Abstract
MicroRNAs (miRNAs) have been demonstrated to play pivotal roles in the pathogenesis of sepsis‐induced acute lung injury (ALI). In this work, we aimed to clarify the potential role and the underlying mechanism of miR‐942‐5p in a lipopolysaccharide (LPS)‐induced A549 cell injury model. The cell injury was evaluated by CCK‐8 assay, flow cytometry and enzyme‐linked immunosorbent assay (ELISA). The expression levels of miR‐942‐5p and tripartite motif‐containing protein 37 (TRIM37) were measured by real‐time PCR and Western blot, and their association was then validated by bioinformatics, luciferase reporter assay and RNA pull‐down assay. We found that the expression of miR‐942‐5p was decreased in LPS‐treated A549 cells. Furthermore, LPS treatment suppressed A549 cell viability, promoted apoptosis and increased the levels of inflammatory cytokines. Conversely, overexpression of miR‐942‐5p increased cell viability, reduced apoptosis and alleviated inflammatory cytokine secretion in the presence of LPS. Moreover, miR‐942‐5p directly targeted TRIM37 by binding to the 3′‐UTR of TRIM37 mRNA. Upregulation of TRIM37 effectively reversed the anti‐apoptotic and anti‐inflammatory effects of miR‐942‐5p in LPS‐induced A549 cells. Our findings suggested that miR‐942‐5p protected against LPS‐induced cell injury through inhibiting apoptosis and inflammation in A549 cells by negatively regulating TRIM37.
Keywords: acute lung injury, lipopolysaccharide, miR‐942‐5p, sepsis, TRIM37
1. INTRODUCTION
Sepsis is a life‐threatening systemic inflammatory response syndrome (SIRS) that could develop into multiple organ dysfunction syndrome (MODS), in which the lung is one of the most vulnerable organs. 1 , 2 Acute lung injury (ALI), and its extreme form, acute respiratory distress syndrome (ARDS), is one of the leading causes of mortality in intensive care units. 3 , 4 When ALI occurs, the injury to alveolar epithelial cells caused by lipopolysaccharide (LPS) may lead to endothelial barrier dysfunction. 5 , 6 , 7 , 8 Therefore, it is of potential clinical importance to protect the damaged alveolar epithelial cells from sepsis‐induced ALI.
MicroRNAs (miRNAs) are a class of previously discovered small non‐coding RNAs that can regulate gene expression at the post‐transcriptional level by specifically targeting 3'UTR of target gene mRNA to inhibit protein translation or to induce mRNA degradation. 9 miRNAs play regulatory roles in diverse physiological and pathological processes, such as cell proliferation and apoptosis, 10 , 11 , 12 , 13 , 14 , 15 tissue differentiation, 16 inflammation 17 , 18 and tumour formation. 10 , 11 , 12 , 13 , 19 In recent years, several lines of evidence suggest the pivotal roles of miRNA in sepsis, such as miR‐223, 20 miR‐133a 21 , 22 and miR‐186. 23 , 24 miRNA‐942‐5p is shown to be involved in many cellular processes, such as tumour cell phenotypes, 6 , 12 , 23 , 24 osteogenic differentiation, 25 , 26 cardiomyocyte apoptosis, 27 and the oxidative stress and inflammatory response of vascular endothelial cells. 28 More importantly, a recent report implies that miR‐942‐5p expression is downregulated in LPS‐induced HK‐2 cells, and participates in inflammation and cell apoptosis, suggesting that miR‐942‐5p might be involved in sepsis‐induced acute kidney injury (AKI). 29 However, whether miR‐942‐5p is implicated in sepsis‐induced ALI is largely unknown.
In the present study, we established a sepsis‐induced ALI cellular model using LPS‐treated alveolar epithelial cell line A549. Moreover, we also investigated the underlying effects of miR‐942‐5p on septic ALI and its possible regulatory mechanism.
2. MATERIALS AND METHODS
2.1. Cell culture, transfection and LPS treatment
Human alveolar type II epithelial A549 cells were obtained from American Type Culture Collection (ATCC). A549 cells were cultured in ATCC‐formulated Kaighn's modification of Ham's F‐12 medium supplemented with 10% foetal bovine serum (Gibco; Thermo Fisher Scientific) at 37°C in an atmosphere of 95% air and 5% CO2. Cell transfection was all performed using Lipofectamine 3000 (Invitrogen; Thermo Fisher Scientific, Waltham, MA, USA) in line with the protocol. miR‐942‐5p mimic and TRIM37 overexpression vector synthesized by RiboBio Co., Ltd were transfected into A549 cells to upregulate miR‐942‐5p and TRIM37 expression respectively. The transfection efficiency was determined after 48‐h transfection. LPS (Sigma‐Aldrich) was used to stimulate cells at 1000 ng/ml for 72 h to construct a cellular model of septic ALI, as previously described. 7 , 8 , 30
2.2. Luciferase reporter and RNA pull‐down assays
For luciferase reporter assay, A549 cells were transfected with miR‐942‐5p mimic or miR‐NC together with the luciferase reporter vectors containing TRIM37‑3'UTR wild or mutant types. The luciferase activities were measured after 48‐h transfection using the Renilla‐Firefly Luciferase Dual Assay Kit (Pierce Biotechnology; Thermo Fisher Scientific).
For biotinylated miRNA pull‐down assay, A549 cells were transfected with biotinylated miR‐942‐5p or scrambled biotinylated probes. The cell lysates were collected at 48 h post‐transfection and then incubated with streptavidin‐coated magnetic beads. The enrichment of TRIM37 mRNA in the co‐precipitated RNAs was determined by real‐time PCR.
2.3. Cell viability assay
Cell Counting Kit‐8 (CCK‐8; Beyotime Biotechnology) was performed to measure the cell viability. Briefly, A549 cells (2 × 103 cells/well) were replated into 96‐well plates with 100 μl culture medium. After incubation for 24 h, the complete medium was replaced with medium containing 10 μl CCK‐8 reagent. The absorbance which revealed cell viability was detected after another 2 h at the wavelength of 450 nm.
2.4. Flow cytometry
After centrifugation in pre‐cooled PBS, the cells were resuspended in PBS and stained with the eBioscience™ Annexin V‐FITC Apoptosis Detection Kit (Invitrogen; Thermo Fisher Scientific) according to the manufacturer's instructions. The number of apoptotic cells was analysed by flow cytometry (Beckman Coulter).
2.5. Enzyme‐linked immunosorbent assay
A549 cells in the different groups were collected and centrifuged at 3,000 × g for 10 min at 4°C. The supernatant was harvested, and the levels of interleukin (IL)‐1β, IL‐6 and tumour necrosis factor‐α (TNF‐α) were measured using the commercial ELISA kits purchased from Beyotime Biotechnology, following the manufacturer's protocol.
2.6. Real‐time PCR
Total RNA of A549 cells was isolated using the TRIzol™ Reagent (Invitrogen; Thermo Fisher Scientific). Equal amount of RNAs were used for reverse transcription using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems; Thermo Fisher Scientific) to product complementary DNA. To quantify the mRNA expression of miR‐942‐5p and TRIM37, real‐time PCRs were performed on the 7500 Fast Real‐Time PCR System (Applied Biosystems; Thermo Fisher Scientific). U6 and GADPH were used as endogenous controls. The primers were miR‐942‐5p, forward: 5′‐AGGGTCTTCTCTGTTTTGGC‐3′, reverse: 5′‐GTTGTGGTTGGTTGGTTTGT‐3′; TRIM37, forward: 5′‐TATGGAGAAATTGCGGGATGC‐3′, reverse: 5′‐GTCAGCCAGCGCCTAATACAG‐3′; U6, forward: 5′‐AAAGCAAATCATCGGACGACC‐3′, reverse: 5′‐GTACAACACATTGTTTCCTCGGA‐3′; GAPDH, forward: 5′‐TGTGGGCATCAATGGATTTGG‐3′, reverse: 5′‐ACACCATGTATTCCGGGTCAAT‐3′.
2.7. Western blot
Total protein of A549 cells isolated with RIPA lysis buffer (Beyotime Biotechnology) was quantified using the BCA Protein Assay Kit (Beyotime Biotechnology). Following electrophoresis, separated proteins were transferred to polyvinylidene fluoride (PVDF) membranes. Then, the membranes were blocked overnight with primary antibodies (1:1000 dilution; Cell Signaling Technology, Boston, MA, USA) targeting TRIM37 (#96167), Bcl‐2 (#3498), Bax (#5023) and GAPDH (#5174) as a loading control, interacted with HRP‐linked anti‐rabbit IgG secondary antibody (#7074; 1:2000 dilution; Cell Signaling Technology) at room temperature for 2 h and developed by SignalFire™ ECL Reagent (#6883; Cell Signaling Technology).
2.8. Statistical analysis
Data were expressed as mean ±standard deviation (SD). Statistical analysis was performed using SPSS 19.0 statistical software (SPSS Inc.). Differences between two groups were assessed by Student's t‐test. Differences between more than two groups were analysed by one‐way analysis of variance test followed by Tukey's post hoc test. p < .05 indicated a statistically significant difference.
3. RESULTS
3.1. LPS reduces miR‐942‐5p expression and increases TRIM37 expression in A549 cells
To investigate the role of miR‐942‐5p in sepsis‐induced ALI, the in vitro cell model was generated by using LPS‐stimulated A549 cells. We found that treatment with 1000 ng/ml of LPS for 72 h led to an obvious downregulation of miR‐942‐5p expression (Figure 1A) and a significant increase in the mRNA (Figure 1B) and protein (Figure 1C) levels of TRIM37.
FIGURE 1.
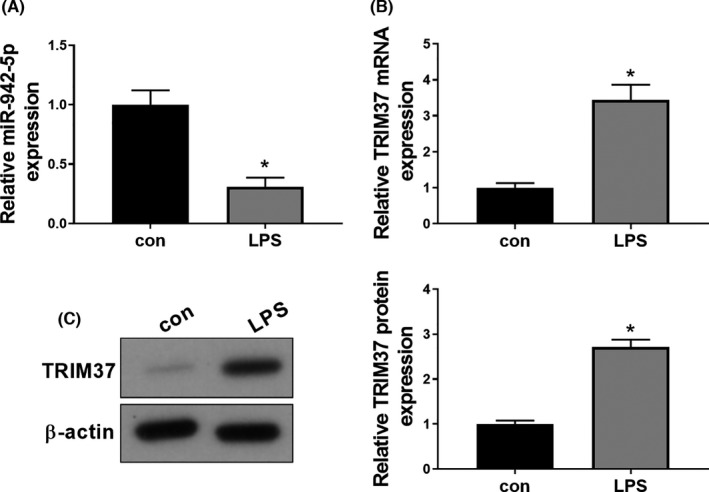
LPS reduces miR‐942‐5p expression and increases TRIM37 expression in A549 cells. After treatment with or without 1000 ng/ml LPS for 72 h, A549 cells were subjected to qRT‐PCR and Western blotting analysis to determine the expression of miR‐942‐5p (A) and TRIM37 (B‐C). Data were expressed as mean ±SD from three independent experiments (n = 3). Differences between two groups were assessed by Student's t‐test. * p < .05
3.2. miR‐942‐5p protects A549 cells against LPS‐induced cell injury
Then, we constructed miR‐942‐5p‐overexpressing A549 cells in the presence of LPS through miR‐942‐5p mimic transfection to further investigate whether miR‐942‐5p was implicated in LPS‐induced cell injury. The transfection efficiency was assessed by qRT‐PCR, showing that the abundance of miR‐942‐5p in A549 cells was markedly elevated by the transfection of miR‐942‐5p mimic (Figure 2A). The potential cytotoxicity of LPS in A549 cells was tested using the CCK‐8 assay. The results showed that LPS notably reduced the cell viability in a time‐dependent manner, while the overexpression of miR‐942‐5p restored cell viability in the presence of LPS (Figure 2B). Flow cytometry further validated that the cell apoptotic rate was abnormally increased upon LPS treatment, whereas the upregulation of miR‐942‐5p obviously diminished LPS‐induced cell apoptosis in A549 cells (Figure 2C). Coincidentally, the anti‐apoptotic protein Bcl‐2 level was inhibited, while the pro‐apoptotic protein Bax was prominently upregulated after LPS treatment when compared to the control group. In contrast, the enforced expression of miR‐942‐5p led to opposite effects (Figure 2D). In addition, the release of proinflammatory cytokines was evaluated by ELISA. Our results elucidated that LPS signally enhanced the levels of TNF‐α, IL‐6 and IL‐1β in A549 cells compared with the untreated cells (Figure 2E‐G).
FIGURE 2.
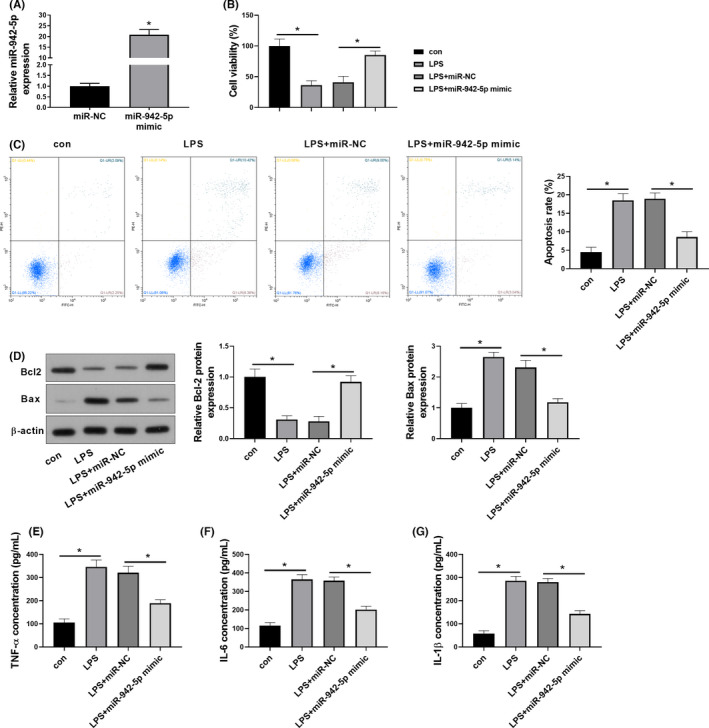
miR‐942‐5p protects A549 cells against LPS‐induced cell injury. A549 cells were transfected with miR‐942‐5p mimic or miR‐NC before the treatment with 1000 ng/ml of LPS for 72 h. (A) The transfection efficiency was determined by qRT‐PCR. (B) CCK‐8 assay was performed to reveal cell viability. (C) Apoptotic rate was analysed by flow cytometry. (D) The expression levels of apoptosis‐related proteins (Bcl‐2 and Bax) were measured by Western blotting. The secretion levels of TNF‐α (E), IL‐6 (F) and IL‐1β (G) were assessed by ELISA. Data were expressed as mean ±SD from three independent experiments (n = 3). Differences between two groups were assessed by Student's t‐test. Differences between more than two groups were analysed by one‐way analysis of variance test followed by Tukey's post hoc test. * p < .05
3.3. TRIM37 is a direct target of miR‐942‐5p in A549 cells
Starbase was used to predict the target gene of miR‐942‐5p and provided the binding sites of miR‐942‐5p with TRIM37 3′‐UTR (Figure 3A). To validate this interaction, luciferase assay was performed in A549 cells and indicated that abundant accumulation of miR‐942‐5p resulted in a reduction of luciferase activity in the TRIM37‐WT group, but not in the TRIM37‐MUT group (Figure 3B). Moreover, there was more enrichment of TRIM37 in the bio‐miR‐942‐5p group as compared to the bio‐NC group, as demonstrated by an RNA pull‐down assay (Figure 3C). Besides, the protein expression of TRIM37 was decreased in A549 cells after the transfection with miR‐942‐5p mimic when compared to the miR‐NC group (Figure 3D).
FIGURE 3.
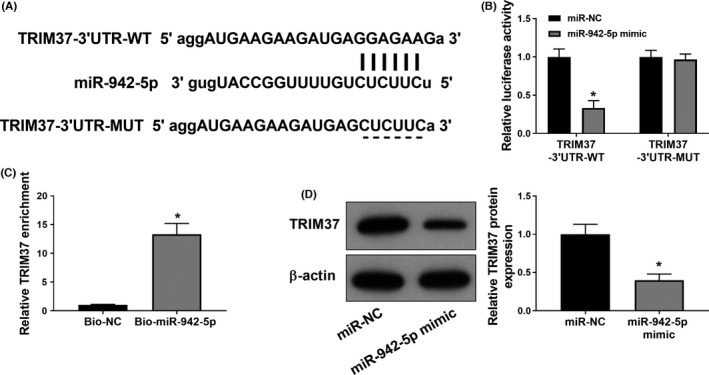
TRIM37 is a direct target of miR‐942‐5p in A549 cells. (A) The predicted binding sites and mutated sites of miR‐942‐5p to the TRIM37 sequence. (B) Luciferase activities of A549 cells co‐transfected with miR‐942‐5p mimic or miR‐NC and luciferase reporters containing TRIM37 3′UTR were analysed in luciferase assay. (C) miR‐942‐5p was labelled with biotin, and then, the interaction of miR‐942‐5p with TRIM37 was detected by RNA pull‐down assay in A549 cells. (D) The levels of TRIM37 transfected with miR‐942‐5p mimic or miR‐NC in A549 cells were analysed by Western blotting. Data were expressed as mean ±SD from three independent experiments (n = 3). Differences between two groups were assessed by Student's t‐test. * p < .05
3.4. TRIM37 overexpression attenuates the protective role of 942‐5p in LPS‐treated A549 cells
To elucidate whether miR‐942‐5p‐induced cell injury inhibition was mediated by targeting TRIM37, we upregulated TRIM37 expression in A549 cells using its specific overexpression vector. Immunoblotting validated the increased protein expression of TRIM37 in A549 cells after the transfection with TRIM37 overexpression vector (Figure 4A). In the presence of LPS, the ectopic expression of TRIM37 alleviated miR‐942‐5p overexpression‐induced the enhancement of cell viability (Figure 4B) and the reduction of cell apoptotic rate (Figure 4C) in A549 cells. Likewise, the restoration of TRIM37 expression counteracted miR‐942‐5p‐mediated the expression of apoptosis‐related proteins in LPS‐treated A549 cells (Figure 4D). Additionally, the introduction of TRIM37 abated the inhibitory effects of miR‐942‐5p on the secretion levels of TNF‐α, IL‐6 and IL‐1β in LPS‐exposed A549 cells (Figure 4E).
FIGURE 4.
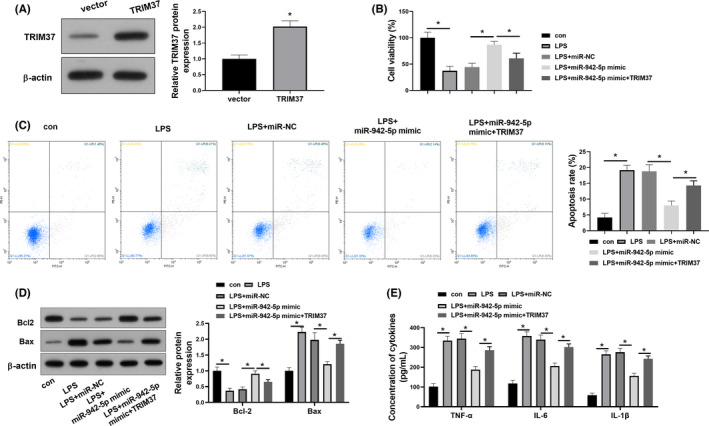
TRIM37 overexpression attenuates the protective role of 942‐5p in LPS‐treated A549 cells. A549 cells were co‐transfected with miR‐942‐5p mimic and TRIM37 before the treatment with 1000 ng/ml of LPS for 72 h. (A) The transfection efficiency of TRIM37 overexpression was determined by Western blotting. (B) CCK‐8 assay was performed to reveal cell viability. (C) Apoptotic rate was analysed by flow cytometry. (D) The expression levels of apoptosis‐related proteins (Bcl‐2 and Bax) were measured by Western blotting. (E) The secretion levels of TNF‐α, IL‐6 and IL‐1β were assessed by ELISA. Data were expressed as mean ±SD from three independent experiments (n = 3). Differences between two groups were assessed by Student's t‐test. Differences between more than two groups were analysed by one‐way analysis of variance test followed by Tukey's post hoc test. * p < .05
4. DISCUSSION
miRNAs are implicated in the pathogenesis of sepsis‐related cardiac, lung, liver and kidney disorders. 21 , 22 , 31 , 32 , 33 This study aimed to ascertain a novel target for the treatment of sepsis‐induced ALI. This study revealed that miR‐942‐5p limited TRIM37 expression and ameliorated LPS‐induced ALI in vitro and inflammatory response in A549 cells.
LPS‐induced A549 cells were established as an in vitro cell model of sepsis‐induced ALI in several studies 14 , 15 , 34 . In the present study, miR‐942‐5p expression in A549 cells was shown to decrease under LPS stimulation, indicating that silencing of miR‐942‐5p might be involved in the pathogenesis of septic ALI. miR‐942‐5p has been found to have diverse biological functions in several diseases. For instance, miR‐942‐5p has been reported as an anti‐oncogene in ovarian cancer 6 and oral squamous cell carcinoma. 10 , 11 , 12 , 13 In addition, Wang et al revealed that miR‐942‐5p was involved in the regulatory roles of lncRNA SOX2‐OT on doxorubicin‐induced cardiomyocyte apoptosis. 27 Following the overexpression of miR‐942‐5p, cell viability was promoted, but cell apoptosis and the production of the pro‐inflammatory cytokines IL‐1β, TNF‐α and IL‐6 were inhibited in LPS‐induced A549 cells. These findings were in agreement with those seen in the previous study that uncovered the anti‐apoptotic and anti‐inflammatory effects of miR‐942‐5p in LPS‐treated renal tubular epithelial HK‐2 cells. 29 Collectively, our data showed that miR‐942‐5p attenuated LPS‐induced ALI by suppressing apoptosis and inflammatory response, indicating that miR‐942‐5p might serve as a therapeutic target for sepsis.
TRIM family proteins, as E3 ubiquitin ligases, have a variety of biological functions in cellular processes including in carcinogenesis, proliferation, apoptosis, differentiation, autophagy and inflammation. 35 , 36 TRIM37 has been previously demonstrated by its regulatory role in inflammatory diseases, such as hepatic fibrosis 37 and acute stroke‐associated pulmonary inflammation. 38 Recently, Chen et al reported that TRIM37 is directly regulated by miR‐944 involved in the regulation of apoptosis and inflammatory response in ALI mice and LPS‐induced WI‐38 cells. 39 In the present study, the mRNA and protein levels of TRIM37 were highly expressed in LPS‐induced A549 cells. Moreover, the binding site between miR‐942‐5p and TRIM37 was predicted using bioinformatics analysis, and then, the predicted binding between them was verified through luciferase reporter and RNA pull‐down assays. Furthermore, the expression of TRIM37 was significantly decreased in A549 cells by the overexpression of miR‐942‐5p, indicating that miR‐942‐5p may direct target and negatively regulate TRIM37. In addition, TRIM37 overexpression reversed miR‐942‐5p‐mediated inhibitory effects on LPS‐induced cell injury. Therefore, it may be implied that miR‐942‐5p exerted the protective roles in LPS‐induced A549 cells by interacting with TRIM37. However, our study still has some limitations. Firstly, the clinical significance of miR‐942‐5p is worth further investigation. Further in vivo study is needed to verify the role of miR‐942‐5p in sepsis using the animal models. Other target genes remain to be identified. Further studies are needed to confirm the role of miR‐942‐5p in other cell lines.
In conclusion, the in vitro data in A549 cells demonstrated that miR‐942‐5p overexpression may exhibit a favourable effect by inhibiting LPS‐induced apoptosis and inflammation via directly targeting TRIM37. These results may suggest that miR‐942‐5p/TRIM37 therapeutic signalling axis might be a promising target for the treatement of patients with sepsis‐induced ALI.
CONFLICT OF INTEREST
The authors declare there is no conflict of interest in this study.
AUTHOR CONTRIBUTIONS
QL conducted most of the experiments and wrote the manuscript; DGZ and HL conducted the experiments and analysed the data; HX designed the study and revised the manuscript. All authors have read and approved the manuscript.
ETHICAL APPROVAL AND CONSENT TO PARTICIPATE
The present study was approved by the ethics committee of Yiyang Central Hospital of Science and Technology.
CONSENT FOR PUBLICATION
All authors agreed the submission and the policy of the journal and copyright.
Lu Q, Zhang D, liu H, Xu H. miR‐942‐5p prevents sepsis‐induced acute lung injury via targeting TRIM37. Int J Exp Path. 2021;102:192–199. 10.1111/iep.12413
Funding information
Not applicable
DATA AVAILABILITY STATEMENT
All data in this study can be obtained by proper request from the authors.
REFERENCES
- 1. Kim WY, Hong SB. Sepsis and acute respiratory distress syndrome: recent update. Tuberc Respir Dis (Seoul). 2016;79:53‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Levitt JE, Matthay MA. The utility of clinical predictors of acute lung injury: towards prevention and earlier recognition. Expert Rev Respir Med. 2010;4:785‐797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Butt Y, Kurdowska A, Allen TC. Acute lung injury: a clinical and molecular review. Arch Pathol Lab Med. 2016;140:345‐350. [DOI] [PubMed] [Google Scholar]
- 4. Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest. 2012;122:2731‐2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Du HL, Zhai AD, Yu H. Synergistic effect of halofuginone and dexamethasone on LPS‐induced acute lung injury in type II alveolar epithelial cells and a rat model. Mol Med Rep. 2020;21:927‐935. [DOI] [PubMed] [Google Scholar]
- 6. Du Z, Wang L, Xia Y. Circ_0015756 promotes the progression of ovarian cancer by regulating miR‐942‐5p/CUL4B pathway. Cancer Cell Int. 2020;20:572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li P, Yao Y, Ma Y, Chen Y. MiR‐150 attenuates LPS‐induced acute lung injury via targeting AKT3. Int Immunopharmacol. 2019;75:105794. [DOI] [PubMed] [Google Scholar]
- 8. Li X, Jamal M, Guo P, et al. Irisin alleviates pulmonary epithelial barrier dysfunction in sepsis‐induced acute lung injury via activation of AMPK/SIRT1 pathways. Biomed Pharmacother. 2019;118:109363. [DOI] [PubMed] [Google Scholar]
- 9. Lu TX, Rothenberg ME. MicroRNA. J Allergy Clin Immunol. 2018;141:1202‐1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang C, Huang Y, Zhang J, Fang Y. MiRNA‐339‐5p suppresses the malignant development of gastric cancer via targeting ALKBH1. Exp Mol Pathol. 2020;115:104449. [DOI] [PubMed] [Google Scholar]
- 11. Wang J, Jiang C, Li N, et al. The circEPSTI1/mir‐942‐5p/LTBP2 axis regulates the progression of OSCC in the background of OSF via EMT and the PI3K/Akt/mTOR pathway. Cell Death Dis. 2020;11:682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang Q, Wu J, Huang H, et al. lncRNA LIFR‐AS1 suppresses invasion and metastasis of non‐small cell lung cancer via the miR‐942‐5p/ZNF471 axis. Cancer Cell Int. 2020;20:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang Q, Yang X, Zhou X, et al. MiR‐3174 promotes proliferation and inhibits apoptosis by targeting FOXO1 in hepatocellular carcinoma. Biochem Biophys Res Comm. 2020;526:889‐897. [DOI] [PubMed] [Google Scholar]
- 14. Zhou F, Chen W, Cui Y, et al. miRNA‐122‐5p stimulates the proliferation and DNA synthesis and inhibits the early apoptosis of human spermatogonial stem cells by targeting CBL and competing with lncRNA CASC7. Aging (Albany NY). 2020;12:25528‐25546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhou H, Wang X, Zhang B. Depression of lncRNA NEAT1 Antagonizes LPS‐Evoked Acute Injury and Inflammatory Response in Alveolar Epithelial Cells via HMGB1‐RAGE Signaling. Mediators Inflamm. 2020;2020:8019467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuse N, Kamio K, Azuma A, et al. Exosome‐derived microRNA‐22 ameliorates pulmonary fibrosis by regulating fibroblast‐to‐myofibroblast differentiation in vitro and in vivo. J Nippon Med Sch. 2020;87:118‐128. [DOI] [PubMed] [Google Scholar]
- 17. Cheng N, Liu C, Li Y, et al. MicroRNA‐223‐3p promotes skeletal muscle regeneration by regulating inflammation in mice. J Biol Chem. 2020;295:10212‐10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Najm A, Masson FM, Preuss P, et al. MicroRNA‐17‐5p reduces inflammation and bone erosions in mice with collagen‐induced arthritis and directly targets the JAK/STAT pathway in rheumatoid arthritis fibroblast‐like synoviocytes. Arthritis Rheumatol. 2020;72:2030‐2039. [DOI] [PubMed] [Google Scholar]
- 19. Ma LL, Liang L, Zhou D, Wang SW. Tumor suppressor miR‐424‐5p abrogates ferroptosis in ovarian cancer through targeting ACSL4. Neoplasma. 2021;68:165‐173. [DOI] [PubMed] [Google Scholar]
- 20. Wu X, Yang J, Yu L, Long D. Plasma miRNA‐223 correlates with risk, inflammatory markers as well as prognosis in sepsis patients. Medicine. 2018;97:e11352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen L, Lu Q, Deng F, et al. miR‐103a‐3p could attenuate sepsis‐induced liver injury by targeting HMGB1. Inflammation. 2020;43:2075‐2086. [DOI] [PubMed] [Google Scholar]
- 22. Chen L, Xie W, Wang L, Zhang X, Liu E, Kou Q. MiRNA‐133a aggravates inflammatory responses in sepsis by targeting SIRT1. Int Immunopharmacol. 2020;88:106848. [DOI] [PubMed] [Google Scholar]
- 23. Li M, Li W, Ren FQ, Zhang ML. miRNA‐186 improves sepsis induced renal injury via PTEN/PI3K/AKT/P53 pathway. Open Med (Wars). 2020;15:254‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li S, Yan G, Liu W, Li C, Wang X. Circ0106714 inhibits tumorigenesis of colorectal cancer by sponging miR‐942‐5p and releasing DLG2 via Hippo‐YAP signaling. Mol Carcinog. 2020;59:1323‐1342. [DOI] [PubMed] [Google Scholar]
- 25. Ji H, Cui X, Yang Y, Zhou X. CircRNA hsa_circ_0006215 promotes osteogenic differentiation of BMSCs and enhances osteogenesis‐angiogenesis coupling by competitively binding to miR‐942‐5p and regulating RUNX2 and VEGF. Aging (Albany NY). 2021;13:10275‐10288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ouyang Z, Tan T, Zhang X, et al. CircRNA hsa_circ_0074834 promotes the osteogenesis‐angiogenesis coupling process in bone mesenchymal stem cells (BMSCs) by acting as a ceRNA for miR‐942‐5p. Cell Death Dis. 2019;10:932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang H, Lin X, Li J, Zeng G, Xu T. Long noncoding RNA SOX2‐OT aggravates doxorubicin‐induced apoptosis of cardiomyocyte by targeting miR‐942‐5p/DP5. Drug Design Dev Ther. 2021;15:481‐492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wan H, You T, Luo W. circ_0003204 regulates cell growth, oxidative stress, and inflammation in ox‐LDL‐induced vascular endothelial cells via regulating miR‐942‐5p/HDAC9 axis. Front Cardiovasc Med. 2021;8:646832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Luo N, Gao HM, Wang YQ, Li HJ, Li Y. MiR‐942‐5p alleviates septic acute kidney injury by targeting FOXO3. Eur Rev Med Pharmacol Sci. 2020;24:6237‐6244. [DOI] [PubMed] [Google Scholar]
- 30. Xi X, Yao Y, Liu N, Li P. MiR‐297 alleviates LPS‐induced A549 cell and mice lung injury via targeting cyclin dependent kinase 8. Int Immunopharmacol. 2020;80:106197. [DOI] [PubMed] [Google Scholar]
- 31. Ahmad S, Ahmed MM, Hasan PMZ, et al. Identification and validation of potential miRNAs, as biomarkers for sepsis and associated lung injury: a network‐based approach. Genes (Basel). 2020;11(11):1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Manetti AC, Maiese A, Paolo MD, De Matteis A, La Russa R, Turillazzi E, Frati P, Fineschi V. MicroRNAs and sepsis‐induced cardiac dysfunction: a systematic review. Int J Mol Sci. 2020;22(1):321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yi HX, Jiang SY, Yu LH, Chen K, Yang ZX, Wu Q. MicroRNA 181a‐2‐3p Alleviates the Apoptosis of Renal Tubular Epithelial Cells via Targeting GJB2 in Sepsis‐Induced Acute Kidney Injury. Mol Cell Biol. 2021;41(7):e0001621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang YM, Ji R, Chen WW, et al. Paclitaxel alleviated sepsis‐induced acute lung injury by activating MUC1 and suppressing TLR‐4/NF‐kappaB pathway. Drug Design Dev Ther. 2019;13:3391‐3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hatakeyama S. TRIM family proteins: roles in autophagy, immunity, and carcinogenesis. Trends Biochem Sci. 2017;42:297‐311. [DOI] [PubMed] [Google Scholar]
- 36. Watanabe M, Hatakeyama S. TRIM proteins and diseases. J Biochem. 2017;161:135‐144. [DOI] [PubMed] [Google Scholar]
- 37. Xie H, Xie D, Zhang J, et al. ROS/NF‐kappaB Signaling Pathway‐Mediated Transcriptional Activation of TRIM37 Promotes HBV‐Associated Hepatic Fibrosis. Mol Ther Nucleic Acids. 2020;22:114‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xiang Y, Zhang S, Lu J, et al. Ginkgolide B protects human pulmonary alveolar epithelial A549 cells from lipopolysaccharide‐induced inflammatory responses by reducing TRIM37‐mediated NF‐kappaB activation. Biotechnol Appl Biochem. 2020;67:903‐911. [DOI] [PubMed] [Google Scholar]
- 39. Chen C, Zhang H, Ge M, Ye J, Li R, Wang D. LncRNA NEAT1 acts as a key regulator of cell apoptosis and inflammatory response by the miR‐944/TRIM37 axis in acute lung injury. J Pharmacol Sci. 2021;145:202‐212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data in this study can be obtained by proper request from the authors.


