Abstract
Introduction:
Enchondroma is a solitary, benign, intramedullary cartilaginous tumor commonly noticed in the phalanges of hands and feet with characteristic radiological features. Its occurrence in aberrant sites with atypical features lead to diagnostic dilemma. Enchondromas which are usually managed non-operatively can mimic other benign and malignant lesions, especially chondrosarcoma.
Case Report:
We report the case of a 31-year-old farmer who presented with long standing inconspicuous pain in his left leg which turned out to be a diaphyseal enchondroma even though it demonstrated aggressive radiological features mimicking a chondrosarcoma. Incisional biopsy was done from the scalloped areas to obtain the correct histological diagnosis. He underwent thorough curettage of the lesion and remains asymptomatic 2 years after the procedure. We attempt to discuss the differentials which the orthopedic surgeon should keep in mind for diaphyseal lesions mimicking enchondroma.
Conclusion:
Though classically found in metaphysis, Enchondromas are not uncommon in diaphysis of long bones. Enchondromas are generally benign, but can cause diagnostic dilemma when they present with aggressive features at rare locations and surgeons should be wary of the differentials. Despite a size of more than 6 cm and evidence of cortical erosion and intramedullary widening, the lesion could still be benign. Early biopsy will help to differentiate Enchondroma from a malignant transformation or malignant tumor.
Keywords: Enchondroma, chondrosarcoma, diaphysis, multilocular, endosteal scalloping, chondroma
Learning Point of the Article:
To understand the characteristic features and differentials to facilitate correct diagnosis and management of Diaphyseal Enchondromas presenting with aggressive radiological features.
Introduction
Enchondroma, also known as Chondroma, is composed of mature hyaline cartilage and arise from residual cartilaginous rests that gets displaced from the growth plate and are trapped in the intramedullary canal [1, 2, 3]. Classical enchondromas are located in a central or eccentric position in the metaphysis (71%) or metadiaphysis (21%) and majority (72%) are within 1.5 cm from the growth plate [2, 4, 5]. Even though, it may be seen inside any bone formed by enchondral ossification, it is considered as the most common primary bone tumor in hands and feet (>50%). Among the long bones, it is reported to occur more frequently in the proximal metaphysis of humerus and distal metaphysis of femur (2.0%) [6]. Rarely, it can occur in the proximal metaphysis of tibia (0.7%), fibula (0.2%) or in flat bones such as pelvis and ribs. Enchondromas have characteristic clinico-radiological features which help in easy identification. However, its occurrence at uncommon sites or with atypical features leads to diagnostic dilemma. We report a case of Enchondroma in the tibial diaphysis with atypical features with respect to location and clinico-radiological findings. We discuss the characteristic features of Enchondroma and compare them with similar lesions which could help clinicians to diagnose correctly.
Case Report
A 31-year-old-farmer came for a casual evaluation with inconspicuous pain over left leg of 2 years’ duration. Pain was the intermittent, dull aching and non-radiating. Patient noted mild non-progressive swelling in the area of pain since 3 months which made him come for the consultation. There was no history of trauma, similar swellings in other parts of body, fever, loss of weight, or comorbidities. Skin over the anterior aspect of the left tibia appeared normal on inspection (Fig. 1a). There was no local warmth, but tenderness and irregularity on the anterior aspect of left tibia was noted with normal range of movements of knee and ankle joints and intact distal neurovascular status. Blood investigations were normal. Anteroposterior and lateral radiographs of the left tibia showed multiloculated lytic lesion of about 7 × 3 cm with sclerosed margin, intermingled with areas of cortical thickening, expansion and destruction, and doubtful intralesional calcification in the diaphysis of tibia (Fig. 1b). However, no periosteal reaction or cortical breach was observed. Magnetic resonance imaging (MRI) showed well-defined intramedullary cystic lesion with moderate scalloping of cortex (Fig. 1c, d, e). It also reported focal geographic area of marrow replacement on T1-weighted images and corresponding high signal intensity on T2-weighted images with lobular margins.
Figure 1.
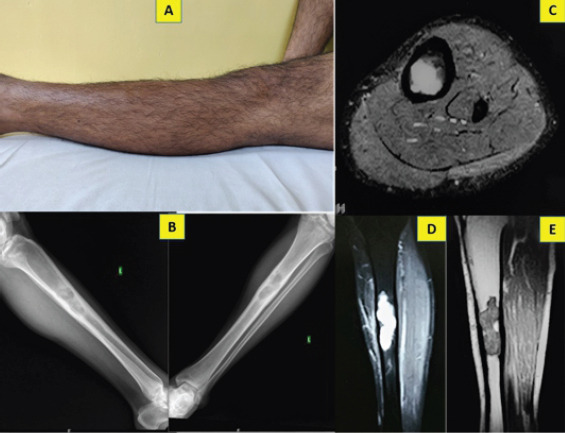
Pre-operative clinical picture of the left leg with no appreciable swelling or skin changes (a); anteroposterior and lateral plain radiographs showing multiloculated, lytic lesion in the diaphysis with cortical thickening and widening of medullary cavity (b); STIR (c, d) and T1-weighted (e) MRI images showing focal area of lobular intramedullary lesion, endosteal scalloping and cortical thickening without extra osseous extension.
Due to the lytic lesion with cortical thickening, erosion, intralesional calcification, moderate scalloping and diaphyseal location we thought of chondrosarcoma, Adamantinoma and Garre’s osteomyelitis. Since the lesion appeared active and to obtain a proper diagnosis, the patient underwent an incisional biopsy from the scalloped region by an anterolateral approach. As the pathologist gave the diagnosis of Enchondroma, the case was further discussed in the tumor board meeting. Despite the aggressive radiological features, as the biopsy specimen did not show any evidence of chondrosarcoma, we went ahead with an extended curettage of the lesion through a 4 cm long cortical window (Fig. 2a, b, c). Since the lesion was large and had the risk of developing pathological fracture, he was advised to undergo bone grafting along with the curettage. However, he declined for the additional procedure.
Figure 2.
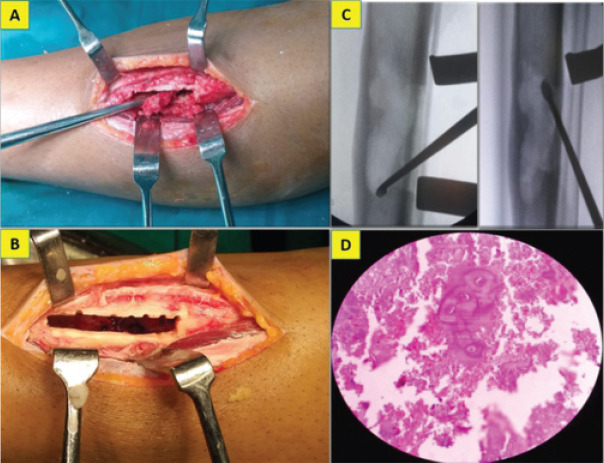
Intraoperative picture showing extended curettage of the lesion (a, b, c) through a cortical window and the pathology slide showing hypo cellularity and lacunar spaces harboring binucleate chondrocytes with intervening chondroid matrix (d).
The gross biopsy specimen appeared greyish brown. Microscopy showed hypocellular chondroid lesion composed of lacunar spaces showing uni-nucleated chondrocytes with small dense nuclei, inconspicuous nucleoli and scant eosinophilic cytoplasm, and occasional bi-nucleated cells with intervening chondroid matrix and focal calcification. Tumor lobules were rimmed by trabeculae of lamellar bone with enclosed marrow showing lymphocytes and plasma cells. No mitotic activity or necrosis was seen (Fig. 2d).
Patient started full weight bearing walking without support after 8 weeks of partial weight bearing. He was kept under regular follow-up and at 2 years after the curettage, he remained asymptomatic with no evidence of local recurrence nor distant metastasis (Fig. 3a, b, c).
Figure 3.
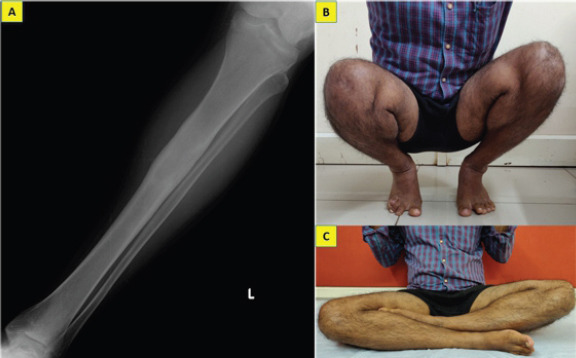
Anteroposterior radiographs of the left tibia taken at 2-year follow-up showing complete healing of the lesion without evidence of recurrence (a) and clinical photograph demonstrating his ability to squat and sit cross-legged normally which are essential in his occupation (b, c).
Discussion
In this unique case, we report the presentation of an atypical enchondroma in the diaphysis of tibia in a young male, which is an uncommon site for occurrence in long bones. Despite the large size of the lesion, the patient underwent only curettage and recovered completely. Enchondroma, which derives its name due to the intramedullary origin is often solitary, though multiple enchrondromatosis may be associated with Ollier’s disease or Maffucci’s syndrome [7]. It accounts for approximately 5% (3–10%) of all bone tumors and 17% (12–24%) of benign bone tumors [8]. It is only second to osteochondroma in frequency. It has no gender predilection and commonly occur in the second decade of life with majority of patients within the 30 and 35-year age group. Our patient also belonged to the susceptible age group. Even though, it can arise sporadically, somatic mutation in isocitrate dehydrogenase (IDH) – 1 and 2 genes have been linked to its occurrence. IDH is an enzymatic component of tricarboxylic acid and mutation in the gene can lead to malfunction and accumulation of D-2-hydroxyglutarate which ultimately leads to inhibition of osteogenic differentiation of mesenchymal stem cells [2, 7]. Most enchondromas are asymptomatic and are picked up incidentally on routine imaging studies. They occasionally cause pain (in around 35% patients) or swelling in the long bones. Our patient had pain over the leg for nearly 2 years and it was only after he noticed some swelling that he thought of seeking medical opinion. However, if the lesion occurs in the small bones of hand and feet or when there are multiple lesions, it can expand and lead to pathological fractures [9]. Rarely, an enchondroma can extend through the cortex and present as an exophytic growth. They are known as enchondroma protuberans and are usually seen sporadically or as part of Ollier’s disease [1].
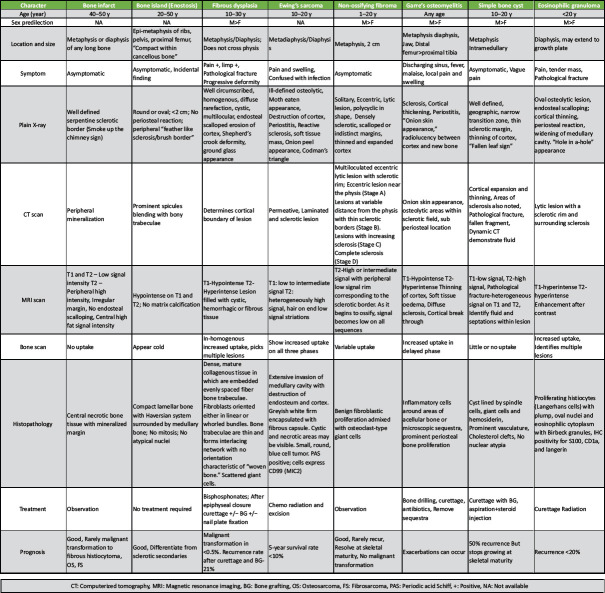
Although characteristic signs to diagnose enchondroma are noted on radiographs, there are overlap of some features with certain diseases and they need to be differentiated from chondrosarcoma, bone infarcts, bone islands, Garre’s sclerosing osteomyelitis, eosinophilic granuloma, fibrous dysplasia and solitary bone cyst (Tables 1, 2) [1, 2, 3, 4, 5, 10, 11, 12, 13].
Table 1.
Differential diagnosis for Enchondroma.
Table 2.
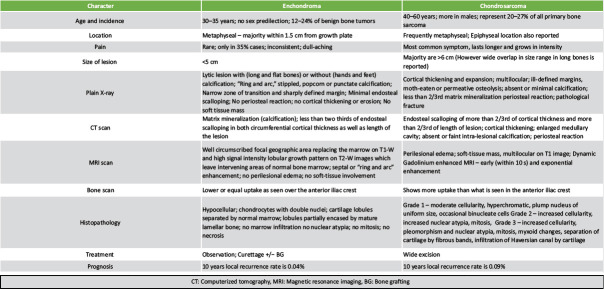
Characteristic differentiating features of enchondroma and chondrosarcoma.
Among all the possibilities, enchondroma needs to be differentiated particularly from chondrosarcoma, as the treatment and prognosis of the two vary a lot (Table 2) [4, 14, 15, 16, 17]. In 2013, the World Health Organization renamed low grade (grade 1) Chondrosarcoma as “atypical cartilaginous tumor” (ACT) [4]. They usually mimic enchondroma and show the ring or stippled calcification similar to enchondroma though they are not sharply circumscribed [4, 10, 11].
Enchondromas are usually <5 cm, have narrow zone of transition with sharply defined margin, mild endosteal scalloping and no cortical destruction, periosteal reaction or soft tissue mass [1, 3, 5, 12]. In hands and feet, the tumor is usually purely lytic without calcification and can show cortical thickening, endosteal scalloping, expansile remodelling and pathological fractures [1].
Thin slice CT scans permit better radiological analysis than plain radiographs as they can appreciate periosteal reaction, endosteal scalloping, cortical breach and soft tissue extension. Murphey et al. based on CT scans had classified endosteal scalloping of the bony cortex with respect to depth and extent of the lesion. Usually enchondromas exhibit less than two-thirds of endosteal scalloping in both circumferential cortical thickness as well as length of the lesion. Tissue samples for biopsy should be taken from these regions to clinch the correct diagnosis [13].
MRI is useful in evaluating soft tissue extension and staging of lesion. In our patient, radiographs showed around 7 cm long multi-lobulated lytic lesions in the diaphysis, areas of cortical thickening and destruction sans intralesional calcification. MRI also showed >50% endosteal scalloping, widening of the medullary cavity and absence of intramedullary calcification. This picture usually mimicked an active lesion or a malignant transformation, despite the absence of periosteal reaction or soft tissue shadow.
Technetium-99 bone scan and Fluro-deoxy-glucose positron emission tomography computerized tomography scan can help in differentiating enchondroma from low and high-grade chondrosarcoma depending on the standardized uptake values. Murphey et al. suggested to compare the radionuclide uptake in the tumor with that over the anterior iliac crest. While majority of cases of chondrosarcoma show increased uptake, enchondromas shows uptake lower or equal to that of anterior iliac crest [4, 13].
Periostin, a stromal-related protein is a biomarker reported to be present in low-grade chondrosarcoma, whereas it is not present in enchondroma. Alpha-methyl acyl-CoA racemase, a mitochondrial and peroxisomal enzyme is expressed in most Enchondromas while it is present only in minority of chondrosarcomas. Further investigation is needed to validate and confirm if these biomarkers are reliable for diagnosis [18].
Enchondromas as mentioned are benign. If there is no pain and the lesion is radiologically inactive, a biopsy is not necessary [19]. However, if radiological investigations show features of aggressive/active lesion, a biopsy is warranted to clinch the diagnosis. Sampling should be done from areas showing endosteal scalloping or cortical thinning as they represent aggressive areas within the tumor [13]. Since we were not sure about the diagnosis and nature of the lesion, we felt it is prudent to do a biopsy and then plan the definitive treatment.
On gross examination, enchondroma which consists of mature hyaline cartilage lobules appear as a well-circumscribed, pale blue, solid lesion with regions of grittiness signifying mineralization with no myxoid changes.
On light microscopy, they are hypocellular and contain chondrocytes with double nuclei. The two important features are cartilage lobules separated by normal marrow and partial encasement by mature lamellar bone [5]. They could be multifocal and rarely have focal necrosis or myxoid changes. However, they do not infiltrate the marrow. Differentiating enchondroma from low-grade chondrosarcoma (ACT) remains difficult as they share similar cellular features [5]. Latter has moderate cellularity and cartilage lobules separated by fibrous tissue rather than bone marrow as in enchondroma [19]. Myxoid changes, infiltration of Haversian canal and cortical erosion favor chondrosarcoma [5].
Majority of the cases of enchondromas which are detected incidentally, of size <5 cm and without pain or radiological evidence of local aggression can be managed non-operatively with serial radiographs and bi-annual MRI scans [6, 14, 20]. MRI is a better modality to assess the growth of the tumor which is an important diagnostic criterion [5, 20, 21].
Lesions which are >5 cm and has associated pain should undergo simple curettage and bone grafting. We too counseled the patient for bone grafting along with curettage, but he was reluctant for the additional procedure. Deckers et al. recommend that small central enchondromas and atypical chondral lesions in long bone should be treated by intralesional curettage and local adjuvant therapy [21]. However, they also report about the complications associated with this modality of treatment such as pathological fracture, infection, and local recurrence. At present, no standardized algorithm for surgical intervention of Enchondroma exists. The timing of surgery has also not shown any significant advantage. Early and delayed surgical intervention was shown to have similar functional outcomes [21].
Compared to an overall risk of <1% in solitary enchondromas, around 5–25% of multiple enchrondromatosis can have malignant transformation to Chondrosarcoma. The incidence of transformation is less in small bone of hand and feet and is highest for lesions in pelvis and shoulder girdle. The 10-year survival rate is around 0.04%. Cortical thickening, endosteal scalloping of >50%, periosteal reaction, soft-tissue mass, and disappearance of calcification in a preexisting enchondroma is highly suggestive of malignant transformation to chondrosarcoma [2, 14, 21]. Our patient also had long lasting pain, recent onset swelling, MRI evidence of cortical thickening, moderate endosteal scalloping and lack of calcification which raised the doubt of malignant transformation.
Conclusion
Although classically found in metaphysis, enchondromas are not uncommon in diaphysis of long bones. Enchondromas are generally benign, but can cause diagnostic dilemma when they present with aggressive features at rare locations and surgeons should be wary of the differentials. Despite a size of more than 6 cm and evidence of cortical erosion and intramedullary widening, the lesion could still be benign. Thorough intralesional curettage would suffice in successfully clearing the atypical enchondromatous lesion.
Clinical Message.
Enchondromas which are generally located in the metaphysis, measuring <5 cm in size and having less than two-third endosteal scalloping can occasionally present as larger lesions with extensive scalloping and periosteal reaction. This should raise the suspicion of chondrosarcoma and biopsy to collect the representative aggressive tissue must be taken from the scalloped area to differentiate the two.
Biography




Footnotes
Conflict of Interest: Nil
Source of Support: Nil
Consent: The authors confirm that informed consent was obtained from the patient for publication of this case report
Declaration of patient consent: The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient's parents have given their consent for patient images and other clinical information to be reported in the journal. The patient's parents understand that his names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
References
- 1.Rasuli B, Gaillard F, Bell DJ. Enchondroma, Radiopaedia, Radiology Reference Article. Available from: https:// www.radiopaedia.org/articles/enchondroma .
- 2.Biondi NL, Varacallo M. Stat Pearls. Treasure Island, FL: Stat Pearls Publishing; 2020. Enchondroma. [Google Scholar]
- 3.Kachnerkar DN. Enchondroma of distal femur treated with curettage and allograft and auto-graft:A rare case study. IOSR J Dent Med Sci. 2017;16:103–6. [Google Scholar]
- 4.Walden MJ, Murphey MD, Vidal JA. Incidental enchondromas of the knee. Am J Roentgenol. 2008;190:1611–5. doi: 10.2214/AJR.07.2796. [DOI] [PubMed] [Google Scholar]
- 5.Jamshidi K, Mirzaei A. Long bone enchondroma vs. low-grade chondrosarcoma:Current concepts review. Shafa Ortho J. 2017;4:e9155. Available from: http://www.shafaorthoj.com/ en/articles/9155.html . [Google Scholar]
- 6.Campanacci DA, Scoccianti G, Franchi A, Roselli G, Beltrami G, Ippolito M, et al. Surgical treatment of central grade 1 chondrosarcoma of the appendicular skeleton. J Orthop Traumatol. 2013;14:101–7. doi: 10.1007/s10195-013-0230-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amary MF, Bacsi K, Maggiani F, Damato S, Halai D, Berisha F, et al. IDH1 and IDH2 mutations are frequent events in central chondrosarcoma and central and periosteal chondromas but not in other mesenchymal tumours. J Pathol. 2011;224:334–43. doi: 10.1002/path.2913. [DOI] [PubMed] [Google Scholar]
- 8.Mulligan ME. How to diagnose enchondroma, bone infarct, and chondrosarcoma. Curr Probl Diagn Radiol. 2019;48:262–73. doi: 10.1067/j.cpradiol.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Quinn RH, Rajani R. Enchondroma- OrthoInfo- AAOS. Available from: https://www.orthoinfo.org/en/ diseases--conditions/enchondroma .
- 10.Geirnaerdt MJ, Hermans J, Bloem JL, Kroon HM, Pope TL, Taminiau AH, et al. Usefulness of radiography in differentiating enchondroma from central grade 1 chondrosarcoma. Am J Roentgenol. 1997;169:1097–104. doi: 10.2214/ajr.169.4.9308471. [DOI] [PubMed] [Google Scholar]
- 11.Kendell SD, Collins MS, Adkins MC, Sundaram M, Unni KK. Radiographic differentiation of enchondroma from low-grade chondrosarcoma in the fibula. Skeletal Radiol. 2004;33:458–66. doi: 10.1007/s00256-004-0791-9. [DOI] [PubMed] [Google Scholar]
- 12.Mahajan S, Bodei L, Castellanos SH, Grewal RK. Enchondroma of tibia as potential false-positive finding on 68Ga-DOTATOC PET/CT scan. Clin Nucl Med. 2019;44:e57–9. doi: 10.1097/RLU.0000000000002360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphey MD, Flemming DJ, Boyea SR, Bojescul JA, Sweet DE, Temple HT. Enchondroma versus chondrosarcoma in the appendicular skeleton:Differentiating features. Radiographics. 1998;18:1213–37. doi: 10.1148/radiographics.18.5.9747616. [DOI] [PubMed] [Google Scholar]
- 14.Herget GW, Strohm P, Rottenburger C, Kontny U, Krauß T, Bohm J, et al. Insights into enchondroma, enchondromatosis and the risk of secondary chondrosarcoma. Review of the literature with an emphasis on the clinical behavior, radiology, malignant transformation and the follow up. Neoplasma. 2014;61:365–78. doi: 10.4149/neo_2014_046. [DOI] [PubMed] [Google Scholar]
- 15.DiCaprio MR, Enneking WF. Fibrous dysplasia. Pathophysiology, evaluation, and treatment. J Bone Joint Surg Am. 2005;87:1848–64. doi: 10.2106/JBJS.D.02942. [DOI] [PubMed] [Google Scholar]
- 16.Angelini A, Mavrogenis AF, Rimondi E, Rossi G, Ruggieri P. Current concepts for the diagnosis and management of eosinophilic granuloma of bone. J Orthop Traumatol. 2017;18:83–90. doi: 10.1007/s10195-016-0434-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balamuth NJ, Womer RB. Ewing's sarcoma. Lancet Oncol. 2010;11:184–92. doi: 10.1016/S1470-2045(09)70286-4. [DOI] [PubMed] [Google Scholar]
- 18.Jeong W, Kim HJ. Biomarkers of chondrosarcoma. J Clin Pathol. 2018;71:579–83. doi: 10.1136/jclinpath-2018-205071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bauer HCF, Brosjö O, Kreicbergs A, Lindholm J. Low risk of recurrence of enchondroma and low-grade chondrosarcoma in extremities:80 patients followed for 2-25 years. Acta Orthop Scand. 1995;66:283–8. doi: 10.3109/17453679508995543. [DOI] [PubMed] [Google Scholar]
- 20.Eriksson AI, Schiller A, Mankin HJ. The management of chondrosarcoma of bone. Clin Orthop Relat Res. 1980;153:44–66. [PubMed] [Google Scholar]
- 21.Deckers C, Schreuder BHW, Hannink G, de Rooy JW, van der Geest IC. Radiologic follow-up of untreated enchondroma and atypical cartilaginous tumors in the long bones:The natural course of chondroid tumors. J Surg Oncol. 2016;114:987–91. doi: 10.1002/jso.24465. [DOI] [PMC free article] [PubMed] [Google Scholar]