Abstract
Alternative sources of fish oil (FO) are one of the major problems in aquaculture; therefore, the goal of the present study was to examine insect (black soldier fly larvae) oil (BSLO) as a potential replacer of fish/soy oil in juvenile rainbow trout (initial average weight of 32 ± 0.15 g) feed. Four diets were formulated wherein FO (control diet) was completely replaced with either soybean oil (SO) or BSLO, and an additional BSLO-based diet supplemented with 1.5% bile acid (BSLO + BA) were fed to the fish for 10 weeks. Growth performance of the BSLO fed group was similar (P > 0.05) to that of the FO and SO fed groups, however, the fish fed BSLO + BA diet registered the lowest growth (P < 0.05). Oil sources did not (P > 0.05) affect the major nutrient content of whole-body, however, the fatty acid composition of the muscle and liver was influenced (P < 0.05), with the highest 14:0, 16:0, and total saturated fatty acid detected in BSLO or BSLO + BA fed trout compared to the others (P < 0.001). No significant differences were observed in eicosapentaenoic acid + docosahexaenoic acid (EPA + DHA) or total n-3 polyunsaturated fatty acid (PUFA) content in muscle among the groups, whereas, the highest EPA:DHA and n-3:n-6 ratios were detected in the FO group. Gene expression for fatty acid binding protein (fabp), fatty acid synthase (fas), and Δ5 desaturase in the liver was lower in FO (P < 0.05), while BSLO + BA registered the highest Δ6 expression (P = 0.006). Supplementation of BA in the BSLO diet increased superoxide dismutase (SOD) and catalase (CAT) activities compared to the other groups (P < 0.05). In conclusion, BSLO could serve as a substitute for FO and SO in rainbow trout diet without negatively impacting growth performance, whole-body composition and nutrient retention, and modulate the expression of fatty acid metabolism-related genes in rainbow trout.
Keywords: Insect oil, Rainbow trout, Growth performance, Fatty acid metabolism, Antioxidant enzyme
1. Introduction
The declining trend in capture fisheries has spurred a steady increase in aquaculture production to meet global demands for seafood. However, the sustainability of aquaculture production is hinged on the development of alternative feed ingredients, as the demand for commercial feed increases, while marine resources used in aquafeed manufacturing are becoming increasingly limited. Marine resources, especially fish meal (FM) and fish oil (FO), are the most commonly used protein and lipid source ingredients in fish feed production, especially for carnivorous species (Turchini et al., 2010). This is due to their essential nutritional contents that support fish growth, health, and the high contents of long-chain omega-3 polyunsaturated fatty acids (n-3 LC-PUFA). The increasing demand for aquafeeds and the need to replace FO has resulted in the use of alternative terrestrial oils as lipid sources in commercial fish feed production, however, most of these oils are not sustainable or are deficient in n-3 highly unsaturated fatty acid (Tocher, 2015; Yang et al., 2020). With increasing global limitation of FO availability for use in aquafeed and demand for human consumption of more oil sources in the near future, it is of great importance to find suitable alternative sources of oils.
Insect meals, especially black soldier fly larvae (BSL) are high in protein (40% to 45%) and lipid (26% to 35%) and have been recognized as a promising feed ingredient (Schiavone et al., 2018; Sheppard et al., 1994; Tran et al., 2015). Several studies have investigated the substitution of FM by BSL meal in different fish species including rainbow trout and turbot (Cardinaletti et al., 2019; Elia et al., 2018; Kroeckel et al., 2012; Renna et al., 2017; Stamer et al., 2014), but little attention has been given to BSL oil for possible use in fish feed (Li et al., 2016; Xu et al., 2021, Xu et al., 2020). BSL oil is a byproduct of meal production and is known to contain high levels of medium-chain fatty acid, especially lauric acid (21% to 49%) (Li et al., 2016), which is beneficial in the reduction of abdominal fat deposition due to their preferential use as an energy substrate (Wang et al., 2015; Li et al., 2016). In addition, the concentration of unsaturated fatty acid (linoleic, 18:2n-6, and linolenic, 18:3n-3) in BSL oil is closely related to that of soybean oil (SO), and this could be effectively utilized by freshwater fish to biosynthesize n-3 and n-6 LC-PUFA due to their endogenous capability (Tocher, 2003). The nutritional modulation and accumulation of LC-PUFA in fish is related to the biosynthesis and decomposition of fatty acids by enzymes associated with fatty acid metabolism and pertinent transcription factors (Yu et al., 2019). Fatty acid synthase (FAS), one of the main lipogenic enzymes, catalyzes the synthesis of long-chain saturated fatty acids (LC-SFA) from acetyl CoA and malonyl-CoA in the presence of nicotinamide adenine dinucleotide phosphate (NADPH) (Dong et al., 2014), while fatty acid-binding proteins (FABP) aid in the transport of fatty acids into cells (Torstensen et al., 2009). The expression of fatty acid elongase and desaturase genes are known to be influenced by the dietary fatty acid composition (Jordal et al., 2005; Zheng et al., 2004) and the fish species. Although studies on the effect of dietary BSL oil (partial replacement) on growth and nutrient deposition in rainbow trout (Dumas et al., 2018), Jian carp (Li et al., 2016) and mirror carp (Xu et al., 2021) have been reported, empirical data on the dietary effect of BSL oil on the expression pattern of fatty acid metabolism-related genes and oxidative antioxidant enzyme response in rainbow trout is yet to be reported.
Dietary lipid fatty acid composition affects the body lipid deposition of fish as was discovered in rainbow trout fed 100% alternative sources of oil as a FO replacer (GÜLER and Yildiz, 2011). Lipids provide fish with essential fatty acids required for growth and development but feeding high level of lipids to fish could result in visceral fat deposition, fatty liver, and induce metabolic distress (Yan et al., 2015). However, BA could be used to improve the efficiency of lipid digestion, thereby reducing excess lipid deposition in fish (Ding et al., 2020; Romano et al., 2020). This was evident in large yellow croaker in which dietary BA enhanced the mRNA expression of carnitine palmitoyltransferase 1 and acyl-CoA oxidase, and reduced liver lipid content (Ding et al., 2020). Furthermore, dietary fatty acids have been reported to influence glucose metabolism (Risérus et al., 2009), and high blood glucose could lead to increased release of free radicals and induce oxidative stress response (Pieme et al., 2017). Based on this, the current study examined BSL oil (with or without BA) as a substitute for FO and SO in diets for rainbow trout. Thus, the objective of this study was to investigate the effect of dietary BSL oil on growth, fatty acid deposition, antioxidant capacity, and expression of fatty acid metabolism-related genes in rainbow trout. This study will help to determine the effectiveness with which rainbow trout utilize BSL oil, as a replacement for FO and SO, to synthesize n-3 HUFA from PUFA and their deposition in muscle.
2. Materials and methods
2.1. Ethics statement
The experimental protocols together with fish handling and sampling were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Idaho (IACUC-2019-76).
2.2. Diets, feeding trial and sample collection
Four isonitrogenous (44% crude protein) and isolipidic (21% crude lipid) experimental diets were formulated, and differed only in their lipid sources (Table 1). The dietary oils were FO, SO, and black soldier fly larvae oil (BSLO) with an additional BSLO-supplemented diet with BA (1.5%; Yamamoto et al., 2007a) (BSLO + BA). All feed ingredients were mixed thoroughly in a Hobart mixer, water was added, and then cold pelleted using a laboratory pellet mill (California Pellet Mill Company, San Francisco, CA, USA) fitted with a 2.4-mm die at the Hagerman Fish Culture Experiment Station (HFCES), University of Idaho, USA. The feeds were dried in a forced-air dryer set at 40 °C until moisture content was reduced to less than 10%, and then stored at ambient temperature until use. The proximate composition of the experimental diets is provided in Table 1. Fatty acids profile of oil (BSLO, FO and SO) is provided in Table 2. The BSFLO used in this study was provided by EnviroFlight, USA.
Table 1.
Ingredients and proximate composition of the trial diets (as-is basis).
| Item | FO | SO | BSLO | BSLO + BA |
|---|---|---|---|---|
| Ingredients, g/kg | ||||
| Fish meal, sardine1 | 240 | 240 | 240 | 240 |
| Poultry meal1 | 102 | 102 | 102 | 102 |
| Soy protein concentrate2 | 60 | 60 | 60 | 60 |
| Wheat gluten meal1 | 50 | 50 | 50 | 50 |
| Corn protein concentrate3 | 60 | 60 | 60 | 60 |
| Blood meal1 | 50 | 50 | 50 | 50 |
| Wheat flour1 | 249 | 249 | 249 | 234 |
| FO1 | 160 | 0 | 0 | 0 |
| SO1 | 0 | 160 | 0 | 0 |
| BSLO4 | 0 | 0 | 160 | 160 |
| Bile salt (Ox-bile)5 | 0 | 0 | 0 | 15 |
| Dicalcium phosphate5 | 12 | 12 | 12 | 12 |
| Choline chloride (60%)5 | 6 | 6 | 6 | 6 |
| Vitamin premix6 | 8 | 8 | 8 | 8 |
| Trace mineral mixture7 | 1 | 1 | 1 | 1 |
| Vitamin C (Stay C-35%)8 | 2 | 2 | 2 | 2 |
| Total |
1,000 |
1,000 |
1,000 |
1,000 |
| Proximate composition, % | ||||
| Moisture | 6.0 | 6.0 | 6.2 | 7.4 |
| Crude protein | 44.8 | 44.5 | 44.1 | 44.4 |
| Crude lipid | 21.0 | 21.7 | 21.4 | 20.9 |
| Ash | 7.6 | 8.4 | 8.6 | 7.8 |
FO = fish oil; SO = soybean oil; BSLO = black soldier fly larvae oil; BA = bile acid.
Rangen Inc., Buhl, ID, USA.
Profine VF, The Solae Company, St. Louis, MO, USA.
Empyreal 75, Cargill Corn Milling, Cargill, Inc., Blair, NE, USA.
EnviroFlight, USA.
Oxbile extract powder (cholic acid: 51.2%), Lot No. 786269, Creative enzymes, USA.
Vitamin premix supplied the following per kg diet: vitamin A, 2.4 mg; vitamin D, 0.15 mg; vitamin E, 267 mg; vitamin K as menadione sodium bisulfite, 20 μg; thiamin as thiamin mononitrate, 32 mg; riboflavin, 64 mg; pyridoxine as pyridoxine-HCl, 64 mg; pantothenic acid as Ca-d-pantothenate, 192 mg; niacin as nicotinic acid, 240 mg; biotin, 0.56 mg; folic acid, 12 mg; vitamin B12, 50 μg; and inositol as meso-inositol, 400 mg.
US Fish and Wildlife Service Trace Mineral Premix #3. It supplied the following (mg/kg diet): Zn (as ZnSO4·7H2O), 75; Mn (as MnSO4), 20; Cu (as CuSO4·5H2O), 1.54; I (as KIO3), 10.
Skretting USA, Tooele, UT, USA.
Table 2.
Fatty acid composition of the total lipid fraction (% of total fatty acids) of insect oil, soy oil and fish oil.
| Item | Name | FO | SO | BSLO |
|---|---|---|---|---|
| C8:0 | Caprylic acid | – | – | 0.02 |
| C10:0 | Capric acid | – | – | 0.92 |
| C12:0 | Lauric acid | – | – | 40.1 |
| C13:0 | Tridecylic acid | – | 0.01 | |
| C14:0 | Myristic acid | 4.51 | – | 9.88 |
| C14:1 | Myristoleic acid | – | – | 0.13 |
| C15:0 | Pentadecylic acid | 0.61 | – | 0.08 |
| C16:0 | Palmitic acid | 15.71 | 11.01 | 13.1 |
| C16:1 | Palmitoleic acid | 5.76 | – | 1.54 |
| C16:2 | Palmitelaidic acid | – | – | 0.36 |
| C17:0 | Heptadecanoic acid | – | – | 0.12 |
| C18:0 | Stearic acid | 3.72 | 3.25 | 2.12 |
| C18:1 | Elaidic acid | 4.34 | – | 0.02 |
| C18:1n-9 | Oleic acid | 19.31 | 25.05 | 12.0 |
| C18:2n-6 | Linoleic acid | 5.71 | 54.55 | 0.01 |
| C18:3n-3 | α-Linolenic acid | 2.17 | 6.21 | – |
| C20:0 | Arachidic acid | 0.24 | – | 0.07 |
| C20:1n-9 | 11-Eicosenoic acid or Gondoic acid | 4.87 | – | 0.07 |
| C20:2n-6 | Eicosadienoic acid | 2.11 | – | – |
| C20:3n-6 | Dihomo-γ-linolenic acid | 0.17 | – | – |
| C20:4n-6 | Arachidonic acid | 0.95 | – | 0.07 |
| C20:3n-3 | Eicosatrienoic acid | 1.19 | – | – |
| C20:5n-3 | Eicosapentaenoic acid | 7.91 | – | – |
| C22:0 | Behenic acid | 0.15 | – | 0.03 |
| C22:1n-9 | Erucic acid | 5.06 | – | 0.05 |
| C22:5n-3 | Docosapentaenoic acid | 2.26 | – | – |
| C22:6n-3 | Docosahexaenoic acid | 10.45 | – | – |
| C24:0 |
Lignoceric acid |
0.08 |
– |
0.32 |
| ΣSFA | 25.02 | 14.26 | 66.77 | |
| ΣMUFA | 39.34 | 25.05 | 13.81 | |
| ΣPUFA | 32.92 | 60.76 | 0.44 | |
| Σn-3 PUFA | 23.98 | 6.21 | – | |
| Σn-6 PUFA | 8.94 | 54.55 | 0.08 | |
| EPA:DHA | 0.76 | – | – | |
| DHA:EPA | 1.32 | – | – | |
| n-3:n-6 PUFA | 2.68 | 0.11 | – | |
| n-6:n-3 PUFA | 0.37 | 8.78 | – |
FO = fish oil; SO = soybean oil; BSLO = black soldier fly oil; SFA = saturated fatty acid; MUFA = monounsaturated fatty acid; PUFA = polyunsaturated fatty acid; EPA = eicosapentaenoic acid; DHA = docosahexaenoic acid; LC-PUFA = long chain polyunsaturated fatty acid.
Twenty-five juvenile rainbow trout with an initial average weight of 32 ± 0.0 g were randomly stocked into each of the twelve 145-L experimental tanks and supplied with 8 L/min of gravity-fed spring water at constant temperature (14 °C). After a 2-week acclimation period on the control diet, each experimental diet was randomly allocated to 3 replicate tanks of rainbow trout following a completely randomized design, and fish were hand-fed to apparent satiation twice daily (09:00 and 17:00), 6 d per week for 10 weeks. The photoperiod was maintained at 14 h of light and 10 h of dark per day using electric timer-controlled fluorescent lights. Fish were weighed (Mettler Toledo: XS32001L, Switzerland) every 30 d to appraise growth rate and feed efficiency. Trout were fasted for 24 h before each measurement to avoid inclusion of ingested feed in the weight measurement. Before the start of the feeding trial, 12 fish were randomly selected for initial whole-body composition analysis.
At the conclusion of the feeding trial, fish from each tank were fasted for 24 h, counted, and batched weighed to determine the final growth response and nutrient utilization indices as specified below. Three fish were randomly selected per tank (n = 9 fish per diet) and anaesthetized with tricaine methanosulfonate (MS-222, 80 mg/L, buffered to pH 7.0) for blood collection using 1 mL hypodermic syringe. The blood was collected via the caudal vein, kept in ice to clot and serum was recovered by centrifugation at 2,000 × g for 10 min at 4 °C. The serum samples were stored at −80 °C and later analysed for glucose and antioxidant enzyme activity assays. Then, the sacrificed fish were dissected to remove various tissues for further analysis. Liver and visceral organs were removed for the determination of hepatosomatic index (HSI) and viscerosomatic index (VSI), respectively. A small section of liver and muscle samples were collected, flash-frozen in liquid nitrogen (N2) and stored at −80 °C until use for fatty acid profile analysis. The liver was further sub-sampled into 1.5-mL tubes (RNAase free, AXYGEN) for gene expression analysis, frozen in liquid N2 and stored as described above. Another 3 fish per tank were collected, euthanized with a lethal dose of MS-222 (300 mg/L), and kept at −20 °C for the determination of whole-body proximate composition.
2.3. Biological response indicators
Using the fish weight and feed consumption data, trout growth performance parameters were calculated from the following formulas to determine the effect of experimental diets:
| Weight gain (g) = Final body weight (g) – Initial body weight (g); |
| Weight gain (%) = [Weight gain (g)/Initial body weight (g)] × 100; |
| Specific growth rate (SGR, %/d) = 100 × [ln Final body weight (g) – ln Initial body weight (g)] /Duration of feeding; |
| Feed intake (g/fish) = Total dry feed given (g)/Number of fish; |
| Feed conversion ratio = Feed consumption (g)/[Final biomass (g) – Initial biomass (g) + Dead fish weight (g)]; |
| Protein efficiency ratio (PER) = Net weight gain (g)/Protein fed (g); |
| Nutrient retention (%) = 100 × Nutrient gain (g) /Nutrient consumed (g); |
| Condition factor (CF, %) = 100 × Weight of fish / (Length of fish)3; |
| HSI (%) = 100 × Wet weight of liver (g)/Whole body weight of fish (g); |
| VSI (%) = 100 × Wet weight of visceral (g)/Whole body weight of fish (g); |
| Survival (%) = 100 × (Total number of fish harvested/Total number of fish stocked) |
2.4. Analysis
2.4.1. Proximate and fatty acid composition
Proximate composition of trout whole-body and experimental diets were conducted per standard AOAC (2000) methods as described previously (Kumar et al., 2020). Briefly, dry matter was determined by drying samples overnight (12 h) in an oven (105 °C) to a constant weight. Crude protein content was determined (total nitrogen × 6.25) by combustion method with a nitrogen determinator (Elementar - rapid N exceed, Germany). Crude fat content was determined using an ANKOM XT 15 extractor (ANKOM Technology, Macedon, NY, USA) with petroleum ether as the extracting solvent. Ash content was determined by incineration at 600 °C in a muffle furnace for 4 h. The fatty acid composition of the oil (BSLO, SO and FO), liver and muscle samples were determined using a modified AOAC method 991.39 (AOAC, 1995). Briefly, samples were dried for 5 to 6 h under an N2 stream at 50 °C (OA-SYS heating system, Organomation Associates, Inc., Berlin, MA, USA). Thereafter, 2 mL of 0.5 mol/L NaOH was added for sample saponification at 70 °C for 60 min. Following sample cooling, free fatty acids were methylated by the addition of 2 mL 14% BF3 (Boron trifluoride, Sigma–Aldrich) in methanol and incubated at 70 °C for 60 min. After the samples cooled, 2 mL of hexane were added, inverted repeatedly for 60 s, and 1 mL of saturated NaCl was added. Samples were again inverted repeatedly for 60 s and then centrifuged at 2,000 × g for 5 min. An aliquot (100 μL) of the clarified hexane extract was diluted in hexane (1:10) and placed into autosampler vials for gas chromatography/mass spectrometry (GC/MS) analysis. The injection mode, helium flow rate, and the column temperature were as described by Overturf et al. (2013).
2.4.2. Hepatic gene expression
Total RNA was isolated from collected liver samples (n = 9 fish per diet) using TRIzol reagent (Invitrogen, USA) extraction method and performed following the manufacturer’s protocol. The RNA purity and quantity of samples were determined by measuring absorbance at 260 and 280 nm (NanoDrop, 2000 spectrophotometer, Thermo Scientific, USA). To ascertain the level of lipid metabolism-related gene expression, a quantitative real-time (RT)-PCR was performed with an ABI QuantStudio Real-Time PCR system using the TaqMan One-Step RT-PCR Master Mix Reagents kit with the procedure provided by ABI (Foster City, CA, USA). The concentration of each reaction was: Master Mix, 1 × (contains AmpliTaq Gold enzyme, dNTP including dUTP, a passive reference, and buffer components); MultiScribe reverse transcriptase, 0.25 U/μL; RNase inhibitor mix, 0.4 U/μL; forward primer 600 nmol/L; reverse primer 600 nmol/L; probe, 250 nmol/L; total RNA, 75 ng. The primer sequences of each gene, accession numbers, and probes are given in Table 3. The β-actin was used as the reference gene. The thermocycler conditions used for the tested genes consisted of 30 min at 48 °C, 10 min at 95 °C, then 40 PCR cycles of 95 °C for 15 s followed by 60 °C for 1 min. After carrying out the melting curve analysis to confirm a single PCR product in each reaction, the comparative CT method was used to determine the relative expression of the target genes (fas, fabp, elongase, Δ5-and Δ6-desaturases), and the values were normalized by 2−ΔΔCT method (Livak and Schmittgen, 2001) with β-actin as the reference gene.
Table 3.
Primers used for one-step quantitative real-time PCR.
| Genes | Gene symbols | Forward primers (5′-3′) | Reverse primers (5′-3′) | Probes | Accession number |
|---|---|---|---|---|---|
| Fatty acid synthase | Fas | GCCAGCGTGCCATGTTC | GGGCCGTTGAAGTCAAAGAA | CCAACCGACTCTCC | XM-021576228 |
| Fatty acid binding protein | Fabp | AGAGAAGTTCTCCCACATCAGAGA | CTTGCTTATCCTGGTGAGGGTT | TCACTGCGGCCTCAAC | XM-020500137.2 |
| Fatty acid elongase | Fae | CTGATGTACTCTTACTATGGGCTCTCT | AACTGAATCAGCTGTACTTGTGTGA | CCTTGCGGCCCTATCT | AY605100 |
| Delta-5- desaturase | CTGGGCCACATCC | CCAAAGCCAGGGCCTCTAG | GCCTCCTGTTCTTCAGCCTCTA | XM-014170388 | |
| Delta-6- desaturase | GACCATGTTTTCACAACGGAACTG | CAGAAGAAGCGAAGGTAG AAAGTCA | ACCACGCCAGATCCA | AY458652 | |
| β-actin | bact | CCCTCTTCCAGCCCTCCT T | AGTTGTAGGTGGTCT CGTGGATA | CCGCAA GACTCC ATACCGA | AF254414 |
2.4.3. Antioxidant enzyme and glucose assay
The superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) enzyme activities in the serum were measured using Cayman chemical assay kit (#706002, #707002, and #703102, respectively). The serum glucose concentration was quantified using a commercial kit (Cayman chemical, #10009582).
2.5. Statistical analysis
All data were tested for normality and homogeneity of variances before one-way ANOVA analysis to test for the significant difference in the means using IBM SPSS version 22 package. When significant difference was observed among the means (F-test), data were subjected to Duncan’s multiple range test to determine specific mean differences at a confidence level of P ≤ 0.05. Principal component analysis (PCA) was also used to relate dietary oil sources with tissue fatty acid composition in order to emphasize differences among the groups. The correlation matrix was used, and the first two principal components were plotted.
3. Results
3.1. Growth performance, feed efficiency, and somatic indices
The BA supplemented group (BSLO + BA) recorded the lowest growth performance (weight gain [g and %] and SGR) compared with other groups (P = 0.009; P = 0.007, respectively), whereas other groups exhibited similar growth performance. Fish grew nearly 6-fold of initial body weight when fed the FO, SO, or BSLO diet (Table 4) for 10 weeks. There was no significant difference noticed in feed intake, feed conversion ratio, or PER values among the tested dietary groups (P > 0.05). Highest VSI was observed in BSLO and BSLO + BA fed fish compared to the lowest values recorded in the FO and SO groups (P = 0.004). No significant differences were observed in HSI, CF, or survival of fish fed different dietary oil sources (P > 0.05).
Table 4.
Growth performance, feed utilization efficiency, and body indices of rainbow trout fed experimental diets for 10 weeks1.
| Item | FO | SO | BSLO | BSLO + BA | P-value |
|---|---|---|---|---|---|
| Final body weight, g | 186.60 ± 5.89a | 180.48 ± 5.48a | 185.14 ± 3.65a | 157.42 ± 2.59b | 0.009 |
| Weight gain2, g | 154.61 ± 5.88a | 148.48 ± 5.48a | 153.14 ± 3.65a | 125.42 ± 2.59b | 0.009 |
| Body weight gain3, % | 483.15 ± 18.39a | 464.00 ± 17.12a | 478.57 ± 11.41a | 391.94 ± 8.10b | 0.009 |
| SGR4, %/d | 2.52 ± 0.05a | 2.47 ± 0.04a | 2.51 ± 0.03a | 2.27 ± 0.02b | 0.007 |
| FI5, g/fish | 151.18 ± 1.15 | 146.29 ± 0.10 | 157.86 ± 1.52 | 140.43 ± 9.96 | 0.435 |
| FCR6 | 0.98 ± 0.03 | 0.99 ± 0.04 | 1.04 ± 0.02 | 1.12 ± 0.06 | 0.258 |
| PER7 | 2.25 ± 0.08 | 2.34 ± 0.06 | 2.20 ± 0.02 | 2.04 ± 0.12 | 0.122 |
| Survival8, % | 100 ± 0.00 | 96.00 ± 0.00 | 96.00 ± 0.00 | 97.33 ± 2.67 | 0.530 |
| CF9, % | 1.48 ± 0.02 | 1.49 ± 0.03 | 1.47 ± 0.03 | 1.39 ± 0.04 | 0.098 |
| HSI10, % | 1.24 ± 0.06 | 1.10 ± 0.02 | 1.20 ± 0.02 | 1.25 ± 0.06 | 0.061 |
| VSI11, % | 10.34 ± 0.56b | 10.37 ± 0.33b | 12.13 ± 0.28a | 11.75 ± 0.40a | 0.004 |
FO = fish oil; SO = soybean oil; BSLO = black soldier fly oil; BA = bile acid.
Data expressed as mean ± SE, n = 3 (each replicate tank was stocked with 25 fish), values along row with different superscripts differ significantly (P < 0.05). Initial average weight of fish is 32 ± 0.0 g.
Weight gain (g) = Final body weight (g) – Initial body weight (g).
Weight gain (%) = [Weight gain (g)/Initial body weight (g)] × 100.
Specific growth rate (SGR, %/d) = 100 × [ln Final body weight (g) – ln Initial body weight (g)]/Duration of feeding.
Feed intake (g/fish) = Total dry feed given (g)/Number of fish.
Feed conversion ratio (FCR) = Feed consumption (g)/[Final biomass (g) – Initial biomass (g) + Dead fish weight (g)].
Protein efficiency ratio (PER) = Net weight gain (g)/Protein fed (g).
Survival (%) = 100 × (Total number of fish harvested/Total number of fish stocked).
Condition factor (CF, %) = 100 × Weight of fish/(Length of fish)3.
Hepatosomatic index (HSI, %) = 100 × Wet weight of liver (g)/Whole body weight of fish (g).
Viscerasomatic index (VSI, %) = 100 × Wet weight of visceral (g)/Whole body weight of fish (g).
3.2. Whole-body composition and nutrient retention
The whole-body composition and nutrient retention of rainbow trout fed different oil sources are shown in Table 5. Crude protein, lipid, and ash contents were not significantly different (P > 0.05); however, fish fed the FO-based diet had the lowest moisture content compared to the SO, BSLO, and BSLO + BA groups (P = 0.018). The efficiency of protein and lipid retentions among the dietary groups was unaffected by the dietary oil sources (P = 0.091 and P = 0.189, respectively).
Table 5.
Whole-body composition and nutrient retention (%, wet-weight basis) of rainbow trout fed experimental diets for 10 weeks1.
| Item | FO | SO | BSLO | BSLO + BA | P-value |
|---|---|---|---|---|---|
| Whole-body composition | |||||
| Moisture | 66.98 ± 0.39b | 68.15 ± 0.27a | 68.37 ± 0.22a | 68.45 ± 0.17a | 0.018 |
| Protein | 16.89 ± 0.12 | 16.72 ± 0.33 | 16.32 ± 0.24 | 16.25 ± 0.18 | 0.235 |
| Lipid | 13.81 ± 0.34 | 13.26 ± 0.27 | 13.57 ± 0.22 | 13.17 ± 0.12 | 0.331 |
| Ash | 2.17 ± 0.02 | 2.12 ± 0.05 | 2.09 ± 0.05 | 2.21 ± 0.07 | 0.431 |
| Retention efficiency | |||||
| Protein retention1 | 39.09 ± 1.27 | 40.38 ± 1.91 | 37.18 ± 0.36 | 34.40 ± 1.85 | 0.091 |
| Lipid retention1 | 71.38 ± 3.01 | 68.39 ± 2.41 | 66.94 ± 1.21 | 62.51 ± 3.25 | 0.189 |
Data expressed as mean ± SE, n = 3, values along row with different superscripts differ significantly (P < 0.05). FO = fish oil; SO = soybean oil; BSLO = black soldier fly oil; BA = bile acid. Initial average weight of fish is 32 ± 0.0 g. Initial whole-body composition of the experimental fish (moisture 75.52%; crude protein 13.40%; crude lipid 7.94%; ash 2.19%).
Nutrient retention (%) = 100 × Nutrient gain (g)/Nutrient consumed (g).
3.3. Fatty acid composition of muscle and hepatic tissue
The fatty composition of the muscle and liver were influenced by the oil sources of the diet (Table 6, Table 7, respectively). The muscle and liver 14:0, 16:0, and total SFA proportion of rainbow trout fed BSLO or BSLO + BA diets were significantly higher than those fed FO and SO (P < 0.001). The highest total MUFA and PUFA proportion in the muscle, which was similar to liver deposition, was observed in fish fed FO and SO diets, respectively (P < 0.001). Dietary SO resulted in higher 18:2n-6 and 18:3n-3 deposition in the liver and muscle compared to other groups, however, the BSLO-based groups had a higher 18:2n-6 deposition than FO-fed fish. The muscle 20:4n-6 deposition in BSLO fish was higher than in FO and SO fed fish while BSLO and BSLO + BA were found to be similar (P < 0.001; Table 6). However, no difference was found in liver 20:4n-6 content of trout fed SO, BSLO, or BSLO + BA, but the FO group recorded significantly lower deposition (P < 0.001; Table 7). Fish fed the FO diet had a significantly higher eicosapentaenoic acid (EPA; 20:5n-3) content in both the muscle and liver than those fed SO and BSLO-based diets (P < 0.001), while EPA in the liver of the BSLO groups was found to be lower than that in SO fed fish. The highest fillet docosahexaenoic acid (DHA; 22:6n-3) deposition and DHA:EPA ratio occurred in the BSLO and BSLO + BA groups followed by SO and FO (Table 6), whereas in the liver, trout fed FO had the highest DHA and the lowest DHA:EPA ratio. The highest ratio of EPA to DHA or n-3:n-6 ratio in both the muscle and liver was detected in FO fed fish (P < 0.001), but no significant difference was recorded among the other dietary groups. In the liver, fish fed FO diet had the highest total n-3 PUFA compared to the other dietary groups (P < 0.001), and no significant difference was found between the SO and BSLO + BA groups. Muscle EPA + DHA showed no differences among the dietary groups (P = 0.233), whereas significant variation was seen in the liver with the highest value observed in FO fish (P < 0.001). Higher total n-6 PUFA content was observed in the SO group followed by BSLO, BSLO + BA, and FO in both the muscle and liver (P < 0.001).
Table 6.
Fatty acid composition of the total lipid fraction (% of total fatty acids) in the muscle of rainbow trout fed experimental diets for 10 weeks1.
| Item | Name | FO | SO | BSLO | BSLO + BA | P-value |
|---|---|---|---|---|---|---|
| Saturates | ||||||
| C14:0 | Myristic acid | 3.35 ± 0.07b | 0.82 ± 0.04c | 4.86 ± 0.18a | 4.71 ± 0.31a | <0.001 |
| C16:0 | Palmitic acid | 14.64 ± 0.81c | 17.84 ± 0.65b | 22.87 ± 0.39a | 23.54 ± 0.51a | <0.001 |
| C18:0 | Stearic acid | 4.96 ± 0.09b | 6.43 ± 0.33a | 5.91 ± 0.10a | 6.21 ± 0.21a | <0.001 |
| ΣSFA | 22.95 ± 0.80c | 25.09 ± 0.90b | 33.65 ± 0.53a | 35.13 ± 0.64a | <0.001 | |
| Monoenes | ||||||
| C16:1n-7 | Palmitoleic acid | 5.23 ± 0.08a | 1.56 ± 0.05c | 3.18 ± 0.08b | 3.24 ± 0.39b | <0.001 |
| C18:1n-9 | Oleic acid | 23.38 ± 0.19a | 17.57 ± 0.52b | 18.64 ± 0.37b | 19.43 ± 1.37b | <0.001 |
| C20:1n-9 | 11-Eicosenoic acid or Gondoic acid | 12.56 ± 0.62a | 1.34 ± 0.03b | 1.04 ± 0.04b | 1.00 ± 0.10b | <0.001 |
| C22:1n-9 | Erucic acid | 5.69 ± 0.78a | ND | ND | ND | <0.001 |
| ΣMUFA | 46.86 ± 1.48a | 20.47 ± 0.52b | 22.86 ± 0.41b | 21.99 ± 0.88b | <0.001 | |
| Polyunsaturate | ||||||
| C18:2n-6 | linoleic acid | 8.50 ± 0.19c | 30.13 ± 1.47a | 18.82 ± 0.44b | 17.41 ± 1.07b | <0.001 |
| C18:3n-3 | α-Linolenic acid | 1.13 ± 0.07b | 4.13 ± 0.17a | 1.28 ± 0.06b | 1.50 ± 0.24b | <0.001 |
| C20:2n-6 | Eicosadienoic acid | 0.48 ± 0.01d | 1.82 ± 0.08a | 1.32 ± 0.03b | 1.09 ± 0.07c | <0.001 |
| C20:3n-6 | Dihomo-γ-linolenic acid | 0.26 ± 0.00c | 1.62 ± 0.09a | 1.46 ± 0.05ab | 1.33 ± 0.07b | <0.001 |
| C20:4n-6 | Arachidonic acid | 0.75 ± 0.03c | 1.78 ± 0.15b | 2.31 ± 0.12a | 2.00 ± 0.23ab | <0.001 |
| C20:5n-3 | Eicosapentaenoic acid | 6.29 ± 0.09a | 1.87 ± 0.15b | 1.90 ± 0.11b | 2.13 ± 0.18b | <0.001 |
| C22:5n-3 | Docosapentaenoic acid | 1.98 ± 0.08a | 0.79 ± 0.05c | 1.02 ± 0.08b | 0.82 ± 0.07bc | <0.001 |
| C22:6n-3 | Docosahexaenoic acid | 11.42 ± 1.10b | 12.78 ± 1.20ab | 15.39 ± 0.67a | 15.43 ± 1.09a | 0.017 |
| ΣPUFA | 30.19 ± 0.79c | 54.44 ± 0.68a | 43.49 ± 0.67b | 42.88 ± 1.18b | <0.001 | |
| EPA + DHA | 17.70 ± 1.15 | 14.65 ± 1.33 | 17.29 ± 0.75 | 17.56 ± 1.24 | 0.233 | |
| EPA:DHA | 0.58 ± 0.04a | 0.15 ± 0.01b | 0.12 ± 0.00b | 0.14 ± 0.01b | <0.001 | |
| DHA:EPA | 1.81 ± 0.16c | 6.82 ± 0.29b | 8.20 ± 0.33a | 7.37 ± 0.39ab | <0.001 | |
| Σn-3 PUFA | 20.81 ± 1.05 | 19.57 ± 1.20 | 19.59 ± 0.76 | 19.17 ± 1.39 | 0.735 | |
| Σn-6 PUFA | 9.38 ± 0.38c | 34.87 ± 1.55a | 23.91 ± 0.29b | 21.71 ± 1.33b | <0.001 | |
| n-3:n-6 ratio | 2.28 ± 0.21a | 0.58 ± 0.06b | 0.82 ± 0.04b | 0.90 ± 0.07b | <0.001 | |
FO = fish oil; SO = soybean oil; BSLO = black soldier fly oil; BA = bile acid; SFA = saturated fatty acid; ND = not detected; MUFA = monounsaturated fatty acid; PUFA = polyunsaturated fatty acid; EPA = eicosapentaenoic acid; DHA = docosahexaenoic acid.
Data expressed as mean ± SE, n = 9; values along row with different superscripts differ significantly (P < 0.05). Initial average weight of fish is 32 ± 0.0 g.
Table 7.
Fatty acid composition of the total lipid fraction (% of total fatty acids) in rainbow trout liver fed experimental diets for 10 weeks1.
| Item | Name | FO | SO | BSLO | BSLO + BA | P-value |
|---|---|---|---|---|---|---|
| Saturates | ||||||
| C14:0 | Myristic acid | 2.04 ± 0.07b | 0.55 ± 0.02c | 4.05 ± 0.26a | 3.60 ± 0.30a | <0.001 |
| C16:0 | Palmitic acid | 15.10 ± 0.26b | 13.79 ± 0.20c | 15.44 ± 0.23ab | 16.07 ± 0.45a | <0.001 |
| C18:0 | Stearic acid | 6.74 ± 0.14c | 8.52 ± 0.12a | 8.01 ± 0.25ab | 7.75 ± 0.17b | <0.001 |
| C20:0 | Arachidic acid/eicosanoic acid | ND | 0.15 ± 0.01a | 0.11 ± 0.01b | 0.12 ± 0.00b | <0.001 |
| ΣSFA | 23.88 ± 0.26b | 23.00 ± 0.22b | 27.60 ± 0.41a | 27.55 ± 0.30a | <0.001 | |
| Monoenes | ||||||
| C16:1n-7 | Palmitoleic acid | 2.64 ± 0.23a | 0.70 ± 0.03b | 2.83 ± 0.27a | 2.28 ± 0.32a | <0.001 |
| C18:1n-9 | Oleic acid | 17.42 ± 1.01a | 12.37 ± 0.33b | 20.54 ± 1.05a | 18.00 ± 1.76a | <0.001 |
| C20:1n-9 | 11-Eicosenoic acid or Gondoic acid | 6.27 ± 0.35a | 1.79 ± 0.08c | 2.90 ± 0.17b | 2.49 ± 0.26b | <0.001 |
| C22:1n-9 | Erucic acid | 1.56 ± 0.13a | 0.10 ± 0.01b | 0.17 ± 0.02b | 0.14 ± 0.01b | <0.001 |
| ΣMUFA | 27.90 ± 1.69a | 14.96 ± 0.41c | 26.44 ± 1.44ab | 22.91 ± 2.30b | <0.001 | |
| Polyunsaturate | ||||||
| C18:2n-6 | Linoleic acid | 3.05 ± 0.11c | 15.84 ± 0.54a | 10.65 ± 0.44b | 10.54 ± 0.27b | <0.001 |
| C18:3n-3 | α-Linolenic acid | 0.34 ± 0.01b | 1.02 ± 0.06a | 0.25 ± 0.02c | 0.22 ± 0.01c | <0.001 |
| C18:3n-6 | γ-Linolenic acid | ND | 0.24 ± 0.01a | 0.16 ± 0.01c | 0.22 ± 0.07b | <0.001 |
| C20:2n-6 | Eicosadienoic acid | 0.84 ± 0.03c | 4.40 ± 0.23a | 2.52 ± 0.08b | 2.37 ± 0.14b | <0.001 |
| C20:3n-6 | Dihomo-γ-linolenic acid | 0.33 ± 0.01d | 2.99 ± 0.10a | 2.53 ± 0.08b | 2.31 ± 0.08c | <0.001 |
| C20:4n-6 | Arachidonic acid | 3.33 ± 0.19b | 7.48 ± 0.34a | 6.36 ± 0.36a | 6.94 ± 0.51a | <0.001 |
| C20:3n-3 | Eicosatrienoic acid | 0.16 ± 0.02b | 0.38 ± 0.01a | 0.10 ± 0.01c | 0.09 ± 0.01c | <0.001 |
| C20:5n-3 | Eicosapentaenoic acid | 7.59 ± 0.21a | 2.48 ± 0.10b | 1.35 ± 0.08c | 1.55 ± 0.11c | <0.001 |
| C22:5n-3 | Docosapentaenoic acid | 1.91 ± 0.04a | 0.65 ± 0.02b | 0.46 ± 0.03c | 0.50 ± 0.04c | <0.001 |
| C22:6n-3 | Docosahexaenoic acid | 30.67 ± 1.40a | 26.56 ± 0.85b | 21.57 ± 1.46c | 24.82 ± 1.75bc | 0.001 |
| ΣPUFA | 48.23 ± 1.57b | 62.04 ± 0.34a | 45.96 ± 1.49b | 49.55 ± 2.07b | <0.001 | |
| EPA + DHA | 38.25 ± 1.53a | 29.03 ± 0.88b | 22.92 ± 1.53c | 26.37 ± 1.85bc | <0.001 | |
| EPA:DHA | 0.25 ± 0.01a | 0.09 ± 0.00b | 0.06 ± 0.00c | 0.06 ± 0.00c | <0.001 | |
| DHA:EPA | 4.04 ± 0.15c | 10.84 ± 0.52b | 16.02 ± 0.53a | 16.07 ± 0.50a | <0.001 | |
| Σn-3 PUFA | 40.68 ± 1.50a | 31.08 ± 0.84b | 23.74 ± 1.56c | 27.16 ± 1.86bc | <0.001 | |
| Σn-6 PUFA | 7.55 ± 0.12c | 30.96 ± 0.54a | 22.22 ± 0.16b | 22.38 ± 0.34b | <0.001 | |
| n-3:n-6 ratio | 5.39 ± 0.17a | 1.01 ± 0.04b | 1.07 ± 0.07b | 1.21 ± 0.08b | <0.001 | |
FO = fish oil; SO = soybean oil; BSLO = black soldier fly oil; BA = bile acid; SFA = saturated fatty acid; MUFA = monounsaturated fatty acid; ND = not detected; PUFA = polyunsaturated fatty acid; EPA = eicosapentaenoic acid; DHA = docosahexaenoic acid.
Data expressed as mean ± SE, n = 9; values along row with different superscripts differ significantly (P < 0.05). Initial average weight of fish is 32 ± 0.0 g.
3.4. Principal component analysis of the liver and muscle fatty acid profile
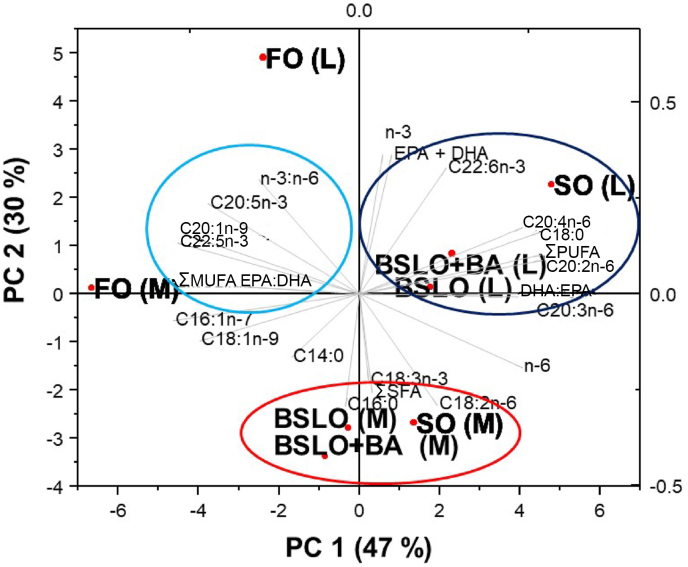
The PCA analysis showed that the two components (PC1 and PC2) represented 77% of the total variance in rainbow trout liver and muscle fatty acid composition (Fig. 1). Several fatty acids and LC-PUFA in the liver and muscle of fish fed either SO or BSLO-based diets were found to be grouped together and are in close proximity to each other, which accounted for 47% of the total variance (PC1). However, FO was found to be separated from other oil sources and accounted for 30% (PC2) of the total variance.
Fig. 1.
Principal component (PC) analysis of fatty acids allocation in the liver (L) and muscle (M) of rainbow trout fed with different dietary oil sources for 10 weeks. FO = fish oil; SO = soybean oil; BSLO = black soldier fly larvae oil; BA = bile acid; EPA = eicosapentaenoic acid; DHA = docosahexaenoic acid. Initial average weight of fish is 32 ± 0.0 g.
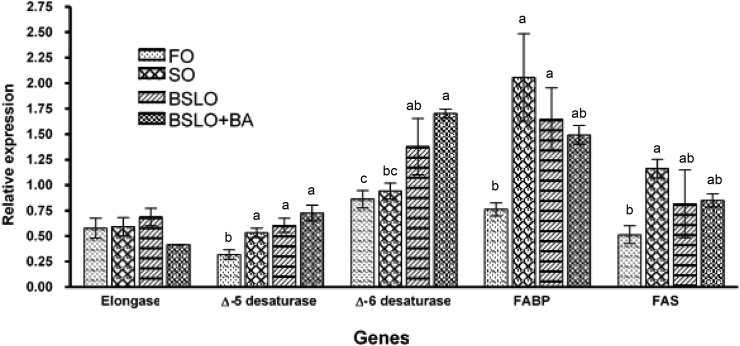
3.5. Expression of hepatic lipid metabolism-related genes
The relative expression of hepatic fabp and fas in rainbow trout fed the different dietary oils is shown in Fig. 2. Fish fed the FO diet had significantly lower fabp expression compared to those given the SO and BSLO diets (P = 0.02) but was similar to the BSLO + BA group (Fig. 2). Hepatic fas expression in trout fed SO was higher than that fed the FO diet; however, no statistically significant difference was observed when compared to fish fed BSLO and BSLO + BA based diets. There were no significant differences (P = 0.37) in the mRNA expression level of fatty acid elongase among the dietary oil groups (Fig. 2). The relative mRNA expression level of hepatic Δ5-desaturase in trout fed the FO diet was significantly lower than those fed the other dietary oils (P = 0.005); whereas, no difference was observed among fish fed the SO, BSLO, or BSLO + BA diets. Expression of the Δ6-desaturase was significantly higher in the BSLO + BA-fed fish, which was similar to the BSLO group but differed significantly from those fed the FO or SO diet (P = 0.006).
Fig. 2.
Relative mRNA expression of hepatic fatty acid metabolism related genes in rainbow trout fed diets containing fish oil (FO), soybean oil (SO), black soldier fly oil (BSLO), or BSLO + bile acid (BA) for 10 weeks. fabp = fatty acid-binding protein; fas = fatty acid synthase. Different lower case letters within the same gene denote statistically significant differences (P < 0.05). Data expressed as means ± SE (n = 9 × 2). Initial average weight of fish is 32 ± 0.0 g.
3.6. Antioxidant enzyme and glucose assay
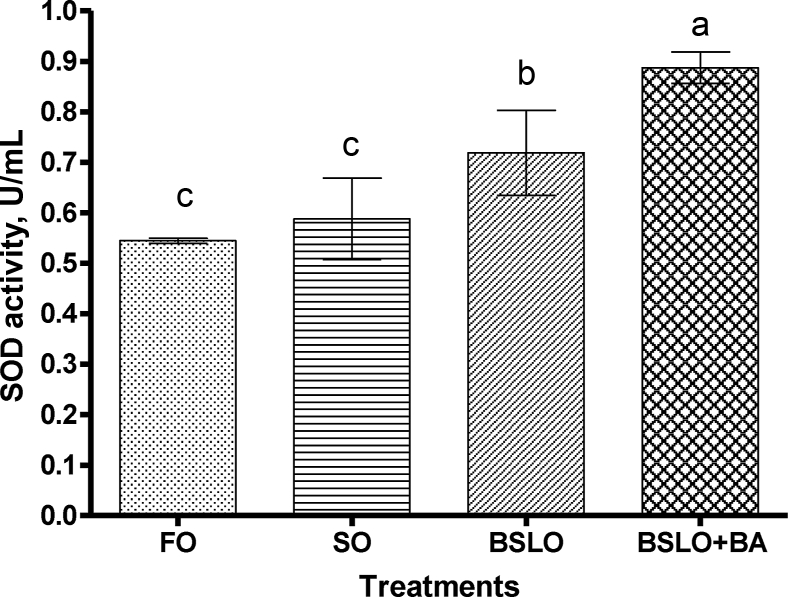
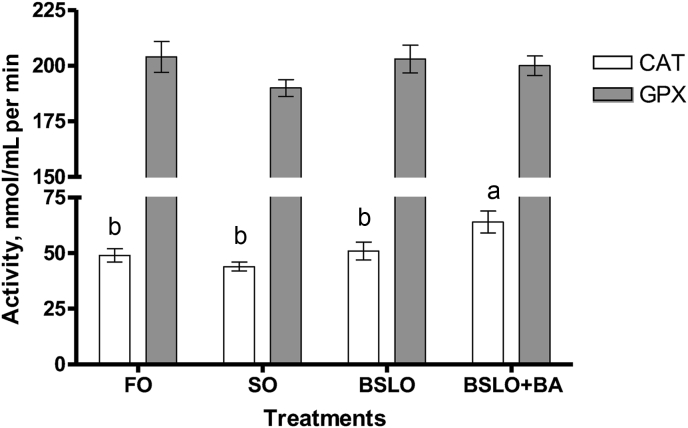
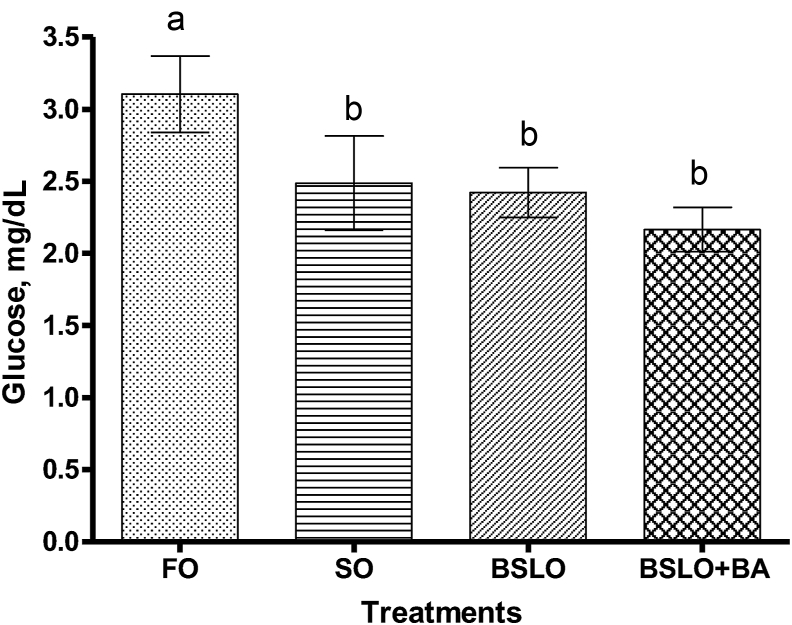
SOD enzyme activity in serum was found to be higher in fish fed the BSLO + BA diet with the lowest activity seen in the FO and SO groups (P < 0.001) (Fig. 3). Serum CAT activity of trout fed the FO, SO and BSLO diets were significantly lower than those fed the BSLO + BA diet (P = 0.03); whereas, no significant difference was recorded in the GPx activity among the dietary groups (Fig. 4). Serum glucose concentration was significantly higher in FO fed fish compared to the SO, BSLO, and BSLO + BA groups (P = 0.001; Fig. 5).
Fig. 3.
Serum superoxide dismutase (SOD) activity in rainbow trout fed diet containing fish oil (FO), soybean oil (SO), black soldier fly oil (BSLO), or BSLO + bile acid (BA) for 10 weeks. Different lower case letters between different dietary groups denote statistically significant differences (P < 0.05). Data expressed as means ± SE (n = 9). Initial average weight of fish is 32 ± 0.0 g.
Fig. 4.
Catalase (CAT) and glutathione peroxidase (GPx) enzyme activities in the serum of rainbow trout fed diets containing fish oil (FO), soybean oil (SO), black soldier fly oil (BSLO), or BSLO + BA (BSLO + bile acid) for 10 weeks. Different lower case letters between different dietary groups denote statistically significant differences (P < 0.05). Data expressed as means ± SE (n = 9). Initial average weight of fish is 32 ± 0.0 g.
Fig. 5.
Serum glucose concentration of rainbow trout fed diets containing fish oil (FO), soybean oil (SO), black soldier fly oil (BSLO), or BSLO + bile acid (BA) for 10 weeks. Different lower case letters between different dietary groups denote statistically significant differences (P < 0.05). Data expressed as means values ± SE (n = 9). Initial average weight of fish is 32 ± 0.0 g.
4. Discussion
The complete replacement of FO and SO with BSLO in the diet of rainbow trout is possible without any significant alteration in the growth performance and nutrient retention efficiency. Our results are consistent with those of Li et al. (2016) in Jian carp in which black solider fly oil was found to have no negative effects on growth response indices when completely replacing SO. Similarly, Dumas et al. (2018) reported that rainbow trout could utilize 10% BSLO without adversely impacting growth or nutrient utilization and deposition. The authors, however, hypothesized that higher dietary inclusion levels could be possible, and this study has revealed that rainbow trout can utilize up to 160 g/kg BSLO. Numerous studies have reported that replacement of FO with an alternative oil in which the essential fatty acid requirements are met does not impair the growth performance of fish (Hixson et al., 2017; Luo et al., 2014; Peng et al., 2017; Turchini et al., 2010). Thus, it can be inferred that the level of BSLO added in this study was not beyond the tolerance level of rainbow trout and the fatty acids requirements were not compromised. Furthermore, the voluntary feed intake among the treatment groups was similar indicating that diet palatability/acceptability was unaffected by oil source, and this further supports the suitability of BSLO as full replacement of FO or SO in rainbow trout diets.
BA or its salt has been reported to enhance growth performance in rainbow trout (Iwashita et al., 2008; Yamamoto et al., 2007b), yellow croaker (Ding et al., 2020), and largemouth bass (Guo et al., 2020) due to their emulsifying effect on lipids for digestion and absorption. On the contrary, supplementation of BA to BSLO in the current study resulted in growth depression, and this noticeable effect could be attributed to the level of BA which appear to be more than the level tolerated by rainbow trout. Excessive addition of BA has been reported to cause growth depression, gallstone formation, and vacuolization of hepatocyte in genetically improved farmed tilapia (GIFT) (Jiang et al., 2018). Also, trout fed the BA-based diet showed a slow response to feeding (visual observation) compared to other dietary groups, even though the variation in feed intake was not statistically significant. The similarity of HSI of fish fed either FO, SO, or BSLO-based diets implies that the 18:3n-3 or n-3 LC-PUFA in the diets were sufficient and consistent with results reported for turbot (Peng et al., 2017) and rainbow trout (Li et al., 2016). However, Teoh and Ng (2016) noted that feeding bleached palm olein to hybrid tilapia caused abnormal fat deposition and higher HSI value, which was linked to deficiency of EFA in the diet. Furthermore, the whole-body lipid content of trout was similar irrespective of the oil source, which is in agreement with Liet al. (2016) where partial or total replacement of SO with BSLO in Jian carp did not affect the whole-body lipid contents. Similarly, replacement of FO with vegetable oil in different fish species does not influence the whole-body contents (Alhazzaa et al., 2011; Nayak et al., 2017; Yang et al., 2020). Nutrient (protein and lipid) retention was similar among experimental diets. The similarity of lipid retention among diets indicates that the quantity of ingested lipid retained by trout was unaffected by the dietary lipid source and that this nutrient met the physiological requirement as noted by Lee et al. (2020). Furthermore, digestible protein intake in excess or below the required levels could contribute to increased lipid storage and reduced protein retention supporting that the protein requirements of rainbow trout in this study were also met.
Substitution of FO with alternative oils has been reported to change the fatty acid composition of fish tissue and quality of the fillet (Peng et al., 2017; Turchini and Francis, 2009; Yang et al., 2020; Yu et al., 2019). In the current study, it was evident that the muscle and liver fatty acid composition mirrored that of the diet with fish fed the SO and BSLO-based diets having the highest 18:2n-6 and 18:3n-3 and 14:0, 16:0, and total SFA, respectively, than trout fed the FO diet. Dietary FO resulted in higher muscle deposition of EPA and a higher n-3:n-6 ratio, but the BSLO-based diet produced the highest DHA which invariably led to similarities in muscle EPA + DHA. The highest deposition of ARA (20:4n-6), EPA (20:5n-3) and DHA (22:6n-3) occurred in the muscle of the BSLO-fed fish despite low dietary levels of 18:2n-6 and 18:3n-3 compared to the SO diet which signifies the high capacity of rainbow trout to biosynthesize LC-PUFA (i.e. ARA, EPA and DHA) from their specific substrate when black soldier fly oil is fed. This was further revealed by Δ6-fatty acid desaturase activity which was higher in the BSLO groups. In a similar study, Liet al. (2016) observed that the levels of ARA and DHA in the muscle of Jian carp fed 100% BSLO were significantly higher than in those fed a SO diet. Also, turbot fed a palm oil-based diet had a higher deposition of ARA and DHA than SO fed fish (Peng et al., 2017). Contrarily, replacement of FO with linseed oil (LO) resulted in a lower whole-body of LC-PUFA in Manchurian trout (Yu et al., 2019). The lower EPA and n-3:n-6 ratio seen in the BSLO groups compared to FO are consistent with other studies where fish were fed vegetable oil (Mu et al., 2020; Peng et al., 2017; Yang et al., 2020; Yu et al., 2019). Furthermore, the close proximity in PCA of fish fed SO and BSLO signifies that BSLO has a comparable fatty acids composition and is suitable for use in place of SO in trout feeds.
FABP are intracellular cytoplasmic proteins that are active in the transport of fatty acids and other lipid-soluble substances through the cytoplasm (Castro et al., 2015; Torstensen et al., 2009). In this study, the relative mRNA expression of the fabp was significantly up-regulated in fish fed the SO and BSLO-based diet compared with the FO group, which indicates increased fatty acid uptake and transport in the hepatic cells. In contrast, the expression of fabp in the white muscle of Atlantic salmon fed a vegetable oil blend was down-regulated (Torstensen et al., 2009), while no significant differences were noticed in the hepatic fabp gene expression of European seabass fed a vegetable oil blend and FO (Castro et al., 2015). The differences noticed in mRNA expression of fabp in this study compared to previous findings could be due to the type of oil used, species of fish, as well as the tissue assayed. FAS plays a crucial role in the synthesis of fatty acids and its activity could be correlated with the dietary saturated fatty acid composition. Peng et al. (2017) reported that substituting FO with palm oil or rapeseed oil resulted in higher fas expression in turbot, and this was related to the higher SFA contents in the diets, which is consistent with our results. Thus, the higher hepatic total SFA contents observed in BSLO-fed groups is related to the up-regulation of fas expression in the liver.
The expression of fatty acid elongase and desaturase genes are known to be influenced by the dietary fatty acid composition (Jordal et al., 2005; Zheng et al., 2004). Different studies have reported increased expression of Δ6-and Δ5-desaturases, and elongase activities when an alternative oil replaced FO in fish diet (Alhazzaa et al., 2011; Bell et al., 2001; Izquierdo et al., 2008; Teoh and Ng, 2016). In the current study, feeding BSLO resulted in increased hepatic and muscle accumulation of 18:2n-6, 20:2n-6, 20:3n-6, 20:4n-6, and muscle 22:6n-3 compared to FO fed fish. Except for 18:2n-6, all other mentioned fatty acids found at higher concentration in the fish tissues were all at low levels in the experimental diets, which indicates that upregulation of Δ6-and Δ5-desaturases in the BSLO-fed groups aided in the biosynthesis of LC-PUFA (Fig. 2). However, expression of fatty acid elongase, responsible for chain elongation, did not differ significantly among the dietary groups; but the BSLO-fed fish recorded the highest numerical value. The higher gene expression of Δ6-and Δ5-desaturase enzymes suggest that the fish made use of the LC-PUFA precursor (18:2n-6 and 18:3n-3) effectively to synthesize and tissue-accumulate greater levels of DHA in the fillet. Similar results were reported in rainbow trout (GÜLER and Yildiz, 2011; Turchini and Francis, 2009), hybrid tilapia (Teoh and Ng, 2016), gilthead seabream larvae (Izquierdo et al., 2008), and Lates calcarifer (Alhazzaa et al., 2011) fed vegetable oil as a substitute for FO. The high level of EPA and DHA in the FO diet could be the reason for decreased mRNA expression levels of Δ5-and Δ6-desaturases in FO fed fish due to a feedback inhibition. High dietary concentrations of EPA and DHA have been reported to inhibit the pathway responsible for LC-PUFA synthesis in fish (Jordal et al., 2005; Teoh and Ng, 2016; Tocher et al., 2000).
Dietary BSLO caused a significant reduction in serum glucose concentration in rainbow trout compared to the FO group, and this is similar to the observation of Dumas et al. (2018) who reported that BSLO might have antihyperglycemic effect in fasted rainbow trout. On the other hand, no effect on glucose concentration was recorded when Jian carp was fed BSLO as replacement for SO (Li et al., 2016), and this may be due to use of plant oil as a control, which possibly has bioactive compounds. SOD, CAT and GPx are important antioxidant defense enzymes which help to protect cells against reactive oxygen species-triggered damage in fish (Fawole et al., 2020; Shamna et al., 2020; Yu et al., 2019). In the present study, the activity of SOD was found to be higher in BSLO + BA fed trout compared to the other dietary groups. The increased activity which indicates some level of oxidative stress may be associated with dietary addition of BA. BA help to improve digestion and absorption capacity of lipids in fish; however, it causes a cytotoxic effect to hepatocytes when supplemented in excess (Jiang et al., 2018), and this may induce production of reactive oxygen species and damage to liver cells. This was further supported by the CAT activity, which was significantly higher in the BSLO + BA group. The function of CAT and GPx enzymes are to decompose hydrogen peroxide formed by the action of SOD to a non-toxic constituent (Yu et al., 2019; Fawole et al., 2020), which is likely the reason for the higher CAT activity observed in BSLO + BA fed fish. Although the SOD activity in BSLO group was higher than those fed FO and SO, however, no effect was noticed on the CAT and GPx activities. Hence, the higher response noticed could not be related to stress, however, this require further study.
In conclusion, dietary black solider fly larvae oil (BSLO) has proven to be a suitable alternative lipid source in rainbow trout diets without adversely affecting growth performance, feed efficiency, nutrient retention and survival. Higher DHA accumulation and DHA:EPA ratio, and similar EPA + DHA and total n-3 PUFA in muscle correlated with up-regulated mRNA expression of genes related to LC-PUFA biosynthesis. Furthermore, the lower serum glucose levels and antioxidant enzyme activities demonstrated that replacement of FO with BSLO did not elicit oxidative-induced stress. Thus, it could be inferred that the addition of 160 g/kg BSLO in place of either FO or SO in the rainbow trout diet did not cause any deleterious effect on the fish performance and muscle deposition of essential fatty acid and could help reduce competition for vegetable oil used in human food, since the majority of the commercial aquafeed industry now makes use of high levels of terrestrial oils in feed production. Notably, it was observed that bile acid supplementation had no impact on the fatty acid deposition nor mRNA gene expression, but a negative impact was observed on growth performance. This observation requires further study to determine if BA at lower supplementation could provide a beneficial effect when added as a supplement in BSLO-based diet.
Author contributions
Vikas Kumar designed the research project, diet formulation and corrected the draft manuscript. Md. Sakhawat Hossain contributed to diet formulation and conducted the feeding trial. Femi J. Fawole, Md. Sakhawat Hossain and Shyam N. Labh contributed to the design and planning of the experiment, fish sampling, proximate composition, fatty acid composition, and gene expression analyses. Femi J. Fawole performed the statistical analysis and wrote the manuscript. Ken Overturf, Brian C. Small, Thomas L. Welker and Ronald W. Hardy, contributed to planning of the experiment and interpretation of results. All authors read the draft, corrected, and approved the final manuscript.
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgements
The authors are grateful to EnviroFlight, USA for donating the BSL oil used for the trial. The authors would also like to thank Michael Becerra, Carol Hoffman and Sara Schwarz at Hagerman Fish Culture Experiment Station, Hagerman, Idaho, USA for their technical support. The authors wish to state that the mention of trade/company names is to convey accuracy and does not represent endorsement by the University of Idaho. The first (Femi J. Fawole) and second (Shyam N. Labh) authors gratefully thanks the J. William Fulbright Scholarship Program for financial support as a Visiting Scholar at Aquaculture Research Institute, University of Idaho, USA, and the United States Department of Agriculture Agricultural Research Service for partially funding the research (Accession numbers: 437676 & 436723).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Alhazzaa R., Bridle A.R., Nichols P.D., Carter C.G. Up-regulated desaturase and elongase gene expression promoted accumulation of polyunsaturated fatty acid (PUFA) but not long-chain PUFA in Lates calcarifer, a tropical euryhaline fish, fed a stearidonic acid-and γ-linoleic acid-enriched diet. J Agric Food Chem. 2011;59(15):8423–8434. doi: 10.1021/jf201871w. [DOI] [PubMed] [Google Scholar]
- AOAC. (Association of Official Analytical Chemists) 16th ed. AOAC; Washington, DC: 1995. Official methods of analysis. [Google Scholar]
- AOAC. (Association of Official Analytical Chemists) 15th ed. AOAC; Arlington, VA: 2000. Official methods of analysis of the association of official analytical chemists. [Google Scholar]
- Bell J.G., McEvoy J., Tocher D.R., McGhee F., Campbell P.J., Sargent J.R. Replacement of fish oil with rapeseed oil in diets of Atlantic salmon (Salmo salar) affects tissue lipid compositions and hepatocyte fatty acid metabolism. J Nutr. 2001;131(5):1535–1543. doi: 10.1093/jn/131.5.1535. [DOI] [PubMed] [Google Scholar]
- Cardinaletti G., Randazzo B., Messina M., Zarantoniello M., Giorgini E., Zimbelli A. Effects of graded dietary inclusion level of full-fat Hermetia illucens prepupae meal in Practical diets for rainbow trout (Oncorhynchus mykiss) Animals. May 17 2019;9(5) doi: 10.3390/ani9050251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro C., Corraze G., Panserat S., Oliva-Teles A. Effects of fish oil replacement by a vegetable oil blend on digestibility, postprandial serum metabolite profile, lipid and glucose metabolism of European sea bass (Dicentrarchus labrax) juveniles. Aquacult Nutr. 2015;21(5):592–603. [Google Scholar]
- Ding T., Xu N., Liu Y., Du J., Xiang X., Xu D. Effect of dietary bile acid (BA) on the growth performance, body composition, antioxidant responses and expression of lipid metabolism-related genes of juvenile large yellow croaker (Larimichthys crocea) fed high-lipid diets. Aquaculture. 2020;518:734768. [Google Scholar]
- Dong G.-F., Zou Q., Wang H., Huang F., Liu X.-C., Chen L. Conjugated linoleic acid differentially modulates growth, tissue lipid deposition, and gene expression involved in the lipid metabolism of grass carp. Aquaculture. 2014;432:181–191. [Google Scholar]
- Dumas A., Raggi T., Barkhouse J., Lewis E., Weltzien E. The oil fraction and partially defatted meal of black soldier fly larvae (Hermetia illucens) affect differently growth performance, feed efficiency, nutrient deposition, blood glucose and lipid digestibility of rainbow trout (Oncorhynchus mykiss) Aquaculture. 2018;492:24–34. [Google Scholar]
- Elia A.C., Capucchio M.T., Caldaroni B., Magara G., Dörr A.J.M., Biasato I. Influence of Hermetia illucens meal dietary inclusion on the histological traits, gut mucin composition and the oxidative stress biomarkers in rainbow trout (Oncorhynchus mykiss) Aquaculture. 2018;496:50–57. doi: 10.1016/j.aquaculture.2018.07.009. [DOI] [Google Scholar]
- Fawole F.J., Adeoye A.A., Tiamiyu L.O., Ajala K.I., Obadara S.O., Oluwaseun G.I. Substituting fishmeal with Hermetia illucens in the diets of African catfish (Clarias gariepinus): effects on growth, nutrient utilization, haemato-physiological response, and oxidative stress biomarker. Aquaculture. 2020:734849. [Google Scholar]
- Güler M., Yildiz M. Effects of dietary fish oil replacement by cottonseed oil on growth performance and fatty acid composition of rainbow trout (Oncorhynchus mykiss) Turk J Vet Anim Sci. 2011;35(3):157–167. [Google Scholar]
- Guo J.-L., Kuang W.-M., Zhong Y.-F., Zhou Y.-L., Chen Y.-J., Lin S.-M. Effects of supplemental dietary bile acids on growth, liver function and immunity of juvenile largemouth bass (Micropterus salmoides) fed high-starch diet. Fish Shellfish Immunol. 2020;97:602–607. doi: 10.1016/j.fsi.2019.12.087. [DOI] [PubMed] [Google Scholar]
- Hixson S.M., Parrish C.C., Xue X., Wells J.S., Collins S.A., Anderson D.M. Growth performance, tissue composition, and gene expression responses in Atlantic salmon (Salmo salar) fed varying levels of different lipid sources. Aquaculture. 2017;467:76–88. [Google Scholar]
- Iwashita Y., Suzuki N., Yamamoto T., Shibata J.-i., Isokawa K., Soon A.H. Supplemental effect of cholyltaurine and soybean lecithin to a soybean meal-based fish meal-free diet on hepatic and intestinal morphology of rainbow trout Oncorhynchus mykiss. Fish Sci. 2008;74(5):1083–1095. [Google Scholar]
- Izquierdo M., Robaina L., Juárez-Carrillo E., Oliva V., Hernández-Cruz C.M., Afonso J.M. Regulation of growth, fatty acid composition and delta 6 desaturase expression by dietary lipids in gilthead seabream larvae (Sparus aurata) Fish Physiol Biochem. 2008;34(2):117–127. doi: 10.1007/s10695-007-9152-7. [DOI] [PubMed] [Google Scholar]
- Jiang M., Wen H., Gou G., Liu T., Lu X., Deng D. Preliminary study to evaluate the effects of dietary bile acids on growth performance and lipid metabolism of juvenile genetically improved farmed tilapia (Oreochromis niloticus) fed plant ingredient-based diets. Aquacult Nutr. 2018;24(4):1175–1183. [Google Scholar]
- Jordal A.-E.O., Torstensen B.E., Tsoi S., Tocher D.R., Lall S.P., Douglas S.E. Dietary rapeseed oil affects the expression of genes involved in hepatic lipid metabolism in Atlantic salmon (Salmo salar L.) J Nutr. 2005;135(10):2355–2361. doi: 10.1093/jn/135.10.2355. [DOI] [PubMed] [Google Scholar]
- Kroeckel S., Harjes A.G.E., Roth I., Katz H., Wuertz S., Susenbeth A. When a turbot catches a fly: evaluation of a pre-pupae meal of the Black Soldier Fly (Hermetia illucens) as fish meal substitute — growth performance and chitin degradation in juvenile turbot (Psetta maxima) Aquaculture. 2012;364–365:345–352. doi: 10.1016/j.aquaculture.2012.08.041. [DOI] [Google Scholar]
- Kumar V., Lee S., Cleveland B.M., Romano N., Lalgudi R.S., Benito M.R. Comparative evaluation of processed soybean meal (EnzoMealTM) vs. regular soybean meal as a fishmeal replacement in diets of rainbow trout (Oncorhynchus mykiss): effects on growth performance and growth-related genes. Aquaculture. 2020;516:734652. [Google Scholar]
- Lee S., Small B.C., Patro B., Overturf K., Hardy R.W. The dietary lysine requirement for optimum protein retention differs with rainbow trout (Oncorhynchus mykiss Walbaum) strain. Aquaculture. 2020;514:734483. [Google Scholar]
- Li S., Ji H., Zhang B., Tian J., Zhou J., Yu H. Influence of black soldier fly (Hermetia illucens) larvae oil on growth performance, body composition, tissue fatty acid composition and lipid deposition in juvenile Jian carp (Cyprinus carpio var. Jian) Aquaculture. 2016;465:43–52. [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Luo L., Xue M., Vachot C., Geurden I., Kaushik S. Dietary medium chain fatty acids from coconut oil have little effects on postprandial plasma metabolite profiles in rainbow trout (Oncorhynchus mykiss) Aquaculture. 2014;420:24–31. [Google Scholar]
- Mu H., Wei C., Zhang Y., Zhou H., Pan Y., Chen J. Impacts of replacement of dietary fish oil by vegetable oils on growth performance, anti-oxidative capacity, and inflammatory response in large yellow croaker Larimichthys crocea. Fish Physiol Biochem. 2020;46(1):231–245. doi: 10.1007/s10695-019-00712-8. [DOI] [PubMed] [Google Scholar]
- Nayak M., Saha A., Pradhan A., Samanta M., Giri S.S. Dietary fish oil replacement by linseed oil: effect on growth, nutrient utilization, tissue fatty acid composition and desaturase gene expression in silver barb (Puntius gonionotus) fingerlings. Comp Biochem Physiol B Biochem Mol Biol. 2017;205:1–12. doi: 10.1016/j.cbpb.2016.11.009. [DOI] [PubMed] [Google Scholar]
- Overturf K., Welker T., Barrows F., Towner R., Schneider R., LaPatra S. Variation in rainbow trout, Oncorhynchus mykiss, to biosynthesize eicosapentaenoic acid and docosahexaenoic acid when reared on plant oil replacement feeds. J World Aquacult Soc. 2013;44(3):326–337. [Google Scholar]
- Peng M., Xu W., Tan P., Du J., Mai K., Zhou H. Effect of dietary fatty acid composition on growth, fatty acids composition and hepatic lipid metabolism in juvenile turbot (Scophthalmus maximus L.) fed diets with required n3 LC-PUFAs. Aquaculture. 2017;479:591–600. [Google Scholar]
- Pieme C.A., Tatangmo J.A., Simo G., Nya P.C.B., Moor V.J.A., Moukette B.M. Relationship between hyperglycemia, antioxidant capacity and some enzymatic and non-enzymatic antioxidants in African patients with type 2 diabetes. BMC Res Notes. 2017;10(1):141. doi: 10.1186/s13104-017-2463-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renna M., Schiavone A., Gai F., Dabbou S., Lussiana C., Malfatto V. Evaluation of the suitability of a partially defatted black soldier fly (Hermetia illucens L.) larvae meal as ingredient for rainbow trout (Oncorhynchus mykiss Walbaum) diets. J Anim Sci Biotechnol. 2017;8:57. doi: 10.1186/s40104-017-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risérus U., Willett W.C., Hu F.B. Dietary fats and prevention of type 2 diabetes. Prog Lipid Res. 2009;48(1):44–51. doi: 10.1016/j.plipres.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano N., Kumar V., Yang G., Kajbaf K., Rubio M.B., Overturf K. Bile acid metabolism in fish: disturbances caused by fishmeal alternatives and some mitigating effects from dietary bile inclusions. Rev Aquacult. 2020;12(3):1792–1817. [Google Scholar]
- Schiavone A., Dabbou S., De Marco M., Cullere M., Biasato I., Biasibetti E. Black soldier fly larva fat inclusion in finisher broiler chicken diet as an alternative fat source. Animal. 2018;12(10):2032–2039. doi: 10.1017/S1751731117003743. [DOI] [PubMed] [Google Scholar]
- Shamna N., Sahu N.P., Sardar P., Fawole F.J., Kumar S. Additional feeding of vitamin–mineral-based nutraceutical to stress-exposed rohu, Labeo rohita, enhances the IGF-1 gene expression and growth. Aquacult Res. 2020;51(7):2649–2666. [Google Scholar]
- Sheppard D.C., Newton G.L., Thompson S.A., Savage S. A value added manure management system using the black soldier fly. Bioresour Technol. 1994;50(3):275–279. [Google Scholar]
- Stamer A., Wessels S., Neidigk R., Hoerstgen-Schwark G. 2014. Black soldier fly (Hermetia illucens) larvae-meal as an example for a new feed ingredients' class in aquaculture diets. [Google Scholar]
- Teoh C.-Y., Ng W.-K. The implications of substituting dietary fish oil with vegetable oils on the growth performance, fillet fatty acid profile and modulation of the fatty acid elongase, desaturase and oxidation activities of red hybrid tilapia, Oreochromis sp. Aquaculture. 2016;465:311–322. [Google Scholar]
- Tocher D.R. Omega-3 long-chain polyunsaturated fatty acids and aquaculture in perspective. Aquaculture. 2015;449:94–107. [Google Scholar]
- Tocher D.R. Metabolism and functions of lipids and fatty acids in teleost fish. Rev Fish Sci. 2003;11(2):107–184. [Google Scholar]
- Tocher D.R., Bell J.G., Dick J.R., Henderson R.J., McGhee F., Michell D. Polyunsaturated fatty acid metabolism in Atlantic salmon (Salmo salar) undergoing parr-smolt transformation and the effects of dietary linseed and rapeseed oils. Fish Physiol Biochem. 2000;23(1):59–73. [Google Scholar]
- Torstensen B., Nanton D., Olsvik P., Sundvold H., Stubhaug I. Gene expression of fatty acid-binding proteins, fatty acid transport proteins (cd36 and FATP) and β-oxidation-related genes in Atlantic salmon (Salmo salar L.) fed fish oil or vegetable oil. Aquacult Nutr. 2009;15(4):440–451. [Google Scholar]
- Tran G., Heuzé V., Makkar H. Insects in fish diets. Anim Front. 2015;5(2):37–44. [Google Scholar]
- Turchini G.M., Francis D.S. Fatty acid metabolism (desaturation, elongation and β-oxidation) in rainbow trout fed fish oil-or linseed oil-based diets. Br J Nutr. 2009;102(1):69–81. doi: 10.1017/S0007114508137874. [DOI] [PubMed] [Google Scholar]
- Turchini G.M., Ng W.-K., Tocher D.R. CRC Press; 2010. Fish oil replacement and alternative lipid sources in aquaculture feeds. [Google Scholar]
- Wang J., Wang X., Li J., Chen Y., Yang W., Zhang L. Effects of dietary coconut oil as a medium-chain fatty acid source on performance, carcass composition and serum lipids in male broilers. Asian-Australas J Anim Sci. 2015;28(2):223. doi: 10.5713/ajas.14.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Ji H., Belghit I., Liland N.S., Wu W., Li X. Effects of black soldier fly oil rich in n-3 HUFA on growth performance, metabolism and health response of juvenile mirror carp (Cyprinus carpio var. specularis) Aquaculture. 2021;533 [Google Scholar]
- Xu X., Ji H., Belghit I., Sun J. Black soldier fly larvae as a better lipid source than yellow mealworm or silkworm oils for juvenile mirror carp (Cyprinus carpio var. specularis) Aquaculture. 2020;527 [Google Scholar]
- Yamamoto T., Goto T., Tanaka N., Furuita H., Sugita T., Suzuki N. Supplemental effects of essential amino acids and bile salts to a high-fat diet containing soybean meal, corn gluten meal and squid meal for rainbow trout Oncorhynchus mykiss. Aquacult Sci. 2007;55(1):115–123. [Google Scholar]
- Yamamoto T., Suzuki N., Furuita H., Sugita T., Tanaka N., Goto T. Supplemental effect of bile salts to soybean meal-based diet on growth and feed utilization of rainbow trout Oncorhynchus mykiss. Fish Sci. 2007;73(1):123–131. [Google Scholar]
- Yan J., Liao K., Wang T., Mai K., Xu W., Ai Q. Dietary lipid levels influence lipid deposition in the liver of large yellow croaker (Larimichthys crocea) by regulating lipoprotein receptors, fatty acid uptake and triacylglycerol synthesis and catabolism at the transcriptional level. PLoS One. 2015;10(6) doi: 10.1371/journal.pone.0129937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G., Jiang W., Chen Y., Hu Y., Zhou Q., Peng M. Effect of oil source on growth performance, antioxidant capacity, fatty acid composition and fillet quality of juvenile grass carp (Ctenopharyngodon idella) Aquacult Nutr. 2020;26(4):1186–1197. [Google Scholar]
- Yu J., Li S., Niu H., Chang J., Hu Z., Han Y. Influence of dietary linseed oil as substitution of fish oil on whole fish fatty acid composition, lipid metabolism and oxidative status of juvenile Manchurian trout, Brachymystax lenok. Sci Rep. 2019;9(1):1–10. doi: 10.1038/s41598-019-50243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X., Tocher D.R., Dickson C.A., Bell J.G., Teale A.J. Effects of diets containing vegetable oil on expression of genes involved in highly unsaturated fatty acid biosynthesis in liver of Atlantic salmon (Salmo salar) Aquaculture. 2004;236(1–4):467–483. [Google Scholar]