Abstract
Gastric inhibitory peptide (GIP) is best known for its role as an incretin hormone in control of blood glucose concentrations. As a classic satiation signal, however, the literature illustrates a mixed picture of GIP involvement with an at best weak anorectic response profile being reported for GIP receptor (GIPR) signaling. Not surprisingly, the pursuit of exploiting the GIP system as a therapeutic target for diabetes and obesity has fallen behind that of the other gastrointestinal-derived incretin, glucagon-like peptide 1 (GLP-1). However, recent discoveries highlighted here support potential therapeutic advantages of combinatorial therapies targeting GIP and GLP-1 systems together, with perhaps the most surprising finding that GIPR agonism may have antiemetic properties. As nausea and vomiting are the most common side effects of all existing GLP-1 pharmacotherapies, the ability for GIP agonism to reduce GLP-1–induced illness behaviors but retain (if not enhance) weight loss and glycemic control may offer a new era in the treatment of obesity and diabetes.
Introduction: The Intersection of Satiety and Nausea
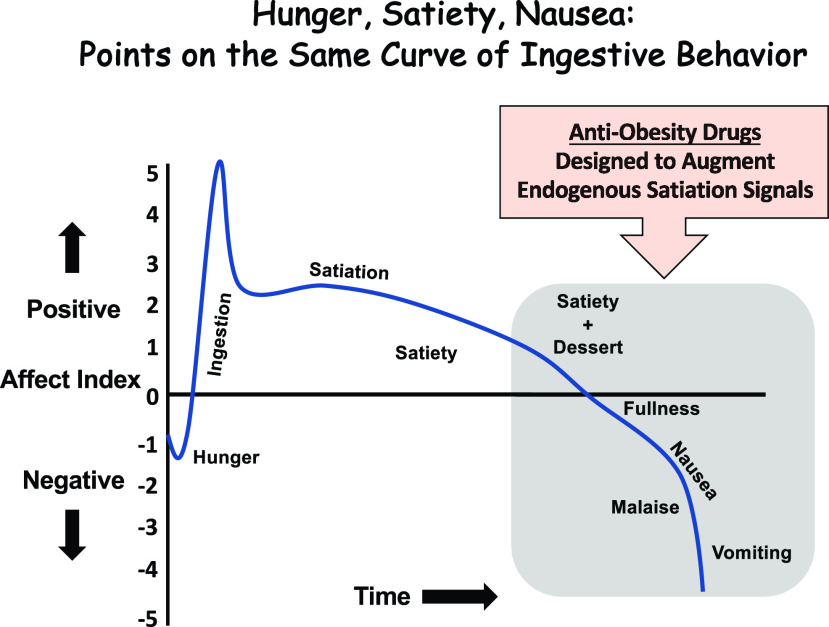
Hunger, satiety, nausea, and emesis are all points along the spectrum of ingestive behavior. This perspective is illustrated in Fig. 1 where arbitrary units of positive and negative affect are ascribed to each of these subjective feelings. Assuming ingestion begins due to energy need (i.e., from internal physiological drive), hunger is clearly a negative affect, one often referred to as hunger pains or pangs. But, rapidly upon ingestion, if the ingredients of the meal are deemed palatable, there is undoubtedly a feed-forward positive hedonic response that drives further meal taking. As the meal progresses, various components of the meal (e.g., macronutrient composition, pH, osmolarity, and total volume) give rise to a multitude of gastrointestinal (GI)-derived satiation signals being released from specialized endocrine cells along the GI tract (review in 1,2). The collective milieu of these satiation signals transmits an orchestrated within-meal intake inhibitory signal to the brain via the vagus nerve and through direct humoral action. If the animal is appropriately responding to these satiation signals, satiety will eventually be achieved and thus meal termination would in theory end with a modest state of positive affect. Humans of course, as is evident in the power of the obesogenic environment pervading society at large, can override internal satiation signals with the inclusion of novel highly palatable foods as drivers for continued consumption during the ongoing meal (i.e., dessert) (3). Meal consumption continues to progress and delay meal termination, but as we pass that balancing point of negative/positive affect with the subjective feeling of fullness, the experience of the meal begins to take on a difficult to define, but clear state of malaise and nausea (4). Ultimately, persistent meal taking beyond comfort could easily lead to emesis as an unmistakable state of negative affect. Importantly, many antiobesity drugs are designed to shift this ingestive behavior curve to the right by either mimicking a satiation signal and/or by engaging the neurotransmitter systems downstream of satiety signaling. Not surprisingly, nausea, emesis, and GI-related adverse events are among the most prevalent side effects of antiobesity pharmacotherapies. The clinical need to prevent negative affect and noxious events for patients seeking to lose weight requires a more inclusive consideration of the neural circuitry governing nausea and emesis than has been devoted in the past. Interestingly, as discussed in more detail below, some surprising new discoveries are showing that the body may already hold a secret in combating illness-like behaviors with a GI-derived hormone, gastric inhibitory peptide (GIP), that is rapidly secreted during the early phases of meal consumption.
Figure 1.
Conceptual graph illustrating that hunger, satiety, and nausea can all be viewed as interconnected points on the same curve of positive/negative affect during a meal.
Neural Substrates Mediating Nausea and Emesis
Nausea and vomiting are among the most frequently occurring symptoms in a myriad of diseases and treatments related to metabolic disorders. Given their prevalence and the severe negative impact on nutritional balance, quality of life, and disease prognosis (5,6), improved understanding and long-term control of nausea and vomiting remain an unmet need in many medical fields such as obesity, diabetes, and oncology. Emesis and nausea are largely controlled by the central nervous system (CNS) (6–10). Three critical nuclei are the nucleus tractus solitarius (NTS), the area postrema (AP), and dorsal motor nucleus of the vagus: adjacent nuclei in the hindbrain collectively termed the dorsal vagal complex, which is essential in the control of nausea, emesis, food intake, cardio-respiratory function, and GI motility (2,6). Because of the many divergent behaviors and physiological functions regulated by this overlapping neurocircuitry, understanding the CNS neural substrates regulating nausea and vomiting has been proven challenging. The most significative advances over the past 20 years culminated with the development of “classic” antiemetics targeting the serotonin type-3 receptor (5-HT3R) and neurokinin-1 receptor (NK1R)11. Indeed, the 5-HT3 receptor antagonist ondansetron (Zofran) and the NK1R antagonist aprepitant (Cinvanti) are both prescribed treatments for chemotherapy-induced nausea and vomiting. Unfortunately, for many diseases and physiological states that can drive nausea and emesis (e.g., cancers, diabetes, cystic fibrosis, pregnancy) it is clear that the 5-HT3R and NK1R antagonists are suboptimal for the total control of malaise (12–14), suggesting that there are other underlying neural substrates involved in the control of nausea and emesis. To that end, recent discoveries are now shedding light on the role of the GIP receptor (GIPR) system in the dorsal vagal complex as playing a role in processing nausea and vomiting. One critical question is whether the same neurons are engaged at different levels of excitation/inhibition, or is there parallel circuitry in the same nuclei?
GIP in Glycemic Control and Energy Balance and Metabolism
GIP is a hormone released from the enteroendocrine cells in the small intestine early during meal ingestion (15). Together with GLP-1, these two incretin hormones serve an important role in priming the β-cells of the pancreas to augment insulin secretion to regulate postprandial glucose levels (16–18). While pharmacotherapies targeting the GLP-1 system have been widely successful for the treatment of type 2 diabetes (T2D) and obesity, the use of GIP analogs as monotherapies has been largely underwhelming in preclinical and clinical studies and even produced some controversial findings (review in 19,20). As such, GIP analogs were initially not pursued as monotherapies to treat diabetes or obesity due to the perception of an overall weak biological effect, in part because of early findings suggesting a GIP resistance in the diabetic condition (21) alongside incongruent results on its hypophagic and body weight–lowering effects (19,22–26). Nonetheless, there is now convincing evidence that the increase in glucose-stimulated insulin secretion following administration of exogenously applied GIP is mediated by direct activation of GIP receptors (GIPR) expressed on pancreatic β-cells (15,27). Additional work is also showing that GIPR signaling has positive actions in bones (i.e., promoting mineral density and inhibiting bone reabsorption) (28), modulates thermogenesis (via direct actions on brown adipose tissue) (29), directly influences fat metabolism (by modulating lipid storage and lipolysis in the white adipose tissue) (30), and contributes to the optimal level of postprandial glucagon secretion via direct α-cell actions (31). These unique actions of GIP, as well as those in combination with GLP-1 described in more detail below, are collectively increasing the exploration of GIP as a therapeutic target.
GLP-1–GIP Coagonists: Improving Glycemic Control and Weight Loss While Reducing Nausea and Emesis
Approximately 30 years ago, the discovery was made that a compound in the venom-laced saliva of the Gila monster shared properties similar to those of human GLP-1, but unlike the endogenous active form of GLP-1 [i.e., GLP-1(7-36)], this compound was resistant to enzymatic degradation by dipeptidyl peptidase IV. This finding initially led to exendin-4 (Ex4) (exenatide) (32), a human GLP-1 receptor agonist. Thereafter, a lipidated GLP-1R agonist, liraglutide, was also introduced for the treatment of diabetes and obesity (18). Not surprisingly, the glycemic and energy balance beneficial effects of Ex4 and liraglutide inspired the creation of second-generation GLP-1 receptor agonists for treating T2D, which include but are not limited to dulaglutide and semaglutide (33). These developments yielded substantial overall metabolic improvements in patients, in comparison with first-generation GLP-1R agonists, that include superior and longer-lasting hypoglycemic actions and greater body weight loss.
Importantly, however, all first- and second-generation GLP-1R agonists are still accompanied by a high incidence of illness-like behaviors as a principal side effect that include nausea and vomiting (34–36). A wealth of literature indicates that a significant portion of the hypophagic effects of current GLP-1R agonists are mediated by GLP-1Rs expressed in the CNS, in particular those in the AP/NTS (37–42). Perhaps not surprising, this hindbrain site of action is also responsible for mediating the illness-like behaviors (e.g., nausea, conditioned taste avoidance, emesis) of systemically delivered GLP-1R agonists (43). Accumulating evidence highlights nausea and emesis as the principal reported side effects of existing GLP-1 therapeutics (44). Industry leaders in the field are also clearly aware of this concern, as a recent report from GlaxoSmithKline concluded that “patients reported that GI-related issues ‘Made me feel sick’ (64.4%) and ‘Made me throw up’ (45.4%) as their top reasons for discontinuation” (12). Consistent with other diseases, there are clear “disparities between patient experiences and physician perceptions,” with a clear need to improve “gaps in physician-patient communication” with regard to GLP-1 therapeutics and incidence of illness. Despite the common dismissive comment to the contrary, these effects are not transient or insignificant, as they lead to discontinuation of treatment in ∼6–10% and reduced dose tolerance in another ∼15% of patients with T2D (45–52). Thus, using conservative numbers, > 20% of patients with T2D in the U.S. cannot benefit fully from existing U.S. Food and Drug Administration–approved GLP-1 therapeutics. Thus, finding an ability to attenuate the nausea/emesis adverse events of GLP-1R agonists without affecting action on β-cells or satiety circuits not only will lead to better patient compliance but also may allow for greater therapeutic tolerability of higher concentrations of GLP-1R agonists to potentially further enhance weight loss and glycemic control. As GLP-1R agonism is now being investigated as a potential pharmacotherapy for an ever-growing number of nonmetabolic diseases affecting the CNS (e.g., cognitive impairments, neurodegeneration, and substance abuse), achieving greater blood-brain barrier penetrance will be imperative with higher concentrations of GLP-1R agonists that can be tolerated if nausea and emesis are blocked so as to enrich GLP-1R ligand access to CNS regions not currently accessed by existing approved dosing regiments.
GIP as an Antiemetic
GIPR activation may have surprising antiemetic effects, as recently described in a patent application filed by Takeda Pharmaceutical Company Limited (53). This finding may contribute in part to the explanation for why the combination of agonists targeting both the GIP and GLP-1 systems has yielded promising results in preclinical models and clinical trials, providing greater body weight loss and better glycemic control than GLP-1R agonism alone (25,54–57). Indeed, in cynomolgus monkeys, GIP/GLP-1 coagonism was shown to be superior in reducing blood glucose levels and increasing plasma insulin compared with equimolar doses of liraglutide (58). The same report showed a reduction in the incidence of gastric-related adverse events for the coagonist compared with GLP-1 monotherapy. These data are further supported by our recent discovery that GIPR agonism was sufficient to block the emetic events by a GLP-1R agonist in the musk shrew (59).
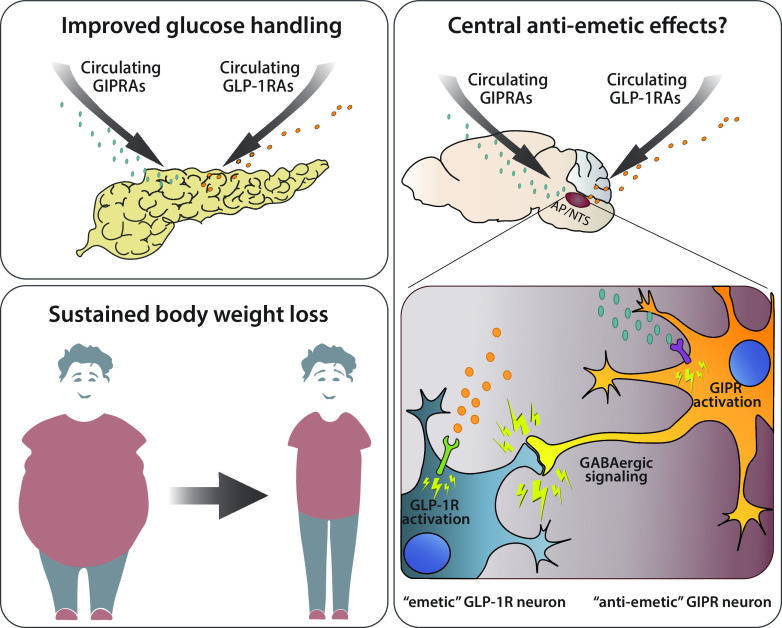
The mechanism(s) mediating the antiemetic actions of GIPR agonism are not known, despite recent work describing the phenotype of GIPR-expressing neurons in the various CNS nuclei involved in the control of energy homeostasis, including the AP and NTS (60,61). Using single-nuclei RNA sequencing of murine AP/NTS tissue, these two recent independent publications (60,61), generated the full transcriptome profile of each individual neuron. Intriguingly, a significant portion of Gad1- and Gad2-expressing neurons (responsible for GABA synthesis), also expressed Gipr, while only a few neurons expressed Glp1r and Gipr (60). These data suggest the presence of two unique and very distinct neuronal circuitries within the AP and highlight the hypothesis of a local inhibitory network within the caudal hindbrain that could be exploited via GIPR activation to reduce hindbrain GLP-1R–mediated emesis and nausea (Fig. 2).
Figure 2.
Overview of the beneficial effects of GIPR agonism in combination with GLP-1–based therapeutics for the treatment of T2D and obesity. GIP/GLP-1 dual treatment improves gluco-regulation while simultaneously promoting sustained body weight loss over time. Additionally, GIPR activation may counteract GLP-1–induced malaise via direct modulation of the AP/NTS circuitry. Given the inhibitory nature of the GIPR-expressing neurons, one can speculate the existence of a local inhibitory network within the caudal hindbrain that could be exploited via GIPR activation to reduce hindbrain GLP-1R–mediated emesis and nausea, thus offering a valuable opportunity of dose modifications increasing the therapeutic window/index. GIPRAs, GIP receptor agonists; GLP-1RAs, GLP-1 receptor agonists.
Conclusions
That the GIP system was initially not heavily pursued as an obesity or diabetes target is not completely surprising given the conflicting literature briefly discussed here. However, the recent discovery that GIPR agonism can block nausea and emesis of GLP-1R agonists should set a precedent to consider whether GIPR agonism can block other drivers of malaise. Indeed, as recently described (53), GIPR agonism was capable of reducing emetic responses that usually occur following gut PYY and cisplatin administrations in beagles and ferrets, respectively. These discoveries should also prompt the field as a whole to reevaluate whether other GI-derived hormones that also appeared to be lackluster in metabolic effects as monotherapies may show promise in food intake/glycemic control/antiemetic potential as a combinatorial target. At least for the GIP system, there is clearly a lot of important unanswered questions with regard to the ability of GIPR agonism to treat nausea and emesis while improving glycemic control.
Article Information
Funding. This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health grants DK021397 (to M.R.H.) and DK112812 (to B.C.D.J.) and Swiss National Science Foundation (SNSF) grant P400PB_186728 (to T.B.).
Duality of Interest. M.R.H. and B.C.D.J. receive research funds from Eli Lilly & Co. for related research. M.R.H. receives research funding from Boehringer Ingelheim. M.R.H., T.B., and B.C.D.J. are owners of Cantius Therapeutics, LLC, which pursues biological work unrelated to the current study. No other potential conflicts of interest relevant to this article were reported.
Funds from Eli Lilly & Co. were not used to support the writing of this article. Funding from Boehringer Ingelheim was not used in support of these studies.
Author Contributions. M.R.H., T.B., and B.C.D.J. prepared the manuscript. All authors serve as guarantors and take responsibility for the integrity of the manuscript.
Prior Presentation. Parts of this work were presented at the 81st Scientific Sessions of the American Diabetes Association, virtual meeting, 25–29 June 2021.
References
- 1. Moran TH, Ladenheim EE, Schwartz GJ. Within-meal gut feedback signaling. Int J Obes Relat Metab Disord 2001;25(Suppl. 5):S39–S41 [DOI] [PubMed] [Google Scholar]
- 2. Grill HJ, Hayes MR. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab 2012;16:296–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rolls BJ. The supersizing of America: portion size and the obesity epidemic. Nutr Today 2003;38:42–53 [DOI] [PubMed] [Google Scholar]
- 4. Linardon J, Tylka TL, Fuller-Tyszkiewicz M. Intuitive eating and its psychological correlates: a meta-analysis. Int J Eat Disord 2021;54:1073–1098 [DOI] [PubMed] [Google Scholar]
- 5. Lacy BE, Parkman HP, Camilleri M. Chronic nausea and vomiting: evaluation and treatment. Am J Gastroenterol 2018;113:647–659 [DOI] [PubMed] [Google Scholar]
- 6. Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med 2008;358:2482–2494 [DOI] [PubMed] [Google Scholar]
- 7. Miller AD, Leslie RA. The area postrema and vomiting. Front Neuroendocrinol 1994;15:301–320 [DOI] [PubMed] [Google Scholar]
- 8. Babic T, Browning KN. The role of vagal neurocircuits in the regulation of nausea and vomiting. Eur J Pharmacol 2014;722:38–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baker PD, Morzorati SL, Ellett ML. The pathophysiology of chemotherapy-induced nausea and vomiting. Gastroenterol Nurs 2005;28:469–480 [DOI] [PubMed] [Google Scholar]
- 10. Horn CC. Measuring the nausea-to-emesis continuum in non-human animals: refocusing on gastrointestinal vagal signaling. Exp Brain Res 2014;232:2471–2481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Basch E, Prestrud AA, Hesketh PJ, et al.; American Society of Clinical Oncology . Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2011;29:4189–4198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sikirica MV, Martin AA, Wood R, Leith A, Piercy J, Higgins V. Reasons for discontinuation of GLP1 receptor agonists: data from a real-world cross-sectional survey of physicians and their patients with type 2 diabetes. Diabetes Metab Syndr Obes 2017;10:403–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sanger GJ, Andrews PL. Treatment of nausea and vomiting: gaps in our knowledge. Auton Neurosci 2006;129:3–16 [DOI] [PubMed] [Google Scholar]
- 14. Sanger GJ, Andrews PLR. A history of drug discovery for treatment of nausea and vomiting and the implications for future research. Front Pharmacol 2018;9:913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007;132:2131–2157 [DOI] [PubMed] [Google Scholar]
- 16. Dupre J, Ross SA, Watson D, Brown JC. Stimulation of insulin secretion by gastric inhibitory polypeptide in man. J Clin Endocrinol Metab 1973;37:826–828 [DOI] [PubMed] [Google Scholar]
- 17. Fehmann HC, Göke R, Göke B. Cell and molecular biology of the incretin hormones glucagon-like peptide-I and glucose-dependent insulin releasing polypeptide. Endocr Rev 1995;16:390–410 [DOI] [PubMed] [Google Scholar]
- 18. Müller TD, Finan B, Bloom SR, et al. Glucagon-like peptide 1 (GLP-1). Mol Metab 2019;30:72–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Finan B, Müller TD, Clemmensen C, Perez-Tilve D, DiMarchi RD, Tschöp MH. Reappraisal of GIP pharmacology for metabolic diseases. Trends Mol Med 2016;22:359–376 [DOI] [PubMed] [Google Scholar]
- 20. Samms RJ, Coghlan MP, Sloop KW. How may GIP enhance the therapeutic efficacy of GLP-1? Trends Endocrinol Metab 2020;31:410–421 [DOI] [PubMed] [Google Scholar]
- 21. Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest 1993;91:301–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McClean PL, Irwin N, Cassidy RS, Holst JJ, Gault VA, Flatt PR. GIP receptor antagonism reverses obesity, insulin resistance, and associated metabolic disturbances induced in mice by prolonged consumption of high-fat diet. Am J Physiol Endocrinol Metab 2007;293:E1746–E1755 [DOI] [PubMed] [Google Scholar]
- 23. Miyawaki K, Yamada Y, Ban N, et al. Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat Med 2002;8:738–742 [DOI] [PubMed] [Google Scholar]
- 24. Boylan MO, Glazebrook PA, Tatalovic M, Wolfe MM. Gastric inhibitory polypeptide immunoneutralization attenuates development of obesity in mice. Am J Physiol Endocrinol Metab 2015;309:E1008–E1018 [DOI] [PubMed] [Google Scholar]
- 25. Killion EA, Wang J, Yie J, et al. Anti-obesity effects of GIPR antagonists alone and in combination with GLP-1R agonists in preclinical models. Sci Transl Med 2018;10: eaat3392. [DOI] [PubMed] [Google Scholar]
- 26. Mroz PA, Finan B, Gelfanov V, et al. Optimized GIP analogs promote body weight lowering in mice through GIPR agonism not antagonism. Mol Metab 2019;20:51–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Khan R, Tomas A, Rutter GA. Effects on pancreatic beta and other islet cells of the glucose-dependent insulinotropic polypeptide. Peptides 2020;125:170201. [DOI] [PubMed] [Google Scholar]
- 28. Stensen S, Gasbjerg LS, Helsted MM, Hartmann B, Christensen MB, Knop FK. GIP and the gut-bone axis - physiological, pathophysiological and potential therapeutic implications. Peptides 2020;125:170197. [DOI] [PubMed] [Google Scholar]
- 29. Beaudry JL, Kaur KD, Varin EM, et al. Physiological roles of the GIP receptor in murine brown adipose tissue. Mol Metab 2019;28:14–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thondam SK, Cuthbertson DJ, Wilding JPH. The influence of glucose-dependent insulinotropic polypeptide (GIP) on human adipose tissue and fat metabolism: implications for obesity, type 2 diabetes and non-alcoholic fatty liver disease (NAFLD). Peptides 2020;125:170208. [DOI] [PubMed] [Google Scholar]
- 31. El K, Gray SM, Capozzi ME, et al. GIP mediates the incretin effect and glucose tolerance by dual actions on α cells and β cells. Sci Adv 2021;7:eabf1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Eng J, Kleinman WA, Singh L, Singh G, Raufman JP. Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom. Further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J Biol Chem 1992;267:7402–7405 [PubMed] [Google Scholar]
- 33. Williams DM, Nawaz A, Evans M. Drug therapy in obesity: a review of current and emerging treatments. Diabetes Ther 2020;11:1199–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wysham C, Blevins T, Arakaki R, et al. Efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD-1). Diabetes Care 2014;37:2159–2167 [DOI] [PubMed] [Google Scholar]
- 35. Ahrén B, Atkin SL, Charpentier G, et al. Semaglutide induces weight loss in subjects with type 2 diabetes regardless of baseline BMI or gastrointestinal adverse events in the SUSTAIN 1 to 5 trials. Diabetes Obes Metab 2018;20:2210–2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pratley RE, Aroda VR, Lingvay I, et al.; SUSTAIN 7 Investigators . Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol 2018;6:275–286 [DOI] [PubMed] [Google Scholar]
- 37. Alhadeff AL, Mergler BD, Zimmer DJ, et al. Endogenous glucagon-like peptide-1 receptor signaling in the nucleus tractus solitarius is Required for food intake control. Neuropsychopharmacology 2017;42:1471–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hayes MR, Leichner TM, Zhao S, et al. Intracellular signals mediating the food intake-suppressive effects of hindbrain glucagon-like peptide-1 receptor activation. Cell Metab 2011;13:320–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kanoski SE, Fortin SM, Arnold M, Grill HJ, Hayes MR. Peripheral and central GLP-1 receptor populations mediate the anorectic effects of peripherally administered GLP-1 receptor agonists, liraglutide and exendin-4. Endocrinology 2011;152:3103–3112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mietlicki-Baase EG, Ortinski PI, Rupprecht LE, et al. The food intake-suppressive effects of glucagon-like peptide-1 receptor signaling in the ventral tegmental area are mediated by AMPA/kainate receptors. Am J Physiol Endocrinol Metab 2013;305:E1367–E1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sisley S, Gutierrez-Aguilar R, Scott M, D’Alessio DA, Sandoval DA, Seeley RJ. Neuronal GLP1R mediates liraglutide’s anorectic but not glucose-lowering effect. J Clin Invest 2014;124:2456–2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Secher A, Jelsing J, Baquero AF, et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J Clin Invest 2014;124:4473–4488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kanoski SE, Rupprecht LE, Fortin SM, De Jonghe BC, Hayes MR. The role of nausea in food intake and body weight suppression by peripheral GLP-1 receptor agonists, exendin-4 and liraglutide. Neuropharmacology 2012;62:1916–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bettge K, Kahle M, Abd El Aziz MS, Meier JJ, Nauck MA. Occurrence of nausea, vomiting and diarrhoea reported as adverse events in clinical trials studying glucagon-like peptide-1 receptor agonists: a systematic analysis of published clinical trials. Diabetes Obes Metab 2017;19:336–347 [DOI] [PubMed] [Google Scholar]
- 45. O’Neil PM, Birkenfeld AL, McGowan B, et al. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet 2018;392:637–649 [DOI] [PubMed] [Google Scholar]
- 46. Capehorn MS, Catarig AM, Furberg JK, et al. Efficacy and safety of once-weekly semaglutide 1.0mg vs once-daily liraglutide 1.2mg as add-on to 1-3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10). Diabetes Metab 2020;46:100–109 [DOI] [PubMed] [Google Scholar]
- 47. Trujillo J. Safety and tolerability of once-weekly GLP-1 receptor agonists in type 2 diabetes. J Clin Pharm Ther 2020;45(Suppl. 1):43–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bergenstal RM, Wysham C, Macconell L, et al.; DURATION-2 Study Group . Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. Lancet 2010;376:431–439 [DOI] [PubMed] [Google Scholar]
- 49. Buse JB, Henry RR, Han J, Kim DD, Fineman MS; Exenatide-113 Clinical Study Group . Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care 2004;27:2628–2635 [DOI] [PubMed] [Google Scholar]
- 50. DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005;28:1092–1100 [DOI] [PubMed] [Google Scholar]
- 51. Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005;28:1083–1091 [DOI] [PubMed] [Google Scholar]
- 52. John LE, Kane MP, Busch RS, Hamilton RA. Expanded use of exenatide in the management of type 2 diabetes. Diabetes Spectr 2007;20:59–63 [Google Scholar]
- 53. Asami T, Nishizawa N, Nida A, et al.; Takeda Pharmaceutical Company Limited . GIP receptor activating peptide. WO 2018/181864 A1, 2018. [Google Scholar]
- 54. Coskun T, Sloop KW, Loghin C, et al. LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: from discovery to clinical proof of concept. Mol Metab 2018;18:3–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nørregaard PK, Deryabina MA, Tofteng Shelton P, et al. A novel GIP analogue, ZP4165, enhances glucagon-like peptide-1-induced body weight loss and improves glycaemic control in rodents. Diabetes Obes Metab 2018;20:60–68 [DOI] [PubMed] [Google Scholar]
- 56. Frias JP, Bastyr EJ 3rd, Vignati L, et al. The sustained effects of a dual GIP/GLP-1 receptor agonist, NNC0090-2746, in patients with type 2 diabetes. Cell Metab 2017;26:343–352.e2 [DOI] [PubMed] [Google Scholar]
- 57. Frias JP, Nauck MA, Van J, et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet 2018;392:2180–2193 [DOI] [PubMed] [Google Scholar]
- 58. Finan B, Ma T, Ottaway N, et al. Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Sci Transl Med 2013;5:209ra151. [DOI] [PubMed] [Google Scholar]
- 59. Geisler C, Borner T, Gaisinsky J, et al. GIP receptor agonism attenuates GLP-1 receptor agonist induced nausea in rodents, 2020. Accessed 5 November 2020. Available from https://onlinelibrary.wiley.com/doi/full/10.1002/oby.23063 [DOI] [PMC free article] [PubMed]
- 60. Zhang C, Kaye JA, Cai Z, Wang Y, Prescott SL, Liberles SD. Area postrema cell types that mediate nausea-associated behaviors. Neuron 2021;109:461–472.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ludwig MQ, Cheng W, Gordian D, et al. A genetic map of the mouse dorsal vagal complex and its role in obesity. Nat Metab 2021;3:530–545 [DOI] [PMC free article] [PubMed] [Google Scholar]