Keywords: channels, hearing loss, synapses, tinnitus, drug development
Abstract
Tinnitus is a pervasive public health issue that affects ∼15% of the United States population. Similar estimates have also been shown on a global scale, with similar prevalence found in Europe, Asia, and Africa. The severity of tinnitus is heterogeneous, ranging from mildly bothersome to extremely disruptive. In the United States, ∼10–20% of individuals who experience tinnitus report symptoms that severely reduce their quality of life. Due to the huge personal and societal burden, in the last 20 yr a concerted effort on basic and clinical research has significantly advanced our understanding and treatment of this disorder. Yet, neither full understanding, nor cure exists. We know that tinnitus is the persistent involuntary phantom percept of internally generated nonverbal indistinct noises and tones, which in most cases is initiated by acquired hearing loss and maintained only when this loss is coupled with distinct neuronal changes in auditory and extra-auditory brain networks. Yet, the exact mechanisms and patterns of neural activity that are necessary and sufficient for the perceptual generation and maintenance of tinnitus remain incompletely understood. Combinations of animal model and human research will be essential in filling these gaps. Nevertheless, the existing progress in investigating the neurophysiological mechanisms has improved current treatment and highlighted novel targets for drug development and clinical trials. The aim of this review is to thoroughly discuss the current state of human and animal tinnitus research, outline current challenges, and highlight new and exciting research opportunities.
CLINICAL HIGHLIGHTS
Although an ancient disorder, in the last 20-30 years, tinnitus has emerged as a serious public health issue, affecting approximately 50 million Americans. Approximately 10-20% of individuals who experience tinnitus report symptoms that severely reduce their quality of life. Among veterans, tinnitus is the number-one service-related disability and impacts more than 2.17 million veterans overall.
Tinnitus is the persistent involuntary subjective phantom percept of internally generated, indistinct, non-verbal noises and tones.
In most cases, tinnitus is initiated by acquired hearing loss and maintained only when this loss is coupled with distinct neuronal changes in auditory and extra-auditory brain networks.
The electrical patterns of activity that are necessary and sufficient for the generation and maintenance of tinnitus are generated within these networks, but their precise geometry and underlying mechanisms still remain unclear.
This review discusses the current state of human and animal tinnitus research, outlines current challenges, and highlights new and exciting research opportunities.
Current treatments, such as sound therapy, amplification, cognitive behavior therapy, and education/counseling, are effective in diminishing awareness of tinnitus or associated distress but do not treat the underlying percept.
This review discusses potential targets for the development of neuroscience-driven tinnitus drugs and therapies, such potassium channel activators and corrective brain plasticity.
Given the heterogeneity of tinnitus, a neuroscience-based precision medicine approach should facilitate clinical trials, treatment, and cure.
1. INTRODUCTION
Tinnitus, commonly referred to as the “ringing in the ears,” is a disorder that likely dates back to the earliest human civilizations, although the earliest documented report of tinnitus is debated (1). In the last 20–30 yr, tinnitus has emerged as a serious public health issue, affecting ∼50 million Americans (2–5). The severity of tinnitus is heterogeneous, ranging from mildly bothersome to extremely disruptive. In the United States, ∼10–20% of individuals who experience tinnitus report symptoms that severely reduce their quality of life (5, 6). Among veterans, tinnitus is the number one service-related disability and impacts more than 2.17 million veterans overall. In fiscal year 2019 alone, 183,145 new veterans entered this pool (7). Due to the huge personal and societal burden, a concerted research effort in recent years has significantly advanced our understanding of this disorder. Yet, neither full understanding, nor cure exists.
Because we believe that the etiological definition is the final stage of knowledge, we will start with an attempt to define tinnitus based on our current knowledge. Our expectation is that this definition and the subsequent sections will help us highlight the scientific advancements, the new challenges, and the opportunities in tinnitus research and treatment.
1.1. Definition
Tinnitus is the persistent involuntary subjective phantom percept of internally generated indistinct nonverbal noises and tones, which in most cases is initiated by acquired hearing loss and maintained only when this loss is coupled with distinct neuronal changes in auditory and extra-auditory brain networks.
Tinnitus is a phantom sound percept, but phantom percepts are not always associated with pathology. In fact, phantom percepts are a fundamental aspect of mindfulness and consciousness. In his essay “Περί Ψυχής” (On The Soul) 2,300 yr ago, Aristotle (8) was the first to formally state that mindfulness is a form of a phantom perception, or at least mindfulness cannot be possible without phantom perceptions (imagination). Aristotle was the first to formally connect the body to the mind, stating that the mind needs phantom percepts for cognition and knowledge and that our body through our sensory organs and sensations provide the initial material to our brain for forming phantom percepts and mental images that ultimately lead to cognition.
To understand tinnitus, it is important to understand the role of phantom percepts. Although we use external stimuli to shape our behavior, our brain is very much relying on its ability to generate internal representations of the world (images), through phantom perceptions. As such, phantom perceptions are essential for our survival and well-being. Namely, our survival depends on the brain’s chief function, which is, in our opinion, to perform an online prediction of reality. To achieve this task, the brain must predict and match reality dynamically. Thus internally generated representations of basic aspects of the outside world allow the brain not to have to recreate the necessary functional geometry for guiding action de novo every time a new stimulus and a new decision has to be made. We propose that this approach reduces the brain’s computational overhead, so that survival (and happiness) become an option. Imagine if we cross a road, and a car is approaching us with a certain speed although the light is green for us. Our brain has to predict the speed of the car, the driver’s intentions, our speed, and then temporally match it to reality, to give our motor system the right command. Moreover, phantom percepts are also essential for our well-being and happiness. When we listen to the Alpine Symphony by Richard Strauss, we can “picture” and enjoy the mountain from our living room at different times of the day, although we only hear sounds. Taken together, our sensory processing and our actions are not working on the premise of an input-output operation, but instead, their functioning is the result of the dynamic processing of internally generated expectations with responses to external stimuli. As such, understanding the underlying mechanisms of tinnitus holds the promise to advance the treatment of the disorder but also fundamental aspects of sensory processing, cognition, and subjectivity.
Tinnitus percepts are involuntary, internally generated percepts. Through a not fully understood mechanism, these internally generated sounds are released to our consciousness and become unwanted percepts that are intrusive and stressful. Unwanted phantom percepts are found in other auditory disorders and other sensory systems. In fact, involuntary auditory hallucinations (often verbal) are a hallmark characteristic of schizophrenia. In the somatosensory system, phantom limb pain, or the perception of pain in a limb that has been amputated or otherwise deafferented, is another example of a phantom perception that is involuntary and can be incredibly disruptive to the patient’s quality of life (9). Remarkably, Tourette’s Syndrome involves phantom percepts with the unintentional release of verbal or motor actions. These symptoms involve involuntary liberation of fixed action patterns leading to continuous drumming of fingers, arms, and legs (10–12). Could tinnitus involve similar involuntary liberation of equivalent auditory common environmental noises stored in our brains for online prediction of reality? Although we do not know the answer to this question, we propose that understanding the mechanisms of pathological phantom perception in analogous disorders may provide powerful insight into the mechanisms of tinnitus.
What leads to the unwanted release of internally generated tinnitus percepts? Internal state is likely malfunctioning in concert with the sensory organ. Could it be that hearing loss due to peripheral damage or aging induce central network maladaptive plasticity that push the mind to an altered internal state, where unwanted release of sound percepts occurs? Although there are tinnitus cases without obvious hearing loss (more details in sect. 3), a major cause of tinnitus is acquired hearing loss. Consistent with this, individuals (and rodents) with tinnitus have some form of peripheral dysfunction/deficit. This deficit may be subclinical and thus not captured by the traditional audiometric test battery. The most popular mechanistic model is that this peripheral deficit leads to compensatory/homeostatic/(mal)adaptive changes in auditory and extra-auditory circuits: the exact form and localization of these changes may vary among individuals with tinnitus (13). However, only very distinct changes, which are currently not fully understood, lead to tinnitus, as not all individuals with hearing loss experience tinnitus.
2. TINNITUS ANIMAL MODELS
The use of animal models to study tinnitus is of great value, as models allow for strict experimental control that is not permissible in humans. In the last several decades of tinnitus research, there have been major advancements in tinnitus animal models that have significantly enhanced the understanding of the pathophysiological mechanisms of tinnitus. Despite the immense value and progress of animal models in tinnitus research, two fundamental hurdles remain: the selection of an animal model that can closely approximate tinnitus as experienced by humans and an assay that accurately and objectively detects tinnitus. The former is challenging, as there are many diverse causes and clinical presentations of tinnitus, while the latter still hinders human research due to the lack of an objective measure of tinnitus. In addition, methods of tinnitus induction and measurement in animals vary widely between studies. In this section, we will briefly review tinnitus induction and assessment in animals. For more detailed reviews on animal models of tinnitus, see Refs. 14–17.
2.1. Tinnitus Induction Methods
The two predominant tinnitus induction methods are exposure to loud sound and administration of ototoxic agents. Exposure to loud sound is often used in animal models, as acoustic trauma and subsequent hearing loss is a major risk factor for tinnitus in humans (5, 18, 19). Noise exposure intensity and duration vary widely among different studies and can result in temporary or permanent auditory threshold shifts. Accordingly, different noise exposure protocols may lead to different neurophysiological changes and likely contribute to conflicting results. While noise exposure and consequent hearing loss is a common cause of tinnitus in humans, key differences exist in the translation from animal studies to humans. One key difference is that hearing loss in animal models typically results from a single high-intensity noise exposure, while hearing loss in humans frequently reflects a lifetime of noise exposure commonly compounded by aging (20, 21) or other biological and lifestyle factors. The age of subjects at the time of noise exposure is also highly variable in animal models. Noise exposures occurring in mice <10 wk of age can have drastically different outcomes on auditory thresholds and recovery of hearing than exposures occurring in older mice (22), perhaps due to critical periods of auditory plasticity. The use of anesthesia during noise exposure is common in many models to reduce distress and discomfort. However, the use of anesthetics during noise exposure protects against noise-induced hearing loss (23, 24) and tinnitus (25). Anesthesia also affects frequency tuning and receptive fields in the auditory cortex (26), thus adding an additional layer of differences in the translation from animal studies to humans. Moreover, the time of day that the noise exposure occurs affects the degree of resulting hearing loss. Mice that are noise exposed at night show less recovery of auditory thresholds than those exposed during the day (27). Finally, significant strain and species-specific differences exist in the vulnerability to noise-induced hearing loss (28, 29) and tinnitus. Notably, in response to noise exposure, mice show much larger threshold shifts than other species, such as the guinea pig, which are much more resilient to acoustic insults (30). Thus careful consideration should be made when choosing noise exposure, anesthesia, species, strain, and age of mice for tinnitus induction experiments.
In terms of ototoxic drugs, sodium salicylate, the active ingredient in aspirin, induces transient tinnitus (∼24–72 h) (31) in nearly all subjects. Given the high proportion of subjects that develop tinnitus and its reversibility, salicylate was instrumental in validating the first operant behavioral model in rodents (32). While salicylate produces a transient phantom percept that is similar to that of chronic tinnitus patients, the underlying mechanism is likely different than tinnitus induced by noise or age-related hearing loss. Briefly, salicylate acts primarily on outer hair cells (OHCs) to reduce electromotility as evidenced by reduced distortion product otoacoustic emissions amplitudes (33). Other possible mechanisms of salicylate-induced tinnitus include auditory cortex hyperactivity (34) via a reduction in GABAergic transmission (35). Despite salicylate’s utility in tinnitus research, high doses must be administered that may produce toxic effects and affect behavior. Salicylate induces transient tinnitus though different mechanisms from noise-induced tinnitus, and these differences should be taken into consideration.
2.2. Tinnitus Behavioral Assays
The difficulty in developing reliable tinnitus assays lies in the nature of the tinnitus percept itself. By definition, tinnitus is the perception of a subjective sound. Thus finding an objective measure of tinnitus remains a challenge. To address this problem, nearly all animal tinnitus assays are predicated on the concept that tinnitus creates the absence or alteration of the perception of silence. Simply put, if an animal is perceiving a constant subjective sound, this animal has an altered perception of silence. Although many variants have been developed, tinnitus animal models can be classified into two main groups: operant or reflexive models.
The first tinnitus animal model was developed by Jastreboff and colleagues (32). This model, and many variants that followed, utilize a lick suppression paradigm in which rats are given restricted access to water and are then trained to lick from a spout during sound presentation. Adult rats are trained, via foot shock, to suppress licking behavior during silent periods. Upon injection of salicylate, rats no longer suppress licking behavior during silent periods, suggesting the perception of a phantom sound (32). Later, Bauer et al. (36, 37) developed a rat model that utilized lever presses for a food reward. An advantage of this model is that it allows for testing of chronic tinnitus for many months after tinnitus induction, as subjects tested with lick-suppression tasks show high levels of behavioral extinction. Additional operant models have been developed in recent years that include conditioned place preference (38) and sound-based avoidance detection (SBAD) in adult mice (39, 40). Both conditioned place preference and SBAD models utilize a behavior apparatus with two chambers and subsequent training of subjects to either move from one chamber to another in response to sound stimuli. In silent trials where no sound is presented, the subject is trained not to move to another chamber. Thus an animal is considered to have behavioral evidence of tinnitus, if, in silent trials, it moves to the other chamber, as if a sound was presented. The key advantage of operant models is that they evaluate whether the animals perceive silence or not, based on how they respond during the silent trials. The major challenges of operant models are the requirements for lengthy training periods and expertise in sophisticated behavioral techniques. Additionally, in many operant models, subjects show extinction of tinnitus behavior over time, which makes longitudinal tinnitus testing difficult. Despite these challenges, operant models are the most appropriate animal models for the study of tinnitus, as they require the involvement of cognition that arises from many cortical and subcortical areas and from auditory and nonauditory brain regions, which is consistent with our current understanding of tinnitus.
A popular tinnitus model circumvents the lengthy and experimentally demanding requirements of operant models. Developed by Turner and colleagues (41), the gap-prepulse inhibition of acoustic startle (GPIAS) is based on the innate prepulse startle reflex inhibition. Briefly, during a lower intensity background sound, a startle reflex is elicited by an abrupt loud sound. When a silent gap is embedded in the background noise preceding the startle stimulus, the reflex is inhibited. The model posits that if an animal has tinnitus, it will be unable to detect the silent gap and will display less inhibition of the acoustic startle reflex. The frequency and amplitude of the background noise in which the gap is embedded can be modulated to approximate the hypothesized tinnitus frequency and intensity in which the animal’s tinnitus have been assumed to “fill in the gap.” In recent years, there have been many critiques of the GPIAS model and the hypothesis of tinnitus “filling in the gap.” Some have reasoned that if tinnitus is truly filling in the gap, then the placement of the gap in the background noise should not affect the inhibition of startle (42). However, inhibition of acoustic startle was observed at some gap latencies but not all (43). Strain-dependent differences can lead to variability in GPIAS measures. For example, CBA mice, commonly used in auditory research due to their good hearing, show reduced inhibition of startle responses and thus are inappropriate for GPIAS use, while other strains such as C57 exhibit greater inhibition of acoustic startle (43), but they show age-dependent hearing loss. Importantly, studies in humans that used a self-report tinnitus frequency matching found that gap detection deficits do not correspond with the reported tinnitus frequencies (44, 45). Yet, despite the previously mentioned limitations, several studies utilized GPIAS and led to significant findings in tinnitus research. For example, new approaches that have shown promise for tinnitus treatment in humans were based on initial animal studies that used GPIAS (46–49). Overall, while the utility of GPIAS has advanced tinnitus research, we propose that operant tinnitus animal models can assess cognitive aspects of tinnitus and thus are more suitable models for determining tinnitus mechanisms in animals.
3. HEARING LOSS AND OTHER CAUSES OF TINNITUS
Hearing loss is a major cause of tinnitus and a substantial public health issue: the World Health Organization estimates over 466 million people globally have severe hearing loss (50). In addition to the individual burden of hearing loss, which includes impaired communication, social isolation, increased prevalence of depression and anxiety, and cognitive decline (51), hearing loss also creates a great global economic burden estimated to be $750 Billion annually (52). Noise-induced hearing loss is a powerful way to induce tinnitus in animal models and is associated with tinnitus in humans (18, 19, 53, 54) (FIGURE 1). A commonly accepted physiological consequence of acute or chronic noise exposure is the death or dysfunction of the sensory receptors of the cochlea, the inner and outer hair cells (IHC and OHCs) (FIGURE 1A). The primary function of OHCs is to mechanically amplify sound vibrations in the cochlea but not transduce sound into neural signals. Conversely, IHCs are the main sensory cells in the cochlea and are deflected by sound-generated fluid pressure waves. Minute deflections of IHCs open mechanosensitive ion channels, which depolarize hair cells and release neurotransmitters onto the dendrites of the spiral ganglion neurons, whose axons, the auditory nerve fibers, propagate sound information to the brain. Exposure to loud sounds damages cochlear hair cells and thus induces permanent elevations of hearing thresholds (55). Exposure to milder sound intensities cause “temporary” threshold shifts that eventually recover to prenoise exposure levels. Although hearing thresholds may only be temporarily affected, this damage can cause permanent physiological and functional hearing deficits. In cases of temporary threshold shifts, there is evidence of swelling of afferent terminals contacting inner hair cells due to glutamate excitotoxicity (56), leading to the loss of “ribbon” synapses, the synapses between IHCs and spiral ganglion neurons (57) (FIGURE 1B). This process has been termed cochlear synaptopathy (57). The loss of these nerve fibers causes a distortion or reduction in the auditory signal that is then relayed to the brain. These peripheral changes are capable of triggering plasticity in central auditory and extra-auditory networks, culminating in the generation of the tinnitus percept. However, untangling the many physiological consequences of noise exposure and peripheral deficits, such as synaptopathy, hair cell loss, temporary, or permanent threshold shifts is no simple task. For example, it remains unclear if synaptopathy itself is sufficient to induce tinnitus in mice. Recent studies suggest that a mouse line that lacks the glutamate aspartate transporter (GLAST), expressed in the supporting cells of the murine cochlea, may be a model of synaptopathy without noise exposure. GLAST knockout (KO) mice lack the glutamate transporter that regulates glutamate levels at the ribbon synapse in the cochlea, where glutamate excitotoxicity underlies synaptopathy at this synapse after noise exposure. The absence of this gene generates synaptopathy in these mice, as evidenced by significant reduction in auditory brainstem response (ABR) wave I amplitudes but similar ABR thresholds to wild-type mice (58). GLAST KO mice do not show GPIAS deficits (43). However, tinnitus was not assessed with an operant model, and KO mice showed more robust salicylate-induced GPIAS deficits, suggesting an enhanced susceptibility to tinnitus. More studies are needed to determine the precise role of synaptopathy in the generation of tinnitus in mice.
FIGURE 1.
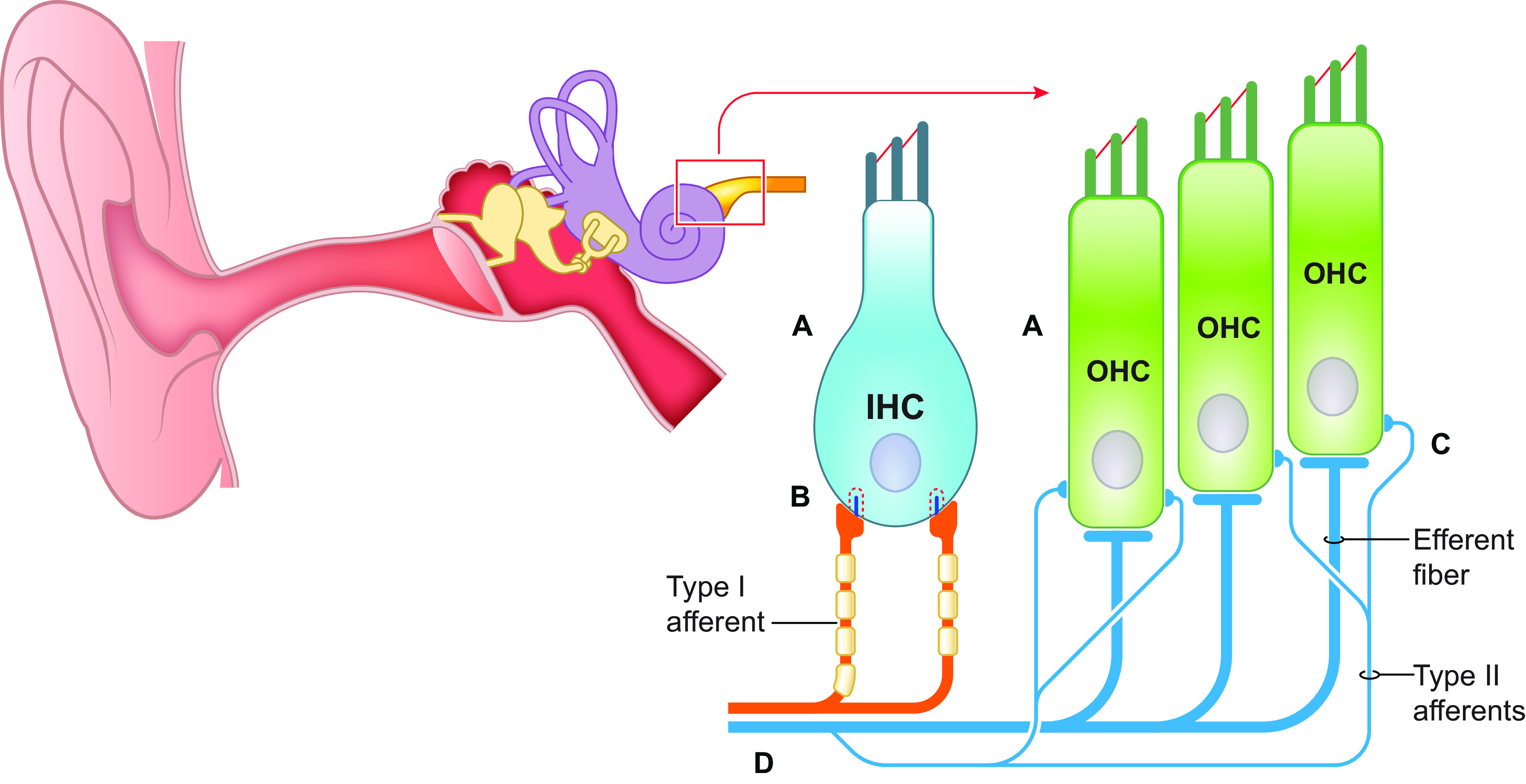
Hearing loss is the most common cause of tinnitus. A: death or dysfunction of cochlear hair cells. B: loss of ribbon synapses. C: hyperexcitability of type II afferents. D: death or dysfunction of auditory nerve fibers. OHC, outer hair cell; IHC, inner hair cell.
In humans, although peripheral dysfunction and hearing loss is a major risk factor for tinnitus, a subset of individuals with tinnitus display no measurable peripheral auditory deficits (59, 60). However, a peripheral dysfunction, such as synaptopathy or high-frequency hearing loss, may not be captured by the traditional audiometric test battery. To support this hypothesis, reduced ABR wave I amplitudes, reflective of auditory nerve activity and thus suggestive of synaptopathy (57), were found in tinnitus subjects with normal thresholds (61, 62). However, differences in ABR wave I amplitudes in tinnitus groups may have been due to unmatched audiometric thresholds at high frequencies (>8 kHz). When matched for auditory thresholds at high frequencies, no relationship was found between synaptopathy and tinnitus (63). Although there is a significant correlation between tinnitus and reported lifetime history of noise exposure (63), the lack of a commonly accepted measure of synaptopathy in humans makes it challenging to causally link subclinical peripheral deficits with tinnitus (63, 64).
So far, peripheral deficits and hearing loss associated with tinnitus have been attributed to the death or dysfunction of cochlear hair cells, synaptopathy, and degeneration of type I spiral ganglion neurons. While type I afferents comprise the vast majority of total cochlear afferents and are primarily responsible for transmitting acoustic information to the central auditory system, type II afferents may be an additional player in the generation of tinnitus (FIGURE 1D). Type II afferents, which account for ∼5% of cochlear afferents, are weakly responsive to sound (65) and activated only by loud stimuli (66). Because of this, their function in the auditory system remains enigmatic. However, recent evidence suggests that type II afferents may convey pain signals resulting from OHC damage (67, 68). Type II afferents are activated by a glutamate-independent mechanism and also express proteins associated with nociception. Furthermore, OHCs respond to noxious (tissue damaging) noise but not innocuous levels of noise. One possible mechanism of type II afferent activation after damaging levels of noise is ATP signaling (69), which is released from nearby supporting cells in response to hair cell damage (68, 70). Activation of metabotropic (P2Y) purinergic receptors by ATP increases type II afferent excitability by the closure of KCNQ-type potassium channels (68, 70). Thus a noise-induced hyperexcitability of type II fibers, due to the closure of KCNQ channels, may underlie the increased sensitivity or intolerance to sounds that are normally not perceived as loud, which is a hallmark of hyperacusis. Alternatively, this mechanism may also be involved in hyperacusis with pain (noxacusis). Although this mechanism has not been directly studied in the context of tinnitus, there is a possibility that it might be shared between tinnitus and hyperacusis/noxacusis pathology. Hyperacusis has been demonstrated to be associated with tinnitus in humans (71, 72). Additionally, the correlation between the two becomes stronger in individuals who self-report more severe tinnitus (71).
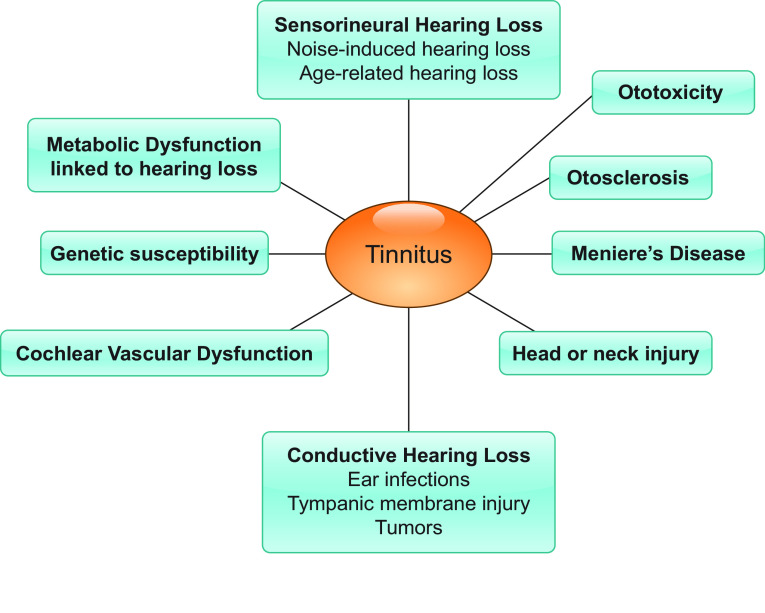
Except for the sensorineural hearing loss described thus far, peripheral damage can take many other forms, such as conductive hearing loss (73) or any obstruction or dysfunction of the middle ear that prevents or disrupts sound from being transmitted to the central auditory pathway. Examples of conductive hearing loss include middle ear infections, glomeric tumors, myoclonus, and Tonic Tensor Tympani Syndrome. In humans and in animals, conductive hearing loss is often modeled by the insertion of an ear plug (74, 75) and produces threshold shifts without damaging the cochlea or auditory nerve. With this procedure, a majority of subjects develop tinnitus that is reversed upon removal of the ear plug (76). Other causes of tinnitus may include, but are not limited to, hearing loss linked to metabolic dysfunction (77), ototoxicity (78, 79), Meniere’s Disease (80), vascular dysfunction (81), genetic factors (discussed in sect. 8), otosclerosis (75), and head and neck injury (82) (FIGURE 2).
FIGURE 2.
Causes of tinnitus.
4. TINNITUS-RELATED CHANGES IN CENTRAL AUDITORY NETWORKS
4.1. Overall Scheme Mechanism
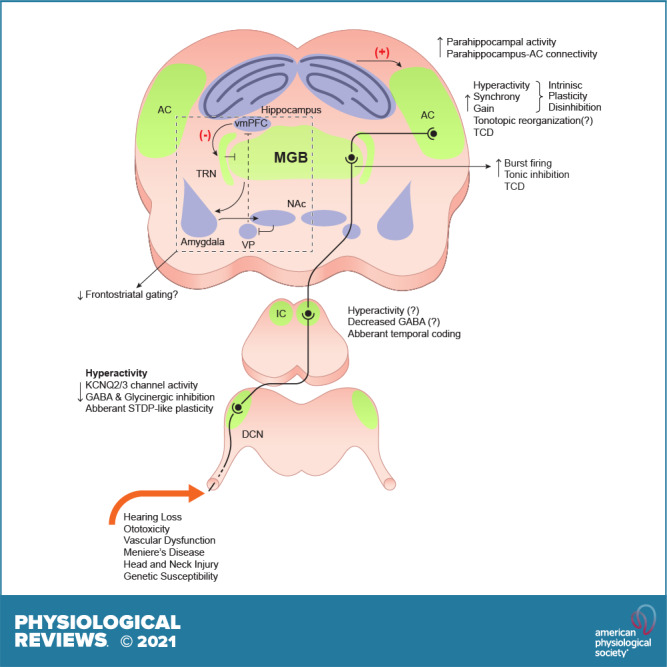
Tinnitus is associated with neuronal hyperactivity [enhanced spontaneous firing rate (SFR)] or hypoactivity (decreased SFR), changes in neuronal transfer functions (gain), changes in tonotopic organization, and changes in neural synchrony. In the next sections, we will discuss tinnitus-related mechanisms in central auditory networks. Overall, animal and human studies suggest that plasticity occurs at different levels of the central auditory system and to different degrees and directions (FIGURES 3–7).
FIGURE 3.
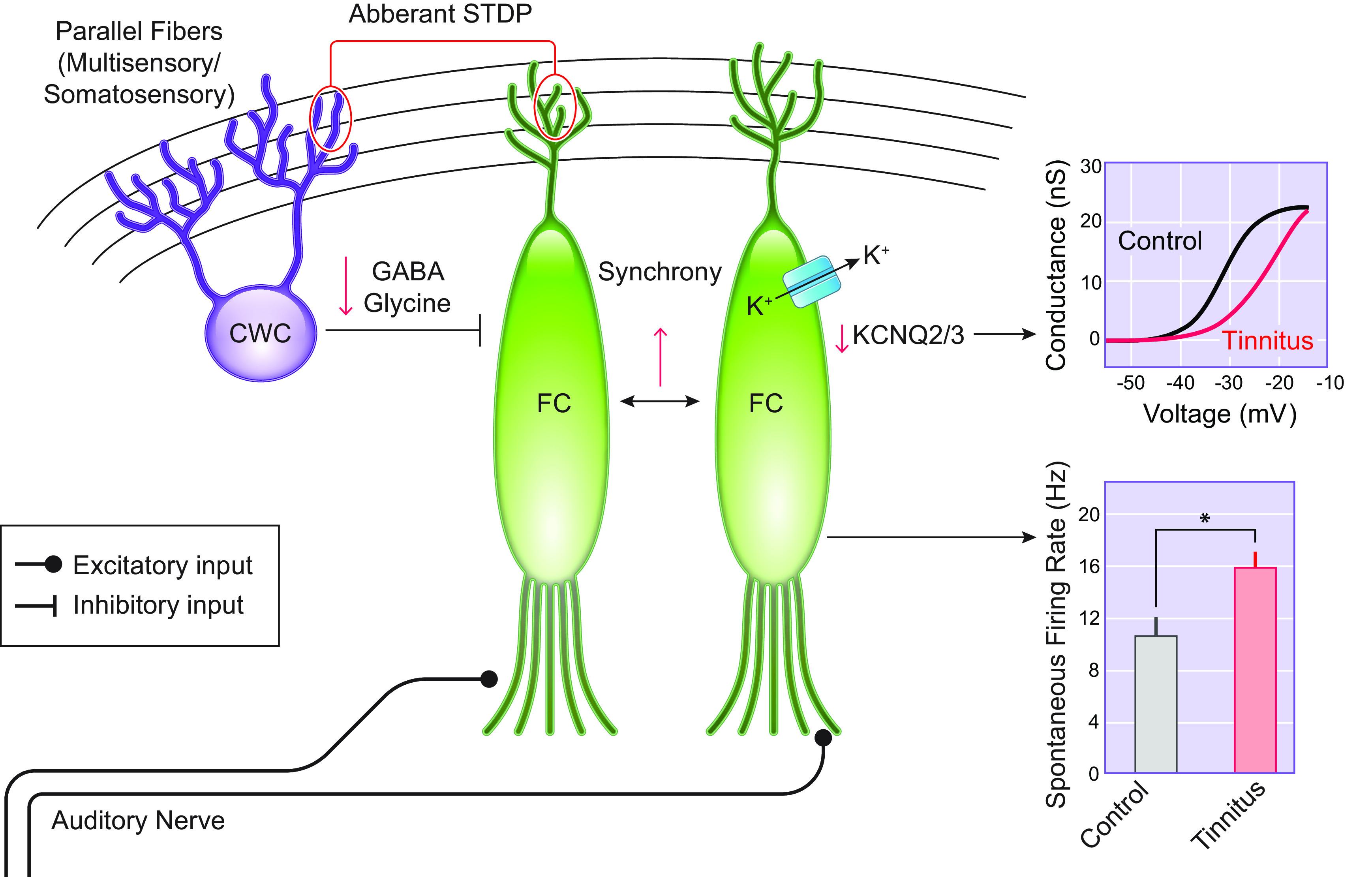
The Dorsal Cochlear Nucleus and Tinnitus Mechanisms. Tinnitus mice display increased spontaneous firing of fusiform cells (FC). This is due to enhanced intrinsic excitability due to a shift in the voltage dependence of KCNQ2/3 channels. Adapted from Ref. 48, with permission from Proceedings of the National Academy of Sciences of the USA. Additional contributors are reductions in GABAergic and glycinergic signaling and aberrant spike timing-dependent plasticity (STDP)-like plasticity. Tinnitus-related changes are depicted in red. CWC, cartwheel cells.
FIGURE 7.
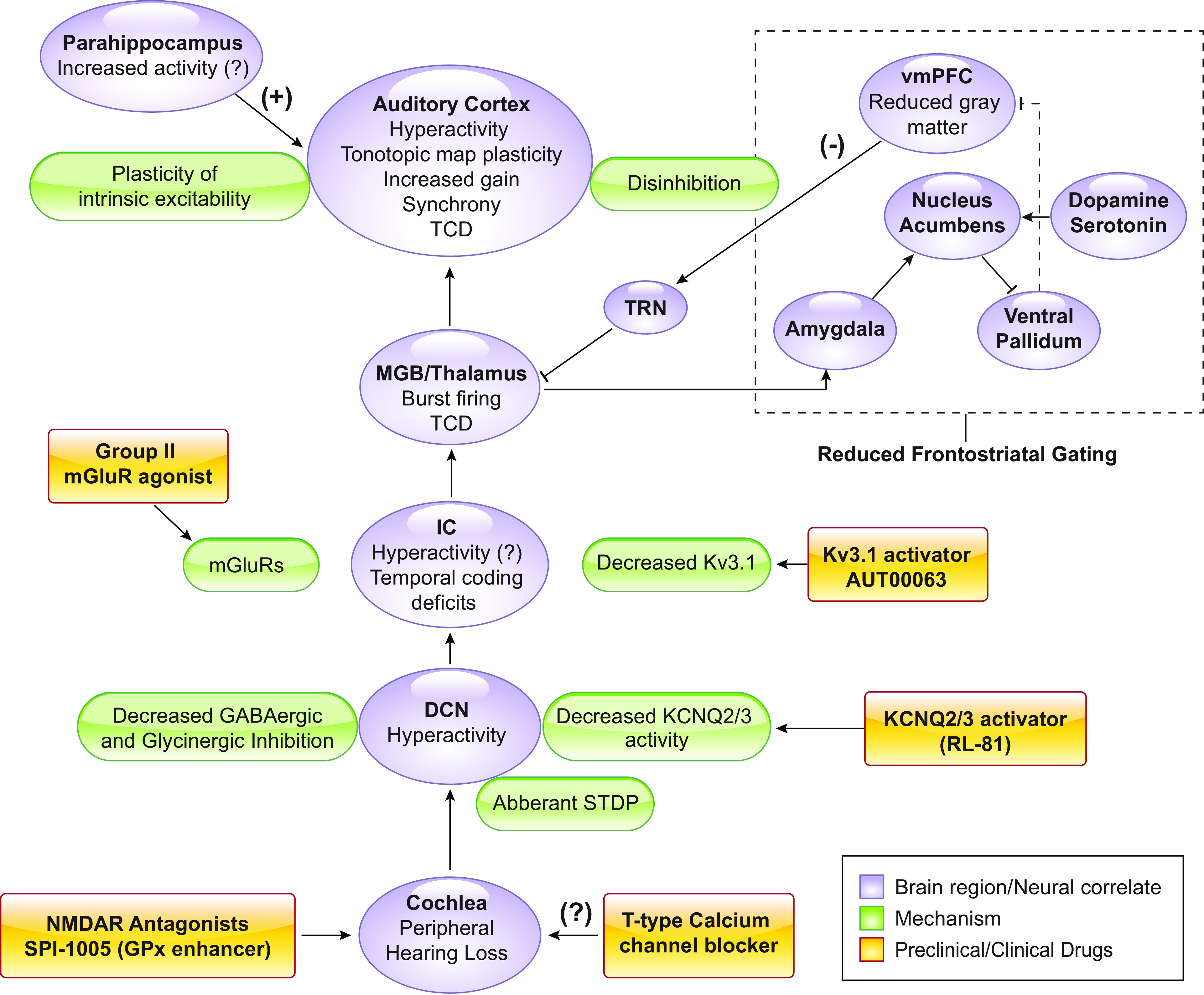
Conclusions. Brain regions and neural correlates of tinnitus (purple), mechanistic underpinnings (green), and preclinical drug development (yellow). TRN, thalamic reticular nucleus; vmPFC, ventromedial prefrontal cortex; STDP, spike timing-dependent plasticity. TCD, thalamocortical dysrhythmia hypothesis; IC, inferior colliculus; mGluR, metabatropic glutamate receptor.
4.2. Auditory Brainstem Plasticity
4.2.1. Dorsal cochlear nucleus.
The dorsal cochlear nucleus (DCN) has been regarded as the site of tinnitus generation but not maintenance, as ablation of the DCN before noise exposure prevents the generation of tinnitus, but tinnitus persists if the DCN is ablated several weeks after noise exposure (83, 84). Early studies in hamsters reported that exposure to loud sound increased the SFR of fusiform cells (85), the principal neurons of the DCN that integrate auditory and multisensory input (86). DCN hyperexcitability after noise exposure has been observed by several independent laboratories, and in many cases, it has been linked specifically to mice with behavioral evidence of tinnitus (48, 87–91). As such, DCN hyperexcitability is likely the most consistent neural correlate of tinnitus in animal models (FIGURE 3).
The underlying mechanisms of tinnitus-related DCN hyperactivity have been of particular interest and have provided novel targets for the development of tinnitus therapeutics. One of the major novel targets are the KCNQ potassium channels. KCNQ channels (KCNQ1-5) are slow-activating, voltage-dependent, noninactivating potassium channels that are open at subthreshold membrane potentials. Due to these properties, they are major modulators of neuronal excitability. Mice with behavioral evidence of tinnitus display reduced KCNQ2/3 currents in fusiform cells (48). The hypofunction of these channels is due to a shift in their voltage dependence, which renders them less likely to open at subthreshold membrane potentials (48). This decrease in KCNQ2/3 channel activity causes the tinnitus-related increases in spontaneous firing rates of fusiform cells (48). Natural recovery of KCNQ2/3 currents is associated with resilience to tinnitus in noise-exposed mice (92). Importantly, administration of retigabine, a KCNQ2-5 channel activator, eliminated the behavioral evidence of noise-induced tinnitus in mice (48). While once a Food and Drug Administration (FDA)-approved antiepileptic drug retigabine was discontinued in 2017 due to severe side effects (93). Given the need for the development of a nontoxic, KCNQ2/3-specific activator for epilepsy and tinnitus, recent studies have led to the synthesis of next-generation KCNQ2/3 channel openers with improved specificity, potency, and metabolic stability (47, 94, 95). Remarkably, these compounds offer an extensive therapeutic window of intervention, as their transient delivery one week after noise exposure mitigates tinnitus in mice (39). As such, these compounds are currently under preclinical development.
While intrinsic plasticity contributes significantly to tinnitus-related hyperexcitability, decreased inhibitory synaptic signaling is an additional contributing mechanism (89, 96). Flavoprotein autofluorescence imaging studies revealed a significant decrease in DCN GABAergic signaling in in mice with behavioral evidence of tinnitus (89). Moreover, downregulation of glycinergic signaling has also been shown to permit fusiform hyperexcitability (96, 97). Finally, in addition to hyperactivity, neuronal synchrony is positively correlated with tinnitus in guinea pigs (98). Together, noise-induced cochlear deafferentation appears to drive homeostatic plasticity in the DCN by a combination of increased intrinsic excitability of fusiform cells as well as a decrease in inhibitory glycinergic and GABAergic neurotransmission.
Fusiform cells receive auditory and multisensory inputs. Multisensory input, originating also from the trigeminal ganglia, is carried via parallel fiber synaptic input. This synaptic pathway shows spike timing-dependent plasticity (STDP) (99, 100), which is a form of long-term synaptic plasticity dependent on the timing of pre- and postsynaptic activity in the same neuron. This STDP mechanism highlighted an additional path for enhancing the excitability of fusiform cell in an activity-dependent manner (101). In fact, recent studies employed an STDP plasticity-related approach to reverse tinnitus activity in the auditory brainstem by using auditory and somatosensory (trigeminal nerve) stimulation to normalize maladaptive DCN plasticity linked to tinnitus (49).
4.2.2. Inferior colliculus.
The inferior colliculus (IC) is an integration site in the auditory pathway, as it receives ascending inputs from the contralateral DCN and sends projections to the auditory thalamus, the medial geniculate body (MGB). The IC is also a site for descending cortical input, originating from auditory cortical layer V (102, 103). Regarding tinnitus-related IC hyperactivity, the results are somewhat contradictory. Some studies have found that IC hyperactivity is correlated with tinnitus behavior in animal models (104–106), but others have observed a less clear distinction between noise-induced hyperactivity and tinnitus-induced hyperactivity (107, 108). Some studies have found no change in SFR in the IC in noise-exposed (109) or tinnitus mice (110). Yet, another study found reduced SFR of IC neurons after noise exposure but did not test for behavioral evidence of tinnitus (111). Some of the variability in these studies may be due to differences in experimental design, as different behavioral assays and different time points after noise exposure were used to measure SFRs. For example, acute responses in IC after noise exposure may be fundamentally different than responses measured two or more weeks after noise exposure (112). Alternatively, the discrepancies in SFR of IC after noise exposure may reflect different mechanisms or sites of plasticity that may underlie subcategories of tinnitus.
In studies where IC hyperactivity was observed, there is evidence for reduced IC GABAergic inhibition after noise exposure (106, 113). Moreover, GAD65, the enzyme responsible for decarboxylation of glutamate to GABA, was significantly reduced in the IC after noise exposure (114). However, other studies report no changes in GABA levels (115). As such, hyperexcitability in the IC may also be due to the increased activity of descending excitatory cortical inputs to the IC. Indeed, there is evidence that the response gain of corticocollicular projections, originating from layer V of auditory cortex and terminating in the IC, display a sustained, increased activity after noise exposure, but tinnitus was not tested in this study (116).
While hyperactivity is one mode of maladaptive plasticity in the IC, changes in the temporal coding of auditory inputs may also underlie aberrant signaling in the auditory pathway, which may subsequently lead to the tinnitus percept. Changes in temporal response properties have been reported in the IC (112), including increased neuronal bursting (104) and a shift in the response pattern from “sustained” to “onset firing” (107). Moreover, in a mouse model of peripheral deafferentation, temporal coding deficits in the IC were partially rescued with application of AUT00063, a compound that increases Kv3.1 potassium currents (117). It remains unclear if temporal coding deficits in the IC are a result of noise exposure and hearing loss or if this aberrant neural activity is present in animals with tinnitus. Nonetheless, this compound ultimately failed to alleviate tinnitus in human subjects in phase II clinical trials (118).
4.3. Thalamic Plasticity
The auditory thalamus (MGB) is considered the gatekeeper of sensory information in the auditory system. The MGB receives input from the IC and projects to auditory cortex and integrates GABAergic inputs from the thalamic reticular nucleus (TRN) and nonauditory inputs from limbic structures and descending inputs from auditory cortex. Thus the MGB is positioned to serve a critical role in tinnitus-related auditory and extra-auditory pathology.
Thalamic neurons have distinct firing patterns or states: they can fire tonically or in bursts (119–121). Single MGB units recorded from unanesthetized rats with behavioral evidence of tinnitus displayed increased burst firing and number of spikes per burst (122). Pathological increases of GABAergic inhibition in the MGB have been hypothesized to initiate burst firing in MGB neurons in animals with behavioral evidence of tinnitus (123, 124). Namely, tinnitus mice display increased levels of tonic inhibition in the MGB (125). Increased tonic inhibition can generate burst firing as hyperpolarization of MGB neurons de-inactivates T-type calcium channels which generate a slow calcium spike, which in turn, can cause the generation of burst firing (126, 127).
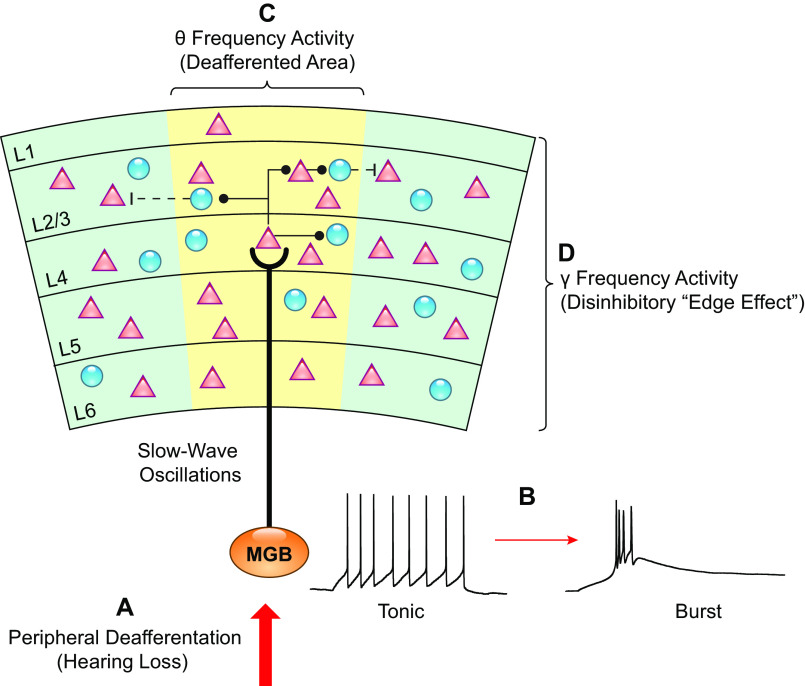
Aberrant firing of MGB neurons after noise exposure has been linked with phantom perceptions, in both tinnitus (127, 128) and chronic or phantom limb pain (129). Aberrant thalamic activity has been proposed to initiate thalamocortical oscillations implicated in tinnitus, as described by the thalamocortical dysrhythmia hypothesis (TCD), (for review, see Refs. 124, 127, 130) (FIGURE 4). Briefly, the TCD hypothesis suggests that peripheral deafferentation shifts the firing mode of thalamic neurons. This can be due to increased hyperpolarization, because of the reduced deafferented input to the MGB, or enhanced tonic inhibition. This hyperpolarization de-inactivates T-type calcium channels, which generate a slow calcium spike, which in turn, generates low frequency recurrent interaction between auditory thalamus and cortex. This low-frequency activity (delta 1–4 Hz and theta 4–8 Hz) is manifested in localized cortical columnar areas. As a result, the inhibitory input to adjacent cortical areas is reduced. The reduced inhibition leads to increased activity in adjacent disinhibited cortex, thus resulting in continuous high-frequency activation in the gamma range (>30Hz), thought to underlie the tinnitus percept (32). Consistent with TCD, slow wave oscillations have been reported to be a neurophysiological correlate of tinnitus in humans (124, 131–134), and perhaps, this is the most consistent neurological marker in human tinnitus studies (13). The TCD hypothesis is an attractive hypothesis, but the detailed mechanisms of TCD in tinnitus have not been studied (13).
FIGURE 4.
Thalamocortical dysrhythmia in tinnitus. A: peripheral deafferentation causes a reduction of input from the peripheral auditory system that is sent to the medial geniculate body (MGB). B: hyperpolarization and decreased excitatory input to the thalamic neurons causes the firing mode to switch from tonic to burst firing due to de-inactivation of T-type calcium channels. C: as a result of the switch to burst firing, low-frequency oscillations are generated and propagated to the auditory cortex. D: low-frequency oscillations in the deafferented area of cortex leads to a reduction of inhibition to adjacent columns generating high frequency oscillations (Edge effect).
4.4 Cortical Plasticity
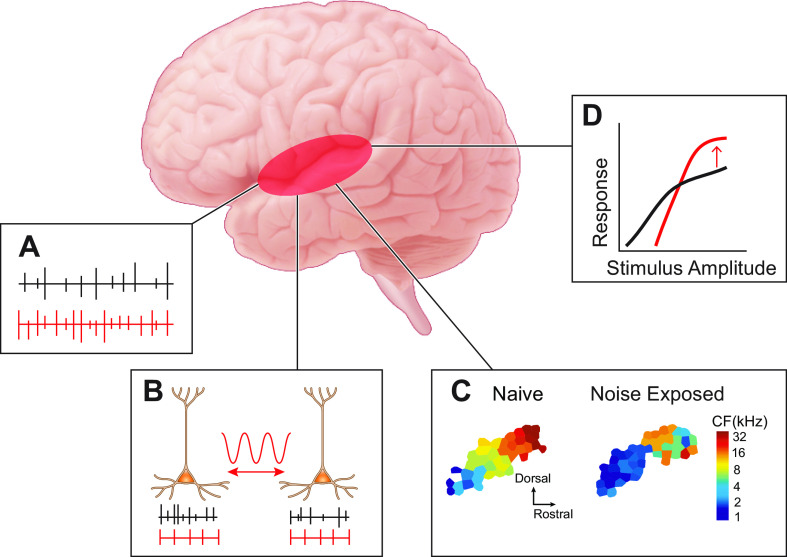
The involvement of auditory cortical plasticity in the pathophysiology of tinnitus has been studied in both human and animal models (135). Despite the differences between animal models of tinnitus and humans, these studies support four main tinnitus correlates: increased SFR, increased neural synchrony, increased gain, and changes in tonotopic map reorganization (FIGURE 5).
FIGURE 5.
Auditory cortex and tinnitus mechanisms. A: increased spontaneous firing rate (SFR). B: increased neural synchrony. C: tonotopic map reorganization. Adapted from Ref. 38, with permission from Proceedings of the National Academy of Sciences of the USA. D: increased response gain. Tinnitus-related changes are depicted in red. CF, characteristic frequency.
Seminal studies in the cat auditory cortex showed that SFRs increased several hours after noise exposure (136) (FIGURE 5A). SFRs were increased in cortical areas of tonotopic reorganization that were tuned to frequencies outside the noise trauma frequencies (137). However, these studies did not differentiate noise-exposed animals with behavioral evidence of tinnitus from noise-exposed animals without tinnitus. Subsequent studies found increased SFRs in animals with behavioral evidence of tinnitus (38, 46, 138). Consistent with these studies, human studies have reported increased neural activity in resting state (139–141), which could be considered as an indirect measure of increased SFRs.
Changes in neural synchrony, broadly defined as the correlated firing of groups of neurons, are cortical correlates of tinnitus. Synchrony is measured on different scales in human and animal studies. Human EEG or magnetoencephalography (MEG) studies measure macroscopic synchrony in cortical oscillations that require large areas of synchronous activity, whereas animal studies measure synchrony on a cellular level. Neural synchrony is enhanced in auditory cortex of anesthetized cats immediately after noise exposure and this enhancement persists after exposure (136) (FIGURE 5B). Synchrony is increased in the reorganized areas of the tonotopic map (137). In rats, increased synchrony was observed after noise exposure compared to controls but was not significantly correlated with behavioral evidence of tinnitus (46). In humans, the noise-induced synchronous activity in auditory cortex is characterized by reduced alpha wave and an enhanced delta (141) and gamma-band activity (142). Enhanced gamma activity has been associated with synchronization of firing in cortical areas (143, 144) and with increased synchronous gain (145). Gamma oscillations are linked to the conscious perception of sensory stimuli and have thus been linked to tinnitus (127). While direct evidence linking enhanced gamma oscillations and tinnitus in humans may be inconsistent (146), gamma oscillations and synchrony remain a component of the TCD model and an area in need of further mechanistic research (13).
Another proposed cortical correlate of tinnitus is tonotopic map reorganization (FIGURE 5C). Tonotopic maps are plastic and respond rapidly to experience. A classic example of this plasticity in the auditory system is the critical period, or a specific temporal window in which the brain is more receptive to experience-dependent changes in tonotopy. During critical periods, mere passive exposure to tones is sufficient to shift cortical frequency representations (147). However, peripheral deafferentation can induce tonotopic plasticity in the adult auditory cortex (38, 46). Unmasking of subthreshold inputs onto cortical neurons by dynamic reductions in inhibition and increases in excitation around the deafferented area is the suggested mechanism for tonotopic map reorganization after peripheral deafferentation (148). Importantly, “corrective” plasticity that reverses map auditory cortex tonotopic reorganization after noise exposure reduced the incidence of tinnitus in rats (46) and in humans (149); thus, supporting the hypothesis that map reorganization may be a causal factor in the generation and maintenance of tinnitus. However, a recent study in mice reported that hearing loss, but not tinnitus, was strongly correlated with map reorganization (150). In the human literature, one study finds that the degree of tonotopic map reorganization is correlated with the degree of tinnitus (151), while another study concludes that tonotopic reorganization is not required for the perception of tinnitus in humans (152). Yet another study showed and that tonotopic map reorganization is related to hearing loss but not to tinnitus (153). Thus it is plausible that tinnitus may be a result of maladaptive tonotopic map plasticity, or incomplete tonotopic plasticity after hearing loss. Taken together, the relationship between map reorganization in tinnitus and hearing loss remains unclear. Additional studies to disentangle the effects of hearing loss and tinnitus on cortical map reorganization will be necessary.
Last but not least, several studies in animals and humans have reported increased cortical responsiveness (gain) to sound after noise exposure (154, 155) and in tinnitus (156, 157) (FIGURE 5D). Gain enhancement is a homeostatic mechanism by which primary auditory cortex increases its responsiveness to sound stimuli to compensate for a reduction of peripheral input. The most likely mechanism for increased gain after peripheral input loss is a reduction in GABAergic inhibition (38, 158). A recent study utilized a novel genetic tool to selectively increase endogenous NMDA receptor (NMDAR) activity in GABAergic neurons and provided support for the role of cortical GABAergic inhibition in the generation of tinnitus (159). Administration of a positive allosteric modulator of NMDARs prevented behavioral evidence of tinnitus in mice (160). The hypothesized mechanism for this therapeutic effect is that the increased activity of GABAergic neurons, induced by the NMDAR agonist, prevents the pathological increase in gain of auditory cortex, thus preventing the development of tinnitus. However, it is a challenge to generate stable cortical activity with high gain and with a general reduction of inhibition. The required tethering of stability and high response gain restricts the possible operative regimes of the cortical network; therefore, we suggest that the current hypothesis that a reduction in general cortical inhibition may not be sufficient to account for the observed gain changes. Luckily, cortical principal neurons and interneurons are very diverse, thus suggesting a cell-specific division-of-labor for achieving this task. For example, it is unknown how the intrinsic, synaptic, or network activity of cortical interneuron subtypes change in tinnitus. The activity of the different cortical interneuron types, such as parvalbumin (PV), somatostatin (SOM), and vasoactive intestinal peptide (VIP) each have a unique effect on cortical output and gain. Recent computational models suggest that PV neurons are critical for maintaining network stability while SOMs may be positioned to regulate principal neuron gain (161). Thus PV and SOMs might be differentially modulated after noise trauma or tinnitus. Understanding the intrinsic, synaptic, and network plasticity in specific classes of principal neurons and interneurons will be key for understanding the underlying mechanisms of gain enhancement in tinnitus.
5. TINNITUS-RELATED EXTRA-AUDITORY NETWORK PLASTICITY
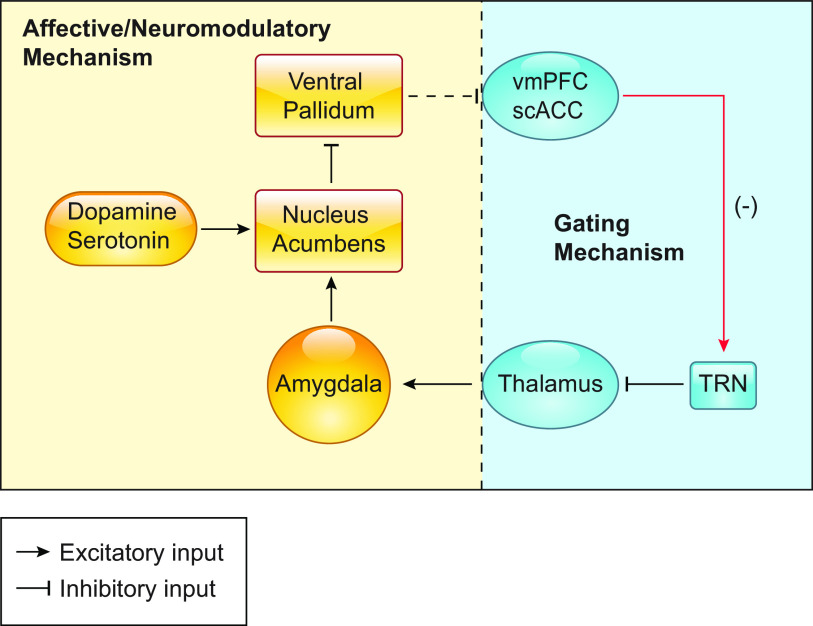
While the majority of tinnitus research has centered on nodes of the auditory pathway, the conscious perception of tinnitus likely involves multiple networks. Nonauditory (extra-auditory) structures play a crucial role in the perception, maintenance, and severity of tinnitus in humans. Tinnitus is highly comorbid with affective disorders, such as depression and anxiety (6, 162–164). Consequently, the interaction between the limbic and auditory centers is of particular interest. While it is possible that affective disorders may be a reaction to the bothersome nature of tinnitus, it has also been hypothesized that dysfunction in the limbic system may play a causal role in generating the perception of tinnitus (165). Rauschecker and colleagues (166) have proposed the frontostriatial gating theory of tinnitus that posits that the nucleus accumbens (NAc) and the ventromedial prefrontal cortex (vmPFC) are central parts of a network that evaluates the relevance and affective value of internally generated tinnitus percepts (FIGURE 6). In control conditions, the limbic thalamus sends input to amygdala and the amygdala send projections to the NAc. The NAc inhibits the ventral pallidum (VP), which in turn inhibits the vmPFC, although this connection is under debate (167). Therefore, when the NAc is active the vmPFC can be disinhibited. The disinhibited (active) vmPFC synapses on GABAergic TRN neurons, which subsequently inhibit the MGB, thus leading to the suppression of unwanted or insignificant percepts (gating). In tinnitus, the observed reduced gray matter in the vmPFC (168) is consistent with this gating hypothesis, as it would presumably lead to less MGB inhibition, and thus, less suppression of the tinnitus percept (166). Moreover, the NAc receives strong modulatory serotonergic and dopaminergic input, which provide a potential mechanism for the valuation process of the tinnitus percept (166). Overall, the frontostriatal gating theory of tinnitus provides several testable hypotheses on the involvement of auditory, extra-auditory, and modulatory networks in the perception and affective value of the tinnitus percept, but the validity and mechanistic underpinnings of this theory remain to be experimentally tested. While tinnitus and emotional distress has been studied in humans (6), there is a significant gap in the animal literature regarding the affective component of tinnitus. For example, relatively little work has utilized traditional models of depression and anxiety, such as open field, forced swim, and elevated plus maze, to study the affective component of tinnitus. This is needed given that most measures of tinnitus in humans primarily quantify the amount of distress or handicap induced by tinnitus. These studies would be particularly useful as they may lead to an understanding of the mechanistic underpinnings of why some humans are more distressed or disabled by their tinnitus than others. In this context, recent work has successfully linked facial expressions to discrete emotional states in mice (169). Application of this approach to study emotional states in mice with tinnitus opens a new area of highly anticipated research in terms of dissecting cellular and synaptic mechanisms associated with bothersome tinnitus. Use of this cutting-edge facial imaging technology has significant advantages over traditional depression or anxiety measures in mice as it allows for simultaneous recording of neural activity while obtaining real-time data of the animal’s emotional state.
FIGURE 6.
Circuit diagram detailing the proposed frontostriatal gating theory of tinnitus. The circuit by which the negative perception of tinnitus and the neuromodulatory inputs may affect the gating of auditory percepts is depicted in yellow. The proposed mechanism by which ventromedial prefrontal cortex (vmPFC) gates auditory perception via the GABAergic the thalamic reticular nucleus (TRN) is depicted in blue. Tinnitus-related changes are depicted in red. scACC, subcallosal anterior cingulate cortex.
Apart from auditory and emotional centers, memory networks, such as the parahippocampal networks have also been involved in the pathophysiology of tinnitus (170). Parahippocampal memory networks are thought to contribute to the maintenance of tinnitus by encoding the memory of the tinnitus percept and subsequently reinforcing involuntary auditory memory and perception (171). Neurons in the parahippocampus increase their firing in response to the presentation of novel stimuli but suppress their responses during subsequent presentations of the same stimuli (172). Suppression of responses in this region is suggested to underlie a habituation to repeated stimuli. A failure to reduce parahippocampal activity during repetitive presentation of the same (internal) stimulus, such as in tinnitus, might lead persistent tinnitus perception. Consistent with this hypothesis, human EEG studies report increased activity in the parahippocampal area of individuals with tinnitus (173). Pathological slow-wave oscillations have been observed in the parahippocampus of individuals with tinnitus and robust (>30 dB) hearing threshold shifts (174). Moreover, functional imaging studies report increased connectivity between parahippocampus and auditory cortex in individuals with tinnitus (175, 176). Finally, transient suppression of amygdalohippocampal complex suppress tonal tinnitus in humans (177). Given these findings, persistent parahippocampal activation and its altered functional connection with the auditory cortex may represent an exciting avenue for animal model studies in the future. Targeted recordings in these brain areas of animals with and without behavioral evidence of tinnitus will be crucial in determining mechanistic underpinnings of this hypothesis.
Perception, including phantom perception, is powerfully modulated by attention. This can be measured when individuals with tinnitus are instructed to attend to a nonauditory task. When attending to another task, most individuals with tinnitus report a decrease in the severity of their tinnitus (178). Many tinnitus sufferers also report that they are often unaware of their tinnitus, until they are in a less demanding sensory environment. These observations point to a critical role of attention in tinnitus (178). Consistent with this, individuals with tinnitus have impaired selective attention on auditory tasks (179, 180). It has been hypothesized that individuals with tinnitus already divert attentional resources to attending to their tinnitus and that their ability to selectively shift attention is impaired (179). Accordingly, a recent study reports that rats with tinnitus show increased attention to sounds within the frequency range of their tinnitus but show impaired selective attention to other sounds, relative to sound-exposed rats without behavioral evidence of tinnitus (181). However, the signaling mechanisms and the networks changes determining how attention mechanisms are pathological and how they are recruited in tinnitus remain unclear.
Predictive coding is a proposed model that may conceptually explain the recruitment of attentional centers in the pathophysiology of tinnitus (182). Briefly, the model posits that sensory systems are organized hierarchically, with lower levels sending sensory information to higher-order structures, and higher order structures sending predictions to the nuclei below. A prediction error is generated when the ascending information is incongruent with predictions from higher structures. This incongruence recruits auditory attention. Although this is an attractive proposal, experimental evidence for this hypothesis is lacking.
Taken together, auditory, emotional, mnemonic, and attention control networks are involved in the generation, maintenance and severity of tinnitus. The exact geometry of the electrical activity that is necessary and sufficient for the generation and maintenance of tinnitus remains unknown.
6. CURRENT TREATMENTS
The clinical treatment of tinnitus is largely clinician-dependent and includes observation, education/counseling, cognitive behavioral therapy (183), amplification (184), tinnitus retraining therapy (185), other sound-based therapies [Neuromonics Oasis or Sanctuary, Serenade, Widex Zen, or Acoustic Coordinated Reset Neuromodulation (186)], or a combination of these approaches, i.e., progressive tinnitus management (187). While some of these treatment modalities are effective in diminishing awareness of tinnitus or associated distress, they can be time-consuming, expensive, and are not covered by insurance and therefore only available to a limited population.
In patients with severe to profound hearing loss, restoration of afferent input via electrical stimulation (cochlear implantation) can suppress tinnitus (188–190). Cochlear implantation has also recently been demonstrated to reduce tinnitus percept in single-sided deafness (191). In the United States, cochlear implantation is currently only FDA-approved for bilateral severe to profound sensorineural hearing loss (SNHL) and the majority of patients with tinnitus are not candidates for this procedure. Single-sided deafness is not an FDA-approved indication for cochlear implantation and therefore not covered by insurance in the US. Recently, some evidence has emerged regarding potential benefits of repetitive transcranial magnetic stimulation (192), vagal nerve stimulation (149, 193), bimodal somatosensory and trigeminal nerve stimulation (49), deep brain stimulation (194), and cortical stimulation (195).
Various medications have also been used to treat tinnitus; however, none are FDA-approved for this indication (196). Sudden onset of tinnitus in the setting of acoustic trauma or idiopathic SNHL is often treated with oral and/or intratympanic steroids. Intravenous lidocaine has been used for tinnitus in the past (197) but is not a viable treatment as the half-life of lidocaine is relatively short and the amelioration of tinnitus lasts only 30 min to a few hours (198). Antidepressants (selective serotonin reuptake inhibitors and tricyclic antidepressants) and anxiolytics are often prescribed off-label, but it is unclear whether they treat the tinnitus or the associated symptoms and there is not sufficient evidence of effectiveness in the absence of depression (199, 200). Carbamazepine and gabapentin have also been used, but with questionable efficacy against tinnitus (201). Thus, given the large population of tinnitus sufferers and the lack of drugs that relieve, the market for tinnitus therapeutics is rapidly evolving with several new compounds in clinical and preclinical development (TABLE 1).
Table 1.
Preclinical and clinical tinnitus drug development
| Company | Drug Candidate | Target | Mechanism of Action | Clinical Phase | Clinical Trial Information |
|---|---|---|---|---|---|
| Auris Medical | AM-101 | NMDAR | Antagonist | 3 | NCT02040194 |
| Otonomy | OTO-313 | NMDAR | Antagonist | 2 | NCT03918109 |
| Sound Pharmaceuticals | SPI-1005 | Glutathione peroxidase (GPx) | Enhancer | 2 | NCT03325790 |
| Autifony Therapeutics, Ltd | AUT000063 | Kv3.1 | Activator | Failed phase 2 | NCT02345031 |
| N/A | RL-81 | KCNQ2/3 | Activator | Preclinical | N/A |
| N/A | Eglumegad | Group II mGluRs | Agonist | Preclinical | N/A |
| Pragma Therapeutics | N/A | T-type calcium channels | Blocker | Preclinical | N/A |
NMDAR, NMDA receptor.
7. CLINICAL AND PRECLINICAL DRUG DEVELOPMENT
Here, we briefly review current clinical and preclinical tinnitus drug candidates. While many clinical trial compounds may be relevant for tinnitus, we chose to include only ones that have tinnitus listed as a specific indication (TABLE 1, for a more comprehensive list, see Ref. 202). We have also summarized tinnitus-related compounds that have been successful in animal models (TABLE 2). The first class of drugs in the FDA pipeline are NMDA receptor (NMDAR) antagonists. Namely, AM101 and OTO-313, produced by Auris Medical and Otonomy, respectively (202). The mechanism of action of these drugs is based on preventing excitotoxicity associated with peripheral hearing loss. Previous studies have shown that noise exposure induces excitotoxic swelling at auditory nerve terminals contacting IHCs (56) and subsequent synaptopathy (57). This process is NMDAR-dependent, as application of NMDAR antagonists prevent excitotoxic damage of IHCs and peripheral deafferentation in rodent models (203, 204). AM101 and OTO-313 are administered via intratympanic injection to target the inner ear. As such, these drugs are designed to target the acute effects of noise exposure, as excitotoxicity and synaptopathy occur soon after noise exposure while death of spiral ganglion neurons occurs many months after the initial insult (57). Accordingly, AM-101 phase 2/3 trials recruited subjects whose tinnitus began less than 3 mo before the trial began (NCT01803646). The applicability to chronic tinnitus and the therapeutic window remains unclear for both of these compounds. Unfortunately, AM-101 failed to demonstrate efficacy in either of the major endpoints and a new phase 2/3 clinical trial is under consideration (205). OTO-313 is in relatively earlier stages of development, but phase 1 data show that the compound is safe and well-tolerated; phase 2 is currently underway (NCT03918109).
Table 2.
Effective treatments in animal models of tinnitus
| Compound | Mechanism of Action | Behavioral Model | References |
|---|---|---|---|
| Retigabine | KCNQ2-5 channel activator | GPIAS | Li et al. (48) |
| SF0034 | KCNQ2/3 channel activator | GPIAS | Kalappa et al. (47) |
| RL-81 | KCNQ 2/3 channel activator | Operant | Marinos et al. (39) |
| Vigabatrin | GABA agonist | Operant | Brozoski et al. (223) |
| NO-711 | GABA agonist | Operant | Yang et al. (38) |
| Gabapentin | GABA agonist | Operant | Bauer and Brozoski (14) |
| Infenprodil | NMDAR antagonist | Operant | Guitton and Dudai (224) |
| AM-101 | NMDAR antagonist | Operant | Bing et al. (203) |
| M8324 | GABAergic NMDAR positive allosteric modulator | GPIAS | Deng et al. (160) |
| PLX3397 | Depletes microglia, downregulates TNF-a | GPIAS | Wang et al. (96) |
NMDAR, NMDA receptor; GPIAS, gap-prepulse inhibition of acoustic startle.
SPI-1005, a compound produced by Sound Pharmaceuticals, has demonstrated promising results in the treatment of tinnitus that is comorbid with Meniere’s Disease (NCT03325790). SPI-1005 is a small molecule designed to increase glutathione peroxidase (GPx) activity (206). Endogenous GPx activity scavenges reactive oxygen species (ROS), and depletion of GPx increases the susceptibility of noise-induced to cochlea damage and hearing loss (207). The cochlea expresses GPx, and noise exposure increases the levels of GPx in the lateral wall 2–4 h after noise exposure (208). Pretreatment or treatment acutely after noise exposure prevented OHC loss and reduced hearing threshold shifts relative to controls (209). The production of ROS is closely linked to inflammation in that ROS production can signal proinflammatory responses (210). While much of this research has been conducted in the cochlea and auditory brainstem nuclei, recent evidence directly links cortical inflammation with tinnitus in rodents (211). Specifically, noise exposure leads to increased expression of proinflammatory cytokines in primary auditory cortex such as: TNF-α, IL-1β, IL-18, and NLRP3. TNF-α knockout mice do not develop noise-induced tinnitus, and infusion of TNF-α in the auditory cortex induces tinnitus. The tinnitus-related excitatory-to-inhibitory imbalance was prevented by pharmacological blockade of TNF-α expression (211).
The next category of preclinical tinnitus drug development programs is potassium channel modulators. The potassium channel family represents a diverse set of ion channels expressed in almost all cell types and that reduce excitability through multiple mechanisms. There is abundant evidence that potassium channels are involved in disorders of hyperexcitability, such as epilepsy (212). Studies in the DCN have provided compelling evidence that the generation of tinnitus in animal models may start in the brain as a noise-induced hypofunction of KCNQ2/3 channels that, in turn, leads to the generation of hyperexcitability in the auditory brainstem (48). Postnoise exposure treatment of mice with the KCNQ2-5 agonist retigabine prevented the development of tinnitus in mice (48). Thus KCNQ2/3 channels provide a promising therapeutic target for the treatment of tinnitus. Retigabine is an FDA-approved drug for the treatment of epilepsy. However, retigabine is no longer a valid candidate drug as its side effects including, urinary retention, skin, and retinal discoloration led to its removal from the market in 2017 (93). These side effects can be attributed to the low specificity among KCNQ 2-5 and the low solubility of its metabolites (93). Since these discoveries, the next generation KCNQ2/3 agonists have been synthesized. The new KCNQ2/3 activators are small molecules that are more selective and potent for KCNQ2/3, and more metabolically stable than retigabine, thus leading to fewer metabolites and side effects (94, 95). Current preclinical efforts are focusing toward developing RL-81, a potent and highly specific and metabolically stable KCNQ2-3 opener (94) that has a wide therapeutic window in mice (39), as a lead candidate for investigational new drug-enabling studies.
Moreover, Kv3.1 potassium channels have also been linked to tinnitus, as hyperactivity and temporal processing deficits are often observed in mouse models of tinnitus. The Kv3.1 channel activator AUT00063 (Autifony Therapeutics Limited) reduced hyperexcitability in the DCN and IC following noise exposure or administration of ototoxic agents (117, 213, 214) but was not tested in animal models of tinnitus. AUT00063 did not significantly reduce tinnitus in humans (118).
There are two additional candidate drugs in preclinical development that target T-type calcium channels and metabatropic glutamate receptors (mGluRs). Gateway Biotechnology is currently developing a drug (GW201) to target T-type calcium channels. Although the mechanism is currently unclear, this compound is thought to reduce reactive oxygen species and prevent inflammation and peripheral damage (202). Finally, modulators of mGluR activity have also been a target of tinnitus drug development. The development of this class of drugs for tinnitus is based on findings from studies of residual inhibition (215). Namely, after a tone is presented, spontaneous firing in the auditory pathway, specifically in the IC, is temporarily suppressed, this process is termed residual inhibition (133). Residual inhibition can be due to suppression of spontaneous firing in central auditory neurons, and metabotropic glutamate receptors (mGluRs) play a critical role in this suppression (215, 216). Application of the group II mGluR agonist Eglumegad (LY354740) suppresses spontaneous firing of neurons in the IC of mice with behavioral evidence of tinnitus (215, 216); thus further development of this drug might lead to a clinical trial for tinnitus.
8. CONCLUSIONS AND FUTURE DIRECTIONS
In recent years, substantial progress has been made in understanding the pathophysiology of tinnitus in humans and in animal models. This work has been instrumental in developing evidence-based tinnitus therapeutics, many of which are in clinical trials or preclinical development stages. Now that several molecular targets have been identified throughout the auditory pathway, high throughput screening tools of new and more effective compounds will be critical to accelerating drug development (TABLE 1).
An interesting and often overlooked aspect of tinnitus pharmacotherapy is the circadian dependence of gene expression in the cochlea and the subsequent effect on drug delivery. For example, a recent study reports that administration of a glucocorticoid is more effective in preventing hearing loss after noise exposure when administered in the daytime (217). We therefore suggest that future drug development studies should consider such factors to increase effectiveness of potential drug interventions for tinnitus.
Moreover, in addition to the acquired causes of tinnitus, genetic causes of tinnitus have recently gained research attention. While specific genes underlying a vulnerability to tinnitus have not been discovered, evidence from a large twin cohort study found a higher concordance of tinnitus in monozygotic twins compared to dizygotic twins, suggesting a genetic contribution to tinnitus (218). A study of Swedish adoptees estimated the heritability of tinnitus to be ∼32% (219); the authors subsequently hypothesize that heritability estimates may increase with cases of severe tinnitus compared to moderate tinnitus (220). Finally, a recent large-scale genome-wide association study conducted in the UK identified 6 gene loci and 27 genes associated with tinnitus (162). While research on the genetic causes of tinnitus is still in its infancy, it represents a promising new avenue for tinnitus research, drug development, and potentially patient stratification.
Significant progress has been made in basic research and preclinical development, but several hurdles and opportunities remain in order to achieve success in clinical trials. One critical step is the development of more nuanced tinnitus classification system. Tinnitus perception can vary widely between individuals. Individuals may experience tinnitus as a pure tone, a buzzing, whooshing, a pulsatile sound, of which the intensity may vary throughout the patient’s life or even during different times of day. Moreover, as described earlier, the causes of tinnitus can vary. Tinnitus may be induced by one or any combination of different factors including conductive or sensorineural hearing loss, vascular irregularities, ototoxicity, genetic factors, and others. There is a mismatch between the diversity of causes and perceptions of tinnitus and the current clinical classification systems. For example, tinnitus is often classified as secondary to another disorder or as primary, or idiopathic tinnitus if no other origin is known. From there, tinnitus can be classified as acute (<6 mo) or chronic (>6 mo). Furthermore, tinnitus can be rated based on level of distress as either bothersome or nonbothersome tinnitus (221). While these classifiers may be useful in the triaging of tinnitus patients in audiology clinics, they are not very useful from a research, clinical trial, or precision medicine perspective. It is unlikely that all tinnitus patients diagnosed with “chronic” tinnitus share similar pathophysiological mechanisms. Thus, it is to be expected that one treatment may not be effective for all. This factor alone, likely accounts for the great variability of outcomes in clinical trials. Another related factor contributing to unsuccessful clinical trials, is the lack of an objective measure of tinnitus. The only available and accepted measures of tinnitus in humans rely on the patient to report the severity or level of distress resulting from their tinnitus or to match their tinnitus pitch or loudness to an external sound (222). We therefore suggest that tinnitus patients can be classified by a neuroscience-based “structure-function” approach, whereby neurophysiological and structural signatures of different types of tinnitus can be determined by functional MRI, EEG, MEG, and ABR measurements assessing central tinnitus-related plasticity in auditory and nonauditory centers. Such approach might reveal a neuroscience-based mechanistic characterization of different types of tinnitus. This understanding will lead to precision medicine approaches and individualized therapies (13). Although current data suggest that there are multiple neurobiological (plasticity) mechanisms associated with the heterogeneous presentation of tinnitus, the alternative hypothesis is that there is a single mechanism or structure common to all the heterogeneous presentations of tinnitus. In either case, a combined structure-function research approach in humans and in animal models is needed to address whether there is a single “tinnitus perception core,” a network that always shows the same tinnitus-specific changes, regardless of the degree of hearing loss, psychological distress, or comorbidities. If this is the case, this would still represent a major breakthrough and suggest a unique target for tinnitus treatment.
Despite the previously mentioned concerns with the animal models of tinnitus, the development and employment of animal models of tinnitus and basic science research have been perhaps the greatest accelerator of tinnitus research and has increased our mechanistic understanding of the disorder. The mechanistic insight on plasticity mechanisms gained by animal models of noise-induced hearing loss and tinnitus, along with the human studies described in previous sections have led to a new dynamic picture of the tinnitus brain. Luckily, there are still many exciting and unexplored research opportunities that are highlighted in this review. Namely, the detailed circuit, cellular and molecular mechanisms underlying TCD, frontostriatal gating, noise-induced gain enhancement are not well-understood. The availability of cutting edge opto- and chemogenetic tools, the increasing availability of transgenic mouse lines makes, and the ability to assess the internal state of rodents hold the promise to unmask the complex mechanisms of tinnitus. Furthering our understanding of these mechanisms will accelerate mechanism-driven tinnitus therapeutics and advance our understanding of cognitive neuroscience mechanisms.
GRANTS
T. Tzounopoulos has received funding from the National Institute on Deafness and Other Communication Disorders (NIDCD) Grant RO1-DC007905 and the U.S. Department of Defense Grant W81XWH-14-1-0117. A. Henton received funding from NIDCD Grant F31-DC017635).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.H. prepared figures; A.H. and T.T. drafted manuscript; A.H. and T.T. edited and revised manuscript; A.H. and T.T. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Elias Aizenman for input on the manuscript title and Dr. Stylianos Kouvaros for example traces in FIGURE 4B.
REFERENCES
- 1.Dietrich S. Earliest historic reference of ‘tinnitus’ is controversial. J Laryngol Otol 118: 487–488, 2004. doi: 10.1258/0022215041615182. [DOI] [PubMed] [Google Scholar]
- 2.Adams PF, Hendershot GE, Marano MA, Centers for Disease Control and Prevention/National Center for Health Statistics. Current estimates from the National Health Interview Survey, 1996. Vital Health Stat 10: 1–203, 1999. [PubMed] [Google Scholar]
- 3.Axelsson A, Ringdahl A. Tinnitus–a study of its prevalence and characteristics. Br J Audiol 23: 53–62, 1989. doi: 10.3109/03005368909077819. [DOI] [PubMed] [Google Scholar]
- 4.McCormack A, Edmondson-Jones M, Somerset S, Hall D. A systematic review of the reporting of tinnitus prevalence and severity. Hear Res 337: 70–79, 2016. doi: 10.1016/j.heares.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Shargorodsky J, Curhan GC, Farwell WR. Prevalence and characteristics of tinnitus among US adults. Am J Med 123: 711–718, 2010. doi: 10.1016/j.amjmed.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 6.Pinto PC, Marcelos CM, Mezzasalma MA, Osterne FJ, de Melo Tavares de Lima MA, Nardi AE. Tinnitus and its association with psychiatric disorders: systematic review. J Laryngol Otol 128: 660–664, 2014. doi: 10.1017/S0022215114001030. [DOI] [PubMed] [Google Scholar]
- 7.U.S. Department of Veterans Affairs. Veterans Benefits Administration Reports Compensation (Online). https://www.benefits.va.gov/REPORTS/abr/. [2020].
- 8.Aristotle. On The Soul. South Bend, IN: Infomotions, Inc., 2000. [Google Scholar]
- 9.Flor H, Nikolajsen L, Staehelin Jensen T. Phantom limb pain: a case of maladaptive CNS plasticity? Nat Rev Neurosci 7: 873–881, 2006. doi: 10.1038/nrn1991,10.1038/nrg1923. doi:. [DOI] [PubMed] [Google Scholar]
- 10.Coffey BJ, Miguel EC, Savage CR, Rauch SL. Tourette's disorder and related problems: a review and update. Harv Rev Psychiatry 2: 121–132, 1994. doi: 10.3109/10673229409017128. [DOI] [PubMed] [Google Scholar]
- 11.Saba PR, Dastur K, Keshavan MS, Katerji MA. Obsessive-compulsive disorder, Tourette's syndrome, and basal ganglia pathology on MRI. J Neuropsychiatry Clin Neurosci 10: 116–117, 1998. doi: 10.1176/jnp.10.1.116. [DOI] [PubMed] [Google Scholar]
- 12.Stern JS. Tourette’s syndrome and its borderland. Pract Neurol 18: 262–270, 2018. doi: 10.1136/practneurol-2017-001755. [DOI] [PubMed] [Google Scholar]
- 13.Tzounopoulos T, Balaban C, Zitelli L, Palmer C. Towards a mechanistic-driven precision medicine approach for tinnitus. J Assoc Res Otolaryngol 20: 115–131, 2019. doi: 10.1007/s10162-018-00709-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brozoski TJ, Bauer CA. Animal models of tinnitus. Hear Res 338: 88–97, 2016. doi: 10.1016/j.heares.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 15.Galazyuk A, Brozoski TJ. Animal models of tinnitus: a review. Otolaryngol Clin North Am 53: 469–480, 2020. doi: 10.1016/j.otc.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayes SH, Radziwon KE, Stolzberg DJ, Salvi RJ. Behavioral models of tinnitus and hyperacusis in animals. Front Neurol 5: 179, 2014. doi: 10.3389/fneur.2014.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von der Behrens W. Animal models of subjective tinnitus. Neural Plast 2014: 741452, 2014. 741452, doi: 10.1155/2014/741452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nondahl DM, Cruickshanks KJ, Huang GH, Klein BE, Klein R, Javier Nieto F, Tweed TS. Tinnitus and its risk factors in the Beaver Dam Offspring Study. Int J Audiol 50: 313–320, 2011. doi: 10.3109/14992027.2010.551220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nondahl DM, Cruickshanks KJ, Wiley TL, Klein BE, Klein R, Chappell R, Tweed TS. The ten-year incidence of tinnitus among older adults. Int J Audiol 49: 580–585, 2010. doi: 10.3109/14992021003753508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernandez KA, Jeffers PW, Lall K, Liberman MC, Kujawa SG. Aging after noise exposure: acceleration of cochlear synaptopathy in “recovered” ears. J Neurosci 35: 7509–7520, 2015. [ doi: 10.1523/JNEUROSCI.5138-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenhall U. The influence of ageing on noise-induced hearing loss. Noise Health 5: 47–53, 2003. [PubMed] [Google Scholar]
- 22.Kujawa SG, Liberman MC. Acceleration of age-related hearing loss by early noise exposure: evidence of a misspent youth. J Neurosci 26: 2115–2123, 2006. [ doi: 10.1523/JNEUROSCI.4985-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chung JW, Ahn JH, Kim JY, Lee HJ, Kang HH, Lee YK, Kim JU, Koo SW. The effect of isoflurane, halothane and pentobarbital on noise-induced hearing loss in mice. Anesth Analg 104: 1404–1408, 2007. doi: 10.1213/01.ane.0000261508.24083.6c. [DOI] [PubMed] [Google Scholar]
- 24.Kim JU, Lee HJ, Kang HH, Shin JW, Ku SW, Ahn JH, Kim YJ, Chung JW. Protective effect of isoflurane anesthesia on noise-induced hearing loss in mice. Laryngoscope 115: 1996–1999, 2005. doi: 10.1097/01.mlg.0000180173.81034.4d. [DOI] [PubMed] [Google Scholar]
- 25.Norman M, Tomscha K, Wehr M. Isoflurane blocks temporary tinnitus. Hear Res 290: 64–71, 2012. doi: 10.1016/j.heares.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 26.Gaese BH, Ostwald J. Anesthesia changes frequency tuning of neurons in the rat primary auditory cortex. J Neurophysiol 86: 1062–1066, 2001. doi: 10.1152/jn.2001.86.2.1062. [DOI] [PubMed] [Google Scholar]
- 27.Meltser I, Cederroth CR, Basinou V, Savelyev S, Lundkvist GS, Canlon B. TrkB-mediated protection against circadian sensitivity to noise trauma in the murine cochlea. Curr Biol 24: 658–663, 2014. doi: 10.1016/j.cub.2014.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gittleman SN, Le Prell CG, Hammill TL. Octave band noise exposure: Laboratory models and otoprotection efforts. J Acoust Soc Am 146: 3800, 2019. doi: 10.1121/1.5133393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohlemiller KK. Mouse methods and models for studies in hearing. J Acoust Soc Am 146: 3668, 2019. doi: 10.1121/1.5132550. [DOI] [PubMed] [Google Scholar]
- 30.Naert G, Pasdelou MP, Le Prell CG. Use of the guinea pig in studies on the development and prevention of acquired sensorineural hearing loss, with an emphasis on noise. J Acoust Soc Am 146: 3743, 2019. doi: 10.1121/1.5132711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Myers EN, Bernstein JM. Salicylate ototoxicity; a clinical and experimental study. Arch Otolaryngol 82: 483–493, 1965. doi: 10.1001/archotol.1965.00760010485006. [DOI] [PubMed] [Google Scholar]
- 32.Jastreboff PJ, Brennan JF, Coleman JK, Sasaki CT. Phantom auditory sensation in rats: an animal model for tinnitus. Behav Neurosci 102: 811–822, 1988. doi: 10.1037//0735-7044.102.6.811. [DOI] [PubMed] [Google Scholar]
- 33.Kujawa SG, Fallon M, Bobbin RP. Intracochlear salicylate reduces low-intensity acoustic and cochlear microphonic distortion products. Hear Res 64: 73–80, 1992. doi: 10.1016/0378-5955(92)90169-n. [DOI] [PubMed] [Google Scholar]
- 34.Wallhäusser-Franke E, Mahlke C, Oliva R, Braun S, Wenz G, Langner G. Expression of c-fos in auditory and non-auditory brain regions of the gerbil after manipulations that induce tinnitus. Exp Brain Res 153: 649–654, 2003. doi: 10.1007/s00221-003-1614-2. [DOI] [PubMed] [Google Scholar]
- 35.Wang HT, Luo B, Zhou KQ, Xu TL, Chen L. Sodium salicylate reduces inhibitory postsynaptic currents in neurons of rat auditory cortex. Hear Res 215: 77–83, 2006. doi: 10.1016/j.heares.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 36.Bauer CA, Brozoski TJ. Assessing tinnitus and prospective tinnitus therapeutics using a psychophysical animal model. J Assoc Res Otolaryngol 2: 54–64, 2001. doi: 10.1007/s101620010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bauer CA, Brozoski TJ, Rojas R, Boley J, Wyder M. Behavioral model of chronic tinnitus in rats. Otolaryngol Head Neck Surg 121: 457–462, 1999. doi: 10.1016/S0194-5998(99)70237-8. [DOI] [PubMed] [Google Scholar]
- 38.Yang S, Weiner BD, Zhang LS, Cho SJ, Bao S. Homeostatic plasticity drives tinnitus perception in an animal model. Proc Natl Acad Sci U S A 108: 14974–14979, 2011. doi: 10.1073/pnas.1107998108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marinos L, Kouvaros S, Bizup B, Hambach B, Wipf P, Tzounopoulos T. Transient delivery of KCNQ2/3-specific channel activator one week after noise trauma mitigates noise-induced tinnitus. J Assoc Res Otolaryngol 22: 127–139, 2021. doi: 10.1007/s10162-021-00786-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zuo H, Lei D, Sivaramakrishnan S, Howie B, Mulvany J, Bao J. An operant-based detection method for inferring tinnitus in mice. J Neurosci Methods 291: 227–237, 2017. doi: 10.1016/j.jneumeth.2017.08.029. [DOI] [PubMed] [Google Scholar]
- 41.Turner JG, Brozoski TJ, Bauer CA, Parrish JL, Myers K, Hughes LF, Caspary DM. Gap detection deficits in rats with tinnitus: a potential novel screening tool. Behav Neurosci 120: 188–195, 2006. doi: 10.1037/0735-7044.120.1.188. [DOI] [PubMed] [Google Scholar]
- 42.Hickox AE, Liberman MC. Is noise-induced cochlear neuropathy key to the generation of hyperacusis or tinnitus? J Neurophysiol 111: 552–564, 2014. doi: 10.1152/jn.00184.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu H, Vikhe PK, Han C, Fabella B, Canlon B, Someya S, Cederroth CR. GLAST deficiency in mice exacerbates gap detection deficits in a model of salicylate-induced tinnitus. Front Behav Neurosci 10: 158, 2016. doi: 10.3389/fnbeh.2016.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boyen K, Başkent D, van Dijk P. The gap detection test: can it be used to diagnose tinnitus? Ear Hear 36: e138–145, 2015. doi: 10.1097/AUD.0000000000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Campolo J, Lobarinas E, Salvi R. Does tinnitus “fill in” the silent gaps? Noise Health 15: 398–405, 2013. doi: 10.4103/1463-1741.121232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Engineer ND, Riley JR, Seale JD, Vrana WA, Shetake JA, Sudanagunta SP, Borland MS, Kilgard MP. Reversing pathological neural activity using targeted plasticity. Nature 470: 101–104, 2011. doi: 10.1038/nature09656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kalappa BI, Soh H, Duignan KM, Furuya T, Edwards S, Tzingounis AV, Tzounopoulos T. Potent KCNQ2/3-specific channel activator suppresses in vivo epileptic activity and prevents the development of tinnitus. J Neurosci 35: 8829–8842, 2015. doi: 10.1523/JNEUROSCI.5176-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li S, Choi V, Tzounopoulos T. Pathogenic plasticity of Kv7.2/3 channel activity is essential for the induction of tinnitus. Proc Natl Acad Sci U S A 110: 9980–9985, 2013. doi: 10.1073/pnas.1302770110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marks KL, Martel DT, Wu C, Basura GJ, Roberts LE, Schvartz-Leyzac KC, Shore SE. Auditory-somatosensory bimodal stimulation desynchronizes brain circuitry to reduce tinnitus in guinea pigs and humans. Sci Transl Med 10: eaal3175, 2018. doi: 10.1126/scitranslmed.aal3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Olusanya BO, Davis AC, Hoffman HJ. Hearing loss: rising prevalence and impact. Bull World Health Organ 97: 646–646a, 2019. doi: 10.2471/blt.19.224683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blazer DG, Tucci DL. Hearing loss and psychiatric disorders: a review. Psychol Med 49: 891–897, 2019. doi: 10.1017/S0033291718003409. [DOI] [PubMed] [Google Scholar]
- 52.World Health Organization. Global Costs of Unaddressed Hearing Loss and Cost-Effectiveness of Interventions: A WHO Report, 2017. Geneva, Switzerland: World Health Organization, 2017. [Google Scholar]
- 53.Bauer CA. Tinnitus. N Engl J Med 378: 1224–1231, 2018. doi: 10.1056/NEJMcp1506631. [DOI] [PubMed] [Google Scholar]
- 54.Roberts LE, Eggermont JJ, Caspary DM, Shore SE, Melcher JR, Kaltenbach JA. Ringing ears: the neuroscience of tinnitus. J Neurosci 30: 14972–14979, 2010. [ doi: 10.1523/JNEUROSCI.4028-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liberman MC, Dodds LW. Single-neuron labeling and chronic cochlear pathology. III. Stereocilia damage and alterations of threshold tuning curves. Hear Res 16: 55–74, 1984. doi: 10.1016/0378-5955(84)90025-x. [DOI] [PubMed] [Google Scholar]
- 56.Robertson D. Functional significance of dendritic swelling after loud sounds in the guinea pig cochlea. Hear Res 9: 263–278, 1983. doi: 10.1016/0378-5955(83)90031-x. [DOI] [PubMed] [Google Scholar]
- 57.Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci 29: 14077–14085, 2009. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tserga E, Damberg P, Canlon B, Cederroth CR. Auditory synaptopathy in mice lacking the glutamate transporter GLAST and its impact on brain activity. Prog Brain Res 262: 245–261, 2021. [ doi: 10.1016/bs.pbr.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nicolas-Puel C, Faulconbridge RL, Guitton M, Puel JL, Mondain M, Uziel A. Characteristics of tinnitus and etiology of associated hearing loss: a study of 123 patients. Int Tinnitus J 8: 37–44, 2002. [PubMed] [Google Scholar]
- 60.Sanchez TG, Medeiros IR, Levy CP, Ramalho JR, Bento RF. Tinnitus in normally hearing patients: clinical aspects and repercussions. Braz J Otorhinolaryngol 71: 427–431, 2005. doi: 10.1016/s1808-8694(15)31194-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gu JW, Herrmann BS, Levine RA, Melcher JR. Brainstem auditory evoked potentials suggest a role for the ventral cochlear nucleus in tinnitus. J Assoc Res Otolaryngol 13: 819–833, 2012. doi: 10.1007/s10162-012-0344-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schaette R, McAlpine D. Tinnitus with a normal audiogram: physiological evidence for hidden hearing loss and computational model. J Neurosci 31: 13452–13457, 2011. doi: 10.1523/JNEUROSCI.2156-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guest H, Munro KJ, Prendergast G, Howe S, Plack CJ. Tinnitus with a normal audiogram: Relation to noise exposure but no evidence for cochlear synaptopathy. Hear Res 344: 265–274, 2017. doi: 10.1016/j.heares.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shim HJ, An YH, Kim DH, Yoon JE, Yoon JH. Comparisons of auditory brainstem response and sound level tolerance in tinnitus ears and non-tinnitus ears in unilateral tinnitus patients with normal audiograms. PLoS One 12: e0189157, 2017. doi: 10.1371/journal.pone.0189157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Robertson D, Sellick PM, Patuzzi R. The continuing search for outer hair cell afferents in the guinea pig spiral ganglion. Hear Res 136: 151–158, 1999. doi: 10.1016/s0378-5955(99)00120-3. [DOI] [PubMed] [Google Scholar]
- 66.Weisz CJ, Lehar M, Hiel H, Glowatzki E, Fuchs PA. Synaptic transfer from outer hair cells to type II afferent fibers in the rat cochlea. J Neurosci 32: 9528–9536, 2012. doi: 10.1523/JNEUROSCI.6194-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Flores EN, Duggan A, Madathany T, Hogan AK, Marquez FG, Kumar G, Seal RP, Edwards RH, Liberman MC, Garcia-Anoveros J. A non-canonical pathway from cochlea to brain signals tissue-damaging noise. Curr Biol 25: 606–612, 2015. doi: 10.1016/j.cub.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu C, Glowatzki E, Fuchs PA. Unmyelinated type II afferent neurons report cochlear damage. Proc Natl Acad Sci U S A 112: 14723–14727, 2015. doi: 10.1073/pnas.1515228112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weisz C, Glowatzki E, Fuchs P. The postsynaptic function of type II cochlear afferents. Nature 461: 1126–1129, 2009. doi: 10.1038/nature08487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Munoz DJ, Kendrick IS, Rassam M, Thorne PR. Vesicular storage of adenosine triphosphate in the guinea-pig cochlear lateral wall and concentrations of ATP in the endolymph during sound exposure and hypoxia. Acta Otolaryngolog 121: 10–15, 2001. doi: 10.1080/000164801300006209. [DOI] [PubMed] [Google Scholar]
- 71.Cederroth CR, Lugo A, Edvall NK, Lazar A, Lopez-Escamez JA, Bulla J, Uhlen I, Hoare DJ, Baguley DM, Canlon B, Gallus S. Association between hyperacusis and tinnitus. J Clin Med 9: 2412, 2020. doi: 10.3390/jcm9082412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hébert S, Fournier P, Noreña A. The auditory sensitivity is increased in tinnitus ears. J Neurosci 33: 2356–2364, 2013. doi: 10.1523/JNEUROSCI.3461-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mills RP, Cherry JR. Subjective tinnitus in children with otological disorders. Int J Pediatr Otorhinolaryngol 7: 21–27, 1984. doi: 10.1016/s0165-5876(84)80050-6. [DOI] [PubMed] [Google Scholar]
- 74.Clarkson C, Antunes FM, Rubio ME. Conductive hearing loss has long-lasting structural and molecular effects on presynaptic and postsynaptic structures of auditory nerve synapses in the cochlear nucleus. J Neurosci 36: 10214–10227, 2016. doi: 10.1523/JNEUROSCI.0226-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nondahl DM, Cruickshanks KJ, Wiley TL, Klein R, Klein BE, Tweed TS. Prevalence and 5-year incidence of tinnitus among older adults: the epidemiology of hearing loss study. J Am Acad Audiol 13: 323–331, 2002. doi: 10.1055/s-0040-1715975. [DOI] [PubMed] [Google Scholar]
- 76.Schaette R, Turtle C, Munro KJ. Reversible induction of phantom auditory sensations through simulated unilateral hearing loss. PLoS One 7: e35238, 2012. e35238doi: 10.1371/journal.pone.0035238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pessin AB, Martins RH, Pimenta WP, Simões AC, Marsiglia A, Amaral AV. Auditory evaluation in patients with type 1 diabetes. Ann Otol Rhinol Laryngol 117: 366–370, 2008. doi: 10.1177/000348940811700507. [DOI] [PubMed] [Google Scholar]
- 78.Cazals Y. Auditory sensori-neural alterations induced by salicylate. Prog Neurobiol 62: 583–631, 2000. doi: 10.1016/s0301-0082(00)00027-7. [DOI] [PubMed] [Google Scholar]
- 79.Stolzberg D, Salvi RJ, Allman BL. Salicylate toxicity model of tinnitus. Front Syst Neurosci 6: 28, 2012. doi: 10.3389/fnsys.2012.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sajjadi H, Paparella MM. Meniere’s disease. Lancet 372: 406–414, 2008. doi: 10.1016/S0140-6736(08)61161-7. [DOI] [PubMed] [Google Scholar]
- 81.Sismanis A. Pulsatile tinnitus. Otolaryngol Clin North Am 36: 389–402, 2003. doi: 10.1016/S0030-6665(02)00169-X. [DOI] [PubMed] [Google Scholar]
- 82.Levine RA. Somatic (craniocervical) tinnitus and the dorsal cochlear nucleus hypothesis. Am J Otolaryngol 20: 351–362, 1999. doi: 10.1016/s0196-0709(99)90074-1. [DOI] [PubMed] [Google Scholar]
- 83.Brozoski TJ, Bauer CA. The effect of dorsal cochlear nucleus ablation on tinnitus in rats. Hear Res 206: 227–236, 2005. doi: 10.1016/j.heares.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 84.Brozoski TJ, Wisner KW, Sybert LT, Bauer CA. Bilateral dorsal cochlear nucleus lesions prevent acoustic-trauma induced tinnitus in an animal model. J Assoc Res Otolaryngol 13: 55–66, 2012. doi: 10.1007/s10162-011-0290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kaltenbach JA, McCaslin DL. Increases in spontaneous activity in the dorsal cochlear nucleus following exposure to high intensity sound: a possible neural correlate of tinnitus. Audit Neurosci 3: 57–78, 1996. [PMC free article] [PubMed] [Google Scholar]
- 86.Shore SE, Vass Z, Wys NL, Altschuler RA. Trigeminal ganglion innervates the auditory brainstem. J Comp Neurol 419: 271–285, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 87.Brozoski TJ, Bauer CA, Caspary DM. Elevated fusiform cell activity in the dorsal cochlear nucleus of chinchillas with psychophysical evidence of tinnitus. J Neurosci 22: 2383–2390, 2002. doi: 10.1523/JNEUROSCI.22-06-02383.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kaltenbach JA, Zhang J, Finlayson P. Tinnitus as a plastic phenomenon and its possible neural underpinnings in the dorsal cochlear nucleus. Hear Res 206: 200–226, 2005. doi: 10.1016/j.heares.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 89.Middleton JW, Kiritani T, Pedersen C, Turner JG, Shepherd GM, Tzounopoulos T. Mice with behavioral evidence of tinnitus exhibit dorsal cochlear nucleus hyperactivity because of decreased GABAergic inhibition. Proc Natl Acad Sci U S A 108: 7601–7606., 2011. doi: 10.1073/pnas.1100223108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pilati N, Large C, Forsythe ID, Hamann M. Acoustic over-exposure triggers burst firing in dorsal cochlear nucleus fusiform cells. Hear Res 283: 98–106, 2012. doi: 10.1016/j.heares.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shore SE, Wu C. Mechanisms of noise-induced tinnitus: insights from cellular studies. Neuron 103: 8–20, 2019. doi: 10.1016/j.neuron.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li S, Kalappa BI, Tzounopoulos T. Noise-induced plasticity of KCNQ2/3 and HCN channels underlies vulnerability and resilience to tinnitus. eLife 4: e07242, 2015. doi: 10.7554/eLife.07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Eskioglou E, Perrenoud MP, Ryvlin P, Novy J. Novel treatment and new drugs in epilepsy treatment. Curr Pharm Des 23: 6389–6398, 2017. doi: 10.2174/1381612823666171024143541. [DOI] [PubMed] [Google Scholar]
- 94.Kumar M, Reed N, Liu R, Aizenman E, Wipf P, Tzounopoulos T. Synthesis and evaluation of potent KCNQ2/3-specific channel activators. Mol Pharmacol 89: 667–677, 2016. doi: 10.1124/mol.115.103200. [DOI] [PubMed] [Google Scholar]
- 95.Liu R, Tzounopoulos T, Wipf P. Synthesis and optimization of Kv7 (KCNQ) potassium channel agonists: the role of fluorines in potency and selectivity. ACS Med Chem Lett 10: 929–935, 2019. doi: 10.1021/acsmedchemlett.9b00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang H, Brozoski TJ, Turner JG, Ling L, Parrish JL, Hughes LF, Caspary DM. Plasticity at glycinergic synapses in dorsal cochlear nucleus of rats with behavioral evidence of tinnitus. Neuroscience 164: 747–759, 2009. doi: 10.1016/j.neuroscience.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang H, Turner JG, Ling L, Parrish JL, Hughes LF, Caspary DM. Age-related changes in glycine receptor subunit composition and binding in dorsal cochlear nucleus. Neuroscience 160: 227–239, 2009. doi: 10.1016/j.neuroscience.2009.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu C, Martel DT, Shore SE. Increased synchrony and bursting of dorsal cochlear nucleus fusiform cells correlate with tinnitus. J Neurosci 36: 2068–2073, 2016. doi: 10.1523/JNEUROSCI.3960-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tzounopoulos T, Kim Y, Oertel D, Trussell LO. Cell-specific, spike timing-dependent plasticities in the dorsal cochlear nucleus. Nat Neurosci 7: 719–725, 2004. doi: 10.1038/nn1272. [DOI] [PubMed] [Google Scholar]
- 100.Tzounopoulos T, Rubio ME, Keen JE, Trussell LO. Coactivation of pre- and postsynaptic signaling mechanisms determines cell-specific spike-timing-dependent plasticity. Neuron 54: 291–301, 2007. doi: 10.1016/j.neuron.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tzounopoulos T. Mechanisms of synaptic plasticity in the dorsal cochlear nucleus: plasticity-induced changes that could underlie tinnitus. Am J Audiol 17: S170–175, 2008. doi: 10.1044/1059-0889(2008/07-0030). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Winer JA. Decoding the auditory corticofugal systems. Hear Res 212: 1–8, 2006. doi: 10.1016/j.heares.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 103.Winer JA, Prieto JJ. Layer V in cat primary auditory cortex (AI): cellular architecture and identification of projection neurons. J Comp Neurol 434: 379–412, 2001. doi: 10.1002/cne.1183. [DOI] [PubMed] [Google Scholar]
- 104.Bauer CA, Turner JG, Caspary DM, Myers KS, Brozoski TJ. Tinnitus and inferior colliculus activity in chinchillas related to three distinct patterns of cochlear trauma. J Neurosci Res 86: 2564–2578, 2008. doi: 10.1002/jnr.21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Niu Y, Kumaraguru A, Wang R, Sun W. Hyperexcitability of inferior colliculus neurons caused by acute noise exposure. J Neurosci Res 91: 292–299, 2013. doi: 10.1002/jnr.23152. [DOI] [PubMed] [Google Scholar]
- 106.Sturm JJ, Zhang-Hooks YX, Roos H, Nguyen T, Kandler K. Noise trauma-induced behavioral gap detection deficits correlate with reorganization of excitatory and inhibitory local circuits in the inferior colliculus and are prevented by acoustic enrichment. J Neurosci 37: 6314–6330, 2017. doi: 10.1523/JNEUROSCI.0602-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Berger JI, Coomber B, Wells TT, Wallace MN, Palmer AR. Changes in the response properties of inferior colliculus neurons relating to tinnitus. Front Neurol 5: 203, 2014. doi: 10.3389/fneur.2014.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Longenecker RJ, Galazyuk AV. Variable effects of acoustic trauma on behavioral and neural correlates of tinnitus in individual animals. Front Behav Neurosci 10: 207, 2016. doi: 10.3389/fnbeh.2016.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shaheen LA, Liberman MC. Cochlear synaptopathy changes sound-evoked activity without changing spontaneous discharge in the mouse inferior colliculus. Front Syst Neurosci 12: 59, 2018. doi: 10.3389/fnsys.2018.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ropp TJ, Tiedemann KL, Young ED, May BJ. Effects of unilateral acoustic trauma on tinnitus-related spontaneous activity in the inferior colliculus. J Assoc Res Otolaryngol 12: 1007–1022, 2014. doi: 10.1007/s10162-014-0488-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Basta D, Ernst A. Erratum to “Noise-induced changes of neuronal spontaneous activity in mice inferior colliculus brain slices”. Neurosci Lett 374: 74–79, 2005. doi: 10.1016/j.neulet.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 112.Berger JI, Coomber B. Tinnitus-related changes in the inferior colliculus. Front Neurol 6: 61, 2015. doi: 10.3389/fneur.2015.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Szczepaniak WS, Moller AR. Evidence of decreased GABAergic influence on temporal integration in the inferior colliculus following acute noise exposure: a study of evoked potentials in the rat. Neurosci Lett 196: 77–80, 1995. doi: 10.1016/0304-3940(95)11851-m. [DOI] [PubMed] [Google Scholar]
- 114.Milbrandt JC, Holder TM, Wilson MC, Salvi RJ, Caspary DM. GAD levels and muscimol binding in rat inferior colliculus following acoustic trauma. Hear Res 147: 251–260, 2000. doi: 10.1016/S0378-5955(00)00135-0. [DOI] [PubMed] [Google Scholar]
- 115.Brozoski T, Odintsov B, Bauer C. Gamma-aminobutyric acid and glutamic acid levels in the auditory pathway of rats with chronic tinnitus: a direct determination using high resolution point-resolved proton magnetic resonance spectroscopy (H-MRS). Front Syst Neurosci 6: 9, 2012. doi: 10.3389/fnsys.2012.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Asokan MM, Williamson RS, Hancock KE, Polley DB. Sensory overamplification in layer 5 auditory corticofugal projection neurons following cochlear nerve synaptic damage. Nat Commun 9: 2468, 2018.doi: 10.1038/s41467-018-05333-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chambers AR, Pilati N, Balaram P, Large CH, Kaczmarek LK, Polley DB. Pharmacological modulation of Kv3.1 mitigates auditory midbrain temporal processing deficits following auditory nerve damage. Sci Rep 7: 17496, 2017. doi: 10.1038/s41598-017-17406-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hall DA, Ray J, Watson J, Sharman A, Hutchison J, Harris P, Daniel M, Millar B, Large CH. A balanced randomised placebo controlled blinded phase IIa multi-centre study to investigate the efficacy and safety of AUT00063 versus placebo in subjective tinnitus: The QUIET-1 trial. Hear Res 377: 153–166, 2019. doi: 10.1016/j.heares.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 119.Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci 4: 2877–2890, 1984. doi: 10.1523/JNEUROSCI.04-11-02877.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Llinás RR, Steriade M. Bursting of thalamic neurons and states of vigilance. J Neurophysiol 95: 3297–3308, 2006. doi: 10.1152/jn.00166.2006. [DOI] [PubMed] [Google Scholar]
- 121.Overton PG, Clark D. Burst firing in midbrain dopaminergic neurons. Brain Res Brain Res Rev 25: 312–334, 1997. doi: 10.1016/s0165-0173(97)00039-8. [DOI] [PubMed] [Google Scholar]
- 122.Kalappa BI, Brozoski TJ, Turner JG, Caspary DM. Single unit hyperactivity and bursting in the auditory thalamus of awake rats directly correlates with behavioural evidence of tinnitus. J Physiol 592: 5065–5078, 2014. doi: 10.1113/jphysiol.2014.278572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Caspary DM, Llano DA. Auditory thalamic circuits and GABAA receptor function: putative mechanisms in tinnitus pathology. Hear Res 349: 197–207, 2017. doi: 10.1016/j.heares.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.De Ridder D, Vanneste S, Langguth B, Llinas R. Thalamocortical dysrhythmia: a theoretical update in tinnitus. Front Neurol 6: 124, 2015. doi: 10.3389/fneur.2015.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sametsky EA, Turner JG, Larsen D, Ling L, Caspary DM. Enhanced GABAA-mediated tonic inhibition in auditory thalamus of rats with behavioral evidence of tinnitus. J Neurosci 35: 9369–9380, 2015. doi: 10.1523/JNEUROSCI.5054-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jahnsen H, Llinas R. Voltage-dependent burst-to-tonic switching of thalamic cell activity: an in vitro study. Arch Ital Biol 122: 73–82, 1984. [PubMed] [Google Scholar]
- 127.Llinas R, Urbano FJ, Leznik E, Ramirez RR, van Marle HJ. Rhythmic and dysrhythmic thalamocortical dynamics: GABA systems and the edge effect. Trends Neurosci 28: 325–333, 2005. doi: 10.1016/j.tins.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 128.De Ridder D, van der Loo E, Vanneste S, Gais S, Plazier M, Kovacs S, Sunaert S, Menovsky T, van de Heyning P. Theta-gamma dysrhythmia and auditory phantom perception. J Neurosurg 114: 912–921, 2011. doi: 10.3171/2010.11.JNS10335. [DOI] [PubMed] [Google Scholar]
- 129.Walton KD, Llinas RR. Frontiers in neuroscience central pain as a thalamocortical dysrhythmia: a thalamic efference disconnection? In: Translational Pain Research: From Mouse to Man, edited by Kruger L, Light AR.. Boca Raton, FL: CRC Press/Taylor & Francis, 2010. [PubMed] [Google Scholar]
- 130.Middleton JW, Tzounopoulos T. Imaging the neural correlates of tinnitus: a comparison between animal models and human studies. Front Syst Neurosci 6: 35, 2012. doi: 10.3389/fnsys.2012.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Adjamian P, Sereda M, Zobay O, Hall DA, Palmer AR. Neuromagnetic indicators of tinnitus and tinnitus masking in patients with and without hearing loss. J Assoc Res Otolaryngol 13: 715–731, 2012. doi: 10.1007/s10162-012-0340-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kahlbrock N, Weisz N. Transient reduction of tinnitus intensity is marked by concomitant reductions of delta band power. BMC Biol 6: 4, 2008. doi: 10.1186/1741-7007-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sedley W, Gander PE, Kumar S, Oya H, Kovach CK, Nourski KV, Kawasaki H, Howard MA 3rd, Griffiths TD. Intracranial mapping of a cortical tinnitus system using residual inhibition. Curr Biol 25: 1208–1214, 2015. doi: 10.1016/j.cub.2015.02.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Weisz N, Muller S, Schlee W, Dohrmann K, Hartmann T, Elbert T. The neural code of auditory phantom perception. J Neurosci 27: 1479–1484, 2007. doi: 10.1523/JNEUROSCI.3711-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Eggermont JJ. The auditory cortex and tinnitu –a review of animal and human studies. Eur J Neurosci 41: 665–676, 2015. doi: 10.1111/ejn.12759. [DOI] [PubMed] [Google Scholar]
- 136.Noreña AJ, Eggermont JJ. Changes in spontaneous neural activity immediately after an acoustic trauma: implications for neural correlates of tinnitus. Hear Res 183: 137–153, 2003. doi: 10.1016/s0378-5955(03)00225-9. [DOI] [PubMed] [Google Scholar]
- 137.Komiya H, Eggermont JJ. Spontaneous firing activity of cortical neurons in adult cats with reorganized tonotopic map following pure-tone trauma. Acta Otolaryngol 120: 750–756, 2000. doi: 10.1080/000164800750000298. [DOI] [PubMed] [Google Scholar]
- 138.Basura GJ, Koehler SD, Shore SE. Bimodal stimulus timing-dependent plasticity in primary auditory cortex is altered after noise exposure with and without tinnitus. J Neurophysiol 114: 3064–3075, 2015. doi: 10.1152/jn.00319.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chen YC, Li X, Liu L, Wang J, Lu CQ, Yang M, Jiao Y, Zang FC, Radziwon K, Chen GD, Sun W, Krishnan Muthaiah VP, Salvi R, Teng GJ. Tinnitus and hyperacusis involve hyperactivity and enhanced connectivity in auditory-limbic-arousal-cerebellar network. eLife 4: e06576, 2015. e06576. doi: 10.7554/eLife.06576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lanting CP, de Kleine E, van Dijk P. Neural activity underlying tinnitus generation: results from PET and fMRI. Hear Res 255: 1–13, 2009. doi: 10.1016/j.heares.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 141.Weisz N, Moratti S, Meinzer M, Dohrmann K, Elbert T. Tinnitus perception and distress is related to abnormal spontaneous brain activity as measured by magnetoencephalography. PLoS Med 2: e153, 2005. doi: 10.1371/journal.pmed.0020153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lorenz I, Müller N, Schlee W, Hartmann T, Weisz N. Loss of alpha power is related to increased gamma synchronization-A marker of reduced inhibition in tinnitus? Neurosci Lett 453: 225–228, 2009. doi: 10.1016/j.neulet.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 143.Gray CM, König P, Engel AK, Singer W. Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature 338: 334–337, 1989. doi: 10.1038/338334a0. [DOI] [PubMed] [Google Scholar]
- 144.Singer W. Neuronal synchrony: a versatile code for the definition of relations? Neuron 24: 49–65, 1999. doi: 10.1016/S0896-6273(00)80821-1. [DOI] [PubMed] [Google Scholar]
- 145.Sedley W, Cunningham MO. Do cortical gamma oscillations promote or suppress perception? An under-asked question with an over-assumed answer. Front Hum Neurosci 7: 595, 2013. doi: 10.3389/fnhum.2013.00595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Eggermont JJ, Tass PA. Maladaptive neural synchrony in tinnitus: origin and restoration. Front Neurol 6: 29, 2015. doi: 10.3389/fneur.2015.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.de Villers-Sidani E, Chang EF, Bao S, Merzenich MM. Critical period window for spectral tuning defined in the primary auditory cortex (A1) in the rat. J Neurosci 27: 180–189, 2007. doi: 10.1523/JNEUROSCI.3227-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Scholl B, Wehr M. Disruption of balanced cortical excitation and inhibition by acoustic trauma. J Neurophysiol 100: 646–656, 2008. doi: 10.1152/jn.90406.2008. [DOI] [PubMed] [Google Scholar]
- 149.Tyler R, Cacace A, Stocking C, Tarver B, Engineer N, Martin J, Deshpande A, Stecker N, Pereira M, Kilgard M, Burress C, Pierce D, Rennaker R, Vanneste S. Vagus nerve stimulation paired with tones for the treatment of tinnitus: a prospective randomized double-blind controlled pilot study in humans. Sci Rep 7: 11960, 2017. 11960. doi: 10.1038/s41598-017-12178-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Miyakawa A, Wang W, Cho SJ, Li D, Yang S, Bao S. Tinnitus correlates with downregulation of cortical glutamate decarboxylase 65 expression but not auditory cortical map reorganization. J Neurosci 39: 9989–10001, 2019. doi: 10.1523/JNEUROSCI.1117-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mühlnickel W, Elbert T, Taub E, Flor H. Reorganization of auditory cortex in tinnitus. Proc Natl Acad Sci U S A 95: 10340–10343, 1998. doi: 10.1073/pnas.95.17.10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Langers DR, de Kleine E, van Dijk P. Tinnitus does not require macroscopic tonotopic map reorganization. Front Syst Neurosci 6: 2, 2012. doi: 10.3389/fnsys.2012.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Koops EA, Renken RJ, Lanting CP, van Dijk P. Cortical tonotopic map changes in humans are larger in hearing loss than in additional tinnitus. J Neurosci 40: 3178–3185, 2020. doi: 10.1523/JNEUROSCI.2083-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chambers AR, Resnik J, Yuan Y, Whitton JP, Edge AS, Liberman MC, Polley DB. Central gain restores auditory processing following near-complete cochlear denervation. Neuron 89: 867–879, 2016. doi: 10.1016/j.neuron.2015.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Syka J, Rybalko N, Popelar J. Enhancement of the auditory cortex evoked responses in awake guinea pigs after noise exposure. Hear Res 78: 158–168, 1994. doi: 10.1016/0378-5955(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 156.Dewey RS, Francis ST, Guest H, Prendergast G, Millman RE, Plack CJ, Hall DA. The association between subcortical and cortical fMRI and lifetime noise exposure in listeners with normal hearing thresholds. Neuroimage 204: 116239, 2020. doi: 10.1016/j.neuroimage.2019.116239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gu JW, Halpin CF, Nam EC, Levine RA, Melcher JR. Tinnitus, diminished sound-level tolerance, and elevated auditory activity in humans with clinically normal hearing sensitivity. J Neurophysiol 104: 3361–3370, 2010. doi: 10.1152/jn.00226.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Resnik J, Polley DB. Fast-spiking GABA circuit dynamics in the auditory cortex predict recovery of sensory processing following peripheral nerve damage. eLife 6: e21452, 2017. doi: 10.7554/eLife.21452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hackos DH, Lupardus PJ, Grand T, Chen Y, Wang TM, Reynen P, Gustafson A, Wallweber HJ, Volgraf M, Sellers BD, Schwarz JB, Paoletti P, Sheng M, Zhou Q, Hanson JE. Positive allosteric modulators of GluN2A-containing NMDARs with distinct modes of action and impacts on circuit function. Neuron 89: 983–999, 2016. doi: 10.1016/j.neuron.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 160.Deng D, Masri S, Yao L, Ma X, Cao X, Yang S, Bao S, Zhou Q. Increasing endogenous activity of NMDARs on GABAergic neurons increases inhibition, alters sensory processing and prevents noise-induced tinnitus. Sci Rep 10: 11969, 2020. doi: 10.1038/s41598-020-68652-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bos H, Oswald AM, Doiron B. Untangling stability and gain modulation in cortical circuits with multiple interneuron classes. bioRxiv, 2020.
- 162.Clifford RE, Maihofer AX, Stein MB, Ryan AF, Nievergelt CM. Novel risk loci in tinnitus and causal inference with neuropsychiatric disorders among adults of European ancestry. JAMA Otolaryngol Head Neck Surg 146: 1015–1025, 2020. doi: 10.1001/jamaoto.2020.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dobie RA. Depression and tinnitus. Otolaryngol Clin North 36: 383–388, 2003. doi: 10.1016/s0030-6665(02)00168-8. [DOI] [PubMed] [Google Scholar]
- 164.Folmer RL, Griest SE, Meikle MB, Martin WH. Tinnitus severity, loudness, and depression. Otolaryngol Head Neck Surg 121: 48–51, 1999. doi: 10.1016/S0194-5998(99)70123-3. [DOI] [PubMed] [Google Scholar]
- 165.Leaver AM, Renier L, Chevillet MA, Morgan S, Kim HJ, Rauschecker JP. Dysregulation of limbic and auditory networks in tinnitus. Neuron 69: 33–43, 2011. doi: 10.1016/j.neuron.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rauschecker JP, May ES, Maudoux A, Ploner M. Frontostriatal gating of tinnitus and chronic pain. Trends Cogn Sci 19: 567–578, 2015. doi: 10.1016/j.tics.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Saunders A, Oldenburg IA, Berezovskii VK, Johnson CA, Kingery ND, Elliott HL, Xie T, Gerfen CR, Sabatini BL. A direct GABAergic output from the basal ganglia to frontal cortex. Nature 521: 85–89, 2015. doi: 10.1038/nature14179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Leaver AM, Seydell-Greenwald A, Turesky TK, Morgan S, Kim HJ, Rauschecker JP. Cortico-limbic morphology separates tinnitus from tinnitus distress. Front Syst Neurosci 6: 21, 2012. doi: 10.3389/fnsys.2012.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dolensek N, Gehrlach DA, Klein AS, Gogolla N. Facial expressions of emotion states and their neuronal correlates in mice. Science 368: 89–94, 2020. doi: 10.1126/science.aaz9468. [DOI] [PubMed] [Google Scholar]
- 170.Shulman A. A final common pathway for tinnitus–the medial temporal lobe system. Int Tinnitus J 1: 115–126, 1995. [PubMed] [Google Scholar]
- 171.De Ridder D, Elgoyhen AB, Romo R, Langguth B. Phantom percepts: tinnitus and pain as persisting aversive memory networks. Proc Natl Acad Sci U S A 108: 8075–8080., 2011. doi: 10.1073/pnas.1018466108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Viskontas IV, Knowlton BJ, Steinmetz PN, Fried I. Differences in mnemonic processing by neurons in the human hippocampus and parahippocampal regions. J Cogn Neurosci 18: 1654–1662, 2006. doi: 10.1162/jocn.2006.18.10.1654. [DOI] [PubMed] [Google Scholar]
- 173.Vanneste S, Plazier M, der Loo E, de Heyning PV, Congedo M, De Ridder D. The neural correlates of tinnitus-related distress. Neuroimage 52: 470–480, 2010. doi: 10.1016/j.neuroimage.2010.04.029. [DOI] [PubMed] [Google Scholar]
- 174.Vanneste S, De Ridder D. Deafferentation-based pathophysiological differences in phantom sound: Tinnitus with and without hearing loss. Neuroimage 129: 80–94, 2016. doi: 10.1016/j.neuroimage.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 175.Maudoux A, Lefebvre P, Cabay JE, Demertzi A, Vanhaudenhuyse A, Laureys S, Soddu A. Connectivity graph analysis of the auditory resting state network in tinnitus. Brain Res 1485: 10–21, 2012. doi: 10.1016/j.brainres.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 176.Schmidt SA, Akrofi K, Carpenter-Thompson JR, Husain FT. Default mode, dorsal attention and auditory resting state networks exhibit differential functional connectivity in tinnitus and hearing loss. PloS one 8: e76488, 2013. doi: 10.1371/journal.pone.0076488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.De Ridder D, Fransen H, Francois O, Sunaert S, Kovacs S, Van De Heyning P. Amygdalohippocampal involvement in tinnitus and auditory memory. Acta Otolaryngol Suppl, 126: 50–53, 2006. doi: 10.1080/03655230600895580. [DOI] [PubMed] [Google Scholar]
- 178.Roberts LE, Husain FT, Eggermont JJ. Role of attention in the generation and modulation of tinnitus. Neurosci Biobehav Rev 37: 1754–1773, 2013. doi: 10.1016/j.neubiorev.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 179.Cuny C, Norena A, El Massioui F, Chery-Croze S. Reduced attention shift in response to auditory changes in subjects with tinnitus. Audiol Neurootol 9: 294–302, 2004. doi: 10.1159/000080267. [DOI] [PubMed] [Google Scholar]
- 180.Trevis KJ, McLachlan NM, Wilson SJ. Cognitive mechanisms in chronic tinnitus: psychological markers of a failure to switch attention. Front Psychol 7: 1262, 2016. doi: 10.3389/fpsyg.2016.01262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Brozoski T, Wisner K, Randall M, Caspary D. Chronic sound-induced tinnitus and auditory attention in animals. Neuroscience 407: 200–212, 2019. doi: 10.1016/j.neuroscience.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 182.Sedley W, Friston KJ, Gander PE, Kumar S, Griffiths TD. An integrative tinnitus model based on sensory precision. Trends Neurosci 39: 799–812, 2016. doi: 10.1016/j.tins.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zenner HP, Vonthein R, Zenner B, Leuchtweis R, Plontke SK, Torka W, Pogge S, Birbaumer N. Standardized tinnitus-specific individual cognitive-behavioral therapy: a controlled outcome study with 286 tinnitus patients. Hear Res 298: 117–125, 2013. doi: 10.1016/j.heares.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 184.Shekhawat GS, Searchfield GD, Stinear CM. Role of hearing aids in tinnitus intervention: a scoping review. J Am Acad Audiol 24: 747–762, 2013. doi: 10.3766/jaaa.24.8.11. [DOI] [PubMed] [Google Scholar]
- 185.Jastreboff PJ, Jastreboff MM. Tinnitus Retraining Therapy (TRT) as a method for treatment of tinnitus and hyperacusis patients. J Am Acad Audiol 11: 162–177, 2000. [PubMed] [Google Scholar]
- 186.Hoare DJ, Adjamian P, Sereda M, Hall DA. Recent technological advances in sound-based approaches to tinnitus treatment: a review of efficacy considered against putative physiological mechanisms. Noise Health 15: 107–116, 2013. doi: 10.4103/1463-1741.110292. [DOI] [PubMed] [Google Scholar]
- 187.Henry JA, Schechter MA, Loovis CL, Zaugg TL, Kaelin C, Montero M. Clinical management of tinnitus using a “progressive intervention” approach. J Rehabil Res Dev 42: 95–116, 2005. doi: 10.1682/jrrd.2005.01.0005. [DOI] [PubMed] [Google Scholar]
- 188.Chang JE, Zeng FG. Tinnitus suppression by electric stimulation of the auditory nerve. Front Syst Neurosci 6: 19, 2012. doi: 10.3389/fnsys.2012.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ito J, Sakakihara J. Suppression of tinnitus by cochlear implantation. Am J Otolaryngol 15: 145–148, 1994. doi: 10.1016/0196-0709(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 190.Valles-Varela H, Royo-Lopez J, Carmen-Samperiz L, Sebastian-Cortes JM, Alfonso-Collado I. The cochlear implant as a tinnitus treatment. Acta Otorrinolaringol Esp 64: 253–257, 2013. doi: 10.1016/j.otorri.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 191.Sladen DP, Frisch CD, Carlson ML, Driscoll CL, Torres JH, Zeitler DM. Cochlear implantation for single-sided deafness: A multicenter study. Laryngoscope 127: 223–228, 2017. doi: 10.1002/lary.26102. [DOI] [PubMed] [Google Scholar]
- 192.Langguth B, De Ridder D. Tinnitus: therapeutic use of superficial brain stimulation. Handb Clin Neurol 116: 441–467, 2013. doi: 10.1016/B978-0-444-53497-2.00036-X. [DOI] [PubMed] [Google Scholar]
- 193.De Ridder D, Vanneste S, Engineer ND, Kilgard MP. Safety and efficacy of vagus nerve stimulation paired with tones for the treatment of tinnitus: a case series. Neuromodulation 17: 170–179, 2014. doi: 10.1111/ner.12127. [DOI] [PubMed] [Google Scholar]
- 194.Cheung SW, Larson PS. Tinnitus modulation by deep brain stimulation in locus of caudate neurons (area LC). Neuroscience 169: 1768–1778, 2010. doi: 10.1016/j.neuroscience.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 195.Friedland DR, Gaggl W, Runge-Samuelson C, Ulmer JL, Kopell BH. Feasibility of auditory cortical stimulation for the treatment of tinnitus. Otol Neurotol 28: 1005–1012, 2007. doi: 10.1097/MAO.0b013e318159ebf5. [DOI] [PubMed] [Google Scholar]
- 196.Langguth B, Elgoyhen AB, Cederroth CR. Therapeutic approaches to the treatment of tinnitus. Annu Rev Pharmacol Toxicol 59: 291–313, 2019. doi: 10.1146/annurev-pharmtox-010818-021556. [DOI] [PubMed] [Google Scholar]
- 197.Duckert LG, Rees TS. Treatment of tinnitus with intravenous lidocaine: a double-blind randomized trial. Otolaryngol Head Neck Surg 91: 550–555, 1983. doi: 10.1177/019459988309100514. [DOI] [PubMed] [Google Scholar]
- 198.Mirz F, Pedersen B, Ishizu K, Johannsen P, Ovesen T, Stødkilde-Jørgensen H, Gjedde A. Positron emission tomography of cortical centers of tinnitus. Hear Res 134: 133–144, 1999. doi: 10.1016/s0378-5955(99)00075-1. [DOI] [PubMed] [Google Scholar]
- 199.Dobie RA. A review of randomized clinical trials in tinnitus. Laryngoscope 109: 1202–1211, 1999. doi: 10.1097/00005537-199908000-00004. [DOI] [PubMed] [Google Scholar]
- 200.Salvi R, Lobarinas E, Sun W. Pharmacological treatments for tinnitus: new and old. Drugs Future 34: 381–400, 2009. doi: 10.1358/dof.2009.034.05.1362442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Aazh H, El Refaie A, Humphriss R. Gabapentin for tinnitus: a systematic review. Am J Audiol 20: 151–158, 2011. doi: 10.1044/1059-0889(2011/10-0041). [DOI] [PubMed] [Google Scholar]
- 202.Dyhrfjeld-Johnsen J, Cederroth CR. Current clinical trials for tinnitus: drugs and biologics. Otolaryngol Clin North Am 53: 651–666, 2020. doi: 10.1016/j.otc.2020.03.012. [DOI] [PubMed] [Google Scholar]
- 203.Bing D, Lee SC, Campanelli D, Xiong H, Matsumoto M, Panford-Walsh R, Wolpert S, Praetorius M, Zimmermann U, Chu H, Knipper M, Rüttiger L, Singer W. Cochlear NMDA receptors as a therapeutic target of noise-induced tinnitus. Cell Physiol Biochem 35: 1905–1923, 2015. doi: 10.1159/000374000. [DOI] [PubMed] [Google Scholar]
- 204.d'Aldin CG, Ruel J, Assié R, Pujol R, Puel JL. Implication of NMDA type glutamate receptors in neural regeneration and neoformation of synapses after excitotoxic injury in the guinea pig cochlea. Intl J Dev Neurosci 15: 619–629, 1997. doi: 10.1016/S0736-5748(96)00116-5. [DOI] [PubMed] [Google Scholar]
- 205.Auris Medical. Press Release (Online). https://ir.aurismedical.com/news-releases/news-release-details/auris-medical-provides-business-update. [2018].
- 206.Holtmaat AJ, Trachtenberg JT, Wilbrecht L, Shepherd GM, Zhang X, Knott GW, Svoboda K. Transient and persistent dendritic spines in the neocortex in vivo. Neuron 45: 279–291, 2005. doi: 10.1016/j.neuron.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 207.Yamasoba T, Nuttall AL, Harris C, Raphael Y, Miller JM. Role of glutathione in protection against noise-induced hearing loss. Brain Res 784: 82–90, 1998. doi: 10.1016/s0006-8993(97)01156-6. [DOI] [PubMed] [Google Scholar]
- 208.Yamasoba T, Harris C, Shoji F, Lee RJ, Nuttall AL, Miller JM. Influence of intense sound exposure on glutathione synthesis in the cochlea. Brain Res 804: 72–78, 1998. doi: 10.1016/s0006-8993(98)00660-x. [DOI] [PubMed] [Google Scholar]
- 209.Kil J, Pierce C, Tran H, Gu R, Lynch ED. Ebselen treatment reduces noise induced hearing loss via the mimicry and induction of glutathione peroxidase. Hear Res 226: 44–51, 2007. doi: 10.1016/j.heares.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 210.Bulua AC, Simon A, Maddipati R, Pelletier M, Park H, Kim KY, Sack MN, Kastner DL, Siegel RM. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). J Exp Med 208: 519–533, 2011. doi: 10.1084/jem.20102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Wang W, Zhang LS, Zinsmaier AK, Patterson G, Leptich EJ, Shoemaker SL, Yatskievych TA, Gibboni R, Pace E, Luo H, Zhang J, Yang S, Bao S. Neuroinflammation mediates noise-induced synaptic imbalance and tinnitus in rodent models. PLoS Biol 17: e3000307, 2019. e3000307doi: 10.1371/journal.pbio.3000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Jentsch TJ. Neuronal KCNQ potassium channels: physiology and role in disease. Nat Rev Neurosci 1: 21–30, 2000. doi: 10.1038/35036198. [DOI] [PubMed] [Google Scholar]
- 213.Anderson LA, Hesse LL, Pilati N, Bakay WMH, Alvaro G, Large CH, McAlpine D, Schaette R, Linden JF. Increased spontaneous firing rates in auditory midbrain following noise exposure are specifically abolished by a Kv3 channel modulator. Hear Res 365: 77–89, 2018. doi: 10.1016/j.heares.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 214.Glait L, Fan W, Stillitano G, Sandridge S, Pilati N, Large C, Alvaro G, Kaltenbach JA. Effects of AUT00063, a Kv3.1 channel modulator, on noise-induced hyperactivity in the dorsal cochlear nucleus. Hear Res 361: 36–44, 2018. doi: 10.1016/j.heares.2018.01.017. [DOI] [PubMed] [Google Scholar]
- 215.Galazyuk AV, Longenecker RJ, Voytenko SV, Kristaponyte I, Nelson GL. Residual inhibition: From the putative mechanisms to potential tinnitus treatment. Hear Res 375: 1–13, 2019. doi: 10.1016/j.heares.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 216.Voytenko SV, Galazyuk AV. mGluRs modulate neuronal firing in the auditory midbrain. Neurosci Lett 492: 145–149, 2011. doi: 10.1016/j.neulet.2011.01.075. [DOI] [PubMed] [Google Scholar]
- 217.Cederroth CR, Park JS, Basinou V, Weger BD, Tserga E, Sarlus H, Magnusson AK, Kadri N, Gachon F, Canlon B. Circadian regulation of cochlear sensitivity to noise by circulating glucocorticoids. Curr Biol 29: 2477–2487, 2019. doi: 10.1016/j.cub.2019.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Maas IL, Brüggemann P, Requena T, Bulla J, Edvall NK, Hjelmborg JVB, Szczepek AJ, Canlon B, Mazurek B, Lopez-Escamez JA, Cederroth CR. Genetic susceptibility to bilateral tinnitus in a Swedish twin cohort. Genet Med 19: 1007–1012, 2017. doi: 10.1038/gim.2017.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Cederroth CR, PirouziFard M, Trpchevska N, Idrizbegovic E, Canlon B, Sundquist J, Sundquist K, Zöller B. Association of genetic vs environmental factors in swedish adoptees with clinically significant tinnitus. JAMA Otolaryngol Head Neck Surg 145: 222–229, 2019. doi: 10.1001/jamaoto.2018.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Trpchevska N, Bulla J, Prada HM, Edvall NK, Lazar A, Mehraei G, Uhlen I, Schlee W, Canlon B, Gallus S, Lopez-Escamez JA, Cederroth CR. Sex-dependent aggregation of tinnitus in Swedish families. J Clin Med 9: 3812, 2020. doi: 10.3390/jcm9123812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Tunkel DE, Bauer CA, Sun GH, Rosenfeld RM, Chandrasekhar SS, Cunningham ER Jr, Archer SM, Blakley BW, Carter JM, Granieri EC, Henry JA, Hollingsworth D, Khan FA, Mitchell S, Monfared A, Newman CW, Omole FS, Phillips CD, Robinson SK, Taw MB, Tyler RS, Waguespack R, Whamond EJ. Clinical practice guideline: tinnitus. JAMA Otolaryngol Head Neck Surg 151: S1–s40, 2014. doi: 10.1177/0194599814545325. [DOI] [PubMed] [Google Scholar]
- 222.Henry JA. Measurement of tinnitus. Otol Neurotol 37: e276–285, 2016. doi: 10.1097/MAO.0000000000001070. [DOI] [PubMed] [Google Scholar]
- 223.Brozoski TJ, Spires TJ, Bauer CA. Vigabatrin, a GABA transaminase inhibitor, reversibly eliminates tinnitus in an animal model. J Assoc Res Otolaryngol 8: 105–118, 2007. doi: 10.1007/s10162-006-0067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Guitton MJ, Dudai Y. Blockade of cochlear NMDA receptors prevents long-term tinnitus during a brief consolidation window after acoustic trauma. Neural Plast 2007: 80904, 2007. doi: 10.1155/2007/80904. [DOI] [PMC free article] [PubMed] [Google Scholar]