Abstract
Background
Ocular toxoplasmosis is an infection caused by Toxoplasma gondii. In South America, the clinical course of ocular toxoplasmosis is more severe than in Europe and North America because virulent strains of the parasite are present. Ocular toxoplasmosis is the leading cause of posterior uveitis and retinochoroiditis in Colombia, requiring timely and appropriate treatment. However, there is no standardized therapy protocol based on economic studies for the country.
Purpose
To compare the cost-effectiveness of four first-line treatment regimens for active ocular toxoplasmosis in immunocompetent adults in Colombia, using the number of averted therapeutic failures as the outcome.
Methods
We performed an economic and cost-effectiveness analysis to compare four first-line treatment regimens for ocular toxoplasmosis from the perspective of a third-party payer (Colombian General System of Social Security in Health). A decision analysis tree was used over a 24-week time horizon, considering only direct costs. Additionally, we performed a discrete sensitivity analysis and a probabilistic sensitivity analysis with 10,000 iterations in the Monte Carlo simulation.
Results
For the base case, trimethoprim/sulfamethoxazole showed 86% effectiveness at a cost of <57 United States Dollars, resulting in the most cost-effective first-line alternative. When performing the probabilistic sensitivity analysis and maintaining the willingness to pay 466.00 United States Dollars, the trimethoprim/sulfamethoxazole regimen remained the most cost-effective alternative.
Conclusion
Ocular toxoplasmosis is a public health issue in Latin America. Despite severe visual consequences for affected patients, there are no standardized treatment guidelines in countries such as Colombia. Our evidence supports the use of trimethoprim/sulfamethoxazole as first-line treatment in Colombia because of its availability and optimal cost-effectiveness performance; it reduces recurrences and complications, while averting therapeutic failure. Furthermore, our evidence can be generalized to other Latin American countries with similar frequencies and severities of Toxoplasma gondii ocular infection and health systems similar to the Colombian system.
Keywords: Ocular toxoplasmosis, Therapy, Costs, Cost-analysis, Immunocompetent patients, Therapeutic failure, Latin America
Ocular toxoplasmosis, therapy, costs, cost-analysis, immunocompetent patients, therapeutic failure, Latin America
1. Introduction
Toxoplasma gondii (Tg) infection is one of the most common zoonoses worldwide. Nearly one-third of the global population has come into contact with this parasite; thus, affected people worldwide represent 25%–30% of the global population [1] and 33% of these affected people exhibit the infection in a chronic form [2].
Ocular toxoplasmosis (OT) can generate irreversible sequelae, especially in Latin America, where the predominant strains of Tg are types I and III, as well as atypical strains. These strains are more aggressive and virulent, compared with strains from north equatorial countries [3]; such infections lead to visual impairment and blindness [4, 5] and have impacts on quality of life [6, 7].
OT represents a public health problem in Colombia because its incidence in immunocompetent patients is threefold greater than the corresponding incidence in European countries [8]. It is estimated that 47% of the Colombian population exhibits anti-Tg IgG antibodies because of contact with the parasite [5]. In 2008, De-la-Torre et al. reported a prevalence of 5.5% for retinochoroidal scars after non-congenital infection, equivalent to approximately 1,000,000 inhabitants; of these, 20% developed visual impairment, including 200,000 cases of unilateral blindness [9]. Furthermore, OT represents the main cause of uveitis in Colombia and the leading cause of posterior uveitis globally [4, 9]. Thus, prompt pharmacological treatment for OT is essential.
OT treatment regimens aim to reduce ocular inflammation, inhibit parasite replication during active retinochoroiditis, and reduce the probability of complications (e.g., retinal and optic nerve damage) [10]. In general, treatment duration is 4–6 weeks. First-line therapies include antiparasitic treatments such as combinations of pyrimethamine (PMT) + sulfadiazine (SDZ), pyrimethamine + sulfadoxine (SDX), trimethoprim-sulfamethoxazole (TMP-SMX), or (TMP-SMX) + oral clindamycin (OC). Corticosteroids can be added to all of these therapies for inflammatory control; folinic acid can also be added to avoid PMT-induced hematological adverse effects [10, 11]. Second-line therapies include azithromycin, OC, and intravitreal clindamycin + dexamethasone; these are recommended as rescue therapy in the event of treatment failure or adverse effects [10, 12].
There has been considerable discussion regarding OT treatment; to our knowledge, there remains no consensus concerning the ideal treatment, and the efficacies of current regimens are controversial [10]. Currently, there is no standardized therapy protocol based on cost-effectiveness parameters for OT in Colombia. However, because the disease has important effects on public health in Colombia, the acquisition of cost-effectiveness information can aid in clinical decision-making and health policy establishment. Therefore, this study was performed to compare the cost-effectiveness of four treatment regimens for active OT, supported by the Colombian General System of Social Security in Health, to determine the most cost-effective alternative that averts therapeutic failures. This information may be useful for other Latin American countries with similar disease circumstances and comparable health systems.
2. Materials and methods
2.1. Design
This study comprised an economic and cost-effectiveness analysis, comparing four OT treatment regimens according to the number of averted therapeutic failures.
2.2. Ethical considerations
All procedures performed in this study were developed in accordance with the ethical standards of the institutional research committee. Furthermore, the study protocol adhered to the ethical principles for human research established by the Helsinki Declaration, the Belmont Report, and Colombian Resolution 008430 from 1993. This work used public information regarding medication prices. Additionally, this study used secondary data and did not involve human interventions nor medical records access.
2.3. Population-sample
The sample population comprised a hypothetical cohort of 10,000 Colombian immunocompetent adults who presented to the Colombian Health System with a confirmed active OT diagnosis; in all patients, infection was not caused by congenital disease [13, 14]. In the analysis of this hypothetical cohort, Holland's clinical criteria were used to establish OT diagnosis in seropositive patients; PCR test results of intraocular fluid samples were used to establish OT diagnosis in patients with atypical disease [9, 15, 16].
2.4. Selection criteria
The inclusion criteria are described in Table 1; they included adults (≥18 years of age), any sex, immunocompetent status, and a diagnosis of OT. The exclusion criteria are also described in Table 1; they included maternal toxoplasmosis infection, congenital toxoplasmosis, and immunodeficiency.
Table 1.
Inclusion and exclusion criteria.
| Inclusion criteria | Definition | Reference |
|---|---|---|
| Adult | In accordance with Colombian Law 27 of 1977, a person who is ≥18 years of age. | [17] |
| Immunocompetent | A person who has a healthy immune system that can respond to an antigen stimulus through an appropriate immune reaction. | [18] |
| OT diagnosis | In accordance with Holland's clinical criteria: White-creamy retinal lesion, together with one or more hyperpigmented retinochoroidal scars in the same eye. Diagnosis should be confirmed with a positive anti-Toxoplasma IgG test result. For atypical presentations that do not lead to a conclusive clinical diagnosis, a PCR test and the Goldmann-Witmer coefficient in intraocular fluid samples can be helpful to confirm the diagnosis (see Villard et al.). |
[15, 16, 19] |
| Exclusion criteria | ||
| Minority of age | In accordance with the Civil Code of Colombia, article 34, a person who is <18 years of age. | [17] |
| Toxoplasmosis during pregnancy |
Tg infection confirmed during pregnancy, according to assessments of Tg-specific IgM and IgG antibodies. Seroconversion from negative to positive anti-Toxoplasma IgG and IgM findings suggests acute infection. A positive IgM or IgG finding with a negative pre-conception IgG finding is considered a recently acquired infection; a positive IgG finding with a positive pre-conception IgG finding suggests previous immunization. |
[13, 20, 21] |
| Congenital toxoplasmosis |
Tg infection due to transplacental parasite transmission after primary maternal infection. This diagnosis is made with indirect (serology) and direct (PCR) methods. Serological diagnosis can be made with a positive IgM finding in the newborn and a low avidity test. Direct PCR detection is performed using amniotic fluid samples. |
[14] |
| Immunodeficiency | Partial or total lack of immune response capacity. | [22] |
Abbreviations: PCR, polymerase chain reaction; Tg, Toxoplasma gondii.
2.5. Model structure
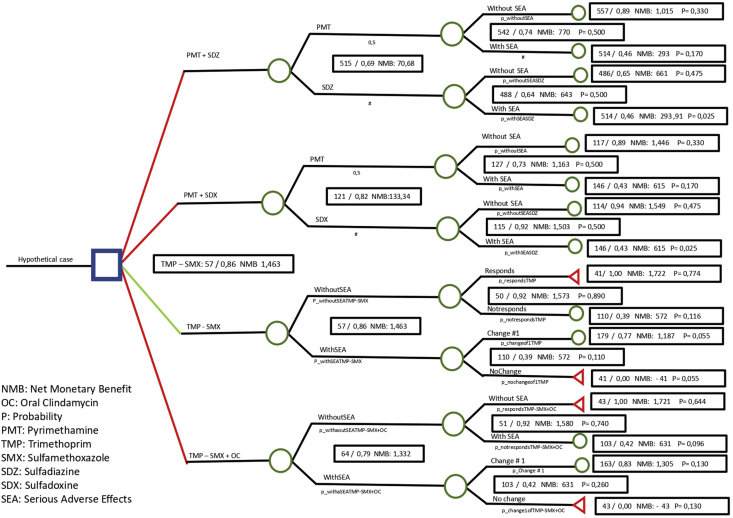
Decision tree analysis modeling was used to compare the four first-line treatment regimens. Decision trees are an essential tool for decision-making and risk analysis because of their easy application. Because these trees are visual representations of data, they are readily understandable and can be used to support rational decision-making [23]. Our model consisted of a classic decision tree from the perspective of the third-party payers (Colombian General System of Social Security in Health) (Figure 1). The decision node (dark blue square) defined the hypothetical cohort. From there, the branches evolved within a series of chance nodes (green circles) representing the probabilities and costs related to the variables included, such as treatment response, therapeutic changes, and therapeutic failure within 24 weeks after initial presentation. The terminal nodes (red triangles) represent the outcomes: number of averted therapeutic failures at each branch.
Figure 1.
Probabilistic sensitivity analysis for first-line treatment regimens–Monte Carlo simulation of incremental cost-effectiveness ratios. In quadrant II, the TMP-SMX regimen (blue) had the best performance: 80.3% of 10,000 simulations were located in the space with higher cost-effectiveness and below the proposed willingness to pay threshold.
2.6. Sources of information
To establish the effectiveness probabilities (Table 2), we performed a systematic literature search, as recommended by Justo N, Espinoza MA, Ratto B, Nicholson M, Rosselli D, Ovcinnikova O, et al [24]. We used MeSH and DeCS terms to search the electronic databases PubMed, Google Scholar, Elsevier, SciELO, and ScienceDirect for the period from January 1992 through July 2021 (Table 3) [5,13].
Table 2.
Probabilities of remission and recurrence for each drug in base case with sensitivity intervals.
| Drugs | Minimum (25%) | Base case probability | Maximum (25%) | Reference |
|---|---|---|---|---|
| Pyrimethamine sulfadiazine | Recurrence: 33.8 | Recurrence: 45 | Recurrence: 56.3 | [36] |
| Remission: 41.3 | Remission: 55 | Remission: 68.8 | ||
| Pyrimethamine sulfadoxine | Recurrence: 6.2 | Recurrence: 8.3 | Recurrence: 10.4 | [37] |
| Remission: 68.8 | Remission: 91,7 | Remission: 114.6 | ||
| TMP-SMX | Recurrence: 9.8 | Recurrence: 13 | Recurrence: 16.3 | [29] |
| Remission: 65.3 | Remission: 87 | Remission: 108.8 | ||
| Oral clindamycin∗ | - | - | - | - |
| - | - | - | ||
| Dexamethasone with intravitreal clindamycin | Recurrence: 9.3 | Recurrence: 12.5 | Recurrence: 15.6 | [32] |
| Remission: 65.6 | Remission: 87.5 | Remission: 109.4 | ||
| Azithromycin | Recurrence: 16 | Recurrence: 21.4 | Recurrence: 26.8 | [38] |
| Remission: 98.3 | Remission: 78.6 | Remission: 58.9 |
Abbreviation: TMP-SMX, trimethoprim/sulfamethoxazole.
To our knowledge, there is no available information regarding remission or recurrence probability with oral clindamycin.
Table 3.
Search strategy for systematic literature review to assess probabilities.
| Database | MeSH terms |
|---|---|
| PubMed | Ocular toxoplasmosis, clindamycin, dexamethasone, pyrimethamine, sulfadiazine, prednisolone, treatment of ocular toxoplasmosis, toxoplasmic retinochoroiditis, remission, prophylaxis, recurrence. |
| Elsevier | Treatment of ocular toxoplasmosis, toxoplasmic retinochoroiditis. |
| SciELO | Treatment of ocular toxoplasmosis, toxoplasmic retinochoroiditis, remission, prophylaxis, recurrence. |
| Google Scholar | Ocular toxoplasmosis, clindamycin, dexamethasone, pyrimethamine, sulfadiazine, prednisolone, treatment of ocular toxoplasmosis, toxoplasmic retinochoroiditis, referral prophylaxis, recurrence. |
| ScienceDirect | Ocular toxoplasmosis, treatment of ocular toxoplasmosis, toxoplasmic retinochoroiditis, remission, prophylaxis, recurrence. |
The data sources for the costs were obtained from Colombian government official reports released from January 2020 through September 2020, including the scale for regulating pharmaceutical product prices used by the Health Ministry (Termómetro de precios de medicamentos- MINSALUD) [25]. Furthermore, to address any possible price gap, we conducted a sensitivity analysis using 25% superior and inferior margins (Table 4). An exception was made with respect to treatment with folinic acid which was excluded from the sensitivity analysis because the typical tablet form was unavailable [25].
Table 4.
Costs (in USD) of drugs used in base case with sensitivity intervals.
| Drug | Minimum (25%) | Base case cost | Maximum (25%) | Reference |
|---|---|---|---|---|
| Pyrimethamine sulfadoxine | 12.5 | 16.69 | 20.87 | [25] |
| Pyrimethamine sulfadiazine | 255.69 | 340.92 | 426.15 | [25] |
| TMP-SMX | 7.98 | 10.64 | 13.3 | [25] |
| Oral clindamycin | 16.19 | 21.59 | 26.99 | [25] |
| Azithromycin | 42.79 | 57.05 | 71.32 | [25] |
| Dexamethasone with intravitreal clindamycin∗ | 1 dose = 392.76 | 1 dose = 393.07 | 1 dose = 393.37 | [25] |
| 2 dose = 393.67 | 2 dose = 393.67 | 2 dose = 394.89 | ||
| 3 dose = 394.58 | 3 dose = 395.50 | 3 dose = 396.41 |
Abbreviations: TMP-SMX, trimethoprim/sulfamethoxazole; USD, United States dollars.
Cost of procedure is included in price; it was obtained by calculating the mean costs from multiple ophthalmological centers in Colombia.
2.7. Base case definition
Based on an expert consensus, we established the base case definition as an immunocompetent adult with active OT, receiving a follow-up consultation at 6-week intervals (for 24 weeks total) to evaluate the response to a first-line treatment regimen. If therapeutic failure or serious adverse effects were identified during the follow-up period, the recommendation was to change the treatment to a second-line regimen. The analysis was made considering a maximum of three regimen changes.
2.8. Treatment regimen definitions
The four treatment regimens evaluated as first-line options were: PMT + SDZ, PMT + SDX, TMP-SMX, and TMP-SMX + OC. Second-line options for treatment changes were intravitreal clindamycin + dexamethasone, oral azithromycin, and OC. Furthermore, systemic corticosteroid (prednisolone) was added to all treatment regimens, except for intravitreal clindamycin + dexamethasone.
2.9. Outcome definition
Therapeutic failure was defined as persistent or worsening inflammation within 6 weeks after treatment initiation or recurrence within 3 months after achievement of inflammation control, in accordance with the Standardized Uveitis Nomenclature [26].
2.10. Sensitivity analysis
A discrete one-way sensitivity analysis was performed to establish model consistency, including superior and inferior margins of 25% on the base case as the minimum and maximum values for effectiveness and costs (Table 5). A probabilistic sensitivity analysis was performed based on the incremental cost-effectiveness ratio, using a Monte Carlo simulation with 10,000 iterations; willingness to pay (WTP) was set at United States dollars (USD) 473, equivalent to the most expensive treatment that the Colombian Ministry of Health is currently paying for OT treatment (i.e., intravitreal clindamycin + dexamethasone, including the injection procedure) [25].
Table 5.
Cost-effectiveness ratios for first-line treatment regimens.
| Regimens | Cost (USD) | INC Cost (USD) | EF | INC EF | ICER | NMB | C/E |
|---|---|---|---|---|---|---|---|
| TMP-SMX | 57.02 | 0.86 | 467.19 | 236336 | |||
| TMP-SMX + OC | 64.38 | 7.36 | 0.79 | 0.07 | 105.73 | -441.42 | 290292 |
| PMT + SDX | 121.57 | 57.18 | 0.82 | 0.03 | 1748.20 | -514.18 | 526378 |
| PMT + SDZ | 515.42 | 451.03 | 0.69 | 0.13 | 4545.29 | -845.22 | 2656736 |
Abbreviations: C/E, cost-effectiveness; EF, effectiveness; ICER: incremental cost-effectiveness ratio; INC cost, incremental cost; INC EF, incremental effectiveness; NMB, net monetary benefits; OC, oral clindamycin; PMT, pyrimethamine; SDX, sulfadoxine; SDZ, sulfadiazine; TMP-SMX: trimethoprim/sulfamethoxazole; USD, United States dollars.
2.11. Model validation
To assure coherence of the algorithms included in the decision tree, all authors initially planned and sketched the intended paths and nodes. Then, two authors independently introduced the data into Tree Age Pro Healthcare software LLC, Williamstown, Massachusetts-USA; a third author made a final revision to assess any disagreements. Because there are no standardized guidelines in Colombia for OT treatment, we conducted an expert consensus assessment (including renowned Colombian ophthalmologists with expertise in OT treatment) to validate the base case definition. To assess the quality and reproducibility of the probabilities included in the model, we included the systematic review search strategy and the evidence table in Table 3. Finally, we performed a series of deterministic and probabilistic sensitivity analyses to validate the model consistency and address uncertainty within the results.
3. Results
When comparing the four first-line treatments, we found that the cost of implementing the TMP-SMX treatment was USD 57.0, with 86% effectiveness. The second most effective regimen was TMP-SMX + OC (79% effectiveness), with a cost of USD 64.38. The third most effective regimen was PMT + SDX (82% effectiveness), with a cost of USD 121.57. The least effective regimen was PMT + SDZ (69% effectiveness), with a cost of USD 515.42. As shown in Table 5, when compared with the other treatment alternatives, TMP-SMX showed the best cost-effectiveness performance, with a mean cost-effectiveness that ranged from seven to 17 percentage points above the other options, as well as the highest value for net monetary benefits.
Concerning second-line treatments that were used in the event of therapeutic failure or severe adverse effects, regardless of changes made in the first-line treatments, intravitreal clindamycin demonstrated the highest effectiveness (87%) among the four regimens, with a cost of 473 USD; thus, it is highly effective as rescue therapy, consistent with previous reports [10, 12].
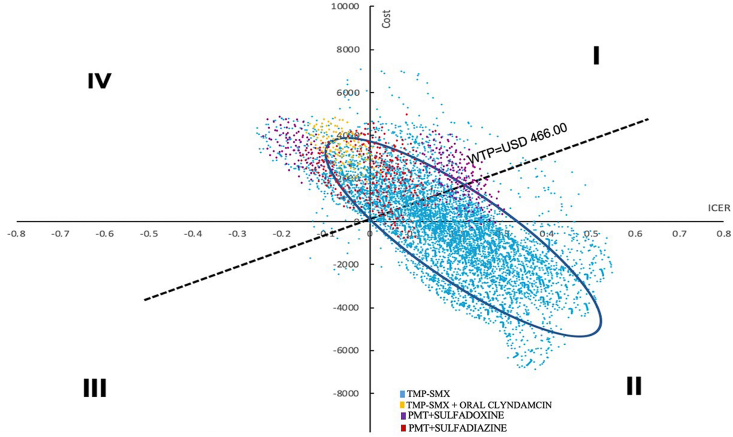
A Monte Carlo simulation was conducted to evaluate the model consistency and the degree of uncertainty involved, including 10,000 iterations. Analysis of the results of the four regimens in a cost-effectiveness chart (Figure 2) revealed that the TMP-SMX regimen is located within a 95% confidence interval in quadrant II where the strategy is considered most effective and least expensive; it exhibits clear dominance with respect to the alternative regimens. The TMP-SMX + OC regimen was located in quadrant IV, indicating that it was the least effective and most expensive strategy with respect to the alternative regimens. Regarding the PMT + SDX and PMT + SDZ regimens most of their iterations were located in quadrant I, indicating that the alternatives were more effective but more expensive; both required a careful evaluation according to the WTP. In our assessments, both alternatives exceeded the proposed WTP threshold.
Figure 2.
Net monetary benefits vs. willingness to pay for first-line treatment regimens. The net monetary benefit was higher for the TMP-SMX regimen than for the other first-line regimens within a wide range of willingness to pay values, indicating that it was the most cost-effective alternative.
4. Discussion
This study investigated the cost-effectiveness of four first-line treatment regimens for OT in Colombia, with the goal of generating evidence for clinical practice and providing decision-making alternatives for the establishment of Colombian Health System policy. Our results indicated that the most cost-effective treatment regimen was TMP-SMX. This antibiotic treatment has been established as an economical alternative, and its effectiveness is similar to the classical therapy for active toxoplasmic retinochoroiditis [27].
A prospective study conducted by Soheilian et al. [28] compared the efficacy of classical therapy versus TMP-SMX for active OT, combined with oral prednisolone for 6 weeks. In that study, retinochoroiditis resolved after antibiotic treatment in all patients; there were no significant differences in lesion size reduction (61% with classical therapy and 59% with TMP-SMX, p = 0.75) or visual acuity. Furthermore, adverse reactions to the two treatment regimens were similar; the recurrence rates after 24 months of follow-up were 10.3% with classical therapy and 10% with TMP-SMX (p = 0.64) [28].
Notably, a reduction of 75% of Tg retinochoroiditis recurrence has been described when using a long-term intermittent TMP-SMX treatment [27]. Similarly, Fernandes Felix et al. [29] conducted a clinical trial that compared the effectiveness of treatment for 1 year with TMP-SMX (800/160 mg) versus placebo in reducing the risk of toxoplasmic retinochoroiditis recurrence. The cumulative probabilities of recurrence in the placebo group at 1, 2, and 3 years of follow-up were 13%, 17.4%, and 20.3%, respectively; the overall probability of recurrence was 0% in the TMP-SMX group [29]. In multiple studies, TMP-SMX has been regarded as a good option for prophylactic therapy, considering that it has few side effects, low cost, good accessibility, and adequate tolerance, even when administered continuously [27, 29, 30, 31]. Our findings support the previous results regarding TMP-SMX cost-effectiveness.
It is important to mention intravitreal clindamycin therapy as an excellent second-line option [32, 33]. However, because intravitreal therapy involves higher costs and there is a preference for systemic treatment as a first-line option, intravitreal clindamycin is recommended as rescue therapy, rather than first-line therapy. Regarding PMT + SDZ, the combined regimen was recently withdrawn from the Colombian market and worldwide. Although this combination was used as the classical therapy, our results support the use of TMP-SMX as the most cost-effective strategy.
This study had some limitations. First, the results are not readily generalizable. The restricted quantity of information, unavailability of some data, and limited access to local databases presented challenges in establishment of the base case. Second, the study was limited by the insufficient information regarding recurrence rate and therapeutic failure in Colombia. Finally, this study only considered OT treatment for immunocompetent adult patients; it did not include pediatric patients with congenital toxoplasmosis, nor did it include immunosuppressed or pregnant patients.
Despite its limitations, to our knowledge, this is the first economic study of cost-effectiveness OT treatment in Colombia. Its value lies in providing evidence for clinical practitioners, local stakeholders, and regulatory agencies regarding the feasibility and benefits of OT treatment options in Colombia. Additionally, it provides insights to support new OT research in Colombia, and it will encourage analogous studies in other Latin American countries with similar disease circumstances and comparable health systems. Additionally, its aim and strategies could be enhanced by additional investigations performed in Colombia, such as the study by Chicaíza Becerra et al. [34], which investigated the cost-effectiveness of diagnosing congenital toxoplasmosis in Colombia.
Finally, the results of this study will be useful for improving quality of life in patients with OT, their families, and society. In particular, visual impairment and blindness, regardless of the degree and age of presentation, can lead to altered neurodevelopment and personality development, as well as difficulties with interpersonal relationships and partial/permanent inability to work [35].
5. Conclusions
This study demonstrated that, in Colombia, the most cost-effective first-line OT treatment regimen was TMP-SMX. Therefore, we recommend using this regimen in immunocompetent patients with OT, based on its availability and good performance. Additionally, this study highlights the need for further research to produce high-quality empirical evidence regarding OT treatment in other Latin American countries with similar disease circumstances and comparable health systems; the results will provide insights that can improve local decision-making and enhance quality of life in affected patients.
Declarations
Author contribution statement
Valentina Álvarez-García, Lorena Rubio-Romero, María Alejandra Maldonado, Marcela Gómez-Suárez and Alejandra de-la-Torre: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We thank Ryan Chastain-Gross, Ph.D., from Edanz (https://www.edanz.com/ac) for editing a draft of this manuscript and Universidad del Rosario for financing the publication charges and proofreading of this article.
References
- 1.Tenter A.M., Heckeroth A.R., Weiss L.M. Toxoplasma gondii: from animals to humans. Int. J. Parasitol. noviembre de 2000;30(12-13):1217–1258. doi: 10.1016/s0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Subauste C.S., Ajzenberg D., Kijlstra A. Review of the series “disease of the year 2011: toxoplasmosis” pathophysiology of toxoplasmosis. Ocul. Immunol. Inflamm. octubre de 2011;19(5):297–306. doi: 10.3109/09273948.2010.605198. [DOI] [PubMed] [Google Scholar]
- 3.Peyron F., Lobry J.R., Musset K., Ferrandiz J., Gomez-Marin J.E., Petersen E. Serotyping of Toxoplasma gondii in chronically infected pregnant women: predominance of type II in Europe and types I and III in Colombia (South America) Microb. Infect. agosto de 2006;8(9-10):2333–2340. doi: 10.1016/j.micinf.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 4.Holland G.N. Ocular toxoplasmosis: a global reassessment. Am. J. Ophthalmol. diciembre de 2003;136(6):973–988. doi: 10.1016/j.ajo.2003.09.040. [DOI] [PubMed] [Google Scholar]
- 5.Gómez-Marín J.E.G., Castaño Osorio J.C., Montoya de Londoño M.T. Toxoplasmosis congenita en Colombia: un problema subestimado de salud pública. Colomb. Méd. 30 de octubre de 2014;26(2):66–70. [Google Scholar]
- 6.de-la-Torre A., González-López G., Montoya-Gutiérrez J.M., Marín-Arango V., Gómez-Marín J.E. Quality of life assessment in ocular toxoplasmosis in a Colombian population. Ocul. Immunol. Inflamm. agosto de 2011;19(4):262–266. doi: 10.3109/09273948.2011.582220. [DOI] [PubMed] [Google Scholar]
- 7.Canamary A.M., Monteiro I.R., Machado Silva M.K.M., Regatieri C.V.S., Silva L.M.P., Casaroli-Marano R.P. Quality-of-Life and psychosocial aspects in patients with ocular toxoplasmosis: a clinical study in a tertiary care hospital in Brazil. Ocul. Immunol. Inflamm. 18 de mayo de 2020;28(4):679–687. doi: 10.1080/09273948.2019.1612453. [DOI] [PubMed] [Google Scholar]
- 8.Palmezano Díaz J.M., Plazas Rey L.K., Rojas Carvajal D. 1 de junio de 2015. Infección por toxoplasma: panorama actual. Spei Domus [Internet]https://revistas.ucc.edu.co/index.php/sp/article/view/1154 [citado 20 de noviembre de 2019];11(22). Disponible en: [Google Scholar]
- 9.De-la-Torre A., López-Castillo C., Gomez-Marin J. Incidence and clinical characteristics in a Colombian cohort of ocular toxoplasmosis. Eye. 2009;23(5):1090. doi: 10.1038/eye.2008.219. [DOI] [PubMed] [Google Scholar]
- 10.De-La-Torre A., Stanford M., Curi A., Jaffe G.J., Gomez-Marin J.E. Therapy for ocular toxoplasmosis. Ocul. Immunol. Inflamm. 2011;19(5):314–320. doi: 10.3109/09273948.2011.608915. [DOI] [PubMed] [Google Scholar]
- 11.Dorangeon P.H., Marx-Chemla C., Quereux C., Fay R., Leroux B., Choisy H. The risks of pyrimethamine-sulfadoxine combination in the prenatal treatment of toxoplasmosis. J. Gynecol. Obstet. Biol. Reprod. (Paris) 1992;21(5):549–556. [PubMed] [Google Scholar]
- 12.Holland G.N., Lewis K.G. An update on current practices in the management of ocular toxoplasmosis. Am. J. Ophthalmol. 2002;134(1):13. doi: 10.1016/s0002-9394(02)01526-x. [DOI] [PubMed] [Google Scholar]
- 13.Rorman E., Zamir C.S., Rilkis I., Ben-David H. Congenital toxoplasmosis--prenatal aspects of Toxoplasma gondii infection. Reprod. Toxicol. Elmsford N. mayo de 2006;21(4):458–472. doi: 10.1016/j.reprotox.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Baquero-Artigao F., del Castillo Martín F., Fuentes Corripio I., Goncé Mellgren A., Fortuny Guasch C., de la Calle Fernández-Miranda M. The Spanish society of pediatric infectious diseases guidelines for the diagnosis and treatment of congenital toxoplasmosis. Pediatria. agosto de 2013;79(2):116.e1–116.e16. doi: 10.1016/j.anpedi.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Villard O., Filisetti D., Roch-Deries F., Garweg J., Flament J., Candolfi E. Comparison of enzyme-linked immunosorbent assay, immunoblotting, and PCR for diagnosis of toxoplasmic chorioretinitis. J. Clin. Microbiol. 2003;41(8):3537–3541. doi: 10.1128/JCM.41.8.3537-3541.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki L.A., Rocha R.J., Rossi C.L. Evaluation of serological markers for the immunodiagnosis of acute acquired toxoplasmosis. J. Med. Microbiol. enero de 2001;50(1):62–70. doi: 10.1099/0022-1317-50-1-62. [DOI] [PubMed] [Google Scholar]
- 17.Colombian Government Law 57/1987 [Internet]. Colombian civil code 1987. https://www.funcionpublica.gov.co/eva/gestornormativo/norma.php?i=39535 Disponible en:
- 18.Fleisher T.A., Rich R.R., Shearer W.T., Schroeder H.W., Frew A.J., Weyand C.M. 2015. Clinical Immunology: Principles and Practice. S.l.: Elsevier/Saunders. [Google Scholar]
- 19.Holland G.N. Ocular toxoplasmosis: a global reassessment. Part II: disease manifestations and management. Am. J. Ophthalmol. enero de 2004;137(1):1–17. [PubMed] [Google Scholar]
- 20.McCabe R., Remington J.S. Toxoplasmosis: the time has come. N. Engl. J. Med. 4 de febrero de 1988;318(5):313–315. doi: 10.1056/NEJM198802043180509. [DOI] [PubMed] [Google Scholar]
- 21.Cancino E, Fontibón M, León H, El Tunal M, Otálora R, Suba M, et al. Secretaría Distrital de Salud de Bogotá, DC Asociación Bogotana de Obstetricia y Ginecología (Asbog). Guía Control Prenat Factores Riesgo.
- 22.Raje N., Dinakar C. Overview of immunodeficiency disorders. Immunol. Allergy Clin. N. Am. noviembre de 2015;35(4):599–623. doi: 10.1016/j.iac.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gardino S.L., Jeruss J.S., Woodruff T.K. Using decision trees to enhance interdisciplinary team work: the case of oncofertility. J. Assist. Reprod. Genet. mayo de 2010;27(5):227–231. doi: 10.1007/s10815-010-9413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Justo N., Espinoza M.A., Ratto B., Nicholson M., Rosselli D., Ovcinnikova O. Real-world evidence in Healthcare decision making: global trends and case studies from Latin America. Value Health. junio de 2019;22(6):739–749. doi: 10.1016/j.jval.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 25.Ministerio de Salud y Protección Social . 2021. TERMÓMETRO DE PRECIOS DE MEDICAMENTOS [Internet]https://www.minsalud.gov.co/salud/MT/Paginas/termometro-de-precios.aspx [citado 10 de marzo de 2021]. Disponible en: [Google Scholar]
- 26.Jabs D.A., Nussenblatt R.B., Rosenbaum J.T. Standardization of uveitis nomenclature (SUN) working group. Standardization of uveitis nomenclature for reporting clinical data. Results of the first International workshop. Am. J. Ophthalmol. septiembre de 2005;140(3):509–516. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silveira C., Belfort R., Muccioli C., Holland G.N., Victora C.G., Horta B.L. The effect of long-term intermittent trimethoprim/sulfamethoxazole treatment on recurrences of toxoplasmic retinochoroiditis. Am. J. Ophthalmol. julio de 2002;134(1):41–46. doi: 10.1016/s0002-9394(02)01527-1. [DOI] [PubMed] [Google Scholar]
- 28.Soheilian M., Sadoughi M.-M., Ghajarnia M., Dehghan M.H., Yazdani S., Behboudi H. Prospective randomized trial of trimethoprim/sulfamethoxazole versus pyrimethamine and sulfadiazine in the treatment of ocular toxoplasmosis. Ophthalmology. 2005;112(11):1876–1882. doi: 10.1016/j.ophtha.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 29.Fernandes Felix J.P., Cavalcanti Lira R.P., Cosimo A.B., Cardeal da Costa R.L., Nascimento M.A., Leite Arieta C.E. Trimethoprim-sulfamethoxazole versus placebo in reducing the risk of toxoplasmic retinochoroiditis recurrences: a three-year follow-up. Am. J. Ophthalmol. octubre de 2016;170:176–182. doi: 10.1016/j.ajo.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Rothova A., Meenken C., Buitenhuis H.J., Brinkman C.J., Baarsma G.S., Boen-Tan T.N. Therapy for ocular toxoplasmosis. Am. J. Ophthalmol. abril de 1993;115(4):517–523. doi: 10.1016/s0002-9394(14)74456-3. [DOI] [PubMed] [Google Scholar]
- 31.Silveira C., Belfort R., Jr., Muccioli C., Abreu M.T., Martins M.C., Victora C. A follow-up study of Toxoplasma gondii infection in southern Brazil. Am. J. Ophthalmol. 2001;131(3):351–354. doi: 10.1016/s0002-9394(00)00830-8. [DOI] [PubMed] [Google Scholar]
- 32.Baharivand N., Mahdavifard A., Fouladi R.F. Intravitreal clindamycin plus dexamethasone versus classic oral therapy in toxoplasmic retinochoroiditis: a prospective randomized clinical trial. Int. Ophthalmol. febrero de 2013;33(1):39–46. doi: 10.1007/s10792-012-9634-1. [DOI] [PubMed] [Google Scholar]
- 33.Soheilian M., Ramezani A., Azimzadeh A., Sadoughi M.M., Dehghan M.H., Shahghadami R. Randomized trial of intravitreal clindamycin and dexamethasone versus pyrimethamine, sulfadiazine, and prednisolone in treatment of ocular toxoplasmosis. Ophthalmology. 2011;118(1):134–141. doi: 10.1016/j.ophtha.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 34.Chicaíza-Becerra L., García-Molina M., Oviedo-Ariza S., Gómez-Marín J.E. Cost effectiveness of various diagnostic strategies for detecting congenital toxoplasmosis in newborns. Infection. 2014;17(2):53–60. [Google Scholar]
- 35.Ministerio de Trabajo . 2014. Manual único para la calificación de la pérdida de la capacidad laboral y ocupacional [Internet]. Decreto 1507.https://www.mintrabajo.gov.co/documents/20147/51963/Manual+Unico+de+Calificaciones+Decreto.pdf/7d224908-ef78-1b90-0255-f62a3e409e4c Disponible en: [Google Scholar]
- 36.Aleixo ALQ do C., Curi A.L.L., Benchimol E.I., Amendoeira M.R.R. Toxoplasmic retinochoroiditis: clinical characteristics and visual outcome in a prospective study. Ngondi JM, editor. PLoS Neglected Trop. Dis. 2 de mayo de 2016;10(5) doi: 10.1371/journal.pntd.0004685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruf B., Schürmann D., Bergmann F., Schüler-Maué W., Grünewald T., Gottschalk H.J. Efficacy of pyrimethamine/sulfadoxine in the prevention of toxoplasmic encephalitis relapses andPneumocystis carinii pneumonia in HIV-infected patients. Eur. J. Clin. Microbiol. Infect. Dis. 1 de mayo de 1993;12(5):325–329. doi: 10.1007/BF01964427. [DOI] [PubMed] [Google Scholar]
- 38.Lashay A., Mirshahi A., Parandin N., Riazi Esfahani H., Mazloumi M., Reza Lashay M. A prospective randomized trial of azithromycin versus trimethoprim/sulfamethoxazole in treatment of toxoplasmic retinochoroiditis. J. Curr. Ophthalmol. junio de 2017;29(2):120–125. doi: 10.1016/j.joco.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.