Abstract
We hypothesize that basal hyperinsulinemia is synergistically mediated by an interplay between increased oxidative stress and excess lipid in the form of reactive oxygen species (ROS) and long-chain acyl-CoA esters (LC-CoA). In addition, ROS production may increase in response to inflammatory cytokines and certain exogenous environmental toxins that mislead β-cells into perceiving nutrient excess when none exists. Thus, basal hyperinsulinemia is envisioned as an adaptation to sustained real or perceived nutrient excess that only manifests as a disease when the excess demand can no longer be met by an overworked β-cell. In this article we will present a testable hypothetical mechanism to explain the role of lipids and ROS in basal hyperinsulinemia and how they differ from glucose-stimulated insulin secretion (GSIS). The model centers on redox regulation, via ROS, and S-acylation–mediated trafficking via LC-CoA. These pathways are well established in neural systems but not β-cells. During GSIS, these signals rise and fall in an oscillatory pattern, together with the other well-established signals derived from glucose metabolism; however, their precise roles have not been defined. We propose that failure to either increase or decrease ROS or LC-CoA appropriately will disturb β-cell function.
Introduction
Advances in diabetes care, focused primarily on controlling blood glucose, have not translated into the anticipated disease reversal or drug development to correct the underlying molecular defects. Consequently, metabolic disorders continue to rise. Current approaches ignore basal hyperinsulinemia, an underappreciated early β-cell defect. The failure of the traditional approaches leads us to consider the possibility that defective glucose-stimulated insulin secretion (GSIS) is a distal problem in the development of type 2 diabetes (T2D) and a logical consequence of sustained earlier abnormalities. Such early changes include the following: exposure to fuel excess, or failure to appropriately store excess fuel, ectopic deposit of lipids, elevated basal insulin secretion, insulin resistance, and chronic low-grade inflammation. The critical role of alternating increases and decreases in Ca2+ and the ATP-to-ADP ratio for GSIS by pancreatic β-cells and the prevailing consensus model of GSIS that implicates KATP channels have previously been reviewed in detail (1–3). The participation and the specific roles of lipid and ROS are more complex and less well defined and generally not included in these models but are, nevertheless, essential for insulin secretion (4–7). Much progress has been made in recent years in our understanding of several key amplification pathways. Like Ca2+ and the ATP-to-ADP ratio, these amplifying signals have both stimulatory and inhibitory impacts on aspects of signal transduction or metabolism that appear to be important for precise regulation of β-cell metabolism and insulin secretion and define a pattern of interdigitation to generate robust oscillatory signals in response to fuel stimulation.
Insulin secretion functions mainly to promote the usage and storage of fuels including glucose and lipids and but also amino acids. Thus, the response of the β-cell and the organism as a whole to glucose alone may be different than that to excess amino or fatty acids or the combined need to store multiple nutrients, which presumably requires responses that are sensitive to single as well as multiple nutrients. It is proposed that when such adaptive responses, induced by excess nutrients, do not achieve efficient fuel storage and successfully decrease circulating fuel levels, the sustained responses and associated stimuli remain and maintain basal and nocturnal hyperinsulinemia. This results in continuous β-cell work that may ultimately exceed β-cell insulin biosynthetic and secretory capacity resulting in impaired GSIS and β-cell failure.
In this article we will consider possible mediators of basal hypersecretion of insulin by the β-cells in the evolution toward obesity and T2D. Although insulin resistance is frequently considered the proximal cause of T2D, no mechanism has been found to explain how this metabolic information is communicated to the β-cell to stimulate basal or nocturnal insulin secretion. Despite half a century of research the sequence of changes preceding T2D is not established, and thus the temporal and causal relationships among obesity, lipid abnormalities, insulin resistance and hyperinsulinemia remain largely undetermined. The problem is likely related to the fact that T2D is a much more heterogenous (8), reversible, environmental, and fuel excess–driven disease than was previously thought a decade ago (9). Acute and chronic effects of glucose and fatty acids on islets have long been studied at high nutrient concentrations for lengths of time in excess of that required to document an effect. Early changes measured after addition of more physiologically relevant smaller nutrient increments are warranted in future studies to unveil sequential causality.
In this article, we hypothesize that basal and nocturnal hyperinsulinemia is a manifestation of β-cell dysfunction at the root of obesity, adaptive insulin resistance, and T2D and propose that understanding its mechanism may facilitate earlier interventions that can prevent or reverse some forms of T2D. Our testable mechanistic model explaining hyperinsulinemia places ROS and lipid signaling molecules in central regulatory roles for promoting basal hypersecretion of insulin. However, these signals can also participate in GSIS to varying degrees together with other important β-cell functions such as the control of insulin biosynthesis. Our model further suggests that these signals for hyperinsulinemia, in the absence of oscillatory glucose metabolism, may not be lowered periodically as they are during GSIS, leading to nonoscillatory sustained secretion that is known to predict β-cell dysfunction (10).
Redox Control and the Dual Role of ROS: β-Cell Dysfunction and Essentiality for Secretion
Excessive calorigenic nutrients increase mitochondrial redox and hence ROS production by the electron transport chain (ETC), whether the fuel is glucose, fat, or amino acids (Fig. 1). Mitochondrial ROS is produced at complex I and III of the ETC in response to fuel in excess of energy needs as a consequence of increases in mitochondrial NADH levels when ADP concentrations are low and in the absence of an increased ATP demand (11). A unique characteristic of β-cells is their relatively low expression of antioxidant enzymes (12,13). This renders β-cells susceptible to oxidative damage on the one hand but, on the other hand, allows ROS levels to remain elevated longer and move from mitochondria to cytosol. Elevated mitochondrial ROS enters the cytosol via aquaporin and the mitochondrial permeability transition pore of the inner mitochondrial membrane and the voltage-dependent anion channel in the outer mitochondrial membrane. Opening of the permeability transition pore (14) allows the efflux of ROS and possibly other molecules to the cytosol (15). A role for cytosolic ROS production during GSIS has also recently been attributed to NADPH oxidase 4 (NOX4) (16). This provides a system in which ROS can act as an indicator of nutrient excess and an effective signal for cytosolic processes including insulin secretion. Indeed, stimulatory glucose increases intracellular H2O2 and superoxide production in rodent islets (17). Variations in both ROS species are glucose concentration–dependent, reaching a maximum at 10–15 mmol/L glucose, with higher glucose levels having less effect (18). Consistent with a direct signaling role for ROS, insulin secretion is also stimulated by either low concentrations of exogenous H2O2 or diethyl maleate, which generates intracellular H2O2 (6,19). Furthermore, provision of ROS scavengers, including cell-permeable catalase or N-acetyl-l-cysteine, decreases H2O2 accumulation and inhibits GSIS (16,20). Finally, Leloup et al. (20) have demonstrated using a detailed pharmacological approach with antioxidants and mitochondrial ETC inhibitors that mitochondrial ROS production is essential for GSIS.
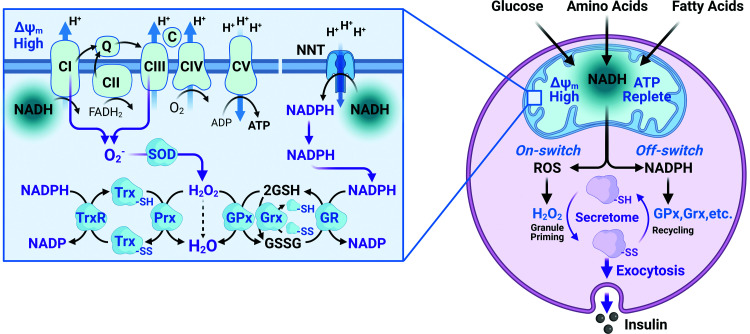
Figure 1.
ROS generation and cycling between mitochondria and cytosol. Left: ROS are produced by complex I and III (CI and CIII) of the ETC in response to increased mitochondrial NADH that donates electrons to the ETC, resulting in superoxide (O2−) production. Superoxide dismutase (SOD) rapidly converts O2− to H2O2. Nicotinamide nucleotide transhydrogenase (NNT) controls mitochondrial ROS levels. NNT spans the inner mitochondrial membrane converting NADH, in excess of energy needs, to NADPH. The reaction is driven by the proton gradient, occurs in the mitochondrial matrix, and is regulated by availability of NADH. In the absence of significant catalase, H2O2 is converted to water by the glutathione peroxidase (GPx) and reduced peroxiredoxin (Prx). The oxidized reactants thus formed—glutathione disulfide (GSSG), peroxiredoxin (PRXSS) (not pictured), and thioredoxin (TrxSS)—use NADPH to restore the reduced state. Glutaredoxin (Grx) reduces proteins by oxidizing glutathione (GSH), which is restored by further NADPH consumption by glutathione reductase (GR). Right: ROS produced in the mitochondria by elevated glucose, FFA, or amino acids impact the cytosolic redox state together with NADPH generated by NNT. Cytosolic H2O2 regulates activity of numerous proteins with accessible reactive cysteines (e.g., the endoplasmic reticulum Ca2+ pump, SERCA). Excess fuel also increases tricarboxylic acid cycle intermediates (citrate, isocitrate, and malate) that are transported into the cytosol and generate NADPH that converts H2O2 to H2O through the actions of peroxidases, resetting the cycle.
The consequences of increases in ROS on various biological processes are concentration dependent, with lower ROS levels serving important signaling functions and high levels potentially causing severe damage. The negative consequences of excess ROS are referred to as oxidative stress, an important risk factor for β-cell dysfunction (21). Acute exposure of isolated mouse islets or INS-1(832/13) cells to oxidative stressors, including arsenite, 4-hydroxynonenal, and methylglyoxal, impairs GSIS (6,19). At the same time, ROS dose dependently stimulates β-cell proliferation at low levels, but at high levels ROS inhibits proliferation (22). Finally, ROS is part of a major communication system that both directly and via the thiol redox state influences hundreds of proteins containing reactive cysteines (23,24) with potentially opposing functions, such as phosphatases and kinases (25–28), including pyruvate kinase (29), which directly regulates KATP channels (3). This complexity makes single target modulation by ROS of pathways implicated in insulin biosynthesis and secretion unlikely. The accessible thiol moiety of cysteine contained in numerous proteins participates in many biologic modifications including sulfenylation and disulfide formation, higher cellular oxidation states, S-acylation, nitrosylation, persulfidation, and other modifications (30). ROS is sensed by reactive thiols as part of the redox chemistry underlying numerous essential cellular processes with a particular impact on phosphatases, SERCA (31), and S-acylated proteins involved in vesicle trafficking (32). SERCA targeting by ROS may also participate in basal hypersecretion by causing ER stress (33) and mitochondrial dysfunction through effects on voltage-dependent anion channel (15). Sustained excess ROS alters the thiol redox state, which is communicated via the circulation throughout the entire body (34). Despite this complexity of ROS signaling, there is a need to define the key targets modified by ROS that are linked to insulin secretion, as so far none have been identified. The potential for complex regulation by multiple or interacting ROS-induced signals must be considered.
Fortunately, ROS are normally tightly controlled and increased levels are usually transient. The large proton gradient between mitochondria and cytosol that drives ATP synthase also drives the nicotinamide nucleotide transhydrogenase (NNT), one of the major enzymes that reversibly converts NADH to NADPH, needed to maintain mitochondrial ROS homeostasis, together with isocitrate dehydrogenase (IDH)-2, glutathione reductases, and peroxidases (35–37) (Fig. 1). Low levels of ROS are produced as a by-product of oxidative phosphorylation during active respiration, but ROS production increases significantly when ATP is sufficient, energy demand is low, and fuel is adequate or excessive, in the basal state (38). Such alternations between active and basal respiration are reflected in oscillatory oxygen consumption, which indeed is well-documented (39,40) and corresponds, as well, with the oscillations in insulin secretion in response to elevations in glucose levels. ROS production has two important consequences. First, it is a sensitive indicator of both fuel and ATP sufficiency. Second, its metabolism explains some of the proton leak under basal conditions (15,36,37). Conversion of NADH to NADPH by NNT uses electrons that could otherwise support ATP production. This appropriate decrease in energy efficiency only occurs under conditions where fuel supply is plentiful and exceeds energy demand and is rapidly reversed in response to increased ADP levels. When fuel supply is low, NADH levels drop, NADPH production ceases, and mitochondrial ROS can no longer be effectively scavenged. The importance of adequate ROS handling is evident in the redundancy of NADPH-generating mechanisms that are present in the mitochondria (Fig. 1) that appear to compensate for the loss of NNT in C57Bl/6J mice. Thus, effective ROS handling is essential to maintain normal β-cell function and only under these conditions can ROS provide a transient and positive signal. Our model has focused on NNT as a major source of the NADPH needed to remove ROS; however, like many critical functions, there are important redundancies including mitochondrial isocitrate dehydrogenase-2 and malic enzyme-3. These enzymes also contribute to NADPH supply, although the relative control strength of NNT, IDH2, and ME1 has not been assessed in islets. Control strength is an important concept where the fractional contribution of each component, determined by incremental inhibition, is considered, as opposed to the essentiality of each component, determined by knockdown experiments.
Redox Control of β-Cell Function
It is proposed that linked redox cycles involve both mitochondria and cytosol as illustrated in Fig. 1. In the mitochondrial redox cycle, fuel in excess of that needed to maintain the ATP-to-ADP ratio is accompanied by decreased oxidative phosphorylation and elevated mitochondrial NADH. Elevated NADH generates ROS via the ETC that is rapidly converted to H2O2 by superoxide dismutase. In addition, elevated NADH at high mitochondrial membrane potential (ΔΨm) is converted to NADPH via NNT. Flux through NNT is driven by the proton gradient, eventually lowering ΔΨm, which favors the flux of electrons from NADH away from NNT to the ETC with resultant restoration of ΔΨm. The consequent increase in ETC flux then lowers NADH and ROS production. If fuel remains excessive the cycle is repeated.
The important signaling molecule, H2O2, equilibrates across the mitochondrial membrane into the cytosol where an NADPH-dependent cytosolic redox cycle is proposed to participate in exocytosis (Fig. 1). H2O2, transported from the mitochondria to the cytosol, oxidizes proteins with reactive cysteines, importantly inhibiting protein serine-threonine and tyrosine phosphatases (28,41,42). Inhibition of phosphatases serves to activate kinases that phosphorylate proteins involved in vesicle trafficking, thus participating in enhancing exocytosis. Indeed, Santo-Domingo et al. (43) have documented phospho-regulation of many elements linked to signal transduction, cytoskeleton organization, vesicle trafficking, and vesicle-mediated exocytosis within the first 5 min following glucose stimulation, although the relevant kinases have not been identified. Elevated fuel-mediated anaplerosis facilitates efflux of malate and isocitrate into the cytosol. Importantly, NADPH generated by cytosolic malic enzyme (ME1), IDH-1, or the pentose phosphate pathway may terminate the ROS signal through conversion of H2O2 to H2O by cytosolic NADPH-dependent peroxidases or amplify the ROS signal through activation of NOX4 (16). Since the products of ME1 and IDH-1 are returned to the mitochondria in exchange for their substrates, control of NADPH production depends on its rate of consumption or the rate of ROS production. The relative contribution of each of these cytosolic NADPH-generating reactions differs among species: ME1 is highest in rat islets and lowest in mouse islets, whereas IDH-1 is highest in mouse islets and both are expressed in human islets. The relative contributions of the pentose shunt and the NADP-dependent dehydrogenases and their potential redundancy have not been explored, and the possibility of variably shared control strength must be considered. Importantly, glucose metabolism controls the essential “off-response” by providing glucose-6-phosphate to the pentose shunt and malate or isocitrate derived from anaplerosis to their cytosolic enzymes. This model predicts that inhibition of the peroxidases fueled by NADPH due to inhibition or knockout of the NADPH-producing enzymes will initially enhance exocytosis, as documented by the Prentki group (44), but may ultimately cause basal hyperinsulinemia and possibly ROS-mediated cell damage.
Experiments to test these redox-mediated signaling pathways have not been performed in β-cells but are entirely feasible. In particular, it is possible to determine the time course and concentration dependence of ROS-mediated effects by modulating the intracellular redox state using extracellular redox clamps (34) to titrate the redox state. This can be done using a range of fixed ratios of β-hydroxybutyrate to acetoacetate, cysteine to cysteine, or glutathione to glutathione disulfide or by direct addition of µmol/L H2O2 to determine effects on insulin secretion, protein phosphorylation, transcriptional activation, and energy metabolism.
The Essential Role of Lipid in GSIS
It is well established that glucose does not stimulate insulin secretion in lipid-depleted islets. Stein, McGarry, and colleagues documented the absence of GSIS in perfused pancreas from starved rats that could be restored by the addition of free fatty acid (FFA) to the perfusate (7). Importantly, intracellular lipids are essential even when glucose is the only fuel provided. Data from the Prentki and Corkey groups have shown that stimulatory glucose increases cytosolic malonyl-CoA in a concentration-dependent manner (45,46). Figure 2 illustrates malonyl-CoA inhibition of CPT1 to block long-chain acyl-CoA ester (LC-CoA) transport into the mitochondria, thus inhibiting β-oxidation. This decreases mitochondrial and increases cytosolic LC-CoA, as well as the formation of lipid products derived from LC-CoA that may impact insulin secretion (45–47). The Prentki laboratory has also provided evidence in support of glucose-responsive triglyceride (TG)/FFA cycling to reesterify FFA and to generate lipids that participate in exocytosis and signal transduction (Fig. 2) (48,49). Such cycling is predicted to increase and decrease cytosolic LC-CoA levels in coordination with glycolytic flux and the cytosolic redox state. The NADH produced during glycolytic flux through GAPDH must be converted back to NAD to allow rapid glycolytic flux. This can be, at least partially, achieved through production of glycerol-3-phosphate (Gro3P) via Gro3P dehydrogenase in response to increases in NADH during oscillatory glycolysis. Gro3P then provides the backbone for TG and other complex lipid formation (Fig. 2). Alternatively, Gro3P can be lost to the cell, via phosphoglycerate phosphatase, or recycled to dihydroxyacetone phosphate, via the Ca2+-dependendent mitochondrial Gro3P oxidase. The relative fluxes through each of these pathways have not yet been determined.
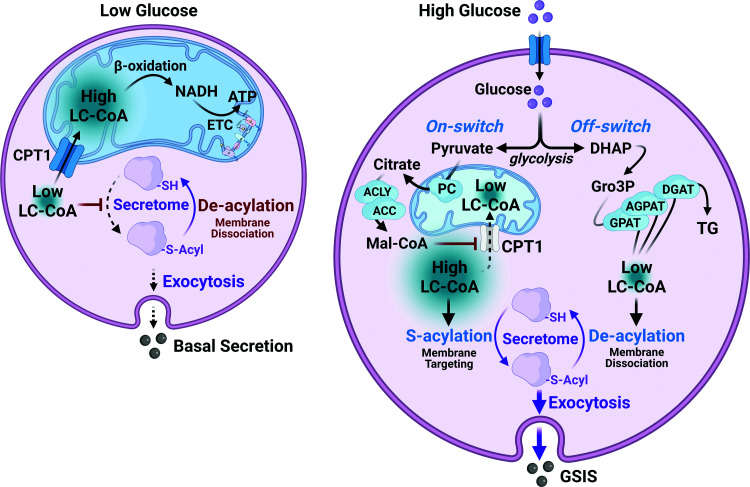
Figure 2.
LC-CoA cycling in response to glucose stimulation. In the basal state (left), energy demand is met by mitochondrial import of LC-CoA and β-oxidation that generates acetyl-CoA and NADH that is consumed by ETC. Glucose metabolism (right) generates pyruvate that enters the mitochondria and forms citrate via pyruvate carboxylase (PC). Citrate is transported into the cytosol leading to increased malonyl-CoA (Mal-CoA). Malonyl-CoA inhibits CPT1 and LC-CoA transport into the mitochondria, preventing β-oxidation and increasing cytosolic LC-CoA–mediated S-acylation that promotes membrane association of the protein secretome. Increased glycolytic flux also increases dihydroxyacetone phosphate (DHAP) and glycerol 3-phosphate (Gro-3P), which complex with LC-CoA, forming complex lipids and lowering LC-CoA to reset the cycle.
Deeney et al. (4) have shown that direct addition of LC-CoA to permeabilized clonal β-cells, in the presence of ATP, stimulates exocytosis, independent of changes in Ca2+. Using islets incubated at basal glucose, it was further demonstrated that LC-CoA increased granule fusion, with consequent increased plasma membrane capacitance that could be prevented by cerulenin, an inhibitor of S-acylation reactions, implying that elevations in LC-CoA are involved in vesicle trafficking to the plasma membrane (Fig. 2). It is of interest that other putative S-acylation inhibitors also inhibit insulin secretion (50,51), although none of these inhibitors are highly specific.
Our model proposes that increases in LC-CoA, S-acylation, and complex lipid products derived from LC-CoA are involved in insulin granule trafficking and exocytosis. The model also provides a mechanism whereby cytosolic LC-CoA can be decreased and increased through complexing with glycolytically derived Gro3P to form diglycerides, monoglycerides, and TGs as key components of TG/FFA cycling (Fig. 2). Indeed, release of glycerol from islets in response to secretory stimuli supports the latter process (18,52). Reesterification of FFA released from TG during lipolysis or following protein deacylation is accomplished by acyl-CoA synthetases and requires ATP. Acyl-CoA synthetases, in addition to LC-CoA, also form AMP and pyrophosphate. Finally, it is important to note that an additional molecule of ATP is lost because Gro3P synthesis occurs before the ATP-generating steps in glycolysis. Thus, it should be noted that lipid cycling is a major energy-consuming process that contributes to the oscillatory lowering of the ATP-to-ADP ratio. Indeed, a significant fraction of glucose is diverted to glycerol (18); however, the percentage of glucose flux diverted to lipid cycling has not yet been determined at different glucose concentrations.
Based on extensive evidence mainly in neural systems, it is hypothesized that an S-acylation/deacylation cycle (reaction below) is required for trafficking of the secretory vesicle between the Golgi and the plasma membrane (53). The key steps in this cycle are illustrated in Fig. 2. Pyruvate, derived from oscillatory glycolysis, is converted in the mitochondria to both acetyl-CoA and oxaloacetate that together form citrate. Citrate is then transported down its concentration gradient from the mitochondria to the cytosol where malonyl CoA is formed via citrate lyase and acetyl-CoA carboxylase. Malonyl-CoA potently inhibits CPT1, which controls LC-CoA entry into the mitochondria, thus decreasing mitochondrial and increasing cytosolic LC-CoA. We propose that the increased cytosolic LC-CoA availability, particularly palmitoyl-CoA, S-acylates proteins involved in vesicle transport and trafficking, similar to what has been documented in neural cells (53).
 |
where SH-Protein is palmitoylated protein, Palm is palmitate, Palm-S-Protein is reduced protein, and CoASH is free CoA. Acylated proteins then associate with the plasma membrane and secretory granule proteins, leading to the documented increase in membrane capacitance (4,54). One or more additional signals including Ca2+, phosphorylated proteins, and ROS may synergistically interact with acylated membrane–associated secretory granules to finally promote fusion and exocytosis. Termination of the LC-CoA–mediated signal results from thioesterase-mediated deacylation. Oscillations in glycolysis, thus, coordinate with oscillations in lipolysis to increase and decrease cytosolic LC-CoA levels. As is the case with ROS, the off-response requires glucose metabolism to provide Gro3P to decrease and incorporate LC-CoA into lipids, thus diverting some glucose carbon from energy production.
The molecular mechanism and specific protein targets involved in LC-CoA–stimulated exocytosis have not been quantitatively determined but are hypothesized to involve S-acylation as well as interactions with ROS, since both target reactive thiols in proteins. In various neural systems, ion channels, vesicle transport and trafficking, and small GTPases have been documented as targets, among many acylation-sensitive proteins (53). Interestingly, vesicle fusion to target membranes requires SNARE complex proteins known to play important roles in β-cell exocytosis. Many of these have been shown to be S-acylated in neurons, including syntaxin, SNAP25, VAMP2, and synaptotagmin-1 (55–58). S-acylation also controls many aspects of protein sorting, membrane localization, and lipid metabolism. Involvement of the commonly acylated proteins has been documented in β-cells—but not whether S-acylation is essential, transient, or regulated during insulin secretion. Proteins involved in vesicle trafficking are modified not solely by S-acylation but also by phosphorylation and other lipid regulation. The molecular details of the roles of lipid in β-cell exocytosis remain an important area for future exploration.
Differences Between GSIS and Basal Secretion
We hypothesize that basal secretion involves some of the same signals that participate in GSIS. The cellular content of both lipid and ROS increase in response to fuel excess, inflammation, and certain exogenous agents. In contrast to starvation where only lipids are elevated but fuels are not excessive, both lipids and ROS are higher in metabolic disease states such as obesity, diabetes, and hepatic steatosis (Fig. 3). Lipid plus ROS inducing increases in basal secretion appropriately serves the function of promoting storage in response to the perception of nutrient excess as well as stimulating insulin-mediated adipocyte TG synthesis. In support of such a concept, β-cell lipid accumulation positively correlates with increased glucose sensitivity and elevated basal insulin secretion (59). However, this process is self-limiting in the absence of glucose, since insulin biosynthesis is not stimulated during such lipid/ROS-mediated secretion (60,61). This mechanism could ultimately deplete insulin stores and induce β-cell exhaustion, as occurs in diabetes.
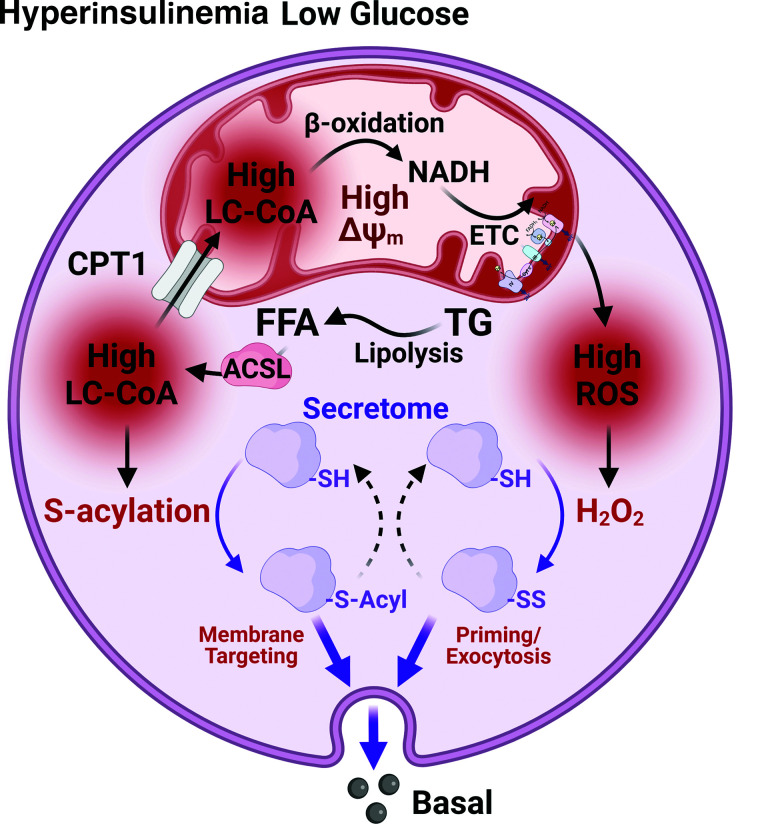
Figure 3.
Basal hyperinsulinemia is driven via the synergistic actions of ROS and LC-CoA on exocytosis. In the basal state in the presence of sustained lipid elevation, FFA are activated to LC-CoA via acyl-CoA synthetases (ACSL). The LC-CoA pool is increased in both mitochondrial and cytosolic compartments, and β-oxidation leads to increased NADH and ROS production. Elevated cytosolic LC-CoA leads to S-acylation, and elevated ROS leads to further activation of the protein secretome. The off-response is absent and insulin secretion prevails.
LC-CoA has also been shown to increase PKC activity in clonal pancreatic β-cells (62). Elevated serum FFA, in addition to increasing LC-CoA in the β-cell, may provide signals through binding to the GPR40 FA receptor, resulting in increased PKC activity (63). FFA have also been shown to increase PKC-sensitive NOX isoforms and increase ROS production in the β-cell (64). Signaling through this pathway may be increased in obesity and contribute to increased basal insulin secretion. Interestingly, when fasting levels of FFA in children and adults were divided into tertiles it was discovered that subjects in the highest tertile exhibited the lowest insulin secretion after an oral glucose tolerance test. Adult subjects in the highest tertile were found to be at a threefold increased risk for impaired glucose tolerance or T2D after 8 years. Male adults in this group also had a significantly higher fasting insulin level (65).
The relative control strength of each of the essential components of GSIS or basal hypersecretion is not known. Control strength may indeed be variable such that lipids, ROS, the ATP-to-ADP ratio, and Ca2+ could each contribute more or less extensively under different conditions. Such shared flexible regulation would permit different degrees of influence of each of the essential cycles to provide adaptive responses to different fuels and fuel combinations with very similar net secretion. Such flexibility also would provide exquisite control over the critical need for β-cells to secrete insulin in order to store all fuels appropriately, including lipids and amino acids. The main difference proposed between basal and GSIS is not so much in the “on” signals but in the absence of the glucose-dependent “off” signals. Assessment of the control strength of each essential component of the signaling cascade can be made by separately determining the percentage decrease in that component needed to diminish insulin secretion. This can be done using partial knockdowns or irreversible inhibitors as illustrated in Alarcon et al. (66). Conversely, the absence of a phenotype may reveal unexpected redundancy and shared control (67).
Resolution of these important opposing influences of LC-CoA in GSIS may be necessary for the alternating stimulatory and inhibitory effects of oscillatory metabolism, whereas basal hypersecretion may result from a failure to remove the stimulatory signals appropriately, thus disrupting the coordinated metabolic signaling involved in GSIS. Testing the role of lipid modulation of exocytosis under basal and GSIS will be greatly facilitated by the enhancement and application of tools that monitor protein S-acylation and deacylation in real time in order to establish its involvement in the sequence of events that occur in β-cells. Novel methodology is now available that can monitor the biochemical exchange of acyl groups on cysteines with defined mass tags. Mass tag labeling reveals site-specific and endogenous levels of protein S-acylation and enables direct visualization of S-acylated protein levels (68). In addition, a tandem labeling and detection method to simultaneously monitor dynamic S-palmitoylation and protein turnover may also be applicable to β-cells (69).
Inhibitory Roles of Lipid
Clearly, physiological levels of FFA bound to physiological levels of albumin usually do not stimulate insulin secretion acutely unless glucose is also present. In fact, during starvation circulating FFA levels more than double but insulin secretion decreases (70,71), since the starvation-induced increase in FFA does not indicate excess fuel. Both FFA and total LC-CoA rise in response to starvation in order to generate enough ATP to maintain energy balance (72). Several possible reasons for diminished secretion in starvation despite the presence of elevated lipids need to be considered. First, in starvation, most of the LC-CoA is localized to the mitochondria since lipid becomes the primary fuel source. Second, sustained elevation of acylated proteins involved in exocytosis, if present, may be subject to further modifications that render them nonfunctional, such as oxidation or ubiquitination. Finally, the process of exocytosis may lack appropriate collaborators such as ATP, Ca2+, or ROS. However, long-term incubation of islets in excess lipid increases membrane capacitance following membrane depolarization, consistent with an association of docked and primed vesicles at the plasma membrane (73) that may be essential but not sufficient to promote insulin secretion.
It has been documented that LC-CoA is the most potent physiological modulator of the KATP channel but it opposes the ability of ATP to close the channel (74–76); however, stimulatory glucose can still close the channel even after exposure to elevated lipids (73). This could be explained by the ability of glucose to decrease cytosolic LC-CoA by converting it to complex lipids via glucose-derived Gro-3P (Fig. 2). Alternatively, activated pyruvate kinase or PEP may displace LC-CoA (3,77).
Finally, LC-CoA modulates the adenine nucleotide translocase and can lower the ATP-to-ADP ratio by inhibiting adenine nucleotide translocase (78,79). Early work by Shrago et al. (80) documented LC-CoA regulation of adenine nucleotide translocase in liver and heart, such that a rise in cytosolic LC-CoA lowers the ATP-to-ADP ratio needed to drive energy production and a decrease in cytosolic LC-CoA has the opposite effect. Such inhibition and activation may also participate in oscillatory oxygen consumption (39); however, the temporal relationships among changes in the cytosolic ATP-to-ADP ratio and flux through the adenine nucleotide translocase have not been determined in β-cells.
In considering solutions to the problems of metabolic dysfunction, it is essential to perform experiments under physiologically relevant conditions in human and rodent model systems. FFA in vivo are always a mixture of saturated and unsaturated FFA, mainly long chain, bound to albumin in a ratio that rarely exceeds 2:1. Selection of model systems may also be important, since responses to physiologically relevant, excess FFA have different effects in juvenile and adult as well as human and mouse islets (81). Treating differences in responses to nonphysiologically relevant stimuli as defects, or the late consequences of inadequate adaptive responses to major stresses, is not likely to be beneficial. Targeting single proteins involved with one of these essential signals is unlikely to be an effective long-term strategy, since they are important in both basal and GSIS and critical functions tend to have redundancies. Resolution of the escalating burdens of metabolic disease requires greater understanding of the earliest events that induce basal insulin hypersecretion in humans as well as consideration of alternative models of potential dysfunction such as impaired off-response.
Summary
In this perspective, a multiplicity of stimulatory and inhibitory interactions are hypothesized to be important and interdependent. We propose that hyperinsulinemia-induced β-cell exhaustion, that begins long before GSIS is impaired, is mediated by elevations in ROS and LC-CoA that do not stimulate insulin biosynthesis. These essential signals, like the other well-established signals derived from glucose metabolism, rise and fall during normal oscillatory GSIS. We hypothesize that failure to decrease ROS and LC-CoA appropriately, specifically, sustained elevations in both ROS and LC-CoA, leads to basal hyperinsulinemia, decreased insulin content, and diminished responsiveness to stimulatory glucose. So while hyperinsulinemia may be initially an adaptation, it manifests as a disease when the excess demand can no longer be met by overworked β-cells. Since not all individuals with basal hyperinsulinemia will develop diabetes, there may be important differences in the capacity of β-cells to respond to stimuli or in the ability to increase β-cell mass in response to demand. Thus, identification of fasting hyperinsulinemia may help identify individuals at greater risk for T2D prior to development of impaired GSIS where interventions may still salvage remaining β-cell function.
Article Information
Acknowledgments. The authors thank Richard Kibbey, James Johnson, and Marc Prentki for useful discussions, helpful suggestions, and critical comments during the development of this article. Figures were created with BioRender (biorender.com).
Funding. M.J.M. acknowledges funding from National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (R01DK113103 and R01DK127637).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
References
- 1. Henquin JC. Regulation of insulin secretion: a matter of phase control and amplitude modulation. Diabetologia 2009;52:739–751 [DOI] [PubMed] [Google Scholar]
- 2. Rorsman P, Ashcroft FM. Pancreatic β-cell electrical activity and insulin secretion: of mice and Men. Physiol Rev 2018;98:117–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lewandowski SL, Cardone RL, Foster HR, et al. Pyruvate kinase controls signal strength in the insulin secretory pathway. Cell Metab 2020;32:736–750.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Deeney JT, Gromada J, Høy M, et al. Acute stimulation with long chain acyl-CoA enhances exocytosis in insulin-secreting cells (HIT T-15 and NMRI beta-cells). J Biol Chem 2000;275:9363–9368 [DOI] [PubMed] [Google Scholar]
- 5. Deeney JT, Tornheim K, Korchak HM, Prentki M, Corkey BE. Acyl-CoA esters modulate intracellular Ca2+ handling by permeabilized clonal pancreatic beta-cells. J Biol Chem 1992;267:19840–19845 [PubMed] [Google Scholar]
- 6. Pi J, Bai Y, Zhang Q, et al. Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes 2007;56:1783–1791 [DOI] [PubMed] [Google Scholar]
- 7. Stein DT, Esser V, Stevenson BE, et al. Essentiality of circulating fatty acids for glucose-stimulated insulin secretion in the fasted rat. J Clin Invest 1996;97:2728–2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fuchsberger C, Flannick J, Teslovichet al. The genetic architecture of type 2 diabetes. Nature 2016;536:41–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sargis RM, Simmons RA. Environmental neglect: endocrine disruptors as underappreciated but potentially modifiable diabetes risk factors. Diabetologia 2019;62:1811–1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. O’Rahilly S, Turner RC, Matthews DR. Impaired pulsatile secretion of insulin in relatives of patients with non-insulin-dependent diabetes. N Engl J Med 1988;318:1225–1230 [DOI] [PubMed] [Google Scholar]
- 11. Murphy MP. How mitochondria produce reactive oxygen species. Biochem J 2009;417:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lenzen S, Drinkgern J, Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med 1996;20:463–466 [DOI] [PubMed] [Google Scholar]
- 13. Lei XG, Vatamaniuk MZ. Two tales of antioxidant enzymes on β cells and diabetes. Antioxid Redox Signal 2011;14:489–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Taddeo EP, Alsabeeh N, Baghdasarian S, et al. Mitochondrial proton leak regulated by cyclophilin D elevates insulin secretion in islets at nonstimulatory glucose levels. Diabetes 2020;69:131–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rottenberg H, Hoek JB. The path from mitochondrial ROS to aging runs through the mitochondrial permeability transition pore. Aging Cell 2017;16:943–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Plecitá-Hlavatá L, Jabůrek M, Holendová B, et al. Glucose-stimulated insulin secretion fundamentally requires H2O2 signaling by NADPH oxidase 4. Diabetes 2020;69:1341–1354 [DOI] [PubMed] [Google Scholar]
- 17. Bindokas VP, Kuznetsov A, Sreenan S, Polonsky KS, Roe MW, Philipson LH. Visualizing superoxide production in normal and diabetic rat islets of Langerhans. J Biol Chem 2003;278:9796–9801 [DOI] [PubMed] [Google Scholar]
- 18. Mugabo Y, Zhao S, Lamontagne J, et al. Metabolic fate of glucose and candidate signaling and excess-fuel detoxification pathways in pancreatic β-cells. J Biol Chem 2017;292:7407–7422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pi J, Zhang Q, Fu J, et al. ROS signaling, oxidative stress and Nrf2 in pancreatic beta-cell function. Toxicol Appl Pharmacol 2010;244:77–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leloup C, Tourrel-Cuzin C, Magnan C, et al. Mitochondrial reactive oxygen species are obligatory signals for glucose-induced insulin secretion. Diabetes 2009;58:673–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Robertson RP, Harmon J, Tran PO, Tanaka Y, Takahashi H. Glucose toxicity in beta-cells: type 2 diabetes, good radicals gone bad, and the glutathione connection. Diabetes 2003;52:581–587 [DOI] [PubMed] [Google Scholar]
- 22. Ahmed Alfar E, Kirova D, Konantz J, Birke S, Mansfeld J, Ninov N. Distinct levels of reactive oxygen species coordinate metabolic activity with beta-cell mass plasticity. Sci Rep 2017;7:3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bak DW, Bechtel TJ, Falco JA, Weerapana E. Cysteine reactivity across the subcellular universe. Curr Opin Chem Biol 2019;48:96–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fowler NJ, Blanford CF, de Visser SP, Warwicker J. Features of reactive cysteines discovered through computation: from kinase inhibition to enrichment around protein degrons. Sci Rep 2017;7:1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Skoko JJ, Attaran S, Neumann CA. Signals getting crossed in the entanglement of redox and phosphorylation pathways: phosphorylation of peroxiredoxin proteins sparks cell signaling. Antioxidants (Basel) 2019;8:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Krylatov AV, Maslov LN, Voronkov NS, et al. Reactive oxygen species as intracellular signaling molecules in the cardiovascular system. Curr Cardiol Rev 2018;14:290–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Go YM, Jones DP. The redox proteome. J Biol Chem 2013;288:26512–26520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang J, Wang X, Vikash V, et al. ROS and ROS-mediated cellular signaling. Oxid Med Cell Longev 2016;2016:4350965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Anastasiou D, Poulogiannis G, Asara JM, et al. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science 2011;334:1278–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Go Y-M, Chandler JD, Jones DP. The cysteine proteome. Free Radic Biol Med 2015;84:227–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Poole LB, Schoneich C. Introduction: what we do and do not know regarding redox processes of thiols in signaling pathways. Free Radic Biol Med 2015;80:145–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Burgoyne JR, Haeussler DJ, Kumar V, et al. Oxidation of HRas cysteine thiols by metabolic stress prevents palmitoylation in vivo and contributes to endothelial cell apoptosis. Faseb J 2012;26:832–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang IX, Ren J, Vadrevu S, Raghavan M, Satin LS. ER stress increases store-operated Ca 2+ entry (SOCE) and augments basal insulin secretion in pancreatic beta cells. J Biol Chem 2020;295:5685–5700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Corkey BE, Deeney JT. The redox communication network as a regulator of metabolism. Front Physiol 2020;11:567796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Albracht SP, Meijer AJ, Rydström J. Mammalian NADH:ubiquinone oxidoreductase (Complex I) and nicotinamide nucleotide transhydrogenase (Nnt) together regulate the mitochondrial production of H(2)O(2)--implications for their role in disease, especially cancer. J Bioenerg Biomembr 2011;43:541–564 [DOI] [PubMed] [Google Scholar]
- 36. Hoek JB, Rydström J. Physiological roles of nicotinamide nucleotide transhydrogenase. Biochem J 1988;254:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mailloux RJ. Mitochondrial antioxidants and the maintenance of cellular hydrogen peroxide levels. Oxid Med Cell Longev 2018;2018:7857251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sharaf MS, Stevens D, Kamunde C. Mitochondrial transition ROS spike (mTRS) results from coordinated activities of complex I and nicotinamide nucleotide transhydrogenase. Biochim Biophys Acta Bioenerg 2017;1858:955–965 [DOI] [PubMed] [Google Scholar]
- 39. Porterfield DM, Corkey RF, Sanger RH, Tornheim K, Smith PJ, Corkey BE. Oxygen consumption oscillates in single clonal pancreatic beta-cells (HIT). Diabetes 2000;49:1511–1516 [DOI] [PubMed] [Google Scholar]
- 40. Gillis KD, Mossner R, Neher E. Protein kinase C enhances exocytosis from chromaffin cells by increasing the size of the readily releasable pool of secretory granules. Neuron 1996;16:1209–1220 [DOI] [PubMed] [Google Scholar]
- 41. Wright VP, Reiser PJ, Clanton TL. Redox modulation of global phosphatase activity and protein phosphorylation in intact skeletal muscle. J Physiol 2009;587:5767–5781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Corcoran A, Cotter TG. Redox regulation of protein kinases. FEBS J 2013;280:1944–1965 [DOI] [PubMed] [Google Scholar]
- 43. Santo-Domingo J, Galindo AN, Cominetti O, et al. Glucose-dependent phosphorylation signaling pathways and crosstalk to mitochondrial respiration in insulin secreting cells. Cell Commun Signal 2019;17:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Guay C, Joly E, Pepin E, et al. A role for cytosolic isocitrate dehydrogenase as a negative regulator of glucose signaling for insulin secretion in pancreatic β-cells. PLoS One 2013;8:e77097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Corkey BE, Glennon MC, Chen KS, Deeney JT, Matschinsky FM, Prentki M. A role for malonyl-CoA in glucose-stimulated insulin secretion from clonal pancreatic beta-cells. J Biol Chem 1989;264:21608–21612 [PubMed] [Google Scholar]
- 46. Prentki M, Vischer S, Glennon MC, Regazzi R, Deeney JT, Corkey BE. Malonyl-CoA and long chain acyl-CoA esters as metabolic coupling factors in nutrient-induced insulin secretion. J Biol Chem 1992;267:5802–5810 [PubMed] [Google Scholar]
- 47. Roduit R, Nolan C, Alarcon C, et al. A role for the malonyl-CoA/long-chain acyl-CoA pathway of lipid signaling in the regulation of insulin secretion in response to both fuel and nonfuel stimuli. Diabetes 2004;53:1007–1019 [DOI] [PubMed] [Google Scholar]
- 48. Prentki M, Corkey BE, Madiraju SRM. Lipid-associated metabolic signalling networks in pancreatic beta cell function. Diabetologia 2020;63:10–20 [DOI] [PubMed] [Google Scholar]
- 49. Prentki M, Madiraju SR. Glycerolipid/free fatty acid cycle and islet β-cell function in health, obesity and diabetes. Mol Cell Endocrinol 2012;353:88–100 [DOI] [PubMed] [Google Scholar]
- 50. Chen S, Ogawa A, Ohneda M, Unger RH, Foster DW, McGarry JD. More direct evidence for a malonyl-CoA-carnitine palmitoyltransferase I interaction as a key event in pancreatic beta-cell signaling. Diabetes 1994;43:878–883 [DOI] [PubMed] [Google Scholar]
- 51. Cheng H, Straub SG, Sharp GW. Protein acylation in the inhibition of insulin secretion by norepinephrine, somatostatin, galanin, and PGE2. Am J Physiol Endocrinol Metab 2003;285:E287–E294 [DOI] [PubMed] [Google Scholar]
- 52. Fex M, Mulder H. Lipases in the pancreatic beta-cell: implications for insulin secretion. Biochem Soc Trans 2008;36:885–890 [DOI] [PubMed] [Google Scholar]
- 53. Chamberlain LH, Shipston MJ. The physiology of protein S-acylation. Physiol Rev 2015;95:341–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Eliasson L, Proks P, Ammälä C, et al. Endocytosis of secretory granules in mouse pancreatic beta-cells evoked by transient elevation of cytosolic calcium. J Physiol 1996;493:755–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kang R, Wan J, Arstikaitis P, et al. Neural palmitoyl-proteomics reveals dynamic synaptic palmitoylation. Nature 2008;456:904–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Prescott GR, Gorleku OA, Greaves J, Chamberlain LH. Palmitoylation of the synaptic vesicle fusion machinery. J Neurochem 2009;110:1135–1149 [DOI] [PubMed] [Google Scholar]
- 57. Wheeler MB, Sheu L, Ghai M, et al. Characterization of SNARE protein expression in beta cell lines and pancreatic islets. Endocrinology 1996;137:1340–1348 [DOI] [PubMed] [Google Scholar]
- 58. Thurmond DC, Gaisano HY. Recent insights into beta-cell exocytosis in type 2 diabetes. J Mol Biol 2020;432:1310–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Erion KA, Berdan CA, Burritt NE, Corkey BE, Deeney JT. Chronic exposure to excess nutrients left-shifts the concentration dependence of glucose-stimulated insulin secretion in pancreatic β-cells. J Biol Chem 2015;290:16191–16201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bollheimer LC, Skelly RH, Chester MW, McGarry JD, Rhodes CJ. Chronic exposure to free fatty acid reduces pancreatic β cell insulin content by increasing basal insulin secretion that is not compensated for by a corresponding increase in proinsulin biosynthesis translation. J Clin Invest 1998;101:1094–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Skelly RH, Bollheimer LC, Wicksteed BL, Corkey BE, Rhodes CJ. A distinct difference in the metabolic stimulus-response coupling pathways for regulating proinsulin biosynthesis and insulin secretion that lies at the level of a requirement for fatty acyl moieties. Biochem J 1998;331:553–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yaney GC, Korchak HM, Corkey BE. Long-chain acyl CoA regulation of protein kinase C and fatty acid potentiation of glucose-stimulated insulin secretion in clonal beta-cells. Endocrinology 2000;141:1989–1998 [DOI] [PubMed] [Google Scholar]
- 63. Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev 2008;29:351–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Oliveira HR, Verlengia R, Carvalho CR, Britto LR, Curi R, Carpinelli AR. Pancreatic beta-cells express phagocyte-like NAD(P)H oxidase. Diabetes 2003;52:1457–1463 [DOI] [PubMed] [Google Scholar]
- 65. Salgin B, Ong KK, Thankamony A, Emmett P, Wareham NJ, Dunger DB. Higher fasting plasma free fatty acid levels are associated with lower insulin secretion in children and adults and a higher incidence of type 2 diabetes. J Clin Endocrinol Metab 2012;97:3302–3309 [DOI] [PubMed] [Google Scholar]
- 66. Alarcon C, Wicksteed B, Prentki M, Corkey BE, Rhodes CJ. Succinate is a preferential metabolic stimulus-coupling signal for glucose-induced proinsulin biosynthesis translation. Diabetes 2002;51:2496–2504 [DOI] [PubMed] [Google Scholar]
- 67. Bauchle CJ, Rohli KE, Boyer CK, et al. Mitochondrial efflux of citrate and isocitrate is fully dispensable for glucose-stimulated insulin secretion and pancreatic islet β-cell function. Diabetes 2021;70:1717–1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Percher A, Ramakrishnan S, Thinon E, Yuan X, Yount JS, Hang HC. Mass-tag labeling reveals site-specific and endogenous levels of protein S-fatty acylation. Proc Natl Acad Sci U S A 2016;113:4302–4307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhang MM, Tsou LK, Charron G, Raghavan AS, Hang HC. Tandem fluorescence imaging of dynamic S-acylation and protein turnover. Proc Natl Acad Sci U S A 2010;107:8627–8632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Cahill GF Jr. The Banting Memorial Lecture 1971. Physiology of insulin in man. Diabetes 1971;20:785–799 [DOI] [PubMed] [Google Scholar]
- 71. Cahill GF Jr. Fuel metabolism in starvation. Annu Rev Nutr 2006;26:1–22 [DOI] [PubMed] [Google Scholar]
- 72. Tubbs PK, Garland PB. Variations in tissue contents of coenzyme A thio esters and possible metabolic implications. Biochem J 1964;93:550–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Olofsson CS, Collins S, Bengtsson M, et al. Long-term exposure to glucose and lipids inhibits glucose-induced insulin secretion downstream of granule fusion with plasma membrane. Diabetes 2007;56:1888–1897 [DOI] [PubMed] [Google Scholar]
- 74. Bränström R, Leibiger IB, Leibiger B, Corkey BE, Berggren PO, Larsson O. Long chain coenzyme A esters activate the pore-forming subunit (Kir6. 2) of the ATP-regulated potassium channel. J Biol Chem 1998;273:31395–31400 [DOI] [PubMed] [Google Scholar]
- 75. Gribble FM, Proks P, Corkey BE, Ashcroft FM. Mechanism of cloned ATP-sensitive potassium channel activation by oleoyl-CoA. J Biol Chem 1998;273:26383–26387 [DOI] [PubMed] [Google Scholar]
- 76. Larsson O, Deeney JT, Bränström R, Berggren PO, Corkey BE. Activation of the ATP-sensitive K+ channel by long chain acyl-CoA. A role in modulation of pancreatic beta-cell glucose sensitivity. J Biol Chem 1996;271:10623–10626 [DOI] [PubMed] [Google Scholar]
- 77. Abulizi A, Cardone RL, Stark R, et al. Multi-tissue acceleration of the mitochondrial phosphoenolpyruvate cycle improves whole-body metabolic health. Cell Metab 2020;32:751–766.e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ciapaite J, Bakker SJ, Diamant M, et al. Metabolic control of mitochondrial properties by adenine nucleotide translocator determines palmitoyl-CoA effects. Implications for a mechanism linking obesity and type 2 diabetes. FEBS J 2006;273:5288–5302 [DOI] [PubMed] [Google Scholar]
- 79. Ciapaite J, van Eikenhorst G, Krab K. Application of modular control analysis to inhibition of the adenine nucleotide translocator by palmitoyl-CoA. Mol Biol Rep 2002;29:13–16 [DOI] [PubMed] [Google Scholar]
- 80. Shrago E, Woldegiorgis G, Ruoho AE, DiRusso CC. Fatty acyl CoA esters as regulators of cell metabolism. Prostaglandins Leukot Essent Fatty Acids 1995;52:163–166 [DOI] [PubMed] [Google Scholar]
- 81. Tong X, Dai C, Walker JT, et al. Lipid droplet accumulation in human pancreatic islets is dependent on both donor age and health. Diabetes 2020;69:342–354 [DOI] [PMC free article] [PubMed] [Google Scholar]