recognition of drug-induced vasculitis. Adequate treatment prevents systemic spreading and a worse prognosis.
References/Further Sources of Information
Jennette JC, Falk RJ: Small-vessel vasculitis. N Engl J Med. 1997;337:1512–23.
Roujeau JC, Stern RS: Severe adverse cutaneous reactions to drugs. N Engl J Med. 1994;331:1272–85.
P066 Assessment of the Severity, Preventability and Causality of Adverse Drug Reactions in Short- and Medium-Stay Psychiatric Inpatient
S. Martin1, M. G. Philipot1, E. Lepoix2, T. Trenque2, P. Pascali1
1Public Mental Health Establishment of Marne, Pharmacy, Chalons en Champagne, France; 2CHU Reims, Pharmacovigilance Pharmacoepidemiology, Reims, France
Background/Introduction: Psychiatric hospitalization, in short and medium-stay units, consists of the overall care of patients in the acute phase to stabilize them and improve their condition to allow their discharge and outpatient care.
Objective/Aim: In this study, we evaluated the causality, severity and preventability of adverse drug reactions (ADRs) mentioned in the medical records of this population of hospitalized patients.
Methods: A retrospective observational study was conducted among hospitalized patients on the 24th of November, 2020, in short- and medium-stay units in our psychiatric hospital. The medical records of each patient were analyzed to identify ADRs that occurred during their hospitalization. For each ADR identified, severity was assessed using the Hartwig scale, preventability was assessed using the French Olivier scale and causality was assessed using the French Arimone method.
Results: Of the 180 patients in our study group, 72 had at least one ADR (median age = 51 years, range 15–85; women: 56%; prescribed drugs = 9 ± 3.5). 149 ADRs were identified: 99.3% were mild or moderate in severity and only one was severe. 87.9% of ADRs were unavoidable and 12.1% were potentially preventable. 85.9% of the ADRs identified were described in the Summary of Product Characteristics of the incriminated molecules. The most common ADRs were sedation/somnolence (35%), asthenia (8.7%) and extrapyramidal syndrome (7.4%). Loxapine (18.1%), diazepam (12.2%) and cyamemazine (8%) were the most involved drugs in the occurrence of ADRs.
Conclusion: The most suspected molecules of causing ADRs have been the neuroleptics and have predominantly affected the central nervous system. This work provides an opportunity to educate medical staff on the appropriate use and monitoring of psychiatric medications to prevent the potential occurrence of ADRs.
References/Further Sources of Information
Arimone Y, Bidault I, Dutertre JP, Gérardin M, Guy C, Haramburu F, Hillaire-Buys D, Meglio C, Penfornis C, Théophile H, Valnet-Rabier MB; Cercle de Réflexion sur l’Imputabilité (CRI). Updating the French method for the causality assessment of adverse drug reactions. Therapie. 2013 Mar-Apr;68(2):69–76. https://doi.org/10.2515/therapie/2013016. Epub 2013 Jun 18. PMID: 23773347.
Olivier P, Caron J, Haramburu F, Imbs JL, Jonville-Béra AP, Lagier G, Sgro C, Vial T, Montastruc JL, Lapeyr-Mestre M. Validation d'une échelle de mesure: exemple de l'échelle française d'évitabilité des effets indésirables médicamenteux [Validation of a measurement scale: example of a French Adverse Drug Reactions Preventability Scale]. Therapie. 2005 Jan-Feb;60(1):39–45. French. https://doi.org/10.2515/therapie:20.
P067 Assessment of Healthcare Facilities Associated with JAKi Drugs Used in Rheumatoid Arthritis Patients in Tuscany, Italy: The LEONARDO
G. Valdiserra1, I. Convertino1, S. Giometto1, V. Lorenzoni2, R. Gini3, G. Turchetti2, E. Fini4, C. Bartolini3, O. Paoletti3, S. Ferraro1, E. Cappello1, C. Blandizzi5, E. Lucenteforte1, M. Tuccori6
1University of Pisa, Department of Clinical and Experimental Medicine, Pisa, Italy; 2Scuola Superiore Sant’Anna, Institute of Management, Pisa, Italy; 3Tuscan Regional Healthcare Agency, Pharmacoepidemiology, Florence, Italy; 4University of Pisa, Medical Specialization School of Pharmacology, Pisa, Italy; 5University of Pisa-University Hospital of Pisa, Department of Clinical and Experimental Medicine-Unit of Adverse Drug Reactions Monitoring, Pisa, Italy; 6University Hospital of Pisa-University of Pisa, Unit of Adverse Drug Reactions Monitoring-Department of Clinical and Experimental Medicine, Pisa, Italy
Background/Introduction: In Europe, JAKi were approved for rheumatoid arthritis (RA) in 2018 [1, 2]. Post-marketing surveillance is crucial to confirm utilization and safety [3].
Objective/Aim: This study is aimed at observing Regional Healthcare Service utilization as proxy of disease burden and safety of new JAKi RA users in the immediate post-approval period by using Tuscan real-world data. We described Emergency Department (ED) accesses and hospitalizations for any causes and RA visits both before and after the treatment initiation.
Methods: We performed a retrospective cohort study using Tuscan data of different healthcare administrative databases, i.e. repositories of drug-supplying, ED admissions, hospitalizations, and specialist visits (EUPAS35746). Two cohorts were selected: (1) the first had a JAKi first-ever supply from January 2018 to December 2019, (2) the second from January 2018 to June 2019. We included Tuscan adult patients with at least 10 years of data before the cohort entry. In the first cohort, we calculated the number of ED accesses and hospitalizations for any causes, and RA visits recorded in the five years preceding the first-ever JAKi supply, while in the second, these events were assessed in the six months of follow-up. The mean time and standard deviation (SD) to events were estimated.
Results: The first cohort included 363 new JAKi-users. The median age was 63 years (interquartile range, IQR 52.5–71.5). Females were 293 (80.7%). Baricitinib was supplied to 285 (78.5%) patients and tofacitinib to 78 (21.5%). The mean number of the investigated events per patient/year slightly increased from the fifth to the first year before the JAKi supply: ED accesses rose from 0.47 to 0.56, hospitalizations from 0.23 to 0.31, and RA visits from 1.02 to 1.19. The second cohort, made up of 220 new JAKi-users, included 179 (81.4%) females. Tofacitinib was supplied to 42 (19.1%) patients while baricitinib to 178 (80.9%). The median age was 62.0 (IQR 53.0–71.0). In the follow-up, 54 (24.5%) patients had ED admissions, 28 (12.7%) hospitalizations, and 38 (17.3%) RA visits. Not all these events were necessarily drug-related. The mean time to the first ED access was 73.5 days (SD 54.1), to hospitalization 89.6 days (SD 54.8), and to RA visit 60.9 days (SD 51.3). The causes most frequently associated with hospitalizations were cardiovascular (69.2%) and musculoskeletal disorders (64.1%), while those reported to ED accesses were injury and poisoning (18.3%), and skin disorders (13.8%).
Conclusion: The healthcare service utilization and related causes were in line with the JAKi safety profile and the underline disease.
References/Further Sources of Information
Olumiant | European Medicines Agency. Accessed February 11, 2021. https://www.ema.europa.eu/en/medicines/human/EPAR/olumiant.
Xeljanz | European Medicines Agency. Accessed February 11, 2021. https://www.ema.europa.eu/en/medicines/human/referrals/xeljanz.
European Medicines Agency. EMA confirms Xeljanz to be used with caution in patients at high risk of blood clots. 2019;31(November). https://www.ema.europa.eu/en/news/ema-confirms-xeljanz-be-used-caution-patients-high-risk-blood-clots.
P068 Dolutegravir-Based ART: Exploring Patient Safety and Acceptability Following National Roll-Out in Uganda
H. Zakumumpa1, F. Kitutu Lecturer2, N. Helen3, D. Nakitto-Kesi Research Officer4, R. Kiguba Senior Lecturer5
1Makerere University, College of Health Sciences, Kampala, Uganda; 2Makerere University-College of Health Sciences, Department of Pharmacy, Kampala, Uganda; 3National Drug Authority of Uganda, Directorate of Product Safety, Kampala, Uganda; 4National Drug Authority of Uganda, Directorate of Product Safety, Kampala, Uganda; 5Makerere University-College of Health Sciences, Department of Pharmacology and Therapeutics, Kampala, Uganda
Background/Introduction: The World Health Organization recommends dolutegravir (DTG) as the backbone for first-line and second-line antiretroviral therapy (ART) worldwide. However, little is known about the acceptability and tolerability of DTG-based ART at routine points-of-care in Uganda.
Objective/Aim: We set out to explore the perceptions of ART clinic managers regarding the acceptability and tolerability of DTG-based ART since national roll-out in March 2018 in Uganda.
Methods: We adopted a qualitative descriptive design involving 49 ART clinic managers and clinicians. Between September 2020 and February 2021, we conducted 22 in-depth interviews with ART clinic managers and clinicians in 12 purposively selected health facilities across Uganda. The selection of study sites ensured diversity in facility ownership-type (public/private), level of service delivery (tertiary/secondary/primary) and the four major geographic sub-regions of Uganda. We conducted three focus group discussions with 27 ART clinicians in the participating facilities. Data were analyzed by thematic approach.
Results: The participants acknowledged that DTG-based ART is well tolerated by the majority of their patients who appreciate the reduced pill burden, perceived less side effects and superior viral load suppression. However, they reported that a number of patients experience adverse drug reactions (ADRs) after being transitioned to DTG. Hyperglycemia is, by far, the most commonly reported suspected ADR associated with DTG-based regimens and was cited in all but two participating facilities. Insomnia, weight gain and reduced libido are among the other frequently cited suspected ADRs. In addition, ART clinic managers perceived some of the suspected ADRs as resulting from drug interactions between dolutegravir and isoniazid. Weak diagnostic capacities and shortage of associated commodities (e.g. glucometers and test kits) were reported as impediments to understanding the full extent of ADRs associated DTG-based ART.
Conclusion: While DTG-based regimens were perceived to be well tolerated by the majority of patients at participating facilities, a number of patients were reported to experience suspected ADRs key among which were hyperglycemia, insomnia and reduced libido. We recommend interventions aimed at strengthening blood glucose monitoring for patients taking DTG-based regimens and precautionary assessment of all patients prior to initiating them on DTG for known risk factors for DTG-related ADRs.
References/Further Sources of Information
Phillips AN, Venter F, Havlir D, Pozniak A, Kuritzkes D, Wensing A, Lundgren JD, De Luca A, Pillay D, Mellors J, Cambiano V. Risks and benefits of dolutegravir-based antiretroviral drug regimens in sub-Saharan Africa: a modelling study. Lancet HIV. 2019 Feb 1;6(2):e116–27.
Dorward J, Hamers RL. Dolutegravir in sub-Saharan Africa: context is crucial. Lancet HIV. 2019 Feb 1;6(2):e72–3.
Zheng A, Kumarasamy N, Huang M, Paltiel AD, Mayer KH, Rewari BB, Walensky RP, Freedberg KA. The cost‐effectiveness and budgetary impact of a dolutegravir‐based regimen as first‐line treatment of HIV infection in India. Journal of the International AIDS Society. 2018 Mar;21(3):e25085.
Lamorde M, Atwiine M, Owarwo NC, Ddungu A, Laker EO, Mubiru F, Kiragga A, Lwanga IB, Castelnuovo B. Dolutegravir-associated hyperglycaemia in patients with HIV. Lancet HIV. 2020 Jul 1;7(7):e461–2.
P069 Drugs and Pregnancy in a Real-Life Setting: Maternal, Fetal and Neonatal Safety Outcomes
C. Scavone1, A. Mascolo1, G. di Mauro1, R. Ruggiero1, C. Rafaniello1, C. Ferrajolo1, L. Sportiello1, F. Rossi1, A. Capuano1
1University of Campania “Luigi Vanvitelli”, Experimental Medicine, Napoli, Italy
Background/Introduction: Pregnant women have been historically representing the frailest population in terms of safety profile of drugs and vaccines, being identified as “vulnerable” population in need of special protections in clinical research.
Objective/Aim: We aim to identify: the amount of ICSRs reporting ADRs related to pregnancy, fetal and neonatal outcomes; drugs most commonly reported as suspected in ICSRs reporting pregnancy, fetal and neonatal outcomes; types ADRs related to pregnancy, fetal and neonatal outcomes.
Methods: We analyzed ICSRs that were sent from January 2001 until March 2021 to the Campania regional Center of Pharmacovigilance (South of Italy) describing the occurrence of ADRs belonging to the SOCs “Pregnancy, puerperium and perinatal conditions”, “Surgical and medical procedures” and “Congenital, familial and genetic disorders”. We also retrieved ICSRs describing the occurrence of any type of ADRs in neonates (aged < 29 days).
Results: From January 2001 to March 2021, 29 ICSRs reported ADRs belonging to the analysed SOCs or described the occurrence of any type of ADRs in neonates. Almost 75% of ICSRs fell into the following categories: spontaneous abortion (n = 9; 31%), congenital disorders (n = 8; 27.5%) and other disorders affecting the mother or the baby (n = 5; 17.2%); the remaining 25% of ICSRs fell into the remaining categories (4 ICSRs in unintended pregnancy, 2 in preterm birth e 1 in ectopic pregnancy). All ADRs were considered to be serious. Almost 90% of ICSRs were reported by healthcare professionals. Drugs most commonly reported as suspected (> 40% of ICSRs) were those indicated for the treatment of autoimmune conditions, including natalizumab (4 ICSRs), methotrexate, ocrelizumab, alemtuzumab and glatiramer acetate (2 ICSRs each one).
Conclusion: The spontaneous reporting system is a valuable tool for the safety monitoring of medicines and vaccines especially in frail population that are normally excluded by the premarketing clinical trials, such as pregnant women and children. In addition, among ADRs that were evaluated in this study, teratogenesis is still of great concern considering that animal studies that focus are rarely adequate to predict human outcomes and that clinical studies in humans systematically exclude pregnant women and ensure that those of childbearing potential do not become pregnant during the period of drug exposure. Therefore, data from ICSRs reporting ADRs occurring in pregnant women represent the main source of information on the safety profile of medicines in this population.
References/Further Sources of Information
Blehar MC, Spong C, Grady C, Goldkind SF, Sahin L, Clayton JA. Enrolling pregnant women: issues in clinical research. Womens Health Issues. 2013;23(1):e39–e45. https://doi.org/10.1016/j.whi.2012.10.003.
European Medicines Agency. Workshop on benefit-risk of medicines used during pregnancy and breastfeeding. https://www.ema.europa.eu/en/events/workshop-benefit-risk-medicines-used-during-pregnancy-breastfeeding. Last access 18-05-2021.
Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin Perinatol. 2015;39(7):512–9. https://doi.org/10.1053/j.semperi.2015.08.003.
P070 The Role of the Pharmaceutical Industry in Enhancing Pharmacovigilance: Perspectives from Asia and the Middle East
I. Abdeldayem1, A. Abdelrahman2, M. Alkhakany3
1Arriello s.r.o., Pharmacovigilance, Prague, Czech Republic; 2Boehringer Ingelheim, Patient Safety and Pharmacovigilance, Dubai, United Arab Emirates; 3Boehringer Ingelheim, TA Analytics-Insights and Excellence, Konigstein, Germany
Background/Introduction: Pharmaceutical industry has a fundamental role in producing effective and safe medicine. However, this strategy was driven by profitability rather than the benefit of human health. Thalidomide tragedy caused worldwide birth defects, which forced the World Medical Association to set standards: for clinical research and post-marketing reporting. The United States Food and Drug Administration released the Kefauver Harris Amendment demanding accurate disclosure of side effects and the proof of efficacy for new medications (1). Although the countries in Asia and the Middle East region are facing many challenges, the healthcare system is still a top priority. The Arab Guidelines for Pharmacovigilance (PV) was published in 2015. The major obstacles to implement the PV concepts were a lack of training and funding. In Asia, PV functionality and legislative framework varied depending on local healthcare ecosystem networks (2). The key drivers to strengthen patient safety are health authorities, pharmaceutical companies, and healthcare professionals. As they should collaborate to understand, the customer’s needs and challenges.
Objective/Aim: To illustrate how the industry can be a key stakeholder in the safety of medicines and provide recommendations to strengthen pharmacovigilance in Asia and the Middle East.
Methods: This comprehensive narrative review described the role of the pharmaceutical industry in developing the culture of pharmacovigilance in Asia and the Middle East.
Results: This review considered the role of pharmaceutical companies is to establish strong local PV systems to detect, monitor, and evaluate the safety of the medicine, and closely collaborate with health authorities and healthcare professionals utilizing different channels. In Asia and the Middle East, networking between health authorities to leverage experiences, knowledge, and share data is still missing. In addition, HCP needs to focus on enhancing awareness and knowledge and educate patients about the importance of sharing safety concerns. Nonetheless, Asia and the Middle East region are missing Patient engagement through actively listening to patient’s concerns and preferences.
Conclusion: In this review, we demonstrated to have efficient drug safety; it is needed to speak one language through the harmonization of PV regulations. Our recommendation is the urgent establishment of communication channels between Health authorities in Asia and the Middle East to share knowledge, experience, and data. Also, we highlight the need for active partnerships between health societies and the pharmaceutical industry for patient awareness campaigns and for optimizing safety reporting. In the latter, digital solutions can offer an innovative approach for such sakes.
References/Further Sources of Information
Walsh, Robin. “A History of The Pharmaceutical Industry.” pharmaphorum, 1 Oct. 2010, pharmaphorum.com/articles/a_history_of_the_pharmaceutical_industry/. Accessed 3 Nov. 2019.
Uppsala Monitoring Centre, Global Pharmacovigilance, members, who program members. 2019. https://www.who-umc.org/global-pharmacovigilance/members/who-programme-members/. Accessed 21 Oct 2019.
P071 Effect of Proper Pharmacovigilance Awareness on Community Pharmacists’ Knowledge, Perception and Impact on Practice in Egypt
S. Bayoumi1, A. El Shafei2, C. Scavone3, A. Capuano4, H. Rostom5
1Eu2P Masters, Pharmacovigilance and Pharmacoepidemiology, Cairo, Egypt; 2Al Azhar University, Professor Assistant of Biochemistry and Molecular Biology-Faculty of Pharmacy, Cairo, Egypt; 3University of Campania “Luigi Vanvitelli”, Pharmacovigilance and Pharmacoepidemiology, Province of Caserta, Italy; 4Campania Regional Center for Pharmacovigilance and Pharmacoepidemiology, Pharmacovigilance and Pharmacoepidemiology, Province of Caserta, Italy; 5ISOP Egypt Chapter, Pharmacovigilance, Cairo, Egypt
Background/Introduction: Even though pharmacists play a key role in pharmacovigilance systems (1, 2, 3), some studies showed that they might have insufficient knowledge about ADRs’ reporting, suggesting the need for educational interventions to increase their role in the reporting process (4,5,6).
Objective/Aim: This study aimed to evaluate the knowledge, perception and the practice of community pharmacists in Egypt towards pharmacovigilance and ADRs’ reporting before and after the commencement of an educational intervention.
Methods: This study was carried out virtually through the online platform “Zoom”. The Study population comprised community pharmacists working at El Ezaby chain pharmacies in Cairo, Egypt. The study was divided in two phases. During the first phase, the pharmacists’ knowledge and perception regarding pharmacovigilance were assessed through a pre and post session electronic questionnaire to measure the effect of an educational intervention on them. During the second phase an electronic ICSR reporting form was sent to the pharmacists and they were asked to implement what they learned in the session in their daily life and to report through this reporting form any ADE they encounter during the following two months. A barriers survey was distributed at the end of the 2 months to determine the factors that prevented reporting.
Results: Sixty pharmacists participated in the study. The median knowledge, perception and total scores before the intervention were 7, 33 and 41, respectively. After the intervention there was a statistically significant increase (p < 0.0001) in the median scores to 9, 40.5 and 49 with a percentage increase of 23.5%, 11.7% and 13.8% in the total scores, respectively. None of the participants have reported any safety reports before the intervention but 10 out of the 60 were able to introduce the reporting practice and submit 19 reports in the 2 months following the intervention. Insufficient information from the patients and not knowing the association between the drug and the ADR were of the highest barriers reported that hinders reporting.
Conclusion: The educational intervention was successfully able to significantly improve the pharmacists’ knowledge and perception towards ADR reporting. It was also successful in introducing the reporting practice to their daily life. However, further education should be continuously provided to improve their behaviours towards ADR reporting and more studies on a wider range of Healthcare Professionals are needed. ADR reporting should be included as part of healthcare professionals’ job description so it is not only a personal preference but an obligation.
References/Further Sources of Information
van Grootheest AC, de Jong-van den Berg LTW. The role of hospital and community pharmacists in pharmacovigilance. Res Soc Adm Pharm. 2005 Mar 1;1(1):126–33.
Leone R, Moretti U, D’Incau P, Conforti A, Magro L, Lora R, et al. Effect of pharmacist involvement on patient reporting of adverse drug reactions: first Italian study. Drug Saf. 2013 Apr 1;36(4):267–76.
Parretta E, Rafaniello C, Magro L, Pittoni AC, Sportiello L, Ferrajolo C, et al. Improvement of patient adverse drug reaction reporting through a community pharmacist-based intervention in the Campania region of Italy. Expert Opin Drug Saf. 2014 Sep 1;13(sup1):21–9.
Vessal, G., Mardani, Z., Mollai, M. Knowledge, attitudes, and perceptions of pharmacists to adverse drug reaction reporting in Iran. Pharmacy World Science. [cited 2020 Oct 16]. https://link.springer.com/article/10.1007%2Fs11096-008-9276-6.
Community pharmacists’ knowledge, behaviors and experiences about adverse drug reaction reporting in Saudi Arabia. Saudi Pharmaceutical Journal [cited 2020 Oct 16]. https://reader.elsevier.com/reader/sd/pii/S1319016413000856?token=A23A9D378774E15C653B0107646BC00E8D6EE7F18DAD9E6FE0E66E0EAF21433D41D4C1B472354D28466E4DB107AC1916.
Suyagh M, Farah D, Abu Farha R. Pharmacist’s knowledge, practice and attitudes toward pharmacovigilance and adverse drug reactions reporting process. Saudi Pharm J SPJ. 2015 Apr;23(2):147–53.
P072 Physician’s Attitude Towards Clinical Pharmacists Advice on the Rational Prescribing of Antibiotics
A. Issac1, J. J. Kochuparambil1, J. Vayalikunnel1
1Mary Queen’s Mission Hospital, Department of Service Excellence and Quality, Kanjirappally, India
Background/Introduction: Rational prescribing of antibiotics is very essential in ensuring patient safety by decreasing the incidence of antibiotic resistance. The clinical pharmacist's opinion on the appropriateness of the antibiotic prescription can reduce the number of drug-related problems.
Objective/Aim: To evaluate the clinical pharmacist interventions on antibiotic therapy and the response of physicians toward these interventions.
Methods: The surveillance study was conducted under the initiative of the clinical pharmacy department in a secondary care hospital in South India from December 2020 to May 2021 to evaluate the appropriateness and rationality of the antibiotic prescription at the Internal Medicine Department. Demographic details of the patients including the age, sex, and medication error (in drug selection, dose, frequency, route of administration) were documented and category of intervention was prospectively recorded. The category, as well as the implementation status of the interventions, were compared with various associated factors using the chi-square test and p-value < 0.05% considered statistically significant.
Results: Of a total of 413 interventions, the overall acceptance rate was 56.1% (232). Frequently observed irrationality in antibiotics prescribing was with improper dosage adjustment in comorbid conditions. The interventions for antibiotic therapy optimization include IV to oral conversion and spectra-based drug selection. The acceptance of clinical pharmacist opinion was positively correlated with the good interpersonal relationship of the physician as well as the evidence from standard references. The acceptance rate was more among the patients with insurance claims with no statistical significance.
Conclusion: The study highlights the importance of evidence-based therapy and multidisciplinary healthcare team workups.
References/Further Sources of Information
USFDA Labels of Antibiotics.
Sharaf N, Al-Jayyousi GF, Radwan E, Shams Eldin SM, Hamdani D, Al-Katheeri H, Elawad K, Habib Sair A. Barriers of appropriate antibiotic prescription at PHCC in Qatar: perspective of physicians and pharmacists. Antibiotics. 2021 Mar;10(3):317.
P073 Development of Learning-Set of Pharmacovigilance for Healthcare Professionals in Brazil: Co-creation Experience Between Different Stakeholders
F. Lourenço1, T. Pinto1, L. Rodrigues2, A. Ribeiro3, M. Perroud4, H. Capucho5, A. Zago6, S. Schiaffino6
1USP-Universidade de São Paulo, Faculdade de Ciências Farmacêuticas, São Paulo, Brazil; 2Boehringer Ingelheim, Patient Safety and Pharmacovigilance, São Paulo, Brazil; 3Ministério da Saúde, REBRATS-Rede Brasileira de Avaliação de Tecnologias em Saúde, São Paulo, Brazil; 4UNICAMP-Universidade Estadual de Campinas, Faculdade de Ciências Médicas, Campinas, Brazil; 5UnB-Universidade de Brasília, Faculdade de Ciências da Saúde, Brasília, Brazil; 6Boehringer Ingelheim, Patient Safety and Pharmacovigilance, Ingelheim, Germany
Background/Introduction: Brazil is among the 10 biggest pharmaceutical markets in the world [1] and local recent publications show that prevalence of adverse events of medicines on Brazilian population is around 6.6% [2] and hospitalizations caused by potential side effects can reach a prevalence of approximately 46% [3]. Despite that, population does not have enough information about rational use of medicines. Besides, there is lack of capacity building among healthcare professionals (HCPs) and health services (HS) about their role in the healthcare with regards to safe use of medicines.
Objective/Aim: HCPs need to be instructed and encouraged to recognize and report adverse events of medicines inside and outside of the HS. With this, they will be able to make communities aware of the importance of taking care and paying special attention on medicine’s use.
Methods: Through a partnership between different stakeholders (pharmaceutical industry, academia, clinical practice), it was built a e-learning-set for HCPs based on the real need of target audience by sharing knowledge and experiences about correct, safe and rational use of medicines.
Results: A 30-h online “Patient Safety and Pharmacovigilance” Course split in 4 modules was offered by Pharmacy College (Universidade de São Paulo) and FIPFARMA (Fundação Instituto de Pesquisas Farmacêuticas) in Nov 2020. There were recorded classes, synchronous meetings and also workshops about the main topics: Overview of Healthcare and Pharmacovigilance (PV); Drug Safety and Management of Drug Utilization; Patient Safety Culture. 51 participants were approved and they expressed the importance of the course to improve adverse event notification flow in HS, enhance quality of existing processes, promote activities of education to other HCPs, including the development of a PV system from scratch in a covid-19 HS.
Conclusion: The e-learning format course was an effective tool to achieve and integrate HCPs in pandemics time, providing knowledge and changing mindset about PV. This initiative demonstrated that co-creation by different stakeholders with one common purpose “patient safety” can make the difference improving people’s live preventing and avoiding any unnecessary risk related to medicines.
References/Further Sources of Information
The IQVIA Institute. The Global Use of Medicine in 2019 and Outlook to 2023 [Internet]; USA; 2019 [updated 2019 Jan 29; cited 2021 Jun 21]. https://www.iqvia.com/insights/the-iqvia-institute/reports/the-global-use-of-medicine-in-2019-and-outlook-to-2023.
Sousa LAO de et al. Prevalência e características dos eventos adversos a medicamentos no Brasil. Cadernos de Saúde Pública [Internet]. 2018;34(4):e00040017. https://doi.org/10.1590/0102-311X00040017. Epub 29 Mar 2018. ISSN 1678-4464.
Varallo FR et al. Possible adverse drug events leading to hospital admission in a Brazilian teaching hospital. Clinics [Internet]. 2014 Mar;69(3):163–7. https://doi.org/10.6061/clinics/2014(03)03. ISSN 1980-5322.
The author(s) meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE).
The authors did not receive payment related to the development of this abstract.
P074 Enhance in the Incidence of Side Effects Reported to a New Regional Pharmacovigilance Center in Morocco, Between 2020 and 2021
A. Tebaa1, M. Mabrouki2, S. El Marnissi2, F. Z. Lasri2, M. Ait El Cadi2
1Moroccan Center of Pharmacovigilance-Rabat-Morocco, Pharmacology, Rabat, Morocco; 2Pharmacology and Toxicology Laboratory-Mohammed V University-Faculty of Medicine and Pharmacy-Rabat-Morocco, Pharmacy-Ibn Sina University Hospital Center, Rabat, Morocco
Background/Introduction: Since the creation of the regional pharmacovigilance center within the IbnSina University Hospital Center (UHC), a pharmacist has been dedicated to this activity, responsible for establishing work procedures, calculating accountability and redaction responses to the notifiers. Thus, the collaboration between the medical services and this regional center to improve the pharmacovigilance system within the UHC. This resulted in an increase in notifications qualitatively and quantitatively
Objective/Aim: Study the impact of setting up a regional center in the UHC, by comparing the incidence of skin adverse reaction notifications between 2020 and 2021.
Methods: A retrospective analysis was performed for the clinical presentation, imputability (French method), severity, evolution, skin adverse reactions reported to the regional center between January 2020 and mid-June 2021.
Results: During the study period, 23 cases were skin adverse reactions (50 and 75% of notifications, in 2020 and 2021 respectively). Using the Medical Dictionary for Regulated Activities (MedDRA) to present the results, up to June 16, 2021, 12 cases were notified, while in the whole of 2020 only 11 cases were notified. There is an increase of 109.09% in just 6 months. The most frequent clinical presentation was, during 2020 and 2021 respectively, maculopapular rash (rash and exanthema) (90.9%, 75%) followed by pruritus (9.1%), (8.33%) and only in 2021 maculopapular lesions (16.66%), with a female preponderance (54.54%) in 2020 and (58.33%) in 2021. The time to onset between skin side effects and drug treatment has revealed that they can develop within a few hours to 14 days in 2020 and up to 9 months of treatment in 2021. The groups of causal drugs common to 2020 and 2021, respectively, were antimicrobials (45.45–25%), uricemic agents (27.27–8.3%), antiepileptics (9–25%), antineoplastics (9–8.3%). In addition, nitrated vasodilators (9%) in 2020 and in 2021, 4-aminoquinolines (8.3%), antidiarrheals (8.3%). The majority of drugs in 2020 and 2021 respectively were administered orally (54.54%), (66.66%), followed by injectables (18.18%), (33.33%), in 2020 (9%) by transdermal route. Polypharmacy was observed in (54.54%) of cases in 2020 versus (83.33%) in 2021, with up to 7 drugs per patient. The imputability (score of I0 to I6), for the majority of skin adverse reactions, was greater than I4, sometimes attainment I6.
Conclusion: The occurrence of skin side effects increased between 2020 and 2021. The establishment of a regional center in the CHU played an important role in improving the rate of notifications, and the pharmacovigilance system.
References/Further Sources of Information
P075 Identifying Priorities for Pharmacovigilance in Low-Resource Settings During the COVID-19 Pandemic: A Community of Practice Approach
R. Walker1, T. Lang1, E. Allen2
1University of Oxford, The Global Health Network, Oxford, UK; 2University of Cape Town, Division of Clinical Pharmacology, Cape Town, South Africa
Background/Introduction: Undertaking effective drug safety monitoring is particularly challenging in low-resource settings due to a lack of infrastructure, weak regulatory systems and poor access to training and education [1]. Given the impact that the COVID-19 pandemic is having upon health systems globally, it is essential to ensure that pharmacovigilance systems in these vulnerable settings have the capacity to address both the exacerbated pre-existing and novel challenges that they now face [2]. This project will harness the membership of an online pharmacovigilance platform, globalpharmacovigilance.org (GPV) to create a ‘community of practice’ (CoP) to address the challenges facing pharmacovigilance during the pandemic[3, 4]. This community can use proven approaches to reach strong and highly democratic consensus on pharmacovigilance priorities, and then provide relevant resources (whether existing or new training, guidance, tools, mentoring) to address the gaps. The online platform will be used by the CoP to share best practices, engage more widely and disseminate outputs and guidance.
Objective/Aim: To identify priorities for pharmacovigilance in low-resource settings during the COVID-19 pandemic and provide resources to address these within a CoP model.
Methods: This project will build on a consensus-gathering methodology developed by The Global Health Network that has been implemented successfully during the pandemic to address wider COVID-19 research priorities. An online survey, distributed via social media, mailing lists and directly on GPV, will be used to identify priority areas for pharmacovigilance improvement in low-resource settings. Participants will then be invited to attend an online open workshop, in which the survey results will be presented and consensus gathered on an area for focus. Attendees will be invited to form a working group(s). Membership will be self-selective to encourage involvement from all experience levels. This working group(s) will then be supported to work together to facilitate the development of (or provision of, if already available elsewhere) pharmacovigilance resources to address the priorities identified.
Results: Results will be presented in the form of a knowledge gap analysis for pharmacovigilance in low-resource settings during the COVID-19 pandemic, consisting of qualitative/quantitative results from the survey and qualitative data from the workshop. Qualitative/quantitative data on the uptake and use of the GPV platform will also be presented.
Conclusion: It is anticipated that findings will help to understand whether and how a CoP may be built and engaged with using an online platform during a pandemic, and then contribute to a priority area for improvement or development within pharmacovigilance at that time.
References/Further Sources of Information
Pharmacovigilance in resource-limited countries. Expert Rev Clin Pharmacol. 2015;8(4):449–60.
The role of pharmacovigilance and ISoP during the global COVID-19 pandemic. Drug Saf. 2020;43(6):511–2.
Cultivating communities of practice: a guide to managing knowledge. Harvard Business Press. 2002.
Global Pharmacovigilance. 2021 [cited 2021 20th June]; https://globalpharmacovigilance.tghn.org/.
P076 Pharmacovigilance of Vaccines: How It Differs from Other Pharmaceuticals?
I. Abdeldayem1, A. Tebaa2, N. AboZaid3
1Arriello s.r.o., Pharmacovigilance Department, Prague, Czech Republic; 2Centre Anti Poison et de Pharmacovigilance du Maroc, Pharmacovigilance Department, Rabat, Morocco; 3The Egyptian Company for Vaccines-Sera and Drugs-Egypt, Pharmacovigilance Department, Cairo, Egypt
Background/Introduction: Vaccines are amid the most reliable means of current medicine that assist in fighting against pandemics and diseases through inducing immunity (1). Routine immunization serves as a crucial benefit to the public health system, introducing one of the most practical tools of decreasing mortality among children, especially those less than five years old. Considering the majority of those vaccinated are children and healthy population, a firm and rigid safety supervision of vaccines is fundamental (2). CIOMS/WHO Working Group defines Vaccine Pharmacovigilance as “the discipline concerned with the detection, assessment, understanding and prevention of adverse events following immunization and other vaccines- or immunization-related problems” (3). Pharmacovigilance of vaccines is varied from that of other pharmaceuticals. Medicines generally treat symptoms of diseases, however vaccines aid in the prevention of diseases in a healthy population. This article provides insights into the background and inner workings of Pharmacovigilance of both vaccines and other Pharmaceuticals.
Objective/Aim: To identify the distinction between vaccines and drugs while operating vaccine pharmacovigilance.
Methods: Comprehensive narrative review explored the pharmacovigilance activities related to vaccines in opposition to other pharmaceuticals, with emphasis on acceptance of adverse events, rechallenge, dechallenge, causality assessment, and benefit/risk assessment.
Results: Although vaccines and other pharmaceuticals are comparable in certain pharmacovigilance aspects, they are significantly different from each other, regarding certain safety monitoring requirements. Vaccines have a short duration of exposure with a long time for response, whereas drugs have a longer duration of exposure with a shorter time for response. For vaccines, minor adverse events are important (can jeopardize acceptance or indicate program error). On the other hand, minor adverse events of drugs are rarely important. Difficulties in determining the causation of adverse events are common to pharmaceuticals and vaccines. This is particularly challenging in the case of vaccines due to absences of information on “dechallenge and rechallenge”. The risk-benefit assessment for vaccines relies upon the prevalence of the disease in the target population, the severity of infectious disease, the risk of transmission, and the recognition of high-risk groups. In general, regulatory tools and risk assessment activities for vaccines are like those of other pharmaceuticals.
Conclusion: Vaccine safety surveillance is vital when establishing that the benefits of immunization are maintained in the interest of public health, which is the absolute objective of the immunization programs.
References/Further Sources of Information
MODULE 4—Pharmacovigilance—WHO Vaccine Safety Basics [Internet]. [cited 2020 Jun 1]. https://vaccine-safety-training.org/pharmacovigilance.html.
Concept paper for a guideline on the conduct of pharmacovigilance for vaccines. European Medicines Agency. 2005. http://www.emea.europa.eu/pdfs/human/phvwp/37200405en.pdf. Accessed 1 Dec 2009.
Pharmacovigilance V. Definition and Application of Terms for Vaccine Pharmacovigilance This report from the Council for International Organizations of Medical Sciences (CIOMS) in collaboration with WHO covers the activities and outputs of the CIOMS/WHO Working Group on [Internet]. 2005 [cited 2020 Jun 1]. http://www.who.int/bookorders.
P077 Acute Relapse Following Vaccination in Multiple Sclerosis Patients: A Nationwide Epidemiologic Study using the French Healthcare Databases
L. Grimaldi1, T. Duchemin2, P. Attias3, Y. Hamon2, A. Buchard4, L. Abenhaim5, Y. Moride6
1Hospital Group Paris-Saclay-Assistance Publique-Hôpitaux de Paris-UFR des Sciences de la Santé-University Paris-Saclay-Paris-France, Department of Pharmacology, Paris, France; 2Re-Meds, Data Analysis, Paris, France; 3Re-Meds, Medical Department, Paris, France; 4Hôpitaux Universitaires de Genève, Psychiatrie Adulte, Geneva, Switzerland; 5London School of Hygiene and Tropical Medicine, Pharmacoepidemiology, London, UK; 6Rutgers, The State University of New Jersey, Pharmacoepidemiology, New Brunswick, USA
Background/Introduction: Multiple sclerosis (MS) is an immune-related disease, it is hypothesized that vaccination may trigger relapse owing to a potential immune attack on the myelin sheath. Evidence of vaccine safety in MS patients and identification of high-risk patients is thus of great relevance to inform clinical practices.
Objective/Aim: To assess the association between vaccination and MS relapse in clinical practice settings
Methods: A case-crossover study was conducted using the French SNDS database, which covers > 66 million lives (99% of the population) from birth to death or emigration, one of the world's largest comprehensive claims database. Database records demographic data, pharmacy dispensing claims, outpatient healthcare encounters, and hospital admissions with primary, secondary and associated ICD10 diagnoses.
Patients with a diagnosis of MS were identified in the database over an 11-year period (1st January 2007–31st December 2017). MS relapse was defined as a hospitalization with an MS-related primary discharge diagnosis or a claim for an outpatient neurologist or ophthalmologist visit followed by a pharmacy dispensing claim for a high-dose corticosteroid within 7 days. Vaccine exposure in the 2 months prior to the relapse date (risk window) was compared to prior exposure in up to 4 control-2 month-time windows per patient. Conditional logistic regression with Generalized Estimating Equation models was used to measure the association between vaccination and relapse accounting for multiple relapses occurrence within an individual and healthcare use. Stratifications by vaccine type or nature, demographic characteristics, number of relapses, time since diagnosis, and disease activity were performed.
Results: A total of 105,170 MS patients with 117,715 relapses were identified. The mean age was 43.5 years [SD 13.5], 71.4% were females, and 53.8% were vaccinated at least once during the study period, distributed as follows: vaccine combinations (36.9%), flu (18.6%), pneumococcus (7.3%), and others (< 5%). The Odds Ratio (OR) for any vaccination was 1.05 [0.99–1.11]. OR were 1.20 [1.05–1.38], 1.03 [0.96–1.11] and 1.03 [0.94–1.14] for bacterial, viral and vaccines combinations, respectively. OR were 1.08 [1.01–1.16] and 0.88 [0.58–1.33] for antigenic and live attenuated vaccines, respectively. Other stratifications yielded similar results.
Conclusion: ORs were very close to the null, suggesting that vaccination, and MS relapses, is not a major safety concern, whatever the MS subpopulation investigated. Compared with previous studies [1] that included a few hundred cases at best, our study’s generalizability and statistical power were much higher to allow for hypothesis-testing.
References/Further Sources of Information
Mailand MT, Frederiksen JL. Vaccines and multiple sclerosis: a systematic review. J Neurol. 2017 Jun;264(6):1035–50.
P078 Leveraging on Community Health Volunteers to Educate the Public on Adverse Events Following Immunization and Dispel Myths and Misconceptions
C. Ambale1, C. Murerwa2
1Machakos County, Health and Emergency Services, Nairobi, Kenya; 2Amref Health Africa, Covid-19 Vaccine Support, Nairobi, Kenya
Background/Introduction: The Covid-19 pandemic has affected the world and caused millions of deaths and morbidities. The discovery of effective vaccines was a welcome relief that was expected to be received with positivity. However, there have been lots of myths and misconceptions around the adverse events caused by the Covid-19 vaccine. This has necessitated more engagement with the community at their level to understand the origin of these myths and help dispel them.
Objective/Aim: To utilize community health volunteers to educate the community on adverse events following immunization (AEFIs).
Methods: This is an ongoing activity to engage CHVs through focused group discussions that elucidate the myths and misconceptions that are rife in the community. Messages were designed to address the most commonly spread rumors against the Covid-19 vaccines. Radio announcements were also prepared and aired to help educate members of the public on AEFis. Contacts of the regulatory authority (RA) pharmacovigilance section were shared with the CHVs for dissemination. The public section for reporting ADRs on the RA portal was explained.
Results: There was increased uptake of the vaccine amongst CHVs and an increase in the number of reports on ADRs. Calls from the CHVs to the RA hotline shared increased and the public was keener on getting the right information on the Covid-19 vaccine.
Conclusion: There needs to be more enhanced and continuous engagement of the public on matters concerning vaccines and more so AEFIs.
References/Further Sources of Information
Bowen F, Newenham-Kahindi A, Herremans I. When suits meet roots: the antecedents and consequences of community engagement strategy. J Bus Ethics. 2010;95:297–318. https://doi.org/10.1007/s10551-009-0360-1.
James V. Lavery, Paulina O. Tinadana, Thomas W. Scott, Laura C. Harrington, Janine M. Ramsey, Claudia Ytuarte-Nuñez, Anthony A. James. Towards a framework for community engagement in global health research. Trends Parasitol. 26(6).
P079 Differences in Reporting of Adverse Drug Reactions Between Consumers and Healthcare Professionals
C. Anton1, R. Sahak2, K. Badyal1
1West Midlands Centre for Adverse Drug Reactions, Pharmacy, Birmingham, UK; 2University of Birmingham, School of Pharmacy, Birmingham, UK
Background/Introduction: Underreporting of ADRs to spontaneous reporting systems is a worldwide and widely recognized obstacle to pharmacovigilance. The addition of consumers to the pool of potential reporters increases the number of spontaneous reports, contributes to earlier detection of important ADRs (1–3), and highlights the effect of ADRs on patients’ quality of life (4). However, differences in the nature of reports have been reported.
Objective/Aim: To identify any differences in reports from consumers and healthcare professionals.
Methods: We identified the suspected drugs reported by both consumers and healthcare professionals reported from the West Midlands area (population 6 million) in the UK from April 2013 to March 2020. Reports were categorised as either consumer or healthcare professional (HCP) and suspect drugs were categorised into their BNF chapter (5). The odds ratio and confidence interval was calculated for each BNF chapter. The number of characters in the narrative describing each reaction was also calculated.
Results: There were 12,391 ADR reports in the study period and 25.2% of these were reported by consumers. The table shows (for the BNF chapters where there was a significant difference [p < 0.05] between HCP and consumer report) the frequency by reporter type and BNF chapter and the odds ratio. An odds ratio < 1 indicates HCP reports were more common than consumer reports for drugs in that BNF chapter. Consumer reports had a longer narrative describing the reaction and this was significantly different [p < 0.0001] from HCP reports.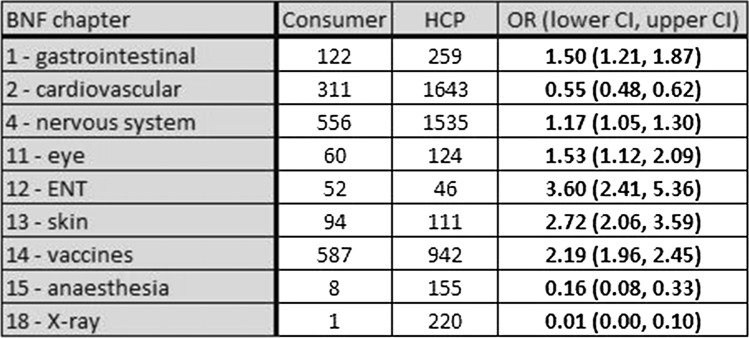
Conclusion: Differences between HCP and consumer ADR reports exist. X-ray drugs, cardiovascular drugs, and anaesthetics are reported more by HCPs, which may be linked to the specialist nature of some of these drugs, with patients unable to recognise the reactions and expecting the HCP to submit the reports. A higher proportion of ADR reports for ENT and dermatological products are reported by consumers. This is might be because patients are more likely to report OTC medication compared to HCPs. Consumers are also more likely to report ADRs to vaccines, which could be linked to minor delayed ADRs, such as a temperature or injection site reaction, which are not worrisome enough to seek medical attention. Narrative length of ADR reports shows consumers are encouraged to describe their reactions in detail particularly the effects on their quality of life and this may account for the higher frequency of reports from consumers to CNS drugs.
References/Further Sources of Information
George C, Nathanson V, Seddon C, Jayesinghe N, Jackson G, Fookes N et al. Reporting adverse drug reactions. A guide for healthcare professionals. BMA Board of Science; 2006 [cited 13 October 2020]. p. 3–16. https://www.isoponline.org/wp-content/uploads/2015/01/BMAreport.pdf.
World Health Organization. The Importance of Pharmacovigilance. Safety Monitoring of medicinal products. World Health Organization; 2000 [cited 15 October 2020]. https://apps.who.int/iris/bitstream/handle/10665/42493/a75646.pdf.;jsessionid=7A21DD626D4E54312D04FC341CEDD167?sequence=1.
Inácio P, Cavaco A, Airaksinen M. The value of patient reporting to the pharmacovigilance system: a systematic review. British Journal of Clinical Pharmacology. 2016 [cited 17 October 2020];83(2):227–46. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5237689/.
Rolfes L, van Hunsel F, Taxis K, van Puijenbroek E. The impact of experiencing adverse drug reactions on the patient’s quality of life: a retrospective cross-sectional study in the Netherlands. Drug Saf [Internet]. 2016 [cited 18 October 2020];39(8):769–76. https://link.springer.com/article/10.1007%2Fs40264-016-0422-0.
Joint Formulary Committee. BNF 80: September 2020-March 2021. London: Pharmaceutical Press. 2019.
P080 The Process of Developing a Pharmacovigilance Policy: Experiences from Nigeria and Eswatini
A. Isah1, R. Ozolua2, A. Opadeyi3, S. Ayinbuomwan3, A. Osakwe4, I. Ali5, P. Bassi6, A. Duga7, S. Nhlabatsi8, F. Bhembe9, S. Magongo10, N. Shongwe11, D. Vambe12, H. Tumwijukye13, F. Cobelens14
1University of Benin/University of Benin Teaching Hospital, Clinical Pharmacology and Therapeutics, Benin City, Nigeria; 2University of Benin, Department of Pharmacology and Toxicology, Benin City, Nigeria; 3University of Benin, Clinical Pharmacology and Therapeutics, Benin City, Nigeria; 4ALine Pharmaceuticals and General Enterprise, A Line Pharmaceuticals and General Enterprise, Nsukka, Nigeria; 5NAFDAC Nigeria, Pharmacovigilance and Postmarketing Surveillance, Abuja, Nigeria; 6University of Abuja, Pharmacology and Therapeutics, Benin City, Nigeria; 7Ministry of Health, National Pharmacovigilance Unit, Matsapha, Swaziland; 8Ministry of Health, National Pharmacovigilance Centre, Matsapha, Swaziland; 9Miniistry of Health, Pharmaceutical Services, Matsapha, Swaziland; 10Ministry of Health, Medicine Regulatory Unit, Matsapha, Swaziland; 11Ministry of Health, Pharmacy, Matsapha, Swaziland; 12Ministry of Health, Programmatic Drug Resistance TB Management Unit, Matsapha, Swaziland; 13Amsterdam Institute for Global Health and Development AIGHD-Amsterdam-Netherlands, AIGHD PAVIA Project, Amsterdam, The Netherlands; 14Amsterdam Institute for Global Health and Development AIGHD-Amsterdam-Netherlands, Academic Medical Centre and AIGHD PAVIA, Amsterdam, The Netherlands
Background/Introduction: The increasing awareness of the importance of medicines safety amongst the population on the African continent necessitated the development of a national policy on pharmacovigilance (PV) that ensures multistakeholder participation of various actors concerned with the use of medicines. Moreover, a consensual document taking into cognisance stakeholders inclusive of the conventional Ministry of Health (MOH), National Medicines Regulatory Authority (NMRA) and Market Authorization Holders (MAH) as well as patients/consumers is likely to embrace issues of medicine safety in-depth without undermining other extant statutory laws and regulations. Such a document is likely to strengthen PV with increased reporting of adverse drug reactions which has been low due to several prevailing factors in resource limited settings. The use of a Standalone PV document will obviate the present situation where PV policies are embedded as terse statements in other health and pharmaceutical policy documents.
Objective/Aim: To present a practical outline for use in the development of a PV Policy document.
Methods: The initial step was an advocacy engagement at government levels on the need for a policy document as a tool to further strengthen PV. The arrow head was the National Drug Safety Advisory Committee (Nigeria) and the PV Unit (Eswatini). Following approval, the responsible organs of Government on Policy development as well as the MOH and the NMRAs carried out further preparatory work.
Multisectoral stakeholders were then identified and invited for a number of intense discussions on various aspects of the PV system. A framework was developed so as to capture the processes and note the provisions for the various elements of the Policy. A zero draft was developed by a core team and further circulated for notification, clarifications, inputs etc. from stakeholders and a general call for comments. Subsequent drafts had the input of other organs in the system to avoid conflicts with extant laws and regulations. Following a final stakeholder engagement, the draft was forwarded to the requisite organs and the MOH for endorsement and thereafter approval was granted by the Executive/Management
Results: The process was used in the development of PV policy documents for Nigeria [2, 3] and Eswatini [4] in 2010 and 2020, respectively.
Conclusion: The consensual development of the policy documents with intense multisectoral stakeholder engagement provides a tool likely to enhance the enabling environment and ensure collaboration of health and non health functionaries in PV activities without undermining statutory laws and regulations.
References/Further Sources of Information
Isah AO, Pal SN, Olsson S, et al. Specific features of medicines safety and pharmacovigilance in Africa. Ther Adv Drug Saf. 2012;3:25–34.
Federal Ministry of Health. Nigerian National pharmacovigilance policy and implementation framework. Abuja, Nigeria, 2012.
Federal Ministry of Heath. Nigerian National Pharmacovigilance Policy and implementation framework—first revision. Abuja, June 2020.
Federal Ministry of Health. Eswatini National Pharmacovigilance Policy and implementation framework. Eswatini, 2021.
P081 The Standalone Pharmacovigilance Policy: A Tool for Engagement and Collaboration of Stakeholders in the Pharmacovigilance System in Resource Limited Settings
A. Isah1, A. Opadeyi1, F. Cobelens2, H. Tumwijukye3
1University of Benin/University of Benin Teaching Hospital, Clinical Pharmacology and Therapeutics, Benin City, Nigeria; 2Amsterdam Institute for Global Health and Development AIGHD-Amsterdam-Netherlands, Academic Medical Centre and AIGHD PAVIA Project, Benin City, The Netherlands; 3Amsterdam Institute for Global Health and Development AIGHD-Amsterdam-Netherlands, AIGHD PAVIA Project, Amsterdam, The Netherlands
Background/Introduction: The establishment of the WHO Program of International Drug Monitoring commenced with countries in Europe and America in 1968 and it was after 30 years that the first African countries were admitted. The less developed countries, Africa constituting a substantial number, with their minimal resources have in the last two to three decades established Pharmacovigilance (PV) systems many of which remain weak and contending with many challenges. Reporting rates of Individual Case Safety Reports (ICSRs) remain low and the trajectory unimpressive. Where policies exist, there are usually terse statements embedded in other pharmaceutical documents.
In order to further strengthen PV in these settings, there is need to develop a policy document to serve as a non-intimidating engaging tool for obtaining commitment from stakeholders and serving as a guide to action in the PV scenario.
Objective/Aim: To discuss the development of a Standalone PV policy document not limited to Ministries of Health (MOHs), National Medicines Regulatory Authorities (NMRAs), Market Authorization Holders (MAHs) and even Health Care Professionals (HCPs) as a tool for advocacy to address the overlooked engagement and collaboration of multiple stakeholders.
Methods: A clear understanding of the PV system, structure and processes is an essential initial step. This is followed by identification and invitation of stakeholders required for the engagement exercise. The process of development requires the involvement of government and other major operators in the health and pharmaceutical systems (MOH, NMRA, MAHs) as well as the public-consumer.
The Policy Framework and Provisions should be duly addressed taking into consideration the various elements ensuring good PV practices and a consensus reached. The zero draft is developed by a core team and is subjected to multiple reviews amongst stakeholders. Following clarifications and inputs from policy organs of government the necessary endorsements, approval is obtained from the executive arm of government.
Results: The application of the above in Nigeria and Eswatini has resulted in the development of National PV Policy documents. The implementation framework accompanying the documents and a recently developed guidance document is facilitating implementation.
Conclusion: The Standalone PV Policy when properly developed following intense stakeholder engagement and applied is likely to serve as a tool for advocacy, increased awareness and strengthening of PV in resource-limited settings without undermining other statutory laws and regulations.
References/Further Sources of Information
Nil.
P082 Impact of Follow-Up Activities on Spontaneous Reports
V. Kara1, G. Powell2, E. Merico3, N. Kaur4, A. Bate1
1GSK, Global Safety, London, UK; 2GSK, Global Safety, Research Triangle-NC, USA; 3Northeast Ohio Medical University, College of Pharmacy, Rootstown-OH, USA; 4Nova Southeastern University, College of Pharmacy, Fort Lauderdale-FL, USA
Background/Introduction: The quality of spontaneous adverse event (AE) reports are often insufficient to perform a proper causality assessment, it is therefore essential to gather additional information to support scientific assessment. This is supported by Pharmacovigilance guidelines [1–4] which require marketing authorization holders to follow up all reports to obtain comprehensive information.
Objective/Aim: The aim of this study was to assess a random sample of spontaneous AE reports to characterise follow-up activities.
Methods: Spontaneous AE reports were randomly selected (n = 1000) between 2018 and 2019 from the GSK Global Safety Database. Reports derived from the literature, patient assistance programmes, and legal cases were excluded. The reports were manually reviewed by HCPs based on a predefined set of variables and characterised using descriptive statistics.
Results: Of the 1000 randomly selected AE reports, 66.5% were medically confirmed, 25.8% were serious, as defined by regulatory reporting, and 84.4% were unlisted AEs as assessed against the company datasheet. The reports were geographically distributed across 58 countries with the majority from the United States (32.1%), Japan (9.7%), Canada (7.8%), Brazil (5.4%), Germany (5.2%), France (5.1%) and the United Kingdom (4.5%).
Consent to follow-up was obtained in 18.4% (n = 184) of reports, with follow-up received subsequently in 68.5% (n = 126) of these reports, with some cases receiving additional further follow-up. For unlisted AEs (n = 844), follow-up was consented in 20.0% of cases (n = 169), and follow-up received in 13.7% (n = 116) of cases while for listed AEs (n = 156), follow-up was consented and received in 9.6% (n = 15) and 6.4% (n = 10) of cases, respectively. Across report types, the overall time to receive follow-up information was 47.4 days (median = 23 days; range = 1–432 days), some cases received multiple follow-ups.
Conclusion: This study showed the capture of follow-up data varies for spontaneous cases, including across different AE report types and highlighted that consent for follow-up is low (18.4%). Follow-up was obtained for more than half of reports (68.5%) when consent was obtained.
References/Further Sources of Information
Guideline: Pharmacovigilance Responsibilities of Medicine Sponsors—Australian Recommendations and Requirements, Version 2.2, Jan-2021. 2021.
21 CFR 314.80—Postmarketing reporting of adverse drug experiences. 2011.
GVP Module VI—Collection, management and submission of reports of suspected adverse reactions to medicinal products (Rev 2), EMA/873138/2011 Rev 2*. 2017.
Reporting Adverse Reactions to Marketed Health Products, EMA/873138/2011 Rev 2*. 2018.
P083 An Assessment of the Clinical Completeness of Spontaneous Adverse Event Reports Pre- and Post-Follow-Up
V. Kara1, G. Powell2, E. Merico3, N. Kaur4, A. Bate1
1GSK, Global Safety, London, UK; 2GSK, Global Safety, Research Triangle-NC, USA; 3Northeast Ohio Medical University, College of Pharmacy, Rootstown-OH, USA; 4Nova Southeastern University, College of Pharmacy, Fort Lauderdale-FL, USA
Background/Introduction: Spontaneous adverse event (AE) reports normally have missing data and are often insufficient to perform a proper causality assessment. Follow up information is collected and should enable better assessment. There is limited evidence describing how follow-up data increase the informativeness and assessability of AE reports.
Objective/Aim: The aim of this study was to assess a random sample of spontaneous AE reports to determine the extent of missing information potentially supporting scientific evaluation between initial reports and those for which follow-up was obtained.
Methods: Spontaneous AE reports were randomly selected (n = 1000) between 2018 and 2019 from the GSK Global Safety Database. Reports derived from the literature, patient assistance programmes, and legal cases were excluded. The reports were manually reviewed by health care professionals against criteria that have been previously included in other methods (completeness [1, 2], clinical quality [3], and pharmacovigilance utility [4]) and are generalisable to data collected by marketing authorization holders. The assessment excluded attributes unique to country-specific database formats (e.g. report title and compliance measures), that do not impact scientific assessment (e.g. patient initials), and that are linked to another datapoint (e.g. start-stop date and time-to-onset). The total number of data attributes reviewed (n = 26) were divided across general information (n = 2), patient information (n = 5), drug-related information (n = 6) and AE-related information (n = 13). Descriptive statistics were used to characterise results.
Results: Across the 1000 reports, 12.7 of 26 attributes were complete at initial receipt. Consent for follow-up was obtained in 18.4% (n = 184) of reports, with follow-up received in 68.5% (n = 126). The most frequently missing data attributes across the 1000 reports are presented in Table 1.
For reports that received follow-up, the baseline was 13.5 of 26 attributes complete; this increased to 15.5 post-follow-up. The most frequently reported data attributes with the most relative increase in proportion post-follow-up included medication history (38%; 48/126), medical history (20%; 26/126), outcome of AE (20%; 26/126), concomitant medications (13%; 16/126), dose/concentration of suspect medication (13%; 16/126), treatment for the AE (12%; 15/126) and lot number (9%; 11/126).
Limitations of this assessment are while the measure of clinical completeness was based on external published criteria and therefore likely to generalise well across all adverse events, there is no universally accepted measure of clinical completeness in the context of pharmacovigilance, and it will vary by drug-AE pair.
Conclusion: This study demonstrates the extent of incompleteness at initial reporting, and that follow-up of AE reports, whilst not always possible, when done, facilitates scientific evaluation.
References/Further Sources of Information
Bergvall T, Noren GN, Lindquist M. vigiGrade: a tool to identify well-documented individual case reports and highlight systematic data quality issues. Drug Saf. 2014;37(1):65–77.
Kumar R, Kumar P, Kalaiselvan V, Kaur I, Singh GN. Best practices for improving the quality of individual case safety reports in pharmacovigilance. Ther Innov Regul Sci. 2016;50(4):464–71.
3Oosterhuis I RL, Ekhart C, Muller A, Härmark L. Development and validating of a clinical documentation tool to assess individual case safety reports in an international setting. Pharmacoepidemiol Drug Saf. 2016;25:224–5.
Munoz MA, Dal Pan GJ, Wei YJ, Delcher C, Xiao H, Kortepeter CM, et al. Towards automating adverse event review: a prediction model for case report utility. Drug Saf. 2020;43(4):329–38.
P084 Adverse Drug Event Surveillance System for Common People: Effective Utilization of a WhatsApp Based Drug Information Platform
J. J. Kochuparambil1, A. Issac1, J. Vayalikunnel1
1Mary Queen’s Mission Hospital, Department of Service Excellence and Quality, Kanjirappally, India
Background/Introduction: Social networks like WhatsApp are being widely used by pharmacovigilance professionals for sharing adverse drug reactions (ADRs) data and by the patients as a new channel to report ADRs to the patient hospital. Because of convenience and acceptance amongst the general public, our WhatsApp Drug Information Platform (WDIP) has been successfully used for this purpose.
Objective/Aim: To characterize the Adverse Drug Events (ADEs) reported through the WhatsApp Drug Information Platform (WDIP) and to evaluate the causality of those events.
Methods: The adverse drug event data received through the WDIP from 25/09/2020 to 25/05/2021 were collected and the Naranjo algorithm scale was used for causality assessment. Confirmed ADRs were classified according to the Wills and Brown method and the severity of the reactions was assessed using the Hartwigs severity scale.
Results: Out of the total of 14 ADEs reported via WDIP, 12 reports were confirmed as ADRs with the occurrence being prominent in females (7). All the reactions were classified as probable. The age of the study participants had a statistically significant association with the occurrence of the events (p < 0.001). The majority of the reactions were classified as type H (10, 83.3%), which indicates that they were not predictable and not potentially preventable. Hartwigs Severity Scale revealed that all the reactions reported were mild. 8 events were advised for drug discontinuation and outpatient clinic review.
Conclusion: Social media communications including WhatsApp messaging could be used as a potential pharmacovigilance tool for collecting and evaluating ADRs.
References/Further Sources of Information
Arulmani R, Rajendran SD, Suresh B. Adverse drug reaction monitoring in a secondary care hospital in South India. British journal of clinical pharmacology. 2008 Feb;65(2):210–6.
Kamel Boulos MN, Giustini DM, Wheeler S. Instagram and WhatsApp in health and healthcare: an overview. Future Internet. 2016 Sep;8(3):37.
P085 Adherence Herbal Medicines: A Prospective Observational Study in Thai Traditional Medicine Service
K. Kornkaew1, B. Wimonanun1, J. Noikeaw1, C. Kongkaew1
1Naresuan University, Faculty of Pharmaceutical Sciences, Phitsanulok, Thailand
Background/Introduction: The reported prevalence of traditional medicine users in Southeast Asia varied from 55% in Singapore to 2% in Indonesia [1]. In Thailand, data from National Health Survey in 2014 showed that 21.9% of the Thai population had used HMs [2]. In contrast, smaller surveys involving a few hundred subjects showed that 29 to 54% were using HMs [3, 4]. However, there is little information about adherence to herbal medicines and determinants that affect adherence to these herbal medicines. Numerous adherence terms, including self-management, compliance, concordance, and persistence, are routinely used to describe patients’ use of prescribed medications over time [5]. Poor adherence to long-term therapies severely compromises the effectiveness of treatment making this a critical issue in population health both from the perspective of quality of life and of health economics. Interventions aimed at improving adherence would provide a significant positive return on investment through primary prevention (of risk factors) and secondary prevention of adverse health outcomes. [6] Related factors such as side effects, treatment complexity and duration of treatment, and patient related factors such as age, education level and marital status influence medication adherence. Patients’ personal beliefs about life, health and medicine are also important factors. [7] Therefore, we conducted a cross-sectional survey in order to investigate herbal medicine adherence in Thai traditional medicine service.
Objective/Aim: The aim of the study was to examine the prevalence rates of adherence to herbal medicines (HMs) in Thai traditional medicine clinic.
Methods: A prospective observational study was undertaken October 2020–February 2021. Adherence HMs were identified within participants of 238 using Thai traditional medicine service. All suspected cases were assessed for reliability by Thai traditional medicine specialists. Problems of non-adherence were categorized according to a model of medication self-management.
Results: After detailed reviews by clinical specialists, 165 cases (69.33%) were deemed to have problems. Categories of problems with prevalence rates were the following: taking (86.67%), organizing (86.06%), monitoring (84.24%), and understanding (55.76%). The most common cause to involve problem of adherence were patients forgot to use/take drug (X2 = 38.304, P-value < 0.001) and no therapeutic drug monitoring (X2 = 38.304, P-value < 0.001)
Conclusion: HMs use was found to be relevant with low medication adherence in more than half of the study population, especially taking and organizing step. This knowledge will support health care providers and policy makers in decision-making on the use of herbal medicine.
References/Further Sources of Information
Peltzer KPS. Utilization and practice of traditional/complementary/alternative medicine (T/CAM) in southeast Asian nations (ASEAN) member states. Stud Ethno-Med. 2015;9:209–18.
Thai traditional and herbal medicine used by Thail houshold [http://www.nso.go.th/sites/2014/DocLib14/A07-05-57-3.pdf].
Satyapan N, Patarakitvanit S, Temboonkiet S, Vudhironarit T, Tankanitlert J. Herbal medicine: affecting factors and prevalence of use among Thai population in Bangkok. J Med Assoc Thail. 2010;93(Suppl 6):S139–44.
Tangkiatkumjai M, Boardman H, Walker DM. Herbal and dietary supplement use in Bangkok: a survey. J Complement Integr Med. 2014; 11:203–11.
Cramer JA, Roy A, Burrell A, Fairchild CJ, Fuldeore MJ, Ollendorf DA, Wong PK. Medication compliance and persistence: terminology and definitions. Value Health. 2008 Jan–Feb;11(1):44-7. https://doi.org/10.1111/j.1524-4733.2007.00213.x. PMID: 18237359.
De Geest S, Sabaté E. Adherence to long-term therapies: evidence for action. Eur J Cardiovasc Nurs. 2003 Dec;2(4):323. https://doi.org/10.1016/S1474-5151(03)00091-4. PMID: 14667488.
Jin J, Sklar GE, Min Sen Oh V, Chuen Li S. Factors affecting therapeutic compliance: A review from the patient's perspective. TherClin Risk Manag. 2008 Feb;4(1):269–86.
Bailey SC, Oramasionwu CU, Wolf MS. Rethinking adherence: a health literacy-informed model of medication self-management. J Health Commun. 2013;18 Suppl 1(Suppl 1):20–30. https://doi.org/10.1080/10810730.2013.825672. PMID: 24093342; PMCID: PMC3814610.
Haynes RB, Yao X, Degani A, Kripalani S, Garg A, McDonald HP. Interventions for enhancing medication adherence (Review). Cochrane Database Syst Rev. 2005;4.
Farber HJ, Capra AM, Finkelstein JA, Lozano P, Quesenberry CP, Jensvold NG, … Lieu, TA. Misunderstanding of asthma controller medications: association with non-adherence. J Asthma, 2003;40:17–25.
P086 Herbal Product-Reporting Questions for Consumer: An International Delphi Study
P. Meesomperm1,2, C. Kongkaew1,3,4, N. Scholfield1,3
1Naresuan University, Research Centre for Safety and Quality in Health, Phitsanulok, Thailand; 2Armed Force Development Command, Medical Division of General Support Office, Bangkok, Thailand; 3Naresuan University, Department of Pharmacy Practice, Phitsanulok, Thailand; 4UCL School of Pharmacy, Research Department of Practice and Policy, London, UK
Background/Introduction: The past decades, herbal products were used for treatment and prevent disease widespread. In contrast, the number of herbal product experience reports were low [1, 2]. Because of limitation of health care professional reporting, consumer reporting will contribute benefit for the reporting system [3]. However, consumer reporting still has limitations, especially complex reporting question [4].
Objective/Aim: This study aims to invent important and appropriated questions on herbal product experience for consumers.
Methods: Modified Delphi technique was used to identify consensus of questions. Expert panels about herbal product and/or pharmacovigilance were sought from the International Society of Pharmacovigilance (ISoP). Two rounds of questionnaires were gathered to analyze the consensus of experts in each question.
Results: Fourteen experts from eleven countries accepted an invitation to participate in the study. The participation and respond rate were 100% both first and second rounds. Eighteen questions from thirty-nine questions were high important and appropriated question and high consensus; three questions about consumer, six questions about herbal products, and nine questions about adverse events. Although there is no high important and high consensus in about reporter information and about perceived effectiveness part, there is no low important and high consensus of any question in this study.
Conclusion: The international Delphi indicated important and appropriated questions for consumer to report herbal product experiences. This could be useful for countries which do not have herbal product reporting to select these questions for consumer’s reporting.
References/Further Sources of Information
Shaw D, Graeme L, Pierre D, Elizabeth W, Kelvin C. Pharmacovigilance of herbal medicine. J Ethnopharmacol. 2012;140(3):513–8.
World Health Organization. Regional Office for South-East Asia. The Pharmacovigilance System for Traditional Medicine in Thailand. World Health Organization. Regional Office for South-East Asia. 2017.
Mukherjee S, Sen S, Kalaiselvan V, Tripathi SK. Consumer reporting of adverse drug reactions: a current perspective. Int J Green Pharm. 2016;10(3):136–42.
Al Dweik R, Stacey D, Kohen D, Yaya S. Factors affecting patient reporting of adverse drug reactions: a systematic review. Br J Clin Pharmacol. 2017;83(4):875–83.
P087 Insulin Analogs and Risk of Solid Tumor Development in Patients Diagnosed with Type 2 Diabetes Mellitus
M. Muñoz1
1Universidad Nacional de Colombia-Hospital Universitario Mayor-Méderi-Universidad del Rosario, Cundinamarca, Bogota D.C., Colombia
Background/Introduction: Non-communicable diseases are those that are not spread from one person to another (1). According to the WHO report, non-communicable diseases represent 70% of the deaths of the world population, this makes non-communicable diseases a public health problem (2). Cancer and diabetes are among the non-communicable diseases that have shown an increase in the last decade. According to reports in the literature, diabetes can predispose a subject to cancer, either due to oxidative stress generated by the pathology or by treatments received to control it. Previous studies have reached different conclusions regarding the increased risk of cancer development and the treatment of insulin analogues as drugs to control diabetes (3–6).
Objective/Aim: To compare the risk of cancer development in patients exposed to treatment with insulin analogues versus other treatments in a high-complexity hospital.
Methods: Observational case-control study in a cohort of patients diagnosed with type 2 diabetes treated in a highly complex hospital from 2012 to 2017. The medical records of the patients were reviewed from the first admission to date on 12/31/2019, development of cancer or death. The person-year calculation was performed for each treatment and an Odds Ratio (OR) and the OR interval were calculated for 95% confidence. The subject who developed a solid tumor after treatment for the control of type 2 diabetes mellitus was counted as a case and the subject who did not develop a solid tumor after treatment was counted as a control.
Results: The medical records of 8313 subjects were reviewed, and after applying the inclusion and exclusion criteria, 37 cases and 542 controls were obtained, it was found that the variables sex and age influence the development of cancer, the OR for sex female it was 2.7; CI [1.3–5.9] and for women in the age range of 60–69 years 2.3; CI [1.01–5.3]. When comparing the analog insulins with the other treatments, an increased risk of cancer development was found when comparing insulin Aspart versus metformin OR = 2.6 CI [1.4–4.7], Insulin Glargine, Detemir, Aspart versus Inhibitors of dipeptidylpeptidase-4 (IDPP-4) with OR of 4.0 CI [1.2–11.7]; 6.1 CI [1.8–19.8]; 8.9 CI [2.9–26.1] respectively and insulin Aspart and Detemir versus sulfonylureas with OR of 3.3, CI [1.7–6.6] and 2.3, CI [1.02–5.1].
Conclusion: Analog insulins such as glargine, detemir and Aspart are associated with an increased risk of solid tumor development in the population studied when compared with other treatments according to the ORs obtained in the results.
References/Further Sources of Information
(OMS) Organización Mundial de la salud. Monitoreo de avances en materia de enfermedades no trasmisibles 2017 [Internet]. Suiza. 2017. p. 7–54. http://apps.who.int/iris/bitstream/10665/259806/1/9789243513027-spa.pdf?ua=1.
(OMS) Organización Mundial de la salud. Global action plan for the prevention and control of noncommunicable diseases 2013-2020 [Internet]. http://apps.who.int/iris/bitstream/10665/94384/1/9789241506236_eng.pdf?ua=1.
Liu S, Li Y, Lin T, Fan X, Liang Y, Heemann U. High dose human insulin and insulin glargine promote T24 bladder cancer cell proliferation via PI3K-independent activation of Akt. Diabetes Res Clin Pract [Internet]. Elsevier; 2018 Apr 17;91(2):177–82. https://doi.org/10.1016/j.diabres.2010.11.009.
Lind M, Fahlén M, Eliasson B, Odén A. The relationship between the exposure time of insulin glargine and risk of breast and prostate cancer: An observational study of the time-dependent effects of antidiabetic treatments in patients with diabetes. Prim Care Diabetes [Internet]. Elsevier; 2018 Apr 17;6(1):53–9. https://doi.org/10.1016/j.pcd.2011.10.004.
Wintrob ZAP, Hammel JP, Khoury T, Nimako GK, Fu H-W, Fayazi ZS, et al. Insulin use, adipokine profiles and breast cancer prognosis. Cytokine [Internet]. 2017;89:45–61. http://www.sciencedirect.com/science/article/pii/S1043466616305579.
Wu JW, Azoulay L, Majdan A, Boivin J-F, Pollak M, Suissa S. Long-term use of long-acting insulin analogs and breast cancer incidence in women with type 2 diabetes. J Clin Oncol [Internet]. American Society of Clinical Oncology; 2017 Sep 27;35(32):3647–53. https://doi.org/10.1200/JCO.2017.73.4491.
P088 Patient Involvement in Pharmacovigilance: Best Practices Identified from a Qualitative Multi-Stakeholder Study Across Europe
F. Van Hunsel1, M. van Hoof1, K. Chinchilla1, L. Härmark1, C. Matos2, P. Inácio3, V. Hall4
1Netherlands Pharmacovigilance Centre Lareb, Signal Detection, ‘s-Hertogenbosch, The Netherlands; 2Instituto Politécnico De Coimbra, ESTESC-Coimbra Health School-Farmácia, Coimbra, Portugal; 3University of Helsinki, Faculty of Pharmacy-Division of Pharmacology and Pharmacotherapy, Helsinki, Finland; 4Universidad de Costa Rica, Facultad de Farmacia, San José, Costa Rica
Background/Introduction: Engaging with patients can add value to pharmacovigilance [1–3]. However, it should be recognized that there is a gap between allowing patients to report and actual patient involvement in pharmacovigilance. Views on best practices from regulators, patient organizations and pharmaceutical companies could be helpful to increase and improve patient involvement in pharmacovigilance.
Objective/Aim: To create a set of best practices in patient involvement in pharmacovigilance based on a qualitative multi-stakeholder study across Europe.
Methods: A qualitative research with semi-structured interviews was performed. Themes for the interview guide and selection of stakeholder groups were based on a literature study. A purposive sampling strategy was used, followed by a snowballing technique. The goal was to capture diversity of stakeholder and countries within the EU to come to a set of best practice recommendations that would be representative for the whole of Europe. Transcribed interviews were analyzed in NVivo® through reflective thematic analysis until data saturation was reached [4]. We adhered to the Consolidated criteria for reporting qualitative research (COREQ) [5].
Results: A total of 20 interviews took place between March and June 2021 with representatives of patient organizations, regulators and pharmaceutical companies on both national and European level. In addition, a supranational organization (Drug Information Association) was also included as a ‘neutral platform’ that fosters conversations between the other stakeholder groups. See Table 1.
The high-level concepts on ‘Best practices in patient involvement’ that emerged from all groups of interviews are shown in Table 1, illustrated by quotes from the interviews. There was a separate theme for Activities that highlighted practical activities that could be linked to the concepts for Best Practices. Some of the activities were highlighted as important by national patient organizations, but not mentioned in the interviews with other stakeholders. Other main themes that were identified were ‘Current situation of patient involvement’, ‘Importance and benefits of patient involvement’ and ‘Definition of Patient Involvement.'
Conclusion: This study found concepts that were mentioned across stakeholder groups that would be beneficial for patient involvement. The needs of (national) patient organizations are highlighted by this study.
References/Further Sources of Information
van Hunsel F, Härmark L, Rolfes L. Fifteen years of patient reporting -what have we learned and where are we heading to? Expert Opin Drug Saf. 2019 Jun;18(6):477–84.
Inácio P. The Value of Patient Reporting of Adverse Drug Reactions to Pharmacovigilance Systems. Thesis, University of Helsinki (Finland), 2018. https://helda.helsinki.fi/handle/10138/242574.
Matos, C. Active involvement of the general public in pharmacovigilance. Thesis, University of Seville (Spain), 2020. https://idus.us.es/handle/11441/98207.
Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101.
Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349–57.
P089 Do Pharmacists’ Spontaneous Reports of Quality Defects Contribute to Product Recalls?
M. Ganso1, A. Said1, M. Schulz1
1Drug Commission of German Pharmacists AMK, Department of Medicine-ABDA-Federal Union of German Associations of Pharmacists, Berlin, Germany
Background/Introduction: Spontaneously reported adverse drug reactions (ADR) are a corner stone in post marketing drug surveillance [1]. In contrast, limited evidence exists for the outcome of reported quality defects of medicinal products. In Germany, pharmacists are legally obliged to report suspected quality defects caused by the manufacturer, but the benefit is uncertain.
Objective/Aim: Our aim was to analyse spontaneously reported quality defects by assessing risk minimization measures (RMM) such as product recalls or corrective and preventive measures (CAPA) taken by the marketing authorization holder (MAH).
Methods: Using the databases for pharmacovigilance and risk information of the Drug Commission of German Pharmacists (AMK), reports with suspected quality defects and related product recalls were identified. The primary outcome was the frequency of reports contributing to recalls or CAPAs implemented by the MAH. The latter information was derived from the MAHs response of single case assessments. Reports received after respective recall publication and reports referring to ADRs were excluded. Additionally, qualitative analysis was performed by the case/non-case method. Cases were defined as reports that contributed to recalls or CAPAs. All other reports of suspected quality defects were non-cases. A reporting odds ratio > 2 with a lower confidence interval > 1 and at least 3 cases were regarded significant.
Results: Between 2014 and 2018, the AMK received 44,570 spontaneous reports in total. In 30,045 (67.4%) pharmacists suspected a quality defect of a medicinal product. Of those, 270 (0.9%) contributed to 126 recalls of specific batches and further 4107 (13.7%) led to the initiation of CAPAs. The following quality defects were significantly associated with product recalls in a descending order: particle contamination in liquid and solid dosage forms > defect dosage forms (also during deblistering) > product mix-up > labelling error and organoleptic deviations. The median time (interquartile range) from first report to published recall was 3 weeks (Q1: 1 week to Q3: 11 weeks).
Conclusion: Spontaneous reports of suspected quality defects caused by the manufacturer enables a continuous surveillance of medicinal products at the pharmacy level. Some types of quality defects were associated with product recalls. However, compared to the high number of spontaneous reports by pharmacists, the rate of recalls or initiated CAPAs is low. Future studies are needed to examine obstacles and limitations in spontaneous reporting outcomes with regard to the initiation of RMM.
References/Further Sources of Information
Aagaard L, Hansen EH. Information about ADRs explored by pharmacovigilance approaches: a qualitative review of studies on antibiotics, SSRIs and NSAIDs. BMC Clin Pharmacol. 2009;9:4.
P090 Local Medical Safety, A New Capability Ensuring the Safe Use of Products at the Local Level
P. Willems1, C. Michielsens1, A. G. Lau2, Corboy3, A. Khedr1
1J&J, Global Medical Safety-International Pharmacovigilance, Beerse, Belgium; 2J&J, Global Medical Safety-International Pharmacovigilance, Singapore, Singapore; 3J&J, Global Medical Safety-International Pharmacovigilance, Titusville, USA
Background/Introduction: Historically, local/affiliate pharmacovigilance units of big pharmaceutical companies were rightly focusing on the performance and compliance of local pharmacovigilance systems. However, at companies like Johnson & Johnson, a vast amount of data is available on the safety profile of the products, often summarized in multiple, separate documents such as aggregate reports and risk management plans. While these reports are submitted to local health authorities and utilized by local stakeholders, there is greater opportunity for the data to be proactively translated by local safety units into safety insights and messaging for local use.
Objective/Aim: Ensuring the proper use of products at the local level through proactive risk minimization activities.
Methods: With a wealth of safety data across all of our products around the world, there was a need to partner at the regional level. To meet this need, Johnson & Johnson created a new capability within the local safety units, Local Medical Safety. Local Medical Safety is the liaison between the local affiliate’s stakeholders and the global safety organization and ensures that global safety data and information are translated into locally applicable, practical, and relevant insights and actions. This practice closes the benefit-risk management loop, linking global to local to ensure the proper and safe use of our products free from avoidable harm. This is achieved by enhancing safety knowledge through product safety profile education, the creation of a knowledge sharing platform to ensure standardized messaging, and actively measuring the outcomes of our risk minimization activities using the data in our safety database (pilot). Through our Interaction Dashboard we’re focusing on the right products and activities.
Results: Through the new Local Medical Safety capability, local safety employees are now integrated in launch activities and country product strategy teams, ensuring consistent, standardized and up-to-date safety messaging and providing expertise on the safety profile of our products. Through our pilot, we’re monitoring the results of our risk minimization activities at the country level enabling the creation of targeted interventions on the country level in close collaboration with our Medical Affairs colleagues.
Conclusion: Local Medical Safety is a new, country based, capability ensuring the safe use of products based on global insights and data.
References/Further Sources of Information
Nap.
P091 Ifosfamide-Induced Encephalopathy: A Case Report of a 15-Year-Old Patient
A. Cherif Chefchaouni1, H. Bechar2, S. El Baraka3, M. Mahdad1, C. Nouibi1, M. J. Belahcen4, Y. Rahali1
1National Institute of Oncology-Ibn Sina University Hospital, Pharmacy, Rabat, Morocco; 2National Institute of Oncology-Ibn Sina University Hospital, Pharmacovigilance Unit, Rabat, Morocco; 3Faculty of Medicine and Pharmacy-Mohammed V University, Pharmacy, Rabat, Morocco; 4National Institute of Oncology-Ibn Sina University Hospital, National Institute of Oncology, Rabat, Morocco
Background/Introduction: Ifosfamide is an alkylating agent that is an effective treatment for solid tumors such as sarcomas. However, its administration can be the cause of encephalopathy which treatment would be based on intravenous methylene blue.
Objective/Aim: In this study, we will report here the case of an encephalopathy induced by ifosfamide in a young patient followed for metastatic clear cell sarcoma of the kidney with pulmonary localization at the National Institute of Oncology in Rabat, Morocco.
Methods: This case report was notified on May 11th 2021 to the hospital's pharmacovigilance unit.
Results: The 15-year-old patient was treated for metastatic clear cell sarcoma of the kidney with pulmonary localization with also involvement of the right femur and the left shoulder. He had no previous pathological history. Ifosfamide was administered as an infusion at the dose of 3641 mg. After the third day of the treatment, the patient developed neurological symptoms such as hallucinations and agitation. The Magnetic resonance imaging (MRI) performed did not show any particular anomalies which confirmed the suspicion of encephalopathy. After these events, it was decided to stop the ifosfamide, to prolong the hospitalization and to administer albumin which constitutes an alternative to methylene blue, given the absence of the latter in our hospital structure and in the Moroccan market. The evolution of the encephalopathy was not very favorable.
Conclusion: According to the different studies performed and our context, methylene blue administration remains the safest means with a more favorable cost-benefit ratio than albumin to treat ifosfamide-induced encephalopathy.
References/Further Sources of Information
None.
P092 Status of Voluntary Reporting of Drug Adverse Events Through Decentralized Pharmacovigilance System in the Republic of Korea
Y. Ko1, A. Suh1, S. Oh1, B. Kim1, S. Shin1, S. Chung2, S. Han3
1Korea Institute of Drug Safety and Risk Management, Office of Drug Safety Information, Anyang-si-Gyeonggi-do, Republic of Korea; 2Korea Institute of Drug Safety and Risk Management, Department of Drug Safety Information, Anyang-si-Gyeonggi-do, Republic of Korea; 3Korea Institute of Drug Safety and Risk Management, President, Anyang-si-Gyeonggi-do, Republic of Korea
Background/Introduction: To promote spontaneous reporting of drug adverse event (AE) in the Republic of Korea, pharmacovigilance system is operated on a decentralized basis. In this system, the Regional Pharmacovigilance Centers (RPVCs), function as the focal point of pharmacovigilance for local hospitals, clinics and pharmacies and collect data from each institution.
Objective/Aim: The purpose of this study is to account for the changes in the number of RPVCs and AE reports over time and to identify the characteristics of AE reports from the RPVCs.
Methods: The annual individual case safety reports (ICSRs) reported to the Korea Adverse Event Reporting System (KAERS) from 1988 to 2020 were analyzed. Additionally, reporting trends of ICSRs collected by the RPVCs in 2020 were characterized by type of sender and therapeutic class of the reported drug.
Results: As the number of RPVCs gradually increased from 3 in 2006 to 28 in 2020, the number of AE reports increased rapidly. The number of AE reports in the Republic of Korea in 2020 is 259,089. Among these reports, 184,861 (71.4%) cases were reported by the RPVCs. Of all the AE reports from RPVCs, 72.3% originated from the centers and 27.7% from local medical institutions. Of all the center reports, the proportion of reports by nurses was the highest among occupations, accounting for 77.5%. Among the local medical institution reports, reporting rates were almost similar, with 49.9% from pharmacies and 49.2% from hospitals and local clinics. In 2020, the most frequently reported therapeutic class in the RPVCs reports was antipyretic anti-inflammatory analgesics, which was different from the therapeutic class most frequently reported by marketing authorization holders.
Conclusion: RPVCs have greatly contributed to pharmacovigilance in the Republic of Korea by playing a pivotal role in expanding the collection and monitoring of drug adverse events. Local clinics, pharmacies, pharmaceutical companies, other related professional groups, consumer organizations as well as the centers should further be encouraged to participate in the voluntary reporting of adverse events.
References/Further Sources of Information
Not applicable.
P093 Implementation of PRAC Periodic Safety Update Report Single Assessment (PSUSA): Recommendations for NAPs at National Regulatory Level in the EU
V. Macolić Šarinić1, A. Amiri1,2
1European Medicines Agency, Human Medicines Division-Pharmacovigilance Office, Amsterdam, The Netherlands; 2Utrecht University, Pharmaceutical Sciences, Utrecht, The Netherlands
Background/Introduction: Through the PSUSA procedure, new safety data provided by marketing authorization holders (MAHs) for a specific active substance is periodically assessed by the Pharmacovigilance Risk Assessment Committee (PRAC) of the European Medicines Agency (EMA). Based on this assessment, the PRAC may provide recommendations with the aim of mitigating potential risks, thereby ensuring that the benefit-risk balance of medicines containing the concerned active substance remains favourable in the post-marketing setting [1,2]. A review of the product information (PI) was performed for a group of medicines to understand the extent to which MAHs comply with the European Union good pharmacovigilance practice (EU-GVP) guidelines with regards to implementing the PRAC PSUSA recommendations at national regulatory level.
Objective/Aim: Describing the extent and the time frame in which the PSUSA recommendations provided by the PRAC are implemented at the national regulatory level in EU Member States (MS).
Methods: A sample of 87 PSUSA reports on nationally authorized products (NAPs) containing active substances with ATC-code L01 (antineoplastic agents), published between July 2012 and December 2020, was retrieved and reviewed from EMA databases [3]. In case the outcome of the procedure was to vary the marketing authorization (MA), the PI of the concerned medicines were appraised to assess (1) compliance to recommendations and (2) time to the full implementation of the recommendations. In this context, 10 EU MS were selected based on their geographic location and area in a way to represent all EU MS in the study. Data-analysis was performed using descriptive statistics.
Results: The extent to which the recommendations were fully implemented were 42.5% for southern, 62.8% for western, 62.7% for northern, 44.9% for eastern and 47.5% for the central part of Europe. On the other hand, the extent of the full implementation seemed to be comparable between small and large MS: 54.1% versus 49.0%. Furthermore, the average time to implementation was 9.3 months for Summaries of Product Characteristics (SmPCs) and 9.4 months for package leaflets (PLs).
Conclusion: The findings show that the extent of the full implementation of the recommendations vary among different EU regions, while the difference between small and large MS is negligible. Overall, a relatively low compliance to the recommendations and a long time to implementation has been observed at the national level for products containing antineoplastic agents. Further investigation is needed to understand the impact of these findings on the overall benefit-risk profile of the medicines assessed and the potential effect on patient safety.
References/Further Sources of Information
Santoro A, Genov G, Spooner A, Raine J, Arlett P. Promoting and protecting public health: how the European Union pharmacovigilance system works [Internet]. 2017 [cited 7 June 2021]. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5606958/.
The European Medicines Agency. Module VII—periodic safety update report (Rev 1) [Internet]. Ema.europa.eu. 2013 [cited 7 June 2021]. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-good-pharmacovigilance-practices-gvp-module-vii-periodic-safety-update-report_en.pdf.
WHOCC - ATC/DDD Index [Internet]. Whocc.no. 2020 [cited 19 June 2021]. https://www.whocc.no/atc_ddd_index/?code=L&showdescription=no.
P094 Adverse Drug Reactions Induced by Methotrexate Among 181 Tunisian Patients with Acute Lymphoblastic Leukemia: Evaluating Its Incidence and Characteristics
B. Ouni1, K. Mansour2, N. Fathallah1, W. Chambeh3, N. Ben sayde3, C. Bensalem1, R. Slim1
1Faculté de Médecine de Sousse, Departement de pharmacologie clinique, Avenue Mohamed Karoui-4000 Sousse Tunisia, Tunisia; 2Faculty of Medicine-University of Monastir-Monastir-Tunisia, Department of Pharmacology, Monastir-Tunisia, Tunisia; 3Service d'hématologie clinique, Hôpital Fahat Hached, Sousse-Tunisia, Tunisia
Background/Introduction: Methotrexate (MTX), an antineoplastic agent, is one of the oldest but effective drugs used for the treatment and maintenance therapy of acute lymphoblastic leukemia (ALL). Frequently used in high-dose, MTX is reported to induce several adverse drugs reactions. As its indications for use increase, more accidental overdoses can be expected and need to be recognized.
Objective/Aim: The purpose of the study was to summarize epidemiological, clinical characteristics of adverse drug reactions induced by MTX in Tunisian Patients with ALL.
Methods: We conducted a retrospective analysis in the clinical Haematology department of Farhat Hached University Hospital of Sousse for over 10 years (from January 2008 to December 2017). Retrospective collection of data was carried out from available clinical records of all patients who were treated by MTX and developed toxicity while its use.
Results: Overall, 181 Patients were enrolled in this study. The mean patient age was about 17.4 years, 50% of them were under 10 years. The majority of these patients were Males with a predominance of 55%. 114 patients were diagnosed as B-cell ALL, and 6 patients were diagnosed as T-cell ALL. The most used Protocols were EORTC (49%), Hyper CVAD 32% and Saint Jude 15%. MTX Toxicity was most noticed at chemotherapy induction phase (67.6%) with 5 g/m2. The median time of toxicity MTX occurrence was 10 days following the MTX administration. Hepatotoxicity was the most common MTX-induced toxicity (56.8%) observed in our study: while transient elevated hepatic enzymes were noticed at 84%, cholestatic hepatitis and mixed hepatitis were noted at 4 and 11.5% respectively. Followed by renal toxicity (acute kidney injury) with an incidence of 16% and cutaneous lesions (13.8%) in which mucositis was the most predominant.6.9%of enrolled patients experienced gastrointestinal disorder mainly presented with vomiting and diarrhea. Finally, 4.4% of subjects developed hematologic disorders and 2.2% of them had neurologic symptoms.
Conclusion: Although his incontestable efficacy, MTX toxicity spectrum is ranging from a minor event to life-threatening reactions. Understanding and monitoring these drug-related reactions may improve patient prognosis and prevent both therapeutic removal due to toxicity as well as fatal outcomes.
References/Further Sources of Information
N/A.
P095 Patent Blue Induced ADRs During Sentinel Lymph Node Detection in Tunisian Patients with Breast Cancer: Incidence and Characteristics
B. Ouni1, K. Mansour2, R. Slim1, I. Bannour3, C. Bensalem1, N. Fathallah1
1Faculté de Médecine de Sousse, Departement de pharmacologie clinique, Avenue Mohamed Karoui-4000 Sousse Tunisia, Tunisia; 2Faculty of Medicine-University of Monastir-Monastir-Tunisia., Department of Pharmacology, Monastir, Tunisia; 3Hôpital Farhat Hached, Service de Maternité de Sousse, Sousse, Tunisia
Background/Introduction: Patent Blue V (PBV) is a colouring agent mainly used to identify the sentinel lymph node during breast cancer surgical treatment preventing eventually total lymphadenectomy. Widely used, adverse drugs reactions related to Patent Blue V are increasingly reported ranging from hypersensitivity reactions (HSR) to pulse oximetry changes.
Objective/Aim: Our study aimed to review the incidence and epidemio-clinical characteristics of adverse drug reactions induced by PBV in Tunisian Patients.
Methods: We conducted a retrospective analysis in the Genecology and rea-anaesthesia departments of Farhat Hached University Hospital of Sousse for over 12 months (from January 2018 to December 2018). We included in this study all patients scheduled for breast surgery with injection of PBV. Patients were screened for past medical history, atopy, and known food and drug allergies. HSR were classified according to The Gell–Coombs hypersensitivity reactions.
Results: Out of 80 patients, who underwent breast cancer surgery with sentinel lymph node detection with PBV, were included in this study. Median age was about 51 years. Four patients reported allergy history to penicillin, latex and lode, but none of them reported allergy to anaesthetics. Four other patients had an atopic history. 13 patients had been previously exposed to PBV with no adverse events notified. Adverse events occurred in 10 procedures out of 80 (12.5%). Grade I HSR with urticaria and blue hives were observed in two patients. 7 patients experienced grade II HSR (including urticaria with either tachycardia or hypotension) and only one patient manifested grade III HSR (cardiovascular collapse requiring treatment with vasoactive drugs). None of our patients had a grade IVHSR. Mean onset time of the reaction was 26 min. All patients had favourable outcomes with no mortality. Median good amelioration of 86 min. Seven patients had received intravenous hydrocortisone. Nine patients required fluid replacement. And 3 patients received intravenous ephedrine infusion. Just one patient needed adrenaline and monitoring. 16 patients manifested decrease in oxygen saturation 20%. Only two patients had skin tests performed 6 weeks later and were positive suggesting immunological HSR.
Conclusion: Although Patent Blue V dye is a valuable surgical tool to detect sentinel lymph node, adverse reactions do occur varying from cutaneous cardiovascular depression to serious Hypersensitivity. It must be considered by surgeons and anaesthesiologists in perioperative hypersensitivity reactions as one of frequently involved agents besides latex, antibiotics and anesthetic drugs. Skin tests with patent blue can be useful to confirm the diagnosis.
References/Further Sources of Information
N/A.
P096 Symptomatic Acute Pancreatitis Induced by Nilotinib
B. Ouni1, K. Mansou2, R. Slim1, B. Achour3, N. BenSayde3, W. Cherif3, W. Chambeh3, C. Bensalem1, A. Khlif3, N. Fathallah1
1Faculté de Médecine de Sousse, Departement de pharmacologie clinique, Avenue Mohamed Karoui-4000 Sousse Tunisia, Tunisia; 2Faculty of Medicine-University of Monastir-Monastir-Tunisia., Department of Pharmacology, Monastir, Tunisia; 3Hôpital Farhat Hached, Service d'hématologie clinique, Sousse, Tunisia
Background/Introduction: Nilotinib (IM), a second-generation inhibitor of the protein tyrosine kinase (PTK), coded by the BCR-ABL gene on the Philadelphia chromosome (Ph), has revolutionized the treatment of chronic myeloid leukemia (CML). This newer agent is reserved for the treatment of Ph+CML resistant/intolerant to imatinib. Nilotinib is known to elicit mild side effects such as pruritus, haematological disorders and an elevation of the pancreatic enzymes but whether or not nilotinib induces symptomatic pancreatitis remains to be elucidated.
Objective/Aim: To highlight the importance of recognizing acute pancreatitis as a possible adverse event associated with nilotinib so that clinicians will be more aware of this potential life-threatening adverse reaction.
Methods: We prescribed a new case of symptomatic acute pancreatitis induced by nilotinib.
Results: A 45-year-old man, with a history of diabetes, had been recently diagnosed with CML and received imatinib for 1 year with no response. Therapy was switched to nilotinib 400 mg twice daily. 4 months later, the patient developed acute abdominal pain radiated to the back, nausea with no fever. Biology testing objected an elevated serum lipase levels of 970 UI/L (NL ≤ 52 UI/L) and Amylase levels of at 626 U/L (NL ≤ 125 UI/L). Serum bilirubin alongside with Liver enzyme levels were within normal limits. Serum pancreatic enzymes were within normal limits the day before nilotinib initiation. Abdominal computed tomography revealed a normal liver, absence of gallstones in the bile duct or biliary strictures, and a normal pancreas. Accordingly, the patient had normal levels of triglycerides and denied alcohol use. His long-term medications included metformin and lithium and were taken for at least 10 years without any reported adverse effects, then were renewed with no recurrence of the patient’s symptoms. Nilotinib was immediately discontinued and treated supportively with IV fluids and pain management. Abdominal pain resolved and serum pancreatic enzymes levels returned to normal 2 weeks later. Correspondingly, this case is classified as possible drug-induced pancreatitis according to the Naranjo causality scale.
Conclusion: Since nilotinib is being utilized more frequently for the treatment of CML, it needs to be applied cautiously performing pancreatic function tests routinely both before and after its administration especially with the life-threatening symptomatic pancreatitis it may cause.
References/Further Sources of Information
N/A.
P097 Characterization of Pediatric Reports in the U.S. Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) from 2010–2020
M. Phan1, C. Cheng1, V. Dang1, E. Wu1, M. Muñoz1
1United States of America Food and Drug Administration, Center for Drug Evaluation and Research-Office of Surveillance and Epidemiology-Department of Pharmacovigilance I, Silver Spring, USA
Background/Introduction: FAERS is a database of adverse event and medication error reports for drugs and therapeutic biologics. It is an essential tool for signal identification in postmarket safety surveillance. Pediatric FAERS reports are relatively understudied and examining recent data can provide important context for evaluating and interpreting FAERS data.
Objective/Aim: Characterize the pediatric FAERS individual case safety reports (ICSRs) and compare trends (1) to adult reports; (2) within pediatric subgroups.
Methods: ICSRs received between January 1, 2010 through December 31, 2020 were identified in FAERS and stratified into the following age groups: neonates (0 to < 1 month), infants (1 month to < 2 years), younger children (2 to < 6 years), older children (6 to < 12 years), adolescents (12 to < 17 years), adults (17–120 years), and null age. Pediatrics were compared to adults. Additional comparisons were made within the pediatric subgroups. Variables of interest included demographic information, reporter variables, reported outcome, suspect product active ingredients (PAIs), and adverse events [coded as Preferred Terms (PTs)]. Categorical variables were described using relative frequencies and descriptive statistics.
Results: This study examined 11,257,558 ICSRs. Pediatric, adult, and null aged patients consisted of 3.14%, 58.04%, and 38.81% ICSRs respectively (Table 1). Compared to adults, pediatrics had higher rates of hospitalization (57.09% vs 28.21%), life threatening (41.13% vs 3.47%), disability (7.56% vs 2.18%), congenital anomaly (2.64% vs 0.04%), and other serious outcomes (58.10% vs 36.61%). Pediatric reports listed multiple PAIs per report more commonly than adults (mean 1.63 vs 1.47 respectively). Out of the top 30 PTs, PAIs, and PT-PAI combinations, more than half of the pediatric terms were unique compared to the adults. Within the pediatric subgroups, neonates had the highest rates of serious outcomes compared to infants, younger children, older children, and adolescents (96.24% vs 79.82% vs 67.89% vs 59.5% vs 52.71%, respectively). Younger pediatric age groups were more likely to have weight information than older age groups but were less likely to include sex or gender information.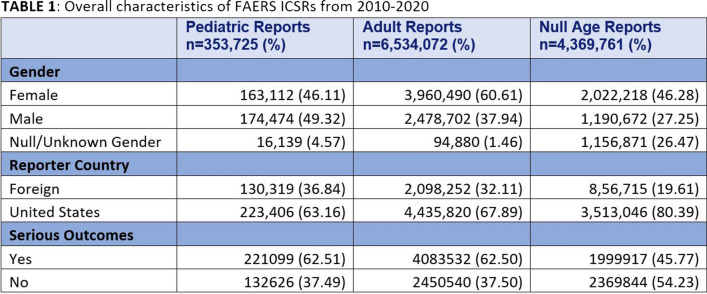
Conclusion: Pediatric FAERS ICSRs have different reporting trends than adult ICSRs. Reporting trends also vary within pediatric subgroups, which highlights the need for unique considerations and approaches for safety surveillance in pediatrics.
References/Further Sources of Information
P098 Exploring the Feasibility of the Uptake of the Med Safety App in ADRs Reporting in Uganda: A Qualitative Study
H. Zakumumpa1
1Makerere University-College of Health Sciences, School of Public Health, Kampala, Uganda
Background/Introduction: In sub-Saharan Africa (SSA) it is estimated that only 10% of adverse drug reactions (ADRs) are reported. The WHO-recommended Med Safety smart phone mobile app which has had considerable implementation success in Western Europe could improve ADRs reporting in SSA by supplementing the dominant paper-based platforms. Besides, health care professionals, the Med Safety app can be utilized by patients to report ADRs directly to authorities. However, there is little research exploring the potential of roll-out of the Med Safety App in resource-limited operational contexts in SSA.
Objective/Aim: This study aimed to understand facilitators and barriers to roll-out of the internet-based Med Safety mobile App in reporting Adverse Drug Reactions (ADRs) among HIV clinicians in Uganda.
Methods: We adopted a qualitative descriptive research design involving 49 participants. Between September and December 2020, we conducted 22 in-depth interviews with HIV clinicians in 12 purposefully selected health facilities across Uganda. We purposely selected HIV clinicians because HIV medications contribute the highest number of ADRs case reports to the National Pharmacovigilance Centre of Uganda. The selection of study sites ensured diversity in facility ownership-type (public/private), level of service delivery (tertiary/secondary/primary) and the four major geographic sub-regions of Uganda. We conducted three focus group discussions with 27 HIV clinicians in the participating facilities. Data were analyzed by thematic approach.
Results: The identified facilitators include a high rate of ownership of smart phones among HIV clinicians, the presence of free wifi connections at select HIV clinics and widely-held positive attitudes towards embracing the mobile app in ADRs reporting. HIV clinicians perceived the mobile app as user-friendly, more efficient, and appreciated the feedback provision in the app. Multiple barriers were cited including heavy workloads, the perceived lengthy registration processes for first-time users of the app, unreliable electricity supply in some parts of Northern Uganda, the high cost of internet data from internet service providers in Uganda, difficulty in recognizing ADRs for newer HIV medications (such as dolutegravir), fear of litigation by patients and the perceived relatively lengthy procedures for reporting on the app.
Conclusion: While several enablers for the roll-out of the Med Safety app in Uganda emerged in our study, several barriers including physical infrastructure constraints such as internet access and electricity supply need to be overcome to improve potential scalability.
References/Further Sources of Information
de Vries ST, Wong L, Sutcliffe A, Houÿez F, Ruiz CL, Mol PG, IMI Web-RADR Work Package 3b Consortium. Factors influencing the use of a mobile app for reporting adverse drug reactions and receiving safety information: a qualitative study. Drug Saf. 2017 May 1;40(5):443–55.
Jeon E, Park HA. Factors affecting acceptance of smartphone application for management of obesity. Healthc Inform Res. 2015;21(2):74–82. https://doi.org/10.4258/hir.2015.21.2.74.
Scheibe M, Reichelt J, Bellmann M, Kirch W. Acceptance factors of mobile apps for diabetes by patients aged 50 or older: a qualitative study. Med 2 0. 2015;4(1):e1. https://doi.org/10.2196/med20.3912.
P099 Proactive Drug Safety Monitoring of Adverse Events Program 2020 Experience
W. Alghamdi1, A. Aldayel1, N. Alfadel1, F. Alharbi1
1Saudi Food and Drug Authority, Pharmacovigilance Drug Safety and Risk Management, Riyadh, Saudi Arabia
Background/Introduction: There are numerous adverse events (AEs) reported globally to the World Health Organization (WHO) global database of individual case safety reports (VigiBase). [1] The proactive drug safety monitoring program was established by the Saudi Food and Drug Authority (SFDA) for post-marketing monitoring of the safety of registered medications.
Objective/Aim: The aim of the program is to assess the relatedness of AEs reported in VigiBase to a group of medications approved by SFDA.
Methods: A list of medications registered by SFDA from 2005 to 2020 was selected. All AEs reports of the selected medications was retrieved from VigiBase. Then, we included only AEs with ≥ two serious reports and reported from ≥ two different sources (clinical study and spontaneous report). A filtration process of three steps (1) Exclusion of non-relevant AEs, (2) Listedness assessment, and (3) Literature search was performed on the retrieved AEs lists.
Results: Out of 122 screened medicinal products, three were deregistered during 2020, and five did not meet the inclusion criteria. A total of 114 medications met the inclusion criteria with 43895 reported AEs. The pharmacological categories of the included medications were classified as 31 antineoplastic agents (27.2%), 17 endocrine and metabolic disease agents (14.9%), 16 anti-infective agents (14%), and 16 immunomodulatory agents (14 %). The first and second step of filtration (exclusion of non-relevant AEs and listedness), yielded 2285 potential signals. The third filtration step (literature search), yielded 250 potentially signals with evidence, and are set for extensive safety evaluation. The majority of these AEs reported by System Organ Class (SOC) were; 28 metabolism and nutrition disorders (11.2%), 26 gastrointestinal disorders (10.4%), 25 blood and lymphatic system disorders (10 %), and 21 cardiac disorders (8.4%). The listedness assessment resulted in requesting of label update for 56 medicines (49%) with 335 AEs.
Conclusion: The pharmacovigilance systems face a tremendous amount of AEs reports with inadequate information. The proactive drug safety monitoring program in SFDA successfully improved the identification of new potential signals with medications use. Regulatory authorities are thus encouraged to develop tools/programs for better signals management.
References/Further Sources of Information
Vigilyze.who-umc.org. 2021. [Online]. https://vigilyze.who-umc.org/.
P100 Medication Errors in Old Patients (MEDEROP): A Retrospective Study of the French Pharmacovigilance Network
M. Guillaumin1, M. Grau2, M. L. Laroche2, M. B. Valnet Rabier1, J-B. Annie-Pierre3
1University Hospital of Besançon, Regional Center of Pharmacovigilance, Besançon, France; 2University Hospital of Limoges, Regional Center of Pharmacovigilance, Limoges, France; 3Regional Center of Pharmacovigilance Network, Lyon, France
Background/Introduction: Medication errors (MEs) are one of the major threats to patient safety, specially in old subjects, particularly concerned because of polypharmacy (1) and multimorbidity (2). Few articles have been published concerning characteristics of medication errors in this population (3). In France, since May 2019, Pharmacovigilance Regional Centre follows medication errors without adverse effects (AE) with product quality root cause, in addition to ME with AE. Thus, our coding method for ME has been harmonized in September 2019, allowing homogenous data. Considering medication error as avoidable, it appears topical to analyze the French pharmacovigilance database (FPD) to describe those ME in old patients in order to better target our public health messages.
Objective/Aim: To describe medication errors with or without adverse effects in people older than 75-year-old.
Methods: We performed a retrospective observational study of ME with or without AE reported to the FPD between 09/01/2019 and 02/29/2020. The variables analyzed were age, typology of the error (stage, nature, cause), typology of the AE, evolution according to classification code system of MedDRA and typology of the pharmacological classes classified using the Anatomical Therapeutic Chemical (ATC) classification code system.
Results: Over the period, 651 ME were registered whatever the age and among, 180 declarations dealing with patients over 75 years old were analyzed. The average age was 86-year-old (76–101) and sex ratio (M/F) was 0.65. There were 151 notifications with AE (84%), 94 of whom were considered serious (62%), 12 (8%) led to death. The principal stage reported for initial error was the administration (64%). Concerning the nature of error, the most common were error of dose/posology/concentration (37%), error of medication (27%), and patient error (14%). The most common drugs involved were nervous system drugs (35.5%) follow up by cardiovascular system drugs (16.2%) then blood and blood forming organs drugs (13.2%). The most reported adverse effect (SOC) were Nervous system disorders (n = 59), Vascular disorders (N = 27), Respiratory, thoracic and mediastinal disorders (N = 22) and Gastrointestinal disorders (N = 19).
Conclusion: This study showed that ME is often associated with serious and potentially harmful AE in older people. The typology of ME, main molecules implicated and common AE occurring allowed by the harmonization of coding, identify some priority for future preventive measures on ME in older adults such as improving the administration stage with much more control for example. We need to collect these data thanks to a pharmacovigilance network implicated in ME and qualify for their coding and analyses.
References/Further Sources of Information
Monégat M, Sermet C. La polymédication: définitions, mesures et enjeux. 2014;8.
Sermet (C), Enquête sur la santé et les soins médicaux 1991–1992. Méthodologie, Rapport CREDES n°965, Mars 1993.
José Joaquín Mira. Medication errors in the older people population. Expert Rev Clin Pharmacol. 2019;12(6):491–4. https://doi.org/10.1080/17512433.2019.1615442.
P101 Development of an Adverse Event Reporting Button for a Veterinary Practice Management System
H. Davies1, S. Smyth1, P. J. Noble1, G. Pinchbeck1, G. Diesel2, M. Pirmohamed3, D. Killick1
1University of Liverpool, Institute of Infection-Veterinary and Ecological Sciences, Neston, UK; 2Pharmacovigilance Unit-VMD, Pharmacovigilance Unit, New Haw-Addlestone-Surrey, UK; 3University of Liverpool, Wolfson Centre for Personalised Medicine-Institute of Systems-Molecular and Integrative Biology, Liverpool, UK
Background/Introduction: In the UK veterinary surgeons and veterinary nurses are encouraged to report adverse events (AEs) to either the UK competent authority (the Veterinary Medicines Directorate (VMD)) or the product manufacturer by their respective Codes of Professional Conduct [1, 2]. However, there is emerging evidence of under-reporting of AEs in the veterinary setting [3, 4]. The ability to report AEs directly from the practice management system has been highlighted as a perceived facilitator to reporting by veterinary professionals [3].
The Small Animal Veterinary Surveillance Network (SAVSNET) at the University of Liverpool collects veterinary electronic health records from first opinion veterinary practices in the UK. In addition to passive data collection, participating veterinary practices are presented with a window at the end of every consultation and professionals are asked to select the main presenting complaint (MPC). This existing infrastructure provides an opportunity for implementation of an AE reporting button to allow direct reporting to the VMD by professionals in practice with the aim of enhancing reporting.
Objective/Aim: To develop and implement an AE reporting button within the SAVSNET MPC window to allow veterinary professionals to report AEs directly to the VMD.
Methods: We developed an AE report form based on the existing VMD online AE reporting form, in collaboration with the VMD. The report form was designed to allow pre-population of animal and drug history from the animal’s health record. Further functionalities included the ability to append recent clinical history with one click and the option to receive a copy of the submitted report for record keeping. A gateway connection was established with the VMD to allow receipt of reports electronically. The button was promoted via advertisements in the veterinary press, social media and a demonstration.
Results: Since launch (18-September-2020) 38 events have been reported via the AE reporting button. 57.9% of reports (n = 22) relate to dogs, 34.3% (n = 13) relate to cats, 5.3% (n = 2) relate to rabbits and 2.6% (n = 1) relate to a human. The majority of reports (57.9%, n = 22) relate to vaccine products.
Conclusion: Implementation of an AE reporting button onto the SAVSNET MPC window has been a technical success. However, COVID-19 may have impacted uptake due to the extra demands on veterinary professionals during this time. Future work will include deploying the button in other practice management systems and further promotion to increase uptake.
References/Further Sources of Information
Royal College of Veterinary Surgeons. Code of Professional Conduct for Veterinary Surgeons [Internet]. 2020 [cited 2021 Jun 29]. https://www.rcvs.org.uk/setting-standards/advice-and-guidance/code-of-professional-conduct-for-veterinary-surgeons/.
Royal College of Veterinary Surgeons. Code of Professional Conduct for Veterinary Nurses [Internet]. 2020 [cited 2021 Jun 29]. https://www.rcvs.org.uk/setting-standards/advice-and-guidance/code-of-professional-conduct-for-veterinary-nurses/.
De Briyne N, Iatridou D, Gopal R, Diesel G, and O’Rourke, D. Veterinary pharmacovigilance in Europe: a survey of veterinary practitioners. Vet Rec Open. 2017;4:e000224. https://doi.org/10.1136/vetreco-2017-000224.
Wilson A, Pinchbeck G, Dean R, McGowan C. Equine influenza vaccination in the UK: current practices may leave horses with suboptimal immunity. Equine Vet J. 2020;00:1–11.
P102 Trends of Digitalization in Pharmacovigilance Process After COVID-19: Pharmaceutical Companies Perspective
G. El-Hefney1, M. El-Warwary2,3, M. A. Elhawary4, S. Bahr5, M. Mounir6, Y. Abdelgayed7, I. N. Ali8, H. Rostom9,10
1Medical Union Pharmaceuticals, Medical Manager, Cairo, Egypt; 2Inspire Pharmaceuticals, Medical Manager, Cairo, Egypt; 3ISoP Egypt Chapter, Vice President, Cairo, Egypt; 4Ain Shams University, Faculty of Pharmacy Researcher, Cairo, Egypt; 5Boehringer Ingelheim, Levant-Iraq and North East Africa Pharmacovigilance Manager, Cairo, Egypt; 6Bayer, Middle East and Maghreb PV Country Head, Cairo, Egypt; 7Amgen, Global Senior Safety Associate, Cairo, Egypt; 8University of Glasgow, Faculty of Pharmacy-Researcher, Glasgow, Scotland, UK; 9MSA University, Clinical Pharmacy-Lecturer, Cairo, Egypt; 10ISoP Egypt Chapter, President, Cairo, Egypt
Background/Introduction: It is recognized that information technology (IT) has entered and transformed the world of health care in a way that enabled it to proceed in work with higher quality, efficiency, and lower cost. This is also applying to the Pharmacovigilance systems and across the pharmaceutical company functions [1]. The most commonly used digital solutions in pharmacovigilance are those related to the processing of Individual Case Safety Reports (ICSRs) and signal management [2]. However, introducing new digital solutions in other pharmacovigilance processes is evolving with the strengthening of the regulatory environment for safety monitoring and risk management of medicinal products, which places a demand on pharmaceutical companies to manage pharmacovigilance activities more efficiently than before [3]. Furthermore, the COVID-19 outbreak has triggered a widespread disruption to everyday life and has created unprecedented challenges for companies to retool their business based on the long-term implications.
Objective/Aim: Egypt Chapter of the International Society of Pharmacovigilance (ISoP) has constructed a survey questionnaire to get a better understanding of the level of digitalization as an opportunity to build upon methods of different pharmacovigilance process within the pharmaceutical companies and the new trends which might be adopted in workflow, sources of information, and attaining pharmacovigilance compliance.
Methods: The ISoP Egypt Chapter designed a self-administered online survey. The participation survey invitations were sent to pharmaceutical companies with a diversity of national, international, and multinational ones. The survey questions were grouped to form seven clusters investigating the trends in digitalization in different pharmacovigilance process including (1) ICSRs and sources of safety information; (2) Risk management and safety communication; (3) Signal management and aggregate reports; (4) Training; (6) Pharmacovigilance interface with other functions; (7) Pharmacovigilance system and PV compliance.
Results: The research is still ongoing; the responses of the Pharmaceutical companies will be analyzed to identify the level of digitalization in their pharmacovigilance process with comparison in between the 6 clusters of the survey. Different trends—if any—between national, international and multinational companies will be highlighted.
Conclusion: The research is still ongoing. An updated version of the abstract with the conclusion based on the results will be presented by this year’s ISoP annual meeting.
References/Further Sources of Information
Ghosh R, Kempf D, Pufko A, Barrios Martinez LF, Davis CM, Sethi S. Automation opportunities in pharmacovigilance: an industry survey. Pharm Med. 2020;34(1):7–18. https://doi.org/10.1007/s40290-019-00320-0.
Lewis DJ, McCallum JF. Utilizing advanced technologies to augment pharmacovigilance systems: challenges and opportunities. Ther Innov Regul Sci. 2020;54(4):888–99. https://doi.org/10.1007/s43441-019-00023-3.
Muñoz MA, Dal Pan GJ, Wei YJ, Delcher C, Xiao H, Kortepeter CM, Winterstein AG. Towards automating adverse event review: a prediction model for case report utility. Drug Saf. 2020;43(4):329–38. https://doi.org/10.1007/s40264-019-00897-0.
P103 Exploring the Safety Profiles of the COVID-19 Vaccines in VigiBase Using vigiGroup: a Novel Method for Clustering ICSRs
N. Erlanson1, J. Barrett1, G. Lucie1, J. Mitchell1, C. Melgarejo1, Q. Y. Yue1
1Uppsala Monitoring Centre, Research and Education, Uppsala, Sweden
Background/Introduction: Disproportionality analysis [1, 2] has been a stalwart of post marketing safety surveillance, however it has some drawbacks. vigiGroup [3, 4] is a novel ICSR clustering method which can complement disproportionality analysis, and we present its first application to prospective monitoring of a drug’s safety profile, by incorporating its use into our surveillance activities of the COVID-19 vaccines.
Objective/Aim: We aim to explore and develop the utilisation of vigiGroup as a tool for prospective monitoring of COVID-19 vaccine safety reports in VigiBase.
Methods: VigiBase receives tens of thousands of adverse event reports per week for all COVID-19 vaccines collectively. Each week, the vigiGroup algorithm has been applied to cluster the accumulated ICSRs of COVID-19 vaccines. The algorithm was applied independently for each vaccine, but also grouping the vaccines by platform. Our multidisciplinary team of pharmacovigilance experts and data scientists have developed powerful data visualisation tools to rapidly explore the evolving safety profile of the vaccines, as well as to strengthen the hypotheses generated with traditional disproportionality analysis.
Results: A number of potential safety signals have been identified directly using the tools we have developed in tandem with the vigiGroup clustering algorithm. These include appendicitis and hearing loss/tinnitus for all COVID-19 vaccines and delayed local reactions to the Moderna vaccine, which were identified in a vigiGroup interface with the aid of imbedded data visualisation tools. Several more signals have been strengthened by further incorporating vigiGroup as a complementary tool to traditional disproportionality analysis. We have moreover demonstrated the efficacy of the algorithm and our tools by recovering labelled adverse reactions, such as hypersensitivity and anaphylaxis, and emerging safety signals, e.g. myocarditis, for all of the vaccines.
Conclusion: The use of vigiGroup clustering has been explored by pharmacovigilance experts at the Uppsala Monitoring Centre and has been found to be a promising new tool for post-marketing drug safety surveillance. The vigiGroup algorithm and data visualisation tools are being iteratively developed as the prospective monitoring of COVID-19 vaccines continues.
References/Further Sources of Information
Norén GN, Hopstadius J, Bate A. Shrinkage observed-to-expected ratios for robust and transparent large-scale pattern discovery. Stat Methods Med Res. 2013 Feb;22(1):57–69.
Bate A, Lindquist M, Edwards IR, Olsson S, Orre R, Lansner A, et al. A Bayesian neural network method for adverse drug reaction signal generation. Eur J Clin Pharmacol. 1998 Jul 1;54(4):315–21.
Norén GN, Meldau E-L, Chandler R. Consensus clustering for case series identification and adverse event profiles in pharmacovigilance. Submitted.
Erlanson N. Improving the speed and quality of an Adverse Event cluster analysis with Stepwise Expectation Maximization and Community Detection [Internet]. 2020 [cited 2021 Jun 10]. http://urn.kb.se/resolve?urn=urn:nbn:se:uu:diva-415476.
P104 Computer Vision-Aided “Fingerprinting” of Adverse Events (AE) Source Documents as a Means for Early Case Prioritization
S. Guerler1, K. Stewart2, G. Serbarinov3, M. Widdowson4, S. Desai3
1Bristol Myers Squibb, WorldWide Patient Safety, Boudry, Switzerland; 2Bristol Myers Squibb, WorldWide Patient Safety, Seattle, USA; 3Bristol Myers Squibb, WorldWide Patient Safety, Summit, USA; 4Bristol Myers Squibb, WorldWide Patient Safety, Stockley Park, UK
Background/Introduction: Integrating cognitive technology in Pharmacovigilance (PV) processes to support safety teams with gained efficiency and intelligent insights is a shared ambition across the industry. In case management, the primary focus is on steps performed by case processors, yet we found that cases spend most of their time in queues, with little hands-on processing time.
Objective/Aim: To develop a solution for intelligent case prioritization and optimized queue management from the very first user step queue, ahead of any source document data extraction by a user or a machine.
Methods: Image recognition was accomplished through a convolutional neural network (CNN) and a novel approach to data preparation and deep learning. First, case source documents were converted into a single image using image processing software. A CNN for image recognition was built using architecture inspired by Abhinav Sagar’s work1. It was trained using PV expert assessment of seriousness, causality and labelling as the ground truth.
A Robotic Process Automation (RPA) solution was built to service the CNN with source documents and transfer its outputs to the global safety database as a basis for a new priority value. Further, the RPA solution is utilized to create pdfs, create new items in the intake module and upload the source documents. Daily performance monitoring of the solution was achieved via a custom-made dashboard.
Results: The solution was deployed into production with a focus on interventional clinical trials. At launch, the machine learning model had achieved an F1 score of 0.72 and a receiver operating characteristics area under the curve (AUC) of 0.75. Retraining of the model with the latest production data is scheduled to occur on a bi-monthly basis.
Conclusion: We have demonstrated that Machine Learning (ML) classification capabilities can support existing PV processes using ML image recognition, here to enable efficient resource utilization. The size of the training dataset impacts the quality of the neural network results. Upstream or downstream processes may have to be redesigned. In this case the RPA, an already established solution, acted as the delivery mechanism to and from the ML to enable a seamless integration from mailbox to global safety database.
References/Further Sources of Information
Sagar A. Deep learning for detecting pneumonia from X-ray images. Towards Data Science. July 26, 2019. Accessed June 3, 2021. https://towardsdatascience.com/deep-learning-for-detecting-pneumonia-from-x-ray-images-fc9a3d9fdba8.
P105 AI-Powered Drug Coding With WHODrug Koda: An Evaluation on Adverse Event Reports
E. L. Meldau1, S. Bista1, E. Rofors2, L. M. Gattepaille1
1Uppsala Monitoring Centre, Research and Education, Uppsala, Sweden; 2Uppsala Monitoring Centre, Products and Services, Uppsala, Sweden
Background/Introduction: Coding medicinal products on adverse event reports (AE) to entries in drug dictionaries, such as WHODrug Global [1], is a time-consuming activity during case processing [2]. Drug coding is repetitive and has large potential for automation, while some cases require trained coding experts. Many organisations are partially automating the drug coding using rules [2, 3] but no advanced AI solutions for drug coding are in broad use.
WHODrug Koda is the Uppsala Monitoring Centre’s automated coding engine developed for coding concomitant drugs in clinical trials. Koda uses coding rules and machine learning to select drug names from WHODrug. Koda can select a drug name with high certainty, suggest a set of drug names to choose from or leave the entry uncoded, when human expertise is required. Drug entries are described by a verbatim and optional information in form of route, indication and country.
Objective/Aim: Evaluate the drug coding performance of WHODrug Koda on adverse event reports from VigiBase, the World Health Organization’s global database of Individual Case Safety Reports, in terms of level of automation and coding quality.
Methods: Koda is evaluated on 4.8 million drug entries with a valid WHODrug coding reported on 2.6 million reports received in VigiBase in 2020, excluding entries with non-latin characters. These contain 907,153 unique inputs. Exact match of verbatim against the drug name in WHODrug is used as baseline. Coding quality is evaluated by comparing high-certainty Koda results to the gold standard and a sample of 200 disagreeing codings was manually assessed by two teams to judge the coding quality.
Results: Koda shows an automation level of 95% with 89% high-certainty coded drug entries and 6% suggested codings; 5% are uncoded. This is significantly higher than our direct-match baseline coding 56% of the drug entries. In 97% of the cases, Koda’s high-certainty predictions agree with the gold-standard coding. Manual evaluation by two teams on the sample of disagreeing codings show that Koda’s predictions are at least as good as the gold standard in 94% and 92% of the samples with an inter-annotator agreement (Cohen’s kappa) score of 0.83.
Conclusion: While Koda was designed for concomitant drug coding in clinical trials, it achieves automation level and coding quality on AE reports in VigiBase comparable to the performance of a previous evaluation on clinical trial data [3]. Koda can resolve non-trivial codings by utilising additional information about the drug.
References/Further Sources of Information
Lagerlund O, Strese S, Fladvad M, Lindquist M. WHODrug: a global, validated and updated dictionary for medicinal information. Ther Innov Regul Sci. 2020 Feb 20:1–7.
Ghosh R, Kempf D, Pufko A, Martinez LF, Davis CM, Sethi S. Automation opportunities in pharmacovigilance: an industry survey. Pharm Med. 2020 Feb;34(1):7–18.
Herrgard S, Gil C, Holst I, Nielsen JH, Ranthe MF, Serif A, et al. Assessment of machine learning methods in coding of concomitant medications in clinical trials. In: PhUSE Connect US, 8–11 March 2020, Orlando, USA. 2020. https://www.lexjansen.com/phuse-us/2020/ml/ML13.pdf.
P106 The Portuguese Survey on Anticoagulated Patients Register (START-Portugal-Register): Preliminary Results
J. Abrantes1,2, D. Mendes1,2, F. Batel-Marques1,2,3
1Association for Innovation and Biomedical Research on Light and Image-AIBILI, Centre for Health Technology Assessment and Drug Research-CHAD, Coimbra, Portugal; 2Drug Safety and Effectiveness Research Network, DruSER.Net, Coimbra, Portugal; 3University of Coimbra, Laboratory of Social Pharmacy and Public Health, School of Pharmacy, Coimbra, Portugal
Background/Introduction: Anticoagulation aims to prevent thrombotic events in patients with predisposing factors or to treat venous thromboembolism. However, such therapy is associated to an increased risk of bleeding [1].
Objective/Aim: To evaluate safety and effectiveness of anticoagulants, and to improve our understanding of the risks and benefits of these therapies by recording and analysing data from patients followed-up in routine clinical practice. This report presents longitudinal follow-up data collected during the first one and half year of the study.
Methods: This is a multicentre prospective cohort study which is enrolling adult patients on anticoagulant treatment, irrespectively of the prescribed drug, the dosage, and the indication for its use. The collected baseline data include demographic and clinical characteristics of patients, risk factors for bleeding and thrombotic complications, laboratory data, indication for treatment, and concomitant drugs. Follow-up data include information regarding the management of anticoagulation therapy, and events or complications occurring during treatment. Data are collected and recorded in electronic Case Report Forms by physicians. In this report, analysis of each patient’s observation period started on the day of inclusion and ended on June 1, 2021 (or sooner if treatment had been discontinued for any reason).
Results: The inclusion of patients in the registry started in January 2020. A total of 22 patients were recruited by 21 physicians from 11 clinical sites. Non valvular atrial fibrillation was the most frequent indication for treatment (n = 17; 77.3%), followed by venous thromboembolism (n = 4; 18.2%), and valvular atrial fibrillation (n = 1; 4.5%). All patients had comorbidities and used concomitant therapies. Nine patients (40.9%) were on apixaban, 7 (31.8%) on edoxaban, and 2 (9.1%) on rivaroxaban. One patient (4.5%) was on low molecular-weight heparin, and 3 (13.6%) were on vitamin k antagonists (VKA). Only two patients switched anticoagulant drug (both VKA by apixaban) during follow-up. Three patients on apixaban reported 5 adverse events: 4 minor bleeding events (i.e., epistaxis (n = 2), decrease in haemoglobin (Hb < 2 g/dL) (n = 1), and microscopic haematuria (n = 1)), and liver abscess with cholangitis (n = 1). Two patients on edoxaban reported 4 adverse events: 1 minor bleeding event (haemorrhage aggravated (n = 1)), renal function aggravated (n = 1), septic shock (n = 1) and fall with cranioencephalic trauma (n = 1). One patient died.
Conclusion: After one and half year of follow-up, no serious thrombotic events were reported, but 13.6% of patients had minor bleeding events. Further participants need to be included to better characterize the benefit-risk profiles of anticoagulation therapies.
References/Further Sources of Information
Antonucci E, Poli D, Tosetto A, et al. The Italian START-Register on anticoagulation with focus on atrial fibrillation. PLoS One. 2015;10(5):e0124719.
P107 Using Network Analysis for Better Signal Detection in Pharmacovigilance
M. Pétervári1,2,3, O. Balogh1, B. Benzcik1, B. Ágg1,3,4, P. Ferdinandy1,3,4
1Semmelweis University, Pharmacology and Pharmacotherapy, Budapest, Hungary; 2Sanovigado Ltd, Budapest, Hungary; 3Hungarian Academy of Sciences, System Pharmacology Research Group, Budapest, Hungary; 4Pharmahungary Group, Szeged, Hungary
Background/Introduction: Previously unknown drug—adverse event relationships are identified during the signal detection procedure in post-marketing phase of the drugs. Over 80% of the detected signals are originating from statistical analysis, however only 2.5% of these signals are prioritised and assessed by PRAC at later stages. This high proportion of false positive findings means a significant burden to the pharmaceutical industry, and the regulatory bodies.
Objective/Aim: In our study we aimed to develop a network-based statistical signal detection method which could contribute to the more efficient identification of previously unknown drug—adverse event relationships.
Methods: We accessed all Individual Case Safety Reports (ICSRs) containing acute myocardial infarction (AMI) from EudraVigilance spontaneous reporting database. We developed a software, called Vigilance, which allowed us to generate networks, and analyze the accessed data. By using various edge weighting algorithms, four different networks were built. After normalizing the different edge weights, we utilized three network-based methods considering the co-reported adverse events and drugs of a drug—adverse event edge. We assessed the efficiency of these new methods on a reference dataset and compared it by receiver operating characteristic (ROC) analysis to the currently used reference Reporting Odds Ratio (ROR) signal detection method.
Results: Over 17 546 ICSRs were accessed which contained AMI. The ICSRs were processed by Vigilance, and four types of networks were generated. In this dataset rofecoxib (2543) and coronary artery disease (2157) were the most frequently co-reported drug and adverse event with AMI. The new signal detection method considering co-reported drugs, performed significantly better compared to the standard ROR method (area under the curve of ROC 0.86 vs 0.72, DeLong-test p < 0.05)
Conclusion: We demonstrated that network-based signal detection methods can perform better compared to the currently used standard methods. However, considering the limitations of this current study, further investigation would be required to assess the full potential of application of network analysis for pharmacovigilance purposes.
References/Further Sources of Information
This study was supported by the National Research, Development and Innovation Office of Hungary (NKFIA; NVKP-16-1-2016-0017 National Heart Program). Mátyás Pétervári was supported by EFOP-3.6.3-VEKOP-16-2017-00009 grant.
P108 A Smartphone Application for the Completion of IMiD Risk Evaluation and Mitigation Strategy (REMS) Patient Surveys: The REMS Companion App
N. Sweeney1, J. Chapman2, J. Varandas3, R. McWilliams4, J. Roldan1, M. L. Chan-Liston2
1Bristol Myers Squibb, WorldWide Patient Safety/US REMS, Summit, USA; 2Bristol Myers Squibb, WorldWide Patient Safety/Global Risk Management, Summit, USA; 3Bristol Myers Squibb, WorldWide Patient Safety/Global Risk Mgmt. Technology, Summit, USA; 4Bristol Myers Squibb, WorldWide Patient Safety/Global Risk Management Operations, Summit, USA
Background/Introduction: Patients who use thalidomide, lenalidomide, and pomalidomide (a class of drugs known as immunomodulators, IMiDs) in the U.S. take mandatory, periodic patient surveys prior to receiving treatment to verify their understanding and confirm appropriate contraception behaviors. The patient survey requirement is an element to assure safe use (ETASU), which is part of a REMS mandated by the U.S. Food and Drug Administration (FDA) for these medications. Historically, most patient surveys have been initiated when REMS-certified pharmacies call patients to complete their pharmacy REMS requirements but cannot proceed because the patient’s survey is still pending. The burden was thus placed on pharmacy staff to transfer patients to a Bristol Myers Squibb (BMS)-Celgene Customer Care representative or to an Interactive Voice Recognition (IVR) system to complete the required patient survey. This process could potentially delay prescription confirmation and is inconvenient for patients. A first-in-class smartphone application (the REMS Companion App) has been developed to help alleviate this burden by notifying patients when surveys are available for confidential completion on their smartphone.
Objective/Aim: The objective of this 9-month pilot study was to validate the REMS Companion App for the purpose of obtaining FDA approval for its use.
Methods: The 9-month pilot included 21 actively enrolled REMS patients who validated the application. Pre- and post-pilot feedback questionnaires were administered to participants to assess smartphone user practices and current REMS interaction.
Results: All patient surveys completed by pilot participants were successfully reconciled, thus allowing the REMS process to continue and their prescriptions to be confirmed at the pharmacy. In addition, a majority of participants opted in for survey notifications; age did not deter patients from utilizing the application (Table 1). Pilot participants’ feedback was overwhelmingly positive. Overall, 95% (19/20) of pilot participants stated that they would continue to utilize the REMS Companion App to complete their patient surveys. Based on these results, the application was approved by the FDA and launched on November 1, 2020 via Google Play and Apple’s App Store. As of June 1, 2021, 8355 patients have downloaded the app and set up an account.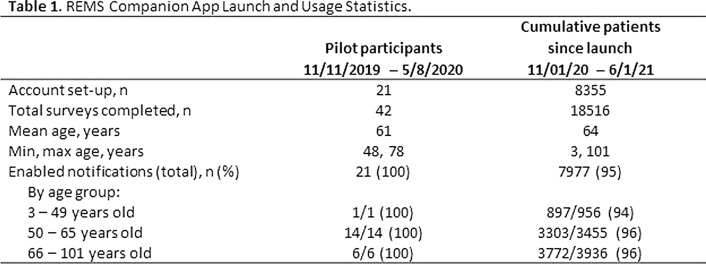
Conclusion: The REMS Companion App is a first-in-class smartphone application that provides an additional, convenient way for patients to complete REMS surveys, thus helping to ensure safe use of medications and reducing the burden on pharmacies.
References/Further Sources of Information
n/a.
P109 Unresolved Gustatory, Olfactory and Auditory Adverse Drug Reactions to Antibiotic Drugs: A Signal Detection Analysis on Publicly Accessible Eudravigilance Data
G. Valdiserra1, S. Ferraro1, M. Fornili2, I. Convertino1, E. Cappello1, E. Lucenteforte2, M. Tuccori3
1University of Pisa, Unit of Pharmacology and Pharmacovigilance-Department of Clinical and Experimental Medicine, Pisa, Italy; 2University of Pisa, Unit of Medical Statistics-Department of Clinical and Experimental Medicine, Pisa, Italy; 3University Hospital of Pisa, Unit of Adverse Drug Reactions Monitoring, Pisa, Italy
Background/Introduction: Unresolved outcomes are frequently reported for gustatory, olfactory and auditory (GOA) ADRs in spontaneous reporting databases [1, 2]. However, such high volume of unresolved GOA ADRs could reflect an under-investigated clinical issue or an intrinsic difficulty in the outcome assessment [3].
Objective/Aim: The primary aim was to demonstrate that unresolved outcomes are reported more frequently for GOA ADRs than for other ADRs to systemic antibiotics. The secondary aim was to identify potential signals of unresolved GOA ADRs for specific classes of antibiotic drugs and for specific antibiotics.
Methods: We extracted the number of ADRs to systemic antibiotics of the Anatomical Therapeutic Chemical (ATC) class J01 from Eudravigilance up to February 2019. We classified ADRs in “non-GOA ADRs” and “GOA ADRs”. ADRs were categorised in three groups according to the outcome: defined, persistent/permanent (unresolved) and undetermined ADRs. We performed disproportionality analyses with the case/non-case methodology, by calculating the crude reporting odds ratio (ROR) and 95% confidence interval (CI). Cases were all persistent/permanent ADRs, and non-cases were defined and undetermined ADRs. For the primary aim, index groups were gustatory or olfactory or auditory ADRs, while reference group included all non-GOA ADRs. For the secondary aim, we performed a disproportionality analysis by using the sub-set of GOA ADRs. Index and reference groups varied with subgroups of ATC class, so that each class was compared with the others. We conducted two sensitivity analyses for each analysis by varying case definition.
Results: We extracted 748,798 ADRs, including 10,770 GOA ADRs. The primary analysis showed that ROR for unresolved gustatory, olfactory and auditory ADRs was 2.68 (95% CI 2.51–2.85), 5.20 (95% CI 4.66–5.81) and 2.64 (95% CI 2.51–2.79), respectively. The secondary analyses detected signals of unresolved gustatory ADRs to doxycycline (ROR 1.69, 95% CI 1.18–2.41, p < 0.05), azithromycin (ROR 2.07, 95% CI 1.58–2.72, p < 0.001) and levofloxacin (ROR 1.59, 95% CI 1.22–2.07, p < 0.05). Signals of auditory unresolved ADRs were found for doxycycline (ROR 1.52, 95% CI 1.09–2.12, p < 0.05), clarithromycin (ROR 1.31, 95% CI 1.09–1.59, p < 0.05) and several aminoglycosides (e.g. ROR 3.49, 95% CI 2.23–5.45, p < 0.001 for amikacin). Signals of olfactory unresolved ADRs were detected for doxycycline (ROR 2.4, 95% CI 1.26–4.58, p < 0.05) and levofloxacin (ROR 1.92, 95% CI 1.28–2.86, p < 0.05). Sensitivity analyses mostly confirmed the results of the main analysis.
Conclusion: In this study, we tested and used an appropriate expected frequency standard to identify signals of unresolved GOA ADRs. This approach allowed the identification of several signals of potential persistent/permanent GOA reactions for antibiotic drugs in the Eudravigilance database.
References/Further Sources of Information
EMA. Disabling and potentially permanent side effects lead to suspension or restrictions of quinolone and fluoroquinolone antibiotics. European Medicines Agency. Published 2018. http://www.ema.europa.eu/contact. Accessed 29 Apr 2019.
Tuccori M, Lapi F, Testi A, Ruggiero E, Moretti U, Vannacci A, Bonaiuti R, Antonioli L, Fornai M, Giustarini G, Scollo C, Corona T, Ferrazin F, Sottosanti L, Blandizzi C. Drug-induced taste and smell alterations. A case/non-case evaluation of an Italian database of spontaneous adverse drug reaction reporting. Drug Saf. 2011;34(10):849–59. https://doi.org/10.2165/11593120-000000000-00000.
Ferraro S, Convertino I, Leonardi L, Blandizzi C, Tuccori M. Unresolved gustatory, olfactory and auditory adverse drug reactions to antibiotic drugs: a survey of spontaneous reporting to Eudravigilance. Expert Opin Drug Saf. 2019;18(12):1245–53. https://doi.org/10.1080/14740338.2019.1676724.
Author’s Index
Authors Presentation No.
Abdeldayem, I. P070, P076
Abdelgayed, Y. P102
Abdelrahman, A. P070
Abenhaim, L. O-011, P077
Abidi, K. P048
Abiodun, A.S. O-001
Abou Taam, M. O-007
AbouDaya, M. P041
AboZaid, N. P076
Abrantes, J. P018, P106
Abu esba, L. P001
Achour, B. P096
Achour, S. P065, P023
Adrien, F. P045
Ágg, B. P107
Aicha, C. P050
Ait El Cadi, M. P074
Al-Fadel, N. P001
Al-hilali, Z. P046
Alakeel, A. P057
Alamri, R. P057
Albalawi, O. O-002
Albalushi, S. P035
Albores Citlali, Z. P016
Aldayel, A. P099
Aldhirgham, T. P001
Alfadel, N. P028, P029, P099
Alghamdi, E. O-010
Alghamdi, W. P028, P029, P099
Alharbi, F. P001, P028, P029, P099
Ali, I. O-001, P080
Ali, I.N. P102
Alilou, M. P049
Alioua, A. P013
Alj, L. P011
Alkhakany, M. P070
Allen, E. P075
Almaghrabi, M. P029
Almagrabi, M. P028
Almutairi, A. P001
Alomair, A. P057
Alomran, M. O-002
Alrwisan, A. P001
Alsagri, G. O-002
Alshammari, T. O-002
Alshuhayb, R. O-010
Althunian, T. O-002
Alves, C. P018
Alzola-Elizondo, U. P037
Ambale, C. P078
Amiri, A. P002, P093
Annie-Pierre, J-B. P100
Anton, C. P046, P079
Aronoff, D. O-006
Asseray, N. O-003
Attias, P. O-011, P077
Attjioui, H. P031
Ayinbuomwan, S. P080
Azzouz, B. P042, P043, P044
Badyal, K. P046, P079
Bahr, S. P102
Bahri, P. O-007, P002, P003
Balabanov, P. O-007
Balogh, O. P107
Bannour, I. P095
Barkat, A. P050
Barrett, J. P103
Bartolini, C. O-005, P067
Bassi, P. P080
Bate, A. O-004, P082, P083
Batel-Marques, F. P018, P106
Batischeva, G. P056
Baux, E. O-003
Bayoumi, S. P071
Beam, A. O-006
Bechar, H. P030, P063, P091
Belahcen, M.J. P030, P091
Ben sayde, N. P094
Benabdallah, G. P022
Bensalem, C. P094, P095, P096
BenSayde, N. P096
Benzcik, B. P107
Bernard, L. O-003
Berrios, E. P017
Bhembe, F. P080
Bista, S. P105
Blandizzi, C. P067
Boivin, P. P045
Bonal, C. O-004
Bond, C. P039
Bouatia, M. P032, P033
Boudina, Y. P049
Brik, Y. P031
Buchard, A. O-011, P077
Bucsa, C. P047, P061
Cappello, E. O-005, P067, P109
Capuano, A. P069, P071
Capucho, H. P073
Carvajal, A. P019, P064
Cazacu, I. P047
Ceballos Villalva, J.C. P054
Chaibi, A. P048, P049, P058
Challa, A. O-006
Chamani, K. O-009
Chambeh, W. P094, P096
Chan-Liston, M.L. P108
Chapman, J. P108
Cheikh, A. P032, P033
Cheng, C. P097
Cherif Chefchaouni, A. P030, P063, P091
Cherif, W. P096
Chinchilla, C. P036
Chinchilla, K. P060, P088
Chung, S. P092
Cobelens, F. O-001, P080, P081
Convertino, I. O-005, P067, P109
Corboy, C. P090
Cosio, J.A. P016
Cunningham, S. P035
Cutler, R. O-001
Dang, V. P097
Davies, H. P101
de Fays, L. O-007
Degremont, A. P036
Dendane, T. P048
Desai, S. P104
Deschanvres, C. O-003
Dey, S. P004, P005, P006, P007, P008, P009, P010
di Mauro, G. P069
Diaz-Tufinio, C. P059
Diesel, G. P101
Doan, D.L. P020
Domingo-Echaburu, S. P037
Dorguin, G. P042
Dreyfus, B. P039
Duchemin, T. O-011, P077
Dufosse, M. P045
Duga, A. P080
Durante-Salmerón, D.A. P059
Ekhart, C. P060
El Baraka, S. P030, P091
El kartouti, A. P023, P065
El Marnissi, S. P074
El Shafei, A. P071
EL-Hefney, G. P102
El-Warwary, M. P102
Elagbaje, C. O-001
Elhawary, M.A. P102
Elq, O. P058
Elrherbi, A. P022
Epaulard, O. O-003
Erlanson, N. P103
Farcas, A. P047, P061
Fathallah, N. P094, P095, P096
Ferdinandy, P. P107
Ferner, R. P046
Ferrajolo, C. P069
Ferraro, S. O-005, P067, P109
Ferreira Da Silva, R. P012
Fini, E. P067
Fornai, M. O-005
Fornili, M. P109
Fouda, M. P057
Fraden, B. O-001
Gandhi, H. P038
Ganso, M. P089
Gattepaille, L.M. P105
Gaxiola, M. P017
Gini, R. O-005, P067
Giometto, S. O-005, P067
Giraudeau, B. O-003
Goldstein, J. O-006
Grant, A. P035
Gras, V. P045
Grau, M. P100
Grimaldi, L. O-011, P077
Guedes, P. P012
Guerler, S. P104
Guillaumin, M. P100
Guimard, T. O-003
Gutiérrez, I. P017, P016
Haasum, Y. P020
Habchane, A. P013
Haguinet, F. O-004
Hall, V. P088
Hamon, Y. O-011, P077
Hamza, S. P048
Han, S. P092
Härmark, L. O-001, P025, P026, P088
Harrison, M. P039
Hedna, K. P051
Helen, N. P068
Hermani, N. P011
Herrera, C.A. O-007
Hult, S. P020
Inácio, P. P088
Inoubli, A. O-007
Isah, A. O-001, P080, P081
Issac, A. P014, P015, P072, P084
Jatau, B. O-001
Jiménez Corona, M.E. P016
Johnson, N. P055
Jokinen, J. P039
Jonville-Bera, A.P. O-003
Jung, M. P044
Junqueira Polónia, J. P012
Kambai Avong, Y. O-001
Kant, A. P025, P026
Kapil, N. P039
Kapoor, V. P062
Kara, V. O-004, P082, P083
Kaur, N. P082, P083
Kawabata, H. P004, P005, P006, P007, P008, P009, P010
Kayode, G.A. O-001
Kharb, P. P062
Khatem, S. P013
Khedr, A. P090
Khlif, A. P096
Kiguba, R. P068
Killick, D. P101
Kim, B. P092
Kitutu, F. P068
Ko, Y. P092
Kochuparambil, J.J. P014, P015, P072, P084
Kongkaew, C. P085, P086
Kornkaew, K. P085
Kratt, C. P039
Lalchandani, V. P039
Lane, S. P052, P053
Lang, T. P075
Laoulaou, B. P043
Laroche, M.L. P100
Lasri, F.Z. P074
Lau, A.G. P090
Lavieri, R. O-006
Le Gouge, A. O-003
Le Turnier, P. O-003
Lecompte, A.S. O-003
Legras, A. O-003
Leila, B. P023
Leon-Curiñaupa, S. P064
Lepoix, E. P043, P066
Lertxundi, U. P037
Lippmann, E. O-006
López-Gamboa, M. P054
Lorenzoni, V. O-005, P067
Lourenço, F. P073
Lucenteforte, E. O-005, P067, P109
Lucie, G. P103
Lum, L. P055
Luque, J. P064
Lynn, E. P052, P053
Mabrouki, M. P074
Macolić Šarinić, V. P002, P003, P093
Magongo, S. P080
Mahaux, O. O-004
Mahdad, M. P091
Mansou, K. P096
Mansour, K. P094, P095
Martin, S. P042, P043, P044, P066
Martínez, H.A. P016
Mascolo, A. P069
Masmoudi, K. P045
Matos, C. P088
Maza, J.A. P016, P017
McIntosh, T. P035
McWilliams, R. P108
Meddah, B. P031
Meesomperm, P. P086
Meftah, A. P032, P033
Meldau, E.L. P105
Melgarejo, C. P103
Mendes, D. P018, P106
Mentzer, R. P055
Merico, E. P082, P083
Michielsens, C. P090
Mitchell, J. P103
Mogosan, C. P047, P061
Mohammed, S. P040
Moinuddeen, S. P004, P005, P006, P007, P008, P009, P010
Monzon, E. O-007
Morato, M. P012
Morel, A. P042, P043, P044
Moride, Y. O-011, P077
Moscol, S. P064
Moukafih, B. P023, P065
Mounir, M. P102
Mousannif, S. P031, P032, P033
Muñoz, M. P087, P097
Murerwa, C. P078
Murillo, A. P017
Mustafa, W.W. P040
Muzalevskaya, E. P056
Nakitto-Kesi, D. P068
Nhlabatsi, S. P080
Nidha, A. P015
Noble, P.J. P101
Noikeaw, J. P085
Noor, A. O-007
Nouibi, C. P063, P091
Oger, E. P036
Oh, S. P092
Okai, J. P002, P003
Oosterhuis, I. P024, P025
Opadeyi, A. O-001, P080, P081
Orain, J. O-003
Orellana-Nuñez, G. P019
Orive, G. P037
Osakwe, A. P080
Oscanoa, T. P019, P064
Osccorima Gonzales, C. P019
Osmont, M.N. P036
Ouedraogo, J.M. P030
Ouni, B. P094, P095, P096
Ozolua, R. P080
Palma-Aguirre, J.A. P059
Palomino-Serna, V. P019
Pan, X. P004, P005, P006, P007, P008, P009, P010
Paoletti, O. O-005, P067
Pasache-Juarez, J. P019
Pascali, P. P066
Penedones, A. P018
Peredo, K. P017
Pérez Barragán, A. P016
Pérez, A. P017
Perova, N. P056
Perroud, M. P073
Peryea, T. O-006
Pesquidou, M.C. O-007
Pétervári, M. P107
Phan, M. P097
Philipot, M.G. P066
Pinchbeck, G. P101
Pinel, S. P042
Pinto, T. P073
Pirmohamed, M. P101
Polard, E. P036
Pont, L. O-001
Powell, G. P082, P083
Pp, S. P004, P005, P006, P007, P008, P009, P010
Puerto, M. P017
Quiroz, A. P017
Radouani, M.A. P050
Rafaniello, C. P069
Rahali, Y. P030, P063, P091
Rahmouni, H. P050
Ramsden, S. O-008
Rashed, A. P041
Rausch, C. P020
Ribeiro-Vaz, I. P012
Ribeiro, A. P073
Richardson, M. P039
Rodrigues, L. P073
Rofors, E. P105
Rojas Velasco, G. P016
Rojas, G. P017
Roldan, J. P108
Rolfes, L. P026
Rosado, F. P017
Rossi, F. P069
Rostom, H. P071, P102
Rudolph, A. P020
Ruggiero, R. P069
Ruiz-Aguilar, D.F. P059
Rusu, A. P047, P061
Sahak, R. P079
Said, A. P089
Saint-Raymond, A. O-007
Salwan, P. P062
Sánchez-Pérez, Á. P037
Scailteux, L.M. P036
Scavone, C. P069, P071
Schiaffino, S. P073
Scholfield, N. P086
Schulz, M. P089
Seekins, D. P039, P055
Sefiani, H. P022
Semenza, C. O-008
Serbarinov, G. P104
Shakir, S. P052, P053
Shen, M. O-006
Shin, S. P092
Shongwe, N. P080
Shuibu, A.T. O-001
Silva, A.M. P012
Slattery, J. P052, P053
Slim, R. P094, P095, P096
Smyth, S. P101
Soulaymani Bencheikh, R. P011, P022, P023, P065
Sportiello, L. P069
Stankovic, M. P027
Stewart, D. P035
Stewart, K. P104
Straus, S. O-007
Suh, A. P092
Sweeney, N. P108
Taavola, H. P020
Takoui, A. P013
Talarmin, J.P. O-003
Tavares, I. P018
Tebaa, A. P011, P022, P023, P031, P032, P033, P065, P074, P076
Tiemersma, E. O-001
Tillati, S. O-005
Tomlin, S. P041
Trenque, T. P042, P043, P044, P066
Tuccori, M. O-005, P067, P109
Tumwijukye, H. O-001, P080, P081
Turchetti, G. O-005, P067
Valdiserra, G. O-005, P067, P109
Valnet Rabier, M.B. P100
Vambe, D. P080
van Balveren, L. P026
van de Koppel, S. P034
van der Kooi, D. P034
van Hoof, M. P088
Van Hunsel, F. P060, P024, P025, P026, P034, P088
van Puijenbroek, E. O-009, P024
Varandas, J. P108
Vayalikunnel, J. P014, P015, P072, P084
Veyrac, G. O-003
Vujovic, S. P027
Vukicevic, V. P027
Waern, M. P051
Walker, R. P075
Weetink, Y. O-009
Widdowson, M. P104
Willems, P. P090
Wimonanun, B. P085
Woerdenbag, H. P034
Wu, E. P097
Younis, M. P040
Yue, Q.Y. P020, P103
Zago, A. P073
Zakumumpa, H. P068, P098
Zaoui, S. P013
Zhang, J. P004, P005, P006, P007, P008, P009, P010, P055
Zhdanova, O. P056
Zheng, J. P004, P005, P006, P007, P008, P009, P010


