Key Points
Question
Is there an association between fetal smallness and adult exercise capacity or cardiac function and structure?
Findings
In this cohort study of 158 young adults, compared with individuals having birth weights within standard reference ranges, individuals born small for gestational age showed limited exercise capacity, with reduced maximal workload and oxygen consumption, and minor differences in right ventricular geometry at rest.
Meaning
These findings provide evidence suggesting that being born small for gestational age may limit exercise capacity in adulthood.
Abstract
Importance
Being born small for gestational age (SGA), approximately 10% of all births, is associated with increased risk of cardiovascular mortality in adulthood, but mechanistic pathways are unclear. Cardiac remodeling and dysfunction occur in fetuses SGA and children born SGA, but it is uncertain whether and how these changes persist into adulthood.
Objective
To evaluate baseline cardiac function and structure and exercise capacity in young adults born SGA.
Design, Setting, and Participants
This cohort study conducted from January 2015 to January 2018 assessed a perinatal cohort born at a tertiary university hospital in Spain between 1975 and 1995. Participants included 158 randomly selected young adults aged 20 to 40 years born SGA (birth weight below the 10th centile) or with intrauterine growth within standard reference ranges (controls). Participants provided their medical history, filled out questionnaires regarding smoking and physical activity habits, and underwent incremental cardiopulmonary exercise stress testing, cardiac magnetic resonance imaging, and a physical examination, with blood pressure, glucose level, and lipid profile data collected.
Exposure
Being born SGA.
Main Outcomes and Measures
Cardiac structure and function assessed by cardiac magnetic resonance imaging, including biventricular end-diastolic shape analysis. Exercise capacity assessed by incremental exercise stress testing.
Results
This cohort study included 81 adults born SGA (median age at study, 34.4 years [IQR, 30.8-36.7 years]; 43 women [53%]) and 77 control participants (median age at study, 33.7 years [interquartile range (IQR), 31.0-37.1 years]; 33 women [43%]). All participants were of White race/ethnicity and underwent imaging, whereas 127 participants (80% of the cohort; 66 control participants and 61 adults born SGA) completed the exercise test. Cardiac shape analysis showed minor changes at rest in right ventricular geometry (DeLong test z, 2.2098; P = .02) with preserved cardiac function in individuals born SGA. However, compared with controls, adults born SGA had lower exercise capacity, with decreased maximal workload (mean [SD], 180 [62] W vs 214 [60] W; P = .006) and oxygen consumption (median, 26.0 mL/min/kg [IQR, 21.5-33.5 mL/min/kg vs 29.5 mL/min/kg [IQR, 24.0-36.0 mL/min/kg]; P = .02). Exercise capacity was significantly correlated with left ventricular mass (ρ = 0.7934; P < .001).
Conclusions and Relevance
This cohort of young adults born SGA had markedly reduced exercise capacity. These results support further research to clarify the causes of impaired exercise capacity and the potential association with increased cardiovascular mortality among adults born SGA.
This cohort study assesses exercise capacity and baseline cardiac structure and function among young adults born with a birth weight below the 10th centile (small for gestational age).
Introduction
Small for gestational age (SGA), defined as being born with a birth weight below the 10th centile, affects 10% of pregnancies and predominantly includes growth-restricted fetuses. For more than 30 years, epidemiological studies1,2,3 have shown a consistent association between SGA and cardiovascular mortality and hypertension in adults. Although the precise mechanisms underlying this association are not fully clarified, prenatal cardiac changes are proposed as a main contributor. Small for gestational age is associated with cardiac remodeling and dysfunction in fetuses,3,4 children,5,6 and preadolescents.7 However, the persistence of the cardiac effects of SGA into adulthood is unclear. A Swedish cohort of 19 young adults born extremely small described slightly smaller ventricular and vascular dimensions with normal function; this cohort study included individuals born SGA at term with severely abnormal fetal aortic blood blow, and the mean (SD) birth weight was 2243 (331) g.8 The Cardiovascular Risk in Young Finns Study,9 including 157 young adults born SGA, showed a subtle increase in heart size with preserved function. Overall, these studies suggest some statistically significant but minor clinical changes in cardiac structure and function in adults born SGA. The aforementioned studies used classical measurements on echocardiography or cardiac magnetic resonance (CMR) imaging but not contemporary techniques, such as statistical shape analysis (SSA), which enables the performance of statistical analyses directly on cardiac structures as a single object and the ability to study regional shape variability in a population. This technique has allowed for the identification of specific ventricular regional remodeling in athletes,10 hypertension in pregnancy,11 adults born preterm,12 and preadolescents born SGA.7 Thus, applying SSA may help to better describe the pattern of cardiac remodeling in adults born SGA.
In addition, all the aforementioned studies evaluated baseline changes, but no tests to challenge the heart were conducted. Evaluating the response to exercise may be relevant to revealing the long-term effects of in utero adverse conditions associated with SGA, particularly in young patients. For instance, Huckstep et al13 showed similar baseline values but a marked reduction of ejection fraction during exercise in young adults born preterm. Similarly, cardiopulmonary exercise testing has been proposed to show pulmonary blood flow maldistribution and length of hospital stay among children with congenital heart disease.14
We postulated that adults born SGA would have very small cardiac changes at rest but reduced exercise capacity. To test this hypothesis, we performed CMR imaging and exercise stress testing among young adults born SGA and control young adults, with the aim of evaluating baseline cardiac structure and function and response to exercise.
Methods
Study Design
This ambispective cohort study included young adults (20-40 years of age) born SGA and control participants with birth weights within standard reference ranges. Potential cases and controls were randomly selected from a perinatal cohort born at a tertiary university hospital in Barcelona, Spain, from 1975 to 1995 (eFigure 1 in the Supplement). Eligible participants who could be found were offered to participate in the study, until the minimum required sample size was reached. Small for gestational age was defined as birth weight below the 10th centile for gestational age, and controls as normal birth weight. Exclusion criteria were neonatal macrosomia, twins, congenital malformation, genetic syndromes, major mental disorder, professional sport practice (men and women in amateur sports were only included if they had not participated in any official competition in the last 2 years), or current pregnancy. The study protocol included medical history, physical examination, blood pressure, questionnaires for smoking15 and physical activity,16 and glucose level and lipid profile tests. The study protocol was approved by the ethics committee at Hospital Clínic and the ethics committee at Fundació Sant Joan de Déu, both in Barcelona, Spain, and written informed consent was obtained for all study participants in a manner consistent with the Declaration of Helsinki.17 Participants received reimbursement (€30-€50 [US $35-$60]) for pocket expenses associated with participating in the research study (eg, transportation or parking).
Cardiac Magnetic Resonance
We performed CMR using a 3-T scanner (Magnetom Trio [Siemens]) with retrospective electrocardiographic gating. Contiguous short-axis cine images covering both ventricles were acquired using a standard steady-state free precession sequence (slice thickness, 8 mm; 2-mm interslice gap) during breath hold. Long-axis cine images of 4-, 3-, and 2-chamber views were also acquired. Flow imaging was performed perpendicular to the main pulmonary artery and to the ascending aorta with a velocity-encoded gradient echo sequence. Finally, delayed enhancement imaging was performed 15 minutes after the administration of 0.15 mmol/kg of body weight of gadobutrol. All images were stored on a digital archive for postprocessing with dedicated software, Argus, version syngo MR E 11 (Siemens Medical Solutions) and Segment, version 3.0 (Medviso AB). All parameters were indexed by body surface area (BSA).
Ventricular Shapes
A 3-dimensional (3-D) model of the whole heart was constructed with in-house customized software using a deformable template to fit the CMR short axis, using a previously described algorithm.18 From this model, the endocardial surface of the right ventricle (RV) and both the endocardium and epicardium of the left ventricle (LV) at the end of diastole were extracted. The 3-D surfaces were scaled by BSA, and positioning variability was removed using Procrustes analysis.19 Ventricular shape differences between those born SGA and controls were assessed by SSA.7,10,20 Statistical shape analysis was used to compute a reference shape for each population (controls and SGA) separately to compare them. Next, the specific remodeling in each individual (biventricular remodeling score) was estimated. The biventricular score was obtained by quantifying the remodeling captured by the biventricular shape models and derived from the logistic regression model that predicts SGA from shapes, taking into account the confounding variables (BSA, age, and sex) (eMethods in the Supplement). Shape analysis was conducted using the point distribution model, which associates each shape with a vector concatenating all the coordinates of the shape vertices.
Exercise Stress Testing
All participants performed standard incremental exercise testing on a cycloergometer (range, 6-999 W) using an Ergoselect 100 (Ergoline). All measurements were made using a breath-by-breath respiratory gas exchange system (Medisoft), with peak exercise measurements as follows: workload, minute ventilation, oxygen consumption (V̇o2), carbon dioxide production (V̇co2), oxygen saturation, blood pressure, and heart rate. Percentage of predicted V̇o2 was calculated according to standard values.21 Oxygen pulse was calculated as V̇o2 divided by heart rate, and respiratory exchange ratio (RER), as peak V̇co2 divided by peak V̇o2.
Main Outcomes and Sample Size Calculation
Prespecified main outcome measures were cardiac structure and function—assessed by CMR—and exercise capacity—assessed by incremental exercise testing. Sample sizes for the main outcome measures were calculated assuming an unknown but equal variance (previous studies suggest that variances among those born SGA and controls are similar), 80% power, 5% α error, 1:1 allocation index. For CMR, a sample of 75 individuals per group was estimated to identify a 3% difference between groups in ejection fraction and assuming an SD of 4.512,22 and a standardized difference of 0.43. For exercise stress testing, a sample of 55 individuals per group was estimated to detect a 15% difference in indexed V̇o2.23,24
Statistical Analysis
Stata IC, version 14.0 (StataCorp LP) and Python 2.7 (Enthought Inc) were used for statistical analysis to assess data collected from January 2015 to January 2018. Normality was tested using the Shapiro-Wilk test. Study groups were described using mean (SD) values, median (interquartile range [IQR]) values, or frequencies and were compared by t test, Wilcoxon-Mann-Whitney test, χ2 test, or Fisher exact test as appropriate. Linear regression was used when the variable met the normality assumption and for those variables that were normalized by logarithmic transformation. Robust regression was applied otherwise. Multivariate linear or robust regressions were fitted to adjust for age, sex, asthma, educational level, smoking habit, gestational age at delivery, breastfeeding, and gestational hypertension. All reported P values are 2-sided, and P < .05 was considered statistically significant.
Ventricular Shape Statistical Analysis
Ventricular shape analysis followed a previously described framework.20 This framework enables identification of the main regional shape pattern that encodes the differences between 2 populations and enables quantification of the amount of that pattern in each individual. Ventricular shape variability between the groups was assessed by principal component analysis applied to the end-diastolic surfaces obtained from CMR. Ten components were kept so that 95% of the shape variability was retained in the model. Partial least-squares–logistic regression was performed in that compact representation of the shape space to find the 3 most discriminative shape patterns between controls and those born SGA that were combined using a logistic regression model. Age, sex, and BSA were added as covariates in this logistic model to correct for potential bias. Statistical significance was established by comparing the cross-validated receiver operating characteristic curve of a model that considered shape and confounders of a simpler model that considered only the confounders using the DeLong method,25 a statistical test for comparing the area under receiver operating characteristic curves of different models. Finally, we used the coefficients associated with the shape of the logistic regression model to derive a remodeling score for each individual.
Results
Perinatal Data and Characteristics at Recruitment
The final numbers of participants included in the analyses were 81 adults born SGA (median age at study, 34.4 years [IQR, 30.8-36.7 years]; 43 women [53%]) and 77 control participants (median age at study, 33.7 years [IQR, 31.0-37.1 years]; 33 women [43%]). All participants were of White race/ethnicity and underwent CMR, whereas 127 participants (80% of the cohort; 66 control and 61 adults born SGA) completed the exercise test. Perinatal and adult characteristics are given in Table 1. By design, compared with controls, adults born SGA showed lower birth weight and birth weight centile with similar gestational age at delivery. Adults born SGA showed lower height, weight, and BSA, with similar body mass index compared with control participants. Both groups presented similar rates of tobacco smoking (eAppendix in the Supplement), sedentary activities, overweight, chronic hypertension, and diabetes. There were no cases of pulmonary hypertension or chronic obstructive pulmonary diseases. Plasma concentrations of glucose, cholesterol, and triglyceride were also similar between the 2 groups.
Table 1. Perinatal and Current Baseline Characteristics of the Study Population.
| Characteristic | No. (%) of group participants | P value | |
|---|---|---|---|
| Control (n = 77) | Born SGA (n = 81) | ||
| Perinatal characteristic | |||
| Birth weight, median (IQR), g | 3380 (3250-3550) | 2570 (2440-2700) | NA |
| Gestational age at delivery, median (IQR), wk | 40 (39-40) | 40 (39-41) | .06 |
| Birth weight centile, median (IQR) | 53 (40-66) | 1 (1-3) | NA |
| Prematurity | 2 (3) | 0 (0) | .14 |
| Gestational hypertension | 1 (1) | 6 (7) | .06 |
| Breastfeedinga | 9 (12) | 17 (21) | .12 |
| Current baseline characteristic | |||
| Age, median (IQR), y | 33.7 (31.0-37.1) | 34.4 (30.8-36.7) | .96 |
| Sex | |||
| Female | 33 (43) | 43 (53) | .20 |
| Male | 44 (57) | 38 (47) | |
| White ethnicity | 77 (100) | 81 (100) | NA |
| Height, median (IQR), m2 | 1.7 (1.7-1.8) | 1.7 (1.6-1.7) | <.001 |
| Weight, median (IQR), kg | 73.3 (60.9-85.0) | 70.0 (57.7-79.1) | .07 |
| BMI, median (IQR) | 24.3 (21.4-26.6) | 24.7 (21.7-28.4) | .48 |
| Body surface area, median (IQR), m2b | 1.9 (1.7-2.0) | 1.8 (1.6-1.9) | .001 |
| Previous familiar history of myocardial infarction | 10 (13) | 7 (9) | .37 |
| Educational level | |||
| Primary | 9 (12) | 8 (10) | .049 |
| Secondary | 3 (4) | 7 (9) | |
| Postsecondary nontertiary | 10 (13) | 20 (26) | |
| Short-cycle tertiary | 7 (9) | 13 (16) | |
| University | 48 (62) | 33 (41) | |
| Smoking habit | 42 (57) | 42 (52) | .67 |
| Overweightc | 33 (43) | 35 (43) | .96 |
| Sedentary time, median (IQR), h | 90.5 (76.5-106.0) | 87.0 (73.0-105.0) | .24 |
| Level of physical activityd | |||
| Low | 35 (48) | 33 (43) | .25 |
| Moderate | 26 (36) | 22 (29) | |
| High | 12 (16) | 21 (28) | |
| Diabetes | 0 (0) | 3 (4) | .38 |
| Asthma | 4 (5) | 9 (11) | .18 |
| Chronic hypertension | 2 (3) | 2 (2) | .96 |
| Blood pressure, median (IQR), mm Hge | |||
| Systolic | 118.7 (110.2-128.7) | 117.5 (109.2-126.7) | .84 |
| Diastolic | 71.5 (66.7-78.2) | 72.0 (63.7-80.2) | .90 |
| Glucose level, median (IQR), mg/dL | 89 (83-97) | 89 (81-96) | .31 |
| Cholesterol level, median (IQR), mg/dL | |||
| LDL | 109 (95-134) | 111 (101-125) | .98 |
| HDL | 46 (35-57) | 47 (39-54) | .47 |
| Triglyceride, median (IQR), mg/dL | 105 (76-150) | 86 (69-123) | .10 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); HDL, high-density lipoprotein; IQR, interquartile range; LDL, low-density lipoprotein; NA, nonapplicable; SGA, small for gestational age.
SI conversion factors: To convert glucose to mmol/L, multiply by 0.0555; LDL and HDL to mmol/L, multiply by 0.0259; and triglyceride to mmol/L, multiply by 0.0113.
Breastfeeding considered present if existed (exclusive or mixed) at any duration.
Body surface area calculated using the Haycock formula.
Overweight defined as BMI above 25.
Basal level of physical activity assessed by the International Physical Activity Questionnaire.16
Blood pressure was obtained at the beginning of the medical evaluation by a trained nurse while the participant was seated after having rested for 5 to 10 minutes.
Baseline Cardiac Structure and Function
The CMR results of the study populations are shown in Table 2 and in eTable 2 and eFigure 2 in the Supplement. Compared with controls, adults born SGA showed similar indexed cardiac dimensions (eg, LV end-diastolic volume/BSA z score mean [SD], 0.3 [1.1] vs 0.1 [0.8]), ventricular sphericity (mean [SD] LV sphericity index for both groups, 0.4 [0.1]), LV mass (mean [SD], 47.8 [11.9] g/m2 vs 45.4 [8.8] g/m2), and relative wall thickness, which was calculated as (posterior LV wall thickness × 2)/end-diastolic LV cavity diameter (median, 2.3% [IQR, 2.0%-2.5%] vs 2.2% [IQR, 1.9%-2.6%]). Cardiac function was mainly preserved in adults born SGA (LV ejection fraction median, 60.2% [IQR, 56.8%-63.6%] vs 59.2% [IQR, 55.7%-61.8%]). No delayed enhancement patterns were detected in CMR images of any individual.
Table 2. Cardiac Magnetic Resonance Results for the Study Populations.
| Measure | Median (IQR) | P value | Adjusted P valuea | |
|---|---|---|---|---|
| Control (n = 77) | Born SGA (n = 81) | |||
| Left morphometry b | ||||
| LVEDV, mean (SD), mL/m2 | 86.6 (12.8) | 83.4 (12.2) | .10 | .27 |
| LVESV, mean (SD), mL/m2 | 34.5 (7.2) | 34.3 (6.5) | .91 | .49 |
| LV base-to-apex length, mm/m2 | 5.0 (4.7-5.3) | 5.0 (4.7-5.5) | .11 | .53 |
| LV basal diameter, mm/m2 | 25.6 (23.8-27.0) | 25.9 (24.1-27.1) | .29 | .72 |
| LV sphericity index, mean (SD)c | 0.4 (0.1) | 0.4 (0.1) | .98 | .62 |
| Relative wall thicknessd | 2.3 (2.0-2.5) | 2.2 (1.9-2.6) | .63 | .62 |
| LV mass, mean (SD), g/m2 | 47.8 (11.9) | 45.4 (8.8) | .25 | .64 |
| Left atrial area, cm2/m2 | 12.6 (11.7-13.7) | 12.3 (10.9-14.3) | .50 | .29 |
| Aortic area, mean (SD), cm2/m2 | 3.6 (0.6) | 3.7 (0.7) | .26 | .66 |
| Right morphometry b | ||||
| RVEDV, mean (SD), mL/m2 | 78.8 (15.1) | 74.1 (13.8) | .04 | .13 |
| RVESV, mL/m2 | 34.4 (28.3-42.9) | 32.1 (27-36.8) | .01 | .03 |
| Right atrial area, mean (SD), cm2/m2 | 11.4 (2.1) | 10.3 (2.0) | .01 | .01 |
| Pulmonary artery area, cm2/m2 | 3.9 (3.4-4.3) | 3.7 (3.3-4.3) | .37 | .11 |
| LV function | ||||
| LV ejection fraction, % | 60.2 (56.8-63.6) | 59.2 (55.7-61.8) | .04 | .01 |
| LV cardiac index, mL/min/m2 | 2.3 (3.0-3.7) | 3.2 (2.9-3.7) | .31 | .16 |
| RV function | ||||
| Right ejection fraction, % | 55.0 (51.6-58.5) | 56.1 (52.9-60.7) | .05 | .06 |
| RV cardiac index, mean (SD), mL/min/m2 | 2.8 (0.5) | 2.8 (0.5) | .98 | .95 |
Abbreviations: EDV, end-diastolic volume; ESV, end-systolic volume; IQR, interquartile range; LV, left ventricle; RV, right ventricle; SGA, small for gestational age.
P value as compared with controls adjusted for age, sex, asthma, educational level, smoking habit, gestational age at delivery, breastfeeding, and gestational hypertension.
Cardiac dimensions, volumes, mass, and output were indexed for body surface area.
Ventricular sphericity indices were calculated as base-to-apex length divided by the basal diameter.
Relative wall thickness was calculated as (posterior LV wall thickness × 2)/end-diastolic LV cavity diameter.
Statistical shape analysis was subsequently applied to find the most discriminating shape pattern between adults born SGA and control participants. Figure 1, the Video, and eFigure 3 in the Supplement illustrate the resulting pattern, which corresponds to minor changes, mainly in the RV, with adults born SGA displaying slight flattening of the basal septum and enlargement of the basal portion of the RV, which subtly increased the basal curvature. The DeLong test was used to compare the cross-validated area under the receiver operating characteristic curve of the SSA model with a simple mode that only considered confounders, establishing statistical significance with z score = 2.2098 and P = .02.
Figure 1. Individual Ventricular Surfaces and Corresponding Biventricular Remodeling Scores in 2 Control Participants and 2 Adults Born Small for Gestational Age (SGA).
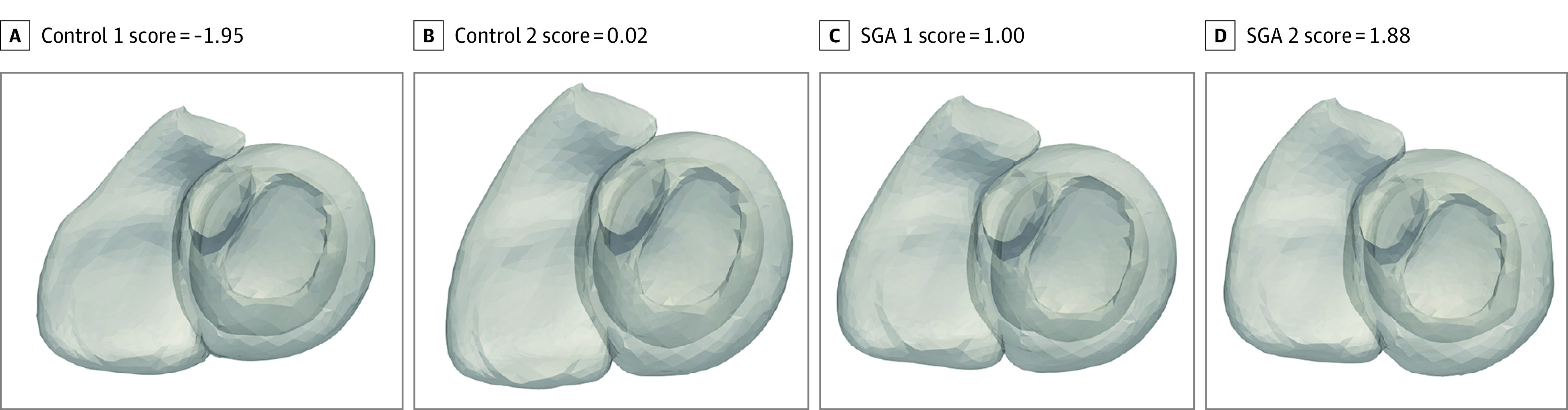
Individuals with higher remodeling scores have a more curved right ventricular outflow tract. The biventricular remodeling score was obtained by quantifying the remodeling captured by the biventricular shape models. It was derived from the logistic regression model that predicts SGA from shapes, taking into account the confounding variables (body surface area, age, and sex) (eMethods in the Supplement).
Video. Ventricular Shape Patterns in Young Adults Born Small for Gestational Age vs Control Patients.
Ventricular shape analysis defined regional cardiac shape patterns that distinguish small for gestational age (SGA) from non-SGA controls. In this video, the shape of the heart changes from the lowest (most normal) to the highest (most SGA) biventricular remodeling score and illustrates that individuals with higher remodeling scores more representative of SGA have an enlarged and more curved right ventricular outflow tract and a flattened basal septum. See text for details.
Exercise Capacity
Adults born SGA achieved lower maximal workload (mean [SD], 214 [60] W vs 180 [62] W) and showed lower oxygen consumption (median V̇o2, 29.5 mL/min/kg [IQR, 24.0-36.0 mL/min/kg] vs 26.0 mL/min/kg [21.5-33.5 mL/min/kg]), ventilation (median, 91.1% [IQR, 71.2%-109.7%] vs 76.9% [60.6%-90.9%), systolic blood pressure (median, 180 mm Hg [IQR, 162-203 mm Hg] vs 171 mm Hg [IQR, 144-186 mm Hg]), and pulse pressure (median, 90 mm Hg [IQR, 75-108 mm Hg] vs 75 mm Hg [IQR, 56-90 mm Hg]) at peak exercise, with oxygen similar saturation (mean [SD], 98% [2%] for both groups) compared with controls (Table 3). In addition, the change in systolic blood pressure and pulse pressure (delta) from baseline to peak exercise was reduced in adults born SGA (eTable 3 in the Supplement).
Table 3. Exercise Capacity at Peak Exercise by Study Population.
| Measure | Median (IQR) | P value | Adjusted P valuea | |
|---|---|---|---|---|
| Control (n = 66) | Born SGA (n = 61) | |||
| Maximal workload, mean (SD), W | 214 (60) | 180 (62) | .001 | .006 |
| Ventilation, % | 91.1 (71.2-109.7) | 76.9 (60.6-90.9) | .001 | .03 |
| V̇o2, mL/min/kg | 29.5 (24.0-36.0) | 26.0 (21.5-33.5) | .04 | .02 |
| V̇o2/LV mass, mean (SD), mL/min/g/m2 | 47.8 (14.0) | 41.7 (11.0) | .02 | .03 |
| V̇o2, mean (SD), % predicted | 85.6 (16.5) | 83.1 (17.7) | .42 | .40 |
| V̇co2, mL/min/kg | 44.4 (38.2-50.1) | 39.2 (33.0-48.9) | .04 | .02 |
| Respiratory exchange ratio, mean (SD)b | 1.4 (0.1) | 1.5 (0.1) | .69 | .90 |
| O2 saturation, mean (SD), % | 98 (2) | 98 (2) | .70 | .16 |
| Heart rate, bpm | 176 (168-185) | 176 (165-184) | .76 | .62 |
| Blood pressure, mm Hg | ||||
| Systolic | 180 (162-203) | 171 (144-186) | .03 | .04 |
| Diastolic | 90 (94-100) | 89 (80-102) | .86 | .55 |
| Pulse pressure, mm Hgc | 90 (75-108) | 75 (56-90) | .008 | .048 |
| O2 pulse, mean (SD), mL/beat/mind | 12.7 (3.9) | 10.6 (3.4) | .003 | .10 |
Abbreviations: bpm, beats per minute; IQR, interquartile range; O2, oxygen; SGA, small for gestational age; V̇co2, carbon dioxide production; V̇o2, oxygen consumption.
P value as compared with controls adjusted by age, sex, body surface area, asthma, educational level, smoking habit, gestational age at delivery, breastfeeding, and gestational hypertension.
Respiratory exchange ratio calculated as V̇co2 divided by V̇o2.
Pulse pressure calculated as systolic blood pressure divided by diastolic blood pressure.
Oxygen pulse calculated as V̇o2 divided by heart rate.
Exercise capacity significantly correlated with LV mass (ρ = 0.7934; P < .001 and P = .046 when adjusted by age, sex, and BSA) in the overall population (Figure 2A). Adults born SGA presented a different regression line and had significantly lower exercise capacity per unit of cardiac mass than controls (F2,96 = 3.312; P = .04 for interaction). Exercise capacity was statistically correlated with the biventricular shape index (ρ = −0.2549; P = .009, and P = .002 when adjusted by age, sex, and BSA) (Figure 2B).
Figure 2. Workload and Oxygen Consumption (V̇o2) at Peak Exercise and Its Association With Left Ventricular (LV) Mass and Biventricular Remodeling Score in the Study Populations.
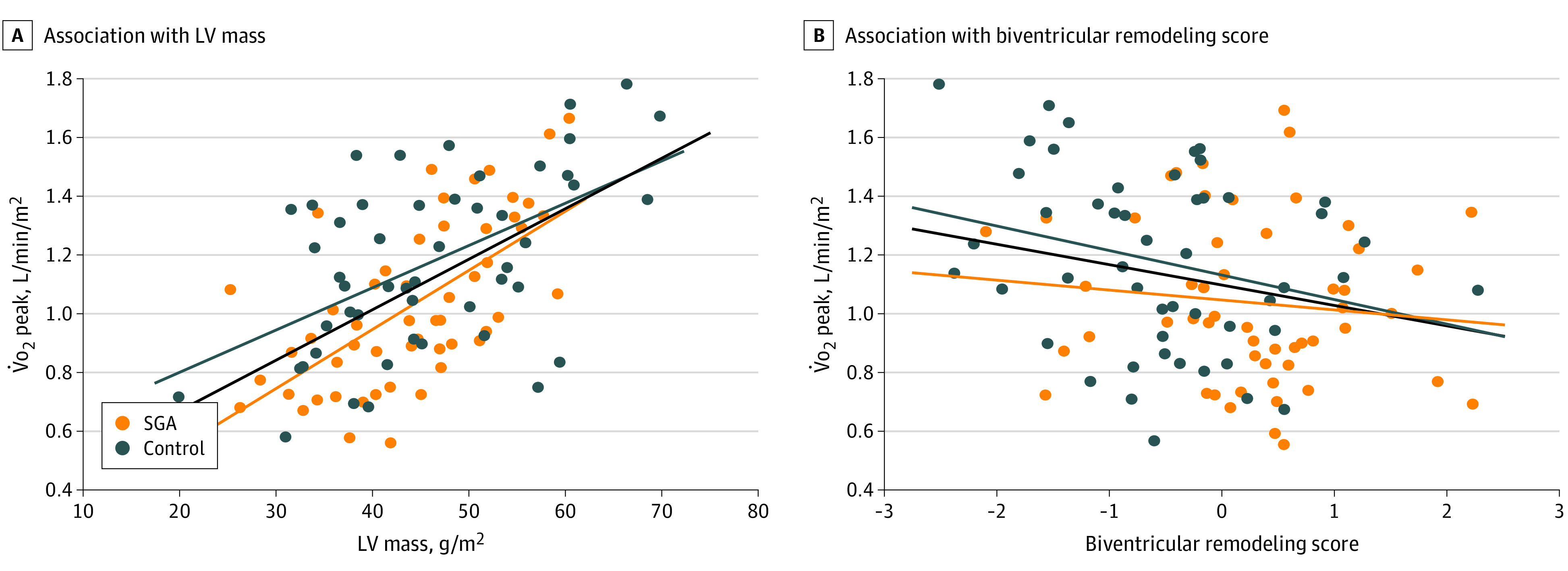
A, Regression line showing a positive association between the peak V̇o2 and the indexed LV mass (ρ = 0.7934; P < .001, and P = .046 when adjusted by age, sex, and body surface area). Adults born small for gestational age (SGA) present a significantly different regression line and have lower exercise capacity per unit of LV mass than controls (P = .04 for interaction). B, Regression line showing an inverse association between the peak V̇o2 and the quantity of biventricular remodeling score by 3-dimensional shape analysis (ρ = −0.2549; P = .009, and P = .002 when adjusted by age, sex, and body surface area).
Discussion
This study found reduced exercise capacity in a cohort of young adults born SGA compared with participants with birth weight within standard reference ranges. Thus, these results provide supporting evidence for a persistent association between being born SGA and cardiac outcomes in adult life.
We found very minor cardiac shape changes compared with control participants, mainly affecting the RV, in adults born SGA, with similar cardiac dimensions and mass and preserved cardiac function under basal conditions. Although some changes were statistically significant, they are unlikely to be clinically relevant given their small magnitude (ie, a 1% difference in LV ejection fraction). The observation of no delayed enhancement patterns is concordant with experimental data suggesting a change of fiber direction without evidence of fibrosis.26 Our results are consistent with previous studies evaluating SGA in adulthood8,9 suggesting that postnatal lifestyle partly compensates for the cardiac remodeling observed in fetuses SGA and children born SGA.3,4,5,6 The RV predominance of the observed changes may reflect the dominance of the right side of the heart during fetal life. Fetal cardiac remodeling is thought to be induced by sustained in utero pressure overload and restriction of oxygen and nutrients secondary to placental insufficiency.3,4 Growth-restricted fetuses present larger, globular, and hypertrophic ventricles with impaired relaxation.3,4,27 Most of these features persist in childhood5,6 and preadolescence.7 The subtle nature of the cardiac changes observed in adulthood may be partially explained by the inclusion of milder cases in adult cohorts. In addition, multiple influences during a lifetime contribute to attenuate or boost the effects of fetal programming changes.
Our results showed decreased exercise capacity among adults born SGA, with mean reductions of 16% in maximal workload and 12% in oxygen consumption at peak exercise. Such differences are noteworthy considering that the study participants were young and healthy. To our knowledge, this is the first study evaluating an incremental exercise test in a cohort of adults born SGA. Previous studies have evaluated the association between SGA and exercise capacity indirectly, with conflicting findings. Whereas some study findings suggest lower levels of leisure time physical activity and increased levels of sedentary behavior among individuals born SGA,28 other study results did not show any association of SGA with physical activity29 or sedentary time.30 The interpretation of previous studies is hampered by the use of definitions of SGA based purely on birth weight28,29,30 and the use of surrogates of physical activity, such as questionnaires or accelerometer or dynamometer metrics. We used centiles for gestational age to select SGA predominantly resulting from fetal growth restriction. Similarly, the use of a standardized exercise stress test allowed for precisely measuring exercise performance. In addition, our results showed that exercise capacity significantly correlated with LV mass and the biventricular remodeling score. Although our study was not designed to establish a causal or temporal relationship between cardiovascular changes and exercise performance among young adults born SGA, we may speculate on its association with altered exercise capacity. During exercise, the increased cardiac output required to satisfy the increased oxygen demand is generated through a combination of stroke volume and heart rate changes. Whereas on the left side of the heart, an increased volume output can be established without much change in LV pressure, the pulmonary circulation is too short to provide volume buffering, resulting in important changes in RV pressure during exercise.31 Indeed, the observed RV basal and septal shape remodeling in adults born SGA shares some similarities with those found in sports-induced remodeling,10,31 although the population born SGA in our study did not exercise more (often on the contrary) than their control counterparts. The observed remodeling may indicate that the RV of adults born SGA would be regularly subjected to higher pressures, suggesting that the pressure increase with moderate exercise would be larger in these adults compared with adults born at normal weight. Overall, this alteration would result in decreased exercise capacity given that higher RV pressures would induce more discomfort for the (untrained) individual. This speculation is further strengthened by our data indicating that adults born SGA have less exercise capacity for the same amount of LV mass compared with control participants, suggesting an overall cardiopulmonary maladaptation to exercise (or potentially altered intrinsic myocardial tissue properties). We also observed reduced peripheral systolic blood pressure and pulse pressure change with exercise, indicating that the whole cardiovascular system performs suboptimally during exercise. These findings are in line with impaired cardiac performance and increased superoxide generation in response to exercise reported in male rats born SGA.32 Overall, these data suggest that poorer exercise capacity may be explained, at least in part, by the cardiovascular changes observed in adults born SGA and warrant further investigation.
For more than 30 years, epidemiological studies have consistently shown that low birth weight is associated with increased adult cardiovascular mortality.1,2,3 However, the mechanisms underlying this association are still unclear. Here, we provided evidence suggestive of suboptimal capacity to exercise among such individuals. If confirmed, these findings open new opportunities for prevention of disease. The implications for public health are important because growth-restricted SGA affects 10% of the population. Individuals born SGA can be easily detected by a clinician conducting a routine clinical history. Defining adults born SGA as a high-risk population may enable personalized information and intervention strategies,33,34,35 with a potential for reducing morbidity. Although it is likely that, as a whole, the association of SGA with the adult cardiovascular mortality rate is not huge (ranging from 1% to 25%), a conservative estimate of 5% attributed risk and 30% improvement may result in reductions of 12 000 deaths annually in the US.
Strengths and Limitations
This study has some strengths and limitations. We studied well-characterized cases selected from delivery and assessed in adulthood. The study comprised a comprehensive cardiac assessment, including ventricular shape analysis. Tobacco smoking status was assessed using a standardized and validated questionnaire, although we acknowledge potential recall bias and limited accuracy of this method. Cardiac structures were indexed to BSA per the European Society Cardiology guidelines. Indeed, absolute cardiac dimensions—not indexed—were predominantly smaller in individuals born SGA, which appears to be associated with their reduced body size because most differences were no longer detected when indexed by BSA (eTable 1 in the Supplement). We acknowledge that other baseline or perinatal factors—such as gestational hypertension, breastfeeding, or educational level—may have influenced the results because they may be associated with cardiovascular risk. In fact, although educational level was significantly different in the 2 study populations, gestational hypertension and breastfeeding were not statistically significant, likely owing to the relatively small sample size. Given the potential influence of confounders, all results were adjusted by age, sex, asthma, educational level, smoking habit, gestational age at delivery, breastfeeding, and gestational hypertension. However, we acknowledge that our study was not designed to assess the influence of other factors. In addition, the changes reported here are subclinical, with most cardiac measurements lying within standard reference ranges. The use of a young adult cohort avoided the interference of other comorbidities but prevented study of any associations with disease. Despite the differences here reported, their longer-term persistence and association with cardiovascular disease remains to be proven. Therefore, the causes of reduced exercise capacity and their potential association with cardiovascular disease in older ages should be further investigated.
Conclusions
In conclusion, this study found that this cohort of young adults born SGA had reduced exercise capacity compared with their counterparts born within standard reference weights. Studies with larger sample sizes and with older participants are required to confirm and better characterize these observations. These results support considering SGA as a risk factor that may benefit from preventive strategies. Given the high prevalence of infants born SGA, targeting lifestyle policies in this group may provide a substantial positive contribution to public health.
eMethods. Methods: Selection of Cases From a Perinatal Registry, Definitions, Cardiac Magnetic Resonance Acquisition and Analysis, and Incremental Exercise Testing Methodology
eAppendix. Results: Smoking Status in the Study Populations
eFigure 1. CONSORT Flow Diagram of the Study
eFigure 2. Baseline Cardiac Structure of Individual Examples
eFigure 3. Population-Derived Representative Reconstructed Ventricular Shapes Depicting a Mean Control and an Extreme SGA Based on the Results of the Statistical Shape Analysis on the Ventricular Surfaces
eTable 1. Non-Indexed Cardiac Dimensions and Volumes by Magnetic Resonance
eTable 2. Cardiac Magnetic Resonance Results According to Standard Deviations From the Mean Reference Values (Z-Scores)
eTable 3. Heart Rate and Blood Pressure Results at Baseline, Anaerobic Threshold and Peak Exercise in the Study Populations
eReferences.
References
- 1.Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ. 1989;298(6673):564-567. doi: 10.1136/bmj.298.6673.564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knop MR, Geng TT, Gorny AW, et al. Birth weight and risk of type 2 diabetes mellitus, cardiovascular disease, and hypertension in adults: a meta-analysis of 7 646 267 participants from 135 studies. J Am Heart Assoc. 2018;7(23):e008870. doi: 10.1161/JAHA.118.008870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crispi F, Miranda J, Gratacós E. Long-term cardiovascular consequences of fetal growth restriction: biology, clinical implications, and opportunities for prevention of adult disease. Am J Obstet Gynecol. 2018;218(2S):S869-S879. doi: 10.1016/j.ajog.2017.12.012 [DOI] [PubMed] [Google Scholar]
- 4.Crispi F, Hernandez-Andrade E, Pelsers MM, et al. Cardiac dysfunction and cell damage across clinical stages of severity in growth-restricted fetuses. Am J Obstet Gynecol. 2008;199(3):254.e1-254.e8. doi: 10.1016/j.ajog.2008.06.056 [DOI] [PubMed] [Google Scholar]
- 5.Sehgal A, Doctor T, Menahem S. Cardiac function and arterial indices in infants born small for gestational age: analysis by speckle tracking. Acta Paediatr. 2014;103(2):e49-e54. doi: 10.1111/apa.12465 [DOI] [PubMed] [Google Scholar]
- 6.Crispi F, Bijnens B, Figueras F, et al. Fetal growth restriction results in remodeled and less efficient hearts in children. Circulation. 2010;121(22):2427-2436. doi: 10.1161/CIRCULATIONAHA.110.937995 [DOI] [PubMed] [Google Scholar]
- 7.Sarvari SI, Rodriguez-Lopez M, Nuñez-Garcia M, et al. Persistence of cardiac remodeling in preadolescents with fetal growth restriction. Circ Cardiovasc Imaging. 2017;10(1):e005270. doi: 10.1161/CIRCIMAGING.116.005270 [DOI] [PubMed] [Google Scholar]
- 8.Bjarnegård N, Morsing E, Cinthio M, Länne T, Brodszki J. Cardiovascular function in adulthood following intrauterine growth restriction with abnormal fetal blood flow. Ultrasound Obstet Gynecol. 2013;41(2):177-184. doi: 10.1002/uog.12314 [DOI] [PubMed] [Google Scholar]
- 9.Arnott C, Skilton MR, Ruohonen S, et al. Subtle increases in heart size persist into adulthood in growth restricted babies: the Cardiovascular Risk in Young Finns Study. Open Heart. 2015;2(1):e000265. doi: 10.1136/openhrt-2015-000265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernardino G, Sanz de la Garza M, Domenech-Ximenos B, et al. Three-dimensional regional bi-ventricular shape remodeling is associated with exercise capacity in endurance athletes. Eur J Appl Physiol. 2020;120(6):1227-1235. doi: 10.1007/s00421-020-04335-3 [DOI] [PubMed] [Google Scholar]
- 11.Boardman H, Lamata P, Lazdam M, et al. Variations in cardiovascular structure, function, and geometry in midlife associated with a history of hypertensive pregnancy. Hypertension. 2020;75(6):1542-1550. doi: 10.1161/HYPERTENSIONAHA.119.14530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewandowski AJ, Augustine D, Lamata P, et al. Preterm heart in adult life: cardiovascular magnetic resonance reveals distinct differences in left ventricular mass, geometry, and function. Circulation. 2013;127(2):197-206. doi: 10.1161/CIRCULATIONAHA.112.126920 [DOI] [PubMed] [Google Scholar]
- 13.Huckstep OJ, Williamson W, Telles F, et al. Physiological stress elicits impaired left ventricular function in preterm-born adults. J Am Coll Cardiol. 2018;71(12):1347-1356. doi: 10.1016/j.jacc.2018.01.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gavotto A, Vandenberghe D, Abassi H, et al. Oxygen uptake efficiency slope: a reliable surrogate parameter for exercise capacity in healthy and cardiac children? Arch Dis Child. 2020;105(12):1167-1174. doi: 10.1136/archdischild-2019-317724 [DOI] [PubMed] [Google Scholar]
- 15.Becoña E, Vázquez FL. The Fagerström test for nicotine dependence in a Spanish sample. Psychol Rep. 1998;83(3, pt 2):1455-1458. [DOI] [PubMed] [Google Scholar]
- 16.Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381-1395. doi: 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed] [Google Scholar]
- 17.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 18.Peters J, Ecabert O, Meyer C, Kneser R, Weese J. Optimizing boundary detection via simulated search with applications to multi-modal heart segmentation. Med Image Anal. 2010;14(1):70-84. doi: 10.1016/j.media.2009.10.004 [DOI] [PubMed] [Google Scholar]
- 19.Dryden IL, Mardia KV. Statistical Shape Analysis, With Applications in R. 2nd ed. John Wiley and Sons; 2016. doi: 10.1002/9781119072492 [DOI] [Google Scholar]
- 20.Bernardino G, Benkarim O, Sanz-de la Garza M, et al. Handling confounding variables in statistical shape analysis—application to cardiac remodelling. Med Image Anal. 2020;65:101792. doi: 10.1016/j.media.2020.101792 [DOI] [PubMed] [Google Scholar]
- 21.Puente-Maestú L, Ortega F, Pedro JG, et al. Prediction equations for maximal aerobic capacity on cycle ergometer for the Spanish adult population. Arch Bronconeumol (Engl Ed). 2020;S0300-2896(20)30375-6. Published online October 10, 2020. doi: 10.1016/j.arbres.2020.09.014 [DOI] [PubMed] [Google Scholar]
- 22.Bellenger NG, Davies LC, Francis JM, Coats AJ, Pennell DJ. Reduction in sample size for studies of remodeling in heart failure by the use of cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2000;2(4):271-278. doi: 10.3109/10976640009148691 [DOI] [PubMed] [Google Scholar]
- 23.Kaminsky LA, Imboden MT, Arena R, Myers J. Reference standards for cardiorespiratory fitness measured with cardiopulmonary exercise testing using cycle ergometry: data from the Fitness Registry and the Importance of Exercise National Database (FRIEND) Registry. Mayo Clin Proc. 2017;92(2):228-233. doi: 10.1016/j.mayocp.2016.10.003 [DOI] [PubMed] [Google Scholar]
- 24.Porszasz J, Blonshine S, Cao R, Paden HA, Casaburi R, Rossiter HB. Biological quality control for cardiopulmonary exercise testing in multicenter clinical trials. BMC Pulm Med. 2016;16:13. doi: 10.1186/s12890-016-0174-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837-845. doi: 10.2307/2531595 [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez-Tendero A, Zhang C, Balicevic V, et al. Whole heart detailed and quantitative anatomy, myofibre structure and vasculature from X-ray phase-contrast synchrotron radiation-based micro computed tomography. Eur Heart J Cardiovasc Imaging. 2017;18(7):732-741. doi: 10.1093/ehjci/jew314 [DOI] [PubMed] [Google Scholar]
- 27.Crispi F, Bijnens B, Sepulveda-Swatson E, et al. Postsystolic shortening by myocardial deformation imaging as a sign of cardiac adaptation to pressure overload in fetal growth restriction. Circ Cardiovasc Imaging. 2014;7(5):781-787. doi: 10.1161/CIRCIMAGING.113.001490 [DOI] [PubMed] [Google Scholar]
- 28.Andersen LG, Angquist L, Gamborg M, et al. ; NordNet Study Group . Birth weight in relation to leisure time physical activity in adolescence and adulthood: meta-analysis of results from 13 Nordic cohorts. PLoS One. 2009;4(12):e8192. doi: 10.1371/journal.pone.0008192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ridgway CL, Brage S, Sharp SJ, et al. Does birth weight influence physical activity in youth? a combined analysis of four studies using objectively measured physical activity. PLoS One. 2011;6(1):e16125. doi: 10.1371/journal.pone.0016125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hildebrand M, Kolle E, Hansen BH, et al. ; International Children’s Accelerometry Database (ICAD) Collaborators . Association between birth weight and objectively measured sedentary time is mediated by central adiposity: data in 10,793 youth from the International Children’s Accelerometry Database. Am J Clin Nutr. 2015;101(5):983-990. doi: 10.3945/ajcn.114.103648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.La Gerche A, Heidbüchel H, Burns AT, et al. Disproportionate exercise load and remodeling of the athlete’s right ventricle. Med Sci Sports Exerc. 2011;43(6):974-981. doi: 10.1249/MSS.0b013e31820607a3 [DOI] [PubMed] [Google Scholar]
- 32.Reyes LM, Kirschenman R, Quon A, Morton JS, Shah A, Davidge ST. Aerobic exercise training reduces cardiac function in adult male offspring exposed to prenatal hypoxia. Am J Physiol Regul Integr Comp Physiol. 2015;309(5):R489-R498. doi: 10.1152/ajpregu.00201.2015 [DOI] [PubMed] [Google Scholar]
- 33.van Deutekom AW, Chinapaw MJ, Vrijkotte TG, Gemke RJ. The association of birth weight and infant growth with physical fitness at 8-9 years of age—the ABCD study. Int J Obes (Lond). 2015;39(4):593-600. doi: 10.1038/ijo.2014.204 [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez-Lopez M, Osorio L, Acosta-Rojas R, et al. Influence of breastfeeding and postnatal nutrition on cardiovascular remodeling induced by fetal growth restriction. Pediatr Res. 2016;79(1-1):100-106. doi: 10.1038/pr.2015.182 [DOI] [PubMed] [Google Scholar]
- 35.Lewandowski AJ, Lamata P, Francis JM, et al. Breast milk consumption in preterm neonates and cardiac shape in adulthood. Pediatrics. 2016;138(1):e20160050. doi: 10.1542/peds.2016-0050 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Methods: Selection of Cases From a Perinatal Registry, Definitions, Cardiac Magnetic Resonance Acquisition and Analysis, and Incremental Exercise Testing Methodology
eAppendix. Results: Smoking Status in the Study Populations
eFigure 1. CONSORT Flow Diagram of the Study
eFigure 2. Baseline Cardiac Structure of Individual Examples
eFigure 3. Population-Derived Representative Reconstructed Ventricular Shapes Depicting a Mean Control and an Extreme SGA Based on the Results of the Statistical Shape Analysis on the Ventricular Surfaces
eTable 1. Non-Indexed Cardiac Dimensions and Volumes by Magnetic Resonance
eTable 2. Cardiac Magnetic Resonance Results According to Standard Deviations From the Mean Reference Values (Z-Scores)
eTable 3. Heart Rate and Blood Pressure Results at Baseline, Anaerobic Threshold and Peak Exercise in the Study Populations
eReferences.


