Abstract
MicroRNAs (miRNAs or miRs) serve essential roles in the pathogenic process of spinal cord injury (SCI). The present study investigated the role of miR‐378‐3p and autophagy‐related 12 (ATG12) in SCI. RT‐qPCR was used to detect the mRNA expression levels of miR‐378‐3p and ATG12. Cell viability and membrane integrity were evaluated using CCK‐8 and LDH assays. For the analysis of the interaction between miR‐378‐3p and ATG12, a dual‐luciferase reporter assay was conducted. The hindlimb function of rats was detected with the Basso, Beattie and Bresnahan score, and the motor deficit index score was used to evaluate nerve function. Using these approaches, it was identified that miR‐378‐3p expression was downregulated, while that of ATG12 was upregulated in SCI tissues and in cells exposed to hypoxia. Hypoxia repressed the expression of miR‐378‐3p via hypoxia‐inducible factor 1‐α. The overexpression of miR‐378‐3p exerted anti‐apoptotic effects on nerve cells by directly repressing ATG12. The infusion of miR‐378‐3p improved hindlimb motor function and the neurological functions of rats with contusion SCI, which contributed to amelioration of functional deficits and the relief of contusion SCI. Therefore, it was concluded that upregulated expression of miR‐378‐3p in PC12 or N2A cells repressed the apoptosis of nerve cells, and the administration of miR‐378‐3p in model rats with contusion SCI improved neurological and motor functions.
Keywords: autophagy‐related 12, microRNA‐378‐3p, spinal cord injury
1. INTRODUCTION
Spinal cord injury (SCI) can damage the motor and sensory functions below the injury site, causing permanent disability in some patients. 1 , 2 It has been reported that the incidence of SCI in China is >60,000 individuals annually. 3 There are few treatment options for SCI, which renders the investigation of its underlying mechanisms of utmost importance. Immediately following SCI, an initial disruption occurs, including mechanical tissue compression, stretching and decreased blood flow with resultant hypoxia, followed by the triggering of a complex set of secondary effects, largely induced by nerve cell apoptosis. 4 , 5 Other evidence from mouse and human investigations has confirmed the existence of a hypoxia zone in the SCI site, with induction of apoptosis surrounding the physical lesion. 6 However, the mechanisms through which hypoxia mediates cell apoptosis have not yet been investigated fully.
MicroRNAs (miRNAs or miRs), endogenously expressed non‐coding RNAs, can modulate gene translational efficiency or stability negatively via targeting the 3′ untranslated region (3′UTR) of mRNAs. 7 Aberrant miRNA expression profiles have been identified in adult rats following SCI. Moreover, further analysis using the bioinformatic approach has revealed that these miRNAs may contribute to inflammation, oxidation and apoptosis at the injury site. 8 Under hypoxic conditions, miR‐378‐3p can exert anti‐apoptotic effects in cardiomyocytes. 9 , 10 Hypoxia‐induced exosomal miR‐378‐3p has been shown to promote H9c2 cell viability and inhibit apoptosis. 11 Another study suggested that miR‐378 can alleviate cerebral ischaemic injury. 12 However, there is limited research available on miR‐378‐3p in the development of SCI.
In addition, another possible component in SCI, suggested by bioinformatic analysis, is that Autophagy‐related 12 (ATG12), a member of the Atg gene family that is known to conjugate with ATG3 to carry out key functions in mitochondrial homeostasis and membrane integrity, 13 (mainly through further binding and inactivating the anti‐apoptotic gene, Bcl‐2, without an apparent role in autophagy, 14 ) might play a role.
In this study, therefore, in summary, we have explored the role of miRNA‐378‐3p in SCI, with emphasis on both the cellular and molecular pathways involved, and on the neurological outcomes.
2. MATERIALS AND METHODS
2.1. Establishment of contusion SCI model
The present study was approved by the Animal Ethics Board of the General Hospital of Northern Theater Command (Shenyang, China). For the detection of miR‐378‐3p and ATG12 expression, a total of 36 adult Sprague Dawley (SD) rats, weighing 250 ± 20 g, were randomly divided into two groups as follows: (a) the contusion SCI group (SCI group) and (b) sham‐operated group (control group). The contusion SCI model was established as previously described. 15 Rats were anaesthetized with isoflurane (2 vol % of the isoflurane for inducing and 0.8 vol % of the isoflurane for maintaining anaesthesia), and no suggestion of pain or discomfort was observed. The skin was incised along the midline of the back, followed by the exposure of the vertebral column. A laminectomy was performed at the T9 level, and the spinal cord was impacted by a vertically dropped metal rod (10 g; 2 cm in diameter) from a height of 25 mm, 16 and the spinal cord segments (10 mm long), including the injury epicentre, were obtained at day 1 (n = 6), day 4 (n = 6) and day 7 (n = 6). The sham‐operated group (control group, n = 18)was subjected to the laminectomy but not received metal rod impact injury.
2.2. Grouping and injection of the recombinant adeno‐associated viruses (rAAVs)
For the injection of rAAVs, another 18 adult SD rats, weighing 250 ± 20 g, were randomly divided into three groups as follows: The sham‐operated group (which received no microinjection), the SCI group injected with AAV‐miR‐NC (AAV‐miR‐NC group) and the SCI group injected with AAV‐miR‐378‐3p (AAV‐miR‐378‐3p group). Precisely, the injection of rAAVs (AAV‐miR‐NC or AAV‐miR‐378‐3p, which were produced and purchased from HanHeng, China) was performed immediately after the establishment of SCI as described above. Specifically, four intraspinal injections of 0.5 μL (1.3 × 1010 vp/ml) each were administered into the left and right spinal cord, 1 mm lateral of the midline at a depth of 1.5 mm, 1.2 mm rostral and 1.2 mm caudal to the injury epicentre (ie the edge of the bruise). The muscles and skin were then closed, and subcutaneous injections of antibiotic (Baytril 5 mg kg‐1) and 5 ml of sterile lactated Ringer's solution were administered. The rats were housed individually and received injections of Baytril, Buprenorphine and lactated Ringer's solution for 3 days following surgery. 17
2.3. Spinal cord function evaluation
The BBB motor rating scale was used to evaluate hindlimb motor function at 7 days postsurgery, 18 which was evaluated by two blind observers, and the mean value of the two observers’ scores was used. A score of 0 indicates no locomotion, and a score of 21 indicates normal motor functions. The motor deficit index (MDI) score was used to evaluate the neurological functions according to the ambulation and placing/stepping responses at the time point of the rAAV injection for 7 days, which was conducted by an observer who was blinded to the group status. If the MDI was <3, the rats were considered not paraplegic; however, an MDI ≥3 indicated that the rats were paraplegic. 19
2.4. Cell culture and exposure to hypoxia
Nerve cell lines, including the N2A and PC12 cells, purchased from the Cell Resource Centre of Peking Union Medical College (Beijing, China), were grown in DMEM (HyClone) supplemented with 100 U/ml penicillin, 100 mg/mL streptomycin and 10% FBS (Gibco; Thermo Fisher Scientific) in a humidified atmosphere with 5% CO2 at 37°C. For exposure to hypoxia, AnaeroPack was used, in which the cells were placed and the oxygen tension in it dropped to <1% O2 within 1 hour. 20
2.5. Cell transfection
miR‐NC (sequence: UCACAACCUCCUAGAAAGAGUAGA), miR‐378‐3p mimics (sequence: ACUGGACUUGGAGUCAGAAGG), ASO‐NC (antisense oligonucleotides (ASO), sequence: UUGUACUACACAAAAGUACUG), ASO‐378‐3p (sequence: ACUGGACUUGGAGUCAGAAGG), si‐NC (target sequence: AATTCTCCGAACGTGTCACGT), si‐HIF1A (target sequence: CGTTGTGAGTGGTATTATT), pcDNA3 (OE‐NC) and pcDNA3.1‐ATG12 (OE‐ATG12) (GenePharma, China) were used for cell transfection at a final concentration of 50 nmol\L or 0.5 ng/µL, they were mixed with Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific) according to the manufacturer's instructions, and then the trypsin‐dispersed cells were added to this mixed medium and incubated for 15 minutes, followed by seeding the cells in 6‐well plate. 21 After 48 hours, the RNA/protein isolation, CCK‐8 and LDH assays, as well as luciferase reporter assay, were performed.
2.6. Cell viability and membrane integrity by CCK‐8 and LDH release
To evaluate PC12 or N2A cell survival and membrane integrity following exposure to hypoxia, CCK‐8 and LDH release assays were performed respectively. Cells, placed in 96‐well plates, were supplemented with 10 μL CCK‐8. The formazan, formed by CCK‐8 reacting with dehydrogenase in active cells, was detected using a microplate reader (Thermo Fisher Scientific) at optical density of 450 nm. The culture medium was used for the LDH detection of LDH activity using CytoTox 96 Non‐Radioactive Cytotoxicity Kit (Promega) with a microplate reader. The percentage of the maximum enzymatic activity of a control sample was calculated as the LDH release rate.
2.7. TUNEL assay
Terminal deoxynucleotidyl transferase‐mediated dUTP nick‐end labelling (TUNEL) assay (Roche Applied Science, Mannheim, Germany) was performed to detect cell apoptosis. The transfected cells were fixed in 4% paraformaldehyde at room temperature, followed by treating with 0.1% Triton ˟‐100 buffer for 5 minutes at 4°C. Then, 3% BSA was used for blocking, and incubated with TUNEL mixture for 1 hours at 37°C, followed by observing using fluorescent microscopy.
2.8. RNA extraction for RT‐qPCR
Spinal cord segments (10 mm long), including the injury epicentre, were obtained. 22 For RNA extraction from spinal cord tissues and cells, TRIzol Reagent (Invitrogen; Thermo Fisher Scientific) was used. The cDNA Synthesis Kit was obtained from Invitrogen to synthesize cDNA, followed by RT‐qPCR using SYBR Mix (ABI) on a 7500 Fast Real‐Time PCR System (Bio‐Rad). For the quantification of the relative expression of miR‐378‐3p, ATG12 and Bax, the 2‐ddCt method was used. U6 and GAPDH were used as loading controls.
2.9. Western blot analysis
Ultrasonicated spinal cord tissues (15 mg) were supplemented with lysis buffer (Beyotime Biotechnology), followed by centrifugation at 4°C for 15 minutes at 11269 g; the supernatants were then collected. The PC12 or N2A cells were treated directly with lysis buffer. The Pierce BCA Kit (Pierce) was used to detect the concentration of the proteins. Equal amounts of protein were then loaded onto 10% SDS‐PAGE for protein separation, followed by transferring the proteins onto PVDF membranes (Invitrogen; Thermo Fisher Scientific) and blocking at room temperature for 1 hour using 5% skimmed milk. After the washing step, the membranes were incubated overnight with primary antibodies, including anti‐ATG12 (1:2,000, #4180T; Cell Signaling Technology), anti‐Bax (1:800, #6A7; Abcam), cleaved caspase‐3 (Asp175) (1:500, #9661; Cell Signaling Technology) and anti‐GAPDH (1:5000, AP0063; Bioworld) antibodies at 4°C. The membranes were incubated with HRP‐bound antibodies for 1 hour, and proteins were visualized by ECL reagents (Millipore).
2.10. Dual‐luciferase reporter assay
Based on bioinformatic prediction database TargetScan (http://www.targetscan.org/vert_72), ATG12 was predicted as a potential target of miR‐378‐3p. To detect the direct regulatory effects of miR‐378‐3p on ATG12, the ATG12 3ʹUTR DNA fragment, which contained the predicted binding site of miR‐378‐3p, was inserted into the pmirGLO vector (Promega), resulting in pmirGLO‐ATG12‐3’UTR, which was then co‐transfected with miR‐378‐3p mimic, or ASO‐378‐3p mimic into N2A cells. The miR‐378‐3p promoter‐luciferase reporter vector was constructed by ligating the pGL3 vector (Promega) with miR‐378‐3p promoter. The Dual‐Luciferase Reporter Assay Kit (Promega) was used to detect the luciferase activity.
2.11. Statistical analysis
Data are presented as the means ± SD, and the data were analysed using Prism v7 (GraphPad, Inc) with Student's t test (two groups) or one‐way ANOVA followed by Tukey's test (more than two groups). A P < .05 was considered to indicate a statistically significant difference.
3. RESULTS
3.1. Expression of miR‐378‐3p and ATG12 levels is altered in tissues from rats with contusion SCI
To investigate the in vivo expression of miR‐378‐3p and ATG12, RT‐qPCR was used and the results showed that the expression of miR‐378‐3p in SCI tissues was downregulated at 1, 4 and 7 days post‐SCI in a time‐dependent manner, compared with the control group (Figure 1A), while the mRNA expression levels of ATG12 were markedly upregulated at 1, 4 and 7 days postcontusion SCI in a time‐dependent manner (Figure 1B). Furthermore, the BBB score at 7 days in the SCI experimental group was significantly decreased compared with that in the control group (Figure 1C), suggesting that the downregulation of miR‐378‐3p and the upregulation of ATG12 were associated with the hindlimb motor functions of the rats with contusion SCI.
FIGURE 1.
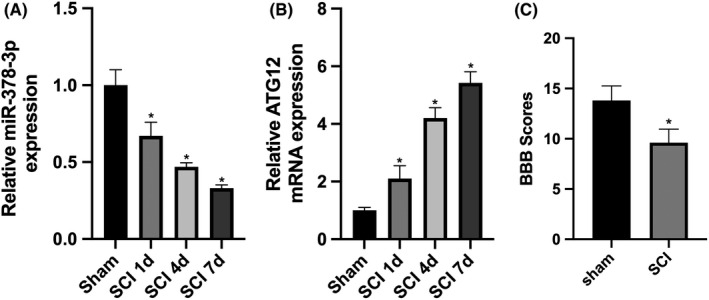
Expression of miR‐378‐3p and ATG12 is altered in tissues of rats with spinal cord injury (SCI). A, RT‐qPCR was used to quantify the expression of miR‐378‐3p in the tissues of control rats and rats with SCI (n = 12). B, RT‐qPCR and Western blot analysis were performed to detect the mRNA and protein expression levels of ATG12 (n = 12) respectively. C, BBB scoring was used to evaluate the hindlimb motor function. Data are reported as the means ± SD. *P < .05 vs control (t test)
3.2. Hypoxia induces the expression of ATG12 by repressing miR‐378‐3p
Since SCI is involved with nearby tissue hypoxia, 23 we speculated that the expression of miR‐378‐3p and ATG12 was affected by hypoxia. The results revealed that exposure to hypoxia repressed miR‐378‐3p expression (Figure 2A) and induced ATG12 expression (Figure 2B) in the PC12 and N2A cells, compared with the control group. Suppression of miR‐378‐3p expression by hypoxia was reversed by transfection with miR‐378‐3p mimics, and the hypoxia‐induced expression of ATG12 was also reversed by transfection with miR‐378‐3p mimics (Figure 2A and B).
FIGURE 2.
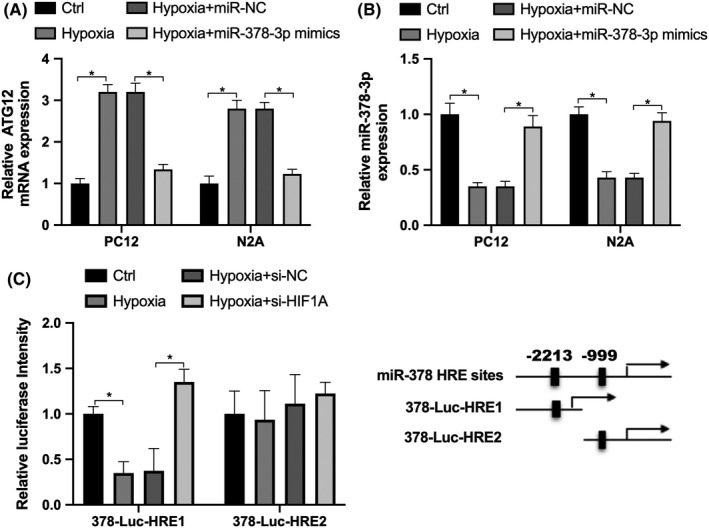
The upregulation of ATG12 induced by hypoxia is mediated via miR‐378‐3p. A, RT‐qPCR was used to detect the expression of miR‐378‐3p in PC12 and N2A cells in the control or hypoxia‐exposed group, or in cells transfected with miR‐NC or miR‐378‐3p mimics. B, Expression of ATG12 in PC12 and N2A cells in the control or hypoxia‐exposed group, or in cells transfected with miR‐NC or miR‐378‐3p mimics. C, The luciferase reporter construct 378‐Luc‐HRE1 or 378‐Luc‐HRE2 was examined in N2A cells in the control (Ctrl), hypoxia, hypoxia+si‐NC or hypoxia‐si‐HIF1A group. Data are reported as the means ± SD. *P < .05 vs control (t test)
We further investigated whether miR‐378‐3p can be directly regulated by the hypoxia‐inducible factor HIF1A, which can bind the consensus sequence 5ʹ‐(A/G)CGTG‐3ʹ within the hypoxia response element (HRE). In the promoter of miR‐378‐3p, we found two potential HRE sites, which were constructed by ligating the pGL3 vector (Promega) with miR‐378‐3p promoter containing two HRE sites respectively (Figure 2C). Then, N2A cells were transfected with small interfering RNA (si‐HIF1A) or negative control (NC) si‐NC. As shown in Figure 2C, hypoxia repressed the luciferase activity of 378‐Luc‐HRE1, while the activity was increased by transfection with si‐HIF1A. However, there was no obvious alteration in the activity of 378‐Luc‐HRE2 in the presence of either hypoxia or si‐HIF1A transfection. These findings indicated that hypoxia repressed the expression of miR‐378‐3p by influencing the activity of the miR‐378‐3p promoter (378‐Luc‐HRE1), resulting in the upregulation of ATG12.
3.3. Overexpression of miR‐378‐3p exerts anti‐apoptotic effects on nerve cells
To investigate the effect of miR‐378‐3p under hypoxia conditions, a CCK‐8 assay was performed, which identified that the inhibition of cell viability induced by hypoxia was reversed by miR‐378‐3p overexpression, while it was exacerbated by miR‐378‐3p knockdown (Figure 3A). We then analysed membrane integrity by LDH release assay, which confirmed that exposure to hypoxia promoted LDH release, which was attenuated by transfection with miR‐378‐3p mimics, while miR‐378‐3p knockdown further promoted the hypoxia‐induced LDH release (Figure 3B). Furthermore, hypoxia markedly increased Bax expression, which was reversed by transfection with miR‐378‐3p mimics, while it was exacerbated by ASO‐378‐3p (Figure 3C). TUNEL assay further confirmed miR‐378‐3p reversed hypoxia‐induced apoptosis (Figure 3D). These results indicated that miR‐378‐3p exerted an anti‐apoptotic effect on N2A cells under hypoxic conditions.
FIGURE 3.
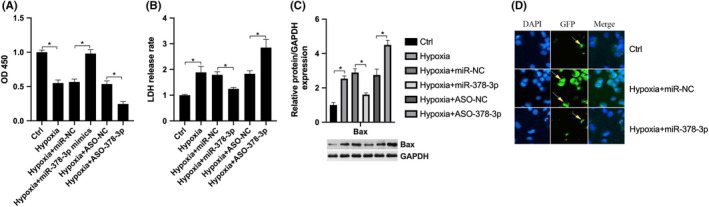
miR‐378‐3p regulates nerve cell viability and apoptosis. A, CCK‐8 assay was performed to analyse the cell viability in PC12 and N2A cells treated with hypoxia, or transfected with miR‐NC, miR‐378‐3p, ASO‐NC or ASO‐378‐3p. B, LDH assay was performed to detect the cell membrane integrity in PC12 and N2A cells exposed to hypoxia, or transfected with miR‐NC, miR‐378‐3p, ASO‐NC or ASO‐378‐3p. C, The mRNA expression of Bax was detected by RT‐qPCR in the PC12 and N2A cells exposed to hypoxia, or transfected with miR‐NC, miR‐378‐3p, ASO‐NC or ASO‐378‐3p. D, TUNEL assay was performed to detect cell apoptosis. Data are reported as the means ± SD. *P < .05 (t test)
3.4. Expression of ATG12 is directly regulated by miR‐378‐3p in N2A cells
Bioinformatic analysis (TargetScan) was performed and predicted that miR‐378‐3p may target ATG12 potentially. To identify this hypothesis, the pmirGLO vector was used for constructing the ATG12‐WT with the seed sequence AGUCCAG of miR‐378‐3p (Figure 4A) and the ATG12 3’UTR‐mut, which mutated the seed sequence AGUCCAG to ACACCTC. Compared with miR‐NC, the overexpression of miR‐378‐3p decreased ATG12 expression (Figure 4B and C), and it inhibited the activity of ATG12‐3’UTR‐WT; no difference was noted in ATG12 3ʹUTR‐mut activity between the two groups (Figure 4D). Compared with ASO‐NC, the knockdown of miR‐378‐3p by ASO‐378‐3p upregulated ATG12 mRNA and protein expression (Figure 4B and C), and it promoted the activity of ATG12‐3’UTR‐WT; no significant difference was observed in ATG12 3’UTR‐mut activity between the two groups (Figure 4D). These results indicate that miR‐378‐3p can directly target the 3’TUR of ATG12 and repress its mRNA and protein expression.
FIGURE 4.
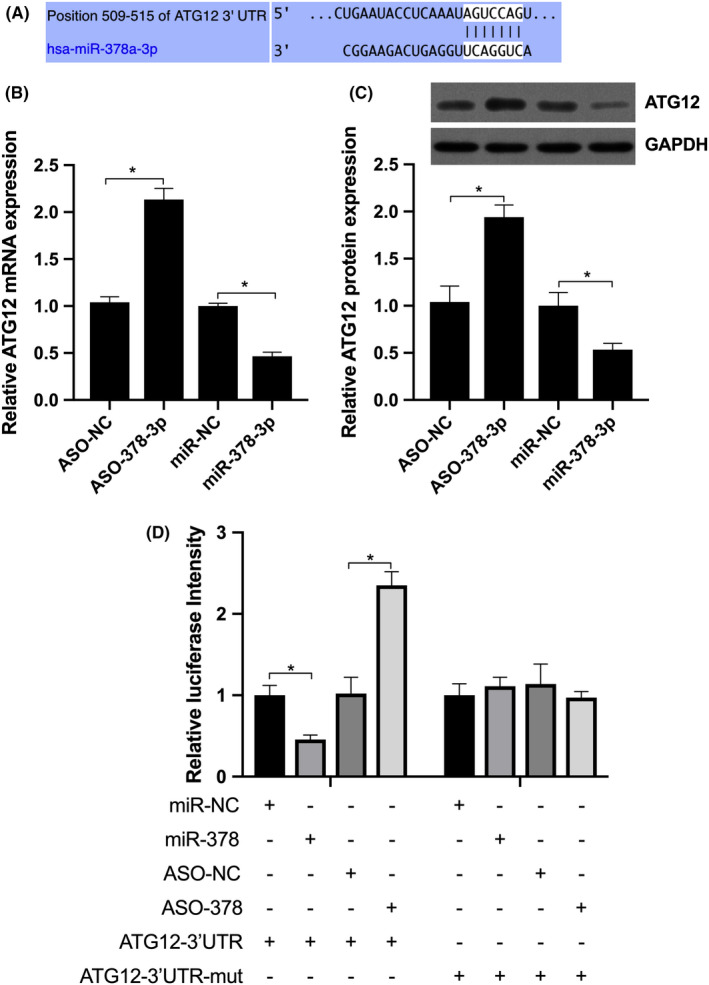
miR‐378‐3p targets ATG12 directly in N2A cells. A, Predicted miR‐378‐3p binding site in the ATG12 3’UTR. B and C, RT‐qPCR and Western blot analysis were performed to detect the mRNA and protein expression levels of ATG12 in N2A cells transfected with miR‐NC, miR‐378‐3p, ASO‐NC or ASO‐378‐3p. D, Relative luciferase activity of ATG12‐3’UTR and ATG12‐3’UTR‐mut in N2A cells transfected with miR‐NC, miR‐378‐3p, ASO‐NC or ASO‐378‐3p NC respectively. Data are reported as the means ± SD. *P < .05 vs NC (t test)
3.5. miR‐378‐3p‐mediated hypoxia‐induced apoptosis is reversed by ATG12 overexpression
We further investigate whether ATG12 mediated downstream effect of miR‐378‐3p. The results showed that in the N2A cells, exposure to hypoxia significantly inhibited cell viability and promoted apoptosis, which was significantly reversed by transfection with miR‐378‐3p mimics, while the effect was further reversed by co‐transfection with OE‐ATG12 (Figure 5A and B). Furthermore, it was found that under hypoxic conditions, miR‐378‐3p inhibited the level of Cle‐caspase‐3, ATG12 overexpression promoted the level of Cle‐caspase‐3, while co‐transfection with miR‐378‐3p mimics and OE‐ATG12 reversed the effects mediated by miR‐378‐3p mimics or OE‐ATG12 respectively (Figure 5C). This suggested that miR‐378‐3p exerted anti‐apoptotic effects via ATG12 under hypoxic conditions.
FIGURE 5.
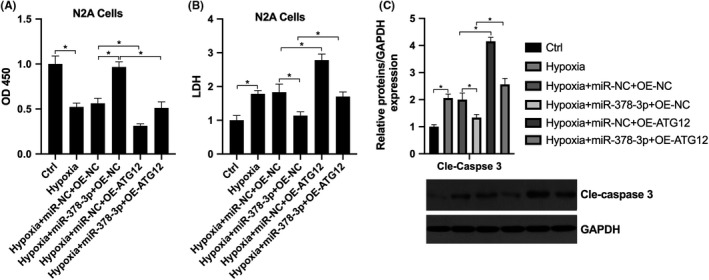
Hypoxia regulates ATG12 through miR‐378‐3p, participating in nerve cell apoptosis. N2A cells were grouped as Ctrl, hypoxia, hypoxia+miR‐NC+OE‐NC, hypoxia+miR‐378‐3p+OE‐NC, hypoxia+miR‐NC+OE‐ATG12 and hypoxia+miR‐378‐3p+OE‐ATG12. A, CCK‐8 assay was performed to detect cell viability; B, LDH assay was performed to detect the membrane integrity; C, Western blot analysis was performed to detect cleaved caspase‐3 expression
3.6. miR‐378‐3p improves motor functions and neurological functions in rats with SCI
To further evaluate the effect of miR‐378‐3p on spinal function in rats with contusion SCI, the MDI scale and BBB score were used. The results illustrated that the decreased MDI and increased BBB scores returned to levels similar to those of the sham‐operated group by AAV‐miR‐378‐3p, suggesting that miR‐378‐3p exerted neuroprotective effects on adult rats with contusion SCI (Figure 6A and B). Compared with AAV‐miR‐NC, the expression of miR‐378‐3p was increased, while ATG12 expression was decreased by AAV‐miR‐378‐3p in the spinal tissue (Figure 6C and D), suggesting that the administration of miR‐378‐3p can improve the motor and neurological functions of rats with contusion SCI.
FIGURE 6.
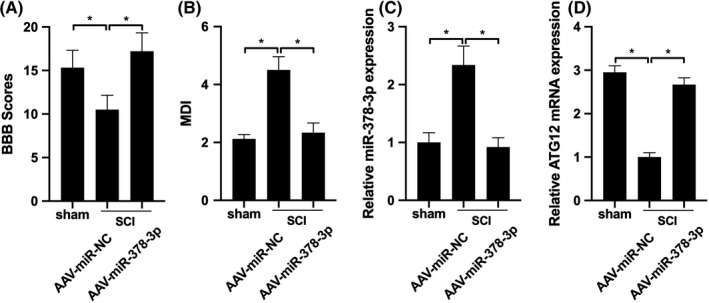
miR‐378‐3p improves motor and neurological functions of rats with SCI. A, MDI and BBB scores were detected in rats with SCI infused with AAV‐miR‐378‐3p (n = 12) or AAV‐miR‐NC (n = 12). B, RT‐qPCR was performed to detect the levels of miR‐378‐3p and ATG12 in spinal tissues. Data are reported as the means ± SD. *P < .05
4. DISCUSSION
The present study demonstrated that miR‐378‐3p expression was downregulated and that of ATG12 was upregulated in tissues of rats with contusion SCI and in nerve cells, suggesting an association between miR‐378‐3p and ATG12 in the pathogenesis of contusion SCI. Hypoxia induced ATG12 expression by directly repressing miR‐378‐3p in N2A cells. The upregulation of ymiR‐378‐3p expression improved neurological and motor functions by downregulating ATG12 expression, which contributed to the amelioration of rats with contusion SCI.
Alterations in miRNAs have been observed in the spinal cord, including three types: upregulated, downregulated and those initially upregulated followed by downregulation following SCI. 24 It was further revealed that the altered miRNAs exert various effects on the regulation of cell growth and apoptosis. 25 It has been reported that the upregulation of miR‐20a persists for at least 1 week. The administration of miR‐20a into normal mouse spinal cord can produce symptoms similar to those of SCI, including neural cell apoptosis, whereas anti‐miR‐20a can decrease apoptosis and ameliorate the functional deficits. 8 In a similar manner, in a previous study, following SCI, miR‐486 expression was induced in 7 days, and miR‐486 administration into the normal spinal cord of mice induced neuronal death, whereas miR‐486 silencing decreased neuronal death and improved motor function. 26 This study suggested that long‐term contusion SCI decreased miR‐378‐3p expression.
The effects of miRNAs on cell apoptosis are exerted due to the changes in their targets, which are associated with apoptotic functions. For example, miR‐146a downregulation induces cell apoptosis by increasing the expression of the pro‐apoptotic protein, caspase‐3. 27 On the contrary, miR‐21 overexpression inhibits neural cell death via the downregulation of the pro‐apoptotic gene, Fas ligand. 28 In this study, it was confirmed that miR‐378‐3p can directly target ATG12. It is generally accepted that ATG12 is a key mediator in promoting the formation of apoptotic vacuoles 29 by conjugating to ATG5 or ATG3, 30 which therefore promotes mitochondrial apoptosis by associating with Bcl‐2. 14 In the present study, we identified that nerve cell apoptosis can be promoted by ATG12, the upregulation of which can rescue miR‐378‐3p–mitigated cell apoptosis under hypoxic conditions. In fact, crosstalk between apoptosis and autophagy has been observed, with Bcl‐2 family proteins functioning as a bridge for the two pathways. 14 A major limitation of this study is that we did not investigate whether the miR‐378‐3p/ATG12 axis is involved in the regulation of autophagy in contusion SCI, which warrants further investigation.
In conclusion, the present study demonstrated that the upregulation of miR‐378‐3p repressed the apoptosis of nerve cells and improved neurological and motor functions by targeting ATG12, which may provide a further basis for the investigation of the molecular mechanisms and therapy of contusion SCI.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
HC and HL performed the in vitro cell line experiment, HC was also a major contributor in the writing of the manuscript. HL, HF and YZ conducted the in vivo rat experiment. XW designed the experiment. All authors read and approved the final manuscript.
ETHICAL APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
Zhang H, Yu H, Yang H, Zhan Y, Liu X. miR‐378‐3p alleviates contusion spinal cord injury by negatively regulating ATG12. Int J Exp Path. 2021;102:200–208. 10.1111/iep.12400
Funding information
The project was supported by the National Natural Science Foundation of Liaoning (No. 20180550948)
DATA AVAILABILITY STATEMENT
The data and materials are available when requested.
REFERENCES
- 1. Hou S, Rabchevsky AG. Autonomic consequences of spinal cord injury. Compr Physiol. 2014;4(4):1419‐1453. [DOI] [PubMed] [Google Scholar]
- 2. Scivoletto G, Tamburella F, Laurenza L, et al. Who is going to walk? A review of the factors influencing walking recovery after spinal cord injury. Front Hum Neurosci. 2014;8:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lin CA, Duan KY, Wang XW, Zhang ZS. MicroRNA‐409 promotes recovery of spinal cord injury by regulating ZNF366. Eur Rev Med Pharmacol Sci. 2018;22(12):3649‐3655. [DOI] [PubMed] [Google Scholar]
- 4. Ek CJ, Habgood MD, Callaway JK, et al. Spatio‐temporal progression of grey and white matter damage following contusion injury in rat spinal cord. PLoS ONE. 2010;5(8):e12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Byrnes KR, Stoica BA, Fricke S, Di Giovanni S, Faden A. Cell cycle activation contributes to post‐mitotic cell death and secondary damage after spinal cord injury. Brain. 2007;130(Pt 11):2977‐2992. [DOI] [PubMed] [Google Scholar]
- 6. Soubeyrand M, Laemmel E, Court C, Dubory A, Vicaut E, Duranteau J. Rat model of spinal cord injury preserving dura mater integrity and allowing measurements of cerebrospinal fluid pressure and spinal cord blood flow. Eur Spine J. 2013;22(8):1810‐1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hammond SM. An overview of microRNAs. Adv Drug Deliv Rev. 2015;87:3‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu NK, Wang XF, Lu QB, Xu XM. Altered microRNA expression following traumatic spinal cord injury. Exp Neurol. 2009;219(2):424‐429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fang J, Song XW, Tian J, et al. Overexpression of microRNA‐378 attenuates ischemia‐induced apoptosis by inhibiting caspase‐3 expression in cardiac myocytes. Apoptosis. 2012;17(4):410‐423. [DOI] [PubMed] [Google Scholar]
- 10. Cheng Y, Zhu P, Yang J, et al. Ischaemic preconditioning‐regulated miR‐21 protects heart against ischaemia/reperfusion injury via anti‐apoptosis through its target PDCD4. Cardiovasc Res. 2010;87(3):431‐439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang J, Ma J, Long K, et al. Overexpression of Exosomal Cardioprotective miRNAs Mitigates Hypoxia‐Induced H9c2 Cells Apoptosis. Int J Mol Sci. 2017;18(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang N, Zhong J, Han S, Li Y, Yin Y, Li J. MicroRNA‐378 Alleviates Cerebral Ischemic Injury by Negatively Regulating Apoptosis Executioner Caspase‐3. Int J Mol Sci. 2016;17(9):1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang Z, Klionsky DJ. Mammalian autophagy: core molecular machinery and signaling regulation. Curr Opin Cell Biol. 2010;22(2):124‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rubinstein AD, Eisenstein M, Ber Y, Bialik S, Kimchi A. The autophagy protein Atg12 associates with antiapoptotic Bcl‐2 family members to promote mitochondrial apoptosis. Mol Cell. 2011;44(5):698‐709. [DOI] [PubMed] [Google Scholar]
- 15. Zhang JF, Wu YC. microRNA‐182‐5p alleviates spinal cord injury by inhibiting inflammation and apoptosis through modulating the TLR4/NF‐κB pathway. Int J Clin Exp Pathol. 2018;11(6):2948‐2958. [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou X, Chen J, Zang H, Chen X, Shao G. MicroRNA‐23b attenuates the H2O2‐induced injury of microglial cells via TAB3/NF‐κB signaling pathway. Int J Clin Exp Pathol. 2018;11(12):5765‐5773. [PMC free article] [PubMed] [Google Scholar]
- 17. Li R, Bao L, Hu W. Expression of miR‐210 mediated by adeno‐associated virus performed T neuroprotective effects on a rat model of acute spinal cord injury. Tissue Cell. 2019;57(4):22‐33. [DOI] [PubMed] [Google Scholar]
- 18. Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12(1):1‐21. [DOI] [PubMed] [Google Scholar]
- 19. Taira Y, Marsala M. Effect of proximal arterial perfusion pressure on function, spinal cord blood flow, and histopathologic changes after increasing intervals of aortic occlusion in the rat. Stroke. 1996;27(10):1850‐1858. [DOI] [PubMed] [Google Scholar]
- 20. Yamamoto Y, Osanai T, Nishizaki F, et al. Matrix metalloprotein‐9 activation under cell‐to‐cell interaction between endothelial cells and monocytes: possible role of hypoxia and tumor necrosis factor‐alpha. Heart Vessels. 2012;27(6):624‐633. [DOI] [PubMed] [Google Scholar]
- 21. Zhao YH, Wang RN, Yang GJ, et al. Optimization of N2a cell transfection mediated by liposome. Chinese J Tissue Eng. Res. 2014;18(29):4669‐4674. [Google Scholar]
- 22. Cemil B, Gokce EC, Kahveci R, et al. Aged Garlic Extract Attenuates Neuronal Injury in a Rat Model of Spinal Cord Ischemia/Reperfusion Injury. J Med Food. 2016;19(6):601‐606. [DOI] [PubMed] [Google Scholar]
- 23. A JC, Li ZY, Long QF, et al. MiR‐379‐5p improved locomotor function recovery after spinal cord injury in rats by reducing endothelin 1 and inhibiting astrocytes expression. Eur Rev Med Pharmacol Sci. 2019;23(22):9738‐9745. [DOI] [PubMed] [Google Scholar]
- 24. Ning B, Gao L, Liu RH, Liu Y, Zhang NS, Chen ZY. microRNAs in spinal cord injury: potential roles and therapeutic implications. Int J Biol Sci. 2014;10(9):997‐1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nieto‐Diaz M, Esteban FJ, Reigada D, et al. MicroRNA dysregulation in spinal cord injury: causes, consequences and therapeutics. Front Cell Neurosci. 2014;8:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jee MK, Jung JS, Choi JI, et al. MicroRNA 486 is a potentially novel target for the treatment of spinal cord injury. Brain. 2012;135(Pt 4):1237‐1252. [DOI] [PubMed] [Google Scholar]
- 27. Aimone JB, Leasure J, Perreau V, Thallmair M. Spatial and temporal gene expression profiling of the contused rat spinal cord. Exp Neurol. 2004;189(2):204‐221. [DOI] [PubMed] [Google Scholar]
- 28. Buller B, Liu X, Wang X, et al. MicroRNA‐21 protects neurons from ischemic death. FEBS J. 2010;277(20):4299‐4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Metlagel Z, Otomo C, Takaesu G, Otomo T. Structural basis of ATG3 recognition by the autophagic ubiquitin‐like protein ATG12. Proc Natl Acad Sci USA. 2013;110(47):18844‐18849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Haller M, Hock AK, Giampazolias E, et al. Ubiquitination and proteasomal degradation of ATG12 regulates its proapoptotic activity. Autophagy. 2014;10(12):2269‐2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data and materials are available when requested.


