Abstract
The hopeful outcomes from 30 years of research in BH3-mimetics have indeed served a number of solid paradigms for targeting intermediates from the apoptosis pathway in a variety of diseased states. Not only have such rational approaches in drug design yielded several key therapeutics, such outputs have also offered insights into the integrated mechanistic aspects of basic and clinical research at the genetics level for the future. In no other area of medical research have the effects of such work been felt, than in cancer research, through targeting the BAX-Bcl-2 protein-protein interactions. With these promising outputs in mind, several mimetics, and their potential therapeutic applications, have also been developed for several other pathological conditions, such as cardiovascular disease and tissue fibrosis, thus highlighting the universal importance of the intrinsic arm of the apoptosis pathway and its input to general tissue homeostasis. Considering such recent developments, and in a field that has generated so much scientific interest, we take stock of how the broadening area of BH3-mimetics has developed and diversified, with a focus on their uses in single and combined cancer treatment regimens and recently explored therapeutic delivery methods that may aid the development of future therapeutics of this nature.
Keywords: Apoptosis, BH-3 mimetics, PUMA-mimetics, Smac-mimetics, Bcl-xL-mimetics, Noxa-mimetics, Mcl1-mimetics, Nanoparticles
Background
Harnessing the potential of apoptosis, as a strategic approach for the eradication of cancer cells, has been an area of intense activity over the last 30 years, ranging from the implementation of death inducing ligands and therapeutics, to engineering synergistic chemical antagonists [1, 2]. What originally started as research aimed at unveiling the mechanistic input of extrinsic and intrinsic signaling pathways in cell demise, has indeed developed towards how such pathways can be therapeutically exploited as the findings have transitioned from a basic- to applied- research setting. Underpinning such developments have unambiguously relied on defining an ever-growing number of molecular signaling networks and their detailed regulatory crosstalk in defining potential axes of regulation that may be amenable for therapeutic intervention [3].
Herein, one critical regulatory event central to triggering apoptosis is the activation of Mitochondrial Outer Membrane Polarization (MOMP) and is the cornerstone of intrinsic pathway activation. How this is achieved at the molecular level, and what factors regulate the thresholds that exist in achieving MOMP, thereby predisposing cells to undergo apoptosis, has been the basis of many excellent studies that have either overlapped or converged on the importance of the Bcl-2 homology (BH) -domain containing proteins [4].
Briefly, the Bcl-2 proteins can be broadly categorized as acting in either a pro-apoptotic or anti-apoptotic manner. Whilst these groups act directly in driving or diminishing apoptosis, a third group of proteins, which are functionally and structurally unique, and when over-expressed can sensitize cells to biochemical cues that induce apoptosis, are the BH3-only proteins (or sensitizer proteins). From these three groups, the BAX (pro-apoptotic) and Bcl-2, Bcl-xL or Mcl1 (anti-apoptotic) proteins have gained the most attention over the recent decades, based on their deregulated expression, significance in the development of a number of cancers and their responsiveness to therapeutics [5]. From these, the importance of BAX protein deregulation in cancer development can stem from the loss-of-function genetic frame-shift mutations, which contribute to the prevalence of a number of solid tumors and leukemias [6–8]. Conversely, in the instance of the Bcl-2 and Bcl-xL anti-apoptotic proteins, their genetically deregulated-over-expression can give rise to similar malignancies, such as B-cell lymphoma, prostate cancer, non-small cell lung cancer, Acute lymphoblastic leukaemia and breast cancer [9–12].
As an alternative regulatory mechanism, protein sub-cellular localization and the coordinated manner in which Bcl-2 protein family members can be regulated by factors from the nucleus, lysosome and mitochondria, has also taken on greater significance over the recent years [13]. Synergistically, the structural composition of the Bcl-2 proteins has unveiled how such proteins come to reside at specific subcellular compartments, and whether each member is regulated by other group members of this family through protein-protein interactions (Table 1) [14].
Table 1.
The Bcl-2 protein family members can be sub-grouped into pro-apoptotic (Pro-), anti-apoptotic (Anti-) and sensitizer (Sen-) members, which originate from distinct genetic loci and encode proteins of varying amino-acid (aa) length and molecular weight (in kilodaltons, kDa). Each member can be localized in the cytoplasm (C), mitochondria (M), endoplasmic reticulum (ER) or the nucleus (N) and can be regulated through its interaction with other protein family members through protein-protein interactions
| Protein | Apoptosis | Gene | Size (kDa) | Location | Protein Partners |
|---|---|---|---|---|---|
| BAX | Pro- | 19q13.33 | 192aa (21.18) | C, M, N, ER | BAK, Bcl-2,Bcl-xL,Mcl1, BID, BIM, NOXA |
| BAK | Pro- | 6p21.31 | 211aa (23.40) | M | BAX, Bcl-2, Bcl-xL, Mcl1, BID |
| Bcl-2 | Anti- | 18q21.33 | 239aa (26.00) | C, M, N, ER | BAX, BAK, Bcl-xL, BID, BIM, BAD, PUMA, NOXA |
| Bcl-xL | Anti- | 20q11.21 | 233aa (26.04) | M, C | BAX, BAK, Bcl-2, BID, BIM BAD, PUMA |
| Mcl1 | Anti- | 1q21.20 | 350aa (37.33) | C, M, N | BAX, BAK, BID, BIM, PUMA, NOXA |
| BID | Pro-/Sen- | 22q11.21 | 195aa (21.99) | C, M | BAX, BAK, Bcl-2, Bcl-xL, Mcl1 |
| BIM | Pro-/Sen- | 2q13.00 | 198aa (22.17) | C, M | BAX, Bcl-2, Bcl-xL, Mcl1, |
| BAD | Pro-/Sen- | 11q13.10 | 168aa (18.39) | C, M | Bcl-2, Bcl-xL |
| PUMA | Pro-/Sen- | 19q13.32 | 193aa (20.53) | M | Bcl-2, Bcl-xL, Mcl1, |
| NOXA | Pro-/Sen- | 9q34.30 | 476aa (50.93) | C, M, N | BAX, Bcl-2, Mcl1, |
| SMAC | Pro-/Sen- | 12q24.31 | 239aa (27.13) | C, M | xIAP1 |
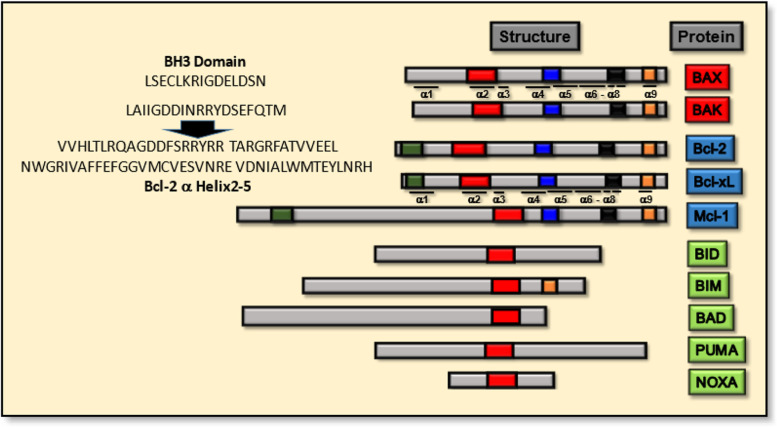
In large, the Bcl-2 proteins are composed of conserved BH1-4 domains and in some instances a transmembrane domain (Fig. 1) [4]. Here, the key structural component of intrinsic importance, which is present in all of the pro-apoptotic Bcl-2 family protein members, is unquestionably the BH3 domain, which is a structure composed of ~ 15 amino acids from α-helix 2, and which interacts with the hydrophobic pocket structure formed by α-helices 2-5 of the anti-apoptosis proteins, such as Bcl-2 protein (Fig. 1) [15].
Fig. 1.
The BH- and helical- domain composition of selected Bcl-2 family pro-apoptotic (red boxes), anti-apoptotic (blue boxes) and BH3-only (green boxes) members. The amino acid sequences of the human BH3-domains from BAX and BAK are highlighted (top left), below which are shown the amino acid sequences of the alpha helices 2–5 from the human Bcl-2 protein. For each of the proteins, the transmembrane domain is highlighted in orange and BH1–4 domains are respectively highlighted in blue, black, red and green for the relevant proteins
Mechanistically, the interplay between all three groups of proteins, based on their relative abundance in the presence of biochemical activation cues, determine whether the two key intermediates BAX and BAK successfully mediate the formation of a Mitochondrial Pore Complex (MPC), denoted by enlarged mitochondrial cristae and the ultimate release of cytochrome c as a prerequisite to caspases -3, -7 and -9 activation and finally followed by DNA fragmentation [16, 17]. Part of this mechanism incorporates the complex regulatory input from TP53 activation and the key mitochondrial proteins NOXA, PUMA and Smac/DIABLO [18, 19]. As a relatively weak and indirect activator of apoptosis, BH3-only NOXA can bind (and inhibit) the anti-apoptotic regulators Mcl1 and Bcl-2A1, thus preventing their suppression of BAX- and BAK- protein activation [20–22]. Alternatively, NOXA has also been reported to bind BAX and activate apoptosis in the absence of a BIM, BID and Bcl-xL expression-dependent manner, thereby regulating apoptosis directly [23, 24]. Similarly, BH3-only PUMA can induce apoptosis through the direct activation of BAX or BAK [25]. As a negative regulator of apoptosis, Mcl1 has been widely reported as being overexpressed in a number of cancers such as lung and breast [26, 27], and which can be destabilized by NOXA, through the ubiquitination pathway [28, 29]. As an integral regulator of apoptosis, PUMA can also mediate activation of apoptosis through a direct association with Mcl1, that is reversible upon NOXA expression (through it binding Mcl1), thus releasing PUMA by a ‘catch and release’ mechanism [30, 31]. Consequently, from a therapeutic standpoint, Mcl1 expression and regulation have taken on increasing levels of importance, particularly from the contribution they make in offering drug resistance to several cancer therapeutics [32–35]. Like NOXA [36] and PUMA [37, 38], Smac/DIABLO [39] is also resident on the cytoplasmic face of the MOM, and which uniquely allows it to fulfill its role as a positive activator of caspases, through it binding and inhibiting the Inhibitor of Apoptosis Proteins (IAPs), from the extrinsic and intrinsic arms of the apoptosis pathway [40].
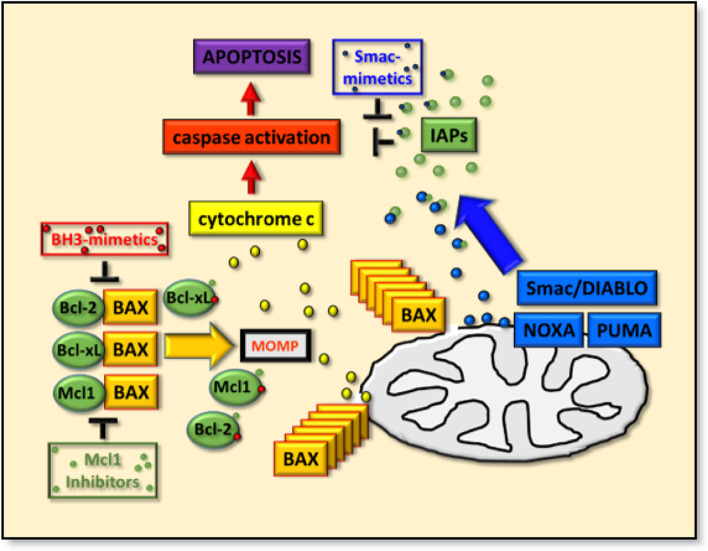
Collectively, the functional significance of such a small handful of regulatory proteins on cell fate cannot be underplayed, as neither can their mechanistic regulation. While some facets of the latter may be steeped in controversy, many aspects of the BH3-domain containing proteins (and their regulation) have nevertheless been fully harnessed throughout the initial formulation of BH3-mimetics, the emerging Smac-mimetics or the Mcl-inhibitors, and their applications as potential cancer therapeutics, thereafter (Fig. 2).
Fig. 2.
The regulation of apoptosis by the BAX protein through mitochondrial outer membrane permeabilization (MOMP), and the modulation of this key steps by therapeutics. Key negative regulator Bcl-2, Bcl-xL and Mcl1 proteins (solid oval green boxes) for BAX (solid orange box) and their apoptosis inducing effects by cytochrome c release (solid yellow box and circles), caspase protein activation (solid red box) and apoptosis (solid purple box) are shown. The mimetics/inhibitors that can target anti−/pro-apoptotic protein interactions are highlighted as BH3-mimetics (outlined red box, red dots) and Mcl1 inhibitors (outlined green box, green dots), which either induce apoptosis of cells as mono-therapeutics or sensitize them to such effects during combined therapeutic targeting. The blue solid boxes (and small circles) highlight mitochondrial Smac/DIABLO, NOXA and PUMA, which bind the Inhibitor of Apoptosis Proteins (small green circles, IAPs) and the interactions of which can be inhibited by Smac-mimetics (blue outlined box and blue dots)
The principles of BH3-mimetics are mechanistically founded on disrupting the interaction of the pro-apoptotic BH3 domain with the hydrophobic pocket of the anti-apoptotic Bcl-2 proteins (such as Bcl-2, Bcl-xL or Mcl1), thus permitting oligomerized BAX (or BAK) to form the MCP [1]. While this approach is largely dependent on labor-intensive rational therapeutic design strategies, the functional significance of such an approach is rewarded with yielding antagonists that have high efficacy, with demonstrated effectiveness at nano-Molar (nM) concentrations, and which have the potential to be used as either single- or combined- therapeutics [5]. Whilst the founding members of the BH3-mimetic drugs did exhibit multiple protein targets, the subsequent pre-clinical studies that have arisen from such findings have identified a number of additional protein targets. Consequently, when targeted collectively with single therapeutics using combined therapeutic regimens at the pre-clinical and clinical levels, such therapeutics are showing a very high level of effectiveness and generating a growing level of interest in how drugs of this nature can be developed further for greater efficacy.
Synonymously, as progress in therapeutic development has come into fruition, so have the delivery methods that can be utilized for efficient drug delivery to offer maximum effects. Of late, such approaches include the use of self-assembling nanofibers and micelles. The importance of such emerging approaches is highlighted by them offering greater scope in permitting the development and delivery of novel therapeutics that may otherwise be limited by their solubility and availability to cancer cells.
As seen in the context of most cancer-related diseases, the development of BH3-mimetics has been driven by them inducing cell death through apoptosis, a morphologically distinct form of death followed by phagocytosis [41]. Herein, we highlight the importance of this therapeutic paradigm, how it has evolved over time through it being applied in targeting additional key regulatory intermediates from the intrinsic apoptosis pathway. As this has given rise to a number of other potential therapeutics, we describe them in the context of how effective they are as single- or combined-therapeutics in preclinical and clinical models. In doing so, we also address the challenges that have arisen, and how some of them can be addressed through key emerging delivery and targeting approaches, so that novel therapeutics of this nature can be given greater effect for the future.
Main text
Basic research and mimetics: a rational drug design strategy
With the contextual origins of BH3-mimetics laying with certain intrinsic pathway intermediate proteins of apoptosis, several approaches have been adopted to develop such therapeutics through specific aspects of rational drug design. For such purposes, the BAX/Bcl-2 regulatory axis has primarily served as a strong foundation to build upon, with particular focus between the 15-amino acid BH3-domain spanning α-helix 2 of BAX [15], and the hydrophobic pocket of Bcl-2 (spanning α-helices 2-5, Fig. 1), for the development of small peptides- and small molecule- inhibitors (SMIs). In furthering such developments, the categorization of genuine mimetics through ‘BH3 profiling’, and whether the potency of candidate agents can induce apoptosis and reduce cell viability in the absence of BAK or BAK expression has also offered good leads in defining therapeutic -authenticity, -specificity and any off-target effects [42–44]. Based on such principles, what has arisen is the diversification of potential antagonists (in both manner and form) and their on-going development and applications in modulating the apoptosis pathway.
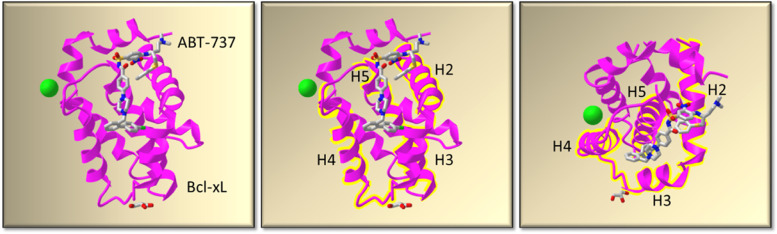
Classically, BH3-mimetics have incorporated engineered peptide inhibitors derived from the BH3 domain of the pro-apoptotic activators or sensitizer proteins and designed to bind the hydrophobic groove of their cognate anti-apoptotic proteins [45–47]. Such, unprecedented developments dispelled the belief that such protein-protein targets were ‘undruggable’ at that juncture. While additional criteria of importance included high-affinity binding to targets (within nM ranges) and a dependency on BAK or BAX induced apoptosis [48], some therapeutic peptides were observed to be toxic, unstable and where penetrability was relatively low [49]. However, some candidates emerged to have great potential in acting as apoptosis inducers or sensitizers, either as single agents or in a combined therapeutic approach. Born from such studies were the design and development of small molecule inhibitors, as shown for ABT-737 in Fig. 3 [50].
Fig. 3.
Co-crystal structure of Bcl-xL and small-molecule inhibitor ABT-737. The interaction of alpha-helices (H) 1-9 from Bcl-xL (pink), in combination with ABT-737 (stick diagram) are highlighted in the presence of a chloride ion (green circle) and glycerol (unlabeled lower stick) in the left panel. Bcl-xL α-helices 2-5 (H2-H5) are highlighted in pink and yellow (middle panel) and in the right panel, are shown when viewed from above
Through implementing a number of alternative approaches, either exclusively or in a combined manner, through utilizing natural library screening, peptide therapeutics and structure-based design, a new era in therapeutic targeting has arisen, with most of it focusing on the intrinsic arm of the apoptosis pathway for the treatment of hematological or solid cancers [51–57]. As highlighted in Table 2, this field has diversified from the original BH3-mimetics (designed to target the anti-apoptosis regulators), to the development of Mcl1-based inhibitors (to help overcome Mcl1-derived resistance), and beyond towards the development of Smac-mimetics, which more specifically target the Inhibitors of Apoptosis Proteins (IAPs).
Table 2.
Promising mimetics and inhibitor therapeutic agents for cancer
| Agent | Type | Origins | Target | Off-Target | Developer | Ref |
|---|---|---|---|---|---|---|
| ABT-737 | BH3-M |
Structure-based design, BAK peptide |
Bcl-2, Bcl-xL | – | Abbott Labs. (IL, USA) | [58] |
| Navitoclax | BH3-M | ABT-737 | Bcl-2, Bcl-xL, Mcl1 | Mcl1 (weak) | Abbott Labs. (IL, USA) | [59] |
|
Gossypol (AT-101) |
BH3-M |
Structure-based design, BIM peptide |
Bcl-2, Bcl-xL, Mcl1 | – | University of Michigan (MI, US) | [60] |
| Obatoclax | BH3-M | In silico docking studies | Bcl-2, Mcl1 | Bcl-xL | University of Montreal (CAN) | [61] |
| Venetoclax | BH3-M | Navitoclax | Bcl-2 | Bcl-xL (weak) | AbbieVie (IL, USA) | [62] |
| Compound 3 | Smac-M |
Smac (AVPI/AVPF peptide sequence) |
xIAP, cIAP1/2 | NF-κB activation | University of Texas (TX, USA) |
[63] [64] |
| APG1387 | Smac-M | Smac (AVPI peptide sequence) | xIAP, cIAP1/2 | – | University of Michigan (MI, USA) | [65] |
| AT-406 | Smac-M | Structure-based design | xIAP, cIAP1/2 | – | University of Michigan (MI, USA) | [66] |
| Compound A | Smac-M | Small molecule screen | xIAP, cIAP1/2 | – | University of Texas (TX, USA) | [63] |
| LC161 | Smac-M | Structure-based design | xIAP, cIAP1/2 | – | Dana-Farber CI (MA, US) | [67] |
| SM-164 | Smac-M | Structure-based design | xIAP | – | University of Michigan (MI, USA) | [68] |
| Birinapant | Smac-M | Smac (AVPI peptide sequence) | cIAP1 | xIAP (weak) | Duke University (NC, USA) | [69] |
| A1210477 | Mcl1-I | High throughput screen | Mcl1 | – |
AbbieVie (IL, USA) Genetech (CA, US) |
[70] [71] |
| AMG-176 | Mcl1-I |
Structure-based design, High throughput screen |
Mcl1 | Bcl-2, Bcl-xL (minimal) | Amgen (CA, USA) | [72] |
| AZD-5991 | Mcl1-I | Structure-based design | Mcl1 | – | AstraZeneca (MA, US) | [73] |
| S63845 | Mcl1-I | In-silico modelling | Mcl1 | – | Institut de Recherches Servier Oncology (FRA) | [74] |
| MIM1 | Mcl1-I | Small molecule screen | Mcl1 | – | Dana-Farber CI (MA, USA) | [75] |
| VU661013 | Mcl1-I | Structure-based design | Mcl1 | BIM-Mcl1 destabilization | Vanderbilt University (TN, USA) |
[76] [77] |
| GDC-0941 | Mcl1-I | In-silico modelling | PI3Kα/δ, Mcl1 | – | Piramed Pharma (UK) | [78] |
BH3-mimetics (-M), Smac-mimetics (-M) and Mcl1 inhibitors (-I) are highlighted along with the techniques utilized for their discovery or origins. For each drug, we show its cognate target and off-target proteins or effects, its developer and the publication describing its development (Ref)
While some agents show off-target effects, such effects are being harnessed advantageously, through devising novel and combined treatment regimens, based on a common vision shared by the scientific communities from the academic and private sectors. Additionally, most classes of therapeutics exhibit a broad protein target range (with non-specific off targets), whilst some have greater specificity (with minimal off-target effects), as seen for Venetoclax and Gossypol (for BH3-mimetics), and numerous agents for Smac-mimetics and Mcl-inhibitors (Table 2).
Consequently, such efforts have given rise to several promising biological agents, reported to induce the death of several cancer cell types in pre-clinical models, and some of which are currently being driven through clinical trials as very promising anti-cancer therapeutics. In the following sub-sections, we aim to review key aspects of the research efforts published over the last 20 years. As an active area of research that has intensely diversified on a number of fruitful tangents, we aim to initially describe the founding members of the BH3-mimetics group and how the outputs from their evaluation have evolved to give rise to promising therapeutics. We will then turn our attention to how the principles of BH3-mimetics design and targeting have been applied to spawn a number of promising mimetics derived from the NOXA, PUMA and Smac proteins. Lastly in this subsection, we focus on the most encouraging BH3-mimetic (Venetoclax) and the emerging Mcl-1 inhibitors, which are reportingly showing notable pre-clinical outcomes.
Bcl-2 protein targeting mimetics
Cell fate is determined by the balance of Bcl-2 anti-apoptotic protein expression in relation to pro-apoptotic BH3 proteins levels, highlighted by the discovery of Bcl-2 being the first anti-apoptotic overexpressed oncogene, as seen in follicular lymphoma [79–81]. Subsequently, 17–18 member proteins have been reported to share at least one of the four Bcl-2 homology domains (BH1-4), in a family of proteins that exhibit anti- or pro- apoptotic expression-dependent effects [82]. As a protein highly expressed in a number of B-cell lymphomas [83, 84], Bcl-2 is expressed in Chronic lymphocytic leukaemia (CLL) [85], Mantle cell lymphoma (MCL) [85–87], Multiple myeloma (t11;14) (MM) [88] or solid tumors [83], and (in a similar manner to Mcl1 expression) is critical for cell survival [89]. Here, high levels of pro-apoptotic protein expression had also been reported [90], thus potentially offering the induction of potent cell death by mono-therapeutic BH3-mimetics acting through disrupting the BH3-domain-hydrophobic groove interaction. Mechanistically, this is permitted through the localization of functionally equivalent BAX or BAK proteins [91] to the outer membrane of the mitochondrion through their α-helix 9 motifs [92–95], where they can homo-oligomerize and mediate the formation of the MPC, thereby inducing the release of cytochrome c and other apoptotic inducing or regulatory factors [96, 97].
-ABT-737
The BH3-mimetic ABT-737, was designed using an NMR structure-based approach, which targeted the BH3-binding hydrophobic groove of Bcl-2, Bcl-xL (Fig. 3) and Bcl-w (with a Ki of 36 nM), whilst binding minimally to Mcl1 or A1 [50, 98, 99]. Its effectiveness was observed against cell lines and patient samples derived from lymphoma, leukemia and in senescence of solid tumors in single [51, 100] or combined therapy approaches [101–103]. However, resistance against the death-inducing effects of ABT-737 was reported in pre-clinical models, mainly due to upregulated Mcl1 protein expression in certain cancer cell types, and the abrogation of which sensitized cells to death, as seen in normal mouse embryonic fibroblast [99, 104], prostate cancer [34] and breast cancer cells [35]. As a potential mechanism for ABT-737 resistance in pre-clinical models, the significance of such a mechanism in clinical studies remains to be explored in greater detail.
Other aspects of drug resistance were also derived from the stress protein Bcl-2-associated athanogene 3 (BAG3), which could stabilize Mcl1 expression, thus contributing to ABT-737 resistance in breast cancer and prostate cells [105]. Conversely, Mcl1 protein levels were observed to be destabilized when A549 and 95-D cells were treated with Nedaplatin, allowing ABT-737 to induce death with greater efficacy [106], thus highlighting the effectiveness of Mcl1 co-inhibition in a viable combined therapeutic strategy, and which was confirmed through ARC-mediated transcriptional down-regulation of Mcl1 expression [107]. Similar ABT-737-enhancing effects have also been reported upon Mcl1 inhibition in retinoblastoma- [108], melanoma- [109], breast-, prostate-, colon- [110] and liver- cancer cells [111]. While such findings, suggestive of ABT-737 synergizing well with other therapeutics (Table 3) are very encouraging (as seen from its therapeutic activity against a diverse repertoire of cell types), poor bioavailability and low aqueous solubility of ABT-737 did present themselves as major obstacles against its further use in the clinic.
Table 3.
The single and combined synergizing effects of ABT-737 on cell line viability and/or apoptosis
| Cancer | Cell Type | Combined | Reduced Viability | Apoptosis | Ref |
|---|---|---|---|---|---|
| AML | ex-vivo samples | 5-azacytidine | synergized | – | [112] |
| Breast | MDA-MB-231R | irradiation | synergized | – | [113] |
| Breast | T47D | cisplatin | synergized | synergized | [114] |
| Breast | MCF-7, ZR-75-1, MDA-MB231 | irradiation | synergized | – | [35] |
| Breast | MDA-MB-435S | VX-680 | synergized | – | [115] |
| Breast (TN) | MDA-MB-231 | docetaxel | synergized | – | [116] |
| CRC | C26, HCT116, LS174T | oxaliplatin | synergized | – | [117] |
| CRC | HT-29, HCT116 | celecoxib | synergized | – | [118] |
| Glioma | LN229, LN18 | bortezomib | synergized | – | [119] |
| HNSCC | UM-22A, UM-22B, 1483 | cisplatin/etopiside | synergized | – | [120] |
| Lung | A549 and H460 lines | perifosine | synergized | synergized | [121] |
| Lung | A549 and 95-D | nedaplatin | synergized | – | [106] |
| Leukaemia | HL-60, U-937, ML-1, MOLT-4 | -single- | reduced | – | [122] |
| Leukaemia (T-cell) | MOLT-4 | resveratrol | synergized | – | [123] |
| Leukaemia | HL-60 | doxorubicin | synergized | – | [124] |
| Liver | HuH-7, HepG2, BEL-7402, SMMC-7721 | norcantharidin | synergized | – | [111] |
| Liver | HepG2 | curcumin | synergized | – | [125] |
| Melanoma | A375, WM852c | bortezomib | synergized | synergized | [109] |
| NSCLC | A549, H460, H1299, H358, H2009, H1703, H596 | cisplatin | – | synergized | [126] |
| Oral | MC-3, HSC-3 | sorafenib | synergized | – | [127] |
| Osteosarcoma | U-2OS | cisplatin | synergized | – | [128] |
| Ovarian/Gastric | SKOV-3, OVCAR-8, SGC-7901 | epothilone B | synergized | – | [129] |
| Ovarian | patient derived organoids | naftopidil | synergized | – | [130] |
| Ovarian | Ovcar-3, Igrov-1 | pitavastatin | synergized | – | [131] |
| Ovarian | A2780, cisA2780, IGROV-1, OVCAR, SK-OV-03, primary and xenograft | carboplatin | synergized | synergized | [132, 133] |
| Prostate | DU 145, LNCaP and PC-3 | ARC | synergized | – | [107] |
| Renal | PV10, KRC/Y,A498, ACHN | TRAIL | synergized | – | [134] |
| Retinoblastoma | Y79, WERI-Rb | -single- | – | – | [108] |
| Thyroid | FTC236, ML1, SW1736, HTh7 | doxorubicin/gemcitabine | synergized | – | [135] |
| Uterine/Cervical | SiHa, CaSki | irradiation | synergized | – | [136] |
| Breast, Colon, Liver, Pancreatic | SW480 and LIM1215, Huh-7 and HepG2, HPAC, MDA-MB-231 | ARC | synergized | synergized | [137] |
| Breast, Colon, Prostate | MDA-MB-231, HT-29, DU145 | methylseleninic acid | synergized | – | [110] |
The cancer types are highlighted in bold (left column), the evaluation of single therapy alone is highlighted by ‘-single-‘, non-synergy is highlighted by ‘-‘and the corresponding studies are referenced in the column on the right (Ref). Abbreviations: TN Triple Negative, CRC Colorectal cancer, HNSCC Head and Neck Squamous Cell Carcinoma, NSCLC Non-small cell lung cancer
-Navitoclax (ABT-263)
In striving towards overcoming bioavailability and solubility issues for ABT-737, its redesign gave rise to orally administered Navitoclax (ABT-263) [59], which demonstrated a Ki for Bcl-2 of < 0.010 nM and could bind albumin with greater affinity for potential additional delivery benefits in the clinic. While the latter was seen as an encouraging observation, such an interaction was unveiled to reduce the availability of Navitoclax for during the treatment of CLL patients [138]. Regardless of the delivery method of choice, Navitoclax is currently being evaluated and pursued as single- or combined- anti-cancer therapeutic [48], as seen from its ability to sensitize cervical cancer cell lines [139] for example, or synergize with a number of therapeutics for hematological malignancies [140] (Table 4). Despite consistent side effects for Navitoclax, which included thrombocytopenia, based on Bcl-xL expression-dependency for platelet survival, it was effective at killing Bcl-2-dependent CLL cancers, just as effectively as ABT-737 [138].
Table 4.
The single- and combined- synergizing, or resistance-inducing effects of Navitoclax on cellular apoptosis
| Cancer | Cell Type | Combined | Apoptosis | Ref |
|---|---|---|---|---|
| AML | Primary cells | dasatinib | – | [141] |
| AML/ALL | Jurkat, Molt-4 | wogonin | synergized | [142] |
| Cervical | SiHa, CaSki | A-1210477 | synergized | [139] |
| CRC | HCT116, DLD1, SW48, HT29, HCT-8 | apigenin | synergized | [143] |
| CRC, Liver | Huh7, HepG2, BEL7402, HCT116, DLD1, AGS | sorafenib | synergized | [144] |
| CRC/Melanoma | Colo-205 | AZD6244 resistance | – | [145] |
| Esophageal | SKGT-4, KATO-TN, YES-6 | fluorouracil | synergized | [146] |
| Esophageal | EC109, HKESC-2, CaES-17 | -single- | – | [147] |
| HNSCC | HN12 | fenretinide | synergized | [148] |
| Liver | Huh7 | TRAIL resistance | – | [149] |
| Lung | H1650 and H1975 | src-inhibitors | synergized | [150] |
| Non-small cell lung | LC2, PC10 | cisplatin | synergized | [151] |
| Small cell lung | H209 | vorinostat | synergized | [152] |
| Lymphoma | DoHH-2 and SuDHL-4 | rapamycin | – | [153] |
| Neuroblastoma | SH-SY5Y and CHLA-119 | norcantharidin | synergized | [154] |
| Prostate | LNCaP and PC3 | paclitaxel | synergized | [155] |
| Prostate | PC3, C4–2B, C4–2, DU145 | MLN2238 | synergized | [156] |
| Ovarian | Numerous | paclitaxel/gemcitabine | synergized | [157] |
| Liver, Prostate, Cervical, CRC, NSCLC | Hep3B, PC3, HCT-116, SW480, and SW620, H1299, SK-BR-3, HeLa | metformin | synergized | [158] |
The cancer types are highlighted in bold (left column), the evaluation of single therapy alone is highlighted by -single-, non-synergy is highlighted by ‘-‘and the corresponding studies are referenced in the column on the right (Ref). Abbreviations: AML Acute Myelogenous Leukemia, ALL Acute Lymphoblastic Lymphoma, CRC Colorectal cancer, HNSCC Head and Neck Squamous Cell Carcinoma, NSCLC Non-small cell lung cancer
Despite such encouraging outcomes, resistance against Navitoclax was also encountered, due to the induction of Mcl1 [159] or Survivin expression [160], and the direct (or indirect) down-regulation of which, were seen as beneficial to Navitoclax efficacy [142, 143]. For example, inhibition of EGF-R mediated Mcl1 induction improved Navitoclax effects in leukemia K562 cells [161], by destabilizing Mcl1 levels through the upregulation of NOXA expression [148, 154]. Conversely, not all instances of resistance were attributed to Mcl1 expression, as reported for cisplatin-treated non-small cell lung cancer cells (NSCLC), which induced cell death independently of Mcl1 expression levels [151].
-Gossypol
Other broad-range BH3-mimetics include Gossypol and its derivatives. Derived from cotton seed extracts and identified using NMR binding assays and Fluorescence Polarization displacement assays, racemic Gossypol directly interacted with Bcl-xL and could also counteract the anti-apoptotic effects of Bcl-2, with an IC50 of 13.2 μM in MCF-7 cells, as a pan-Bcl-2 inhibitor [52]. Gossypol also inhibits growth and induces apoptosis in several other cell types, such as H1975, H441 and A549 lung cells as a mono-therapeutic in a dose-dependent manner, while also reducing H1975 xenograft growth in mice [162]. It can inhibit EGFR-L858R/T790M signaling, proliferation and migration of NSCLC cells [163], induce death of prostate cancer DU-145 and PC-3 cells [164, 165] and ovarian SKOV3 cancer cells [166]. Fortuitously, racemic Gossypol can also behave as a NOXA-like BH3-mimetic, by selectively promoting apoptosis of cancer cells from the bladder [167], breast [168, 169] and prostate [170], when administered as a mono-therapeutic. Mechanistically, Gossypol can reduce cellular viability upon p53 activation, as seen in LAPC4, PC-3, and DU-145 prostate cancer (PC) cells [171], through ER stress and autophagy in hepatocellular carcinoma (HCC) cells [172], and oxidative stress, as seen in ovarian and MM cells [166, 173]. As a combined therapeutic, it has been reported to induce autophagy and apoptosis in a cell type-dependent manner [174] or exclusively autophagy in melanoma cells [175]. Alternatively, the R(-)-Gossypol enantiomer AT-101, has also been encouragingly reported to reduce invasiveness of rat MLL PC cells [176] and induce mitophagy in U87MG and U343 glioma cells [177]. Mechanistically, Gossypol and AT-101 may contribute to cell death by binding and inhibiting Mcl1, resulting in the sensitization of several cell lines to other therapeutics or through it stabilizing NOXA expression, as in gastric, breast and nasopharyngeal cell lines [178, 179]. Collectively, racemic Gossypol or its enantiomer are showing good potential as anti-cancer agents which induce cell death through a variety of mechanisms, and in a variety of cell line-based models. They can be effective as a single- or combined- therapeutics (Table 5), but the activity of which may be limited through the levels of toxicity that arise from administering increasing doses [183].
Table 5.
The combined synergizing effects of Gossypol and its derivative (AT-101) against certain cancers and cell types
| Mimetic | Cancer | Cell Type | Combined | Apoptosis | Ref |
|---|---|---|---|---|---|
| Gossypol | Glioblastoma | Diff13–20, TS13–20 | temozolomide resistance | – | [180] |
| Gossypol | CML | K562 | imatinib | – | [181] |
| Gossypol | Colon | HT-29 cells, HCT116, RKO | fluorouracil | – | [182] |
| Gossypol | Nasopharyngeal, Breast, Gastric | MCF-7, YC116, CNE2 | gemcitabine | – | [183] |
| Gossypol | Ovarian | OVCAR-3 and MDAH-2774 | zoledronic acid | – | [184] |
| Gossypol | Thoracic | H460, TE2, H211 | TRAIL | – | [185] |
| AT-101 | Bladder | UM-UC2, UM-UC9 | gemcitabine, carboplatin | synergized | [167] |
| AT-101 | Breast | SKBR-3, MDA-MB-453 | trastuzumab | – | [186] |
| AT-101 | Pancreatic | BxPC-3 | genistein | – | [187] |
| AT-101 | Prostate | PC-3 and xenograft | radiation | – | [188] |
| AT-101 | Prostate | PC-3 xenograft | docetaxel | synergized | [170] |
| AT-101 | Prostate | DU145, PC-3 | sorafenib | – | [174] |
| AT-101 | Prostate | VCaP | bicalutamide | – | [189] |
The therapeutic type is highlighted in bold (left column), non-synergy is highlighted by ‘-‘and the corresponding studies are referenced in the right column (Ref). Abbreviations: CML Chronic Myelogenous Leukemia
Based on toxicity effects, alternative Gossypol derivatives and analogues have been developed, based on the structural binding properties of the BIM protein BH3-domain with the Bcl-2 protein, namely TW-37 (Compound 5), and which can also bind Bcl-xL and Mcl1 with respective Ki’s of 1110 and 260 nM [60]. As emerging alternatives to Gossypol, their further evaluation in model systems and potential use as single or combined therapeutics are awaited with great eagerness.
-Obatoclax
Another promising BH3-mimetic, specific for all Bcl-2 proteins includes the rationally-designed and prodiginine-related Obatoclax, which binds the mitochondrially-associated Bcl-2 protein and all pro-survival Bcl-2 proteins with a Ki of 220 nM [61, 190], outlining its suitability for the treatment of hematological malignancies and solid tumors [191, 192]. Good evidence to support this has been derived from xenograft models, where its successful use as a single agent against thyroid cancer, small cell lung cancer and colorectal cancer development has been reported, and efficacy of which can be enhanced when used as a combined therapeutic in limited instances with cisplatin, MEK inhibition, doxorubicin [193, 194], bortezomib, carfilzomib or AZD2281, as highlighted in Table 6.
Table 6.
The combined synergizing effects of Obatoclax on cellular apoptosis
| Cancer | Cell Type | Combined | Apoptosis | Ref |
|---|---|---|---|---|
| AML | U937, HL-60, MV4–11 | sorafenib | – | [195] |
| Bladder | HT1197 | paclitaxel | – | [196] |
| Bladder | T24, TCCSuP, 5637 | cisplatin | – | [197] |
| Cholangiocarcinoma | KMCH, KMBC, TFK, | TRAIL | – | [198] |
| Colon | HCT116, HCT-8, | fluorouracil | – | [199] |
| Esophageal | CaES-17 | MG132 | – | [200] |
| Glioblastoma | Patient samples | SAHA, LBH589 | – | [201] |
| Small cell lung | H82, H526, DMS79, H196, H1963, H69 | bortezomib and carfilzomib | synergized | [202] |
| Non-small cell Lung | LoVo, RKO, HCT116 | oxaliplatin resistance | – | [203] |
| Neuroblastoma | SK-N-DZ, IGR-NB8 |
hydroxychloroquine/cisplatin/ doxorubicin |
– | [204] |
| Pancreas | BxPC-3 | gemcitabine | – | [205] |
| Pancreas | PANC-1 and BxPC-3 | TRAIL | – | [206] |
| Pancreas | BxPC-3, HPAC | chloroquine | – | [207] |
| Pancreas | BxPC-3, HPAC, MIAPaCa-2, PANC-1, AsPC-1, CFPAC-1 | AZD2281 | synergized | [208] |
| Thyroid | KTC-1, BCPAP | LY3009120/vemurafenib resistance | – | [209] |
The cancer types are highlighted in bold (left column), non-synergy is highlighted by ‘-‘and the corresponding studies are referenced in the column on the right (Ref). Abbreviations: AML Acute Myelogenous Leukemia
Moreover, Obatoclax has completed phase I trials for several cancers with encouraging efficacy, but with notable side effects [191, 192].
NOXA-mimetics
As to be expected NOXA expression plays a relatively restricted role in determining cell fate and tumor progression in the presence and absence of therapeutic treatments against LC [210–213], leukemias [214, 215], rhabdomyosarcoma [216, 217], PC [218], OC [219], colorectal cancer (CRC) [220, 221], melanoma [222] and MM [223]. As a protein up-regulated in CLL, NOXA can interact with Mcl1 and neutralize its anti-apoptotic activity [224], thus offering good justification for the development of NOXA-like BH3-mimetics, and particularly for CLL therapy [225]. Here, Mcl1-derived inhibitors had been reported to be sufficient to induce apoptosis through their specific targeting of NOXA. While encouraging, such an approach may come with limitations, seeing as anti-apoptotic proteins (such as NOXA) are embedded in the mitochondrial membrane and more susceptible to changes in their conformation, and which may consequently respond differently to the affinity of mimetics in comparison to BH3-mimetics which are otherwise directed at soluble targets [14].
PUMA-mimetics
The BH3-only protein PUMA, is frequently down-regulated in a number of tumors [26], and which may contribute to chemoresistance and tumor progression in conjunction with other protein factors, based on the phenotype of PUMA knockout-mice lacking spontaneous oncogenesis [226]. Nevertheless, PUMA-derived helices in the form of SAHBs [227], which have the capacity to bind BAX or Bcl-xL/Mcl1 as dual anti-apoptosis inhibitors that induce BAX activation, have shown some encouraging outputs in overcoming chemoresistance in neuroblastoma cells. As in the instance of BH3-mimetics, the starting point here has been the design of peptide inhibitors, which can potentially take up a helical conformation upon binding the hydrophobic groove of BAX, Bcl-xL or Mcl1 [228, 229], or through them being generated as stapled peptides [228].
SMAC-mimetics
The human Inhibitor of Apoptosis (IAP) family of proteins are composed of eight members, each of which encode a Baculoviral IAP Repeat domain (BIR) and several of which are over-expressed in hematological malignancies and solid tumors [230–232]. While each member can regulate cell survival in response to a number of signaling cues, cIAPs and XIAPs have direct anti-apoptotic roles [233, 234] and offer chemoresistance when over-expressed in cancer cells [230]. Mechanistically, they can be targeted through homodimers of Smac binding the BIR domain and destabilizing the IAP protein through IAP auto-ubiquitination and degradation [235–238]. Consequently, cells can be sensitized to the apoptosis-inducing effects of TRAIL with PI3K or MAPK inhibition [239, 240] and etoposide or paclitaxel [241] in combined therapeutic approaches. As mono-therapeutics, Smac-mimetics can act through preventing inhibitory IAP binding to caspases -3, -7, -9 [242–245] and thus potentiating the effects of any death-inducing signals [230]. Therefore the loss of Smac expression can negatively impact the full execution of apoptosis, as seen with some cancer patients encoding high levels of Smac expression, and which can be correlated with a better prognosis [230]. Consequently, targeting the Smac-IAP interaction has been explored as a therapeutic strategy through the use of SMIs, peptides [230], LAso Smac-mimetics or Smac anti-sense oligonucleotides, and all of which showed varying degrees of success. For example, Smac-mimetics induced the degradation of cIAPs -1, -2 and -3 via the proteasome, leading to NIK activation, NF-κB activation, and up regulation of TNF-α expression and subsequently cell death in an autocrine- or paracrine- manner, in treated fibrosarcoma, colorectal and melanoma cell lines [235, 238]. Generally speaking, Smac-mimetics can be as little as 4 aa long and which can target BIR repeats -3 and -4 of the three cIAPs [246]. While they have shown good effects against specific solid tumors and leukemias as mono-therapeutics [247], they can also sensitize cells to death in combined therapeutic regimens, as seen with temozolomide [248], cytarabine [249], prednisolone [250] and even the pro-inflammatory cytokine TNF-α [251, 252], in a variety of cell types (Table 7).
Table 7.
The combined synergizing effects of Smac-mimetic (or IAP inhibitor) agents against selected cancers and cell types
| Agents | Cancer | Cell Type | Combined | Ref |
|---|---|---|---|---|
| APG-1387 | Liver | HepG2 and HCCLM3 | TNF-α, TRAIL | [253] |
| APG-1387 | Ovarian | SKOV3 | TNF-α | [254] |
| AT-406 | Osteosarcoma | AT-406, Xenograft | doxorubicin | [255] |
| AZ58 | Bladder | UMUC-6, UMUC-12, and UMUC-18 | gemcitabine, cisplatin | [256] |
| Birinapant | AML | MLL-ENL AML | emricasan | [257] |
| Birinapant | Breast | SUM190, SUM149 | TRAIL | [69] |
| Birinapant | Head and Neck | UM-SCC-46 and -11B xenograft | radiation | [258] |
| Birinapant | Non-small cell Lung | LKB1- and KRAS-mutated | ralimetinib | [259] |
| Birinapant | Ovarian | CAOV3, OVCAR4, SKOV3, OVCAR8, OV90, 1A9 | docetaxel | [260] |
| Birinapant | Ovarian | OCAR3, OVCAR8 | carboplatin/ paclitaxel | [261] |
| BV6 | AML | 51% primary AML cells | cytarabine | [262] |
| BV6 | Glioblastoma | – | temozolomide | [263] |
| BV6 | CRC | SW480, HT-29, HCT-15 | radiation | [264] |
| BV6 | Glioblastoma | A172, T98G | temozolomide | [265] |
| BV6 | Multiple | HT1080, HeLa, Jurkat, L363, MMI, OPM2, RPMI, HT29 | TNF-α, TRAIL | [266] |
| BV6 | Renal | CaKi1, KTCTL26, 786O, KTCTL30, KTCTL2 | interferon-α | [267] |
| JP-1201 | CRC | HT-29 | radiation | [268] |
| JP-1201 | Pancreas | Xenograft MIA PaCa-2 | gemcitabine | [269] |
| LCL161 | B-Cell Lymphoma | Xenograft Raji/4RH | rituximab, gemcitabine, vinorelbine | [270] |
| LCL161 | Breast | MCF7-TamC3 | tamoxifen | [271] |
| LCL 161 | HNSCC | human cell culture, xenograft | radiation | [272] |
| LCL161 | HNSCC | PCI-1, PCI-9, PCI-13, PCI-52, PCI-68 | FAS-L | [273] |
| SM-164 | Breast | (SK-BR3) and (MDA-MB-468 | radiation | [274] |
| SM-164 | Breast, Prostate, Colon | Cell lines | TRAIL | [275] |
| SM-164 | Pancreatic | Panc-1, AsPC-1, BxPC-3 | gemcitabine | [276] |
| compound A | Bladder | UC-9. UC-14, RT4 v1, RT4 v6 | TRAIL | [277] |
| compound 3 | Pancreas/CRC | Panc-1 and HCT116 | doxorubicin | [278] |
| Debio 1143 | Lung | LLC-OVA | radiotherapy | [279] |
| SH122 | Prostate | DU145, CL1 | TRAIL | [280] |
| SW IV-134 | Pancreatic | PANC-1, CFPAC-1, BxPC-3, AsPC-1, MIA PaCa-2 | gemcitabine | [281] |
The therapeutics types (Agents) are highlighted in bold (left column) and the corresponding studies are referenced in the column on the right (Ref). Abbreviations: AML Acute Myelogenous Lymphoma, CRC Colorectal cancer, HNSCC Head and Neck Squamous Cell Carcinoma
Smac-mimetics or IAP inhibitors have also shown effectiveness with death receptor agonists in a variety of cell types, as seen against TRAIL in -CLL [282], -ALL [283] and -cholangiocarcinoma cell types [284], or with radiation-induced death of glioblastoma cells [285]. Similarly, signal transduction inhibitors have been reported to improve efficacy of treatments when administered in combination with Smac-mimetics, as seen with 5-Aza against AML cells [286, 287], tyrosine kinase inhibitors against leukemia cells [67, 288], CD95 agonist antibodies against leukemia cells [289], or Smac-mimetics and TNF-α against alveolar epithelial cells [290]. Lastly, some Smac-mimetics exhibit synergistic effects with Birinapant-induced death of liver cancer cells [291], triple-negative BC cells (TNBC) [292], ovarian cancer (OC) cells [260] and primary AML cells in response to BV6 [262].
-Venetoclax (ABT-199)
Venetoclax was developed through rational design approaches as a high-affinity antagonist for Bcl-2 (with a Ki < 0.010 nM) and a 4000 fold lower affinity binding for Bcl-xL, to help overcome thrombocytopenia side effects derived from off-target Bcl-xL inhibition, and a common feature associated with ABT-737 and Navitoclax treatments [62]. While Venetoclax could induce therapeutic resistance by up-regulating Bcl-xL and Mcl1 expression in some instances [293], it has shown encouraging results as a single agent for treating acute lymphocytic leukemia (ALL) [294], head and neck squamous cell carcinoma (HNSCC) [295] and neuroblastoma [296, 297] in pre-clinical models. Through structure-based design, other ABT-737-derived SMIs targeting Bcl-2 and Bcl-xL, have also arisen in the form of BM-957 and BM-1197, which showed improved solubility, pharmokinetic properties and tumor regression capabilities [298, 299], as single therapeutics against AML [300]. More specifically, such agents spared platelets [62] and to avoid therapeutic resistance, Gemcitabine was reported to effectively decrease Mcl1 expression, while enhanced Bcl-2 expression (in pancreatic cancer cells), could be efficiently targeted by Venetoclax through it beneficially enhancing expression of BIM [301]. Similarly, Venetoclax has shown encouraging results for synergizing with a growing array of therapeutics in pre-clinical models for several cancer cell types, as a well-tolerated combined therapeutic (Table 8).
Table 8.
The combined synergizing effects of Venetoclax on cellular apoptosis
| Cancer | Cell Type | Combined | Apoptosis | Ref |
|---|---|---|---|---|
| ALL | LOUCY cell line | doxorubicin, l-asparaginase, and dexamethasone | – | [302] |
| AML | Primary cells and U937 | daunorubicin or cytarabine | – | [303] |
| AML | KOPT-K1 | S63845 | synergized | [304] |
| AML | MV4–11 and MOLM-13, KG-1a, U937, and THP-1 | triptolide | synergized | [305] |
| AML | Jurkat and Molt4 | gemcitabine | synergized | [306] |
| AML | Molm14 and OCI-AML3 | VS-4718 | – | [307] |
| AML/MDS/CMML | Ex-vivo samples | 5-azacytidine | – | [112] |
| Breast | 23 T Xenografts and MCF7 | tamoxifen, AZD8055 | – | [308] |
| CML | KCL22 | imatinib | sensitized | [309] |
| CRC | Xenograft and RKO cell line | LZT-106 | synergized | [310] |
| Diffuse BCL and FL | Cell lines and TMD8 xenograft model | ibrutinib | synergized | [311] |
| Leukemia, Lymphoma | SU-DHL-4, OCI-Ly1 199R, SC-1199R and BCl and FL primary samples | A-1592668 and analogue A-1467729 | synergized | [312] |
| Nasopharyngeal | CNE-2, 5-8F | S63845 | synergized | [293] |
| MM | OPM2, H929 | THZ1 | synergized | [313] |
| MM | U266, KMS11, OPM2, RPMI8226 and KMS28-PE | flavopiridol | synergized | [314] |
| Pancreatic | MIA Paca-2 xenograft | gemcitabine | – | [301] |
| Soft Tissue Sarcoma | Rhabdomyosarcoma, SW982 (synovial sarcoma) cells or primary cells | bortezomib | synergized | [315] |
The cancer types are highlighted in bold (left column), non-synergy is highlighted by ‘-‘and the corresponding studies are referenced in the column on the right (Ref). Abbreviations: AML Acute Myelogenous Leukemia, ALL Acute Lymphoblastic Lymphoma, MDS Myelodysplastic syndrome, CMML Chronic myelomonocytic leukemia, BCL B-cell lymphoma, FL Follicular Lymphoma, MM Multiple myeloma
Mcl1-inhibitors
As the first homologue of Bcl-2 found to be overexpressed in a number of hematological malignancies such as MM [316], and which conferred anti-apoptotic effects under normal conditions [317–320], Mcl1 expression was also reported to contribute to chemoresistance, thus highlighting its suitability to be targeted in combined therapeutic approaches. Normally, Mcl1 is localized to the MOM [321], ER, nucleus [322, 323], and mitochondrial matrix [321] in conjunction with its spliced variants Mcl-1S [324–326] and Mcl-1ES [327]. As a protein that is essential for survival of a number of normal cell types [328] it has been reported to be over-expressed in a number of other cancers, such as B- and T- non-Hodgkin’s lymphoma [329], and solid tumors, such as hepatocellular carcinoma (HCC) [330], esophageal squamous cell carcinoma (SCC) [331] and breast cancer (BC) [332].
Based on these properties of Mcl1 expression, Mcl1-inhibitors have been eagerly pursued, giving rise to the discovery of Prodigiosin for example, which is a natural compound that targets the hydrophobic groove of Mcl1 with good specificity [333]. Based on the normal role of Mcl1 for hematopoietic stem cell survival [334] and oxidative phosphorylation [321], the development of most Mcl1 antagonists has been seen to offer potential limitations when used as a mono-therapeutic.
Alternative Mcl1-specific mimetics or inhibitors are also showing their usefulness as mono- or combined- therapeutics, as seen with S63845-mediated apoptosis of MM and NSCLC, gastric cancer (GC), PC, [74] and T-cell acute lymphoblastic leukaemia (T-ALL) cells [304] or ABT-199 and S63845 co-treatments in cervical cancer cells [335]. Other, promising Mcl1-specific candidates as combined therapeutics include, A-1210477 (Ki, 0.45 nM) [70], AZD5991 (Ki, 0.13 nM) [73] and AMG-176 (Ki, 0.13 nM) [72], AM8621 (Ki, 0.06 nM) [72], S64315 (Ki, 0.048 nM) [336] and VU661013 (Ki, 97 pM) [76], as outlined in Table 9.
Table 9.
The combined synergizing effects of Mcl1-inhibitors against selected cancer and cell types
| Agents | Cancer | Cell Type | Combined | Ref |
|---|---|---|---|---|
| A1210477 | AML | THP-1 U937 | venetoclax | [337] |
| A1210477 | Breast | MDA-MB-231 cells | TRAIL | [338] |
| A1210477 | Cervical | SiHa and CaSki | navitoclax | [139] |
| A1210477 | CML | K562, K562/R | EE-84 | [339] |
| A1210477 | CRC | RKO, HT29, A375 | cobimetinib | [340] |
| A1210477 | DLBCL | U-2946 | navitoclax | [341] |
| A1210477 | PC, GC, NSCLC, MM | BxPC-3, EJ-1, H23, and OPM-2 | navitoclax | [70] |
| A1210477 | HNSCC | PCI15B, Detroit 562, MDA686LN, and HN30 | navitoclax | [342] |
| A1210477 | nHL | SU-DHL-4, WSU-NHL, WSU-DLCL2, KARPAS-422 | venetoclax | [343] |
| AMG-176 | CLL | Patient samples (5) | venetoclax | [344] |
| AZD-5991 | AML | OCI-AML3 and MCL-1-OE Molm13 and MV4–11 | venetoclax | [345] |
| AZD5991 | MM | NCI-H929 | bortezomib, venetoclax | [73] |
| GDC-0941 | Breast | MDA-MB-231, SKBR3 | ABT-737 | [346] |
| Mim1 | Glioblastoma | U87mg | temozolomide | [347] |
| Mim1 | Melanoma | C32 melanoma cells | dacarbazine | [348] |
| Mim1 | MM | Colo829 | dacarbazine | [349] |
| S63845 | AML | OCI-AML3, MOLM-13, OCI-AML2 | trametinib/HDM201 | [350] |
| S63845 | AML | Primary samples | venetoclax | [351] |
| S63845 | AML | MOML-13, SKM-1, | trametinib | [352] |
| S63845 | AML | Cell lines and primary cells | venetoclax | [353] |
| S63845 | Breast | SK-BR-3 | docetaxel, trastuzumab, lapatinib | [354] |
| S63845 | CRC | HCT116 | regorafenib | [355] |
| S63845 | Mantle cell lymphoma | Patient-derived xenografts | venetoclax | [356] |
| S63845 | Melanoma | Patient samples | navitoclax | [357] |
| S63845 | Melanoma | MeWo | TRAIL resistance | [358] |
| S63845 | Myeloma | U266 xenograft | venetoclax | [359] |
| S63845 | MM | MOL-P8, OPM-2, NCI-H929 | venetoclax, bortezomib | [360] |
| S63845 | MM | RPMI-8226 xenograft | venetoclax | [361] |
| S63845 | Nasopharyngeal carcinoma | CNE-2, 5-8F | venetoclax | [293] |
| S63845 | T-ALL | Zebrafish T-ALL cells | venetoclax | [304] |
| VU661013 | AML | MV-4-11, AML-001/2, patient xenografts | venetoclax | [76] |
| VU661013 | Breast | HCC1428, MCF7, T47D | navitoclax | [362] |
The therapeutic types (Agents) are highlighted in bold (left column) and their corresponding studies referenced in the column on the right (Ref). Abbreviations: CML Chronic Myelogenous Leukemia, CRC Colorectal cancer, DLBCL Diffuse large B-Cell Lymphoma, PC Prostate Cancer, GC Gastric Cancer, NSCLC Non-small cell lung cancer, MM Multiple myeloma, HNSCC Head and Neck Squamous Cell Carcinoma, nHL Non-Hodgkin’s Lymphoma, CLL Chronic lymphocytic leukemia, AML Acute Myelogenous Lymphoma, T-ALL T-cell acute lymphoblastic leukemia
From this group, AZD5991 showed promising effects [363, 364], either as a monotherapy or as a combined agent [73], to treat a number of hematologic and solid tumor cell lines. It also showed combined effectiveness against biomarker-specific and Venetoclax-resistant AML cells, highlighting its usefulness for cell line- or biomarker- specific cancers [365]. Of similar interest is AZD0466, which can also be administered using nanoparticle technology as novel delivery method [366]. Alternatively, S63845 [74] is also showing good promise, although its optimal use is not fully
defined [367], but nevertheless, can be used as a monotherapy against T-ALL [304] using MOLT-3, RPMI-8402 or neuroblastoma patient cells [297] and can be delivered using nanoparticles to improve remission and therapeutic indices [368].
As drug resistance can be mediated by Mcl1 up-regulation in a number of cancer types [369], which can be sensitized to death upon Mcl1 down-regulation (as in the instance of some ABT-737 co-treatments) [99], Mcl1 inhibitors are therefore viewed as having better potential for combined chemotherapeutic treatments. Alternative approaches that induce Mcl1 proteasomal degradation (by GDC-094 treatments, for example), have also been effective in overcoming resistance, thus allowing ABT-737 to have greater effects against BC cells [346] and lung cancer (LC) cells [106]. Similar effects have also been reported for Mcl1 inhibition and TRAIL co-stimulation of BC cells [338]. Conversely, stabilization of Mcl1 protein by BAG3 and survival of BC and PC cells under ABT-737 stimulatory conditions has also been reported [105], highlighting a central but indirect and positive regulatory mechanism for the Mcl1 protein as a therapeutic resistance factor. In a similar manner, the interaction of Mcl1 with other protein regulators cannot go ignored and may help in overcoming ABT-737 resistance, as seen from the NOXA protein interacting with Mcl1 [211, 370]. Here, enhancing NOXA levels, suppressed the anti-apoptotic actions of Mcl1 and override resistance to ABT-737, with Vorinostat [152], Vinblastine [371], Bortezomib [109], Dinaciclib [372] co-treatments in SCLC, CLL and melanoma cells.
Transcriptional repression of Mcl1 expression has also been reported as an effective approach to overcoming drug resistance as reported in the instance of HCC sensitization to ABT-737 and Norcantharidin co-treatments [111]. Alternatively, inhibition of Mcl1 can also be induced through mitochondrial stress or under Obatoclax stimulatory conditions, which can initiate autophagy-dependent necroptosis as an alternative to cell death by apoptosis [373].
As mentioned, of emerging interest are studies being performed in the presence of specific genetic biomarkers [351]. For example, AML primary samples with an IDH2–140 mutation were more sensitive to Venetoclax as a single agent, whereas samples with a FLT3-ITD mutation were more resistant, which is an effect that could be reversed with S63845 co-treatments [351]. Similarly, AMG-176 [72] has also been shown to be effective as a monotherapy directed against Mcl1 in CLL lymphocyte patient samples [344] and in combination with Venetoclax [344]. Lastly, stapled peptides can also activate and promote BAX/BAK/BIM proteins [374], which also validates findings from previously published structural studies [375, 376]. For example BimS2A, a hydrocarbon stapled BIM BH3-peptide can override Mcl1 mediated drug resistance in cell lines [377] only when Bcl-xL is absent or neutralized [378]. Additionally, Mcl1-specific MIM1 was identified in a stapled peptide-based screen [75], which showed good efficacy as a monotherapy against colo-829 melanoma cells, and which synergized the death inducing effects of ABT-737 or Decarbazine [349, 379]. When taken with reports that the Mcl1 hydrophobic groove is more rigid than the hydrophobic groove of Bcl-xL [14], targeting Mcl1 may be permitted with greater specificity and affinity and which may even offer some very promising outcomes with minimal non-specific side effects towards Bcl-xL. Consequently, induced Mcl1 expression can potently enhance drug resistance in cancer cells treated with BH3-mimetics and Mcl1 inhibitors are showing their true potential in over-coming this when utilized in combined- therapeutic treatments, in pre-clinical studies.
To summarize, a growing number of BH3-mimetics have been designed and show good efficacy for inducing cell death at nM quantities. Here, compounds such as ABT-737, Obatoclax and Gossypol, have even been reported to show broader specificity by displacing anti-apoptotic proteins Bcl-2, Bcl-xL and Mcl1, thus enhancing their effectiveness at inducing or sensitizing cells to apoptosis in a number of hematological cancers and solid tumors [51, 61, 100, 190, 380–382]. However, such studies have also indirectly unveiled the importance of Bcl-xL in platelet homeostasis, thus inspiring alternative targeting strategies, and which arrived at the further development of Navitoclax- and Venetoclax- derivatives, which eliminate Bcl-xL protein levels through protein degradation [383, 384]. Limitations to one side, such stoic examples offer great promise as therapeutics, the development of which have also encouraged avenues of research to overcome important considerations, such as off-target effects, bioavailability, and solubility.
In view of the surge in efforts at developing promising mimetics at the preclinical level, one central question that has been raised originates from how well basic-research efforts have developed in the direction of translational medicine. Although the Venetoclax and Mcl1-inhibitor paradigms lay strong foundations for other mimetics to developmentally follow suit by (at the pre-clinical and clinical level), a significant number of the resulting therapeutics still remain to be evaluated in the clinic. Therefore, in the following sections we highlight what progress has been made over the last 5–10 years in bringing the most promising aspects of specific therapeutic strategies towards fruition, as seen from published clinical trials.
BH3-mimetics and Bcl-2 protein inhibition: a clinical perspective
-ABT-737
Although ABT-737 showed promising therapeutic properties in pre-clinical cell line-, primary cell- and animal-models against cancers over-expressing Bcl-2 or Bcl-xL [51], to date only two clinical trials for ABT-737 have been publicized (www.clinicaltrials.gov). These addressed the effects of platinum combined with ABT-737 against ovarian cancer (NCT01440504) and the use of ABT-737 ex-vivo on apoptosis of platelets in idiopathic thrombocytopenic purpura patients co-treated with Eltrombopag and Romiplostim (NCT00902018, [385]). While the findings from the former study remain to be published, in the latter study (NCT00902018), 8 h and 3 h treatments of ABT-737 sensitized patient platelets to apoptosis, and Eltrombopag pre-treatment for 1 week gave rise to therapeutic resistance towards ABT-737, possibly due to a recorded increase in the ratio of Bcl-xL:BAX proteins or enhanced Akt signaling and Mcl1 expression in patient samples. As the prototypical BH3-mimetic [51], ABT-737 did encouragingly enter clinical trial phases I/II, but poor solubility and oral bioavailability [59, 386] did offer limitations in its dose adjustments during combined or single treatment approaches [387].
-Navitoclax (ABT-263)
As the orally available analogue of ABT-737, promising findings from phase I/II clinical trials with Navitoclax have been recorded for its safe tolerance [388] against various types of cancer [389–391]. To date, 34 clinical trial studies have been registered (www.cliniclatrials.gov), of which 17 have been completed and 12 published in depth (Table 10).
Table 10.
Selected phase I clinical trials conducted with Navitoclax as a single or combined therapeutic in untreated and pre-treated patients with Docetaxel (DOC), Erlotinib (ERLO), Gemcitabine (GEM), Carboplatin (CARB), Paclitaxel (PAC), Etopiside (ETOP), Cisplatin (CISP) against Advanced Solid Tumors (AST), non-small cell lung cancer (NSCLC), Prostate Cancer (PC), Squamous cell carcinoma (SCC) and lymphoid malignancies (LM) for Maximum Tolerated Doses (MTD) outcomes
| Navitoclax | Combined | Patients | Disease | Outcomes | ORR | Stabilized | Ref |
|---|---|---|---|---|---|---|---|
| NCT00888108 | DOC | 39/41 Pre-Treated | AST | MTD | 4/35 PR | – | [392] |
| NCT01009073 | ERLO | – | NSCLC, PC, SCC | MTD | 0% ORR | 27% | [393] |
| NCT00887757 | GEM | Pre-Treated | ST | MTD | 0% ORR | 54% | [394] |
| NCT00891605 | CARB/PAC | – | ST | TERMINATED | 5.3% PR | 36.80% | [395] |
| NCT00878449 | ETOP/CISP | Untreated (14 days) | SCLC | MTD | – | – | [396] |
| NCT00445198 | -single- | Pre-Treated | NSCLC, ST | 0% | 1/47 PR | 22.8% (13 m) | [390] |
| NCT00406809 | -single- | – | LM | MTD | 10/46 PR | – | [389, 397] |
The clinical trials reference numbers highlighted in bold (left column), the evaluation of a single therapy alone is highlighted by -single-. Objective Response Rates (ORR) and Partial Responses (PR) are expressed as responding patient numbers/numbers assessed, or as percentages (%). Disease stabilization (Stabilized) effects (as percentage responders) are highlighted in months (m). The corresponding references for the studies are highlighted in the column on the right (Ref)
As a single agent, it has been tested with CLL (NCT01557777), platinum resistant ovarian cancer (NCT02591095) and in combination with other drugs, against hematological and solid cancers [192] such as Rifampicin (NCT01121133, [398]), and Ketoconazole (NCT01021358). Results for some of these trials are eagerly awaited. Among the hematological neoplasms, Navitoclax was evaluated in combination with Rituximab in phases I/II clinical trial studies, conducted in patients with relapsed or refractory CD20+ lymphoid malignancies and patients with B-cell CLL with no prior treatment (NCT01087151), respectively. Here, combinations of Navitoclax and Rituximab were tolerated and showed significant synergistic effects in both settings [399, 400]. Additionally, Navitoclax showed encouraging disease stabilization properties as a single therapeutic or in combination with Gemcitabine in NSCLC and solid tumor patients, although side effects remained as on-going concerns. In this context, and more recently among hematological cancer patients, Venetoclax was evaluated in combination with Navitoclax (NCT03181126) to determine safety and pharmokinetic properties in a phase I trial for relapsed or refractory ALL or LL patients and which offered more encouraging outcomes, highlighting promising potential for BH3 mimetics to be used in combination with each other in addition with pre-existing therapeutics.
-Gossypol
AT-101, the orally available enantiomer of racemic Gossypol, showed acceptable anti-cancer properties in a range of models (Table 11), and which led to it being evaluated further in clinical trials.
Table 11.
Selected phase I-II clinical trials conducted with AT-101 as a single (−single-) or combined therapeutic on untreated/pre-treated patients with Carboplatin (CARB), Paclitaxel (PAC), Cisplatin (CIS), Etoposide (ETOP), Luteinizing Hormone Receptor Hormone (LHRH) agonist, Bicalutamide (BIC) against Advanced Solid Tumors (AST), Giant Cell Glioblastoma (GCG), Adrenocortical (ADC), Solid Tumors (ST), Small Cell lung Cancer (SCLC) and Metastatic Prostate Cancer (MPC) are highlighted for Maximum Tolerated Doses (MTD). Side effects are abbreviated as ADP (Abdominal Pain), Neut (Neutropenia), Throm (Thrombocytopenia), Gastrointestinal symptoms (GI), Fatigue (FAT), Anemia (Anem) and Nausea (Nau). Objective Response Rates (ORR), Complete Responses (CR), Partial Responses (PR), Prostate-Specific Antigen levels (PSA) and percentage patients (%) from the whole group experiencing disease stabilization effects are also highlighted (Stab.). The clinical trials reference numbers are highlighted in bold (left column), and the corresponding references highlighted in the column on the right (Ref). Unavailable data is highlighted by ‘-‘
| AT-101 | Phase | Patients | Patients | Combined | Disease | Adverse Effects | ORR | Stab. | Ref |
|---|---|---|---|---|---|---|---|---|---|
| NCT00891072 | I | 24 | Pre-treated | CARB/PAC | AST | ADP/Neut/Throm | 4.16% CR; 16.66% PR | 33% | [401] |
| NCT00540722 | II | 56 | Untreated (3 wks) | -single- | GCG | GI/FAT | – | – | – |
| NCT00848016 | II | 29 | – | -single- | ADC | Anem/Naus/FAT | – | – | – |
| NCT00544596 | I | 27 | Untreated (4 wks) | CIS/ETOP | ST/SCLC | – | – | – | – |
| NCT00773955 | II | 14 | Pre-treated | -single- | SCLC | Anem/GI | 0% CR; 0% PR | – | – |
| NCT00666666 | II | 55 | Untreated (4 wks) | LHRH/BIC | MPC | Anem/GI/AT | 18–60% Decreased PSA | – | – |
To date, 29 clinical trial studies have been registered (www.clinicaltrials.gov), 17 completed and 5 of which have been published in detail (Table 11). As a mono-therapeutic, it has been tested in several phase II trials addressing its efficacy in NSCLC (NCT00773955), adrenocortical cancer (NCT00848016) and giant cell glioblastoma (NCT00540722) with significant adverse gastrointestinal effects (such as diarrhoea and nausea) or thrombocytopenia and neutropenia, with little significant benefits. Nevertheless, testing has advanced to determine efficacy in relation to genetic biomarker expression, such as the Bcl-2 family of BH3-proteins and the results for which are eagerly awaited (NCT00540722). AT-101 has also been tested in combined therapeutic approaches in trials for solid tumors and hematological cancers such as SCLC (NCT00397293, NCT00544596), NSCLC (NCT00544960), CLL (NCT00286780), relapsed or refractory SCLC (NCT00397293) and with androgen ablation therapy in PC (NCT00666666). While some of the studies are yet to report their findings, AT-101 with androgen ablation did encouragingly reduce circulating levels of Prostate-Specific Antigen (PSA) in PC patients (NCT00666666). Based on AT-101 binding Mcl1, further work in this area may address how this agent synergizes with other well-established (or promising) BH3-mimetics, or in overcoming enhanced Mcl1-expression mediated Venetoclax resistance.
-Obatoclax
As an antagonist that binds the BH3-domain of apoptotic proteins, Obatoclax has been reported to induce cell death, cell arrest and autophagy in leukemia and lymphoma cell lines [190, 402–407]. To date, 20 clinical trials for Obatoclax have been registered (www.clinicaltrials.gov), of which 12 have been completed, and 8 of which have been described in detail (Table 12).
Table 12.
Selected phase I-II clinical trials conducted with Obatoclax as a single (−single-) or combined therapeutic in untreated/pre-treated or non-refractory(−)/refractory (Refrac) patients with Carboplatin (CARB), Etoposide (ETOP) or Topotecan (TOPOT) against extensive-stage small cell lung cancer (es-SCLC), Myelodysplastic Syndrome (MDS), Hodgkin’s Lymphoma (HL), Myelofibrosis (MFS), advanced Chronic Lymphocytic Leukemia (a-CLL), and Hematologic Malignancies (HM). Adverse effects are abbreviated as Neut (Neutropenia), Anem (Anemia), Euph (Euphoria), Dizz (Dizziness), Naus (Nausea), Atax (Ataxia) and Throm (Thrombocytopenia). Disease stabilization effects on patient numbers (expressed as a percentage (%) of the whole group or as positive responders/group size) are highlighted in weeks (>wks). Unavailable data for disease stabilization effects is highlighted by ‘-‘. The clinical trials reference numbers highlighted in bold (left column) and their corresponding references highlighted in the columns on the right (Ref)
| Obatoclax | Phase | Patients | Refrac | Combined | Disease | Adverse Effects | Stabilization | Ref |
|---|---|---|---|---|---|---|---|---|
| NCT00684918 | I/II | Untreated | – | -single- | AML | Neut | 4/19 for 11 cycles | [408] |
| NCT00682981 | II | Untreated | – | CARB/ETOP | es-SCLC | Neut/Anem | – | [409] |
| NCT00413114 | II | Untreated | – | -single- | MDS | Euph/Naus | 50% (> 12 wks) | [410] |
| NCT00359892 | II | – | Yes | -single- | HL | Euph/Dizz | 38% (> 8 wks) | [411] |
| NCT00521144 | II | Pre-treated | Yes | TOPOT | SCLC | Throm/Neut/Anem/Atax | 56% (Phase II) | [412] |
| NCT00360035 | II | Pre-treated | – | -single- | MFS | Atax/Anem/Throm | – | [413] |
| NCT00600964 | I/II | Pre-treated | Yes (22/26) | -single- | a-CLL |
Atax/Euph/Anem/ Throm |
– | [414] |
| NCT00438178 | I | N/A | Yes | -single- | HM | Neut/Anem/Throm | – | [415] |
Obatoclax has been tested in a number of phase I/II trials against SCLC (NCT00682981, NCT00521144), NSCLC (NCT00405951), Hodgkin’s Lymphoma (NCT00359892), MCL (NCT00407303), CLL (NCT00600964), and AML (NCT00684918), with marginally-encouraging outcomes as a single agent or in a limited number of combined studies with common side effects. This may be attributed to it beneficially targeting Bcl-xL (in addition to Bcl-2 and Mcl1) to differing degrees (at a fixed dose), albeit with limited clinical benefits (such as disease stabilization) as a dual-acting drug. Alternatively, in utilizing a combined therapeutic approach, mono-therapeutics (exclusively specific for Bcl-2, Bcl-xL or Mcl1), are being seen to have the advantage of being optimized in doses, to target each of these components more accurately based on the degree of resistance encountered, and may (in some respects) be a more fruitful approach [191, 192, 410, 416, 417]. Based on the benefits arising from Obatoclax stabilizing the progression of certain cancers as a mono-therapeutic, there does therefore exist some potential in how it may be optimized further for this purpose, and possibly thereafter in combined therapeutic regimens.
SMAC-mimetics
As an important emerging therapeutic group with great potential based on pre-clinical studies, 10 clinical studies for the Smac-mimetic LCL-161 have been registered to date (www.clinicaltrials.gov), of which 6 have been completed and 3 of which have been described in detail (Table 13).
Table 13.
Selected phase I-II clinical trials conducted with LC-161 as a single (−single-) or combined therapeutic in pre-treated or refractory (Refrac) patients with Paclitaxel (PAC), against Advanced Solid Tumor (AST) diseases (Dis.) and Outcomes for Maximum Tolerated Doses (MTD) are highlighted. Adverse effects (Adv. Effects) are highlighted as Neut (Neutropenia), Gastrointestinal symptoms (GI), Diarrhoea (Diar), Nausea (Nau), Vomiting (Vom) and Anemia (Anem). Objective Response Rates (ORR), Partial Responses (PR), Progressive Disease (PD) and disease stabilization effects (Stabil.) are highlighted as percentage (%) positive-responders. The clinical trials reference numbers highlighted in bold (left column) and their corresponding references highlighted in the columns on the right (URL/Ref). Biomarker assessments (Bio.M) are highlighted and unavailable data is highlighted by ‘-‘
| LCL-161 | Phase | Patient | Refrac | Combined | Dis. | Outcomes | Adv. Effects | ORR | Stabil. | Bio.M | URL/Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NCT01240655 | Ib | Pre-treated | Yes | PAC | AST | MTD | Neut/GI | 27.6% PR; 25% PD | 36.8% | – | www.novctrd.com |
| NCT01968915 | I/II | – | – |
-single−/ PAC |
AST | – | Neut/Diar | DISC. | – | – | www.novctrd.com |
| NCT01098838 | I/II | Pre-treated | – | -single- | AST | MTD |
Naus/Vom/ Anem |
0% ORR | 19% | cIAP | [418] |
Additionally, phase I studies of TL32711, LCL-161 and HGS1029 Smac-mimetics have been conducted (orally and intravenously), in patients with solid tumors and lymphoma, revealing mixed outcomes ranging from being well-tolerated to inducing cytokine release syndrome. As expected, the latter can be explained through clinical biomarker studies showing the degradation of cIAP and up-regulation of NF-κB activation and pro-inflammatory cytokine expression [418–421]. Further optimization of such approaches may help overcome such side effects. Nevertheless, LCL-161 does exhibit disease stabilization (Table 13), in relation to other initial phase I trials evaluating alternative Smac-mimetics, that show encouraging evidence of significant anti-tumor activity, as seen with GDC-0917 as a monotherapy for patients with OC or MALT-lymphoma, HGS1029 against colon cancer and Debio1143 against melanoma metastases [418]. From the perspective of a combined therapeutic, the effects of Smac-mimetics can be enhanced further upon the co-stimulation of cells with death inducing ligands such as TNF-α and TRAIL, and is also an area being developed through inducing their expression with oncolytic viruses and immunomodulatory adjuvants [422].
Venetoclax (ABT-199)
As the most promising therapeutic from the BH3-mimetics group in targeting the Bcl-2-BAX or -BAK axis of apoptosis regulation, to date 331 clinical trials have been registered testing Venetoclax, and of which 27 have been completed (www.clinicaltrials.gov). As seen in Tables 12, 14 studies have been published in detail, where Venetoclax was seen to exhibit a relatively good safety profile, leading to its evaluation in trials with combined agents and in patients with specific genetic biomarker aberrations.
Table 14.
Selected phase I-III clinical trials conducted with Venetoclax (V) as a single (−single-) or combined therapeutic in untreated/pre-treated or non-refractory(−)/refractory patients (Refrac) with Mivebresib (Miv), Rituximab and Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone (R-CHOP), Bendamustine (BEND) Rituximab (RIT) Obinutuzumab (OBIN), Cytarabine (CYT), Ibrutinib (IBU) Rituximab (RIT), Bortezomib (BORT) and Dexamethasone (DEX) against Acute Myelogenous Leukemia (AML), Large B-cell Lymphoma (L-BCL), Follicular non-Hodgkin’s Lymphoma (FnHL), Chronic Lymphocytic Leukemia (CLL), Non-Hodgkin’s Lymphoma (NHL), Multiple Myeloma (MM). Objective Response Rates (ORR), Complete Responses (CR) and Partial Responses (PR), expressed as percentage responders (%) are highlighted for disease progression (R/R) patients against R/R with 1/L (1 year treatment) patients. The clinical trials reference numbers highlighted in bold (left column) and the corresponding references highlighted in the column on the right (Ref). Studies where patients were profiled (and their numbers) are highlighted in the Biomarkers column as percentages (%) and ‘-‘indicates no profiling
| Venetoclax | Phase | Combined | Patients | Refrac | Disease | ORR, CR, PR | Biomarkers | Ref |
|---|---|---|---|---|---|---|---|---|
| NCT02391480 | I/II | Miv | Pre-treated | Yes | AML | 6.66% CR; 6.66% PR | HEXIM1, DCXR, ITD/TKD, PTPN11 | [423, 424] |
| NCT02055820 | II | R-CHOP | Pre-treated | – | L-BCL | – | Bcl-2, MYC | [425] [426] |
| NCT02187861 | II |
BEND(B)/ RIT (R) |
Untreated (28 d) | Yes | FnHL |
75% V + BR; 69% BR (untreated) 4% V + R (non-Refrac) + 19% V + R (Refrac) |
Bcl-2/ Mcl1 |
[427] |
| NCT02265731 | II | RIT (R) | Pre-treated | Yes | CLL |
100% (V, ORR); 66.7% (V + R, ORR) 16.7% (V, CR) + 50% (V + R, CR) |
– | [428] |
| NCT01685892 | Ib | OBIN | Untreated | Yes | CLL |
95% (R/R, ORR); 100% (1/L, ORR) 37%, (R/R, CR) + 78% (1/L, CR) |
IGHV, P53, B2 MG, CD38 | [429] |
| NCT02287233 | Ib/II | CYT | Untreated | – | AML | 62% CR | – | [430] |
| NCT02756897 | II | IBRUTINIB | Untreated | – | CLL | 88% CR | – | [431] |
| NCT01594229 | Ib | BEND/RIT | – | Yes | NHL | 65% ORR (30%, CR; 35%, PR) | Bcl-2 | [432] |
| NCT03755947 | III | RIT | Pre-treated | Yes | 17p−/TP53−/IGHV-CLL | 92.3% ORR; (17.48%, CR) | – | [433–439] |
| NCT01794507 | Ib | BORT/DEX | Pre-treated | Yes | MM | 67% ORR | Bcl-2 Bcl-xL, Mcl1, | [440] |
| NCT01889186 | II | -single- | – | Yes | del17p-CLL | 70% ORR | 17P del (50.3%) | [441–443] |
| NCT01994837 | II | -single- | Pre-treated | – | AML | 19% ORR (6% CR) | Bcl-2, Bcl-xL, BH3 Profiling, Mcl1 | [444] |
| NCT01328626 | I | -single- | – | Yes | CLL | 79% ORR (20% CR) | 17Pdel | [435, 443] |
As seen, Venetoclax is showing itself to be a very promising therapeutic, against Bcl-2-dependent cancer cells such as CLL, acute leukemias, breast cancer, myc-driven lymphomas [293–295], and through successful phase I-III trials for acute myeloid leukemia (AML), CLL, MM and non-Hodgkin’s lymphoma (NHL) [296], it was ultimately awarded US Food and Drug Administration (US FDA) and European Medicines Agency (EMA) approval for treating CLL patients harboring the 17p or p53 mutations [445].
As a mono-therapeutic, Venetoclax has also been reported to be effective against other lymphomas with high Bcl-2 expression levels, as in MCL, myeloma, refractory AML [444], follicular lymphoma and some diffuse large B-cell lymphomas [446], whereby the dose administered could be correlated with expression levels of Mcl1 and Bcl-xL [447]. Over the last 3 years a number of follow-up trials have been published, aimed at defining its use against drug resistance, with some very encouraging outcomes based on its combined use with other therapeutics such as DNA damaging agents, anti-CD20 antibodies (such as Rituximab), hyper-methylating agents, kinase inhibitors, Mdm2 inhibitors, proteasome inhibitors, and in conjunction with inhibitors targeting the anti-apoptotic proteins Bcl-xL and Mcl1 [448]. At a time when there are a plethora of on-going clinical trials involving Venetoclax (www.clinicaltrials.gov), its combined use with Rituximab is showing some very promising outcomes with complete remissions recorded for 51% of relapsed CLL patients [449], and it is also leading the way for use with patients encoding specific genetic biomarker aberrations (NCT02391480, NCT02055820, NCT02187861, NCT01685892, NCT01594229, NCT01794507, NCT01994837, NCT01328626), and where serious side effects (such as neutropenia and thrombocytopenia) are being managed with the growth factor prophylaxes (NCT02265731, NCT02055820, NCT01889186). Collectively, such findings encouragingly highlight the potential of Venetoclax as a developing model for the treatment of hematological cancers, and thus paving the way for its further testing on solid tumors.
Mcl1-inhibitors
Although genetically elevated Mcl1 levels have been reported in many tumor types [26], it has been implicated in therapeutic resistance of breast and lung cancers [450, 451], with some studies showing that Mcl1 expression can mediate resistance to Navitoclax or Venetoclax [70, 99, 452], Gemcitabine, Vincristine and Taxols [453–455]. To date 36 clinical trials have been registered utilizing Mcl1 inhibitors (www.clinicaltrials.gov.uk), amongst which 10 have been completed, 2 withdrawn, 4 terminated and 8 are still recruiting. Of the completed trials related to targeting the Mcl1 protein (Table 15), S64315 has been tested as a single agent against MM and DLBCL (NCT02992483) in untreated relapsed/refractory patients to establish maximum tolerated doses and the results for which are to be published.
Table 15.
Selected phase I clinical trials conducted with MIK655 or S64315 as a single (S) therapeutic, on untreated, and refractory (Refrac) diseases, such as Multiple Myeloma (MM), Diffuse Large Cell B-Lymphoma (DLBCL), Acute Myeloid Leukemia (AML) and Myelodysplastic Syndrome (AST) patients, for Maximum Tolerated Dose (MTD) are highlighted. Adverse effects (Adv. Effects) are highlighted as Nausea (Nau), Familial Neutropenia (F-Neut) and Diarrhoea (Diar). The clinical trials reference numbers are highlighted in bold (left column) and the Objective Response Rates (ORR) expressed as the percentage of patients who responded positively (%). Unavailable data is highlighted by ‘-‘
| MIK655/S64315 | Phase | Single | Patients | Refrac | Disease | Outcomes | Adv. Effects | ORR |
|---|---|---|---|---|---|---|---|---|
| NCT02992483 | I | S | Untreated | Yes | MM, DLBCL | MTD | – | Unpublished |
| NCT02979366 | I | S | Untreated (14 days) | Yes | AML, MDS | MTD | Nau/F-Neut/Diar | 0% ORR |
Similarly, trials for S64315 efficacy have also been conducted in AML and MDS patients, and which showed no significant benefits outside of side effects such as nausea and familial neutropenia (NCT02979366). Consequently, concerns regarding the safety of Mcl1 inhibition as a single agent, have arisen and are founded on the dependency of Mcl1 expression for the normal survival and growth of cells, as seen for mouse cardiomyocytes [456], hepatocytes [457] and neurons [458]. In support of this viewpoint, Mcl1+/− mice should mimic a phenotype consistent with 50% inhibition of Mcl1, but they appear normal and healthy, thus supporting the belief that Mcl1 may also function independently of BH3-domain protein binding and regulation during cellular death [321, 459]. Another challenge for the development of specific Mcl1 inhibitors has emerged from the rigidity of the Mcl1 protein hydrophobic pocket and competitiveness for this posed by alternative high-affinity endogenous protein binding partners [460]. Nevertheless, several highly potent (sub-nanomolar) and selective inhibitors have emerged very recently, and one of which (UMI-77) shows excellent potential against pancreatic cell lines in vitro and in xenograft pre-clinical models [461]. Similarly, pre-clinical findings for the inhibitor S63845 have been very impressive [74], against several cancer types including melanoma, leukemia, lymphoma, and other solid cancers either as a single or combined agent, and further work here will indeed help in extending these findings towards clinical studies.
In summary, while serious side effects and solubility issues had been reported for the founding therapeutic member ABT-737, as seen from the findings presented in Tables 10, 11, 12, 13, 14, 15, derivatives of this appear to be showing incredible promise in treating hematological and solid cancers. With concerted efforts being made in the clinic to address side effects, the members that are showing steady progress from phases I to III, include Venetoclax and Obatoclax, with progress from studies evaluating Mcl1- and Smac- mimetics (or inhibitors) maintaining steady momentum. When coupled with evaluating patients for genetic biomarker mutation profiling, a very clear and interesting picture is coming into focus with regards to how some of these mimetics may have greater efficacy and potential in a personalized medicine context, and as encouragingly seen from clinical trials highlighted in Table 14.
Mimetics: current considerations for solubility and delivery
In light of the above developing areas for therapeutic mimetics, key areas for therapeutic delivery have also been given greater consideration, particularly in response to solubility and delivery limitations arising from the ABT-737 paradigm. Classically, while therapeutic affinity for the target protein is of paramount importance when designing an intervention strategy, additional factors affecting therapeutic availability, longevity, toxicity, and penetrability are also being given greater collective importance. In the next section, we selectively outline and evaluate the recent progress that has been reported in the areas of SMI and peptide therapeutics delivery, with a view to highlighting their usefulness for mimetic bioavailability and solubility, which (from the ABT-737 paradigm) were serious considerations that hindered the immediate evaluation of such a promising therapeutic.
As many SMIs are hydrophobic, which may present solubility and bioavailability challenges, the recently demonstrated successes of cell-penetrating peptide (CPP) nanoparticles [462] or nanofibers [463, 464] are emerging as synergistic alternatives for therapeutic solubilization and the delivery of therapeutic cargo. Briefly, the combined use of monomeric CPP amphiphilic peptides with cholesterol can self-assemble, forming a shell-structured nanoparticle, that contains a hydrophobic cholesterol core and a hydrophilic cationic exterior, which can enhance solubility, loading, delivery, and uptake of potential therapeutics [462]. Similarly, the potential of supramolecular nanofiber technology in this context is evidenced through them being able to enhance the incorporation of Paclitaxel from 6.8 to 41% [465], from a self-assembling amphiphilic peptide monomer [466]. Moreover, such applications can be extended to incorporate anti-inflammatories [467], multi-drug combinations [468] and imaging agents [469, 470], which collectively highlight the potential of such approaches.
While, CPPs can enhance cargo delivery of drugs using nanostructures [462, 471, 472], reduced specificity, resulting toxicity and reduced half-life can present challenges, as with most delivery systems of this nature [473, 474]. In the context of delivering BH3-mimetics, one good example which demonstrates how some of these issues of specificity and delivery can be overcome, was published by Schnorenberg et al. (2019), who utilized a BIM BH3-mimetic peptide amphiphile nanostructure to facilitate uptake for triggering the death of Hela and MEF cells [475]. Interestingly, a cathepsin B cleavage signal was also incorporated between the mimetic and hydrophobic tail to permit release of the mimetic at the site of enhanced ECM turnover, or within cells following endosomal and lysosomal uptake [475].
Importantly, the use of supra-molecular peptide delivery systems is being seen to offer certain advantages over classical delivery systems, such as high-concentration delivery of peptides, stabilization of peptide secondary structure, protection from proteolysis within the circulatory system, increased half-life, delivery of multiple therapeutics and enhanced uptake to the endosomal and lysosomal compartments [476, 477]. While carrier peptides or CPPs can be utilized to help aide solubility and penetrability, an alternative targeting approach worth highlighting is the use of Elastin-like polypeptides (ELPs), which can form aggregates when heated externally and which can selectively accumulate in tumors [478–480]. Such an approach exploits the increased permeability of tumor vasculature during regional hyperthermia, during which a greater accumulation of macromolecular drug carriers loaded with single or combined therapeutics is permitted [481–483]. This permits enhanced cytotoxicity [484], which can be optimised further through CPP tagging for delivery to cells in vitro or in vivo [485–488]. While some peptides may require certain conformations to achieve full inhibitory activity or cellular penetrability, pH dependent membrane insertion (pHLIPS) may offer additional potential benefits, from the perspective of correcting structurally disorganized peptide therapeutics to adopt a favorable α-helical structure during their delivery and thereafter [489–491].
Lastly, the use of phospholipids [492] for self-assembling micelles and the delivery of safe and non-immunogenic, hydrophobic drugs such as cyclosporine A [493], Paclitaxel [494] and peptides [495], can minimize the degradation or aggregation of cargo [496–499]. Encouragingly, such approaches can enhance therapeutic efficacy through reduced extravasation from the circulation, while simultaneously reducing adverse off-target effects [495, 500]. However, such an approach does come with limitations that cannot go ignored, due to potentially diminished availability of the helical peptide therapeutic moiety, through masking and immunogenic effects from PEG, or limitations in peptide cargo lengths of 17–36 aa [501, 502]. Nevertheless, as a way of highlighting the potential of this approach, Gossypol loaded micelles combined with A549 cells and radiation therapy in a xenograft mouse model [503], showed 7x greater efficacy against tumors than Gossypol alone [504]. Moreover, biomimetic poly(lactic-coglycolic acid) (PLGA) combined with red blood-cell membrane (RBCm) as nanoparticles loaded with Obatoclax mesylate for the treatment of non-small-cell lung cancer, also showed significant success in prolonging drug circulation in the presence of a reduced immune response against the particles [505].
Conclusions
In summary, while ABT-737 was discovered as an authentic BH3-mimetic, derivatives such as Navitoclax and Venetoclax hold great potential in treating hematological cancers and even solid tumors when administered with classical and conventional anti-cancer drugs, and the evidence for which is firmly grounded on basic research efforts at the pre-clinical level. In this context, Smac-mimetics have also emerged as being promising candidates for treating a range of cancer types, as seen from their combined compatibility with a broader range of anti-cancer drugs in pre-clinical models. However, the most promising potential therapeutics in pre-clinical models appear to be the Mcl1 inhibitors, particularly when administered in combination with Venetoclax, Navitoclax, death inducing ligands and conventional anti-cancer drugs.
Through extending such findings by way of clinical trials, the therapeutic efficacy of the most promising anti-cancer drugs are coming into fruition, either as single or as combined agents. While resistance has always served to hinder the development of many therapeutics for cancer, the off-target effects of such agents are becoming increasingly clear, and in some instances are also being harnessed through the development of dual-activity inhibitors, or as agents that can be synergistically utilized to overcome any resistance effects. From the above findings, the best example of this is highlighted by the effectiveness of Mcl1 inhibitors, when combined with the drugs Navitoclax or Venetoclax as co-therapeutics, and the findings from which are also being effectively aligned with specific genetic biomarkers of interest. Lastly, of emerging interest, in this context, are the BH3-mimetics Obatoclax and Gossypol, and Smac-mimetics, the clinical importance of which are eager awaited.
As the focus of this article has revolved around the regulation of the intrinsic arm of apoptosis, the effects of the above mimetics and inhibitors towards other cell death-inducing off-target effects, cannot be ignored. Such an assertion arises from what contribution these therapeutics may offer in minimizing the protective effects of pro-survival proteins, associated with the ER stress-response [403, 506], autophagy [507, 508], necroptosis [406] or cell cycle deregulation [509, 510]. When considered alongside how technologies have developed to improve solubility and the delivery of therapeutics, and thereby improving their efficacy, the initial limitations surrounding candidates (such as ABT-737), based on lack of solubility and availability, can be addressed with greater optimism. Such developments indeed offer greater certainty in pursuing mimetics as therapeutics and which follow a strongly-validated paradigm. From an equally important perspective, the presence of clinical side effects, such as thrombocytopenia have always appeared as a major source of concern. As mentioned, such effects can be minimized through molecular approaches, as in the instance of re-engineering Navitoclax to DT2216, and which acts through degrading Bcl-xL [383], thus reducing its off-target effects. Moreover, complimentary approaches through the use of prophylaxes are also being implemented, as effective management strategies within the clinic.
When considered together, the previous 20-30 years of mimetics research have indeed come a long way, whilst occupying a vast area of development in cancer treatment, and which lay very strong technological foundations for the future development of novel single-, combined- or dual- action therapeutics, with personalized medicine being well positioned at the forefront of such endeavors.
Abbreviations
- ALL
Acute lymphoblastic leukaemia
- AML
Acute myelogenous leukemia
- BAX
Bcl-2-associated protein X
- BAK
Bcl-2 antagonist/killer
- BC
Breast Cancer
- BOK
Bcl-2 ovarian killer
- Bcl-2
B cell lymphoma-2
- Bcl-xL
B cell lymphoma extra-large
- BH domain
Bcl-2 homology domain
- Bid
Bcl-2 interacting domain death agonist
- Bim
Bcl-2 interacting mediator of cell death
- BIR
Baculovirus IAP Repeat
- CAD
Caspase-activated DNAase
- CLL
Chronic lymphocytic leukaemia
- CPP
Cell penetrating peptide
- DLBCL
Diffuse large B-cell lymphoma
- ER
Endoplasmic reticulum
- HNSCC
Head and neck squamous cell carcinoma
- Mcl1
Myeloid cell leukemia-1 protein
- EGF-R
Epidermal growth factor-receptor
- GC
Gastric Cancer
- cIAP
Cellular inhibitor of apoptosis
- MCL
Mantle cell lymphoma
- MPC
Mitochondrial pore complex
- MOMP
Mitochondrial outer membrane permeabilization
- NF-κB
Nuclear factor kappa B
- NHL
Non-Hodgkin’s lymphoma
- NSCLC
Non-small cell lung cancer
- MM
Multiple myeloma
- PARP
Poly ADP-ribose polymerase
- PC
Prostate Cancer
- PROTAC
Proteolysis targeting chimera
- pHLIPS
pH-Low Insertion Peptide
- Puma
p53-upregulated modulator of apoptosis
- SAHBs
Stabilized alpha-helix of BCL-2
- SCLC
Small cell carcinoma
- Smac/DIABLO
Second mitochondria-derived activator of caspases
- SMI
Small molecule inhibitor
- T-ALL
T-cell acute lymphoblastic leukaemia
- TNF-α
Tumor necrosis factor-alpha
- TRAIL
Tumor necrosis factor-related apoptosis-inducing ligand
Authors’ contributions
Conception, research theme and efforts, writing, graphics, and text layout were formulated by SMS and PAT. Editing and discussions (ONFC, MVK and AAZ). The author(s) read and approved the final manuscript.
Funding
This work was funded by the ‘University of Surrey and University of Surrey Health Partners’.
Availability of data and materials
All data and materials cited herein are available upon request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All Authors consent to the submission and publication of this manuscript.
Competing interests
The authors highlight no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Paul A. Townsend, Email: p.townsend@surrey.ac.uk
Surinder M. Soond, Email: s.soond@surrey.ac.uk
References
- 1.Delbridge AR, Strasser A. The BCL-2 protein family, BH3-mimetics and cancer therapy. Cell Death Differ. 2015;22(7):1071–1080. doi: 10.1038/cdd.2015.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams JM, Cory S. The BCL-2 arbiters of apoptosis and their growing role as cancer targets. Cell Death Differ. 2018;25(1):27–36. doi: 10.1038/cdd.2017.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashkenazi A, Fairbrother WJ, Leverson JD, Souers AJ. From basic apoptosis discoveries to advanced selective BCL-2 family inhibitors. Nat Rev Drug Discov. 2017;16(4):273–284. doi: 10.1038/nrd.2016.253. [DOI] [PubMed] [Google Scholar]
- 4.Soond SM, Kozhevnikova MV, Savvateeva LV, Townsend PA, Zamyatnin AA., Jr Intrinsically connected: therapeutically targeting the cathepsin proteases and the Bcl-2 family of protein substrates as co-regulators of apoptosis. Int J Mol Sci. 2021;22(9):4669. doi: 10.3390/ijms22094669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merino D, Kelly GL, Lessene G, Wei AH, Roberts AW, Strasser A. BH3-mimetic drugs: blazing the Trail for new Cancer medicines. Cancer Cell. 2018;34(6):879–891. doi: 10.1016/j.ccell.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Rampino N, Yamamoto H, Ionov Y, Li Y, Sawai H, Reed JC, et al. Somatic frameshift mutations in the BAX gene in colon cancers of the microsatellite mutator phenotype. Science. 1997;275(5302):967–969. doi: 10.1126/science.275.5302.967. [DOI] [PubMed] [Google Scholar]
- 7.Zhang M, Zheng J, Nussinov R, Ma B. Oncogenic mutations differentially affect Bax monomer, dimer, and oligomeric pore formation in the membrane. Sci Rep. 2016;6:33340. doi: 10.1038/srep33340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shibata MA, Liu ML, Knudson MC, Shibata E, Yoshidome K, Bandey T, et al. Haploid loss of bax leads to accelerated mammary tumor development in C3(1)/SV40-TAg transgenic mice: reduction in protective apoptotic response at the preneoplastic stage. EMBO J. 1999;18(10):2692–2701. doi: 10.1093/emboj/18.10.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaiser U, Schilli M, Haag U, Neumann K, Kreipe H, Kogan E, et al. Expression of bcl-2--protein in small cell lung cancer. Lung Cancer. 1996;15(1):31–40. doi: 10.1016/0169-5002(96)00568-5. [DOI] [PubMed] [Google Scholar]
- 10.Delbridge AR, Grabow S, Strasser A, Vaux DL. Thirty years of BCL-2: translating cell death discoveries into novel cancer therapies. Nat Rev Cancer. 2016;16(2):99–109. doi: 10.1038/nrc.2015.17. [DOI] [PubMed] [Google Scholar]
- 11.Schott AF, Apel IJ, Nunez G, Clarke MF. Bcl-XL protects cancer cells from p53-mediated apoptosis. Oncogene. 1995;11(7):1389–1394. [PubMed] [Google Scholar]
- 12.Findley HW, Gu L, Yeager AM, Zhou M. Expression and regulation of Bcl-2, Bcl-xl, and Bax correlate with p53 status and sensitivity to apoptosis in childhood acute lymphoblastic leukemia. Blood. 1997;89(8):2986–2993. [PubMed] [Google Scholar]
- 13.Campbell KJ, SWG T. Targeting BCL-2 regulated apoptosis in cancer. Open Biol. 2018;8(5):180002. doi: 10.1098/rsob.180002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shamas-Din A, Brahmbhatt H, Leber B, Andrews DW. BH3-only proteins: orchestrators of apoptosis. Biochim Biophys Acta. 2011;1813(4):508–520. doi: 10.1016/j.bbamcr.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 15.Lomonosova E, Chinnadurai G. BH3-only proteins in apoptosis and beyond: an overview. Oncogene. 2008;27(Suppl 1):S2–19. doi: 10.1038/onc.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flores-Romero H, Ros U, Garcia-Saez AJ. Pore formation in regulated cell death. EMBO J. 2020;39(23):e105753. doi: 10.15252/embj.2020105753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91(4):479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 18.Schuler M, Green DR. Mechanisms of p53-dependent apoptosis. Biochem Soc Trans. 2001;29(Pt 6):684–688. doi: 10.1042/0300-5127:0290684. [DOI] [PubMed] [Google Scholar]
- 19.Ren D, Tu HC, Kim H, Wang GX, Bean GR, Takeuchi O, et al. BID, BIM, and PUMA are essential for activation of the BAX- and BAK-dependent cell death program. Science. 2010;330(6009):1390–1393. doi: 10.1126/science.1190217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Villunger A, Labi V, Bouillet P, Adams J, Strasser A. Can the analysis of BH3-only protein knockout mice clarify the issue of 'direct versus indirect' activation of Bax and Bak? Cell Death Differ. 2011;18(10):1545–1546. doi: 10.1038/cdd.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, et al. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8(3):705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- 22.Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE, et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315(5813):856–859. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- 23.Chen HC, Kanai M, Inoue-Yamauchi A, Tu HC, Huang Y, Ren D, et al. An interconnected hierarchical model of cell death regulation by the BCL-2 family. Nat Cell Biol. 2015;17(10):1270–1281. doi: 10.1038/ncb3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du H, Wolf J, Schafer B, Moldoveanu T, Chipuk JE, Kuwana T. BH3 domains other than Bim and bid can directly activate Bax/Bak. J Biol Chem. 2011;286(1):491–501. doi: 10.1074/jbc.M110.167148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gallenne T, Gautier F, Oliver L, Hervouet E, Noel B, Hickman JA, et al. Bax activation by the BH3-only protein Puma promotes cell dependence on antiapoptotic Bcl-2 family members. J Cell Biol. 2009;185(2):279–290. doi: 10.1083/jcb.200809153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan J, et al. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;463(7283):899–905. doi: 10.1038/nature08822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warr MR, Shore GC. Unique biology of Mcl-1: therapeutic opportunities in cancer. Curr Mol Med. 2008;8(2):138–147. doi: 10.2174/156652408783769580. [DOI] [PubMed] [Google Scholar]
- 28.Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, et al. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19(11):1294–1305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhong Q, Gao W, Du F, Wang X. Mule/ARF-BP1, a BH3-only E3 ubiquitin ligase, catalyzes the polyubiquitination of Mcl-1 and regulates apoptosis. Cell. 2005;121(7):1085–1095. doi: 10.1016/j.cell.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 30.Nakajima W, Tanaka N. Synergistic induction of apoptosis by p53-inducible Bcl-2 family proteins Noxa and Puma. J Nippon Med Sch. 2007;74(2):148–157. doi: 10.1272/jnms.74.148. [DOI] [PubMed] [Google Scholar]
- 31.Nakajima W, Tanaka N. Noxa induces apoptosis in oncogene-expressing cells through catch-and-release mechanism operating between Puma and Mcl-1. Biochem Biophys Res Commun. 2011;413(4):643–648. doi: 10.1016/j.bbrc.2011.09.036. [DOI] [PubMed] [Google Scholar]
- 32.Varadarajan S, Vogler M, Butterworth M, Dinsdale D, Walensky LD, Cohen GM. Evaluation and critical assessment of putative MCL-1 inhibitors. Cell Death Differ. 2013;20(11):1475–1484. doi: 10.1038/cdd.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hussain SR, Cheney CM, Johnson AJ, Lin TS, Grever MR, Caligiuri MA, et al. Mcl-1 is a relevant therapeutic target in acute and chronic lymphoid malignancies: down-regulation enhances rituximab-mediated apoptosis and complement-dependent cytotoxicity. Clin Cancer Res. 2007;13(7):2144–2150. doi: 10.1158/1078-0432.CCR-06-2294. [DOI] [PubMed] [Google Scholar]
- 34.Masilamani AP, Dettmer-Monaco V, Monaco G, Cathomen T, Kuckuck I, Schultze-Seemann S, et al. An Anti-PSMA Immunotoxin Reduces Mcl-1 and Bcl2A1 and specifically induces in combination with the bad-like BH3 Mimetic ABT-737 apoptosis in prostate cancer cells. Cancers (Basel) 2020;12(6):1648. doi: 10.3390/cancers12061648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu H, Schiff DS, Lin Y, Neboori HJ, Goyal S, Feng Z, et al. Ionizing radiation sensitizes breast cancer cells to Bcl-2 inhibitor, ABT-737, through regulating Mcl-1. Radiat Res. 2014;182(6):618–625. doi: 10.1667/RR13856.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oda E, Ohki R, Murasawa H, Nemoto J, Shibue T, Yamashita T, et al. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science. 2000;288(5468):1053–1058. doi: 10.1126/science.288.5468.1053. [DOI] [PubMed] [Google Scholar]
- 37.Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell. 2001;7(3):683–694. doi: 10.1016/s1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- 38.Yu J, Zhang L, Hwang PM, Kinzler KW, Vogelstein B. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol Cell. 2001;7(3):673–682. doi: 10.1016/s1097-2765(01)00213-1. [DOI] [PubMed] [Google Scholar]
- 39.Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102(1):33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 40.Philchenkov A, Miura K. The IAP protein family, SMAC mimetics and Cancer treatment. Crit Rev Oncog. 2016;21(3–4):185–202. doi: 10.1615/CritRevOncog.2016017032. [DOI] [PubMed] [Google Scholar]
- 41.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fraser C, Ryan J, Sarosiek K. BH3 profiling: a functional assay to measure apoptotic priming and dependencies. Methods Mol Biol. 1877;2019:61–76. doi: 10.1007/978-1-4939-8861-7_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Villalobos-Ortiz M, Ryan J, Mashaka TN, Opferman JT, Letai A. BH3 profiling discriminates on-target small molecule BH3 mimetics from putative mimetics. Cell Death Differ. 2020;27(3):999–1007. doi: 10.1038/s41418-019-0391-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhola PD, Ahmed E, Guerriero JL, Sicinska E, Su E, Lavrova E, et al. High-throughput dynamic BH3 profiling may quickly and accurately predict effective therapies in solid tumors. Sci Signal. 2020;13(636):eaay1451. doi: 10.1126/scisignal.aay1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cosulich SC, Worrall V, Hedge PJ, Green S, Clarke PR. Regulation of apoptosis by BH3 domains in a cell-free system. Curr Biol. 1997;7(12):913–920. doi: 10.1016/s0960-9822(06)00410-6. [DOI] [PubMed] [Google Scholar]
- 46.Holinger EP, Chittenden T, Lutz RJ. Bak BH3 peptides antagonize Bcl-xL function and induce apoptosis through cytochrome c-independent activation of caspases. J Biol Chem. 1999;274(19):13298–13304. doi: 10.1074/jbc.274.19.13298. [DOI] [PubMed] [Google Scholar]
- 47.Chin JW, Schepartz A. Design and evolution of a miniature Bcl-2 binding protein. Angew Chem Int Ed Engl. 2001;40(20):3806–3809. doi: 10.1002/1521-3773(20011015)40:20<3806::AID-ANIE3806>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 48.Billard C. BH3 mimetics: status of the field and new developments. Mol Cancer Ther. 2013;12(9):1691–1700. doi: 10.1158/1535-7163.MCT-13-0058. [DOI] [PubMed] [Google Scholar]
- 49.Baell JB, Huang DC. Prospects for targeting the Bcl-2 family of proteins to develop novel cytotoxic drugs. Biochem Pharmacol. 2002;64(5–6):851–863. doi: 10.1016/s0006-2952(02)01148-6. [DOI] [PubMed] [Google Scholar]
- 50.Lee EF, Czabotar PE, Smith BJ, Deshayes K, Zobel K, Colman PM, et al. Crystal structure of ABT-737 complexed with Bcl-xL: implications for selectivity of antagonists of the Bcl-2 family. Cell Death Differ. 2007;14(9):1711–1713. doi: 10.1038/sj.cdd.4402178. [DOI] [PubMed] [Google Scholar]
- 51.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435(7042):677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 52.Kitada S, Leone M, Sareth S, Zhai D, Reed JC, Pellecchia M. Discovery, characterization, and structure-activity relationships studies of proapoptotic polyphenols targeting B-cell lymphocyte/leukemia-2 proteins. J Med Chem. 2003;46(20):4259–4264. doi: 10.1021/jm030190z. [DOI] [PubMed] [Google Scholar]
- 53.Wang JL, Liu D, Zhang ZJ, Shan S, Han X, Srinivasula SM, et al. Structure-based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells. Proc Natl Acad Sci U S A. 2000;97(13):7124–7129. doi: 10.1073/pnas.97.13.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Degterev A, Lugovskoy A, Cardone M, Mulley B, Wagner G, Mitchison T, et al. Identification of small-molecule inhibitors of interaction between the BH3 domain and Bcl-xL. Nat Cell Biol. 2001;3(2):173–182. doi: 10.1038/35055085. [DOI] [PubMed] [Google Scholar]
- 55.Tzung SP, Kim KM, Basanez G, Giedt CD, Simon J, Zimmerberg J, et al. Antimycin a mimics a cell-death-inducing Bcl-2 homology domain 3. Nat Cell Biol. 2001;3(2):183–191. doi: 10.1038/35055095. [DOI] [PubMed] [Google Scholar]
- 56.Enyedy IJ, Ling Y, Nacro K, Tomita Y, Wu X, Cao Y, et al. Discovery of small-molecule inhibitors of Bcl-2 through structure-based computer screening. J Med Chem. 2001;44(25):4313–4324. doi: 10.1021/jm010016f. [DOI] [PubMed] [Google Scholar]
- 57.Kutzki O, Park HS, Ernst JT, Orner BP, Yin H, Hamilton AD. Development of a potent Bcl-x(L) antagonist based on alpha-helix mimicry. J Am Chem Soc. 2002;124(40):11838–11839. doi: 10.1021/ja026861k. [DOI] [PubMed] [Google Scholar]
- 58.Park CM, Oie T, Petros AM, Zhang H, Nimmer PM, Henry RF, et al. Design, synthesis, and computational studies of inhibitors of Bcl-XL. J Am Chem Soc. 2006;128(50):16206–16212. doi: 10.1021/ja0650347. [DOI] [PubMed] [Google Scholar]
- 59.Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68(9):3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 60.Wang G, Nikolovska-Coleska Z, Yang CY, Wang R, Tang G, Guo J, et al. Structure-based design of potent small-molecule inhibitors of anti-apoptotic Bcl-2 proteins. J Med Chem. 2006;49(21):6139–6142. doi: 10.1021/jm060460o. [DOI] [PubMed] [Google Scholar]
- 61.Nguyen M, Marcellus RC, Roulston A, Watson M, Serfass L, Murthy Madiraju SR, et al. Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci U S A. 2007;104(49):19512–19517. doi: 10.1073/pnas.0709443104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19(2):202–208. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 63.Li L, Thomas RM, Suzuki H, De Brabander JK, Wang X, Harran PG. A small molecule Smac mimic potentiates TRAIL- and TNFalpha-mediated cell death. Science. 2004;305(5689):1471–1474. doi: 10.1126/science.1098231. [DOI] [PubMed] [Google Scholar]
- 64.Bai L, Chen W, Chen W, Wang X, Tang H, Lin Y. IKKbeta-mediated nuclear factor-kappaB activation attenuates smac mimetic-induced apoptosis in cancer cells. Mol Cancer Ther. 2009;8(6):1636–1645. doi: 10.1158/1535-7163.MCT-09-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lu J, Bai L, Sun H, Nikolovska-Coleska Z, McEachern D, Qiu S, et al. SM-164: a novel, bivalent Smac mimetic that induces apoptosis and tumor regression by concurrent removal of the blockade of cIAP-1/2 and XIAP. Cancer Res. 2008;68(22):9384–9393. doi: 10.1158/0008-5472.CAN-08-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cai Q, Sun H, Peng Y, Lu J, Nikolovska-Coleska Z, McEachern D, et al. A potent and orally active antagonist (SM-406/AT-406) of multiple inhibitor of apoptosis proteins (IAPs) in clinical development for cancer treatment. J Med Chem. 2011;54(8):2714–2726. doi: 10.1021/jm101505d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weisberg E, Ray A, Barrett R, Nelson E, Christie AL, Porter D, et al. Smac mimetics: implications for enhancement of targeted therapies in leukemia. Leukemia. 2010;24(12):2100–2109. doi: 10.1038/leu.2010.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun H, Nikolovska-Coleska Z, Lu J, Meagher JL, Yang CY, Qiu S, et al. Design, synthesis, and characterization of a potent, nonpeptide, cell-permeable, bivalent Smac mimetic that concurrently targets both the BIR2 and BIR3 domains in XIAP. J Am Chem Soc. 2007;129(49):15279–15294. doi: 10.1021/ja074725f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Allensworth JL, Sauer SJ, Lyerly HK, Morse MA, Devi GR. Smac mimetic Birinapant induces apoptosis and enhances TRAIL potency in inflammatory breast cancer cells in an IAP-dependent and TNF-alpha-independent mechanism. Breast Cancer Res Treat. 2013;137(2):359–371. doi: 10.1007/s10549-012-2352-6. [DOI] [PubMed] [Google Scholar]
- 70.Leverson JD, Zhang H, Chen J, Tahir SK, Phillips DC, Xue J, et al. Potent and selective small-molecule MCL-1 inhibitors demonstrate on-target cancer cell killing activity as single agents and in combination with ABT-263 (navitoclax) Cell Death Dis. 2015;6:e1590. doi: 10.1038/cddis.2014.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bruncko M, Wang L, Sheppard GS, Phillips DC, Tahir SK, Xue J, et al. Structure-guided design of a series of MCL-1 inhibitors with high affinity and selectivity. J Med Chem. 2015;58(5):2180–2194. doi: 10.1021/jm501258m. [DOI] [PubMed] [Google Scholar]
- 72.Caenepeel S, Brown SP, Belmontes B, Moody G, Keegan KS, Chui D, et al. AMG 176, a selective MCL1 inhibitor, is effective in hematologic Cancer models alone and in combination with established therapies. Cancer Discov. 2018;8(12):1582–1597. doi: 10.1158/2159-8290.CD-18-0387. [DOI] [PubMed] [Google Scholar]
- 73.Tron AE, Belmonte MA, Adam A, Aquila BM, Boise LH, Chiarparin E, et al. Discovery of Mcl-1-specific inhibitor AZD5991 and preclinical activity in multiple myeloma and acute myeloid leukemia. Nat Commun. 2018;9(1):5341. doi: 10.1038/s41467-018-07551-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kotschy A, Szlavik Z, Murray J, Davidson J, Maragno AL, Le Toumelin-Braizat G, et al. The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature. 2016;538(7626):477–482. doi: 10.1038/nature19830. [DOI] [PubMed] [Google Scholar]
- 75.Stewart ML, Fire E, Keating AE, Walensky LD. The MCL-1 BH3 helix is an exclusive MCL-1 inhibitor and apoptosis sensitizer. Nat Chem Biol. 2010;6(8):595–601. doi: 10.1038/nchembio.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ramsey HE, Fischer MA, Lee T, Gorska AE, Arrate MP, Fuller L, et al. A novel MCL1 inhibitor combined with Venetoclax rescues Venetoclax-resistant acute myelogenous leukemia. Cancer Discov. 2018;8(12):1566–1581. doi: 10.1158/2159-8290.CD-18-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee T, Bian Z, Zhao B, Hogdal LJ, Sensintaffar JL, Goodwin CM, et al. Discovery and biological characterization of potent myeloid cell leukemia-1 inhibitors. FEBS Lett. 2017;591(1):240–251. doi: 10.1002/1873-3468.12497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Folkes AJ, Ahmadi K, Alderton WK, Alix S, Baker SJ, Box G, et al. The identification of 2-(1H-indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-t hieno[3,2-d]pyrimidine (GDC-0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancer. J Med Chem. 2008;51(18):5522–5532. doi: 10.1021/jm800295d. [DOI] [PubMed] [Google Scholar]
- 79.Cleary ML, Smith SD, Sklar J. Cloning and structural analysis of cDNAs for bcl-2 and a hybrid bcl-2/immunoglobulin transcript resulting from the t(14;18) translocation. Cell. 1986;47(1):19–28. doi: 10.1016/0092-8674(86)90362-4. [DOI] [PubMed] [Google Scholar]
- 80.Tsujimoto Y, Cossman J, Jaffe E, Croce CM. Involvement of the bcl-2 gene in human follicular lymphoma. Science. 1985;228(4706):1440–1443. doi: 10.1126/science.3874430. [DOI] [PubMed] [Google Scholar]
- 81.Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335(6189):440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- 82.Cory S, Huang DC, Adams JM. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003;22(53):8590–8607. doi: 10.1038/sj.onc.1207102. [DOI] [PubMed] [Google Scholar]
- 83.Correia C, Lee SH, Meng XW, Vincelette ND, Knorr KL, Ding H, et al. Emerging understanding of Bcl-2 biology: implications for neoplastic progression and treatment. Biochim Biophys Acta. 2015;1853(7):1658–1671. doi: 10.1016/j.bbamcr.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yip KW, Reed JC. Bcl-2 family proteins and cancer. Oncogene. 2008;27(50):6398–6406. doi: 10.1038/onc.2008.307. [DOI] [PubMed] [Google Scholar]
- 85.Tessoulin B, Papin A, Gomez-Bougie P, Bellanger C, Amiot M, Pellat-Deceunynck C, et al. BCL2-family dysregulation in B-cell malignancies: from gene expression regulation to a targeted therapy biomarker. Front Oncol. 2018;8:645. doi: 10.3389/fonc.2018.00645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tracey L, Perez-Rosado A, Artiga MJ, Camacho FI, Rodriguez A, Martinez N, et al. Expression of the NF-kappaB targets BCL2 and BIRC5/Survivin characterizes small B-cell and aggressive B-cell lymphomas, respectively. J Pathol. 2005;206(2):123–134. doi: 10.1002/path.1768. [DOI] [PubMed] [Google Scholar]
- 87.Li Y, Bouchlaka MN, Wolff J, Grindle KM, Lu L, Qian S, et al. FBXO10 deficiency and BTK activation upregulate BCL2 expression in mantle cell lymphoma. Oncogene. 2016;35(48):6223–6234. doi: 10.1038/onc.2016.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moreaux J, Klein B, Bataille R, Descamps G, Maiga S, Hose D, et al. A high-risk signature for patients with multiple myeloma established from the molecular classification of human myeloma cell lines. Haematologica. 2011;96(4):574–582. doi: 10.3324/haematol.2010.033456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gong JN, Khong T, Segal D, Yao Y, Riffkin CD, Garnier JM, et al. Hierarchy for targeting prosurvival BCL2 family proteins in multiple myeloma: pivotal role of MCL1. Blood. 2016;128(14):1834–1844. doi: 10.1182/blood-2016-03-704908. [DOI] [PubMed] [Google Scholar]
- 90.Del Gaizo MV, Letai A. BH3 profiling--measuring integrated function of the mitochondrial apoptotic pathway to predict cell fate decisions. Cancer Lett. 2013;332(2):202–205. doi: 10.1016/j.canlet.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292(5517):727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nechushtan A, Smith CL, Hsu YT, Youle RJ. Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J. 1999;18(9):2330–2341. doi: 10.1093/emboj/18.9.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ. Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol. 1997;139(5):1281–1292. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moldoveanu T, Liu Q, Tocilj A, Watson M, Shore G, Gehring K. The X-ray structure of a BAK homodimer reveals an inhibitory zinc binding site. Mol Cell. 2006;24(5):677–688. doi: 10.1016/j.molcel.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 95.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9(1):47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 96.Dewson G, Kratina T, Sim HW, Puthalakath H, Adams JM, Colman PM, et al. To trigger apoptosis, Bak exposes its BH3 domain and homodimerizes via BH3:groove interactions. Mol Cell. 2008;30(3):369–380. doi: 10.1016/j.molcel.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 97.George NM, Evans JJ, Luo X. A three-helix homo-oligomerization domain containing BH3 and BH1 is responsible for the apoptotic activity of Bax. Genes Dev. 2007;21(15):1937–1948. doi: 10.1101/gad.1553607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Labi V, Grespi F, Baumgartner F, Villunger A. Targeting the Bcl-2-regulated apoptosis pathway by BH3 mimetics: a breakthrough in anticancer therapy? Cell Death Differ. 2008;15(6):977–987. doi: 10.1038/cdd.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10(5):389–399. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10(5):375–388. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 101.High LM, Szymanska B, Wilczynska-Kalak U, Barber N, O'Brien R, Khaw SL, et al. The Bcl-2 homology domain 3 mimetic ABT-737 targets the apoptotic machinery in acute lymphoblastic leukemia resulting in synergistic in vitro and in vivo interactions with established drugs. Mol Pharmacol. 2010;77(3):483–494. doi: 10.1124/mol.109.060780. [DOI] [PubMed] [Google Scholar]
- 102.Oakes SR, Vaillant F, Lim E, Lee L, Breslin K, Feleppa F, et al. Sensitization of BCL-2-expressing breast tumors to chemotherapy by the BH3 mimetic ABT-737. Proc Natl Acad Sci U S A. 2012;109(8):2766–2771. doi: 10.1073/pnas.1104778108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Song JH, Kandasamy K, Zemskova M, Lin YW, Kraft AS. The BH3 mimetic ABT-737 induces cancer cell senescence. Cancer Res. 2011;71(2):506–515. doi: 10.1158/0008-5472.CAN-10-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen S, Dai Y, Harada H, Dent P, Grant S. Mcl-1 down-regulation potentiates ABT-737 lethality by cooperatively inducing Bak activation and Bax translocation. Cancer Res. 2007;67(2):782–791. doi: 10.1158/0008-5472.CAN-06-3964. [DOI] [PubMed] [Google Scholar]
- 105.Boiani M, Daniel C, Liu X, Hogarty MD, Marnett LJ. The stress protein BAG3 stabilizes Mcl-1 protein and promotes survival of cancer cells and resistance to antagonist ABT-737. J Biol Chem. 2013;288(10):6980–6990. doi: 10.1074/jbc.M112.414177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang C, Li YL, Weng X, Li LY, Zhou MX, Zhang DY, et al. Nedaplatin enhanced apoptotic effects of ABT-737 in human cancer cells via Mcl-1 inhibition. Oncol Lett. 2016;12(5):4195–4202. doi: 10.3892/ol.2016.5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pandit B, Gartel AL. New potential anti-cancer agents synergize with bortezomib and ABT-737 against prostate cancer. Prostate. 2010;70(8):825–833. doi: 10.1002/pros.21116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Allaman-Pillet N, Oberson A, Munier F, Schorderet DF. The Bcl-2/Bcl-XL inhibitor ABT-737 promotes death of retinoblastoma cancer cells. Ophthalmic Genet. 2013;34(1–2):1–13. doi: 10.3109/13816810.2011.615077. [DOI] [PubMed] [Google Scholar]
- 109.Reuland SN, Goldstein NB, Partyka KA, Smith S, Luo Y, Fujita M, et al. ABT-737 synergizes with Bortezomib to kill melanoma cells. Biol Open. 2012;1(2):92–100. doi: 10.1242/bio.2011035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yin S, Dong Y, Li J, Fan L, Wang L, Lu J, et al. Methylseleninic acid potentiates multiple types of cancer cells to ABT-737-induced apoptosis by targeting Mcl-1 and bad. Apoptosis. 2012;17(4):388–399. doi: 10.1007/s10495-011-0687-9. [DOI] [PubMed] [Google Scholar]
- 111.Zhang S, Li G, Ma X, Wang Y, Liu G, Feng L, et al. Norcantharidin enhances ABT-737-induced apoptosis in hepatocellular carcinoma cells by transcriptional repression of Mcl-1. Cell Signal. 2012;24(9):1803–1809. doi: 10.1016/j.cellsig.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 112.Bogenberger JM, Delman D, Hansen N, Valdez R, Fauble V, Mesa RA, et al. Ex vivo activity of BCL-2 family inhibitors ABT-199 and ABT-737 combined with 5-azacytidine in myeloid malignancies. Leuk Lymphoma. 2015;56(1):226–229. doi: 10.3109/10428194.2014.910657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li JY, Li YY, Jin W, Yang Q, Shao ZM, Tian XS. ABT-737 reverses the acquired radioresistance of breast cancer cells by targeting Bcl-2 and Bcl-xL. J Exp Clin Cancer Res. 2012;31:102. doi: 10.1186/1756-9966-31-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen ZJ, Zhang B, Pan SH, Zhao HM, Zhang Y. Feng WH et al: [Bcl-2 inhibitor ABT-737 enhances the cisplatin-induced apoptosis in breast cancer T47D cells] Zhonghua Zhong Liu Za Zhi. 2011;33(12):891–895. [PubMed] [Google Scholar]
- 115.Choi JE, Woo SM, Min KJ, Kang SH, Lee SJ, Kwon TK. Combined treatment with ABT-737 and VX-680 induces apoptosis in Bcl-2- and c-FLIP-overexpressing breast carcinoma cells. Oncol Rep. 2015;33(3):1395–1401. doi: 10.3892/or.2015.3728. [DOI] [PubMed] [Google Scholar]
- 116.Hwang E, Hwang SH, Kim J, Park JH, Oh S, Kim YA, et al. ABT-737 ameliorates docetaxel resistance in triple negative breast cancer cell line. Ann Surg Treat Res. 2018;95(5):240–248. doi: 10.4174/astr.2018.95.5.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Raats DA, de Bruijn MT, Steller EJ, Emmink BL, Borel-Rinkes IH, Kranenburg O. Synergistic killing of colorectal cancer cells by oxaliplatin and ABT-737. Cell Oncol (Dordr) 2011;34(4):307–313. doi: 10.1007/s13402-011-0026-8. [DOI] [PubMed] [Google Scholar]
- 118.Huang S, Sinicrope FA. Celecoxib-induced apoptosis is enhanced by ABT-737 and by inhibition of autophagy in human colorectal cancer cells. Autophagy. 2010;6(2):256–269. doi: 10.4161/auto.6.2.11124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Premkumar DR, Jane EP, DiDomenico JD, Vukmer NA, Agostino NR, Pollack IF. ABT-737 synergizes with bortezomib to induce apoptosis, mediated by bid cleavage, Bax activation, and mitochondrial dysfunction in an Akt-dependent context in malignant human glioma cell lines. J Pharmacol Exp Ther. 2012;341(3):859–872. doi: 10.1124/jpet.112.191536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li R, Zang Y, Li C, Patel NS, Grandis JR, Johnson DE. ABT-737 synergizes with chemotherapy to kill head and neck squamous cell carcinoma cells via a Noxa-mediated pathway. Mol Pharmacol. 2009;75(5):1231–1239. doi: 10.1124/mol.108.052969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shen J, Xu L, Zhao Q. Perifosine and ABT-737 synergistically inhibit lung cancer cells in vitro and in vivo. Biochem Biophys Res Commun. 2016;473(4):1170–1176. doi: 10.1016/j.bbrc.2016.04.035. [DOI] [PubMed] [Google Scholar]
- 122.Opydo-Chanek M, Rak A, Cierniak A, Mazur L. Combination of ABT-737 and resveratrol enhances DNA damage and apoptosis in human T-cell acute lymphoblastic leukemia MOLT-4 cells. Toxicol in Vitro. 2017;42:38–46. doi: 10.1016/j.tiv.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 123.Opydo-Chanek M, Mazur L. Comparison of in vitro antileukemic activity of obatoclax and ABT-737. Tumour Biol. 2016;37(8):10839–10849. doi: 10.1007/s13277-016-4943-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ugarenko M, Nudelman A, Rephaeli A, Kimura K, Phillips DR, Cutts SM. ABT-737 overcomes Bcl-2 mediated resistance to doxorubicin-DNA adducts. Biochem Pharmacol. 2010;79(3):339–349. doi: 10.1016/j.bcp.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 125.Zheng R, You Z, Jia J, Lin S, Han S, Liu A, et al. Curcumin enhances the antitumor effect of ABT-737 via activation of the ROS-ASK1-JNK pathway in hepatocellular carcinoma cells. Mol Med Rep. 2016;13(2):1570–1576. doi: 10.3892/mmr.2015.4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kim EY, Jung JY, Kim A, Chang YS, Kim SK. ABT-737 synergizes with cisplatin bypassing aberration of apoptotic pathway in non-small cell lung Cancer. Neoplasia. 2017;19(4):354–363. doi: 10.1016/j.neo.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kim LH, Shin JA, Jang B, Yang IH, Won DH, Jeong JH, et al. Sorafenib potentiates ABT-737-induced apoptosis in human oral cancer cells. Arch Oral Biol. 2017;73:1–6. doi: 10.1016/j.archoralbio.2016.08.034. [DOI] [PubMed] [Google Scholar]
- 128.Zhang F, Yu X, Liu X, Zhou T, Nie T, Cheng M, et al. ABT-737 potentiates cisplatin-induced apoptosis in human osteosarcoma cells via the mitochondrial apoptotic pathway. Oncol Rep. 2017;38(4):2301–2308. doi: 10.3892/or.2017.5909. [DOI] [PubMed] [Google Scholar]
- 129.Li YL, Sun J, Hu X, Pan YN, Yan W, Li QY, et al. Epothilone B induces apoptosis and enhances apoptotic effects of ABT-737 on human cancer cells via PI3K/AKT/mTOR pathway. J Cancer Res Clin Oncol. 2016;142(11):2281–2289. doi: 10.1007/s00432-016-2236-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Florent R, Weiswald LB, Lambert B, Brotin E, Abeilard E, Louis MH, et al. Bim, Puma and Noxa upregulation by Naftopidil sensitizes ovarian cancer to the BH3-mimetic ABT-737 and the MEK inhibitor Trametinib. Cell Death Dis. 2020;11(5):380. doi: 10.1038/s41419-020-2588-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.De Wolf E, De Wolf C, Richardson A. ABT-737 and pictilisib synergistically enhance pitavastatin-induced apoptosis in ovarian cancer cells. Oncol Lett. 2018;15(2):1979–1984. doi: 10.3892/ol.2017.7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Witham J, Valenti MR, De-Haven-Brandon AK, Vidot S, Eccles SA, Kaye SB, et al. The Bcl-2/Bcl-XL family inhibitor ABT-737 sensitizes ovarian cancer cells to carboplatin. Clin Cancer Res. 2007;13(23):7191–7198. doi: 10.1158/1078-0432.CCR-07-0362. [DOI] [PubMed] [Google Scholar]
- 133.Jain HV, Meyer-Hermann M. The molecular basis of synergism between carboplatin and ABT-737 therapy targeting ovarian carcinomas. Cancer Res. 2011;71(3):705–715. doi: 10.1158/0008-5472.CAN-10-3174. [DOI] [PubMed] [Google Scholar]
- 134.Song JH, Kandasamy K, Kraft AS. ABT-737 induces expression of the death receptor 5 and sensitizes human cancer cells to TRAIL-induced apoptosis. J Biol Chem. 2008;283(36):25003–25013. doi: 10.1074/jbc.M802511200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Broecker-Preuss M, Becher-Boveleth N, Muller S, Mann K. The BH3 mimetic drug ABT-737 induces apoptosis and acts synergistically with chemotherapeutic drugs in thyroid carcinoma cells. Cancer Cell Int. 2016;16:27. doi: 10.1186/s12935-016-0303-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shen HP, Wu WJ, Ko JL, Wu TF, Yang SF, Wu CH, et al. Effects of ABT-737 combined with irradiation treatment on uterine cervical cancer cells. Oncol Lett. 2019;18(4):4328–4336. doi: 10.3892/ol.2019.10755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bhat UG, Pandit B, Gartel AL. ARC synergizes with ABT-737 to induce apoptosis in human cancer cells. Mol Cancer Ther. 2010;9(6):1688–1696. doi: 10.1158/1535-7163.MCT-09-0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vogler M, Furdas SD, Jung M, Kuwana T, Dyer MJ, Cohen GM. Diminished sensitivity of chronic lymphocytic leukemia cells to ABT-737 and ABT-263 due to albumin binding in blood. Clin Cancer Res. 2010;16(16):4217–4225. doi: 10.1158/1078-0432.CCR-10-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lian BSX, Yek AEH, Shuvas H, Abdul Rahman SF, Muniandy K, Mohana-Kumaran N. Synergistic anti-proliferative effects of combination of ABT-263 and MCL-1 selective inhibitor A-1210477 on cervical cancer cell lines. BMC Res Notes. 2018;11(1):197. doi: 10.1186/s13104-018-3302-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ackler S, Mitten MJ, Foster K, Oleksijew A, Refici M, Tahir SK, et al. The Bcl-2 inhibitor ABT-263 enhances the response of multiple chemotherapeutic regimens in hematologic tumors in vivo. Cancer Chemother Pharmacol. 2010;66(5):869–880. doi: 10.1007/s00280-009-1232-1. [DOI] [PubMed] [Google Scholar]
- 141.Kivioja JL, Thanasopoulou A, Kumar A, Kontro M, Yadav B, Majumder MM, et al. Dasatinib and navitoclax act synergistically to target NUP98-NSD1(+)/FLT3-ITD(+) acute myeloid leukemia. Leukemia. 2019;33(6):1360–1372. doi: 10.1038/s41375-018-0327-2. [DOI] [PubMed] [Google Scholar]
- 142.Polier G, Giaisi M, Kohler R, Muller WW, Lutz C, Buss EC, et al. Targeting CDK9 by wogonin and related natural flavones potentiates the anti-cancer efficacy of the Bcl-2 family inhibitor ABT-263. Int J Cancer. 2015;136(3):688–698. doi: 10.1002/ijc.29009. [DOI] [PubMed] [Google Scholar]
- 143.Shao H, Jing K, Mahmoud E, Huang H, Fang X, Yu C. Apigenin sensitizes colon cancer cells to antitumor activity of ABT-263. Mol Cancer Ther. 2013;12(12):2640–2650. doi: 10.1158/1535-7163.MCT-13-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li J, Chen Y, Wan J, Liu X, Yu C, Li W. ABT-263 enhances sorafenib-induced apoptosis associated with Akt activity and the expression of Bax and p21((CIP1/WAF1)) in human cancer cells. Br J Pharmacol. 2014;171(13):3182–3195. doi: 10.1111/bph.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sale MJ, Cook SJ. The BH3 mimetic ABT-263 synergizes with the MEK1/2 inhibitor selumetinib/AZD6244 to promote BIM-dependent tumour cell death and inhibit acquired resistance. Biochem J. 2013;450(2):285–294. doi: 10.1042/BJ20121212. [DOI] [PubMed] [Google Scholar]
- 146.Chen Q, Song S, Wei S, Liu B, Honjo S, Scott A, et al. ABT-263 induces apoptosis and synergizes with chemotherapy by targeting stemness pathways in esophageal cancer. Oncotarget. 2015;6(28):25883–25896. doi: 10.18632/oncotarget.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lin QH, Que FC, Gu CP, Zhong DS, Zhou D, Kong Y, et al. ABT-263 induces G1/G0-phase arrest, apoptosis and autophagy in human esophageal cancer cells in vitro. Acta Pharmacol Sin. 2017;38(12):1632–1641. doi: 10.1038/aps.2017.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Britt EL, Raman S, Leek K, Sheehy CH, Kim SW, Harada H. Combination of fenretinide and ABT-263 induces apoptosis through NOXA for head and neck squamous cell carcinoma treatment. PLoS One. 2019;14(7):e0219398. doi: 10.1371/journal.pone.0219398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang G, Zhan Y, Wang H, Li W. ABT-263 sensitizes TRAIL-resistant hepatocarcinoma cells by downregulating the Bcl-2 family of anti-apoptotic protein. Cancer Chemother Pharmacol. 2012;69(3):799–805. doi: 10.1007/s00280-011-1763-0. [DOI] [PubMed] [Google Scholar]
- 150.Sakuma Y, Tsunezumi J, Nakamura Y, Yoshihara M, Matsukuma S, Koizume S, et al. ABT-263, a Bcl-2 inhibitor, enhances the susceptibility of lung adenocarcinoma cells treated with Src inhibitors to anoikis. Oncol Rep. 2011;25(3):661–667. doi: 10.3892/or.2010.1123. [DOI] [PubMed] [Google Scholar]
- 151.Matsumoto M, Nakajima W, Seike M, Gemma A, Tanaka N. Cisplatin-induced apoptosis in non-small-cell lung cancer cells is dependent on Bax- and Bak-induction pathway and synergistically activated by BH3-mimetic ABT-263 in p53 wild-type and mutant cells. Biochem Biophys Res Commun. 2016;473(2):490–496. doi: 10.1016/j.bbrc.2016.03.053. [DOI] [PubMed] [Google Scholar]
- 152.Nakajima W, Sharma K, Hicks MA, Le N, Brown R, Krystal GW, et al. Combination with vorinostat overcomes ABT-263 (navitoclax) resistance of small cell lung cancer. Cancer Biol Ther. 2016;17(1):27–35. doi: 10.1080/15384047.2015.1108485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ackler S, Xiao Y, Mitten MJ, Foster K, Oleksijew A, Refici M, et al. ABT-263 and rapamycin act cooperatively to kill lymphoma cells in vitro and in vivo. Mol Cancer Ther. 2008;7(10):3265–3274. doi: 10.1158/1535-7163.MCT-08-0268. [DOI] [PubMed] [Google Scholar]
- 154.Wang X, Gu Z, Li G, Zhang S, Cao Z, Yang Z, et al. Norcantharidin enhances ABT-263-mediated anticancer activity in neuroblastoma cells by upregulation of Noxa. Oncol Rep. 2014;32(2):716–722. doi: 10.3892/or.2014.3228. [DOI] [PubMed] [Google Scholar]
- 155.Wang C, Huang SB, Yang MC, Lin YT, Chu IH, Shen YN, et al. Combining paclitaxel with ABT-263 has a synergistic effect on paclitaxel resistant prostate cancer cells. PLoS One. 2015;10(3):e0120913. doi: 10.1371/journal.pone.0120913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wei X, Zhou P, Lin X, Lin Y, Wu S, Diao P, et al. MLN2238 synergizes BH3 mimetic ABT-263 in castration-resistant prostate cancer cells by induction of NOXA. Tumour Biol. 2014;35(10):10213–10221. doi: 10.1007/s13277-014-2333-y. [DOI] [PubMed] [Google Scholar]
- 157.Wong M, Tan N, Zha J, Peale FV, Yue P, Fairbrother WJ, et al. Navitoclax (ABT-263) reduces Bcl-x(L)-mediated chemoresistance in ovarian cancer models. Mol Cancer Ther. 2012;11(4):1026–1035. doi: 10.1158/1535-7163.MCT-11-0693. [DOI] [PubMed] [Google Scholar]
- 158.Li X, Li B, Ni Z, Zhou P, Wang B, He J, et al. Metformin synergizes with BCL-XL/BCL-2 inhibitor ABT-263 to induce apoptosis specifically in p53-defective Cancer cells. Mol Cancer Ther. 2017;16(9):1806–1818. doi: 10.1158/1535-7163.MCT-16-0763. [DOI] [PubMed] [Google Scholar]
- 159.Wang B, Ni Z, Dai X, Qin L, Li X, Xu L, et al. The Bcl-2/xL inhibitor ABT-263 increases the stability of Mcl-1 mRNA and protein in hepatocellular carcinoma cells. Mol Cancer. 2014;13:98. doi: 10.1186/1476-4598-13-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lee EY, Gong EY, Shin JS, Moon JH, Shim HJ, Kim SM, et al. Human breast cancer cells display different sensitivities to ABT-263 based on the level of survivin. Toxicol in Vitro. 2018;46:229–236. doi: 10.1016/j.tiv.2017.09.023. [DOI] [PubMed] [Google Scholar]
- 161.Lee YC, Wang LJ, Huang CH, Chiou JT, Shi YJ, Chang LS. Inhibition of EGFR pathway promotes the cytotoxicity of ABT-263 in human leukemia K562 cells by blocking MCL1 upregulation. Biochem Pharmacol. 2020;178:114047. doi: 10.1016/j.bcp.2020.114047. [DOI] [PubMed] [Google Scholar]
- 162.Xu J, Zhu GY, Cao D, Pan H, Li YW. Gossypol overcomes EGFR-TKIs resistance in non-small cell lung cancer cells by targeting YAP/TAZ and EGFR(L858R/T790M) Biomed Pharmacother. 2019;115:108860. doi: 10.1016/j.biopha.2019.108860. [DOI] [PubMed] [Google Scholar]
- 163.Wang Y, Lai H, Fan X, Luo L, Duan F, Jiang Z, et al. Gossypol inhibits non-small cell lung Cancer cells proliferation by targeting EGFR(L858R/T790M) Front Pharmacol. 2018;9:728. doi: 10.3389/fphar.2018.00728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Huang YW, Wang LS, Chang HL, Ye W, Dowd MK, Wan PJ, et al. Molecular mechanisms of (−)-gossypol-induced apoptosis in human prostate cancer cells. Anticancer Res. 2006;26(3A):1925–1933. [PubMed] [Google Scholar]
- 165.Zhang M, Liu H, Tian Z, Griffith BN, Ji M, Li QQ. Gossypol induces apoptosis in human PC-3 prostate cancer cells by modulating caspase-dependent and caspase-independent cell death pathways. Life Sci. 2007;80(8):767–774. doi: 10.1016/j.lfs.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 166.Wang J, Jin L, Li X, Deng H, Chen Y, Lian Q, et al. Gossypol induces apoptosis in ovarian cancer cells through oxidative stress. Mol BioSyst. 2013;9(6):1489–1497. doi: 10.1039/c3mb25461e. [DOI] [PubMed] [Google Scholar]
- 167.Macoska JA, Adsule S, Tantivejkul K, Wang S, Pienta KJ, Lee CT. (−)gossypol promotes the apoptosis of bladder cancer cells in vitro. Pharmacol Res. 2008;58(5–6):323–331. doi: 10.1016/j.phrs.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 168.Gilbert NE, O'Reilly JE, Chang CJ, Lin YC, Brueggemeier RW. Antiproliferative activity of gossypol and gossypolone on human breast cancer cells. Life Sci. 1995;57(1):61–67. doi: 10.1016/0024-3205(95)00243-y. [DOI] [PubMed] [Google Scholar]
- 169.Ye W, Chang HL, Wang LS, Huang YW, Shu S, Sugimoto Y, et al. Induction of apoptosis by (−)-gossypol-enriched cottonseed oil in human breast cancer cells. Int J Mol Med. 2010;26(1):113–119. [PubMed] [Google Scholar]
- 170.Meng Y, Tang W, Dai Y, Wu X, Liu M, Ji Q, et al. Natural BH3 mimetic (−)-gossypol chemosensitizes human prostate cancer via Bcl-xL inhibition accompanied by increase of Puma and Noxa. Mol Cancer Ther. 2008;7(7):2192–2202. doi: 10.1158/1535-7163.MCT-08-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Volate SR, Kawasaki BT, Hurt EM, Milner JA, Kim YS, White J, et al. Gossypol induces apoptosis by activating p53 in prostate cancer cells and prostate tumor-initiating cells. Mol Cancer Ther. 2010;9(2):461–470. doi: 10.1158/1535-7163.MCT-09-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhang G, Wang Z, Chen W, Cao Y, Wu J, Qiang G, et al. Dual effects of gossypol on human hepatocellular carcinoma via endoplasmic reticulum stress and autophagy. Int J Biochem Cell Biol. 2019;113:48–57. doi: 10.1016/j.biocel.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 173.Xu R, Tian E, Tang H, Liu C, Wang Q. Proteomic analysis of gossypol induces necrosis in multiple myeloma cells. Biomed Res Int. 2014;2014:839232. doi: 10.1155/2014/839232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lian J, Ni Z, Dai X, Su C, Smith AR, Xu L, et al. Sorafenib sensitizes (−)-gossypol-induced growth suppression in androgen-independent prostate cancer cells via Mcl-1 inhibition and Bak activation. Mol Cancer Ther. 2012;11(2):416–426. doi: 10.1158/1535-7163.MCT-11-0559. [DOI] [PubMed] [Google Scholar]
- 175.Jang GH, Lee M. BH3-mimetic gossypol-induced autophagic cell death in mutant BRAF melanoma cells with high expression of p21Cip(1) Life Sci. 2014;102(1):41–48. doi: 10.1016/j.lfs.2014.02.036. [DOI] [PubMed] [Google Scholar]
- 176.Huang YW, Wang LS, Dowd MK, Wan PJ. Lin YC: (−)-gossypol reduces invasiveness in metastatic prostate cancer cells. Anticancer Res. 2009;29(6):2179–2188. [PubMed] [Google Scholar]
- 177.Meyer N, Zielke S, Michaelis JB, Linder B, Warnsmann V, Rakel S, et al. AT 101 induces early mitochondrial dysfunction and HMOX1 (heme oxygenase 1) to trigger mitophagic cell death in glioma cells. Autophagy. 2018;14(10):1693–1709. doi: 10.1080/15548627.2018.1476812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Etxebarria A, Landeta O, Antonsson B, Basanez G. Regulation of antiapoptotic MCL-1 function by gossypol: mechanistic insights from in vitro reconstituted systems. Biochem Pharmacol. 2008;76(11):1563–1576. doi: 10.1016/j.bcp.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 179.Mallick DJ, Eastman A. AT101 [(−)-Gossypol] Selectively Inhibits MCL1 and Sensitizes Carcinoma to BH3 Mimetics by Inducing and Stabilizing NOXA. Cancers (Basel) 2020;12(8):2298. doi: 10.3390/cancers12082298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kim HY, Lee BI, Jeon JH, Kim DK, Kang SG, Shim JK, et al. Gossypol Suppresses Growth of Temozolomide-Resistant Glioblastoma Tumor Spheres. Biomolecules. 2019;9(10):595. doi: 10.3390/biom9100595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Meng Y, Li Y, Li J, Li H, Fu J. Liu Y et al: (−)gossypol and its combination with imatinib induce apoptosis in human chronic myeloid leukemic cells. Leuk Lymphoma. 2007;48(11):2204–2212. doi: 10.1080/10428190701583991. [DOI] [PubMed] [Google Scholar]
- 182.Yang D, Qu J, Qu X, Cao Y, Xu L, Hou K, et al. Gossypol sensitizes the antitumor activity of 5-FU through down-regulation of thymidylate synthase in human colon carcinoma cells. Cancer Chemother Pharmacol. 2015;76(3):575–586. doi: 10.1007/s00280-015-2749-0. [DOI] [PubMed] [Google Scholar]
- 183.Wong FY, Liem N, Xie C, Yan FL, Wong WC, Wang L, et al. Combination therapy with gossypol reveals synergism against gemcitabine resistance in cancer cells with high BCL-2 expression. PLoS One. 2012;7(12):e50786. doi: 10.1371/journal.pone.0050786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Atmaca H, Gorumlu G, Karaca B, Degirmenci M, Tunali D, Cirak Y, et al. Combined gossypol and zoledronic acid treatment results in synergistic induction of cell death and regulates angiogenic molecules in ovarian cancer cells. Eur Cytokine Netw. 2009;20(3):121–130. doi: 10.1684/ecn.2009.0159. [DOI] [PubMed] [Google Scholar]
- 185.Yeow WS, Baras A, Chua A, Nguyen DM, Sehgal SS, Schrump DS, et al. Gossypol, a phytochemical with BH3-mimetic property, sensitizes cultured thoracic cancer cells to Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand. J Thorac Cardiovasc Surg. 2006;132(6):1356–1362. doi: 10.1016/j.jtcvs.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 186.Bulut G, Atmaca H, Karaca B. Trastuzumab in combination with AT-101 induces cytotoxicity and apoptosis in Her2 positive breast cancer cells. Future Oncol. 2020;16(3):4485–4495. doi: 10.2217/fon-2019-0521. [DOI] [PubMed] [Google Scholar]
- 187.Mohammad RM, Wang S, Banerjee S, Wu X, Chen J, Sarkar FH. Nonpeptidic small-molecule inhibitor of Bcl-2 and Bcl-XL, (−)-gossypol, enhances biological effect of genistein against BxPC-3 human pancreatic cancer cell line. Pancreas. 2005;31(4):317–324. doi: 10.1097/01.mpa.0000179731.46210.01. [DOI] [PubMed] [Google Scholar]
- 188.Xu L, Yang D, Wang S, Tang W, Liu M. Davis M et al: (−)-gossypol enhances response to radiation therapy and results in tumor regression of human prostate cancer. Mol Cancer Ther. 2005;4(2):197–205. [PubMed] [Google Scholar]
- 189.McGregor N, Patel L, Craig M, Weidner S, Wang S, Pienta KJ. AT-101 (R-(−)-gossypol acetic acid) enhances the effectiveness of androgen deprivation therapy in the VCaP prostate cancer model. J Cell Biochem. 2010;110(5):1187–1194. doi: 10.1002/jcb.22633. [DOI] [PubMed] [Google Scholar]
- 190.Konopleva M, Watt J, Contractor R, Tsao T, Harris D, Estrov Z, et al. Mechanisms of antileukemic activity of the novel Bcl-2 homology domain-3 mimetic GX15-070 (obatoclax) Cancer Res. 2008;68(9):3413–3420. doi: 10.1158/0008-5472.CAN-07-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Goard CA, Schimmer AD. An evidence-based review of obatoclax mesylate in the treatment of hematological malignancies. Core Evid. 2013;8:15–26. doi: 10.2147/CE.S42568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Vogler M. Targeting BCL2-proteins for the treatment of solid Tumours. Adv Med. 2014;2014:943648. doi: 10.1155/2014/943648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Li J, Viallet J, Haura EB. A small molecule pan-Bcl-2 family inhibitor, GX15-070, induces apoptosis and enhances cisplatin-induced apoptosis in non-small cell lung cancer cells. Cancer Chemother Pharmacol. 2008;61(3):525–534. doi: 10.1007/s00280-007-0499-3. [DOI] [PubMed] [Google Scholar]
- 194.Champa D, Russo MA, Liao XH, Refetoff S, Ghossein RA, Di Cristofano A. Obatoclax overcomes resistance to cell death in aggressive thyroid carcinomas by countering Bcl2a1 and Mcl1 overexpression. Endocr Relat Cancer. 2014;21(5):755–767. doi: 10.1530/ERC-14-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Rahmani M, Aust MM, Attkisson E, Williams DC, Jr, Ferreira-Gonzalez A, Grant S. Inhibition of Bcl-2 antiapoptotic members by obatoclax potently enhances sorafenib-induced apoptosis in human myeloid leukemia cells through a Bim-dependent process. Blood. 2012;119(25):6089–6098. doi: 10.1182/blood-2011-09-378141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Jimenez-Guerrero R, Gasca J, Flores ML, Perez-Valderrama B, Tejera-Parrado C, Medina R, et al. Obatoclax and paclitaxel synergistically induce apoptosis and overcome paclitaxel resistance in urothelial cancer cells. Cancers (Basel) 2018;10(12):490. doi: 10.3390/cancers10120490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Steele TM, Talbott GC, Sam A, Tepper CG, Ghosh PM, Vinall RL. Obatoclax, a BH3 Mimetic, Enhances Cisplatin-Induced Apoptosis and Decreases the Clonogenicity of Muscle Invasive Bladder Cancer Cells via Mechanisms That Involve the Inhibition of Pro-Survival Molecules as Well as Cell Cycle Regulators. Int J Mol Sci. 2019;20(6):1285–300. doi: 10.3390/ijms20061285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Mott JL, Bronk SF, Mesa RA, Kaufmann SH, Gores GJ. BH3-only protein mimetic obatoclax sensitizes cholangiocarcinoma cells to Apo2L/TRAIL-induced apoptosis. Mol Cancer Ther. 2008;7(8):2339–2347. doi: 10.1158/1535-7163.MCT-08-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Gariboldi MB, Taiana E, Bonzi MC, Craparotta I, Giovannardi S, Mancini M, et al. The BH3-mimetic obatoclax reduces HIF-1alpha levels and HIF-1 transcriptional activity and sensitizes hypoxic colon adenocarcinoma cells to 5-fluorouracil. Cancer Lett. 2015;364(2):156–164. doi: 10.1016/j.canlet.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 200.Zhao XY, Lin QH, Que FC, Gu CP, Yu L. Liu SW: [synergistic anti-tumor effect of obatoclax and MG-132 in esophageal cancer cell line CaES-17] Nan Fang Yi Ke Da Xue Xue Bao. 2016;36(4):506–513. [PubMed] [Google Scholar]
- 201.Berghauser Pont LM, Spoor JK, Venkatesan S, Swagemakers S, Kloezeman JJ, Dirven CM, et al. The Bcl-2 inhibitor Obatoclax overcomes resistance to histone deacetylase inhibitors SAHA and LBH589 as radiosensitizers in patient-derived glioblastoma stem-like cells. Genes Cancer. 2014;5(11–12):445–459. doi: 10.18632/genesandcancer.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Yin YP, Shi WH, Deng K, Liu XL, Li H, Lv XT, et al. Combinations of proteasome inhibitors with obatoclax are effective for small cell lung cancer. Acta Pharmacol Sin. 2021;42(8):1298–310. doi: 10.1038/s41401-020-00544-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Crawford N, Chacko AD, Savage KI, McCoy F, Redmond K, Longley DB, et al. Platinum resistant cancer cells conserve sensitivity to BH3 domains and obatoclax induced mitochondrial apoptosis. Apoptosis. 2011;16(3):311–320. doi: 10.1007/s10495-010-0561-1. [DOI] [PubMed] [Google Scholar]
- 204.Cournoyer S, Addioui A, Belounis A, Beaunoyer M, Nyalendo C, Le Gall R, et al. GX15-070 (Obatoclax), a Bcl-2 family proteins inhibitor engenders apoptosis and pro-survival autophagy and increases Chemosensitivity in neuroblastoma. BMC Cancer. 2019;19(1):1018. doi: 10.1186/s12885-019-6195-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hou XF, Li S, Wu C, Li K, Xu SN, Wang JF. Effects of obatoclax combined with gemcitabine on the biological activity of pancreatic cancer cells under hypoxic conditions. Mol Med Rep. 2018;18(1):495–501. doi: 10.3892/mmr.2018.8932. [DOI] [PubMed] [Google Scholar]
- 206.Huang S, Okumura K, Sinicrope FA. BH3 mimetic obatoclax enhances TRAIL-mediated apoptosis in human pancreatic cancer cells. Clin Cancer Res. 2009;15(1):150–159. doi: 10.1158/1078-0432.CCR-08-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wang G, Chen S, Edwards H, Cui X, Cui L, Ge Y. Combination of chloroquine and GX15-070 (obatoclax) results in synergistic cytotoxicity against pancreatic cancer cells. Oncol Rep. 2014;32(6):2789–2794. doi: 10.3892/or.2014.3525. [DOI] [PubMed] [Google Scholar]
- 208.Chen S, Wang G, Niu X, Zhao J, Tan W, Wang H, et al. Combination of AZD2281 (Olaparib) and GX15-070 (Obatoclax) results in synergistic antitumor activities in preclinical models of pancreatic cancer. Cancer Lett. 2014;348(1–2):20–28. doi: 10.1016/j.canlet.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 209.Wei WJ, Sun ZK, Shen CT, Song HJ, Zhang XY, Qiu ZL, et al. Obatoclax and LY3009120 efficiently overcome Vemurafenib resistance in differentiated thyroid cancer. Theranostics. 2017;7(4):987–1001. doi: 10.7150/thno.17322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hauck P, Chao BH, Litz J, Krystal GW. Alterations in the Noxa/Mcl-1 axis determine sensitivity of small cell lung cancer to the BH3 mimetic ABT-737. Mol Cancer Ther. 2009;8(4):883–892. doi: 10.1158/1535-7163.MCT-08-1118. [DOI] [PubMed] [Google Scholar]
- 211.Nakajima W, Hicks MA, Tanaka N, Krystal GW, Harada H. Noxa determines localization and stability of MCL-1 and consequently ABT-737 sensitivity in small cell lung cancer. Cell Death Dis. 2014;5:e1052. doi: 10.1038/cddis.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Sakakibara-Konishi J, Oizumi S, Kikuchi J, Kikuchi E, Mizugaki H, Kinoshita I, et al. Expression of Bim, Noxa, and Puma in non-small cell lung cancer. BMC Cancer. 2012;12:286. doi: 10.1186/1471-2407-12-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Yan J, Zhong N, Liu G, Chen K, Liu X, Su L, et al. Usp9x- and Noxa-mediated Mcl-1 downregulation contributes to pemetrexed-induced apoptosis in human non-small-cell lung cancer cells. Cell Death Dis. 2014;5:e1316. doi: 10.1038/cddis.2014.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Smit LA, Hallaert DY, Spijker R, de Goeij B, Jaspers A, Kater AP, et al. Differential Noxa/Mcl-1 balance in peripheral versus lymph node chronic lymphocytic leukemia cells correlates with survival capacity. Blood. 2007;109(4):1660–1668. doi: 10.1182/blood-2006-05-021683. [DOI] [PubMed] [Google Scholar]
- 215.Zhang LN, Li JY, Xu W. A review of the role of Puma, Noxa and Bim in the tumorigenesis, therapy and drug resistance of chronic lymphocytic leukemia. Cancer Gene Ther. 2013;20(1):1–7. doi: 10.1038/cgt.2012.84. [DOI] [PubMed] [Google Scholar]
- 216.Marshall AD, Picchione F, Geltink RI, Grosveld GC. PAX3-FOXO1 induces up-regulation of Noxa sensitizing alveolar rhabdomyosarcoma cells to apoptosis. Neoplasia. 2013;15(7):738–748. doi: 10.1593/neo.121888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ramirez-Peinado S, Alcazar-Limones F, Lagares-Tena L, El Mjiyad N, Caro-Maldonado A, Tirado OM, et al. 2-deoxyglucose induces Noxa-dependent apoptosis in alveolar rhabdomyosarcoma. Cancer Res. 2011;71(21):6796–6806. doi: 10.1158/0008-5472.CAN-11-0759. [DOI] [PubMed] [Google Scholar]
- 218.Diallo JS, Aldejmah A, Mouhim AF, Peant B, Fahmy MA, Koumakpayi IH, et al. NOXA and PUMA expression add to clinical markers in predicting biochemical recurrence of prostate cancer patients in a survival tree model. Clin Cancer Res. 2007;13(23):7044–7052. doi: 10.1158/1078-0432.CCR-07-1224. [DOI] [PubMed] [Google Scholar]
- 219.Lin C, Zhao XY, Li L, Liu HY, Cao K, Wan Y, et al. NOXA-induced alterations in the Bax/Smac axis enhance sensitivity of ovarian cancer cells to cisplatin. PLoS One. 2012;7(5):e36722. doi: 10.1371/journal.pone.0036722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Conti A, Majorini MT, Elliott R, Ashworth A, Lord CJ, Cancelliere C, et al. Oncogenic KRAS sensitizes premalignant, but not malignant cells, to Noxa-dependent apoptosis through the activation of the MEK/ERK pathway. Oncotarget. 2015;6(13):10994–11008. doi: 10.18632/oncotarget.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Jansson AK, Emterling AM, Arbman G, Sun XF. Noxa in colorectal cancer: a study on DNA, mRNA and protein expression. Oncogene. 2003;22(30):4675–4678. doi: 10.1038/sj.onc.1206655. [DOI] [PubMed] [Google Scholar]
- 222.Mukherjee N, Lu Y, Almeida A, Lambert K, Shiau CW, Su JC, et al. Use of a MCL-1 inhibitor alone to de-bulk melanoma and in combination to kill melanoma initiating cells. Oncotarget. 2017;8(29):46801–46817. doi: 10.18632/oncotarget.8695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kelly KR, Espitia CM, Mahalingam D, Oyajobi BO, Coffey M, Giles FJ, et al. Reovirus therapy stimulates endoplasmic reticular stress, NOXA induction, and augments bortezomib-mediated apoptosis in multiple myeloma. Oncogene. 2012;31(25):3023–3038. doi: 10.1038/onc.2011.478. [DOI] [PubMed] [Google Scholar]
- 224.Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17(3):393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 225.Chen LS, Balakrishnan K, Gandhi V. Inflammation and survival pathways: chronic lymphocytic leukemia as a model system. Biochem Pharmacol. 2010;80(12):1936–1945. doi: 10.1016/j.bcp.2010.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Erlacher M, Labi V, Manzl C, Bock G, Tzankov A, Hacker G, et al. Puma cooperates with Bim, the rate-limiting BH3-only protein in cell death during lymphocyte development, in apoptosis induction. J Exp Med. 2006;203(13):2939–2951. doi: 10.1084/jem.20061552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Edwards AL, Gavathiotis E, LaBelle JL, Braun CR, Opoku-Nsiah KA, Bird GH, et al. Multimodal interaction with BCL-2 family proteins underlies the proapoptotic activity of PUMA BH3. Chem Biol. 2013;20(7):888–902. doi: 10.1016/j.chembiol.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2(3):183–192. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- 229.Day CL, Smits C, Fan FC, Lee EF, Fairlie WD, Hinds MG. Structure of the BH3 domains from the p53-inducible BH3-only proteins Noxa and Puma in complex with Mcl-1. J Mol Biol. 2008;380(5):958–971. doi: 10.1016/j.jmb.2008.05.071. [DOI] [PubMed] [Google Scholar]
- 230.Fulda S, Vucic D. Targeting IAP proteins for therapeutic intervention in cancer. Nat Rev Drug Discov. 2012;11(2):109–124. doi: 10.1038/nrd3627. [DOI] [PubMed] [Google Scholar]
- 231.Condon SM, Mitsuuchi Y, Deng Y, LaPorte MG, Rippin SR, Haimowitz T, et al. Birinapant, a smac-mimetic with improved tolerability for the treatment of solid tumors and hematological malignancies. J Med Chem. 2014;57(9):3666–3677. doi: 10.1021/jm500176w. [DOI] [PubMed] [Google Scholar]
- 232.Zender L, Spector MS, Xue W, Flemming P, Cordon-Cardo C, Silke J, et al. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125(7):1253–1267. doi: 10.1016/j.cell.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.de Almagro MC, Vucic D. The inhibitor of apoptosis (IAP) proteins are critical regulators of signaling pathways and targets for anti-cancer therapy. Exp Oncol. 2012;34(3):200–211. [PubMed] [Google Scholar]
- 234.Obexer P, Ausserlechner MJ. X-linked inhibitor of apoptosis protein - a critical death resistance regulator and therapeutic target for personalized cancer therapy. Front Oncol. 2014;4:197. doi: 10.3389/fonc.2014.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P, et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 2007;131(4):669–681. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 236.Bertrand MJ, Milutinovic S, Dickson KM, Ho WC, Boudreault A, Durkin J, et al. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell. 2008;30(6):689–700. doi: 10.1016/j.molcel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 237.Mahoney DJ, Cheung HH, Mrad RL, Plenchette S, Simard C, Enwere E, et al. Both cIAP1 and cIAP2 regulate TNFalpha-mediated NF-kappaB activation. Proc Natl Acad Sci U S A. 2008;105(33):11778–11783. doi: 10.1073/pnas.0711122105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Vince JE, Wong WW, Khan N, Feltham R, Chau D, Ahmed AU, et al. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell. 2007;131(4):682–693. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 239.Fulda S, Wick W, Weller M, Debatin KM. Smac agonists sensitize for Apo2L/TRAIL- or anticancer drug-induced apoptosis and induce regression of malignant glioma in vivo. Nat Med. 2002;8(8):808–815. doi: 10.1038/nm735. [DOI] [PubMed] [Google Scholar]
- 240.Carter BZ, Milella M, Tsao T, McQueen T, Schober WD, Hu W, et al. Regulation and targeting of antiapoptotic XIAP in acute myeloid leukemia. Leukemia. 2003;17(11):2081–2089. doi: 10.1038/sj.leu.2403113. [DOI] [PubMed] [Google Scholar]
- 241.Arnt CR, Chiorean MV, Heldebrant MP, Gores GJ, Kaufmann SH. Synthetic Smac/DIABLO peptides enhance the effects of chemotherapeutic agents by binding XIAP and cIAP1 in situ. J Biol Chem. 2002;277(46):44236–44243. doi: 10.1074/jbc.M207578200. [DOI] [PubMed] [Google Scholar]
- 242.Silke J, Hawkins CJ, Ekert PG, Chew J, Day CL, Pakusch M, et al. The anti-apoptotic activity of XIAP is retained upon mutation of both the caspase 3- and caspase 9-interacting sites. J Cell Biol. 2002;157(1):115–124. doi: 10.1083/jcb.200108085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Bratton SB, Walker G, Srinivasula SM, Sun XM, Butterworth M, Alnemri ES, et al. Recruitment, activation and retention of caspases-9 and -3 by Apaf-1 apoptosome and associated XIAP complexes. EMBO J. 2001;20(5):998–1009. doi: 10.1093/emboj/20.5.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Wu G, Chai J, Suber TL, Wu JW, Du C, Wang X, et al. Structural basis of IAP recognition by Smac/DIABLO. Nature. 2000;408(6815):1008–1012. doi: 10.1038/35050012. [DOI] [PubMed] [Google Scholar]
- 245.Ekert PG, Silke J, Hawkins CJ, Verhagen AM, Vaux DL. DIABLO promotes apoptosis by removing MIHA/XIAP from processed caspase 9. J Cell Biol. 2001;152(3):483–490. doi: 10.1083/jcb.152.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Eckhardt I, Roesler S, Fulda S. Identification of DR5 as a critical, NF-kappaB-regulated mediator of Smac-induced apoptosis. Cell Death Dis. 2013;4:e936. doi: 10.1038/cddis.2013.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Fulda S. Smac mimetics as IAP antagonists. Semin Cell Dev Biol. 2015;39:132–138. doi: 10.1016/j.semcdb.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 248.Wagner L, Marschall V, Karl S, Cristofanon S, Zobel K, Deshayes K, et al. Smac mimetic sensitizes glioblastoma cells to Temozolomide-induced apoptosis in a RIP1- and NF-kappaB-dependent manner. Oncogene. 2013;32(8):988–997. doi: 10.1038/onc.2012.108. [DOI] [PubMed] [Google Scholar]
- 249.Chromik J, Safferthal C, Serve H, Fulda S. Smac mimetic primes apoptosis-resistant acute myeloid leukaemia cells for cytarabine-induced cell death by triggering necroptosis. Cancer Lett. 2014;344(1):101–109. doi: 10.1016/j.canlet.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 250.Belz K, Schoeneberger H, Wehner S, Weigert A, Bonig H, Klingebiel T, et al. Smac mimetic and glucocorticoids synergize to induce apoptosis in childhood ALL by promoting ripoptosome assembly. Blood. 2014;124(2):240–250. doi: 10.1182/blood-2013-05-500918. [DOI] [PubMed] [Google Scholar]
- 251.Probst BL, Liu L, Ramesh V, Li L, Sun H, Minna JD, et al. Smac mimetics increase cancer cell response to chemotherapeutics in a TNF-alpha-dependent manner. Cell Death Differ. 2010;17(10):1645–1654. doi: 10.1038/cdd.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133(4):693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 253.Chen Z, Chen J, Liu H, Dong W, Huang X, Yang D, et al. The SMAC mimetic APG-1387 sensitizes immune-mediated cell apoptosis in hepatocellular carcinoma. Front Pharmacol. 2018;9:1298. doi: 10.3389/fphar.2018.01298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Li BX, Wang HB, Qiu MZ, Luo QY, Yi HJ, Yan XL, et al. Novel smac mimetic APG-1387 elicits ovarian cancer cell killing through TNF-alpha, Ripoptosome and autophagy mediated cell death pathway. J Exp Clin Cancer Res. 2018;37(1):53. doi: 10.1186/s13046-018-0703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Kamata E, Kawamoto T, Ueha T, Hara H, Fukase N, Minoda M, et al. Synergistic effects of a Smac mimetic with doxorubicin against human osteosarcoma. Anticancer Res. 2017;37(11):6097–6106. doi: 10.21873/anticanres.12058. [DOI] [PubMed] [Google Scholar]
- 256.Lee EK, Jinesh GG, Laing NM, Choi W, McConkey DJ, Kamat AM. A Smac mimetic augments the response of urothelial cancer cells to gemcitabine and cisplatin. Cancer Biol Ther. 2013;14(9):812–822. doi: 10.4161/cbt.25326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Brumatti G, Ma C, Lalaoui N, Nguyen NY, Navarro M, Tanzer MC, et al. The caspase-8 inhibitor emricasan combines with the SMAC mimetic birinapant to induce necroptosis and treat acute myeloid leukemia. Sci Transl Med. 2016;8(339):339ra369. doi: 10.1126/scitranslmed.aad3099. [DOI] [PubMed] [Google Scholar]
- 258.Eytan DF, Snow GE, Carlson S, Derakhshan A, Saleh A, Schiltz S, et al. SMAC mimetic Birinapant plus radiation eradicates human head and neck cancers with genomic amplifications of cell death genes FADD and BIRC2. Cancer Res. 2016;76(18):5442–5454. doi: 10.1158/0008-5472.CAN-15-3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Colombo M, Marabese M, Vargiu G, Broggini M, Caiola E. Activity of Birinapant, a SMAC mimetic compound, Alone or in Combination in NSCLCs With Different Mutations. Front Oncol. 2020;10:532292. doi: 10.3389/fonc.2020.532292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Noonan AM, Cousins A, Anderson D, Zeligs KP, Bunch K, Hernandez L, et al. Matrix drug screen identifies synergistic drug combinations to augment smac mimetic activity in ovarian cancer. Cancers (Basel) 2020;12(12):3784. doi: 10.3390/cancers12123784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Hernandez LF, Dull AB, Korrapati S, Annunziata CM. Smac-mimetic enhances antitumor effect of standard chemotherapy in ovarian cancer models via caspase 8-independent mechanism. Cell Death Discov. 2021;7(1):134. doi: 10.1038/s41420-021-00511-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Lueck SC, Russ AC, Botzenhardt U, Schlenk RF, Zobel K, Deshayes K, et al. Smac mimetic induces cell death in a large proportion of primary acute myeloid leukemia samples, which correlates with defined molecular markers. Oncotarget. 2016;7(31):49539–49551. doi: 10.18632/oncotarget.10390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Seyfrid M, Marschall V, Fulda S. Reactive oxygen species contribute toward Smac mimetic/temozolomide-induced cell death in glioblastoma cells. Anti-Cancer Drugs. 2016;27(10):953–959. doi: 10.1097/CAD.0000000000000412. [DOI] [PubMed] [Google Scholar]
- 264.Hehlgans S, Oppermann J, Reichert S, Fulda S, Rodel C, Rodel F. The SMAC mimetic BV6 sensitizes colorectal cancer cells to ionizing radiation by interfering with DNA repair processes and enhancing apoptosis. Radiat Oncol. 2015;10:198. doi: 10.1186/s13014-015-0507-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Marschall V, Fulda S. Smac mimetic-induced upregulation of interferon-beta sensitizes glioblastoma to temozolomide-induced cell death. Cell Death Dis. 2015;6:e1888. doi: 10.1038/cddis.2015.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.El-Mesery M, Shaker ME, Elgaml A. The SMAC mimetic BV6 induces cell death and sensitizes different cell lines to TNF-alpha and TRAIL-induced apoptosis. Exp Biol Med (Maywood) 2016;241(18):2015–2022. doi: 10.1177/1535370216661779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Reiter M, Eckhardt I, Haferkamp A, Fulda S. Smac mimetic sensitizes renal cell carcinoma cells to interferon-alpha-induced apoptosis. Cancer Lett. 2016;375(1):1–8. doi: 10.1016/j.canlet.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 268.Huerta S, Gao X, Livingston EH, Kapur P, Sun H, Anthony T. In vitro and in vivo radiosensitization of colorectal cancer HT-29 cells by the smac mimetic JP-1201. Surgery. 2010;148(2):346–353. doi: 10.1016/j.surg.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 269.Dineen SP, Roland CL, Greer R, Carbon JG, Toombs JE, Gupta P, et al. Smac mimetic increases chemotherapy response and improves survival in mice with pancreatic cancer. Cancer Res. 2010;70(7):2852–2861. doi: 10.1158/0008-5472.CAN-09-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Runckel K, Barth MJ, Mavis C, Gu JJ, Hernandez-Ilizaliturri FJ. The SMAC mimetic LCL-161 displays antitumor activity in preclinical models of rituximab-resistant B-cell lymphoma. Blood Adv. 2018;2(23):3516–3525. doi: 10.1182/bloodadvances.2018018168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Chang YC, Kondapuram SK, Yang TH, Syed SB, Cheng SM, Lin TY, et al. The SMAC mimetic LCL161 is a direct ABCB1/MDR1-ATPase activity modulator and BIRC5/Survivin expression down-regulator in cancer cells. Toxicol Appl Pharmacol. 2020;401:115080. doi: 10.1016/j.taap.2020.115080. [DOI] [PubMed] [Google Scholar]
- 272.Yang L, Kumar B, Shen C, Zhao S, Blakaj D, Li T, et al. LCL161, a SMAC-mimetic, preferentially Radiosensitizes human papillomavirus-negative head and neck squamous cell carcinoma. Mol Cancer Ther. 2019;18(6):1025–1035. doi: 10.1158/1535-7163.MCT-18-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Brands RC, Herbst F, Hartmann S, Seher A, Linz C, Kubler AC, et al. Cytotoxic effects of SMAC-mimetic compound LCL161 in head and neck cancer cell lines. Clin Oral Investig. 2016;20(9):2325–2332. doi: 10.1007/s00784-016-1741-3. [DOI] [PubMed] [Google Scholar]
- 274.Yang D, Zhao Y, Li AY, Wang S, Wang G, Sun Y. Smac-mimetic compound SM-164 induces radiosensitization in breast cancer cells through activation of caspases and induction of apoptosis. Breast Cancer Res Treat. 2012;133(1):189–199. doi: 10.1007/s10549-011-1752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Lu J, McEachern D, Sun H, Bai L, Peng Y, Qiu S, et al. Therapeutic potential and molecular mechanism of a novel, potent, nonpeptide, Smac mimetic SM-164 in combination with TRAIL for cancer treatment. Mol Cancer Ther. 2011;10(5):902–914. doi: 10.1158/1535-7163.MCT-10-0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Zhou B, Zhang J, Chen G, You L, Zhang TP, Zhao YP. Therapy of Smac mimetic SM-164 in combination with gemcitabine for pancreatic cancer. Cancer Lett. 2013;329(1):118–124. doi: 10.1016/j.canlet.2012.10.039. [DOI] [PubMed] [Google Scholar]
- 277.Metwalli AR, Khanbolooki S, Jinesh G, Sundi D, Shah JB, Shrader M, et al. Smac mimetic reverses resistance to TRAIL and chemotherapy in human urothelial cancer cells. Cancer Biol Ther. 2010;10(9):885–892. doi: 10.4161/cbt.10.9.13237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Yang C, Ran Q, Zhou Y, Liu S, Zhao C, Yu X, et al. Doxorubicin sensitizes cancer cells to Smac mimetic via synergistic activation of the CYLD/RIPK1/FADD/caspase-8-dependent apoptosis. Apoptosis. 2020;25(5–6):441–455. doi: 10.1007/s10495-020-01604-6. [DOI] [PubMed] [Google Scholar]
- 279.Tao Z, McCall NS, Wiedemann N, Vuagniaux G, Yuan Z, Lu B. SMAC mimetic Debio 1143 and ablative radiation therapy synergize to enhance antitumor immunity against lung Cancer. Clin Cancer Res. 2019;25(3):1113–1124. doi: 10.1158/1078-0432.CCR-17-3852. [DOI] [PubMed] [Google Scholar]
- 280.Dai Y, Liu M, Tang W, Li Y, Lian J, Lawrence TS, et al. A Smac-mimetic sensitizes prostate cancer cells to TRAIL-induced apoptosis via modulating both IAPs and NF-kappaB. BMC Cancer. 2009;9:392. doi: 10.1186/1471-2407-9-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Hashim YM, Vangveravong S, Sankpal NV, Binder PS, Liu J, Goedegebuure SP, et al. The targeted SMAC mimetic SW IV-134 is a strong enhancer of standard chemotherapy in pancreatic cancer. J Exp Clin Cancer Res. 2017;36(1):14. doi: 10.1186/s13046-016-0470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Loeder S, Zenz T, Schnaiter A, Mertens D, Winkler D, Dohner H, et al. A novel paradigm to trigger apoptosis in chronic lymphocytic leukemia. Cancer Res. 2009;69(23):8977–8986. doi: 10.1158/0008-5472.CAN-09-2604. [DOI] [PubMed] [Google Scholar]
- 283.Fakler M, Loeder S, Vogler M, Schneider K, Jeremias I, Debatin KM, et al. Small molecule XIAP inhibitors cooperate with TRAIL to induce apoptosis in childhood acute leukemia cells and overcome Bcl-2-mediated resistance. Blood. 2009;113(8):1710–1722. doi: 10.1182/blood-2007-09-114314. [DOI] [PubMed] [Google Scholar]
- 284.Fingas CD, Blechacz BR, Smoot RL, Guicciardi ME, Mott J, Bronk SF, et al. A smac mimetic reduces TNF related apoptosis inducing ligand (TRAIL)-induced invasion and metastasis of cholangiocarcinoma cells. Hepatology. 2010;52(2):550–561. doi: 10.1002/hep.23729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Vellanki SH, Grabrucker A, Liebau S, Proepper C, Eramo A, Braun V, et al. Small-molecule XIAP inhibitors enhance gamma-irradiation-induced apoptosis in glioblastoma. Neoplasia. 2009;11(8):743–752. doi: 10.1593/neo.09436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Steinhart L, Belz K, Fulda S. Smac mimetic and demethylating agents synergistically trigger cell death in acute myeloid leukemia cells and overcome apoptosis resistance by inducing necroptosis. Cell Death Dis. 2013;4:e802. doi: 10.1038/cddis.2013.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Carter BZ, Mak PY, Mak DH, Shi Y, Qiu Y, Bogenberger JM, et al. Synergistic targeting of AML stem/progenitor cells with IAP antagonist birinapant and demethylating agents. J Natl Cancer Inst. 2014;106(2):djt440. doi: 10.1093/jnci/djt440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Weisberg E, Kung AL, Wright RD, Moreno D, Catley L, Ray A, et al. Potentiation of antileukemic therapies by Smac mimetic, LBW242: effects on mutant FLT3-expressing cells. Mol Cancer Ther. 2007;6(7):1951–1961. doi: 10.1158/1535-7163.MCT-06-0810. [DOI] [PubMed] [Google Scholar]
- 289.Foster FM, Owens TW, Tanianis-Hughes J, Clarke RB, Brennan K, Bundred NJ, et al. Targeting inhibitor of apoptosis proteins in combination with ErbB antagonists in breast cancer. Breast Cancer Res. 2009;11(3):R41. doi: 10.1186/bcr2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Hao Q, Tang H. Interferon-gamma and Smac mimetics synergize to induce apoptosis of lung cancer cells in a TNFalpha-independent manner. Cancer Cell Int. 2018;18:84. doi: 10.1186/s12935-018-0579-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Ding J, Qin D, Zhang Y, Li Q, Li Y, Li J. SMAC mimetic birinapant inhibits hepatocellular carcinoma growth by activating the cIAP1/TRAF3 signaling pathway. Mol Med Rep. 2020;21(3):1251–1257. doi: 10.3892/mmr.2020.10908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Lalaoui N, Merino D, Giner G, Vaillant F, Chau D, Liu L, et al. Targeting triple-negative breast cancers with the Smac-mimetic birinapant. Cell Death Differ. 2020;27(10):2768–2780. doi: 10.1038/s41418-020-0541-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Wang Y, Wang Y, Fan X, Song J, Wu H, Han J, et al. ABT-199-mediated inhibition of Bcl-2 as a potential therapeutic strategy for nasopharyngeal carcinoma. Biochem Biophys Res Commun. 2018;503(3):1214–1220. doi: 10.1016/j.bbrc.2018.07.027. [DOI] [PubMed] [Google Scholar]
- 294.Diaz-Flores E, Comeaux EQ, Kim KL, Melnik E, Beckman K, Davis KL, et al. Bcl-2 is a therapeutic target for Hypodiploid B-lineage acute lymphoblastic leukemia. Cancer Res. 2019;79(9):2339–2351. doi: 10.1158/0008-5472.CAN-18-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Guy JB, Espenel S, Louati S, Gauthier A, Garcia MA, Vial N, et al. Combining radiation to EGFR and Bcl-2 blockade: a new approach to target cancer stem cells in head and neck squamous cell carcinoma. J Cancer Res Clin Oncol. 2021;147(7):1905–1916. doi: 10.1007/s00432-021-03593-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Bate-Eya LT, den Hartog IJ, van der Ploeg I, Schild L, Koster J, Santo EE, et al. High efficacy of the BCL-2 inhibitor ABT199 (venetoclax) in BCL-2 high-expressing neuroblastoma cell lines and xenografts and rational for combination with MCL-1 inhibition. Oncotarget. 2016;7(19):27946–27958. doi: 10.18632/oncotarget.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Bierbrauer A, Jacob M, Vogler M, Fulda S. A direct comparison of selective BH3-mimetics reveals BCL-XL, BCL-2 and MCL-1 as promising therapeutic targets in neuroblastoma. Br J Cancer. 2020;122(10):1544–1551. doi: 10.1038/s41416-020-0795-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Chen J, Zhou H, Aguilar A, Liu L, Bai L, McEachern D, et al. Structure-based discovery of BM-957 as a potent small-molecule inhibitor of Bcl-2 and Bcl-xL capable of achieving complete tumor regression. J Med Chem. 2012;55(19):8502–8514. doi: 10.1021/jm3010306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Bai L, Chen J, McEachern D, Liu L, Zhou H, Aguilar A, et al. BM-1197: a novel and specific Bcl-2/Bcl-xL inhibitor inducing complete and long-lasting tumor regression in vivo. PLoS One. 2014;9(6):e99404. doi: 10.1371/journal.pone.0099404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Pan R, Hogdal LJ, Benito JM, Bucci D, Han L, Borthakur G, et al. Selective BCL-2 inhibition by ABT-199 causes on-target cell death in acute myeloid leukemia. Cancer Discov. 2014;4(3):362–375. doi: 10.1158/2159-8290.CD-13-0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Zhou Y, Liu H, Xue R, Tang W, Zhang S. BH3 mimetic ABT-199 enhances the sensitivity of gemcitabine in pancreatic Cancer in vitro and in vivo. Dig Dis Sci. 2018;63(12):3367–3375. doi: 10.1007/s10620-018-5253-7. [DOI] [PubMed] [Google Scholar]
- 302.Peirs S, Matthijssens F, Goossens S, Van de Walle I, Ruggero K, de Bock CE, et al. ABT-199 mediated inhibition of BCL-2 as a novel therapeutic strategy in T-cell acute lymphoblastic leukemia. Blood. 2014;124(25):3738–3747. doi: 10.1182/blood-2014-05-574566. [DOI] [PubMed] [Google Scholar]
- 303.Niu X, Zhao J, Ma J, Xie C, Edwards H, Wang G, et al. Binding of released Bim to Mcl-1 is a mechanism of intrinsic resistance to ABT-199 which can be overcome by combination with Daunorubicin or Cytarabine in AML cells. Clin Cancer Res. 2016;22(17):4440–4451. doi: 10.1158/1078-0432.CCR-15-3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Li Z, He S, Look AT. The MCL1-specific inhibitor S63845 acts synergistically with venetoclax/ABT-199 to induce apoptosis in T-cell acute lymphoblastic leukemia cells. Leukemia. 2019;33(1):262–266. doi: 10.1038/s41375-018-0201-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Shi YF, Liu L, He LL, Ye J, Lin ZJ, Yuan DL, et al. Combining triptolide with ABT-199 is effective against acute myeloid leukemia through reciprocal regulation of Bcl-2 family proteins and activation of the intrinsic apoptotic pathway. Cell Death Dis. 2020;11(7):555. doi: 10.1038/s41419-020-02762-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Xiufeng Z, Haijun Z, Silei B, Manman D, Yong Z, Lian Y, et al. Co-operation of ABT-199 and gemcitabine in impeding DNA damage repair and inducing cell apoptosis for synergistic therapy of T-cell acute lymphoblastic leukemia. Anti-Cancer Drugs. 2019;30(2):138–148. doi: 10.1097/CAD.0000000000000702. [DOI] [PubMed] [Google Scholar]
- 307.Wang X, Mak PY, Mu H, Tao W, Rao A, Visweswaran R, et al. Combinatorial inhibition of focal adhesion kinase and BCL-2 enhances antileukemia activity of Venetoclax in acute myeloid leukemia. Mol Cancer Ther. 2020;19(8):1636–1648. doi: 10.1158/1535-7163.MCT-19-0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Vaillant F, Merino D, Lee L, Breslin K, Pal B, Ritchie ME, et al. Targeting BCL-2 with the BH3 mimetic ABT-199 in estrogen receptor-positive breast cancer. Cancer Cell. 2013;24(1):120–129. doi: 10.1016/j.ccr.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 309.Ko TK, Chuah CT, Huang JW, Ng KP, Ong ST. The BCL2 inhibitor ABT-199 significantly enhances imatinib-induced cell death in chronic myeloid leukemia progenitors. Oncotarget. 2014;5(19):9033–9038. doi: 10.18632/oncotarget.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Yu Z, Du J, Zhao Y, Gao Y, Li Y, Zhao K, et al. A novel kinase inhibitor, LZT-106, downregulates Mcl-1 and sensitizes colorectal cancer cells to BH3 mimetic ABT-199 by targeting CDK9 and GSK-3beta signaling. Cancer Lett. 2021;498:31–41. doi: 10.1016/j.canlet.2020.10.001. [DOI] [PubMed] [Google Scholar]
- 311.Kuo HP, Ezell SA, Schweighofer KJ, Cheung LWK, Hsieh S, Apatira M, et al. Combination of Ibrutinib and ABT-199 in diffuse large B-cell lymphoma and follicular lymphoma. Mol Cancer Ther. 2017;16(7):1246–1256. doi: 10.1158/1535-7163.MCT-16-0555. [DOI] [PubMed] [Google Scholar]
- 312.Phillips DC, Jin S, Gregory GP, Zhang Q, Xue J, Zhao X, et al. A novel CDK9 inhibitor increases the efficacy of venetoclax (ABT-199) in multiple models of hematologic malignancies. Leukemia. 2020;34(6):1646–1657. doi: 10.1038/s41375-019-0652-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Zhang Y, Zhou L, Bandyopadhyay D, Sharma K, Allen AJ, Kmieciak M, et al. The covalent CDK7 inhibitor THZ1 potently induces apoptosis in multiple myeloma cells in vitro and in vivo. Clin Cancer Res. 2019;25(20):6195–6205. doi: 10.1158/1078-0432.CCR-18-3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Zhou L, Zhang Y, Sampath D, Leverson J, Dai Y, Kmieciak M, et al. Flavopiridol enhances ABT-199 sensitivity in unfavourable-risk multiple myeloma cells in vitro and in vivo. Br J Cancer. 2018;118(3):388–397. doi: 10.1038/bjc.2017.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Muenchow A, Weller S, Hinterleitner C, Malenke E, Bugl S, Wirths S, et al. The BCL-2 selective inhibitor ABT-199 sensitizes soft tissue sarcomas to proteasome inhibition by a concerted mechanism requiring BAX and NOXA. Cell Death Dis. 2020;11(8):701. doi: 10.1038/s41419-020-02910-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Zhang B, Gojo I, Fenton RG. Myeloid cell factor-1 is a critical survival factor for multiple myeloma. Blood. 2002;99(6):1885–1893. doi: 10.1182/blood.v99.6.1885. [DOI] [PubMed] [Google Scholar]
- 317.Kozopas KM, Yang T, Buchan HL, Zhou P, Craig RW. MCL1, a gene expressed in programmed myeloid cell differentiation, has sequence similarity to BCL2. Proc Natl Acad Sci U S A. 1993;90(8):3516–3520. doi: 10.1073/pnas.90.8.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Lomo J, Smeland EB, Krajewski S, Reed JC, Blomhoff HK. Expression of the Bcl-2 homologue Mcl-1 correlates with survival of peripheral blood B lymphocytes. Cancer Res. 1996;56(1):40–43. [PubMed] [Google Scholar]
- 319.Zhou P, Qian L, Bieszczad CK, Noelle R, Binder M, Levy NB, et al. Mcl-1 in transgenic mice promotes survival in a spectrum of hematopoietic cell types and immortalization in the myeloid lineage. Blood. 1998;92(9):3226–3239. [PubMed] [Google Scholar]
- 320.Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426(6967):671–676. doi: 10.1038/nature02067. [DOI] [PubMed] [Google Scholar]
- 321.Perciavalle RM, Stewart DP, Koss B, Lynch J, Milasta S, Bathina M, et al. Anti-apoptotic MCL-1 localizes to the mitochondrial matrix and couples mitochondrial fusion to respiration. Nat Cell Biol. 2012;14(6):575–583. doi: 10.1038/ncb2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Fujise K, Zhang D, Liu J, Yeh ET. Regulation of apoptosis and cell cycle progression by MCL1. Differential role of proliferating cell nuclear antigen. J Biol Chem. 2000;275(50):39458–39465. doi: 10.1074/jbc.M006626200. [DOI] [PubMed] [Google Scholar]
- 323.Jamil S, Mojtabavi S, Hojabrpour P, Cheah S, Duronio V. An essential role for MCL-1 in ATR-mediated CHK1 phosphorylation. Mol Biol Cell. 2008;19(8):3212–3220. doi: 10.1091/mbc.E07-11-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Bingle CD, Craig RW, Swales BM, Singleton V, Zhou P, Whyte MK. Exon skipping in Mcl-1 results in a bcl-2 homology domain 3 only gene product that promotes cell death. J Biol Chem. 2000;275(29):22136–22146. doi: 10.1074/jbc.M909572199. [DOI] [PubMed] [Google Scholar]
- 325.Bae J, Leo CP, Hsu SY, Hsueh AJ. MCL-1S, a splicing variant of the antiapoptotic BCL-2 family member MCL-1, encodes a proapoptotic protein possessing only the BH3 domain. J Biol Chem. 2000;275(33):25255–25261. doi: 10.1074/jbc.M909826199. [DOI] [PubMed] [Google Scholar]
- 326.Morciano G, Giorgi C, Balestra D, Marchi S, Perrone D, Pinotti M, et al. Mcl-1 involvement in mitochondrial dynamics is associated with apoptotic cell death. Mol Biol Cell. 2016;27(1):20–34. doi: 10.1091/mbc.E15-01-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Kim JH, Sim SH, Ha HJ, Ko JJ, Lee K, Bae J. MCL-1ES, a novel variant of MCL-1, associates with MCL-1L and induces mitochondrial cell death. FEBS Lett. 2009;583(17):2758–2764. doi: 10.1016/j.febslet.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 328.Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347(6220):1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 329.Fernandez-Marrero Y, Spinner S, Kaufmann T, Jost PJ. Survival control of malignant lymphocytes by anti-apoptotic MCL-1. Leukemia. 2016;30(11):2152–2159. doi: 10.1038/leu.2016.213. [DOI] [PubMed] [Google Scholar]
- 330.Sieghart W, Losert D, Strommer S, Cejka D, Schmid K, Rasoul-Rockenschaub S, et al. Mcl-1 overexpression in hepatocellular carcinoma: a potential target for antisense therapy. J Hepatol. 2006;44(1):151–157. doi: 10.1016/j.jhep.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 331.Lin J, Fu D, Dai Y, Lin J, Xu T. Mcl-1 inhibitor suppresses tumor growth of esophageal squamous cell carcinoma in a mouse model. Oncotarget. 2017;8(70):114457–114462. doi: 10.18632/oncotarget.18772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Campbell KJ, Dhayade S, Ferrari N, Sims AH, Johnson E, Mason SM, et al. MCL-1 is a prognostic indicator and drug target in breast cancer. Cell Death Dis. 2018;9(2):19. doi: 10.1038/s41419-017-0035-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Hosseini A, Espona-Fiedler M, Soto-Cerrato V, Quesada R, Perez-Tomas R, Guallar V. Molecular interactions of prodiginines with the BH3 domain of anti-apoptotic Bcl-2 family members. PLoS One. 2013;8(2):e57562. doi: 10.1371/journal.pone.0057562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Opferman JT, Iwasaki H, Ong CC, Suh H, Mizuno S, Akashi K, et al. Obligate role of anti-apoptotic MCL-1 in the survival of hematopoietic stem cells. Science. 2005;307(5712):1101–1104. doi: 10.1126/science.1106114. [DOI] [PubMed] [Google Scholar]
- 335.Abdul Rahman SF, Muniandy K, Soo YK, Tiew EYH, Tan KX, Bates TE, et al. Co-inhibition of BCL-XL and MCL-1 with selective BCL-2 family inhibitors enhances cytotoxicity of cervical cancer cell lines. Biochem Biophys Rep. 2020;22:100756. doi: 10.1016/j.bbrep.2020.100756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Szlavik Z, Csekei M, Paczal A, Szabo ZB, Sipos S, Radics G, et al. Discovery of S64315, a potent and selective Mcl-1 inhibitor. J Med Chem. 2020;63(22):13762–13795. doi: 10.1021/acs.jmedchem.0c01234. [DOI] [PubMed] [Google Scholar]
- 337.Luedtke DA, Niu X, Pan Y, Zhao J, Liu S, Edwards H, et al. Inhibition of Mcl-1 enhances cell death induced by the Bcl-2-selective inhibitor ABT-199 in acute myeloid leukemia cells. Signal Transduct Target Ther. 2017;2:17012. doi: 10.1038/sigtrans.2017.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.De Blasio A, Pratelli G, Drago-Ferrante R, Saliba C, Baldacchino S, Grech G, et al. Loss of MCL1 function sensitizes the MDA-MB-231 breast cancer cells to rh-TRAIL by increasing DR4 levels. J Cell Physiol. 2019;234(10):18432–18447. doi: 10.1002/jcp.28479. [DOI] [PubMed] [Google Scholar]
- 339.Song S, Kim S, El-Sawy ER, Cerella C, Orlikova-Boyer B, Kirsch G, et al. Anti-leukemic properties of aplysinopsin derivative ee-84 alone and combined to bh3 mimetic a-1210477. Mar Drugs. 2021;19(6):285. doi: 10.3390/md19060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Kawakami H, Huang S, Pal K, Dutta SK, Mukhopadhyay D, Sinicrope FA. Mutant BRAF upregulates MCL-1 to confer apoptosis resistance that is reversed by MCL-1 antagonism and Cobimetinib in colorectal Cancer. Mol Cancer Ther. 2016;15(12):3015–3027. doi: 10.1158/1535-7163.MCT-16-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Quentmeier H, Drexler HG, Hauer V, MacLeod RA, Pommerenke C, Uphoff CC, et al. Diffuse large B cell lymphoma cell line U-2946: model for MCL1 inhibitor testing. PLoS One. 2016;11(12):e0167599. doi: 10.1371/journal.pone.0167599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Ow TJ, Fulcher CD, Thomas C, Broin PO, Lopez A, Reyna DE, et al. Optimal targeting of BCL-family proteins in head and neck squamous cell carcinoma requires inhibition of both BCL-xL and MCL-1. Oncotarget. 2019;10(4):494–510. doi: 10.18632/oncotarget.26563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Phillips DC, Xiao Y, Lam LT, Litvinovich E, Roberts-Rapp L, Souers AJ, et al. Loss in MCL-1 function sensitizes non-Hodgkin's lymphoma cell lines to the BCL-2-selective inhibitor venetoclax (ABT-199) Blood Cancer J. 2015;5:e368. doi: 10.1038/bcj.2015.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Yi X, Sarkar A, Kismali G, Aslan B, Ayres M, Iles LR, et al. AMG-176, an Mcl-1 antagonist, shows preclinical efficacy in chronic lymphocytic leukemia. Clin Cancer Res. 2020;26(14):3856–3867. doi: 10.1158/1078-0432.CCR-19-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Carter BZ, Mak PY, Tao W, Warmoes M, Lorenzi PL, Mak D, et al. Targeting MCL-1 dysregulates cell metabolism and leukemia-stroma interactions and resensitizes acute myeloid leukemia to BCL-2 inhibition. Haematologica. 2020; 10.3324/haematol.2020.260331. [DOI] [PMC free article] [PubMed]
- 346.Zheng L, Yang W, Zhang C, Ding WJ, Zhu H, Lin NM, et al. GDC-0941 sensitizes breast cancer to ABT-737 in vitro and in vivo through promoting the degradation of Mcl-1. Cancer Lett. 2011;309(1):27–36. doi: 10.1016/j.canlet.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 347.Respondek M, Beberok A, Rok J, Rzepka Z, Wrzesniok D, Buszman E. MIM1, the Mcl-1 - specific BH3 mimetic induces apoptosis in human U87MG glioblastoma cells. Toxicol in Vitro. 2018;53:126–135. doi: 10.1016/j.tiv.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 348.Respondek M, Beberok A, Rzepka Z, Rok J, Wrzesniok D. Mcl-1 inhibitor induces cells death in BRAF-mutant Amelanotic melanoma trough GSH depletion, DNA damage and cell cycle changes. Pathol Oncol Res. 2020;26(3):1465–1474. doi: 10.1007/s12253-019-00715-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Respondek M, Beberok A, Rzepka Z, Rok J, Wrzesniok D. MIM1 induces COLO829 melanoma cell death through mitochondrial membrane breakdown, GSH depletion, and DNA damage. Fundam Clin Pharmacol. 2020;34(1):20–31. doi: 10.1111/fcp.12503. [DOI] [PubMed] [Google Scholar]
- 350.Seipel K, Schmitter K, Bacher U, Pabst T. Rationale for a combination therapy consisting of MCL1- and MEK-Inhibitors in Acute Myeloid Leukemia. Cancers (Basel) 2019;11(11):1779. doi: 10.3390/cancers11111779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Grundy M, Balakrishnan S, Fox M, Seedhouse CH, Russell NH. Genetic biomarkers predict response to dual BCL-2 and MCL-1 targeting in acute myeloid leukaemia cells. Oncotarget. 2018;9(102):37777–37789. doi: 10.18632/oncotarget.26540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Seipel K, Kopp B, Bacher U, Pabst T. BMI1-Inhibitor PTC596 in Combination with MCL1 Inhibitor S63845 or MEK Inhibitor Trametinib in the Treatment of Acute Leukemia. Cancers (Basel). 2021;13(3):581–96. doi: 10.3390/cancers13030581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Hormi M, Birsen R, Belhadj M, Huynh T, Cantero Aguilar L, Grignano E, et al. Pairing MCL-1 inhibition with venetoclax improves therapeutic efficiency of BH3-mimetics in AML. Eur J Haematol. 2020;105(5):588–596. doi: 10.1111/ejh.13492. [DOI] [PubMed] [Google Scholar]
- 354.Merino D, Whittle JR, Vaillant F, Serrano A, Gong JN, Giner G, et al. Synergistic action of the MCL-1 inhibitor S63845 with current therapies in preclinical models of triple-negative and HER2-amplified breast cancer. Sci Transl Med. 2017;9(401). 10.1126/scitranslmed.aam7049. [DOI] [PubMed]
- 355.Song X, Shen L, Tong J, Kuang C, Zeng S, Schoen RE, et al. Mcl-1 inhibition overcomes intrinsic and acquired regorafenib resistance in colorectal cancer. Theranostics. 2020;10(18):8098–8110. doi: 10.7150/thno.45363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Prukova D, Andera L, Nahacka Z, Karolova J, Svaton M, Klanova M, et al. Cotargeting of BCL2 with Venetoclax and MCL1 with S63845 is synthetically lethal in vivo in relapsed mantle cell lymphoma. Clin Cancer Res. 2019;25(14):4455–4465. doi: 10.1158/1078-0432.CCR-18-3275. [DOI] [PubMed] [Google Scholar]
- 357.Mukherjee N, Skees J, Todd KJ, West DA, Lambert KA, Robinson WA, et al. MCL1 inhibitors S63845/MIK665 plus Navitoclax synergistically kill difficult-to-treat melanoma cells. Cell Death Dis. 2020;11(6):443. doi: 10.1038/s41419-020-2646-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Sarif Z, Tolksdorf B, Fechner H, Eberle J. Mcl-1 targeting strategies unlock the proapoptotic potential of TRAIL in melanoma cells. Mol Carcinog. 2020;59(11):1256–1268. doi: 10.1002/mc.23253. [DOI] [PubMed] [Google Scholar]
- 359.Seiller C, Maiga S, Touzeau C, Bellanger C, Kervoelen C, Descamps G, et al. Dual targeting of BCL2 and MCL1 rescues myeloma cells resistant to BCL2 and MCL1 inhibitors associated with the formation of BAX/BAK hetero-complexes. Cell Death Dis. 2020;11(5):316. doi: 10.1038/s41419-020-2505-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Wong KY, Chim CS. Venetoclax, bortezomib and S63845, an MCL1 inhibitor, in multiple myeloma. J Pharm Pharmacol. 2020;72(5):728–737. doi: 10.1111/jphp.13240. [DOI] [PubMed] [Google Scholar]
- 361.Algarin EM, Diaz-Tejedor A, Mogollon P, Hernandez-Garcia S, Corchete LA, San-Segundo L, et al. Preclinical evaluation of the simultaneous inhibition of MCL-1 and BCL-2 with the combination of S63845 and venetoclax in multiple myeloma. Haematologica. 2020;105(3):e116–e120. doi: 10.3324/haematol.2018.212308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Williams MM, Elion DL, Rahman B, Hicks DJ, Sanchez V, Cook RS. Therapeutic inhibition of Mcl-1 blocks cell survival in estrogen receptor-positive breast cancers. Oncotarget. 2019;10(52):5389–5402. doi: 10.18632/oncotarget.27070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Lu X, Liang H, Orvig C, Chen ZF. Peptide and Small Molecule Inhibitors Targeting Myeloid Cell Leukemia 1 (Mcl-1) as Novel Antitumor Agents. Curr Mol Med. 2021;21(5):426–39. doi: 10.2174/1566524020666200929121016. [DOI] [PubMed] [Google Scholar]
- 364.Wei AH, Roberts AW, Spencer A, Rosenberg AS, Siegel D, Walter RB, et al. Targeting MCL-1 in hematologic malignancies: rationale and progress. Blood Rev. 2020;44:100672. doi: 10.1016/j.blre.2020.100672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Zhang H, Nakauchi Y, Kohnke T, Stafford M, Bottomly D, Thomas R, et al. Integrated analysis of patient samples identifies biomarkers for venetoclax efficacy and combination strategies in acute myeloid leukemia. Nat Cancer. 2020;1(8):826–839. doi: 10.1038/s43018-020-0103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Arulananda S, O'Brien M, Evangelista M, Jenkins LJ, Poh AR, Walkiewicz M, et al. A novel BH3-mimetic, AZD0466, targeting BCL-XL and BCL-2 is effective in pre-clinical models of malignant pleural mesothelioma. Cell Death Discov. 2021;7(1):122. doi: 10.1038/s41420-021-00505-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Letai A. S63845, an MCL-1 selective BH3 mimetic: another arrow in our quiver. Cancer Cell. 2016;30(6):834–835. doi: 10.1016/j.ccell.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 368.Bala Tannan N, Manzari MT, Herviou L, Da Silva FM, Hagen C, Kiguchi H, et al. Tumor-targeted nanoparticles improve the therapeutic index of BCL2 and MCL1 dual inhibition. Blood. 2021;137(15):2057–2069. doi: 10.1182/blood.2020008017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Michels J, Obrist F, Vitale I, Lissa D, Garcia P, Behnam-Motlagh P, et al. MCL-1 dependency of cisplatin-resistant cancer cells. Biochem Pharmacol. 2014;92(1):55–61. doi: 10.1016/j.bcp.2014.07.029. [DOI] [PubMed] [Google Scholar]
- 370.Tromp JM, Geest CR, Breij EC, Elias JA, van Laar J, Luijks DM, et al. Tipping the Noxa/Mcl-1 balance overcomes ABT-737 resistance in chronic lymphocytic leukemia. Clin Cancer Res. 2012;18(2):487–498. doi: 10.1158/1078-0432.CCR-11-1440. [DOI] [PubMed] [Google Scholar]
- 371.Bates DJ, Danilov AV, Lowrey CH, Eastman A. Vinblastine rapidly induces NOXA and acutely sensitizes primary chronic lymphocytic leukemia cells to ABT-737. Mol Cancer Ther. 2013;12(8):1504–1514. doi: 10.1158/1535-7163.MCT-12-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Jane EP, Premkumar DR, Cavaleri JM, Sutera PA, Rajasekar T, Pollack IF. Dinaciclib, a cyclin-dependent kinase inhibitor promotes proteasomal degradation of Mcl-1 and enhances ABT-737-mediated cell death in malignant human glioma cell lines. J Pharmacol Exp Ther. 2016;356(2):354–365. doi: 10.1124/jpet.115.230052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Sulkshane P, Teni T. BH3 mimetic Obatoclax (GX15-070) mediates mitochondrial stress predominantly via MCL-1 inhibition and induces autophagy-dependent necroptosis in human oral cancer cells. Oncotarget. 2017;8(36):60060–60079. doi: 10.18632/oncotarget.11085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Garner TP, Lopez A, Reyna DE, Spitz AZ, Gavathiotis E. Progress in targeting the BCL-2 family of proteins. Curr Opin Chem Biol. 2017;39:133–142. doi: 10.1016/j.cbpa.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Robin AY, Krishna Kumar K, Westphal D, Wardak AZ, Thompson GV, Dewson G, et al. Crystal structure of Bax bound to the BH3 peptide of Bim identifies important contacts for interaction. Cell Death Dis. 2015;6:e1809. doi: 10.1038/cddis.2015.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Garner TP, Reyna DE, Priyadarshi A, Chen HC, Li S, Wu Y, et al. An autoinhibited dimeric form of BAX regulates the BAX activation pathway. Mol Cell. 2016;63(3):485–497. doi: 10.1016/j.molcel.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Hadji A, Schmitt GK, Schnorenberg MR, Roach L, Hickey CM, Leak LB, et al. Preferential targeting of MCL-1 by a hydrocarbon-stapled BIM BH3 peptide. Oncotarget. 2019;10(58):6219–6233. doi: 10.18632/oncotarget.27262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Lee EF, Czabotar PE, van Delft MF, Michalak EM, Boyle MJ, Willis SN, et al. A novel BH3 ligand that selectively targets Mcl-1 reveals that apoptosis can proceed without Mcl-1 degradation. J Cell Biol. 2008;180(2):341–355. doi: 10.1083/jcb.200708096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Kritzer JA. The secret of MIM: a novel, MCL-1-specific small molecule. Chem Biol. 2012;19(9):1082–1083. doi: 10.1016/j.chembiol.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 380.Trudel S, Li ZH, Rauw J, Tiedemann RE, Wen XY, Stewart AK. Preclinical studies of the pan-Bcl inhibitor obatoclax (GX015-070) in multiple myeloma. Blood. 2007;109(12):5430–5438. doi: 10.1182/blood-2006-10-047951. [DOI] [PubMed] [Google Scholar]
- 381.Zhang M, Liu H, Guo R, Ling Y, Wu X, Li B, et al. Molecular mechanism of gossypol-induced cell growth inhibition and cell death of HT-29 human colon carcinoma cells. Biochem Pharmacol. 2003;66(1):93–103. doi: 10.1016/s0006-2952(03)00248-x. [DOI] [PubMed] [Google Scholar]
- 382.Certo M, Del Gaizo MV, Nishino M, Wei G, Korsmeyer S, Armstrong SA, et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9(5):351–365. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 383.Khan S, Zhang X, Lv D, Zhang Q, He Y, Zhang P, et al. A selective BCL-XL PROTAC degrader achieves safe and potent antitumor activity. Nat Med. 2019;25(12):1938–1947. doi: 10.1038/s41591-019-0668-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Dutta S, Ryan J, Chen TS, Kougentakis C, Letai A, Keating AE. Potent and specific peptide inhibitors of human pro-survival protein Bcl-xL. J Mol Biol. 2015;427(6 Pt B):1241–1253. doi: 10.1016/j.jmb.2014.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Mitchell WB, Pinheiro MP, Boulad N, Kaplan D, Edison MN, Psaila B, et al. Effect of thrombopoietin receptor agonists on the apoptotic profile of platelets in patients with chronic immune thrombocytopenia. Am J Hematol. 2014;89(12):E228–E234. doi: 10.1002/ajh.23832. [DOI] [PubMed] [Google Scholar]
- 386.Brinkmann K, Kashkar H. Targeting the mitochondrial apoptotic pathway: a preferred approach in hematologic malignancies? Cell Death Dis. 2014;5:e1098. doi: 10.1038/cddis.2014.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Suvarna V, Singh V, Murahari M. Current overview on the clinical update of Bcl-2 anti-apoptotic inhibitors for cancer therapy. Eur J Pharmacol. 2019;862:172655. doi: 10.1016/j.ejphar.2019.172655. [DOI] [PubMed] [Google Scholar]
- 388.Xiong H, Pradhan RS, Nada A, Krivoshik AP, Holen KD, Rhodes JW, et al. Studying navitoclax, a targeted anticancer drug, in healthy volunteers--ethical considerations and risk/benefit assessments and management. Anticancer Res. 2014;34(7):3739–3746. [PubMed] [Google Scholar]
- 389.Wilson WH, O'Connor OA, Czuczman MS, LaCasce AS, Gerecitano JF, Leonard JP, et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol. 2010;11(12):1149–1159. doi: 10.1016/S1470-2045(10)70261-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Gandhi L, Camidge DR, Ribeiro de Oliveira M, Bonomi P, Gandara D, Khaira D, et al. Phase I study of Navitoclax (ABT-263), a novel Bcl-2 family inhibitor, in patients with small-cell lung cancer and other solid tumors. J Clin Oncol. 2011;29(7):909–916. doi: 10.1200/JCO.2010.31.6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Roberts AW, Seymour JF, Brown JR, Wierda WG, Kipps TJ, Khaw SL, et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J Clin Oncol. 2012;30(5):488–496. doi: 10.1200/JCO.2011.34.7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Puglisi M, Molife LR, de Jonge MJ, Khan KH, Doorn LV, Forster MD, et al. A phase I study of the safety, pharmacokinetics and efficacy of navitoclax plus docetaxel in patients with advanced solid tumors. Future Oncol. 2021;17(21):2747–2758. doi: 10.2217/fon-2021-0140. [DOI] [PubMed] [Google Scholar]
- 393.Tolcher AW, LoRusso P, Arzt J, Busman TA, Lian G, Rudersdorf NS, et al. Safety, efficacy, and pharmacokinetics of navitoclax (ABT-263) in combination with erlotinib in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2015;76(5):1025–1032. doi: 10.1007/s00280-015-2883-8. [DOI] [PubMed] [Google Scholar]
- 394.Cleary JM, Lima CM, Hurwitz HI, Montero AJ, Franklin C, Yang J, et al. A phase I clinical trial of navitoclax, a targeted high-affinity Bcl-2 family inhibitor, in combination with gemcitabine in patients with solid tumors. Investig New Drugs. 2014;32(5):937–945. doi: 10.1007/s10637-014-0110-9. [DOI] [PubMed] [Google Scholar]
- 395.Vlahovic G, Karantza V, Wang D, Cosgrove D, Rudersdorf N, Yang J, et al. A phase I safety and pharmacokinetic study of ABT-263 in combination with carboplatin/paclitaxel in the treatment of patients with solid tumors. Investig New Drugs. 2014;32(5):976–984. doi: 10.1007/s10637-014-0116-3. [DOI] [PubMed] [Google Scholar]
- 396.Pietanza MC, Rudin CM. Novel therapeutic approaches for small cell lung cancer: the future has arrived. Curr Probl Cancer. 2012;36(3):156–173. doi: 10.1016/j.currproblcancer.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.de Vos S, Leonard JP, Friedberg JW, Zain J, Dunleavy K, Humerickhouse R, et al. Safety and efficacy of navitoclax, a BCL-2 and BCL-XL inhibitor, in patients with relapsed or refractory lymphoid malignancies: results from a phase 2a study. Leuk Lymphoma. 2021;62(4):810–818. doi: 10.1080/10428194.2020.1845332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Yang J, Pradhan RS, Rosen LS, Graham AM, Holen KD, Xiong H. Effect of rifampin on the pharmacokinetics, safety and tolerability of navitoclax (ABT-263), a dual inhibitor of Bcl-2 and Bcl-XL , in patients with cancer. J Clin Pharm Ther. 2014;39(6):680–684. doi: 10.1111/jcpt.12193. [DOI] [PubMed] [Google Scholar]
- 399.Roberts AW, Advani RH, Kahl BS, Persky D, Sweetenham JW, Carney DA, et al. Phase 1 study of the safety, pharmacokinetics, and antitumour activity of the BCL2 inhibitor navitoclax in combination with rituximab in patients with relapsed or refractory CD20+ lymphoid malignancies. Br J Haematol. 2015;170(5):669–678. doi: 10.1111/bjh.13487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Kipps TJ, Eradat H, Grosicki S, Catalano J, Cosolo W, Dyagil IS, et al. A phase 2 study of the BH3 mimetic BCL2 inhibitor navitoclax (ABT-263) with or without rituximab, in previously untreated B-cell chronic lymphocytic leukemia. Leuk Lymphoma. 2015;56(10):2826–2833. doi: 10.3109/10428194.2015.1030638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Stein MN, Goodin S, Gounder M, Gibbon D, Moss R, Portal D, et al. A phase I study of AT-101, a BH3 mimetic, in combination with paclitaxel and carboplatin in solid tumors. Investig New Drugs. 2020;38(3):855–865. doi: 10.1007/s10637-019-00807-2. [DOI] [PubMed] [Google Scholar]
- 402.Vogler M, Weber K, Dinsdale D, Schmitz I, Schulze-Osthoff K, Dyer MJ, et al. Different forms of cell death induced by putative BCL2 inhibitors. Cell Death Differ. 2009;16(7):1030–1039. doi: 10.1038/cdd.2009.48. [DOI] [PubMed] [Google Scholar]
- 403.Wroblewski D, Jiang CC, Croft A, Farrelly ML, Zhang XD, Hersey P. OBATOCLAX and ABT-737 induce ER stress responses in human melanoma cells that limit induction of apoptosis. PLoS One. 2013;8(12):e84073. doi: 10.1371/journal.pone.0084073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Heidari N, Hicks MA, Harada H. GX15-070 (obatoclax) overcomes glucocorticoid resistance in acute lymphoblastic leukemia through induction of apoptosis and autophagy. Cell Death Dis. 2010;1:e76. doi: 10.1038/cddis.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Urtishak KA, Edwards AY, Wang LS, Hudome A, Robinson BW, Barrett JS, et al. Potent obatoclax cytotoxicity and activation of triple death mode killing across infant acute lymphoblastic leukemia. Blood. 2013;121(14):2689–2703. doi: 10.1182/blood-2012-04-425033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Basit F, Cristofanon S, Fulda S. Obatoclax (GX15-070) triggers necroptosis by promoting the assembly of the necrosome on autophagosomal membranes. Cell Death Differ. 2013;20(9):1161–1173. doi: 10.1038/cdd.2013.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Su JC, Chen KF, Chen WL, Liu CY, Huang JW, Tai WT, et al. Synthesis and biological activity of obatoclax derivatives as novel and potent SHP-1 agonists. Eur J Med Chem. 2012;56:127–133. doi: 10.1016/j.ejmech.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 408.Schimmer AD, Raza A, Carter TH, Claxton D, Erba H, DeAngelo DJ, et al. A multicenter phase I/II study of obatoclax mesylate administered as a 3- or 24-hour infusion in older patients with previously untreated acute myeloid leukemia. PLoS One. 2014;9(10):e108694. doi: 10.1371/journal.pone.0108694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Langer CJ, Albert I, Ross HJ, Kovacs P, Blakely LJ, Pajkos G, et al. Randomized phase II study of carboplatin and etoposide with or without obatoclax mesylate in extensive-stage small cell lung cancer. Lung Cancer. 2014;85(3):420–428. doi: 10.1016/j.lungcan.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 410.Arellano ML, Borthakur G, Berger M, Luer J, Raza A. A phase II, multicenter, open-label study of obatoclax mesylate in patients with previously untreated myelodysplastic syndromes with anemia or thrombocytopenia. Clin Lymphoma Myeloma Leuk. 2014;14(6):534–539. doi: 10.1016/j.clml.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 411.Oki Y, Copeland A, Hagemeister F, Fayad LE, Fanale M, Romaguera J, et al. Experience with obatoclax mesylate (GX15-070), a small molecule pan-Bcl-2 family antagonist in patients with relapsed or refractory classical Hodgkin lymphoma. Blood. 2012;119(9):2171–2172. doi: 10.1182/blood-2011-11-391037. [DOI] [PubMed] [Google Scholar]
- 412.Paik PK, Rudin CM, Pietanza MC, Brown A, Rizvi NA, Takebe N, et al. A phase II study of obatoclax mesylate, a Bcl-2 antagonist, plus topotecan in relapsed small cell lung cancer. Lung Cancer. 2011;74(3):481–485. doi: 10.1016/j.lungcan.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Parikh SA, Kantarjian H, Schimmer A, Walsh W, Asatiani E, El-Shami K, et al. Phase II study of obatoclax mesylate (GX15-070), a small-molecule BCL-2 family antagonist, for patients with myelofibrosis. Clin Lymphoma Myeloma Leuk. 2010;10(4):285–289. doi: 10.3816/CLML.2010.n.059. [DOI] [PubMed] [Google Scholar]
- 414.O'Brien SM, Claxton DF, Crump M, Faderl S, Kipps T, Keating MJ, et al. Phase I study of obatoclax mesylate (GX15-070), a small molecule pan-Bcl-2 family antagonist, in patients with advanced chronic lymphocytic leukemia. Blood. 2009;113(2):299–305. doi: 10.1182/blood-2008-02-137943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Schimmer AD, O'Brien S, Kantarjian H, Brandwein J, Cheson BD, Minden MD, et al. A phase I study of the pan bcl-2 family inhibitor obatoclax mesylate in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14(24):8295–8301. doi: 10.1158/1078-0432.CCR-08-0999. [DOI] [PubMed] [Google Scholar]
- 416.Zhang L, Ming L, Yu J. BH3 mimetics to improve cancer therapy; mechanisms and examples. Drug Resist Updat. 2007;10(6):207–217. doi: 10.1016/j.drup.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Goy A, Hernandez-Ilzaliturri FJ, Kahl B, Ford P, Protomastro E, Berger M. A phase I/II study of the pan Bcl-2 inhibitor obatoclax mesylate plus bortezomib for relapsed or refractory mantle cell lymphoma. Leuk Lymphoma. 2014;55(12):2761–2768. doi: 10.3109/10428194.2014.907891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Infante JR, Dees EC, Olszanski AJ, Dhuria SV, Sen S, Cameron S, et al. Phase I dose-escalation study of LCL161, an oral inhibitor of apoptosis proteins inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2014;32(28):3103–3110. doi: 10.1200/JCO.2013.52.3993. [DOI] [PubMed] [Google Scholar]
- 419.Ikeda F, Deribe YL, Skanland SS, Stieglitz B, Grabbe C, Franz-Wachtel M, et al. SHARPIN forms a linear ubiquitin ligase complex regulating NF-kappaB activity and apoptosis. Nature. 2011;471(7340):637–641. doi: 10.1038/nature09814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Gerlach B, Cordier SM, Schmukle AC, Emmerich CH, Rieser E, Haas TL, et al. Linear ubiquitination prevents inflammation and regulates immune signalling. Nature. 2011;471(7340):591–596. doi: 10.1038/nature09816. [DOI] [PubMed] [Google Scholar]
- 421.Tokunaga F, Nakagawa T, Nakahara M, Saeki Y, Taniguchi M, Sakata S, et al. SHARPIN is a component of the NF-kappaB-activating linear ubiquitin chain assembly complex. Nature. 2011;471(7340):633–636. doi: 10.1038/nature09815. [DOI] [PubMed] [Google Scholar]
- 422.Beug ST, Tang VA, LaCasse EC, Cheung HH, Beauregard CE, Brun J, et al. Smac mimetics and innate immune stimuli synergize to promote tumor death. Nat Biotechnol. 2014;32(2):182–190. doi: 10.1038/nbt.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Borthakur G, Odenike O, Aldoss I, Rizzieri DA, Prebet T, Chen C, et al. A phase 1 study of the pan-bromodomain and extraterminal inhibitor mivebresib (ABBV-075) alone or in combination with venetoclax in patients with relapsed/refractory acute myeloid leukemia. Cancer. 2021;127(16):2943–2953. doi: 10.1002/cncr.33590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Piha-Paul SA, Sachdev JC, Barve M, LoRusso P, Szmulewitz R, Patel SP, et al. First-in-human study of Mivebresib (ABBV-075), an Oral Pan-inhibitor of Bromodomain and extra terminal proteins, in patients with relapsed/refractory solid tumors. Clin Cancer Res. 2019;25(21):6309–6319. doi: 10.1158/1078-0432.CCR-19-0578. [DOI] [PubMed] [Google Scholar]
- 425.Morschhauser F, Feugier P, Flinn IW, Gasiorowski R, Greil R, Illes A, et al. A phase 2 study of venetoclax plus R-CHOP as first-line treatment for patients with diffuse large B-cell lymphoma. Blood. 2021;137(5):600–609. doi: 10.1182/blood.2020006578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Zelenetz AD, Salles G, Mason KD, Casulo C, Le Gouill S, Sehn LH, et al. Venetoclax plus R- or G-CHOP in non-Hodgkin lymphoma: results from the CAVALLI phase 1b trial. Blood. 2019;133(18):1964–1976. doi: 10.1182/blood-2018-11-880526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Zinzani PL, Flinn IW, Yuen SLS, Topp MS, Rusconi C, Fleury I, et al. Venetoclax-rituximab with or without bendamustine vs bendamustine-rituximab in relapsed/refractory follicular lymphoma. Blood. 2020;136(23):2628–2637. doi: 10.1182/blood.2020005588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Izutsu K, Yamamoto K, Kato K, Ishikawa T, Fukuhara N, Terui Y, et al. Phase 1/2 study of venetoclax, a BCL-2 inhibitor, in Japanese patients with relapsed or refractory chronic lymphocytic leukemia and small lymphocytic lymphoma. Int J Hematol. 2021;113(3):370–380. doi: 10.1007/s12185-020-03024-3. [DOI] [PubMed] [Google Scholar]
- 429.Flinn IW, Gribben JG, Dyer MJS, Wierda W, Maris MB, Furman RR, et al. Phase 1b study of venetoclax-obinutuzumab in previously untreated and relapsed/refractory chronic lymphocytic leukemia. Blood. 2019;133(26):2765–2775. doi: 10.1182/blood-2019-01-896290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Wei AH, Strickland SA, Jr, Hou JZ, Fiedler W, Lin TL, Walter RB, et al. Venetoclax combined with low-dose Cytarabine for previously untreated patients with acute myeloid leukemia: results from a phase Ib/II study. J Clin Oncol. 2019;37(15):1277–1284. doi: 10.1200/JCO.18.01600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Jain N, Keating M, Thompson P, Ferrajoli A, Burger J, Borthakur G, et al. Ibrutinib and Venetoclax for first-line treatment of CLL. N Engl J Med. 2019;380(22):2095–2103. doi: 10.1056/NEJMoa1900574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.de Vos S, Swinnen LJ, Wang D, Reid E, Fowler N, Cordero J, et al. Venetoclax, bendamustine, and rituximab in patients with relapsed or refractory NHL: a phase Ib dose-finding study. Ann Oncol. 2018;29(9):1932–1938. doi: 10.1093/annonc/mdy256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Seymour JF, Kipps TJ, Eichhorst B, Hillmen P, D'Rozario J, Assouline S, et al. Venetoclax-rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2018;378(12):1107–1120. doi: 10.1056/NEJMoa1713976. [DOI] [PubMed] [Google Scholar]
- 434.Cramer P, von Tresckow J, Bahlo J, Robrecht S, Langerbeins P, Al-Sawaf O, et al. Bendamustine followed by obinutuzumab and venetoclax in chronic lymphocytic leukaemia (CLL2-BAG): primary endpoint analysis of a multicentre, open-label, phase 2 trial. Lancet Oncol. 2018;19(9):1215–1228. doi: 10.1016/S1470-2045(18)30414-5. [DOI] [PubMed] [Google Scholar]
- 435.Roberts AW, Davids MS, Pagel JM, Kahl BS, Puvvada SD, Gerecitano JF, et al. Targeting BCL2 with Venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374(4):311–322. doi: 10.1056/NEJMoa1513257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Barr PM, Robak T, Owen C, Tedeschi A, Bairey O, Bartlett NL, et al. Sustained efficacy and detailed clinical follow-up of first-line ibrutinib treatment in older patients with chronic lymphocytic leukemia: extended phase 3 results from RESONATE-2. Haematologica. 2018;103(9):1502–1510. doi: 10.3324/haematol.2018.192328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Byrd JC, Brown JR, O'Brien S, Barrientos JC, Kay NE, Reddy NM, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371(3):213–223. doi: 10.1056/NEJMoa1400376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Cartron G, de Guibert S, Dilhuydy MS, Morschhauser F, Leblond V, Dupuis J, et al. Obinutuzumab (GA101) in relapsed/refractory chronic lymphocytic leukemia: final data from the phase 1/2 GAUGUIN study. Blood. 2014;124(14):2196–2202. doi: 10.1182/blood-2014-07-586610. [DOI] [PubMed] [Google Scholar]
- 439.Farooqui MZ, Valdez J, Martyr S, Aue G, Saba N, Niemann CU, et al. Ibrutinib for previously untreated and relapsed or refractory chronic lymphocytic leukaemia with TP53 aberrations: a phase 2, single-arm trial. Lancet Oncol. 2015;16(2):169–176. doi: 10.1016/S1470-2045(14)71182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Moreau P, Chanan-Khan A, Roberts AW, Agarwal AB, Facon T, Kumar S, et al. Promising efficacy and acceptable safety of venetoclax plus bortezomib and dexamethasone in relapsed/refractory MM. Blood. 2017;130(22):2392–2400. doi: 10.1182/blood-2017-06-788323. [DOI] [PubMed] [Google Scholar]
- 441.Stilgenbauer S, Eichhorst B, Schetelig J, Coutre S, Seymour JF, Munir T, et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: a multicentre, open-label, phase 2 study. Lancet Oncol. 2016;17(6):768–778. doi: 10.1016/S1470-2045(16)30019-5. [DOI] [PubMed] [Google Scholar]
- 442.Stilgenbauer S, Eichhorst B, Schetelig J, Hillmen P, Seymour JF, Coutre S, et al. Venetoclax for patients with chronic lymphocytic leukemia with 17p deletion: results from the full population of a phase II pivotal trial. J Clin Oncol. 2018;36(19):1973–1980. doi: 10.1200/JCO.2017.76.6840. [DOI] [PubMed] [Google Scholar]
- 443.Roberts AW, Ma S, Kipps TJ, Coutre SE, Davids MS, Eichhorst B, et al. Efficacy of venetoclax in relapsed chronic lymphocytic leukemia is influenced by disease and response variables. Blood. 2019;134(2):111–122. doi: 10.1182/blood.2018882555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Konopleva M, Pollyea DA, Potluri J, Chyla B, Hogdal L, Busman T, et al. Efficacy and biological correlates of response in a phase II study of Venetoclax monotherapy in patients with acute myelogenous leukemia. Cancer Discov. 2016;6(10):1106–1117. doi: 10.1158/2159-8290.CD-16-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Leverson JD, Sampath D, Souers AJ, Rosenberg SH, Fairbrother WJ, Amiot M, et al. Found in translation: how preclinical research is guiding the clinical development of the BCL2-selective inhibitor Venetoclax. Cancer Discov. 2017;7(12):1376–1393. doi: 10.1158/2159-8290.CD-17-0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Davids MS, Roberts AW, Seymour JF, Pagel JM, Kahl BS, Wierda WG, et al. Phase I first-in-human study of Venetoclax in patients with relapsed or refractory non-Hodgkin lymphoma. J Clin Oncol. 2017;35(8):826–833. doi: 10.1200/JCO.2016.70.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Roberts AW, Huang D. Targeting BCL2 with BH3 mimetics: basic science and clinical application of Venetoclax in chronic lymphocytic leukemia and related B cell malignancies. Clin Pharmacol Ther. 2017;101(1):89–98. doi: 10.1002/cpt.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Ruefli-Brasse A, Reed JC. Therapeutics targeting Bcl-2 in hematological malignancies. Biochem J. 2017;474(21):3643–3657. doi: 10.1042/BCJ20170080. [DOI] [PubMed] [Google Scholar]
- 449.Seymour JF, Ma S, Brander DM, Choi MY, Barrientos J, Davids MS, et al. Venetoclax plus rituximab in relapsed or refractory chronic lymphocytic leukaemia: a phase 1b study. Lancet Oncol. 2017;18(2):230–240. doi: 10.1016/S1470-2045(17)30012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Zhang H, Guttikonda S, Roberts L, Uziel T, Semizarov D, Elmore SW, et al. Mcl-1 is critical for survival in a subgroup of non-small-cell lung cancer cell lines. Oncogene. 2011;30(16):1963–1968. doi: 10.1038/onc.2010.559. [DOI] [PubMed] [Google Scholar]
- 451.Xiao Y, Nimmer P, Sheppard GS, Bruncko M, Hessler P, Lu X, et al. MCL-1 is a key determinant of breast Cancer cell survival: validation of MCL-1 dependency utilizing a highly selective small molecule inhibitor. Mol Cancer Ther. 2015;14(8):1837–1847. doi: 10.1158/1535-7163.MCT-14-0928. [DOI] [PubMed] [Google Scholar]
- 452.Yecies D, Carlson NE, Deng J, Letai A. Acquired resistance to ABT-737 in lymphoma cells that up-regulate MCL-1 and BFL-1. Blood. 2010;115(16):3304–3313. doi: 10.1182/blood-2009-07-233304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Wei SH, Dong K, Lin F, Wang X, Li B, Shen JJ, et al. Inducing apoptosis and enhancing chemosensitivity to gemcitabine via RNA interference targeting Mcl-1 gene in pancreatic carcinoma cell. Cancer Chemother Pharmacol. 2008;62(6):1055–1064. doi: 10.1007/s00280-008-0697-7. [DOI] [PubMed] [Google Scholar]
- 454.Wertz IE, Kusam S, Lam C, Okamoto T, Sandoval W, Anderson DJ, et al. Sensitivity to antitubulin chemotherapeutics is regulated by MCL1 and FBW7. Nature. 2011;471(7336):110–114. doi: 10.1038/nature09779. [DOI] [PubMed] [Google Scholar]
- 455.Akagi H, Higuchi H, Sumimoto H, Igarashi T, Kabashima A, Mizuguchi H, et al. Suppression of myeloid cell leukemia-1 (Mcl-1) enhances chemotherapy-associated apoptosis in gastric cancer cells. Gastric Cancer. 2013;16(1):100–110. doi: 10.1007/s10120-012-0153-6. [DOI] [PubMed] [Google Scholar]
- 456.Thomas RL, Roberts DJ, Kubli DA, Lee Y, Quinsay MN, Owens JB, et al. Loss of MCL-1 leads to impaired autophagy and rapid development of heart failure. Genes Dev. 2013;27(12):1365–1377. doi: 10.1101/gad.215871.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Vick B, Weber A, Urbanik T, Maass T, Teufel A, Krammer PH, et al. Knockout of myeloid cell leukemia-1 induces liver damage and increases apoptosis susceptibility of murine hepatocytes. Hepatology. 2009;49(2):627–636. doi: 10.1002/hep.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Arbour N, Vanderluit JL, Le Grand JN, Jahani-Asl A, Ruzhynsky VA, Cheung EC, et al. Mcl-1 is a key regulator of apoptosis during CNS development and after DNA damage. J Neurosci. 2008;28(24):6068–6078. doi: 10.1523/JNEUROSCI.4940-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Opferman JT, Kothari A. Anti-apoptotic BCL-2 family members in development. Cell Death Differ. 2018;25(1):37–45. doi: 10.1038/cdd.2017.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Lee EF, Czabotar PE, Yang H, Sleebs BE, Lessene G, Colman PM, et al. Conformational changes in Bcl-2 pro-survival proteins determine their capacity to bind ligands. J Biol Chem. 2009;284(44):30508–30517. doi: 10.1074/jbc.M109.040725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Abulwerdi F, Liao C, Liu M, Azmi AS, Aboukameel A, Mady AS, et al. A novel small-molecule inhibitor of mcl-1 blocks pancreatic cancer growth in vitro and in vivo. Mol Cancer Ther. 2014;13(3):565–575. doi: 10.1158/1535-7163.MCT-12-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Ji T, Ding Y, Zhao Y, Wang J, Qin H, Liu X, et al. Peptide assembly integration of fibroblast-targeting and cell-penetration features for enhanced antitumor drug delivery. Adv Mater. 2015;27(11):1865–1873. doi: 10.1002/adma.201404715. [DOI] [PubMed] [Google Scholar]
- 463.Soukasene S, Toft DJ, Moyer TJ, Lu H, Lee HK, Standley SM, et al. Antitumor activity of peptide amphiphile nanofiber-encapsulated camptothecin. ACS Nano. 2011;5(11):9113–9121. doi: 10.1021/nn203343z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Ben-Nun Y, Fichman G, Adler-Abramovich L, Turk B, Gazit E, Blum G. Cathepsin nanofiber substrates as potential agents for targeted drug delivery. J Control Release. 2017;257:60–67. doi: 10.1016/j.jconrel.2016.11.028. [DOI] [PubMed] [Google Scholar]
- 465.Zhang P, Cheetham AG, Lin YA, Cui H. Self-assembled tat nanofibers as effective drug carrier and transporter. ACS Nano. 2013;7(7):5965–5977. doi: 10.1021/nn401667z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Lin R, Cheetham AG, Zhang P, Lin YA, Cui H. Supramolecular filaments containing a fixed 41% paclitaxel loading. Chem Commun (Camb) 2013;49(43):4968–4970. doi: 10.1039/c3cc41896k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Chen Z, Xing L, Fan Q, Cheetham AG, Lin R, Holt B, et al. Drug-bearing supramolecular filament hydrogels as anti-inflammatory agents. Theranostics. 2017;7(7):2003–2014. doi: 10.7150/thno.19404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Mazza M, Hadjidemetriou M, de Lazaro I, Bussy C, Kostarelos K. Peptide nanofiber complexes with siRNA for deep brain gene silencing by stereotactic neurosurgery. ACS Nano. 2015;9(2):1137–1149. doi: 10.1021/nn5044838. [DOI] [PubMed] [Google Scholar]
- 469.Lock LL, Li Y, Mao X, Chen H, Staedtke V, Bai R, et al. One-component supramolecular filament hydrogels as Theranostic label-free magnetic resonance imaging agents. ACS Nano. 2017;11(1):797–805. doi: 10.1021/acsnano.6b07196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Lock LL, Reyes CD, Zhang P, Cui H. Tuning cellular uptake of molecular probes by rational Design of Their Assembly into supramolecular Nanoprobes. J Am Chem Soc. 2016;138(10):3533–3540. doi: 10.1021/jacs.6b00073. [DOI] [PubMed] [Google Scholar]
- 471.Li Y, Lock LL, Wang Y, Ou SH, Stern D, Schon A, et al. Bioinspired supramolecular engineering of self-assembling immunofibers for high affinity binding of immunoglobulin G. Biomaterials. 2018;178:448–457. doi: 10.1016/j.biomaterials.2018.04.032. [DOI] [PubMed] [Google Scholar]
- 472.Zhang X, Xu X, Li Y, Hu C, Zhang Z, Gu Z. Virion-like membrane-breaking nanoparticles with tumor-activated cell-and-tissue dual-penetration conquer impermeable cancer. Adv Mater. 2018;30(27):e1707240. doi: 10.1002/adma.201707240. [DOI] [PubMed] [Google Scholar]
- 473.Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33(9):941–951. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Newcomb CJ, Sur S, Ortony JH, Lee OS, Matson JB, Boekhoven J, et al. Cell death versus cell survival instructed by supramolecular cohesion of nanostructures. Nat Commun. 2014;5:3321. doi: 10.1038/ncomms4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Schnorenberg MR, Bellairs JA, Samaeekia R, Acar H, Tirrell MV, JL LB. Activating the intrinsic pathway of apoptosis using bim bh3 peptides delivered by peptide amphiphiles with endosomal release. Materials (Basel) 2019;12(16):2567. doi: 10.3390/ma12162567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Missirlis D, Teesalu T, Black M, Tirrell M. The non-peptidic part determines the internalization mechanism and intracellular trafficking of peptide amphiphiles. PLoS One. 2013;8(1):e54611. doi: 10.1371/journal.pone.0054611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Acar H, Ting JM, Srivastava S, LaBelle JL, Tirrell MV. Molecular engineering solutions for therapeutic peptide delivery. Chem Soc Rev. 2017;46(21):6553–6569. doi: 10.1039/c7cs00536a. [DOI] [PubMed] [Google Scholar]
- 478.Meyer DE, Kong GA, Dewhirst MW, Zalutsky MR, Chilkoti A. Targeting a genetically engineered elastin-like polypeptide to solid tumors by local hyperthermia. Cancer Res. 2001;61(4):1548–1554. [PubMed] [Google Scholar]
- 479.Dreher MR, Liu W, Michelich CR, Dewhirst MW, Chilkoti A. Thermal cycling enhances the accumulation of a temperature-sensitive biopolymer in solid tumors. Cancer Res. 2007;67(9):4418–4424. doi: 10.1158/0008-5472.CAN-06-4444. [DOI] [PubMed] [Google Scholar]
- 480.Liu W, Dreher MR, Furgeson DY, Peixoto KV, Yuan H, Zalutsky MR, et al. Tumor accumulation, degradation and pharmacokinetics of elastin-like polypeptides in nude mice. J Control Release. 2006;116(2):170–178. doi: 10.1016/j.jconrel.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 481.Issels RD. Regional hyperthermia combined with systemic chemotherapy of locally advanced sarcomas: preclinical aspects and clinical results. Recent Results Cancer Res. 1995;138:81–90. doi: 10.1007/978-3-642-78768-3_10. [DOI] [PubMed] [Google Scholar]
- 482.Feyerabend T, Steeves R, Wiedemann GJ, Richter E, Robins HI. Rationale and clinical status of local hyperthermia, radiation, and chemotherapy in locally advanced malignancies. Anticancer Res. 1997;17(4B):2895–2897. [PubMed] [Google Scholar]
- 483.van Vulpen M, Raaymakers BW, de Leeuw AA, van de Kamer JB, van Moorselaar RJ, Hobbelink MG, et al. Prostate perfusion in patients with locally advanced prostate carcinoma treated with different hyperthermia techniques. J Urol. 2002;168(4 Pt 1):1597–1602. doi: 10.1016/S0022-5347(05)64527-2. [DOI] [PubMed] [Google Scholar]
- 484.Issels RD. Regional hyperthermia in high-risk soft tissue sarcomas. Curr Opin Oncol. 2008;20(4):438–443. doi: 10.1097/CCO.0b013e3283025e50. [DOI] [PubMed] [Google Scholar]
- 485.Dinca A, Chien WM, Chin MT. Intracellular delivery of proteins with cell-penetrating peptides for therapeutic uses in human disease. Int J Mol Sci. 2016;17(2):263. doi: 10.3390/ijms17020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Morris MC, Depollier J, Mery J, Heitz F, Divita G. A peptide carrier for the delivery of biologically active proteins into mammalian cells. Nat Biotechnol. 2001;19(12):1173–1176. doi: 10.1038/nbt1201-1173. [DOI] [PubMed] [Google Scholar]
- 487.Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285(5433):1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- 488.Cao G, Pei W, Ge H, Liang Q, Luo Y, Sharp FR, et al. In vivo delivery of a Bcl-xL fusion protein containing the TAT protein transduction domain protects against ischemic brain injury and neuronal apoptosis. J Neurosci. 2002;22(13):5423–5431. doi: 10.1523/JNEUROSCI.22-13-05423.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Andreev OA, Engelman DM, Reshetnyak YK. Targeting acidic diseased tissue: new technology based on use of the pH (low) insertion peptide (pHLIP) Chim Oggi. 2009;27(2):34–37. [PMC free article] [PubMed] [Google Scholar]
- 490.Andreev OA, Engelman DM, Reshetnyak YK. pH-sensitive membrane peptides (pHLIPs) as a novel class of delivery agents. Mol Membr Biol. 2010;27(7):341–352. doi: 10.3109/09687688.2010.509285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Deacon JC, Engelman DM, Barrera FN. Targeting acidity in diseased tissues: mechanism and applications of the membrane-inserting peptide, pHLIP. Arch Biochem Biophys. 2015;565:40–48. doi: 10.1016/j.abb.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Banerjee A, Onyuksel H. Peptide delivery using phospholipid micelles. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2012;4(5):562–574. doi: 10.1002/wnan.1185. [DOI] [PubMed] [Google Scholar]
- 493.Guo J, Wu T, Ping Q, Chen Y, Shen J, Jiang G. Solubilization and pharmacokinetic behaviors of sodium cholate/lecithin-mixed micelles containing cyclosporine a. Drug Deliv. 2005;12(1):35–39. doi: 10.1080/10717540590889691. [DOI] [PubMed] [Google Scholar]
- 494.Onyuksel H, Jeon E, Rubinstein I. Nanomicellar paclitaxel increases cytotoxicity of multidrug resistant breast cancer cells. Cancer Lett. 2009;274(2):327–330. doi: 10.1016/j.canlet.2008.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Lim SB, Rubinstein I, Sadikot RT, Artwohl JE, Onyuksel H. A novel peptide nanomedicine against acute lung injury: GLP-1 in phospholipid micelles. Pharm Res. 2011;28(3):662–672. doi: 10.1007/s11095-010-0322-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Krishnadas A, Onyuksel H, Rubinstein I. Interactions of VIP, secretin and PACAP(1-38) with phospholipids: a biological paradox revisited. Curr Pharm Des. 2003;9(12):1005–1012. doi: 10.2174/1381612033455206. [DOI] [PubMed] [Google Scholar]
- 497.Onyuksel H, Sejourne F, Suzuki H, Rubinstein I. Human VIP-alpha: a long-acting, biocompatible and biodegradable peptide nanomedicine for essential hypertension. Peptides. 2006;27(9):2271–2275. doi: 10.1016/j.peptides.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 498.Kuzmis A, Lim SB, Desai E, Jeon E, Lee BS, Rubinstein I, et al. Micellar nanomedicine of human neuropeptide Y. Nanomedicine. 2011;7(4):464–471. doi: 10.1016/j.nano.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Banerjee A, Onyuksel H. Human pancreatic polypeptide in a phospholipid-based micellar formulation. Pharm Res. 2012;29(6):1698–1711. doi: 10.1007/s11095-012-0718-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Reubi JC. Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocr Rev. 2003;24(4):389–427. doi: 10.1210/er.2002-0007. [DOI] [PubMed] [Google Scholar]
- 501.Liu S, Pan J, Liu J, Ma Y, Qiu F, Mei L, et al. Dynamically PEGylated and borate-coordination-polymer-coated Polydopamine nanoparticles for synergetic tumor-targeted, Chemo-Photothermal Combination Therapy. Small. 2018;14(13):e1703968. doi: 10.1002/smll.201703968. [DOI] [PubMed] [Google Scholar]
- 502.Choi JY, Gupta B, Ramasamy T, Jeong JH, Jin SG, Choi HG, et al. PEGylated polyaminoacid-capped mesoporous silica nanoparticles for mitochondria-targeted delivery of celastrol in solid tumors. Colloids Surf B Biointerfaces. 2018;165:56–66. doi: 10.1016/j.colsurfb.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 503.Tomoda K, Chiang HC, Kozak KR, Kwon GS. Injectable (−)-gossypol-loaded Pluronic P85 micelles for cancer chemoradiotherapy. Int J Radiat Biol. 2017;93(4):402–406. doi: 10.1080/09553002.2016.1257833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Tomoda K, Chiang C, Kozak KR, Kwon GS. Examination of gossypol-Pluronic micelles as potential Radiosensitizers. AAPS J. 2015;17(6):1369–1375. doi: 10.1208/s12248-015-9809-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Chen S, Ren Y, Duan P. Biomimetic nanoparticle loading obatoclax mesylate for the treatment of non-small-cell lung cancer (NSCLC) through suppressing Bcl-2 signaling. Biomed Pharmacother. 2020;129:110371. doi: 10.1016/j.biopha.2020.110371. [DOI] [PubMed] [Google Scholar]
- 506.Oakes SA, Scorrano L, Opferman JT, Bassik MC, Nishino M, Pozzan T, et al. Proapoptotic BAX and BAK regulate the type 1 inositol trisphosphate receptor and calcium leak from the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2005;102(1):105–110. doi: 10.1073/pnas.0408352102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Maiuri MC, Criollo A, Tasdemir E, Vicencio JM, Tajeddine N, Hickman JA, et al. BH3-only proteins and BH3 mimetics induce autophagy by competitively disrupting the interaction between Beclin 1 and Bcl-2/Bcl-X(L) Autophagy. 2007;3(4):374–376. doi: 10.4161/auto.4237. [DOI] [PubMed] [Google Scholar]
- 508.Pedro JM, Wei Y, Sica V, Maiuri MC, Zou Z, Kroemer G, et al. BAX and BAK1 are dispensable for ABT-737-induced dissociation of the BCL2-BECN1 complex and autophagy. Autophagy. 2015;11(3):452–459. doi: 10.1080/15548627.2015.1017191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Koehler BC, Scherr AL, Lorenz S, Elssner C, Kautz N, Welte S, et al. Pan-Bcl-2 inhibitor obatoclax delays cell cycle progression and blocks migration of colorectal cancer cells. PLoS One. 2014;9(9):e106571. doi: 10.1371/journal.pone.0106571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Zhong D, Gu C, Shi L, Xun T, Li X, Liu S, et al. Obatoclax induces G1/G0-phase arrest via p38/p21(waf1/Cip1) signaling pathway in human esophageal cancer cells. J Cell Biochem. 2014;115(9):1624–1635. doi: 10.1002/jcb.24829. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and materials cited herein are available upon request.