Abstract
Objective
Newborns with Trisomy 21 (T21) often require NICU hospitalization. Oxygen desaturations are frequently observed in these infants, even in the absence of congenital heart defects (CHD). We hypothesized that T21 NICU patients have more hypoxemia than those without T21.
Design
All infants with T21 without significant CHD discharged home from the NICU between 2009–2018 were included (n=23). Controls were matched 20:1 for gestational age and length of stay. We compared daily severe hypoxemia events (SpO2<80% for ≥10 seconds) for the whole NICU stay and the pre-discharge week.
Results
Infants with T21 showed significantly more daily hypoxemia events during their entire NICU stay (median 10 versus 7, p=0.0064), and more so in their final week (13 versus 7, p=0.0008).
Conclusion
NICU patients with T21 without CHD experience more severe hypoxemia events than controls, particularly in the week before discharge. Whether this hypoxemia predicts or contributes to adverse outcomes is unknown.
Introduction:
Newborn infants with Trisomy 21 (T21) frequently require admission to the Neonatal Intensive Care Unit (NICU) for a variety of surgical and non-surgical conditions (1–5). Hypoxemia spells are frequently observed in these infants even in absence of congenital cardiac defects. Obstructive apnea and hypoventilation have been reported in infants and children with T21 (6), possibly reflecting airway hypotonia and differences in autonomic nervous system control of breathing (7), as well as anatomical differences (8). Infants with T21 may also have difficulty with oral feeding associated with oxygen desaturation (9). Despite these observations and the potential importance of intermittent hypoxemia events during the neonatal period, there is a dearth of research into hypoxemia in NICU patients with T21.
We sought to test the hypothesis that infants with T21 have more severe hypoxemic events (SpO2<80% for at least 10 seconds) during their stay in the NICU compared to those without T21, and that this difference persists in the final week prior to discharge home. As in our previous work (10,11) we focused on these more severe events since frequency of SpO2 <80% during the neonatal period has been linked to neurodevelopmental impairment (12).
Methods:
We performed a retrospective analysis of pulse oximetry-derived peripheral oxygen saturation (SpO2) in infants with and without Trisomy 21. All infants with T21 admitted at less than 60 days of age to the University of Virginia (UVA) NICU from 2009 to 2018 with archived bedside monitor SpO2 data were candidates for inclusion. Each T21 infant was matched with twenty infants without T21, of similar gestational age and approximate length of NICU stay. Infants were excluded from the T21 and control groups if they died prior to NICU discharge, were transferred to another location prior to discharge home, or had significant congenital cardiac defects, congenital diaphragmatic hernia, or received extracorporeal membrane oxygenation treatment. For the purpose of this study, significant congenital cardiac defects were defined as those requiring surgical or transcatheter repair in the first 6 months of life. Infants with patent ductus arteriosus or septal defects not requiring repair within 6 months of birth were not excluded. This study was approved by the Institutional Review Board of the University of Virginia with waiver of consent due to the retrospective, non-interventional nature of the analyses.
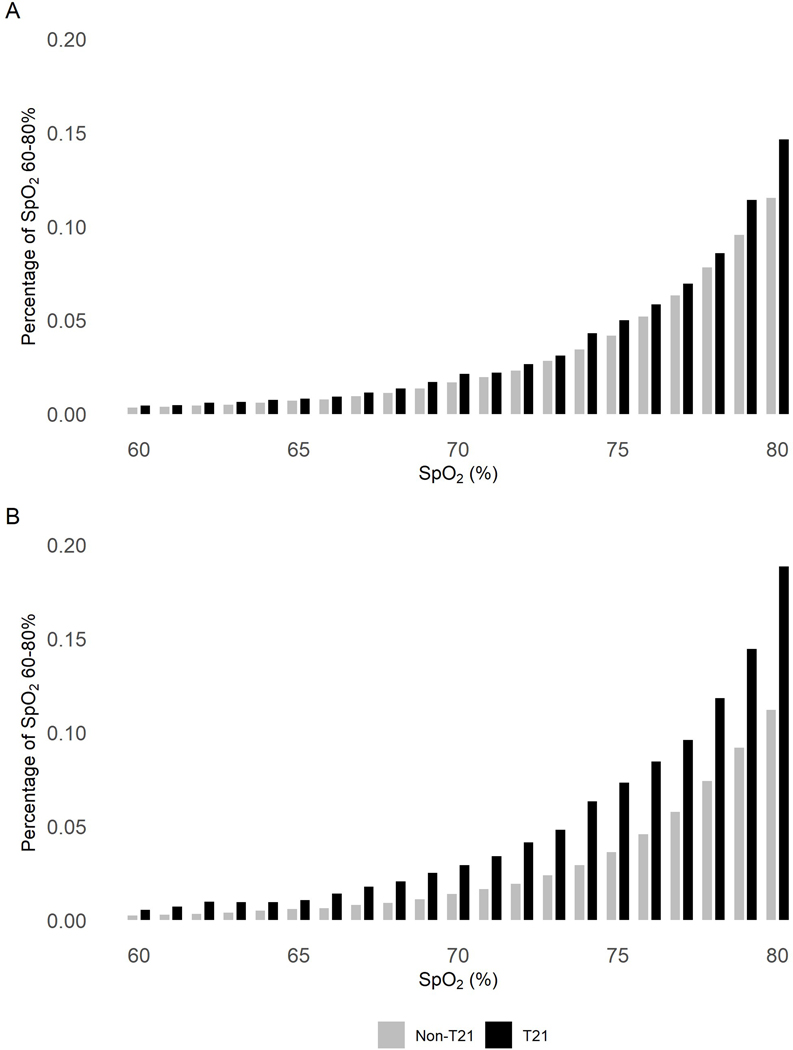
Demographic and clinical data were extracted from a NICU database, including the diagnosis of apnea. SpO2 data from pulse oximeters (Masimo technology) are routinely collected for all NICU patients at UVA since 2009, using BedMaster technology (Excel Medical, Jupiter, FL). SpO2 averaging time was 8 seconds and sampling rate 0.5 Hz (every 2 seconds), and the recording technology and averaging times were unchanged during the course of the study. For summary analysis in Figure 2, SpO2 values were binned by percentage point, and counts in each bin were divided by the infant’s total number of SpO2 measurements to arrive at a normalized proportion. For counts of intermittent hypoxemia in Figure 3, events were defined as SpO2 <80% for at least 10 seconds.
Figure 2: Distribution of all SpO2 values 60–80%.
Pulse oximeter SpO2 values measured every 2 seconds were analyzed (A) through the entire NICU stay and (B) in the final NICU week before discharge home. Rates of each SpO2 value from 60%–80% are shown, normalized by amount of data available per infant so that infants with more data points recorded are not over-represented in the distribution, for infants with T21 (black bars) or without T21 (gray bars). SpO2 data were available for 23 infants with T21 and 460 infants without T21 for the whole NICU stay, and for 22 infants with T21 and 411 infants without T21 for the final week.
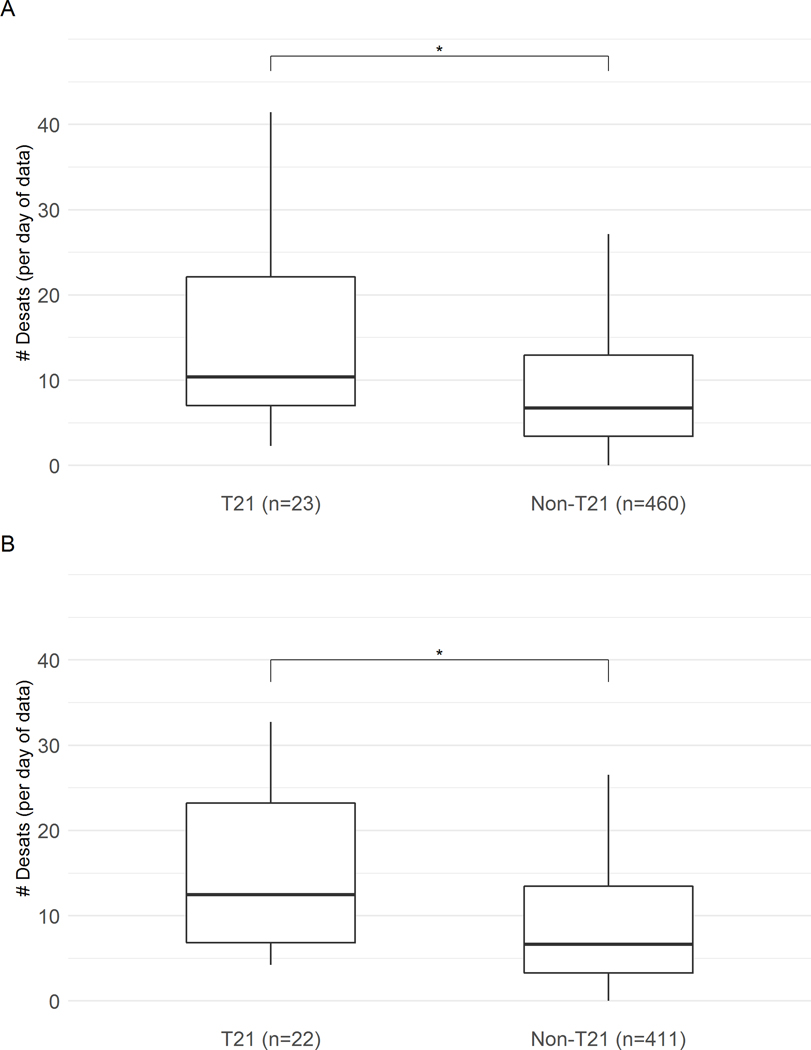
Figure 3: Number of events of desaturation <80% per day for infants with and without T21.
Pulse oximeter SpO2 values measured every 2 seconds were analyzed (A) through the entire NICU stay and (B) in the final NICU week before discharge home. Daily number of desaturation events with SpO2 <80% for at least 10 seconds are shown, normalized by amount of data available, for infants with T21 (left) or without T21 (right). Box boundaries are 25th and 75th percentiles, whiskers are 5th and 95th percentiles, and horizontal line is median events per day. Outliers are not shown. *p<0.05 T21 versus controls
Data are shown as median (25th percentile, 75th percentile) or n (%) unless otherwise specified. Statistical testing included Fisher’s exact test for categorical variables and Mann-Whitney tests for continuous variables, with p<0.05 designated as statistically significant. Statistical analyses were performed using MATLAB and R version 3.6.3.
Results:
In the 10 years of the study, 95 infants with T21 were admitted to the UVA NICU. Of these, 72 were excluded either due to significant cardiac malformations, death or transfer prior to discharge home, or unavailability of SpO2 data. The 23 infants with T21 were matched 1:20 with control infants of similar gestational age and length of stay. Demographic and clinical variables of the T21 and control cohorts are shown in Table 1.
Table 1:
Demographics of T21 and control infants
| T21 | Non-T21 | ||
|---|---|---|---|
| (n = 23) | (n = 460) | p = | |
| Gestational age (weeks) | 36 (34,38) | 36 (34,38) | 0.97 |
| Birthweight (kilograms) | 2.38 (1.96, 2.79) | 2.64 (2.04, 3.20) | 0.32 |
| Male | 16 (70%) | 268 (58%) | 0.39 |
| White | 21 (91%) | 390 (85%) | 0.55 |
| Black or African American | 1 (4%) | 60 (13%) | 0.34 |
| Hispanic | 3 (13%) | 33 (7%) | 0.4 |
| Maternal age (years) | 34 (28,38) | 27 (23,32) | 0.001 |
| Days on mechanical ventilation | 0 (0,1) | 0 (0,1) | 0.61 |
| Days on CPAP | 0 (0,4) | 0 (0,3) | 0.92 |
| Days on nasal cannula | 0 (0,8) | 0 (0,3) | 0.95 |
| Length of stay (days) | 19 (12,24) | 18 (10,26) | 0.99 |
| Day of age at discharge | 20 (14,27) | 18 (11,28) | 0.48 |
| Home with pulse oximeter, no O2 | 1 (4%) | 8 (2%) | 0.36 |
| Home with O2 | 5 (22%) | 16 (3%) | 0.002 |
| Days SpO2 data per infant | 13 (7,20) | 12 (6,19) | 0.74 |
| Total days SpO2 data analyzed | 327 | 7,170 | na |
Median (25th, 75th percentile) or n (%) are shown
Gestational age for infants with and without T21 was closely matched at 36 (34,38) weeks, median (25th,75th percentile). NICU length of stay was 19 (12,24) days for infants with T21 and 18 (10,26) days for controls. Infants with T21 were more likely to be born at an outside hospital and transferred to our NICU at an older age (the 75th percentile of age at admission was 4 days versus 1 day), but this was not statistically significant. Maternal age for the infants with T21 was significantly higher: median 34 (28,38) years old versus 27 (23,32), p=0.001. There were no significant differences between groups with respect to ethnicity, race, gender, 5-minute Apgar scores or number of days on mechanical ventilation, continuous positive airway pressure, or nasal cannula oxygen. Apnea was diagnosed in 53 (12%) of the control infants and none of the infants with T21. There was no significant difference in the number of infants who were discharged home with tube feeds (4% of both groups) or pulse oximeter without home oxygen (4% of infants with T21 versus 2% of controls, p=0.358). Significantly more infants in the T21 group were discharged home with oxygen (22%) compared to control infants (3%), p = 0.002 (all with pulse oximeters).
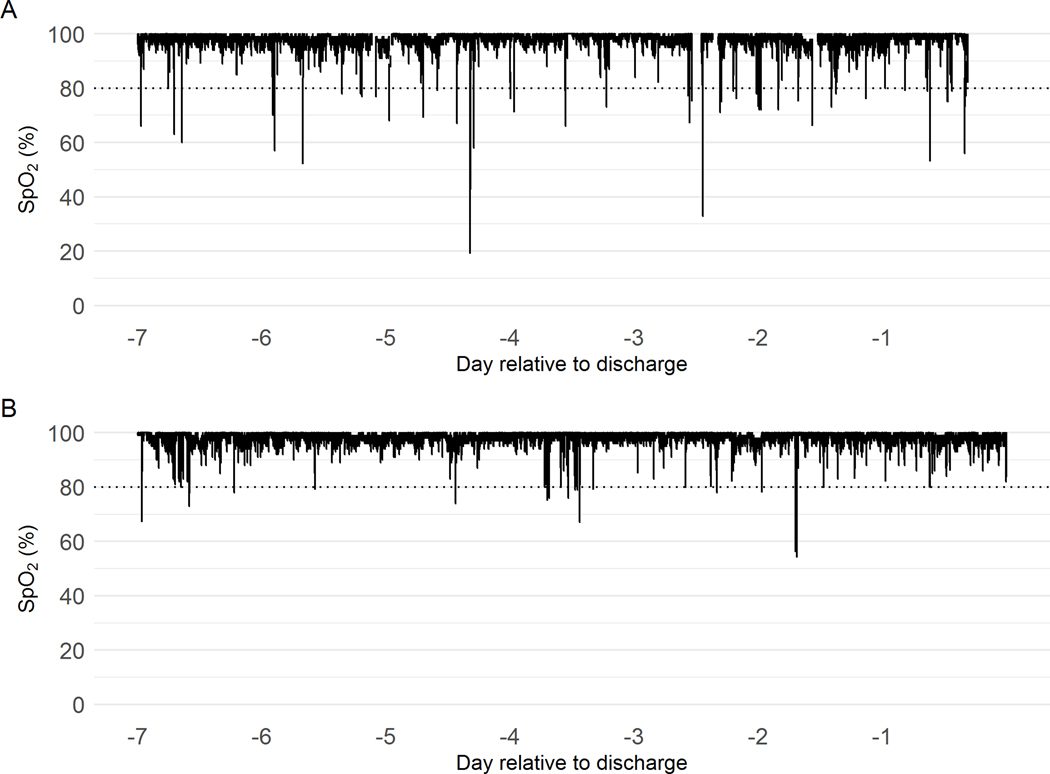
In total, over 175,000 hours of SpO2 data were analyzed, with a median of 13 days for infants with T21 and 12 days for control infants. Mean SpO2 for the whole NICU stay was 96.6.% for infants with T21 and 97.8% for controls (p=0.004). In the final week of the NICU stay, mean SpO2 was 96.8% for infants with T21 and 98.2% for controls (p=0.002). Infants with T21 had significantly more hypoxemia events of SpO2 <80% for at least 10 seconds. Figure 1 shows representative examples of SpO2 data the week before discharge from an infant with T21 and a control infant of the same gestational and chronologic ages. Figure 2 is a histogram showing the fraction of values of SpO2 in the very low range, 60–80%, over the entire NICU stay and in the final week prior to discharge home. In the final NICU week, infants with T21 spent 0.91% of the time with SpO2 <80% whereas control infants spent only 0.49% of the time with SpO2 this low.
Figure 1: SpO2 trend in a representative infant with T21 and without T21.
Pulse oximeter SpO2 values for the final week in the NICU for a representative infant with T21 (A) and without T21 (B).
Figure 3 shows that the number of hypoxemia events was significantly higher for infants with T21 compared to controls during the whole NICU stay, and the difference was even greater in the final NICU week. Through the entire stay, infants with T21 had a median of 10 (7, 23) events/day versus 7 (3, 13) events/day for control infants, p=0.0064. In the final week before discharge home, there was an even greater difference in number of hypoxemia events in infants with versus controls (median 13 (7, 24) events/day versus 7 (3,14), p=0.0008).
Discussion:
Many newborn infants with Trisomy 21 spend time in the NICU due to cardiorespiratory instability, feeding issues, or other medical or surgical conditions requiring specialized care. We found that these infants, even in the absence of significant congenital cardiac malformations, have more severe intermittent hypoxemia events compared to control infants.
Experienced NICU clinicians generally recognize that some infants with T21 have frequent oxygen desaturation events, particularly during oral feeding. In a report of 121 infants with T21, a low oxygen saturation profile was the most common cause for admission to the NICU either directly after birth or in transfer (2). Our study is the first, to our knowledge, to quantitate severe hypoxemia events in infants with T21 throughout the NICU stay. We matched infants for gestational age (median 36 weeks), and none of the infants with T21 were diagnosed with apnea. We used a rigorous definition of hypoxemia, SpO2 <80% for at least 10 seconds, as we have done in our prior work (10). Of note, a multicenter study found that prolonged events of SpO2<80% in the neonatal period have been linked to neurodevelopmental impairment in preterm infants(12). Although milder degrees of intermittent hypoxemia were not studied, the overall mean SpO2 was also significantly lower in infants with T21. We found that, for control infants, severe hypoxemia events <80% continued at about the same daily rate by the final week before discharge home from the NICU. In contrast, those with T21 had more desaturation events in the final week in the NICU. Home oxygen therapy was prescribed for 16% of NICU infants with T21 and only 3% of controls. A similar finding was published in a case-control study of infants in over 100 neonatal units, in which 11% of infants with T21 and 3% of control infants were prescribed home oxygen (3).
The design of our study does not allow us to account for the many variables potentially contributing to oxygen desaturation in NICU patients, but we can speculate on cardiac, pulmonary, and neurologic etiologies as well as potential consequences of chronic intermittent hypoxemia in infants with T21. From a cardiac perspective, some infants may have had intermittent right to left intra- or extra-cardiac shunting, since a number had echocardiographic evidence of a patent ductus arteriosus or patent foramen ovale. Most infants with T21 in our cohort had a single echocardiogram performed on the day of birth, to rule out significant cardiac malformations, and we are unable to determine whether and when the ductus arteriosus closed. As far as cardiac consequences of the desaturation events we describe, evolution of pulmonary hypertension is a risk for individuals with T21 (16–20) and the extent to which acute intermittent hypoxemia events during the neonatal period worsens this remains speculative (21). Chronic intermittent hypoxemia, such as that occurring in the setting of obstructive sleep apnea in older individuals with T21, impacts sympathetic/parasympathetic balance (22) which can also contribute to chronic cardiovascular dysfunction.
From a pulmonary perspective, our cohort of infants with and without T21 had a similar (short) duration of mechanical ventilation and continuous positive airway pressure dependence suggesting that hypoxemia differences were not due to acute lung injury (23). Of note, abnormalities of lung development are well described in individuals with Down Syndrome and likely play a role in unstable oxygenation. In a mouse model of Down syndrome, increased intermittent hypoxemia events occur at an early age, without evidence of a cardiac etiology (24). Lung growth and structural abnormalities are not infrequently seen in autopsies of individuals with T21. Alveolar simplification, prominent brochopulmonary anastamoses, and other observed lung abnormalities would be expected to contribute not only to hypoxemia but also to evolution of pulmonary hypertension (16,20,25,26).
Neurologic differences may also contribute to hypoxemia events in infants with T21. In our cohort, many infants with T21 were described in the medical record as having desaturations associated with dyscoordinated oral feeding, likely related at least in part to low muscle tone. This may be a reason for the increase in hypoxemia in the week prior to discharge, as infants get fewer feedings via nasogastric tube and more by mouth. Neck and airway hypotonia may also contribute to the occurrence of obstructive apnea which is well described in older infants and children with T21 (27–31). Ventilatory control has also been reported to be unstable in children with T21 as shown by increased loop gain(32). Hypoxemia events due to obstructive apnea have been reported to lead to less robust catecholamine release in children with T21 compared to typically developing children, and this dampened sympathetic activation may contribute to reduced ventilatory response (33). Whether intermittent hypoxemia in the neonatal period impacts neurodevelopmental outcomes is also not known, but obstructive sleep apnea has been associated with more severe cognitive impairments in children and adults with Down syndrome (34).
Individuals with T21 have increased expression of antioxidant enzymes such as superoxide dismutase, encoded on chromosome 21, which would seem to be protective against detrimental effects of reoxygenation after acute intermittent hypoxia events. On the other hand, they also have evidence of increased oxidative stress throughout life. (13) Hypoxemia and reoxygenation can also lead to inflammation(2), and children with T21 have been reported to have increased inflammatory cytokines compared to typically developing children (14). Interestingly, a recent meta-analysis of 41 published reports on in vitro bioenergetics reported that cells from individuals with Down Syndrome versus controls are in a “pseudohypoxic state,” in that their metabolites resemble those associated with hypoxia even when their supply of oxygen is not disrupted (15). This suggests a potential fundamental alteration in cell metabolism that may be associated with a variety of functional deficits, even in absence of hypoxemia.
Conclusion:
NICU patients with T21 without significant congenital heart defects experience up to twice as much severe hypoxemia (SpO2 < 80% for > 10 seconds) as those without T21, with the largest difference in the week prior to discharge home. More investigation is needed to determine the natural history and consequences of varying degrees of intermittent hypoxemia in individuals with T21, including neurocognitive and cardiorespiratory effects, and the best therapeutic strategies to minimize hypoxemia and improve outcomes.
Acknowledgments
Funding Information: NICHD R01HD072071
Footnotes
Disclosures: None of the authors have any conflicts or disclosures related to this work.
Bibliography
- 1.Boghossian NS, Hansen NI, Bell EF, Stoll BJ, Murray JC, Laptook AR, et al. Survival and morbidity outcomes for very low birth weight infants with Down syndrome. Pediatrics. 2010. December;126(6):1132–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin T, Smith A, Breatnach CR, Kent E, Shanahan I, Boyle M, et al. Infants Born with Down Syndrome: Burden of Disease in the Early Neonatal Period. J Pediatr. 2018;193:21–26. [DOI] [PubMed] [Google Scholar]
- 3.Mann JP, Statnikov E, Modi N, Johnson N, Springett A, Morris JK. Management and outcomes of neonates with down syndrome admitted to neonatal units. Birth Defects Res Part A Clin Mol Teratol. 2016. June;106(6):468–474. [DOI] [PubMed] [Google Scholar]
- 4.Ergaz-Shaltiel Z, Engel O, Erlichman I, Naveh Y, Schimmel MS, Tenenbaum A. Neonatal characteristics and perinatal complications in neonates with Down syndrome. Am J Med Genet A. 2017. May;173(5):1279–1286. [DOI] [PubMed] [Google Scholar]
- 5.McAndrew S, Acharya K, Nghiem-Rao TH, Leuthner S, Clark R, Lagatta J. NICU management and outcomes of infants with trisomy 21 without major anomalies. J Perinatol. 2018. May 25;38(8):1068–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waters KA, Castro C, Chawla J. The spectrum of obstructive sleep apnea in infants and children with Down Syndrome. Int J Pediatr Otorhinolaryngol. 2020. February;129:109763. [DOI] [PubMed] [Google Scholar]
- 7.Wong W, Rosen D. Isolated mild sleep-associated hypoventilation in children with Down syndrome. Arch Dis Child. 2017. April 13;102(9):821–824. [DOI] [PubMed] [Google Scholar]
- 8.Colvin KL, Yeager ME. What people with Down Syndrome can teach us about cardiopulmonary disease. Eur Respir Rev. 2017. January;26(143). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stanley MA, Shepherd N, Duvall N, Jenkinson SB, Jalou HE, Givan DC, et al. Clinical identification of feeding and swallowing disorders in 0–6 month old infants with Down syndrome. Am J Med Genet A. 2019;179(2):177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagraj VP, Sinkin RA, Lake DE, Moorman JR, Fairchild KD. Recovery from bradycardia and desaturation events at 32 weeks corrected age and NICU length of stay: an indicator of physiologic resilience? Pediatr Res. 2019. July 4;86(5):622–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fairchild KD, Nagraj VP, Sullivan BA, Moorman JR, Lake DE. Oxygen desaturations in the early neonatal period predict development of bronchopulmonary dysplasia. Pediatr Res. 2019;85(7):987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poets CF. Intermittent hypoxia and long-term neurological outcome: How are they related? Semin Fetal Neonatal Med. 2020. April;25(2):101072. [DOI] [PubMed] [Google Scholar]
- 13.Lott IT. Antioxidants in Down syndrome. Biochim Biophys Acta. 2012. May;1822(5):657–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huggard D, Kelly L, Ryan E, McGrane F, Lagan N, Roche E, et al. Increased systemic inflammation in children with Down syndrome. Cytokine. 2020;127:154938. [DOI] [PubMed] [Google Scholar]
- 15.Pecze L, Randi EB, Szabo C. Meta-analysis of metabolites involved in bioenergetic pathways reveals a pseudohypoxic state in Down syndrome. Mol Med. 2020. November 9;26(1):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bush D, Galambos C, Ivy DD, Abman SH, Wolter-Warmerdam K, Hickey F. Clinical Characteristics and Risk Factors for Developing Pulmonary Hypertension in Children with Down Syndrome. J Pediatr. 2018. July 17;202:212–219.e2. [DOI] [PubMed] [Google Scholar]
- 17.Shah PS, Hellmann J, Adatia I. Clinical characteristics and follow up of Down syndrome infants without congenital heart disease who presented with persistent pulmonary hypertension of newborn. J Perinat Med. 2004;32(2):168–170. [DOI] [PubMed] [Google Scholar]
- 18.Galambos C, Minic AD, Bush D, Nguyen D, Dodson B, Seedorf G, et al. Increased Lung Expression of Anti-Angiogenic Factors in Down Syndrome: Potential Role in Abnormal Lung Vascular Growth and the Risk for Pulmonary Hypertension. PLoS One. 2016. August 3;11(8):e0159005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weijerman ME, van Furth AM, van der Mooren MD, van Weissenbruch MM, Rammeloo L, Broers CJ, et al. Prevalence of congenital heart defects and persistent pulmonary hypertension of the neonate with Down syndrome. Eur J Pediatr. 2010. October;169(10):1195–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cua CL, Blankenship A, North AL, Hayes J, Nelin LD. Increased incidence of idiopathic persistent pulmonary hypertension in Down syndrome neonates. Pediatr Cardiol. 2007. August;28(4):250–254. [DOI] [PubMed] [Google Scholar]
- 21.Ramani M, Bradley WE, Dell’Italia LJ, Ambalavanan N. Early exposure to hyperoxia or hypoxia adversely impacts cardiopulmonary development. Am J Respir Cell Mol Biol. 2015. May;52(5):594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horne RSC, Sakthiakumaran A, Bassam A, Thacker J, Walter LM, Davey MJ, et al. Children with Down syndrome and sleep disordered breathing have altered cardiovascular control. Pediatr Res. 2020. November 23; [DOI] [PubMed]
- 23.Martin RJ, Di Fiore JM, Macfarlane PM, Wilson CG. Physiologic basis for intermittent hypoxic episodes in preterm infants. Adv Exp Med Biol. 2012;758:351–358. [DOI] [PubMed] [Google Scholar]
- 24.Das D, Medina B, Baktir MA, Mojabi FS, Fahimi A, Ponnusamy R, et al. Increased incidence of intermittent hypoxemia in the Ts65Dn mouse model of Down syndrome. Neurosci Lett. 2015. September 14;604:91–96. [DOI] [PubMed] [Google Scholar]
- 25.Cooney TP, Thurlbeck WM. Pulmonary hypoplasia in Down’s syndrome. N Engl J Med. 1982. November 4;307(19):1170–1173. [DOI] [PubMed] [Google Scholar]
- 26.Bush D, Abman SH, Galambos C. Prominent Intrapulmonary Bronchopulmonary Anastomoses and Abnormal Lung Development in Infants and Children with Down Syndrome. J Pediatr. 2017;180:156–162.e1. [DOI] [PubMed] [Google Scholar]
- 27.Simpson R, Oyekan AA, Ehsan Z, Ingram DG. Obstructive sleep apnea in patients with Down syndrome: current perspectives. Nat Sci Sleep. 2018. September 13;10:287–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee C-F, Lee C-H, Hsueh W-Y, Lin M-T, Kang K-T. Prevalence of Obstructive Sleep Apnea in Children With Down Syndrome: A Meta-Analysis . J Clin Sleep Med. 2018. May 15;14(5):867–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Southall DP, Stebbens VA, Mirza R, Lang MH, Croft CB, Shinebourne EA. Upper airway obstruction with hypoxaemia and sleep disruption in Down syndrome. Dev Med Child Neurol. 1987. December;29(6):734–742. [DOI] [PubMed] [Google Scholar]
- 30.Goffinski A, Stanley MA, Shepherd N, Duvall N, Jenkinson SB, Davis C, et al. Obstructive sleep apnea in young infants with Down syndrome evaluated in a Down syndrome specialty clinic. Am J Med Genet A. 2015. February;167A(2):324–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coverstone AM, Bird M, Sicard M, Tao Y, Grange DK, Cleveland C, et al. Overnight pulse oximetry for evaluation of sleep apnea among children with trisomy 21. J Clin Sleep Med. 2014. December 15;10(12):1309–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siriwardhana LS, Nixon GM, Davey MJ, Mann DL, Landry SA, Edwards BA, et al. Children with down syndrome and sleep disordered breathing display impairments in ventilatory control. Sleep Med. 2020. December 8;77:161–169. [DOI] [PubMed] [Google Scholar]
- 33.O’Driscoll DM, Horne RSC, Davey MJ, Hope SA, Anderson V, Trinder J, et al. Cardiac and sympathetic activation are reduced in children with Down syndrome and sleep disordered breathing. Sleep. 2012. September 1;35(9):1269–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Breslin J, Spanò G, Bootzin R, Anand P, Nadel L, Edgin J. Obstructive sleep apnea syndrome and cognition in Down syndrome. Dev Med Child Neurol. 2014. July;56(7):657–664. [DOI] [PubMed] [Google Scholar]