Abstract
Patients undergoing evaluation for solid organ transplantation often have a history of malignancy. Although the cancer has been treated in these patients, the benefits of transplantation needs to be balanced against the risk of tumor recurrence, especially in the setting of immunosuppression. Prior guidelines of when to transplant patients with a prior treated malignancy do not take in to account current staging, disease biology, or advances in cancer treatments. To develop contemporary recommendations, the American Society of Transplantation held a consensus workshop to perform a comprehensive review of current literature regarding cancer therapies, cancer stage specific prognosis, the kinetics of cancer recurrence, and the limited data on the effects of immunosuppression on cancer-specific outcomes. This document contains prognosis based on contemporary treatment and transplant recommendations for breast, colorectal, anal, urological, gynecological, non-small cell lung cancers. This conference and consensus documents aim to provide recommendations to assist in the evaluation of patients for solid organ transplantation given a history of a pre transplant malignancy.
Introduction
The primary barrier for consideration of solid-organ transplantation (SOT) in patients with pre-transplant malignancy (PTM) is the concern that immunosuppression amplifies the risk of cancer recurrence, potentially impacting post-transplant mortality. While it is clear that immunosuppression administered to SOT recipients is associated with an increased likelihood of de novo cancer1, clinical evidence on the safety of immunosuppression in the circumstance of PTM is limited.
The most utilized guidelines for the selection of patients with PTM for SOT were extrapolated from recommendations made for potential renal transplant recipients.2 In most cases, a minimum of two years between cancer treatment and SOT was advised. Two-year waiting times were recommended even for cancers with extremely low or zero risk of recurrence, such as ductal carcinoma in situ of the breast. For cancers at increased risk of recurrence, even longer wait times of two to five or greater than five years were recommended, with little or no supporting data. Historical data on transplant recipients with PTM obtained from the Israel Penn International Transplant Tumor Registry reported a 21% overall risk of cancer recurrence following SOT, and higher rates in certain, high-risk malignancies.3 This information formed the basis for previous recommendations.
Contemporary, population-based studies have reported lower cancer recurrence rates than the original registry provided4, although poorer outcomes persist in those with PTM.5,6 Recent studies also indicate a higher incidence of all-cause mortality in SOT recipients with PTM than those without, but the cause of mortality is not entirely linked to recurrence of the cancer.5,7 However, despite these increased risks, overall patient survival may still be superior to what would be anticipated without transplantation and may approach acceptable transplant-specific outcomes. In addition, newer therapies may improve outcomes for recurrences.
As improvements in cancer therapies result in better prognosis and survival, more individuals with a history of cancer are likely to present with a need for SOT. In fact, SOT in patients with PTM has increased substantially in recent decades (<1% in 1994 to 8.3% in 2016 for kidney transplant recipients).7 The risk of cancer recurrence and the possibility for worse outcome following SOT must be weighed against the benefit the patient will receive from the transplant (life-saving vs. life-prolonging), while also considering the potential alternatives (e.g. dialysis and ventricular assist devices) (Fig. 1).
Figure 1.
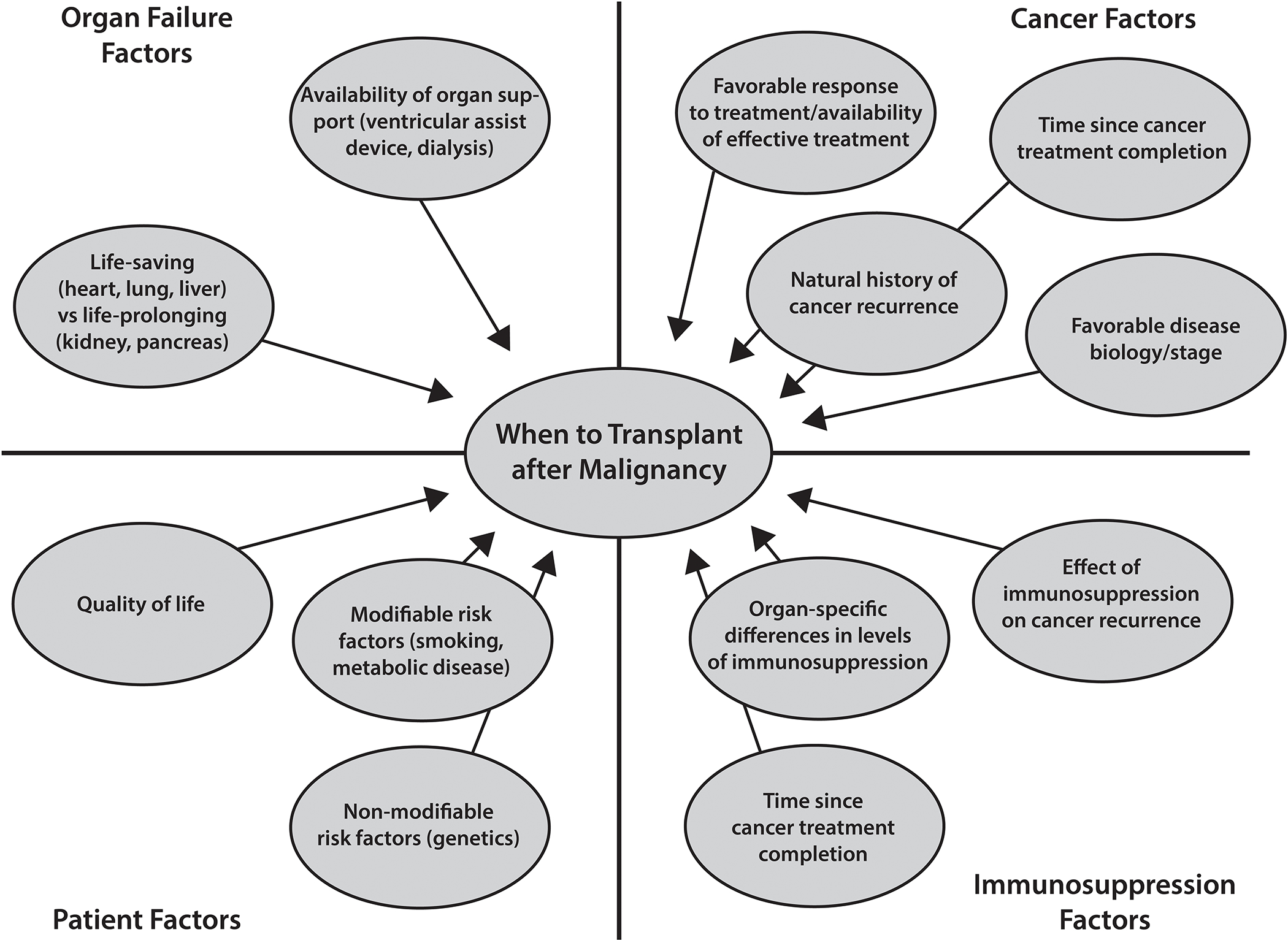
Potential factors to Consider When Evaluating a Patient with a PTM for Transplantation.
The risk of cancer recurrence may also vary depending on the organ transplanted and the immunosuppression regimen used. For example, lung recipients historically carry the greatest risk as they are often under the influence of the highest immunosuppression. Transplantation of a patient who later dies of cancer recurrence, rather than a patient without cancer, may result in loss of an organ. Therefore, it is imperative to establish reasonable and updated recommendations to assist practitioners in selecting the appropriate transplant candidates with PTM in a safe and consistent manner.
Purpose and Scope of Consensus
Our goal is to assist transplant practitioners in determining suitability and timing of transplantation after a successfully treated malignancy. The recommendations presented here are limited to commonly encountered solid organ cancers, including breast, colorectal, anal, urological, gynecological, non-small cell lung cancers. Hematological cancers and melanoma are discussed in a separate manuscript. The type of solid organ transplant needed may significantly affect recipient candidacy, due to both variability in wait list mortality and degree of immunosuppression expected post-transplant. Further, it is important to consider the limitations of this document; while comprehensive, the recommendations cannot account for every clinical situation or the needs of each individual patient.
Methods
To address the unmet needs in our field, the AST held a consensus workshop on September 29–30, 2019 in Dallas-Fort Worth, Texas. The Malignancy and Transplantation Meeting convened transplant physicians (including surgeons, medical specialists, and anesthesiologists) along with experts in surgical and medical oncology, and cancer epidemiology to review the timing of SOT after successful treatment of a PTM. The resulting recommendations are based on current literature regarding contemporary cancer therapies, cancer stage-specific prognosis, the kinetics of cancer recurrence in the general population, and the limited data on the effects of immunosuppression on cancer-specific outcomes. There are significant gaps in knowledge and most of the data are extrapolated from the general population, therefore, the authors have made the best recommendations with these limited data.
There were over 30 participants in attendance at the meeting, where three experts in each of the fields of breast, colorectal, urological, gynecological, and lung cancer presented summaries of these diseases and their relation to transplantation. After the presentations, the opinion of the oncology experts within each field were discussed as a panel and consensus agreements were then made (modified Delphi method), with the general consideration that a five-year cancer survival rate of near 80% to be an acceptable benchmark before proceeding with transplantation. The stage-based survival rate, disease biology, and recurrence kinetics were considered when making waiting time recommendations. Writing groups for each cancer consisted of the three cancer-specific experts and two or more transplant physicians.
This is a consensus document rather than a guideline; thus, levels of evidence were not graded. Instead, a comprehensive literature review and consensus expert opinion are presented. This manuscript is a work product of the American Society of Transplantation’s Liver and Intestinal Community of Practice. The recommendations are not to omit the valuable input oncologists play in appropriately selecting those to be transplant candidates, and we encourage ongoing discussions with our oncology colleagues.
Breast Cancer
Background and Staging
Breast cancer encompasses a group of genetically distinct diseases, each with significantly variable approaches to management, treatment, and prognosis. Over 50,000 new cases of ductal carcinoma in situ (DCIS) and 250,000 new cases of invasive disease are diagnosed annually in the US.8 Given the excellent prognosis for many women with early stage breast cancer, it is reasonable to assume that the treatment for breast cancer will often result in “cure”.9–11 Currently, one in 38 women will die from breast cancer in the United States, but this number is decreasing.9 The latest American Joint Committee on Cancer (AJCC) staging manual recently refined prognostic staging groups by including the traditional tumor, node, and metastasis, as well as tumor biomarkers (ER=estrogen receptor, PR=progesterone receptor, and HER2=human epidermal growth factor receptor 2), tumor grade, and tumor genomic testing (e.g., Oncotype DX). These changes have led to more women being diagnosed with stage I disease.12
Ductal Carcinoma in situ
DCIS should be considered a precursor to breast cancer. The traditional measures for assessing risk of recurrence for DCIS are similar to those used in invasive breast cancer: age, residual tumor/margin width, grade, histology, tumor size, and menopausal status. None of these characteristics, however, provides a quantitative assessment of recurrence risk, leading to a significant gap in our understanding of the clinical significance of a diagnosis of DCIS and optimal approaches to treatment.
Therapy
Changes in treatment paradigms have made the algorithm for prognostication much more diverse.12,13 Most women with non-metastatic breast cancer will undergo breast surgery and surgical evaluation of the axillary nodes. For women who undergo a partial mastectomy, most will also receive radiation therapy, while post-mastectomy radiation is often reserved for those with large tumors and positive nodes. If the tumor is hormone receptor positive, endocrine therapy (such as tamoxifen or aromatase inhibitors) is typically recommended for 5–10 years. Chemotherapy is the most variable component of treatment, and numerous factors are considered, including tumor size, nodal status, receptor status, and genomic testing.
Transplant Recommendations
Low Risk Breast Cancer
Several tools can help predict which women are most likely to develop recurrences and potentially die from their disease.14–16 For example, Oncotype DX stratifies women with early stage, ER+/HER2− breast cancer into subgroups that are associated with risk of recurrence. For women in the low risk subgroup, their five-year risk of recurrence (distant or local-regional) is <2%.14,17,18 In contrast, women with ER− disease have a significant spike in breast cancer deaths within the first 2–3 years (peak annual mortality rate of 7.5% at 1–2 years), but that peak annual mortality rate sharply declines to 4% or less by 4 years after diagnosis.19 In general, better prognoses are associated with negative nodes, small tumor size (<1 cm), and stage I disease.19
The consensus recommendation is that women with low risk disease such as DCIS and stage I breast cancer, should be considered transplant candidates after completion of all standard treatments (such as surgery, radiation, and/or non-endocrine systemic therapy), with no additional waiting time (Table 1). Endocrine therapy is often continued for 5–10 years after completion of other therapies and should not affect the decision on when to transplant, as these medications are well tolerated with few significant side effects. For women with stage II disease, the five-year overall survival is 78–83%.18 Therefore, these patients could be considered for transplantation after a disease-free interval of 1–2 years after all treatments have been completed. Prior to transplant, obtaining a mammogram is recommended.
Table 1.
Recommended wait time for SOT candidates with a prior history of breast cancer.
| Risk/Stage | 5-year Disease Specific Survival18,19 | Time Interval to Transplant | Additional Considerations |
|---|---|---|---|
|
LOW RISK DCIS Stage I |
97–99% | No wait time necessary* | -Hormone receptor negative disease may have a slightly higher risk of recurrence in the first 2–3 years |
|
INTERMEDIATE RISK Stage II |
90–99% |
1–2 years
NED* |
-Hormone receptor negative disease may have a slightly higher risk of recurrence in the first 2–3 years |
|
HIGH RISK Stage III |
66–97% |
3–5 years
NED* |
-Hormone receptor negative disease may have a slightly higher risk of recurrence in the first 2–3 years -Inflammatory breast cancer likely has a higher risk of recurrence and worse survival |
|
PROHIBITIVE RISK Stage IV |
32–38% | Not a SOT candidate |
After completion of all standard treatments. Endocrine therapy does not need to be completed prior to transplant, as this is an oral medication that is fairly well tolerated with few serious side effects and often continues for 5–10 years.
Standard oncologic treatments are based on those recommended in the NCCN (National Comprehensive Cancer Network) Breast Cancer guidelines (www.nccn.org). Breast cancer stages are based on the prognostic stage groups specified in the AJCC’s Staging Manual, 8th edition. Anatomic stage groups are not necessarily equivalent to the corresponding prognostic stage groups and should not be applied here. DCIS: ductal carcinoma in situ, NED: no evidence of disease
High Risk Breast Cancer
Patients with advanced stage breast cancer (stage III) have five-year survival rates ranging from 50–70%18. However, most recurrences will occur within the first three years. As such, after a disease-free interval of 3–5 years after all treatments have been completed, these patients could be considered as transplant candidates.
Inflammatory breast cancer represents one of the most aggressive presentations of breast cancer.20 Median survival for women with inflammatory breast cancer is approximately 2.9 years, and the overall five-year survival is <55%.21,22 Similarly, all women with metastatic disease have a poor prognosis, with a median overall survival of two to three years.23–25 Therefore, these patients generally should not be considered as transplant candidates.
Colorectal Cancer
Background and Staging
Colorectal cancer (CRC) is the third most common cancer worldwide, and several factors determine its treatment and prognosis.26 These factors are largely contained in the AJCC staging criteria.27 Recently, the AJCC staging classification has been refined to account for new prognostic factors and subcategorization of the stage groups, with an emphasis on histopathologic and molecular features. For example, molecular classification of CRC has identified defects in DNA mismatch repair, and epigenetic DNA hypomethylation and CpG Island hypermethylation. These distinctions are important, as mismatch repair defect tumors have been associated with markedly improved prognosis, whereas CpG Island hypermethylation tumors associated with BRAF mutations have markedly worse survival.28,29 However, additional prognostic factors that are not currently included in the overall staging classification include presence of tumor deposits, perineural invasion, lymphatic or vascular invasion, high-grade, or signet ring and mucinous histology. The most recent addition to the list of prognostic classifiers is circulating tumor DNA (ctDNA). In the setting of advanced disease, ctDNA is emerging as a highly sensitive marker of treatment response and holds great promise for the detection of minimal residual disease.30,31 Such information may have great utility for post-surgical treatment decision-making, including transplantation.
Therapy
Most newly diagnosed CRC patients present with locoregional disease stage. For these patients, surgical resection remains central to their treatment. Multimodal treatment with less invasive approaches results in better outcomes. Following surgery for colon cancer, survival is excellent for early stage tumors (91% 5 year survival), and adjuvant chemotherapy is recommended for those patients with stage III disease as well as patients with high-risk stage II disease. However, following curative-intent surgical treatments, between 5–40% of patients in this intermediate group will develop cancer recurrence, with approximately 80% identified within the first 3 years, and nearly all recurrences identified by 5 years upon completion of treatment.32
Historically, rectal cancer was treated with abdominoperineal resection until sphincter sparing procedures became refined and treatment included neoadjuvant therapies.33 At this time, there is increasing interest in total neoadjuvant therapy to improve systemic disease management and potential for organ preservation, i.e., treatment without surgery at all. With the introduction of non-operative treatment of rectal cancer, transplant considerations have become more challenging in these patients, as there is increasing confusion about when the patient with rectal cancer is considered “cancer free”. Today, patients treated non-operatively for rectal cancer undergo surveillance for at least five years.34
Transplant Recommendations
There is a paucity of data on transplantation of patients with a known history of treated CRC. In 1993 and 1997, Israel Penn reported on 38 and 53 patients with CRC who underwent transplantation, respectively. The recurrence rate in these studies was 21%, with 63% resulting in death. In addition, late recurrences (>5 years post-cancer treatment) were common (27%).35,36 Of patients with recurrence, only 13% had been treated for their CRC within two years prior to transplantation, while the remaining 87% of recurrences occurred in patients that were transplanted 3–6 years post-malignancy.35 This delay in recurrence is concerning, considering that most recurrences in the general population occur within three years, with very few (<1%) occurring >5 years post-cancer treatment.37 However, these data derive from a different cancer treatment and transplant era and are limited by unknown complete staging.
Given modern treatment options and improved prognosis in the current era, expert consensus suggests that a patient with a history of fully treated colon cancer may be considered for transplantation within 1–2 years for low risk disease and 3–5 years for higher risk disease (Table 2). A patient with a history of surgically treated rectal cancer may be considered for transplantation with similar timeframes (Table 3). Patients who have not undergone surgical resection will require multidisciplinary discussion of the individual scenario.
Table 2.
Recommended wait time for SOT candidates with a prior history of colon cancer.
| Risk/Stage | Recurrence Free Survival 5-year41,46 | Time Interval to Transplant | Additional Considerations |
|---|---|---|---|
|
LOW RISK Stage I (T1 or T2, N0, M0) |
91% | 1 year |
Low risk features: - MSI without BRAF mutation High risk features: - LVI or PNI - Mucinous or Signet Histology - Poorly differentiated histology - Bowel obstruction - Tumor perforation - <12 lymph nodes examined *Tumor deposits considered as N+ disease *Consider chemotherapy prior to transplantation for high-risk stage II disease *Patients with stage III disease should complete chemotherapy |
|
LOW INTERMEDIATE RISK Stage II (T3, N0, M0) |
72% | 2 years, consider longer if high-risk features present | |
|
HIGH INTERMEDIATE RISK
Stage II (T4, N0, M0) Stage III (Any T, N+, M0) |
3 years, 5 years if high-risk features present | ||
|
HIGH RISK Stage IV (Any T, Any N, M+) |
13% | 5 years NED | SOT not recommended prior to 5 years; see special consideration regarding resectable CRC metastasis |
RFS: recurrence free survival; LVI: lymphovascular invasion; PVI: perineural invasion; MSI: microsatellite instability; CT: computed tomography; CAP: chest, abdomen and pelvis; CEA: Carcinoembryonic antigen; NED: no evidence of disease
Table 3.
Recommended wait time for SOT candidates with a prior history of rectal cancer.
| Risk/Stage | Recurrence Free Survival 5-year41,46 | Time Interval to Transplant | Additional Considerations |
|---|---|---|---|
|
LOW RISK Stage I (T1 or T2, N0, M0) Full oncologic resection |
85%–88% | 1 year, consider 2 years if high-risk features present |
Low risk features: - MSI without BRAF mutation - Upper 1/3 rectum or rectosigmoid High risk features: - LVI or PNI - Mucinous or Signet Histology - Poorly differentiated histology - Bowel obstruction - Tumor perforation - <12 lymph nodes examined - Lower 1/3 of rectum - Incomplete mesorectal excision *Tumor deposits considered as N+ disease *Patients with stage II & III disease should complete trimodaility treatment (chemoradiotherapy, surgery and chemotherapy) unless elimination of one of these is deemed appropriate after multidisciplinary discussion *For patients who have undergone preoperative radiotherapy, response to treatment is highly prognostic. Complete and nearly complete responders have much lower risk for recurrence than those with poor response |
|
LOW INTERMEDIATE RISK Stage I (T1, N0, M0) Local Excision |
78%–88% | 2 years | |
|
HIGH INTERMEDIATE RISK Stage II (T3 or T4, N0, M0) Stage III (Any T, N+, M0) |
70% | 3 years, 5 years if high-risk features present | |
|
HIGH RISK Stage IV (Any T, Any N, M+) |
14% | 5 years NED | SOT not recommended prior to 5 years; see special consideration regarding resectable CRC metastasis |
RFS: recurrence free survival; LVI: lymphovascular invasion; PVI: perineural invasion; MSI: microsatellite instability; CT: Computed tomography; CAP: chest, abdomen and pelvis; CEA: Carcinoembryonic antigen; NED: no evidence of disease
Special consideration for colorectal liver metastasis and transplantation
Recent advances in medical and surgical treatments of colorectal liver metastases (CRLM) have allowed for an important expansion in resectability and life expectancy in this population.38 For patients with insufficient liver remnant (precluding liver resection) and absence of extra-hepatic involvement, liver transplantation may be an option since the total hepatectomy will remove all viable disease.39,40 Recently published data show that with strict selection criteria, overall survival after liver transplantation at one and five years are 100% and 83%, respectively.41 Therefore, in selected patients, there appears to be a possible benefit of liver transplantation for unresectable CRLM in select cases. This data and experience is limited and clinical trials are ongoing.
Anal Cancer
Squamous cell anal carcinoma accounts for a small (<3%) proportion of digestive system cancers. Anal cancer risk in transplant patients is of particular interest, due to the relationship between immunosuppression and the inability to clear human papilloma virus (HPV) infections.42 No data exist on patients with preexisting anal cancer at time of transplantation, but data from the general population suggests a 5-year survival below 70% with invasive anal squamous cell cancer.43 Considering the risk of aggressive anal lesions after immunosuppression, the consensus expert panel recommends transplantation can proceed in patients with a history of invasive, HPV related anal cancer after a 5-year disease-free interval. Patients with non-invasive anal lesions require careful consideration before transplanting due to the increased risk for progression of these lesions. Aggressive surveillance practice would be warranted after transplant.
Urological Malignancies
Prostate Cancer
Autopsy studies have identified prostate cancer in 20–30% of men in their 30s, 30–50% in their 50s and 50–70% in their 70s, with 50% being ‘high-grade’ (Gleason≥7).44 Despite the high prevalence, only 3% of US men die from prostate cancer and the overwhelming majority of these cancers are never destined to become clinically evident. Surveillance of newly diagnosed low or intermediate-risk cases without immediate treatment is common and associated with a 10-year cancer-specific survival of >95%.45
In many large studies of men with solid organ transplants, there is no worrisome signal that immunosuppression increases the risk of a clinically meaningful prostate cancer,46–48 recurrence following previous treatment,49 or five-year cancer-specific mortality (<1%) after a post-transplant diagnosis of prostate cancer.49,50 Accordingly, approximately two-thirds of kidney transplant programs allow surveillance of prostate cancer prior to transplantation.51 Population-based data suggest that surveillance in men with prostatae cancer who are being considered for transplant has become more common, without any apparent long-term adverse cancer-specific consequences.47
For men diagnosed with prostate cancer during a transplant evaluation and electing treatment, multinomial predictive tools (e.g., cancer of the prostate (CAPRA), nomograms) are available to predict the likelihood of cancer-specific death over the next 15 years. Even for the highest possible risk profile within ‘intermediate-risk’ prostate cancer (PSA=19 ng/ml, Gleason 4+3=7, T3a, margin-positive, node-negative), likelihood of a cancer-specific death within 15 years of treatment is <5%. Our recommended waiting time and management guidelines after a diagnosis of prostate cancer are listed in Table 4.
Table 4:
Recommended wait time for SOT candidates with a prior history of prostate cancer.
| Risk/Stage | Survival60, 62, 64 | Time Interval to Transplant | Additional Considerations |
|---|---|---|---|
|
| |||
| VERY LOW RISK | <1% risk of mets/death over 15 years | None | Surveillance is strongly recommended |
| - PSA< 10 ng/ml | |||
| - 3 or fewer cores of Gleason 6 (grade group 1); no greater than 50% of individual core | Extenuating circumstances may require treatment | ||
| - T1c-T2a | |||
|
| |||
| LOW RISK | ~2–3% risk of mets/death over 15 years | None | Surveillance is strongly recommended |
| - PSA< 10 ng/ml | |||
| - Gleason 6 (not meeting very low-risk criteria) | Extenuating circumstances may require treatment | ||
| - T1c-T2a | |||
|
| |||
| LOW-VOLUME INTERMEDIATE RISK | <5% risk of mets/death over 15 years | If surveillance, no wait time | Surveillance or treatment, depending on patient and cancer characteristics |
| - One of the following criteria: PSA > 10 ng/ml, Gleason 7 (grade group 2 or 3), T2b | If treatment initiated, and nomogram (www.nomograms.org) predicts cancer-specific death over the next 15 years <10%, no wait time | ||
|
| |||
| HIGH-VOLUME INTERMEDIATE RISK, HIGH RISK or VERY HIGH RISK | 20–70% risk of mets/death over 15 years | If treatment initiated, and nomogram predicts cancer-specific death over the next 15 years <10%, no wait time | Treatment |
| - PSA >20 ng/ml or high-volume Gleason 7 or any Gleason 8–10, T3 | |||
|
| |||
| METASTATIC CASTRATION-SENSITIVE | Median survival ~ 5–6 years | If stable disease for 2 years with prolonged estimated life expectancy, may consider transplant | Best systemic therapy +/− local treatment |
|
| |||
| METASTATIC CASTRATION-RESISTANT | Median survival 2–3 years | Not a SOT candidate | Best systemic therapy |
PSA: Prostate specific antigen
Renal Cell Carcinoma (RCC)
The majority of renal masses detected in patients being considered for transplantation are incidental and ≤4cm, considered a small renal mass (SRM).52 Most SRMs are RCC (75–80%), the majority are low grade (85%), and risk of metastasis at presentation is <2%.53 Following treatment of a SRM, the three-year probability of metastases is ≤2%.53 Nephrectomy remains the standard approach for SRM treatment for patients on a transplant waiting list. However, active surveillance of SRMs (solid and cystic) is a safe, standard-of-care option in the general population.54,55 The majority demonstrate slow (<0.3cm/year) or no growth, low risk of future metastases (1–2%), and low rates of stage progression (<10%).55 Long-term safety data of surveillance in patients being considered for transplant is lacking and nephrectomy (radical/partial) remains the most popular treatment prior to transplantation.56 Biopsy is often helpful to guide management decisions since a significant minority of SRMs are benign or cancers with negligible metastatic potential. Tumor size predicts probability of cancer and aggressive histology.57
Nephrectomy in patients with organ failure has significant risk of post-operative complications that may outweigh the benefit of surgery, in light of the low risk of disease progression.58 Therefore, in the context of a life-saving transplant, (e.g., heart, lung, liver) surveillance should be considered in SRM (<3cm). Following a successful transplant and outcome, the post-transplant nephrectomy can be performed 3–6 months post-transplant with superior outcomes.58 In non-immunosuppressed patients on surveillance, the American Society of Clinical Oncology guidelines consider tumor growth >0.5 cm/ year or tumor size >4 cm to be an indicator for intervention.52 In patients on surveillance awaiting heart/lung/liver transplant, and in patients with ablated renal tumors, no data exist on whether increased immunosuppression has detrimental effects. Consequently, recommendation is for definitive management post-transplantation, and nephrectomy of ablated renal masses with enhancement or growth. Table 5 outlines the disease-free survival by stage as well as our recommendations on wait time following treatment.57,59,60
Table 5:
Recommended wait time for SOT candidates with a prior history of renal cell carcinoma.
| Stage | Recurrence free survival 5-year69,73,74,75 | Time Interval to Transplant |
|---|---|---|
|
| ||
| T1a (≤4cm), N0, M0 | 95–98% | No wait time |
|
| ||
| T1b (>4cm ≤7cm), N0, M0 | 91% for FG 1/2 | FG 1–2: no wait time |
| 80–82% for FG 3/4 | FG 3–4: 1–2 years | |
|
| ||
| T2 (7–10cm), N0, M0 | 80% | 2 years |
|
| ||
| T3, N0, M0 | 43–80% | Minimum of 2 years, then reassess |
|
| ||
| T4, N0, M0 | 28–55% | Minimum of 2 years, then reassess |
|
| ||
| Any T, Node positive, Metastatic disease | 0–32% | Not a candidate (if solitary metastasis + resected, tumor board discussion on candidacy) |
|
| ||
| Any T with sarcomatoid and/or rhabdoid histologic features | 15–27% | Not a SOT candidate |
|
| ||
| Collecting duct or Medullary RCC | <10% | Not a SOT candidate |
RCC: renal cell carcinoma; FG: Fuhrman grade (Grade 1: Inconspicuous nucleoli at ×400 magnification and basophilic, Grade 2: Clearly visible nucleoli at ×400 magnification and eosinophilic, Grade 3: Clearly visible nucleoli at ×100 magnification, Grade 4: Extreme pleomorphism or rhabdoid and/or sarcomatoid morphology)
Bladder Cancer
Five-year survival with bladder cancer is 77%, with 10-year survival at 70%.61 Although the recurrence rate is extremely high for patients with localized bladder cancer, the progression is extremely low. Therefore, the proposed wait times for patients with non-muscle invasive bladder cancer (NMIBC) are based on the understanding that most recurrences can be salvaged with local resection, but since progression is rare, the bladder can remain intact. Patients with low risk NMIBC should undergo surveillance for at least six months to determine recurrence kinetics (Table 6). If there is no recurrence within six months, transplant can be considered, as the risk of progression is extremely low (ranging from 1–2% over five years) despite a recurrence rate of up to 28% at five years.62 For patients with intermediate risk NMIBC, the risk of progression remains low, although the risk of recurrence is slightly higher. Again, recurrences can be managed, and a wait time of six months is recommended. For patients with high risk NMIBC, the risk of progression is significantly higher upon diagnosis (approximately 18% at five years),63,64 and the timing of transplant remains controversial. However, a waiting time of at least two years is generally advised after local control and intravesical therapy.49 Based on conditional recurrence/progression models, the risk of recurrence is only 7–18% and the risk of progression is only 4–6% if there is no evidence of disease for two years after diagnosis.62
Table 6:
Recommended wait time for SOT candidates with a prior history of bladder cancer.
| Bladder Cancer History | 2-year Local Recurrence from Baseline Trans Urethral Resection of Bladder Tumor77, 80, 81 | Time Interval to Transplant |
|---|---|---|
| NMIBC low risk* | 19% | 6 months |
| Intermediate risk** | 39% | 6 months |
| high risk*** | 38%*** | 2 years |
| MIBC, post radical cystectomy | 25–37% | 2 years |
| MIBC, post chemoradiation | 25–30% (10 year) | Not a SOT candidate |
NMIBC: non-muscle invasive bladder cancer; MIBC: muscle invasive bladder cancer
Low risk* - solitary, ≤ 3 cm, low grade, Ta tumor, absence of carcinoma in situ (CIS)
Intermediate risk** - solitary tumor > 3 cm, recurrence within 12 months with low grade Ta tumor, multifocal low-grade Ta tumor, low grade T1 tumor, or high-grade tumor < 3 cm
High risk*** - any CIS, high grade Ta tumor > 3 cm, high grade T1 tumor, multifocal high-grade Ta tumor, any recurrent high-grade Ta tumor, CIS, variant histology, lymphovascular invasion, high grade prostatic urethral involvement, recurrence after BCG intravesical therapy. Although 2-year recurrence rate is lower than intermediate risk, the progression rate to muscle invasion is higher.
For patients with muscle-invasive bladder cancer (MIBC) treated with radical cystectomy, most recurrences occur within two years of surgery and can either occur locally, within the remaining urinary tract, or be metastatic. Beyond two years, the recurrence rate is low65 and, therefore, consideration may be given to transplantation in patients with at least no evidence of disease two years after radical cystectomy. In fact, a two-year disease-free survival rate is an adequate surrogate for five-year overall survival.66 However, in patients with MIBC treated with a bladder sparing approach utilizing chemoradiation, there remains a substantial lifetime risk of local recurrence with NMIBC (30%) or MIBC (25%). Therefore, these patients should be considered for solid organ transplantation on a case-by case basis.
Gynecologic cancers
Background and Staging
Gynecologic cancers have impacted over 100,000 women in the US in 2019, and will be the cause of death in over 33,000.61 Among these cancers, those emanating from the uterus are the most common, but cancers of the ovary remain the most fatal. The incidence of lower genital track cancers in women is lower but still was the cause of death in almost 7000 women in 2019. Unlike most solid tumors, these cancers are staged using the International Federation of Gynecologic Oncology classification, which relies on surgical findings and has been consistently demonstrated to be prognostic.
Therapy
For women with newly diagnosed high-risk stage IA disease to IIIC ovarian cancer, curative treatment requires surgical therapy and adjuvant chemotherapy. The goal of surgery is complete resection of disease; when that is not possible, neoadjuvant chemotherapy is indicated.67 For women with a mutation in BRCA1 or BRCA2 and those whose tumor shows evidence of homologous recombination deficiency, data support the use of further treatment beyond chemotherapy, using a poly(ADP) ribose phosphorylase (PARP) inhibitor.68,69
For women with endometrial disease, the vast majority will be diagnosed with low stage, grade 1 endometrioid cancer.70 These cancers are most often cured with surgical treatment alone, with radiation therapy reserved for certain high-risk features, such as lymphovascular invasion or deep myometrial invasion.71 Women with grade 2 or 3 endometrioid or serous carcinomas may present with later stages of disease. These patients will often require multi-modality therapy for curative intent treatment, which may consist of surgery, radiation therapy, and/or adjuvant chemotherapy.72 The Cancer Genome Atlas has led to the recognition of at least four clinically distinct phenotypes of endometrial cancer: DNA-polymerase-ε (POLE) ultramutated; microsatellite instability hypermutated; copy-number low; and copy-number high. Among these phenotypes, POLE-mutant tumors (comprising approximately 10% of endometrioid tumors) appear to be associated with significantly better progression-free survival, while those with copy-number high tumors have the least favorable prognosis.73
For women with cervical cancer, surgery is reserved for those without evidence of bulky cervical disease (primary cervical lesion 4cm or larger) or other advanced features (e.g., local invasion beyond the uterus). For these women, surgery can be curative, though adjuvant therapy may be indicated if high-risk features are present.74,75 For those with locally advanced disease, chemoradiation is the standard of care.76
Transplant Recommendations
There is minimal literature on survival and risk of cancer recurrence after transplant in patients with pre-transplant gynecological malignancy.6 Recommendations for the most common types of endometrial, ovarian, and cervical cancer were stratified by the risk of recurrence: low, intermediate, and high (Table 7).
Table 7:
Recommended wait time for SOT candidates with a prior history of gynecological cancer.
| 5-year Recurrence Risk92,93,94 | Type and Stage | Time Interval to Transplant |
|---|---|---|
|
| ||
| LOW RISK | Stage IA/IB, grade 1–2 endometrial cancer without lymph-vascular space invasion |
No waiting period after completion of primary treatment |
| <5% risk of recurrence | Stage IA/IB/IC Grade 1–2 epithelial ovarian cancer |
|
| Stage IA1, IA2 squamous/adenocarcinoma of the cervix | ||
|
| ||
| INTERMEDIATE RISK | Stage I/II endometrial cancer + risk factors* |
2–3 years after completion of treatment |
| 5–15% risk of recurrence | Stage IB squamous/adenocarcinoma of the cervix | |
|
| ||
| HIGH RISK | Serous, clear cell, or carcinosarcoma of uterus (All stages) Stage III grade 1–3 endometrioid cancer of the uterus |
5 years after completion of treatment |
| >30% risk of recurrence | Stage II/III epithelial ovarian cancer |
|
| Stage II/III squamous cell/adenocarcinoma cervical cancer | ||
|
| ||
| VERY HIGH RISK | Stage IV endometrial cancer (all grades) | Not a SOT candidate |
| >80% chance of recurrence | Recurrent or metastatic endometrial cancer |
|
| Stage IV epithelial ovarian cancer (any grade) | ||
| Recurrent ovarian cancer |
||
| Stage IV squamous cell/adenocarcinoma of the cervix Metastatic or recurrent cervical cancer |
||
Risk factors: Older age, lymph-vascular space invasion, grade 2 or 3 endometrioid, deeply invasive tumor
Patients at low risk of recurrence can be considered at any time after completion of primary treatment. Patients at intermediate risk of recurrence have a five-year disease-specific survival that exceeds 90%, with the greatest risk of disease recurrence in the first two years.77 As a result, one should consider transplant if no evidence of disease at least 2–3 years after completion of therapy. Patients at high risk of recurrence include patients with advanced uterine ovarian or cervical cancer. Although some patients with ovarian cancer are cured, more than half will relapse in the first two years of follow-up.78 However, women who are candidates for a Poly (ADP) ribose phosphorylase (PARP) inhibitor can extend progression-free survival by three years or longer with maintenance therapy.68,69 For women with high-risk endometrial cancers, approximately 40% will relapse within the first three years.79 For women with stage III cervical cancer, the rate of progression free survival is 80% at four years.74 Taken together, transplant should only be considered if the patient is without disease recurrence for at least 3–5 years after primary treatment.
Non-small Cell Lung Cancer
Background and Staging
Non-small cell lung cancer (NSCLC) remains the leading cause of cancer-related mortality in the United States, with more annual deaths than breast, prostate, and colorectal cancers combined.80 While curative therapy remains elusive in advanced disease, early stage disease can be cured by surgical resection and/or radiation therapy. In a large study of over 23,000 patients diagnosed with NSCLC between 1996–2007, 16.4% were alive 5 years following diagnosis.81 This increases the prospect of a NSCLC survivor seeking a solid organ transplant. NSCLC staging is based on tumor size and location, extent and location of lymph node involvement, and presence of distant metastases. Molecular information is not currently factored into the AJCC 8th edition staging manual, but may impact precision-based risk stratification in the future.82
Therapy
For early-stage NSCLC, surgical resection is the preferred strategy.83 Adjuvant chemotherapy is recommended to treat micro-metastatic disease for some stage IB tumors (≥4cm) and for all stage II and III NSCLC. While adjuvant therapy has traditionally consisted of chemotherapy and/or radiation therapy, immunotherapy in the form of checkpoint blockade is rapidly evolving, but remains in clinical trials currently.84
Stage III NSCLC encompasses a heterogeneous group of patients. Patients with limited nodal (N1) involvement may be candidates for upfront surgical resection followed by adjuvant chemotherapy and/or radiation. Those with more advanced nodal (N2) involvement are treated with neoadjuvant therapy (chemotherapy or chemoradiotherapy) prior to surgery, given improved survival with this approach.85 Patients with more advanced nodal (N3) involvement are generally not considered surgical candidates, but are treated with chemoradiotherapy followed by consolidation immunotherapy. Other modalities, such as stereotactic body radiation therapy (SBRT) or hypo-fractionated radiation therapy, can be utilized for patients unable to tolerate resection.
Most recurrences in NSCLC occur in the first two years following definitive treatment, however, recurrence can occur as far out as five years in as many as one third of patients.86,87 Additionally, a second primary lung cancer occurs at a rate of about two per 100 patient years88. Local recurrence after SBRT is rare and will generally occur in the first two years after treatment. The most common pattern of failure is development of distant disease. Most patients treated with SBRT (60–100%) will have radiographic changes that range from diffuse consolidation to patchy ground glass opacities89. Thus, assessing for local recurrence on imaging can be difficult. PET can differentiate benign radiographic changes from possible tumor recurrence but inflammatory changes from SBRT can be FDG avid for more than 12 months after therapy90. Tissue should be obtained prior to transplant consideration if a lesion remains suspicious.
Pre-existing lung cancer may not be diagnosed before lung transplant due to the overlapping radiographic findings of cancer and end-stage lung disease. The overall incidence of lung cancer in explanted lungs has increased to 2.5% in recent years.91 A retrospective institution review of explanted lungs found the median survival time for those with node negative disease (stage I NSCLC) was 27 months, and those with node positive disease (advanced NSCLC) had a median survival of seven months.92
Transplant Recommendations
Deciding whether a patient can be listed for transplant following NSCLC diagnosis depends on the stage of disease, history of curative therapy, and, for thoracic transplant recipients, the extent of complexity in the thorax due to prior radiation and/or surgery. Although lung transplant guidelines seem to suggest a five-year observation window,93 there are some specific considerations for NSCLC that inform the selection process for solid organ transplant candidacy (Table 8). The main message from this table is that early stage disease that has responded to treatment may be considered for transplantation after three years with significant caution. It is also worth noting that the effects of checkpoint inhibition pre-transplant may have unintended immunological consequences post-transplant.94 Furthermore, the cancer control/remission through the use of checkpoint inhibitors may dissipate and lead to relapse when immunosuppression is introduced after transplantation. There are limited data regarding the timing of or the use of checkpoint inhibitors prior to transplantation, however, it is an area of interest and currently under investigation. In addition, checkpoint inhibitors use in the post-transplant patient population is being considered in selected patients. Recently, two systematic reviews have summarized the use of checkpoint inhibitor therapies for treatment of skin, liver and lung cancers after kidney, heart, or liver transplantation.95,96 Although beyond the scope of this consensus review, these studies highlight the consideration that the immunological checkpoint inhibition for cancer therapy must be weighed against the risk of organ rejection and potential graft loss.
Table 8:
Recommended wait time for SOT candidates with a prior history of lung cancer.
| Stage | Tumor and Node | 5-Year Survival (%)101,102 | Work-up Pre-SOT | Time Interval to Transplantation | Additional Considerations |
|---|---|---|---|---|---|
| I | T1aN0 | 92 | PET-CT; consider biopsy post SBRT | ≥3 years | |
| T1bN0 | 83 | PET-CT; consider biopsy post SBRT | ≥3 years | ||
| T1cN0 | 77 | PET-CT; consider biopsy post SBRT | 3–5 years | 5-year recurrence-free survival is safest | |
| IB | T2aN0 | 68 | PET-CT | 5 years | |
| IIA | T2bN0 | 60 | PET-CT | 5 years | |
| IIB | T3N0 | 53 | PET-CT | 5 years | |
| IIIA | 36 | PET-CT | 5 years | Special caution with N2 disease | |
| IIIB | 26 | N/A | N/A | Not a SOT candidate | |
| IIIC | 13 | N/A | N/A | Not a SOT candidate | |
| IVA | 10 | N/A | N/A | Not a SOT candidate | |
| IVB | 0 | N/A | N/A | Not a SOT candidate |
SOT: solid organ transplantation; PET-CT: positron emission tomography - computed tomography; SBRT: stereotactic body radiation therapy
Conclusions
Pre-transplant malignancy is increasingly common in patients with end-stage organ disease undergoing evaluation for SOT and can affect post-transplant outcomes. Given the advances in the contemporary treatment of cancer with improved patient survival, an updated consensus document on when to transplant patients with PTM was deemed a high priority by the AST. Recognizing the paucity of data surrounding the recurrence of solid organ malignancies after transplantation, this conference and consensus document aimed to update the recommendations for proceeding with SOT given a history of a PTM. In order to improve the strength of these recommendations, future goals are to create a multi-institutional database to collect cancer- and transplant-specific outcomes on patients transplanted using these recommendations. In addition, future areas of research should focus on appropriate cancer surveillance and decreasing modifiable risk factors for cancer recurrence after transplant in a patient with a PTM.
Acknowledgements
The authors, on behalf of the American Society of Transplantation, thank Sanofi Pharmaceuticals for generously supporting the Malignancy and Transplantation Meeting, held on September 29 – 30, 2019, in Dallas-Fort Worth. The conference sponsor did not have any role in the study design, collection of literature, interpretation of data, the writing of the report, or the decision to submit the paper for publication. We would also like to thank Dr. Eric Engels for his contribution and critical feedback. In addition, we would like to thank Madeline Hall and the UW Department of Surgery Communications & Marketing division for assistance with the figure creation.
Abbreviations:
- (AJCC)
American Joint Committee on Cancer
- (AST)
American Society of Transplantation
- (ctDNA)
Circulating tumor DNA
- (CRC)
Colorectal cancer
- (CRLM)
Colorectal liver metastases
- (POLE)
DNA-polymerase-ε
- (DCIS)
Ductal Carcinoma in situ
- (ER)
Estrogen receptor
- (HR)
Hazzard Ratio
- (HER2)
Human epidermal growth factor receptor 2
- (HPV)
Human papilloma virus
- (MIBC)
Muscle-invasive bladder cancer
- (NMIBC)
Non-muscle invasive bladder cancer
- (NSCLC)
Non-small cell lung cancer
- (PARP)
Poly (ADP) ribose phosphorylase
- (PTM)
Pre-transplant malignancy
- (PR)
Progesterone receptor
- (RCC)
Renal cell carcinoma
- (SRM)
Small renal mass
- (SOT)
Solid-organ transplantation
- (SBRT)
Stereotactic body radiation therapy
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by The American Journal of Transplantation.
References
- 1.Noone AM, Pfeiffer RM, Dorgan JF, et al. Cancer-attributable mortality among solid organ transplant recipients in the United States: 1987 through 2014. Cancer. 2019;125(15):2647–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kasiske BL, Cangro CB, Hariharan S, et al. The evaluation of renal transplantation candidates: clinical practice guidelines. Am J Transplant. 2001;1 Suppl 2:3–95. [PubMed] [Google Scholar]
- 3.Penn I Evaluation of the candidate with a previous malignancy. Liver Transpl Surg. 1996;2(5 Suppl 1):109–113. [PubMed] [Google Scholar]
- 4.Acuna SA, Huang JW, Dossa F, Shah PS, Kim SJ, Baxter NN. Cancer recurrence after solid organ transplantation: A systematic review and meta-analysis. Transplant Rev (Orlando). 2017;31(4):240–248. [DOI] [PubMed] [Google Scholar]
- 5.Acuna SA, Sutradhar R, Kim SJ, Baxter NN. Solid Organ Transplantation in Patients With Preexisting Malignancies in Remission: A Propensity Score Matched Cohort Study. Transplantation. 2018;102(7):1156–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brattstrom C, Granath F, Edgren G, Smedby KE, Wilczek HE. Overall and cause-specific mortality in transplant recipients with a pretransplantation cancer history. Transplantation. 2013;96(3):297–305. [DOI] [PubMed] [Google Scholar]
- 7.Livingston-Rosanoff D, Foley DP, Leverson G, Wilke LG. Impact of Pre-Transplant Malignancy on Outcomes After Kidney Transplantation: United Network for Organ Sharing Database Analysis. J Am Coll Surg. 2019;229(6):568–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Cancer Society: Breast Cancer Facts & Figures 2019–2020. 2019; https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts-and-figures-2019-2020.pdf. Accessed 10/30/2019.
- 9.How Common Is Breast Cancer? 2018; https://www.cancer.org/cancer/breast-cancer/about/how-common-is-breast-cancer.html. Accessed 5/16/2018.
- 10.Du XL, Fox EE, Lai D. Competing causes of death for women with breast cancer and change over time from 1975 to 2003. Am J Clin Oncol. 2008;31(2):105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdel-Qadir H, Austin PC, Lee DS, et al. A Population-Based Study of Cardiovascular Mortality Following Early-Stage Breast Cancer. JAMA Cardiol. 2017;2(1):88–93. [DOI] [PubMed] [Google Scholar]
- 12.Plichta JK, Ren Y, Thomas SM, et al. Implications for Breast Cancer Restaging Based on the 8th Edition AJCC Staging Manual. Ann Surg. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giuliano AE, Connolly JL, Edge SB, et al. Breast Cancer-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(4):290–303. [DOI] [PubMed] [Google Scholar]
- 14.Sparano JA, Gray RJ, Makower DF, et al. Prospective Validation of a 21-Gene Expression Assay in Breast Cancer. The New England journal of medicine. 2015;373(21):2005–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cardoso F, van’t Veer LJ, Bogaerts J, et al. 70-Gene Signature as an Aid to Treatment Decisions in Early-Stage Breast Cancer. N Engl J Med. 2016;375(8):717–729. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Schnabel CA, Schroeder BE, et al. Breast Cancer Index Identifies Early-Stage Estrogen Receptor-Positive Breast Cancer Patients at Risk for Early- and Late-Distant Recurrence. Clinical Cancer Research. 2013;19(15):4196–4205. [DOI] [PubMed] [Google Scholar]
- 17.Sparano JA, Gray RJ, Makower DF, et al. Adjuvant Chemotherapy Guided by a 21-Gene Expression Assay in Breast Cancer. N Engl J Med. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiss A, Chavez-MacGregor M, Lichtensztajn DY, et al. Validation Study of the American Joint Committee on Cancer Eighth Edition Prognostic Stage Compared With the Anatomic Stage in Breast Cancer. JAMA Oncol. 2018;4(2):203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Narod SA, Giannakeas V, Sopik V. Time to death in breast cancer patients as an indicator of treatment response. Breast Cancer Research and Treatment. 2018;172(3):659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dawood S, Merajver SD, Viens P, et al. International expert panel on inflammatory breast cancer: consensus statement for standardized diagnosis and treatment. Ann Oncol. 2011;22(3):515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hance KW, Anderson WF, Devesa SS, Young HA, Levine PH. Trends in inflammatory breast carcinoma incidence and survival: the surveillance, epidemiology, and end results program at the National Cancer Institute. J Natl Cancer Inst. 2005;97(13):966–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fouad TM, Barrera AMG, Reuben JM, et al. Inflammatory breast cancer: a proposed conceptual shift in the UICC-AJCC TNM staging system. Lancet Oncol. 2017;18(4):e228–e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiely BE, Soon YY, Tattersall MH, Stockler MR. How long have I got? Estimating typical, best-case, and worst-case scenarios for patients starting first-line chemotherapy for metastatic breast cancer: a systematic review of recent randomized trials. J Clin Oncol. 2011;29(4):456–463. [DOI] [PubMed] [Google Scholar]
- 24.Dafni U, Grimani I, Xyrafas A, Eleftheraki AG, Fountzilas G. Fifteen-year trends in metastatic breast cancer survival in Greece. Breast Cancer Res Treat. 2010;119(3):621–631. [DOI] [PubMed] [Google Scholar]
- 25.Lobbezoo DJ, van Kampen RJ, Voogd AC, et al. Prognosis of metastatic breast cancer: are there differences between patients with de novo and recurrent metastatic breast cancer? Br J Cancer. 2015;112(9):1445–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69(5):363–385. [DOI] [PubMed] [Google Scholar]
- 27.Amin MB, American Joint Committee on Cancer., American Cancer Society. AJCC cancer staging manual. Eight edition / editor-in-chief, Amin Mahul B., MD, FCAP; editors, Edge Stephen B., MD, FACS and 16 others; Gress Donna M., RHIT, CTR - Technical editor; Meyer Laura R., CAPM - Managing editor. ed. Chicago IL: American Joint Committee on Cancer, Springer; 2017. [Google Scholar]
- 28.Kuipers EJ, Grady WM, Lieberman D, et al. Colorectal cancer. Nat Rev Dis Primers. 2015;1:15065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Markowitz SD, Bertagnolli MM. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med. 2009;361(25):2449–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tie J, Cohen JD, Wang Y, et al. Circulating Tumor DNA Analyses as Markers of Recurrence Risk and Benefit of Adjuvant Therapy for Stage III Colon Cancer. JAMA Oncol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Li L, Cohen JD, et al. Prognostic Potential of Circulating Tumor DNA Measurement in Postoperative Surveillance of Nonmetastatic Colorectal Cancer. JAMA Oncol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snyder RA, Hu CY, Cuddy A, et al. Association Between Intensity of Posttreatment Surveillance Testing and Detection of Recurrence in Patients With Colorectal Cancer. Jama. 2018;319(20):2104–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benson AB, Venook AP, Al-Hawary MM, et al. Rectal Cancer, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16(7):874–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith JJ, Strombom P, Chow OS, et al. Assessment of a Watch-and-Wait Strategy for Rectal Cancer in Patients With a Complete Response After Neoadjuvant Therapy. JAMA Oncol. 2019;5(4):e185896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Penn I The effect of immunosuppression on pre-existing cancers. Transplantation. 1993;55(4):742–747. [DOI] [PubMed] [Google Scholar]
- 36.Penn I Evaluation of transplant candidates with pre-existing malignancies. Ann Transplant. 1997;2(4):14–17. [PubMed] [Google Scholar]
- 37.Kobayashi H, Mochizuki H, Sugihara K, et al. Characteristics of recurrence and surveillance tools after curative resection for colorectal cancer: a multicenter study. Surgery. 2007;141(1):67–75. [DOI] [PubMed] [Google Scholar]
- 38.Siegel RL, Miller KD, Jemal A. Colorectal Cancer Mortality Rates in Adults Aged 20 to 54 Years in the United States, 1970–2014. JAMA. 2017;318(6):572–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hagness M, Foss A, Line PD, et al. Liver transplantation for nonresectable liver metastases from colorectal cancer. Ann Surg. 2013;257(5):800–806. [DOI] [PubMed] [Google Scholar]
- 40.Toso C, Pinto Marques H, Andres A, et al. Liver transplantation for colorectal liver metastasis: Survival without recurrence can be achieved. Liver Transpl. 2017;23(8):1073–1076. [DOI] [PubMed] [Google Scholar]
- 41.Dueland S, Syversveen T, Solheim JM, et al. Survival Following Liver Transplantation for Patients With Nonresectable Liver-only Colorectal Metastases. Ann Surg. 2020;271(2):212–218. [DOI] [PubMed] [Google Scholar]
- 42.Leeds IL, Fang SH. Anal cancer and intraepithelial neoplasia screening: A review. World J Gastrointest Surg. 2016;8(1):41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kabarriti R, Brodin NP, Ohri N, et al. Human papillomavirus, radiation dose and survival of patients with anal cancer. Acta Oncol. 2019;58(12):1745–1751. [DOI] [PubMed] [Google Scholar]
- 44.Zlotta AR, Egawa S, Pushkar D, et al. Prevalence of prostate cancer on autopsy: cross-sectional study on unscreened Caucasian and Asian men. J Natl Cancer Inst. 2013;105(14):1050–1058. [DOI] [PubMed] [Google Scholar]
- 45.Hamdy FC, Donovan JL, Lane JA, et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N Engl J Med. 2016;375(15):1415–1424. [DOI] [PubMed] [Google Scholar]
- 46.Engels EA, Pfeiffer RM, Fraumeni JF Jr., et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306(17):1891–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liauw SL, Ham SA, Das LC, et al. Prostate Cancer Outcomes Following Solid-Organ Transplantation: A SEER-Medicare Analysis. J Natl Cancer Inst. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stockle M, Junker K, Fornara P. Low-risk Prostate Cancer Prior to or After Kidney Transplantation. Eur Urol Focus. 2018;4(2):148–152. [DOI] [PubMed] [Google Scholar]
- 49.Boissier R, Hevia V, Bruins HM, et al. The Risk of Tumour Recurrence in Patients Undergoing Renal Transplantation for End-stage Renal Disease after Previous Treatment for a Urological Cancer: A Systematic Review. Eur Urol. 2018;73(1):94–108. [DOI] [PubMed] [Google Scholar]
- 50.Carvalho JA, Nunes P, Dinis PJ, et al. Prostate Cancer in Renal Transplant Recipients: Diagnosis and Treatment. Transplant Proc. 2017;49(4):809–812. [DOI] [PubMed] [Google Scholar]
- 51.Gin GE, Pereira JF, Weinberg AD, et al. Prostate-specific antigen screening and prostate cancer treatment in renal transplantation candidates: A survey of U.S. transplantation centers. Urol Oncol. 2016;34(2):57 e59–13. [DOI] [PubMed] [Google Scholar]
- 52.Finelli A, Ismaila N, Bro B, et al. Management of Small Renal Masses: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2017;35(6):668–680. [DOI] [PubMed] [Google Scholar]
- 53.Umbreit EC, Shimko MS, Childs MA, et al. Metastatic potential of a renal mass according to original tumour size at presentation. BJU Int. 2012;109(2):190–194; discussion 194. [DOI] [PubMed] [Google Scholar]
- 54.Bahouth Z, Halachmi S, Meyer G, Avitan O, Moskovitz B, Nativ O. The natural history and predictors for intervention in patients with small renal mass undergoing active surveillance. Adv Urol. 2015;2015:692014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chandrasekar T, Ahmad AE, Fadaak K, et al. Natural History of Complex Renal Cysts: Clinical Evidence Supporting Active Surveillance. J Urol. 2018;199(3):633–640. [DOI] [PubMed] [Google Scholar]
- 56.Beksac AT, Paulucci DJ, Sfakianos JP, et al. Trends in management of the small renal mass in renal transplant recipient candidates: A multi-institutional survey analysis. Urol Oncol. 2017;35(8):529 e517–529 e522. [DOI] [PubMed] [Google Scholar]
- 57.Bhindi B, Thompson RH, Lohse CM, et al. The Probability of Aggressive Versus Indolent Histology Based on Renal Tumor Size: Implications for Surveillance and Treatment. Eur Urol. 2018;74(4):489–497. [DOI] [PubMed] [Google Scholar]
- 58.Schmitges J, Trinh QD, Sun M, et al. Higher perioperative morbidity and in-hospital mortality in patients with end-stage renal disease undergoing nephrectomy for non-metastatic kidney cancer: a population-based analysis. BJU Int. 2012;110(6 Pt B):E183–190. [DOI] [PubMed] [Google Scholar]
- 59.Patard JJ, Kim HL, Lam JS, et al. Use of the University of California Los Angeles integrated staging system to predict survival in renal cell carcinoma: an international multicenter study. J Clin Oncol. 2004;22(16):3316–3322. [DOI] [PubMed] [Google Scholar]
- 60.Stephenson AJ, Chetner MP, Rourke K, et al. Guidelines for the surveillance of localized renal cell carcinoma based on the patterns of relapse after nephrectomy. J Urol. 2004;172(1):58–62. [DOI] [PubMed] [Google Scholar]
- 61.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 62.Soukup V, Capoun O, Cohen D, et al. Risk Stratification Tools and Prognostic Models in Non-muscle-invasive Bladder Cancer: A Critical Assessment from the European Association of Urology Non-muscle-invasive Bladder Cancer Guidelines Panel. Eur Urol Focus. 2018. [DOI] [PubMed] [Google Scholar]
- 63.Oddens J, Brausi M, Sylvester R, et al. Final results of an EORTC-GU cancers group randomized study of maintenance bacillus Calmette-Guerin in intermediate- and high-risk Ta, T1 papillary carcinoma of the urinary bladder: one-third dose versus full dose and 1 year versus 3 years of maintenance. Eur Urol. 2013;63(3):462–472. [DOI] [PubMed] [Google Scholar]
- 64.Sylvester RJ, Brausi MA, Kirkels WJ, et al. Long-term efficacy results of EORTC genito-urinary group randomized phase 3 study 30911 comparing intravesical instillations of epirubicin, bacillus Calmette-Guerin, and bacillus Calmette-Guerin plus isoniazid in patients with intermediate- and high-risk stage Ta T1 urothelial carcinoma of the bladder. Eur Urol. 2010;57(5):766–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19(3):666–675. [DOI] [PubMed] [Google Scholar]
- 66.Sonpavde G, Khan MM, Lerner SP, et al. Disease-free survival at 2 or 3 years correlates with 5-year overall survival of patients undergoing radical cystectomy for muscle invasive bladder cancer. J Urol. 2011;185(2):456–461. [DOI] [PubMed] [Google Scholar]
- 67.Wright AA, Bohlke K, Armstrong DK, et al. Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology Clinical Practice Guideline. Gynecol Oncol. 2016;143(1):3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gonzalez-Martin A, Pothuri B, Vergote I, et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N Engl J Med. 2019;381(25):2391–2402. [DOI] [PubMed] [Google Scholar]
- 69.Moore K, Colombo N, Scambia G, et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N Engl J Med. 2018;379(26):2495–2505. [DOI] [PubMed] [Google Scholar]
- 70.Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15(1):10–17. [DOI] [PubMed] [Google Scholar]
- 71.Creasman WT, Morrow CP, Bundy BN, Homesley HD, Graham JE, Heller PB. Surgical pathologic spread patterns of endometrial cancer. A Gynecologic Oncology Group Study. Cancer. 1987;60(8 Suppl):2035–2041. [DOI] [PubMed] [Google Scholar]
- 72.Bandera C, Dizon DS. Contemporary Approaches to High-risk, Early-Stage Serous Endometrial Cancer: Clinical Equipoise Persists. Am J Clin Oncol. 2019;42(2):107–108. [DOI] [PubMed] [Google Scholar]
- 73.Cancer Genome Atlas Research N, Kandoth C, Schultz N, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peters WA 3rd, Liu PY, Barrett RJ 2nd, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18(8):1606–1613. [DOI] [PubMed] [Google Scholar]
- 75.Sedlis A, Bundy BN, Rotman MZ, Lentz SS, Muderspach LI, Zaino RJ. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: A Gynecologic Oncology Group Study. Gynecol Oncol. 1999;73(2):177–183. [DOI] [PubMed] [Google Scholar]
- 76.Chemoradiotherapy for Cervical Cancer Meta-analysis C. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: individual patient data meta-analysis. Cochrane Database Syst Rev. 2010(1):CD008285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Group AES, Blake P, Swart AM, et al. Adjuvant external beam radiotherapy in the treatment of endometrial cancer (MRC ASTEC and NCIC CTG EN.5 randomised trials): pooled trial results, systematic review, and meta-analysis. Lancet. 2009;373(9658):137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Clamp AR, James EC, McNeish IA, et al. Weekly dose-dense chemotherapy in first-line epithelial ovarian, fallopian tube, or primary peritoneal carcinoma treatment (ICON8): primary progression free survival analysis results from a GCIG phase 3 randomised controlled trial. Lancet. 2019;394(10214):2084–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Matei D, Filiaci V, Randall ME, et al. Adjuvant Chemotherapy plus Radiation for Locally Advanced Endometrial Cancer. N Engl J Med. 2019;380(24):2317–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 81.Kanitkar AA, Schwartz AG, George J, Soubani AO. Causes of death in long-term survivors of non-small cell lung cancer: A regional Surveillance, Epidemiology, and End Results study. Ann Thorac Med. 2018;13(2):76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–99. [DOI] [PubMed] [Google Scholar]
- 83.Howington JA, Blum MG, Chang AC, Balekian AA, Murthy SC. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e278S–e313S. [DOI] [PubMed] [Google Scholar]
- 84.Califano R, Gomes F, Ackermann CJ, Rafee S, Tsakonas G, Ekman S. Immune checkpoint blockade for non-small cell lung cancer: What is the role in the special populations? Eur J Cancer. 2019;125:1–11. [DOI] [PubMed] [Google Scholar]
- 85.Ramnath N, Dilling TJ, Harris LJ, et al. Treatment of stage III non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e314S–e340S. [DOI] [PubMed] [Google Scholar]
- 86.Taylor MD, Nagji AS, Bhamidipati CM, et al. Tumor recurrence after complete resection for non-small cell lung cancer. Ann Thorac Surg. 2012;93(6):1813–1820; discussion 1820–1811. [DOI] [PubMed] [Google Scholar]
- 87.Sasaki H, Suzuki A, Tatematsu T, et al. Prognosis of recurrent non-small cell lung cancer following complete resection. Oncol Lett. 2014;7(4):1300–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rice D, Kim HW, Sabichi A, et al. The risk of second primary tumors after resection of stage I nonsmall cell lung cancer. Ann Thorac Surg. 2003;76(4):1001–1007; discussion 1007–1008. [DOI] [PubMed] [Google Scholar]
- 89.Verstegen NE, Lagerwaard FJ, Hashemi SM, Dahele M, Slotman BJ, Senan S. Patterns of Disease Recurrence after SABR for Early Stage Non-Small-Cell Lung Cancer: Optimizing Follow-Up Schedules for Salvage Therapy. J Thorac Oncol. 2015;10(8):1195–1200. [DOI] [PubMed] [Google Scholar]
- 90.Bradley J Radiographic response and clinical toxicity following SBRT for stage I lung cancer. J Thorac Oncol. 2007;2(7 Suppl 3):S118–124. [DOI] [PubMed] [Google Scholar]
- 91.Panchabhai TS, Arrossi AV, Patil PD, et al. Unexpected Neoplasms in Lungs Explanted From Lung Transplant Recipients: A Single-Center Experience and Review of Literature. Transplant Proc. 2018;50(1):234–240. [DOI] [PubMed] [Google Scholar]
- 92.Grewal AS, Padera RF, Boukedes S, et al. Prevalence and outcome of lung cancer in lung transplant recipients. Respir Med. 2015;109(3):427–433. [DOI] [PubMed] [Google Scholar]
- 93.Weill D, Benden C, Corris PA, et al. A consensus document for the selection of lung transplant candidates: 2014--an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2015;34(1):1–15. [DOI] [PubMed] [Google Scholar]
- 94.Nordness MF, Hamel S, Godfrey CM, et al. Fatal hepatic necrosis after nivolumab as a bridge to liver transplant for HCC: Are checkpoint inhibitors safe for the pretransplant patient? Am J Transplant. 2020;20(3):879–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fisher J, Zeitouni N, Fan W, Samie FH. Immune checkpoint inhibitor therapy in solid organ transplant recipients: A patient-centered systematic review. J Am Acad Dermatol. 2020;82(6):1490–1500. [DOI] [PubMed] [Google Scholar]
- 96.Abdel-Wahab N, Safa H, Abudayyeh A, et al. Checkpoint inhibitor therapy for cancer in solid organ transplantation recipients: an institutional experience and a systematic review of the literature. J Immunother Cancer. 2019;7(1):106. [DOI] [PMC free article] [PubMed] [Google Scholar]


