Key Points
Question
In patients with proximal anterior circulation occlusion stroke presenting in the extended window, are rates of favorable outcomes at 90 days comparable in the patients selected for thrombectomy with noncontrast computed tomography vs patients selected with computed tomography perfusion or magnetic resonance imaging?
Findings
In a multicenter cohort of 1604 patients in the extended window with large-vessel occlusion, patients selected by noncontrast computed tomography had comparable clinical and safety outcomes with patients selected by computed tomography perfusion or magnetic resonance imaging.
Meaning
These findings suggest noncontrast computed tomography alone may be used as an alternative to advanced imaging in selecting patients with late-presenting large-vessel occlusion for mechanical thrombectomy.
This cohort study compares the clinical outcomes of patients with stroke who presented 6 to 24 hours after symptom onset and were selected for mechanical thrombectomy by noncontrast computed tomography vs those selected by computed tomography perfusion or magnetic resonance imaging.
Abstract
Importance
Advanced imaging for patient selection in mechanical thrombectomy is not widely available.
Objective
To compare the clinical outcomes of patients selected for mechanical thrombectomy by noncontrast computed tomography (CT) vs those selected by computed tomography perfusion (CTP) or magnetic resonance imaging (MRI) in the extended time window.
Design, Setting, and Participants
This multinational cohort study included consecutive patients with proximal anterior circulation occlusion stroke presenting within 6 to 24 hours of time last seen well from January 2014 to December 2020. This study was conducted at 15 sites across 5 countries in Europe and North America. The duration of follow-up was 90 days from stroke onset.
Exposures
Computed tomography with Alberta Stroke Program Early CT Score, CTP, or MRI.
Main Outcomes and Measures
The primary end point was the distribution of modified Rankin Scale (mRS) scores at 90 days (ordinal shift). Secondary outcomes included the rates of 90-day functional independence (mRS scores of 0-2), symptomatic intracranial hemorrhage, and 90-day mortality.
Results
Of 2304 patients screened for eligibility, 1604 patients were included, with a median (IQR) age of 70 (59-80) years; 848 (52.9%) were women. A total of 534 patients were selected to undergo mechanical thrombectomy by CT, 752 by CTP, and 318 by MRI. After adjustment of confounders, there was no difference in 90-day ordinal mRS shift between patients selected by CT vs CTP (adjusted odds ratio [aOR], 0.95 [95% CI, 0.77-1.17]; P = .64) or CT vs MRI (aOR, 0.95 [95% CI, 0.8-1.13]; P = .55). The rates of 90-day functional independence (mRS scores 0-2 vs 3-6) were similar between patients selected by CT vs CTP (aOR, 0.90 [95% CI, 0.7-1.16]; P = .42) but lower in patients selected by MRI than CT (aOR, 0.79 [95% CI, 0.64-0.98]; P = .03). Successful reperfusion was more common in the CT and CTP groups compared with the MRI group (474 [88.9%] and 670 [89.5%] vs 250 [78.9%]; P < .001). No significant differences in symptomatic intracranial hemorrhage (CT, 42 [8.1%]; CTP, 43 [5.8%]; MRI, 15 [4.7%]; P = .11) or 90-day mortality (CT, 125 [23.4%]; CTP, 159 [21.1%]; MRI, 62 [19.5%]; P = .38) were observed.
Conclusions and Relevance
In patients undergoing proximal anterior circulation mechanical thrombectomy in the extended time window, there were no significant differences in the clinical outcomes of patients selected with noncontrast CT compared with those selected with CTP or MRI. These findings have the potential to widen the indication for treating patients in the extended window using a simpler and more widespread noncontrast CT–only paradigm.
Introduction
The DAWN and DEFUSE 3 trials were 2 landmark stroke trials that changed the paradigm of care for patients with large-vessel occlusion stroke who present within 6 to 24 hours from symptom onset (defined as time last seen well [TLSW]), opening the indications of endovascular treatment for this extended time window.1,2 Advanced imaging with magnetic resonance imaging (MRI) or computed tomography perfusion (CTP) demonstrating clinical or tissue mismatch were the mainstay of triage entry for these 2 studies and are currently recommended in the American Stroke Association and European Stroke Organization guidelines for the selection of these patients.3,4
Access to acute MRI or CTP is not readily available or performed across many stroke centers in the US5 or globally.6,7 A correlation between the Alberta Stroke Program Early CT Score (ASPECTS) on noncontrast CT (NCCT) and CTP has been demonstrated in several studies,8 with CT being potentially more sensitive than relative cerebral blood flow for irreversible injury in the later time window.9 However, there is known interrater variability in the interpretation of ASPECTS.10
An important notion is to distinguish triage performed to identify patients who can benefit from treatment (the appropriate goal of imaging) and triage performed to identify patients who may have better outcomes than others (an inappropriate goal in the context of individual medical care). Better clinical outcomes can be observed among patients selected on optimal CTP parameters, but this selection may improve overall statistics at the cost of denying care to many patients who could benefit.9,11 Moreover, CTP is vulnerable to infarct core overestimation, which may inappropriately exclude patients from treatment.11,12 It is not well established whether advanced neuroimaging is indispensable or if NCCT may be sufficient for identifying patients with large-vessel occlusion likely to benefit from endovascular thrombectomy who present in the extended window.
The objective of this study was to evaluate clinical outcomes in patients with proximal anterior circulation occlusion stroke presenting in the extended time window who were selected by NCCT and CT angiography, compared with patients selected via advanced imaging with CTP or MRI. The prespecified hypothesis was that there were no significant differences in the outcomes of patients selected for thrombectomy using NCCT compared with patients selected with advanced imaging with CTP or MRI.
Methods
Ethics
Local institutional review board or ethics committee approval was obtained from all sites. Written informed consent was waived because of the retrospective nature of this study and because the research was considered no more than minimal risk. This was an investigator-initiated study. The study funder had no role in the study design, analysis, management, or writing of this report. The corresponding author (T.N.N.) and lead statistician (M.M.Q.) had access to all data in the study. Anonymized data are available on reasonable request to the corresponding author.
Study Population
The CT for Late Endovascular Reperfusion (CLEAR) study (NCT04096248) was a multicenter cohort study of consecutive patients with proximal anterior circulation stroke undergoing mechanical thrombectomy (MT) in the extended time window, defined as a period from TLSW to arterial puncture between 6 to 24 hours. Patients were recruited at 15 sites across 5 countries in Europe and North America between January 1, 2014, and December 31, 2020. Pretreatment imaging protocols are listed in eTable 1 in the Supplement. A description of the change in protocol imaging selection across the study period is reported in eTable 2 in the Supplement. ASPECTS was site adjudicated based on the last NCCT image before thrombectomy.
The study cohort for this analysis included consecutive patients meeting the following criteria: baseline National Institutes of Health Stroke Scale (NIHSS) scores of 6 or more, occlusion of the internal carotid artery or proximal middle cerebral artery (M1/M2 segments), prestroke modified Rankin Scale (mRS) scores of 0 to 2, and TLSW to treatment of 6 to 24 hours. These criteria mirror the broader range of inclusion of patients encompassing both the DAWN and DEFUSE3 trials, with the addition of M2 occlusions. A subset of patients in the CLEAR study were included in the DAWN study (2 sites, up to 45 patients) or the DEFUSE-3 study (3 sites, 18 patients).
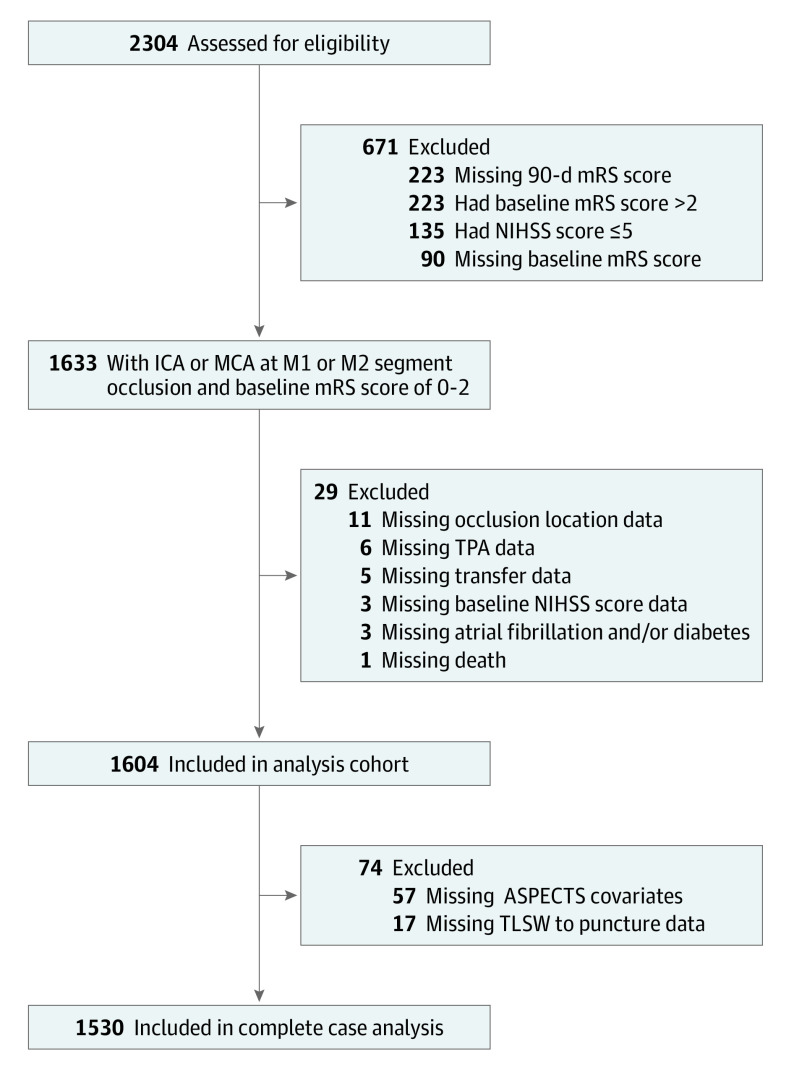
The CLEAR study cohort was categorized according to the imaging modality used to select the patient for endovascular therapy: NCCT with CT angiography, CTP, or MRI. If both NCCT and CTP were used, the patient was classified as selected by CTP. If both CTP and MRI were used, the patient was classified as selected by MRI. Patients presenting with large-vessel occlusion stroke presenting in an early time window (0 to <6 hours from TLSW), prestroke baseline mRS scores of 3 to 5, or large-vessel occlusion of the posterior circulation were excluded (Figure 1).
Figure 1. Study Flow Diagram.
ASPECTS indicates Alberta Stroke Program Early Computed Tomography Score; ICA, internal carotid artery; MCA, middle cerebral artery; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; TLSW, time last seen well; TPA, tissue plasminogen activator.
Outcome Variables
The primary end point was the distribution of mRS score at 90 days (ordinal shift analysis). The secondary clinical outcomes included the rate of 90-day functional independence (mRS scores of 0-2) as well as workflow metrics including TLSW to arterial puncture, time from door to puncture, and postprocedure successful reperfusion (defined as grade 2b or 3 [>50% of the affected territory] on the modified Treatment in Cerebral Infarction [mTICI] scale). A standard approach to mRS assessment was used (eTable 3 in the Supplement). The safety end points included postprocedural symptomatic intracranial hemorrhage (as defined in the European Cooperative Acute Stroke Study III: intracranial hemorrhage that is associated with deterioration in NIHSS of 4 or more points and the main reason for neurological deterioration) and 90-day mortality.13
Statistical Analysis
For the continuous variables, we tested the normal distribution of the data with the Shapiro-Wilk test. As the normality tests showed significant results, the data were deemed nonnormal, and hence, continuous data are presented as median (IQR) values. Nonparametric Kruskal-Wallis tests were used to assess for differences in continuous variables by imaging modality. For categorical variables, χ2 tests (or Fisher exact tests, when appropriate) were used to examine differences in the distribution by imaging modality.
For the outcome of functional independence at 90 days, a logistic regression model was used to estimate the likelihood of functional independence (mRS scores of 0-2). Clustering by sites was accounted for using a generalized estimating equation. The model was fitted using PROC GENMOD in SAS version 9.4 (SAS Institute), with the specifications of logit link function and binomial distribution. An independent correlation structure was assumed for the within-site clustering of patients. The independent correlation structure had the smallest quasilikelihood independence criterion value. Crude and adjusted odds ratios (ORs) with 95% CIs were obtained for each parameter of interest. The following characteristics were selected as covariates of interest a priori: age, NIHSS score at baseline, sex, baseline mRS scores, hypertension, atrial fibrillation, diabetes, transfer status, intravenous thrombolysis, baseline ASPECTS, site of occlusion, and TLSW to arterial puncture.
For the distribution of 90-day mRS scores (ordinal mRS shift), a multinomial ordinal logistic regression was applied to estimate a 1-point shift toward the lowered ordered value, indicating a better outcome. The model was fitted using PROC GENMOD in SAS version 9.4 with the cumulative logit link function and multinomial distribution. An independent correlation structure was assumed for the within-site clustering of patients.
All statistical computations were performed on SAS version 9.4. All tests were 2-sided, and a P value less than .05 was considered significant.
Results
There were 2304 patients with internal carotid artery or proximal middle cerebral artery occlusions recruited in the CLEAR study, of whom 1768 had a premorbid mRS score of 0 to 2. Of these, 1604 patients had baseline NIHSS scores of 6 or more and descriptive data of the key variables, constituting the primary analysis cohort (median [IQR] age, 70 [59-80] years; 848 women [52.9%]; Figure 1). Patients were selected for MT on the basis of the following modalities: NCCT with angiography (n = 534), CTP (n = 752), and MRI (n = 318). Computed tomography angiography was performed in 1303 of 1334 patients (98%), whereas MRI and magnetic resonance angiography were the primary modalities for large-vessel occlusion selection in 270 patients. Data variables are summarized in Table 1.
Table 1. Baseline Characteristics, Metrics, and Outcomes of Patients in the 6-24–Hour Window, According to Imaging Modality Selection for Thrombectomy.
| Characteristic | Patients, No. (%) | P value | |||
|---|---|---|---|---|---|
| Overall | Computed tomographya | Computed tomography perfusion | Magnetic resonance imaging | ||
| Total patients, No. | 1604 | 534 | 752 | 318 | NA |
| Demographics and clinical characteristics | |||||
| Age, median (IQR), y | 70 (58.5-80) | 71 (58-81) | 69 (58-80) | 71.5 (61-80) | .20 |
| Baseline NIHSS score, median (IQR) | 16 (12-20) | 17 (13-21) | 16 (11-19) | 16 (12-21) | <.001 |
| Sex | |||||
| Male | 756 (47.1) | 261 (48.9) | 346 (46.0) | 149 (46.9) | .59 |
| Female | 848 (52.9) | 273 (51.1) | 406 (54.0) | 169 (53.1) | |
| Baseline mRS | |||||
| 0 | 1033 (64.4) | 335 (62.7) | 482 (64.1) | 216 (67.9) | .41 |
| 1 | 345 (21.5) | 114 (21.4) | 170 (22.6) | 61 (19.2) | |
| 2 | 226 (14.1) | 85 (15.9) | 100 (13.3) | 41 (12.9) | |
| Hypertension | |||||
| No | 470 (29.3) | 149 (27.9) | 208 (27.7) | 113 (35.5) | .02 |
| Yes | 1134 (70.7) | 385 (72.1) | 544 (72.3) | 205 (64.5) | |
| Atrial fibrillation | |||||
| No | 1077 (67.1) | 343 (64.2) | 533 (70.9) | 201 (63.2) | .01 |
| Yes | 527 (32.9) | 191 (35.8) | 219 (29.1) | 117 (36.8) | |
| Diabetes | |||||
| No | 1220 (76.1) | 405 (75.8) | 563 (74.9) | 252 (79.3) | .31 |
| Yes | 384 (23.9) | 129 (24.2) | 189 (25.1) | 66 (20.8) | |
| Transfer status | |||||
| Local | 598 (37.3) | 172 (32.2) | 330 (43.9) | 96 (30.2) | <.001 |
| Transferred | 1006 (62.7) | 362 (67.8) | 422 (56.1) | 222 (69.8) | |
| Intravenous tissue-type plasminogen activator | |||||
| No | 1251 (78.0) | 408 (76.4) | 661 (87.9) | 182 (57.2) | <.001 |
| Yes | 353 (22.0) | 126 (23.6) | 91 (12.1) | 136 (42.8) | |
| Clot location and imaging | |||||
| ASPECTS, median (IQR)b | 8 (7-9) | 8 (7-9) | 8 (7-9) | 8 (6-9) | .03 |
| Site of occlusion | |||||
| Middle cerebral artery | |||||
| M1 | 906 (56.5) | 300 (56.2) | 430 (57.2) | 176 (55.4) | <.001 |
| M2 | 272 (17.0) | 73 (13.7) | 160 (21.3) | 39 (12.3) | |
| Internal carotid artery | 426 (26.6) | 161 (30.2) | 162 (21.5) | 103 (32.4) | |
| Time metrics and procedural factors | |||||
| TLSW to puncture, median (IQR), hb | 11.5 (8.3-15.0) | 10.4 (7.8-14.4) | 11.3 (8.4-15.2) | 12.4 (9.4-15.4) | <.001 |
| TLSW to computed tomography, median (IQR), hb | 10.3 (7.3-14.1) | 9.4 (6.6-13.3) | 10.5 (7.3-14.4) | 10.9 (8.0-14.3) | <.001 |
| Modified Treatment in Cerebral Infarction scoreb | |||||
| 0-2a | 205 (12.8) | 59 (11.1) | 79 (10.6) | 67 (21.1) | <.001 |
| 2b-3 | 1394 (87.2) | 474 (88.9) | 670 (89.5) | 250 (78.9) | |
| General anesthesiab | |||||
| No | 1255 (79.9) | 389 (74.5) | 602 (82.5) | 264 (83.0) | .001 |
| Yes | 315 (20.1) | 133 (25.5) | 128 (17.5) | 54 (17.0) | |
| Clinical outcomes | |||||
| Discharge NIHSS score, median (IQR)b | 7 (3-16) | 7 (3-17) | 6 (2-14) | 11 (3-19) | <.001 |
| Patients with 90-d mRS score, No. | 3 (1-5) | 3 (2-5) | 3 (1-5) | 3 (2-5) | .23 |
| 90-d mRS score | .21 | ||||
| 0-2 | 676 (42.1) | 220 (41.2) | 333 (44.3) | 123 (38.7) | |
| 3-6 | 928 (57.9) | 314 (58.8) | 419 (55.7) | 195 (61.3) | |
| Symptomatic intracranial hemorrhageb | .11 | ||||
| No | 1478 (93.7) | 476 (91.9) | 700 (94.2) | 302 (95.3) | |
| Yes | 100 (6.3) | 42 (8.1) | 43 (5.8) | 15 (4.7) | |
| Mortality, 90 d | |||||
| No | 1258 (78.4) | 409 (76.6) | 593 (78.9) | 256 (80.5) | .38 |
| Yes | 346 (21.6) | 125 (23.4) | 159 (21.1) | 62 (19.5) | |
Abbreviations: ASPECTS, Alberta Stroke Program Early Computed Tomography Score; mRS, modified Rankin Scale; NA, not applicable; NIHSS, National Institutes of Health Stroke Scale; TLSW, time last seen well.
Of 1604 patients, magnetic resonance imaging–magnetic resonance angiography was the primary imaging modality of selection for large-vessel occlusion in 270 patients. Computed tomography angiography was performed in 1303 of 1334 patients (98%).
ASPECTS, TLSW to puncture, TLSW to computed tomography, modified Treatment in Cerebral Infarction score, general anesthesia, NIHSS score prior to discharge, and symptomatic intracranial hemorrhage were missing for 57, 20, 143, 5, 34, 201, and 26 patients, respectively.
There was no difference in age, sex, baseline mRS score, and diabetes across the 3 modalities. Median (IQR) baseline NIHSS scores were marginally higher in the NCCT-selected group than the CTP-selected and MRI-selected groups, respectively (NCCT, 17 [13-21]; CTP, 16 [11-19]; MRI, 16 [12-21]; P < .001). Patients selected by NCCT had higher rates of hypertension (NCCT, 385 [72.1%]; CTP, 544 [72.3%]; MRI, 205 [64.5%]; P = .02) and atrial fibrillation (NCCT, 191 [35.8%]; CTP, 219 [29.1%]; MRI, 117 [36.8%]; P = .01) and more frequently presented as transfers (NCCT, 362 [67.8%]; CTP, 422 [56.1%]; MRI, 222 [69.8%]; P < .001) (Table 1). Intravenous thrombolysis was more frequent in patients selected by MRI (136 [42.8%]), followed by NCCT (126 [23.6%]) and CTP (91 [12.1%]; P < .001).
The median (IQR) ASPECTS score was 8 (7-9) across the 3 groups. Internal carotid artery occlusion was more common in the MRI and NCCT groups compared with the CTP group (103 [32.4%] vs 161 [30.2%] vs 162 [21.5%], respectively; P < .001). The M2 occlusions were more common in the CTP than CT and MRI groups (160 [21.3%] vs 73 [13.7%] vs 39 [12.3%], respectively; P < .001).
Time Metrics
Time last seen well to puncture was shorter in patients who underwent NCCT selection (median [IQR], 10.4 [7.8-14.4] hours) compared with CTP selection (median [IQR], 11.3 [8.4-15.2] hours) and MRI selection (median [IQR], 12.4 [9.4-15.4] hours; P < .001). In a subgroup analysis of patients who presented directly to the endovascular center (n = 598) with available door arrival time (n = 484), there were shorter door-to-puncture times for NCCT (median [IQR],76 [50-107] minutes) compared with CTP (median [IQR], 93 [72-118] minutes) and MRI (median [IQR], 98 [78-135] minutes; P < .001).
Procedural Outcome
Successful reperfusion (mTICI scores 2b-3) was more common in the NCCT group (474 [88.9%]) and CTP group (670 [89.5%]) compared with the MRI group (250 [78.9%]; P < .001). General anesthesia was more commonly used in the NCCT group (133 [25.5%]) than the CTP group (128 [17.5%]) and MRI group (54 [17.0%]; P = .001).
Clinical and Safety Outcomes
Discharge NIHSS scores were lower among patients selected with NCCT (median [IQR], 7 [3-17]) and CTP (median [IQR], 6 [2-14]) than those undergoing MRI (median [IQR], 11 [3-19]; P < .001). Overall, symptomatic intracranial hemorrhage was present in 6.3% (n = 100) of this cohort and was similar between the 3 imaging modalities (NCCT, 42 [8.1%]; CTP, 43 [5.8%]; MRI, 15 [4.7%]; P = .11). In addition, mortality at 90 days was similar between the 3 groups (NCCT, 125 [23.4%]; CTP, 159 [21.1%]; MRI, 62 [19.5%]; P = .38) (Table 1).
Analysis of 90-Day Functional Independence
A 90-day mRS score of 0 to 2 was observed in 220 patients selected by NCCT (41.2%), 333 selected by CTP (44.3%), and 123 selected by MRI (38.7%) (Table 1). The crude and adjusted odds of independent outcomes (mRS score 0-2) by imaging modality, baseline characteristics, and metrics are presented in Table 2. Of the 1604 patients in the primary cohort, complete information for multivariate analysis was available in 1530 patients (NCCT, 480; CTP, 735; MRI, 315). In the multivariable analysis, after adjusting for these factors, odds of functional independence at 90 days (mRS scores, 0-2) was similar between patients selected by CTP and NCCT (adjusted OR [aOR], 0.90 [95% CI, 0.70-1.16]; P = .42). However, there were lower odds of independent outcomes for patients selected by MRI than those selected by NCCT (aOR, 0.79 [95% CI, 0.63-0.98]; P = .03). Among other factors, age, baseline NIHSS score, baseline mRS score, diabetes, transfer status, and baseline ASPECTS were associated with independent outcomes at 90 days (Table 2).
Table 2. Univariate and Multivariate Analysis of Imaging Modality, Baseline Characteristics, and Metrics With Good Outcomea.
| Characteristic | Univariate | Multivariateb | ||
|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | |
| Imaging modality | ||||
| Computed tomography | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Computed tomography perfusion | 1.08 (0.83-1.42) | .56 | 0.90 (0.70-1.16) | .42 |
| Magnetic resonance imaging | 0.84 (0.63-1.13) | .25 | 0.79 (0.63-0.98) | .03 |
| Age | 0.97 (0.97-0.98) | <.001 | 0.97 (0.97-0.98) | <.001 |
| Baseline National Institutes of Health Stroke Scale score | 0.89 (0.87-0.90) | <.001 | 0.90 (0.89-0.91) | <.001 |
| Female | 0.85 (0.69-1.05) | .14 | 0.90 (0.70-1.17) | .44 |
| Baseline Modified Rankin Scale score | ||||
| 0 | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 1 | 0.58 (0.45-0.75) | <.001 | 0.70 (0.53-0.92) | .01 |
| 2 | 0.32 (0.21-0.49) | <.001 | 0.40 (0.24-0.66) | <.001 |
| Hypertension | 0.63 (0.54-0.74) | <.001 | 0.84 (0.67-1.06) | .14 |
| Atrial fibrillation | 0.67 (0.56-0.81) | <.001 | 1.07 (0.88-1.29) | .53 |
| Diabetes | 0.71 (0.57-0.88) | .002 | 0.72 (0.55-0.93) | .01 |
| Transfer | 0.80 (0.70-0.92) | .001 | 0.79 (0.65-0.96) | .02 |
| Intravenous tissue-type plasminogen activator | 0.95 (0.75-1.21) | .70 | 1.14 (0.83-1.56) | .43 |
| Alberta Stroke Program Early Computed Tomography Score | 1.15 (1.10-1.21) | <.001 | 1.17 (1.11-1.24) | <.001 |
| Site of occlusion | ||||
| Middle cerebral artery M1 | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Middle cerebral artery M2 | 1.57 (1.21-2.02) | .001 | 1.31 (0.97-1.77) | .08 |
| Internal carotid artery | 0.78 (0.64-0.94) | .01 | 0.88 (0.72-1.08) | .22 |
| Time last seen well to puncture | 1.0 (0.99-1.01) | .96 | 1.0 (0.99-1.01) | .75 |
A good outcome was defined as a 90-day modified Rankin Scale score of 0 to 2.
A total of 1530 patients had complete information on all the factors in the multivariate model.
Analysis of 90-Day Ordinal mRS Shift
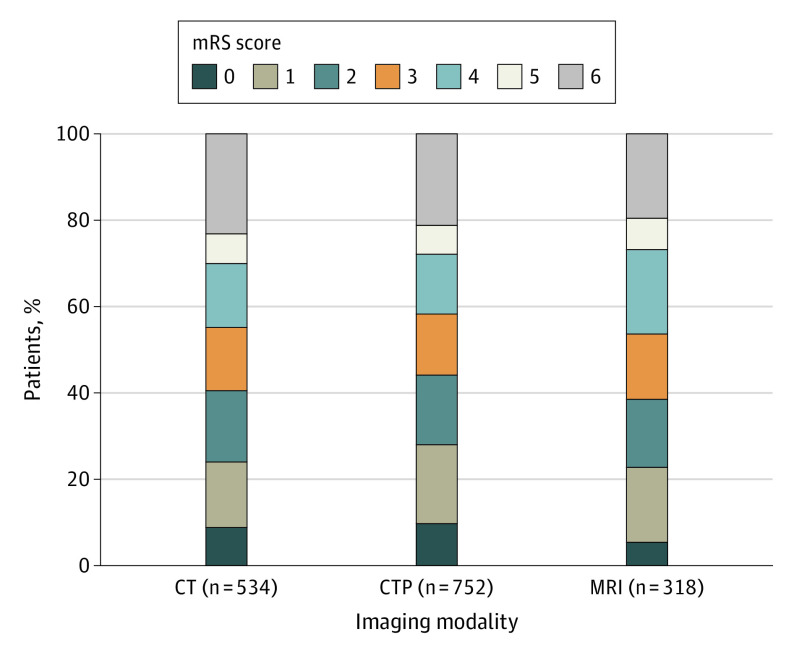
In the multivariable analysis, after adjusting for the mentioned factors, there was no difference in 90-day ordinal mRS shift between patients selected by NCCT vs CTP (aOR, 0.95 [95% CI, 0.77-1.17]; P = .64) or patients selected by NCCT vs MRI (aOR, 0.95 [95% CI, 0.80-1.13]; P = .55; Table 3; Figure 2). On multivariate analysis, increasing age (OR, 0.97 [95% CI, 0.96-0.99]; P < .001), higher baseline NIHSS scores (OR, 0.91 [95% CI, 0.89-0.92]; P < .001), higher baseline mRS scores (ORs: score of 1, 0.68 [95% CI, 0.54-0.86]; P = .001; score of 2, 0.48 [95% CI, 0.34-0.65]; P < .001), diabetes (on univariate analysis only; OR, 0.77 [95% CI, 0.62-0.96]; P = .02), internal carotid artery occlusion (OR, 0.83 [95% CI, 0.69-1.0]; P = .049), and having been transferred (OR, 0.79 [95% CI, 0.67-0.92]; P = .002) were associated with decreased odds of a 1-point shift toward the lowered ordered value, indicating a worse prognosis. Conversely, an increasing ASPECTS score (OR, 1.18 [95% CI, 1.13-1.24]; P < .001) was associated with increased odds of a 1-point shift, indicating an improved prognosis.
Table 3. Univariate and Multivariate Analysis of Imaging Modality, Baseline Characteristics, and Metrics With 90-Day Ordinal Modified Rankin Scale Score Shift.
| Characteristic | Univariate | Multivariatea | ||
|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | |
| Imaging modality | ||||
| Computed tomography | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Computed tomography perfusion | 1.13 (0.92-1.38) | .24 | 0.95 (0.77-1.17) | .64 |
| Magnetic resonance imaging | 0.95 (0.75-1.19) | .64 | 0.95 (0.80-1.13) | .55 |
| Age | 0.97 (0.96-0.98) | <.001 | 0.97 (0.96-0.99) | <.001 |
| Baseline National Institutes of Health Stroke Scale score | 0.89 (0.88-0.91) | <.001 | 0.91 (0.89-0.92) | <.001 |
| Female | 0.87 (0.73-1.05) | .14 | 0.97 (0.78-1.20) | .75 |
| Baseline Modified Rankin Scale score | ||||
| 0 | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 1 | 0.56 (0.45-0.70) | <.001 | 0.68 (0.54-0.86) | .001 |
| 2 | 0.36 (0.27-0.48) | <.001 | 0.48 (0.34-0.65) | <.001 |
| Hypertension | 0.65 (0.55-0.75) | <.001 | 0.89 (0.74-1.07) | .23 |
| Atrial fibrillation | 0.64 (0.56-0.74) | <.001 | 1.0 (0.87-1.14) | .94 |
| Diabetes | 0.77 (0.62-0.96) | .02 | 0.81 (0.65-1.01) | .06 |
| Transfer | 0.78 (0.71-0.86) | <.001 | 0.79 (0.67-0.92) | .002 |
| Intravenous tissue-type plasminogen activator | 0.92 (0.77-1.11) | .38 | 1.08 (0.86-1.36) | .51 |
| Alberta Stroke Program Early Computed Tomography Score | 1.15 (1.10-1.20) | <.001 | 1.18 (1.13-1.24) | <.001 |
| Site of occlusion | ||||
| Middle cerebral artery M1 | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Middle cerebral artery M2 | 1.47 (1.21-1.79) | <.001 | 1.16 (0.93-1.44) | .19 |
| Internal carotid artery | 0.74 (0.63-0.87) | <.001 | 0.83 (0.69-1.0) | .049 |
| Time last seen well to puncture | 1.0 (1.0-1.0) | .88 | 1.0 (0.99-1.0) | .67 |
A total of 1530 patients had complete information on all the factors in the multivariate model.
Figure 2. Distribution of 90-Day Modified Rankin Scale Score (mRS) in Patients Presenting in the Window 6 to 24 Hours After Time Last Seen Well With Internal Carotid Artery and Middle Cerebral Artery M1/M2 Occlusions, by Imaging Modality.
Scores range from 0 to 6, with 0 indicating no symptoms; 1, no clinically significant disability; 2, slight disability (the patient is able to look after their own affairs without assistance but unable to carry out all previous activities); 3, moderate disability (patient requires some help but is able to walk unassisted); 4, moderately severe disability (patient is unable to attend to bodily needs without assistance and unable to walk unassisted); 5, severe disability (patient requires constant nursing care and attention); and 6, death. After adjustment of confounders, there was no difference in 90-day ordinal mRS shift between patients selected by computed tomography (CT) vs CT perfusion (CTP) (adjusted odds ratio, 0.95 [95% CI, 0.77-1.17]; P = .64) or CT vs magnetic resonance imaging (adjusted odds ratio, 0.95 [95% CI, 0.8-1.13]; P = .55).
Sensitivity Analysis of Local Patients
In a sensitivity analysis confined to patients who arrived locally and were not transferred (n = 598), odds of functional independence at 90 days (mRS scores, 0-2) were similar on the multivariate analysis between patients selected by NCCT vs CTP (aOR, 0.71 [95% CI, 0.42-1.21]; P = .21), and patients selected by NCCT vs MRI (aOR, 0.69 [95% CI, 0.36-1.33]; P = .27; eTable 4 in the Supplement). Similarly, there was no difference in ordinal mRS shifts between patients selected by NCCT vs CTP (aOR, 0.74 [95% CI, 0.46-1.18]; P = .21) or patients selected by NCCT vs MRI (aOR, 0.78 [95% CI, 0.54-1.12]; P = .18) for those arriving locally.
Sensitivity Analysis of Time Associations
In a sensitivity analysis on the association of time as a continuous variable from 2014 to 2020 with available data in 1425 patients, odds of a good outcome at 90 days (mRS scores 0-2) were no different between patients selected by NCCT vs CTP (aOR, 0.88 [95% CI, 0.67-1.15]; P = .35) and patients selected by NCCT vs MRI (aOR, 0.85 [95% CI, 0.64-1.14]; P = .29). Similarly, no significant differences were observed in ordinal mRS shift between patients selected by NCCT vs CTP (aOR, 0.92 [95% CI, 0.75-1.12]; P = .39) and those selected by NCCT vs MRI (aOR, 1.01 [95% CI, 0.85-1.21]; P = .92).
Analysis of Reperfusion
In a separate analysis, patients who were reperfused (mTICI 2b/3) had better odds of good clinical outcome compared with those who were not reperfused (mTICI 0, 1, or 2a) (aOR, 6.31 [95% CI, 4.45-8.95]; P < .001). Of the 3 imaging modalities, the odds of a favorable outcome with reperfusion were highest in patients selected with MRI (aOR, 8.9 [95% CI, 6.7-11.9]; P < .001) followed by NCCT (aOR, 6.1 [95% CI, 2.2-16.5]; P < .001) and CTP (aOR, 5.1 [95% CI, 2.9-9.2]; P < .001; eTable 5 in the Supplement).
Discussion
The CLEAR study demonstrated that in patients with stroke due to occlusion of the internal carotid or proximal middle cerebral artery (M1/M2 segments) presenting within 6 to 24 hours from TLSW and undergoing mechanical thrombectomy, favorable functional outcomes defined by both ordinal shift and dichotomized analysis of the mRS scores at 90 days were equivalent for patients selected by NCCT compared with patients selected by CTP or MRI. The rate of functional independence at 90 days among patients in the CLEAR study who were treated using NCCT was comparable with that of patients treated in the DAWN and DEFUSE-3 trials. There was no evidence of a greater risk of symptomatic hemorrhage or death according to the imaging modality used in patient selection. Notably, door-to-puncture time, a workflow metric of stroke care,14 was shorter in patients selected by NCCT than CTP and MRI. To our knowledge, this is the largest multicenter study to date assessing selection of patients in the extended time window with NCCT compared with CTP or MRI.
These findings have the potential to support the adoption of a more pragmatic selection of patients for MT in the extended window, simply based on NCCT and proximal anterior circulation large-vessel occlusion. This selection could occur as an alternative to CTP-based or MRI-based selection paradigms, the second of which are not widely available across the globe,7 associated with potential treatment delays,15,16 cost, contrast load, radiation exposure, and resource use. On the other hand, it is important to note that management of patients in the NCCT group in the CLEAR study were not aligned with the American Stroke Association or European Stroke Organization guidelines.
In the extended time window, the concept of clinical core mismatch was used to guide patient selection in the DAWN trial,1 with additional and controversial criteria17 for study entry based on age and core infarct volume. The DEFUSE-3 trial relied on perfusion imaging assessment for the qualification of mismatch and study eligibility.2 While our study did not prespecify study entry based on clinical-core mismatch, NCCT-based selection in this analysis led to equivalent outcomes compared with patients selected by CTP or MRI. Two factors may explain the equivalence of outcome by the different imaging modality selection. First, prior reports demonstrated a moderate correlation between NCCT ASPECTS and CTP core volumes,8,18 with a stronger correlation in patients in the extended (6-24–hour) vs early (0-6–hour) time window.19 Second, among similar ASPECTS levels, the presence of clinical core mismatch does not decline over time, even when presenting in the extended time window.20
The rates of 90-day functional independence in our study were numerically but not significantly lower in the NCCT compared with the CTP group (41.2% vs 44.3%; P = .21). This apparent difference disappeared on multivariate analysis, which is likely explained by higher NIHSS scores, transfers, and internal carotid artery occlusions in the NCCT compared with the CTP group. On multivariate dichotomized mRS score analysis, patients selected by NCCT had higher rates of 90-day functional independence compared with patients selected by MRI (41.2% vs 38.7%; aOR, 0.79 [95% CI, 0.63-0.98]; P = .03), which was likely explained by a higher rate of reperfusion in the NCCT compared with the MRI group (88.9% vs 78.9%; P < .001). In a reperfusion sensitivity analysis, patients who achieved reperfusion had greater odds of good outcomes than patients who were not reperfused. There was a higher likelihood of functional independence in patients who were reperfused and selected by MRI compared with CT or CTP.
The findings in our study are concordant with other studies. The Heidelberg group evaluated thrombectomy of patients in the extended window using Highly Effective Reperfusion Using Multiple Endovascular Devices (HERMES)–like criteria and found equivalent outcomes in patients treated in the extended window with an ASPECTS-based selection paradigm.21 The Trevo multicenter registry evaluated the association of imaging modality with thrombectomy outcomes in both the early and extended windows.7 Computed tomography perfusion did not confer better 90-day outcomes in the extended window than NCCT. In a 2-site analysis of patients in the extended time window in Switzerland, MT was effective in the presence of a clinical-ASPECTS mismatch as defined by the DAWN study criteria but not effective in its absence.22 Another study suggested that NCCT-ASPECTS–based selection paradigms for late MT have similar 90-day outcomes as the DAWN and DEFUSE-3 studies.23 In a network of stroke centers in Houston, Texas, hospitals with NCCT as the initial imaging choice were more likely to perform MT than hospitals with NCCT and CTP as the primary approach, without any difference in clinical outcomes.24
While this study did not specify patient inclusion based on ASPECTS thresholds, most sites used ASPECTS of 6 or more to treat patients in the extended window (eTable 1 in the Supplement). The median (IQR) NCCT ASPECTS of 8 (7-9) in this study reflects that most patients selected for MT had high NCCT ASPECTS. As the IQR for ASPECTS score ranged from 7 to 9 in this cohort, this suggests an ASPECTS of 7 or more may be considered if one were to select patients with NCCT for thrombectomy in the extended window. Whether one should select patients with lower NCCT ASPECTS is unknown, because these patients were not well represented in our study.
In the absence of data on the number of patients denied thrombectomy and their outcomes, we could only compare aggregate results of patients who were ultimately treated. While the end results were similar, this does not mean that the same patients were selected in or out, and all triage methods may be inaccurate. Outside randomized trials, this may be impossible to determine. Two randomized trials are in progress to provide more definitive evidence of a simplified imaging protocol in the extended window: the MR CLEAN LATE trial (Endovascular Treatment of Acute Ischemic Stroke in the Netherlands for Late Arrivals; ISRCTN19922220) and the RESILIENT-Extended trial (Randomization of Endovascular Treatment in Acute Ischemic Stroke in the Extended Time Window; NCT04256096).
Strengths
The strength of our study was the large number of patients across multiple centers and countries. To decrease bias, we excluded patients with missing data to conduct a complete case analysis with the final cohort of 1530 patients.
Limitations
Our study was limited to patients with baseline mRS scores of 0 to 2, occlusion of the internal carotid or proximal middle cerebral artery (M1/M2 segments), with median (IQR) ASPECTS of 8 (7-9). Hence our study’s findings cannot be applied to other patients.
The retrospective design of our study may have led to selection bias, thereby limiting the generalization of results. While we encouraged enrollment of consecutive patients in the study period, this was not monitored. There was no independent imaging core laboratory in this study. Differences in imaging interpretation, selection paradigms, and automatic CTP processing software across the multiple centers could introduce bias. We did not measure core volumes across the patients selected by CTP or MRI, other than by their CT or diffusion-weighted imaging–adjudicated ASPECTS scores. Collateral score was not measured; most sites reported that CT angiography collaterals were not routinely used in their imaging triage (eTable 1 in the Supplement). Thus, we could only compare various imaging modalities without knowing the explicit criteria used to select patients. In this analysis, we have data for patients treated; we do not know the proportions of patients who were excluded by the triage process, nor could we compare treatment effect sizes, given the lack of patients who were medically managed.
Conclusions
In patients with large-vessel occlusion of the internal carotid artery and proximal middle cerebral artery (M1/M2 segments) undergoing MT in the time window of 6 to 24 hours after TLSW, selection by NCCT yielded no significant difference in clinical and safety outcomes compared with advanced imaging with CTP or MRI. These findings have the potential to widen the indication for treating patients in the extended window using the simpler, less costly, and easier to implement NCCT imaging as an alternative to CTP or MRI.
eTable 1. Neuroimaging triage protocols of sites in the CLEAR Study
eTable 2. Selection criteria for late window (6-24h) thrombectomy between 2014 to 2020)
eTable 3. Modified Rankin Score Assessment
eTable 4. Analysis of imaging modality with good outcome: Local patients
eTable 5. mTICI reperfusion and 90-day mRS by imaging modality
References
- 1.Nogueira RG, Jadhav AP, Haussen DC, et al. ; DAWN Trial Investigators . Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11-21. doi: 10.1056/NEJMoa1706442 [DOI] [PubMed] [Google Scholar]
- 2.Albers GW, Marks MP, Kemp S, et al. ; DEFUSE 3 Investigators . Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378(8):708-718. doi: 10.1056/NEJMoa1713973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Powers WJ, Rabinstein AA, Ackerson T, et al. ; American Heart Association Stroke Council . 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49(3):e46-e110. doi: 10.1161/STR.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 4.Turc G, Bhogal P, Fischer U, et al. European Stroke Organisation (ESO)—European Society for Minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischaemic stroke endorsed by Stroke Alliance for Europe (SAFE). Eur Stroke J. 2019;4(1):6-12. doi: 10.1177/2396987319832140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim Y, Lee S, Abdelkhaleq R, et al. Utilization and availability of advanced imaging in patients with acute ischemic stroke. Circ Cardiovasc Qual Outcomes. 2021;14(4):e006989. doi: 10.1161/CIRCOUTCOMES.120.006989 [DOI] [PubMed] [Google Scholar]
- 6.Kane I, Whiteley WN, Sandercock PAG, Wardlaw JM. Availability of CT and MR for assessing patients with acute stroke. Cerebrovasc Dis. 2008;25(4):375-377. doi: 10.1159/000120688 [DOI] [PubMed] [Google Scholar]
- 7.Nogueira RG, Haussen DC, Liebeskind D, et al. ; Trevo Registry and DAWN Trial Investigators . Stroke imaging selection modality and endovascular therapy outcomes in the early and extended time windows. Stroke. 2021;52(2):491-497. doi: 10.1161/STROKEAHA.120.031685 [DOI] [PubMed] [Google Scholar]
- 8.Demeestere J, Garcia-Esperon C, Garcia-Bermejo P, et al. Evaluation of hyperacute infarct volume using ASPECTS and brain CT perfusion core volume. Neurology. 2017;88(24):2248-2253. doi: 10.1212/WNL.0000000000004028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siegler JE, Messé SR, Sucharew H, et al. Noncontrast CT versus perfusion-based core estimation in large vessel occlusion: the Blood Pressure after Endovascular Stroke Therapy Study. J Neuroimaging. 2020;30(2):219-226. doi: 10.1111/jon.12682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farzin B, Fahed R, Guilbert F, et al. Early CT changes in patients admitted for thrombectomy: intrarater and interrater agreement. Neurology. 2016;87(3):249-256. doi: 10.1212/WNL.0000000000002860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nogueira RG, Ribó M. Endovascular treatment of acute stroke. Stroke. 2019;50(9):2612-2618. doi: 10.1161/STROKEAHA.119.023811 [DOI] [PubMed] [Google Scholar]
- 12.García-Tornel Á, Campos D, Rubiera M, et al. Ischemic core overestimation on computed tomography perfusion. Stroke. 2021;52(5):1751-1760. doi: 10.1161/STROKEAHA.120.031800 [DOI] [PubMed] [Google Scholar]
- 13.Hacke W, Kaste M, Bluhmki E, et al. ; ECASS Investigators . Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317-1329. doi: 10.1056/NEJMoa0804656 [DOI] [PubMed] [Google Scholar]
- 14.Sun CHJ, Ribo M, Goyal M, et al. Door-to-puncture: a practical metric for capturing and enhancing system processes associated with endovascular stroke care, preliminary results from the rapid reperfusion registry. J Am Heart Assoc. 2014;3(2):e000859. doi: 10.1161/JAHA.114.000859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheth KN, Terry JB, Nogueira RG, et al. Advanced modality imaging evaluation in acute ischemic stroke may lead to delayed endovascular reperfusion therapy without improvement in clinical outcomes. J Neurointerv Surg. 2013;5(suppl 1):i62-i65. doi: 10.1136/neurintsurg-2012-010512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meinel TR, Kaesmacher J, Mosimann PJ, et al. Association of initial imaging modality and futile recanalization after thrombectomy. Neurology. 2020;95(17):e2331-e2342. doi: 10.1212/WNL.0000000000010614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raymond J, Fahed R, Roy D, Darsaut TE. The 2018 ter Brugge lecture: problems with the introduction of innovations in neurovascular care. Can J Neurol Sci. 2019;46(2):151-158. doi: 10.1017/cjn.2018.391 [DOI] [PubMed] [Google Scholar]
- 18.Haussen DC, Dehkharghani S, Rangaraju S, et al. Automated CT perfusion ischemic core volume and noncontrast CT ASPECTS (Alberta Stroke Program Early CT Score): correlation and clinical outcome prediction in large vessel stroke. Stroke. 2016;47(9):2318-2322. doi: 10.1161/STROKEAHA.116.014117 [DOI] [PubMed] [Google Scholar]
- 19.Nannoni S, Ricciardi F, Strambo D, et al. Correlation between ASPECTS and core volume on CT perfusion: impact of time since stroke onset and presence of large-vessel occlusion. AJNR Am J Neuroradiol. 2021;42(3):422-428. doi: 10.3174/ajnr.A6959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desai SM, Tonetti DA, Molyneaux BJ, et al. Interaction between time, ASPECTS, and clinical mismatch. J Neurointerv Surg. 2020;12(9):911-914. doi: 10.1136/neurintsurg-2020-015921 [DOI] [PubMed] [Google Scholar]
- 21.Nagel S, Herweh C, Pfaff JAR, et al. Simplified selection criteria for patients with longer or unknown time to treatment predict good outcome after mechanical thrombectomy. J Neurointerv Surg. 2019;11(6):559-562. doi: 10.1136/neurintsurg-2018-014347 [DOI] [PubMed] [Google Scholar]
- 22.Nannoni S, Kaesmacher J, Ricciardi F, et al. ASPECTS-based selection for late endovascular treatment: a retrospective two-site cohort study. Int J Stroke. 2021;17474930211009806. doi: 10.1177/17474930211009806:17474930211009806 [DOI] [PubMed] [Google Scholar]
- 23.Bouslama M, Haussen DC, Rodrigues G, Barreira C, Frankel M, Nogueira RG. Novel selection paradigms for endovascular stroke treatment in the extended time window. J Neurol Neurosurg Psychiatry. 2021;jnnp-2020-325284. doi: 10.1136/jnnp-2020-325284 [DOI] [PubMed] [Google Scholar]
- 24.Lopez-Rivera V, Abdelkhaleq R, Yamal J-M, et al. Impact of initial imaging protocol on likelihood of endovascular stroke therapy. Stroke. 2020;51(10):3055-3063. doi: 10.1161/STROKEAHA.120.030122 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Neuroimaging triage protocols of sites in the CLEAR Study
eTable 2. Selection criteria for late window (6-24h) thrombectomy between 2014 to 2020)
eTable 3. Modified Rankin Score Assessment
eTable 4. Analysis of imaging modality with good outcome: Local patients
eTable 5. mTICI reperfusion and 90-day mRS by imaging modality