Abstract
Background
Since the emergency use approval of different types of COVID-19 vaccines, several safety concerns have been raised regarding its early and delayed impact on the nervous system.
Objective
This study aims to systematically review the reported cases of CNS demyelination in association with COVID-19 vaccination, which has not been performed, to our knowledge.
Methods
A systematic review was performed by screening published articles and preprints of cases of CNS demyelination in association with COVID-19 vaccines in PubMed, SCOPUS, EMBASE, Google Scholar, Ovid and medRxiv databases, until September 30, 2021. This study followed PRISMA guidelines. Descriptive findings of reported cases were reviewed and stratified by demographic and clinical findings, diagnostic work-up, management, and overall outcome.
Results
A total of 32 cases were identified, with female predominance (68.8%) and median age of 44 years. Eleven cases were reported after Pfizer vaccine, 8 following AstraZeneca vaccine, 6 following Moderna, 5 following Sinovac/ Sinopharm vaccines, and one following each of Sputnik and Johnson&Johnson vaccines. The majority of cases (71.8%) occurred after the first dose of the vaccine, with neurological symptoms manifesting after a median of 9 days. The most common reported presentations were transverse myelitis (12/32) and MS-like pictures (first diagnosis or a relapse) in another 12/32 cases, followed by ADEM- like (5/32), and NMOSD- like (3/32) presentations. History of a previous immune-mediated disease was reported in 17/32 (53.1%) cases. The mRNA-based vaccines resulted in the greatest number of demyelinating syndromes (17/32), followed by viral vector vaccines (10/32), and inactivated vaccines (5/32). Most MS-like episodes (9/12) were triggered by mRNA-based vaccines, while TM occurred following both viral vector and mRNA-based vaccines. Management included high dose methylprednisolone, PLEX, IVIg, or a combination of those, with a favorable outcome in the majority of case; marked/complete improvement (25/32) or stabilized/ partial recovery in the remaining cases.
Conclusion
This systematic review identified few cases of CNS demyelination following all types of approved COVID-19 vaccines so far. Clinical presentation was heterogenous, mainly following the first dose, however, half of the reported cases had a history of immune-mediated disease. Favorable outcome was observed in most cases. We suggest long-term post-marketing surveillance for these cases, to assess for causality, and ensure the safety of COVID-19 vaccines.
Keywords: COVID-19, SARS-CoV-2, Vaccine, Demyelinating disease, Multiple sclerosis, Transverse myelitis
1. Introduction
Coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), had a devastating impact on public health, global economy, and social life worldwide. In response, there has been an unprecedented effort for the rapid development of vaccines, as the most effective tool in reducing morbidity and mortality (World Health Organisation, 2021).
Despite the challenges related to the development of the vaccine, an emergency use approval has been granted for COVID-19 vaccines by the end of 2020, by different regulatory authorities around the world before the completion of conventional phases of clinical trials.
Currently, there are four types of vaccines against COVID-19; whole virus (live attenuated, inactivated), nucleic acid (mRNA, DNA), viral vector (non-replicating, replicating), and protein-based (subunit, virus-like particle) vaccines. Whole virus vaccines use a weakened or inactivated form of SARS-CoV-2 to trigger protective immunity; the nucleic acid vaccines introduce mRNA or DNA coding for SARS-CoV-2 spike protein into the cells, to induce cells to produce antibodies; viral vector vaccines use a chemically weakened virus (e.g. adenovirus) to insert the code for SARS-CoV-2 antigens into the cells; while protein subunit vaccines are based on the Spike protein or its antigenic fragments (Nagy and Alhatlani, 2021).
As of July 2021, there are 18 approved COVID-19 vaccines in use around the world, 184 COVID-19 vaccine candidates in pre-clinical development, and 105 in clinical development (Ndwandwe and Wiysonge, 2021). Although initial data on efficacy and safety were encouraging, several concerns have been raised regarding its immediate, intermediate, and long-term sequelae. The commonly reported adverse events are usually mild and self-limited, including injection site reaction, headache, fever, fatigue, and myalgia (Hernández et al., 2021). However, and as global vaccination advanced, several cases of neurological syndromes have been reported in temporal relationship with the vaccination, although causality could not be made with absolute certainty (Goss et al., 2021; Lu et al., 2021a).
Interim reports of safety data from the clinical trials of several approved vaccines have been published. Recombinant ChAdOX1 nCoV-19 vaccine had been associated with three instances of acute transverse myelitis (ATM) during the trial phase (Mahase, 2020; Ling et al., 2021). Moreover, Centers for Disease Control (CDC)’s Vaccine Adverse Event Reporting System (VAERS) reported neurological complications in relation to Pfizer-BioNTech, Moderna and Johnson & Johnson's COVID-19 vaccines in 254 cases (2.69%); of which, 9 had ATM, and 6 had acute disseminated encephalomyelitis (ADEM) (Goss et al., 2021).
In literature, a wide variety of autoimmune neurological syndromes have been reported following different types of viral vaccinations. The most commonly reported vaccines that were associated with CNS demyelination were influenza, human papilloma virus (HPV), hepatitis A or B, rabies, measles, and rubella (Karussis and Petrou, 2014a).
As it is crucial to evaluate the long-term post-marketing safety data, particularly events affecting the nervous system, we systematically reviewed the current literature of reported cases of CNS demyelination post-COVID-19 vaccination. This review described their clinical, laboratory, and imaging findings, in addition to their diagnostic work-up and management, which has not been performed, to the best of our knowledge.
2. Methods
2.1. Design
This systematic review was carried out in accordance with Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines. Data from PubMed, SCOPUS, EMBASE, Google Scholar, Ovid, and medRxiv databases were searched. We aimed to identify relevant articles reporting any form of CNS demyelination in association with any type of approved COVID-19 vaccines, until September 30, 2021.
2.2. Search strategy
A pre-specified searching strategy consisted of a variation of keywords of relevant medical subject headings (MeSH) and keywords, including: “COVID-19”, “SARS-CoV-2”, “vaccine”, “vaccination”, “mRNA vaccine”, “AstraZeneca COVID-19 vaccine”, “ChAdOx1 nCoV-19 vaccine”, “AZD1222 vaccine”, “Janssen COVID-19 vaccine”, “Johnson & Johnson COVID-19 vaccine”, “Ad26.COV2 vaccine”, “Pfizer-BioNTech COVID-19 vaccine”, “BNT162b2”, “Moderna COVID-19 vaccine”, “ Sinovac COVID-19 vaccine”, “demyelinating disease”, “demyelination”, “acute disseminated encephalomyelitis”, “transverse myelitis”, “multiple sclerosis”, “neuromyelitis optica”. Moreover, we hand-searched additional relevant articles that were referenced in the included studies.
2.3. Inclusion criteria
We included all peer-reviewed publications and preprints that reported any form of CNS demyelination in association with any type of COVID-19 vaccines, including but not limited to case reports and case series that met the following criteria: (i) reports of early or delayed CNS demyelination after COVID-19 vaccine; (ii) reports of possible association of cases fulfilling the diagnostic criteria of multiple sclerosis (MS), transverse myelitis (TM), neuromyelitis optica spectrum disorder (NMOSD), or myelin oligodendrocyte glycoprotein antibody disease (MOGAD), and COVID-19 vaccines; and (iii) studies published in English.
2.4. Exclusion criteria
Reports that lacked supporting imaging findings, laboratory, or clinical evidence of CNS demyelination after vaccination were excluded from this study. We also excluded review papers, viewpoints, commentaries, and editorials, unless reporting a case of demyelination. Reports of CNS demyelination during clinical trials were also excluded due to lack of clinical data. The review was restricted to studies published in English.
2.5. Data extraction
Titles and abstracts of all identified studies were screened for relevance by the two reviewers, followed by full-text screening of the deemed eligible articles. The same reviewers then extracted data on the following parameters: article title, authors, publication year, age and gender of the patients, COVID-19 vaccine related information, onset of neurological symptoms, findings of neurological examination, MRI findings, laboratory work-up, CSF analysis, treatment, and clinical outcome.
2.6. Statistical analysis
Qualitative data were described in percentages and numbers. Quantitative data were described using range (minimum and maximum), and median. Significance of the obtained results was judged at the 5% level, but it could not be calculated due to insufficient data. A meta-analysis was planned to evaluate the association of the demographic findings, clinical, radiological and laboratory findings and outcomes, but it could not be performed due to lack of sufficient data.
3. Results
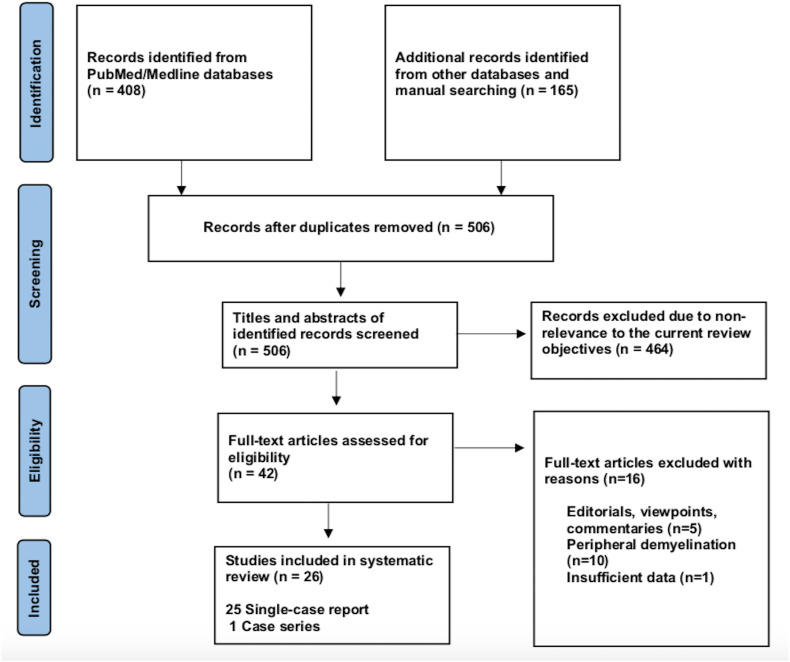
Our systematic search resulted in an initial number of 506 of potentially relevant articles, following duplicates removal. Articles were screened by title and abstract, and 42 articles were deemed eligible, after applying the inclusion/exclusion criteria to the full-text documents. Of which, 25 single-case reports and 1 case-series were included in the final systematic review. The flowchart for the study selection is shown in Fig. 1 .
Fig. 1.
Flowchart of literature inclusion in accordance with PRISMA guidelines.
3.1. Demographic data
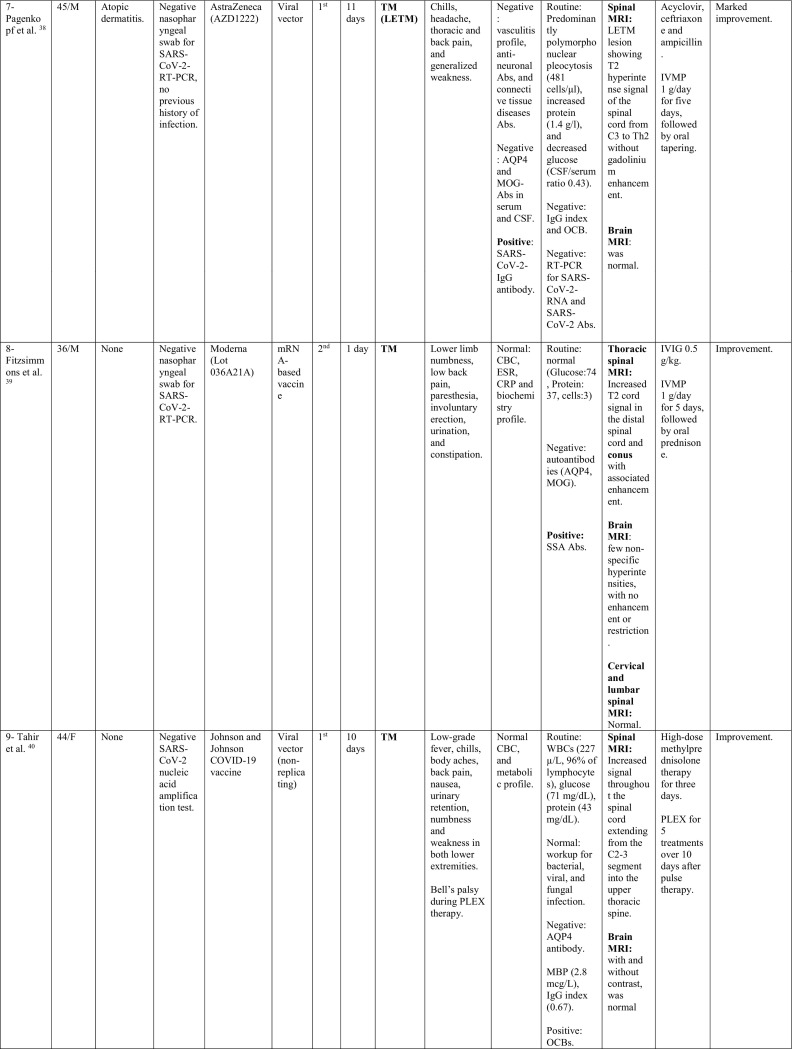
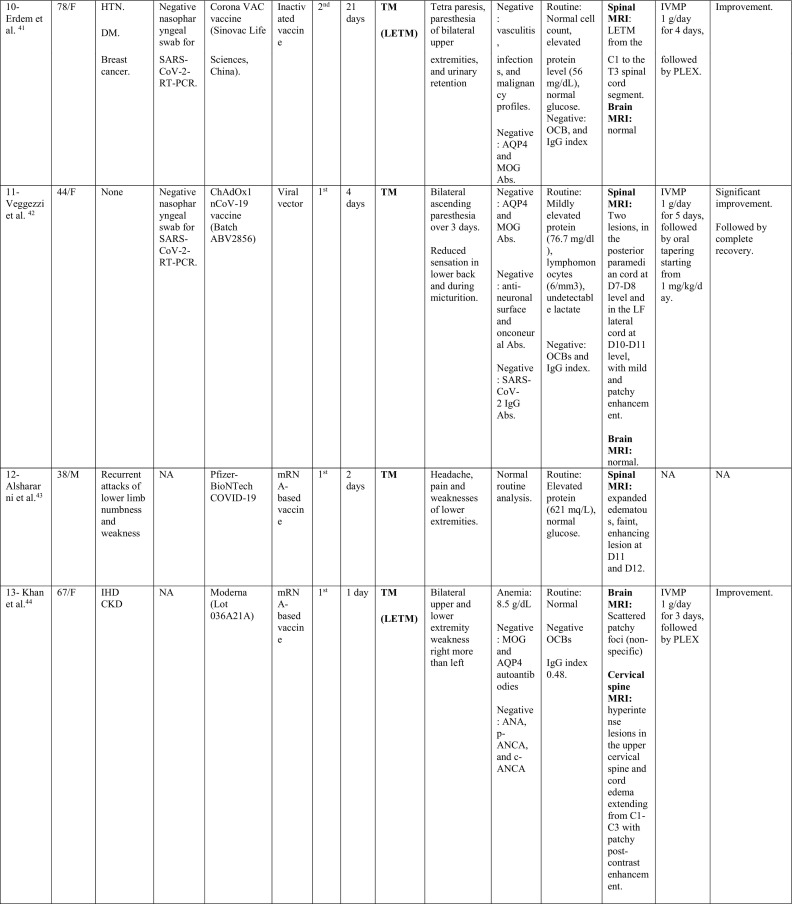
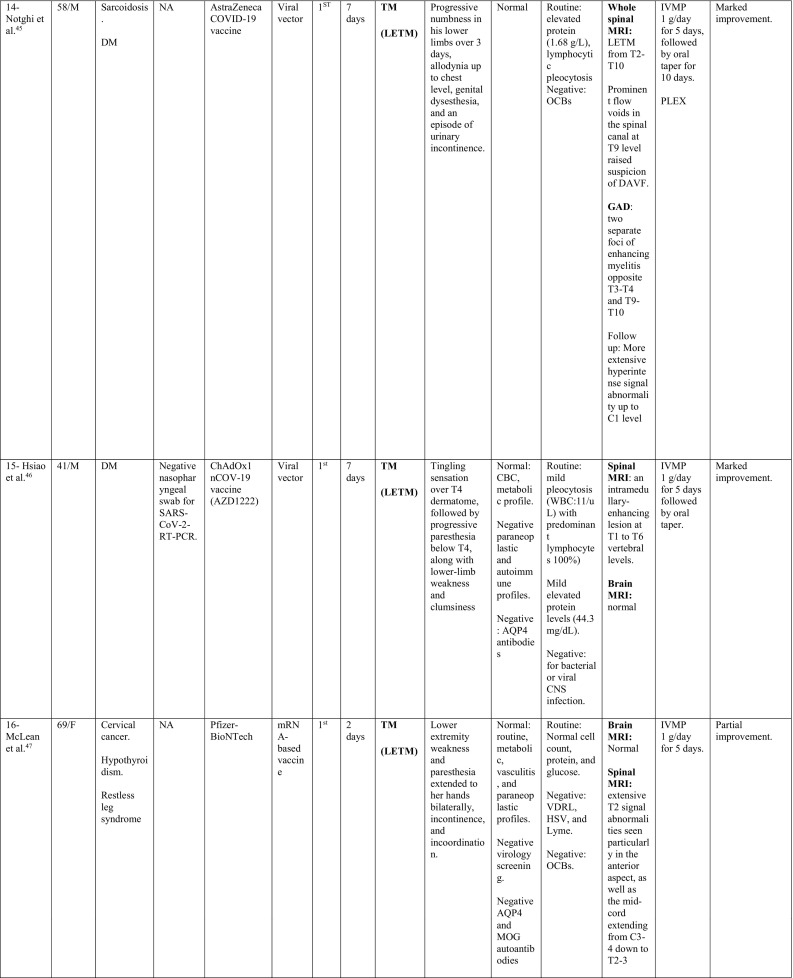
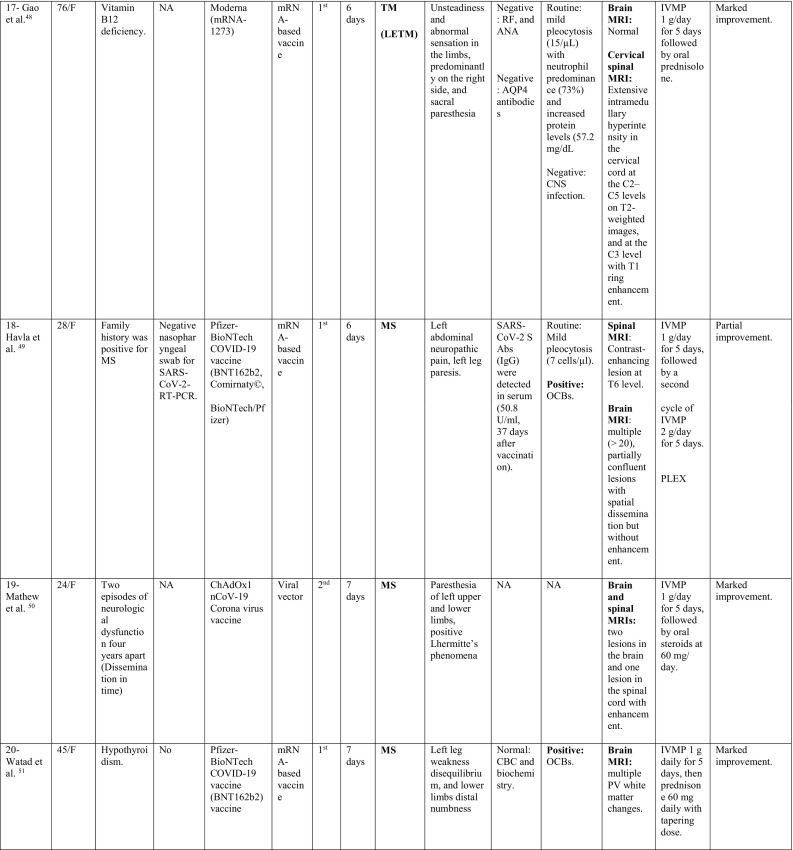
A total of 32 cases were reported as of September 30, 2021; 11 cases occurred following Pfizer-BioNTech vaccine, 8 following Oxford-AstraZeneca vaccine, 6 following Moderna, 5 following Sinovac/ Sinopharm vaccines and one following each of Sputnik and Johnson & Johnson vaccines. The majority of cases were females; 22/32 (68.8%), with a ratio of (2.2:1). The reported cases came from 12 countries; 11 cases from USA, 4 from Italy, 3 from Germany; 2 from India, Iran, China, Taiwan and Turkey; and 1 case from each of UK, Israel, Bangladesh, and KSA.
The median age of patients was 44 years (24–78 years), while the median duration between vaccination and onset of clinical symptoms was 9 days (1–30 days). In 23/32 patients (71.8%), the onset of neurological symptoms followed the first dose of the vaccine, while only 9/32 (28.1%) developed the symptoms after the second dose.
During their current illness, a negative nasopharyngeal swab for SARS-CoV-2-RT-PCR was reported in 13/32 cases (40.6%). Meanwhile, 3 cases reported no previous history of COVID-19 infection, while the data was missing for the remaining cases.
Interestingly, 17/32 (53.1%) had history of a previously diagnosed immune-mediated condition; 7 patients had MS, one had CIS suggestive of MS (Patient 28), 2 had a history of recurrent neurological symptoms; one was diagnosed as MS in the current illness (Patient 19), while the other was diagnosed as TM (Patient 12), 3 reported a history of thyroid dysfunction (Hashimoto's thyroiditis in Patient 1 and hypothyroidism in Patient 16 and Patient 20), 1 (Patient 3) had post-infectious rhombencephalitis, 1 (Patient 7) had atopic dermatitis, 1 had sarcoidosis (Patient 14), and 1 had Sjogren's disease (Patient 32).
In addition, one patient (Patient 18) reported a family history of MS, one had a history of cancer breast (Patient 10) and one had a history of cervical cancer (Patient 16).
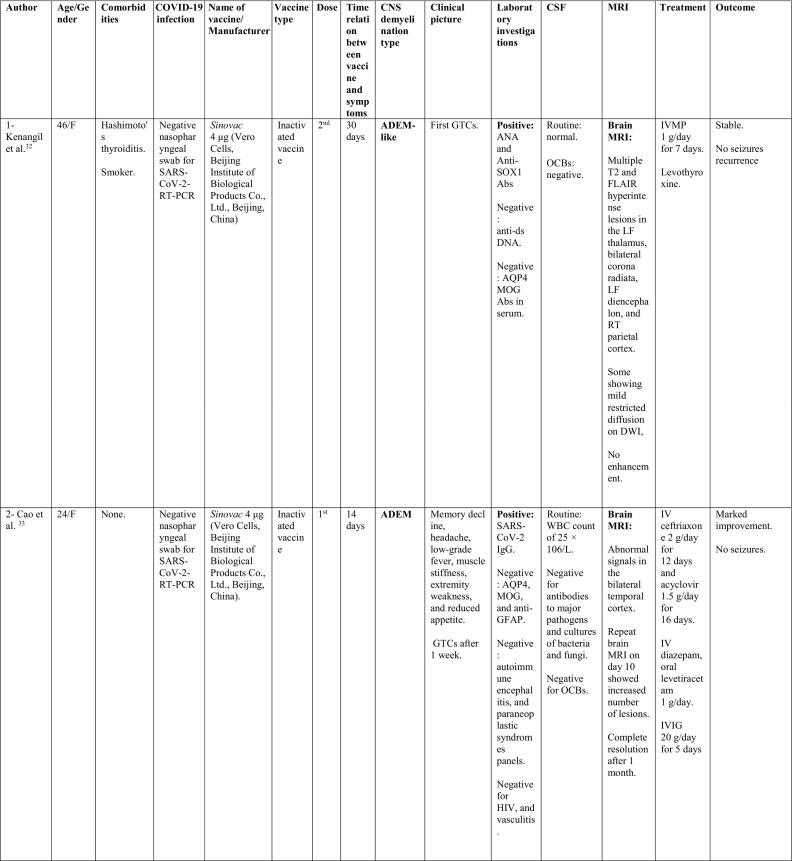
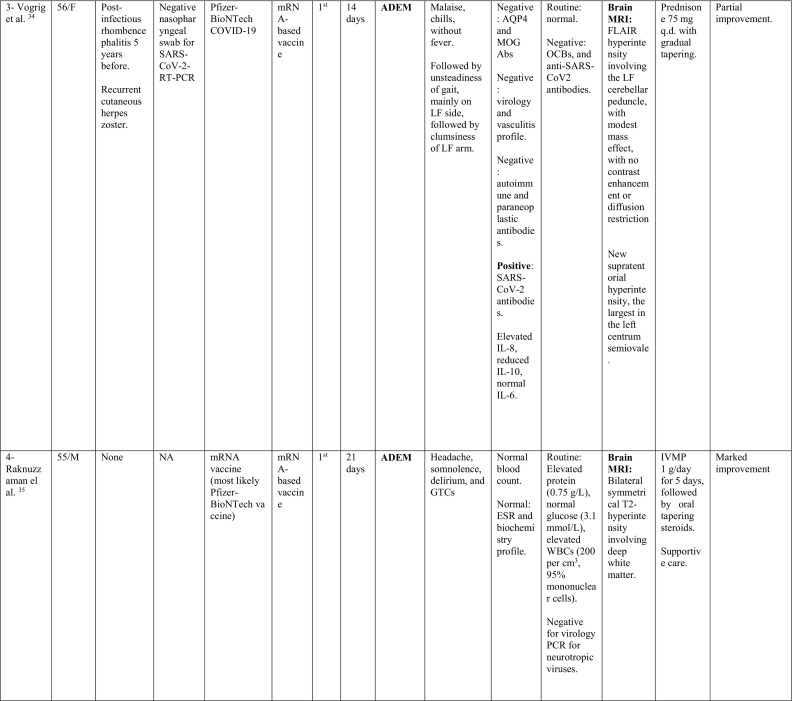
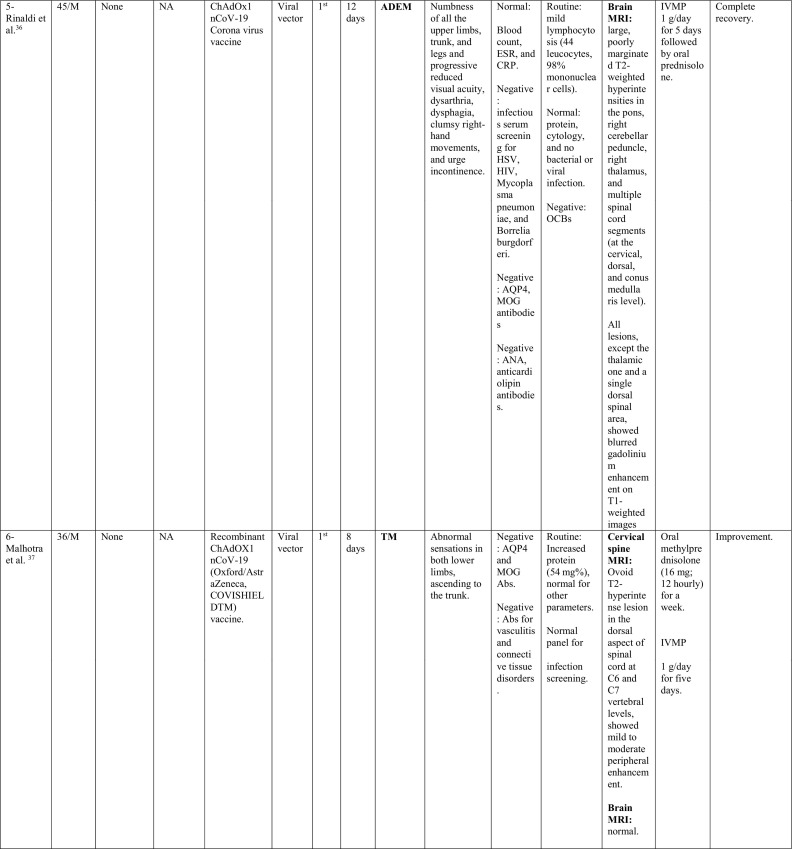
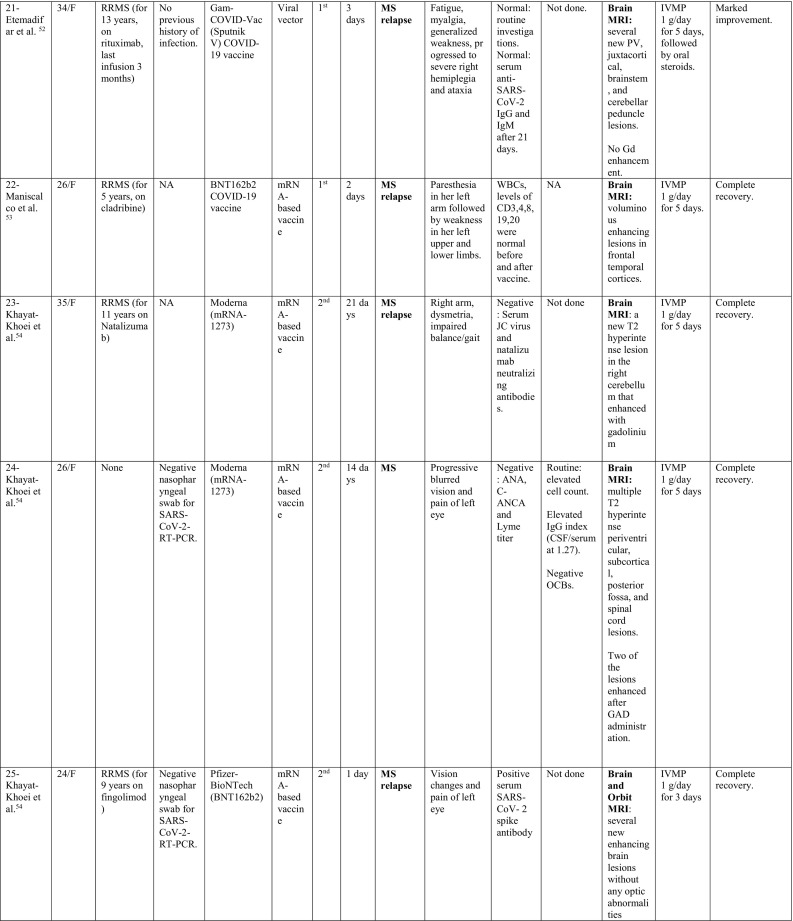
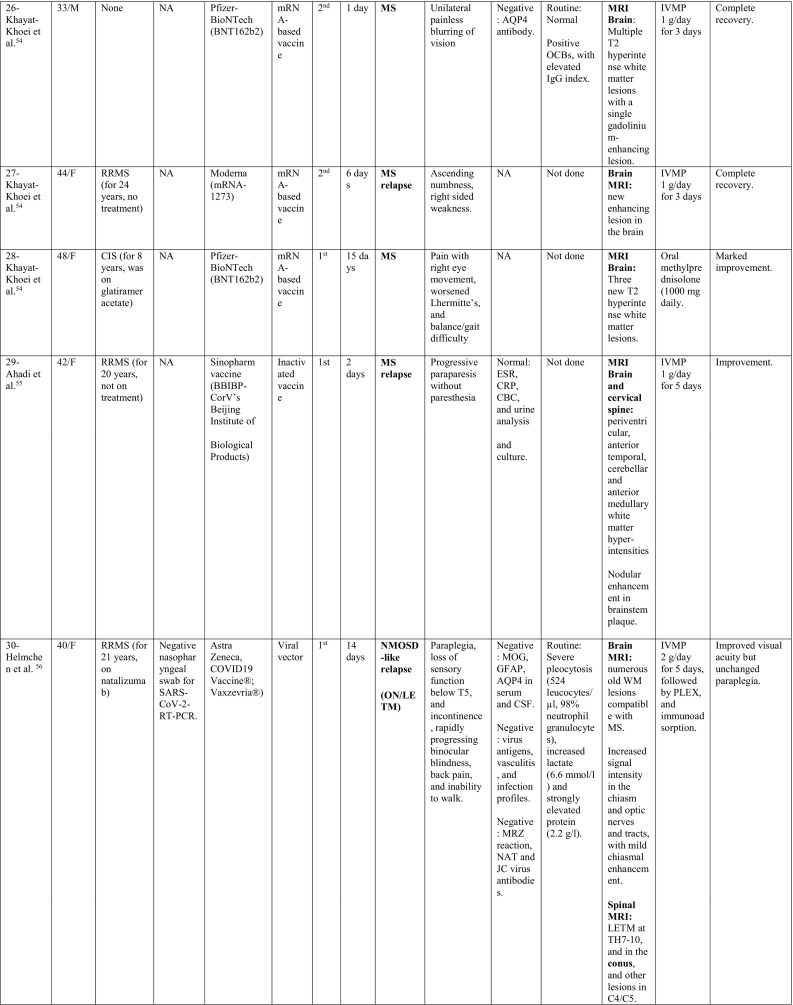
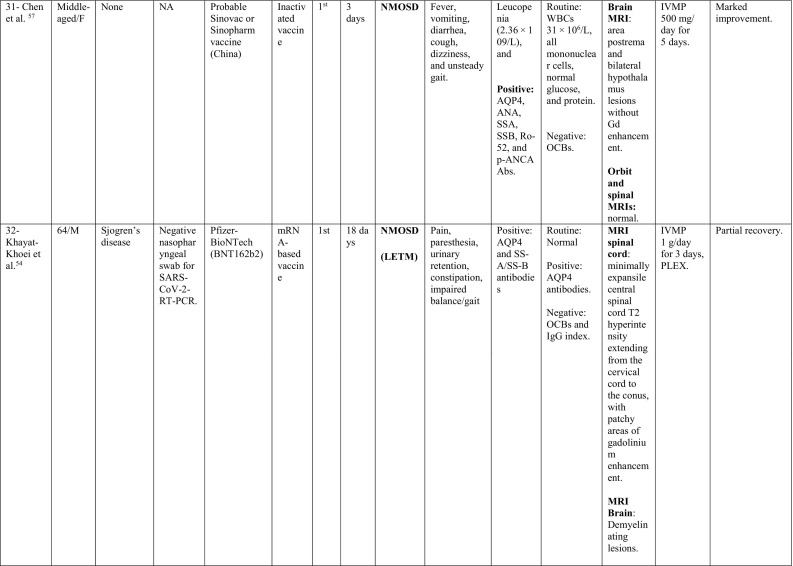
A summary of the clinical characteristics is presented in Table 1 .
Table 1.
Characteristics of cases presenting with CNS demyelination in relation to COVID-19 vaccines (Alshararni, 2021; Cao et al., 2021; Chen et al., 2021; Erdem et al., 2021; Etemadifar et al., 2021; Fitzsimmons and Nance, 2021; Gao et al., 2021; Havla et al., 2021; Helmchen et al., 2021; Hsiao et al., 2021; Khan et al., 2021; Khayat-Khoei et al., 2021; Maniscalco et al., 2021; Mathew and John, 2021; Malhotra et al., 2021; McLean and Trefts, 2021; Notghi et al., 2021; Ozgen Kenangil et al., 2021; Pagenkopf and Südmeyer, 2021; Raknuzzaman et al., 2021; Rinaldi et al., 2021; Seyed Ahadi et al., 2021; Tahir et al., 2021; Vegezzi et al., 2021; Vogrig et al, 2021; Watad et al., 2021).
COVID-19: Coronavirus disease 2019, SARS-CoV-2: severe acute respiratory syndrome- related coronavirus 2, RT-PCR: reverse transcriptase polymerase chain reaction, NP: nasopharyngeal, Abs: antibodies, Q.D: quarter in die (four times a day), GTCs: Generalized tonic- clonic convulsions, ANA: antinuclear antibody, SOX-1: SRY-BOX Transcription Factor 1, AQP4: aquaporin 4, MOG: myelin oligodendrocyte glycoprotein, LF: left, RT: right, CSF: cerebrospinal fluid, HIV: human immunodeficiency virus, IV: intravenous, IgG: immunoglobulin G, MRI: magnetic resonance imaging, FLAIR: fluid attenuated inversion recovery, DW: diffusion weighted, mRNA: messenger ribonucleic acid,
IVMP: intravenous methyl prednisolone, TM: transverse myelitis, LETM: longitudinally extensive transverse myelitis, OCBs: oligoclonal bands, ADEM: acute disseminated encephalomyelitis, Anti- ds DNA: anti- double stranded DNA, MMSE: Mini-Mental State Examination, GFAP: glial fibrillary acidic protein, MS: multiple sclerosis, WM: white matter, IVIG: intravenous immunoglobulins, GCS: Glasgow coma scale, ESR: erythrocyte sedimentation rate, NMOSD: neuromyelitis optica spectrum disorder, PLEX: plasma exchange, CBC: complete blood count, ESR: erythrocyte sedimentation rate, CRP: C-reactive protein, p-ANCA: WBC: white blood count, OCBs: oligoclonal bands, IgG index: immunoglobulin G, PV: periventricular, GFAP: glial fibrillary acidic protein, MRZ: measles, rubella, zoster, CIS: clinically isolated syndrome, DAVF: dural arteriovenous fistulae, IHD: ischemic heart disease.
3.2. Clinical, laboratory and radiological data
When looking at the clinical pictures, the most common presentations were transverse myelitis (12/32), and MS- like (first diagnosis or relapse) pictures (12/32), followed by ADEM- like (5/32), and NMOSD- like (3/18) presentations.
Transverse myelitis presentation was reported in 12 patients, 6 males and 6 females, with a median age of 44.5 years (36–78 years). There was a median interval of 6.5 days (1−21) between receiving the vaccine and onset of symptoms. CSF analysis in this group showed pleocytosis in 6/12 patients with a median of 15 cells (6–481 cells/μl). Lymphocytes were the predominant cells in 4/6, while polymorphonuclear cells predominated in 2 patients. In addition, high protein level was reported in 8/12 cases, with a median of 0.596 g/L (0.44–1.68 g/L).
On spinal MRIs, simultaneous thoracic and cervical spinal cord involvement was reported in 6/12 cases, followed by isolated thoracic cord affection in 5/12 patients. On the other hand, isolated cervical cord was noted in 2 patients, while conus medullaris involvement was reported in only one patient. Longitudinally extensive transverse myelitis (LETM) was the main radiological feature in 7/12 patients, while short segment involvement was reported in 5/12. In patient 8, conus pathology was part of a lesion involving the thoracic cord, however, serological testing for AQP4 and MOG antibodies was negative. Brain MRI was abnormal in only two of the myelitis patients with non-specific hyperintensities showing neither restriction nor contrast enhancement.
MS-like presentation was reported in 12 patients, of which, 6 had a well-established diagnosis of MS prior to vaccination, and the vaccine was hypothesized to have triggered a relapse, while 4 patients experienced their first episode post-vaccination. Additionally, one patient had a history of previous episodes of neurologic dysfunction without receiving a diagnosis of MS (Patient 19) and one patient (Patient 28) was diagnosed as CIS suggestive of MS. Eleven were females as opposed to only one male. Their median age was 33.5 years (24–48 years). The median interval between vaccination and symptoms development was 6 days (1–21 days). Half of the patients (6/12) developed their symptoms after the 1st dose of vaccination. Brain MRI depicted demyelinating lesions in all patients (12/12), while spinal cord involvement was reported in (3/12) patients (Patients 18, 19 and 24), was unremarkable in one (Patient 29) and not assessed in the rest. The diagnosis of MS was supported by positive oligoclonal bands (OCBs) in 3/12 patients (Patients 18, 20 and 26).
ADEM-like presentation was reported in 5 patients (3 females, 2 males), with a median age of 46 years (24–56 years). The time interval to symptom development was longer than other presentations, with a median of 14 days (12–30 days).
Generalized tonic-clonic seizures (GTCS) were reported in 3/5 patients, while confusion and headache were seen in 2/5 patients. Serological testing for AQP4 and MOG antibodies was negative in 4 patients and was not tested in the fifth. CSF analysis showed pleocytosis in 3/5 patients (Patient 2, 4 and 5), while elevated protein was reported in only one patient (0.75 g/L). Moreover, OCBs were tested in 4/5 patients and were negative.
All reported MRI lesions were supratentorial. Some lesions showed contrast enhancement (Patient 5). Additionally, spinal cord was involved in the form of multifocal lesions in one case (Patient 5).
NMOSD-like presentation was reported in 3 cases. One of them (Patient 30) was previously diagnosed with MS, and was on natalizumab for 21 years, when she suffered simultaneous optic neuritis and LETM. Even though she tested negative for AQP4 antibody in serum and CSF, her imaging features were more in favor of NMOSD rather than MS. Moreover, her CSF showed severe pleocytosis (542 cells/μl) with neutrophils predominance and markedly elevated protein (2.2 g/L). Unfortunately, testing for MOG antibody and OCBs in CSF was not reported. The other patient (Patient 31) presented by the characteristic area postrema syndrome that was supported by MRI data and positive serology for AQP4 antibodies. CSF analysis had elevated white blood cells count of (31 cells/μl), with mononuclear cells as the predominant type. The third patient (Patient 32) was a 64-year-old male with a history of Sjogren's disease. His presentation suggested spinal cord involvement which was supported radiologically by extensive cord involvement from cervical region to conus. Serological testing was positive for AQP4 antibodies. Brain MRI showed demyelinating patches as well.
3.3. Treatment and outcome
When addressing the management plans, treatment comprised high dose methylprednisolone (with or without oral tapering), PLEX, IVIG, or a combination of those. Antiviral/ antibiotics were also given in few cases. Fortunately, interventions achieved either marked/complete improvement in 25/32 (78.1%) cases, stabilized or resulted in partial recovery in the rest.
Finally, to further characterize the cases, we classified them based on the received vaccine type ( Table 2 ). CNS demyelination was reported after all types of vaccines in the literature. The mRNA-based vaccines resulted in the greatest number of demyelinating syndromes (17/32), followed by viral vector vaccines (10/32) as opposed to 5 cases following inactivated vaccines. Similarly, most MS-like episodes (9/12) were triggered by mRNA-based vaccines.
Table 2.
Classification of cases based on the received vaccine type.
| Vaccine | Number of demyelination cases | Median age (years) | Median interval (days) | TM | ADEM-like | MS- like | NMOSD- like | Immune- mediate conditions |
|---|---|---|---|---|---|---|---|---|
| mRNA (Pfizer, Moderna) | 17 | 44 (24–76) | 6 (1–21) | 5 | 2 | 9 | 1 | 10 |
| Viral vector (Astra, Sputnik, J&J) | 10 | 42.5 (24–58) | 7.5 (3–14) | 6 | 1 | 2 | 1 | 5 |
| Inactivated (Sinopharm, Sinovac) | 5 | 44 (24–78) | 14 (2−30) | 1 | 2 | 1 | 1 | 2 |
However, in TM cases, viral vector vaccines were associated with demyelination in 6/12 cases, followed by mRNA-based vaccines (5/12), and only one case following inactivated vaccines. Furthermore, the median interval between receiving inactivated COVID-19 vaccines and symptom development was longer; 14 days, as opposed to 7.5 and 6 days for viral vector and mRNA vaccines, respectively.
Data regarding the second dose of vaccination, for those who developed symptoms after the first dose, was available for only 9/23 cases; 4 cases did not adhere to the vaccination schedule, 3 cases switched from viral vector to an mRNA vaccine, and 2 cases received the second dose without any new symptoms.
4. Discussion
Since the early 1800s, vaccines have been the most efficient solution in preventing viral infections. However, in rare occasions, vaccines induced unexpected inflammatory reactions, or has been associated with manifest autoimmune diseases, within a short period of time following their administration (Lu et al., 2021b).
In order to be defined as vaccine-induced, the WHO had suggested certain criteria to be met (Wraith et al., 2003), including: (1) temporal relationship (vaccination must precede the occurrence of the event), (2) consistency of evidence (similar or same results generated by studies using different methods in different settings), (3) strength of association (statistical significance to demonstrate that it was not a chance occurrence), (4) specificity (vaccine is the only cause of the event), and (5) biological plausibility and coherence (there must be a biologically plausible mechanism between cause and effect). Such strict criteria were rarely met in the majority of cases in the literature, similar to our review, making inference of causation a challenge. In literature, few post-vaccination autoimmune diseases were firmly and reliably considered as vaccine-associated, such as GBS cases following 1976 swine influenza vaccine. However, other suspected associations, such as the hepatitis B vaccine and MS, and HPV vaccine and ADEM, have not been strongly confirmed (Salemi and D'Amelio, 2010).
A PubMed search from 1979 to 2013 by Karussis and colleagues (Karussis and Petrou, 2014a), revealed 71 documented cases of post-vaccination CNS demyelination. The most reported vaccines were influenza, HPV, and hepatitis A or B vaccines. Symptoms usually appeared within 2 weeks (mean: 14.2 days), however, delayed presentation (4 weeks and up to 5 months post-vaccination) has been reported. The commonest clinical presentations were optic neuritis, multifocal disseminated demyelination, TM, and encephalitis. Furthermore, Agmon-Levin and colleagues (Agmon-Levin et al., 2009) reported only 37 cases, from 1970 to 2009, in association with several vaccines, including hepatitis B, measles-mumps-rubella, and diphtheria-tetanus-pertussis.
The concern of a potential association of CNS autoimmune inflammation and COVID-19 vaccines has been recently raised, as cases began to unfold. According to the interim analysis of four randomized controlled trials of ChAdOx1 nCoV-19 vaccine (AZD1222), 3 cases of ATM were reported, from 11, 636 participants included. One case was considered to be an idiopathic demyelination that is possibly related to the vaccine, while the other two were most likely a pre-existing or previously unrecognized MS (Voysey et al., 2021). However, in a recent review of 11 COVID-19 vaccine candidates (Lu et al., 2021a), the preliminary official data from the vaccine manufactures and the drug authorities suggested that neurologic adverse events were rare, and cases of CNS demyelination were reported in association with viral vector vaccine only.
In our review, CNS demyelination was reported following all types of approved COVID-19 vaccines (no protein-based vaccine was approved at the time of writing). Neurological symptoms appeared within the first 1–2 weeks in most cases. Females comprised the majority of cases, which agrees with data in literature, where around 85% of immune-mediated diseases affects women (Angum et al., 2020). This has been attributed to greater immune responses against foreign and self-antigens in women compared to men. Furthermore, more than half of the cases had history of probable or definite autoimmune diseases, which could make them liable to increased risk of developing other immune-mediated diseases (Somers et al., 2009).
The mRNA-based vaccines resulted in the greatest number of demyelinating syndromes (53.1%), followed by viral vector vaccines (31.2%), as opposed to (15.6%) following inactivated vaccines. Similarly, 75% of MS-like episodes were triggered by mRNA-based vaccines. However, it should be noted that more patients with immune-mediated diseases received mRNA-based vaccines compared to the other types combined (10 vs 7 cases, respectively). Furthermore, TM cases were associated with both viral vector and mRNA-based vaccines (50% vs 41.6%, respectively), contrary to earlier published data, which limited TM to viral vector vaccines only (Lu et al., 2021a).
As of September 2021, VAERS database had 328 reports of suspected cases of TM worldwide, following all types of vaccines. However, a recent analysis of VAERS data revealed no increased risk of neuroautoimmune adverse events from COVID-19 vaccines compared to other vaccines (von Csefalvay, 2021).
At the time of writing, 6.4 billion doses of COVID-19 vaccines have been given, and 2.7 billion were fully vaccinated. Considering the annual incidence of TM of 1.34 to 4.60/ million in general population, 24.6/ million in cases of acquired demyelination (Beh et al., 2013), and 0.5/million in COVID-19 patients (Román et al., 2021), the current incidence of post-vaccination TM would be considered low.
Moreover, few cases of ADEM were reported, given that ADEM is the prototype and one of the most common white matter diseases associated with vaccines. ADEM is usually monophasic with a widely variable clinical presentation and favorable outcome, which was seen in our cases (Karussis and Petrou, 2014a).
Six MS patients had clinical relapses, while another 6 were newly diagnosed with MS following vaccination. Vaccines had long been incriminated in the development of MS, or in triggering MS relapses. However, pooled analysis from multiple studies found no sufficient evidence to support a causal relationship between the onset of MS and various common vaccinations (Farez and Correale, 2011). Moreover, a 2017 systematic review of more than 50 articles (Mailand and Frederiksen, 2017) found no increased risk in developing MS and in relapses after vaccination.
As regards to COVID-19, Achiron and colleagues (Achiron et al., 2021) found no increased risk of relapse activity in MS patients who received BNT162b2 vaccine as well. The relapse rate was higher following the first dose than the second dose (2.1% and 1.6%, respectively), which was similar to the rate in non-vaccinated patients during the corresponding period. However, in our review, MS relapse rate occurred following first and second doses equally. Moreover, they found that relapse rate was slightly higher in younger patients and in those treated with immunomodulatory drugs. However, their review was limited to cases who received BNT162b2 COVID-19 vaccine only, with a relatively short follow-up period.
Interestingly, NMOSD-like presentation was reported in 3 cases, raising the possibility of cross-reactivity between the used viral antigens and aquaporin-4. This predisposition to the spinal cord and the optic nerves has also been reported following other vaccines (e.g. HPV) vaccine (Karussis and Petrou, 2014b).
A favorable outcome was noted in the majority of the reported cases, which was similar to the findings of a recent nationwide study (García-Grimshaw et al., 2021), where most patients with neurological complications experienced complete recovery within days to weeks without long-term sequalae.
The exact mechanism of demyelination after COVID-19 vaccines remains poorly understood, however, it is postulated that a combination of vaccine-related factors, in addition to susceptibility of the patients, could be involved. Molecular mimicry represents one of the main immunopathogenic factors, where similarity between the proteins of the viruses used for the vaccination and self-antigens (e.g. myelin) triggers an undesired immune-response (García-Grimshaw et al., 2021).
In ChAdOx1 vaccine (AZD1222); a SARS-CoV-2 structural surface vector glycoprotein antigen (spike protein; nCoV-19) gene is included in a replication-deficient chimpanzee adenovirus, which could be a possible trigger of demyelination (CDC, 2021; Knoll and Wonodi, 2021).
Another important factor is the pathogenic role of immunologic adjuvants (substances that are used to enhance the antigen-specific immune responses), which can mimic evolutionarily conserved molecules activating both the innate and adaptive immune systems (Vera-Lastra et al., 2013). The mRNA vaccine exhibits a property of self-adjuvantation, where the mRNA acts as both antigen and adjuvant. A theoretical risk of inducing an autoimmune reaction could be related to activation of toll-like receptors TLR7 and TLR8, resulting in type I interferon production, and eliciting robust T and B cell responses, thus activating bystander autoreactive lymphocytes (Velikova and Georgiev, 2021). This bystander activation, along with macrophages secreting cytokines, can results in local inflammation and the recruitment of additional T-helper cells (Aharon-Maor and Shoenfeld, 2000).
Other etiologies include vaccine-related factors such as the type, dose and the route of administration (Velikova and Georgiev, 2021), in addition to a possible immunological and genetic susceptibility of the patients.
Finally, in a recent review (Ismail and Salama, 2021), 102 cases of CNS demyelination were reported in association with COVID-19 infection, from January 1, 2020, until June 15, 2021, while only 32 cases in association with COVID-19 vaccine were reported in a 10-month period. This is in line with robust evidence in literature, which documents a substantially higher risk of demyelination following infections, compared to the different types of vaccines (García-Grimshaw et al., 2021; De Martino et al., 2013). Although non-negligible, COVID-19 vaccine-associated CNS demyelination is still relatively low, and the benefits of vaccinations surpass the potential risks of CNS inflammation.
4.1. Limitations
This systematic review has some limitations. Since all available literature were reported as single case reports and one case series so far, there could be some sort of reporting and/or publication bias. In addition, we presented the reports as a set of cases for practical reasons, however, one should be wary of interpreting the data as coming from a uniform cohort, and inferring direct causality from the anecdotal data provided. Moreover, the small number of reported cases, the heterogeneity of clinical data, or the incomplete work-up in some cases, hindered the ability to perform a meta-analysis. Despite these shortcomings, the current review represents the first preliminary data on the association of COVID-19 vaccination and CNS demyelination, which can help future research.
5. Conclusion
In this review, CNS demyelination occurred following all types of approved COVID-19 vaccines. Clinical presentation was heterogenous, including TM, MS, ADEM and NMOSD. Symptoms occurred within 1–2 weeks, mainly following the first dose of vaccine. Interestingly, more than half of the reported cases had history of immune-mediated diseases. A favorable outcome was observed in the majority of cases after treatment. Currently, the world is facing the largest mass vaccination campaign in history, and cases of demyelination will inevitably occur, either directly following vaccination, or by chance. However, the incidence appears to be low, in comparison to demyelination following COVID-19 infection. Although association does not always imply causation, long-term post-marketing surveillance for cases of demyelination is warranted, to assess for causality, and ensure the safety of COVID-19 vaccines.
Funding
No funding received.
Declaration of Compeitng Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Achiron A., Dolev M., Menascu S., Zohar D.-N., Dreyer-Alster S., Miron S., et al. COVID-19 vaccination in patients with multiple sclerosis: what we have learnt by February 2021. Mult. Scler. 2021 May;27(6):864–870. doi: 10.1177/13524585211003476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agmon-Levin N., Kivity S., Szyper-Kravitz M., Shoenfeld Y. Transverse myelitis and vaccines: a multi-analysis. Lupus. 2009 Nov;18(13):1198–1204. doi: 10.1177/0961203309345730. [DOI] [PubMed] [Google Scholar]
- Aharon-Maor A., Shoenfeld Y. The good, the bad and the ugly of vaccination. Isr. Med. Assoc. J. 2000 Mar;2(3):225–227. [PubMed] [Google Scholar]
- Alshararni Ali. Acute transverse Myelitis associated with COVID-19 vaccine: a case report. IJRPS. 2021 Jul 26;12(3):2083–2087. [Google Scholar]
- Angum F., Khan T., Kaler J., Siddiqui L., Hussain A. The prevalence of autoimmune disorders in women: a narrative review. Cureus. 2020 May 13;12(5):e8094. doi: 10.7759/cureus.8094. https://www.cureus.com/articles/31952-the-prevalence-of-autoimmune-disorders-in-women-a-narrative-review [Internet]. [cited 2021 Sep 2]; Available from: (PMID: 32542149; PMCID: PMC7292717) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beh S.C., Greenberg B.M., Frohman T., Frohman E.M. Transverse Myelitis. Neurol. Clin. 2013 Feb;31(1):79–138. doi: 10.1016/j.ncl.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L., Ren L., et al. Acta Neurol. Belg. 2021 Feb 1 doi: 10.1007/s13760-021-01608-2. [Internet]. [cited 2021 Sep 2]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC . US Department of Health and Human Services, CDC; Atlanata, GA: 2021. Vaccine Adverse Event Reporting System (VARES) Accessed August 15, 2021. 2021. [Google Scholar]
- Chen S., Fan X.-R., He S., Zhang J.-W., Li S.-J. Watch out for neuromyelitis optica spectrum disorder after inactivated virus vaccination for COVID-19. Neurol. Sci. 2021 Jun 29 doi: 10.1007/s10072-021-05427-4. [Internet]. [cited 2021 Sep 2]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Csefalvay C. VAERS data reveals no increased risk of neuroautoimmune adverse events from COVID-19 vaccines [Internet] Inf. Dis. (except HIV/AIDS) 2021 Jun doi: 10.1101/2021.06.13.21258851. [cited 2021 Sep 2]. Available from: [DOI] [Google Scholar]
- De Martino M., Chiappini E., Galli L. Vaccines and autoimmunity. Int. J. Immunopathol. Pharmacol. 2013 Apr;26(2):283–290. doi: 10.1177/039463201302600201. [DOI] [PubMed] [Google Scholar]
- Erdem N.Ş., Demirci S., Özel T., Mamadova K., Karaali K., Çelik H.T., et al. Acute transverse myelitis after inactivated COVID-19 vaccine. Ideggyogy Sz. 2021;74(7–8):273–276. doi: 10.18071/isz.74.0273. [DOI] [PubMed] [Google Scholar]
- Etemadifar M., Sigari A.A., Sedaghat N., Salari M., Nouri H. Acute relapse and poor immunization following COVID-19 vaccination in a rituximab-treated multiple sclerosis patient. Human Vacc. Immunotherap. 2021 May;20:1–3. doi: 10.1080/21645515.2021.1928463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farez M.F., Correale J. Immunizations and risk of multiple sclerosis: systematic review and meta-analysis. J. Neurol. 2011 Jul;258(7):1197–1206. doi: 10.1007/s00415-011-5984-2. [DOI] [PubMed] [Google Scholar]
- Fitzsimmons W., Nance C.S. Sudden Onset of Myelitis after COVID-19 Vaccination: An Under-Recognized Severe Rare Adverse Event. SSRN J. 2021 https://www.ssrn.com/abstract=3841558 [Internet]. [cited 2021 Sep 2]; Available from: [Google Scholar]
- Gao J.-J., Tseng H.-P., Lin C.-L., Shiu J.-S., Lee M.-H., Liu C.-H. Acute transverse myelitis following COVID-19 vaccination. Vaccines. 2021 Sep 10;9(9):1008. doi: 10.3390/vaccines9091008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Grimshaw M., Ceballos-Liceaga S.E., Hernández-Vanegas L.E., Núñez I., Hernández-Valdivia N., Carrillo-García D.A., et al. Systemic and Neurologic Adverse Events Among 704,003 First-Dose Recipients of the Pfizer-Biontech (BNT162b2) mRNA COVID-19 Vaccine in Mexico. SSRN J. 2021;229:108786. doi: 10.1016/j.clim.2021.108786. https://www.ssrn.com/abstract=3816489 [Internet]. [cited 2021 Sep 2]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss A.L., Samudralwar R.D., Das R.R., Nath A. ANA investigates: neurological complications of COVID -19 vaccines. Ann. Neurol. 2021 May;89(5):856–857. doi: 10.1002/ana.26065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havla J, Schultz Y, Zimmermann H, Hohlfeld R, Danek A, Kümpfel T. First manifestation of multiple sclerosis after immunization with the Pfizer-BioNTech COVID-19 vaccine. J. Neurol. [Internet]. 2021 Jun 11 [cited 2021 Sep 2]; Available from: doi: 10.1007/s00415-021-10648-w. [DOI] [PMC free article] [PubMed]
- Helmchen C., Buttler G.M., Markewitz R., Hummel K., Wiendl H., Boppel T. Acute bilateral optic/chiasm neuritis with longitudinal extensive transverse myelitis in longstanding stable multiple sclerosis following vector-based vaccination against the SARS-CoV-2. J. Neurol. 2021 Jun 15 doi: 10.1007/s00415-021-10647-x. [Internet]. [cited 2021 Sep 2]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández A.F., Calina D., Poulas K., Docea A.O., Tsatsakis A.M. Safety of COVID-19 vaccines administered in the EU: should we be concerned? Toxicol. Rep. 2021;8:871–879. doi: 10.1016/j.toxrep.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao Y.-T., Tsai M.-J., Chen Y.-H., Hsu C.-F. Acute transverse myelitis after COVID-19 vaccination. Medicina. 2021 Sep 25;57(10):1010. doi: 10.3390/medicina57101010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail I.I., Salama S. Association of CNS demyelination and COVID-19 infection: an updated systematic review. J. Neurol. 2021 Aug 12 doi: 10.1007/s00415-021-10752-x. [Internet]. [cited 2021 Sep 2]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karussis D., Petrou P. The spectrum of post-vaccination inflammatory CNS demyelinating syndromes. Autoimmun. Rev. 2014 Mar;13(3):215–224. doi: 10.1016/j.autrev.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Karussis D., Petrou P. The spectrum of post-vaccination inflammatory CNS demyelinating syndromes. Autoimmun. Rev. 2014 Mar;13(3):215–224. doi: 10.1016/j.autrev.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Khan E., Shrestha A.K., Colantonio M.A., Liberio R.N., Sriwastava S. Acute transverse myelitis following SARS-CoV-2 vaccination: a case report and review of literature. J. Neurol. 2021 Sep 5 doi: 10.1007/s00415-021-10785-2. [Internet]. [cited 2021 Oct 11]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khayat-Khoei M., Bhattacharyya S., Katz J., Harrison D., Tauhid S., Bruso P., et al. COVID-19 mRNA vaccination leading to CNS inflammation: a case series. J. Neurol. 2021 Sep 4:1–14. doi: 10.1007/s00415-021-10780-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll M.D., Wonodi C. Oxford–AstraZeneca COVID-19 vaccine efficacy. Lancet. 2021 Jan;397(10269):72–74. doi: 10.1016/S0140-6736(20)32623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Y., Zhong J., Luo J. Safety and effectiveness of SARS-CoV-2 vaccines: A systematic review and meta-analysis. J. Med. Virol. 2021 Jul 26;93(12):6486–6495. doi: 10.1002/jmv.27203. PMID: 34264528; PMCID: PMC8426880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Xiong W., Mu J., Zhang Q., Zhang H., Zou L., et al. The potential neurological effect of the COVID-19 vaccines: a review. Acta Neurol. Scand. 2021 Jul;144(1):3–12. doi: 10.1111/ane.13417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Xiong W., Mu J., Zhang Q., Zhang H., Zou L., et al. Neurological side effects of COVID-19 vaccines are rare. Acta Neurol. Scand. 2021 Jul;144(1):111–112. doi: 10.1111/ane.13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahase E. Covid-19: Oxford researchers halt vaccine trial while adverse reaction is investigated. BMJ. 2020 Sep;9:m3525. doi: 10.1136/bmj.m3525. [DOI] [PubMed] [Google Scholar]
- Mailand M.T., Frederiksen J.L. Vaccines and multiple sclerosis: a systematic review. J. Neurol. 2017 Jun;264(6):1035–1050. doi: 10.1007/s00415-016-8263-4. [DOI] [PubMed] [Google Scholar]
- Malhotra H.S., Gupta P., Prabhu V., Kumar Garg R., Dandu H., Agarwal V. COVID-19 vaccination-associated myelitis. QJM: Int. J. Med. 2021 Mar 31;114(8):591–593. doi: 10.1093/qjmed/hcab069. PMID: 33787891; PMCID: PMC8083508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniscalco G.T., Manzo V., Di Battista M.E., Salvatore S., Moreggia O., Scavone C., et al. Severe multiple sclerosis relapse after COVID-19 vaccination: a case report. Front. Neurol. 2021 Aug 10;12 doi: 10.3389/fneur.2021.721502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew T., John S.K. COVID-19 vaccine (ChAdOx1 nCoV-19 Corona virus vaccine (Recombinant)) – COVISHIELD related MS relapse. Neuroimmunol. Reports. 2021 Dec;1:100006. [Google Scholar]
- McLean P., Trefts L. Transverse myelitis 48 hours after the administration of an mRNA COVID 19 vaccine. Neuroimmunol. Reports. 2021 Dec;1:100019. [Google Scholar]
- Nagy A., Alhatlani B. An overview of current COVID-19 vaccine platforms. Comput. Struct. Biotechnol. J. 2021;19:2508–2517. doi: 10.1016/j.csbj.2021.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndwandwe D., Wiysonge C.S. COVID-19 vaccines. Curr. Opin. Immunol. 2021 Aug;71:111–116. doi: 10.1016/j.coi.2021.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notghi A.A., Atley J., Silva M. Lessons of the month 1: longitudinal extensive transverse myelitis following AstraZeneca COVID-19 vaccination. Clin. Med. 2021 Sep;21(5):e535–e538. doi: 10.7861/clinmed.2021-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozgen Kenangil G., Ari B.C., Guler C., Demir M.K. Acute disseminated encephalomyelitis-like presentation after an inactivated coronavirus vaccine. Acta Neurol. Belg. 2021 Aug;121(4):1089–1091. doi: 10.1007/s13760-021-01699-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagenkopf C., Südmeyer M. A case of longitudinally extensive transverse myelitis following vaccination against Covid-19. J. Neuroimmunol. 2021 Sep;358:577606. doi: 10.1016/j.jneuroim.2021.577606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raknuzzaman M., Jannaty T., Hossain M.B., Saha B., Dey S.K., Shahidullah M. Post Covid19 vaccination acute disseminated encephalomyelitis: a case report in Bangladesh. Int. J. Med. Sci. Clin. Res. Stud. 2021;1(03):31–36. [Google Scholar]
- Rinaldi V., Bellucci G., Romano A., Bozzao A., Salvetti M. ADEM after ChAdOx1 nCoV-19 vaccine: a case report. Mult. Scler. 2021 Sep;30 doi: 10.1177/13524585211040222. 135245852110402. [DOI] [PubMed] [Google Scholar]
- Román G.C., Gracia F., Torres A., Palacios A., Gracia K., Harris D. Acute transverse myelitis (ATM):clinical review of 43 patients with COVID-19-associated ATM and 3 post-vaccination ATM serious adverse events with the ChAdOx1 nCoV-19 vaccine (AZD1222) Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.653786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salemi S., D’Amelio R. Could autoimmunity be induced by vaccination? Int. Rev. Immunol. 2010 Jun;29(3):247–269. doi: 10.3109/08830181003746304. [DOI] [PubMed] [Google Scholar]
- Seyed Ahadi M., Ghadiri F., Ahraian M.A., Naser Moghadasi A. Acute attack in a patient with multiple sclerosis 2 days after COVID vaccination: a case report. Acta Neurol. Belg. 2021 Aug 11 doi: 10.1007/s13760-021-01775-2. [Internet]. [cited 2021 Oct 11]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers E.C., Thomas S.L., Smeeth L., Hall A.J. Are individuals with an autoimmune disease at higher risk of a second autoimmune disorder? Am. J. Epidemiol. 2009 Jan 6;169(6):749–755. doi: 10.1093/aje/kwn408. [DOI] [PubMed] [Google Scholar]
- Tahir N., Koorapati G., Prasad S., Jeelani H.M., Sherchan R., Shrestha J., et al. SARS-CoV-2 vaccination-induced transverse Myelitis. Cureus. 2021 Jul 25;13(7):e16624. doi: 10.7759/cureus.16624. https://www.cureus.com/articles/63667-sars-cov-2-vaccination-induced-transverse-myelitis [Internet]. [cited 2021 Sep 2]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegezzi E., Ravaglia S., Buongarzone G., Bini P., Diamanti L., Gastaldi M., et al. Acute myelitis and ChAdOx1 nCoV-19 vaccine: casual or causal association? J. Neuroimmunol. 2021 Oct;359:577686. doi: 10.1016/j.jneuroim.2021.577686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velikova T., Georgiev T. SARS-CoV-2 vaccines and autoimmune diseases amidst the COVID-19 crisis. Rheumatol. Int. 2021 Mar;41(3):509–518. doi: 10.1007/s00296-021-04792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera-Lastra O., Medina G., Cruz-Dominguez M.D.P., Jara L.J., Shoenfeld Y. Autoimmune/inflammatory syndrome induced by adjuvants (Shoenfeld’s syndrome): clinical and immunological spectrum. Expert. Rev. Clin. Immunol. 2013 Apr;9(4):361–373. doi: 10.1586/eci.13.2. [DOI] [PubMed] [Google Scholar]
- Vogrig A., Janes F., Gigli G.L., Curcio F., Negro I.D., D’Agostini S., et al. Acute disseminated encephalomyelitis after SARS-CoV-2 vaccination. Clin. Neurol. Neurosurg. 2021 Sep;208:106839. doi: 10.1016/j.clineuro.2021.106839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021 Jan;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watad A., De Marco G., Mahajna H., Druyan A., Eltity M., Hijazi N., et al. Immune-mediated disease flares or new-onset disease in 27 subjects following mRNA/DNA SARS-CoV-2 vaccination. Vaccines. 2021 Apr 29;9(5):435. doi: 10.3390/vaccines9050435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organisation WHO Coronavirus Disease (COVID-19) Dashboard. [Internet] 2021. https://covid19.who.int/ [cited 2021 Sep 1]. Available from:
- Wraith D.C., Goldman M., Lambert P.-H. Vaccination and autoimmune disease: what is the evidence? Lancet. 2003 Nov;362(9396):1659–1666. doi: 10.1016/S0140-6736(03)14802-7. [DOI] [PubMed] [Google Scholar]