Abstract
Purpose:
The number of cancer survivors worldwide is growing, with over 15.5 million cancer survivors in the United States alone – a figure expected to double in the coming decades. Cancer survivors face unique health challenges as a result of their cancer diagnosis and the impact of treatments on their physical and mental well-being. For example, cancer survivors often experience declines in physical functioning and quality of life, while facing an increased risk of cancer recurrence and all-cause mortality compared to persons without cancer. The 2010 American College of Sports Medicine Roundtable was among the first reports to conclude that cancer survivors could safely engage in enough exercise training to improve physical fitness and restore physical functioning, enhance quality of life, and mitigate cancer-related fatigue.
Methods:
A second Roundtable was convened in 2018 to advance exercise recommendations beyond public health guidelines and toward prescriptive programs specific to cancer type, treatments and/or outcomes.
Results:
Overall findings retained the conclusions that exercise training and testing was generally safe for cancer survivors and that every survivor should “avoid inactivity”. Enough evidence was available to conclude that specific doses of aerobic, combined aerobic plus resistance training, and/or resistance training could improve common cancer-related health outcomes, including anxiety, depressive symptoms, fatigue, physical functioning, and health-related quality of life. Implications for other outcomes, such as peripheral neuropathy and cognitive functioning, remain uncertain.
Conclusions:
The proposed recommendations should serve as a guide for the fitness and health care professional working with cancer survivors. More research is needed to fill remaining gaps in knowledge in order to better serve cancer survivors, as well as fitness and healthcare professionals, to improve clinical practice.
Keywords: cancer survivorship, exercise prescription, safety
1. INTRODUCTION
In the last decade, the United States has seen a 27% decline in cancer deaths due to early detection and improved treatments for cancer. In turn, the number of cancer survivors is growing, with over 15.5 million cancer survivors in the United States - a figure that is expected to double by 2040(1). Improved prognosis has created a growing need to address the unique health issues facing cancer survivors that result from the disease, its treatment, and related comorbid conditions. For example, the symptom of fatigue can persist in 25% of cancer survivors many years after their treatment has ended and contributes to difficulty returning to work, independent living and poor quality of life(2). Furthermore, risk of developing of heart disease may be elevated by some cancer treatments and cardiovascular mortality is emerging as a major competing cause of death in cancer survivors along with cancer recurrence(3, 4). Cancer is also a disease strongly linked with aging, and almost half of survivors are older than 70 years(5). The adverse synergistic effects of age, cancer treatment, and related sequelae increase the total burden of cancer. Historically, clinicians advised cancer patients to rest and to avoid physical activity, but early exercise research in the 1990’s and 2000’s challenged this advice.
In 2010 the American College of Sports Medicine convened a Roundtable meeting composed of a team of clinical and research experts in the field of cancer and exercise to develop the first set of exercise guidelines for cancer survivors(6). Drawing on studies mainly in breast and prostate cancer survivors, the key findings from this review were that exercise training was generally safe and well tolerated during and after cancer treatment and could elicit improvements in some health outcomes. There was sufficient evidence to conclude that exercise could improve physical fitness, physical functioning, quality of life, and cancer-related fatigue. However, given the limited number of rigorously designed studies at that time, there was insufficient evidence to inform specific exercise prescriptions for any of these outcomes nor by cancer site or treatment type. Thus, the 2010 Roundtable recommendations largely followed the 2008 Physical Activity Guidelines for adults with chronic conditions to aim for at least 150 minutes per week of aerobic activity, two or more days a week of resistance training, and stretch major muscles groups daily when possible, with specific exercise testing and program modifications based on health status and cancer and treatment-related side effects(7). At a minimum, survivors were urged to “avoid inactivity” and be as physically active as possible(6).
The 2010 ACSM recommendations came with the expectation that they would be updated as the evidence grew and indeed, since that landmark publication the number of randomized controlled exercise trials in cancer survivors has increased by 281% (PubMed Search completed March 2018) to over 2500 published randomized controlled trials. Moreover, an increasing number of calls for the integration of exercise into clinical cancer care have since been issued (8–11). Thus, in 2018 the ACSM International, Multidisciplinary Roundtable on Physical Activity and Cancer Prevention and Control was convened to bring together an international group of exercise and rehabilitation professionals and organizations with the goal to update recommendations based on current evidence. The Roundtable meeting took place on March 12–13, 2018 in San Francisco, California, USA, with 40 representatives from twenty organizations across the world who came together to sponsor and attend this meeting (Table 1). Roundtable members were invited to participate based on their clinical and scientific expertise and were asked to contribute in one or more of the following areas: 1) Role of exercise in cancer prevention and control; 2) Efficacy of exercise to improve cancer-related health outcomes (acute, late and long-term effects); and, 3) Translation of evidence into the clinical and community settings. The outcome of the work in each of these areas would be three separate, but related publications. The manuscripts were circulated to professional organizations for review and to obtain their official endorsement.
Table 1.
Participating organizations
| American College of Sports Medicine (ACSM) *, † | Exercise and Sports Science Australia *, † |
| American Cancer Society (ACS) *, † | German Union for Health Exercise and Exercise Therapy (DVGS) *, † |
| American Academy of Physical Medicine and Rehabilitation (AAPMR)*, † | MacMillan *, † |
| American Physical Therapy Association (APTA)* | National Cancer Institute (U.S.) |
| American Congress of Rehabilitation Medicine | National Comprehensive Cancer Network † |
| American College of Lifestyle Medicine † | Royal Dutch Society for Physical Therapy (KVDP) *, † |
| Canadian Society for Exercise Physiology *, † | Society for Behavioral Medicine † |
| Centers for Disease Control † | Sunflower Wellness *, † |
| Commission on Accreditation of Rehabilitation Facilities † |
Legend
Roundtable Partner Organizations
Organizations that provided official endorsement
This paper will update evidence-based guidelines for exercise testing, prescription and delivery in cancer survivors. As it was acknowledged that the general exercise recommendations put forward in 2010 may be unachievable for cancer survivors with physical limitations and that benefits may come from less exercise, a particular goal of the 2018 Roundtable was to develop more granular exercise prescriptions for distinct cancer-related health outcomes to better guide fitness and other healthcare professionals who train or care for cancer survivors. In the following sections we: 1) describe the evidence review process and decisions for generating exercise prescriptions for specific cancer-related health outcomes; 2) provide evidence-based FITT (frequency, intensity, time, type) prescriptions for outcomes with sufficient evidence (as outlined below); and 3) provide updates to the 2010 guidelines around exercise testing and training, including special considerations and safety precautions, specific to cancer survivors. We conclude by acknowledging the limitations of this latest Roundtable and suggest directions for future updates.
2. UPDATE TO EVIDENCE-INFORMED EXERCISE PRESCRIPTIONS
2.1. Overview
Two a priori decisions were made by consensus at the Roundtable Meeting. The first was to develop a list of cancer-related health outcomes with a high degree of clinical relevance for which exercise may have therapeutic benefit (BOX 1). The second was to focus the review of evidence primarily on traditional modalities of exercise including aerobic, resistance or combined aerobic plus resistance training on relevant health outcomes. A brief discussion on other modalities of exercise (e.g., yoga) is provided at the end of the paper. Three additional decisions were made by the writing team early on in the writing process where it was agreed upon by consensus that: 1) components of physical fitness (e.g., aerobic capacity, muscular strength/endurance) would not be categorized as cancer-health related outcomes, but would be used to evaluate the adaptability and responsiveness of cancer survivors to specific modes of exercise training; 2) exercise prescriptions would only be generated for outcomes where there was sufficient evidence on the efficacy of exercise to improve a given outcome; 3) beyond each outcome, the exercise prescriptions could not be further specified by tumor type, phase of treatment or type of treatment due to the lack of sufficient evidence to do so in a robust manner. The implications of these limitations are discussed later in the paper.
Box 1. List of common acute, long term and late effects of cancer for review of evidence for therapeutic efficacy of exercise and subsequent exercise prescriptions.
| • Anxiety |
| • Bone health |
| • Cardiotoxicity |
| • Chemotherapy-induced peripheral neuropathy |
| • Cognitive function |
| • Depressive symptoms |
| • Falls |
| • Fatigue |
| • Health-related quality of life |
| • Lymphedema |
| • Nausea |
| • Pain |
| • Physical function |
| • Sexual function |
| • Sleep |
| • Treatment tolerance |
2.2. Methodology for Evidence Review
To efficiently evaluate and provide a rich synthesis of the evidence, a review of published randomized controlled trials, systematic reviews and meta-analyses for cancer-related health outcomes (Box 1) using Medline/PubMed, EMBASE, the Cochrane Central Register of Controlled Trials, CINAHL, the Physical Therapy Evidence Database (PEDro), and the Cochrane Collaboration. A search was conducted from June-August 2018, for papers published as of June 1, 2018, using standardized search terms for cancer and exercise, in combination with search terms for the list of key cancer-related health outcomes (Supplemental Materials). For each outcome, two writing team members reviewed the resulting systematic reviews and meta-analyses to identify the most recent, relevant, and high quality publications that could facilitate evaluation of the state of science around efficacy of exercise for a particular outcome. If no systematic reviews and meta-analyses were identified, the available RCTs were reviewed.
A decision framework adapted from the Dutch Physical Activity Guidelines(12) was applied to first determine whether or not there was sufficiently strong evidence to conclude that exercise improved specific outcomes and in turn, warrant generation of an evidence-based FITT prescription (Figure 1). Evidence for a given outcome was judged to be strong when there were a substantial number of RCTs (≥5), the aggregate sample size was large (n>150), and the beneficial effect of exercise was observed consistently across studies. For outcomes with a smaller number of RCTs (<5) or where the overall effect of exercise was inconsistently observed or null, the level of evidence for an exercise benefit was judged as insufficient. For such cases, a FITT prescription was not generated and only a summary of current evidence and research recommendations were provided.
FIGURE 1:
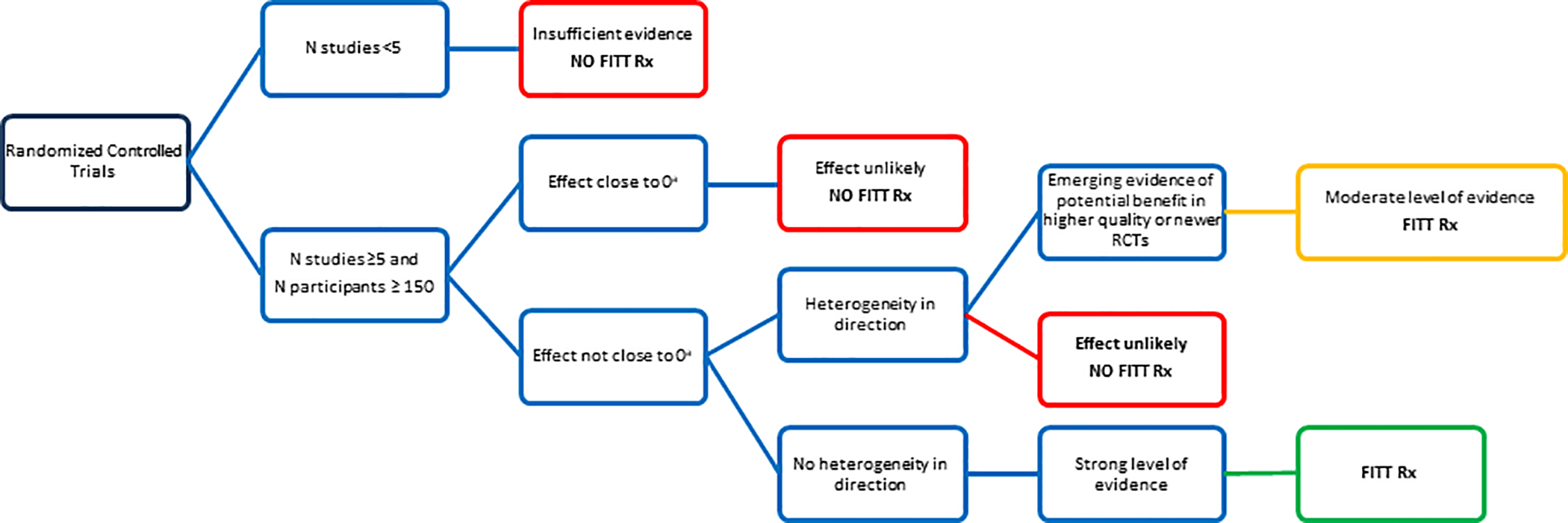
Decision tree on the level of evidence for effects (RCT) sufficient to merit an exercise prescription (FITT Rx). aAdapted with permission from Weggemans RM, Backx FJG, Borghouts L, et al. The 2017 Dutch Physical Activity Guidelines. Int J Behav Nutr Phys Act. 2018;15(1):58.
During the review process, authors felt that an intermediate category should be considered for outcomes with >5 RCTs and an aggregate sample >150, yet heterogeneity for evidence of an effect in cancer survivors. In these cases, if there were a consistent number of high quality RCTs (designed and powered on the outcome of interest as the primary endpoint) and that reported findings that were congruent with established evidence on the same outcome in non-cancer populations, the evidence of exercise benefit for these outcomes was judged as moderate and a FITT prescription was generated. However, more research would be needed to confirm the exercise recommendation for these outcomes in cancer survivors.
The next step was to define exercise prescriptions that conformed to the FITT formula and outlined the type and dose of exercise expected to improve a given outcome. To streamline the process, the evidence used to inform each exercise prescription was derived from high quality systematic reviews and meta-analyses which also often provided separate analyses on efficacy by FITT components to identify the most optimal exercise type, time, intensity or frequency to improve a given outcome. However, if the quality of systematic reviews was deemed low (AMSTAR rating <7), individual high-quality RCTs were used to derive the prescription. FITT prescriptions and accompanying summary of the literature, which informed them, were presented to the full writing team during a series of conference calls for discussion and expert consensus (September – October 2018).
2.3. Strong Evidence
For the following cancer-related health outcomes, there was consensus that benefit has been consistently demonstrated and a FITT prescription was developed. Details of each FITT prescription, including any unique considerations, by outcome are provided in Table 2. When interpreting and applying a prescription, the exercise professional should be mindful that the evidence is often disproportionately from trials in a single cancer type (i.e., breast), but the prescription is assumed to generalize across cancer types unless otherwise specified. In addition, studies often did not specifically target enrollment of individuals with the poorest initial values of an outcome (e.g, high fatigue, low bone density) thus the efficacy of a FITT prescription may or may not generalize to cancer survivors in greatest need.
Table 2.
Cancer-related health outcome with sufficient evidence for development of FITT prescription
| Outcome | Type† | Intensity | Duration (min) or Sets (reps) | Frequency (sessions per week) | Length (weeks) | Setting (supervised, home-based or combination) | Dose response | Special considerations | Evidence primarily from these cancer types. |
|---|---|---|---|---|---|---|---|---|---|
| Anxiety | Aerobic | 60–80% HRmax 60–80% VO2max RPE 13–15 |
30–60 | 3 | 12 | Supervised more effective | Moderate to vigorous may be more effective than light to moderate | Not known | Breast (majority), prostate, colorectal, gynecological (ovarian, endometrial, cervical), head and neck, lung, hematological cancer |
| Resistance | Efficacy not demonstrated | NA | NA | NA | NA | NA | NA | ||
| Aerobic + Resistance | 60–80% HRmax 60–80% VO2max RPE 13–15 |
20–40 | 2–3 | 6–12 | Supervised or combination of supervised & home-based | None observed | Not known | ||
| 65–85% 1RM | 2 sets 8–12 reps |
2–3 | 6–12 | ||||||
| Depressive Symptoms | Aerobic | 60–80% HRmax 60–80% VO2max RPE 13–15 |
30–60 | 3 | 12 | Supervised more effective | Benefit up to 180 min/wk | Not known | Breast (majority), prostate, colorectal, hematological |
| Resistance | Efficacy not demonstrated | NA | NA | NA | NA | NA | NA | ||
| Aerobic + Resistance | 60–80% HRmax 60–80% VO2max RPE 13–15 |
20–40 | 3 | 12 | Supervised or combination of supervised & home-based | None observed | Not known | ||
| 65–85% 1RM | 2 sets 8–12 reps |
2–3 | 6–12 | ||||||
| Fatigue | Aerobic | 65% HRmax 45% VO2max RPE 12 |
30 | 3 | 12 | Supervised and unsupervised appear similarly effective | No dose response by intensity; possible > benefits with ↑ duration & length of program | No evidence of benefits from light intensity | Breast (majority), prostate, mixed |
| Resistance | 60% 1RM RPE 12 |
2 sets 12–15 reps |
2 | 12 | Supervised and unsupervised appear similarly effective | None observed | Not known | ||
| Aerobic + Resistance | 65% HRmax 45% VO2max RPE 12 |
30 | 3 | 12 | Supervised and unsupervised appear similarly effective | None observed | Not known | ||
| 60% 1RM RPE 12 |
2 sets 12–15 reps |
2 | 12 | ||||||
| Health-related Quality of Life | Aerobic | 60–80% HRmax RPE 11–13 |
30 | 2–3 | 12 | Supervised more effective | None observed | NA | Breast (majority), prostate, colorectal, lung, head and neck, bladder, gynecological, mixed, hematological cancer, hematopoietic stem cell transplant |
| Resistance | 60–75% 1 RM RPE 13–15 |
2–3 sets 8–15 reps |
2–3 | 12 | Supervised or combination of supervised & home-based | None observed | NA | ||
| Aerobic + Resistance | 60–80% HRmax RPE 11–13 |
20–30 | 2–3 | 12 | Supervised more effective | None observed | AT & RT combined most effective | ||
| 60–80% 1RM RPE 12–14 |
2 sets 8–15 reps |
2–3 | 12 | ||||||
| Lymphedema* | Aerobic | NA | NA | NA | NA | NA | NA | Generally safe, as no significant increase in number of lymphedema-related adverse events reported in RCT of aerobic exercise | Breast cancer-related lymphedema only |
| Resistance | 60–70% 1RM RPE 15 |
1–3 sets 8–15 reps |
2–3 | 52 | All of the interventions reviewed started with supervision to teach the exercises. | NA | Start resistance a low weight and progress slowly | ||
| Aerobic + Resistance | NA | NA | NA | NA | NA | NA | NA | ||
| Physical Function | Aerobic | 60–85% HRmax 60–85% VO2max RPE 12–13 |
30–60 | 3 | 8–12 | Supervised more effective | If unsupervised, requires higher weekly exercise expenditure (unclear what the threshold is) | NA | Breast (majority), prostate, colorectal, lung, bladder, head and neck, hematological cancer, hematopoietic stem cell transplant |
| Resistance | 60–75% 1RM RPE 13–15 |
2 sets 8–12 reps |
2–3 | 8–12 | Limited evidence to determine benefit of unsupervised | NA | NA | ||
| Aerobic + Resistance | 60–85% HRmax 60–85% VO2max RPE 12–13 |
20–40 | 3 | 8–12 | Both supervised & home-base suitable in older adults | NA | Community-based interventions that met in groups & used behaviour change strategies may produce larger effects in older adults | ||
| 60–75% 1RM RPE 13–15 |
2 sets 8–12 reps |
2–3 | 8–12 |
Mode: For aerobic activity, this includes walking, cycling ergometer and other forms of traditional aerobic exercise; for resistance, this includes machine-based and free weights.
Legend
recommendation for breast cancer-related lymphedema only.
Abbreviations: NA, not applicable.
2.3.1. Anxiety
A dose of moderate intensity aerobic training three times per week for 12 weeks or twice weekly combined aerobic plus resistance training for 6–12 weeks can significantly reduce anxiety in cancer survivors during and after treatment(13–17). Based on sufficient evidence, it does not appear that resistance training alone reduces anxiety. There is not sufficient evidence to determine whether or not there is a dose response relationship between exercise intensity and changes in anxiety. Improvements in anxiety appear to be greater in supervised training programs or those having a larger supervised component than those that are predominantly unsupervised or home-based.
2.3.2. Depressive Symptoms.
Moderate intensity aerobic training performed three times per week and for at least 12 weeks or twice weekly combined aerobic plus resistance training lasting 6–12 weeks can significantly reduce depressive symptoms in cancer survivors during and after treatment(18, 19). Based on sufficient evidence, resistance training alone does not seem to be effective for this outcome. Based on results from high quality trials of aerobic training, there may be a dose-response effect where higher volumes of aerobic exercise (90 min vs. up to 180 min/week) leads to better symptom reduction. Improvements in depressive symptoms appear to be greater in supervised training programs or those having a larger supervised component than those that are predominantly unsupervised or home-based.
2.3.3. Fatigue
For training programs that last at least 12 weeks, engaging in moderate intensity aerobic training three times per week can significantly reduce cancer-related fatigue both during and after treatment(20–23). Moderate intensity combined aerobic plus resistance training sessions performed 2–3 times per week or twice weekly moderate intensity resistance training may also be effective(21, 24–26), and the latter particularly in prostate cancer(27). The effect of exercise was strongest for moderate to vigorous intensity exercise whereas the effect for low intensity training was weak and this level of exercise is unlikely to reduce fatigue(22, 24, 26, 28). Whether or not more exercise translates to less cancer-related fatigue remains unclear, though there is suggestive evidence that the reductions in fatigue are greater with exercise sessions longer than 30 minutes and programs longer than 12 weeks compared to less exercise(29). There is insufficient evidence for a linear dose response since going beyond 150 minutes per week of aerobic exercise does not appear to result in the greatest reductions in fatigue. The efficacy of exercise for this outcome appears to be independent of the level of supervision and/or setting for training(21, 27, 30).
2.3.4. Health-related Quality of Life (HRQoL)
Combined moderate intensity aerobic and resistance exercise performed 2–3 times per week for at least 12 weeks results in improvements in HRQoL both during and after treatment(17, 31, 32). The benefit of combined aerobic plus resistance training programs appears more potent than programs consisting of only aerobic or resistance training(32). Though enough evidence favored the efficacy of exercise to improve HRQoL, it should be noted that this particular outcome is a construct that encompasses many factors and may have a more variable response than by individual domains. For example, improvements seems to be more robust if the physical functioning domain of HRQoL is the primary outcome and this will be covered separately. Improvements in HRQoL appear to be greater in supervised training programs or those having a larger supervised component than those that are predominantly unsupervised or home-based.
2.3.5. Lymphedema
There is a history of clinical recommendations to refrain from aerobic or resistance training in order to avoid onset or exacerbation of lymphedema(33, 34). For this particular outcome, an evidence-based exercise prescription is designed for safety or no harm, versus the benefit of exercise to prevent lymphedema or improve lymphedema symptoms, and limited to addressing upper extremity breast cancer-related lymphedema(35–37). For resistance training, a general progressive program focused on large muscle groups performed 2–3 times per week, with the principle of “start low, progress slow” is safe when supervised by a fitness professional(38–42). Insufficient evidence exists to conclude whether or not starting a resistance training program without supervised instruction is safe for women with or at risk for lymphedema after breast cancer. To date, there is insufficient evidence to draw conclusions for aerobic exercise. In general, aerobic exercise seems to be safe, with no significant increase in number of lymphedema-related adverse events reported in studies investigating aerobic exercise(17). Based on preliminary evidence(43), the effects seen in breast cancer may not translate for lymphedema following head and neck, bladder, melanoma, gynecologic, or other cancer sites.
2.3.6. Physical Function
Moderate intensity aerobic training, resistance training or combined aerobic plus resistance training performed three times weekly for 8–12 weeks can significantly improve self-reported physical function(31, 32, 44). Broadly, supervised exercise appears to be more effective than unsupervised or home-based interventions(32), although unsupervised programs may be effective in older cancer survivors(44). Further, there is some evidence to suggest that if the intervention is unsupervised, physical function could improve with higher weekly energy expenditure (MET-hr/week) but not weekly exercise duration (min/week)(32). It should be noted that these results are based on self-reported physical function, not objective measures where the evidence base on these outcomes remains immature and more challenging to aggregate due to the variation and limitations of assessment techniques.
2.4. Moderate Evidence
2.4.1. Bone Health
Two recent systematic reviews in cancer survivors concluded that across all trials the evidence for exercise to improve bone health is inconsistent(45, 46), though RCTs that were designed with bone health as the primary outcome were largely consistent with the exercise recommendations in the ACSM Position Stand for exercise and bone health(47). In cancer, the majority of evidence is derived from trials in breast and prostate cancer patients in the post-adjuvant treatment setting, which indicates that a one-year supervised program of combined moderate-vigorous intensity resistance plus high impact training (i.e., exercise that generates ground reaction forces above 3–4 times body weight) performed 2–3 days per week is the most consistently efficacious modality of exercise to improve bone health (e.g., slow loss or slightly improve bone mineral density at the lumbar spine and hip). In contrast, aerobic training, particularly walking, does not appear to provide a sufficient stimulus to improve bone outcomes, results that are consistent with RCTs in persons without cancer. There is insufficient evidence to determine if resistance training alone improves bone outcomes.
In non-cancer populations, whether or not resistance plus impact training programs are safe for individuals with osteoporosis remains controversial(48), thus at this time this exercise prescription may not be safe for cancer survivors with bone fragility associated with osteoporosis or bony metastases in the hip or spine (see section 5). Furthermore, it may not be appropriate for individuals with joint/orthopedic issues and/or stability problems who may be better served by an exercise program aimed at reducing fall risk. Further research is needed to confirm that this recommendation is effective and safe for cancer survivors.
2.4.2. Sleep
Two recent systematic reviews in cancer survivors(49, 50) provided mixed evidence for overall sleep quality, indicating either a positive effect of walking(49), or no effect of exercise(50). Four recent RCTs not included in either systematic review have shown consistent evidence of small to moderate effect sizes on overall sleep quality for aerobic training(51), in addition to walking specifically(52, 53) and one study reported evidence of benefit for resistance training(31). In non-cancer populations, there is strong evidence that moderate to vigorous intensity aerobic training is associated with better overall sleep quality in the general population(54) and there was some evidence showing benefit for specific characteristics of sleep, such as total sleep time, sleep onset latency, and sleep efficiency. Overall, moderate intensity aerobic training, particularly walking, 3–4 times per week, for 30–40 minutes per session over 12 weeks is recommended.
2.5. Insufficient Evidence
Insufficient evidence for a specific outcome does not mean that cancer survivors facing these cancer-related health outcomes will not benefit in other ways from engaging in physical activity or should remain sedentary. Rather there is insufficient evidence showing that exercise is beneficial for these specific outcomes based on current evidence and this creates an obvious gap in knowledge the research community must fill.
2.5.1. Cardiotoxicity
The effect of exercise to prevent or ameliorate cardiotoxicity is an emerging field of research and promising results for a protective effect of exercise in animal models and some novel evidence in humans for cardiac function(55), including measures of left ventricular function(55), and vascular endothelial function, measured as flow-mediated dilation(56). More research is needed to understand the impact of various types of cancer, cancer treatments and exercise prescriptions on both cardiac and vascular function.
2.5.2. Chemotherapy induced peripheral neuropathy
To date, there are too few high quality trials to interpret the potential of the benefits of exercise for preventing and/or managing chemotherapy-induced peripheral neuropathy and related side effects, such as balance impairment and falls(57). In general, exercise appeared safe in the few published studies, however, the degree of improvement varied for this outcome, and other related outcomes, such as mobility and balance. Future research should include well controlled exercise interventions with chemotherapy-induced peripheral neuropathy as a primary outcome, using both patient-reported outcomes and objective assessment of neuropathy, balance and mobility, and also rigorously evaluate the safety of training in this group due to the known risk of falls.
2.5.3. Cognitive function
While promising results from animal studies are emerging for a protective effect of aerobic training on cancer treatment related changes in cognitive function, to date, the evidence in humans is limited(58). The majority of human studies, so far, have been conducted in breast cancer survivors, using self-reported measures of cognitive function, and report inconsistent results. While there is compelling evidence of a positive effect of exercise on cognitive function in older adults and other clinical populations(59), specific to cancer, more research is needed in cancer survivors where cognitive function is the primary outcome, and this outcome is assessed by both self-reported and standardized objective measures of cognitive function.
2.5.4. Falls
There are no randomized controlled trials to date in cancer survivors with falls as a primary endpoint. There are several challenges to this type of research, including the relatively rare occurrence of falls and the large sample and time needed to observe a change in falls from an intervention. Similarly, the causes of falls associated with cancer treatment have not been fully characterized and may be due to more than an acceleration of the risks associated with age-related falls (i.e., muscle weakness and poor balance), but may also be due to treatment-related toxicities such as hearing loss, ataxia, peripheral neuropathies and fatigue, creating a challenge to develop new exercise-based approaches to fall prevention. In the absence of any evidence-based fall prevention studies in cancer survivors, it seems reasonable to consider standard fall prevention exercise approaches that reduce the risk of age-related falls for cancer survivors with a fall history to at least reduce the risk of falls that may be associated with advanced age(60, 61).
2.5.5. Nausea
While a commonly reported benefit of exercise during chemotherapy, there is limited data from high quality trials with nausea as a primary endpoint to support this finding outside of an early study demonstrating a reduction by Winningham et al.(62). Future research could examine the effect of well controlled exercise interventions on nausea in highly emetogenic regimens to determine if there is an effect. These studies should account for antiemetic use, duration and intensity of nausea, and function.
2.5.6. Pain
To date most published controlled trials in cancer survivors have examined non-specific pain, and included pain as a secondary outcome, which limits the interpretation of the research. There is early evidence from two high-quality controlled trials where pain was the primary outcome that a combined home-based aerobic plus supervised resistance training intervention in women with breast cancer significantly reduced arthralgia associated with aromatase inhibiter therapy(63) and a supervised resistance training intervention focused on the upper extremity significantly reduced in shoulder pain in individuals with head and neck cancer(64). However, more research is needed specifically focused on cancer-related pain. Survivors may be able to exercise with pain that is tolerable and not worsened by exercise; however, modification or omission of individual exercises that exacerbate pain may be necessary.
2.5.7. Sexual Function
The majority of research to date has been in men with prostate cancer(65, 66) and there is insufficient evidence from controlled trials investigating the effect of exercise on sexual function during or after cancer therapy. Early promising results for a positive effect of exercise on sexual function among prostate cancer patients treated with androgen deprivation therapy has been reported in some trials(67, 68) but not others(69). While there is compelling evidence of a positive effect of exercise on sexual function in the general population, for both women(70) and men(71), the significant effects of cancer therapies on hormonal and anatomical/functional changes (i.e., nerve sparing versus non-nerve sparing surgeries in prostate cancer) preclude the ability to extrapolate those findings to the cancer population.
2.5.8. Treatment Tolerance
Treatment tolerance (i.e., completion of or adherence to planned therapy) is a complex outcome that likely varies by cancer type, treatment modality (i.e., radiation therapy, chemotherapy, hormone therapy, immunotherapy), and even specific drug(s) and protocols. Consequently, determining the effects of exercise on “treatment tolerance” is a challenging goal and may make it difficult to achieve a high degree of generalizability. A recent systematic review of exercise and chemotherapy completion rate concluded that, while promising, the evidence for an exercise benefit to chemotherapy tolerance is insufficient(72). The effects of exercise on treatment tolerance for radiation therapy, hormonal therapy (i.e., aromatase inhibitors or androgen deprivation therapy), targeted therapies or immunotherapy is currently unknown. To understand the effect of exercise on treatment tolerance, studies would need to be conducted for each combination of cancer type and treatment regimen.
2.6. Limitations
The FITT prescriptions provided for several outcomes aim to serve as guidelines for fitness and healthcare professionals working with cancer survivors. However, the development of these guidelines had some key limitations that should be kept in mind when working with individual clients. The majority of available literature is still in the most common cancers, namely early stage breast cancer and prostate cancers, which limits the ability to extrapolate to other cancer types or advanced cancers. Though, it could be reasonably assumed that in the absence of any unique safety concerns for survivors of other types of cancer that the efficacy of exercise on various outcomes would be similar for survivors with early stage cancers other than breast and prostate cancer. However, differences among cancer survivors by cancer type are known to exist (i.e., demographics, prognosis, treatments received and associated side effects) and further, there is very little information regarding the feasibility, safety or benefits of exercise in individuals living with advanced cancer. More research is needed to advance the level of specificity available in the exercise oncology literature to serve a broader range of cancer types and stages(73). In addition, there has historically been incomplete reporting of compliance to a prescribed FITT program in published studies, likely because tracking and quantifying compliance can be burdensome (74–76). Thus, it remains possible that some proportion of cancer survivors may not be able to tolerate the evidence-based FITT, as explicit reporting of adjustments to the exercise prescription (i.e., dose modification) based on tolerance of individuals has been limited. Thus, the fitness or healthcare professional should monitor for early signs of poor tolerance to training and adjust the dose of exercise accordingly even if this means dropping below recommended training volumes. Furthermore, understanding the specific efficacy of exercise for a particular outcome is hampered by the fact that the majority of research to date does not consider the training principle of initial values by limiting enrollment to individuals experiencing the specific outcome of interest (i.e., those with sleep issues or high level of fatigue), rarely examines potential moderators of the exercise response (i.e., baseline functional capacity), and often draws conclusions about outcomes that are secondary to the intended design of the study. Finally, a further understanding of dose response is limited by a paucity of trials that directly compare two or more levels of exercise training (e.g., high versus low intensity exercise) on cancer-related outcomes(77) or compare settings (e.g., supervised versus home-based)75. Clearly, more research to address these knowledge gaps is warranted so that recommendations can continue to improve in scope and specificity.
2.7. Other exercise modes
There is also increasing interest in the safety and efficacy of types of exercise that fall outside of traditional modes of aerobic and resistance training. In a recent systematic review of the role of yoga in symptoms management for cancer survivors, yoga both during and after cancer treatment was reported to improve quality of life and fatigue, while further research is needed to confirm the observed potential to improve sleep, depressive symptoms, anxiety/distress, and cancer-related cognitive change(78). However, the literature is reflective of practices for many different types of yoga, including those that incorporate non-exercise features such as breathing or meditation and which use a wide variety of prescriptions (e.g., frequency, duration, with/without home practice), making it difficult to generate a definitive prescription. While there is also insufficient evidence at this time for a definitive prescription around safety and efficacy for other types of exercise for cancer survivors, such as dragon boating(79, 80), recreational sports(81), wall/rock climbing(82), triathalon(83), or high intensity interval training(84), research is on-going.
3. EFFECTS OF CANCER TREATMENT AND ADVERSE EFFECTS RELEVANT TO EXERCISE
To best evaluate a cancer survivor’s exercise tolerance and prescribe a safe and effective exercise program, it is necessary for fitness professionals to know about the type and extent (i.e. stage) of cancer a person has. Fitness professionals must also be familiar with the common treatment approaches to cancer, the side effects and symptoms these treatments can cause, and the subsequent impact on exercise tolerance (Table 3). The treatment approach used will differ by type of cancer, stage of disease, cancer subtype, patient health and many other considerations. Treatment modalities may include a combination of surgery, radiation, and systemic therapies, including cytotoxic chemotherapy, newer targeted agents including immune checkpoint inhibitors, and hormonal therapy. When individuals are on active cancer treatment, working closely with the oncology treatment team is recommended, as treatment approaches change frequently and understanding the side effects of newer treatments continues to evolve.
Table 3.
Potential impact of cancer treatments on exercise tolerance and safety
| Surgery | Chemotherapy | Radiation | Anti-hormonal therapy (surgical or pharmaceutical) | Targeted Therapy or Immunotherapy a | ||
|---|---|---|---|---|---|---|
| Cardiovascular changes | Cardiac damage or increased CVD risk | √ | √ | √ | √ | |
| Endocrine changes | ||||||
| Worsening bone health | √ | √ | √ | |||
| Changes in body composition (weight gain) | √ | √ | ||||
| Changes in body composition (weight loss/muscle mass loss) | √ | √ | √ | √ | √ | |
| Gastrointestinal changes | ||||||
| Nausea | √ | √ | ||||
| Diarrhea | √ | √ | √ | |||
| Altered GI function | √ | √ | √ | √ | ||
| Immune changes | Impaired immune function and/or anemia | √ | √ | √ | √ | |
| Metabolic changes | ||||||
| Development/worsening of metabolic syndrome | √ | √ | √ b | |||
| Neurological changes | Peripheral Neuropathy | √ | ||||
| Cognitive changes | √ (brain surgery) | √ | √ | √ | ||
| Pulmonary changes | Altered lung function or pneumonitis | √ (lung surgery) | √ | √ | ||
| Skin changes | ||||||
| Redness, irritation | √ | |||||
| Rashes | √ | √ | ||||
| Reduced ROM | √ (by healing at surgical site) | √ | ||||
| Fatigue | √ | √ | √ | √ | √ | |
| Lymphedema c | √ | √ | ||||
| Pain | General | √ | √ | √ | √ | √ |
| Myalgia/Arthralgia | √ | √ | √ |
Adapted from Schmitz et al. ACSM 2010 Roundtable
Legend
Depends on type or target of agent
Especially common with PI3kinase inhibitors
Can occur in any type of cancer when and where lymph nodes are surgically resected and/or radiation over lymph nodes.
Abbreviations: CVD, cardiovascular disease; GI, Gastrointestinal.
The impact of cancer treatment on exercise tolerance may further depend upon the pre-diagnosis health and functional capacity of the individual. Furthermore, fitness professionals should be aware of and respectful of the fact that individuals diagnosed with cancer commonly have many concerns, such as life expectancy, employment issues, and family matters, that may limit prioritization of exercise in their lives.
4. MEDICAL CLEARANCE & EXERCISE TESTING
Both the diagnosis of cancer and curative cancer treatments may affect the underlying safety of exercise training. Guidance for the indications of medical clearance prior to exercise testing and/or training, as well as how exercise testing should be adapted for cancer survivors, can be useful for creating a safe and effective exercise prescription. Where this information is available elsewhere and to avoid redundancy, the reader will be referred to specific publications.
4.1. Medical Clearance Prior to Exercise
Given the diversity of tumor types and side effect of different cancer treatments, including the potential acceleration of cardiovascular disease (CVD), the question of whether or not cancer survivors require medical clearance (i.e., approval from a medical professional to engage in exercise) prior to starting an exercise program is always relevant. Recently, the ACSM updated its pre-participation exercise guidelines for all persons in an attempt to reduce barriers to exercise by removing a requirement for medical clearance for individuals whose risk of an adverse cardiac event during exercise are low, including exercise naïve persons(85). Pre-participation guidelines for evaluating the need for medical clearance for non-cancer comorbidities should be applied in cancer survivors to minimize risks of adverse exercise-related events. The ACSM pre-participation guidelines do not explicitly address risks for adverse events and/or injury during exercise that are specific to the adverse effects of cancer treatment. Therefore, we have referred to the National Comprehensive Cancer Network (NCCN) Survivorship Guidelines(8) to frame recommendations for when medical clearance and/or further medical evaluation by a medical professional is indicated, as well as the level of supervision during exercise training for cancer survivors to ensure safety based on the disease and treatment related side effects (Table 4).
Table 4.
Adapted National Comprehensive Cancer Network Triage Approach Based on Risk of Exercise-Induced Adverse Events
| Description of Patients | Evaluation, Prescription and Programming Recommendations |
|---|---|
| No comorbidities | No further pre-exercise medical evaluation* Follow general exercise recommendations |
| Peripheral neuropathy, arthritis/musculoskeletal issues, poor bone health (e.g., osteopenia or osteoporosis), lymphedema | Recommend pre-exercise medical evaluation* Modify general exercise recommendations based on assessments Consider referral to trained personnel† |
| Lung or abdominal surgery, ostomy, cardiopulmonary disease, ataxia, extreme fatigue, severe nutritional deficiencies, worsening/changing physical condition (i.e., lymphedema exacerbation), bone metastases | Pre-exercise medical evaluation* and clearance by physician prior to exercise Referral to trained personnel† |
Legend
Rehabiliation specialists (i.e., physical therapists, occupational therapists, physiatrists) and certified exercise physiologists (i.e., American College of Sports Medicine Certified Clinical Exercise Physiologist (ACSM-CEP), Canadian Society for Exercise Physiology Certified Exercise Physiologist (CSEP-CEP), Exercise & Sport Science Australia Accredited Exercise Physiologist (ESSA-AEP)).
Medical evaluation – per NCCN guidelines for specific symptoms and side effects
4.2. Exercise testing
Ideally, cancer survivors should receive a comprehensive assessment of all components of health-related physical fitness (i.e., cardiorespiratory fitness, muscle strength and endurance, body composition and flexibility), with some specific cancer-specific considerations (BOX 2), in order to individualize an exercise prescription. However, requiring a comprehensive physical fitness assessment prior to starting exercise may create an unnecessary barrier to starting activity. For this reason, no assessments are required to start low intensity aerobic training (i.e., walking or cycling), resistance training with gradual progression, or a flexibility program in most survivors. Medical clearance may still be indicated as previously described depending on exercise and health history and presence of cardiovascular, renal or metabolic symptoms(85).
BOX 2. Exercise testing recommendations:
| • Standard exercise testing methods are generally appropriate for patients with cancer who do not require medical clearance or who have been medically cleared for exercise with the following considerations: |
| • Be aware of a survivor’s health history, comorbid chronic diseases and health conditions, and any general exercise contraindications before commencing health-related fitness assessments or designing the exercise prescription(85). |
| • Be familiar with the most common toxicities associated with cancer treatments including increased risk for fractures and cardiovascular events, along with neuropathies or musculoskeletal morbidities related to specific types of treatment |
| • Health-related fitness assessments may be valuable for evaluating the degree to which components of fitness have been affected by cancer-related fatigue or other commonly experienced symptoms that impact function(106) |
| • In principle, there is no evidence that the level of medical supervision required for symptom-limited or maximal cardiopulmonary exercise testing need to be different for patients with cancer than for other populations(85). |
| • The evidence-based literature indicates 1-RM testing is safe among survivors of breast and prostate cancer without bony metastases(6) |
| • Among patients with bony metastases or known or suspected osteoporosis routine assessments of muscle strength and/or endurance involving musculature that attaches to and/or acts on a skeletal site that contains bone lesions should be avoided(107). For example, 1-RM testing for leg strength (e.g, leg press) should be avoided in patients who have bony metastases in the proximal femur (i.e., hip) or vertebrae. Other sites where lesions are absent could be tested. In this example, if the patient had no lesions in the upper body, 1-RM for a chest press or 1-RM for a seated row might be feasible, given no other contraindications. Medical clearance from a physician (i.e., orthopedic or radio-oncology) may be mandatory depending on scope of practice or protocols at a specific site/facility. |
| • Older survivors and/or survivors treated with neurotoxic chemotherapy (typical for breast, colon, lung, ovarian cancers) may especially benefit from a standard assessment of balance and mobility to assess fall risk(108) |
| • CVD has become a competing cause of morbidity and mortality for survivors of cancer with a favorable prognosis(109). Given the potential for underlying CVD, cancer survivors should be screened for evident or underlying CVD using the ACSM pre-participation guidelines (see below) and if implicated have a cardiopulmonary exercise test prior to beginning an exercise program(110). |
5. EXERCISE SAFETY & TRAINING TOLERANCE
5.1. Safety of exercise training
The overall conclusion from the 2010 Roundtable was that exercise is generally safe for cancer survivors(6) and this has not changed based on the majority of studies conducted since that time. It should be recognized that the majority of available evidence on the safety and efficacy of exercise during and following cancer treatment is derived from RCTs of supervised and/or home-based prescribed exercise, and trials in breast cancer survivors(17, 31, 32). Hence the individuals enrolled in studies commonly meet pre-specified eligibility criteria for age, comorbidities, physical ability, largely based out of academic and/or medical centers, and were willing to participate in research. This often results in a sample that is healthier or with higher physical function and exercise motivation that may not fully generalize to the broader population of cancer survivors. Depending on the nature and extent of a survivor’s presenting problems they may not be able to adequately and/or safely engage in the levels of exercise outlined in this recommendation. In these cases, we again refer to the NCCN guidelines (Table 4). Physical therapy or medical evaluation might be a bridge to inform appropriate modifications to an individual’s exercise program and/or correct toxicities, impairments and limitations that prevent a survivor from working toward recommended levels of exercise.
5.2. Exercise Tolerance
Exercise has well established health benefits in persons without cancer, thus a key consideration in exercise trials has been whether or not cancer survivors can tolerate the doses of exercise known or hypothesized to effectively improve physical fitness and in turn, associated cancer-related outcomes. Research to date supports the potential of cancer survivors to respond positively to an exercise training stimulus by improving individual components of physical fitness, including cardiorespiratory fitness (i.e., VO2peak)(86), muscular strength and endurance(87, 88), body composition(89). However, an individual’s response to a given exercise stimulus may vary due to the direct effects of cancer treatments on physiological systems (i.e., anemia), side effects of cancer treatment (i.e., cancer-related fatigue may lower exercise tolerance), or demographics factors (i.e., age)(90). Furthermore, during active treatment an individual’s ability to tolerate exercise may fluctuate from day to day or week to week. Understanding of these interactions is a topic of ongoing research, especially with the emergence of novel therapies.
Specific to cardiorespiratory fitness, during chemotherapy treatment there is a well-documented decline in cardiorespiratory fitness, as measured by VO2peak or 6-minute walk test(4). Randomized trials of aerobic exercise during adjuvant chemotherapy demonstrate a preservation of, or an improvement in, cardiorespiratory fitness, especially in those with low initial values(86), while others report better improvement in those with higher initial values(91). Aerobic exercise during adjuvant chemotherapy does not appear to stimulate greater production of red blood cells(92), so improvements in cardiorespiratory fitness are contingent on other central (i.e., cardiac function, plasma volume) and peripheral adaptations (i.e., improved vascularization and mitochondrial enzyme function)(93).
Specific to muscular strength, loss of muscle strength and endurance is common due to deconditioning or as a side effect of cancer treatment. For example, androgen deprivation therapy (ADT), which is commonly used as treatment for prostate cancer, results in an abrupt loss of lean body mass accompanied by a reduction in muscle strength and endurance(94, 95). In the absence of the anabolic drive from testosterone, men on ADT may not be able to build lean mass in response to resistance training; however, several trials that have employed resistance training, or combined with aerobic training, have reported small, but statistically significant improvements in lean body mass after 12–36 weeks of training(96) While sarcopenia is related to muscle weakness and contributes to poor functioning in older adults, neuromuscular contributions explain up to 50% of variation in muscle strength in older adults, thus resistance training in the setting of ADT or deconditioning may still effectively improve muscle strength in the absence of gains in muscle mass(97).
Specific to body composition, maintenance of body weight can be difficult during treatment for some cancers, where loss of weight and lean body mass is a common concern, such as advanced colon, lung cancer and pancreatic cancer(98); whereas weight gain can be a common side effect of chemotherapy and anti-estrogen therapy for breast cancer or anti-androgen therapy for prostate cancer(99). Moreover, obesity is a risk factor for multiple cancers, including postmenopausal breast, renal cell and endometrial cancer, thus these survivors are more likely to be overweight or obese at the time of diagnosis(100). In cases where weight and lean body mass loss may be a side effect of treatment, the fitness professional needs to ensure that exercise training is not creating an excess energy deficit (i.e., energy expenditure exceeds adequate dietary energy and nutrient intake) that contributes to weight loss and can aggravate fatigue(101, 102). Working with a trained oncology dietician who can advise on dietary modifications that would support adequate fuel availability and replacement during and post exercise, respectively, may be prudent. For cases where survivors may be prone to weight gain and/or obesity, the exercise professional should be aware of the safety considerations related to exercise, including orthopedic limitations and cardiovascular disease risk(85, 103). If weight loss is implicated in the health goals for these individuals, it may be prudent for the exercise professional and/or survivor to partner with a registered dietician to provide dietary recommendations that can complement an exercise program.
Specific to musculoskeletal flexibility, surgery can result in temporary or more permanent reductions in joint range of motion, and extensibility of muscle, tendon, fascia and skin. Exercise professionals should be aware of surgical sites and if abnormal movement patterns are observed, adapt the proposed movements to avoid placing abnormal strain on other body structures and consider referral to physical therapy in efforts to address restrictions.
6. IMPLEMENTING FITT PRESCRIPTIONS IN PRACTICE
Based on the current literature, an effective exercise prescription that most consistently addresses health-related outcomes experienced due to a cancer diagnosis and cancer treatment includes moderate intensity aerobic training at least 3 times per week, for at least 30 minutes, for at least 8–12 weeks. The addition of resistance training to aerobic training, at least 2 times per week, using at least 2 sets of 8–15 repetitions at least 60% of one repetition maximum, appears to results in similar benefits (BOX 3). Exercise programs that only prescribe resistance training are also efficacious at improving most health-related outcomes, though for some specific outcomes the evidence is either insufficient or suggestive that resistance training alone may not be enough (e.g., depressive symptoms). Exercise programs that were supervised appear to be more effective than strictly unsupervised or home-based programs, though it is unclear whether or not this is because a higher dose of exercise may be better achieved with supervised training or from other attributes of this setting (i.e., more attention, motivation, reinforcement, selection bias). While a variety of professionals delivered supervised interventions in the research literature (e.g., exercise physiologists, certified exercise instructors, nurses, physical therapists) determining the type of professional that could maximize outcomes was beyond the scope of this paper and the available evidence.
Box 3: Expected patient benefits from exercise training by mode.
| Aerobic | Resistance | Aerobic plus Resistance |
|---|---|---|
| Reduced anxiety Fewer depressive symptoms Less fatigue Better QoL Improved perceived physical function |
Less fatigue Better QoL No risk of exacerbating lymphedema Improved perceived physical function |
Reduced anxiety Fewer depressive symptoms Less fatigue Better QoL Improved perceived physical function |
However, the fitness professional should be prepared to an create exercise program that meet their clients’ needs. A customized program may not yet resemble or reach the exercise programs recommended in these guidelines, such that a goal may be to strive toward preparing the client to engage in recommended types and levels of exercise over their lifetime as outlined in the 2018 Physical Activity Guidelines for Americans(54). There is consistent observational evidence that engaging in physical activity following a cancer diagnosis reduces the risk of cancer-specific and all-cause mortality for individuals diagnosed with early stage breast, colorectal and prostate cancer(104). Special considerations and modifications to exercise programs have been adapted from the NCCN guidelines (Table 5). Finally, as part of the ACSM Roundtable efforts, oncologists are being asked to “Assess, Advise, and Refer”, in order to connect cancer survivors to the most appropriate available exercise programming. A registry of programming is available at www.exerciseismedicine.org/movethruca (105).
Table 5.
Exercise programming considerations for specific cancer survivors
| Consideration | Recommendations. |
|---|---|
| Bone loss / bone metastases: | • Avoid contraindicated movements that place an excessively high load on fragile skeletal sites. These include the following: high-impact loads, hyperflexion or hyperextension of the trunk, flexion or extension of the trunk with added resistance, and dynamic twisting motion • Specific guidance on how to modify exercise programs based on the site of bony lesions is provided elsewhere(107, 111) • Preventing falls must also be a goal of therapy, since falls play an important role in fracture etiology (112). • Be aware of signs and symptoms of bone metastases in survivors, as well as common locations where these occur (i.e., spinal vertebrae, ribs, humerus, femur, pelvis). Bone pain can be an initial sign of skeletal metastases thus, exercise trainers should refer survivors who report pain back to the medical team for clinical evaluation prior to continuing exercise |
| Lymphedema |
• To date, there is insufficient evidence to support or refute this clinical advice to wear a compression garment during exercise to prevent or reduce symptoms of breast cancer-related upper body lymphedema. Therefore, it is recommended that exercise professionals provide this information as part of client education and defer to an individual client’s preference regarding use of a compression sleeve. • Being overweight or deconditioned have been associated with a higher risk of developing cancer-related lymphedema in observational studies, at this time there is insufficient evidence that weight loss or improving aerobic fitness can lower the risk of developing cancer-related lymphedema(113). • |
| Older adults |
• Physical problems reported by cancer survivors, such as cognitive difficulty, neuropathy, sarcopenia, muscle weakness, slowing, and fatigue, may be similar to those of older people without cancer, but cancer treatment can accelerate these declines(114–116) • Exercise professionals will need to combine ACSM guidelines on exercise programming for older adults(117) with the recommendations in this publication. • Integrate fitness and functional assessments prior to beginning an exercise program to more accurately determine baseline functional abilities. |
| Ostomy | • Empty ostomy bag before starting exercise • Weight lifting/resistance exercises should start with low resistance and progress slowly under the guidance of trained exercise professionals. People with an ostomy may be at an increased risk of parastomal hernia. To regulate intra-abdominal pressure, correct lifting technique and good form is required. Avoid use of a Valsalva maneuver(118, 119). • Modify any core exercises which cause excessive intra-abdominal pressure, namely a feeling of pressure or observed bulging of the abdomen. • Those with an ileostomy are at increased risk of dehydration. Get medical advice on ways to maintain optimum hydration prior, during and after exercise. • Those doing contact sports or where there is a risk of a blow to the ostomy may wish to wear an ostomy protector/shield. |
| Peripheral Neuropathy | • Stability, balance, and gait should be assessed before engaging in exercise; consider balance training as indicated • Consider alternative aerobic exercise (stationary biking, water exercise) rather than walking if neuropathy affects stability or use treadmill with safety handrails • Resistance training recommendations: ○ Monitor discomfort in hands when using hand-held weights ○ Consider using dumbbells with soft/rubber coating, and/or wear padded gloves ○ Consider resistance machines over free weights(120) |
| Stem cell transplantation | • Home-based exercise encouraged • A full recovery of the immune system recommended before return to gym facilities with the general public • Start with light intensity, short durations but high frequency and progress slowly • Exercise volume (intensity and duration) should be adapted on a daily basis based on the individual’s presentation |
| Symptom Clusters |
• Symptoms and side effects of cancer treatment rarely appear in isolation; rather, symptom clusters are the norm (i.e., fatigue, pain, sleep disturbance), especially during cancer treatment and in those with advanced disease(121). • Exercise professionals must be aware of this complexity and be prepared to refer clients/patients back to the medical team (i.e., rehabilitation or oncology physician, general practitioner, or nurse) for review and management of symptoms when safety concerns develop or when target symptom (e.g., fatigue) is not responding as expected. |
| Sun Safety | • In addition to melanoma survivors(122), survivors of cancer at other primary sites may be at increased risk for secondary skin cancers(123) • Exercise professionals should recommend that cancer survivors engage in sun protective practices when exercising outdoors(124). |
7. SUMMARY AND FUTURE DIRECTIONS
The 2018 ACSM Roundtable recommendations were made possible due to the increase in the availability of high quality randomized controlled trials of exercise in cancer survivors published after the 2010 recommendations were issued. This allowed for the development of more specific evidence-based exercise prescription to improve common side effects of a cancer diagnosis and treatment, namely anxiety, depressive symptoms, fatigue, health-related quality of life, and physical function, along with safety of exercise training in persons with or at risk of breast-cancer related lymphedema. Future research is needed to determine the efficacy of exercise to improve other outcomes, including those identified here under the emerging or insufficient evidence categories. In addition, the literature remains insufficient for further detailing prescriptions according to cancer type, timing of treatment, and/or types of treatment, while exercise prescriptions were rarely based on studies that directly compared varying FITT components, such as a head-to-head trial of low versus high intensity training. Thus, as the evidence base continues to grow in other cancer sites and keeps pace with the evolution of cancer treatment, as well as trial designs broaden to include multiple treatment arms, the next generation of exercise prescriptions could have the specificity to move exercise oncology toward the same goal as precision oncology where treatment is matched to the specific characteristics of a person’s cancer.
Supplementary Material
Acknowledgements:
Funding for the Roundtable was provided by: American College of Sports Medicine,, American Cancer Society, American Academy of Physical Medicine and Rehabilitation, American Physical Therapy Association, Canadian Society for Exercise Physiology, Exercise and Sports Science Australia, German Union for Health Exercise and Exercise Therapy (DVGS), McMillan, Royal Dutch Society for Physical Therapy (KVDP), Sunflower Wellness.
References:
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Bower JE. Management of cancer-related fatigue. Clin Adv Hematol Oncol. 2006;4(11):828–9. [PubMed] [Google Scholar]
- 3.Curigliano G, Cardinale D, Dent S, Criscitiello C, Aseyev O, Lenihan D, et al. Cardiotoxicity of anticancer treatments: Epidemiology, detection, and management. CA Cancer J Clin. 2016;66(4):309–25. [DOI] [PubMed] [Google Scholar]
- 4.Scott JM, Nilsen TS, Gupta D, Jones LW. Exercise Therapy and Cardiovascular Toxicity in Cancer. Circulation. 2018;137(11):1176–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271–89. [DOI] [PubMed] [Google Scholar]
- 6.Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvao DA, Pinto BM, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409–26. [DOI] [PubMed] [Google Scholar]
- 7.Department of Human and Health Services. Physical Activity Guidelines for Americans. Department of Health and Human Services, 2008. [Google Scholar]
- 8.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology - Survivorship. 2018;Version 2.2018.
- 9.Cormie P, Atkinson M, Bucci L, Cust A, Eakin E, Hayes S, et al. Clinical Oncology Society of Australia position statement on exercise in cancer care. Med J Aust. 2018;209(4):184–7. [DOI] [PubMed] [Google Scholar]
- 10.Segal R, Zwaal C, Green E, Tomasone JR, Loblaw A, Petrella T. Exercise for people with cancer: a clinical practice guideline. Curr Oncol. 2017;24(1):40–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62:242–74. [DOI] [PubMed] [Google Scholar]
- 12.Weggemans RM, Backx FJG, Borghouts L, Chinapaw M, Hopman MTE, Koster A, et al. The 2017 Dutch Physical Activity Guidelines. Int J Behav Nutr Phys Act. 2018;15(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mishra SI, Scherer RW, Geigle PM, Berlanstein DR, Topaloglu O, Gotay CC, et al. Exercise interventions on health-related quality of life for cancer survivors. The Cochrane database of systematic reviews. 2012;8:CD007566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mishra SI, Scherer RW, Snyder C, Geigle PM, Berlanstein DR, Topaloglu O. Exercise interventions on health-related quality of life for people with cancer during active treatment. Cochrane Database Syst Rev. 2012(8):CD008465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Persoon S, Kersten MJ, van der Weiden K, Buffart LM, Nollet F, Brug J, et al. Effects of exercise in patients treated with stem cell transplantation for a hematologic malignancy: a systematic review and meta-analysis. Cancer Treat Rev. 2013;39(6):682–90. [DOI] [PubMed] [Google Scholar]
- 16.Zhou Y, Zhu J, Gu Z, Yin X. Efficacy of Exercise Interventions in Patients with Acute Leukemia: A Meta-Analysis. PLoS One. 2016;11(7):e0159966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lahart IM, Metsios GS, Nevill AM, Carmichael AR. Physical activity for women with breast cancer after adjuvant therapy. Cochrane Database Syst Rev. 2018;1:CD011292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown JC, Huedo-Medina TB, Pescatello LS, Ryan SM, Pescatello SM, Moker E, et al. The efficacy of exercise in reducing depressive symptoms among cancer survivors: a meta-analysis. PLoS One. 2012;7(1):e30955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Craft LL, Vaniterson EH, Helenowski IB, Rademaker AW, Courneya KS. Exercise effects on depressive symptoms in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2012;21(1):3–19. Epub 2011/11/10. doi: 10.1158/1055-9965.EPI-11-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puetz TW, Herring MP. Differential effects of exercise on cancer-related fatigue during and following treatment: a meta-analysis. Am J Prev Med. 2012;43(2):e1–24. [DOI] [PubMed] [Google Scholar]
- 21.Tomlinson D, Diorio C, Beyene J, Sung L. Effect of exercise on cancer-related fatigue: a meta-analysis. Am J Phys Med Rehabil. 2014;93(8):675–86. [DOI] [PubMed] [Google Scholar]
- 22.Meneses-Echavez JF, Gonzalez-Jimenez E, Ramirez-Velez R. Effects of Supervised Multimodal Exercise Interventions on Cancer-Related Fatigue: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Biomed Res Int. 2015;2015:328636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Vulpen JK, Peeters PH, Velthuis MJ, van der Wall E, May AM. Effects of physical exercise during adjuvant breast cancer treatment on physical and psychosocial dimensions of cancer-related fatigue: A meta-analysis. Maturitas. 2016;85:104–11. [DOI] [PubMed] [Google Scholar]
- 24.Brown JC, Huedo-Medina TB, Pescatello LS, Pescatello SM, Ferrer RA, Johnson BT. Efficacy of exercise interventions in modulating cancer-related fatigue among adult cancer survivors: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2011;20(1):123–33. [DOI] [PubMed] [Google Scholar]
- 25.Cramp F, Byron-Daniel J. Exercise for the management of cancer-related fatigue in adults. The Cochrane database of systematic reviews. 2012;11:CD006145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Juvet LK, Thune I, Elvsaas IKO, Fors EA, Lundgren S, Bertheussen G, et al. The effect of exercise on fatigue and physical functioning in breast cancer patients during and after treatment and at 6 months follow-up: A meta-analysis. Breast. 2017;33:166–77. [DOI] [PubMed] [Google Scholar]
- 27.Keogh JW, MacLeod RD. Body composition, physical fitness, functional performance, quality of life, and fatigue benefits of exercise for prostate cancer patients: a systematic review. J Pain Symptom Manage. 2012;43(1):96–110. [DOI] [PubMed] [Google Scholar]
- 28.Mustian KM, Alfano CM, Heckler C, Kleckner AS, Kleckner IR, Leach CR, et al. Comparison of Pharmaceutical, Psychological, and Exercise Treatments for Cancer-Related Fatigue: A Meta-analysis. JAMA Oncol. 2017;3(7):961–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meneses-Echavez JF, Gonzalez-Jimenez E, Ramirez-Velez R. Supervised exercise reduces cancer-related fatigue: a systematic review. J Physiother. 2015;61(1):3–9. [DOI] [PubMed] [Google Scholar]
- 30.McMillan EM, Newhouse IJ. Exercise is an effective treatment modality for reducing cancer-related fatigue and improving physical capacity in cancer patients and survivors: a meta-analysis. Appl Physiol Nutr Metab. 2011;36(6):892–903. [DOI] [PubMed] [Google Scholar]
- 31.Buffart LM, Kalter J, Sweegers MG, Courneya KS, Newton RU, Aaronson NK, et al. Effects and moderators of exercise on quality of life and physical function in patients with cancer: An individual patient data meta-analysis of 34 RCTs. Cancer Treat Rev. 2017;52:91–104. [DOI] [PubMed] [Google Scholar]
- 32.Sweegers MG, Altenburg TM, Chinapaw MJ, Kalter J, Verdonck-de Leeuw IM, Courneya KS, et al. Which exercise prescriptions improve quality of life and physical function in patients with cancer during and following treatment? A systematic review and meta-analysis of randomised controlled trials. Br J Sports Med. 2018;52(8):505–13. [DOI] [PubMed] [Google Scholar]
- 33.Erickson VS, Pearson ML, Ganz PA, Adams J, Kahn KL. Arm edema in breast cancer patients. J Natl Cancer Inst. 2001;93(2):96–111. [DOI] [PubMed] [Google Scholar]
- 34.Petrek JA, Pressman PI, Smith RA. Lymphedema: current issues in research and management. CA Cancer J Clin. 2000;50(5):292–307. [DOI] [PubMed] [Google Scholar]
- 35.Singh B, Disipio T, Peake J, Hayes SC. Systematic Review and Meta-Analysis of the Effects of Exercise for Those With Cancer-Related Lymphedema. Arch Phys Med Rehabil. 2016;97(2):302–15 e13. [DOI] [PubMed] [Google Scholar]
- 36.Keilani M, Hasenoehrl T, Neubauer M, Crevenna R. Resistance exercise and secondary lymphedema in breast cancer survivors-a systematic review. Support Care Cancer. 2016;24(4):1907–16. [DOI] [PubMed] [Google Scholar]
- 37.Nelson NL. Breast Cancer-Related Lymphedema and Resistance Exercise: A Systematic Review. J Strength Cond Res. 2016;30(9):2656–65. [DOI] [PubMed] [Google Scholar]
- 38.Ahmed RL, Thomas W, Yee D, Schmitz KH. Randomized controlled trial of weight training and lymphedema in breast cancer survivors. J Clin Oncol. 2006;24(18):2765–72. [DOI] [PubMed] [Google Scholar]
- 39.Courneya KS, Segal RJ, Mackey JR, Gelmon K, Reid RD, Friedenreich CM, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol. 2007;25(28):4396–404. [DOI] [PubMed] [Google Scholar]
- 40.Schmitz KH, Ahmed RL, Troxel A, Cheville A, Smith R, Lewis-Grant L, et al. Weight lifting in women with breast-cancer-related lymphedema. N Engl J Med. 2009;361(7):664–73. [DOI] [PubMed] [Google Scholar]
- 41.Schmitz KH, Ahmed RL, Troxel AB, Cheville A, Lewis-Grant L, Smith R, et al. Weight lifting for women at risk for breast cancer-related lymphedema: a randomized trial. JAMA. 2010;304(24):2699–705. [DOI] [PubMed] [Google Scholar]
- 42.Kilbreath SL, Refshauge KM, Beith JM, Ward LC, Lee M, Simpson JM, et al. Upper limb progressive resistance training and stretching exercises following surgery for early breast cancer: a randomized controlled trial. Breast Cancer Res Treat. 2012;133(2):667–76. [DOI] [PubMed] [Google Scholar]
- 43.Katz E, Dugan NL, Cohn JC, Chu C, Smith RG, Schmitz KH. Weight lifting in patients with lower-extremity lymphedema secondary to cancer: a pilot and feasibility study. Arch Phys Med Rehabil. 2010;91(7):1070–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Swartz MC, Lewis ZH, Lyons EJ, Jennings K, Middleton A, Deer RR, et al. Effect of Home- and Community-Based Physical Activity Interventions on Physical Function Among Cancer Survivors: A Systematic Review and Meta-Analysis. Arch Phys Med Rehabil. 2017;98(8):1652–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dalla Via J, Daly RM, Fraser SF. The effect of exercise on bone mineral density in adult cancer survivors: a systematic review and meta-analysis. Osteoporos Int. 2018;29(2):287–303. [DOI] [PubMed] [Google Scholar]
- 46.Fornusek CP, Kilbreath SL. Exercise for improving bone health in women treated for stages I-III breast cancer: a systematic review and meta-analyses. J Cancer Surviv. 2017;11(5):525–41. [DOI] [PubMed] [Google Scholar]
- 47.Kohrt WM, Bloomfield SA, Little KD, Nelson ME, Yingling VR, American College of Sports M. American College of Sports Medicine Position Stand: physical activity and bone health. Med Sci Sports Exerc. 2004;36(11):1985–96. [DOI] [PubMed] [Google Scholar]
- 48.Watson SL, Weeks BK, Weis LJ, Harding AT, Horan SA, Beck BR. High-intensity exercise did not cause vertebral fractures and improves thoracic kyphosis in postmenopausal women with low to very low bone mass: the LIFTMOR trial. Osteoporos Int. 2019. Epub 2019/01/07. doi: 10.1007/s00198-018-04829-z. [DOI] [PubMed] [Google Scholar]
- 49.Chiu HY, Huang HC, Chen PY, Hou WH, Tsai PS. Walking improves sleep in individuals with cancer: a meta-analysis of randomized, controlled trials. Oncology Nursing Forum. 2015;42(2):E54–62. [DOI] [PubMed] [Google Scholar]
- 50.Mercier J, Savard J, Bernard P. Exercise interventions to improve sleep in cancer patients: A systematic review and meta-analysis. Sleep Medicine Reviews. 2017;36:43–56. [DOI] [PubMed] [Google Scholar]
- 51.Rogers LQ, Courneya KS, Oster RA, Anton PM, Robbs RS, Forero A, et al. Physical Activity and Sleep Quality in Breast Cancer Survivors: A Randomized Trial. Medicine & Science in Sports & Exercise. 2017;49(10):2009–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen HM, Tsai CM, Wu YC, Lin KC, Lin CC. Effect of walking on circadian rhythms and sleep quality of patients with lung cancer: a randomised controlled trial. Br J Cancer. 2016;115(11):1304–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roveda E, Vitale JA, Bruno E, Montaruli A, Pasanisi P, Villarini A, et al. Protective Effect of Aerobic Physical Activity on Sleep Behavior in Breast Cancer Survivors. Integr Cancer Ther. 2017;16(1):21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Physical Activity Guidelines Advisory Committee. 2018. Physical Activity Guidelines Advisory Committee Scientific Report. In: Department of Health and Human Services, editor. Washington, DC: 2018. [Google Scholar]
- 55.Chen JJ, Wu PT, Middlekauff HR, Nguyen KL. Aerobic exercise in anthracycline-induced cardiotoxicity: a systematic review of current evidence and future directions. Am J Physiol Heart Circ Physiol. 2017;312(2):H213–H22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beaudry RI, Liang Y, Boyton ST, Tucker WJ, Brothers RM, Daniel KM, et al. Meta-analysis of Exercise Training on Vascular Endothelial Function in Cancer Survivors. Integr Cancer Ther. 2018;17(2):192–9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Duregon F, Vendramin B, Bullo V, Gobbo S, Cugusi L, Di Blasio A, et al. Effects of exercise on cancer patients suffering chemotherapy-induced peripheral neuropathy undergoing treatment: A systematic review. Crit Rev Oncol Hematol. 2018;121:90–100. [DOI] [PubMed] [Google Scholar]
- 58.Campbell KL, Zadravec K, Bland KA, Chesley E, Wolf F, Janelsins M. The effect of exercise on cancer-related cognitive impairment and applications for physical therapy: A systematic review of randomized clinical trials. Phys Ther. Under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Northey JM, Cherbuin N, Pumpa KL, Smee DJ, Rattray B. Exercise interventions for cognitive function in adults older than 50: a systematic review with meta-analysis. Br J Sports Med. 2018;52(3):154–60. [DOI] [PubMed] [Google Scholar]
- 60.Centres for Disease Control. CDC Compendium of Effective Fall Interventions: What Works for Community-Dwelling Older Adults, 3rd ed. 2015. [Google Scholar]
- 61.Guirguis-Blake JM, Michael YL, Perdue LA, Coppola EL, Beil TL, Thompson JH. Interventions to Prevent Falls in Community-Dwelling Older Adults: A Systematic Review for the US Preventive Services Task Force. U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews. Rockville, MD, 2018. [PubMed] [Google Scholar]
- 62.Winningham ML, MacVicar MG. The effect of aerobic exercise on patient reports of nausea. Oncol Nurs Forum. 1988;15(4):447–50. [PubMed] [Google Scholar]
- 63.Irwin ML, Cartmel B, Gross CP, Ercolano E, Li F, Yao X, et al. Randomized exercise trial of aromatase inhibitor-induced arthralgia in breast cancer survivors. J Clin Oncol. 2015;33(10):1104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McNeely ML, Parliament MB, Seikaly H, Jha N, Magee DJ, Haykowsky MJ, et al. Effect of exercise on upper extremity pain and dysfunction in head and neck cancer survivors: a randomized controlled trial. Cancer. 2008;113(1):214–22. [DOI] [PubMed] [Google Scholar]
- 65.Yunfeng G, Weiyang H, Xueyang H, Yilong H, Xin G. Exercise overcome adverse effects among prostate cancer patients receiving androgen deprivation therapy: An update meta-analysis. Medicine (Baltimore). 2017;96(27):e7368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bourke L, Smith D, Steed L, Hooper R, Carter A, Catto J, et al. Exercise for Men with Prostate Cancer: A Systematic Review and Meta-analysis. Eur Urol. 2016;69(4):693–703. [DOI] [PubMed] [Google Scholar]
- 67.Cormie P, Chambers SK, Newton RU, Gardiner RA, Spry N, Taaffe DR, et al. Improving sexual health in men with prostate cancer: randomised controlled trial of exercise and psychosexual therapies. BMC Cancer. 2014;14:199.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cormie P, Newton RU, Taaffe DR, Spry N, Joseph D, Akhlil Hamid M, et al. Exercise maintains sexual activity in men undergoing androgen suppression for prostate cancer: a randomized controlled trial. Prostate Cancer Prostatic Dis. 2013;16(2):170–5.. [DOI] [PubMed] [Google Scholar]
- 69.Hojan K, Kwiatkowska-Borowczyk E, Leporowska E, Gorecki M, Ozga-Majchrzak O, Milecki T, et al. Physical exercise for functional capacity, blood immune function, fatigue, and quality of life in high-risk prostate cancer patients during radiotherapy: a prospective, randomized clinical study. Eur J Phys Rehabil Med. 2016;52(4):489–501. [PubMed] [Google Scholar]
- 70.Stanton AM, Handy AB, Meston CM. The Effects of Exercise on Sexual Function in Women. Sex Med Rev. 2018;6(4):548–57. [DOI] [PubMed] [Google Scholar]
- 71.Gerbild H, Larsen CM, Graugaard C, Areskoug Josefsson K. Physical Activity to Improve Erectile Function: A Systematic Review of Intervention Studies. Sex Med. 2018;6(2):75–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bland KA, Zadravec K, Landry T, Weller S, Meyers L, KL. C. Impact of exercise on chemotherapy completion rate: a systematic review of the evidence and recommendations for future exercise oncology research. Crit Rev Oncol Hematol. In press. Epub February 16, 2019. doi: 10.1016/j.critrevonc.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 73.Courneya KS. Physical activity and cancer survivorship: a simple framework for a complex field. Exerc Sport Sci Rev. 2014;42(3):102–9. [DOI] [PubMed] [Google Scholar]
- 74.Campbell KL, Neil SE, Winters-Stone KM. Review of exercise studies in breast cancer survivors: attention to principles of exercise training. Br J Sports Med. 2011. [DOI] [PubMed] [Google Scholar]
- 75.Winters-Stone KM, Neil SE, Campbell KL. Attention to principles of exercise training: a review of exercise studies for survivors of cancers other than breast. Br J Sports Med. 2014;48(12):987–95. [DOI] [PubMed] [Google Scholar]
- 76.Neil-Sztramko SE, Winters-Stone KM, Bland KA, Campbell KL. Updated systematic review of exercise studies in breast cancer survivors: attention to the principles of exercise training. Br J Sports Med. 2017. [DOI] [PubMed] [Google Scholar]
- 77.Courneya KS, McKenzie DC, Mackey JR, Gelmon K, Friedenreich CM, Yasui Y, et al. Effects of exercise dose and type during breast cancer chemotherapy: multicenter randomized trial. J Natl Cancer Inst. 2013;105(23):1821–32. [DOI] [PubMed] [Google Scholar]
- 78.Danhauer SC, Addington EL, Cohen L, Sohl SJ, Van Puymbroeck M, Albinati NK, et al. Yoga for Symptom Management in Oncology: A Review of the Evidence Base and Future Directions for Research. Cancer. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McKenzie DC. Abreast in a boat--a race against breast cancer. CMAJ. 1998;159(4):376–8. [PMC free article] [PubMed] [Google Scholar]
- 80.McDonough MH, Patterson MC, Weisenbach BB, Ullrich-French S, Sabiston CM. The difference is more than floating: factors affecting breast cancer survivors’ decisions to join and maintain participation in dragon boat teams and support groups. Disabil Rehabil. 2018:1–9. [DOI] [PubMed] [Google Scholar]
- 81.Uth J, Hornstrup T, Christensen JF, Christensen KB, Jorgensen NR, Schmidt JF, et al. Efficacy of recreational football on bone health, body composition, and physical functioning in men with prostate cancer undergoing androgen deprivation therapy: 32-week follow-up of the FC prostate randomised controlled trial. Osteoporosis International 2016;27(4):1507–18. Epub 2015/11/18. doi: 10.1007/s00198-015-3399-0. [DOI] [PubMed] [Google Scholar]
- 82.Crawford JJ, Vallance JK, Holt NL, Bell GJ, Steed H, Courneya KS. A Pilot Randomized, Controlled Trial of a Wall Climbing Intervention for Gynecologic Cancer Survivors. Oncol Nurs Forum. 2017;44(1):77–86. [DOI] [PubMed] [Google Scholar]
- 83.Ng AV, Cybulski AN, Engel AA, Papanek PE, Sheffer MA, Waltke LJ, et al. Triathlon training for women breast cancer survivors: feasibility and initial efficacy. Support Care Cancer. 2017;25(5):1465–73. [DOI] [PubMed] [Google Scholar]
- 84.Dolan LB, Campbell K, Gelmon K, Neil-Sztramko S, Holmes D, McKenzie DC. Interval versus continuous aerobic exercise training in breast cancer survivors--a pilot RCT. Support Care Cancer. 2016;24(1):119–27. [DOI] [PubMed] [Google Scholar]
- 85.Riebe D, Ehrman JK, Liguori G, Magal M, editors. ACSM’s Guidelines fo Exercise Testing and Prescription 10th ed. Philadelphia, PA: Wolters Kluwer; 2018. [Google Scholar]
- 86.Scott JM, Zabor EC, Schwitzer E, Koelwyn GJ, Adams SC, Nilsen TS, et al. Efficacy of Exercise Therapy on Cardiorespiratory Fitness in Patients With Cancer: A Systematic Review and Meta-Analysis. J Clin Oncol. 2018;36(22):2297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fuller JT, Hartland MC, Maloney LT, Davison K. Therapeutic effects of aerobic and resistance exercises for cancer survivors: a systematic review of meta-analyses of clinical trials. Br J Sports Med. 2018;52(20):1311. [DOI] [PubMed] [Google Scholar]
- 88.Cheema BS, Kilbreath SL, Fahey PP, Delaney GP, Atlantis E. Safety and efficacy of progressive resistance training in breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat. 2014;148(2):249–68. [DOI] [PubMed] [Google Scholar]
- 89.Padilha CS, Marinello PC, Galvao DA, Newton RU, Borges FH, Frajacomo F, et al. Evaluation of resistance training to improve muscular strength and body composition in cancer patients undergoing neoadjuvant and adjuvant therapy: a meta-analysis. J Cancer Surviv. 2017;11(3):339–49. [DOI] [PubMed] [Google Scholar]
- 90.Sweegers MG, Altenburg TM, Brug J, May AM, van Vulpen JK, Aaronson NK, et al. Effects and moderators of exercise on muscle strength, muscle function and aerobic fitness in patients with cancer: a meta-analysis of individual patient data. Br J Sports Med. 2018. [DOI] [PubMed] [Google Scholar]
- 91.Buffart LM, Sweegers MG, May AM, Chinapaw MJ, van Vulpen JK, Newton RU, et al. Targeting Exercise Interventions to Patients With Cancer in Need: An Individual Patient Data Meta-Analysis. J Natl Cancer Inst. 2018;110(11):1190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dolan LB, Gelmon K, Courneya KS, Mackey JR, Segal RJ, Lane K, et al. Hemoglobin and aerobic fitness changes with supervised exercise training in breast cancer patients receiving chemotherapy. Cancer epidemiology, biomarkers & prevention 2010;19(11):2826–32. [DOI] [PubMed] [Google Scholar]
- 93.Lakoski SG, Eves ND, Douglas PS, Jones LW. Exercise rehabilitation in patients with cancer. Nat Rev Clin Oncol. 2012;9(5):288–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Galvao DA, Newton RU, Taaffe DR, Spry N. Can exercise ameliorate the increased risk of cardiovascular disease and diabetes associated with ADT? Nat Clin Pract Urol. 2008;5(6):306–7. [DOI] [PubMed] [Google Scholar]
- 95.Galvao DA, Taaffe DR, Spry N, Joseph D, Turner D, Newton RU. Reduced muscle strength and functional performance in men with prostate cancer undergoing androgen suppression: a comprehensive cross-sectional investigation. Prostate Cancer Prostatic Dis. 2009;12(2):198–203. [DOI] [PubMed] [Google Scholar]
- 96.Gardner JR, Livingston PM, Fraser SF. Effects of Exercise on Treatment-Related Adverse Effects for Patients With Prostate Cancer Receiving Androgen-Deprivation Therapy: A Systematic Review. Journal of Clinical Oncology. 2014;32(4):335–46. [DOI] [PubMed] [Google Scholar]
- 97.Winters-Stone KM, Dobek JC, Bennett JA, Dieckmann NF, Maddalozzo GF, Ryan CW, et al. Resistance training reduces disability in prostate cancer survivors on androgen deprivation therapy: evidence from a randomized controlled trial. Arch Phys Med Rehabil. 2015;96(1):7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer-associated cachexia. Nat Rev Dis Primers. 2018;4:17105. [DOI] [PubMed] [Google Scholar]
- 99.Demark-Wahnefried W, Schmitz KH, Alfano CM, Bail JR, Goodwin PJ, Thomson CA, et al. Weight management and physical activity throughout the cancer care continuum. CA Cancer J Clin. 2018;68(1):64–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.World Cancer Research Fund/American Institute for Cancer Research. Diet, Physical Activity and Cancer: A Global Perspective. 2018.
- 101.Arends J, Baracos V, Bertz H, Bozzetti F, Calder PC, Deutz NEP, et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin Nutr. 2017;36(5):1187–96. [DOI] [PubMed] [Google Scholar]
- 102.Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36(1):11–48. [DOI] [PubMed] [Google Scholar]
- 103.American College of Sports M, Armstrong LE, Casa DJ, Millard-Stafford M, Moran DS, Pyne SW, et al. American College of Sports Medicine position stand. Exertional heat illness during training and competition. Med Sci Sports Exerc. 2007;39(3):556–72. [DOI] [PubMed] [Google Scholar]
- 104.Patel AV. American College of Sports Medicine Roundtable Report on Physical Activity, Sedentary Behavior, and Cancer Prevention and Control. Med Sci Sports Exerc. 2019;In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schmitz KH, al. E. Exercise Is Medicine in Oncology: Moving Through Cancer CA Cancer J Clin in press. [Google Scholar]
- 106.McNeely ML, Courneya KS. Exercise programs for cancer-related fatigue: evidence and clinical guidelines. J Natl Compr Canc Netw. 2010;8(8):945–53. [DOI] [PubMed] [Google Scholar]
- 107.Galvao DA, Taaffe DR, Spry N, Cormie P, Joseph D, Chambers SK, et al. Exercise Preserves Physical Function in Prostate Cancer Patients with Bone Metastases. Med Sci Sports Exerc. 2018;50(3):393–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Panel on Prevention of Falls in Older Persons AGS, British Geriatrics S. Summary of the Updated American Geriatrics Society/British Geriatrics Society Clinical Practice Guideline for Prevention of Falls in Older Persons. Journal of the American Geriatrics Society. 2011;59(1):148–57. [DOI] [PubMed] [Google Scholar]
- 109.Patnaik JL, Byers T, DiGuiseppi C, Dabelea D, Denberg TD. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast cancer research : BCR. 2011;13(3):R64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Riebe D, Franklin B, Thompson P, Garber C, Whitfield G, Magal M, et al. Updating ACSM’s Recommendations for Exercise Preparticipation Health Screening. Medicine & Science in Sports & Exercise. 2015;47(11):2473–9. [DOI] [PubMed] [Google Scholar]
- 111.Rief H, Petersen LC, Omlor G, Akbar M, Bruckner T, Rieken S, et al. The effect of resistance training during radiotherapy on spinal bone metastases in cancer patients - a randomized trial. Radiother Oncol. 2014;112(1):133–9. [DOI] [PubMed] [Google Scholar]
- 112.Frost HM. Should fracture risk-of-fracture analyses include another major risk factor? The case for falls. J Clin Densitom. 2001;4:381–3. [DOI] [PubMed] [Google Scholar]
- 113.Paskett ED, Dean JA, Oliveri JM, Harrop JP. Cancer-related lymphedema risk factors, diagnosis, treatment, and impact: a review. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(30):3726–33. [DOI] [PubMed] [Google Scholar]
- 114.Clough-Gorr KM, Stuck AE, Thwin SS, Silliman RA. Older breast cancer survivors: geriatric assessment domains are associated with poor tolerance of treatment adverse effects and predict mortality over 7 years of follow-up. J Clin Oncol. 2010;28(3):380–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Klepin HD, Geiger AM, Tooze JA, Newman AB, Colbert LH, Bauer DC, et al. Physical performance and subsequent disability and survival in older adults with malignancy: results from the health, aging and body composition study. J Am Geriatr Soc. 2010;58(1):76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Maccormick RE. Possible acceleration of aging by adjuvant chemotherapy: a cause of early onset frailty? Med Hypotheses. 2006;67(2):212–5. [DOI] [PubMed] [Google Scholar]
- 117.American College of Sports M, Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, Minson CT, Nigg CR, et al. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41(7):1510–30. [DOI] [PubMed] [Google Scholar]
- 118.UK AoSCN. ACSN Stoma Care National Clinical Guidelines. 2016.
- 119.Esper P. Symptom clusters in individuals living with advanced cancer. Semin Oncol Nurs. 2010;26(3):168–74. [DOI] [PubMed] [Google Scholar]
- 120.Streckmann F, Zopf EM, Lehmann HC, May K, Rizza J, Zimmer P, et al. Exercise intervention studies in patients with peripheral neuropathy: a systematic review. Sports Med. 2014;44(9):1289–304. [DOI] [PubMed] [Google Scholar]
- 121.Ward Sullivan C, Leutwyler H, Dunn LB, Miaskowski C. A review of the literature on symptom clusters in studies that included oncology patients receiving primary or adjuvant chemotherapy. J Clin Nurs. 2018;27(3–4):516–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tucker MA, Elder DE, Curry M, Fraser MC, Pichler V, Zametkin D, et al. Risks of Melanoma and Other Cancers in Melanoma-Prone Families over 4 Decades. J Invest Dermatol. 2018;138(7):1620–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Donin N, Filson C, Drakaki A, Tan HJ, Castillo A, Kwan L, et al. Risk of second primary malignancies among cancer survivors in the United States, 1992 through 2008. Cancer. 2016;122(19):3075–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lau SC, Chen L, Cheung WY. Protective skin care behaviors in cancer survivors. Curr Oncol. 2014;21(4):e531–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


