Abstract
We present a case study article demonstrating successful implementation of ultrasound guided extra cardiac vagus nerve stimulation during cardioneuroablation.
To our knowledge it is first published description of this technique, as most ECVS are done in the internal jugular vein bulb area. This method allows for reduction of fluoroscopy time, and most importantly reproducible vagus nerve capture especially after full bi-nodal (sinus and atrioventricular) cardioneuroablation when stimulation of vagus nerve may not give any effect in the heart. This article includes a case study with “dual component” atrioventricular block, where functional component is cured with cardioneuroablation, but structural (PR elongation) remains after procedure.
Keywords: Cardioneuroablation, Neuromodulation, ECANS, Vagus nerve stimulation, Ultrasound guided
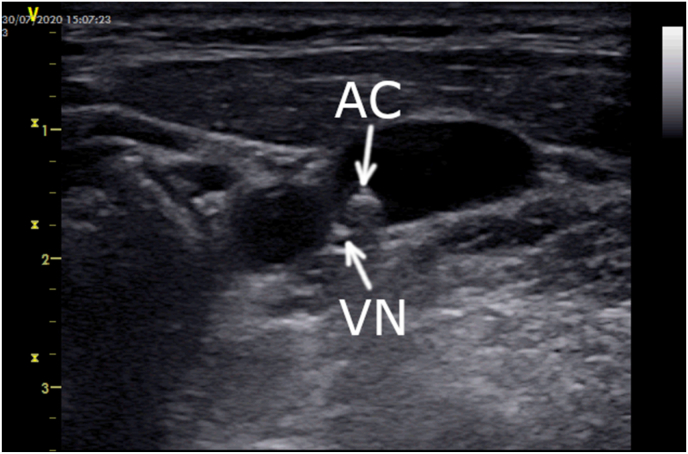
High-output extracardiac vagus nerve stimulation (ECANS) allows transvenous vagus nerve (VN) pacing through the internal jugular vein (IJV) (Figure A) to guide cardioneuroablation (CNA) [[1], [2], [3]] The procedure is performed under general anesthesia. Stimulation activates the parasympathetic nervous system, induces cardioinhibitory reflex and ipsilateral lacrimation, and causes ipsilateral head turning via accessory nerve stimulation. The type of the cardioinhibitory reflex depends on whether the left or the right nerve is being stimulated. It also varies depending on stimulation of different longitudinal nerve fibers. Stimulation of the left VN elicits sinus depression (asystole or bradycardia) more often than atrioventricular block (AVB), whereas stimulation of the right VN induces AVB rather than sinus depression. Although the superior bulb of the IJV (Fig. 1) has been recommended as a preferable site for ECANS [3], the approach has several limitations (eg, radiation exposure, difficulty in left-sided cannulation, unselective cranial nerve stimulation, or lack of confirmation of post-CNA VN capture). The limitations of ECANS might be eliminated by the use of ultrasound guidance, especially noninvasive and repeated imaging of transvascular VN contact and capture. Therefore, nonfluoroscopic ultrasound-guided real-time imaging of the VN nex to the cervical IJV for ECANS is clinically valuable. A linear array transducer (probe frequency, 13 MHz) is used in the long-axis (Video 1) and short-axis (Video 2) views. The ablation catheter is rotated to place the distal tip directly above the VN, thus stretching the IJV wall to the VN (Fig. 2). In this mid-cervical position, little-to-no head tilt is observed. Owing to the direct visualization of the VN, ultrasound-guided ECANS is reproducible and repetitive and achieves a consistent capture of the VN with similar pacing voltage (1 V per 1 Kg up to a maximum of 70 V) as the superior bulb area. Moreover, it is less burdensome and reduces fluoroscopy exposure. In our experience, this technique enables a more reliable assessment of CNA as well as repetitive ECANS in patients with the unusual course of the IJV and VN or any other limiting factors (see Fig. 3).
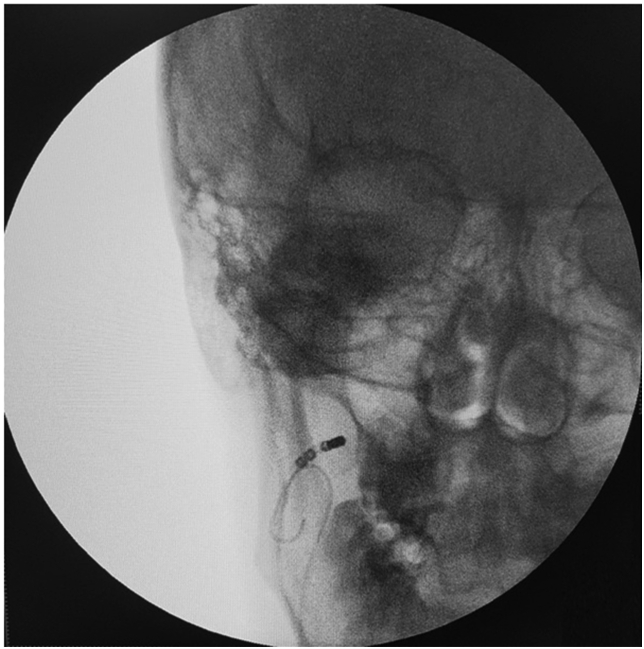
Fig. 1.
A catheter position for right extracardiac vagus nerve stimulation (ECANS) with the standard upper cervical approach. An additional guiding wire is introduced to guide the positioning of a steerable mapping catheter.
Fig. 2.
A catheter position for ultrasound-guided right ECANS using the middle cervical approach.
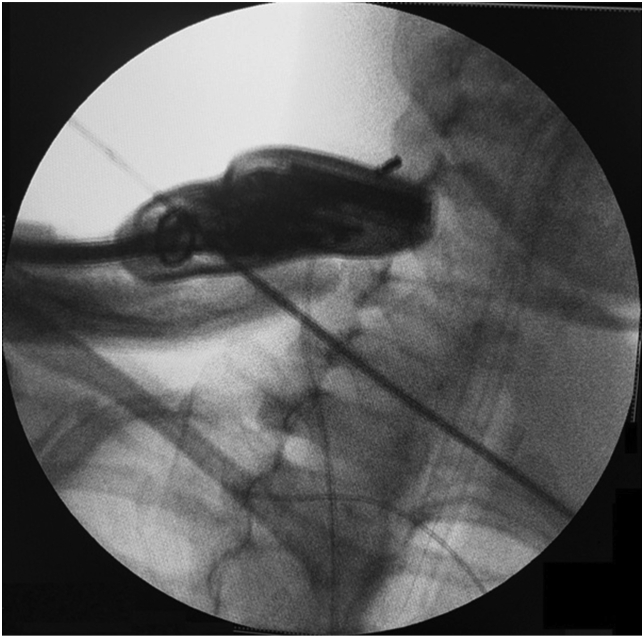
Fig. 3.
Ultrasound-guided vagus nerve stimulation in the mid-cervical position.
A 35-year-old woman with a single-lead dual-chamber pacemaker (VDD) implanted at the age of 19 was referred for an electrophysiology consultation to consider pacemaker extraction before her second pregnancy. Before device implantation, she was diagnosed with persistent first-degree and second-degree Mobitz type I and II as well as third-degree AVB. At the time of planned unit replacement (elective replacement indicator), we considered continuation of permanent pacing due to suspicion of the neurocardiogenic etiology of AV conduction disturbances.
After shared decision making, the patient was referred for several tests before CNA. Optimization of pacing parameters resulted in only 1%–4% ventricular pacing under the rate of 40 bpm. Holter monitoring revealed several daytime episodes of second-degree Mobitz type I AVB with sinus rhythm (60–70 bpm). Persistent PR-interval prolongation over 300 ms was noted. A 12-lead electrocardiogram showed sinus rhythm (72 bpm) with first-degree AVB (PR, 380 ms) and narrow QRS (110 ms) with incomplete right bundle branch block. Tilt testing with beat-to-beat blood pressure monitoring confirmed mixed syncope. Following atropine test (intravenous bolus, 0.02 mg/kg), sinus rhythm accelerated, first-degree AVB persisted (PR, 300 ms), while second- and third-degree AVB resolved. Therefore, the coincidence of structural and partially functional first-degree AVB with functional sinus bradycardia and second-degree/third-degree AVB was revealed. (see Fig. 5)
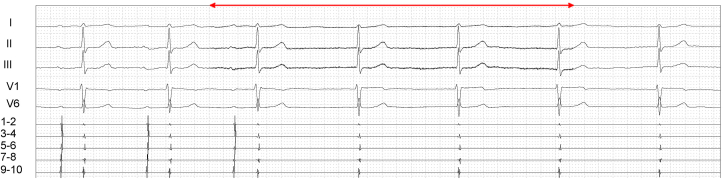
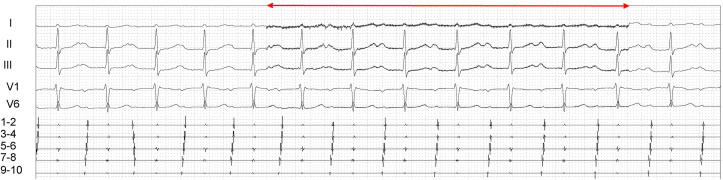
Fig. 5.
Outcomes of ultrasound-guided right ECANS using the middle cervical approach before CNA. The red line represents active ECANS. ECANS showed a sinus pause of 9.4 seconds with junctional rhythm below 40 bpm, and ECANS during proximal coronary sinus stimulation (CI, 600 ms) resulted in atrioventricular block with variable conduction (2:1, 3:1), with 2-s ventricular pauses.
Right upper ECANS and ultrasound-guided middle cervical ECANS were performed (Fig. 5), inducing sinus asystole (9,4s) with junctional escape rhythm, below 40bpm, and during proximal coronary sinus stimulation (CI 600 ms) advanced AVB was induced with 2:1–3:1 conduction. Ultrasound views were achieved according to current guidelines [4]. Left upper and middle cervical ECANS was not possible due to vein occlusion. During initial electrophysiological study baseline measurements were noted: AH: 315 ms, PR: 360 ms, HV: 65 ms, WP: 130bpm (460 ms). Biatrial, binodal CNA was performed (Fig. 4). In our center, we perform CNA first in the left atrium. The ganglionated plexi (GP) were identified using the anatomic method, and in order of applying radiofrequency energy those were GP near the left superior pulmonary vein (left lateral ridge), left inferior pulmonary vein, and right superior pulmonary vein - P point. Then, we moved to the right atrium, ablating the GP in the roof of the coronary sinus and in superior vena cava. Of note, very little PR-interval shortening was achieved. Finally, we ablated the GP in the superior vena cava, with sinus rhythm acceleration from 70 to 85 bpm. After the procedure PR was 320 ms and WP 150bpm. Acute effectiveness of the procedure was confirmed by right ECANS and ultrasound-guided right ECANS (Fig. 6), which showed no changes in sinus rhythm and no AVB with proximal coronary sinus pacing (CI, 600 ms). Atropine test resulted in no change in the PR interval and only slight acceleration of heart rate from 80 to 88 bpm. One month after CNA, tilt test results were negative, heart rate after atropine infusion increased to 90 bpm (12%), and the PR interval was between 270 and 330 ms. Holter monitoring showed PR-interval prolongation to 280 ms, no episodes of advanced AVB, and ventricular pacing below 30 bpm. A month after CNA, the system was removed. An insertable loop recorder was implanted in an asymptomatic patient with only persistent first-degree AVB to validate the efficacy of the procedure and monitor the patient during planned pregnancy. Ten months after the procedure, the patient remains symptom free.
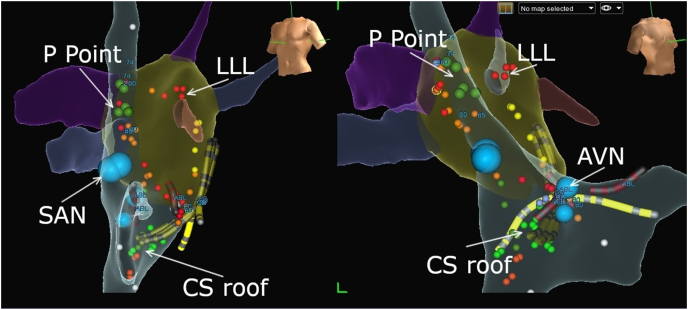
Fig. 4.
Three-dimensional electroanatomical mapping for biatrial applications (left-left anterior oblique and right-right anterior oblique views) for cardioneuroablation (CNA). Yellow – a decapolar catheter is located between the right ventricle, His region, and right atrium. Blue dots represent sinoatrial and atrioventricular nodes. CNA was performed using the anatomical approach, focusing on the area of the left atrial ganglionated plexi as well as the coronary sinus roof and ostium, with minimal applications in the superior vena cava.
Fig. 6.
Outcomes of ultrasound-guided right ECANS using the middle cervical approach after CNA. The red line represents active ECANS. Only the right vagus nerve was available due to inability to cannulate the left internal jugular vein (probably due to lead-related venous occlusion).
In line with a previous report [5], our case proves that CNA is effective and safe for functional AVB and that ECANS can be used to define procedural endpoints. The coincidence of structural and functional AVBs may be present. Ultrasound-guided catheter navigation in the IJV and ultrasound-guided ECANS may guide CNA, especially in patients with a single site available due to difficult vein anatomy and lead adhesions.
Declaration of competing interest
Sebastian Stec is the coauthor of several patents for diagnostic and ablation catheters and a stockholder of Medinice S.A.; No products of the Medinice S.A. were used during the reported procedure.
Other authors have no conflict to declare.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ipej.2021.06.008.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Aksu T., Guler T.E., Yalin K. Catheter ablation of bradyarrhythmia: from the beginning to the future. Am J Med Sci. 2018;355:252–265. doi: 10.1016/j.amjms.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 2.Piotrowski R., Baran J., Kułakowski P. Cardioneuroablation using an anatomical approach: a new and promising method for the treatment of cardioinhibitory neurocardiogenic syncope. Kardiol Pol. 2018;76:1736–1738. doi: 10.5603/KP.a2018.0200. [DOI] [PubMed] [Google Scholar]
- 3.Pachon M.J.C., Pachon M.E.I., Santillana P.T.G. Simplified method for vagal effect evaluation in cardiac ablation and electrophysiological procedures. JACC Clin Electrophysiol. 2015;1:451–460. doi: 10.1016/j.jacep.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 4.Troianos C.A., Hartman G.S., Glas K.E. Councils on intraoperative echocardiography and vascular ultrasound of the American society of echocardiography. Guidelines for performing ultrasound guided vascular cannulation: recommendations of the American society of echocardiography and the society of cardiovascular anesthesiologists. J Am Soc Echocardiogr. 2011;24:1291–1318. doi: 10.1016/j.echo.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 5.Stec S., Dobaj Ł., Śledź A. Cardioneuroablation for management of cardioinhibitory vasovagal syncope and pacemaker complications. HeartRhythm Case Rep. 2020;6:531–534. doi: 10.1016/j.hrcr.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.