Abstract
Prohibitin-1 (PHB) is a multifunctional protein previously reported to be important for adipocyte function. PHB is expressed on the surface of adipose cells, where it interacts with a long-chain fatty acid (LCFA) transporter. Here, we show that mice lacking PHB in adipocytes (PHB adipocyte [Ad]-knockout [KO]) have a defect in fat tissue accumulation despite having larger lipid droplets in adipocytes due to reduced lipolysis. Although PHB Ad-KO mice do not display glucose intolerance, they are insulin resistant. We show that PHB Ad-KO mice are lipid intolerant due to a decreased capacity of adipocytes for LCFA uptake. Instead, PHB Ad-KO mice have increased expression of GLUT1 in various tissues and use glucose as a preferred energy source. We demonstrate that PHB Ad-KO mice have defective brown adipose tissue, are intolerant to cold, and display reduced basal energy expenditure. Systemic repercussions of PHB inactivation in adipocytes were observed in both males and females. Consistent with lower cellular mitochondrial content and reduced uncoupling protein 1 protein expression, brown adipocytes lacking PHB display decreased proton leak and switch from aerobic metabolism to glycolysis. Treatment of differentiating brown adipocytes with small molecules targeting PHB suppressed mitochondrial respiration and uncoupling. Our results demonstrate that PHB in adipocytes is essential for normal fatty acid uptake, oxidative metabolism, and adaptive thermogenesis. We conclude that PHB inhibition could be investigated as an approach to altering energy substrate utilization.
Introduction
An important function of adipose tissue (AT) is the maintenance of energy balance and healthy metabolism (1). Adipocytes are the lipid-storing cells of AT, dysfunction of which results in type 2 diabetes. Prohibitin-1 (PHB) is a multifunctional protein found in various cellular compartments and also secreted and is reported to be important for normal adipocyte function (2). Studies based on small-molecule ligands of PHB modifying its function have helped to map the diverse functions of this protein in metabolic, inflammatory, cardiac, connective tissue, and neurological diseases, as well as in cancer (3). A key insight on adipose function of PHB was the mouse model overexpressing PHB from the aP2 promoter, which resulted in increased adiposity and impaired glucose homeostasis despite elevated mitochondrial biogenesis (4). By screening for molecules that home to white AT (WAT), we previously identified a peptide (sequence KGGRAKD) that binds to PHB on the surface of adipose endothelium (5). In the search for PHB function in WAT endothelium, we have identified annexin A2 (ANX2), as a PHB-binding protein containing the amino acid sequence KGRRAED mimicked by the WAT-homing peptide KGGRAKD. We have shown that ANX2 and PHB are found in a protein complex present in the cell membrane lipid rafts of both mouse and human WAT (6). We subsequently demonstrated that PHB and ANX2 are found in complex with the fatty acid (FA) translocase CD36 and demonstrated that the ANX2-PHB interaction supports long-chain FA (LCFA) uptake by adipocytes in cell culture (7). However, the importance of PHB in adipose physiology still remained unclear due to the lack of in vivo adipocyte loss-of-function models.
The ability of adipocytes in WAT to store and metabolize lipids predetermines susceptibility to metabolic diseases (1). Visceral AT (VAT) mainly stores lipids, and its excessive expansion, inflammation, and dysfunction in obesity is linked with the metabolic syndrome (8). In contrast, subcutaneous AT (SAT) can protect from metabolic disease through generation of mitochondria-rich adipocytes specialized to activate lipolysis and burn lipids through adaptive thermogenesis (9). Like brown AT (BAT), beige adipocytes in SAT express uncoupling protein 1 (UCP1), which leaks protons to uncouple substrate oxidation from ATP synthesis (10). BAT and beige adipocytes both activate energy expenditure and can counteract the metabolic consequences of obesity in mice (1). A possible function of PHB in BAT has not been investigated.
Based on our previous findings (7), we hypothesized that PHB promotes LCFA uptake in WAT. In addition, based on the known mitochondrial function of PHB (4), we also hypothesized that it regulates metabolic activity and may be particularly important for BAT function. Here, we tested these hypotheses by analyzing mice lacking PHB specifically in adipocytes and by using pharmacological PHB modulators.
Research Design and Methods
Animal Experiments
All animal experimentations were approved by the University of Texas Health Animal Care and Use Committee. Mice were housed in the animal facility with a 12-h light/dark cycle at a temperature of 22–24°C with free access to water and diet. PHB fl/fl mice (11) and Apn-Cre mice (The Jackson Laboratory, stock 010803) were used for crosses. For diet-induced obesity induction, mice were fed 58 kcal% (fat) diet (Research Diets, D12331). Body composition was measured using EchoMRI-100T (Echo Medical Systems). Indirect calorimetry studies were performed with the Oxymax (Columbus Instruments) Comprehensive Lab Animal Monitoring System (CLAMS), as described previously (12). Food intake and spontaneous locomotor activity were quantified over 2 days at the same time. The core body temperature was determined by using a MicroTherma 2 K High Precision Type K Thermocouple Meter (ThermoWorks, THS-221-092,)/RET-3 Rectal Probe (Braintree Scientific), as described previously (12). Cold tolerance/adaptive thermogenesis was measured upon placing mice into environmental chamber IS33SD (Powers Scientific) as previously described (13). For glucose tolerance test, glucose (2 g/kg body wt) was injected i.p. into overnight-fasted mice. For insulin tolerance test, insulin (0.6 units/kg body wt) was injected i.p. into mice fasted 4 h. Blood glucose concentration was measured with a OneTouch Ultra glucometer. Intravenous fat tolerance tests were performed by injecting overnight-fasted mice with 100 μL of intralipid 20% fat emulsion. Blood from a tail vein was measured for triglyceride using the EnzyChrom Triglyceride Assay Kit (BioAssay System, cat. no. ETGA-200). Lipolysis was induced by i.p. injection of isoproterenol (10 mg/kg). Plasma free FA (FFA) levels were measured with the EnzyChrom Free Fatty Assay Kit (BioAssay System, cat. no. EFFA-100).
Cell Lines and Culture Assays
For stromal cell isolation, outer ears and SAT pads of 8-week-old mice were excised, minced, and digested in 0.5 mg/mL collagenase type I (Worthington Biochemical) and 2.5 mg/mL of dispase (Roche, cat. no. 04942078001) solution in a shaking bath for 1 h at 37°C. The cell suspension was filtered through a 70-µm cell strainer (Thomas Scientific, cat. no. 1181X53), followed by centrifugation (360g) for 5 min at room temperature. Pelleted cells were plated in 100-mm Petri dishes in DMEM/10% FBS. Brown preadipocyte cells (13) were cultured in DMEM/10% FBS. For white adipogenesis induction, cells grown to confluence were cultured in medium containing 1.7 μmol/L insulin/0.5 mmol/L 3-isobutyl-1-methylxanthine (IBMX), 1 μmol/L dexamethasone, and 5 μmol/L pioglitazone for 3 days, and 1.7 μmol/L insulin afterward, as described previously (12). For brown adipogenesis induction, cells grown to confluence were cultured in medium containing 50 nmol/L insulin/0.5 mmol/L IBMX, 1 μmol/L dexamethasone, 1 nmol/L 3,5,3′-triiodothyronine (T3), and 5 μmol/L rosiglitazone for 3 days, and 50 nmol/L insulin with 1 nmol/L T3 afterward. For FA uptake, adipocytes from differentiated ear fibroblast cells were treated with 2 μmol/L BODIPY (4,4-difluoro-4-bora-3a,4a-diaza-s-indacene)-C16 for 30 min, fixed and stained with Hoechst 33258 (Invitrogen, H3569), and imaged as previously described (7). LCFA uptake by adipocytes was quantified with the QBT Assay (Molecular Devices) as previously described (7). PHB ligands were diluted as described previously (Jl130 at 0.1 μmol/L, Mel56 at 10 μmol/L, and SA1m at 10 μmol/L) (3,14,15) in beige adipocyte-inducing medium. The Seahorse XF Cell Mito Stress Test Kit (Agilent Technologies, cat. no. 103015-100) was used to analyze mitochondrial respiration in beige adipocytes treated with PHB inhibitors for 2 h. The oxygen consumption rate (OCR) was measured upon successive treatment with 1 mmol/L oligomycin, 1 mmol/L FCCP (carbonyl cyanide-p-trifluoromethoxyphenylhydrazone), and 0.5 mmol/L rotenone/antimycin A.
Quantitative Real-Time RT-PCR
Total RNA was extracted using TRIzol Reagent (Life Technologies, 15596018). cDNAs were generated using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, 4368814). PCR reactions were performed on a CFX96 Real-Time System C1000 Touch thermal cycler (Bio-Rad) using Q-PCR Master Mix (GenDEPOT, Q5600-005). Expression of mouse Phb, Ucp1, and Cox IV was normalized to 18S RNA. Primers were as follows: Ucp1, 5'-TCTCAGCCGGCTTAATGACTG-3' and 5'-GGCTTGCATTCTGACCTTCAC-3'; Cox IV, 5'-CTGCCCGGAGTCTGGTAATG-3' and 5'-CAGTCAACGTAGGGGGTCATC-3'; Phb, 5'-GCATTGGCGAGGACTATGAT-3' and 5'-CTCTGTGAGGTCATCGCTCA-3'; and 18S RNA, 5'-AAGTCCCTGCCCTTTGTACACA-3' and 5'-GATCCGAGGGCCTCACTAAAC-3'.
Cell and Tissue Analysis
Paraformaldehyde-fixed cells and formalin-fixed paraffin-embedded tissue sections were analyzed by immunofluorescence, as described previously (12). Upon blocking, primary (4°C, 12 h) and secondary (room temperature, 1 h) antibody incubations were done. Antibodies, diluted in PBS with 0.05% Tween 20, were as follows: anti-UCP1 (1:400; Alpha Diagnostic, UCP-11A), anti-PHB (1:100; Invitrogen, cat. no. MA5-32000), anti-perilipin-1 (1:200; Abcam, cat. no. ab61682), and phosphorylated (activated) hormone-sensitive lipase (pHSL) (1:100; Cell Signaling, cat. no. 4126). Donkey Alexa 488-conjugated (1:200) and Cy3-conjugated (1:300) IgG were from Jackson ImmunoResearch. MitoTracker Deep Red (Thermo Fisher, cat. no. M22426) was used (200 nmol/L) to stain live cells for 30 min prior to fixation. Nuclei were stained with Hoechst 33258 (Invitrogen, H3569). Images were acquired with a confocal Leica TCS SP5 microscope/LAS AF software (Leica) or Carl Zeiss upright ApoTome Axio Imager Z1/ZEN2 Core Imaging software. Adipocyte size was quantified by measuring cell area with the Adiposoft plug-in of ImageJ software.
Immunoblotting
Whole-cell lysates were prepared in radioimmunoprecipitation assay buffer and analyzed as described previously (12). AT lysates were separated by SDS-PAGE and subsequently analyzed by immunoblotting. The following antibodies were used: anti-UCP1 (1:5,000; Sigma-Aldrich, U6382), anti-PHB (1:1,000; Invitrogen, cat. no. MA5-32000), and anti–β-actin (1:5,000; Abcam, ab8226). The signal was detected using the Odyssey CLx imaging system (LI-COR Biosciences) and quantified with ImageJ analysis software.
Statistical Analysis
All statistical analyses were performed with GraphPad Prism 6 software. Experimental results are shown as mean ± SEM. Two-tailed Student unpaired t tests were performed to littermates unless otherwise indicated. P < 0.05 was considered significant.
Data and Resource Availability
Additional data and critical resources supporting the reported findings, methods, and conclusions will be available upon request.
Results
AT Abnormality in Mice With PHB-Knockout Adipocytes
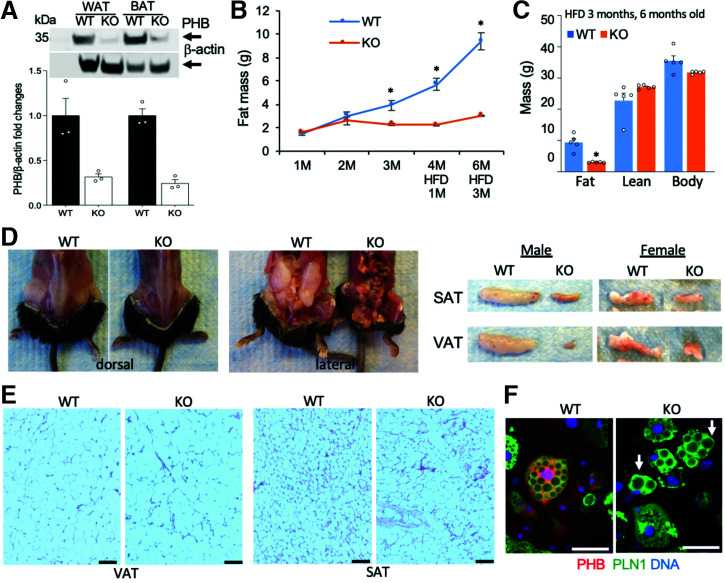
To create a model with a knockout (KO) of PHB in adipocytes (PHB Ad-KO), we used an adiponectin-Cre (Apn-Cre) strain with adipocyte-specific Cre expression (16). We crossed it with the Phb1fl/fl strain (11) in which the Phb gene is flanked by loxP recombination sites. Apn-Cre;Phbfl/fl (PHB Ad-KO) and Cre-negative Phbfl/fl (wild-type [WT]) littermates were then compared. PHB KO was confirmed by Western blot of extracts from WAT and BAT, which demonstrated a marked reduction in protein expression (Fig. 1A). Residual expression remained as expected due to PHB expression in other cell types (stroma, endothelium, and leukocytes). We then phenotyped male PHB Ad-KO and WT littermates. EchoMRI analysis demonstrated a reduced fat body mass accumulation in KO mice (Fig. 1B), which by 3 months of age was detectable for males and females (Fig. 1A). While high-fat diet (HFD) feeding expectedly induced obesity development in WT mice (Fig. 1B), adiposity failed to increase in the KO littermates (Fig. 1C). Analysis of internal organs demonstrated both subcutaneous and visceral lipodystrophy in PHB Ad-KO males (Fig. 1D) and females (Supplementary Fig. 1B). There was no significant effect of PHB Ad-KO on lean body mass (Fig. 1C) or on appetite (Supplementary Fig. 1C) or locomotor activity (Supplementary Fig. 1D). Analysis of WAT hematoxylin-eosin (H-E)-stained WAT sections revealed that PHB Ad-KO mice have significantly larger adipocytes in both VAT and SAT compared with WT littermates (Fig. 1E and Supplementary Fig. 1E).
Figure 1.
PHB Ad-KO mice are lipodystrophic despite adipocyte hypertrophy. A: Western blotting of extracts from WAT and BAT confirms the loss of PHB expression in PHB Ad-KO male mice compared with WT littermates. Immunoblotting: β-actin as control. Graph: band intensity quantification. B: EchoMRI measurements on male mice raised for 3 months (M) on chow and 3 months of HFD reveals reduced fat mass accumulation in PHB Ad-KO littermates (n = 5 mice). C: EchoMRI measurements on male mice from B show a lack of difference in lean body mass. D: Images demonstrate subcutaneous and visceral lipodystrophy in PHB Ad-KO male littermates. Resected SAT and VAT (one side) are compared on the right for males and females. E: H-E staining of sections reveals larger adipocytes in VAT and SAT of PHB Ad-KO male mice compared with WT littermates. F: Ear-derived fibroblasts subjected to white adipogenesis for 8 days demonstrate accumulation of large lipid droplets (arrows) in PHB Ad-KO adipocytes. Immunofluorescence shows that PHB expression is lost in PHB Ad-KO adipocytes expressing perilipin-1 (PLN1). Scale bar: 50 µm. In all panels, data plotted are mean ± SEM. *P < 0.05 (Student t test).
To investigate how PHB loss affects lipid droplets, we induced adipogenesis in stromal cells isolated from mouse SAT and ears. Lipid-laden adipocytes differentiated from cells of both WT and PHB Ad-KO mice (Supplementary Fig. 1F), and there was no difference in adipogenesis marker expression (Supplementary Fig. 1G). However, consistent with a previous report (17), KO of the Phb gene in 3T3-L1 preadipocytes (Supplementary Fig. 1H) resulted in an inhibition of the adipogenic transcription program (Supplementary Fig. 1I) and delayed lipid droplet accumulation (Supplementary Fig. 1J). This indicates that while PHB regulates adipogenesis, its deletion in mature adipocytes in PHB Ad-KO mice does not preclude lipid droplet maintenance. Consistent with WAT adipocyte hypertrophy, cultured PHB Ad-KO adipocytes, confirmed to lack PHB by immunofluorescence, accumulated lipid droplets that tended to be larger than in WT adipocytes (Fig. 1F). This indicated that PHB also plays a role in lipid droplet maintenance after adipogenesis.
Lipid Metabolism in PHB Ad-KO Mice
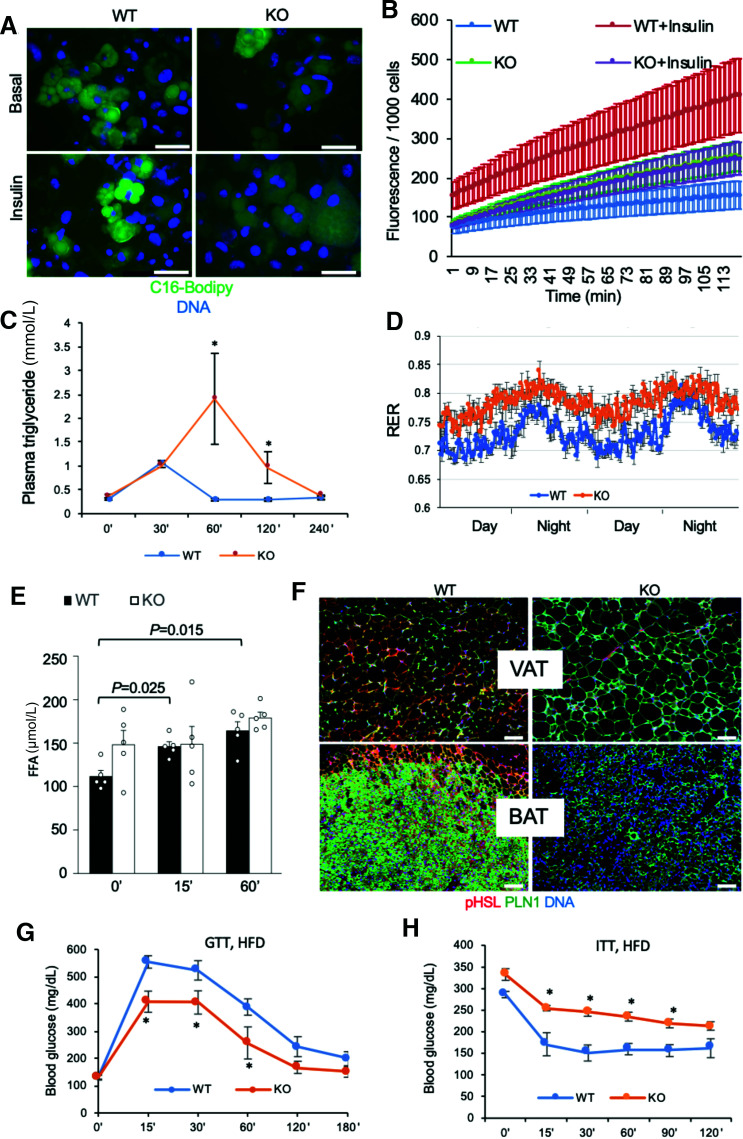
To find an explanation to the WAT phenotype of PHB Ad-KO mice, we analyzed lipid metabolism. First, we analyzed cultured adipocytes for the ability to uptake a fluorophore-labeled palmitic acid, BODIPY-C16 FA. This revealed a marked defect of PHB-null adipocytes to uptake LCFA (Fig. 2A). Quantification of this defect was performed by the QBT assay with a fluorophore-labeled FA. This confirmed the efficiency of insulin-induced LCFA uptake by PHB-null adipocytes (Fig. 2B). To confirm these findings in vivo, we performed a lipid tolerance test on mice. Upon i.v. LCFA infusion, the time course of triglyceride blood concentration demonstrated a delay in clearance for PHB Ad-KO littermates compared with WT (Fig. 2C). Consistent with reduced LCFA uptake, indirect calorimetry analysis of mice in metabolic chambers demonstrated that PHB Ad-KO mice had increased respiratory exchange ratio (RER) (Fig. 2D), indicating lower use of lipid as an energy source. Nonfasting circulating FFA levels were higher in PHB Ad-KO littermates, consistent with a decreased ability of adipocytes to uptake and use them (Fig. 2E). Liver histopathology revealed steatosis in PHB-Ad KO mice (Supplementary Fig. 2A). Triglyceride content was also quantified and was significantly higher for liver and skeletal muscle of PHB-Ad KO mice (Supplementary Fig. 2B). This indicates that inefficient adipocyte FA uptake in these mice results in increased ectopic lipid deposition.
Figure 2.
Lipid metabolism defect and insulin resistance in PHB Ad-KO mice. A: Uptake of BODIPY-C16 FA (green) added to cell culture medium (2 μmol/L) by WT and PHB-null adipocytes nonstimulated or stimulated with insulin (200 nmol/L). B: QBT assay demonstrates that insulin-induced LCFA uptake by PHB-null adipocytes is significantly lower than by WT adipocytes (n = 5 wells). C: Triglyceride blood concentration analysis upon i.v. intralipid infusion into prestarved mice shows a delay in clearance for PHB Ad-KO littermates compared with WT (n = 6). D: RER measured by indirect calorimetry over 2 days demonstrates an increase in PHB Ad-KO male mice. E: Plasma concentration of FFA after isoproterenol injection is elevated at baseline and not further induced in PHB Ad-KO mice (n = 5). F: Immunofluorescence on VAT and BAT sections reveals lower expression of pHSL in adipocytes expressing perilipin-1 (PLN1). Scale bar: 50 µm. G: Glucose tolerance test (GTT) in male mice raised on the HFD. After overnight fasting, littermates (n = 5) were injected with glucose (1 g/kg body wt i.p.), and glucose in blood was measured. H: Insulin tolerance test (ITT) in male mice raised on the HFD. After 4 h fasting, littermates (n = 5) were injected with insulin (0.6 units/kg body wt i.p.), and glucose in blood was measured. In all panels, plotted data are mean ± SEM. *P < 0.05 (Student t test).
As a potential mechanistic explanation for the increased adipocyte size despite a decrease in LCFA uptake, we investigated lipolysis. Upon its induction by injection of a β-adrenergic agonist isoproterenol, circulating FFA levels were expectedly increased in WT mice. In contrast to WT mice, the induction by isoproterenol was not observed in PHB Ad-KO littermates (Fig. 2E). Consistent with this, pHSL expression was virtually undetectable in both white and brown adipocytes of PHB Ad-KO mice (Fig. 2F). Because HSL is phosphorylated by cAMP-dependent protein kinase (PKA), we measured PKA activity with pPKA substrate antibodies. Our data indicate relatively low PKA activity in SAT, VAT, and BAT of PHB-KO AT mice, elucidating the mechanism for reduced lipolysis (Supplementary Fig. 2C). This indicates that PHB-KO adipocyte hypertrophy results from a lipolysis defect.
Glucose Metabolism in PHB Ad-KO Mice
The RER increase observed in male (Fig. 2D) and female (Supplementary Fig. 3A) PHB Ad-KO mice suggested their switch to glucose as the main energy substrate. In accord with this, immunofluorescence analysis expression of GLUT1 was higher in WAT and BAT as well as in liver and skeletal muscle of PHB Ad-KO mice (Supplementary Fig. 3B). There was a trend for higher nonfasting glucose levels in PHB Ad-KO male littermates, suggesting an increase in gluconeogenesis (Supplementary Fig. 3C). Analysis of male mice raised on chow did not reveal a difference in fasting glucose clearance rates (Supplementary Fig. 3D). Analysis of PHB Ad-KO females raised on chow also revealed increased steady-state glucose levels and normal fasting glucose clearance (Supplementary Fig. 3F and G). Interestingly, upon HFD feeding, PHB Ad-KO mice displayed a higher glucose tolerance than WT littermates (Fig. 2G). However, both male and female PHB Ad-KO littermates displayed a significantly lower insulin tolerance when raised not only on HFD (Fig. 2H) but also on chow (Supplementary Fig. 3E and H). Combined, our data indicate that adipocyte PHB deficiency leads to systemic upregulation of glucose metabolism and insulin resistance.
Brown AT and Adaptive Thermogenesis in PHB Ad-KO Mice
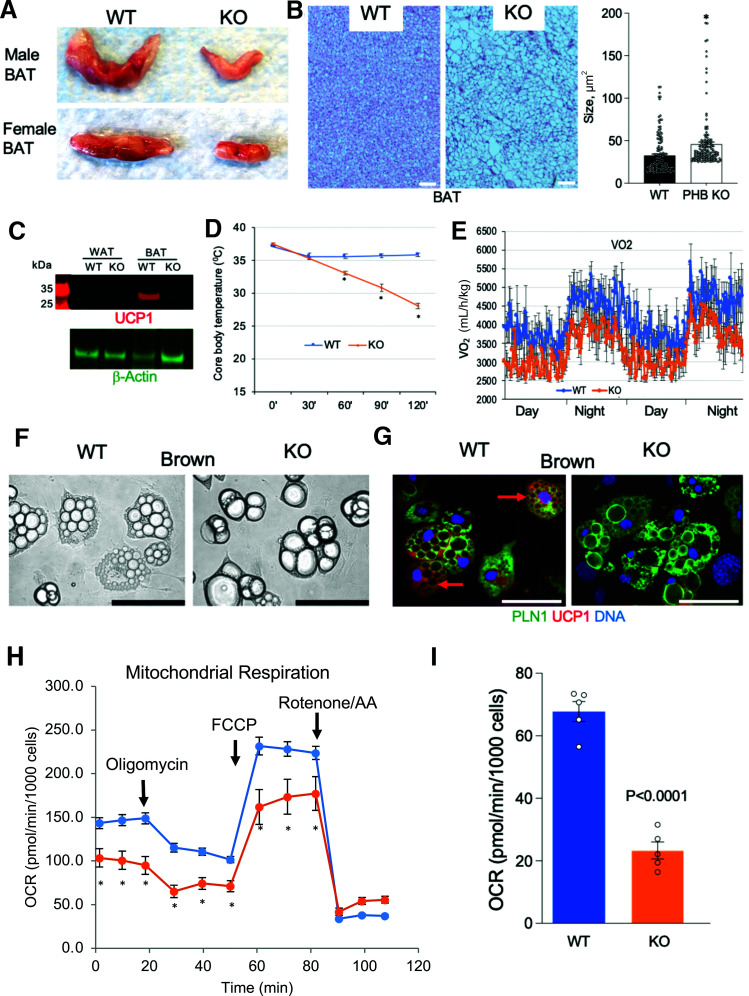
Thermogenic activity of BAT relies on both lipolysis and mitochondrial function. Based on the lipolysis defect revealed here (Fig. 2E and F) and previous PHB implication in mitochondrial biogenesis (4), we analyzed BAT. Interscapular AT was drastically smaller in both male and female PHB Ad-KO mice compared with WT littermates (Fig. 3A). Its WAT-like appearance indicated a lower mitochondrial content. H-E staining of interscapular AT sections confirmed its whitening in PHB Ad-KO mice (Fig. 3B), with larger lipid droplets being consistent with deficient lipolysis. Western blotting of protein extracts demonstrated that UCP1 expression in BAT of PHB Ad-KO mice was undetectable (Fig. 3C). As expected for the lack of this key uncoupling protein, core body temperature measurement in mice placed at 4°C indicated a significantly reduced cold tolerance of PHB Ad-KO males (Fig. 3D) and females (Supplementary Fig. 4A). Consistent with this, VO2 measured by indirect calorimetry revealed a reduction of nighttime and daytime energy expenditure in PHB Ad-KO males (Fig. 3E) and females (Supplementary Fig. 4B).
Figure 3.
BAT adaptive thermogenesis defect in PHB Ad-KO mice. A: Appearance of resected interscapular BAT in WT and PHB Ad-KO male and female littermates. B: H-E staining of sectioned BAT from WT and PHB Ad-KO littermates. Graph: adipocyte size quantification. C: Western blotting of extracts from WAT and BAT demonstrates the loss of UCP1 expression in BAT of PHB Ad-KO mice. Immunoblotting: β-actin as loading control. D: Body temperature maintenance in mice placed at 4°C, indicating reduced cold tolerance of PHB Ad-KO mice (n = 5). E: Indirect calorimetry data showing reduced VO2 by PHB Ad-KO mice. F: Ear-derived fibroblasts subjected to brown adipogenesis for 8 days demonstrate accumulation of larger lipid droplets (arrows) in PHB-KO adipocytes. G: Immunofluorescence on cells from F shows that UCP1 expression (arrows) is not induced in PHB Ad-KO adipocytes expressing perilipin-1 (PLN1). Scale bar: 50 µm. H: XF Cell Mito Stress assay used to analyze mitochondrial respiration in cells from PHB Ad-KO and WT mice subjected to brown adipogenesis. OCR, measured upon successive treatment with oligomycin, FCCP (carbonyl cyanide-p-trifluoromethoxyphenylhydrazone), and rotenone/antimycin A (AA), demonstrates lower basal and induced mitochondrial function in PHB-KO adipocytes. I: Oligomycin-resistant OCR, reflecting ATP-uncoupled respiration, is lower in PHB-KO adipocytes. In all panels, plotted data are mean ± SEM. *P < 0.05 (Student t test).
Mitochondrial Dysfunction in PHB-KO Adipocytes
In addition to UCP1 expression loss in BAT, RT-PCR analysis revealed lower cyclooxygenase (COX) IV gene expression in PHB Ad-KO BAT and WAT (Supplementary Fig. 4C). Reduction in this nuclear-encoded enzyme essential for electron transport may account for mitochondrial dysfunction. In addition, there was a significant reduction of mitochondrial content in both BAT and WAT (Supplementary Fig. 4D). We used a cell culture model to investigate the consequences of mitochondrial defect in PHB-KO adipocytes. UCP1 expression was observed in WT but not in PHB-KO brown adipocytes differentiated from stromal cells (Fig. 3F and G). Notably, differentiated PHB-KO cells had larger lipid droplets, indicative of them differentiating into white rather than brown adipocytes (Fig. 3F and G). The Seahorse XF Cell Mito Stress Assay was then performed on these cells to analyze mitochondrial respiration. Lower basal and induced OCR revealed a defect in mitochondrial respiration of PHB-KO adipocytes (Fig. 3H). Consistent with the lack of UCP1, oligomycin-resistant OCR was lower in PHB-KO adipocytes, indicating a decreased proton leak (Fig. 3I). Importantly, the extracellular acidification rate, measured by the XF Cell Mito Stress Assay, was significantly higher for PHB-KO adipocytes (Supplementary Fig. 4E). These data indicate that the cells switch from mitochondria-dependent oxidative phosphorylation to glycolysis in the absence of PHB.
Pharmacological PHB Targeting Block With Brown Adipocyte Differentiation and Function
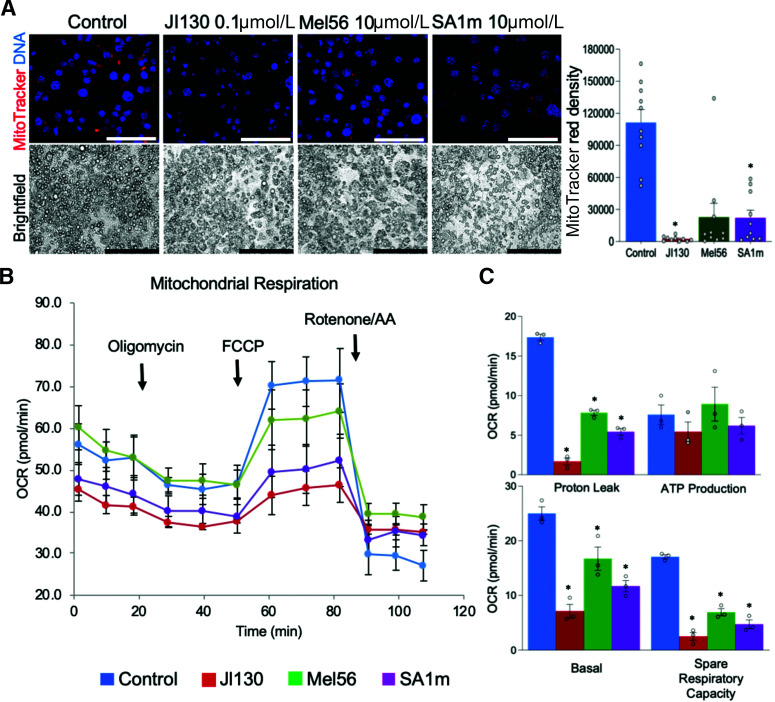
Finally, we investigated the involvement of PHB in different aspects of brown adipocyte biology. We used mouse immortalized brown preadipocytes (13) induced to undergo brown adipogenesis. For PHB targeting, we used the previously characterized PHB small-molecule ligands JI130, Mel56, and SA1m (3,14,15). Treatment of preadipocytes during adipogenesis induction did not interfere with differentiation, as evident from lipid droplet formation (Fig. 4A). However, MitoTracker staining revealed a notably lower signal in cells treated with each PHB ligand compared with control adipocytes (Fig. 4A). This indicated that PHB is important for mitochondrial biogenesis in brown adipocytes. Consistent with this, the Seahorse Real-Time ATP rate assay demonstrated a decrease in mitochondrial respiration (Supplementary Fig. 5A) and in extracellular acidification (Supplementary Fig. 5B), with JI130 having the strongest effect compared with the other two PHB small molecules. There was also a shift to glycolytic ATP production (Supplementary Fig. 5C) and a decrease in uncoupled respiration (Supplementary Fig. 5D) in treated cells. We then treated immortalized brown preadipocyte-derived adipocytes with the same PHB modulators after the completion of brown differentiation. The Seahorse XF Cell Mito Stress Assay revealed that JI130 strongly decreased both basal and induced OCR, while Mel56 and SA1m also had lower effects (Fig. 4B). All three compounds significantly decreased proton leak as well as spare respiration capacity (Fig. 4C). However, neither compound significantly affected ATP production, confirming that cells switch from mitochondria-dependent oxidative phosphorylation to glycolysis in the absence of PHB.
Figure 4.
PHB modulators suppress mitochondrial respiration and uncoupling. A: Mouse brown preadipocytes were treated with indicated PHB ligands during brown adipogenesis induction for 8 days. Brightfield images demonstrate comparable adipocyte differentiation in control and treated cells. MitoTracker staining reveals lower mitochondrial activity in adipocytes upon PHB modulator treatment. Graph: red fluorescence quantification. Scale bar: 50 µm. B: Mouse brown preadipocytes were induced to undergo brown adipogenesis for 8 days and then treated with indicated PHB ligands. Seahorse XFe24/Flux Assay demonstrates a decrease of mitochondrial respiration upon treatment with each PHB modulator. C: Analysis of data from B reveals a decrease in basal and spare respiration and in uncoupling, but not in ATP production, upon treatment with each PHB modulator. Plotted date are mean ± SEM; *P < 0.05 (Student t test).
Discussion
Our analysis of PHB Ad-KO mice reveals their defect in fat mass accumulation despite normal food intake. Adipocyte hypoplasia accounts for this lipodystrophy because adipocyte size is increased in WAT of PHB Ad-KO mice. While we confirm the previously reported (17) requirement of PHB for initiation of adipogenesis, in PHB Ad-KO mice adipocytes differentiate normally because PHB deletion is driven by expression of Apn, a late adipogenesis gene. Our data show that inactivation of PHB after adipogenesis leads to hypertrophy of mature adipocytes due to a reduction in PKA and HSL activity resulting in decreased lipolysis. This could be explained by reduced activity of adrenergic receptors in “whitened” PHB-deficient adipocytes because PKA activity is regulated by cAMP, downstream of G protein-coupled receptors activation. Although PHB Ad-KO mice do not display glucose intolerance, both males and females have increased levels of blood glucose and are insulin resistant. Diabetes development in these mice occurs despite a systemic increase in GLUT expression. Our results indicate that the drastic disbalance in substrate utilization in PHB Ad-KO mice is due to their lipid intolerance. This is supported by the notion that PHB Ad-KO mice fed an HFD clear glucose better than WT mice, suggesting that increased lipid load further boosts glucose utilization. Lipid intolerance of PHB Ad-KO mice, resulting in steatosis, could be explained by a decreased capacity of adipocytes for LCFA uptake, LCFA oxidation, or both. By using cell culture models, we demonstrate a defect in lipid uptake by PHB-KO adipocytes. This is consistent with the phenotypes reported for CD36 KO (18) and ANX2 KO (7) mice. Our data confirm that PHB, in complex with CD36, regulates FA uptake and contributes to lipid accumulation in AT.
As expected, the metabolic consequences of adipocyte PHB overexpression (4) are opposite to the phenotype we describe in PHB Ad-KO mice. Importantly, our data reveal a previously unappreciated function of PHB in brown adipocytes, pointing to the role of PHB in FA oxidation. A marked defect in BAT observed in PHB Ad-KO mice results in their cold intolerance and reduced energy expenditure. The reduced mitochondrial content in the AT of PHB Ad-KO mice is consistent with our results from PHB-KO cells, as well as WT cells treated with PHB ligands. The apparent role of PHB in mitochondrial biogenesis and function is consistent with results from PHB overexpression models (4). We show that, brown adipocytes lacking PHB have a decreased proton leak, consistent with a reduced UCP1 protein expression. PHB-null cells also have lower mitochondrial respiration, consistent with a reduced expression of the mitochondrial transporter COX IV. Upon treatment of differentiated brown adipocytes with pharmacological PHB modulators, a decrease in mitochondrial respiration and uncoupling was similarly observed. Importantly, glycolysis was induced in PHB-KO or PHB-inhibited adipocytes in compensation for the oxidative defect. Combined, our results demonstrate that PHB in adipocytes is essential for normal lipid metabolism and adaptive thermogenesis and that its adipocyte dysfunction derails AT function, energy substrate utilization, and leads to metabolic dysfunction.
Our previous studies discovered that PHB is expressed on the surface of adipocytes and endothelial cells selectively in AT. We have used a PHB-homing peptide, KGGRAKD, to direct an apoptosis-inducing peptide d(KLAKLAK)2 to WAT in an experimental approach to obesity reversal (5). WAT vascular-targeting capacity of the hunter-killer CKGGRAKDC–d(KLAKLAK)2 peptide has been validated in mouse, rat, and nonhuman primates and has shown antiobesity effects (19,20). This study suggests a possibility that the antiobesity effect could result in part due to the loss of PHB function executed by cells expressing it in ATs.
Article Information
Acknowledgments. The authors thank Shelly Lu (University of California, Los Angeles) for providing Phb1fl/fl mice.
Funding. This work was supported by National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases grant 2R01DK088131 to M.G.K.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Authors Contributions. Z.G., A.C.D., C.F., and A.D. designed and performed the experiments, analyzed data, and edited the manuscript. Z.G., L.D., and M.G.K. conceived and designed the experiments, analyzed data, and wrote the manuscript. M.G.K. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.14932665.
References
- 1. Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell 2014;156:20–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ande SR, Nguyen KH, Nyomba BLG, Mishra S. Prohibitin in adipose and immune functions. Trends Endocrinol Metab 2016;27:531–541 [DOI] [PubMed] [Google Scholar]
- 3. Wang D, Tabti R, Elderwish S, et al. Prohibitin ligands: a growing armamentarium to tackle cancers, osteoporosis, inflammatory, cardiac and neurological diseases. Cell Mol Life Sci 2020;77:3525–3546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ande SR, Nguyen KH, Padilla-Meier GP, Wahida W, Nyomba BL, Mishra S. Prohibitin overexpression in adipocytes induces mitochondrial biogenesis, leads to obesity development, and affects glucose homeostasis in a sex-specific manner. Diabetes 2014;63:3734–3741 [DOI] [PubMed] [Google Scholar]
- 5. Kolonin MG, Saha PK, Chan L, Pasqualini R, Arap W. Reversal of obesity by targeted ablation of adipose tissue. Nat Med 2004;10:625–632 [DOI] [PubMed] [Google Scholar]
- 6. Staquicini FI, Cardó-Vila M, Kolonin MG, et al. Vascular ligand-receptor mapping by direct combinatorial selection in cancer patients. Proc Natl Acad Sci U S A 2011;108:18637–18642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Salameh A, Daquinag AC, Staquicini D, et al. Prohibitin/annexin 2 interaction regulates fatty acid transport in adipose tissue. JCI Insight 2016;1:e86351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sun K, Tordjman J, Clément K, Scherer PE. Fibrosis and adipose tissue dysfunction. Cell Metab 2013;18:470–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kajimura S, Spiegelman BM, Seale P. Brown and beige fat: physiological roles beyond heat generation. Cell Metab 2015;22:546–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 2004;84:277–359 [DOI] [PubMed] [Google Scholar]
- 11. Ko KS, Tomasi ML, Iglesias-Ara A, et al. Liver-specific deletion of prohibitin 1 results in spontaneous liver injury, fibrosis, and hepatocellular carcinoma in mice. Hepatology 2010;52:2096–2108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gao Z, Daquinag AC, Fussell C, et al. Age-associated telomere attrition in adipocyte progenitors predisposes to metabolic disease. Nat Metab 2020;2:1482–1497 [DOI] [PubMed] [Google Scholar]
- 13. Gao Z, Daquinag AC, Su F, Snyder B, Kolonin MG. PDGFRα/PDGFRβ signaling balance modulates progenitor cell differentiation into white and beige adipocytes. Development 2018;145:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Djehal A, Krayem M, Najem A, et al. Targeting prohibitin with small molecules to promote melanogenesis and apoptosis in melanoma cells. Eur J Med Chem 2018;155:880–888 [DOI] [PubMed] [Google Scholar]
- 15. Perron A, Nishikawa Y, Iwata J, et al. Small-molecule screening yields a compound that inhibits the cancer-associated transcription factor Hes1 via the PHB2 chaperone. J Biol Chem 2018;293:8285–8294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eguchi J, Wang X, Yu S, et al. Transcriptional control of adipose lipid handling by IRF4. Cell Metab 2011;13:249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ande SR, Xu Z, Gu Y, Mishra S. Prohibitin has an important role in adipocyte differentiation. Int J Obes 2012;36:1236–1244 [DOI] [PubMed] [Google Scholar]
- 18. Febbraio M, Abumrad NA, Hajjar DP, et al. A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J Biol Chem 1999;274:19055–19062 [DOI] [PubMed] [Google Scholar]
- 19. Barnhart KF, Christianson DR, Hanley PW, et al. A peptidomimetic targeting white fat causes weight loss and improved insulin resistance in obese monkeys. Sci Transl Med 2011;3:108ra112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim DH, Woods SC, Seeley RJ. Peptide designed to elicit apoptosis in adipose tissue endothelium reduces food intake and body weight. Diabetes 2010;59:907–915 [DOI] [PMC free article] [PubMed] [Google Scholar]