Abstract
T1-hyperintense urine can be an incidental finding on MRI with many potential causes, such as prior gadolinium administration or hematuria. This is the case of a 33-year-old female with a history of sickle cell disease complicated by iron overload secondary to chronic transfusions, who has been on multiple different iron chelation regimens. Due to persistent iron overload despite various treatments, the patient was started on a new iron chelation regimen that utilized a protocol involving inpatient admission for high dose IV deferoxamine. While admitted for the administration of this regimen, the patient underwent an MRI due to acute on chronic hip pain; this MRI demonstrated an incidental finding of T1-hyperintense urine. There was no evidence found to suggest that this T1-hyperintense urine was caused by prior gadolinium administration, hematuria, or other typical causes of T1-hyperintensity. This incidental finding was thought to have been caused by the usage of deferoxamine; to our knowledge, there is no previous literature discussing this association. Therefore, the findings of this case report demonstrate that this medication is an important item to keep in mind while evaluating the differential diagnosis of T1-hyperintense urine.
Keywords: Deferoxamine, MRI, Urine, T1-hyperintensity
Introduction
Deferoxamine is an iron chelating agent, and it is used to treat both acute iron toxicity and chronic iron overload [1]. It binds to iron that is free from any iron carrying proteins and forms the octahedral iron complex known as ferrioxamine, which is excreted in both urine and bile to decrease iron levels [1]. We will describe a case in which the use of IV deferoxamine for iron chelation in a patient with sickle cell disease was thought to cause an incidental finding of T1-hyperintense urine on MRI of the pelvis.
Case Report
This patient is a 33-year-old female with a significant past medical history of sickle cell disease complicated by transient ischemic attack, iron overload secondary to chronic transfusion, avascular necrosis, moyamoya with progressive cerebrovascular disease, and recurrent thrombosis. The patient was admitted to the hospital for 48 hours of chelation therapy with high dose IV deferoxamine (Desferal, Novartis Pharma Stein AG, Stein, Switzerland) [2]. This patient has an extensive history of recurrent sickle cell crises. Due to minimal improvement in hemoglobin levels and increased crises despite treatment, the patient began receiving simple transfusions every four weeks; the patient went on to develop iron overload secondary to these transfusions. Aggressive iron chelation was attempted with multiple different regimens, including subcutaneous deferoxamine, deferasirox (Exjade, Novartis Pharma Stein AG, Stein, Switzerland) [3], and a newer formulation of deferasirox (Jadenu, Novartis Pharma Stein AG, Stein, Switzerland) [4]. Due to persistent iron overload, the patient also began IV deferoxamine infusions; these were eventually discontinued due to lack of improvement of iron overload. The patient began exchange transfusions in addition to deferasirox. Deferiprone (Ferriprox, Apotex Inc., Toronto, Ontario, Canada) [5] was later started as an alternative oral iron chelator to deferasirox. Following this change, deferoxamine was again added for combination therapy due to continued iron overload. In more recent months, a new deferoxamine regimen was started. This regimen requires hospital admission every 14-28 days for high dose IV deferoxamine.
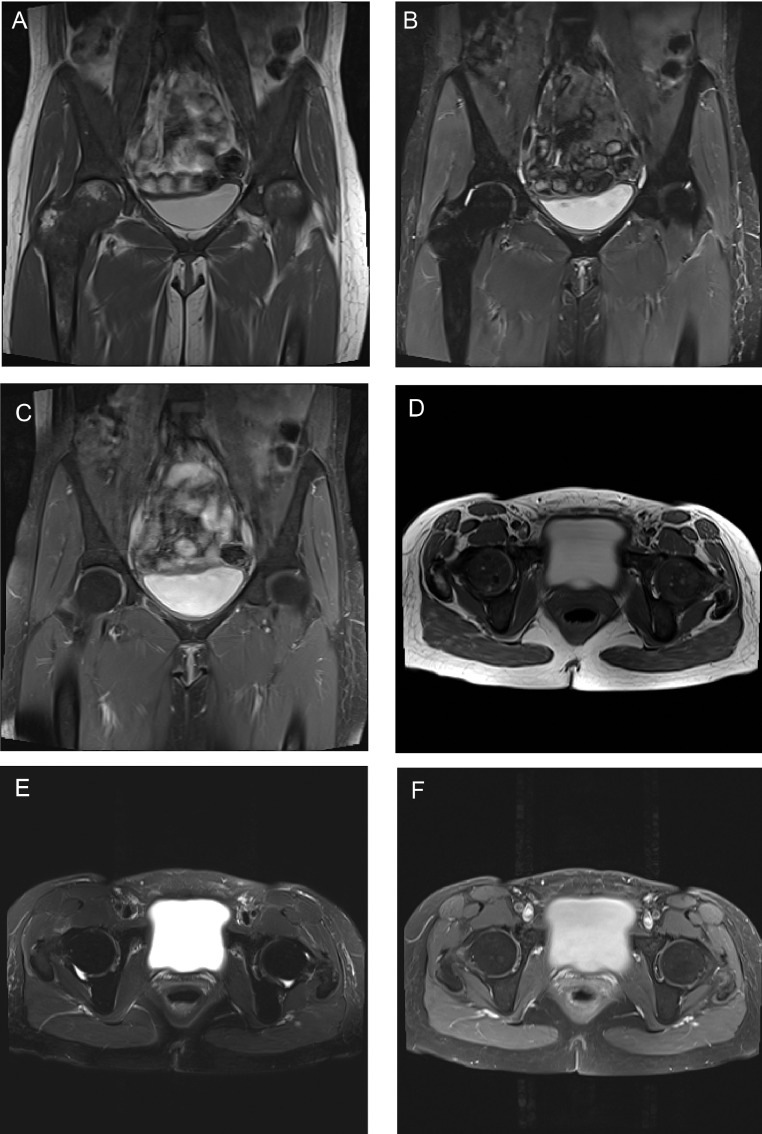
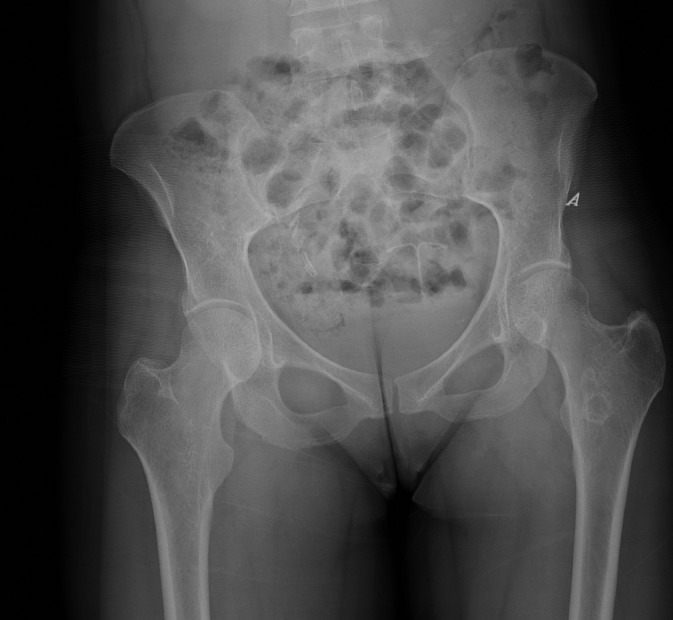
According to this newest regimen, the patient was admitted for 48 hours of chelation therapy with high dose IV deferoxamine 800mg/hr (15mg/kg/hr) as a continuous infusion. During this treatment, deferiprone was held. Throughout the admission, her vital signs remained stable, and she was afebrile. Initial laboratory evaluation was significant for a white blood cell count of 11.83, with a maximum of 11.98, but this normalized throughout the admission. Otherwise, laboratory evaluation revealed chronic findings of anemia and iron overload, with hemoglobin of 7.8, iron of 160, and iron saturation of 116 early in the admission. Her hemoglobin did downtrend to as low as 6.8, but she remained asymptomatic and received no transfusions during her chelation therapy. This decrease in hemoglobin was thought to be dilutional and IV fluids were stopped, after which the hemoglobin remained stable. Laboratory values were otherwise noncontributory. During the admission, she noted that she had been having worsening hip pain over the previous few days that was not like her usual sickle cell pain. She described mid to low back pain, along with left pelvic pain; she stated that most of the time her chronic pain is in the chest, ribs, and right hip. On physical exam, she was noted to have tenderness in multiple areas, including the lumbosacral spine, left pubic bone, L2 region radiating to the right flank, and left posterior superior iliac spine. Physical exam was otherwise unremarkable. The decision was made to obtain an MRI to assess for worsening hip integrity due to her history of avascular necrosis. An MRI of the pelvis with and without contrast was performed on day two of her hospitalization. The exam was performed on a Siemens Skyra 3T MRI (Siemens AG, Munich, Germany). The MRI images revealed diffuse T1-hypointense and T2-hypointense bone marrow signal consistent with her known sickle cell disease and chronic iron overload (Fig. 1). Otherwise, no acute findings were identified, and there was no evidence of avascular necrosis (Fig. 1). On this MRI, it was also noted that there was an incidental finding of intrinsically T1-hyperintense urine (Fig. 1: A, D). An AP radiograph of the pelvis was performed on the same day, and this showed no abnormal hyperdensity that could explain the T1-hyperintense urine (Fig. 2).
Fig. 1.
(A) Coronal T1-weighted (TR 500 TE 10), (B) STIR (TR 4000 TE 44 TI 220) and (C) postcontrast fat-saturated T1-weighted (TR 643 TE 10), and (D) axial T1 (TR 700 TE 10), (E) T2-weighted with fat-saturation (TR 4000 TE 88) and (F) post-contrast fat-saturated T1-weighted (TR 650 TE 10) images demonstrating intrinsic T1-hyperintensity of the urine (A, D). Note also diffuse T1- and T2-hypointensity of the bone marrow due to chronic sickle cell disease and iron overload.
Fig. 2.
AP radiograph of the pelvis from the same day demonstrating no abnormal hyperdensity in the bladder.
Discussion
Urine normally demonstrates low T1 intensity and high T2 intensity on MRI [6]. The differential diagnosis for T1-hyperintense urine could include hematuria, prior gadolinium administration, or the presence of certain macromolecules or protein in the urine, which could be caused by things such as neoplasm, infection, stones, obstruction, medications, or diet [6,7]. This patient had a urinalysis on the same day as the MRI that was negative for hemoglobin, indicating that hematuria was unlikely to be the cause of the T1-hyperintense urine. The urinalysis in total was unremarkable, making an acute pathology such as infection or obstruction less likely as well. Foran et al. noted in their study that if a patient has received gadolinium in the 36 hours before the current MRI, then the T1-hyperintense urine is likely due to gadolinium excretion in the urine [7]; if the patient has a recent MRI and has received gadolinium more than 36 hours before the current MRI, then the T1-hyperintense urine could be caused by delayed gadolinium excretion in the setting of decreased renal function [7]. This patient's last MRI was about 10.5 months prior to this current MRI; she also had normal eGFR and no elevation in creatinine upon initial laboratory evaluation, indicating no renal impairment. Therefore, prior gadolinium administration is unlikely to be the cause of the T1-hyperintense urine in this case. The interpreting radiologist also confirmed with the technologist that IV gadolinium was not administered prior to the non-fat saturated T1-weighted acquisition, and the medication record in the electronic medical record corroborated this.
In the absence of any of these potential causes of this incidental finding, and because deferoxamine is known to be renally excreted [1], we find it most likely that this incidental finding is secondary to deferoxamine usage. To our knowledge, there is no literature regarding IV deferoxamine usage and its possible relation to T1-hyperintense urine. A study by Babos et al. evaluated potential oral contrast agents for gastrointestinal tract MRI and noted that iron-deferoxamine solution displayed positive T1 enhancement [8]. With this knowledge, it seems most likely that deferoxamine led to the T1-hyperintense urine displayed on this patient's MRI. This association is important to keep in mind when evaluating the potential causes of incidentally found T1-hyperintense urine.
Conclusion
To our knowledge, this is the first study to report the potential association between IV deferoxamine usage and T1-hyperintensity of the urine on MRI. Because deferoxamine is renally excreted [1], and previous literature has demonstrated that deferoxamine compounds can display positive T1 enhancement [8], it is likely that deferoxamine can cause this incidental finding of T1-hyperintense urine on MRI. Knowing this association is important to prevent the T1-hyperintensity from being interpreted as a pathologic finding.
Footnotes
Competing Interests: none.
Patient Consent: for case reports, our institution does not require informed consent.
References
- 1.IBM Watson Health.(n.d.). DEFEROXAMINE. IBM Micromedex®. Retrieved August 10, 2021, from https://www-micromedexsolutions-com.medjournal.hmc.psu.edu:2200/micromedex2/librarian/CS/F07FAA/ND_PR/evidencexpert/ND_P/evidencexpert/DUPLICATIONSHIELDSYNC/CCA37D/ND_PG/evidencexpert/ND_B/evidencexpert/ND_AppProduct/evidencexpert/ND_T/evidencexpert/PFActionId/evidencexpert.DoIntegratedSearch?SearchTerm=deferoxamine&UserSearchTerm=deferoxamine&SearchFilter=filterNone&navitem=searchALL#.
- 2.Label (PDF): Desferal. 2011, August 16th. U.S. Food and Drug Administration. Retrieved August 20th, 2021 from https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/016267s050lbl.pdf.
- 3.Label (PDF): EXJADE. 2005, November 2nd. U.S. Food and Drug Administration. Retrieved August 20th, 2021 from https://www.accessdata.fda.gov/drugsatfda_docs/label/2005/021882lbl.pdf.
- 4.Jadenu (deferasirox) tablets: printed labeling (PDF).2015, March 30th. U.S. Food and Drug Administration. Retrieved August 20th, 2021 from https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/206910Orig1s000Lbl.pdf.
- 5.Label (PDF): FERRIPROX (deferiprone) tablets, for oral use. 2020, February 20th. U.S. Food and Drug Administration. Retrieved August 20th, 2021 from https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/021825s007lbl.pdf.
- 6.Rosenkrantz A.B., Niver B.E., Kopec M., Berkman D.S., Lepor H., Babb J.S. T1 hyperintensity of bladder urine at prostate MRI: Frequency and comparison with urinalysis findings. Clin Imag. 2011;35(3):203–207. doi: 10.1016/j.clinimag.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Foran P., Hwang S., Mazaheri Y., Panicek D.M. High-signal bladder urine at T1-weighted MR imaging performed 1-7 days after a prior gadolinium-enhanced MRI: prevalence and correlation with renal function. BJR Open. 2019;1(1) doi: 10.1259/bjro.20180030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Babos M., Schwarcz A., Randhawa M.S., Marton B., Kardos L., Palkó A. In vitro evaluation of alternative oral contrast agents for MRI of the gastrointestinal tract. Euro J Radiol. 2008;65(1):133–139. doi: 10.1016/j.ejrad.2007.03.025. [DOI] [PubMed] [Google Scholar]