Abstract
Introduction
Cardiac autonomic system modulation by endocardial ablation targeting atrial ganglionated plexi (GP) is an alternative strategy in selected patients with severe functional bradyarrhythmias, although no consensus exists on the best ablation strategy. The aim of this study was to evaluate if a simplified approach by a purely anatomical guided ablation of just the atrial right GP is enough for the treatment of these patients.
Methods
We prospectively enrolled patients with significant functional bradyarrhythmias and performed endocardial ablation purely guided by 3D electroanatomic mapping directed at the atrial right GP and accessed parameters of parasympathetic modulation and recurrence of bradyarrhythmias.
Results
Thirteen patients enrolled (76.9% male, median age 51, 42–63 years). After ablation, a median RR interval shortening of 28.3 (25.6–40.3)% occurred (1111, 937.5–1395.4 ms to 722.9, 652.2–882.4 ms, p = 0.0002). The AH interval also shortened (19, 10.5–35.7%) significantly after the procedure (115, 105–122 ms to 85, 71–105 ms, p = 0.0023) as well as Wenckebach cycle length (11.1, 5.9–17.8% shortening) from 450, 440–510 ms to 430, 400–460 ms, p = 0.0127. On 24-h Holter monitoring there was significant increase in heart rates (HR) of patients after ablation (minimal HR increased from 34 (26–43)bpm to 49 (43–56)bpm, p = 0,0102 and mean HR from 65 (47–72)bpm to 78 (67–87)bpm, p = 0.0004). No patients had recurrence of symptoms or significant bradyarrhythmias during a median follow-up of 8.4 months.
Conclusions
A purely anatomic guided procedure directed only at the atrial right ganglionated plexi seems to be enough as a therapeutic approach for cardioneuroablation in selected patients with significant functional bradyarrhythmias.
Keywords: Bradyarrhythmias, Parasympathetic, Modulation, Ablation, Ganglionated plexi, Cardioneuroablation
1. Introduction
Bradyarrhythmias manifested as sinus arrest, sinus bradycardia, brady-tachy syndrome, transient atrioventricular (AV) block or cardioinhibitory syncope can be associated with autonomic imbalance, for which parasympathetic drive predominates. In these cases, specially in younger patients, this poses a conundrum when it comes to decisions whether and how to treat. Increasing evidence, also states a role of vagal tone in some patients with atrial fibrillation (AF), as parasympathetic stimulation is known to shorten atrial effective refractory period and increases the probability of multiple reentrant circuits in the atrial myocardium, therefore increasing stability of atrial fibrillation [[1], [2], [3]]. An increasing number of studies [[3], [4], [5], [6], [7], [8], [9]] have convincingly shown that parasympathetic cardiac modulation, also known as cardioneuroablation, is an alternative and effective therapeutic method for the treatment of these patients, although there is no standardized technique or endpoint criteria to assess therapeutic success.
The objective of this study was to understand if performing a simplified, purely anatomic guided ablation aiming only at the atrial right GP proves to be a valid and successful strategy to perform cardioneuroablation in patients with significant functional bradyarrhythmias.
2. Methods
2.1. Study population
Between February 2017 and October 2019, we prospectively enrolled 13 patients in three hospital centers, with documented episodes of severe functional bradyarrhythmias suggestive of vagal etiology (sinus bradycardia and/or arrest, brady-tachy syndrome, transient AV block and cardioinhibitory syncope). Functional bradyarrhythmias were acknowledged after exclusion of reversible causes, such as negative chronotropic drugs, ionic disorders, thyroid dysfunction, cardiac ischemia, obstructive sleep apnea, intrinsic sinus or AV node disease and in patients engaged in competitive sports, after deconditioning. Intrinsic sinus and AV node disease were ruled out after assessment of a positive chronotropic response on 24-h Holter monitoring and on exercise treadmill test, with absence of exercise induced AV block and when bradyarrhythmias were suggestive to occur in a vagal setting as during sleep, post meals or prolonged standing. Patients with suspected vagal syncope underwent tilt testing that exhibited syncope with predominant cardioinhibitory response. An ECG was performed in all patients and the HR and PQ interval was measured. Patients with persistent abnormal ECG apart from sinus bradycardia, first-degree or second-degree Mobitz type I AV block or with abnormal transthoracic echocardiogram were excluded.
Included patients were either symptomatic (syncope/pre-syncope related to bradycardia) or had severe bradycardia with an indication for pacing regardless of the absence of symptoms as outlined in the current European Society of Cardiology Guidelines for the management of bradyarrhythmias [10].
2.2. Baseline electrophysiological study
The procedure was performed in fasting state, under general anesthesia in 5 patients (standard procedure in one center) for which propofol and atracurium were used during the study. In the remaining procedures, patients were under moderate conscious sedation with propofol and remifentanil and monitored with bispectral index (BIS®), kept at levels between 65 and 70. Three right femoral vein punctures allowed introduction of a 7F diagnostic decapolar catheter in the coronary sinus, a 6F diagnostic quadripolar catheter at His location and one 8.5F SL1® sheath (Abbot Inc.) Baseline electrophysiological study was performed using a multichannel cardiac recorder for bipolar intracardiac electrograms with filtered signals at 30–300 Hz (EP WorkMate Claris System®, Abbott Medical and LabSystem Pro®, Boston Scientific), displaying 3 surface ECG leads (DII, aVF and V1) and with programmed digital stimulation (EP-4® Cardiac Stimulator, Abbott Medical and Qubic Stim, Biotronik). Baseline intervals were measured, and sinoatrial node function was evaluated by measuring RR intervals and AV node function was assessed by measuring Wenckebach cycle length (WBCL) (atrial decremental stimulation at which Wenckebach AV block occurs).
2.3. GP mapping and ablation in the left atrium and AF ablation
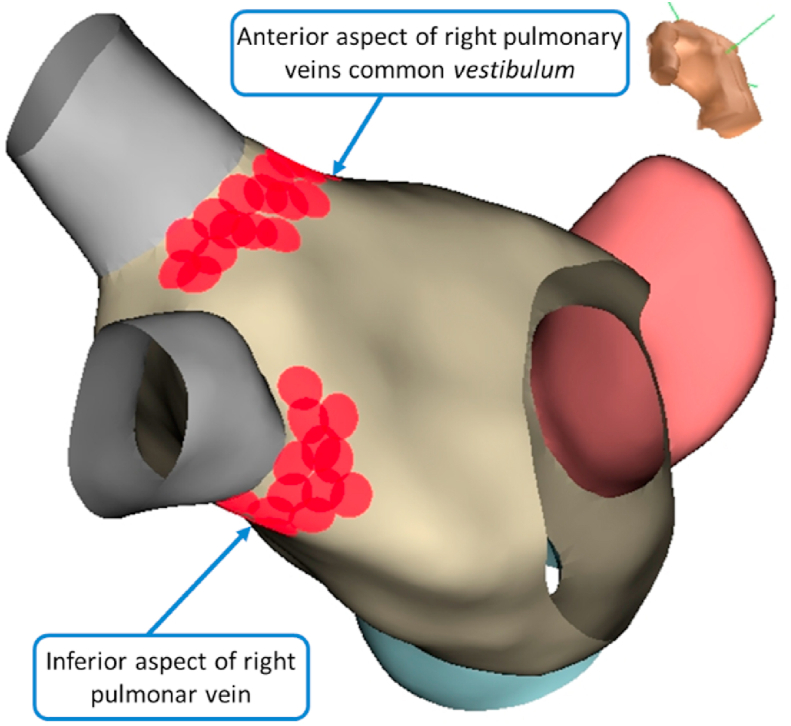
We performed endocardial electroanatomic mapping of the left atria (LA) for anatomic geometry reconstruction with NAVx Ensite Precision® (Abbot Inc.) or Carto3 system® (Biosense Webster Inc.) using a bidirectional irrigated catheter with 4 mm (FlexAbility®, Abbot Inc), a 3.5 mm (ThermoCool SmartTouch® or Navistar RMT ThermoCool®, Biosense Webster Inc.) or a circular mapping catheter in patients who underwent atrial fibrillation ablation (Advisor®, Abbot Inc and LASSO® Biosense Webster, Inc.). We started the procedure by mapping in the LA after transseptal puncture with a Brockenbrough® needle (Medtronic Inc.) under fluoroscopic guidance. After the puncture a bolus o 10 000 IU of heparin was administrated and targeted activated clot time (ACT) was maintained between 300 and 400 s and monitored every 30 min after achieving a value of 300 s. We identified the pulmonary vein (PV) ostia, left atrial appendage and mitral valve annulus. In the reconstructed anatomy, we marked the empiric anatomic location of the atrial right GP site as previously described in the literature [1,4,5,9,11] (Fig. 1): the anterior right GP in the common vestibulum of the anterior aspect of the right pulmonary veins and the inferior right GP in the inferior aspect of the inferior pulmonary vein).
Fig. 1.
Electroanatomic 3D endocardial mapping showing cluster ablation lesions set at right anterior and inferior GP, approach from the left atrium.
For ablation of the GP, we used only an anatomical approach aiming at previously described empiric GP sites. Radiofrequency (RF) lesions were performed for 30 s with 25W (20W in the inferior aspect of the right inferior pulmonary vein) with a set temperature of 43 °C, approximately, 10 mm from the respective PV in a cloud-like shape. The endpoint of the ablation was the deployment of lesions in all pre-specified ablation targets with the disappearance of local electrograms, aiming at shortening RR and AH intervals and Wenckebach Cycle Length (WBCL).
For patients with an indication for AF ablation, the used technique was PV isolation (PVI), with RF circumferential lesions at PV antrum (around 10–15 mm from PV ostia) with 20W for 20 s in the posterior wall and 25W for 30 s in the remaining walls, with a limit temperature of 43 °C and aiming at abolishment of local electrograms to <0,1 mV. The endpoint for PV isolation success was the disappearance or dissociation of PV potentials and the achievement of bidirectional block between PV and LA. If necessary, additional RF lesions were made at conduction gap sites.
2.4. GP mapping and ablation in the right atrium
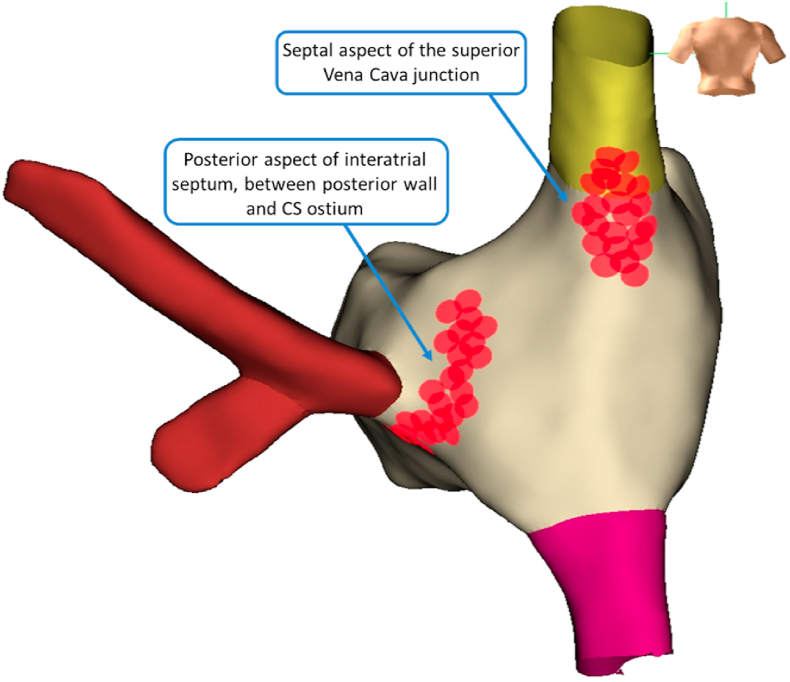
After LA ablation, the catheter was withdrawn to the right atrium (RA) and the anatomy of the RA was obtained. The superior and inferior vena cava, the interatrial septum and coronary sinus ostium were identified. In the RA (Fig. 2) we marked the empiric location of the right aspect of GP, opposite to the area of the LA GP ablation (Fig. 3), at the septal aspect of the superior vena cava junction and the posterior aspect of the interatrial septum (between the posterior wall and the coronary sinus ostia) [1]. In the RA we made RF lesions for 30 s at 35W and a temperature of 43 °C. The endpoints were as on the LA, the deployment of lesions in all pre-specified ablation sites at the specified described parameters.
Fig. 2.
Electroanatomic 3D endocardial mapping showing cluster ablation lesions set from a right atrium approach, at the superior vena cava junction and posterior aspect of interatrial septum.
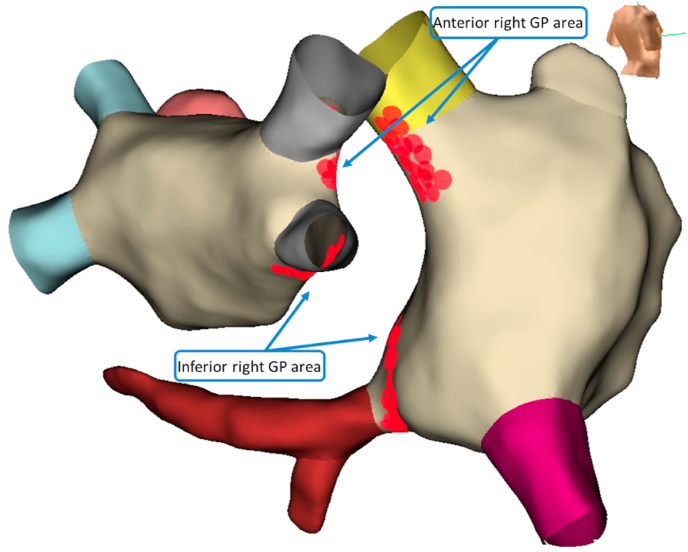
Fig. 3.
(Representative figure). Electroanatomic 3D endocardial mapping showing cluster ablation lesions in the opposite areas of LA and RA, targeting anterior right and inferior right GP.
2.5. Post ablation electrophysiological study
The basic intervals, RR intervals and WBCL were measured using the same approach as in the baseline electrophysiologic study.
2.6. Follow up
Immediate complications of the procedure were assessed, namely vascular access complication, pericardial effusion, stroke or arrhythmias. All patients were scheduled for presential follow-up at 30 days with ECG with measurement of HR and PQ interval. At the six-month visit and every six months afterwards a 24-h Holter monitoring was performed. Three patients had an implantable cardiac monitor (ICM) implanted (for which two were implanted at the same admission of the ablation procedure, at physician discretion). Antiarrhythmic drugs were maintained for at least 3 months in patients with AF and ceased afterwards if no recurrences were detected.
2.7. Statistical analysis
All analysis was performed using version 12 of STATA® (Statistics Data Analysis). Data is presented as median and lower and upper quartiles (Q1-Q3) for continuous variables and as absolute number and percentage for categorical variables.
After testing for normality of the population with the Shapiro-Wilk test, continuous variables were compared with the use of the t-test. A value of p < 0.05 was considered statistically significant.
2.8. Ethics disclosure
This study complies within declaration of Helsinki rules. All patients signed the informed consent and the study was approved by the ethical committee of the three hospitals.
3. Results
3.1. Study population
We enrolled 13 patients, 10 males (76.9%), with a median age of 51 (42–63) years. Nine patients (69.2%) were symptomatic (syncope/pre-syncope related to bradyarrhythmia) and the remaining four patients were asymptomatic (#2, #6, #10 and #11) (Table 1). The diagnosis was transient high-grade AV block in three patients (one had also sinus arrest), cardioinhibitory syncope in two patients and sinus arrest in eight patients. The mean ECG heart rate (HR) was 55 (45–61), beats per/minute (bpm) (Table 2) and the PQ interval on 12 lead ECG among patients was 180 (170–220) ms. Four out of eight patients with sinus arrest had also sinus bradycardia and four had brady-tachy syndrome with paroxysmal atrial fibrillation (Table 1).
Table 1.
Baseline Clinical Characteristics of the cohort.
| Patient | Gender | Age (years) | Diagnosis | Symptoms | AFib | Other |
|---|---|---|---|---|---|---|
| 1 | Male | 51 | SB and Sinus Arrest | Syncope | No | Athlete |
| 2 | Female | 34 | High grade AVB | None | No | – |
| 3 | Male | 55 | SB and Sinus Arrest | Syncope | Yes | Athlete |
| 4 | Male | 58 | SB and Sinus Arrest | Pre syncope | Yes | – |
| 5 | Female | 69 | Cardioinhibitory syncope | Syncope | Yes | – |
| 6 | Female | 63 | Sinus Arrest | None | No | OSA |
| 7 | Male | 70 | SB and Sinus Arrest | Pre syncope | Yes | – |
| 8 | Male | 48 | SB | Pre syncope | No | Athlete; PM explantation (device infection) |
| 9 | Male | 20 | Sinus Arrest | Syncope | No | Athlete |
| 10 | Male | 42 | Sinus Arrest and high grade AVB | None | No | Athlete |
| 11 | Male | 47 | Sinus Arrest | None | No | – |
| 12 | Male | 19 | Cardioinhibitory syncope | Syncope | No | – |
| 13 | Male | 68 | High grade AVB | Pre Syncope | No | Athlete |
(SB – Sinus Bradycardia; AVB – Atrioventricular Block; OSA – Obstructive Sleep Apnea; PM – Pacemaker).
Table 2.
Electrocardiographic characteristics of the cohort, before and after ablation.
| Patient | Longest Pause (ms) | Basal ECG HR (bpm) | 24h-Holter minimal HR, pre Abl (bpm) | 24h-Holter minimal HR, post Abl (bpm) | 24h-holter mean HR, pre Abl (bpm) | 24h-holter mean HR, post Abl (bpm) | PQ interval, pre Abl (ms) | PQ interval, post Abl (ms) |
|---|---|---|---|---|---|---|---|---|
| 1 | 8320 | 45 | 21 | 70 | 66 | 91 | 240 | 160 |
| 2 | 7000 | 58 | 45 | 60 | 65 | 93 | 180 | 180 |
| 3 | 4000 | 40 | 26 | 50 | 44 | 81 | 220 | 180 |
| 4 | 5350 | 76 | 36 | 51 | 63 | 78 | 180 | 180 |
| 5 | 10000 | 55 | 43 | 48 | 62 | 67 | 190 | 175 |
| 6 | 13200 | 88 | 39 | 43 | 82 | 83 | 170 | 150 |
| 7 | 3300 | 35 | 46 | 41 | 72 | 73 | 160 | 145 |
| 8 | 4320 | 42 | 26 | 31 | 42 | 66 | 160 | 150 |
| 9 | 3300 | 61 | 14 | 62 | 76 | 89 | 160 | 135 |
| 10 | 3650 | 45 | 12 | 46 | 42 | 60 | 250 | 200 |
| 11 | 9000 | 75 | 42 | 42 | 63 | 67 | 230 | 160 |
| 12 | 18000 | 60 | 52 | 56 | 78 | 87 | 180 | 160 |
| 13 | 10000 | 49 | 42 | 43 | 47 | 77 | 180 | 170 |
(ms – milliseconds; ECG – Electrocardiogram; HR – Heart Rate; bpm – beats per minute; Abl – Ablation).
The longest pause (either for sinus arrest or AV block) was 7000 (4000–10000) ms. Regarding assessment on 24-h Holter monitoring, minimal HR was 34 (26–43) bpm and mean HR 65 (47–72) bpm.
3.2. Baseline electrophysiological study
All patients were in sinus rhythm at the day of the procedure. The measured AH interval was 115 (105–122) ms and HV interval were 45 (45–52) ms. The measured RR intervals were 1111 (937.5–1395.4) ms and the WBCL 450 (440–510) ms.
3.3. GP mapping and ablation and AF ablation
In all patients, successful access to LA and performance of 3D electroanatomic mapping was possible, with identification of PV ostia and GP specified anatomic location sites. Completion of the lesion subset prespecified to ablate right GP was undertook in all patients, with approaches from the left and right atria. In the four patients with documented paroxysmal atrial fibrillation, PV isolation was done and with achievement of bidirectional block in all patients.
3.4. Post ablation electrophysiological study
Measured AH interval (85, 71–105 ms) significantly decreased after GP ablation (p = 0.0023), with a shortening of 19 (10,5–35,7) %. No differences were observed in HV interval (45, 45–50 ms, p = 0.107). After ablation, RR intervals were 722.9 (652.2–882.4) ms, significantly reduced (p = 0.0002), and with a shortening of 28.3 (25.6–40.3) % (Fig. 4). We also encountered a significant difference between pre and post ablation WBCL (430, 400–460 ms, p = 0.0127) with a correspondent shortening of 11.1 (5-9 – 17.8) % (Table 3). These results showed to be consistent among the subset of patients without PVI, regarding AH interval decrease (p = 0,0099), reduction in RR intervals (p = 0,0016) and shortening of WBCL (p = 0,0202).
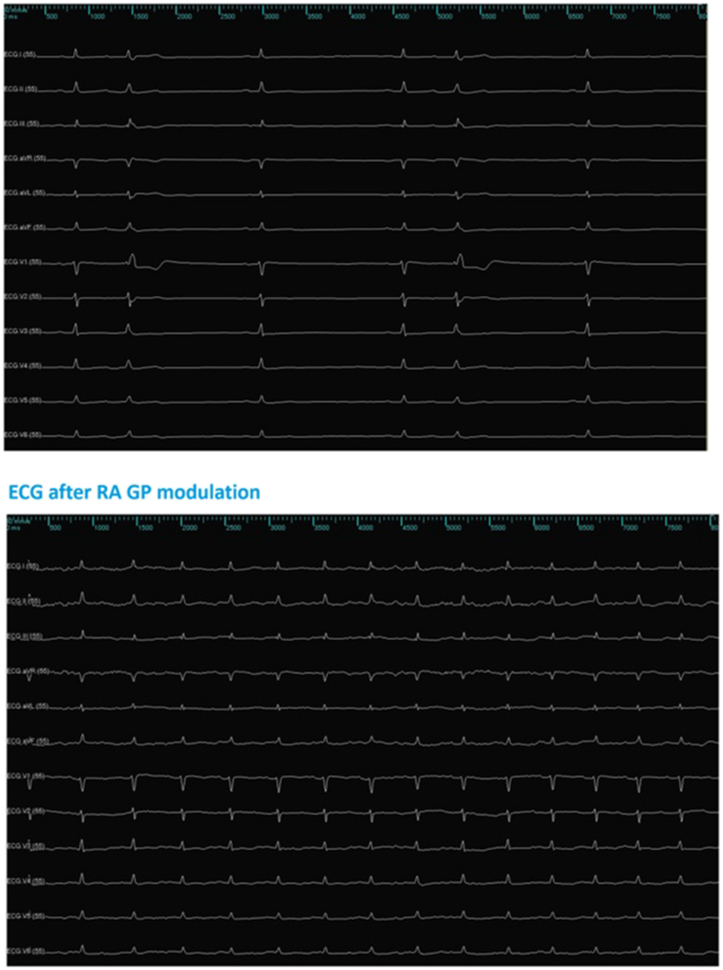
Fig. 4.
Effect on RR intervals after ablation of GP from a right atrium approach at the posterior aspect of the interatrial septum, patient #5; superior image – basal RR intervals; inferior image – RR intervals after ablation.
Table 3.
Electrophysiological characteristics of the cohort, before and after ablation and follow-up.
| Patient | RR pre Abl (ms) | RR post Abl (ms) | RR shortening (%) | HR Accl (bpm) | AH pre Abl (ms) | AH post Abl (ms) | AH shortening (%) | WBCL pre Abl (ms) | WBCL post Abl (ms) | WBCL shortening (%) | PVI | Symptoms after Abl | FUP (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1395 | 1000 | 28.3 | 17 | 130 | 50 | 61,5 | 450 | 400 | 11.1 | No | No | 19.2 |
| 2 | 1035 | 600 | 42.0 | 42 | 115 | 115 | 0 | 540 | 440 | 18.5 | No | No | 8.4 |
| 3 | 1200 | 800 | 33.3 | 25 | 110 | 70 | 36,4 | 460 | 430 | 6.5 | Yes | No | 9.7 |
| 4 | 732 | 652 | 10.9 | 10 | 115 | 115 | 0 | 450 | 440 | 2.2 | Yes | No | 6.0 |
| 5 | 1539 | 606 | 60.6 | 60 | 125 | 110 | 12 | 480 | 460 | 4.2 | Yes | No | 6.5 |
| 6 | 1395 | 1017 | 27.1 | 16 | 105 | 85 | 19 | 420 | 400 | 4.8 | No | No | 7.3 |
| 7 | 1395 | 833 | 40.3 | 29 | 95 | 80 | 15,8 | 510 | 480 | 5.9 | Yes | No | 6.3 |
| 8 | 984 | 723 | 26.5 | 22 | 95 | 85 | 10,5 | 800 | 600 | 25.0 | No | No | 36.4 |
| 9 | 769 | 690 | 10.3 | 9 | 95 | 71 | 25,3 | 450 | 370 | 17.8 | No | No | 35.3 |
| 10 | 1818 | 923 | 49.2 | 32 | 140 | 90 | 35,7 | 1000 | 620 | 38.0 | No | No | 27.7 |
| 11 | 934 | 698 | 25.6 | 22 | 122 | 50 | 59,0 | 400 | 340 | 15.0 | No | No | 23.4 |
| 12 | 882 | 600 | 32.0 | 32 | 115 | 93 | 19,1 | 440 | 410 | 6.8 | No | No | 7.1 |
| 13 | 1111 | 882 | 20.6 | 14 | 115 | 105 | 8,7 | 440 | 370 | 15.9 | No | No | 6.3 |
(RR – RR intervals; ms – milliseconds; HR Accl – Heart Rate acceleration; AH – A to His interval; WBCL – Wenckebach cycle length; Abl – Ablation; PVI – Pulmonary Vein Isolation; FUP – Follow Up).
3.5. Follow up
No immediate or delayed complications of the procedure were observed.
The effect of parasympathetic modulation led to a significant increase of HR to 80 (65–85) bpm, p = 0.0016 and a reduction of PQ intervals in 12 lead ECG at 30 days to 160 (150–180) ms, p = 0.0023. On 24-h Holter monitoring there was a significant increase in minimal HR to 49 (43–56) bpm, p = 0,0102 and in mean HR to 78 (67–87) bpm, p = 0,0004 (Table 2). In the subset of patients that didn't underwent PVI, these results maintained statistical significance (p = 0,0099 for PQ reduction; p = 0,0304 and p = 0,0014 regarding increase in minimal and mean HR on 24-h Holter monitoring respectively).
After a median follow-up of 8.4 (6.5–23.4) months, no patient had recurrence of symptoms and there was no recurrence of sinus arrest among the cohort. One patient referred for ablation for transient high-grade AV block showed paroxysmal 2nd degree Mobitz type I AV block (patient #10) and the remaining patients had no evidence of recurring AV block. None of the patients needed pacemaker implant. No significant atrial arrhythmias or AF recurrence were detected during follow-up apart for one patient without previously documented AF with ICM that had 1-h episode of AF at two months post ablation (patient #6).
4. Discussion
Although current guidelines recommend pacemaker implant in patients with severe symptomatic bradyarrhythmias, there is evidence that some of these disorders are related to functional imbalance in cardiac autonomic nervous system with a predominant excess of vagal tone [10]. Cardiac parasympathetic drive occurs through efferent signals from vagal fibers, connected to postganglionic cells within atrial walls or in para-cardiac ganglia, mainly by decreasing automatism, excitability, and conductivity. Cardiac modulation of parasympathetic GP abolishes the influence of excessive parasympathetic autonomic influence [4]. Even though GP are located in epicardial fat pads, the extensive network of intramural atrial micro ganglia [2,7,12] renders endocardial ablation effective. Various reports [4,5,7,12] have shown that modulation of cardiac parasympathetic system supports this approach as an alternative strategy for these patients, particularly at younger ages for whom pacemaker implant is unwanted due to its potential lifelong complications. Similarly, in patients with vagally related AF in whom increased parasympathetic tone has an important role in initiation and perpetuation of AF episodes, cardioneuroablation in addition to PVI seems to confer increased arrhythmia free survival [2,13].
The evaluation of sinus and AV node intrinsic disease can be aided non-invasively with Holter monitoring and exercise treadmill test, as these are validated surrogates of nodal disease, in spite the limitations of the last because of increased balance of sympathetic versus parasympathetic tone during exercise. Maintained circadian variation of HR and normal chronotropic response to exercise are excellent surrogates of normal intrinsic sinus and AV node function. Previous studies have found comparable accuracy of Holter monitoring and treadmill exercise test with intrinsic HR measurements after pharmacological autonomic blockade. In spite, this method is not infallible. On the other hand, interpreting the measurement of baseline sinus node recovery time (SNRT) performed at electrophysiological study may be difficult, as pacing suppression can be a normal phenomenon even in patients with normal sinus node function and because of existent overlap in recovery times between patients with normal and abnormal sinus node function. Additionally, it has sensitivity of only 70% [14]. Furthermore, performing SNRT without pharmacological blockade can be misleading and not reflect the intrinsic properties of sinus and AV node. This way, it was a decision of the authors to evaluate patients non-invasively before the inclusion in the study and to not perform SNRT. We do understand that choosing this protocol may be prone to limitations.
In our study we included patients with suspected parasympathetic driven bradyarrhythmias, that showed adequate chronotropic response on non-invasive evaluation which otherwise would have indication for pacemaker implant due to the severity of the clinical symptoms or the rhythm disorder. Our results show that cardioneuroablation of the parasympathetic system is effective in treating functional bradyarrhythmias. It has shown good results in the immediate period after ablation with increase in HR, in shortening of AH intervals and WBCL and on follow up, as none of the patients had recurrence of symptoms or severe bradyarrhythmias.
So far, targeting GP for cardioneuroablation has been quite demanding, as most of previous studies have used complex methodologies that require appropriate specific systems for identification of GP site. In spite feasible and reproducible, this can lead to extensive ablation in the atria, predisposing patients to iatrogenic atrial arrhythmias [15].
Previous works [3,5,8,9,16,17] have shown that GP ablation can be achieved either using high frequency stimulation (HFS) (from epicardial or endocardial sides) to identify the GP or with an anatomical approach at known GP sites. Some works [3,9,12,18] have posteriorly shown that the sensitivity of HFS is low (21–71%) for the identification of GP sites and for assessment of the success of ablation. It is increasingly supported in the literature, the superiority of the anatomical approach over the HFS methodology due do the inconsistent parasympathetic response of the later. Besides, it allows avoidance of additional dedicated catheters and generators to perform HFS and spares the awake patient the unpleasant procedure [7,17,19]. Our work pursued a simplified methodology to perform cardioneuroablation, using an anatomical approach with 3D electroanatomic mapping and geometry reconstruction and with further evaluation of the effects produced by parasympathetic modulation. This was done having patients under general anesthesia or objectivated moderate sedation to outcome the possible effect of sympathetic drive due to procedure stress caused to the patients. With this method the GP described location was easily accessible, as was precise delivery of radiofrequency lesions with homogenization of the created scars and reducing potential additional procedure complications, without compromising success. Likewise, endpoints of the procedure are not standardized and there is no current consensus on endpoints for successful procedures. The most used assessment of parasympathetic modulation by achieving a blunted HR response with atropine administration after ablation has low value because of the increase in sympathetic tone [8,19]. So, in our study the endpoint of ablation was, by anatomical deployment of lesions in pre specified sites and with pre specified settings, to evaluate changes electrophysiological parameters, concerning shortening of RR and AH intervals and Wenckebach Cycle Length.
Anatomic and physiological studies demonstrate that parasympathetic drive is predominantly located in the GP between the right superior pulmonary vein and right atria (which has most influence in cardiac parasympathetic innervation) and that the group of fibers located between the inferior vena cava and the right and left atria plays a major role in AV nodal innervation [4]. Increasing clinical evidence, have also shown that the most important modification of parasympathetic autonomic response during ablation occurs by targeting the right GP and some authors additionally claim predominant modulation from the right atria side of GP, namely during ablation of the right side of the interatrial septum along the coronary sinus ostium [1,5,20]. We used a simple and consistent method for GP ablation aiming at just the right GP with anatomical guidance. Our results have shown that this strategy is feasible and effective, with less scar creation. In our patients, approaching the right GP alone seemed to be enough leading to abolishment of vagal cardiac tone, with significant shortening of RR and AH intervals and WBCL acutely. Also, we report a significant increase in minimal and mean heart rate 30 days after cardioneuroablation, rendering patients asymptomatic with persistent effect in the medium-term. This data demonstrates that in the absence of standardized hard endpoints for GP ablation, an anatomic guided procedure for parasympathetic modulation and for simplification purposes, with creation of a lesion subset in pre specified anatomic areas is enough.
4.1. Study limitations
This is a study with a small number of patients and therefore larger prospective studies are needed to validate the strategy of performing ablation of only the right GP with a purely anatomic technique. Interestingly, it would be of value a larger study aiming at ablation of just the interatrial septum, for it seems to be the most effective site for cardiac parasympathetic denervation among these patients.
It is important to stress that careful selection of patients is crucial, and certainty related to functional origin of bradyarrhythmias key to the success of the procedure. Additionally, it should be kept in mind that this technique of cardioneuroablation may prove insufficient if all related areas are not ablated, as observed by the recurrence of 2nd degree type I AV block in one patient of the cohort referred for transient high-grade AV block. We underline that the used technique in this study was partial, as we only aimed at the right GP and the acute degree of denervation was assessed with the use of indirect methods and not with vagal stimulation, reasons for which relapses can be due to the used protocol with a partial technique and not because of cardioneuroablation failure.
In spite no immediate effect on HR was noted during PVI in the 4 patients that had ablation for AF, we cannot exclude a possible additional effect of concurrent inadvertent left GP ablation in these patients. However, in the subset of 9 patients without PVI, the study results maintained significance throughout all measured parameters after cardioneuroablation.
5. Conclusions
In this study, our results show that cardioneuroablation is an effective alternative therapy in selected patients with severe functional bradyarrhythmias and that a simplified purely anatomic ablation strategy seems to be a valid approach.
Funding
No funding was received for this study.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
The study is preprinted on Authorea for peer review and can be accessed at https://doi.org/10.22541/au.159363425.56570181.
Declaration of competing interest
We wish to submit an original work entitled “Anatomic guided ablation of the atrial right ganglionated plexi is enough for cardiac autonomic modulation in patients with significant bradyarrhythmias” for consideration by Indian Pacing and Electrophysiology Journal. The author state that they have not any conflicts of interest and no funding was received for this study.
Acknowledgements
The authors wish to thank Lia Marques (Abbot Inc.) for the help in assembling 3D electroanatomic maps.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
References
- 1.Caló L., Rebecchi M., Sciarra L., Luca L., Fagagnini A., Zuccaro L. Catheter ablation of right atrial ganglionated plexi in patients with vagal paroxysmal atrial fibrillation. Circ Arrhythm Electrophysiol. 2012;5:22–31. doi: 10.1161/CIRCEP.111.964262. [DOI] [PubMed] [Google Scholar]
- 2.Choi E., Zhao Y., Everett T., Chen P. Ganglionated plexi as neuromodulation targets for atrial fibrillation. J Cardiovasc Electrophysiol. 2017;28:1485–1491. doi: 10.1111/jce.13319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scanavacca M., Pisani C., Hachul D., Lara S., Hardy C., Darrieux F. Selective atrial vagal denervation guided by evoked vagal reflex to treat patients with paroxysmal atrial fibrillation. Circulation. 2006;114:876–885. doi: 10.1161/CIRCULATIONAHA.106.633560. [DOI] [PubMed] [Google Scholar]
- 4.Pachon J., Pachon E., Pachon J., Lobo T., Pachon M., Vargas R. ‘‘Cardioneuroablation:’’ a new treatment for neurocardiogenic syncope, functional AV block and sinus dysfunction using catheter RF-ablation. Europace. 2005;7:1–13. doi: 10.1016/j.eupc.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Rivarola E., Hachul D., Wu T., Pisani C., Hardy C., Raimundi F. Targets and end points in cardiac autonomic denervation procedures. Circ Arrhythm Electrophysiol. 2017;10e004638:1–9. doi: 10.1161/CIRCEP.116.004638. [DOI] [PubMed] [Google Scholar]
- 6.Mulpuru S., Shen W. Selective modulation of the cardiac autonomic nervous system, A new strategy for treatment of cardioinhibitory syncope. Circ Arrhythm Electrophysiol. 2017;10e004994:1–3. doi: 10.1161/CIRCEP.117.004994. [DOI] [PubMed] [Google Scholar]
- 7.Sun W., Zheng L., Qiao Y., Shi R., Hou B., Wu L. Catheter ablation as a treatment for vasovagal syncope: long-term outcome of endocardial autonomic modification of the left atrium. Am Heart Assoc. 2016;5e003471:1–10. doi: 10.1161/JAHA.116.003471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pachon J., Pachon E., Santillana T., Lobo T., Pachon C., Pachon J. Simplified method for vagal effect evaluation in cardiac ablation and electrophysiological procedures. J Am Coll Cardiol EP. 2015;1:451–460. doi: 10.1016/j.jacep.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Lemery R., Birnie D., Tang A., Green M., Gollob M. Feasibility study of endocardial mapping of ganglionated plexuses during catheter ablation of atrial fibrillation. Heart Rhythm. 2006;3:387–396. doi: 10.1016/j.hrthm.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Brignole M., Auricchio A., Baron-Esquivias G., Bordachar P., Boriani G., Breithardt O. ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J. 2013;34:2281–2329. doi: 10.1093/eurheartj/eht150. 2013. [DOI] [PubMed] [Google Scholar]
- 11.Katritsis G., Katritsis D. Cardiac autonomic denervation for ablation of atrial fibrillation. Arrhythmia Electrophysiol Rev. 2014;3:113–115. doi: 10.15420/aer.2014.3.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stavrakis S., Po S. Ganglionated plexi ablation: physiology and clinical applications. Arrhythmia Electrophysiol Rev. 2017;6:186–190. doi: 10.15420/aer2017.26.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pokushalov E., Romanov A., Artyomenko S., Shirokova N., Karashov A., Katritsis D., Karashov A. Ganglionated plexi ablation directed by high-frequency stimulation and complex fractionated atrial electrograms for paroxysmal atrial fibrillation. PACE (Pacing Clin Electrophysiol) 2012;35:776–884. doi: 10.1111/j.1540-8159.2012.03392.x. [DOI] [PubMed] [Google Scholar]
- 14.Fogoros R. fourth ed. Blackwell Publishing; Pittsburgh PA: 2006. Electrophisiologic testing; pp. 61–69. [Google Scholar]
- 15.Qin M., Zeng C., Liu X. The cardiac autonomic nervous system: a target for modulation of atrial fibrillation. Clin Cardiol. 2019;42:644–652. doi: 10.1002/clc.23190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piotrowski R., Baran J., Kulakowski P. Cardioneuroablation using an anatomical approach: a new and promising method foir the treatment of cardioinhibitory neurocardiogenic syncope. Kardiol Pol. 2018;76:1736–1738. doi: 10.5603/KP.a2018.0200. [DOI] [PubMed] [Google Scholar]
- 17.Katritis D., Giazitzoglou E., Sougiannis D., Goumas N., Paxinos G., Camm A. Anatomic approach for ganglionated plexi ablation in patients with paroxysmal atrial fibrillation. Am J Cardiol. 2008;102:330–334. doi: 10.1016/j.amjcard.2008.03.062. [DOI] [PubMed] [Google Scholar]
- 18.Pokushalov E., Romanov A., Shugayev P., Artyomenko S., Shirokova N., Turov D. Selective ganglionated plexi ablation for paroxysmal atrial fibrillation. Heart Rhythm. 2009;6:1257–1264. doi: 10.1016/j.hrthm.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 19.Osório T., Paparella G., Stec S., Chierchia G., Asmundis C. Cardiac parasympathetic modulation in the setting of radiofrequency ablation for atrial fibrillation. Arch Med Sci. 2019:84717. doi: 10.5114/aoms.2019.84717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu F., Zheng L., Liang E., Ding L., Wu L., Chen G. Right anterior ganglionated plexus: the primary target of cardioneuroablation? Heart Rhythm. 2019;16:1545–1551. doi: 10.1016/j.hrthm.2019.07.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.
The study is preprinted on Authorea for peer review and can be accessed at https://doi.org/10.22541/au.159363425.56570181.