Abstract
Changes in expression or activation of various metalloproteases including matrix metalloproteases (Mmp), a disintegrin and metalloprotease (Adam) and a disintegrin and metalloprotease with thrombospondin motif (Adamts), and their endogenous inhibitors (tissue inhibitors of metalloproteases, Timp), have been shown to be critical for ovulation in various species from studies in past decades. Some of these metalloproteases such as Adamts1, Adamts9, Mmp2, and Mmp9 have also been shown to be regulated by luteinizing hormone (LH) and/or progestin, which are essential triggers for ovulation in all vertebrate species. Most of these metalloproteases also express broadly in various tissues and cells including germ cells and somatic gonad cells. Thus, metalloproteases likely play roles in gonad formation processes comprising primordial germ cell (PGC) migration, development of germ and somatic cells, and sex determination. However, our knowledge on the functions and mechanisms of metalloproteases in these processes in vertebrates is still lacking. This review will summarize our current knowledge on the metalloproteases in ovulation and gonad formation with emphasis on PGC migration and germ cell development.
Keywords: metalloprotease, Mmp, Adam, Adamts, ovulation, primordial germ cell, PGC, folliculogenesis, gonad formation, gonad differentiation, sex determination, zebrafish
Introduction
The formation of a functional ovary and the release of a fertilizable oocyte during ovulation is essential for animals to reproduce. Ovulation is a dynamic tissue remodeling process, which involves cleavage of extracellular matrix (ECM), rupture of basal membranes, follicular layers, and capillary vessels, release of mature oocytes, repairing lesions; and remodeling of ECM and ovarian structures (Tsafriri, 1995; Duffy et al., 2019). Formation of a functional ovary or testis is also a vigorous remodeling process, whereby the mature organ develops from a bipotential gonad via primordial germ cell (PGC) migration, proliferation, apoptosis, transformation, and development (Ungewitter & Yao, 2013; Spiller et al., 2017; Kossack & Draper, 2019). These processes are regulated by various signaling molecules and multiple metalloproteases including matrix metalloproteases (Mmp), a disintegrin and metalloprotease (Adam) and a disintegrin and metalloprotease with thrombospondin motif (Adamts), and their endogenous inhibitors (tissue inhibitors of metalloproteases, Timp). These zinc-dependent metalloproteases play a critical role in the cleavage of ECM proteins and releasing of membrane-bound signaling molecules and receptors. Excellent reviews covering various facets of ovulation have been published over the years and readers are recommended for their examination (Ohnishi et al., 2005; Curry & Smith, 2006; Russell & Robker, 2007; Richards, 2018; Duffy et al., 2019; Takahashi et al., 2019). Metalloproteases express in a wide range of organs and tissues, including testis and have been shown to be critical to semen and sperm production, and the integrity of testicular structures and sperm in animals (Baumgart et al., 2002; Choi et al., 2015; Belardin et al., 2019). Due to limited space, readers are referred to these reviews (Le Magueresse-Battistoni, 2008; Cho, 2012; Asgari et al., 2021). The present review will focus on functions and expression of individual metalloproteases in ovarian follicle development and ovulation, conservation and difference among various species, and future directions. The review is centered around a few vertebrate models largely due to availabilities of literature. Whenever possible, I will include invertebrates and other animal species where involvements of metalloproteases in ovulation or gonad formation have been reported. I will use nomenclature conventions of fish for depictions of proteins (e.g., Adamts1), transcripts/genes (e.g., adamts1) to avoid potential confusions.
Metalloproteases are synthesized in the latent form (zymogen or proenzyme) that require in situ activation for their activities to allow physiological processes such as ovulation to occur. The activity of each metalloprotease depends on 1) de novo synthesis; and 2) in situ activation. Except for a couple of gelatinases, most metalloproteases lack a sensitive bioassay for examining their activities in vivo or in vitro. Almost all cells/tissues contain multiple metalloproteases, which also make bioassay and interpretation of in vivo results difficult. In addition, majority of metalloprotease knockouts have no clear reproductive phenotypes (Table 1). A few in vitro characterizations such as cleavage assay on the enzyme activation using recombinant proteins in test tubes are very useful for identification of substrates/ligands and cleavage sites but have limited implications in term of physiological functions and activation of each metalloprotease in native cells/tissues/processes in vivo. So, it is not surprising that majority of studies so far were focused on expression changes of metalloproteases. To include a broad range of metalloprotease candidates for their possible involvements in ovulation and gonad formation, I assessed the expression changes of each metalloprotease candidate during these two processes and attempted to include activation and knockout results when they are available. At the conclusion, I will describe limitations and suggest future directions.
Table 1.
A partial list of major reproductive processes (PGC migration, folliculogenesis, fertility, or ovulation) likely regulated or affected by representative metalloproteases in female animals.
Notes: reference with * is a study using knockout approach. No expected phenotype from knockouts is likely due to that same substrate is targeted by several metalloproteases. Loss of one or two metalloproteases is likely to be compensated in the knockouts.
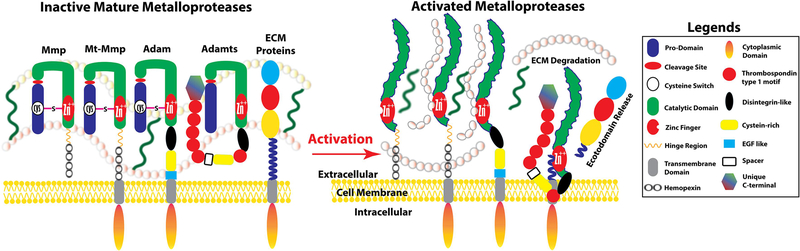
Matrix metalloproteases (Mmps) are a family of zinc-dependent proteases, part of the Metzincins superfamily, can cleave extracellular proteins and signaling molecules. Most Mmps are secreted proteases, and a few are membrane anchored proteases. In addition, Mmps also can act intracellularly (Bassiouni et al., 2021). Mmps are involved in modifying matrix structure, growth factor availability and cell surface signal molecules. They then in turn affect development, growth, cellular differentiation, proliferation, and apoptosis (Page-McCaw et al., 2007). The first member, Mmp1, was discovered in 1962 by Jerome Gross and Charles Lapiere, when they observed enzymatic activity of tadpole tail in degradation of collagen matrix (Gross & Lapiere, 1962). There are 28 related enzymes belonging to Mmps in vertebrates of which 24 genes are found in humans (Murphy & Nagase, 2008). In zebrafish, 26 protease genes have been identified as Mmp-orthologs (Huxley-Jones et al., 2007; Wyatt et al., 2009). Each mature Mmp protein is initially synthesized as an inactive form (pro-enzyme) with three common domains: pro-peptide, catalytic and a hemopexin-like C-terminal domain (Nagase et al., 2005; Cui et al., 2017; Fig. 1). The pro-peptide domain contains a cysteine residue that interacts with the catalytic zinc in the active site, preventing its binding to the substrate and consequently keeping the Mmp inactive, and therefore is designated as “The Cysteine Switch” (Van Wart & Birkedal-Hansen, 1990). Once pro-peptide domain is proteolytically removed, Mmp becomes active (Nagase et al., 1990; Fig. 1). Several endogenous inhibitors including four tissue inhibitors of metalloproteases (Timp-1, Timp-2, Timp-3, and Timp-4), α2-Macroglobulin, and β-Amyloid precursor protein could also inhibit activities of Mmps via binding of hemopexin domain at c-terminal of Mmps (Murphy, 2011). These endogenous tissue inhibitors also inhibit activities of Adam or Adamts described below. Therefore, activity of each metalloprotease is regulated no less than three different levels: 1) regulation of transcription by growth factors, cytokines, and hormones; 2) activation of synthesized mature but inactive pro-enzyme (i.e. pro-domain cleavage); and 3) removal of their specific inhibitors (i.e., Timps). In some cases, Timp not only inhibits activity of individual metalloprotease, but also forms binding complex with the metalloprotease to facilitate the docking and cleavage of metalloprotease by other metalloproteases (Lu et al., 2004).
Figure 1. Schematic illustration of major domains of Mmp, Adam and Adamts.
Each mature metalloproteinase protein is synthesized in a latent form (proenzyme) that require in situ activation. Signal peptide is not shown. Activity and binding to the substrates of each mature metalloprotease is inhibited by the prodomain and a cystine switch (in the case of Mmp or Adam) and bindings of endogenous tissue inhibitors of metalloproteases (Timps, not shown), whereas Adamts in general lack the cystine switch. Cleavage of cystine and prodomain and removal of Timps are required for the activation of these metalloproteases.
Members of Adams family are single-pass transmembrane proteins and belong to the Metzincins superfamily. The metalloprotease domain of Adams is similar to Mmps metalloprotease domain, but Adams also contain a unique integrin receptor-binding disintegrin domain. Therefore, Adams were named for these two functional domains (a disintegrin and metalloprotease) (Black & White, 1998; Schlondorff & Blobel, 1999). The first member of Adam family, Adam1, was discovered as a sperm surface protein important for sperm-egg membrane fusion in the fertilization of guinea pig (Primakoff et al., 1987). The Adam family has 20 Adam members in human, whereas 22 adam genes have been identified in zebrafish (Huxley-Jones et al., 2007; Bahudhanapati et al., 2015). All mature Adam proteins share several domains including a prodomain, a metalloprotease domain, a disintegrin domain, a cysteine-rich domain, an EGF-like domain, a transmembrane domain, and a cytoplasmic tail (Black & White, 1998; Schlondorff & Blobel, 1999; Fig. 1). The Adams family has been implicated in the control of membrane fusion, cytokine and growth factor shedding, cell migration, cell adhesion, cell-cell and cell-matrix interactions, and signal transduction (Black, 2002; Liu et al., 2006). As Adamts described below, there is no sensitive bioassay for analyzing activity or activation of Adam in vivo.
The Adamts family is a subgroup of secreted zinc metalloproteases with several distinct domains separated from classical Mmp and Adam families (Tang and Hong, 1999; Apte, 2004; Porter et al., 2005). Members of the Adamts family share several distinct protein modules, including a pro-peptide domain, a metalloprotease domain, a disintegrin domain, a cysteine-rich region, and several repeats of thrombospondin type 1 (TSP1) motif. Some Adamts members have a unique C-terminal module (Fig. 1). The presence of TSP1 motifs is unique to Adamts family and is not found in Adam or Mmp family. Thrombospondin is a major constituent of platelet α-granules but is also secreted by a wide variety of epithelial and mesenchymal cells. Levels of thrombospondin are correlated with developmental changes in developing embryos and response to injury in adults (Bornstein,1992). Unlike Mmps or Adams, members of Adamts in general lack the ‘cysteine switch’ that controls activation of the pro-enzyme (Fig. 1), though Adamts15 seems to have the “cystine switch” (Kelwick et al., 2015). Interaction of propeptide domain with metalloprotease domain or binding of Timps to Adamts inhibits activities of the pro-enzyme (Kelwick et al., 2015). The first member, Adamts1, was cloned in mice and cattle as a gene suggested to be associated with inflammation and development (Colige et al., 1997; Kuno et al., 1997). The Adamts family has 19 highly structurally and sequentially conserved members in vertebrates (Brunet et al., 2015). Adamts enzymes cleave large proteoglycans such as aggrecan, versican, brevican and neurocan. They have been demonstrated to have important roles in connective tissue organization, coagulation, inflammation, arthritis, angiogenesis and cell migration (Apte, 2004; Kelwick et al., 2015).
Metalloproteases in germ cell migration and gonadal formation
Gonad formation is a remarkable tissue remodeling process. All vertebrates first form a bipotential gonad before adopting a male or female fate (McLaren, 1988; Koopman et al., 1991; Gubbay et al., 1992; Hawkins et al., 1992; Polanco and Koopman, 2007). The formation of a functional gonad begins with long-distance migration of PGCs to the presumptive gonad (Raz, 2002; Pearson et al., 2016; Piprek et al., 2018). In mammals, PGCs are specified and segregated early in gastrulation. This occurs at around six days post coitum (dpc) in mice (Lawson et al., 1999). Approximate four days later (around 10dpc), the gonadal primordium starts to develop from bilateral thickenings of the coelomic epithelium (Kusaka et al., 2010) and by 11.5 dpc proliferating PGCs have settled into the genital ridge (Harikae et al., 2013). Testis’s fate is determined by the expression of Sry, which initiates the differentiation of Sertoli cells and sequester PGCs inside testis cords by 12.5 dpc in mice (McLaren, 1988; Koopman et al., 1991). In the absence of the Sry gene, the competing ovarian signaling pathways will develop bipotential gonad into ovaries (Gubbay et al., 1992; Hawkins et al., 1992).
Zebrafish PGCs are also set aside early during gastrulation. PGCs complete their migration before 24 hours post fertilization (hpf) and migrate to the presumptive gonad located along the coelomic wall (Hartung et al., 2014; Kossack and Draper, 2019; Carver et al., 2021; Figs. 2&3). During the first week post fertilization (wpf), these PGCs (~30) are kept in a quiescent state and numbers of PGC do not change. In the following two weeks (2–3 wpf), germ cells and somatic gonad cells are actively proliferating and develop into an ovary-like bipotential gonad containing stage IA perinuclear-stage and stage IB oocytes. Two-types of stage IA divisions have been observed in medaka (Saito et al., 2007). Type I divisions generate individual daughter cells, while Type II divisions amplify and generate multiple daughter cells within a cyst. These stage IA oocytes are still connected to each other via an incompletely divided cell membrane, are forming multiple cyst-like structure, and appear in groups surrounded by pre-granulosa cells. As oocyte grow, some cysts are going to break down into individual stage IB follicle equivalent to primordial/primary follicles in mammals. Stage IB follicle will form when each oocyte is surrounded by single layer of granulosa cells (Takahashi, 1977; Saito et al., 2007; Tzung et al., 2015; Fig. 2), while some stage IA will go through programmed cell death and loss during the processes (Pepling and Spradling, 1998; Kloc et al., 2004; Saito et al., 2007; Lei and Spradling, 2013; Fig. 2). Thereafter, gonadal differentiation and primary sex determination start around three weeks when some immature oocytes (stage IA and IB follicles) begin to undergo apoptosis in presumptive males, whereas immature oocytes (stage IA & IB follicles) continue to grow (into stage II, III) and proliferate in presumptive females (Uchida et al., 2002). The transitioning phase of testis development and primary sex determination is typically over by ~ 30 days post fertilization (dpf), when majority of oocytes have been cleared from gonads that already differentiate as testes (Uchida et al., 2002; Fig. 2).
Figure 2. Schematic illustration of conserved processes for folliculogenesis and programmed cell death of ovarian follicles in animals.
Metalloproteases induce changes of intrafollicular structures and/or signaling which can lead to survival or programmed cell deaths of ovarian follicles in all stages of folliculogenesis. Major events or processes of oogenesis/folliculogenesis, and times of occurrence in zebrafish, mice and human are illustrated. For simplicity, only three representative metalloproteases are used as examples. Certain transcriptional or growth factors are well known to function in these processes (not shown). Adamts9 is expressed in the early germ cells. Adamts9 knockout (adamts9−/−) caused female-to-male sex conversion in adult zebrafish (see Carter al., 2019 for detail). Under normal conditions, an excessive programmed cell death of ovarian follicles is required for male sex determination in zebrafish that occurs around 4 weeks post fertilization (wpf) in early juveniles (around the red Stop sign in the figure). Thereafter, the gonadal sex is maintained throughout the life in wildtype (adamts9+/+) zebrafish. However, disproportionate loss of ovarian follicles during the development, in the juveniles or adults all could lead to females-to-males sex conversion in zebrafish (see Dranow et al., 2013; Dai et al., 2015; Tzung et al., 2015 for detail). In our Adamts9 knockout (adamts9−/−) zebrafish, unwarranted losses of ovarian follicles were still evident in the ovaries collected from middle-juvenile stage of zebrafish at eight-week post fertilization (wpf). A red arrowhead (in image D) indicates a leftover huge hole likely due to loss of a germ cell. A white arrowhead indicates that a stage IB oocyte is undergoing programmed death (image D). (A-C), Representative confocal images of Adamts9 expression in the early germ cells of gonads from an established transgenic zebrafish line Tg (adamts9:EGFP, Carver et al., 2021). A). a representative image of a pair of gonads from four wpf zebrafish; B). a part of six wpf ovary; C). a stage IB oocyte at six wpf (also see Carver et al., 2021 for additional detail). Similar expression of native Adamts9 protein in the germ cells of early stages was also confirmed by immunostaining using specific antibodies (data not shown). (D & E), Representative confocal images of ovaries at eight wpf. D), a representative image of Adamts9 knockout (−/−) ovary from adamts9−/− zebrafish at eight wpf; E), a representative image of a normal ovary from wildtype (+/+, adamts9+/+) sibling at 8wpf, in which stage I were developed further into stage II oocytes. In contrast, all follicles in Adamts9 knockout were arrested at early stage of stage IB. (F). Representative ovaries from 3-month old adult Adamts9 knockout (−/−) or wildtype (+/+) sibling, which were raised in enriched environments with low fish density and enhanced feeding. Enrichment completely rescued minor deficiencies in growth and development, but still could not rescue the deficiency in folliculogenesis and fertility. Ovaries in Adamts9 knockout (−/−) remain underdeveloped, Adamts9 knockout females are anovulatory and infertile, while ovaries in their siblings of Adamts9 wildtype (+/+) developed normally and ovulated. White arrowheads indicate ovulated ooctyes. Scale bars, A&B, 200 μm; C, 10 μm; D&E, 50 μm; F, 2mm. The apoptosis carton within the figure was modifed from an original carton with a permission from Dr. Vinita Bharat at Stanford who was the original creator of the carton.
Figure 3. Delay in primordial germ cells (PGCs) migration in Adamts9 knockout.
However, Adamts9 knockout (adamts9−/−) has no effect on the survival and proliferation of PGC during early development in zebrafish (see Carver et al., 2021 detail).
It is evident that migration of PGCs, degradation of unnecessary parts of the bipotential gonads, and formation of testes or ovaries require various signaling molecules and modification of ECM. Germ cells and gonadal somatic cells not only contribute to the content of the ECM but can also receive signals from the ECM through mechanical and chemical means, which are essential for cells to respond to their environments and key for gonadal sex development and differentiation (Fröjdman et al., 1989; Mackay, 2000). There is some evidence that metalloproteases are expressed in developing gonads in human (Robinson et al., 2001), mice (Chen et al., 2007; Díez-Torre et al., 2013; Piprek et al., 2018), chicken (Huh & Jung, 2013), and zebrafish (Mazzoni & Quagio-Grassiotto, 2020). The secretion of Mmp2, Mmp9 and four Timps was found in developing testes and ovaries in human fetuses. Mmp1, Mmp2, Mmp9 and Timps were localized to the interstitium and the tubules of the testis. Mmp9 and Timp4 were abundant in both Sertoli cells and gonocytes. In the ovary, all four Timps and Mmp1, Mmp2 and Mmp9 were localized to the oogonium/oocyte cytoplasm in the gonads of human fetuses (Robinson et al., 2001). Higher expression of Mt1-Mmp (Mmp14), Mmp2, Mmp11, Mmp9, and Timp were also found in migrating PGCs and genital ridge somatic cells in mice (Díez-Torre et al., 2013). Piprek and colleagues studied the expression of ECM enzymes during the sexual differentiation in mice. They found that most of these enzymes were expressed in supporting and interstitial/stromal cells, and a few enzymes were expressed in the germ cells in mice (Piprek et al., 2018). In zebrafish, at least three Mmps were found to be expressed in the somatic cells that were located ahead of the PGCs in the migration route after organogenesis and maintained throughout the mesentery but not expressed in the PGCs in zebrafish. Once PGCs reached the gonadal ridge, both the PGCs and somatic cells expressed Mmps (Mazzoni & Quagio-Grassiotto, 2020). Several matrix metalloproteases (Mmps) had also been implicated in testis development in chicken (Huh and Jung et al., 2013). Treatment of Mmps inhibitors caused accumulation of ECM and led to cells dispersion and disappearance of testis cords, whereas Mmps activator 4-aminophenylmercuric acetate (APMA) caused ECM loss and a complete disruption of the gonad structure in mouse gonads. In addition, these inhibitor or activators also increased apoptosis and the loss of germ cells, decreased Sertoli cell markers Sox9 and Amh, but did not cause sex reversal in mice (Piprek et al., 2019). However, functional studies of metalloproteases in gonad formation are still rare. A few natural mutations have been identified affecting ECM leading to defects in folliculogenesis, gonadal development, or sex determination, presumably due to 1) these natural mutations could not be passed to offspring due to embryonic lethality or parents’ infertility; 2) compensation from other members of metalloproteases.
In the knockout of Adamts9 ortholog, gon-1 in C. elegans, germ cells did not migrate, and the gonad developed as a disorganized mass of somatic and germ line tissues (Blelloch et al., 1999; Blelloch & Kimble, 1999; Nishiwaki et al., 2000). Gon-1 helped migration of distal tip cells by degrading extracellular matrix components. In Gon-1 mutants, the adult gonad was severely disorganized with no arm extension and no recognizable somatic structure. The developmental defects in gon-1 mutants were limited to the gonad. Other cells, tissues, and organs developed normally in C. elegans (Blelloch et al., 1999; Blelloch & Kimble, 1999; Nishiwaki et al., 2000). In Drosophila, the knockout of Adamts9 ortholog, AdamtTS-A caused the mis-migration of collective cells, including germ cells (Ismat et al., 2013). These studies of Adamts9 orthologs in C. elegans and Drosophila suggest this protease may release a signal or clear a path for PGC to migrate correctly in invertebrates. However, partly due to embryonic lethality in the knockouts of Drosophila and mouse models (Enomoto et al., 2010; Ismat et al., 2013; Nandadasa et al., 2015; Tharmarajah et al., 2018), the underlying mechanisms of Adamts9 in PGC migration, gonad development and formation is still unclear.
We found that Adamts9 was expressed in the germ cells of stage I immature follicles, but the expression was greatly reduced or disappeared in the germ cells of the growing or mature follicles (stages III or V) in zebrafish (Carver et al., 2021; Fig. 2). During the embryonic development, zygotic expression of adamts9 increased significantly around eight somite stage (~10 hpf) in wildtype zebrafish. A few hours later, we found a delay in PGC migration at 15 and 24 hpf in the Adamts9 knockout (adamts9−/−) zebrafish (Carver et al., 2021; Fig. 3). In wildtype siblings, all PGCs completed their migration to gonadal ridge around 24hpf (Weidinger et al., 2002; Carver et al., 2021; Fig. 3), whereas PGCs would take 48 hours to migrate to the gonadal ridge in Adamts9 knockout zebrafish (Carver et al., 2021; Fig. 3). Our results suggest a conserved function of Adamts9 in germ cell migration among vertebrates and invertebrates. Our results also suggest that Adamts9 is not essential in the germ cell migration in the zebrafish as demonstrated in C. elegans (Blelloch et al., 1999; Blelloch & Kimble, 1999; Nishiwaki et al., 2000). The role of Adamts9 in germ cell migration in zebrafish is likely to be shared by other proteases since members of metalloproteases are dramatically expanded in vertebrates (Brunet et al. 2015).
It is well established that the number of germ cells is important for gonadal development and sex determination in zebrafish (Dranow et al., 2013; Dai et al., 2015; Tzung et al., 2015). A threshold number of germ cells is required for ovarian development. The depletion of germ cells or ovarian follicles in the development or the adult would cause female-to-male conversion (Dranow et al., 2013; Dai et al., 2015; Tzung et al., 2015). Intriguingly, most Adamts9 knockout adult zebrafish developed as males (Carter et al., 2019; Figs 2&4). Therefore, we hypothesized that the unusual development of ovaries and severe male biased sex ratio found in Adamts9 knockout zebrafish was due to deficiencies in the germ cell migration, apoptosis, proliferation of PGCs or germ cells during primary sex determination. Surprisingly, we found Adamts9 had no effects in the numbers of germ cells during PGC migration (Carver et al., 2021). Thus, the prospect of PGC migration defects as a possible cause for sex reversal in Adamts9 knockout is low. Further research in our lab identified another surprising fact: Adamts9 knockout has no effects on sex ratio after the primary sex determination, but significantly reduced the number and size of stage IB follicles in Adamts9 knockout at 5 wpf (right after primary sex determination) and affected long-term viability of stage IB follicles in zebrafish (Fig. 2; Carver and Zhu, unpublished). Therefore, the likely cause of severe male sex ratio biases in Adamts9 knockout adult zebrafish is not due to PGC migration or primary sex determination, but due to defects in early folliculogenesis, which lead to reduced viability of ovarian follicles, programmed germ cell death, and finally sex reversal in adult zebrafish. The processes and signaling that control folliculogenesis, especially in early stages of development, are highly conserved among different species (Fig. 2). Therefore, our Adamts9 knockout provide a new opportunity for examining functions and mechanisms of metalloproteases in folliculogenesis, germ cell development, follicle losses and sex reversal in animals. Elucidation of underlying mechanisms of Adamts9 in these processes and additional proteases in PGCs migration are ongoing studies in the lab.
Figure 4. A proposed model for metalloproteases’ actions in ovarian follicles.
I used Adamts9 as an example for illustration. Metalloproteases can promote or inhibit intrafollicular signaling via cleavage of ECM proteins including cell surface receptors and/or their ligands. Regulation ECM and intrafollicular signaling determines survival and programmed cell deaths of ovarian follicles.
Metalloproteases in ovulation
Ovulation is a well-studied physiological process that releases a fertilizable oocyte from surrounding follicular cells. This process is initiated by luteinizing hormone (LH) that triggers multiple signaling pathways including progestin and prostaglandin signaling, and finally activates downstream metalloproteases, eventually leading to changes in ECM and rupture of follicular layers (Richards, 2018; Takahashi et al., 2019). The components of ECM have been studied extensively in the ovaries of various mammalian species and a few fish species using in situ, immunohistochemistry and PCR (Irving-Rodgers & Rodgers 2006, Berkholtz et al. 2006, Curry & Smith 2006; Thomé et al. 2010; Kato et al. 2010). Various ECM proteins including collagen, aggrecan, versican, fibronectin and laminin were found in the ovary of various mammalian and fish species (Rodgers et al. 2003; Horiguchi et al., 2008; Kato et al., 2010; Takahashi et al., 2019). Collagen I, IV, versican and laminin were thought to be major constituents of ECM proteins in ovaries of mammals and fish (Lind et al. 2006b; Santos et al. 2008; Thomé et al. 2010; Kato et al. 2010). Regulation of the ECM is critically important not only for the regulation of ovarian cell morphology and release of mature oocytes, but also for paracrine and endocrine signaling, differentiation, proliferation, and apoptosis of gonad somatic cells and germ cells (Woodruff and Shea, 2007). At ovulation the follicular basal lamina and extracellular collagens were degraded, presumably by metalloproteases (Smith et al., 1999; Curry & Smith, 2006). The involvement of metalloproteases including members of Mmp, Adam, and Adamts families, have been examined in various species (Robker et al., 2000; Richards, 2005, 2018; Brown et al., 2006; Sriraman et al., 2008; Peluffo et al., 2011; Takahashi et al., 2013, 2019; Duffy et al., 2019). Most of our knowledge on the candidates and involvements of metalloproteases are from studies of hormonal regulations or changes of these metalloproteases during ovulation in various animal species. It is important to note that I will focus on the most likely metalloproteases that may have critical roles in the ovulation in various species, and to discuss whether their roles in the ovulation are conserved across different species.
Adamts1 in ovulation
Proteoglycans such as aggrecan and versican were identified as main substrates of Adamts1 (Kuno et al., 2000; Rodriguez-Manzaneque et al., 2002; Rodríguez-Baena, 2018). Versican isoforms were the main proteoglycans regulated by follicle stimulating hormone (FSH) and testosterone but not by nuclear progestin receptor (Pgr) in rat and mice ovaries (Russell et al., 2003). Adamts1 could also cleave thrombospondin-1 and thrombospondin-2 (Lee et al., 2006), and ligands of the epidermal growth factor (EGF) receptor such as pro-HBEGF (heparin-binding EGF-like growth factor) or pro-amphiregulin (Liu et al., 2006). Novel substrates such as syndecan 4, TFPI-2, semaphorin 3C, nidogen-1, −2, desmocollin-3, dystroglycan, mac-2, gelatin and TGF-α were also identified (Satz-Jacobowitz & Hubmacher, 2021). Adamts1 was implicated in inflammatory processes (Kuno et al., 1997; Rodríguez-Baena, 2018). Studies using Adamts1 knockout mice showed that this protease was important for normal growth, organogenesis and fertility (Shindo et al., 2000; Mittaz et al., 2004; Brown et al. 2010; Table 1).
Expression studies of adamts1 have suggested the involvement of Adamts1 in ovulation and fertility in mammals. Transcripts of adamts1 were upregulated in the preovulatory follicles of several mammalian species including mice (Robker et al., 2000; Shindo et al., 2000; Shozu et al., 2005), human (Freimann et al., 2005), horse (Boerboom et al., 2003; Brown et al., 2006), and cattle (Fortune et al., 2009; Sayasith et al., 2013, Table 1). This upregulation was partly dependent on the expression of Pgr in the granulosa cells (Robker et al., 2000). However, increase of adamts1 transcript was only observed after ovulation in rhesus monkey, which suggests Adamts1 may not play a role in ovulation for them (Peluffo et al., 2011). The studies of Adamts1 in non-mammalian species is limited. A recent study in zebrafish found that adamts1 transcript increased significantly in the preovulatory follicular cells. However, the expression of adamts1 transcript was not affected in pgr−/− zebrafish in comparison to those in their wildtype sibling. The adamts1 transcripts were highest in stage I immature follicles and gradually decreased as follicles grew, which suggests additional roles for Adamtsl in early folliculogenesis (Liu et al., 2018). A more recent study also confirmed that progestin did not stimulate the expression of adamts1 transcripts in zebrafish follicles (Baker & Van Der Kraak, 2021). Interestingly, adamts1 expression was upregulated by prostaglandins (PGE2) specifically via a prostaglandin receptor subtype (EP4) (Baker & Van Der Kraak, 2021).
Knockout of Adamtsl in mice suggests an important role of this protease as a downstream effector of Pgr in ovulation. Homozygous Adamtsl knockouts were subfertile, producing litters four-to five-fold less than control littermates, partially due to failed rupture of some large follicles (Shozu et al., 2005). It should be noted that the subfertility was observed in the global Adamtsl knockout mice. Retarded growth and follicular cell development were also observed in this global Adamtsl knockout mice, which also likely contributed to the observed subfertility (Thai and Iruela-Arispe, 2002; Shozu et al., 2005). Nevertheless, Adamtsl knockout mice were subfertile and had less severe phenotypes than Pgr knockout mice, which could not ovulate and therefore had no litters. This indicates that other Pgr regulated proteases may also contribute to ovulation.
Adamts9 in ovulation
In COS-l cells transfected with human adamts9, this protease could proteolytically cleaved bovine versican and aggrecan core proteins at the Glu44l–Ala442 bond of versican Vl and the Glul77l–Alal772 bond of aggrecan, respectively (Somerville et al., 2003). Recently, Adamts9 was also found to bind fibronectin dimers and fibrils directly through multiple sites in both molecules. An Adamts9 cleavage at Gly2196-Leu2197, which was also target of other proteases, was identified (Wang et al., 2019). In Adamts9 deficient mice, reduced cleavage of versican was observed (Kern et al., 2010; Dubail et al., 2014). Aggrecan, versican and fibronectin are currently considered to be the main substrates of Adamts9 (Somerville et al., 2003; Wang et al., 2019; Satz-Jacobowitz and Hubmacher, 2021). As more novel substrates have been identified for Adamts1, it is likely that many new substrates of Adamts9 will be discovered in the future.
Adamts9 is another member of Adamts family likely to be critical for ovulation, as Adamts9 was regulated by LH and progestin, and dramatically increased its expression during ovulation both in fish and mammalian species (Peluffo et al., 2011; Wissing et al., 2014; Liu et al.,2017, 2018, 2020; Table 1). Transcripts of adamts9 were found to be upregulated in preovulatory follicles or granulosa cells following hCG treatment in macaque and human (Peluffo et al., 2011; Wissing et al., 2014). A synthetic Pgr agonist, R5020, could reverse the inhibitory effect of mifepristone (RU486) on the LH induced expression of adamts1 and adamts9 mRNA in granulosa cells of cattle (Fortune et al. 2009; Willis et al., 2017). In zebrafish, we found that adamts9 was the downstream target of LH and progestin. Expression of adamts9 in ovarian follicles was regulated by LH and progestin both in vivo and in vitro. Transcripts and proteins of Adamts9 increased dramatically in preovulatory follicular cells during ovulation, and the expression of Adamts9 proteins and transcripts were significantly low in Pgr knockout zebrafish (Liu et al., 2017, 2018, 2020; Carver et al., 2021).
The knockout of Adamts9 ortholog, gon-1 in C. elegans survived normally, but led to infertility mainly due to defects in primordial germ cells migration and formation of non-functional gonads (Blelloch et al., 1999; Blelloch & Kimble, 1999; Nishiwaki et al., 2000). Recently, male biased gonadal sex ratio and female infertility were reported in Adamts9 knockout zebrafish (Carter et al., 2019, Figs 2&4). However, Adamts9’s role in ovulation in vertebrates have not been firmly established by the knockout studies, due to Adamts9 knockout mice dying before gastrulation (Enomoto et al., 2010). Furthermore, overwhelming adult Adamts9 knockout zebrafish were males, and a few Adamts9 knockout females have underdeveloped ovaries and infertile (<7% females in Adamts9 KO zebrafish vs. 33% females in wildtype and heterozygous siblings, Carter et al., 2019, Fig. 2).
Mmp2 in ovulation
Mmp2, also known as gelatinase A, has a wide range of substrates including collagen, elastin, endothelin, fibroblast growth factor, Mmp9, Mmp13, plasminogen, and Tgf-β (Bassiouni et al., 2021; DeCoux et al., 2014).
Mmp2 is another metalloprotease that most likely causes follicular rupture during ovulation in various animal species. Increases of mmp2 transcripts or type IV collagenolysis were reported in periovulatory follicles of women (Puistola et al., 1986; Baka et al., 2010), rats (Reich et al., 1991; Curry et al., 1992, 2001; Bagavandoss, 1998; Liu et al., 1999), monkeys (Chaffin and Stouffer, 1999), medaka (Ogiwara et al., 2005), mice (Nagaraja et al., 2010), cat (Fujihara et al., 2016; Kehoe et al., 2021), and Ciona intestinalis (an Ascidiacea, Matsubara et al. 2019). There was a progressive increase in follicular Mmp2 production during the periovulatory period in sheep. Mmp2 was localized within connective tissue strands that extend into the substance of the corpus luteum and form an infrastructure for thecal invasion and neovascularization (Gottsch et al., 2000). The transcripts, proteins and/or activities of Mmp2 were also upregulated by FSH or LH in growing or preovulatory follicles in chicken (Wolak et al., 2021), brook trout (Crespo et al., 2013), rat (Jo et al., 2004), and cow (Imai et al., 2003). Knockdown Mmp2, but not Mmp1, caused anovulation and subfertility in Drosophila (Deady et al., 2015). However, other studies suggested that Mmp2 was not the key acute regulator for the changes in follicle shape immediately prior to ovulation (Riley et al., 2004; Sessions et al., 2009). No changes of Mmp2 were found throughout follicular development in mares, although the predominant gelatinase in follicular fluid was Mmp2 (Riley et al., 2001). We found that mmp2 transcripts were high in immature follicles, but low in preovulatory follicles and decreased prior to ovulation in zebrafish (Liu et al., 2018). We also found the expression of mmp2 transcript was not different in ovarian follicles between Pgr knockout and wildtype zebrafish (Liu et al., 2018), It was also reported that Mmp2 was not the target of Pgr during ovulation in mice (Robker et al. 2000). An obligate function for Mmp2 in ovulation may be species dependent. Indeed, mice or zebrafish deficient in Mmp2 had normal fertility (Itoh et al., 1997; Hayashidani et al., 2003; Kok et al., 2015, Table 1).
Mmp9 in ovulation
Mmp9, known as gelatinase B, also have a wide range of substrates including collagen, elastin, aggrecan, gelatin, laminin and a variety of non-ECM substrates such as casein, plasminogen, and Tgf-β1 (Bassiouni et al., 2021; Skjøt-Arkil et al., 2012; Kobayashi et al., 2014).
Transcripts of mmp9 and its gelatinase activity increased in the preovulatory follicles of chicken (Hrabia et al. 2019), pigs (Kim et al., 2014), mares (Riley et al., 2001), rats (Curry et al., 2001), cattle (Goldberg et al., 1996; Zhao and Luck, 1996), rhesus macaque (Peluffo et al., 2011), women (Duncan et al., 1998), and Ciona intestinalis (Matsubara et al. 2019). LH and FSH were shown to be important for maintaining and increasing of Mmp9 transcript, protein, and its enzymatic activities in vitro in porcine granulosa cells (Kim and Yoon, 2020). We also found that mmp9 transcript was high in immature follicles, and low in mature follicles in zebrafish (Liu et al., 2018). Interestingly, the expression of mmp9 transcript increased significantly in the follicular cells of preovulatory follicles, and this increase was significantly suppressed in Pgr knockout zebrafish (Liu et al. 2018).
In contrast, it was found that LH suppressed Mmp9 proteins in rhesus monkeys (Young and Stouffer, 2004). Both FSH and IGF1 inhibited mmp2 and mmp9 mRNA expression in vitro in bovine granulosa cells. The specific down regulation by the gonadotropic hormones FSH and IGF1 in vitro suggests that excess Mmp2 and Mmp9 expression is neither required nor desired for follicle development in cattle (Portela et al., 2009). The mmp9 transcript was induced by Tgf-β1 but not by FSH, LH, progesterone, or estrogen in chicken (Zhu et al., 2014). The expression of Mmp9 in ovaries of PGR knockout mice was like that in their wild-type littermates (Robker et al., 2000). Both Mmp9 knockout mice and zebrafish were fertile (Itoh et al., 1999; Silva et al., 2020, Table 1), which suggest Mmp9 is not essential for ovulation in mice and zebrafish.
Other proteases and their endogenous inhibitors in ovulation.
Adam8 was found to be a downstream target of Pgr in murine ovary (Sriraman et al., 2008). Both Adam8 mRNA and protein were expressed in granulosa cells and cumulus cells of periovulatory follicles. The adam8 expression was also significantly stimulated in the follicles by hCG and greatly reduced in Pgr KO mice. Basal activities of adam8 promoters could be increased with co-expression of Pgr and its co-activators (Sriraman et al., 2008). We found a significant increase of adam8b expression in preovulatory follicles of wildtype zebrafish, while this increase was blocked in Pgr KO (pgr−/−) zebrafish (Liu et al., 2017, 2018, Table 1) that failed to ovulate (Zhu et al., 2015). Transcripts of adam 17 showed a transit increase in granulosa and theca interna cells of bovine preovulatory follicles following treatments of hCG or forskolin, and this increase was dependent on the response elements of forskolin in promoter region of adam17 (Sayasith & Sirois, 2015). The mmp19 and timp1 transcripts were localized in the granulosa and thecal-interstitial cells of large preovulatory or ovulating follicles, and significantly increased (5–10 fold) following hCG treatment in mice. It was suggested that the increase Mmp19 was involved in ECM degradation that occurred during follicular rupture and that Timp1 could have a role in terminating Mmp19 activity after ovulation (Hägglund et al., 1999). Similar gonadotropin induced mmp19 expression was also reported in at granulosa cells of rats and rhesus macaques (Jo & Curry, 2004; Peluffo et al., 2011), but not theca-interstitial cells in rats, suggesting a role of this proteinase during follicular growth, ovulation, and luteal regression (Jo & Curry, 2004). Estrogen is considered as an inhibitory factor for oocyte maturation and ovulation. Interestingly, Mmp19 was identified as a downstream target of ERβ, and downregulated in ERKO mouse ovaries (Nalvarte et al., 2016.). The expression of mmp23 (a membrane-anchored Mmp) transcripts was spatially and temporally regulated by gonadotropin during follicular development in rats (Ohnishi et al., 2001). However, the functions of Mmp23 in folliculogenesis and ovulation are still unclear (Ohnishi et al., 2005, Table 1).
An increase in mmp1, mmp2, mmp7, timp1, timp2 transcripts was found within 12 hours after hCG administration in macaque. Interestingly, mmp9 mRNA did not increase until 36 hours after hCG treatment, immediately prior to ovulation (Chaffin and Stouffer, 1999). Trilostane, an inhibitor of 3β-hydroxysteroid dehydrogenase could inhibit hCG stimulated expression of mmp1, mmp2, mmp7, timp1, timp2 transcripts, while transcripts of mmp1 and timp1 were comparable to those in control in combined treatments of progestin (R5020), hCG and trilostane (Chaffin and Stouffer, 1999). Administration of hCG in dominant follicles rapidly increased transcripts of mmp1, mmp10, mmp19, adamts4, adamts9, and adamts15 in rhesus monkey (Peluffo et al., 2011). No changes in Mmp2, Mmp9, or Timp2 protein expression were found in dominant follicles during the ovulatory phases in women (Lind et al., 2006a). Transcripts of mmp10 increased significantly between the preovulatory and early ovulatory periods and remained elevated at the late ovulatory phase in granulosa cells in human ovulatory follicles. In contrast, transcripts of mmp11 decreased significantly after hCG treatment in both granulosa and thecal cells in human (McCord et al., 2012). Increases of mmp1, mmp19, adamts1, and adamts9 transcripts were also found in the granulosa cells in human in response to hCG stimulation (Rosewell et al., 2015).
Studies of plasmin/plasminogen also suggest their involvements in the ovulation, activation of other Mmps, and degradation of ECM in mammals (Curry and Smith, 2006; Liu et al., 2004; Murdoch and McDonnel, 2002; Ohnishi et al., 2005; Peluffo et al. 2011; Ogiwara et al., 2012). However, double knockout of two main plasminogen activators only decreased ovulation by 26%, suggesting that plasmin/plasminogen contribute to ovulation but are not essential (Leonardsson et al., 1995, Table 1). Cathepsin L, a lysosomal cysteine protease member of the papain family was also shown to be induced by progestin in preovulatory follicles of rodents (Robker et al., 2000).
In medaka, at least five distinct proteases (Plau1, plasmin, Mmp2, Mmp14 and Mmp15) was shown to be responsible for the degradation ECM during ovulation. However, Mmp15 was the only protease that is drastically induced in the granulosa cells of preovulatory follicles by LH (Ogiwara et al., 2012; Hagiwara et al., 2014). Transcripts of mmp2, mmp9, adamts1,plg, and timp2 were also found to be up-regulated by LH in vitro in brown trout (Crespo et al., 2013). Significant increase of adam8b, adamts1, adamts8a, adamts9, and mmp9 transcripts in the follicular cells were also observed in the preovulatory follicles of zebrafish (Liu et al., 2017, 2018). Takahashi and colleagues (Takahashi et al., 2019) proposed a “two-step ECM hydrolysis mechanism’ model for follicle rupture based on changes of expression and activities of proteases during ovulation in medaka. In this model, two key proteases (Plaul & Mmp2) are constantly synthesized and released, but their activities are relentlessly blocked by protease inhibitors, Pai1 for Plau1 and Timp2b for Mmp2. In the 1st step of this ECM hydrolysis model, expression and secretion of Pai1 (inhibitor for Plau1) from granulosa cells decrease considerably approximately 7 hours prior to the ovulation, thus releasing Plau1 from the inhibition. Active Plau1 starts converting membrane-bound plasminogen to the active enzyme plasmin, which in turn hydrolyzes laminin, a major ECM component of basement membrane in follicles. Around 3 hours before ovulation with sufficient degradation of laminin, the activity of Plau1 and conversion of plasminogen to plasmin are blocked again by Pai1 due to resumed synthesis and secretion of Pai1. Mmp2 is constitutively synthesized in the oocyte as its inactive precursor proMmp2. ProMmp2 is then activated by membrane-bound Mmp14 (also known as Mt1-Mmp) which is expressed constitutively on the cell surface of the oocyte. However, Timp2b, which is also produced and secreted from the oocyte to the extracellular space of the follicle, binds to Mmp2 to suppress the enzyme activity until 3 hours prior to ovulation. In the 2nd step of this model, Timp2b in the extracellular space of the periovulatory follicle is drastically reduced around 3 hours before ovulation. Removing inhibition of Timp2b on Mmp2 permits the active Mmp2 to degrade collagen type IV in the basement membrane, and Mmp15 (also known as Mt2-Mmp) to cleave collagen I in the extracellular space. Interaction of Mmp15 with collagen I is achievable only after the breakdown of the basement membrane by Plasmin and Mmp2 (Takahashi et al., 2019).
It is clear that interplay of multiple proteases for degrading various ECM is required for the rupture of follicular layers and extracellular membranes in order to release ovulated oocytes. The biological effects of these proteases are dependent on de novo production, proteolytic activation and endogenous tissue inhibitor of matrix metalloprotease (Timp) concentrations. Studies of changes and hormonal regulations of various proteases in different animal species have increased our knowledge on the candidates of proteases involved. However, significant number of these studies only examined changes of transcripts, which may not reflect changes of proteins and enzymatic activities. So far, knockout studies of most metalloproteases in mice or zebrafish have provided little information on the functions of these proteases (Table 1), mainly due to knockouts either dying in utero or exhibiting non-observable defects in ovulation (Carmeliet et al. 1993; Peschon et al. 1998; Vu and Werb 2000; Kelly et al. 2005; Enomoto et al. 2010).
Summary and Future Directions
Intensive interests and research in past decades have shown changes of various metalloproteases during ovulation in various species. Our understanding is also advanced by hormone regulation of these metalloproteases and differential expression in the knockouts vs. wildtype for metalloproteases including Adamts1, Adamts9 and others. Unlike 100% anovulation and infertility found in Pgr KOs in the rat, mouse, and zebrafish (Lydon et al., 1995; Zhu et al., 2015; Kubota et al., 2016; Tang et al., 2016), none of metalloprotease knockout could completely block ovulation. A few of these metalloprotease knockouts are embryonic lethal, while no obvious reproductive defects in the ovulation or fertility were reported for most metalloprotease knockouts (Table 1). It is well known that same substrate or even same cleavage site could be the target of several metalloproteases (Wang et al., 2019). Loss of one or even two metalloproteases could be compensated by other metalloproteases. Therefore, no observable deficiency in the knockouts only suggests that specific metalloprotease is not essential for the process; however, does not suggest that the protease has no function in the reproductive process such as ovulation. On the other hand, subfertility observed in Adamts1 global knockout mice also needs to be interpreted with caution. Most of these metalloproteases including Adamtsl are expressed broadly in various tissues, which also have effects in the processes other than reproduction such as growth and development of other vital organs including the brain and heart. To further explore roles and mechanisms of these metalloproteases in ovulation, additional tools such as conditional knockouts, specific antibodies for ECM proteins, transgenic reporter lines and sensitive in vivo bioassay that allow studying dynamic changes of ECM and signaling molecules need to be developed.
Our knowledge on the roles and mechanisms of metalloproteases in PGC migration, gonad formation and sex differentiation, especially in vertebrates is still lacking. The fast generation of knockouts, availability of transgenic lines that label PGCs, gonad somatic cells and specific ECM molecules with fluorescent proteins and nearly transparent embryos made zebrafish an excellent vertebrate model for studying germ cell migration at a high resolution within a live organism (Raz, 2002; Dumstrei et al., 2004). Transgenic zebrafish lines in different genetic backgrounds in addition to Adamts9 knockout, and additional new research reagents will permit studying the roles and mechanisms of various metalloproteases and signaling molecules in PGC migration and gonad development starting from the earliest stages of their development through gonadal differentiation or sex reversal in adult animals. New research reagents, new models and new knowledge for the gonadal formation and ovulation are likely to be generated from these future studies.
Highlights.
Adamts9 has a role in PGC migration in zebrafish.
Adamts9 is critical for the development and maintenance of ovarian follicles.
Metalloprotease expression including Adamts1, Adamts9, Mmp2, and Mmp9 change during gonadal development and ovulation.
Post-translational activation of metalloproteases is critical for their functions.
Lack of clear reproductive phenotypes in most metalloprotease knockouts is likely due to multiple proteases complementing each other in their ECM targeting.
Acknowledgments
Funding
This work was supported by the NIH GM100461 to YZ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abdul-Majeed S, Mell B, Nauli SM, Joe B. 2014. Cryptorchidism and infertility in rats with targeted disruption of the Adamts16 locus. PLoS One. 9, e100967. doi: 10.1371/journal.pone.0100967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apte SS 2004. A disintegrin-like and metalloprotease (reprolysin type) with thrombospondin type 1 motifs: the ADAMTS family. Int. J. Biochem. Cell Biol. 36, 981–985. [DOI] [PubMed] [Google Scholar]
- 3.Asgari R, Mansouri K, Abdolmaleki A, Mitra Bakhtiari M. 2021. (in press) Association of matrix metalloproteases with male reproductive functions; with focus on MMP2, 7, and 9. Mate Gene. 10.1016/j.mgene.2021.100906 [DOI] [Google Scholar]
- 4.Bagavandoss P 1998. Differential distribution of gelatinases and tissue inhibitor of metalloprotease-1 in the rat ovary. J Endocrinol 158, 221–228. [DOI] [PubMed] [Google Scholar]
- 5.Baka S, Zourla K, Kouskouni E, Makrakis E, Demeridou S, Tzanakaki D, Hassiakos D, Creatsas G. 2010. Matrix metalloproteases 2 and 9 and their tissue inhibitors in the follicular fluid of patients with polycystic ovaries undergoing in vitro fertilisation. In Vivo. 24, 293–296. [PubMed] [Google Scholar]
- 6.Baker SJC, Van Der Kraak G. 2021. ADAMTS1 is regulated by the EP4 receptor in the zebrafish ovary. Gen CompEndocrinol. 311, 113835. doi: 10.1016/j.ygcen.2021.113835. [DOI] [PubMed] [Google Scholar]
- 7.Bahudhanapati H, Bhattacharya S, Wei S. 2015. Evolution of vertebrate Adam genes; duplication of testicular Adams from ancient Adam9/9-like loci. PLoS One. 10, e0136281. doi: 10.1371/journal.pone.0136281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barseghyan H, Symon A, Zadikyan M, Almalvez M, Segura EE, Eskin A, Bramble MS, Arboleda VA, Baxter R, Nelson SF, Délot EC, Harley V, Vilain E. 2018. Identification of novel candidate genes for 46,XY disorders of sex development (DSD) using a C57BL/6J-Y POS mouse model. Biol Sex Differ. 9, 8. doi: 10.1186/s13293-018-0167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartlett JD, Beniash E, Lee DH, Smith CE. 2004. Decreased mineral content in MMP-20 null mouse enamel is prominent during the maturation stage. J Dent Res. 83, 909–13. doi: 10.1177/154405910408301204. [DOI] [PubMed] [Google Scholar]
- 10.Bassiouni W, Ali MAM, Schulz R. 2021. (in press). Multifunctional intracellular matrix metalloproteinases: implications in disease. FEBS J. doi: 10.1111/febs.15701. [DOI] [PubMed] [Google Scholar]
- 11.Baumgart E, Lenk SV, Loening SA & Jung K 2002. Quantitative differences in matrix metalloprotease (MMP)-2, but not in MMP-9, tissue inhibitor of metalloprotease (TIMP)-1 or TIMP-2, in seminal plasma of normozoospermic and azoospermic patients. Hum. Reprod. Oxf. Engl 17, 2919–2923. [DOI] [PubMed] [Google Scholar]
- 12.Beck IM, Rückert R, Brandt K, Mueller MS, Sadowski T, Brauer R, Schirmacher P, Mentlein R, Sedlacek R. 2008. MMP19 is essential for T cell development and T cell-mediated cutaneous immune responses. PLoS One. 3, e2343. doi: 10.1371/journal.pone.0002343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belardin LB, Antoniassi MP, Camargo M, Intasqui P, Fraietta R, Bertolla RP. 2019. Semen levels of matrix metalloprotease (MMP) and tissue inhibitor of metallorproteinases (TIMP) protein families members in men with high and low sperm DNA fragmentation. Sci Rep. 2019 9:903. doi: 10.1038/s41598-018-37122-4. Erratum in: Sci Rep. 9,10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berkholtz CB, Lai BE, Woodruff TK, Shea LD. 2006. Distribution of extracellular matrix proteins type I collagen, type IV collagen, fibronectin, and laminin in mouse folliculogenesis. Histochem Cell Biol. 126, 583–92. doi: 10.1007/s00418-006-0194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhyan SB, Wee Y, Liu Y, Cummins S, Zhao M. 2019. Integrative analysis of common genes and driver mutations implicated in hormone stimulation for four cancers in women. PeerJ. 7, e6872. doi: 10.7717/peerj.6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Black RA. 2002. Tumor necrosis factor-alpha converting enzyme. Int J Biochem Cell Biol 34, 1–5. doi: 10.1016/s1357-2725(01)00097-8. [DOI] [PubMed] [Google Scholar]
- 17.Black RA, White JM.1998. ADAMs: Focus on the protease domain. Curr. Opin. Cell. Biol 10, 654–659. [DOI] [PubMed] [Google Scholar]
- 18.Blelloch R, Kimble J. 1999. Control of organ shape by a secreted metalloprotease in the nematode Caenorhabditis elegans. Nature. 399, 586–90. [DOI] [PubMed] [Google Scholar]
- 19.Blelloch R, Anna-Arriola SS, Gao D, Li Y, Hodgkin J, Kimble J. 1999. The gon-1 gene is required for gonadal morphogenesis in Caenorhabditis elegans. Dev Biol. 216, 382–93. [DOI] [PubMed] [Google Scholar]
- 20.Boerboom D, Russell DL, Richards JS, Sirois J. 2003. Regulation of transcripts encoding ADAMTS-1 (a disintegrin and metalloprotease with thrombospondin-like motifs-1) and progesterone receptor by human chorionic gonadotropin in equine preovulatory follicles. J Mol Endocrinol. 31, 473–85. doi: 10.1677/jme.0.0310473. [DOI] [PubMed] [Google Scholar]
- 21.Bornstein P 1992. Thrombospondins: structure and regulation of expression. FASEB J. 6, 3290–3299. doi: 10.1096/fasebj.6.14.1426766. [DOI] [PubMed] [Google Scholar]
- 22.Brown HM, Dunning KR, Robker RL, Pritchard M, Russell DL. 2006. Requirement for ADAMTS-1 in extracellular matrix remodeling during ovarian folliculogenesis and lymphangiogenesis. Dev Biol. 300, 699–709. doi: 10.1016/j.ydbio.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 23.Brown HM, Dunning KR, Robker RL, Boerboom D, Pritchard M, Lane M, Russell DL. 2010. ADAMTS1 cleavage of versican mediates essential structural remodeling of the ovarian follicle and cumulus-oocyte matrix during ovulation in mice. Biol Reprod. 83, 549–57. doi: 10.1095/biolreprod.110.084434. [DOI] [PubMed] [Google Scholar]
- 24.Brunet FG et al. 2015. The evolutionary conservation of the a disintegrin-like and metalloprotease domain with thrombospondin-1 motif metzincins across vertebrate species and their expression in teleost zebrafish. BMC Evol. Biol 15, doi: 10.1186/s12862-015-0281-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carmeliet P, Kieckens L, Schoonjans L, Ream B, van Nuffelen A, Prendergast G, et al. 1993. Plasminogen activator inhibitor-1 gene-deficient mice. I Generation by homologous recombination and characterization. J Clin Invest. 92, 2746–55. doi: 10.1172/JCI116892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carmeliet P, Schoonjans L, Kieckens L, Ream B, Degen J, Bronson R, De Vos R, van den Oord JJ, Collen D, Mulligan RC. 1994. Physiological consequences of loss of plasminogen activator gene function in mice. Nature. 368, 419–24. doi: 10.1038/368419a0. [DOI] [PubMed] [Google Scholar]
- 27.Carter NJ, Roach ZA, Byrnes MM, Zhu Y. 2019. Adamts9 is necessary for ovarian development in zebrafish. Gen Comp Endocrinol. 277, 130–140. doi: 10.1016/j.ygcen.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Carver JJ, He Y, Zhu Y. 2021. Delay in primordial germ cell migration in adamts9 knockout zebrafish. Sci Rep 11, 8545. 10.1038/s41598-021-88024-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caterina JJ, Yamada S, Caterina NC, Longenecker G, Holmbäck K, Shi J, Yermovsky AE, Engler JA, Birkedal-Hansen H. 2000. Inactivating mutation of the mouse tissue inhibitor of metalloproteinases-2(Timp-2) gene alters proMMP-2 activation. J Biol Chem. 275, 26416–22. doi: 10.1074/jbc.M001271200. [DOI] [PubMed] [Google Scholar]
- 30.Chaffin CL, Stouffer RL. 1999. Expression of matrix metalloproteases and their tissue inhibitor messenger ribonucleic acids in Macaque periovulatory granulosa cells: time course and steroid regulation. Biol Reprod 61,14–21. [DOI] [PubMed] [Google Scholar]
- 31.Charlton-Perkins M, Almeida AD, MacDonald RB, Harris WA. 2019. Genetic control of cellular morphogenesis in Müller glia. Glia. 67,1401–1411. doi: 10.1002/glia.23615. Epub 2019 Mar 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen L, Nakai M, Belton RJ Jr, Nowak RA. 2007. Expression of extracellular matrix metalloprotease inducer and matrix metalloproteases during mouse embryonic development. Reproduction. 133, 405–14. doi: 10.1530/rep.1.01020 [DOI] [PubMed] [Google Scholar]
- 33.Cho C 2012. Testicular and epididymal ADAMs: expression and function during fertilization. Nat Rev Urol. 9, 550–60. doi: 10.1038/nrurol.2012.167. [DOI] [PubMed] [Google Scholar]
- 34.Choi H, Han C, Jin S, Kwon JT, Kim J, Jeong J, Kim J, Ham S, Jeon S, Yoo YJ, Cho C. 2015. Reduced Fertility and Altered Epididymal and Sperm Integrity in Mice Lacking ADAM7. Biol Reprod. 93, 70. doi: 10.1095/biolreprod.115.130252. [DOI] [PubMed] [Google Scholar]
- 35.Colgin DC, Murdoch WJ. 1997. Evidence for a role of the ovarian surface epithelium in the ovulatory mechanism of the sheep: secretion of urokinase-type plasminogen activator. Anim Reprod Sci. 47,197–204. doi: 10.1016/s0378-4320(97)00011-0. [DOI] [PubMed] [Google Scholar]
- 36.Colige A, Li SW, Sieron AL, Nusgens BV, Prockop DJ, Lapiere CM. 1997. cDNA cloning and expression of bovine procollagen I N-proteinase: a new member of the superfamily of zinc-metalloproteases with binding sites for cells and other matrix components. Proc. Natl. Acad. Sci. USA, 94, 2374–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crespo D, Pramanick K, Goetz FW, Planas JV. 2013. Luteinizing hormone stimulation of in vitro ovulation in brook trout (Salvelinus fontinalis) involves follicle contraction and activation of proteolytic genes. Gen Comp Endocrinol. 188, 175–82. doi: 10.1016/j.ygcen.2013.02.031. [DOI] [PubMed] [Google Scholar]
- 38.Cui N, Hu M, Khalil RA. 2017. Biochemical and biological attributes of matrix metalloproteinases. Prog Mol Biol Transl Sci. 147, 1–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Curry TE Jr, Mann JS, Huang MH, Keeble SC. 1992. Gelatinase and proteoglycanase activity during the periovulatory period in the rat. Biol Reprod 46, 256–264. [DOI] [PubMed] [Google Scholar]
- 40.Curry TE Jr, Song L, Wheeler SE. 2001. Cellular localization of gelatinases and tissue inhibitors of metalloproteases during follicular growth, ovulation, and early luteal formation in the rat. Biol Reprod. 65, 855–865. doi: 10.1095/biolreprod65.3.855. [DOI] [PubMed] [Google Scholar]
- 41.Curry TE Jr, Smith MF. 2006. Impact of extracellular matrix remodeling on ovulation and the folliculo-luteal transition. Semin Reprod Med. 24, 228–241. [DOI] [PubMed] [Google Scholar]
- 42.Dai X, Jin X, Chen X, He J & Yin Z 2015. Sufficient numbers of early germ cells are essential for female sex development in zebrafish. PLoS One. 10, e0117824. doi: 10.1371/journal.pone.0117824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deady LD, Shen W, Mosure SA, Spradling AC, Sun J. 2015. Matrix metalloprotease 2 is required for ovulation and corpus luteum formation in Drosophila. PLoS Genet. 11, e1004989. doi: 10.1371/journal.pgen.1004989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DeCoux A, Lindsey ML, Villarreal F, Garcia RA, Schulz R. 2014. Myocardial matrix metalloproteinase-2: inside out and upside down. J Mol Cell Cardiol. 77, 64–72. doi: 10.1016/j.yjmcc.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Vos IJHM, Tao EY, Ong SLM, Goggi JL, Scerri T, Wilson GR, ow CGM L, Wong ASW, Grussu D, Stegmann APA, van Geel M, Janssen R, Amor DJ, Bahlo M, Dunn NR, Carney TJ, Lockhart PJ, Coull BJ, van Steensel MAM. 2018. Functional analysis of a hypomorphic allele shows that MMP14 catalytic activity is the prime determinant of the Winchester syndrome phenotype. Hum Mol Genet. 27, 2775–2788. doi: 10.1093/hmg/ddy168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Díez-Torre A, Díaz-Núñez M, Eguizábal C, Silván U, J Aréchaga J. 2013. Evidence for a role of matrix metalloproteases and their inhibitors in primordial germ cell migration. Andrology 1, 779–86. doi: 10.1111/j.2047-2927.2013.00109.x. [DOI] [PubMed] [Google Scholar]
- 47.Dranow DB, Tucker RP, Draper BW. 2013. Germ cells are required to maintain a stable sexual phenotype in adult zebrafish. Dev Biol. 376, 43–50. doi: 10.1016/j.ydbio.2013.01.016 [DOI] [PubMed] [Google Scholar]
- 48.Dubail J, Aramaki-Hattori N, Bader HL, Nelson CM, Katebi N, Matuska B, Olsen BR, Apte SS. 2014. A new Adamts9 conditional mouse allele identifies its non-redundant role in interdigital web regression. Genesis. 52, 702–12. doi: 10.1002/dvg.22784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duffy DM, Ko C, Jo M, Brannstrom M, Curry TE. 2019. Ovulation: parallels with inflammatory processes. Endocr Rev. 40, 369–416. doi: 10.1210/er.2018-00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dumstrei K, Mennecke R, Raz E. 2004. Signaling pathways controlling primordial germ cell migration in zebrafish. J Cell Sci. 117, 4787–95. [DOI] [PubMed] [Google Scholar]
- 51.Duncan WC, McNeilly AS, Illingworth PJ. 1998. The effect of luteal “rescue” on the expression and localization of matrix metalloproteases and their tissue inhibitors in the human corpus luteum. J Clin Endocrinol Metab. 83, 2470–8. doi: 10.1210/jcem.83.7.4950. [DOI] [PubMed] [Google Scholar]
- 52.Enomoto H, Nelson CM, Somerville RP, Mielke K, Dixon LJ, Powell K, Apte SS. 2010. Cooperation of two ADAMTS metalloproteases in closure of the mouse palate identifies a requirement for versican proteolysis in regulating palatal mesenchyme proliferation. Development. 137, 4029–38. doi: 10.1242/dev.050591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Espey LL, Yoshioka S, Russell DL, Robker RL, Fujii S, Richards JS. 2000. Ovarian expression of a disintegrin and metalloproteinase with thrombospondin motifs during ovulation in the gonadotropin-primed immature rat. Biol Reprod. 62,1090–5. doi: 10.1095/biolreprod62.4.1090. [DOI] [PubMed] [Google Scholar]
- 54.Foley CJ, Fanjul-Fernández M, Bohm A, Nguyen N, Agarwal A, Austin K, Koukos G, Covic L, López-Otín C, Kuliopulos A. 2014. Matrix metalloprotease 1a deficiency suppresses tumor growth and angiogenesis. Oncogene. 33, 2264–72. doi: 10.1038/onc.2013.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fortune JE, Willis EL, Bridges PJ, Yang CS. 2009. The periovulatory period in cattle: progesterone, prostaglandins, oxytocin and ADAMTS proteases. Anim Reprod. 6, 60–71. [PMC free article] [PubMed] [Google Scholar]
- 56.Freimann S, Ben-Ami I, Dantes A, Armon L, Ben Ya’cov-Klein A, Ron-El R, Amsterdam A. 2005. Differential expression of genes coding for EGF-like factors and ADAMTS1 following gonadotropin stimulation in normal and transformed human granulosa cells. Biochem Biophys Res Commun. 333, 935–943. doi: 10.1016/j.bbrc.2005.04.177. [DOI] [PubMed] [Google Scholar]
- 57.Fröjdman K, Paranko J, Kuopio T, Pelliniemi LJ. 1989. Structural proteins in sexual differentiation of embryonic gonads. Int J Dev Biol. 33, 99–103. [PubMed] [Google Scholar]
- 58.Fujihara M, Yamamizu K, Wildt DE, Songsasen N. 2016. Expression pattern of matrix metalloproteinases changes during folliculogenesis in the cat ovary. Reprod Domest Anim. 51, 717–25. doi: 10.1111/rda.12736. [DOI] [PubMed] [Google Scholar]
- 59.Glasson SS, Askew R, Sheppard B, Carito BA, Blanchet T, Ma HL, Flannery CR, Kanki K, Wang E, Peluso D, Yang Z, Majumdar MK, Morris EA. 2004. Characterization of and osteoarthritis susceptibility in ADAMTS-4-knockout mice. Arthritis Rheum. 50, 2547–58. doi: 10.1002/art.20558. [DOI] [PubMed] [Google Scholar]
- 60.Gottsch ML, Van Kirk EA, Murdoch WJ. 2000. Tumour necrosis factor α up-regulates matrix metalloprotease-2 activity in periovulatory ovine follicles: metamorphic and endocrine implications. Reprod Fertil Dev. 12,75–80. [DOI] [PubMed] [Google Scholar]
- 61.Goldberg MJ, Moses MA, Tsang PC. 1996. Identification of matrix metalloproteases and metalloprotease inhibitors in bovine corpora lutea and their variation during the estrous cycle. J Anim Sci. 74, 849–857. [DOI] [PubMed] [Google Scholar]
- 62.Gross J and Lapiere CM. 1962. Collagenolytic activity in amphibian tissues: a tissue culture assay. Proc Natl Acad Sci USA 48, 1014–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gubbay J, Vivian N, Economou A, Jackson D, Goodfellow P and Lovell-Badge R. 1992. Inverted repeat structure of the Sry locus in mice. Proc. Natl. Acad. Sci. USA. 89, 7953–7957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hägglund AC, Ny A, Liu K, Ny T. 1996. Coordinated and cell-specific induction of both physiological plasminogen activators creates functionally redundant mechanisms for plasmin formation during ovulation. Endocrinology. 137, 5671–7. doi: 10.1210/endo.137.12.8940398 [DOI] [PubMed] [Google Scholar]
- 65.Hägglund AC, Ny A, Leonardsson G, Ny T. 1999. Regulation and localization of matrix metalloproteinases and tissue inhibitors of metalloproteinases in the mouse ovary during gonadotropin-induced ovulation. Endocrinology. 140, 4351–8. doi: 10.1210/endo.140.9.7002 [DOI] [PubMed] [Google Scholar]
- 66.Hagiwara A, Ogiwara K, Katsu Y & Takahashi T 2014. Luteinizing hormone-induced expression of Ptge4b, a prostaglandin E2 receptor indispensable for ovulation of the medaka Oryzias latipes, is regulated by a genomic mechanism involving nuclear protestin receptor. Biology of Reproduction 90, 1–14. 10.1095/biolreprod.113.115485. [DOI] [PubMed] [Google Scholar]
- 67.Hagiwara A, Ogiwara K, Sugama N, Yamashita M, Takahashi T. 2020. Inhibition of medaka ovulation by gap junction blockers due to its disrupting effect on the transcriptional process of LH-induced Mmp15 expression. Gen Comp Endocrinol. 288, 113373. doi: 10.1016/j.ygcen.2019.113373. [DOI] [PubMed] [Google Scholar]
- 68.Hawkins JR, Taylor A, Berta P, Levilliers J, van Der Auwere B. and Goodfellow PN. 1992. Mutational analysis of SRY: Nonsense and missense mutations in XY sex reversal. Hum. Genet. 88, 471–474. [DOI] [PubMed] [Google Scholar]
- 69.Harikae K, Miura K, Kanai Y. 2013. Early gonadogenesis in mammals: significance of long and narrow gonadal structure. Dev Dyn. 242, 330–8. doi: 10.1002/dvdy.23872. [DOI] [PubMed] [Google Scholar]
- 70.Hayashidani S, Tsutsui H, Ikeuchi M, Shiomi T, Matsusaka H, Kubota T, Imanaka-Yoshida K, Itoh T, Takeshita A. 2003. Targeted deletion of MMP-2 attenuates early LV rupture and late remodeling after experimental myocardial infarction. Am J Physiol Heart Circ Physiol. 285, H1229–35. doi: 10.1152/ajpheart.00207.2003. [DOI] [PubMed] [Google Scholar]
- 71.Hartung O, Forbes MM, Marlow FL. 2014. Zebrafish vasa is required for germ-cell differentiation and maintenance. Mol Reprod Dev. 81, 946–61. doi: 10.1002/mrd.22414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, Mankani M, Robey PG, Poole AR, Pidoux I, Ward JM, Birkedal-Hansen H. 1999. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 99, 81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- 73.Hrabia A, Wolak D, Kwaśniewska M, Kieronska A, Socha JK, Sechman A. 2019. Expression of gelatinases (MMP-2 and MMP-9) and tissue inhibitors of metalloproteases (TIMP-2 and TIMP-3) in the chicken ovary in relation to follicle development and atresia. Theriogenology. 125, 268–276. doi: 10.1016/j.theriogenology.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 74.Huh MI, Jung JC. 2013. Expression of matrix metalloprotease-13 (MMP-13) in the testes of growing and adult chicken. Acta Histochem. 115, 475–80. doi: 10.1016/j.acthis.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 75.Horiguchi M, Fujimori C, Ogiwara K, Moriyama A, Takahashi T. 2008. Collagen type-I alpha1 chain mRNA is expressed in the follicle cells of the medaka ovary. Zoolog Sci. 25, 937–45. doi: 10.2108/zsj.25.937. [DOI] [PubMed] [Google Scholar]
- 76.Huxley-Jones J, Clarke TK, Beck C, Toubaris G, Robertson DL, Boot-Handford RP. 2007. The evolution of the vertebrate metzincins; insights from Ciona intestinalis and Danio rerio. BMC Evol Biol. 7, 63. doi: 10.1186/1471-2148-7-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Imai K, Khandoker M, Yonai M, Takahashi T, Sato T, Hasegawa Y, Hashizume K. 2003. Matrix metalloproteases-2 and −9 activities in bovine follicular fluid of different-sized follicles: relationship to intra-follicular inhibin and steroid concentrations. Domest Anim Endocrinol 24,171–183. [DOI] [PubMed] [Google Scholar]
- 78.Irving-Rodgers HF, Rodgers RJ. 2006. Extracellular matrix of the developing ovarian follicle. Semin Reprod Med. 24, 195–203. doi: 10.1055/s-2006-948549. [DOI] [PubMed] [Google Scholar]
- 79.Ismat A, Cheshire AM, Andrew DJ. 2013. The secreted AdamTS-A metalloprotease is required for collective cell migration. Development. 140, 1981–1993. doi: 10.1242/dev.087908 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Itoh T, Ikeda T, Gomi H, Nakao S, Suzuki T, Itohara S. 1997. Unaltered secretion of beta-amyloid precursor protein in gelatinase A (matrix metalloproteinase 2)-deficient mice. J Biol Chem. 272, 22389–92. doi: 10.1074/jbc.272.36.22389. [DOI] [PubMed] [Google Scholar]
- 81.Itoh T, Tanioka M, Matsuda H, Nishimoto H, Yoshioka T, Suzuki R, Uehira M. 1999. Experimental metastasis is suppressed in MMP-9-deficient mice. Clin Exp Metastasis. 17, 177–81. doi: 10.1023/a:1006603723759. [DOI] [PubMed] [Google Scholar]
- 82.Jo M, Thomas LE, Wheeler SE, Curry TE Jr. 2004. Membrane type 1-matrix metalloprotease (MMP)-associated MMP-2 activation increases in the rat ovary in response to an ovulatory dose of human chorionic gonadotropin. Biol Reprod. 70,1024–32. doi: 10.1095/biolreprod.103.023499. [DOI] [PubMed] [Google Scholar]
- 83.Jo M, Curry TE Jr. 2004. Regulation of matrix metalloproteinase-19 messenger RNA expression in the rat ovary. Biol Reprod. 71, 1796–806. doi: 10.1095/biolreprod.104.031823. [DOI] [PubMed] [Google Scholar]
- 84.Kassim SY, Gharib SA, Mecham BH, Birkland TP, Parks WC, McGuire JK. 2007. Individual matrix metalloproteinases control distinct transcriptional responses in airway epithelial cells infected with Pseudomonas aeruginosa. Infect Immun. 75, 5640–50. doi: 10.1128/IAI.00799-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kato Y, Ogiwara K, Fujimori C, Kimura A, Takahashi T. 2010. Expression and localization of collagen type IV alpha1 chain in medaka ovary. Cell Tissue Res. 340, 595–605. doi: 10.1007/s00441-010-0969-5. [DOI] [PubMed] [Google Scholar]
- 86.Kehoe S, Jewgenow K, Johnston PR, Mbedi S, Braun BC. 2021. Signaling pathways and mechanistic cues highlighted by transcriptomic analysis of primordial, primary, and secondary ovarian follicles in domestic cat. Sci Rep. 11, 2683. doi: 10.1038/s41598-021-82051-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kelly K, Hutchinson G, Nebenius-Oosthuizen D, Smith AJ, Bartsch JW, Horiuchi K, et al. 2005. Metalloprotease-disintegrin ADAM8: expression analysis and targeted deletion in mice. Dev Dyn. 232, 221–31. doi: 10.1002/dvdy.20221 [DOI] [PubMed] [Google Scholar]
- 88.Kelwick R, Desanlis I, Wheeler GN, Edwards DR. 2015. The ADAMTS (A Disintegrin and Metalloproteinase with Thrombospondin motifs) family. Genome Biol. 16, 113. doi: 10.1186/s13059-015-0676-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kern CB, Wessels A, McGarity J, Dixon LJ, Alston E, Argraves WS, Geeting D, Nelson CM, Menick DR, Apte SS. 2010. Reduced versican cleavage due to Adamts9 haploinsufficiency is associated with cardiac and aortic anomalies. Matrix Biol. 29, 304–16. doi: 10.1016/j.matbio.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kloc M, Bilinski S, Etkin LD. 2004. The Balbiani body and germ cell determinants: 150 years later. Curr Top Dev Biol. 59, 1–36. doi: 10.1016/S0070-2153(04)59001-4. [DOI] [PubMed] [Google Scholar]
- 91.Kok FO, Shin M, Ni CW, Gupta A, Grosse AS, van Impel A, Kirchmaier BC, Peterson-Maduro J, Kourkoulis G, Male I, DeSantis DF, Sheppard-Tindell S, Ebarasi L, Betsholtz C, Schulte-Merker S, Wolfe SA, Lawson ND. 2015. Reverse genetic screening reveals poor correlation between morpholino-induced and mutant phenotypes in zebrafish. Dev Cell. 32, 97–108. doi: 10.1016/j.devcel.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kossack ME, Draper BW. 2019. Genetic regulation of sex determination and maintenance in zebrafish (Danio rerio). Curr Top Dev Biol. 134,119–149. doi: 10.1016/bs.ctdb.2019.02.004. Epub 2019 Mar 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kusaka M, Katoh-Fukui Y, Ogawa H, Miyabayashi K, Baba T, Shima Y, Sugiyama N, Sugimoto Y, Okuno Y, Kodama R, Iizuka-Kogo A, Senda T, Sasaoka T, Kitamura K, Aizawa S, Morohashi K. 2010. Abnormal epithelial cell polarity and ectopic epidermal growth factor receptor (EGFR) expression induced in Emx2 knockout embryonic gonads. Endocrinology. 151, 5893–904. doi: 10.1210/en.2010-0915. [DOI] [PubMed] [Google Scholar]
- 94.Kim SH, Kang CW, Min KS, Yoon JT. 2014. Matrix metalloproteases are important for follicular development in normal and miniature pigs. Biotechnol Lett 36, 1187–96. doi: 10.1007/s10529-014-1474-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim SH, Yoon JT. 2020. Matrix metallopeptidases regulate granulosa cell remodeling through the hormone signaling pathway. J Adv Vet Anim Res. 7, 367–373. doi: 10.5455/javar.2020.g430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kobayashi T, Kim H, Liu X, Sugiura H, Kohyama T, Fang Q, Wen FQ, Abe S, Wang X, Atkinson JJ, Shipley JM, Senior RM, Rennard SI. 2014. Matrix metalloproteinase-9 activates TGF-β and stimulates fibroblast contraction of collagen gels. Am J Physiol Lung Cell Mol Physiol. 306, L1006–15. doi: 10.1152/ajplung.00015.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Koopman P, Gubbay J, Vivian N, Goodfellow P. and Lovell-Badge R. 1991. Male development of chromosomally female mice transgenic for Sry. Nature 351, 117–121. [DOI] [PubMed] [Google Scholar]
- 98.Kubota K, Cui W, Dhakal P, Wolfe MW, Rumi MA, Vivian JL, Roby KF, Soares MJ. 2016. Rethinking progesterone regulation of female reproductive cyclicity. Proc Natl Acad Sci U S A. 113, 4212–7. doi: 10.1073/pnas.1601825113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kuno K, Kanada N, Nakashima E, Fujiki F, Ichimura F, Matsushima K. 1997. Molecular cloning of a gene encoding a new type of metalloprotease-disintegrin family protein with thrombospondin motifs as an inflammation associated gene. J Biol Chem. 272, 556–62. doi: 10.1074/jbc.272.1.556. [DOI] [PubMed] [Google Scholar]
- 100.Kuno K, Okada Y, Kawashima H, Nakamura H, Miyasaka M, Ohno H, Matsushima K. 2000. ADAMTS-1 cleaves a cartilage proteoglycan, aggrecan. FEBS Lett. 478, 241–5. [DOI] [PubMed] [Google Scholar]
- 101.Lawson KA, Dunn NR, Roelen BA, Zeinstra LM, Davis AM, Wright CV, Korving JP, Hogan BL. 1999. Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev.13, 424–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Le Magueresse-Battistoni B 2008. Proteases and their cognate inhibitors of the serine and metalloprotease subclasses, in testicular physiology. Adv Exp Med Biol. 636, 133–53. doi: 10.1007/978-0-387-09597-4_8. [DOI] [PubMed] [Google Scholar]
- 103.Lee NV, Sato M, Annis DS, Loo JA, Wu L, Mosher DF, Iruela-Arispe ML. 2006. ADAMTS1 mediates the release of antiangiogenic polypeptides from TSP1 and 2. EMBO J. 25, 5270–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lei L, Spradling AC. 2013. Mouse primordial germ cells produce cysts that partially fragment prior to meiosis. Development. 140, 2075–81. doi: 10.1242/dev.093864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Leonardsson G, Peng XR, Liu K, Nordstrom L, Carmeliet P, Mulligan R, Collen D, Ny T. 1995. Ovulation efficiency is reduced in mice that lack plasminogen activator gene function: functional redundancy among physiological plasminogen activators. Proc Natl Acad Sci USA. 92, 12446–12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lind AK, Dahm-Kähler P, Weijdegård B, Sundfeldt K, Brännström M. 2006a. Gelatinases and their tissue inhibitors during human ovulation: increased expression of tissue inhibitor of matrix metalloprotease-1. Mol Hum Reprod. 12, 725–36. doi: 10.1093/molehr/gal086. [DOI] [PubMed] [Google Scholar]
- 107.Lind AK, Weijdegård B, Dahm-Kähler P, Mölne J, Sundfeldt K, Brännström M. 2006b. Collagens in the human ovary and their changes in the perifollicular stroma during ovulation. Acta Obstet GynecolScand. 85, 1476–84. doi: 10.1080/00016340601033741. [DOI] [PubMed] [Google Scholar]
- 108.Liu DT, Brewer MS, Chen S, Hong W, Zhu Y. 2017. Transcriptomic signatures for ovulation in vertebrates. Gen Comp Endocrinol. 247, 74–86. doi: 10.1016/j.ygcen.2017.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu DT, Carter NJ, Wu XJ, Hong WS, Chen SX, Zhu Y. 2018. Progestin and nuclear progestin receptor are essential for upregulation of metalloprotease in zebrafish preovulatory follicles. Front Endocrinol (Lausanne). 9, 517. doi: 10.3389/fendo.2018.00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu DT, Hong WS, Chen SX, Zhu Y. 2020. Upregulation of adamts9 by gonadotropin in preovulatory follicles of zebrafish. Mol Cell Endocrinol. 499, 110608. doi: 10.1016/j.mce.2019.110608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu K, Wahlberg P, Ny T. 1998. Coordinated and cell-specific regulation of membrane type matrix metalloprotease 1 (MT1-MMP) and its substrate matrix metalloprotease 2 (MMP-2) by physiological signals during follicular development and ovulation. Endocrinology. 139, 4735–8. doi: 10.1210/endo.139.11.6431. [DOI] [PubMed] [Google Scholar]
- 112.Liu K, Olofsson JI, Wahlberh P, Ny T. 1999. Distinct expression of gelatinase A [matrix metalloprotease (MMP)-2], collagenase-3 (MMP-13), membrane type MMP 1 (MMP-14), and tissue inhibitor of MMPs type 1 mediated by physiological signals during formation and regression of the rat corpus luteum. Endocrinology 140, 5330–5338. [DOI] [PubMed] [Google Scholar]
- 113.Liu K, Wahlberg P, Leonardsson G, Hägglund AC, Ny A, Bodén I, Wibom C, Lund LR, Ny T. 2006. Successful ovulation in plasminogen-deficient mice treated with the broad-spectrum matrix metalloproteinase inhibitor galardin. Dev Biol. 295, 615–22. doi: 10.1016/j.ydbio.2006.03.046 [DOI] [PubMed] [Google Scholar]
- 114.Liu PC, Liu X, Li Y, Covington M, Wynn R, Huber R, Hillman M, Yang G, Ellis D, Marando C, Katiyar K, Bradley J, Abremski K, Stow M, Rupar M, Zhuo J, Li YL, Lin Q, Burns D, Xu M, Zhang C, Qian DQ, He C, Sharief V, Weng L, Agrios C, Shi E, Metcalf B, Newton R, Friedman S, Yao W, Scherle P, Hollis G, Burn TC. 2006. Identification of ADAM10 as a major source of HER2 ectodomain sheddase activity in HER2 overexpressing breast cancer cells. Cancer Biol Ther. 5, 657–64. doi: 10.4161/cbt.5.6.2708. [DOI] [PubMed] [Google Scholar]
- 115.Liu YJ, Xu Y, Yu Q. 2006. Full-length ADAMTS-1 and the ADAMTS-1 fragments display pro- and antimetastatic activity, respectively. Oncogene. 25, 2452–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu YX, Liu K, Feng Q, Hu ZY, Liu HZ, Fu GQ, Li YC, Zou RJ, Ny T. 2004.Tissue-type plasminogen activator and its inhibitor plasminogen activator inhibitor type 1 are coordinately expressed during ovulation in the rhesus monkey. Endocrinology. 145, 1767–1775. [DOI] [PubMed] [Google Scholar]
- 117.Livermore C, Warr N, Chalon N, Siggers P, Mianné J, Codner G, Teboul L, Wells S, Greenfield A. 2019. Male mice lacking ADAMTS-16 are fertile but exhibit testes of reduced weight. Sci Rep. 9, 17195. doi: 10.1038/s41598-019-53900-0. Lu KV, [DOI] [PMC free article] [PubMed] [Google Scholar]; Jong KA, Rajasekaran AK, Cloughesy TF, Mischel PS. 2004. Upregulation of tissue inhibitor of metalloproteinases (TIMP)-2 promotes matrix metalloproteinase (MMP)-2 activation and cell invasion in a human glioblastoma cell line. Lab Invest. 84, 8–20. doi: 10.1038/sj.labinvest.3700003. [DOI] [PubMed] [Google Scholar]
- 118.Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA Jr, Shyamala G, Conneely OM, O’Malley BW. 1995. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev 9, 2266–78. doi: 10.1101/gad.9.18.2266 [DOI] [PubMed] [Google Scholar]
- 119.Mackay S 2000. Gonadal development in mammals at the cellular and molecular levels. Int Rev Cytol. 200, 47–99. doi: 10.1016/s0074-7696(00)00002-4. [DOI] [PubMed] [Google Scholar]
- 120.Masson R, Lefebvre O, Noël A, Fahime ME, Chenard MP, Wendling C, Kebers F, LeMeur M, Dierich A, Foidart JM, Basset P, Rio MC. 1998. In vivo evidence that the stromelysin-3 metalloproteinase contributes in a paracrine manner to epithelial cell malignancy. J Cell Biol. 140, 1535–41. doi: 10.1083/jcb.140.6.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Matsubara S, Shiraishi A, Osugi T, Kawada T, Satake H. 2019.The regulation of oocyte maturation and ovulation in the closest sister group of vertebrates. Elife. 8, e49062. doi: 10.7554/eLife.49062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mazzoni TS, Irani Quagio-Grassiotto I. 2020.Presence of the matrix metalloproteases during the migration of the primordial germ cells in zebrafish gonadal ridge. Cell Tissue Res,. 383, 707–722. doi: 10.1007/s00441-020-03288-5. [DOI] [PubMed] [Google Scholar]
- 123.McCord LA, Li F, Rosewell KL, Brännström M, Curry TE. 2012. Ovarian expression and regulation of the stromelysins during the periovulatory period in the human and the rat. Biol Reprod. 86,78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.McLaren A 1988. Somatic and germ-cell sex in mammals. Philos. Trans.R. Soc. Lond 322, 3–9. [DOI] [PubMed] [Google Scholar]
- 125.Mittaz L, Russell DL, Wilson T, Brasted M, Tkalcevic J, Salamonsen LA, Hertzog PJ, Pritchard MA. 2004. ADAMTS1 is essential for the development and function of the urogenital system. Biol Reprod. 70, 1096–105. [DOI] [PubMed] [Google Scholar]
- 126.Murdoch WJ, Van Kirk EA, Murdoch J. 1999. Plasmin cleaves tumor necrosis factor alpha exodomain from sheep follicular endothelium: implication in the ovulatory process. Biol Reprod. 60, 1166–71. doi: 10.1095/biolreprod60.5.1166. [DOI] [PubMed] [Google Scholar]
- 127.Murdoch WJ, McDonnel AC. 2002. Roles of the ovarian surface epithelium in ovulation and carcinogenesis. Reproduction. 123, 743–750. [DOI] [PubMed] [Google Scholar]
- 128.Murphy G 2011. Tissue inhibitors of metalloproteinases. Genome Biol. 12, 233. doi: 10.1186/gb-2011-12-11-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Murphy G and Nagase H. 2008. Progress in matrix metalloproteinase research. Mol Aspects Med. 29, 290–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nagase H, Enghild JJ, Suzuki K, Salvesen G. 1990. Stepwise activation mechanisms of the precursor of matrix metalloproteinase 3 (stromelysin) by proteinases and (4-aminophenyl)mercuric acetate. Biochemistry 29, 5783–5789. [DOI] [PubMed] [Google Scholar]
- 131.Nagase H, Visse R, Murphy G. 2005. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 69, 562–73. doi: 10.1016/j.cardiores.2005.12.002 [DOI] [PubMed] [Google Scholar]
- 132.Nagaraja AK, Middlebrook BS, Rajanahally S, Myers M, Li Q, Matzuk MM, Pangas SA. 2010. Defective gonadotropin-dependent ovarian folliculogenesis and granulosa cell gene expression in inhibin-deficient mice. Endocrinology. 151, 4994–5006. doi: 10.1210/en.2010-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nalvarte I, Töhönen V, Lindeberg M, Varshney M, Gustafsson JÅ, Inzunza J. 2016. Estrogen receptor β controls MMP-19 expression in mouse ovaries during ovulation. Reproduction. 151, 253–9. doi: 10.1530/REP-15-0522. [DOI] [PubMed] [Google Scholar]
- 134.Nandadasa S, Nelson CM, Apte SS. 2015. ADAMTS9-mediated extracellular matrix dynamics regulates umbilical cord vascular smooth muscle differentiation and rotation. Cell Rep. 11, 1519–28. doi: 10.1016/j.celrep.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nishiwaki K, Hisamoto N, Matsumoto, KA 2000. Metalloprotease disintegrin that controls cell migration in Caenorhabditis elegans. Science, 288, 2205–2208 (2000). [DOI] [PubMed] [Google Scholar]
- 136.Niu BY, Lan XM, Yan XH, Di SW, Wang Y, Li HT, Xiong YZ, Wang Xb. 2013. Characterization of Porcine Matrix Metalloproteinase 23 (pMMP-23) Gene and Its Association with Litter Size Traits. J Integrative Agriculture, 12, 103–109. [Google Scholar]
- 137.Ny A, Leonardsson G, Hagglund AC, Hagglòf P, Ploplis VA, Carmeliet P, Ny T. 1999. Ovulation in plasminogen-deficient mice. Endocrinology. 140, 5030–5. doi: 10.1210/endo.140.11.7113. [DOI] [PubMed] [Google Scholar]
- 138.Ogiwara K, Takano N, Shinohara M, Murakami M, Takahashi T. 2005. Gelatinase A and membrane-type matrix metalloproteinases 1 and 2 are responsible for follicle rupture during ovulation in the medaka. Proc Natl Acad Sci U S A. 102, 8442–7. doi: 10.1073/pnas.0502423102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ogiwara K, Minagawa K, Takano N, Kageyama T, Takahashi T. 2012. Apparen involvement of plasmin in early-stage follicle rupture during ovulation in medaka. Biol Reprod 86, 113. doi: 10.1095/biolreprod.111.093880. [DOI] [PubMed] [Google Scholar]
- 140.Ogiwara K, Hagiwara A, Rajapakse S, Takahashi T. 2015. The role of urokinas plasminogen activator and plasminogen activator inhibitor-1 in follicle rupture during ovulation i the teleost medaka. Biol Reprod. 92,10. doi: 10.1095/biolreprod.114.121442. [DOI] [PubMed] [Google Scholar]
- 141.Ogiwara K, Takahashi T. 2019. Nuclear Progestin Receptor Phosphorylation by Cdk9 Is Required for the Expression of Mmp15, a Protease Indispensable for Ovulation in Medaka. Cells 8, 215. doi: 10.3390/cells8030215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ohnishi J, Ohnishi E, Jin M, Hirano W, Nakane D, Matsui H, Kimura A, Sawa H Nakayama K, Shibuya H, Nagashima K, Takahashi T. 2001. Cloning and characterization of a ra ortholog of MMP-23 (matrix metalloproteinase-23), a unique type of membrane-anchored matrix metalloproteinase and conditioned switching of its expression during the ovarian follicular development. Mol Endocrinol. 15, 747–64. doi: 10.1210/mend.15.5.0638. [DOI] [PubMed] [Google Scholar]
- 143.Ohnishi J, Ohnishi E, Shibuya H, Takahashi T. 2005. Functions for proteinases in the ovulatory process. Biochim Biophys Acta. 1751, 95–109. doi: 10.1016/j.bbapap.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 144.Robinson LL, Sznajder NA, Riley SC, Anderson RA. 2001. Matrix metalloproteases and tissue inhibitors of metalloproteases in human fetal testis and ovary. Mol Hum Reprod. 7, 641002D8. doi: 10.1093/molehr/7.7.641. [DOI] [PubMed] [Google Scholar]
- 145.Rodríguez-Baena FJ, Redondo-García S, Peris-Torres C, Martino-Echarri E, Fernández-Rodríguez R, Plaza-Calonge MDC, Anderson P, Rodríguez-Manzaneque JC. 2018. ADAMTS1 protease is required for a balanced immune cell repertoire and tumour inflammatory response. Sci Rep. 8, 13103. doi: 10.1038/s41598-018-31288-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Page-McCaw A, Ewald AJ, Werb Z. 2007. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol 8, 221–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pearson JR, Zurita F, Tomás-Gallardo L, Díaz-Torres A, Díaz de la Loza Mdel C, Franze K, Martín-Bermudo MD, González-Reyes A. 2016. ECM-regulator timp is required for stem cell niche organization and cyst production in the Drosophila ovary. PLoS Genet. 12, e1005763. doi: 10.1371/journal.pgen.1005763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pei W, Xu L, Huang SC, Pettie K, Idol J, Rissone A, Jimenez E, Sinclair JW, Slevin C, Varshney GK, Jones M, Carrington B, Bishop K, Huang H, Sood R, Lin S, Burgess SM. 2018. Guided genetic screen to identify genes essential in the regeneration of hair cells and other tissues. NPJ Regen Med. 3,11. doi: 10.1038/s41536-018-0050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Peluffo MC, Murphy MJ, Baughman ST, Stouffer RL, Hennebold JD. 2011. Systematic analysis of protease gene expression in the rhesus macaque ovulatory follicle: metalloproteinase involvement in follicle rupture. Endocrinology. 152, 3963–74. doi: 10.1210/en.2011-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Peng XR, Hsueh AJ, Ny T. 1993.Transient and cell-specific expression of tissue-type plasminogen activator and plasminogen-activator-inhibitor type 1 results in controlled and directed proteolysis during gonadotropin-induced ovulation. Eur J Biochem. 214, 147–56. doi: 10.1111/j.1432-1033.1993.tb17907.x. [DOI] [PubMed] [Google Scholar]
- 151.Pendás AM, Folgueras AR, Llano E, Caterina J, Frerard F, Rodríguez F, Astudillo A, Noël A, Birkedal-Hansen H, López-Otín C. 2004. Diet-induced obesity and reduced skin cancer susceptibility in matrix metalloproteinase 19-deficient mice. Mol Cell Biol. 24, 5304–13. doi: 10.1128/MCB.24.12.5304-5313.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pepling ME, Spradling AC. 1998. Female mouse germ cells form synchronously dividing cysts. Development. 125, 3323–8. [DOI] [PubMed] [Google Scholar]
- 153.Peschon JJ, Slack JL, Reddy P, Stocking KL, Sunnarborg SW, Lee DC, Russell WE, Castner BJ, Johnson RS, Fitzner JN, Boyce RW, Nelson N, Kozlosky CJ, Wolfson MF, Rauch CT, Cerretti DP, Paxton RJ, March CJ, Black RA. 1998. An essential role for ectodomain shedding in mammalian development. Science 282, 128120134. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- 154.Piprek RP, Kolasa M, Podkowa D, Kloc M, Kubiak JZ. 2018. Transcriptional profiling validates involvement of extracellular matrix and proteinases genes in mouse gonad development. Mech Dev. 149, 9–19. doi: 10.1016/j.mod.2017.11.001 [DOI] [PubMed] [Google Scholar]
- 155.Piprek RP, Kloc M, Kubiak JZ. 2019. Matrix metalloprotease-dependent regulation of extracellular matrix shapes the structure of sexually differentiating mouse gonads. Differentiation 106, 23–34. doi: 10.1016/j.diff.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 156.Polanco JC, Koopman P. 2007. Sry and the hesitant beginnings of male development. Dev Biol. 302,13–24. [DOI] [PubMed] [Google Scholar]
- 157.Porter S, Clark TM, Kevorkian L, Edwards DR. 2005. The ADAMTS metalloproteases. Biochem J. 386, 15–27. doi: 10.1042/BJ20040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Portela VM, Veiga A, Price CA. 2009. Regulation of MMP2 and MMP9 metalloproteases by FSH and growth factors in bovine granulosa cells. Genet Mol Biol. 32, 516–20. doi: 10.1590/S1415-47572009005000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Primakoff P, Hyatt H, Tredick-Kline J. 1987. Identification and purification of a sperm surface protein with a potential role in sperm-egg membrane fusion. J Cell Biol. 104, 141–9. doi: 10.1083/jcb.104.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Puistola U, Salo T, Martikainen H, Ronnberg L. 1986. Type IV collagenolytic activity in human preovulatory follicular fluid. Fertil Steril 45, 5782013580. [DOI] [PubMed] [Google Scholar]
- 161.Puttabyatappa M, Jacot TA, Al-Alem LF, Rosewell KL, Duffy DM, Brännström M, Curry TE Jr. 2014. Ovarian membrane-type matrix metalloproteinases: induction of MMP14 and MMP16 during the periovulatory period in the rat, macaque, and human. Biol Reprod. 91, 34. doi: 10.1095/biolreprod.113.115717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Raz E 2002. Primordial germ cell development in zebrafish. Semin Cell Dev Biol. 13, 489002D95. [DOI] [PubMed] [Google Scholar]
- 163.Reich R, Daphna-Iken D, Chun SY, Popliker M, Slager R, Adelmann-Grill BC, Tsafriri A. 1991. Preovulatory changes in ovarian expression of collagenases and tissue metalloprotease inhibitor messenger ribonucleic acid: role of eicosanoids. Endocrinology 129, 186920131875. [DOI] [PubMed] [Google Scholar]
- 164.Richards JS. 2005. Ovulation: new factors that prepare the oocyte for fertilization. Mol Cell Endocrinol. 234, 75–9. doi: 10.1016/j.mce.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 165.Richards JS, Hernandez-Gonzalez I, Gonzalez-Robayna I, Teuling E, Lo Y, Boerboom D, Falender AE, Doyle KH, LeBaron RG, Thompson V, Sandy JD. 2005. Regulated expression of ADAMTS family members in follicles and cumulus oocyte complexes: evidence for specific and redundant patterns during ovulation. Biol Reprod. 72, 1241–55. doi: 10.1095/biolreprod.104.038083. [DOI] [PubMed] [Google Scholar]
- 166.Richards JS. 2018. From follicular development and ovulation to ovarian cancers: An unexpected journey. Vitam Horm. 107, 453–472. doi: 10.1016/bs.vh.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 167.Riley SC, Gibson AH, Leask R, Mauchline DJ, Pedersen HG, Watson ED. 2001. Secretion of matrix metalloproteases 2 and 9 and tissue inhibitor of metalloproteases into follicular fluid during follicle development in equine ovaries. Reproduction. 121, 553–60. [PubMed] [Google Scholar]
- 168.Riley SC, Thomassen R, Bae SE, Leask R, Pedersen HG, Watson ED. 2004. Matrix metalloprotease-2 and −9 secretion by the equine ovary during follicular growth and prior to ovulation. Anim Reprod Sci. 81, 329–39. doi: 10.1016/j.anireprosci.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 169.Robker RL, Russell DL, Espey LL, Lydon JP, O’Malley BW, Richards JS. 2000. Progesterone-regulated genes in the ovulation process: ADAMTS-1 and cathepsin L proteases Proc Natl Acad Sci U S A. 97, 4689–4694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rodgers RJ, Irving-Rodgers HF, Russell DL. 2003. Extracellular matrix of the developing ovarian follicle. Reproduction. 126, 415–24. doi: 10.1530/rep.0.1260415. [DOI] [PubMed] [Google Scholar]
- 171.Rodriguez-Manzaneque JC, Westling J, Thai SN, Luque A, Knauper V, Murphy G, Sandy JD, Iruela-Arispe ML. 2002. ADAMTS1 cleaves aggrecan at multiple sites and is differentially inhibited by metalloproteinase inhibitors. Biochem Biophys Res Commun. 293, 501–8. [DOI] [PubMed] [Google Scholar]
- 172.Rosewell KL, Al-Alem L, Zakerkish F, McCord L, Akin JW, Chaffin CL, Brännström M, Curry TE Jr. 2015. Induction of proteinases in the human preovulatory follicle of the menstrual cycle by human chorionic gonadotropin. Fertil Steril. 103, 826–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Roth W, Deussing J, Botchkarev VA, Pauly-Evers M, Saftig P, Hafner A, Schmidt P, Schmahl W, Scherer J, Anton-Lamprecht I, Von Figura K, Paus R, Peters C. 2000. Cathepsin L deficiency as molecular defect of furless: hyperproliferation of keratinocytes and pertubation of hair follicle cycling. FASEB J. 14, 2075–86. doi: 10.1096/fj.99-0970com. [DOI] [PubMed] [Google Scholar]
- 174.Russell DL, Ochsner SA, Hsieh M, Mulders S, Richards JS. 2003. Hormone-regulated expression and localization of versican in the rodent ovary. Endocrinology. 144, 1020–31. doi: 10.1210/en.2002-220434. [DOI] [PubMed] [Google Scholar]
- 175.Russell DL, Robker RL. 2007. Molecular mechanisms of ovulation: co-ordination through the cumulus complex. Hum Reprod Update. 13, 289–312. doi: 10.1093/humupd/dml062. [DOI] [PubMed] [Google Scholar]
- 176.Saito D, Morinaga C, Aoki Y, Nakamura S, Mitani H, Furutani-Seiki M, Kondoh H, Tanaka M. 2007. Proliferation of germ cells during gonadal sex differentiation in medaka: Insights from germ cell-depleted mutant zenzai. DevBiol. 310, 280–90. doi: 10.1016/j.ydbio.2007.07.039. [DOI] [PubMed] [Google Scholar]
- 177.Salverson TJ, McMichael GE, Sury JJ, Shahed A, Young KA. 2008. Differential expression of matrix metalloproteinases during stimulated ovarian recrudescence in Siberian hamsters (Phodopus sungorus). Gen Comp Endocrinol. 155, 749–61. doi: 10.1016/j.ygcen.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Santos HB, Sato Y, Moro L, Bazzoli N, Rizzo E. 2008. Relationship among follicular apoptosis, integrin beta1 and collagen type IV during early ovarian regression in the teleost Prochilodus argenteus after induced spawning. Cell Tissue Res. 332, 159–70. doi: 10.1007/s00441-007-0540-1. [DOI] [PubMed] [Google Scholar]
- 179.Satz-Jacobowitz B, Hubmacher D. 2021. The quest for substrates and binding partners: A critical barrier for understanding the role of ADAMTS proteases in musculoskeletal development and disease. Dev Dyn. 250, 8–26. doi: 10.1002/dvdy.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sayasith K, Lussier J, Sirois J. 2013. Molecular characterization and transcriptional regulation of a disintegrin and metalloprotease with thrombospondin motif 1 (ADAMTS1) in bovine preovulatory follicles. Endocrinology. 154, 2857–69. doi: 10.1210/en.2013-1140. [DOI] [PubMed] [Google Scholar]
- 181.Sayasith K, Sirois J. 2015. Molecular characterization of a disintegrin and metalloprotease-17 (ADAM17) in granulosa cells of bovine preovulatory follicles. Mol Cell Endocrinol. 411, 49–57. doi: 10.1016/j.mce.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 182.Schlondorff J, Blobel CP. 1999. Metalloprotease-disintegrins: Modular proteins capable of promoting cell-cell interactions and triggering signals by protein-ectodomain shedding. J. Cell. Sci. 112, 3603–3617. [DOI] [PubMed] [Google Scholar]
- 183.Sessions DR, Vick MM, Fitzgerald BP. 2009. Characterization of matrix metalloprotease-2 and matrix metalloprotease-9 and their inhibitors in equine granulosa cells in vivo and in vitro. J Anim Sci. 87, 3955–66. doi: 10.2527/jas.2009-2088. [DOI] [PubMed] [Google Scholar]
- 184.Shindo T, Kurihara H, Kuno K, Yokoyama H, Wada T, Kurihara Y, Imai T, Wang Y, Ogata M, Nishimatsu H, Moriyama N, Oh-hashi Y, Morita H, Ishikawa T, Nagai R, Yazaki Y, Matsushima K. 2000. ADAMTS-1: a metalloprotease-disintegrin essential for normal growth, fertility, and organ morphology and function. J Clin Invest. 105, 1345–52. doi: 10.1172/JCI8635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Shozu M, Minami N, Yokoyama H, Inoue M, Kurihara H, Matsushima K, Kuno K. 2005. ADAMTS-1 is involved in normal follicular development, ovulatory process and organization of the medullary vascular network in the ovary. J Mol Endocrinol. 35, 343–55. doi: 10.1677/jme.1.01735. [DOI] [PubMed] [Google Scholar]
- 186.Silva NJ, Nagashima M, Li J, Kakuk-Atkins L, Ashrafzadeh M, Hyde DR, Hitchcock PF 2020. Inflammation and matrix metalloprotease 9 (Mmp-9) regulate photoreceptor regeneration in adult zebrafish. Glia 68, 1445–1465. doi: 10.1002/glia.23792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Skjøt-Arkil H, Clausen RE, Nguyen QH, Wang Y, Zheng Q, Martinez FJ, Hogaboam CM, Han M, Klickstein LB, Larsen MR, Nawrocki A, Leeming DJ, Karsdal MA. 2012. Measurement of MMP-9 and −12 degraded elastin (ELM) provides unique information on lung tissue degradation. BMC Pulm Med. 12, 34. doi: 10.1186/1471-2466-12-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Smith MF, McIntush EW, Ricke WA, Kojima FN, Smith GW. 1999. Regulation of ovarian extracellular matrix remodelling by metalloproteinases and their tissue inhibitors: effects on follicular development, ovulation and luteal function. J Reprod Fertil Suppl. 54, 367–81. [PubMed] [Google Scholar]
- 189.Smokovitis A, Kokolis N and Alexaki-Tzivanidou E.1988. The plasminogen activator activity is markedly increased mainly at the area of the rupture of the follicular wall at the time of ovulation. Animal Reproduction Science 16, 285–294. [Google Scholar]
- 190.Somerville RP, Longpre JM, Jungers KA, Engle JM, Ross M, Evanko S, Wight TN, Leduc R, Apte SS. 2003. Characterization of ADAMTS-9 and ADAMTS-20 as a distinct ADAMTS subfamily related to Caenorhabditis elegans GON-1. J Biol Chem. 278, 9503–13. doi: 10.1074/jbc.M211009200. [DOI] [PubMed] [Google Scholar]
- 191.Spiller C, Koopman P, Bowles J. 2017. Sex Determination in the Mammalian Germline. Annu Rev Genet. 51, 265–285. doi: 10.1146/annurev-genet-120215-035449. [DOI] [PubMed] [Google Scholar]
- 192.Sriraman V, Eichenlaub-Ritter U, Bartsch JW, Rittger A, Mulders SM, Richards JS. 2008. Regulated expression of ADAM8 (a disintegrin and metalloprotease domain 8) in the mouse ovary: evidence for a regulatory role of luteinizing hormone, progesterone receptor, and epidermal growth factor-like growth factors. Biol Reprod. 78, 1038–48. doi: 10.1095/biolreprod.107.066340. [DOI] [PubMed] [Google Scholar]
- 193.Stanton H, Rogerson FM, East CJ, Golub SB, Lawlor KE, Meeker CT, Little CB, Last K, Farmer PJ, Campbell IK, Fourie AM, Fosang AJ. 2005. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature. 434, 648–52. doi: 10.1038/nature03417. [DOI] [PubMed] [Google Scholar]
- 194.Szabova L, Son MY, Shi J, Sramko M, Yamada SS, Swaim WD, Zerfas P, Kahan S, Holmbeck K. 2010. Membrane-type MMPs are indispensable for placental labyrinth formation and development. Blood. 116, 5752–61. doi: 10.1182/blood-2009-10-249847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Takahashi H 1977. Juvenile Hermaphroditism in the Zebrafish, Brachydanio rerio Bulletin of the Faculty of Fisheries, Hokkaido University; 28, 57–65. [Google Scholar]
- 196.Takahashi T, Fujimori C, Hagiwara A, Ogiwara K. 2013. Recent advances in the understanding of teleost medaka ovulation: the roles of proteases and prostaglandins. Zoolog Sci. 30, 239–47. doi: 10.2108/zsj.30.239. [DOI] [PubMed] [Google Scholar]
- 197.Takahashi T, Hagiwara A, Ogiwara K. 2019. Follicle rupture during ovulation with an emphasis on recent progress in fish models. Reproduction. 157, R1–R13. doi: 10.1530/REP-18-0251. [DOI] [PubMed] [Google Scholar]
- 198.Tang BL, Hong W. 1999. ADAMTS: a novel family of proteases with an ADAM protease domain and thrombospondin 1 repeats. FEBS Lett. 445, 223–5. doi: 10.1016/s0014-5793(99)00119-2. [DOI] [PubMed] [Google Scholar]
- 199.Tang H, Liu Y, Li J, Yin Y, Li G, Chen Y, Li S, Zhang Y, Lin H, Liu X, Cheng CH. 2016. Gene knockout of nuclear progesterone receptor provides insights into the regulation of ovulation by LH signaling in zebrafish. Sci Rep. 6, 28545. doi: 10.1038/srep28545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Thai SN, Iruela-Arispe ML. 2002. Expression of ADAMTS1 during murine development. Mech Dev. 115, 181–5. doi: 10.1016/s0925-4773(02)00115-6. [DOI] [PubMed] [Google Scholar]
- 201.Tharmarajah G, Eckhard U, Jain F, Marino G, Prudova A, Urtatiz O, Fuchs H, de Angelis MH, Overall CM, Van Raamsdonk CD. 2018. Melanocyte development in the mouse tail epidermis requires the Adamts9 metalloprotease. Pigment Cell Melanoma Res. 31, 693–707. doi: 10.1111/pcmr.12711. [DOI] [PubMed] [Google Scholar]
- 202.Thomé R, dos Santos HB, Sato Y, Rizzo E, Bazzoli N. 2010. Distribution of laminin β2, collagen type IV, fibronectin and MMP-9 in ovaries of the teleost fish. J Mol Histol. 41, 215–24. doi: 10.1007/s10735-010-9281-7. [DOI] [PubMed] [Google Scholar]
- 203.Tsafriri A, Bicsak TA, Cajander SB, Ny T, Hsueh AJ. 1989. Suppression of ovulation rate by antibodies to tissue-type plasminogen activator and alpha 2-antiplasmin. Endocrinology. 124, 415–21. doi: 10.1210/endo-124-1-415. [DOI] [PubMed] [Google Scholar]
- 204.Tsafriri A 1995. Ovulation as a tissue remodelling process. Proteolysis and cumulus expansion. Adv Exp Med Biol. 377, 121–40. doi: 10.1007/978-1-4899-0952-7_8. [DOI] [PubMed] [Google Scholar]
- 205.Tzung KW, Goto R, Saju JM, Sreenivasan R, Saito T, Arai K, Yamaha E, Hossain MS, Calvert ME, Orbán L. 2015. Early depletion of primordial germ cells in zebrafish promotes testis formation. Stem Cell Reports. 4, 61–73. doi: 10.1016/j.stemcr.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Uchida D, Yamashita M, Kitano T, Iguchi T. 2002. Oocyte apoptosis during the transition from ovary-like tissue to testes during sex differentiation of juvenile zebrafish. J Exp Biol. 205, 711–8. [DOI] [PubMed] [Google Scholar]
- 207.Ungewitter EK, Yao HH. 2013. How to make a gonad: cellular mechanisms governing formation of the testes and ovaries. Sex Dev. 7, 7–20. doi: 10.1159/000338612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Van Wart HE, Birkedal-Hansen H. 1990. The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc Natl Acad Sci U S A. 87, 5578–82. doi: 10.1073/pnas.87.14.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Vos MC, van der Wurff AA, Last JT, de Boed EA, Smeenk JM, van Kuppevelt TH, Massuger LF. 2014. Immunohistochemical expression of MMP-14 and MMP-2, and MMP-2 activity during human ovarian follicular development. Reprod Biol Endocrinol. 12,12. doi: 10.1186/1477-7827-12-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM, Werb Z. 1998. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 93, 411–22. doi: 10.1016/s0092-8674(00)81169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Vu TH, Werb Z. 2000. Matrix metalloproteases: effectors of development and normal physiology. Genes Dev. 14, 2123–33. doi: 10.1101/gad.815400 [DOI] [PubMed] [Google Scholar]
- 212.Wang LW, Nandadasa S, Annis DS, Dubail J, Mosher DF, Willard BB, Apte SS. 2019. A disintegrin-like and metalloproteinase domain with thrombospondin type 1 motif 9 (ADAMTS9) regulates fibronectin fibrillogenesis and turnover. J Biol Chem. 294, 9924–9936. doi: 10.1074/jbc.RA118.006479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wang Z, Juttermann R, Soloway PD. 2000. TIMP-2 is required for efficient activation of proMMP-2 in vivo. J Biol Chem. 275, 26411–5. doi: 10.1074/jbc.M001270200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Weidinger G, Wolke U, Köprunner M, Thisse C, Thisse B, Raz E. 2002. Regulation of zebrafish primordial germ cell migration by attraction towards an intermediate target. Development. 129, 25–36. [DOI] [PubMed] [Google Scholar]
- 215.Willis EL, Bridges PJ, Fortune JE. 2017. Progesterone receptor and prostaglandins mediate luteinizing hormone-induced changes in messenger RNAs for ADAMTS proteases in theca cells of bovine periovulatory follicles. Mol Reprod Dev. 84, 55–66. doi: 10.1002/mrd.22761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wilson CL, Heppner KJ, Labosky PA, Hogan BL, Matrisian LM. 1997. Intestinal tumorigenesis is suppressed in mice lacking the metalloproteinase matrilysin. Proc Natl Acad Sci U S A. 94, 1402–7. doi: 10.1073/pnas.94.4.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wissing ML, Kristensen SG, Andersen CY, Mikkelsen AL, Host T, Borup R, Grondahl ML. 2014. Identification of new ovulation-related genes in humans by comparing the transcriptome of granulosa cells before and after ovulation triggering in the same controlled ovarian stimulation cycle. Hum Reprod. 29, 997–1010. [DOI] [PubMed] [Google Scholar]
- 218.Wolak D, Hrabia A. 2021. Alternations in the expression of selected matrix metalloproteases (MMP-2, −9, −10, and −13) and their tissue inhibitors (TIMP-2 and −3) and MMP-2 and −9 activity in the chicken ovary during pause in laying induced by fasting. Theriogenology 161, 176–186. doi: 10.1016/j.theriogenology.2020.12.005. [DOI] [PubMed] [Google Scholar]
- 219.Woodruff TK, Shea LD. 2007. The role of the extracellular matrix in ovarian follicle development. Reprod Sci. 14(8 Suppl), 6–10. doi: 10.1177/1933719107309818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Wright WW, Smith L, Kerr C, Charron M. 2003. Mice that express enzymatically inactive cathepsin L exhibit abnormal spermatogenesis. Biol Reprod. 68, :680–7. doi: 10.1095/biolreprod.102.006726. [DOI] [PubMed] [Google Scholar]
- 221.Wyatt RA, Keow JY, Harris ND, Haché CA, Li DH, Crawford BD. 2009. The zebrafish embryo: a powerful model system for investigating matrix remodeling. Zebrafish. 6, 347–54. doi: 10.1089/zeb.2009.0609. [DOI] [PubMed] [Google Scholar]
- 222.Young KA, Stouffer RL. 2004. Gonadotropin and steroid regulation of matrix metalloproteases and their endogenous tissue inhibitors in the developed corpus luteum of the rhesus monkey during the menstrual cycle. Biol Reprod. 70, 244–52. doi: 10.1095/biolreprod.103.022053. [DOI] [PubMed] [Google Scholar]
- 223.Zack MD, Melton MA, Stock JL, Storer CE, Barve RA, Minnerly JC, Weiss DJ, Stejskal JA, Tortorella MD, Turk JR, Shevlin KM, Malfait AM. 2009. Reduced incidence and severity of experimental autoimmune arthritis in mice expressing catalytically inactive A disintegrin and metalloproteinase 8 (ADAM8). Clin Exp Immunol. 158, 246–56. doi: 10.1111/j.1365-2249.2009.04009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Zhao Y, Luck MR. 1996. Bovine granulosa cells express extracellular matrix proteins and their regulators during luteinization in culture. Reprod Fertil Dev. 8, 259–266. [DOI] [PubMed] [Google Scholar]
- 225.Zhu G, Kang L, Wei Q, Cui X, Wang S, Chen Y, Jiang Y. 2014. Expression and regulation of MMP1, MMP3, and MMP9 in the chicken ovary in response to gonadotropins, sex hormones, and TGFB1. Biol Reprod. 90, 57. doi: 10.1095/biolreprod.113.114249. [DOI] [PubMed] [Google Scholar]
- 226.Zhu Y, Liu D, Shaner ZC, Chen S, Hong W, Stellwag EJ. 2015. Nuclear progestin receptor (pgr) knockouts in zebrafish demonstrate role for pgr in ovulation but not in rapid non-genomic steroid mediated meiosis resumption. Front Endocrinol (Lausanne). 6, 37. doi: 10.3389/fendo.2015.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]